Abstract
Background:
Normative changes in right ventricular (RV) structure and function have not been characterized in the context of treatment-associated functional recovery (RVFnRec). The aim of this study is to assess the clinical relevance of a proposed RVFnRec definition.
Methods:
We evaluated 63 incident patients with PAH by right heart catheterization and cardiac MRI (CMR) at diagnosis and CMR and invasive cardiopulmonary exercise (CPET) following treatment (~11 months). Sex, age, ethnicity matched healthy control subjects (n=62) with one-time CMR and non-invasive CPET were recruited from the PVDOMICS project. We examined therapeutic CMR changes relative to the evidence-based peak oxygen consumption (VO2peak)>15mL/kg/min to define RVFnRec by receiver operating curve analysis. Afterload was measured in the as mean pulmonary artery pressure, resistance, compliance, and elastance.
Results:
A drop in RV end-diastolic volume of −15 mL best defined RVFnRec (AUC 0.87, P=0.0001) and neared upper 95% CI RVEDV of controls. This cutoff was met by 22/63 (35%) of patients which was reinforced by freedom from clinical worsening, RVFnRec 1/21 (5%) versus no RVFnRec 17/42, 40%, (log rank P=0.006). A therapy-associated increase of 0.8 mL/mmHg in compliance had the best predictive value of RVFnRec (AUC 0.76, CI 0.64–0.88, P=0.001). RVFnRec patients had greater increases in stroke volume, and cardiac output at exercise.
Conclusions:
RVFnRec defined by RVEDV therapeutic decrease of −15mL predicts exercise capacity, freedom from clinical worsening, and nears normalization. A therapeutic improvement of compliance is superior to other measures of afterload in predicting RVFnRec. RVFnRec is also associated with increased RV output reserve at exercise.
Keywords: pulmonary hypertension, right ventricular remodeling, right ventricular afterload, treatment effect
Introduction:
Adaptation of the right ventricle (RV) to high afterload is the primary determinant of a patient’s symptoms and survival in PAH, making it a focus of research by expert working groups1,2. As afterload increases in PAH, the RV remodels with hypertrophy resulting in adaptive maintenance of cardiac output. Eventually, maladaptive processes occur such as myocardial interstitial fibrosis, detrimental myocyte intracellular and molecular changes3,4, and alterations in coronary flow5 leading to impaired systolic and diastolic function. When RV afterload is acutely reversed, for example with lung transplantation, there is often complete reversibility of these RV maladaptive changes6. In the pre-combination therapy era, the magnitude of afterload lowering was not large enough to appreciate substantial changes in RV structure7. However, combination therapy results in significant improvements in afterload8 making RV “reverse remodeling” a realistic therapeutic goal.
Reverse remodeling of the left ventricle (LV) is characterized by “normative” (returning toward normal structure and function) changes. But, also the left ventricular, cellular, and molecular processes associated with reverse remodeling have been well described9. Relative to the LV, characterization of RV reverse remodeling is in its infancy. Current definitions7 have relied upon the relationship to freedom from clinical worsening10 but moreover, they lack a substantive pathophysiological link of the consequences of pulmonary vascular load on RV function. In the absence of longitudinal RV cellular and molecular data, a formal definition of RV reverse remodeling is premature. However, RV functional recovery (RVFnRec) can be characterized by therapeutically associated “normative” imaging changes paired with peak exercise data (VO2peak) indicative of the RV stress response. To date, studies have not included a well-matched control group, so characterization of “normative” change is unknown. In addition, most studies thus far have used echocardiography to describe RVFnRec, whereas cardiac MRI (CMR) may be more sensitive to treatment-related changes11.
It is generally accepted that a large drop in afterload is required for RV reverse remodeling (or RVFnRec), but the most affected parameter of afterload is unknown7. Both steady (i.e., pulmonary vascular resistance, PVR) and pulsatile (i.e., pulmonary arterial compliance, Ca) components are important contributors to afterload in the pulmonary circulation12. Effective pulmonary arterial elastance (Ea), a composite measurement of total PVR and Ca, is also proposed as a more comprehensive way of representing afterload13. Lastly, parameters obtained during exercise such as alpha (α) distensibility, which describes the curvilinearity of the PA pressure-flow relationship, may give us insight into pulmonary vascular reserve/recruitment and the state of the distal vasculature14. No study has yet investigated these parameters longitudinally from PAH diagnosis to determine their impact on reverse remodeling.
In the present study, we aim to systematically define RVFnRec by CMR in a group of treatment naïve PAH patients relative to a VO2peak threshold and clinical outcome. We evaluate the degree of normative RV therapeutic changes relative to matched healthy controls. We further assess the best parameter of RV afterload for predicting RVFnRec. Lastly, we examine if afterload and RV output reserve at exercise add to our understanding of RVFnRec.
Methods:
Subjects
The data that support the findings of this study are available from the corresponding author upon reasonable request. Sixty-three incident treatment naive patients with World Symposium of PH (WSPH) Group I PAH gave an informed consent to participate in the study, which was approved by the institutional review board at the University of Arizona (IRB #1100000621). The patients enrolled as part of a prospective protocolized study which is open to all incident patients (See supplemental methods and Figure S1). The diagnosis of PAH was established by dedicated PAH providers with invasive confirmation by right heart catheterization based on updated guidelines15. Patients were included if they had right heart catheterization and CMR at baseline (pre-treatment) and invasive cardiopulmonary exercise testing (iCPET) and CMR at > 6 months of follow-up. All patients were placed on PAH goal-directed therapy per current guidelines at the time. See supplemental methods for details on therapeutic strategy. Patients were categorized by therapeutic strategy based on the European Respiratory Society/European Society of Cardiology (ERS/ESC) guidelines of 2015 at which goal-directed mono or sequential therapy was replaced by up-front combination therapy16. We selected a cohort of patients all placed on parenteral treprostinil (± oral therapy) to optimize the possibility of seeing large changes in afterload (and thus, RVFnRec)17. Ninety-six healthy control subjects were recruited for the NHLBI Redefining Pulmonary Hypertension through Pulmonary Vascular Disease Phenomics (PVDOMICS)18. These control subjects underwent extensive testing including CMR and non-invasive CPET at a single time point. Sixty-two age, sex, and ethnicity matched controls from across all clinical sites were used for this analysis with steering committee approval. The United States Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) score 2.0 score19 was calculated and presented at baseline and follow-up for the PAH patients.
Cardiac MRI
All image analysis was performed using CVI42, Circle Cardiovascular Imaging® by TA (at Arizona) and DK (at the PVDOMICS Core, the Cleveland Clinic), expert cardiac MRI cardiologists with >10 years’ experience in CMR. Fifty controls and PAH subjects with overlap (between PVDOMICS and the UA registry) were analyzed by both TA and DK and used to assess inter-reader variability in RV volumes (see supplemental methods). Cardiac MRI imaging was performed as described previously20. Briefly, standard volumetric measurements were made from short axis cine projections for CMR. Contour smoothing was done to include trabeculations in end-systole (ESV) and diastole (EDV). Stroke volume (SV) was calculated as EDV – ESV and RVEF as [(SV/EDV) × 100]. Additional methods are available in the online supplement.
Right Heart Catheterization and Invasive Cardiopulmonary Exercise Testing
After study inclusion, a pulmonary artery catheter was advanced via the antecubital vein for measurements of pulmonary artery pressure (systolic, mean, and diastolic PAP), RV pressure, right atrial pressure (RAP), wedged PAP (PCWP) and cardiac output (direct Fick). PVR is calculated as mPAP-PCWP divided by direct Fick cardiac output (C.O.) and expressed as WU (mmHg*min*L−1) or mmHg*sec*mL−1 where indicated. Ca was calculated as stroke volume (SV) from direct Fick C.O. divided by PA pulse pressure and expressed as mL*mmHg−1. Effective pulmonary arterial elastance (Ea) was calculated as sRVP/SV21 expressed as mmHg*mL−1. Distensibility (α), expressed as % change in vessel diameter/mmHg distending pressure, was calculated by methods previously described14,22. All stages of a single subject’s mPAP, PCWP, and C.O. were used to calculate a single α value. RC time, the product of PVR and Ca, was expressed as seconds.
Our comprehensive resting and exercise catheterization protocol has been previously published23. Briefly, after obtaining supine resting measurements, the patient was placed in full upright position with an electronic fluoroscopy chair. Fluoroscopy was used to re-zero at left atrial level. A cycle ergometer was positioned below the patient. The patient then proceeded with exercise at predetermined workload based on their level of dyspnea23 for steady-state two-minute stages until respiratory exchange ratio (RER) ~ 1.1. PAP, PCWP, RAP, arterial and venous O2 content (CaO2, CvO2) were obtained during the last 30 seconds of each stage. Metabolic cart analysis (Vyaire Medical™, Mettawa, IL) was used for simultaneous collection gas exchange and lung volume. Hemodynamics presented were averaged over 3 respiratory cycles in accordance with current guidelines at rest and exercise24. The control patients underwent an identical protocol in the non-invasive exercise lab (without a catheter in place).
Definition of RV Functional Recovery
Since exercise VO2peak is physiologically meaningful (dependent on RV function) and prognostic, the guideline25 and evidence (clinical worsening) confirmed26 cutoff of >15 mL/kg/min was chosen to identify potential CMR cutoffs indicative of RVFnRec. Exercise data is presented only for the follow-up visit as many patients (N=55 without iCPET, N=8 with iCPET) were not exercised at baseline due to safety considerations. We examined therapeutically associated change in RV size (RVESV and EDV, % change RV volumes) and function (RVEF) relative to this VO2peak cutoff at follow-up. The Youden index (sensitivity + specificity - 1) was used to define the best cutoffs in RV volumes (absolute and relative change from baseline) and RVEF (change from baseline). Receiver operating characteristic (ROC) curves are used to display optimal cut-offs. Resulting RVFnRec groups were then examined relative to freedom from clinical worsening. Clinical worsening was defined as death, transplant, or all-cause hospitalization. Freedom from clinical worsening was evaluated using Kaplan-Meier Plots and the log-rank test using the follow-up visit (second assessment) as T0. A similar procedure (Youden index and ROC analysis) was used to define the best cutoffs for mPAP, Ca, PVR, Ea, and α at follow-up and change from baseline to follow-up for predicting RVFnRec.
Statistical Analysis
Continuous data are expressed as mean ± standard deviation or median [25,75 percentile]. Categorical data are expressed as counts and percentages. The number of PAH patients was chosen based primarily on data availability given strict inclusion/exclusion criteria. However, based on previously published data in prostacyclin treated patients, we would expect 100% power to detect a mean difference of 7±5.4 WU PVR difference between RVFnRec and no RVFnRec groups (R.B. personal communication, 9/25/22 based on ref.17. Kruskal-Wallis test for continuous and ordinal variables or Fisher exact test for categorical variables were used to test baseline differences. Normality was assessed with the Shapiro-Wilk test. Longitudinal differences were evaluated with Wilcoxon signed-rank (within group) or repeated measures analysis of variance (ANOVA)(between group). Variables were log-transformed in cases of non-normal distribution. Linear or non-linear regression was conducted where appropriate after testing assumptions. Binary logistic regression was done to assess the relationship of treatment type with RVFnRec. Statistical analyses were performed using SPSS software (version 28.0, IBM, Armonk, NY) and SigmaPlot (version 14.5, Systat©, San Jose, CA). Statistical tests were 2-sided, and a p-value <0.05 was considered statistically significant. P values and 95% CIs presented in this report have not been adjusted for multiplicity, and therefore, inferences drawn from these statistics may not be reproducible.
Results:
Patient Characteristics
Sixty-three treatment naïve PAH and 62 control subjects were prospectively enrolled. Figure S2 is a STROBE (strengthening the reporting of observational studies in epidemiology) diagram describing subject eligibility. Patients were predominantly female and PAH subjects were majority idiopathic (Table 1). The median time between CMR and right heart catheterization was 7 [range −11–30] days for both baseline and follow-up. At baseline, the patients had very advanced PAH, characterized by low cardiac index and significantly elevated afterload. CMR demonstrated nearly twice the RVEDVI and depressed RVEF relative to controls. Therapeutic improvements in RVEF were accompanied by improvements in 6-minute walk, PVR, pulmonary compliance, cardiac output, and BNP at 11±3 months follow-up (Table 1).
Table 1.
Demographics and clinical data, cardiac MRI, and resting hemodynamics by assessment time by PAH or control cohorts.
| PAH (n=63) | Control (n=62) | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | P-value* | P-value† | |||
| Age (years) | 51.2±12.7 | 50.0±14.02 | 0.31 | |||
| Sex (N/% Female) | 51 (81) | 49 (79) | 0.82 | |||
| BMI (kg/m2) | 31.0±8.3 | 27.3±5.3 | 0.003 | |||
| Ethnicity (N/% Hispanic) | 18 (28) | 14 (23) | 0.13 | |||
| 6 Minute Walk Distance (M) | 284[115,362] | 355[204,434] | <0.001 | 547[479,600] | <0.001 | |
| BNP (pg/dL) | 503[127.5,852.5] | 70[27,155] | <0.001 | 52[28.5,89.0] | <0.001 | |
| Cardiac MRI | RVEDVI (mL/m2) | 110.1[92.2,136.1] | 100.8[80.7,128.0] | 0.12 | 69.8[59.2,78.8] | <0.001 |
| RVESVI (mL/m2) | 77.6[60,103.8] | 63.6[51.9,85.1] | <0.001 | 29.9[23.8,36.1] | <0.001 | |
| RVEF (%) | 27.4±9.3 | 35.0±9.8 | <0.001 | 57.12±7.29 | <0.001 | |
| RV Mass (gm/m2) | 46.1±15.2 | 45.0±22.8 | 0.65 | 10.8±3.4 | <0.001 | |
| RV Mass/EDV (gm/mL) | 0.41±0.12 | 0.41±0.14 | 1.0 | 0.17±0.05 | <0.001 | |
| Hemodynamics | RAP (mmHg) | 11±7 | 4±4 | <0.001 | ||
| mPAP (mmHg) | 55.4±10.4 | 39.2±9.5 | <0.001 | |||
| PCWP (mmHg) | 9.2±4.4 | 7.3±3.1 | <0.001 | |||
| Cardiac Index (L/min/m2) | 2.02±0.63 | 2.9±0.99 | <0.001 | |||
| PVR (WU) | 11.8[9.45,15.9] | 6.1[4.79,7.98] | <0.001 | |||
| Compliance (mL/mmHg) | 0.99[0.77,1.35] | 1.74[1.30,2.33] | <0.001 | |||
| PA Elastance (mmHg/mL) | 1.52[1.19,1.99] | 0.89[0.70,1.28] | <0.001 | |||
Values are mean ± SD, median [P25,P75], or N (%). BMI, body mass index; BNP, brain natriuretic peptide; MRI, magnetic resonance imaging; RVESVI, right ventricular end-systolic volume index; RVEDVI, right ventricular end-diastolic volume index; RVEF, right ventricular ejection fraction; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure; PVR, pulmonary vascular resistance. P values calculated as follows: Kruskal-Wallis test for continuous and ordinal variables or Wilcoxon signed-rank non-parametric test for related samples, Fisher exact test for categorical variables.
P-value within PAH follow-up versus baseline;
P value control versus PAH at follow-up.
Right Ventricular Functional Recovery
As demonstrated in Figure 1A–B, VO2peak at follow-up was correlated with changes in RVEDV and RVEF between baseline and follow-up. 22/63 (35%) patients had VO2peak>15mL/kg/min at follow-up with 20 (91% of the 22) having a change in both RVEDV and RVEF meeting the cutoff defined by the highest Youden index (0.74 at −15mL and 0.51 at 4% change, respectively) while 2 (9%) met only one of these cutoffs. Of the 41/63 (65%) of patients with VO2peak≤15mL/kg/min at follow-up, 22 (54% of the 41) were below both cutoffs, 15 (37%) had RVEF above the 4% change but below the RVEDV cutoff, whereas 4 (9%) met both the RVEF and RVEDV cutoffs. ROC analysis (Figure 1C) confirmed changes in RVEDV were superior to changes in RVEF in the prediction of VO2peak at follow-up. Indexing RVEDV to BSA (RVEDVI) was not more predictive than RVEDV (Youden 0.65 at −10.4 mL/m2). Change in RVESV and relative change in RVESV and EDV were not more predictive than change in RVEDV (Table S1). All PAH patients who achieved the high VO2peak cutoff at follow-up demonstrated improvements in both RVESV and RVEDV, whereas some patients below the VO2peak cutoff had improvements in RVESV alone (Figure S3).
Figure 1. Defining right ventricular functional recovery.
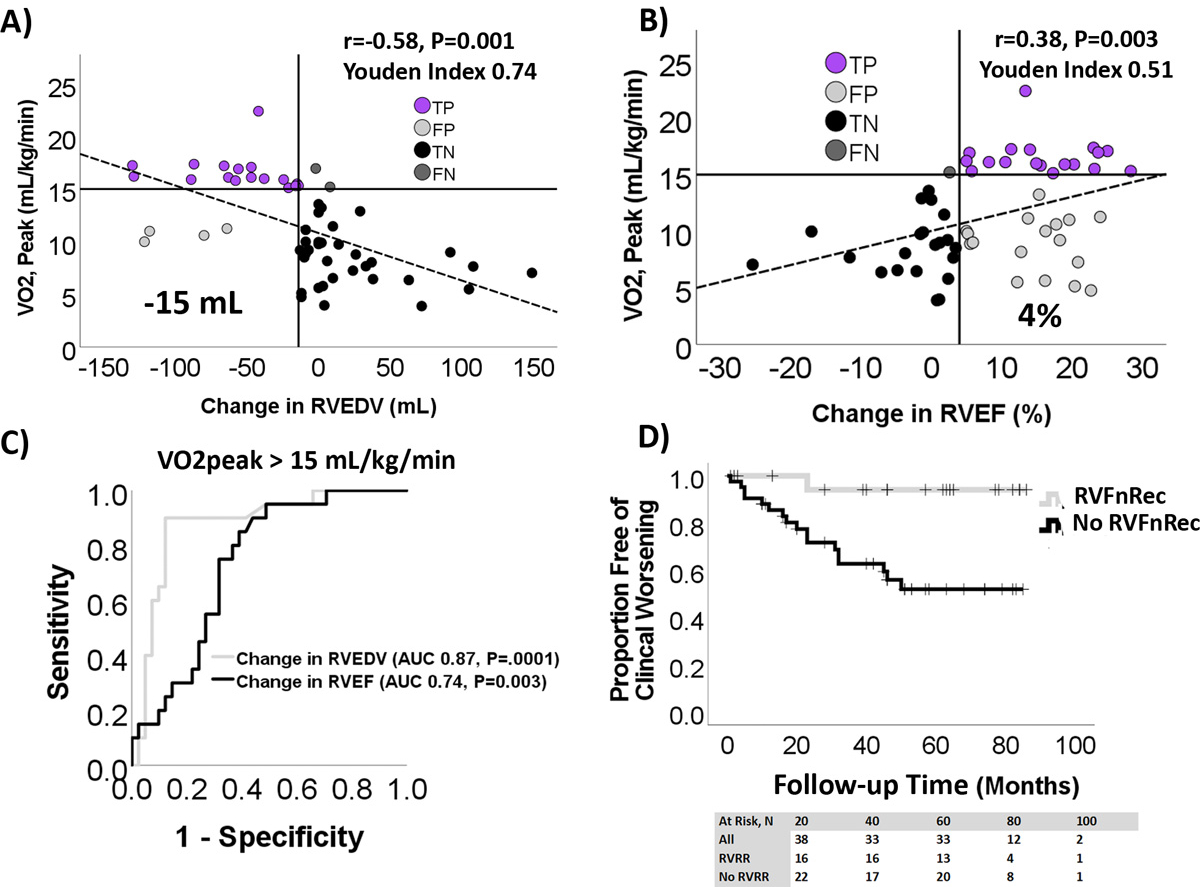
A change in RV end-diastolic volume (RVEDV) (A) and RV ejection fraction (RVEF) (B) from baseline to follow-up both correlated with peak oxygen consumption, VO2peak, at follow-up. However, a change in RVEDV better discriminated high exercise capacity (VO2peak >15mL/kg/min) than did a change in RV ejection fraction (RVEF). Vertical solid lines indicate best Youden index of 0.72 at an RVEDV of −15mL or 0.51 for RVEF at +4%. Based on these cutoffs, the number of true positives (TP) and true (TN) and false (FN) negatives were similar in RVEDV and RVEF. However, the number of false positives (FP) was higher for RVEF. C) Receiver operating characteristic (ROC) analysis predicting VO2peak>15 mL/kg/min shows a change in RVEDV of −15mL is more predictive than RVEF +4mL as well. D) Kaplan-Meier plot of RVFnRec and no RVFnRec by clinical worsening shows higher freedom from clinical worsening in the RVFnRec group.
22/63 (35%) of patients met criteria for RVFnRec using a drop of −15 mL in RVEDV. Using this definition of RVFnRec, the average length of follow up was 50±28 and 38±25 months from their follow-up visit (second assessment) for the RVFnRec and no RVFnRec groups, respectively. All-cause mortality was 1 (5%) and 10 (24%) and clinical worsening was 1 (5%) and 17 (41%) in the RVFnRec and no RVFnRec groups, respectively. As shown in Figure 1D, this definition of RVFnREc also resulted in a significant difference in time to clinical worsening, P=0.006. There were no significant baseline predictors of RVFnRec although this group was represented by a higher proportion of females (91 versus 76%, P=0.14) (Table 2). There were significantly more patients in the RVFnRec group treated with up-front combination therapy versus the no RVFnRec group (50 versus 24%, P=0.01, Table 2 and Figure S4). Treatment approach differences were due in part to a difference between groups in time of enrollment (RVFnRec median enrollment date 4/2017 [3/2014–5/2021] versus no RVFnRec 5/2018 [11/2013–12/2021]). Up-front combination therapy was associated with odds-ratio of 10.4 (CI 1.9–56.6, P=0.007) of RVFnRec relative to goal-directed sequential therapy. There was a trend to higher number of triple-combination therapy in the RVFnRec versus the no RVFnRec cohorts, 8/13 (62%) versus 10/29 (35%)(P=0.10), respectively (Table 2). There were significant improvements in functional class, 6-minute walk distance, REVEAL 2.0, RV volumes and EF in the RVFnRec group by follow-up at 11±3 months compared to the no RVFnRec group at 11±4 months (Table 2 and 3). Although both groups had an increase in LVEDV, only the RVFnRec group had an improvement in LVEF (Table 3).
Table 2.
Demographics and clinical data, multiparametric risk score, and standard resting hemodynamics by assessment time by RV functional recovery (RVFnRec) or no RV functional recovery (No RVFnRec) cohorts.
| Baseline | Follow-Up | P value* | Baseline | Follow-Up | P-value* | P-value† | ||
|---|---|---|---|---|---|---|---|---|
| Age (years) | 54.1±9.4 | 49.7±14.0 | 0.10 | |||||
| Sex (N/% Female) | 19 (91) | 32 (76) | 0.14 | |||||
| BMI (kg/m2) | 31.4±10.5 | 30.9±7.1 | 0.41 | |||||
| WSPH Category, N (%) | CHD | 1 (4.8) | 1 (2.4) | 0.90 | ||||
| CTD | 4 (19.0) | 11 (26.2) | ||||||
| Drug/Toxin | 3 (14.3) | 8 (19.0) | ||||||
| HIV | 0 (0) | 1 (2.4) | ||||||
| iPAH | 12 (57.1) | 20 (47.6) | ||||||
| PoPH | 1 (4.8) | 1 (2.4) | ||||||
| Functional Class | I/II | 1 (4.5) | 12(54.5) | <0.001 | 0 | 12 (35) | <0.001 | 0.013 |
| Risk Scores, N (%) | REVEAL 2.0 Low Risk | 1 (4.8) | 14 (91.0) | <0.001 | 7 (16.7) | 20 (47.6) | 0.005 | 0.01 |
| REVEAL 2.0 Intermediate Risk | 7 (33.3) | 2 (9.0) | 12 (28.6) | 11 (26.2) | ||||
| REVEAL 2.0 High Risk | 13 61.9) | 0 (0) | 23 (54.8) | 11 (26.2) | ||||
| 6 Minute Walk Distance (M) | 267±117 | 412±101 | <0.001 | 252±129 | 274±141 | 0.11 | ||
| Treatment, N (%) | Monotherapy TRE (prior to 2015) | 9 (41) | 12 (29.3) | 0.025 | ||||
| Diuretics, N (%) | Loop Diuretics | 6 (27.3) | 12 (29.3) | 0.22 | ||||
| Hemodynamics | RAP (mmHg) | 11±6 | 3.0±3.0 | <0.001 | 11±7 | 5±4 | <0.001 | 0.08 |
Values are mean ± SD, median [P25,P75], or N (%). ADO, aldosterone; BMI, body mass index; CHD, congenital heart disease; CTD, connective tissue disease; HIV, human immunodeficiency virus; iPAH, idiopathic pulmonary arterial hypertension; PoPH, portopulmonary hypertension; REVEAL, United States Registry to Evaluate Early and Long-Term PAH Disease Management; ERS, European Respiratory Society; BNP, brain natriuretic peptide; MRI, magnetic resonance imaging; RVESVI, right ventricular end-systolic volume index; RVEDVI, right ventricular end-diastolic volume index; RVEF, right ventricular ejection fraction; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure; PVR, pulmonary vascular resistance; TRE, treprostinil. P values calculated as follows: Kruskal-Wallis test for continuous and ordinal variables or Wilcoxon signed-rank non-parametric test for related samples (within cohort) or repeated measures ANOVA (between cohort), Fisher exact test for categorical variables.
P value baseline versus follow-up within RVFnRec or no RVFnRec cohorts;
P-value for the between cohort difference between baseline and follow-up.
Table 3.
Longitudinal Cardiac MRI features of the RV functional recovery (RVFnRec) and no recovery (No RVFnRec) cohorts.
| Baseline | Follow-Up | Mean Change | Relative Change | P-value* | Baseline | Follow-Up | Mean Change | Relative Change | P-value* | P-Value† | P-Value‡ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
RVEDVI
(mL/m2) |
119.5 [104.0,145.7] |
92.3 [75.5,102.7] |
−34.5±24.6 | −25.5±14.1 | <0.001 | 105 [87.5,131.8] |
114.0 [88.2,159.0] |
8.7±26.5 | 9.0±26.8 | 0.04 | <0.001 | <0.001 |
|
RVESVI
(mL/m2) |
92.3 [74.5,105.1] |
35.5 [31.0,40.0] |
−37.6±18.2 | −39.6±13.3 | <0.001 | 71.2 [57.4,101.9] |
73.2 [57.5,123.8] |
1.6±25.1 | 6.2±37.7 | 0.88 | <0.001 | <0.001 |
| RVEF (%) | 26.6±6.7 | 43.0±7.4 | 15.7±7.1 | 55.7±35.9 | <0.001 | 27.7±10.3 | 32.0±8.7 | 4.4±11.0 | 32.4±62.8 | 0.02 | <0.001 | 0.12 |
|
RVMI
(gm/m2) |
44.6 [34.1,51.3] |
35.4 [29.8,40.5] |
−1.1±28.2 | −2.1±41.0 | 0.12 | 50.0 [29.4,60.5] |
43.9 [33.0,59.2] |
−1.2±13.0 | 0.2±30.6 | 0.50 | 0.94 | 0.89 |
|
RV M/V
(gm/mL) |
0.4 [0.3,0.52] |
0.37 [0.33,0.45 |
0.1±0.1 | 22.0±33.7 | 0.45 | 0.42 [0.3,0.6] |
0.34 [0.30,0.50] |
−0.04±0.1 | −7.2±22.8 | 0.61 | 0.06 | 0.07 |
| RV SV/ESV | 0.37±0.1 | 0.72±0.3 | 0.34±0.2 | 98.0±66.8 | <0.001 | 0.41±0.2 | 0.5±0.2 | 0.1±0.2 | 51.4±93.9 | 0.04 | <0.001 | 0.04 |
|
LVEDVI
(mL/m2) |
47.5 [44.8,59.7] |
66.3 [51.5,75.9] |
10.7±16.9 | 28.6±44.3 | 0.006 | 49.0 [39.5,54.9] |
63.8 [55.1,76.2] |
16.8±15.4 | 35.4±32.4 | <0.001 | 0.22 | 0.56 |
|
LVESVI
(mL/m2) |
25.0 [17.1,26.9] |
26.6 [17.7,36.4] |
1.7±9.9 | 12.6±46.0 | 0.21 | 22.0 [15.3,27.8] |
26.6 [23.2,32.8] |
8.3±9.3 | 46.8±52.4 | <0.001 | 0.02 | <0.001 |
| LVEF (%) | 54.0 [46.2,61.3] |
56.8 [54.8,68.3] |
7.2±9.9 | 15.6±19.9 | 0.01 | 54.0 [46.9,67.3] |
57.1 [53.3,60.8] |
−1.3±11.5 | −0.2±19.8 | 0.68 | 0.01 | 0.01 |
|
LVMI
(gm/m2) |
36.8±6.1 | 42.4±26.3 | 22.0±5.1 | 62.3±13.2 | 0.28 | 45.0±16.9 | 42.3±18.7 | −3.5±19.8 | 1.1±52.0 | 0.73 | 0.12 | 0.15 |
|
TR volume
(mL) |
3.0 [0,14.0] |
7.0 [0,21.8] |
7.6±10.9 | 0.23 | 6.0 [0,16] |
5.0 [0,16.0] |
−0.8±16.4 | 1.0 | 0.4 | |||
|
TR fraction
(%) |
0.0 [0,24.6] |
9.8 [0,33.6] |
10.1±18.5 | 0.04 | 5.0 [0,32] |
8.2 [0,27.7] |
−2.5±21.4 | 0.004 | 0.2 |
Values are mean ± SD, median [P25,P75], or N (%). RVEDVI, right ventricular end-diastolic volume index; RVESVI, right ventricular end-systolic volume index; RVEF, right ventricular ejection; RVMI, right ventricular mass index; RV M/V, right ventricular mass/volume; RV SV/ESV, right ventricular stroke volume/end-systolic volume; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; LVEF, left ventricular ejection-fraction; LVMI, left ventricular mass index; TR volume, tricuspid regurgitant volume; TR fraction, tricuspid regurgitant volume fraction (relative to stroke volume) fraction. P values calculated as follows: Wilcoxon signed-rank non-parametric test for related samples (within cohort) or repeated measures ANOVA (between cohort).
P-value for within cohort change from baseline to follow-up;
p value for between cohort change baseline to follow-up;
p-value for between group relative change (as % baseline value) baseline to follow-up.
As shown in Figure 2A, only the RVFnRec group demonstrated a therapeutic change in RVEDV that nears “normalization” of RVEDVI at follow-up at the upper 95% confidence interval (CI) of healthy controls (90 mL/m2). −15 mL decrease in RVEDV identifies most of the patients included in this confidence interval. Although most of both the RVFnRec(20/22, 91%) and no RVFnRec (32/41, 78%) groups met the SVI lower 95% CI of controls (30 mL/m2), only the RVFnRec group neared the upper and lower 95% CI of controls for RVEDVI (11/22, 50% versus 10/41, 24%, respectively) and RVEF (8/22, 35%) versus 2/41, 5%, respectively) at follow-up (Figure 2B). Despite near normalization of EDV and RVEF among the RVFnRec group, relative wall thickness (RV mass/volume) at follow-up remained approximately twice that of controls, RVFnRec 0.43±0.13 gm/mL versus control 0.21±0.05 gm/mL (Figure 2C, Table 1 and 2). Table S2 compares RV volume and mass in PAH and control subjects with previously published control reference values.
Figure 2. Near normalization of right ventricular volume in functional recovery.
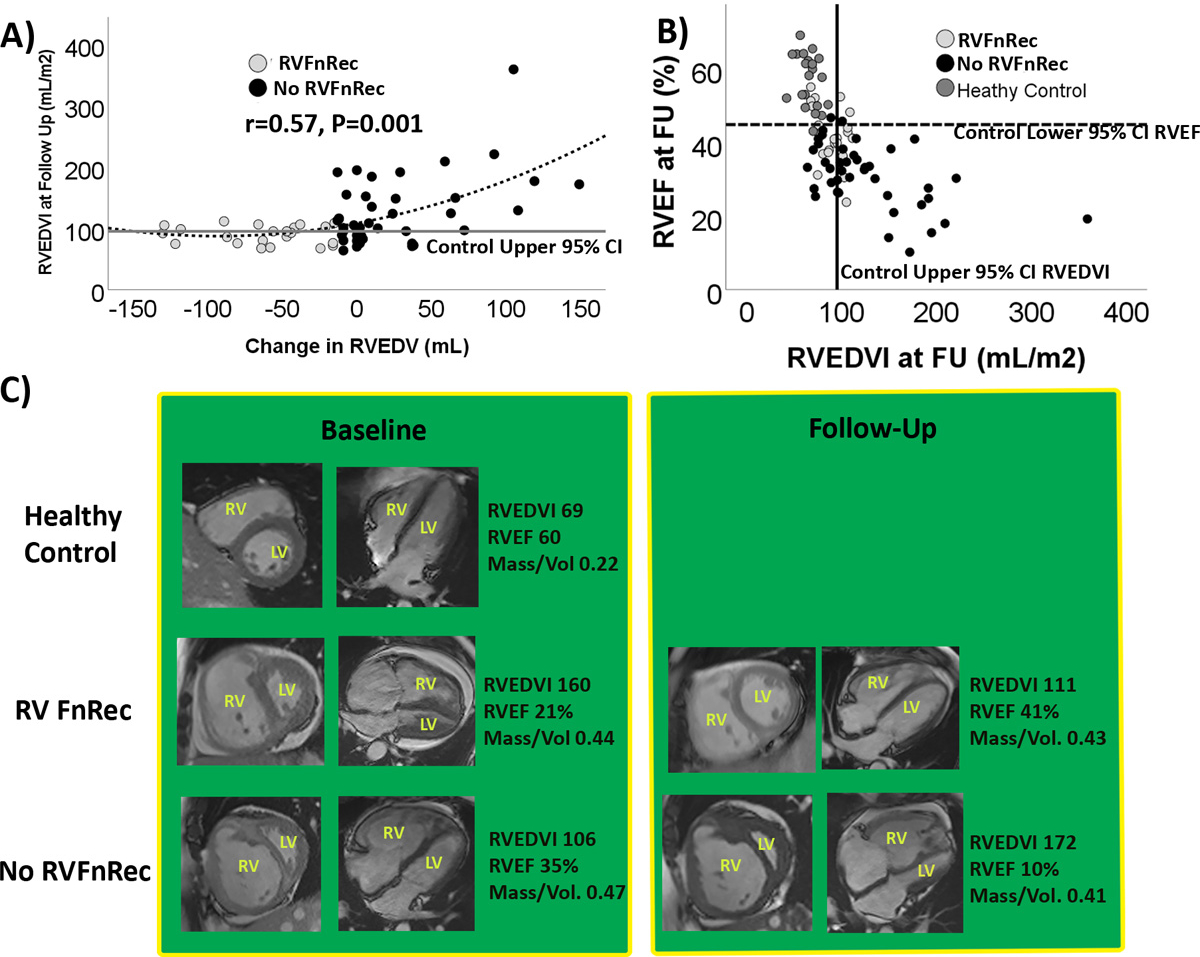
A) The treatment related change in RV end-diastolic volume (RVEDV) relative to RVEDV index at follow-up in the functional recovery (RVFnRec) and no RVFnRec patients. Only RVFnRec patients achieve a therapeutic change in RVEDV that nears normalization at follow-up. B) RVEF and RVEDVI at follow-up in the RVFnRec, no RVFnRec, and healthy controls. Solid vertical line indicates the upper 95% CI and the dark horizontal line the lower 95% CI for RVEDV and RVEF in the control cohort, respectively. C) Treatment related changes in short (first column) and long (second column) axis cardiac MRI images at Baseline and Follow-up. Substantial, improvements in RV volume and ejection fraction are seen in the RVFnRec group. However, relative wall thickness (mass/volume) remained almost twice the healthy control value in both the RVFnRec and no RVFnRec groups.
RV Afterload Parameters Associated with RV Functional Recovery
Patients with RVFnRec had larger absolute and relative (to baseline) drops in mPAP, and Ca, as well as relative changes in PVR, versus the no RVFnRec group by follow-up (Table 4). The absolute change in PVR and Ea and relative changes in Ea were not significant (Table 4). A greater proportion of RVFnRec than no RVFnRec patients had moved to the “steep” portion of the PVR-Ca curve (Figure 3A) by follow-up. 9/63 (~14%) of PAH subjects had near normalization of PVR (~3WU) (Figure 3A). The accuracy of the change in the absolute value, change in relative value (as % baseline), and absolute value at follow-up were assessed as shown in Figure 3B and S5A–B. The value giving the best AUC 0.76 (95% CI 0.64–0.88, P<0.0001) was change in Ca at a cutoff of 0.8 mL/mmHg yielding a sensitivity of 85% and a specificity of 72% for RVFnRec. Changes in mPAP, PVR, and Ca were associated with changes in RVEDV (Figure 3C–E) and RVEF (Figure S5C–E) with Ca being the most highly predictive.
Table 4.
Longitudinal parameters of afterload in the RV functional recovery (RVFnRec) and no recovery (No RVFnRec) cohorts.
| Baseline | Follow-up | Mean Change | Relative Change (%) | P-value* | Baseline | Follow-up | Mean Change | Relative Change (%) | P-value* | P value† | P value‡ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
mPAP
(mmHg) |
55.6±11.8 | 34.7±8.4 | −20.9±12.0 | −35.8±18.2 | <0.001 | 54.8±10 | 41.5±9.4 | −13.3±8.2 | −23.6±13.8 | <0.001 | 0.004 | 0.004 |
| PVR (WU) | 11.2 [9.1,14.7] |
5.1 [3.7,7.0] |
−7.7±6.4 | −53.2±21.4 | <0.001 | 12.7 [9.5,16.1] |
6.9 [5.4,10] |
−5.3±5.4 | −33.3±37.5 | <0.001 | 0.14 | 0.028 |
|
Compliance
(mL/mmHg) |
1.0 [1.2,2.0] |
2.1 [1.7,2.7] |
1.4±0.9 | 174.8±146.9 | <0.001 | 1.0 [0.8,1.4] |
1.4 [1.1,2.1] |
0.6±0.8 | 71.3±83.3 | <0.001 | 0.001 | <0.001 |
|
PA Elastance
(mmHg/mL) |
1.5 [1.2,2.0] |
0.8 [0.7,1.1] |
−.7±0.6 | −35.5±37.9 | <0.001 | 1.5 [1.1,2.0] |
0.9 [0.8,1.3] |
−0.7±0.9 | −31.2±35.6 | <0.001 | 0.94 | 0.67 |
Values are mean ± SD, median [P25,P75], or N (%). mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; PA, pulmonary artery. P values calculated as follows: Wilcoxon signed-rank non-parametric test for related samples (within cohort) or repeated measures ANOVA (between cohort).
P-value for cohort change from baseline to follow-up;
P value for between cohort mean change baseline to follow-up;
P-value for between group relative change (as % baseline value) baseline to follow-up.
Figure 3. Afterload changes predicting right ventricular functional recovery.
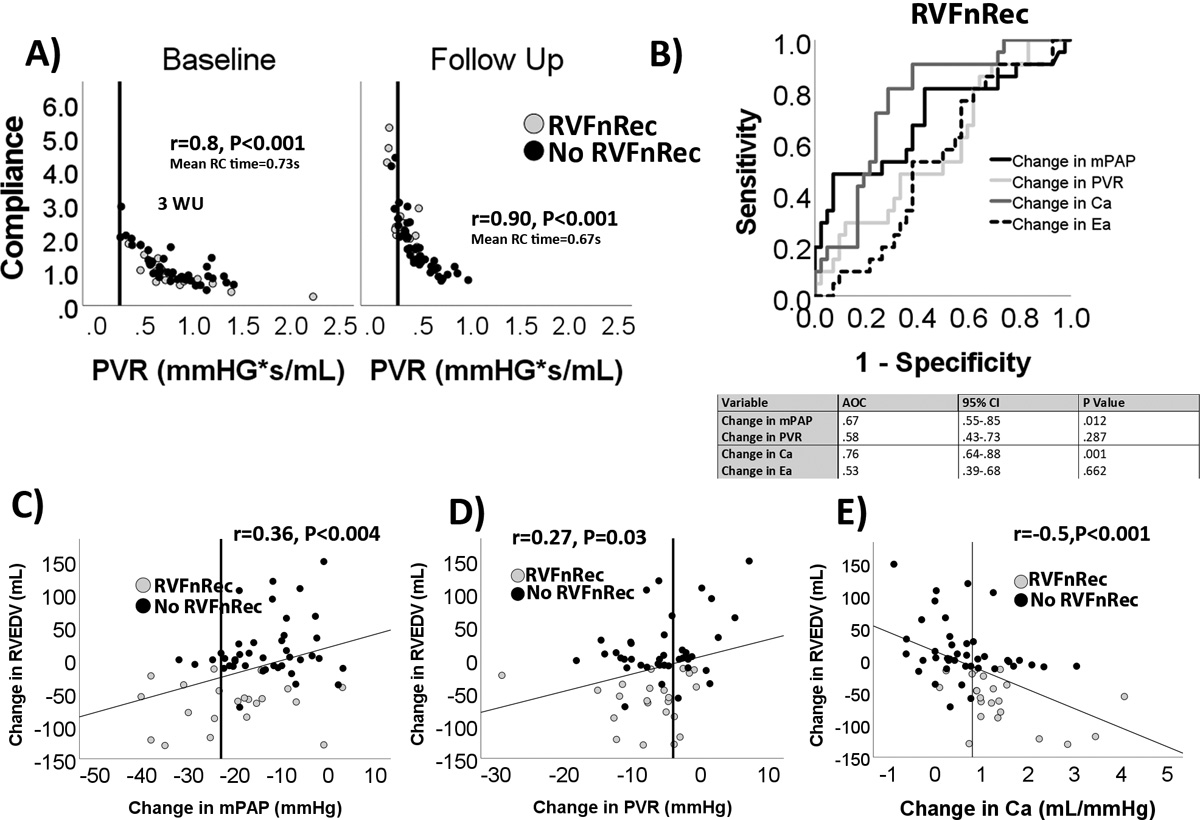
(A) non-linear relationship between pulmonary vascular resistance (PVR) and compliance (Ca) at baseline and follow-up. Most RVFnRec patients had moved to the steep portion of the curve by follow-up. Solid line indicates an upper normal PVR of <3 WU. (B) receiver operating curve analysis of longitudinal changes between baseline and follow-up in mean pulmonary artery pressure (mPAP), PVR, Ca, and effective pulmonary elastance (Ea). Changes in Ca were the most closely related afterload parameter to changes in RV end-diastolic volume (RVEDV) (C-E). Vertical line indicates the highest (“best”) Youdin index cutoff for each afterload parameter.
The Relationship of RV Functional Recovery to Exercise Afterload and RV Output Reserve
The RVFnRec patients had higher VO2peak predicted, mPAP, cardiac output, and stroke volume at exercise than did the no RVFnRec patients at follow-up (Figure 4 and Table S3). The VO2peak was much lower than matched controls, however. Ventilatory efficiency index for carbon dioxide (Ve/VCO2) was lower (better) in the RVFnRec group. The mPAP-cardiac output relationship from rest to peak exercise, was similar in RVFnRec versus the no RVFnRec group (Figure 4B). α distensibility was also similar between RVFnRec and no RVFnRec groups (0.17±0.15 versus 0.19±0.3 %/mmHg, P=NS, respectively) and much lower than predicted normal values of 1.4 (range 0.77–2.34) %/mmHg14(Figure 4D). Using exercise afterload parameters did not improve the accuracy of resting values at follow-up to predict RVFnRec (Figure S6).
Figure 4. The physiological significance of right ventricular functional recovery through exercise at follow-up.
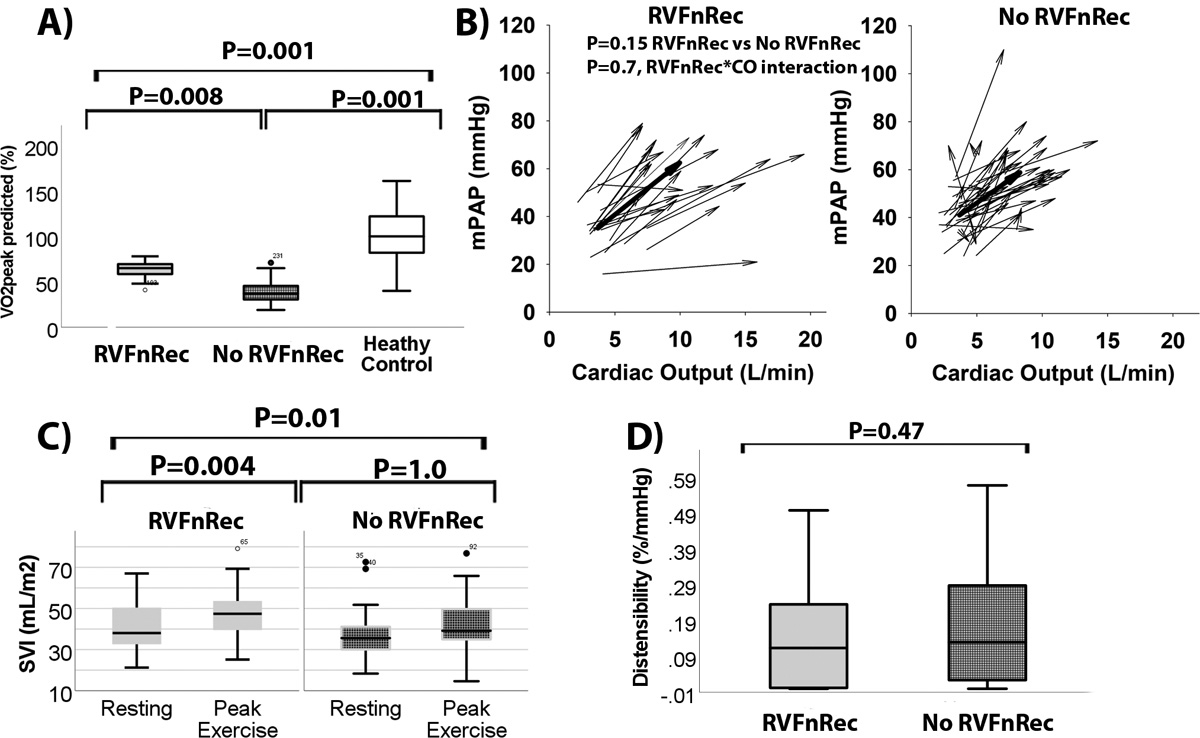
Peak predicted oxygen consumption was higher in RVFnRec than no RVFnRec but both were well below normal matched controls (A). The linear pressure-flow relationship of RVFnRec patients and no RVFnRec patients was similar (B). The arrowheads represent peak and the tails resting mPAP/cardiac output. The thick arrow represents the median vector in each group. Despite similar mPAP/cardiac output, the cardiac output at peak was higher (B) and the change in stroke volume index (SVI) (C) was higher in RVFnRec than that of no RVFnRec patients. Pulmonary vascular α distensibility was similar between groups (D). Taken together (B-D) these findings indicate similar RV afterload at exercise but improved RV output reserve in the RVFnRec cohort.
Discussion:
This study is the first to demonstrate in treatment naïve PAH patients that 1) a −15 mL decrease in RVEDV is a useful CMR definition of RVFnRec associated with exercise capacity (VO2peak) and freedom from clinical worsening, 2) an increase in resting Ca of 0.8 mL/mmHg is superior to other afterload parameters in predicting RVFnRec, and 3) RVFnRec is associated with high exercise RV output reserve.
Although therapeutic improvements in RV size and function add independently prognostic information to well validated risk scores (such as REVEAL 2.0 and ERS risk score) in PAH10,27, the lack of a consensus definition of RV recovery hampers its use as a therapeutic goal and a clinical trial endpoint. As normalization of RV size and function would be an optimal goal, defining the limits of “normal” is challenging given reference populations have varied by age, sex, race/ethnicity, body mass index, and acquisition/reading methodology28–30. We have attempted to mitigate these challenges by enrolling a control group matched for age, sex, and race/ethnicity. Although many of our patients met the CMR 95% confidence interval in the controls, normalization appears to be an unrealistic definition of RVFnRec. However, complete normalization while a laudable goal may be unnecessary for meaningful RV functional recovery. Unlike definitions of RV reverse remodeling which rely on the absence of clinical worsening alone10, we thought it important to examine RVFnRec by CMR cutoffs relating to clinical improvement and exercise capacity. The VO2peak chosen at 15 mL/kg/min is dependent on cardiac output (RV functional reserve) in PAH26 and is derived from guidelines25 and evidence26. We confirmed the findings of others by showing changes in RVEDV are related to freedom from clinical worsening in the RVFnRec group.
There is consensus that RVEF is consistently related to mortality1 and therapeutic improvements in RVEF are related to a drop in afterload10,31. Studies enrolling patients on oral monotherapy or sequential therapy result in minimal afterload reduction. In these studies, improvement in RVEF occurs by reductions in RVESV but with minimal change in RVEDV27,31–33. Early therapeutic changes in RVESV result from improvements in afterload and RV diastolic function20. But RV work efficiency does not improve (vis a vis improved RV-PA coupling) until there is a drop in RVEDV with larger afterload reduction (~7WU or >45% drop in PVR)34. In fact, we demonstrate that a drop in both RVESV and RVEDV are highly associated with an acceptable (VO2peak >15mL/kg/min) exercise capacity. We attempted to optimize the probability of large changes in afterload by employing an aggressive therapeutic strategy based on parenteral prostacyclin7,17. A drop in RVEDV may signify a physiological shift from both heterometric (Frank-Starling) and homeometric (contractility) adaptation to maintain stroke volume to a more energetically optimized condition of homeometric adaptation alone35. Although improvements in RVEF from decreased RVESV represent a formidable short-term therapeutic goal, the increased myocardial efficiency and reduced wall stress accompanied by decreasing RVEDV should represent the ultimate long-term goal35.
Clinical observation indicates that biventricular maladaptive remodeling in PH is reversible with rapid hemodynamic normalization such as lung transplantation6 and pulmonary thomboendarterectomy (PTE) for chronic thromboembolic PH (CTEPH)36. Even RV fibrosis appears reversible in experimental PAH when afterload is gradually but completely normalized37. We have shown for the first time that biventricular normative changes occur particularly in up-front prostacyclin with oral combination therapy resulting in a significant and gradual drop in afterload. Only patients with RVFnRec demonstrated large reductions in RV volume and improvements in RV and LVEF. Therefore, a reduction in RVEDV may be requisite to an improvement in the LV maladaptive changes, such as reduced LVEF36, seen with PAH and CTEPH. Although RVEDV shows near “normalization” relative to controls, normalization of RVEF and mass/volume was less common. RV relative wall thickness (mass/volume) was maintained more than double that of controls likely reflecting reduced but persistently high load. In this context, the present definition of RVFnRec is near the prognostically significant cut-point of 0.47 gm/mL38. The combination of RVFnRec and a high RV mass/volume appears physiologically significant during exercise where these patients can maintain higher cardiac output and stroke volume (RV output reserve) as compared to those without RVFnRec despite similar PAP39,40.
As recently reviewed7, knowledge gaps exist on which afterload parameter predicts RVFnRec. Although the pulmonary impedance spectrum is the gold standard of afterload measurement, it requires simultaneous pressure and flow measurement in the frequency domain making it difficult to measure and interpret12. We have therefore chosen a comprehensive list of frequently measured afterload parameters in clinical medicine. Consistent with previously published data, we found that large changes in PVR (~>50%) are necessary for RVFnRec10. However, pulmonary compliance was significantly more predictive of RVFnRec than measures of steady load such as PVR and the composite measure of load, Ea. Studies have demonstrated that compliance has a more consistent relationship with outcome41–43 and RV function44,45 than does PVR. Also, experimental46 and clinical47 evidence indicates that compliance plays a significant role in maintaining optimal RV-PA coupling even at high PVR. Nevertheless, we found only a mild correlation between all standard load parameters and RV function. This finding may indicate that some afterload parameters not measured, like wave reflection48, or load-independent factors, like possible direct prostacyclin-mediated effects on the RV such as reduced myocardial inflammation49 or coronary microcirculatory effects50, may play a role in RVFnRec. It is possible that RVFnRec may represent a more comprehensive therapeutic target, encompassing both load dependent and independent effects, than targeting load (i.e., PVR) alone.
We found that resting therapeutic improvements in afterload dissipate quickly during exercise as high PAP relative to cardiac output is seen even in patients with RVFnRec. Likely, the drop in load seen at rest is negated at exercise as less affected pulmonary vessels meet maximal recruitment and distension as evidenced by very low α distensibility and high mPAP-C.O. slope in both groups. This observation suggests that pulmonary vascular remodeling is still likely advanced in RVFnRec despite such significant improvements in resting measurements of afterload. The demands on the RV by high afterload at exercise may explain why RVFnRec patients maintain nearly twice the relative RV wall thickness (mass/volume) to that of controls despite near normalization of ventricular volume.
Our study has several limitations. It is a single center PAH cohort and therefore the results require validation amongst a larger population and preferably multiple centers. Validation is particularly important given that our ROC cutoff was assessed only at two time points (baseline and follow-up) and therefore does not account for intrinsic variability during the course of disease. For example, the RVEDV and Ca cutoffs may only be applicable to patients with advanced PAH. Also, our study assessed VO2peak only at follow-up and not the change in VO2 from diagnosis. However, the change in 6-minute walk distance from baseline to follow-up confirms a substantial difference in functional capacity between RVfnRec groups. Also, the feasibility of MRI is difficult at some centers where echocardiography is preferable. Future studies should validate our findings against echocardiographic metrics such as those recently proposed7.
Conclusions
Our data suggest that defining RVFnRec as a therapeutic normative −15 mL drop in RVEDV appears to be clinically useful. Among standard measures of afterload, an increase in Ca of 0.8 mL/mmHg by follow-up best predicts RVFnRec although factors outside afterload alone may be at play. We have shown that our definition of RVFnRec corresponds with near normalization of RV volume, but relative wall thickness remains high. The high relative wall thickness may be needed for output reserve in the face of high afterload at exercise.
Supplementary Material
Clinical Perspective:
What is new?
Right ventricular functional recovery (RVFnRec) represents a novel endpoint of therapeutic success in PAH. We define RVFnRec as treatment associated normative RV changes related to function (peak oxygen consumption). Normative RV imaging changes are compared to a well phenotyped age, sex, and race/ethnicity matched healthy control cohort from the PVDOMICS project. Previous studies have focused on RV ejection fraction improvements. However, we show that changes in RVEDV are perhaps more important in that improvements in LV function also occur. Lastly, RVFnRec is best predicted by improvements in pulmonary artery compliance versus pulmonary vascular resistance, a more often cited metric of RV afterload.
What are the clinical implications?
RVFnRec represents a potential non-invasive assessment of clinical improvement and therapeutic response. Clinicians with access to cardiac MRI can obtain a limited scan (i.e., ventricular volumes) before and after treatment. Future study should examine echocardiographic correlates of RVFnRec.
Acknowledgements:
We would like to thank Andrew Swift (Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Western Bank, Sheffield, UK) for sharing his CMR predicted reference equations with us. We would like to graciously acknowledge the assistance of the UAHD Biorepository at the University of Arizona. We would also like to acknowledge the many PH subjects of the UA registry/biorepository, the PVDOMICS study, and coordinators who have dedicated significant effort in the academic pursuit to further our field.
Sources of funding:
The PVDOMICS study received grants U01 HL125218 (PI: E.B. Rosenzweig), U01 HL125205 (PI: R.P. Frantz), U01 HL125212 (PI: A.R. Hemnes), U01 HL125208 (PI: F.P. Rischard), U01 HL125175 (PI: P.M.Hassoun), U01 HL125215 (PI: J.A. Leopold), and U01 HL125177 (PI: G.J. Beck and S.C. Erzurum) from the NHLBI and the Pulmonary Hypertension Association. The PAH cohort is a continuously enrolling (since 1/21) cohort at UA of treatment naïve patients. This registry/biorepository is funded directly by the University of Arizona.
Author Disclosures:
Dr Rischard has consulting relationships with Bayer and receives research support from Ismed, United Therapeutics, Bayer, Acceleron, Merck and Janssen. Dr. Naeije reports relationships including consultancies, speakers fees and membership of advisory boards with AOP Orphan Pharmaceuticals, Johnson & Johnson, Lung Biotechnology Corporation and United Therapeutics. Dr. Garcia, is CEO and founder of Aqualung Therapeutics Corporation. Dr Frantz has consulting, steering committee, and advisory board relationships with Altavant Sciences, Bayer, Gossamer Bio, Janssen, Shouti, France Foundation, IQVIA, Tenax, UpToDate, and United Therapeutics. Dr. Hassoun has served as consultant for Merck. Dr. Hemnes has consulting relationships with Janssen, Merck, Gossamer, Bio, United Therapeutics, Bayer, Tenax. She owns stock in Tenax therapeutics and she has received research support from NHLBI and CMREF. Dr. Hill is the chair for an Aerovate steering committee. He is a consultant for Bellerophon and Liquidia and Merck. Dr. Horn has consulting relationships with biotronik and AADI biosciences. She serves on the DSMB for SoniVie and V-waves Ltd. She receives research support from Merck and Cereno. Dr. Leopold Abbott has served as consultant for Aria. She has research support form Astella Pharma and United Therapeutics. Dr. Rosenzweig has received consulting fees from Acceleron for a scientific advisory board meeting; and her institution receives grant support from Bayer, United Therapeutics, Janssen, and SonVie. Dr. Tang served as consultant for Sequana Medical, Cardiol Therapeutics, Genomics plc, Zehna Therapeutics, Renovacor, WhiteSwell, Kiniksa, Boston Scientific, and CardiaTec Biosciences and has received honorarium from Springer Nature and American Board of Internal Medicine. Dr. Bernardo, Vanderpool, Kwon, Acharya, Ms. Park, Mr. Katrynuik, Dr. Insel, Kubba, Badagliacca, Larive, Beck, Erzurum, and Ms. Wilcox have no disclosures.
Abbreviations
- ANOVA
analysis of variance
- BNP
brain natriuretic peptide
- Ca
pulmonary vascular compliance
- CI
confidence interval
- CMR
cardiac MRI
- CTEPH
chronic thromboembolic pulmonary hypertension
- EDV
end-diastolic volume
- EF
ejection fraction
- ERS/ESC
European Respiratory Society/European Society of Cardiology
- ESV
end-systolic volume
- Ea
effective pulmonary arterial elastance
- LV
Left ventricle
- PA
pulmonary artery
- PAH
pulmonary arterial hypertension
- PAP
pulmonary artery pressure
- PCWP
pulmonary capillary wedge pressure
- PVR
pulmonary vascular resistance
- RAP
right atrial pressure
- REVEAL
United States Registry to Evaluate Early and Long-Term PAH Disease Management
- RV
right ventricle
- RVFnRec
RV functional recovery
- sRVP
systolic RV pressure
- SV
stroke volume
- PTE
thromboendarterectomy
- WSPH
World Symposium of Pulmonary Hypertension
- VO2peak
peak oxygen consumption
Footnotes
References
- 1.Leopold JA, Kawut SM, Aldred MA, Archer SL, Benza RL, Bristow MR, Brittain EL, Chesler N, DeMan FS, Erzurum SC, et al. Diagnosis and Treatment of Right Heart Failure in Pulmonary Vascular Diseases: A National Heart, Lung, and Blood Institute Workshop. Circ Heart Fail. 2021;14:e000069. doi: 10.1161/CIRCHEARTFAILURE.120.007975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, Hemnes AR, Kawut SM, Kline JA, Kolb TM, et al. Assessment of Right Ventricular Function in the Research Setting: Knowledge Gaps and Pathways Forward. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2018;198:e15–e43. doi: 10.1164/rccm.201806-1160ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rain S, Bos Dda S, Handoko ML, Westerhof N, Stienen G, Ottenheijm C, Goebel M, Dorfmuller P, Guignabert C, Humbert M, et al. Protein changes contributing to right ventricular cardiomyocyte diastolic dysfunction in pulmonary arterial hypertension. J Am Heart Assoc. 2014;3:e000716. doi: 10.1161/JAHA.113.000716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu S, Kokkonen-Simon KM, Kirk JA, Kolb TM, Damico RL, Mathai SC, Mukherjee M, Shah AA, Wigley FM, Margulies KB, et al. Right Ventricular Myofilament Functional Differences in Humans With Systemic Sclerosis-Associated Versus Idiopathic Pulmonary Arterial Hypertension. Circulation. 2018;137:2360–2370. doi: 10.1161/CIRCULATIONAHA.117.033147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Wolferen SA, Marcus JT, Westerhof N, Spreeuwenberg MD, Marques KM, Bronzwaer JG, Henkens IR, Gan CT, Boonstra A, Postmus PE, et al. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J. 2008;29:120–127. doi: 10.1093/eurheartj/ehm567 [DOI] [PubMed] [Google Scholar]
- 6.Gorter TM, Verschuuren EAM, van Veldhuisen DJ, Hoendermis ES, Erasmus ME, Bogaard HJ, Vonk Noordegraaf A, Berger RMF, van Melle JP, Willems TP. Right ventricular recovery after bilateral lung transplantation for pulmonary arterial hypertensiondagger. Interact Cardiovasc Thorac Surg. 2017;24:890–897. doi: 10.1093/icvts/ivx025 [DOI] [PubMed] [Google Scholar]
- 7.Vizza CD, Lang IM, Badagliacca R, Benza RL, Rosenkranz S, White RJ, Adir Y, Andreassen AK, Balasubramanian V, Bartolome S, et al. Aggressive Afterload Lowering to Improve the RV: A New Target for Medical Therapy in PAH? Am J Respir Crit Care Med. 2021. doi: 10.1164/rccm.202109-2079PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Alto M, Badagliacca R, Argiento P, Romeo E, Farro A, Papa S, Sarubbi B, Russo MG, Vizza CD, Golino P, et al. Risk Reduction and Right Heart Reverse Remodeling by Upfront Triple Combination Therapy in Pulmonary Arterial Hypertension. Chest. 2020;157:376–383. doi: 10.1016/j.chest.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 9.Boulet J, Mehra MR. Left Ventricular Reverse Remodeling in Heart Failure: Remission to Recovery. Structural Heart. 2021;5:466–481. doi: 10.1080/24748706.2021.1954275 [DOI] [Google Scholar]
- 10.Badagliacca R, Poscia R, Pezzuto B, Papa S, Reali M, Pesce F, Manzi G, Gianfrilli D, Ciciarello F, Sciomer S, et al. Prognostic relevance of right heart reverse remodeling in idiopathic pulmonary arterial hypertension. J Heart Lung Transplant. 2018;37:195–205. doi: 10.1016/j.healun.2017.09.026 [DOI] [PubMed] [Google Scholar]
- 11.Spruijt OA, Di Pasqua MC, Bogaard HJ, van der Bruggen CE, Oosterveer F, Marcus JT, Vonk-Noordegraaf A, Handoko ML. Serial assessment of right ventricular systolic function in patients with precapillary pulmonary hypertension using simple echocardiographic parameters: A comparison with cardiac magnetic resonance imaging. J Cardiol. 2017;69:182–188. doi: 10.1016/j.jjcc.2016.02.019 [DOI] [PubMed] [Google Scholar]
- 12.Saouti N, Westerhof N, Postmus PE, Vonk-Noordegraaf A. The arterial load in pulmonary hypertension. Eur Respir Rev. 2010;19:197–203. doi: 10.1183/09059180.00002210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513 [DOI] [PubMed] [Google Scholar]
- 14.Lau EMT, Chemla D, Godinas L, Zhu K, Sitbon O, Savale L, Montani D, Jais X, Celermajer DS, Simonneau G, et al. Loss of Vascular Distensibility During Exercise Is an Early Hemodynamic Marker of Pulmonary Vascular Disease. Chest. 2016;149:353–361. doi: 10.1378/chest.15-0125 [DOI] [PubMed] [Google Scholar]
- 15.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1–13. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 17.Badagliacca R, Papa S, Manzi G, Miotti C, Luongo F, Sciomer S, Cedrone N, Fedele F, Naeije R, Vizza CD. Usefulness of Adding Echocardiography of the Right Heart to Risk-Assessment Scores in Prostanoid-Treated Pulmonary Arterial Hypertension. JACC Cardiovasc Imaging. 2020;13:2054–2056. doi: 10.1016/j.jcmg.2020.04.005 [DOI] [PubMed] [Google Scholar]
- 18.Hemnes AR, Leopold JA, Radeva MK, Beck GJ, Abidov A, Aldred MA, Barnard J, Rosenzweig EB, Borlaug BA, Chung WK, et al. Clinical Characteristics and Transplant-Free Survival Across the Spectrum of Pulmonary Vascular Disease. J Am Coll Cardiol. 2022;80:697–718. doi: 10.1016/j.jacc.2022.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benza RL, Gomberg-Maitland M, Elliott CG, Farber HW, Foreman AJ, Frost AE, McGoon MD, Pasta DJ, Selej M, Burger CD, et al. Predicting Survival in Patients With Pulmonary Arterial Hypertension: The REVEAL Risk Score Calculator 2.0 and Comparison With ESC/ERS-Based Risk Assessment Strategies. Chest. 2019;156:323–337. doi: 10.1016/j.chest.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 20.Vanderpool RR, Desai AA, Knapp SM, Simon MA, Abidov A, Yuan JX, Garcia JGN, Hansen LM, Knoper SR, Naeije R, et al. How prostacyclin therapy improves right ventricular function in pulmonary arterial hypertension. Eur Respir J. 2017;50:1–4. doi: 10.1183/13993003.00764-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tedford RJ, Hsu S, Kass DA. Letter by Tedford Regarding Article, “Effective Arterial Elastance in the Pulmonary Arterial Circulation: Derivation, Assumptions, and Clinical Applications”. Circ Heart Fail. 2020;13:e007081. doi: 10.1161/CIRCHEARTFAILURE.120.007081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naeije R, Vanderpool R, Dhakal BP, Saggar R, Saggar R, Vachiery JL, Lewis GD. Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med. 2013;187:576–583. doi: 10.1164/rccm.201211-2090CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang WHW, Wilcox JD, Jacob MS, Rosenzweig EB, Borlaug BA, Frantz RP, Hassoun PM, Hemnes AR, Hill NS, Horn EM, et al. Comprehensive Diagnostic Evaluation of Cardiovascular Physiology in Patients With Pulmonary Vascular Disease: Insights From the PVDOMICS Program. Circ Heart Fail. 2020;13:e006363. doi: 10.1161/CIRCHEARTFAILURE.119.006363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovacs G, Herve P, Barbera JA, Chaouat A, Chemla D, Condliffe R, Garcia G, Grunig E, Howard L, Humbert M, et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J. 2017;50:1–18. doi: 10.1183/13993003.00578-2017 [DOI] [PubMed] [Google Scholar]
- 25.Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano-Subias P, Ferrari P, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 26.Badagliacca R, Papa S, Poscia R, Valli G, Pezzuto B, Manzi G, Torre R, Gianfrilli D, Sciomer S, Palange P, et al. The added value of cardiopulmonary exercise testing in the follow-up of pulmonary arterial hypertension. J Heart Lung Transplant. 2019;38:306–314. doi: 10.1016/j.healun.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 27.Lewis RA, Johns CS, Cogliano M, Capener D, Tubman E, Elliot CA, Charalampopoulos A, Sabroe I, Thompson AAR, Billings CG, et al. Identification of Cardiac Magnetic Resonance Imaging Thresholds for Risk Stratification in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2020;201:458–468. doi: 10.1164/rccm.201909-1771OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawut SM, Lima JA, Barr RG, Chahal H, Jain A, Tandri H, Praestgaard A, Bagiella E, Kizer JR, Johnson WC, et al. Sex and race differences in right ventricular structure and function: the multi-ethnic study of atherosclerosis-right ventricle study. Circulation. 2011;123:2542–2551. doi: 10.1161/CIRCULATIONAHA.110.985515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawel-Boehm N, Hetzel SJ, Ambale-Venkatesh B, Captur G, Francois CJ, Jerosch-Herold M, Salerno M, Teague SD, Valsangiacomo-Buechel E, van der Geest RJ, et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J Cardiovasc Magn Reson. 2020;22:87. doi: 10.1186/s12968-020-00683-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foppa M, Arora G, Gona P, Ashrafi A, Salton CJ, Yeon SB, Blease SJ, Levy D, O’Donnell CJ, Manning WJ, et al. Right Ventricular Volumes and Systolic Function by Cardiac Magnetic Resonance and the Impact of Sex, Age, and Obesity in a Longitudinally Followed Cohort Free of Pulmonary and Cardiovascular Disease. Circulation: Cardiovascular Imaging. 2016;9: e003810. doi: 10.1161/CIRCIMAGING.115.003810 [DOI] [PubMed] [Google Scholar]
- 31.van de Veerdonk MC, Huis In TVAE, Marcus JT, Westerhof N, Heymans MW, Bogaard HJ, Vonk-Noordegraaf A. Upfront combination therapy reduces right ventricular volumes in pulmonary arterial hypertension. Eur Respir J. 2017;49:1–11. doi: 10.1183/13993003.00007-2017 [DOI] [PubMed] [Google Scholar]
- 32.Peacock AJ, Crawley S, McLure L, Blyth KG, Vizza CD, Poscia R, Francone M, Iacucci I, Olschewski H, Kovacs G, et al. Changes in right ventricular function measured by cardiac magnetic resonance imaging in patients receiving pulmonary arterial hypertension-targeted therapy: the EURO-MR study. Circ Cardiovasc Imaging. 2014;7:107–114. doi: 10.1161/CIRCIMAGING.113.000629 [DOI] [PubMed] [Google Scholar]
- 33.Vonk Noordegraaf A, Channick R, Cottreel E, Kiely DG, Marcus JT, Martin N, Moiseeva O, Peacock A, Swift AJ, Tawakol A, et al. The REPAIR Study: Effects of Macitentan on RV Structure and Function in Pulmonary Arterial Hypertension. JACC Cardiovasc Imaging. 2022;15:240–253. doi: 10.1016/j.jcmg.2021.07.027 [DOI] [PubMed] [Google Scholar]
- 34.Vanderpool RR, Hunter KS, Insel M, Garcia JGN, Bedrick EJ, Tedford RJ, Rischard FP. The Right Ventricular-Pulmonary Arterial Coupling and Diastolic Function Response to Therapy in Pulmonary Arterial Hypertension. Chest. 2021;161:1048–1059. doi: 10.1016/j.chest.2021.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The Relationship Between the Right Ventricle and its Load in Pulmonary Hypertension. J Am Coll Cardiol. 2017;69:236–243. doi: 10.1016/j.jacc.2016.10.047 [DOI] [PubMed] [Google Scholar]
- 36.Hardziyenka M, Campian ME, Reesink HJ, Surie S, Bouma BJ, Groenink M, Klemens CA, Beekman L, Remme CA, Bresser P, et al. Right ventricular failure following chronic pressure overload is associated with reduction in left ventricular mass: evidence for atrophic remodeling. J Am Coll Cardiol. 2011;57:921–928. doi: 10.1016/j.jacc.2010.08.648 [DOI] [PubMed] [Google Scholar]
- 37.Boehm M, Tian X, Mao Y, Ichimura K, Dufva MJ, Ali K, Dannewitz Prosseda S, Shi Y, Kuramoto K, Reddy S, et al. Delineating the molecular and histological events that govern right ventricular recovery using a novel mouse model of pulmonary artery de-banding. Cardiovasc Res. 2020;116:1700–1709. doi: 10.1093/cvr/cvz310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badagliacca R, Poscia R, Pezzuto B, Nocioni M, Mezzapesa M, Francone M, Giannetta E, Papa S, Gambardella C, Sciomer S, et al. Right ventricular remodeling in idiopathic pulmonary arterial hypertension: adaptive versus maladaptive morphology. J Heart Lung Transplant. 2015;34:395–403. doi: 10.1016/j.healun.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 39.Chaouat A, Sitbon O, Mercy M, Ponçot-Mongars R, Provencher S, Guillaumot A, Gomez E, Selton-Suty C, Malvestio P, Regent D. Prognostic value of exercise pulmonary haemodynamics in pulmonary arterial hypertension. European Respiratory Journal. 2014;44:704–713. [DOI] [PubMed] [Google Scholar]
- 40.Grünig E, Tiede H, Enyimayew EO, Ehlken N, Seyfarth H-J, Bossone E, D’Andrea A, Naeije R, Olschewski H, Ulrich S. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation. 2013;128:2005–2015. [DOI] [PubMed] [Google Scholar]
- 41.Thenappan T, Prins KW, Pritzker MR, Scandurra J, Volmers K, Weir EK. The Critical Role of Pulmonary Arterial Compliance in Pulmonary Hypertension. Ann Am Thorac Soc. 2016;13:276–284. doi: 10.1513/AnnalsATS.201509-599FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghio S, D’Alto M, Badagliacca R, Vitulo P, Argiento P, Mule M, Tuzzolino F, Scelsi L, Romeo E, Raineri C, et al. Prognostic relevance of pulmonary arterial compliance after therapy initiation or escalation in patients with pulmonary arterial hypertension. International journal of cardiology. 2017;230:53–58. doi: 10.1016/j.ijcard.2016.12.099 [DOI] [PubMed] [Google Scholar]
- 43.Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, Housten T, Champion HC, Lechtzin N, Wigley FM, et al. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:252–260. doi: 10.1164/rccm.200912-1820OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevens GR, Garcia-Alvarez A, Sahni S, Garcia MJ, Fuster V, Sanz J. RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovasc Imaging. 2012;5:378–387. doi: 10.1016/j.jcmg.2011.11.020 [DOI] [PubMed] [Google Scholar]
- 45.Bellofiore A, Wang Z, Chesler NC. What does the time constant of the pulmonary circulation tell us about the progression of right ventricular dysfunction in pulmonary arterial hypertension? Pulm Circ. 2015;5:291–295. doi: 10.1086/680358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fourie PR, Coetzee AR, Bolliger CT. Pulmonary artery compliance: its role in right ventricular-arterial coupling. Cardiovasc Res. 1992;26:839–844. doi: 10.1093/cvr/26.9.839 [DOI] [PubMed] [Google Scholar]
- 47.Bellofiore A, Dinges E, Naeije R, Mkrdichian H, Beussink-Nelson L, Bailey M, Cuttica MJ, Sweis R, Runo JR, Keevil JG, et al. Reduced haemodynamic coupling and exercise are associated with vascular stiffening in pulmonary arterial hypertension. Heart. 2017;103:421–427. doi: 10.1136/heartjnl-2016-309906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oakland H, Joseph P, Naeije R, Elassal A, Cullinan M, Heerdt PM, Singh I. Arterial load and right ventricular-vascular coupling in pulmonary hypertension. J Appl Physiol (1985). 2021;131:424–433. doi: 10.1152/japplphysiol.00204.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dewachter C, Belhaj A, Rondelet B, Vercruyssen M, Schraufnagel DP, Remmelink M, Brimioulle S, Kerbaul F, Naeije R, Dewachter L. Myocardial inflammation in experimental acute right ventricular failure: Effects of prostacyclin therapy. J Heart Lung Transplant. 2015;34:1334–1345. doi: 10.1016/j.healun.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 50.Frump AL, Bonnet S, de Jesus Perez VA, Lahm T. Emerging role of angiogenesis in adaptive and maladaptive right ventricular remodeling in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2018;314:L443–L460. doi: 10.1152/ajplung.00374.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hagger D, Condliffe R, Woodhouse N, Elliot CA, Armstrong IJ, Davies C, Hill C, Akil M, Wild JM, Kiely DG. Ventricular mass index correlates with pulmonary artery pressure and predicts survival in suspected systemic sclerosis-associated pulmonary arterial hypertension. Rheumatology (Oxford). 2009;48:1137–1142. doi: 10.1093/rheumatology/kep187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


