Abstract
Macrocyclic peptides have become increasingly important in the pharmaceutical industry. We present a detailed computational investigation of the reaction mechanism of the recently developed “CyClick” chemistry to selectively form imidazolidinone cyclic peptides from linear peptide aldehydes, without using catalysts or directing groups. We conducted computational mechanistic to investigate the effects of intramolecular hydrogen bonds (IMHBs) in promoting a kinetically facile zwitterionic mechanism in “CyClick” of pentapeptide aldehyde AFGPA. Our DFT calculations highlighted the importance of IMHB in pre-organization of the resting state, stabilization of the zwitterion intermediate, and the control of the product stereoselectivity. Furthermore, we have also identified that the low ring strain energy promotes the “CyClick” of hexapeptide aldehyde AAGPFA to form a thermodynamically more stable 15+5 imidazolidinone cyclic peptide product. In contrast, large ring strain energy suppresses “CyClick” reactivity of tetra peptide aldehyde AFPA from forming the 9+5 imidazolidinone cyclic peptide product.
Keywords: Reaction Mechanism; DFT Calculation; Macrocyclic peptide, Stereoselectivity
Graphical Abstract
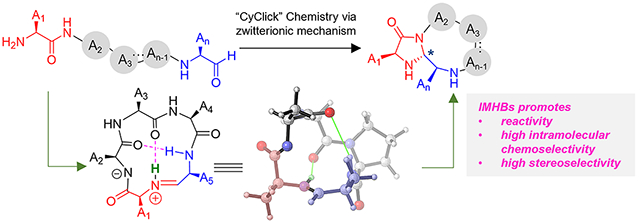
This work presents a detailed computational investigation on reaction mechanisms of the macrocyclization of linear peptide aldehydes to selectively form imidazolidinone cyclic peptides. Our work demonstrates that intramolecular hydrogen bonds (IMHBs) act as transient internal factors to control stereoselectivity by promoting a kinetically facile zwitterionic mechanism.
Introduction
Since the discovery of insulin, peptide drugs have become an indispensable part of the modern pharmaceutical industry due to their low toxicity and high potency.[1],[2] Among all peptide drug candidates, macrocyclic peptides received increasing attention in recent years.[3] Cyclization of linear peptides reduces their conformational freedom and thus increases their protein binding affinity [4],[5], structural stability [6],[7], and improved cellular penetration [8] compared with their linear counterparts. In the past two decades, the FDA approved 18 cyclic peptide new drugs [9], exemplified by antibiotic daptomycin [10],[11], anticancer agent romidepsin [12],[13], lupus nephritis voclosporin [14], and gastrointestinal disorder drug linaclotide [15],[16] (Figure 1a). Despite the great potential of cyclic peptides, there exists only a handful of methods for the selective formation of head-to-tail cyclic peptides. [17],[18]. As presented in the review by C. J. White and A. K. Yudin, controlled macrocyclization of all-l and all-d peptides are carried out at low concentrations to avoid intermolecular reaction and nearly always requires either a ‘internal’ conformational element, or an ‘external’ catalyst [19] or directing group to achieve controlled peptide macrocyclization. For example, previous studies by Ye and co-workers indicated that the use of metal ions promote head-to-tail cyclic product selectivity by bringing the N- and C- termini in close proximity [20]. Alternatively, Yudin and co-workers used Ugi multi-component condensation reaction [21] to prepare a zwitterionic intermediate, which undergoes selective macrocyclization [22] [23] (Figure 1b).
Figure 1.
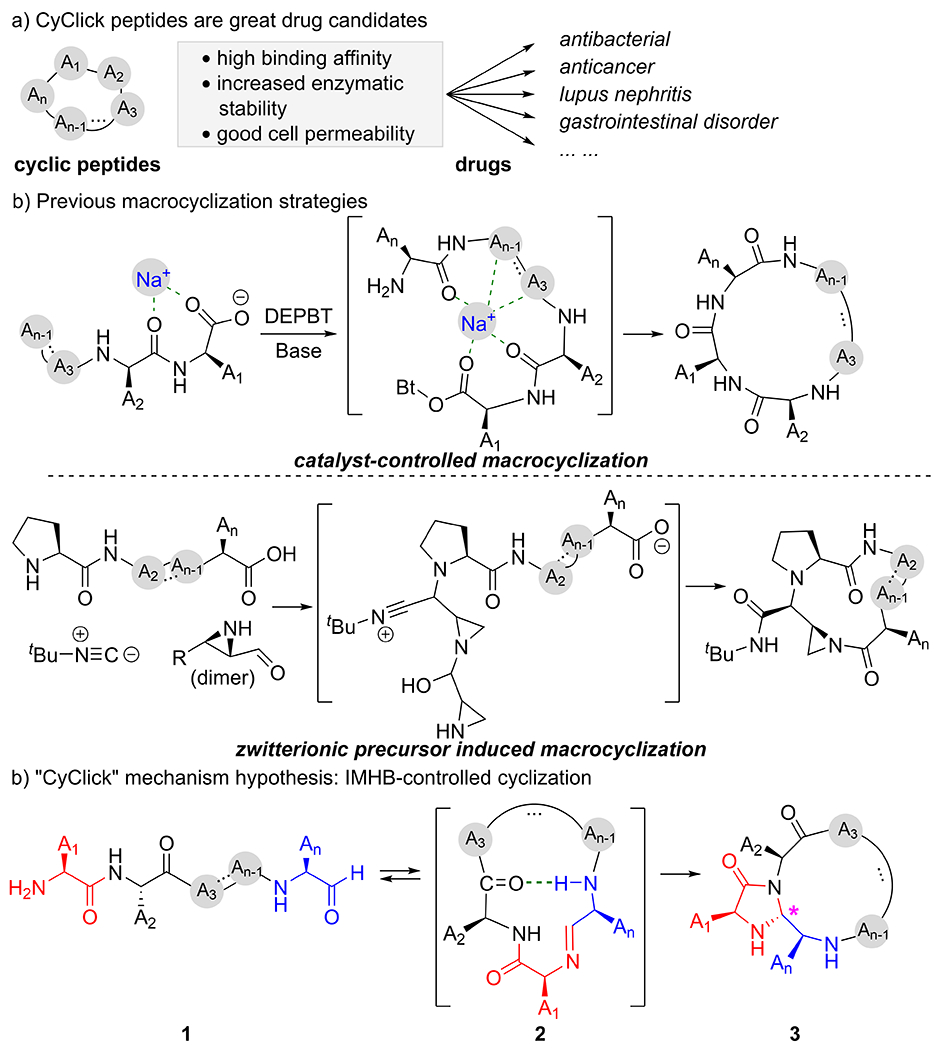
a) Characteristics of cyclic peptides as promising drug candidates; b) previous examples of using metal ion catalysts or zwitterionic precursors for conformational pre-organization to facilitate head-to-tail peptide macrocyclization; c) proposed mechanism, IMHB controlled exclusive intramolecular nature of “CyClick” reaction
In 2019, the Raj group published a novel exclusively intramolecular “CyClick” strategy for peptide macrocyclization [24]. Under mild reaction conditions, the “CyClick” chemistry generates imidazolidinone-cyclic peptides (3) from all-l linear peptide aldehydes (1) with high chemoselectivity and stereoselectivity at very high concentrations (Figure 1c). CyClick chemistry does not form any intermolecular dimers or oligomers even at relatively high concentrations. Since the “CyClick” chemistry does not use any catalyst, nor a directing group, the reaction mechanism and the origin of high intramolecular selectivity is of great interest. Considering the importance of intramolecular hydrogen bonding in confining the conformational flexibility of macrocyclic peptides [25],[26], we postulated that intramolecular hydrogen bond (IMHB) may be responsible for promoting the “CyClick” intramolecular cyclization by stabilizing the cyclic imine intermediate (2) over the linear imine intermediate. Although we found multiple experimental studies recognizing the importance of intramolecular hydrogen bonding in controlling the selectivity of macrocyclization of linear peptides [27],[28],[29],[30],[31], limited computational investigations of the IMHB conformation-directed macrocyclization are available in literature [23],[32], presumably due to the challenging conformational flexibility of peptides [33]. In this work, we present a detailed computational investigation explaining the exclusive intramolecular nature of CyClick chemistry, the origin of high chemo- and stereoselectivity, and the effect of length of linear peptides on “CyClick” reactivity.
Results and Discussion
We conducted detailed computational and experimental investigations to determine the mechanism of the CyClick reaction. We initially investigated experimentally a “CyClick” reaction of a small linear pentapeptide AFGPA 4 that occurs in H2O:DMF (1:1) solvent at room temperature to generate a 12-membered imidazolidinone cyclic peptide 6 (R) (7%) after 16 h we also observed the formation of cyclic-imine intermediate (5%) that was confirmed by its reduction with sodium cyanoborohydride (SI Figure S13). When 7 equivalents of DMAP were added, we noticed a significant increase in the yields of 12-membered cyclic peptide 6 (R) (52%) in 16 h with a high stereoselectivity of >95% de (SI Figure S14). Consistent with the work from our previous publication, no intermolecular reactions leading to dimers or oligomers (Scheme 1).
Scheme 1.
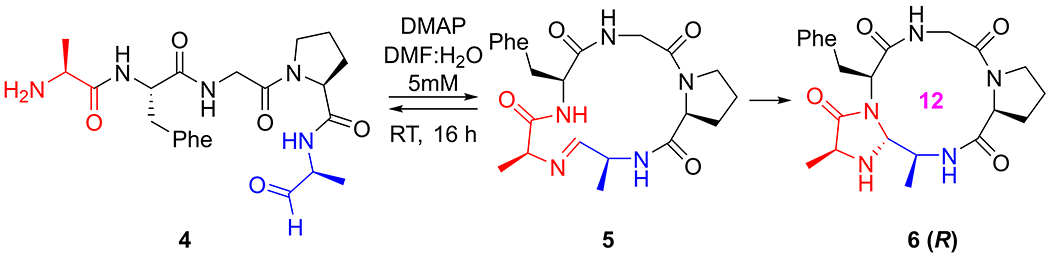
CyClick reaction on linear peptide aldehyde 4 (AFGPA) selectively generates a 12-Membered imidazolidinone cyclic peptide 6 (R).
Computations on the same reaction are summarized in Figure 2, which shows the computed reaction coordinate profile of CyClick reaction of linear peptide aldehyde 4, AFGPA, to selectively form the 12+5 imidazolidinone cyclic peptide 6 (R). To save computation time, we used a model substrate 4’, AAGPA, in which the Phe residue in 4 is replaced by an Ala residue. The formation of a cyclic imine intermediate, 7 is exergonic by 12.1 kcal/mol. Since the formation of cyclic imine intermediate 7 is reversible we assume that the formation of cyclic imine intermediate 7 is not the rate-limiting step. As demonstrated in our conformational analysis, we observed that the cyclic imine intermediate 7 is relatively structurally rigid. We only identified six conformers within the 3.0 kcal/mol window. The second lowest energy conformation of 7 is 2.2 kcal/mol higher in energy compared to the lowest energy conformation shown in Figure 3, which is stabilized by a key transannular IMHB (See SI for detailed conformational analysis). The most favorable reaction pathway of the second cyclization reaction of 7 is a zwitterionic pathway. The intramolecular hydrogen transfer transition state TS1 has a relatively low kinetic barrier of only 17.7 kcal/mol, and formation of the zwitterionic intermediate 8 is endergonic by 16.0 kcal/mol with respect to the resting state 7. The subsequent collapse of the zwitterion (TS2-R) selectively forms the imidazolidinone cyclic peptide product 6’ (R). Along the zwitterionic pathway, the C‒N bond formation step (TS2-R) is the rate-limiting transition state, with a total free energy barrier of 20.0 kcal/mol with respect to cyclic imine intermediate 7. The formation of the product 6’ (R) is exergonic by 3.9 kcal/mol, with respect to 7. Alternatively, the S-selective stereoisomeric pathway (dashed black pathway) via TS2-S requires significantly higher free energy barrier of 36.1 kcal/mol.
Figure 2.
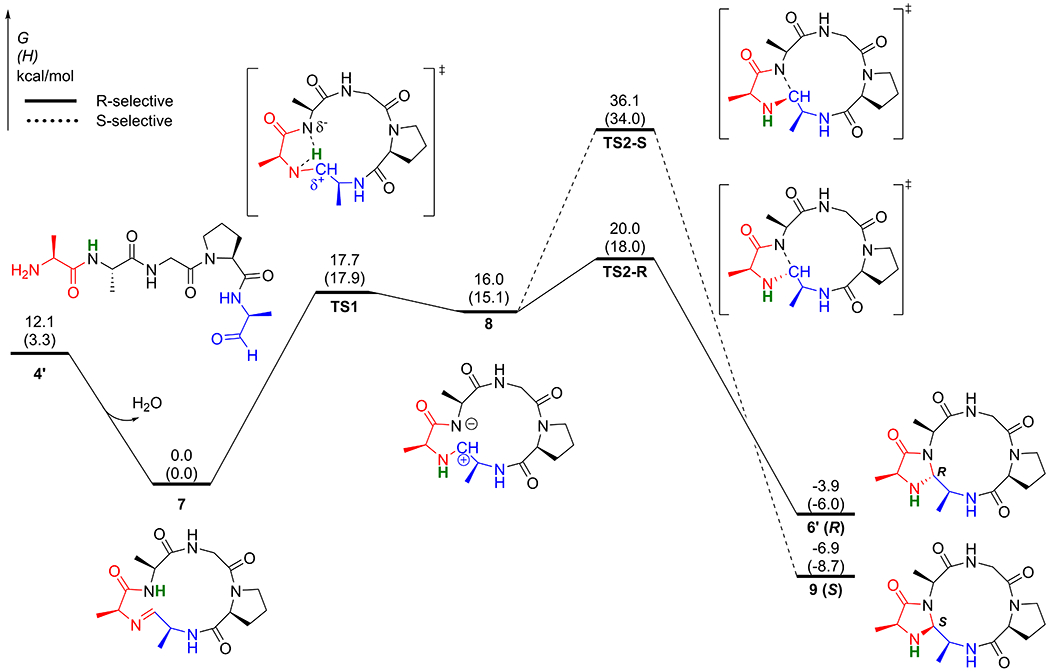
Reaction coordinate profile of intramolecular CyClick reaction of 4’ (AAGPA)
Figure 3.
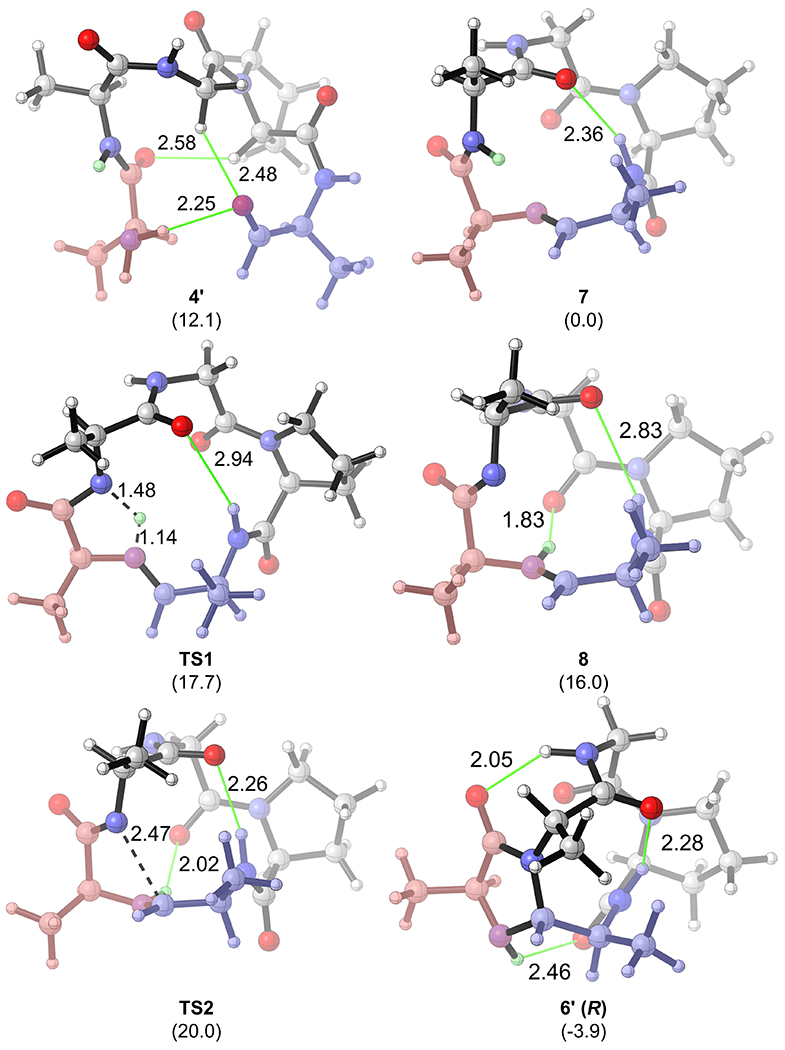
Geometries of intermediates and transition states along the zwitterionic pathway of intramolecular “CyClick” of 4’ (Computed Gibbs free energy ΔG in kcal/mol)
Overall, we identify that the zwitterionic pathway is the most favorable reaction pathway for the intramolecular “CyClick” of 4. The resting state is the cyclic imine intermediate 7, and the rate-limiting transition state is the second cyclization TS2. The computed low free energy barrier and high R-stereoselectivity are consistent with the experimental observations reported in Scheme 1.
Closer evaluation of the DFT optimized geometries of intermediates and transition states along the zwitterionic pathway revealed the vital roles IMHB played in promoting “CyClick” reaction (Figure 3). First, in the lowest energy conformation of 4’, three IMHBs pre-organize the substrate and put the N-terminus in close proximity with the aldehyde C-terminus, which should facilitate the first cyclization reaction to form cyclic imine 7. Second, a transannular IMHB in 7 pre-organizes the macrocycle backbone into a relative rigid conformation, which persists along the subsequent hydrogen transfer transition state TS1, the zwitterionic intermediate 8, and the rate-limiting TS2-R. Finally, a second transannular IMHB between the cationic iminium N‒H and the backbone carbonyl group stabilizes the zwitterionic 8 (1.83Å) and the rate-limiting TS2-R (2.02Å). Our Hirshfeld charge analysis further validates the zwitterionic nature of intermediate 8 (See SI for details). With stabilization from the two IMHB, formation of the zwitterionic intermediate 8 is only endergonic by 16.0 kcal/mol. In comparison, the acid-base reaction between a model N-ethylpropionamide and N-ethylpropan-1-imine to form the amide conjugate anion and the iminium cation is endergonic by 23.2 kcal/mol, suggesting that IMHBs stabilizes the zwitterion intermediate 8 by around 7.2 kcal/mol.
IMHBs also contribute to the origin of stereoselectivity in the intramolecular “CyClick” of 4’. Our computational study predicts that TS2-S has a free energy barrier (37.2 kcal/mol) that is significantly higher than that of TS2-R (20.0 kcal/mol) (Figure 4). The predicted high R-stereoselectivity is in excellent agreement with experimental observation. Further analysis of the DFT optimized molecular geometries of the stereoselectivity-determining transition states reveal that because of a key transannular IMHB, the low energy TS2-R adopts a less distorted conformation as the cyclic imine resting state 7 (Edist = 2.1 kcal/mol), while the TS2-S adapts a more distorted macrocycle backbone conformation (Edist = 9.3 kcal/mol). On top of the lower distortion energy, the second transannular IMHB between the cationic iminium N‒H and the backbone carbonyl group (2.02Å) further stabilizes TS2-R. By contrast, the iminium N‒H in TS2-S points away from the macrocycle backbone and is not stabilized by IMHB. The iminium cation also adopts a more stable trans conformation in the lower energy TS2-R.
Figure 4.
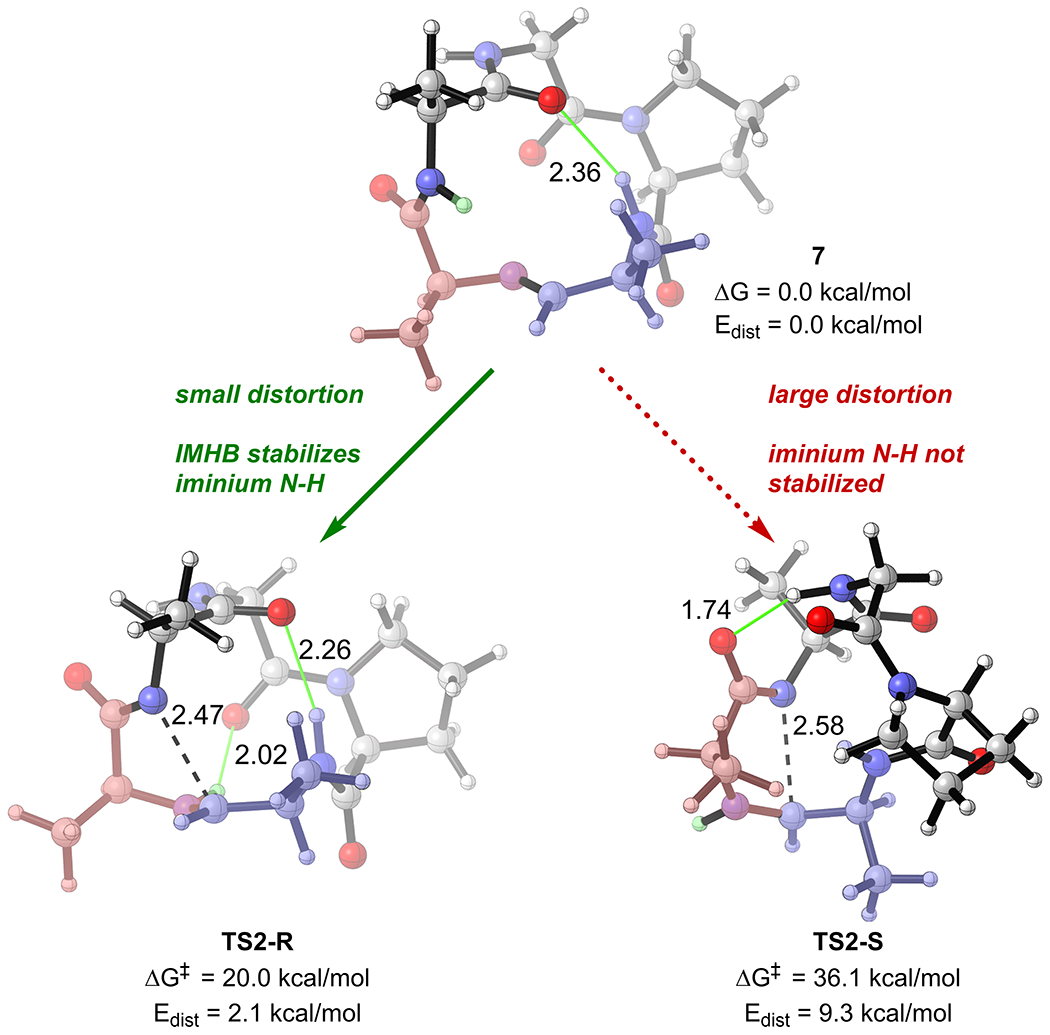
Origin of R-stereoselectivity in intramolecular “CyClick” of 4’
We also studied the effect of dimethylaminopyridine (DMAP) on the “CyClick” reaction. Experimentally, addition of DMAP increases the yield of 6’ (R) by 45%. The computed reaction coordinate profile (Figure 5a) indicates that in presence of the organic base DMAP, the CyClick mechanism is unchanged, but the intramolecular hydrogen atom transfer (TS3) to form zwitterionic intermediate 12 is stabilized by a weak van der Waals complex with DMAP (Figure 5b). The rate-limiting transition state remains the collapse of the zwitterion (TS4). In comparison, the DMAP assisted zwitterionic pathway (dashed red pathway) is less favored. Both zwitterion intermediate 13 and the subsequent TS5 have higher free energy barriers (ΔG13 = 24.9 kcal/mol, ΔG‡TS5 = 29.3 kcal/mol). DMAP does not promote the kinetic barrier of the “CyClick” reaction, but the imidazolidinone cyclic peptide product (14) is thermodynamically stabilized (ΔG14 = − 6.4 kcal/mol) compared to that without DMAP (ΔG6’ = − 3.9 kcal/mol), and a higher yield is observed in this reversible reaction.
Figure 5.
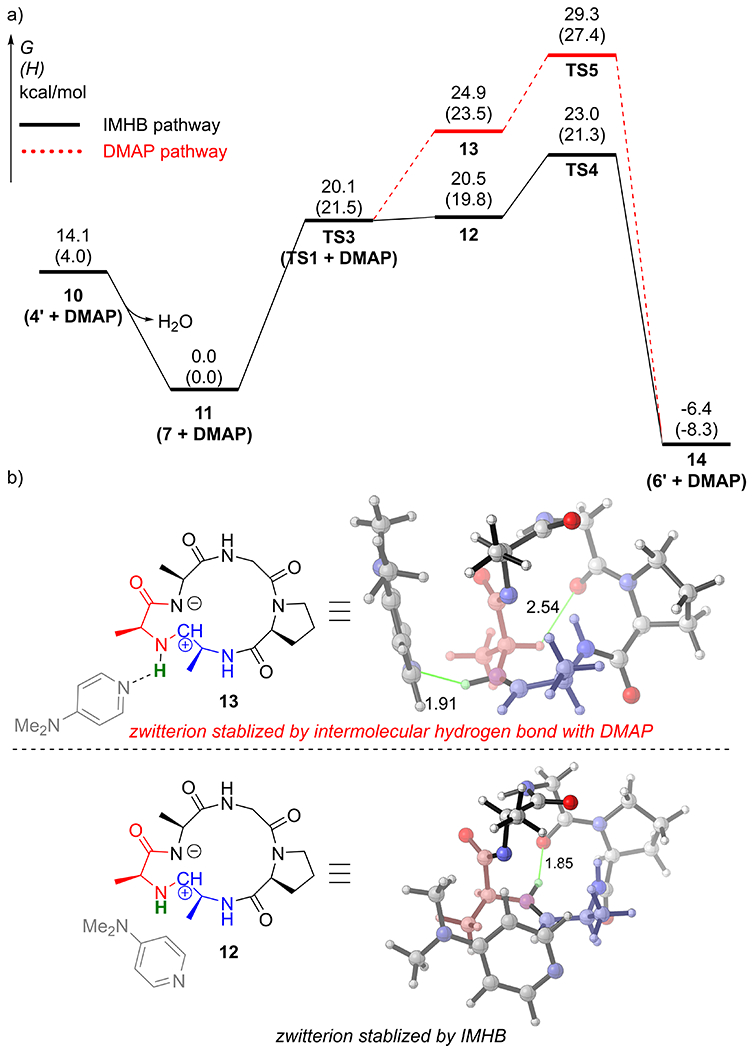
a) Computed reaction coordinate profile of intramolecular CyClick reaction of 4’ (AAGPA) with DMAP additive; b) Effect of DMAP and IMHB in stabilizing the zwitterion intermediate
We have also studied the competing intermolecular CyClick reaction of 4’ (AAGPA) to form linear peptide oligomers (Figure 6). To save computational time, we used a model substrate 4’’ to model the C-terminus of 4’. The computed reaction coordinate profile indicates that the formation of the imine intermediate 15 is only slightly exergonic by 2.1 kcal/mol. The hydrogen transfer transition state TS6 has a free energy barrier of 21.7 kcal/mol with respect to the resting state 15. The formation of the zwitterionic intermediate 16 via intramolecular hydrogen atom transfer (TS6) is endergonic by 17.2 kcal/mol. The subsequent rate-limiting cyclization transition state TS7 has a relatively higher kinetic barrier of 25.3 kcal/mol, which is 5.3 kcal/mol higher than that of the intramolecular cyclization (ΔG‡TS2-R = 20.0 kcal/mol).
Figure 6.
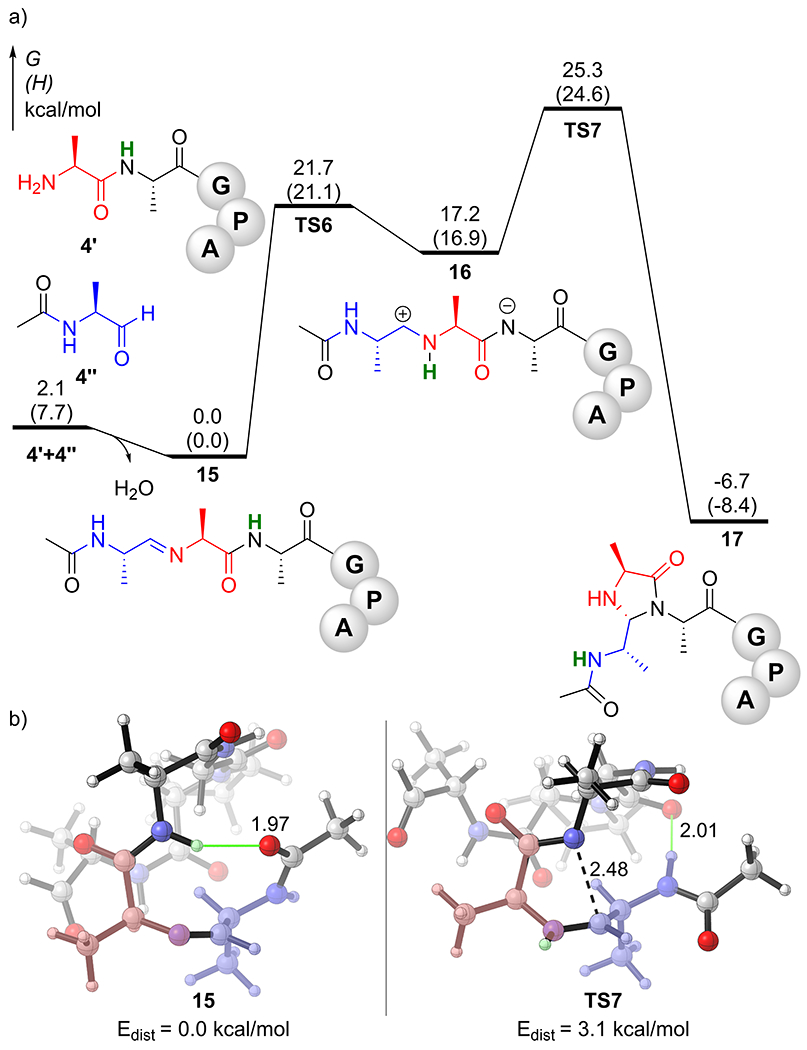
a) Computed reaction coordinate profile of intermolecular “CyClick” of 4’; b) DFT optimized geometries of resting state 15 and rate-limiting transition state TS7
This computed reaction coordinate profile predicts that the intermolecular CyClick reaction is considerably slower than the intramolecular CyClick reaction, which is in good agreement with the experimentally observed exclusive intramolecular chemoselectivity. Previous experimental analysis from the Raj group [24] showed the formation of intramolecular cyclic product occurs even at very high concentrations of peptide (100 mM). Analysis of the DFT optimized molecular geometries revealed that although IMHB exists in both the resting state 15 and the rate-limiting TS7, the cationic iminium N‒H in TS7 points away from any hydrogen bond acceptors. Moreover, the peptide backbone of TS7 is slightly more distorted (Edist = 3.1 kcal/mol), than the pre-organized macrocyclic peptide backbone in the intramolecular TS2-R (Edist = 2.1 kcal/mol).
Overall, we conclude that IMHBs are essential in the “CyClick” cyclization of 4’ to a) pre-organize the linear substrate and promote the first cyclization to form the cyclic imine intermediate; b) stabilize cationic iminium N‒H in the zwitterionic intermediate and the rate-limiting second cyclization transition state; c) promotes R-stereoselectivity and intramolecular chemoselectivity.
With a good understanding of the CyClick reaction mechanism of the 12+5 substrate, we subsequently studied the effect of the length of linear peptide aldehyde on the CyClick reaction experimentally and computationally. We also computed the reaction coordinate profile of the “CyClick” reaction of 18’ (AAGPAA) and 21’ (AAPA) [34], which form the 15+5 and 9+5 imidazolidinone cyclic peptides 20’ and 23’, respectively.
Experimentally, the cyclization of linear hexa-peptide aldehyde, 18 (AAGPFA), which is the linear peptide aldehyde substrate with one additional Ala residue compared with 4. In H2O:DMF (1:1) and 7 equivalents of DMAP at room temperature, we observed the 15-membered cyclic peptide product with 45% conversion (Scheme 2a, SI Figure S15). Alternatively, the tetrapeptide 21 (AFPA), which is the linear peptide aldehyde substrate with one less glycine compared with 4, did not lead to the formation of any 9-membered macrocycle in 16 h (Scheme 2a, SI Figure S16).
Scheme 2.
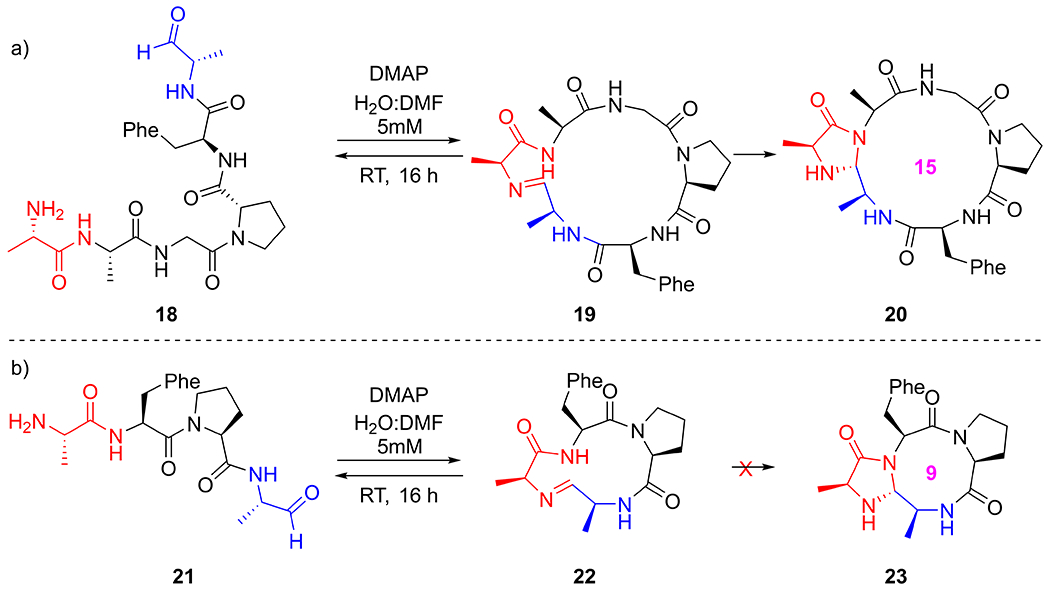
CyClick reaction on linear peptide aldehyde a) 18 (AAGPFA) selectively generates a 15-membered imidazolidinone cyclic peptide 20; b) 21 (AFPA) does not generate 9-membered imidazolidinone cyclic peptide 23
The “CyClick” of 18’ (AAGPAA) along the zwitterionic pathway (Figure 7) is similar to reaction mechanism presented in earlier sections. The formation of the cyclic imine intermediate 24 is exergonic by 12.4 kcal/mol. The subsequent hydrogen transfer transition state (ΔG‡TS5 = 18.3 kcal/mol) leads to the formation of the zwitterionic intermediate 25. The rate-limiting transition state is the second cyclization TS9-R with a free energy barrier of 20.6 kcal/mol. The formation of the product 15+5 imidazolidinone cyclic peptide 20’ is exergonic by 9.5 kcal/mol, with respect to cyclic imine intermediate. The S-selective TS9-S has a kinetic barrier of 25.2 kcal/mol. Overall, we predict that the CyClick reaction of 18’ will readily take place at room temperature to selectively form 20’, which agrees with the experimental observations reported in Scheme 2.
Figure 7.
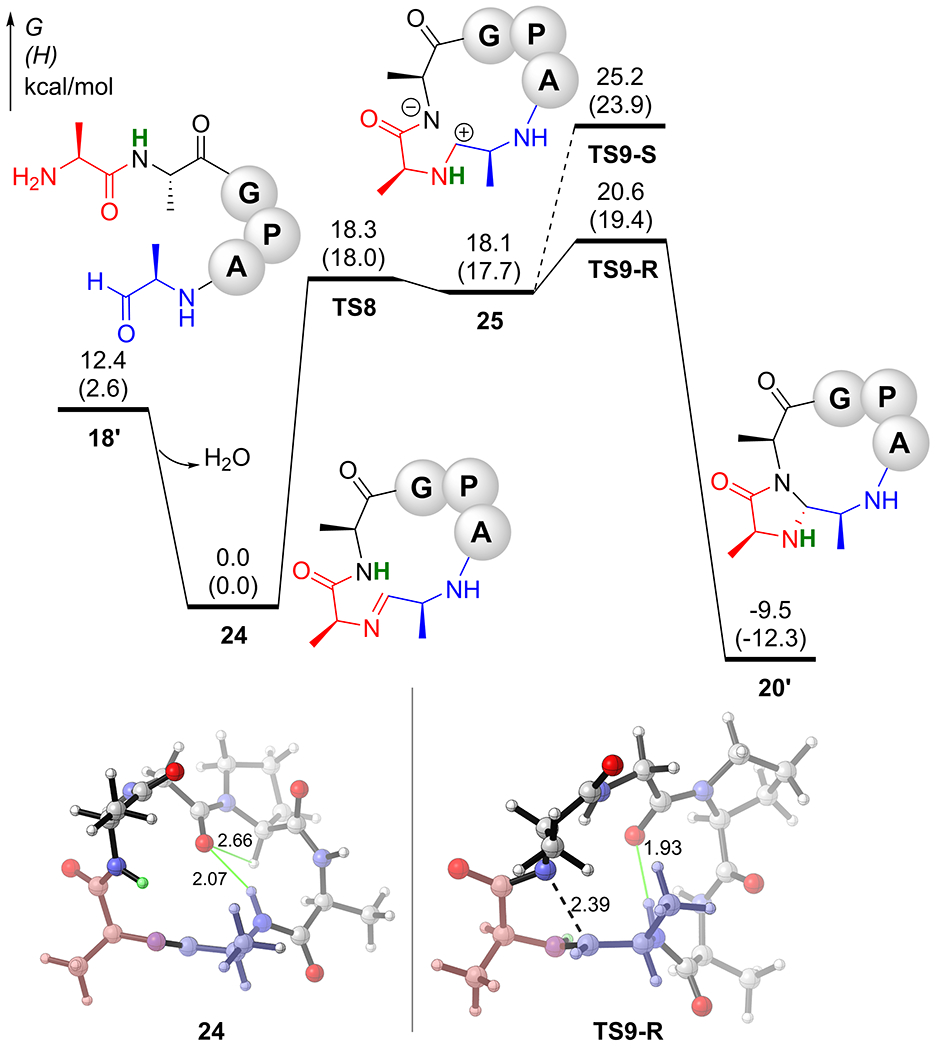
Reaction coordinate profile of CyClick reaction of 18’ (AAGPAA)
We also modeled the hypothetical “CyClick” of 21’ (AAPA), which is the linear peptide aldehyde substrate with one less glycine compared with 4’ (Figure 8). The computed reaction coordinate profile suggests that the rate-limiting transition state for CyClick of 21’ is the hydrogen transfer transition state TS10. Consistent with the experimental observation, the computed free energy barrier (ΔG‡TS10 = 33.5 kcal/mol) is very high so that the reaction should not take place at room temperature. The formation of the 9+5 imidazolidinone cyclic peptide 23’ is exergonic by only 1.9 kcal/mol.
Figure 8.
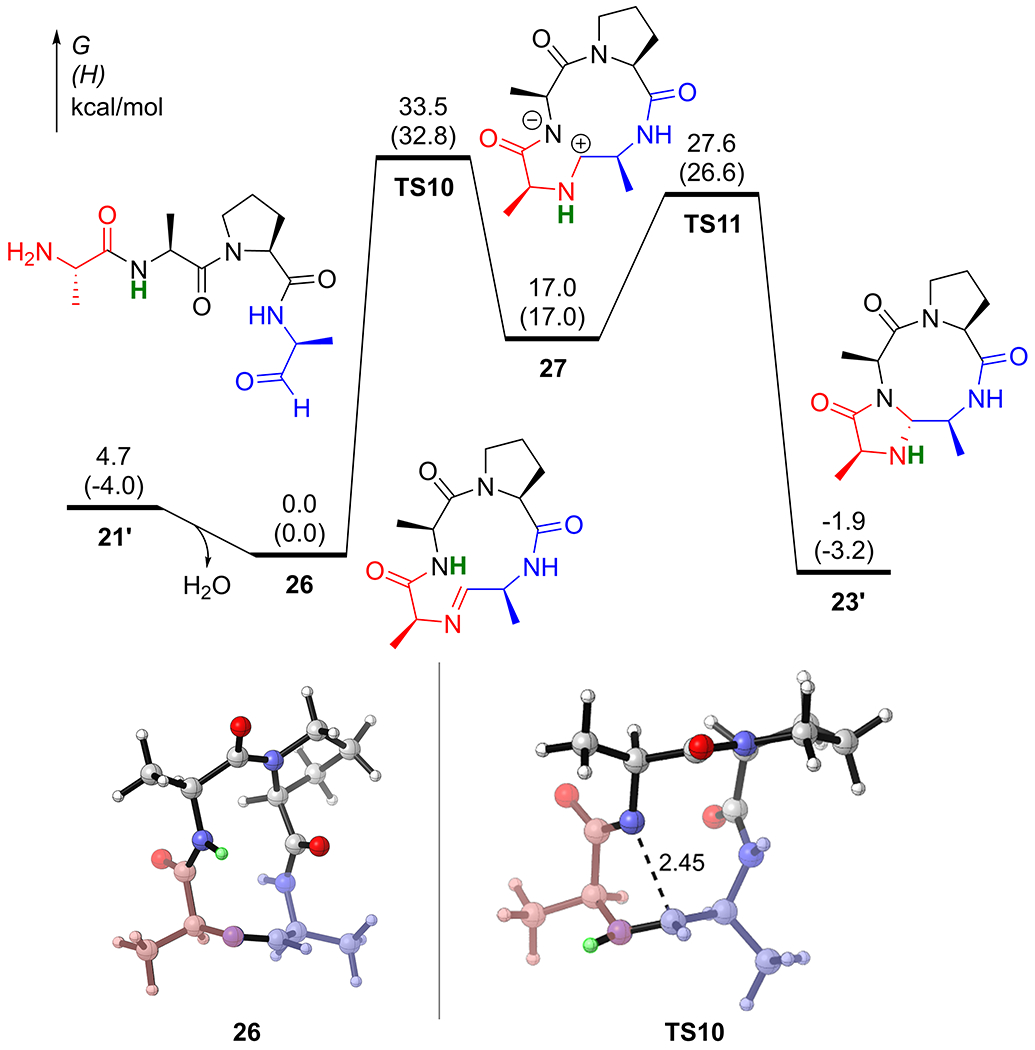
Reaction coordinate profile of CyClick reaction of 21’ (AAPA)
The ring sizes of imidazolidinone-peptide bicyclic products have a significant effect on their thermodynamic stability. The computed heat of reaction to form the imidazolidinone-peptide product decreases as the ring size increases (Figure 9). The 20’ (n =3) is the most stable product (ΔH20’= −12.3 kcal/mol), while the 23’ (n =1) is the lease stable (ΔH23’ = −3.2 kcal/mol), with respect to their corresponding cyclic imine resting states. Calculation of the ring strain with a model homodesmotic reaction (Qstrain) [35] revealed that 23’ is thermodynamically less stable than 6’-R and 20’ because of its larger ring strain energy (Qstrain, n=1 = 14.3 kcal/mol).
Figure 9.
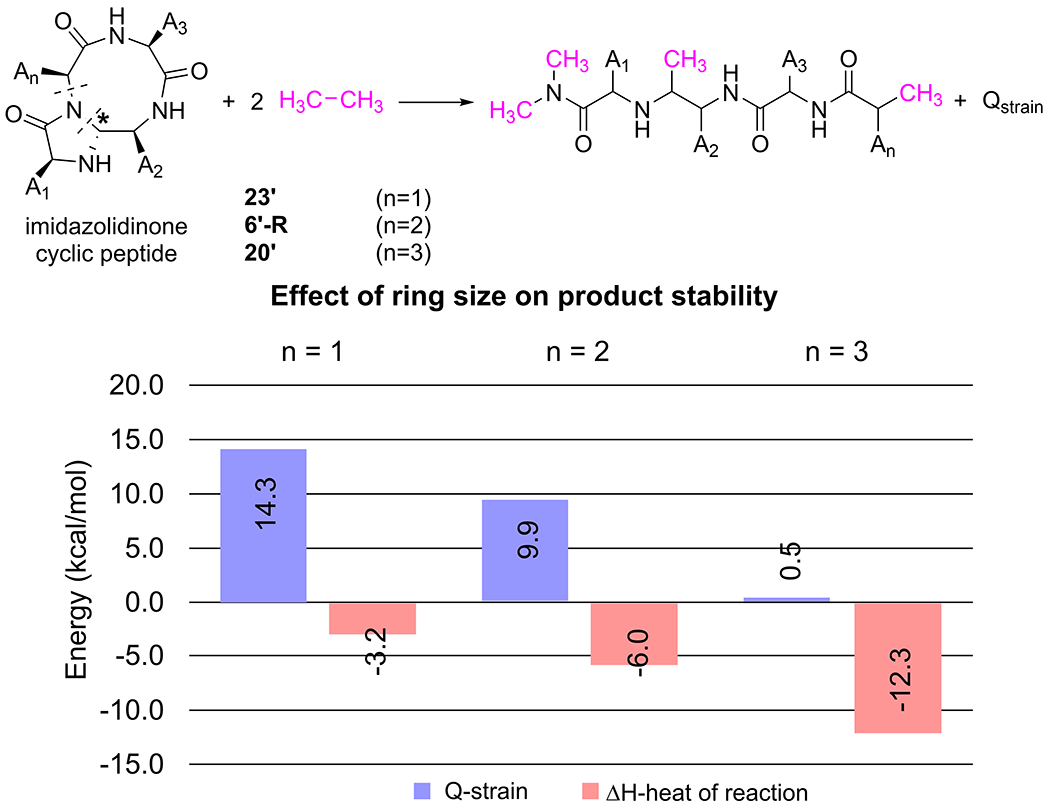
Effect of ring size on the stability of the product imidazolidinone-cyclic peptide
Conclusion
We have presented a detailed mechanistic investigation on the “CyClick” reaction. DFT calculations identify that the “CyClick” mechanism involves a reversible formation of the cyclic imine resting state, followed by rate-limiting cyclization transition state to form the imidazolidinone cyclic product. Along the favored zwitterionic pathway, intramolecular hydrogen bonding (IMHB) is shown to play an essential role in promoting the reactivity, intramolecular chemoselectivity, and stereoselectivity in “CyClick” reactions to form cyclic peptides. Two transannular IMHBs stabilize the key zwitterionic intermediate and the rate-determining cyclization transition state, consequently promoting the “CyClick” reactivity. The IMHB between the iminium N‒H and backbone carbonyl also controls the intramolecular chemoselectivity and R-stereoselectivity. Our calculations show that longer linear peptide aldehydes form thermodynamically more stable imidazolidinone-cyclic peptide due to favorable ring strain in the final product.
Supplementary Material
Acknowledgements
This research was supported by NIH (Grant No. 1R35GM133719-01) and Fellowship from Sloan Foundation granted to M.R. and the National Science Foundation (CHE-2153972) to K.N.H. This work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation grant number ACI-1548562. We thank Jiefu (Jeffrey) Yu for his help in bench-marking computational methods.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].Fosgerau K; Hoffmann T Peptide therapeutics: current status and future directions. Drug. Discov 2015, 20, 122–128. [DOI] [PubMed] [Google Scholar]
- [2].Muttenthaler M; King GF; Adams DJ; Alewood PF Trends in peptide drug discovery. Nat. Rev. Drug. Discov, 2021, 20, 309–325. [DOI] [PubMed] [Google Scholar]
- [3].Zorzi A; Deyle K; Heinis C Cyclic peptide therapeutics: past, present and future. Curr. Opin. Chem. Biol 2017, 38, 24–29. [DOI] [PubMed] [Google Scholar]
- [4].Sohrabi C; Foster A; Tavassoli A Methods for generating and screening libraries of genetically encoded cyclic peptides in drug discovery. Nat. Rev. Chem, 2020, 4, 90–101. [DOI] [PubMed] [Google Scholar]
- [5].Angelini A; Cendron L; Chen S; Touati J; Winter G; Zanotti G; Heinis C Bicyclic peptide inhibitor reveals large contact interface with a protease target, ACS Chem. Biol, 2012, 7, 817–821. [DOI] [PubMed] [Google Scholar]
- [6].Gentilucci L; De Marco R; Cerisoli L Chemical Modifications Designed to Improve Peptide Stability: Incorporation of Non-Natural Amino Acids, Psuedo-Peptide Bonds, and Cyclization. Curr. Pharm. Des 2010, 16, 3185–3203. [DOI] [PubMed] [Google Scholar]
- [7].Craik DJ. Seamless proteins tie up their loose ends, Science, 2006, 311, 1563–1564 [DOI] [PubMed] [Google Scholar]
- [8].Dougherty PG; Sahni A; Pei D Understanding cell penetration of cyclic peptides. Chem. Rev 2019, 119, 10241–10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang H; Chen S Cyclic peptide drugs approved in the last two decades (2001-2021). RSC. Chem. Biol 2022, 3, 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tedesco KL; Rybak MJ Daptomycin. Pharmacotherapy, 2004, 24, 41–57. [DOI] [PubMed] [Google Scholar]
- [11].Heidary M; Khosravi AD; Khoshnood S; Nasiri MJ; Soleimani S; Goudarzi M Daptomycin. J. Antimicrob. Chemother, 2018, 73, 1–11. [DOI] [PubMed] [Google Scholar]
- [12].Bertino EM; Otterson GA Romidepsin: a novel histone deacetylase inhibitor for cancer. Expert. Opin. Investig. Drugs, 2011. 20, 1151–1158. [DOI] [PubMed] [Google Scholar]
- [13].Campas-Moya C Romidepsin for the treatment of cutaneous T-cell lymphoma. Drugs Today, 2009, 45, 787–795. [DOI] [PubMed] [Google Scholar]
- [14].Rovin BH; Solomons N; Pendergraft III WF; Dooley MA; Tumlin J; Romero-Diaz J; Lysenko L; Navarra SV; Huizinga R,B; AURA-LV Study Group. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int, 2019, 95, 219–231. [DOI] [PubMed] [Google Scholar]
- [15].Rao S; Lembo AJ; Shiff SJ; Lavins BJ; Currie MG; Jia XD; Shi K; MacDougall J,E; Shao J,Z; Eng P; Fox S,M; Schneier H,A; Kurtz C,B; Johnston JM A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am. J. Gastroenterol, 2012, 107, 1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lembo AJ; Schneier HA; Shiff SJ; Kurtz CB; MacDougall JE; Jia XD; Shao J,Z; Lavins B,J; Currie M,G; Fitch D,A; Jeglinski B,I; Eng P; Fox S,M; Johnston JM Two randomized trials of linaclotide for chronic constipation. N. Engl. J. Med, 2011, 365, 527–536. [DOI] [PubMed] [Google Scholar]
- [17].Hill TA; Shepherd NE; Diness F; Fairlie DP Constraining cyclic peptides to mimic protein structure motifs. Angew. Chem. Int. Ed 2014, 53, 13020–13041. [DOI] [PubMed] [Google Scholar]
- [18].Lau YH; de Andrade P; Wu Y; Spring DR Peptide stapling techniques based on different macrocyclisation chemistries. Chem. Soc. Rev 2015, 44, 91–102. [DOI] [PubMed] [Google Scholar]
- [19].White CJ; Yudin AK Contemporary strategies for peptide macrocyclization. Nat. Chem 2011, 3, 509–524. [DOI] [PubMed] [Google Scholar]
- [20].Liu M; Tang YC; Fan KQ; Jiang X; Lai LH; Ye YH Cyclization of several linear penta - and heptapeptides with different metal ions studied by CD spectroscopy. J. Pept. Res, 2005, 65, 55–64. [DOI] [PubMed] [Google Scholar]
- [21].Demharter A; Hörl W; Herdtweck E; Ugi I Synthesis of Chiral 1, 1′-Iminodicarboxylic Acid Derivatives from α-Amino Acids, Aldehydes, Isocyanides, and Alcohols by the Diastereoselective Five-Center - Four-Component Reaction. Angew. Chem. Int. Ed, 1996. 35, 173–175. [Google Scholar]
- [22].Hili R; Rai V; Yudin AK Macrocyclization of Linear Peptides Enabled by Amphoteric Molecules. J. Am. Chem. Soc, 2010, 132, 2889–2891. [DOI] [PubMed] [Google Scholar]
- [23].Belding L; Zaretsky S; Yudin AK; Dudding T A Mechanistic Model for the Aziridine Aldehyde-Driven Macrocyclization of Peptides. J. Org. Chem 2018, 83, 9119–9124. [DOI] [PubMed] [Google Scholar]
- [24].Adebomi V; Cohen RD; Wills R; Chavers HAH; Martin GE; Raj M CyClick chemistry for the synthesis of cyclic peptides. Angew. Chem. Int. Ed 2019. 58, 19073–19080. [DOI] [PubMed] [Google Scholar]
- [25].Caron G; Kihlberg J; Ermondi G Intramolecular hydrogen bonding: An opportunity for improved design in medicinal chemistry. Med. Res. Rev, 2019, 39, 1707–1729. [DOI] [PubMed] [Google Scholar]
- [26].Rezai T; Yu B; Millhauser GL; Jacobson MP; Lokey RS Testing the conformational hypothesis of passive membrane permeability using synthetic cyclic peptide diastereomers. J Am Chem Soc. 2006, 128, 2510–2511. [DOI] [PubMed] [Google Scholar]
- [27].Blankenstein J; Zhu J Conformation-Directed Macrocyclization Reactions, Eur. J. Org. Chem 2005, 1949–1964 [Google Scholar]
- [28].Ciabatti R; Kettenring J,K; Winters G; Tuan G; Zerilli L; Cavalleri B Ramoplanin (A-16686), A New Glycolipodepsipeptide Antibiotic. III. Structure Elucidation. J. Antibiot 1989, 42, 254–267. [DOI] [PubMed] [Google Scholar]
- [29].Jiang W; Wanner J; Lee RJ; Bounaud PY; Boger DL J. Am. Chem. Soc 2003, 125, 1877–1887. [DOI] [PubMed] [Google Scholar]
- [30].Bu X; Wu X; Xie G; Guo Z Synthesis of tyrocidine A and its analogues by spontaneous cyclization in aqueous solution. Org. Lett 2002, 4, 2893–2895. [DOI] [PubMed] [Google Scholar]
- [31].Bu X; Wu X; Ng NLJ; Mak CK; Qin C; Guo Z Synthesis of gramicidin S and its analogues via an on-resin macrolactamization assisted by a predisposed conformation of the linear precursors. J. Org. Chem 2004, 69, 2681–2685. [DOI] [PubMed] [Google Scholar]
- [32].Li Y; Li F; Zhu Y; Li X; Zhou Z; Liu C; Zhang W; Tang. DFT Study on Reaction Mechanisms of Cyclic Dipeptide Generation. Struct. Chem 2016, 27, 1165–1173. [Google Scholar]
- [33].Zaretsky Z; Hickey JL; Denis MA; Scully CCG; Roughton AL; Tantillo DJ; Lodewyk MW; Yudin AK Predicting Cyclic Peptide Chemical Shifts Using Quantum Mechanical Calculations. Tetrahedron, 2014, 70, 7655–7663 [Google Scholar]
- [34].For computational efficiency, we replaced the Phe (F) amino acid in 18 and 21 with Ala (A) in the model peptides 18’ and 21’.
- [35].Wheeler SE; Houk KN; Schleyer PVR; Allen WD A hierarchy of homodesmotic reactions for thermochemistry. J. Am. Chem. Soc 2009, 131, 2547–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


