Abstract
Objective:
Type 2 diabetes (T2D) and obesity are global epidemics leading to excess cardiovascular disease (CVD). This study investigates standard and novel cardiac MRI parameters to detect subclinical cardiac and central vascular dysfunction in inactive people with and without T2D.
Methods:
Physically inactive age and BMI-similar premenopausal women and men with (n=22) and without (n=34, controls with overweight/obesity (CWO)) uncomplicated T2D were compared to an age- and sex-similar reference control (RC) cohort (n=20). Left ventricular (LV) structure, function, and aortic stiffness were assessed by MRI. Global arterial pulse wave velocity (PWV) was assessed using carotid to femoral applanation tonometry. Regional PWV was measured via 2D phase-contrast (PC) MRI and 4D flow MRI.
Results:
Global arterial PWV did not differ between CWO and T2D. 2D PC-MRI PWV in the ascending aorta was higher in people with T2D compared to CWOs (p<0.01). 4D flow PWV in the thoracic aorta was higher in CWO (p<0.01), and T2D (p<0.001) compared to RC. End-diastolic volume, end-systolic volume, stroke volume, and cardiac output were lower in CWO and T2D groups compared to RC.
Conclusions:
Subclinical changes in arterial stiffening and cardiac remodeling in inactive CWO and T2D compared to RC support obesity and/or physical inactivity as determinants of incipient CVD complications in uncomplicated T2D. Future studies should determine the mechanistic causes of the CVD complications in greater detail in order to create therapeutic targets.
Keywords: T2D, Obesity, Aortic Stiffness, Heart Failure, Pulse wave velocity, Cardiac MRI, Physical inactivity
Graphical Abstract
Introduction
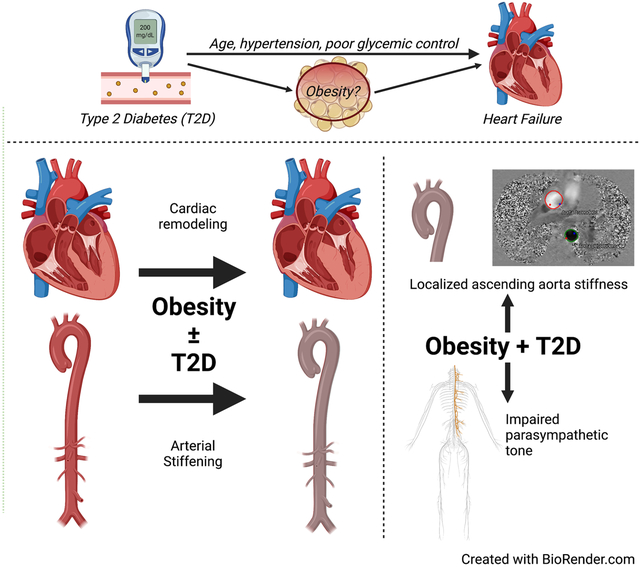
People with type 2 diabetes (T2D) or obesity have greater than twice the risk of developing heart failure (HF) than people without T2D or obesity [1,2]. For women with T2D, the risk is even higher [3]. Excess HF risk persists after adjusting for other risk factors such as age, hypertension, cholesterol, and coronary artery disease [1,4]. Failure of conventional cardiovascular (CV) risk reduction to prevent HF progression supports the idea that factors beyond traditional CV risk factors contribute to increased HF risk and mortality in people with obesity and/or T2D. However, it is not known whether the presence of both T2D and obesity leads to similar or additive risk of incident HF.
People with obesity or T2D have increased arterial stiffness, which is independently associated with increased CV morbidity and mortality [5]. Arterial stiffness increases with increasing BMI [6], while arterial stiffening in people with T2D occurs with short disease duration and can be associated with, or be independent of, atherosclerosis [5]. Regardless of the underlying process, central arterial stiffening increases cardiac afterload increases with compensatory cardiac hemodynamics that can remain subclinical for decades prior to manifesting as incipient HF.
Arterial stiffness appears to have a strong relationship with cardiorespiratory fitness, further modified by age and comorbid conditions [7]. Multiple forms of exercise intervention including isometric training [8], high intensity interval training [9], continuous aerobic exercise training and Nordic walking [10] have been shown to reduce aortic stiffness. Other reports suggest that comorbid obesity interferes with the beneficial impact of exercise on aortic stiffness in older adults[11]. We and others have reported exercise intolerance, aortic stiffness, and subclinical cardiac dysfunction in inactive middle-aged people with overweight/obesity and T2D consistent with changes seen with early HF [12,13]. Thus, more reliable detection of sub-clinical functional biomarkers of cardiac and central arterial changes are needed to: 1) identify people at risk for HF progression, 2) understand whether there are differences in HF onset and progression in T2D vs obesity, and 3) to evaluate preventative treatment efficacy and durability.
Acquisition of pulse wave velocity (PWV) as a proxy for vascular stiffness can be obtained with multiple non-invasive modalities. Higher PWV is associated with increased vascular stiffness, and most larger cohorts have employed tonometry [14–16]. Alternatively, cardiac magnetic resonance imaging (CMR) can characterize stiffness across more focal regions of the central vasculature [17]. For example, 2D phase contrast (PC) magnetic resonance imaging (MRI) can provide ‘regional’ PWV covering the ascending aorta, aortic arch, or proximal descending aorta. Of note, the ascending aorta and portions of the arch are regions that are not covered by applanation tonometry. Furthermore, 4D Flow MRI can provide additional regional information across larger portions of the thoracic aorta [18–20]. CMR can also complement information regarding vascular stiffness with measurements of cardiac structure and function to enable assessment of ventricular vascular coupling [21].
This report presents baseline data from an interventional study examining the impact of aerobic exercise training on cardiac and vascular parameters. Few such data are published in this age range comparing the effects of physical inactivity and obesity with or without T2D on cardiac and vascular function. In this baseline analysis, we tested the hypothesis that CMR would detect subclinical cardiac and central vascular dysfunction in middle-age inactive adults with overweight/obesity with and without uncomplicated T2D.
Methods
Ethical approval
This study was approved by the University of Colorado Anschutz Medical Campus Institutional Review Board (COMIRB 17–0356) and written informed consent was obtained from all participants. The study conformed to the standards set by the Declaration of Helsinki and was registered on Clinicaltrials.gov (NCT03419195). Reference control data were shared under a data use agreement (NU#7180) and written informed consent was obtained from all age- and sex-similar participants as approved by Northwestern University’s Internal Review Board (STU00204434).
Study Design
Male and female participants between the ages of 30–55 were recruited from the Rocky Mountain Veterans Affairs Medical Center and the University of Colorado and by local advertisement. Participants were enrolled in the “Research in Endothelial and Cardiovascular Health, Diabetes and Exercise and the Role of Sex” study investigating exercise training in persons with and without T2D. This manuscript reports the baseline (pre-exercise intervention) demographic, clinical, cardiac, and vascular results of the cohort, which included 22 physically inactive individuals with overweight/obesity and T2D, and 34 physically inactive controls with overweight/obesity (CWO). Physical inactivity was defined as engaging in one or fewer formal exercise bouts/week. Overweight/obesity status was defined as body mass index (BMI) 25–40. For the participants with overweight/obesity and T2D, the following inclusion criteria were used: T2D controlled by diet +/− insulin secretagogues (sulfonylureas or glinides), metformin, or glucose absorption blockers (acarbose); total HbA1c ≤9% on current therapy; all other antihyperglycemic therapies were excluded due to potential CV impact. To limit non-diabetes-related confounding factors on exercise capacity, additional exclusions are detailed on Clinicaltrials.gov (NCT03419195). For the participants with overweight/obesity but no diabetes, an HbA1c <5.7% was required. For both the groups with obesity, with or without diabetes, pregnant or breastfeeding women were excluded and participants were selected to be physically inactive, defined as engaging in one or fewer formal exercise bouts/week, and for women, to be of premenopausal status.
Participants were screened at baseline to rule out clinically evident CVD. Insulin sensitivity was assessed using a hyperinsulinemic euglycemic clamp (female participants were tested on days 5–7 of their menstrual cycles). Resting echocardiography, standard CMR including structural cine acquisitions, 4D Flow MRI and 2D PC-MRI were performed (as described in a later section). The full protocol including the exercise intervention is available on Clinicaltrials.gov (NCT03419195).
Insulin sensitivity
The hyperinsulinemic euglycemic clamp to measure insulin sensitivity via glucose infusion rate (GIR) was performed as previously reported [22]. Briefly, a single stage clamp was performed with an infusion of 80mU/m2 /min−1 insulin and 20% dextrose infusion with GIR calculated over the last 30 minutes of the clamp [22,23].
Tonometry Pulse Wave Velocity
Arterial stiffness was assessed in a fasting state via measurement of PWV (SphygmoCor CP system, AtCor Medical, Itasca, IL, USA) according to methods previously described [24,25] (Figure 1).
Figure 1.
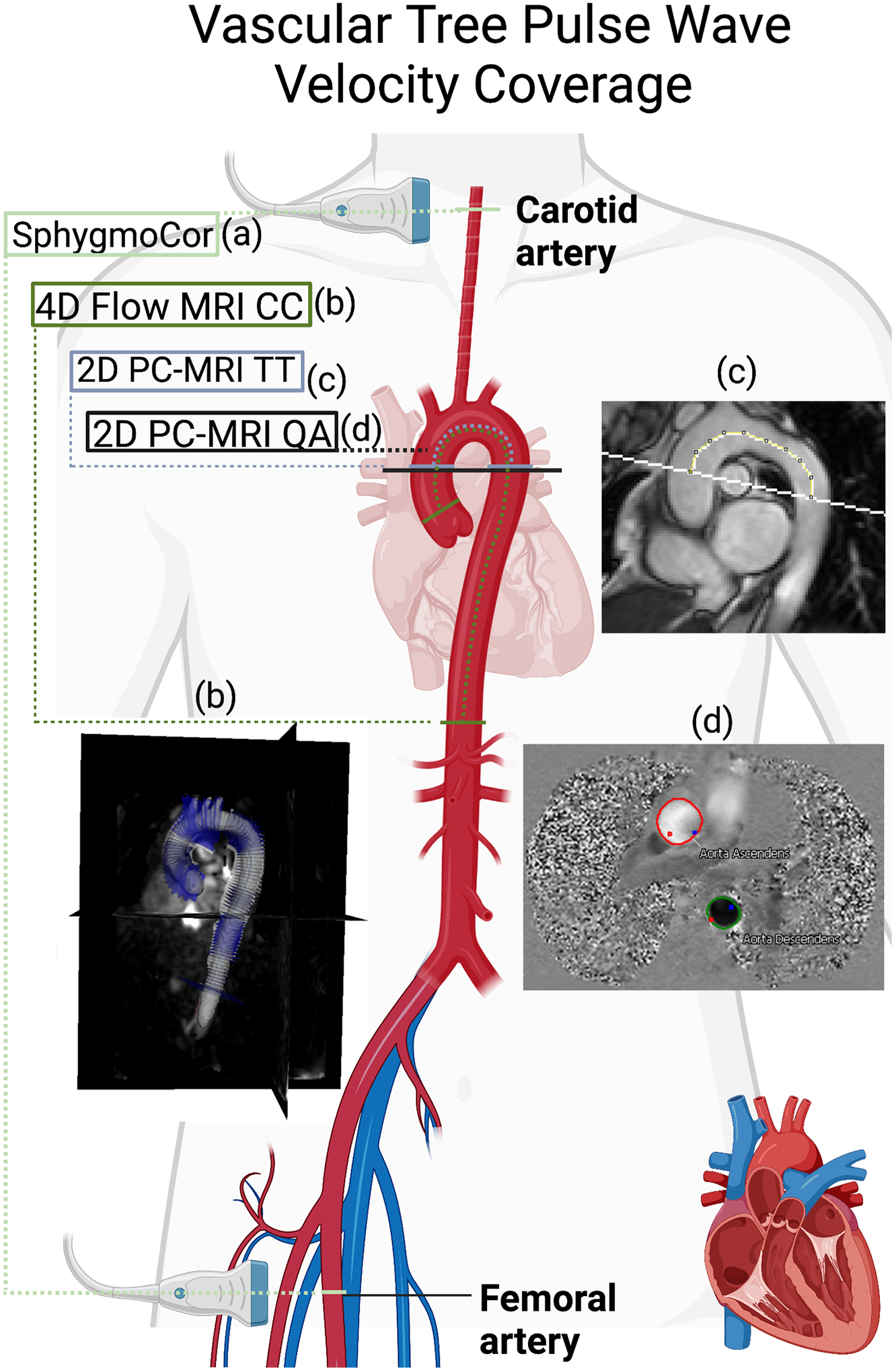
Anatomical coverage of tools for measuring arterial stiffness (a) SphygmoCor-derived carotid-femoral tonometry pulse wave velocity (PWV). (b) 4D flow MRI PWV calculated via cross-correlation of multiple planes between ascending and distal descending aorta. (c) 2D phase contrast (PC)-MRI PWV calculated via the transit-time approach between ascending and proximal descending aorta. (d) 2D PC-MRI PWV calculated via the flow-area approach (QA) in the ascending aorta and proximal descending aorta. Created with BioRender.com.
CMR Protocol
CMR was performed on a 3T Siemens Skyra (Erlangen, Germany). Balanced steady-state free precession cine sequences of the heart 4-chamber and 2-chamber views were obtained as previously described [26]. Blood flow measurements were obtained via two approaches: 1) a 2D fast low angle shot gradient echo phase-contrast sequence (2D PC-MRI) bisecting the ascending and descending aorta at the level of the right pulmonary artery, and 2) 4D flow MRI of the thoracic aorta and heart ventricles. The 2D PC-MRI pulse sequence parameters were as follows: temporal resolution=14–28 ms, echo time (TE)=2.2–3.4 ms, flip angle (FA)=20°, spatial resolution=0.82–1.56 × 0.82–1.56 mm2, slice thickness=6mm, velocity encoding (venc)=150–250 cm/s. The retrospectively-ECG gated, free-breathing 4D flow MRI was acquired in a sagittal 3D volume with a respiratory navigator located at the lung–liver interface [27] with temporal resolution=38.4–40.8ms, TE=2.3–2.6 ms; repetition time=4.8–5.1 ms, FA=7°, field of view=400–440 × 260–330 × 80–160 mm3, spatial resolution=2.20–2.75 × 2.20–2.75 × 2.40–3.20 mm3, velocity encoding (venc)=150 cm/s.
CMR Postprocessing
Left Ventricle (LV) structure - global strain, volume, and thickness:
Analysis of CMR-derived global LV strain indexes were performed using feature-tracking with CVI42 (Circle, Calgary) and semi-automatic contouring of the endocardial and epicardial borders. End-diastolic and end-systolic volumes (EDV and ESV, respectively) were calculated in CVI42 using bSSFP cine images of the long-axis, short-axis, and four-chamber-axis images. These measures were indexed to body surface area (EDVi and ESVi). LV wall thickness was measured perpendicular to the LV long axis in a short axis view.
Vessel distensibility:
2D PC-MRI was used to measure relative area change ((Dmax-Dmin)/Dmax) and normalized to pulse pressure.
PWV (Figure 1):
Three methods of PWV calculation were obtained from the 2D PC-MRI and 4D Flow MRI scans to capture the characteristics of local, regional, and central arterial stiffness. 2D flow-area PWV (local): Local PWV was computed based on the flow-area (QA) approach [28] in both the ascending aorta (AA) and descending aorta (DA) segments. For each location, time-resolved regions of interest were drawn in the cross-sectional lumen on 2D PC-MR images using CVI42. The change in flow versus change in area (dQ/dA) was used to assess regional PWV by computing dQ/dA slope from early systolic data points with in-house MATLAB code (Mathworks, Natick, MA, USA)[28]. 2D transit-time PWV (regional): The flow waveforms of the AA and DA from the 2D PC-MRI were used to compute PWV based on the transit time (TT) approach[16]. A 2D ‘candy cane’ view of the aortic arch was used to measure the path length of the aortic arch between AA and DA using ImageJ (Version 1.51w, Bethesda, MD, USA). The path length was divided by the transit time to compute the 2D transit-time PWV. 4D flow PWV via cross-correlation (central): Images were pre-processed and corrected for eddy currents and velocity aliasing using in-house software[21]. The aorta was segmented in 3D with Slicer (Version 4.10.2 r28257)[29]. As previously described, a 4D flow PWV algorithm was employed to investigate the regional stiffness of the proximal aorta (aortic root to renal arteries) [19].
To obtain further insight into the effect of obesity on CMR imaging biomarkers, a database of 100 controls collected by co-investigator Dr. Alex Barker was queried to obtain 20 age-matched reference control (RC) males and females with no self-reported history of CVD, obesity or diabetes (ages 40–49, BMI<30) [19,30] to compare to the participants with overweight/obesity with and without uncomplicated T2D. The MRI-vendor, imaging protocol, and analysis code for the reference controls was identical to that used for the participants with overweight/obesity and T2D, except that 2D QA PC-MRI was not performed in this group[19]. However, physical activity assessment was not available in the RC control group.
Statistical analysis
Data are presented as mean ± SD and median (1st and 3rd quartiles). For individual measurements, the D’Agostino-Pearson test was used to confirm normal distribution of data. An unpaired t test or Mann-Whitney test was used accordingly to evaluate differences between people with T2D and overweight controls. For comparison between T2D, CWO, and RC groups, one-way ANOVA or Kruskal-Wallis where appropriate and multiple-comparisons tests were utilized to determine the effect of obesity and diabetes on CV health. The level of statistical significance was set at p-value <0.05. All analyses were performed using GraphPad (Version 8.4.3, San Diego, CA, USA).
Results
Demographics and Clinical Phenotyping
Participant clinical characteristics are shown in Table 1. The LC, CWO and T2D cohorts were well-matched for sex and age. Consistent with the study design, the CWO and T2D cohorts had significantly higher BMI than RCs. CWO and T2D cohorts were well-matched for body composition, lipid profiles, and blood pressure. GIR, HbA1c, and glucose levels were significantly different between the T2D and CWO cohorts, as expected (p<0.01).
Table 1.
Demographics and Cardiovascular Phenotype.
| Clinical measures | RC | CWO | T2D | n (RC, CWO, T2D) |
|---|---|---|---|---|
| % Female | 45% | 59% | 48% | (20, 34, 22) |
| Age (years) | 43.0 (41.0 – 45.0) | 44.2 (38.1 – 48.6) | 46.4 (36.9 – 51.9) | (20, 34, 22) |
| Body mass index (kg/m2) | 25.4 ± 4.5 *** | 32.1 ± 5.4 | 34.1 ± 5.3 | (20, 34, 22) |
| Body surface area (m2) | 1.90 ± 0.24 *** | 2.10 ± 0.22 | 2.18 ± 0.23 | (20, 34, 22) |
| Lean body mass (kg) | 52.7 ± 9.8 | 57.7 ± 12.2 | (~, 34, 22) | |
| Body Fat % | 39.8 ± 6.2 | 38.0 ± 7.4 | (~, 34, 22) | |
| GIR (mL/kg-min) | 6.1 ± 2.4 | 4.2 ± 2.3 ** | (~, 31, 20) | |
| HbA1c (%) | 5.3 ± 0.3 | 6.9 ± 0.9 *** | (~, 34, 22) | |
| Insulin (μU/mL) in serum | 8.8 ± 4.7 | 10.5 ± 9.8 | (~, 33, 22) | |
| Glucose at scan time (mg/dL) | 88.1 ± 18.2 | 136.1 ± 59.8 *** | (~, 25, 17) | |
| Adiponectin (μg/mL) | 9.7 ± 5.1 | 7.5 ± 4.0 | (~, 28, 20) | |
| LDL-C (mg/dL) | 114.7 ± 30.7 | 109.8 ± 30.2 | (~, 34, 22) | |
| HDL-C(mg/dL) | 46.2 ± 8.3 | 44.7 ± 8.2 | (~, 34, 22) | |
| Triglycerides (mg/dL) | 130.3 ± 77.3 | 186.1 ± 161.5 | (~, 34, 22) | |
| Systolic BP (mmHg) | 117.6 ± 9.1 | 121.7 ± 7.2 | (~, 34, 22) | |
| Diastolic BP (mmHg) | 84.2 ± 7.1 | 86.8 ± 5.3 | (~, 34, 22) | |
| Pulse Pressure (mmHg) | 33.4 ± 6.4 | 33.4 ± 9.9 | (~, 34, 22) |
Data are presented as mean ± SD or median (1st and 3rd quartiles). RC=reference control, CWO=controls with overweight/obesity, T2D=participants with type 2 diabetes, GIR=Glucose Infusion Rate, LDL-C=low density lipoprotein cholesterol, HDL-C=high density lipoprotein cholesterol, BP=blood pressure.
Significantly different from BMI-similar control group with p<0.05.
p<0.01,
p<0.001.
For reference controls, all differences are between it and both study groups. GIR = glucose infusion rate. BP= blood pressure.
Arterial Stiffness
Global arterial PWV measured by SphygmoCor did not differ between CWO and T2D. PWV measured by 2D QA PC-MRI in the ascending aorta was significantly higher in people with T2D compared to CWOs (p<0.01) (Table 2). 4D flow PWV in the thoracic aorta was significantly higher in CWO (p<0.01), and T2D (p<0.001) compared to RC (Figure 2, Table 2). Of note, given the study design, the RC group did not have results for PWV measured by SphygmoCor or 2D QA PC-MRI. Results from regional measurements of PWV along the vascular tree are illustrated in the diagrams next to each measure (Figure 2).
Table 2.
Arterial Stiffness Measures
| Arterial/Aortic measures | RC | CWO | T2D | n (RC, CWO, T2D) |
|---|---|---|---|---|
| ‘Sectional’ Arterial measurements | ||||
| Global: SphygmoCor PWV (m/s) | 7.7 ± 1.3 | 8.5 ± 1.6 | (~, 31, 20) | |
| Central: 4D Flow PWV (m/s) | 6.5 ± 1.2 *** | 8.2 ± 1.9 | 8.9 ± 2.6 | (20, 30, 18) |
| Regional: 2D TT PC-MRI PWV (m/s) | 5.8 ± 1.6 | 5.6 ± 1.5 | (~, 29, 18) | |
| Ascending Aorta Measurements | ||||
| 2D QA PC-MRI PWV, AA (m/s) | 3.1 ± 0.9 | 4.1 ± 1.6 ** | (~, 27, 16) | |
| RAC, AA (%) | 19.8 ± 6.4 | 16.6 ± 6.6 | (~, 29,16) | |
| Distensibility, AA (10–3 mm Hg−1) | 6.1 ± 2.7 | 5.0 ± 2.1 | (~, 29,16) | |
| WRI, AA (Ratio, 0–1) | 0.29 ± 0.15 | 0.32 ± 0.15 | (~, 29,16) | |
| Descending Aorta Measurements | ||||
| 2D QA PC-MRI PWV, DA (m/s) | 2.3 ± 0.6 | 2.8 ± 1.4 | (~, 29, 18) | |
| RAC, DA (%) | 28.4 ± 7.5 | 26.2 ± 10.3 | (~, 29, 18) | |
| Distensibility, DA (10–3 mm Hg−1) | 9.1 ± 3.7 | 7.9 ± 2.9 | (~, 29, 18) | |
| WRI, DA (Ratio, 0–1) | 0.21 ± 0.16 | 0.22 ± 0.12 | (~, 29, 18) | |
Data are presented as mean ± SD. RC=reference control, CWO=controls with overweight/obesity, T2D=participants with type 2 diabetes, PWV= pulse wave velocity, QA= flow-area, TT=transit-time, AA=Ascending aorta, DA=Descending aorta, PC=phase contrast.
Significantly different from BMI-similar control group with p<0.05.
p<0.01,
p<0.001.
For reference controls, all differences are between it and both study groups. AA = ascending aorta. DA = descending aorta. PC-MRI = phase contrast magnetic resonance imaging. PWV = pulse wave velocity. TT = transit time approach. QA = flow area approach. RAC = relative area change. WRI = wave reflection index.
Figure 2.
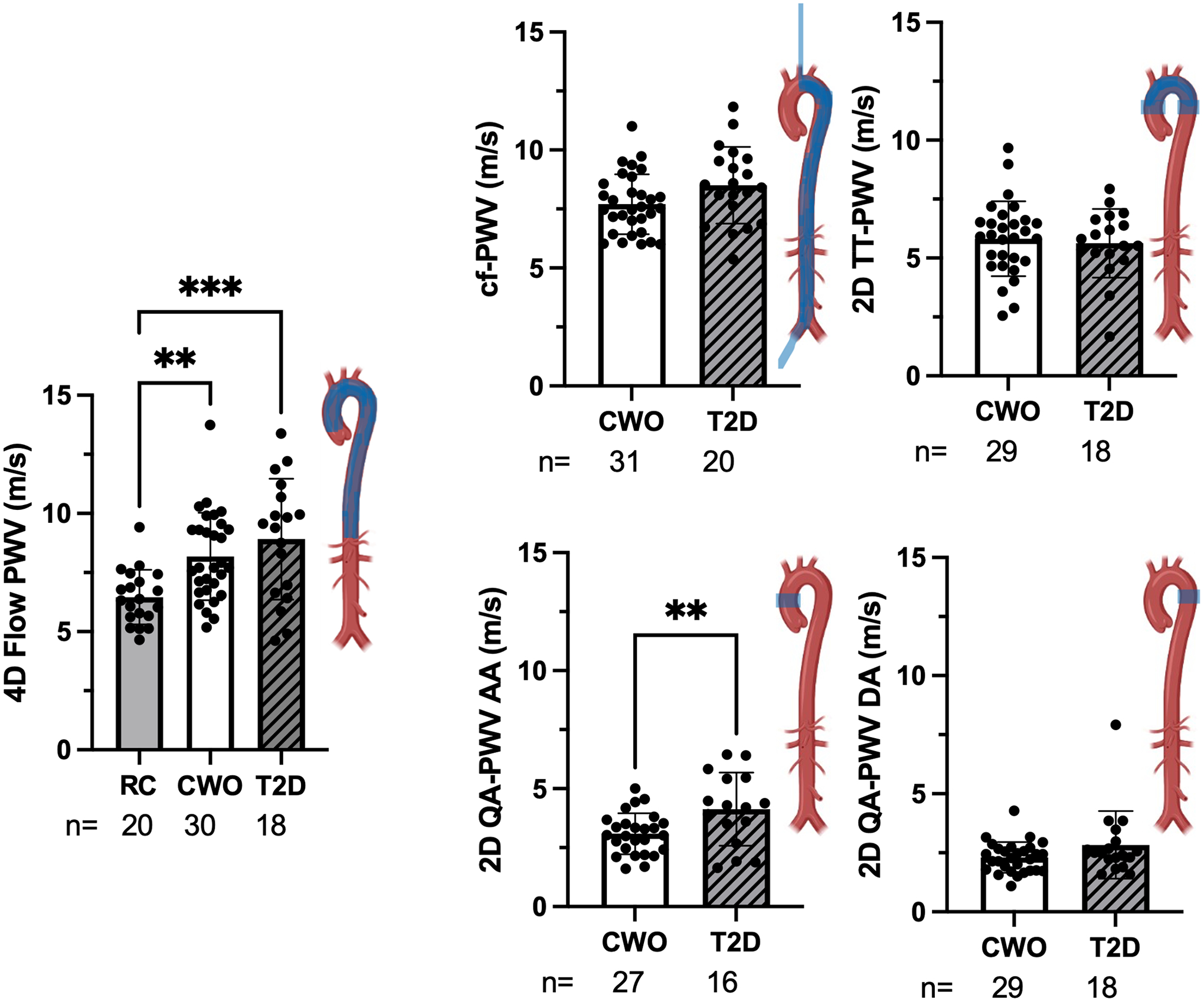
Arterial stiffness: Region assessed per diagram. RC=reference control, CWO=controls with overweight/obesity, T2D=participants with type 2 diabetes, PWV=pulse wave velocity, cf=carotid-femoral, QA=flow-area, TT=transit-time, AA=Ascending aorta, DA=Descending aorta. *Significantly different from BMI-similar control group with p<0.05. **p<0.01, ***p<0.001.
Left Ventricular Cardiac Measures
Baseline cardiac volumes and indexed volumes in the CWO and the T2D groups were significantly lower than the RC group. There were no differences in ejection fraction between the groups. BSA-indexed EDV (EDVi) was significantly lower in people with T2D compared to the CWO and RC groups (Table 3). Differences in indexed LV volume as a function of cohort were observed (Table 3, Figure 3).
Table 3.
Cardiac function in the three study groups.
| Left Ventricle Cardiac Measures | RC | CWO | T2D | n (RC, CWO, T2D) |
|---|---|---|---|---|
| ESV (mL) | 61.5 ± 16.8 *** | 47.1 ± 13.9 | 41.8 ± 10.9 | (20, 33, 22) |
| EDV (mL) | 145.4 ± 31.5 *** | 103.9 ± 22.7 | 90.6 ± 21.4 | (20, 33, 22) |
| ESVi (mL/m2) | 32.4 ± 7.8 *** | 21.8 ± 7.6 | 19.3 ± 5.0 | (20, 33, 22) |
| EDVi (mL/m2) | 76.7 ± 16.1 *** | 48.3 ± 14.0 | 41.7 ± 9.7 * | (20, 33, 22) |
| Ejection Fraction (Ratio, 0–1) | 0.58 ± 0.04 | 0.55 ± 0.07 | 0.53 ± 0.10 | (20, 33, 22) |
| Stroke Volume (mL) | 83.9 ± 16.2 *** | 55.1 ± 16.5 | 46.7 ± 18.7 | (20, 33, 22) |
| Heart Rate (1/min) | 63.0 ± 11.1 *** | 77.8 ± 10.6 | 80.3 ± 14.3 | (20, 34, 22) |
| Cardiac Output (L/min) | 5.20 ± 0.94 *** | 4.24 ± 1.23 | 3.64 ± 1.37 | (20, 33, 22) |
| Septal wall thickness (mm) | 9.6 ± 2.0 | 10.4 ± 2.0 | (~, 33, 22) | |
| Free Wall Thickness (mm) | 8.6 ± 2.4 | 9.1 ± 2.2 | (~, 33, 22) | |
| Radial Peak strain (%) | 27.5 ± 5.8 | 25.8 ± 4.7 | (~, 33, 22) | |
| Circumferential Peak Strain (%) | 18.2 ± 2.6 | 18.2 ± 2.6 | (~, 33, 22) | |
| Longitudinal Peak Strain (%) | 14.3 ± 2.8 | 12.9 ± 2.5 | (~, 31, 22) |
Data are presented as mean ± SD. RC=reference control, CWO=controls with overweight/obesity, T2D=participants with type 2 diabetes, ESV=end-systolic volume, EDV= end-diastolic volume, ESVi=ESV indexed to body surface area, EDVi=EDV indexed to body surface area.
Significantly different from BMI-similar control group with p<0.05.
p<0.01,
p<0.001.
For reference controls, all differences are between itself and both study groups.
Figure 3.
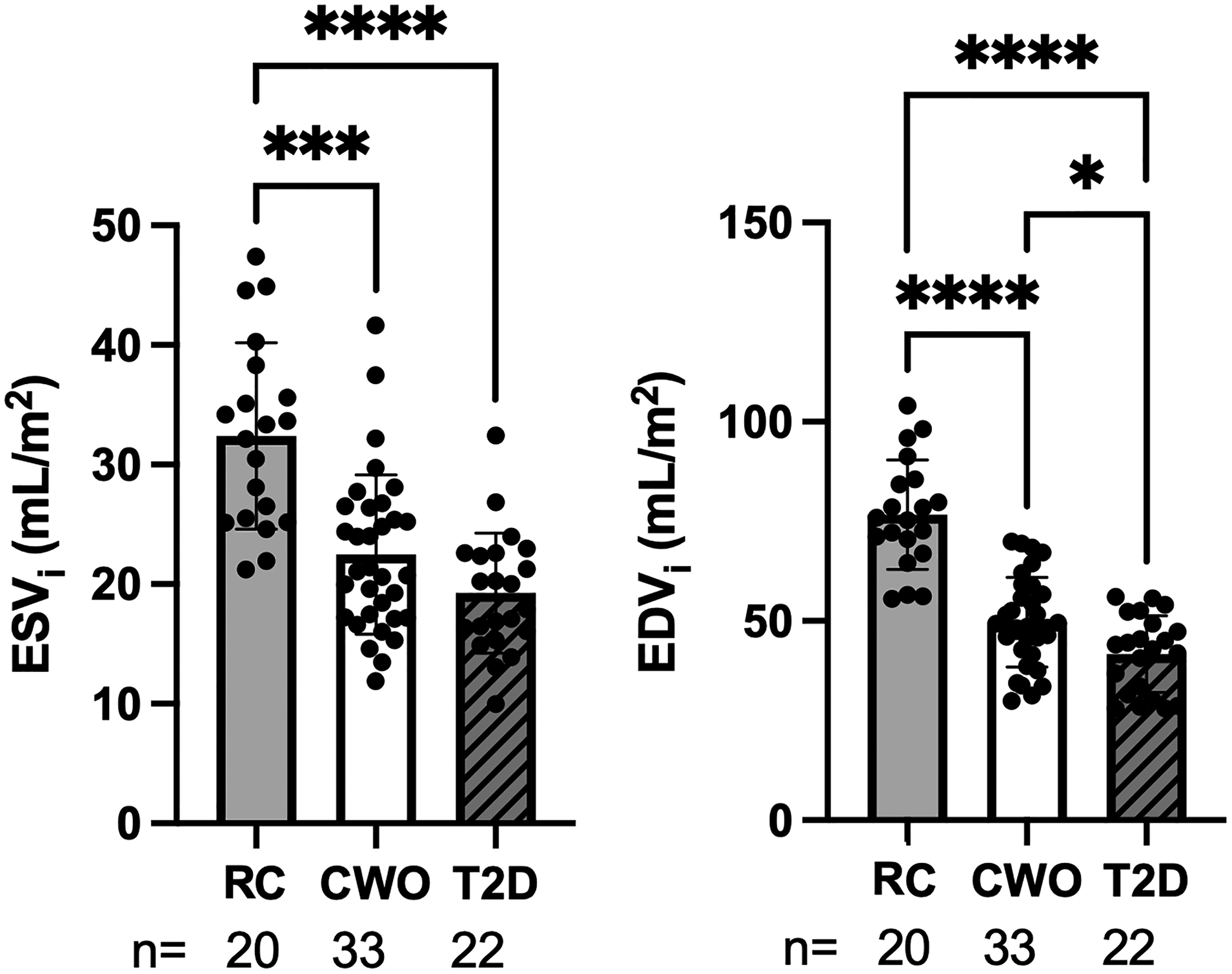
Left ventricle end-systolic and end-diastolic volume indexed to body surface area. RC=reference control, CWO=controls with overweight/obesity, T2D=participants with type 2 diabetes. EDVi=EDV indexed to body surface area, ESVi=ESV indexed to body surface area. *Significantly different from BMI-similar control group with p<0.05. **p<0.01, ***p<0.001, ****p<0.0001.
Discussion
In this study, we measured baseline clinical, cardiac, and central vascular status (using SphygmoCor and CMR) in a carefully recruited, physically inactive population selected for the purpose of studying the effects of exercise on CV health in the presence of obesity with and without uncomplicated, well controlled T2D. We found subclinical changes in arterial stiffness and cardiac remodeling in physically inactive CWO and T2D, as compared to RC. These findings support obesity and/or physical inactivity as determinants of incipient CVD complications in uncomplicated T2D. Notably, compared to the RC group, both CWO and T2D groups had significant differences in diastolic LV volumes and regional vascular parameters of increased arterial stiffness, as shown in previous studies [31,32]. Our findings suggest a greater role of obesity than has previously been reported.
Previous reports comparing people with obesity with and without T2D reported differences by diabetes status [33]; in contrast, few changes specific to the diabetes group were observed in this analysis [33]. Specifically, participants with T2D demonstrated significant differences groups only in EDVi and 2D QA-PWV in the ascending aorta compared to the CWO. Similar changes in the CWO and T2D groups support a model wherein obesity and/or physical inactivity are the primary drivers of cardiac and central arterial changes in these groups, when compared to age-similar reference controls. These findings are clinically pertinent given the advent of GLP-1 receptor and dual agonist therapies that demonstrate efficacy for weight loss and CV risk reduction, as well as SGLT-2 inhibitors which demonstrate modest weight loss and efficacy for prevention and treatment of heart failure.
Arterial stiffness is a well-accepted predictor of CV disease and mortality [34,35]. The current study comprehensively examined systemic and regional PWV markers, characterizing PWV in unique and overlapping regions of the vasculature. Tonometry measures vascular stiffness along the path from the carotid artery to the femoral artery (bypassing the proximal ascending aorta) and is perhaps the most ‘global’ PWV measurement widely available. In contrast, 4D Flow and 2D TT PC-MRI PWV measurements cover ‘regional’ sections of the thoracic aorta and aortic arch, respectively. The most location-specific PWV measurement was obtained with the 2D QA PC-MRI acquisition consisting of a single plane measurement through the ascending and descending aorta. It is important to note that these methods of measuring PWV are not interchangeable in that the QA method is more focal compared to SphygmoCor or 4D flow [14,16] and that the coverage provided by some PWV techniques overlap in area. The use of multiple modalities to assess arterial stiffness presents an opportunity for detecting regional differences not detected by global tonometric assessment.
Assessment of PWV over ‘regional’ sections of the aorta, revealed differences amongst the three cohorts. Compared to RC, the T2D and CWO cohorts had significantly greater aortic stiffness, as measured by 4D PWV. In a recent report of individuals with HF with preserved ejection fraction, arterial stiffness correlated with visceral adipose tissue, consistent with our observations with obesity alone [36]. A Chinese population study reported similar associations between arterial stiffness measured by brachial-ankle PWV and obesity [37]. Arterial stiffness as measured by 2D QA PC-MRI PWV in T2D and CWO significantly differed between the two study groups only in the ascending aorta. This finding is important given the role the relatively compliant ascending aorta plays to dampen flow and pressure waveforms during contraction (via the Windkessel effect). In our previous report in youth with type 1 diabetes (T1D), we observed selected changes in PWV and wall shear stress in the descending aorta only; interestingly, these abnormalities were responsive to treatment with metformin[37]. Proximal aortic stiffness might represent a source of excess afterload and impaired cardiac perfusion, contributing to the observed cardiac function abnormalities.
Inconsistent with our own previous work and others, there were no significant differences in SphygmoCor cfPWV between T2D and CWO study groups [24,38,39]. This may be because our participants with T2D in the present study were younger than previous studies, had a shorter duration of diabetes, or that the CWO and T2D groups had higher BMI than previous studies suggesting that systemic aortic stiffness is promoted by overweight/obesity, considering that these two groups were BMI-similar. Notably, the physiological coverage of cfPWV does not include the ascending aorta, while 2D QA PC-MRI measured-PWV focuses exclusively on the ascending aorta or descending aorta [40]. This difference in anatomic specificity of PWV methods may explain the lack of difference of global cfPWV found between people with T2D and CWO study groups despite a difference being detected on local 2D QA PC-MRI. This regional finding has potential clinical significance as most studies employ tonometry as the measure of aortic stiffness and thus may miss subtle findings of increased aortic stiffness that could be detected using CMR. We did not have cfPWV in our normative RC group for comparison, however, previous reports have shown improvement in cfPWV with weight loss [41].
Our finding of elevated arterial stiffness in PWV in both the CWO and T2D groups when compared to RC agrees with previous work finding decreased distensibility as a marker of stiffness in both the ascending and descending aorta of people with T2D and obesity [37,42]. We speculate that the greater aortic stiffness in people with relatively uncomplicated T2D and obesity is an initial marker of vascular disease in this population. Ongoing work with this cohort will test whether this finding is improved with aerobic exercise training as has been reported in some studies to differ by obesity status [11].
Cardiac Measures
Our group previously reported abnormal LV global circumferential strain and septal and posterior wall thickness in adolescents with T2D compared to overweight controls [43]. In this study, we observed no difference in strain between adults with T2D and CWO. Both the T2D and CWO groups had significantly smaller ESV and EDV compared to age-matched RC, consistent with the findings of Gulsin et al [13,44]. While the literature endorses increased rates of HF in diabetes [12], the early change in cardiac structure appears to be more closely related to obesity than T2D in this study. Participants in the T2D cohort had excellent glycemic control and well-managed cholesterol and blood pressure in contrast to our prior adolescent group who were reported to have poor control and a disproportionately high rate of microvascular and CV complications [45]. Nevertheless, our finding that the T2D group had a smaller EDVi than the CWO group suggests an interaction between diabetes and cardiac remodeling. This finding agrees with reduced LV volumes found in young adults with diabetes observed in prior work [42].
Ventricular-vascular Coupling
Previous studies have shown that aortic stiffness was an independent predictor of concentric LV remodeling in people with T2D [42]. We report aortic stiffness and lower LV volumes in people with overweight/obesity with and without T2D compared to RCs. It is theorized that aortic stiffening precedes LV remodeling by disturbing the arterial-ventricular interaction by augmenting ventricular afterload [46]. The unique region of increased arterial stiffness in people with T2D was only found via the most ‘local’ measure in the proximal ascending aorta, 2D PC-MRI QA PWV. The ascending aorta serves as a compliance chamber for systemic circulation, so it is positioned to contribute to cardiac workload. Decreased ascending aorta distensibility correlates with LV wall thickness in older adults [47]. We recently reported greater aortic stiffening in youth with T1D; these same youth have CMR and echocardiographic features of diastolic dysfunction (manuscript in preparation). Of note, subclinical evidence of cardiac and vascular remodeling in T1D is responsive to metformin [48] and bromocriptine [49] consistent with reversible structural changes. The parent study is addressing the response of these endpoints to an exercise intervention, which we anticipate will be positive based on reports in older people with T2D [23,32,50].
Limitations
This report represents a descriptive analysis. As the age of the cohort is younger than in prior publications and the breadth of the CMR studies and post-processing expand upon published findings, we believe these descriptive data are an important addition to the literature. Noise and artifacts prevented analysis of MRI data in some participants, thus not all endpoints are available in all study participants. Research registry RC values were from individuals in the U.S. with no history of CV disease, obesity or diabetes [51]. While the inclusion criteria for the CWO and T2D groups included physical inactivity, physical activity data were not available for the RC cohort, so we expected a range of physical activity. There was some overlap in BMI between the RC and T2D/OWC cohorts, as RC cohort included participants with BMI <30 while T2D/OWC cohorts included those with BMI 25–40. Additionally, people with T2D have a greater exposure to medical therapy with metformin and ACEi/ARB than controls which may interact with our endpoints though no statistical difference was noted based on medication exposure.
Conclusions
Physically inactive pre-menopausal women and age-similar men with overweight/obesity with and without T2D have greater aortic stiffness and evidence of cardiac remodeling compared to RC participants. This finding suggests that obesity and/or inactivity predominate in development of subclinical cardiac and vascular dysfunction in uncomplicated T2D. In comparison to CWO, participants with T2D have significantly greater ascending aortic stiffness and smaller EDVi.. Measuring changes in central arterial stiffness detected by CMR may present an opportunity for early detection of incipient HF, and inform early intervention via treatment of T2D and obesity with cardioprotective weight loss agents.
Acknowledgments
We are appreciative of Dr. John Rice, PhD for his assistance with statistical analysis. We are grateful to our study participants and would also like to thank the staff at the Clinical and Translational Research Center (CTRC) within the Colorado Clinical and Translational Sciences Institute (CCTSI) for their assistance with implementing the study protocol. All data, excluding patient identifiers, will be available for discovery, validation, retrieval and analysis one-year post study completion/publication as permitted by federal law.
Conflicts of Interest and Source of Funding:
The authors have no conflicts of interest to declare. Research reported in this publication was supported, in part, by the National Institutes of Health’s National Institute on Aging, Grant Number P30AG059988 and K01AG080070. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Other funding includes the VA Clinical Merit CX001532 (JEBR), VA Merit BX002046, NIH-5T32HL007171, VA CDA2 BX004533 (RLS), NIH-NCATS, UL1TR001082 (JEBR, JGR, RLS), Ludeman Family Center for Women’s Health Research (RLS, JGR, JEBR, DE), ADA Cardiovascular Metabolism Fellowship Award 1-21-CMF-003 (LA), NIH pre-doctoral support T32DK120520 (DE), P30 DK116073 (JEBR), NIH/NCATS Colorado CTSA Grant Number KL2 TR002534 (EKE), AHA853697 (EKE), and ADA Junior Faculty Award 7-11-JF-42 (IES).
Footnotes
Previous Presentations: American Diabetes Association Annual Meetings 2021 (poster), 2022 (poster); Society for Cardiovascular Magnetic Resonance Annual Scientific Session 2021 (poster)
Clinical Trial Registration: Cardiovascular Mechanisms of Exercise Intolerance in Diabetes and the Role of Sex (NCT03419195)
References
- 1.Kenny HC, Abel ED. Heart Failure in Type 2 Diabetes Mellitus. Circ Res 2019; 124:121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ndumele CE, Matsushita K, Lazo M, Bello N, Blumenthal RS, Gerstenblith G, et al. Obesity and Subtypes of Incident Cardiovascular Disease. J Am Heart Assoc 2016; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chadalavada S, Jensen MT, Aung N, Cooper J, Lekadir K, Munroe PB, et al. Women With Diabetes Are at Increased Relative Risk of Heart Failure Compared to Men: Insights From UK Biobank. Front Cardiovasc Med 2021; 8:658726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Echouffo-Tcheugui JB, Xu H, DeVore AD, Schulte PJ, Butler J, Yancy CW, et al. Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: Findings from Get With The Guidelines-Heart Failure registry. Am Heart J 2016; 182:9–20. [DOI] [PubMed] [Google Scholar]
- 5.Tian X, Zuo Y, Chen S, Zhang Y, Zhang X, Xu Q, et al. Hypertension, Arterial Stiffness, and Diabetes: a Prospective Cohort Study. Hypertension 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, et al. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens 2005; 23:1839–1846. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto Y, Okamoto T. Peripheral Arterial Stiffness is Associated with Maximal Oxygen Uptake in Athletes. International journal of sports medicine 2023. [DOI] [PubMed] [Google Scholar]
- 8.Edwards JJ, Jalaludeen N, Beqiri A, Wiles JD, Sharma R, O’Driscoll JM. The effect of isometric exercise training on arterial stiffness: A randomized crossover controlled study. Physiol Rep 2023; 11:e15690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taha MM, Aneis YM, Hasanin ME, Felaya EE, Aldhahi MI, Abdeen HAA. Effect of high intensity interval training on arterial stiffness in obese hypertensive women: a randomized controlled trial. Eur Rev Med Pharmacol Sci 2023; 27:4069–4079. [DOI] [PubMed] [Google Scholar]
- 10.Marcal IR, Cotie LM, Ribeiro I, Reed JL. The Effects of Different Exercise Modalities and Intensities on Arterial Stiffness in Patients With Coronary Artery Disease. Journal of cardiopulmonary rehabilitation and prevention 2023. [DOI] [PubMed] [Google Scholar]
- 11.Brinkley TE, Leng I, Bailey MJ, Houston DK, Hugenschmidt CE, Nicklas BJ, et al. Effects of Exercise and Weight Loss on Proximal Aortic Stiffness in Older Adults With Obesity. Circulation 2021; 144:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boonman-de Winter LJM, Rutten FH, Cramer MJM, Landman MJ, Liem AH, Rutten GEHM, et al. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 2012; 55:2154–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulsin GS, Athithan L, McCann GP. Diabetic cardiomyopathy: prevalence, determinants and potential treatments. Ther Adv Endocrinol Metab 2019; 10:2042018819834869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinaglia T, Bensalah MZ, Bollache E, Kachenoura N, Soulat G, Boutouyrie P, et al. Differential impact of local and regional aortic stiffness on left ventricular remodeling: a cardiovascular magnetic resonance study. J Hypertens 2018; 36:552–559. [DOI] [PubMed] [Google Scholar]
- 15.Weber T, Wassertheurer S, Hametner B, Parragh S, Eber B. Noninvasive methods to assess pulse wave velocity: comparison with the invasive gold standard and relationship with organ damage. J Hypertens 2015; 33:1023–1031. [DOI] [PubMed] [Google Scholar]
- 16.Weir-McCall JR, Khan F, Cassidy DB, Thakur A, Summersgill J, Matthew SZ, et al. Effects of inaccuracies in arterial path length measurement on differences in MRI and tonometry measured pulse wave velocity. BMC Cardiovasc Disord 2017; 17:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heier M, Stensæth KH, Brunborg C, Seljeflot I, Margeirsdottir HD, Hanssen KF, et al. Increased arterial stiffness in childhood onset diabetes: a cardiovascular magnetic resonance study. European Heart Journal - Cardiovascular Imaging 2017; 19:694–700. [DOI] [PubMed] [Google Scholar]
- 18.Stankovic Z, Allen BD, Garcia J, Jarvis KB, Markl M. 4D flow imaging with MRI. Cardiovasc Diagn Ther 2014; 4:173–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarvis K, Scott MB, Soulat G, Elbaz MSM, Barker AJ, Carr JC, et al. Aortic Pulse Wave Velocity Evaluated by 4D Flow MRI Across the Adult Lifespan. J Magn Reson Imaging 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarvis K, Soulat G, Scott M, Vali A, Pathrose A, Syed AA, et al. Investigation of Aortic Wall Thickness, Stiffness and Flow Reversal in Patients With Cryptogenic Stroke: A 4D Flow MRI Study. J Magn Reson Imaging 2020; 53:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bock J, Kreher BW, Hennig J, Markl M. Optimized pre-processing of time-resolved 2 D and 3 D Phase Contrast MRI data. 2007.
- 22.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. American Journal of Physiology-Endocrinology and Metabolism 1979; 237:E214. [DOI] [PubMed] [Google Scholar]
- 23.Regensteiner JG, Bauer TA, Reusch JEB. Rosiglitazone Improves Exercise Capacity in Individuals With Type 2 Diabetes. Diabetes Care 2005; 28:2877–2883. [DOI] [PubMed] [Google Scholar]
- 24.Scalzo RL, Rafferty D, Schauer I, Huebschmann AG, Cree-Green M, Reusch JEB, et al. Sitagliptin improves diastolic cardiac function but not cardiorespiratory fitness in adults with type 2 diabetes. J Diabetes Complications 2019; 33:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens 1998; 16:2079–2084. [DOI] [PubMed] [Google Scholar]
- 26.Schäfer M, Browning J, Schroeder JD, Shandas R, Kheyfets VO, Buckner JK, et al. Vorticity is a marker of diastolic ventricular interdependency in pulmonary hypertension. Pulm Circ 2016; 6:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyverfeldt P, Bissell M, Barker AJ, Bolger AF, Carlhäll C-J, Ebbers T, et al. 4D flow cardiovascular magnetic resonance consensus statement. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 2015; 17:72–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schäfer M, Ivy DD, Abman SH, Barker AJ, Browne LP, Fonseca B, et al. Apparent Aortic Stiffness in Children With Pulmonary Arterial Hypertension: Existence of Vascular Interdependency? Circulation Cardiovascular imaging 2017; 10:e005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 2012; 30:1323–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott MB, Huh H, van Ooij P, Chen V, Herrera B, Elbaz M, et al. Impact of age, sex, and global function on normal aortic hemodynamics. Magn Reson Med 2020; 84:2088–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldi J, Aoina J, Whalley G, Carrick-Ranson G, Walsh H, O’Shaughnessy H, et al. The Effect of Type 2 Diabetes on Diastolic Function. Med Sci Sports Exerc 2006; 38:1384–1388. [DOI] [PubMed] [Google Scholar]
- 32.Regensteiner JG, Bauer TA, Reusch JEB, Quaife RA, Chen MY, Smith SC, et al. Cardiac dysfunction during exercise in uncomplicated type 2 diabetes. Med Sci Sports Exerc 2009; 41:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gulsin GS, Brady E, Marsh AM, Squire G, Htike ZZ, Wilmot EG, et al. Clinical associations with stage B heart failure in adults with type 2 diabetes. Ther Adv Endocrinol Metab 2021; 12:20420188211030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic Pulse-Wave Velocity and Its Relationship to Mortality in Diabetes and Glucose Intolerance. Circulation 2002; 106:2085–2090. [DOI] [PubMed] [Google Scholar]
- 35.Laurent Sp, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic Stiffness Is an Independent Predictor of All-Cause and Cardiovascular Mortality in Hypertensive Patients. Hypertension 2001; 37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 36.Hu W, Zhang H, Liu Z, Duan Q, Liu J, Dong Q, et al. Relationship between adipose tissue distribution and arterial stiffness in HFpEF. Nutrition 2022; 102:111726. [DOI] [PubMed] [Google Scholar]
- 37.Lin L, Wang L, Du R, Hu C, Lu J, Wang T, et al. Arterial Stiffness, Biomarkers of Liver Fat, and the Development of Metabolic Dysfunction in Metabolically Healthy Population: A Prospective Study. Frontiers in Cardiovascular Medicine 2022; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scalzo RL, Bauer TA, Harrall K, Moreau K, Ozemek C, Herlache L, et al. Acute vitamin C improves cardiac function, not exercise capacity, in adults with type 2 diabetes. Diabetol Metab Syndr 2018; 10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scalzo RL, Moreau KL, Ozemek C, Herlache L, McMillin S, Gilligan S, et al. Exenatide improves diastolic function and attenuates arterial stiffness but does not alter exercise capacity in individuals with type 2 diabetes. J Diabetes Complications 2017; 31:449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salvi P, Scalise F, Rovina M, Moretti F, Salvi L, Grillo A, et al. Noninvasive Estimation of Aortic Stiffness Through Different Approaches. Hypertension 2019; 74:117–129. [DOI] [PubMed] [Google Scholar]
- 41.Petersen KS, Blanch N, Keogh JB, Clifton PM. Effect of weight loss on pulse wave velocity: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol 2015; 35:243–252. [DOI] [PubMed] [Google Scholar]
- 42.Gulsin GS, Swarbrick DJ, Hunt WH, Levelt E, Graham-Brown MPM, Parke KS, et al. Relation of Aortic Stiffness to Left Ventricular Remodeling in Younger Adults With Type 2 Diabetes. Diabetes 2018; 67:1395–1400. [DOI] [PubMed] [Google Scholar]
- 43.Bjornstad P, Truong U, Dorosz JL, Cree-Green M, Baumgartner A, Coe G, et al. Cardiopulmonary Dysfunction and Adiponectin in Adolescents With Type 2 Diabetes. J Am Heart Assoc 2016; 5:e002804–e002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bilak JM, Gulsin GS, McCann GP. Cardiovascular and systemic determinants of exercise capacity in people with type 2 diabetes mellitus. Ther Adv Endocrinol Metab 2021; 12:2042018820980235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Group TS, Bjornstad P, Drews KL, Caprio S, Gubitosi-Klug R, Nathan DM, et al. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med 2021; 385:416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohyama Y, Ambale-Venkatesh B, Noda C, Chugh AR, Teixido-Tura G, Kim J-Y, et al. Association of Aortic Stiffness With Left Ventricular Remodeling and Reduced Left Ventricular Function Measured by Magnetic Resonance Imaging. Circ Cardiovasc Imaging 2016; 9:e004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soulat G, Gencer U, Kachenoura N, Villemain O, Messas E, Boutouyrie P, et al. Changes in segmental pulse wave velocity of the thoracic aorta with age and left ventricular remodelling. An MRI 4D flow study. J Hypertens 2020; 38:118–126. [DOI] [PubMed] [Google Scholar]
- 48.Bjornstad P, Schäfer M, Truong U, Cree-Green M, Pyle L, Baumgartner A, et al. Metformin Improves Insulin Sensitivity and Vascular Health in Youth With Type 1 Diabetes Mellitus. Circulation 2018; 138:2895–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schafer M, Browne LP, Truong U, Bjornstad P, Tell S, Snell-Bergeon J, et al. Bromocriptine Improves Central Aortic Stiffness in Adolescents With Type 1 Diabetes: Arterial Health Results From the BCQR-T1D Study. Hypertension 2023; 80:482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinto TE, Gusso S, Hofman PL, Derraik JGB, Hornung TS, Cutfield WS, et al. Systolic and Diastolic Abnormalities Reduce the Cardiac Response to Exercise in Adolescents With Type 2 Diabetes. Diabetes Care 2014; 37:1439–1446. [DOI] [PubMed] [Google Scholar]
- 51.Berhane H, Scott M, Elbaz M, Jarvis K, McCarthy P, Carr J, et al. Fully automated 3D aortic segmentation of 4D flow MRI for hemodynamic analysis using deep learning. Magn Reson Med 2020; 84:2204–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]


