Abstract
Screening of compounds comprising an 8-substituted guanine revealed that 8-aminoguanosine and 8-aminoguanine cause diuresis/natriuresis/glucosuria yet decrease potassium excretion. Subsequent investigations demonstrated that 8-aminoguanosine’s effects are mediated by its metabolite 8-aminoguanine. The mechanism by which 8-aminoguanine causes diuresis/natriuresis/glucosuria involves inhibition of purine nucleoside phosphorylase (PNPase), which increases renal interstitial inosine levels. Additional evidence suggests that inosine, via indirect or direct adenosine A2B receptor activation, increases renal medullary blood flow which enhances renal excretory function. Likely, 8-aminoguanine has pleiotropic actions that also alter renal excretory function. Indeed, the antikaliuretic effects of 8-aminoguanine are independent of PNPase inhibition. 8-Aminoguanine is an endogenous molecule; nitrosative stress leads to production of biomolecules containing 8-nitroguanine moieties. Degradation of these biomolecules releases 8-nitroguanosine and 8-nitro-2’-deoxyguanosine which are converted to 8-aminoguanine. Also, guanosine and guanine per se may contribute to 8-aminoguanine formation. 8-Aminoinosine, 8-aminohypoxanthine and 8-aminoxanthine likewise induce diuresis/natriuresis/glucosuria, yet do not reduce potassium excretion. Thus, there are several pharmacologically active 8-aminopurines with nuanced effects on renal excretory function. Chronic treatment with 8-aminoguanine attenuates hypertension in deoxycorticosterone/salt rats, prevents strokes and increases lifespan in Dahl SS rats on a high salt diet and attenuates the metabolic syndrome in rats; 8-aminoguanosine retards progression of pulmonary hypertension in rats and anemia and organ damage in sickle cell mice. 8-Aminoguanine reverses age-associated lower urinary tract dysfunction and retinal degeneration. 8-Aminopurines represent a new class of agents (and potentially endogenous factors) that have beneficial effects on the cardiovascular system and kidneys and may “turn back the clock” in age-associated diseases.
Keywords: 8-aminoguanine, purine nucleoside phosphorylase, A2B receptors, inosine, hypertension, stroke, aging
Introduction.
In 1929, Drury and Szent-Gyorgi published their findings on the cardiovascular effects of adenosine1. This benchmark article initiated a >90 year-long multinational research effort to elucidate the pharmacological actions of adenosine, an effort that has led to the discovery of mechanisms of adenosine formation, metabolism, receptors, transporters, signal transduction pathways and preclinical and clinical pharmacology2. This trove of knowledge has culminated in adenosinergic drugs for nuclear stress testing3, conversion of paroxysmal supraventricular tachycardia3 and treatment of Parkinson’s disease4, with many additional clinical targets in development3 including immune checkpoint inhibition for cancer therapy5.
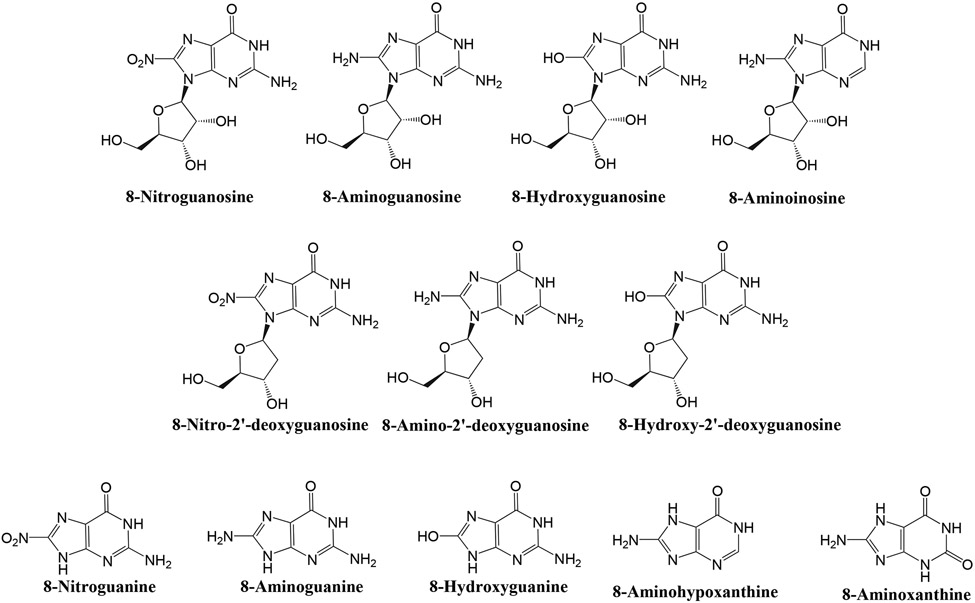
Guanosine has a chemical structure and an evolutionary history similar to that of adenosine, suggesting that guanosine, like adenosine, may have been co-opted by evolution for cellular signaling. If so, guanosine, like adenosine, may hold promise for clinical applications. However, unlike adenosine pharmacology, the pharmacology of guanosine has received limited attention. These considerations motivated our research program on guanosine pharmacology. Another motivation for investigating guanosine pharmacology was the evidence that the guanine moiety in some biomolecules can be chemically modified to yield a family of 8-substituted guanine and guanosine derivatives. In this regard, reactive nitrogen species (RNS), such as peroxynitrite (ONOO−), promote nitration of (i.e, introduction of -NO2 into) endogenous molecules. Moreover, reactive oxygen species (ROS), such as superoxide anion (O2·−), foster hydroxylation of (i.e., insertion of -OH into) biomolecules. Importantly, the 8-position of guanine moieties within biomolecules is particularly prone to nitration6-8 and hydroxylation7,9, and subsequent degradation of nitrated and hydroxylated biomolecules such as RNA, DNA and guanine nucleotides theoretically could release 8-nitroguanosine, 8-nitro-2’-deoxyguanosine, 8-hydroxyguanosine and 8-hydroxy-2’-deoxyguanosine (see Figure 1 for structures of relevant 8-substituted purines). Hypothetically, 8-nitro groups could be enzymatically reduced to generate 8-aminoguanosine and 8-amino-2’-deoxyguanosine, and conceivably purine nucleoside phosphorylase (PNPase) might metabolize any of these aforementioned compounds to yield 8-hydroxyguanine, 8-nitroguanine or 8-aminoguanine10. (Note: PNPase should not be confused with polynucleotide phosphorylase1 (PNPT1), an enzyme that is sometimes referred to as PNPase and is involved in mRNA degradation.) In support of these concepts, investigators have reported the presence in tissues or urine of 8-nitroguanosine11, 8-aminoguanosine12, 8-hydroxyguanosine13, 8-nitroguanine6, 8-hydroxyguanine14 and 8-hydroxy-2’-deoxyguanosine15.
Figure 1: Chemical structures of selected 8-substituted purines.
8-Aminoguanosine and 8-Aminoguanine Affect Renal Excretory Function.
Despite the likelihood that a family of naturally occurring 8-substituted guanosine and guanine derivatives exist in vivo, until recently little to nothing was known concerning the pharmacology of these potentially endogenous compounds. Hence, we were motivated to examine in rats the acute effects of intravenous administration of 8-nitroguanosine, 8-nitroguanine, 8-hydroxyguanosine, 8-hydroxyguanine, 8-hydroxy-2’-deoxyguanosine, 8-aminoguanosine and 8-aminoguanine (and for comparison vehicle, guanosine, guanine and amiloride) on the cardiovascular and renal systems16.
Unlike other 8-substituted purines tested, we noted that 8-aminoguanosine and 8-aminoguanine triggered large increases in urine volume (~ 4-fold) and sodium (~ 20-fold) and glucose (~ 12-fold) excretion yet decreased potassium excretion (~ −70%). These effects were similar in magnitude to those of a matched dose of amiloride, with the exception that amiloride did not significantly alter glucose excretion. Our findings support the conclusion that these two 8-aminopurines may be useful drugs or endogenous factors that regulate renal function.
8-Aminoguanosine’s Action on Renal Function is Mediated by 8-Aminoguanine.
Decades prior to our investigation of the renal effects of 8-substituted purines, Osborne and Barton had shown that 8-aminoguanosine is metabolized to 8-aminoguanine by PNPase10. Thus, we tested whether 8-aminoguanosine’s effects on renal excretory function are due to its conversion to 8-aminoguanine17. In support of this concept, we observed that intravenous 8-aminoguanosine and 8-aminoguanine caused similar increases in renal interstitial levels of 8-aminoguanine, yet neither compound increased renal interstitial levels of 8-aminoguanosine. When infused directly into the left renal artery, 8-aminoguanine elicited diuresis/natriuresis/glucosuria by the ipsilateral kidney, with little effect on the contralateral kidney. By contrast, intrarenal infusions of 8-aminoguanosine did not elicit diuresis/natriuresis/glucosuria in either the ipsilateral or contralateral kidney; however, 8-aminoguanosine did reduce potassium excretion in the ipsilateral kidney. These findings support the conclusion that the diuretic, natriuretic and glucosuric effects of 8-aminoguanosine are mediated by its systemic metabolism to 8-aminoguanine. Although 8-aminoguanosine does have direct antikaliuretic effects, likely even this response is mediated mostly by 8-aminoguanine since systemic administration of 8-aminoguanosine induces antikaliuresis despite the fact that renal interstitial levels of 8-aminoguanosine do not increase and urine levels only briefly increase yet rapidly return to near basal levels.
8-Aminoguanine’s Mechanism of Action (MOA).
The studies described above left unanswered a key question: What is 8-aminoguanine’s MOA on renal excretory function? Hence, we endeavored to explore in depth 8-aminoguanine’s MOA.
To test whether the effects of 8-aminoguanine on renal excretory function could be attributed to “macro” hemodynamic changes, we examined the acute effects of intravenous injections of 8-aminoguanine on blood pressure, heart rate, total renal blood flow (RBF) and glomerular filtration rate (GFR)16,17; acutely 8-aminoguanine did not alter blood pressure or heart rate yet slightly decreased total RBF and modestly decreased GFR. The effects on total RBF and GFR are consistent with activation of tubuloglomerular feedback due to increased delivery of NaCl to the macula densa18. We also tested the effects of 8-aminoguanine on plasma aldosterone and observed that 8-aminoguanine did not alter aldosterone levels17.
Because 8-aminoguanine has structural commonalities with inhibitors of the epithelial sodium channel (ENaC) and Na+/H+ exchangers, as well as with antagonists of the adenosine A1 receptor19, we explored whether 8-aminoguanine’s MOA involves inhibition of these molecular targets. To test whether 8-aminoguanine inhibits ENaC, we examined the effects of 8-aminoguanine on amiloride-sensitive short-circuit currents in immortalized mouse collecting duct cells19; 8-aminoguanine did not alter amiloride-sensitive short-circuit currents. To test whether 8-aminoguanine blocks Na+/H+ exchangers, we examined the effects of 8-aminoguanine on the intracellular pH of human proximal tubular epithelial cells19; 8-aminoguanine did not alter intracellular pH of proximal tubular epithelial cells. To test whether 8-aminoguanine’s MOA involves antagonism of A1 receptors, we tested in vivo whether a diuretic/natriuretic dose of 8-aminoguanine blocks activation of A1 receptors by the selective A1 agonist 2-chloro-N6-cyclopentyladenosine19; 8-aminoguanine did not block A1 receptors.
Since 8-aminoguanine suppresses potassium excretion, we hypothesized that 8-aminoguanine might block the renal outer medullary potassium channel (ROMK, aka Kir1.1). To test this, ROMK mRNA was injected into oocytes and ROMK potassium currents were measured in the absence and presence of a ROMK inhibitor; 8-aminoguanine did not attenuate ROMK currents (unpublished data).
Rac1 activates mineralocorticoid receptors independent of aldosterone20-22 and some guanosine analogues inhibit Rac123. Thus, we examined the effects of 8-aminoguanine on Rac1 activity in mouse collecting duct cells. Rac1 activity was somewhat reduced by high concentrations of 8-aminoguanine, but the reduction was modest (decreased by only ~ 25%)19.
Because 8-aminoguanine has been reported to be a PNPase inhibitor, we performed a kinetic analysis of the effects of 8-aminoguanine on human recombinant PNPase using inosine as a substrate while measuring hypoxanthine as product. These experiments confirmed that 8-aminoguanine is an inhibitor of PNPase (Ki, 2.8 μmol/L)24. Consistent with this finding, we observed that a natriuretic dose of 8-aminoguanine increased and decreased, respectively, urinary excretion of inosine and hypoxanthine19.
Next, we compared in rats the renal effects of intravenous doses of 9-deazaguanine (synthetic PNPase inhibitor) versus 8-aminoguanine (using doses that should have provided similar inhibition of PNPase by the two inhibitors)19. 8-Aminoguanine and 9-deazaguanine similarly increased urine volume and sodium and glucose excretion yet only 8-aminoguanine reduced potassium excretion. However, intravenous administration of Nsc23766, a Rac1 inhibitor, decreased potassium excretion to a level similar to that achieved by 8-aminoguanine; however, Nsc23766 did not increase urine volume or sodium or glucose excretion19.
Together, the above findings indicate that the MOA of 8-aminoguanine on urine volume and sodium and glucose excretion cannot be attributed to changes in blood pressure, heart rate, total RBF, GFR, aldosterone release or inhibition of ENaC, Na+/H+ exchangers, A1 receptors or ROMK. However, these results do support the conclusion that the diuretic/natriuretic/glucosuric effects of 8-aminoguanine are mediated by inhibition of PNPase. Although we have some evidence that inhibition of Rac1 participates in the antikaliuresis induced by 8-aminoguanine, circumspection is advised since 8-aminoguanine only mildly inhibits Rac1; more research is required to clarify how 8-aminoguanine reduces potassium excretion.
The MOA of 8-aminoguanine’s effects on urine volume and sodium and glucose excretion were further explored by combining studies using intravenous 8-aminoguanine, intrarenal artery infusions of PNPase substrates (inosine and guanosine), renal microdialysis, ultra-performance liquid chromatography-tandem mass spectrometry (UPMC-MS/MS) for measurement of purines, selective adenosine-receptor ligands, adenosine receptor knockout rats, laser doppler blood flow analysis, cultured renal microvascular smooth muscle cells, HEK293 cells expressing A2B receptors and homogeneous time-resolved fluorescence assay for adenylyl cyclase activity25.
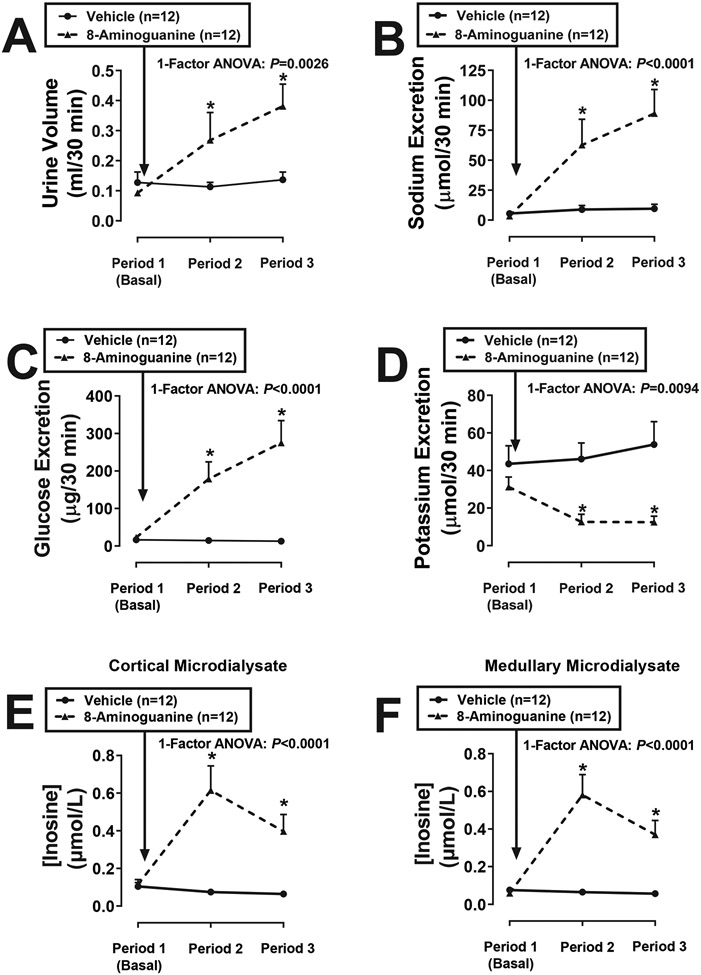
We confirmed that intravenous 8-aminoguanine caused diuresis, natriuresis, glucosuria and antikaliuresis (Figure 2) and that these effects were associated with large increases in renal cortical and medullary interstitial levels of the PNPase substrate inosine (Figure 2). Intravenous 8-aminoguanine also increased renal cortical and medullary interstitial levels of the PNPase substrate guanosine; however, the increases in inosine were larger than the increases in guanosine25. Moreover, we observed that intrarenal infusions of inosine, but not guanosine, caused diuresis, natriuresis and glucosuria, but not antikaliuresis25. We also established that an effective dose of 8-aminoguanine increased renal interstitial levels of inosine as much as an effective dose of exogenous inosine; this supports the concept that inosine mediates the renal excretory effects of 8-aminoguanine. Together, these findings suggest that inosine mediates, at least in part, the effects of 8-aminoguanine on urine volume and sodium and glucose excretion; however, the effects of 8-aminoguanine on potassium excretion are independent of inosine.
Figure 2: Effects of 8-aminoguanine on renal excretory function and renal cortical and medullary microdialysate levels of inosine.
Timed collections of urine from the ureter and renal cortical and medullary microdialysates were obtained from anesthetized rats from 0-30 min (Period 1), 40-70 min (Period 2) and 85-115 min (Period 3) into the protocol. Rats received an intravenous injection of 8-aminoguanine (33.5 μmoles/kg) immediately after Period 1. Urine volumes (A), excretion rates of sodium (B), glucose (C) and potassium (D) and cortical (E) and medullary (F) concentrations of inosine were determined. Values are means and SEMs for the indicated sample size (n). For data points without error bars, the error bars are shorter than the size of the symbol. ANOVA, analysis of variance; *P<0.05 vs Period 1. Reproduced from Hypertension 2023;80:981-994 (Open Access).
There is evidence that inosine can activate adenosine receptors26-33. Therefore, we examined the effects of 8-aminoguanine on renal excretory function in rats in which either adenosine A1, A2A or A2B receptors were knocked out. 8-Aminoguanine caused diuresis, natriuresis and glucosuria in A1 and A2A knockout rats yet 8-aminoguanine was ineffective in A2B knockout rats25. Likewise, inosine’s effects on urine volume and sodium and glucose excretion were eliminated in A2B knockout rats25, and intrarenal infusions of BAY 60-6583 (an A2B agonist) triggered diuresis, natriuresis and glucosuria25. These results support the conclusion that an inosine/A2B receptor pathway mediates in part the effects of 8-aminoguanine on urine volume and sodium and glucose excretion.
The literature regarding inosine’s effects on adenosine receptors is inconsistent26,28-32,34, and the concept that inosine activates adenosine receptors is controversial. Therefore, we examined this issue further in HEK293 cells expressing A2B receptors. Here, inosine activated adenylyl cyclase (as assessed by measuring 3’,5’-cAMP using a highly sensitive homogeneous time-resolved fluorescence assay), a response that was blocked by the A2B antagonist MRS 175425. Moreover, in renal microvascular smooth muscle cells, both 8-aminoguanine and an alternative PNPase inhibitor, forodesine, increased extracellular levels of inosine and 3’,5’-cAMP25; the effects of 8-aminoguanine and forodesine on 3’,5’-cAMP were absent in renal microvascular smooth muscle cells obtained from A2B knockout rats25. It appears that inosine can activate the A2B receptor/adenylyl cyclase axis, particularly in renovascular smooth muscle cells; however, whether this effect is direct or indirect requires further investigation.
Renal microvascular smooth muscle cells regulate medullary blood flow (MBF), and even modest increases in MBF can promote large increases in renal excretory function35-37. The mechanism by which increases in MBF increase renal excretory function remains controversial; however, the prevailing hypothesis is that increases in MBF elevate renal interstitial hydrostatic pressure within the encapsulated kidney which activates mechanisms in the proximal tubule that inhibit reabsorption by proximal tubular epithelial cells38. This suggests that 8-aminoguanine might increase renal excretory function in part via activating A2B receptors leading to increases in MBF. In support of this hypothesis, we observed that 8-aminoguanine and BAY 60-6583 increased MBF and that the MBF response to 8-aminoguanine was abolished by an A2B, but not an A2A, receptor antagonist25. The fact that 8-aminoguanine does not increase total RBF yet increases MBF suggests redistribution of blood flow to the renal medulla. Our hypothesis is congruent with previous reports that: 1) activation of A2 receptors in the renal medulla increases sodium excretion by augmenting MBF39; 2) in the kidney, A2B receptors are predominantly expressed in the renal vasculature40; and 3) A2B receptors are the A2 receptor subtype that mediates renal vasodilation41,42.
MBF affects sodium excretion, and glucose and sodium transport in proximal tubules are coupled by SGLT243; however, whether MBF affects glucose excretion remains an open question. Moreover, A2B receptors in the renal inner medullary collecting duct epithelium increase chloride excretion; this is mediated by cystic fibrosis transmembrane conductance regulator chloride channels44. In addition, A2B receptors activate vacuolar ATPase-dependent proton secretion in renal medullary type A intercalated cells45. Together, the evidence suggests that increases in MBF may account for some, but not all, of the renal excretory effects of 8-aminoguanine. Other effects of A2B receptors or pleiotropic actions of 8-aminoguanine that directly affect renal tubular transport may be involved in 8-aminoguanine’s MOA.
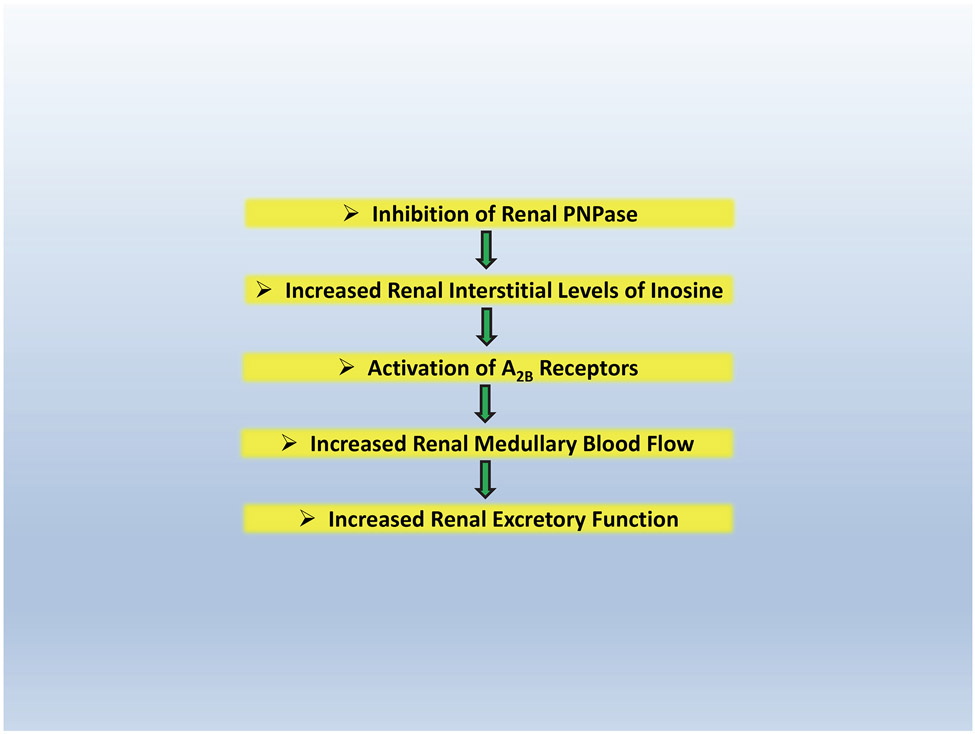
In summary (see Figure 3), we propose that, at least in part, 8-aminoguanine induces diuresis, natriuresis and glucosuria by inhibiting PNPase which increases renal interstitial levels of inosine which, via A2B receptor activation, increases renal excretory function, perhaps in part by increasing MBF (a process which is known to inhibit transport in the proximal tubule). However, we remain open to the possibility that other signaling pathways in the proximal tubules or other nephron segments contribute to the pharmacology of this 8-aminopurine.
Figure 3: Possible mechanism by which 8-aminoguaine increases the excretion of urine, sodium and glucose.
At least in part, 8-aminoguanine induces diuresis, natriuresis and glucosuria by inhibiting PNPase which increases renal interstitial levels of inosine which, via A2B receptor activation, increases renal excretory function, perhaps in part by increasing medullary blood flow. However, it is likely that other signaling pathways contribute to the pharmacology of 8-aminoguanine specifically and 8-aminopurines in general.
8-Aminoguanine is an Endogenous Compound.
Utilizing UPLC-MS/MS, we detected 8-aminoguanine in the urine of 27 out of 30 Sprague-Dawley rats and 10 out of 10 Dahl salt-sensitive (Dahl SS) rats46. Using renal microdialysis to sample the renal interstitial compartment, we detected 8-aminoguanine in the renal microdialysate of 20 out of 30 Sprague-Dawley rats and 10 out of 10 Dahl SS rats46. In Sprague-Dawley rats, the urine concentrations of 8-aminoguanine ranged from less than the detection limit to 1.3 μmol/L (mean of 0.2 μmol/L); and in Dahl SS rats, the urine concentrations of 8-aminoguanine ranged from 0.6 to 2.5 μmol/L (mean of 1.3 μmol/L; P<0.00001, Dahl SS vs. Sprague-Dawley). In many rats, particularly Dahl SS, urine concentrations of 8-aminoguanine were near the Ki of 8-aminoguanine for inhibiting PNPase, which has been variably estimated as between 0.210 and 2.824 μmol/L. These data support the conclusion that 8-aminoguanine is an endogenous compound that exists in some animals at biologically active levels.
Pathways of 8-Aminoguanine Biosynthesis.
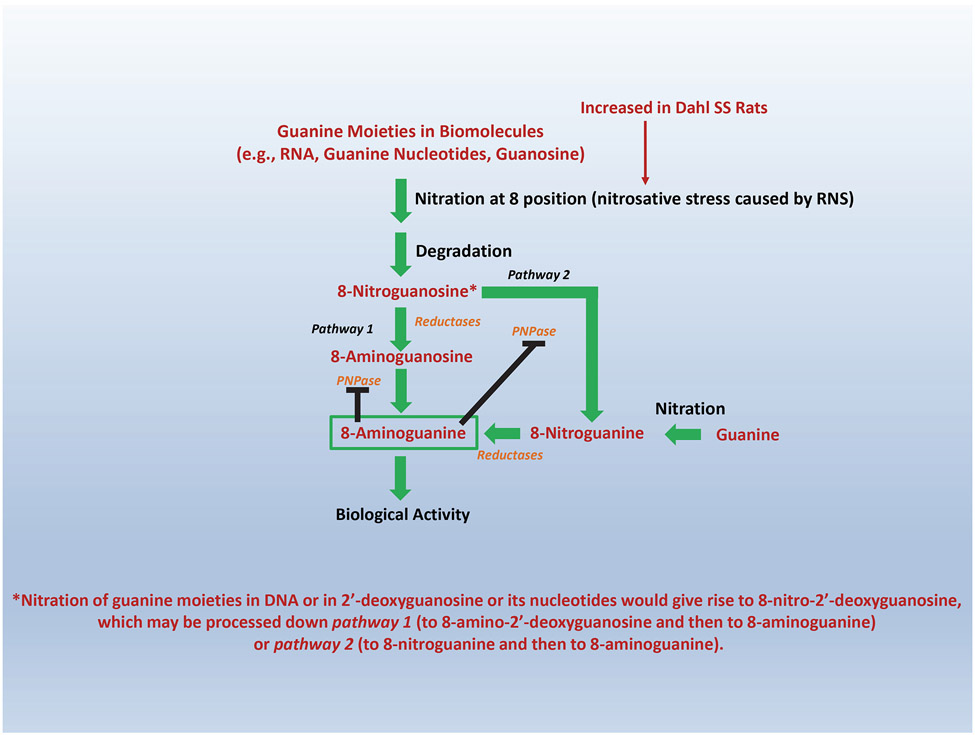
Since 8-nitroguanosine is naturally occurring46, we considered that 8-aminoguanine may arise from two biosynthetic pathways46: pathway 1, 8-nitroguanosine→8-aminoguanosine→8-aminoguanine; and pathway 2, 8-nitroguanosine→8-nitroguanine→8-aminoguanine. In support of the existence of both pathways, we observed that in Sprague-Dawley rats 8-nitroguanosine infusions elevated kidney levels of 8-nitroguanine, 8-aminoguanosine and 8-aminoguanine and that infusions of 8-nitroguanine increased 8-aminoguanine levels46. We also found that recombinant PNPase converted 8-nitroguanosine to 8-nitroguanine and 8-aminoguanosine to 8-aminoguanine46, findings supporting the view that both pathways are feasible. Moreover, we observed that the PNPase inhibitor forodesine decreased the metabolism of 8-nitroguanosine by pathway 2 and shunted metabolism of 8-nitroguanosine to 8-aminoguanosine (pathway 1)46. Infusions of 8-nitroguanosine also increased kidney levels of 8-nitroguanine, 8-aminoguanosine and 8-aminoguanine in Dahl rats46. These results suggest that in both Sprague-Dawley and Dahl rats both pathways 1 and 2 mediate, at least in part, the in vivo production of 8-aminoguanine. In vivo, both 8-nitroguanosine and 8-nitroguanine are reduced to 8-aminoguanosine and 8-aminoguanine, respectively. The reductase(s) responsible for reducing 8-nitropurines to 8-aminopurines in vivo is (are) unknown. Nonetheless, Chen et al. have reported that 8-nitroguanine, in free form or in DNA, is reduced by lipoyl dehydrogenase47 and by hemin and hemoproteins48, thus further supporting plausibility for pathways 1 and 2.
RNS (e.g., peroxynitrite) mediate nitration of guanosine in RNA and guanine nucleotides. It is likely, therefore, that subsequent release of 8-nitroguanosine from these nitrated biomolecules is a major source of 8-nitroguanosine6 that feeds into both pathways 1 and 2. In addition, free guanosine can undergo peroxynitrite-induced nitration to produce 8-nitroguanosine6. Moreover, free guanine may undergo nitration to yield 8-nitroguanine which can enter pathway 2 to produce 8-aminoguanine46. It is also conceivable that 8-nitro-2’-deoxyguanosine contributes to 8-aminoguanine production – although we have not yet studied this possibility. In this regard, nitration of 2’-deoxyguanosine in DNA (followed by degradation of DNA) or nitration of free 2’-deoxyguanosine (or its nucleotides followed by degradation) would give rise to 8-nitro-2’-deoxyguanosine. This substrate likely would be converted to 8-aminoguanine via pathways similar to that described for 8-nitroguanosine. The most likely biochemical pathways generating 8-aminoguanine are summarized in Figure 4. Since both pathways 1 and 2 utilize PNPase and since 8-aminoguanine is a PNPase inhibitor, this biochemical system has a “built-in” negative feedback mechanism that would prevent over-production of 8-aminoguanine in a given biophase (Figure 4).
Figure 4: Biochemical pathways for 8-aminoguanine production in vivo.
Nitrosative stress (which is increased in Dahl SS Rats) caused by reactive nitrogen species (RNS; e.g., peroxynitrite) results in nitration at position 8 of guanine moieties in biomolecules such as RNA, guanine nucleotides and guanosine. Degradation of RNA and guanine nucleotides leads to the formation of 8-nitroguanosine and nitration of guanosine directly generates 8-nitroguanosine. 8-Nitroguanosine can be converted to 8-aminoguanine via Pathway 1 (reduction to 8-aminoguanosine followed by purine nucleoside phosphorylase (PNPase)-mediated phosphorolysis to 8-aminoguanine) or Pathway 2 (PNPase-mediated phosphorolysis to 8-nitroguanine followed by reduction to 8-aminoguaine). In addition, “free” guanine may undergo direct nitration to produce 8-nitroguanine that enters Pathway 2. Moreover, nitration of guanine moieties in DNA or in 2’-deoxyguanosine or its nucleotides would produce 8-nitro-2’-deoxyguanosine (after degradation of DNA or 2’-deoxyguanosine nucleotides; yet directly from 2’-deoxyguanosine), which may be processed by Pathway 1 or 2 to 8-aminoguanine. The critical involvement of PNPase activity for completion of both Pathway 1 and 2 provides an effective mechanism (solid dark lines denoting feedback inhibition) for preventing over-production of 8-aminoguanine in any given biophase.
We also determined that the higher renal levels of 8-aminoguanine in Dahl rats were not caused by changes in pathways 1 and 2. Rather, these differences were due to greater levels of endogenous 8-nitroguanosine in Dahl rats46. This conclusion is consistent with evidence that Dahl rats likely have elevated levels of peroxynitrite49-52 and that a peroxynitrite donor increases kidney levels of 8-aminoguanine46.
Evidence for a Family of Bioactive 8-Aminopurines.
8-Aminoinosine is similar in structure to 8-aminoguanosine, and 8-aminohypoxanthine is similar in structure to 8-aminoguanine. Therefore, we considered that 8-aminoinosine and 8-aminohypoxanthine, like 8-aminoguanine, may affect renal function. In support of this concept, studies by us and others show that 8-aminoinosine24,53 and 8-aminohypoxanthine24,54, like 8-aminoguanine24,55, inhibit PNPase; however, the Ki for 8-aminoinosine and 8-aminohypoxanthine against PNPase is approximately 10-fold higher than the corresponding Ki for 8-aminoguanine24. 8-Aminoinosine per se is a competitive substrate24, yet is also converted in vivo to 8-aminohypoxanthine, which is a competitive inhibitor of PNPase. Thus, 8-aminoinosine inhibits PNPase not only directly, but also indirectly via its conversion to 8-aminohypoxanthine. When administered at equal doses to intact rats, 8-aminoinosine and 8-aminohypoxanthine decrease the urinary ratios of guanine-to-guanosine and hypoxanthine-to-inosine, thus confirming PNPase inhibition in vivo24. Moreover, 8-aminoinosine and 8-aminohypoxanthine, like 8-aminoguanine, cause diuresis, natriuresis and glucosuria24. In some respects, however, the effects of 8-aminoinosine and 8-aminohypoxanthine on renal excretory function differ from those of 8-aminoguanine. For example, the glucosuric effects of 8-aminohypoxanthine and 8-aminoinosine are less than those of 8-aminoguanine24, and 8-aminoinosine and 8-aminohypoxanthine do not reduce potassium excretion24. Since administration of 8-aminoinosine increases 8-aminohypoxanthine excretion, part of the effects of 8-aminoinosine on renal function are likely mediated by its conversion to 8-aminohypoxanthine24. We also have observed that 8-aminohypoxanthine is metabolized by xanthine oxidase to 8-aminoxanthine24 and that 8-aminoxanthine exerts diuretic, natriuretic and glucosuric effects (unpublished observations). These findings suggest that some of the effects of 8-aminohypoxanthine on renal excretory function are mediated via its conversion to 8-aminoxanthine.
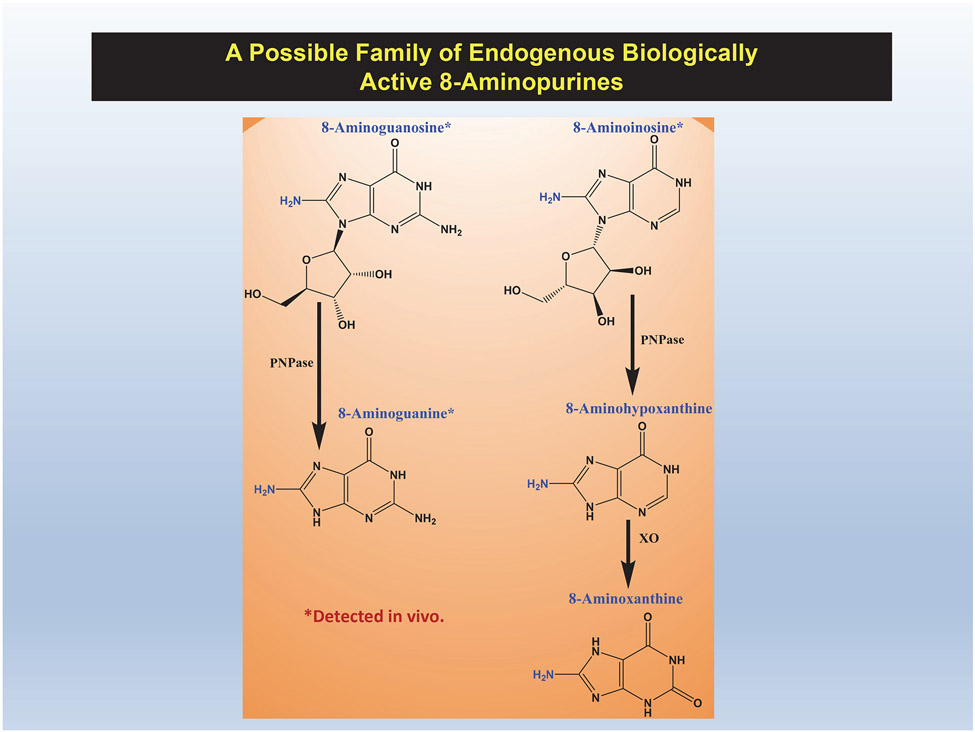
There is evidence that 8-aminoinosine, like 8-aminoguanosine and 8-aminoguanine, is an endogenous 8-aminopurine. Using ultra-performance liquid chromatography-tandem mass spectrometry, we monitored in urine the mass transition of 8-aminoinosine’s precursor ion (284 m/z) to its primary product ion (152 m/z) and observed a chromatographic peak with the identical retention time as authentic 8-aminoinosine24. Whether 8-aminohypoxanthine and 8-aminoxanthine are also endogenous 8-aminopurines is at this writing unknown. Also, we have not yet characterized the effects of 8-aminoxanthine on PNPase. Figure 5 illustrates the five compounds that may comprise an endogenous family of biologically active 8-aminopurines and shows their interconversions. We hypothesize that all of these compounds share the core MOA of inhibiting PNPase; nonetheless, we leave open the possibility that each 8-aminopurine has unique pharmacological features.
Figure 5: 8-Aminopurines may comprise a family of endogenous and biologically active agents.
8-Aminoguanosine, 8-aminoguanine, 8-aminoinosine, 8-aminohypoxanthine and 8-aminoxanthine cause diuresis, natriuresis and glucosuria. 8-Aminoguanosine and 8-aminoguanine also cause antikaliuresis. 8-Aminoguanosine and 8-aminoinosine are metabolized to 8-aminoguanine and 8-aminohypoxanthine, respectively, by purine nucleotide phosphorylase (PNPase), and 8-aminohypoxanthine is converted to 8-aminoxanthine by xanthine oxidase (XO). Thus far, 8-aminoguanosine, 8-aminoguanine and 8-aminoinosine have been detected in vivo.
8-Aminoguanine Attenuates Deoxycorticosterone Acetate-Salt (DOCA-salt) Hypertension in Rats.
Since at least some 8-aminopurines are formed in vivo, it is possible that 8-aminopurines are endogenous natriuretic factors that modulate sodium excretion and arterial blood pressure. Moreover, the natriuretic actions of 8-aminopurines suggest that these compounds may have utility for treating salt-induced hypertension. To test this concept, we instrumented rats for continuous measurement of arterial blood pressure using radiotelemetry. After obtaining baseline blood pressure data for 2 weeks, we treated rats orally with either 8-aminoguanine or 8-aminoguanosine (5 mg/kg/day) for approximately 2 weeks and then induced DOCA-salt hypertension (unilateral nephrectomy + 1% saline as drinking water + subcutaneous DOCA). Before induction of DOCA-salt hypertension, neither 8-aminoguanosine nor 8-aminoguanine altered baseline blood pressure16. However, both 8-aminopurines attenuated the long-term (49 days) increase in blood pressure induced by DOCA-salt administration16. These results indicate that 8-aminopurines may be useful antihypertensive drugs and could conceivably contribute to blood pressure regulation.
8-Aminoguanine Prevents Strokes and Extends Lifespan in Dahl Salt-Sensitive Rats.
We also investigated, using radiotelemetry monitoring of arterial blood pressure, the chronic effects of oral 8-aminoguanine (10 mg/kg/day) in another model of salt-induced hypertension, namely the Dahl SS rat. On a low salt diet (0.3%), chronic treatment with 8-aminoguanine did not affect blood pressure. However, when placed on a high salt diet (8%), blood pressure increased more slowly in Dahl SS rats receiving 8-aminoguanine versus untreated Dahl SS rats56. Also, in 8-aminoguanine-treated Dahl SS rats, strokes were not observed and the median survival time on an 8% salt diet was >50% longer compared to the survival time in untreated Dahl SS rats, all of which apparently died of strokes56.
8-Aminoguanine and the Metabolic Syndrome.
The metabolic syndrome consists of a grouping of medical conditions that include hypertension, obesity, dyslipidemia and insulin resistance57. In developed countries, the metabolic syndrome is a major health challenge due to its complications that include cardiovascular diseases58, kidney diseases58, stroke59 and pulmonary hypertension60. Therefore, we were motivated to investigate the utility of 8-aminoguanine in two rodent models of the metabolic syndrome, namely obese ZSF161 and ZDSD56 rats (see Tofovic and Jackson62 and Peterson and coworkers63 for descriptions of obese ZSF1 and ZDSD rats, respectively).
In order to evaluate whether 8-aminopurines reduce the severity of the metabolic syndrome, 36-week old male, hypertensive, diabetic, obese ZSF1 rats were treated for 8 weeks with either tap water or tap water containing 8-aminoguanosine (20 mg/kg/day)61. Here we used 8-aminoguanosine because this prodrug is much more soluble in water than is 8-aminoguanine and therefore provides a more reliable treatment at this higher dose. Compared to their lean littermates, obese animals had reduced ratios of inosine-to-hypoxanthine concentrations in urine and increased levels of xanthine in urine, suggesting increased PNPase and xanthine oxidase activity, respectively. In obese ZSF1 rats, 8 weeks of treatment with 8-aminoguanosine elevated urine levels of inosine and guanosine and markedly reduced urine levels of vasculotoxic and pro-oxidant hypoxanthine/xanthine/guanine, strongly indicating effective inhibition of PNPase. This rebalancing of the purine metabolism was associated with improved glucose homeostasis (i.e., reduced HbA1c, polyuria and glycosuria), decreased elevated systemic blood pressure, reduced kidney damage (i.e., reduced albuminuria) and reversal of right ventricle (RV) hypertrophy and diastolic dysfunction.
Obese ZSF1 rats have a malfunctioning leptin receptor system. Because this biochemical defect is rare in humans, obese ZSF1 rats might not model accurately the metabolic syndrome in humans. Therefore, we also examined the effects of 8-aminopurines in Zucker Diabetic-Sprague Dawley (ZDSD) rats56, a model of the metabolic syndrome that has a functioning leptin pathway. Here we prepared ZDSD rats for radiotelemetry measurements of arterial blood pressure; and after 1 week of baseline measurements, we randomized animals to either normal drinking water or 8-aminoguanine (10 mg/kg/day) in drinking water. After approximately 2 weeks, rats were provided 1% salt in the drinking water, and after 4 weeks this treatment was augmented with a diabetogenic diet for another 4 weeks. 8-Aminoguanine significantly reduced arterial blood pressure, particularly when salt intake was increased, an effect that was sustained even on a diabetogenic diet. At the end of the study, rats were placed in metabolic cages; it was noted that 8-aminoguanine decreased both water intake and urine output. Also, 8-aminoguanine significantly reduced glycosylated hemoglobin and improved kidney and heart histopathology.
8-Aminoguanosine and Angioprolifertive Pulmonary Arterial Hypertension.
In obese ZSF1 rats, 8-aminoguanosine reduced RV peak systolic pressure, RV end-diastolic pressure and RV tau, findings that suggest a beneficial effect of this 8-aminopurine on the pulmonary circulation. To test this concept, we studied the effects of 8-aminoguanosine on progression of angioproliferative pulmonary arterial hypertension in female rats64. Female rats were injected with Sugen 5416 (a vascular endothelial growth factor receptor antagonist, 20 mg/kg, s.c.) and exposed to hypoxia for 3 weeks. Sugen 5416 combined with hypoxia is known to induce severe angioproliferative pulmonary hypertension65. Animals were returned to normoxia for 3 weeks before hemodynamic and histopathological analyses were conducted (SuHx group). On Day 14, a subset of SuHx rats begin to receive 8-aminoguanosine (20 mg/kg/day in drinking water). Control animals were injected with vehicle and received tap water. A single dose of Sugen 5416 and exposure to hypoxia produced severe pulmonary arterial hypertension, occluded pulmonary vessels, plexiform lesions and necrotizing arteritis. 8-Aminoguanosine decreased RV peak systolic pressure, end-diastolic pressure and hypertrophy and improved RV contractility. Notably, 8-aminoguanosine also reduced the number of occlusive and plexiform lesions and prevented development of necrotizing arteritis.
8-Aminoguanosine and Sickle Cell Disease.
PNPase converts guanosine to guanine, inosine to hypoxanthine and deoxyguanosine to guanine66. In turn, guanine is metabolized to xanthine by guanase67. Thus, PNPase diminishes guanosine and inosine levels, which have well documented anti-inflammatory68-70, anti-ischemic70-73 and anti-thrombotic74-76 effects, while generating large amounts of downstream hypoxanthine and xanthine, which are pro-oxidant and vasculotoxic77. Red blood cells (RBCs) are rich in PNPase66, and patients with sickle cell disease (SCD) have increased PNPase release/activity, accelerated guanosine/inosine metabolism and increased production of vasculotoxic hypoxanthine/xanthine77. Based on this information, we hypothesized that PNPase plays a pathogenic role in SCD by promoting RBC sickling, hemolytic angioproliferation, cell aggregation and end organ damage. The corollary of this hypothesis is that PNPase inhibition may confer beneficial effects in SCD.
To test our hypothesis, we investigated the safety and efficacy of intermediate-term (8 weeks) and long-term (20 weeks) treatment of Townes sickle mice (SS) and their non-sickling controls (AA) with 8-aminoguanosine (60 mg/kg/day administered ad libitum in the drinking water)78. We obtained metabolic cage measurements with blood and urine collection at multiple intervals. Urine samples were analyzed for purines by UPLC-MS/MS.
8-Aminoguanosine was well tolerated by AA and SS mice with no signs of toxicity. In both AA and SS mice, 8-aminoguanosine treatment resulted in a 40- to 50-fold increase in urinary 8-aminoguanine concentrations. Treatment of SS mice with 8-aminoguanosine reduced urinary levels of hypoxanthine and increased the inosine-to-hypoxanthine ratio in urine. Moreover, 8-aminoguanosine increased urine levels of guanosine, reduced urine levels of guanine and increased the guanosine-to-guanine ratio in urine. These findings indicated efficacious inhibition of PNPase in mice treated with 8-aminoguanosine.
Notably, treatment of SS mice with 8-aminoguanosine for 8 weeks significantly reduced hemoglobinuria and albuminuria, attenuated both left and right ventricular hypertrophy, reduced hepatomegaly and improved RV contractility. Also, 8-aminoguanosine tended to reduce lung weights and splenomegaly in SS mice. Treatment of SS mice with 8-aminoguanosine for 20 weeks significantly inhibited age-related reductions in both hemoglobin and hematocrit levels, attenuated hemoglobinuria and albuminuria and significantly reduced splenomegaly and hepatomegaly. Semi-quantitative histopathological analysis revealed reduced ischemic and fibrotic injury and reduced iron deposition in the spleen and liver of SS mice treated with 8-aminoguanosine. Taken together, our findings in SS mice indicate that 8-aminoguanosine has potential as a safe, effective and inexpensive treatment for SCD.
8-Aminoguanine and Aging.
The primary focus of this review relates to the effects of 8-aminopurines on the cardiovascular and renal systems; however, we have observed beneficial effects of these unique compounds that extend beyond these systems. We discuss these findings here because the anti-aging mechanisms engaged by 8-aminopurines in other organ systems likely apply to the cardiovascular and renal systems as well.
There is growing evidence that 8-aminopurines may have anti-aging/reverse aging actions that hold promise for restoring organ form and function. Here, we focus on emerging data supporting the hypothesis that 8-aminopurines may be useful for reversing age-associated diseases of the lower urinary tract (LUT) as well as age-related retinal degeneration. Both of these conditions are life altering in the elderly.
8-Aminoguanine may be geroprotective, with respect to the LUT, by redirecting purine metabolism in the bladder away from uro-damaging and ROS-generating hypoxanthine/xanthine79 and favoring instead the accumulation of uro-protective inosine and guanosine28,80-82 . To test this hypothesis, we assessed LUT function and structure in aged (>25 months) male and female Fischer 344 rats randomized to oral treatment with 8-aminoguanine (5 mg/kg/day; in drinking water) or vehicle for 6 weeks83. The LUT of aged rats exhibited multiple abnormalities including tactile insensitivity, vascular remodeling within the bladder, reduced collagen fiber tortuosity in the bladder, increased bladder stiffness, abnormal bladder smooth muscle cell morphology, swelling and degeneration of mitochondria in both smooth (bladder and urethra) and striated (external urethral sphincter) muscle cells and increased levels of uro-damaging purine metabolites in the LUT83,84. Treatment of aged rats with 8-aminoguanine restored all evaluated histological, ultrastructural and physiological abnormalities toward that of a younger state83,84. Because PNPase inhibition blocks metabolism of inosine to hypoxanthine and guanosine to guanine, likely the uro-protective effects of 8-aminoguanine are mediated by increased bladder levels of uro-protective inosine and guanosine and reductions in uro-damaging hypoxanthine and xanthine. These findings demonstrate that 8-aminoguanine has translational potential for treating age-associated LUT dysfunctions and resultant syndromes in humans.
Reduced visual acuity due to retinal thinning is another common age-related morbidity that, like LUT dysfunction, greatly compromises quality of life in the elderly85. With increasing human lifespans, development of an oral drug that delays or reverses age-related vision decline is a major unmet medical need. Our discovery that 8-aminoguanine restores the aged bladder to a more youthful state motivated us to evaluate whether 8-aminoguanine can also reverse age-related retinal degeneration.
As with the bladder, the Fischer 344 rat is a classic model for investigating age-related retinal degeneration. In this regard, aged Fisher 344 rats exhibit thinning of the whole retina with reduced visual function. Accordingly, we studied the effects of chronic oral treatment with 8-aminoguanine (5 mg/kg/day in drinking water) in aged F344 rats86. Treatment with 8-aminoguanine was initiated at 22 months of age and continued for 8 or 17 weeks. Electroretinography (ERG) and optical coherence tomography (OCT) studies were conducted at baseline and at 7 or 16 weeks into treatment, and retinae were removed at 8 or 17 weeks of treatment for UPLC-MS/MS analyses, RNA-seq, immunoblots and immunohistochemistry.
Compared to untreated rats, at 8 weeks into treatment aged F344 rats had thicker retinae and higher numbers of nuclei in both the outer nuclear layer and retinal ganglion cell (RGC) layer suggesting preserved photoreceptor and RGC cells. Moreover, the 8-aminoguanine-treated aged rats had improved rod and cone functions as evidenced by higher scotopic and photopic ERG responses. Also, treatment with 8-aminoguanine led to thicker outer segments, higher rhodopsin levels and more PNA+ cone cells, suggesting restored rod homeostasis and more cone cells. The retinal bioavailablity of 8-aminoguanine was evidenced by the purine metabolome analysis showing increased 8-aminoguanine concentrations in the retinae of 8-aminoguanine-treated rats. Compared to controls, biomarkers of tissue oxidative damage (i.e., malondialdehyde and 8-hydroxydeoxyguanosine) in retinae were significantly reduced in aged rats treated with 8-aminoguanine. 8-Aminoguanine also significantly decreased retinal CD68+, IBA1+ microglia/macrophages. RNA-seq and transcriptome analyses revealed reduced expression of proinflammatory genes in microglia. Importantly, treatment of aged rats with 8-aminoguanine for 17 weeks (now 26 months of age) preserved all retinal layers with considerable ERG responses compared to controls that showed severe retinal deterioration with no ERG responses.
Safety and Toxicity.
One concern regarding chronic administration of 8-aminopurines is adverse effects related to over-suppression of the purine salvage pathway. The metabolism of inosine, deoxyinosine, guanosine and deoxyguanosine by PNPase produces hypoxanthine and guanine that can be converted to inosine monophosphate and guanosine monophosphate by hypoxanthine-guanine phosphoribosyltransferase66. Guanosine monophosphate can be phosphorylated to yield GTP and inosine monophosphate can be converted to AMP and then phosphorylated to yield ATP66. Thus, severe inhibition of PNPase by 8-aminopurines would be expected to impair this purine salvage mechanism.
Another concern regarding chronic administration of 8-aminopurines is T-cell depletion66. T-cell depletion by PNPase deficiency is likely due to the accumulation of deoxyguanosine, which is normally degraded to guanine by PNPase66. Excessive accumulation of deoxyguanosine results in an excessive level of deoxyguanosine triphosphate, which inhibits ribonucleotide reductase resulting in depletion of deoxynucleoside triphosphates and thus impairment of DNA synthesis, particularly in T-cells66.
Although complete PNPase deficiency due to inactivating mutations in both PNPase alleles results in immunodeficiency, neurological disorders and autoimmune diseases66, fortunately partial inhibition of PNPase does not – heterozygotic parents of homozygotic offspring are normal despite significantly lower PNPase activity66. Indeed, it is estimated that only 8 to 11% of normal PNPase activity is required for near-normal immunity87, and only 1.5% of normal PNPase activity – as measured in RBCs - is required for survival88. This provides for a safe therapeutic window in which 8-aminopurines can be dosed to attenuate, but not eliminate, PNPase activity. This conclusion is supported by our observations that in Dahl SS rats on an 8% salt diet, chronic treatment with 8-aminoguanosine or 8-aminoguanine (10 mg/kg/day for 40 days) did not cause detectable drug related histopathology in the liver, kidneys, heart, brain, aorta or adrenal gland (unpublished findings). The fact that naturally occurring 8-aminopurines are much less potent (μM potency) than other synthetic PNPase inhibitors (e.g., forodesine; pM to nM potency) may be a desirable feature of 8-aminopurines in that the therapeutic objective here is to moderately suppress PNPase activity, rather than abolish such activity. For chronic administration of PNPase inhibitors to treat diseases discussed herein, safety is paramount.
Unknowns and Future Directions.
Research related to 8-aminopurines for cardiovascular and renal diseases and diseases of aging is in its infancy. There are many questions, opportunities and details yet to be addressed, and discoveries yet to be made. Clearly, 8-aminoguanine is unique among the 8-aminopurines in that 8-aminoguanine markedly suppresses potassium excretion, while other 8-aminopurines do not. This effect of 8-aminoguanine is not due to inhibition of PNPase, a conclusion suggesting that this particularly 8-aminopurine has pharmacological actions yet to be elucidated.
As noted herein, evidence strongly suggests that 8-aminoguanosine and 8-aminoguanine are endogenous 8-aminopurines – particularly in Dahl SS rats. Although there is some evidence that 8-aminoinosine is also produced in vivo, additional studies are required to confirm this and to determine whether 8-aminohypoxanthine and 8-aminoxanthine too are endogenous 8-aminopurines. If 8-aminopurines are a family of endogenous factors, this raises the important question as to whether these potentially endogenous PNPase inhibitors play (patho)physiological roles.
We have recently described two pathways for the biosynthesis of 8-aminoguanine46; it is conceivable - perhaps even likely - that gut microorganisms also produce 8-aminopurines via these pathways. Given the increasing awareness that a healthy cardiovascular system is dependent on chemical processes within gut microorganisms89, we believe that the role of the gut microbiota in the production of 8-aminopurines is a research area ripe for exploration.
There is also an increasing awareness that systemic inflammation drives hypertension and cardiovascular and renal diseases90. As mentioned, inhibition of PNPase selectively reduces T-cell number due to accumulation of deoxyguanosine. These realizations motivate the question: What is the role of the anti-inflammatory effects of 8-aminopurines in vascular biology?
Concluding Remarks.
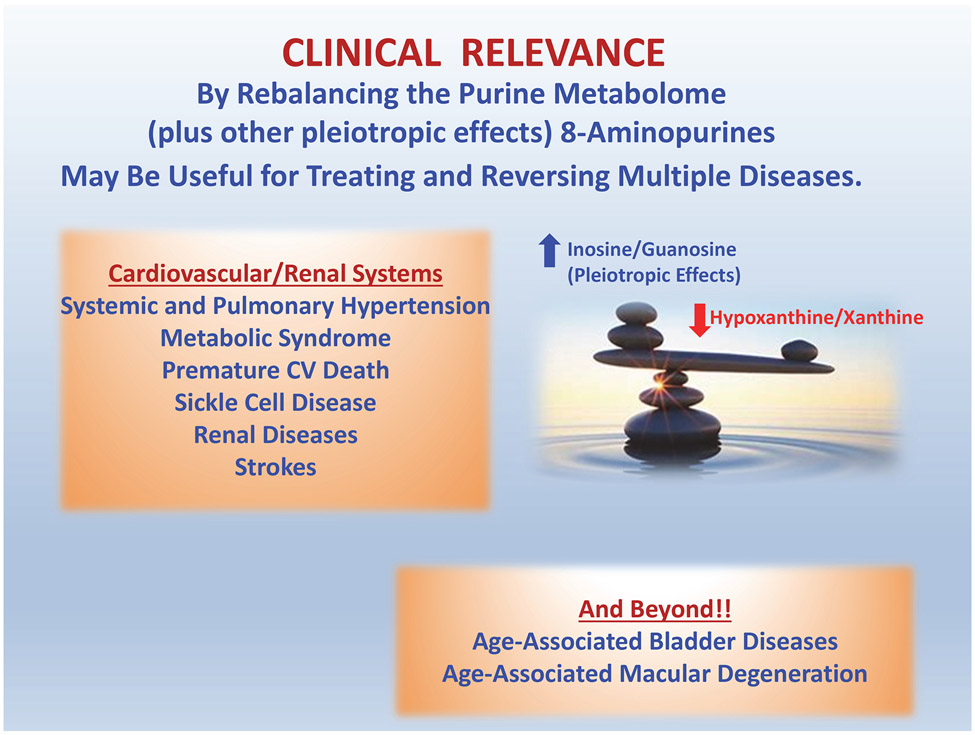
8-Aminopurines have potential as a novel class of drugs for treatment of systemic hypertension, pulmonary hypertension, strokes, renal disorders, sickle cell disease and age-related degeneration of the lower urinary tract and retinae (Figure 6). Likely, at least some 8-aminopurines are endogenous factors that are produced by specific biochemical pathways and may play important roles in (patho)physiology. The mechanism of action of 8-aminopurines entails in part inhibition of PNPase leading to the accumulation of tissue protective purines, such as inosine and guanosine, and reduction in the levels of tissue damaging purines, such as hypoxanthine and xanthine. Hopefully, this line of research will translate into effective treatments for diseases that affect the majority of humankind.
Figure 6: Clinical relevance of the 8-aminopurine hypothesis.
Our studies indicate that 8-aminopurines (particularly 8-aminoguanosine and 8-aminoguanine) may be useful for treating diseases of the cardiovascular and renal systems as well as diseases associated with aging. Although the diuretic, natriuretic and glucosuric effects of 8-aminoguanine have been linked to inosine, it is likely that increases in “protective purines” (such as inosine and guanosine) and decreases in “damaging purines” (such as hypoxanthine and xanthine) also participate in the beneficial actions of this novel class of agents (i.e., 8-aminopurines “rebalance the purine metabolome”). Yet to be discovered pleiotropic effects of 8-aminopurines may also contribute to the mechanism of action of this drug class.
SOURCES OF FUNDING
This work was supported by grants from the National Institutes of Health [HL109002, DK135076, AG056944] and by the Richard King Mellon Foundation.
Footnotes
Disclosures: EKJ, SPT, YC and LAB are inventors (assignee, University of Pittsburgh) of issued or pending patents related to medical uses of 8-aminopurines.
REFERENCES
- 1.Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. Journal of Physiology-London. 1929;68:213–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Effendi WI, Nagano T, Kobayashi K, Nishimura Y. Focusing on Adenosine Receptors as a Potential Targeted Therapy in Human Diseases. Cells. 2020;9. doi: 10.3390/cells9030785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson KA, Tosh DK, Jain S, Gao ZG. Historical and current adenosine receptor agonists in preclinical and clinical development. Front Cell Neurosci. 2019;13:124. doi: 10.3389/fncel.2019.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mori A, Chen JF, Uchida S, Durlach C, King SM, Jenner P. The pharmacological potential of adenosine A2A receptor antagonists for treating Parkinson's disease. Molecules (Basel, Switzerland). 2022;27. doi: 10.3390/molecules27072366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103:13132–13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohshima H, Sawa T, Akaike T. 8-Nitroguanine, a product of nitrative DNA damage caused by reactive nitrogen species: formation, occurrence, and implications in inflammation and carcinogenesis. Antioxid Redox Signal. 2006;8:1033–1045. doi: 10.1089/ars.2006.8.1033 [DOI] [PubMed] [Google Scholar]
- 7.Szabo C, Ohshima H. DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide. 1997;1:373–385. doi: 10.1006/niox.1997.0143 [DOI] [PubMed] [Google Scholar]
- 8.Yermilov V, Rubio J, Ohshima H. Formation of 8-nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Lett. 1995;376:207–210. doi: 10.1016/0014-5793(95)01281-6 [DOI] [PubMed] [Google Scholar]
- 9.Misiaszek R, Crean C, Joffe A, Geacintov NE, Shafirovich V. Oxidative DNA damage associated with combination of guanine and superoxide radicals and repair mechanisms via radical trapping. J Biol Chem. 2004;279:32106–32115. doi: 10.1074/jbc.M313904200 [DOI] [PubMed] [Google Scholar]
- 10.Osborne WR, Barton RW. A rat model of purine nucleoside phosphorylase deficiency. Immunology. 1986;59:63–67. [PMC free article] [PubMed] [Google Scholar]
- 11.Akaike T, Okamoto S, Sawa T, Yoshitake J, Tamura F, Ichimori K, Miyazaki K, Sasamoto K, Maeda H. 8-Nitroguanosine formation in viral pneumonia and its implication for pathogenesis. Proc Natl Acad Sci U S A. 2003;100:685–690. doi: 10.1073/pnas.0235623100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sodum RS, Nie G, Fiala ES. 8-Aminoguanine: a base modification produced in rat liver nucleic acids by the hepatocarcinogen 2-nitropropane. Chem Res Toxicol. 1993;6:269–276. doi: 10.1021/tx00033a004 [DOI] [PubMed] [Google Scholar]
- 13.Park EM, Shigenaga MK, Degan P, Korn TS, Kitzler JW, Wehr CM, Kolachana P, Ames BN. Assay of excised oxidative DNA lesions: isolation of 8-oxoguanine and its nucleoside derivatives from biological fluids with a monoclonal antibody column. Proc Natl Acad Sci U S A. 1992;89:3375–3379. doi: 10.1073/pnas.89.8.3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraga CG, Shigenaga MK, Park JW, Degan P, Ames BN. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990;87:4533–4537. doi: 10.1073/pnas.87.12.4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam PM, Mistry V, Marczylo TH, Konje JC, Evans MD, Cooke MS. Rapid measurement of 8-oxo-7,8-dihydro-2'-deoxyguanosine in human biological matrices using ultra-high-performance liquid chromatography-tandem mass spectrometry. Free Radic Biol Med. 2012;52:2057–2063. doi: 10.1016/j.freeradbiomed.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson EK, Gillespie DG, Mi Z. 8-Aminoguanosine and 8-aminoguanine exert diuretic, natriuretic, glucosuric, and antihypertensive activity. J Pharmacol Exp Ther. 2016;359:420–435. doi: 10.1124/jpet.116.237552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson EK, Mi Z. 8-Aminoguanosine exerts diuretic, natriuretic, and glucosuric activity via conversion to 8-aminoguanine, yet has direct antikaliuretic effects. J Pharmacol Exp Ther. 2017;363:358–366. doi: 10.1124/jpet.117.243758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briggs JP, Schnermann J. The tubuloglomerular feedback mechanism: functional and biochemical aspects. Annu Rev Physiol. 1987;49:251–273. doi: 10.1146/annurev.ph.49.030187.001343 [DOI] [PubMed] [Google Scholar]
- 19.Jackson EK, Mi Z, Kleyman TR, Cheng D. 8-Aminoguanine induces diuresis, natriuresis, and glucosuria by inhibiting purine nucleoside phosphorylase and reduces potassium excretion by inhibiting Rac1. J Am Heart Assoc. 2018;7:e010085. doi: 10.1161/jaha.118.010085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K-i, Yoshida S, Kawarazaki W, Takeuchi M, Ayuzawa N, Miyoshi J, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor–dependent pathway. J Clin Investig. 2011;121:3233–3243. doi: 10.1172/JCI43124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370. doi: 10.1038/nm.1879 [DOI] [PubMed] [Google Scholar]
- 22.Wynne BM, Samson TK, Moyer HC, van Elst HJ, Moseley AS, Hecht G, Paul O, Al-Khalili O, Gomez-Sanchez C, Ko B, et al. Interleukin 6 mediated activation of the mineralocorticoid receptor in the aldosterone-sensitive distal nephron. Am J Physiol Cell Physiol. 2022;323:C1512–c1523. doi: 10.1152/ajpcell.00272.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huh JY, Son DJ, Lee Y, Lee J, Kim B, Lee HM, Jo H, Choi S, Ha H, Chung M-H. 8-Hydroxy-2-deoxyguanosine prevents plaque formation and inhibits vascular smooth muscle cell activation through Rac1 inactivation. Free Radic Biol Med. 2012;53:109–121. doi: 10.1016/j.freeradbiomed.2012.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson EK, Menshikova EV, Ritov VB, Mi Z, Birder LA. 8-Aminoinosine and 8-aminohypoxanthine inhibit purine nucleoside phosphorylase and exert diuretic and natriuretic activity. J Pharmacol Exp Ther. 2022;382:135–148. doi: 10.1124/jpet.122.001221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson EK, Gillespie DG, Mi Z. 8-Aminoguanine and its actions on renal excretory function. Hypertension. 2023;80:981–994. doi: 10.1161/hypertensionaha.122.20760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Rocha Lapa F, da Silva MD, de Almeida Cabrini D, Santos AR. Anti-inflammatory effects of purine nucleosides, adenosine and inosine, in a mouse model of pleurisy: evidence for the role of adenosine A2 receptors. Purinergic Signal. 2012;8:693–704. doi: 10.1007/s11302-012-9299-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Oliveira ED, Schallenberger C, Bohmer AE, Hansel G, Fagundes AC, Milman M, Silva MD, Oses JP, Porciuncula LO, Portela LV, et al. Mechanisms involved in the antinociception induced by spinal administration of inosine or guanine in mice. Eur J Pharmacol. 2016;772:71–82. doi: 10.1016/j.ejphar.2015.12.034 [DOI] [PubMed] [Google Scholar]
- 28.Doyle C, Cristofaro V, Sack BS, Lukianov SN, Schäfer M, Chung YG, Sullivan MP, Adam RM. Inosine attenuates spontaneous activity in the rat neurogenic bladder through an A2B pathway. Sci Rep. 2017;7:44416. doi: 10.1038/srep44416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doyle C, Cristofaro V, Sullivan MP, Adam RM. Inosine - a multifunctional treatment for complications of neurologic injury. Cell Physiol Biochem. 2018;49:2293–2303. doi: 10.1159/000493831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welihinda AA, Kaur M, Greene K, Zhai Y, Amento EP. The adenosine metabolite inosine is a functional agonist of the adenosine A2A receptor with a unique signaling bias. Cell Signal. 2016;28:552–560. doi: 10.1016/j.cellsig.2016.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welihinda AA, Kaur M, Raveendran KS, Amento EP. Enhancement of inosine-mediated A2AR signaling through positive allosteric modulation. Cell Signal. 2018;42:227–235. doi: 10.1016/j.cellsig.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/s0006-2952(00)00570-0 [DOI] [PubMed] [Google Scholar]
- 33.Hasko G, Kuhel DG, Nemeth ZH, Mabley JG, Stachlewitz RF, Virag L, Lohinai Z, Southan GJ, Salzman AL, Szabo C. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol. 2000;164:1013–1019. doi: 10.4049/jimmunol.164.2.1013 [DOI] [PubMed] [Google Scholar]
- 34.Valada P, Hinz S, Vielmuth C, Lopes CR, Cunha RA, Müller CE, Lopes JP. The impact of inosine on hippocampal synaptic transmission and plasticity involves the release of adenosine through equilibrative nucleoside transporters rather than the direct activation of adenosine receptors. Purinergic Signal. 2022. doi: 10.1007/s11302-022-09899-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowley AW Jr., Mori T, Mattson D, Zou AP. Role of renal NO production in the regulation of medullary blood flow. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1355–1369. doi: 10.1152/ajpregu.00701.2002 [DOI] [PubMed] [Google Scholar]
- 36.Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2003;284:R13–27. doi: 10.1152/ajpregu.00321.2002 [DOI] [PubMed] [Google Scholar]
- 37.Roman RJ, Zou AP. Influence of the renal medullary circulation on the control of sodium excretion. Am J Physiol. 1993;265:R963–973. doi: 10.1152/ajpregu.1993.265.5.R963 [DOI] [PubMed] [Google Scholar]
- 38.Cowley AW Jr., Abe M, Mori T, O'Connor PM, Ohsaki Y, Zheleznova NN. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am J Physiol Renal Physiol. 2015;308:F179–197. doi: 10.1152/ajprenal.00455.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou AP, Nithipatikom K, Li PL, Cowley AW. Role of renal medullary adenosine in the control of blood flow and sodium excretion. Am J Physiol Regul Integr Comp Physiol. 1999;276:R790–R798. doi: 10.1152/ajpregu.1999.276.3.R790 [DOI] [PubMed] [Google Scholar]
- 40.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng M-G, Navar LG. Afferent arteriolar vasodilator effect of adenosine predominantly involves adenosine A2B receptor activation. Am J Physiol Renal Physiol. 2010;299:F310–315. doi: 10.1152/ajprenal.00149.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooper SL, Wragg ES, Pannucci P, Soave M, Hill SJ, Woolard J. Regionally selective cardiovascular responses to adenosine A2A and A2B receptor activation. FASEB J. 2022;36:e22214. doi: 10.1096/fj.202101945R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braunwald E. Gliflozins in the management of cardiovascular disease. N Engl J Med. 2022;386:2024–2034. doi: 10.1056/NEJMra2115011 [DOI] [PubMed] [Google Scholar]
- 44.Rajagopal M, Pao AC. Adenosine activates A2b receptors and enhances chloride secretion in kidney inner medullary collecting duct cells. Hypertension. 2010;55:1123–1128. doi: 10.1161/hypertensionaha.109.143404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Battistone MA, Nair AV, Barton CR, Liberman RN, Peralta MA, Capen DE, Brown D, Breton S. Extracellular adenosine stimulates vacuolar ATPase-dependent proton secretion in medullary intercalated cells. J Am Soc Nephrol. 2018;29:545–556. doi: 10.1681/asn.2017060643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson EK, Menshikova EV, Ritov VB, Gillespie DG, Mi Z. Biochemical pathways of 8-aminoguanine production in Sprague-Dawley and Dahl salt-sensitive rats. Biochem Pharmacol. 2022;201:115076. doi: org/ 10.1016/j.bcp.2022.115076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen HJ, Chen YM, Chang CM. Lipoyl dehydrogenase catalyzes reduction of nitrated DNA and protein adducts using dihydrolipoic acid or ubiquinol as the cofactor. Chem Biol Interact. 2002;140:199–213. doi: 10.1016/s0009-2797(02)00019-4 [DOI] [PubMed] [Google Scholar]
- 48.Chen HJ, Chang CM, Chen YM. Hemoprotein-mediated reduction of nitrated DNA bases in the presence of reducing agents. Free Radic Biol Med. 2003;34:254–268. doi: 10.1016/s0891-5849(02)01246-7 [DOI] [PubMed] [Google Scholar]
- 49.Gonick HC, Cohen AH, Ren Q, Saldanha LF, Khalil-Manesh F, Anzalone J, Sun YY. Effect of 2,3-dimercaptosuccinic acid on nephrosclerosis in the Dahl rat. I. Role of reactive oxygen species. Kidney Int. 1996;50:1572–1581. doi: 10.1038/ki.1996.473 [DOI] [PubMed] [Google Scholar]
- 50.Zhou MS, Hernandez Schulman I, Pagano PJ, Jaimes EA, Raij L. Reduced NAD(P)H oxidase in low renin hypertension: link among angiotensin II, atherogenesis, and blood pressure. Hypertension. 2006;47:81–86. doi: 10.1161/01.HYP.0000197182.65554.c7 [DOI] [PubMed] [Google Scholar]
- 51.Mori T, O'Connor PM, Abe M, Cowley AW Jr. Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension. 2007;49:1336–1341. doi: 10.1161/hypertensionaha.106.085811 [DOI] [PubMed] [Google Scholar]
- 52.Taylor NE, Glocka P, Liang M, Cowley AW Jr. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension. 2006;47:692–698. doi: 10.1161/01.HYP.0000203161.02046.8d [DOI] [PubMed] [Google Scholar]
- 53.Rabuffetti M, Rinaldi F, Lo Bianco A, Speranza G, Ubiali D, de Moraes MC, Rodrigues Pereira da Silva LC, Massolini G, Calleri E, Lavecchia A. Discovery of a novel inhibitor of human purine nucleoside phosphorylase by a simple hydrophilic interaction liquid chromatography enzymatic assay. ChemMedChem. 2021;16:1325–1334. doi: 10.1002/cmdc.202000874 [DOI] [PubMed] [Google Scholar]
- 54.Stoeckler JD, Cambor C, Kuhns V, Chu SH, Parks RE Jr. Inhibitors of purine nucleoside phosphorylase, C(8) and C(5') substitutions. Biochem Pharmacol. 1982;31:163–171. doi: 10.1016/0006-2952(82)90206-4 [DOI] [PubMed] [Google Scholar]
- 55.Chern JW, Lee HY, Chen CS, Shewach DS, Daddona PE, Townsend LB. Nucleosides. 5. Synthesis of guanine and formycin B derivatives as potential inhibitors of purine nucleoside phosphorylase. J Med Chem. 1993;36:1024–1031. doi: 10.1021/jm00060a010 [DOI] [PubMed] [Google Scholar]
- 56.Jackson EK, Tofovic SP. Methods for treatment using small molecule potassium-sparing diuretics and natriuretics. US 10,729711 B2. 2020;1–82 [Google Scholar]
- 57.Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.Cir.0000140677.20606.0e [DOI] [PubMed] [Google Scholar]
- 58.Ferreira JP, Verma S, Fitchett D, Ofstad AP, Lauer S, Zwiener I, George J, Wanner C, Zinman B, Inzucchi SE. Metabolic syndrome in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a post hoc analyses of the EMPA-REG OUTCOME trial. Cardiovasc Diabetol. 2020;19:200. doi: 10.1186/s12933-020-01174-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang F, Liu L, Zhang C, Ji S, Mei Z, Li T. Association of metabolic syndrome and its components with risk of stroke recurrence and mortality: A meta-analysis. Neurology. 2021;97:e695–e705. doi: 10.1212/wnl.0000000000012415 [DOI] [PubMed] [Google Scholar]
- 60.Satoh T, Wang L, Espinosa-Diez C, Wang B, Hahn SA, Noda K, Rochon ER, Dent MR, Levine AR, Baust JJ, et al. Metabolic syndrome mediates ROS-miR-193b-NFYA-dependent downregulation of soluble guanylate cyclase and contributes to exercise-induced pulmonary hypertension in heart failure with preserved ejection fraction. Circulation. 2021;144:615–637. doi: 10.1161/circulationaha.121.053889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tofovic SP, Hu J, Jackson E. Purine nucleoside phosphorylase inhibition attenuates the metabolic syndrome and associated pulmonary hypertension and right ventricular dysfunction. In: American Thoracic Society 2019 International Conference. Dallas, TX; 2019:A4197. [Google Scholar]
- 62.Tofovic SP, Jackson EK. Rat models of the metabolic syndrome. In: Goligorsky MS, ed. Methods in Molecular Medicine, volume 86: Renal Disease: Techniques and Protocols. Humana Press; 2003:29–46. doi: org/ 10.1385/1-59259-392-5:29 [DOI] [PubMed] [Google Scholar]
- 63.Peterson RG, Jackson CV, Zimmerman K, de Winter W, Huebert N, Hansen MK. Characterization of the ZDSD Rat: A Translational Model for the Study of Metabolic Syndrome and Type 2 Diabetes. J Diabetes Res. 2015;2015:487816. doi: 10.1155/2015/487816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tofovic SP, Hu J, Milosevic J, Jackson EK. Inhibition of purine nucleoside phosphorylase retards the progression of angioproliferative pulmonary hypertension in female rats. In: American Thoracic Society 2018 International Conference. San Diego, CA; 2018:A2883. [Google Scholar]
- 65.Tofovic SP, Jackson EK. Estradiol metabolism: Crossroads in pulmonary arterial hypertension. Int J Mol Sci. 2020;21:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bzowska A, Kulikowska E, Shugar D. Purine nucleoside phosphorylases: properties, functions, and clinical aspects. Pharmacol Ther. 2000;88:349–425. doi: 10.1016/s0163-7258(00)00097-8 [DOI] [PubMed] [Google Scholar]
- 67.Rathbone MP, Middlemiss PJ, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F. Trophic effects of purines in neurons and glial cells. Prog Neurobiol. 1999;59:663–690. doi: 10.1016/s0301-0082(99)00017-9 [DOI] [PubMed] [Google Scholar]
- 68.Liaudet L, Mabley JG, Pacher P, Virag L, Soriano FG, Marton A, Hasko G, Deitch EA, Szabo C. Inosine exerts a broad range of antiinflammatory effects in a murine model of acute lung injury. Ann Surg. 2002;235:568–578. doi: 10.1097/00000658-200204000-00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liaudet L, Mabley JG, Soriano FG, Pacher P, Marton A, Haskó G, Szabó C. Inosine reduces systemic inflammation and improves survival in septic shock induced by cecal ligation and puncture. Am J Respir Crit Care Med. 2001;164:1213–1220. doi: 10.1164/ajrccm.164.7.2101013 [DOI] [PubMed] [Google Scholar]
- 70.Bellaver B, Souza DG, Bobermin LD, Goncalves CA, Souza DO, Quincozes-Santos A. Guanosine inhibits LPS-induced pro-inflammatory response and oxidative stress in hippocampal astrocytes through the heme oxygenase-1 pathway. Purinergic Signal. 2015;11:571–580. doi: org/ 10.1007/s11302-015-9475-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dal-Cim T, Ludka FK, Martins WC, Reginato C, Parada E, Egea J, Lopez MG, Tasca CI. Guanosine controls inflammatory pathways to afford neuroprotection of hippocampal slices under oxygen and glucose deprivation conditions. J Neurochem. 2013;126:437–450. doi: org/ 10.1111/jnc.12324 [DOI] [PubMed] [Google Scholar]
- 72.Szabó G, Stumpf N, Radovits T, Sonnenberg K, Gerö D, Hagl S, Szabó C, Bährle S. Effects of inosine on reperfusion injury after heart transplantation. Eur J Cardiothorac Surg. 2006;30:96–102. doi: 10.1016/j.ejcts.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 73.Wakai A, Winter DC, Street JT, O'Sullivan RG, Wang JH, Redmond HP. Inosine attenuates tourniquet-induced skeletal muscle reperfusion injury. J Surg Res. 2001;99:311–315. doi: 10.1006/jsre.2001.6192 [DOI] [PubMed] [Google Scholar]
- 74.Fuentes E, Alarcón M, Astudillo L, Valenzuela C, Gutiérrez M, Palomo I. Protective mechanisms of guanosine from Solanum lycopersicum on agonist-induced platelet activation: role of sCD40L. Molecules (Basel, Switzerland). 2013;18:8120–8135. doi: 10.3390/molecules18078120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fuentes E, Pereira J, Mezzano D, Alarcón M, Caballero J, Palomo I. Inhibition of platelet activation and thrombus formation by adenosine and inosine: studies on their relative contribution and molecular modeling. PLoS One. 2014;9:e112741. doi: 10.1371/journal.pone.0112741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsiao G, Lin KH, Chang Y, Chen TL, Tzu NH, Chou DS, Sheu JR. Protective mechanisms of inosine in platelet activation and cerebral ischemic damage. Arterioscler Thromb Vasc Biol. 2005;25:1998–2004. doi: 10.1161/01.ATV.0000174798.25085.d6 [DOI] [PubMed] [Google Scholar]
- 77.Bilan VP, Schneider F, Novelli EM, Kelley EE, Shiva S, Gladwin MT, Jackson EK, Tofovic SP. Experimental intravascular hemolysis induces hemodynamic and pathological pulmonary hypertension: association with accelerated purine metabolism. Pulm Circ. 2018;8:2045894018791557. doi: 10.1177/2045894018791557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tofovic SP, Jackson EK, Ihrig LL, Mutchler S, Novelli EM. Purine nucleoside phosphorylase inhibition attenuates the progression of anemia and organ damage in sickle cell mice. Blood. 2022;140:2519–2520. doi: 10.1182/blood-2022-15978436480218 [DOI] [Google Scholar]
- 79.Kim YJ, Ryu HM, Choi JY, Cho JH, Kim CD, Park SH, Kim YL. Hypoxanthine causes endothelial dysfunction through oxidative stress-induced apoptosis. Biochem Biophys Res Commun. 2017;482:821–827. doi: 10.1016/j.bbrc.2016.11.119 [DOI] [PubMed] [Google Scholar]
- 80.Chung YG, Seth A, Doyle C, Franck D, Kim D, Cristofaro V, Benowitz LI, Tu DD, Estrada CR, Mauney JR, et al. Inosine improves neurogenic detrusor overactivity following spinal cord injury. PLoS One. 2015;10:e0141492. doi: 10.1371/journal.pone.0141492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu F, Yao L, Yuan J, Liu H, Yang X, Qin W, Wu G, Yang L, Wang H, Takahashi N, et al. Protective effects of inosine on urinary bladder function in rats with partial bladder outlet obstruction. Urology. 2009;73:1417–1422. doi: 10.1016/j.urology.2008.10.032 [DOI] [PubMed] [Google Scholar]
- 82.Li S, Juan YS, Kogan BA, Mannikarottu A, Leggett R, Schuler C, Levin RM. Effects of inosine on response to in vitro hypoxia in absence of substrate on bladder dysfunction in adult rats. Urology. 2009;73:661–664. doi: 10.1016/j.urology.2008.10.024 [DOI] [PubMed] [Google Scholar]
- 83.Birder LA, Wolf-Johnston A, Wein AJ, Cheng F, Grove-Sullivan M, Kanai AJ, Watson AM, Stoltz D, Watkins SC, Robertson AM, et al. Purine nucleoside phosphorylase inhibition ameliorates age-associated lower urinary tract dysfunctions. JCI Insight. 2020;5:e140109. doi: 10.1172/jci.insight.140109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Birder LA, Wolf-Johnston A, Wein AJ, Grove-Sullivan M, Stoltz D, Watkins S, Newman D, Dmochowski RR, Jackson EK. A uro-protective agent with restorative actions on urethral and striated muscle morphology. World J Urol. 2021;39:2685–2690. doi: 10.1007/s00345-020-03492-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Campello L, Singh N, Advani J, Mondal AK, Corso-Díaz X, Swaroop A. Aging of the retina: Molecular and metabolic turbulences and potential interventions. Annu Rev Vis Sci. 2021;7:633–664. doi: 10.1146/annurev-vision-100419-114940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clinger O, Xi Y, Vats A, Wolf-Johnson A, Birder L, Jackson E, Sahel JA, Chen Y. Potent retinal protection by oral administration of a purine metabolite against age-related retinal degeneration and RHO-associated retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2022;63:1777 – F0326–1777 – F0326. [Google Scholar]
- 87.Grunebaum E, Campbell N, Leon-Ponte M, Xu X, Chapdelaine H. Partial purine nucleoside phosphorylase deficiency helps determine minimal activity required for immune and neurological development. Front Immunol. 2020;11:1257. doi: 10.3389/fimmu.2020.01257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mak TW, Saunders ME. 24 - Primary Immunodeficiencies. In: Mak TW, Saunders ME, eds. The Immune Response. Burlington: Academic Press; 2006:751–783. doi: org/ 10.1016/B978-012088451-3.50026-0 [DOI] [Google Scholar]
- 89.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van Beusecum JP, Moreno H, Harrison DG. Innate immunity and clinical hypertension. J Hum Hypertens. 2022;36:503–509. doi: 10.1038/s41371-021-00627-z [DOI] [PMC free article] [PubMed] [Google Scholar]