Abstract
HIV-1 Rev is an essential regulatory protein that transports unspliced and partially spliced viral mRNAs from the nucleus to the cytoplasm for the expression of viral structural proteins. During its nucleo-cytoplasmic shuttling Rev interacts with several host proteins to use the cellular machinery for the advantage of the virus. Here we report the 3.5 Å cryo-EM structure of a 4.8 MDa Rev-tubulin ring-complex. Our structure shows that Rev’s Arginine rich motif (ARM) binds to both the acidic surfaces and the C-terminal tails of α/β-tubulin. The Rev-tubulin interaction is functionally homologous to that of Kinesin-13, potently destabilizing microtubules at sub-stoichiometric levels. Expression of Rev in astrocytes and HeLa cells shows that it can modulate the microtubule cytoskeleton within the cellular environment. These results show a previously undefined regulatory role of Rev.
Graphical Abstract
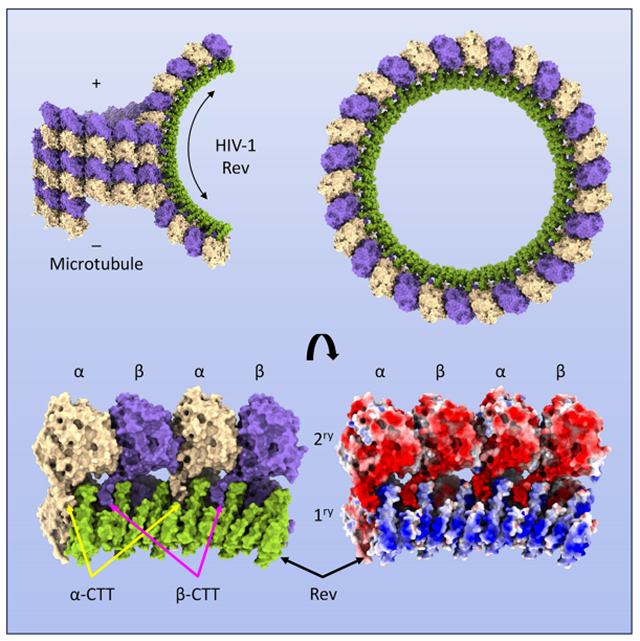
eTOC Blurb
HIV-1 Rev is an essential regulatory protein that transports viral mRNAs from the nucleus to the cytoplasm. Eren et al. report that Rev also depolymerizes microtubules in vitro and in vivo, and describe the cryo-EM structure of the ring-like complexes that ensue, highlighting a previously unrecognized regulatory role for Rev.
INTRODUCTION
Microtubules (MTs) form a dynamic network of filaments that are involved in many cellular processes, including maintenance of cell structure, regulation of cell polarity, intracellular cargo transport, chromosomal segregation, and mitosis 1. MTs are composed of polar chains of α- and ß-tubulin heterodimers (protofilaments of microtubules) arranged with α-tubulin exposed at the minus- and ß-tubulin exposed at the plus-end 2,3. MT minus-ends are anchored at specific subcellular locations, mainly the centrosomes or microtubule organizing centers (MTOCs) and their plus-ends point toward the cell periphery 4,5. The minus-ends are relatively stable whereas the plus-ends are in a state of flux between polymerization and depolymerization due to the “dynamic instability” of α- and ß-tubulin polymers 6. MT dynamics are controlled by Microtubule associated proteins (MAPs) which are mostly structural proteins that bind and stabilize microtubules 7. Cargo transport on MTs depends on the motor proteins (Dynein and Kinesin) that use the energy from ATP to carry cargo around the cell along microtubule tracks 8.
MTs, MAPs and motor proteins play several important roles in HIV infection 9–12. In early infection, HIV utilizes MTs for trafficking, viral core uncoating, possibly reverse transcription, and transport of the Pre-Integration Complex (PIC) to the nucleus. These events involve MAP1 in tethering the capsid to microtubules, EB1 and Kif4 in binding HIV-1 matrix (MA) protein to MTs, Dynein and Kinesin-1 to effect trafficking, and MAP4 in microtubule stabilization 9. Less is known about the role of microtubules in late infection but there is ample evidence that HIV targets microtubules with one of its two key regulatory proteins, Tat (Trans-activator of transcription). Tat binds to microtubules through its core domain and regulates their dynamics, primarily by stabilizing them in various ways 13–16 but also inhibiting their polymerization 17 triggering apoptosis and neurodegeneration. In addition, Tat uses MTs to internalize CD127 for proteasomal degradation to reduce Interleukin-7 signaling and impair CD8 T-cell proliferation and function 18. Tat also has numerous and diverse other functions. It interacts with promotors and transcription factors to regulate many cellular genes and is involved in post-transcriptional gene regulation through microRNAs; it alters the expression and function of multiple cytokines including upregulation of pro-inflammatory cytokines IFN-γ and TNF-α; it is involved in the generation of reactive oxygen species (ROS), activation of the Jnk, Erk1/2, and Akt pathways, and subsequent activation of NF-κB; it alters cell membrane composition, permeability, and Ca2+ regulation; and it alters the structure and function of endolysosomes, disrupting their membranes and raising their pH, thereby decreasing their activity (reviewed in 19–21).
Another HIV-1 key regulatory protein that interacts with MTs is Rev (Regulator of expression of virion proteins), a small (13 kDa) RNA-binding protein 22. In its canonical role, Rev binds to and oligomerizes on the Rev response element (RRE), a highly structured 351-nt region on viral transcripts, where it functions as an adaptor for Crm1 and Ran-GTP 23–27. The complex consisting of viral mRNA, Rev, Crm1, and Ran-GTP (and other partners) is then exported through the nuclear pore to the cytoplasm in a manner that evades the host default splicing of transcripts. Once in the cytoplasm, Ran-GTP hydrolysis dissociates the complex and Rev is then reimported into the nucleus by Importin-β (Impβ) for further rounds of viral mRNA export, thereby enabling transition to the late phase of the replication cycle. In addition to its nuclear export function, Rev is reported to stabilize viral transcripts through inhibition of instability sequences (INS), although the exact mechanism remains unclear, as well as to directly inhibit viral mRNA splicing, possibly through spliceosome suppression 28. Other than its canonical role few regulatory functions have been identified for Rev, and those that have are closely related to its primary function and surrounded by uncertainty. Specifically, unlike Tat which has numerous roles, and whose interaction with microtubules is well documented, there is little in the literature regarding Rev other than its role in nuclear export of viral transcripts. This great difference is surprising, given that Tat and Rev are the two key regulators of HIV, encoded from the same locus, that both are small basic proteins, with helical and unstructured regions, and that viral proteins often have multiple functions.
We have previously observed by negative stain electron microscopy and image averaging, an interaction between Rev and microtubules that results in the rapid formation of laterally paired, 30-fold symmetric rings consisting of 15 tubulin heterodimers on the outside and Rev on the inside 22. From their appearance, it was deduced that the outer surface of the rings corresponded to the luminal surface of the tubulin protofilament. Similar rings with 32-fold symmetry or 28-fold symmetry were also observed in smaller quantities. Given that the rings failed to stack, i.e., polymerize, it was assumed that the individual tubulin rings were anti-parallel to one another and joined by symmetrically arranged Rev dimers. Such rings were observed to form from both dimeric and filamentous Rev and dimeric tubulin and microtubules stabilized with Taxol, suggesting that the interaction was not mediated entirely by charge interactions. Although the structure of Rev was not known at the time, a sequence similarity between the N-terminal domain of Rev and the motor domain of the Kin-I kinesins hinted at a common binding site 22.
Here, we present the Rev-tubulin complex structure to 3.5 Å resolution by cryo-EM. Contrary to expectations the complex is asymmetric, with Rev primarily bound to the negatively charged surface of one of the tubulin rings by its Arginine rich motif (ARM). Interaction of Rev with the other ring involves the ARM and the Carboxy-terminal tails (CTTs) of α/β tubulin. The laterally paired rings are oriented parallel to each other. The complexes fail to stack not because the rings are anti-parallel and therefore forming a closed complex, but rather due to conformational changes in the α- and β-tubulin subunits. The complexes shed light on the mechanism of MT depolymerization by Rev. In addition, we compare how Rev and kinesins depolymerize MTs, and that this is not mediated by the previously observed local sequence similarity between the two proteins but rather due to Rev’s ability to oligomerize. Finally, we propose a regulatory role for Rev in MT modification.
RESULTS
Structure of the Rev-tubulin complex
Rev is a 116 residue, basic (pI = 9.2) protein with helical and unstructured regions. The N-terminal domain includes two helical regions (H1, residues 12-25 and H2, 34-64) that form a hairpin while the C-terminal domain is considered to be essentially unstructured (Figure S1A) 29–32. The N-terminal hairpins pair with one another near their open ends through A-A and B-B interfaces (Figure S1B and C). Rev uses both of these interfaces to dimerize and form higher order oligomers. Both interfaces allow a remarkable degree of flexibility in the angle between the monomers, ranging from small increments to 180° degree rotations, apparently occurring in a ball-and-socket manner, as seen in X-ray crystallographic and cryo-EM structures 29,31,33. Together with a third (C-C) interface located at the turn between the helices, these interfaces allow Rev to self-associate in a wide variety of ways 33,34. In the absence of other binding partners (either protein or RNA) Rev readily polymerizes into long hollow tubes 11-14 nm in outside diameter with a continuously variable helical lattice 33,35,36.
The interaction between Rev and tubulin, in the form of MTs or heterodimers, results in the immediate and rapid formation of ring-like complexes (Figure S2A–D). Rev can depolymerize MTs from both ends, and unlike tubulin depolymerizing kinesins it does not require any energy coupling (Figure S2E). Formation of these ring-like complexes requires Rev oligomerization and is inhibited in the presence of a Rev binding Fab 31 which prevents Rev oligomerization from the B-B interface (Figure S2F). In addition, while a C-terminally truncated Rev (Rev 1-59) can still depolymerize microtubules 22, the ARM itself or soluble arginine has no ability to induce microtubule depolymerization or ring formation (Figure S2G and H) showing that Rev requires both α-helical domains for its depolymerization activity, and the interaction is not merely non-specific.
We solved the structure of Rev-tubulin complexes by cryo-EM to 3.5 Å resolution (Figure 1, Table S1 and Figure S3). The structure shows that the complexes consist of two laterally paired single protofilament tubulin rings on the outside and a single Rev ring on the inside (Figure 1A). The tubulin is oriented such that the luminal surface in microtubules faces outwards. The tubulin heterodimers are arranged parallel and in register relative to each other. The majority of the tubulin rings have C15 symmetry and consist of 15 heterodimers in each ring with 12° intradimer and 24° interdimer rotations in the plane of the ring (Figure 1A). Subsets of particles form C14 and C16 rings as well (Figure S3A and B).
Figure 1. Structure of the Rev-tubulin complex.
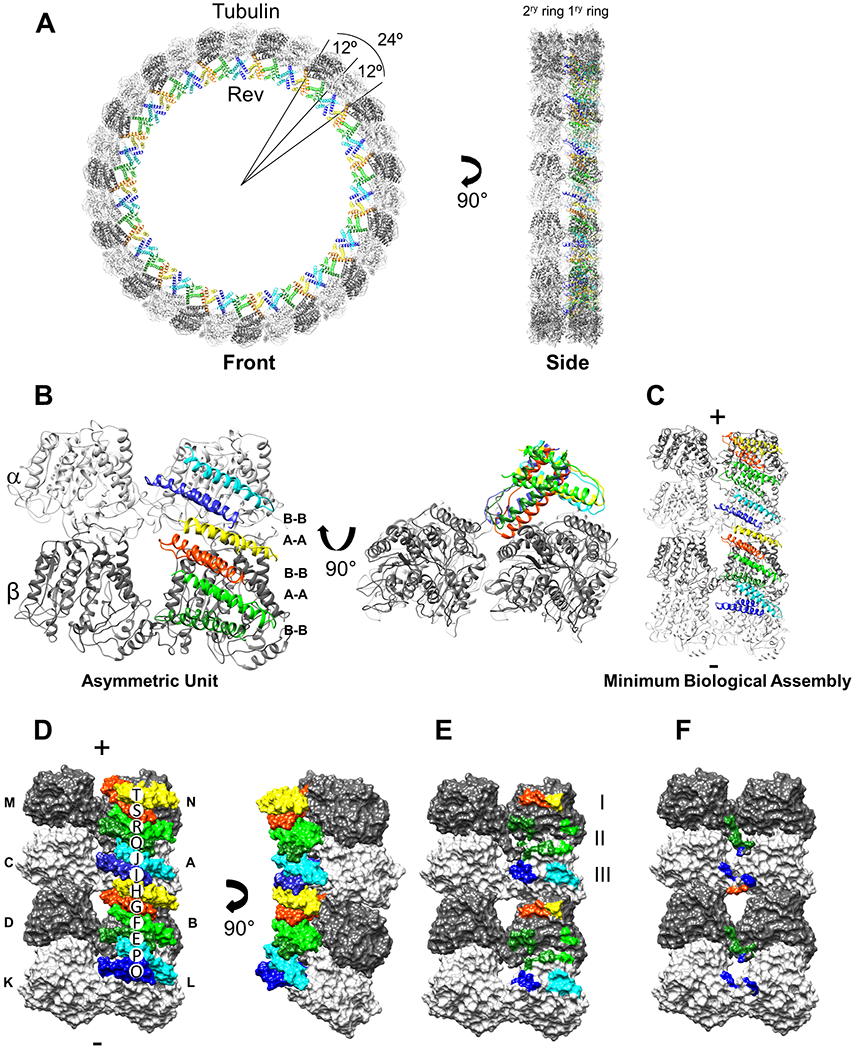
(A) The Rev-tubulin ring-complex viewed from front (left) and side (right) with β-tubulin (dark gray), α-tubulin (light gray), and the Rev monomers in different colors, all depicted as ribbons. (B) The asymmetric unit (ASU), as viewed from the center of the ring with the plus-end up, relative to a microtubule (left), and the ASU viewed towards the plus-end (right). Rev monomers: E, dark green; F, light green; G, orange; H, yellow; I, dark blue; and J, cyan. (C) The minimal biological assembly (MBA), consisting of α-β-tubulin heterodimers, as viewed from the center of the ring with the plus-end up, with the subunits colored as above. (D) The MBA rendered as a surface as viewed from the center of the ring (left) and viewed from the side of the primary ring (right). Rev and tubulin subunits are labeled uniquely and the chain IDs match the deposited structure (PDB ID: 7U0F). (E) The MBA with the Rev monomers removed and their contact areas on tubulin colored accordingly, as viewed form the center of the ring. Areas I, II, III indicated (see text). (F) The MBA with the contact areas of the Rev monomers on the CTTs of the adjacent tubulin subunits colored accordingly.
The structure shows 90 Rev monomers are predominantly bound to the inner surface (i.e., the outer surface of a protofilament) of only one of the tubulin rings (Figure 1A). Hereafter we refer to this ring (and its subunits) as the primary one, and to the adjacent ring (and its subunits) as the secondary one (Figure 1A). Rev monomers associate through homotypic (A-A and B-B) interactions such that in the asymmetric unit (ASU; involving only the tubulin interdimer interface) there are six Rev chains and four tubulin chains (Figure 1B); in what we term the minimum biological assembly (MBA; involving four complete tubulin heterodimers, and both interdimer and intradimer interfaces) there are twelve Rev chains and eight tubulin chains, and Rev assembles on two sequential tubulin heterodimers (Figure 1C and D). The Rev monomers are all arranged with their basic H2 helices in contact with the acidic exterior surfaces of the tubulin subunits of the primary ring (Figure 2).
Figure 2. Charge and hydrophobic interactions between Rev and tubulin.
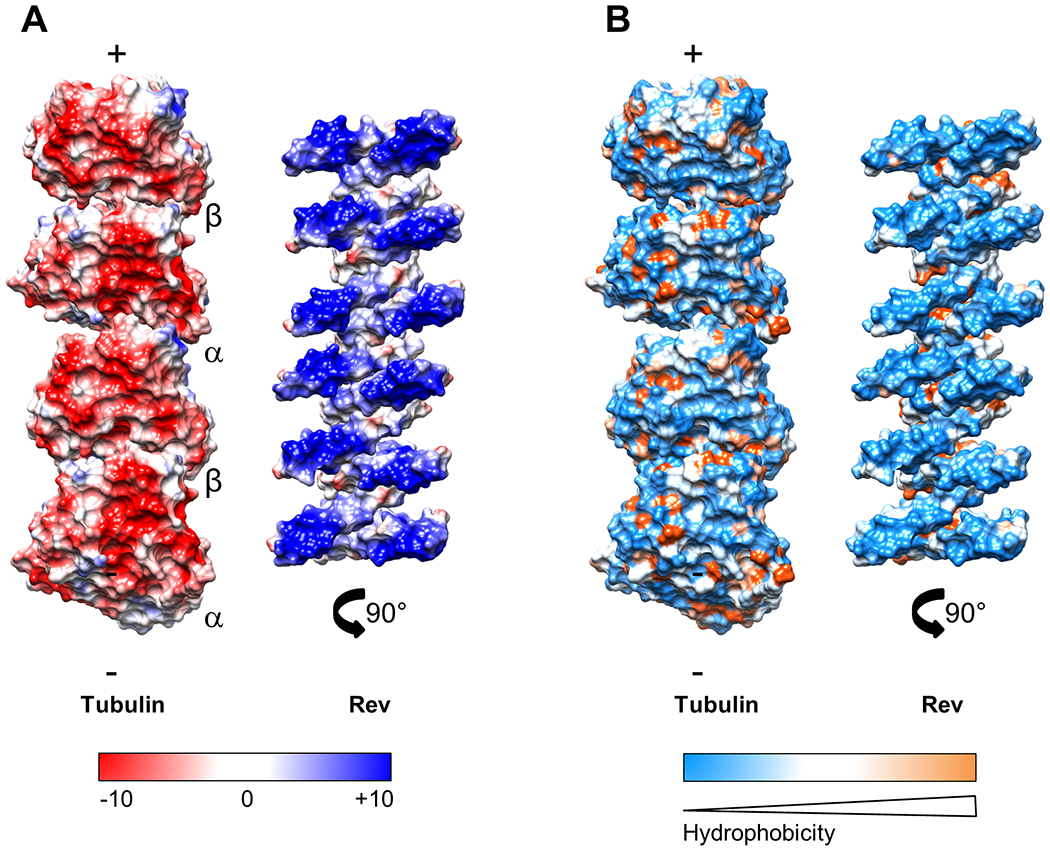
(A) Surface-charge representation of the tubulin and Rev components of the ring-complex; Red, negative; blue, positive; white neutral. The color ranges from −10 (red) to +10 (blue) kcal/(mol·e) at 298 °K. The tubulin (left), as viewed from the center of the ring. The Rev assembly (right) rotated to show the face interacting with tubulin. (B) Surface-hydrophobicity representation of the tubulin and Rev components of the ring-complex; blue, hydrophilic; orange, hydrophobic; and white neutral (Kyte-Doolittle hydropathy scale). The Rev-Rev interaction is hydrophobic, the Rev-tubulin interaction is charge-mediated.
In the ASU, four Rev monomers (E, F, G, and H) are bound to the primary β-subunit and two monomers (I and J) are bound to the primary α-subunit (Figure 1B). Together, they form three sets of contact patches (E-F, G-H, and I-J) on tubulin (Figure 1B, D and E). The E-F and I-J monomer pairs make similar contacts with the β- and α-subunits, respectively, whereas the G-H monomer pair makes a different contact near the tubulin interdimer interface. In the MBA, the O-P, E-F, and G-H (and the I-J, Q-R, and S-T) monomer pairs also make three sets of contacts on their respective heterodimers, however, the set of contacts across the intradimer interface is slightly different from that across the interdimer interface (Figure 1C, D and E). In particular, while the E-F (and Q-R) monomer pairs, situated at the intradimer interfaces of the MBA, contact both the α- and β-subunits, the G-H monomer pair, situated near the interdimer interface, contacts only the β-subunit (Figure 1D and E). The insertion of the E-F (and Q-R) H2 helices into the intradimer interfaces, and no such insertion of the G-H H2 helices into the interdimer interface (Figure 1D and E), may limit curvature at the intradimer interfaces. Together with the inherently greater ability of tubulin to undergo interdimer vs intradimer curvature, as observed in other curved tubulin structures formed with either Kinesin (e.g., PDB: 3EDL, 6BBN, and 6B0C), Stathmin (e.g., PDB: 4I4T, 5IYZ, and 5LXT), or Cryptophycin (e.g., PDB: 7LXB, 7M18, and 7M20), this may account for the 12° intradimer and 24° interdimer angles observed between subunits of the rings. Presumably lateral interactions account for the similar angles in the secondary ring 37–43.
The interactions of the individual Rev monomers with the primary tubulin ring are different in area and energy (Figure 1E, Table 1). Of the three monomer pairs in the ASU, the E-F pair has the smallest contact area (between the two monomers a total of 165 Å2) and the least predicted interaction energy (−5.9 kcal/mol), due to the minimal involvement of the F monomer. The G-H pair has a larger contact area (445 Å2) and greater predicted interaction energy (−10.2 kcal/mol). The I-J pair has the largest area (485 Å2) and a predicted interaction energy of −14.5 kcal/mol. The total predicted interaction energy is therefore approximately −30 kcal/mol for the β-α subunits in the ASU. The predicted interaction energy is similar for the heterodimers in the MBA. This, in part, explains the curvature induced in tubulin at both the interdimer and intradimer sites.
Table 1.
Rev-tubulin interactions.
| Chain | No. Residues | Chain | No. Residues | Area (Å2)1 | Energy (kcal/mol)2 |
|---|---|---|---|---|---|
| Primary interfaces 3 | |||||
| E | 5 | α-L4 | 8 | 78.0 | −3.27 |
| E | 5 | β-B | 7 | 140.6 | −3.87 |
| F | 1 | α-L | 3 | 49.3 | +0.16 |
| F | 2 | β-B | 3 | 24.5 | −2.04 |
| G | 8 | β-B | 9 | 293.8 | −7.34 |
| H | 4 | β-B | 4 | 151.2 | −2.89 |
| I | 7 | α-A | 7 | 187.3 | −6.40 |
| J | 7 | α-A | 10 | 297.6 | −8.13 |
| O | 7 | α-L | 7 | 186.9 | −6.40 |
| P | 7 | α-L | 10 | 298.3 | −8.13 |
| Q | 5 | α-A | 8 | 77.6 | −3.27 |
| Q | 5 | β-N | 7 | 141.3 | −3.87 |
| R | 1 | α-A | 3 | 50.2 | +0.16 |
| R | 2 | β-N | 3 | 24.4 | −2.04 |
| S | 8 | β-N | 9 | 293.6 | −7.34 |
| T | 4 | β-N | 4 | 152.8 | −2.89 |
| Secondary interfaces 3 | |||||
| E | 13 | β-D | 13 | 480.1 | −5.05 |
| G | 7 | α-C | 6 | 144.1 | −1.93 |
| I | 9 | α-C | 7 | 223.8 | −7.63 |
| I | 5 | β-M | 2 | 54.7 | −2.85 |
| Q | 13 | β-M | 12 | 478.9 | −5.05 |
| O | 5 | β-D | 1 | 54.1 | −2.85 |
| O | 9 | α-K | 7 | 223.9 | −7.63 |
Interface residues and areas were determined with PDBePISA.
Interaction energies were estimated with FoldX. Accuracy is limited by the resolution of the cryo-EM data (3.5 Å). With high-resolution X-ray crystallographic data, FoldX is reported to be accurate to within 0.5 kcal/mol.
Primary interfaces are defined as contacts between Rev H2 and the primary tubulin ring. Secondary interfaces are defined as associations between Rev H2 and the C-terminal tails of the α- and β-tubulin subunits in the adjacent ring, with the understanding that the latter are less are less well ordered.
Chains are α- or β-tubulin subunits with chain IDs as given in Figure 1.
The Rev ARM (H2, 34-50) binds viral RNA, more specifically the Stem IIB of RRE in the nucleus 30,32,44,45. The ARM has been suggested to interact specifically with several human proteins such as histone chaperones B23 and Nap1, and RRE can compete with these proteins for binding to Rev 46,47. A more recent study showed that the ARM also interacts with Importin-β (Impβ) in the cytoplasm 48. In the ring structure Rev dimers form a V shape with a 125° dihedral angle and the ARM is oriented towards the tubulin (Figure 1B, Figure S5). Although, both microtubule depolymerization and ring formation require Rev-Rev interactions through hydrophobic A-A and B-B surfaces, the interaction between Rev and tubulin is unusual in the sense that it is primarily electrostatic in nature and involves the interactions between the Rev ARM and charged residues (glutamic acid, aspartic acid, arginine etc.) of β- and α-tubulin (Figure S5). The presence of arginine residues (from Rev) and glutamic/aspartic residues (from tubulin) at the interaction interface suggest the formation of hydrogen-bonds (HB) and salt-bridges (SB). We analyzed these by molecular dynamics (MD) simulations with an elongated ASU (involving 4 tubulin heterodimers and 10 Rev monomers). MD simulation analysis shows that in a ring arrangement collectively hundreds of HBs and SBs can form between Rev-tubulin pairs which explains the strong interaction of the complex (Figure S6 and S7). In addition, as expected from the structure, MD confirms that the highest number HBs from between 1ry α-tubulin-Rev J and 1ry β-tubulin-Rev G pairs, while Rev E forms a substansial number of HBs with both tubulin subunits.
One of the more interesting features of the Rev-tubulin rings is the presence of the secondary ring even though its acidic α-helical regions are not in tight contact with the Rev monomers. Although tubulin CTTs have not been observed in most X-ray crystal and cryo-EM structures due to their flexible nature, here we observe some densities for both the secondary ring β- and α-tubulin CTT main chains (Figure S3E). The structure shows that the CTTs are stabilized by their interactions with Rev, and while the Rev monomers are situated on the primary tubulin subunits, they also engage in lateral interactions with the CTTs of the adjacent tubulin subunits (Figure 3). Tubulin CTTs, in general, are negatively charged and highly flexible 49. Removal of the CTTs of β- and α-tubulin by proteolytic digestion influences the activity of the molecular motors Dynein, Kinesin-1 and MCAK 49,50. The Rev-tubulin CTT interactions are particularly evident in the MBA where there are two such sets of interactions between the acidic CTTs and the basic H2 helices of the alternate Rev monomers (Figure 1F, 3 and Table 1). The total predicted interaction energy between the Rev monomers on a heterodimer in the primary ring and a heterodimer in the secondary ring is approximately −15 kcal/mol (Table 1). This, in part, explains the lateral association of the two tubulin rings. Collectively, through both primary and secondary interactions, Rev binds to tubulin with a total predicted energy of approximately −45 kcal/mol per two adjacent heterodimers. This may explain its ability to rapidly depolymerize even Taxol stabilized microtubules into paired rings even at sub-stoichiometric ratios (Figure S2D). The removal of CTTs by substisilin treatment did not prevent Rev-tubulin interactions or microtubule depolymerization (Figure 4). However, the nature of the complex seems to differ such that addition of Rev to tubulin without CTTs triggers the formation of stacks of single rings instead of double rings. This result supports our structure, which shows that the interaction of the 2ry ring with Rev is mostly mediated by Rev-CTT interactions.
Figure 3. Rev interactions with the secondary tubulin ring.
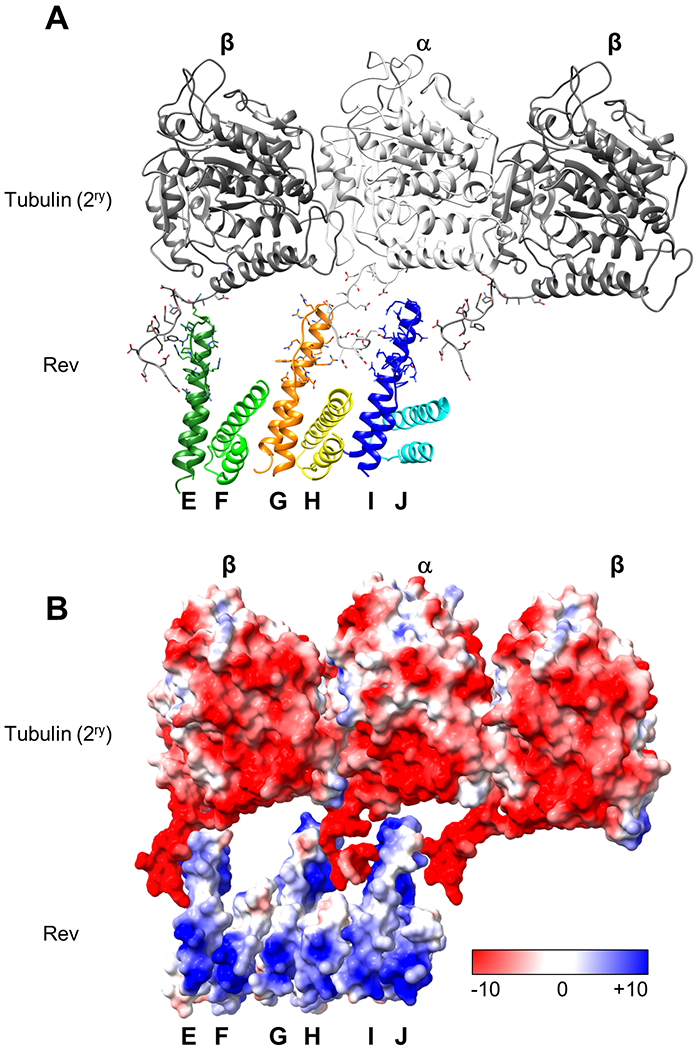
(A) Interaction of Rev monomers in the ASU with the tubulin heterodimer and the adjacent β-tubulin C-termini. β-tubulin is colored in dark gray, and α-tubulin is colored in light gray. ASU Rev monomers are shown in different colors (as coded in Figure 1). (B) Surface-charge representation of the tubulin (in 2ry ring) and Rev; Red, negative; blue, positive; white neutral. The color ranges from −10 (red) to +10 (blue) kcal/(mol·e) at 298 °K
Figure 4. Effect of CTT removal on Rev-tubulin ring formation.
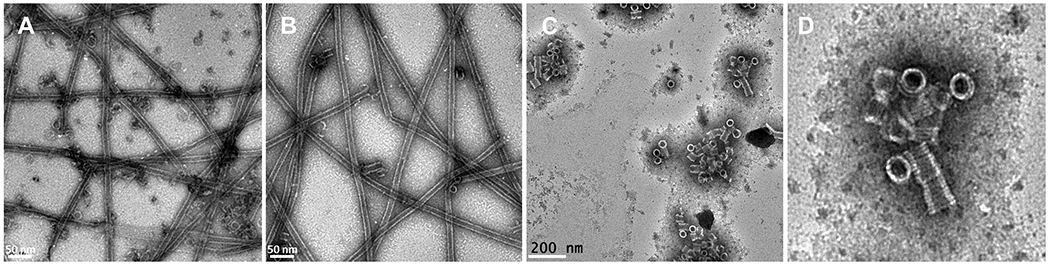
(A) Representative negative stain image of Taxol-tubulin + Rev (B) Subtilisin treated Taxol-tubulin + Rev (C) Subtilisin treated tubulin + Rev. (D) Close-up view of stacked single rings (from panel C) formed by Subtilisin treated tubulin + Rev.
Comparison of Rev and Kinesin-13 interactions with tubulin
The Kinesin-13 (Kin-13) family is a subfamily of kinesin motor proteins which transport cargo around the cell along the MTs with the help of ATP-hydrolysis 51. Unlike motile kinesins which couple ATP hydrolysis to unidirectional movement along the MT tracks, Kin-13s couple ATP hydrolysis to tubulin depolymerization at both MT ends and control MT dynamics 37,52. The neck region and the motor domain of Kin-13s are important for MT depolymerization 37,41,53,54.
Rev behaves in a manner similar to Kin-13s and can depolymerize MTs from both ends. Both Kin-13s and Rev form tubulin-protein ring complexes, however, whereas Kin-13 induces the formation of single-rings Rev induces double-rings 37,54,55. Also, while the Kin-13 Kif2A induces three points of curvature α1β1 (15.3°), β1α2 (15.8°), and α2β2 (12.1°) 42 , as indicated above, Rev induces two points of curvature (12° intradimer and 24° interdimer). Rev and Kin-13s share little sequence identity except some local similarity between Rev H2 and Kin13 α-H4 22 but their binding to tubulin has some structural similarities. A comparison between the recent structure of KLP10A (a Kin-13 from Drosophila melanogaster, PDB: 6B0C) and Rev in complex with tubulin shows that both the KLP10A-tubulin binding interface and the Rev-tubulin binding interface have been divided into three major regions: Area I, consisting of interactions with β-tubulin; Area II, consisting of interactions with α- and β-tubulin at the intradimer interface; and Area III, consisting of interactions with α- and β-tubulin at the interdimer interface 37 (Figure 5). Contacting Area III, the conserved kinesin Loop 2 (L2) KVD sequence is essential for depolymerization as mutations of these residues abolish this activity 37,56. In KLP10 structure, L2 interacts with the β-tubulin H11-H12 loop, the α-tubulin S7-H8 loop, and α-tubulin H12. In particular, conserved residues K317, V318, and D319 interact with α-E434, α-D431, α-V435 and β-K392. Targeting the same area, the Rev monomer J interacts predominantly with α-tubulin H12 glutamic and aspartic acid residues including α-E434 and α-D431. Rev-J R35 forms both hydrogen bonds and salt bridges with α-E434 and Rev-J R39 forms a salt bridge with α-D431 (Figure 5). As indicated above, the J-monomer has the largest area and predicted energy of interaction with the tubulin primary ring of any single monomer in the ASU, suggesting that it is a key contact. Collectively over its three sites, KLP10A has a predicted interaction energy with a tubulin heterodimer of approximately −17 kcal/mol compared to approximately −30 kcal/mol for Rev (i.e., not considering the additional approximately −15 kcal/mol from lateral interactions in which kinesin does not engage).
Figure 5. Comparison of Rev and Kinesin-13 interactions with tubulin.

(A) The MBA, as viewed from the side of the primary ring; α-tubulin, light gray; β-tubulin, dark gray. Rev monomers are colored as in Figure 1. Three areas of contact with the heterodimer (I, II, and III) are indicated. (B) Kin-13 KLP10A (PDB: 6B0C) bound to tubulin as shown from the same point of view as in (A). KLP10A is shown in magenta and the three contact areas are indicated. (C) KLP10A-tubulin aligned to Rev-tubulin. Tubulin contacts made by 6 Rev monomers are similar to contacts made by a KLP10A monomer. (D) Close-up view of Area III. KLP10A L2 contacts on α-tubulin are shown in orange and on β-tubulin are shown in brown. Conserved L2 loops KVD are shown. Rev contacts on α-tubulin are shown in green. Residues that interact both with KLP10A and Rev are shown in blue.
Structural relationship between rings and microtubules
In the KLP10A-tubulin complex structure, KLP10A and tubulin form a spiral around a 15-protofilament microtubule. The conformations of the two α-subunits in the Rev-tubulin ASU are similar to one another (RMSD = 1.3 Å), as are the conformations of the two β-subunits (RMSD = 1.4 Å). They are more like their counterparts in the outer tubulin spiral (average RMSD = 2.2 Å) than to those in the inner microtubule (average RMSD = 2.6 Å) in PDB: 6B0C, reflecting the highly similar curvature of the Rev-tubulin ring and the KLP10A-tubulin spiral (both have 15 heterodimers in one turn and an outer diameter of ca. 470 Å).
Comparison of the tubulin dimers in the Rev-tubulin complex with those in the inner microtubule of the KLP10A-tubulin complex reveals several differences in their arrangements. Alignment of the Rev-tubulin ring to the microtubule at the primary β-subunit—to model an interaction at the microtubule plus-end—shows that the ring is oriented at an angle of 78.4° (CCW) relative to the microtubule surface, as viewed towards the plus-end. Similarly, alignment of the ring at the primary α-subunit—to model an interaction at the microtubule minus-end—shows that the ring here is oriented at an angle of 71.8° (CCW) relative to the microtubule surface (Figure 6A). In both cases, the succeeding and preceding primary heterodimers, respectively, are curved out of the microtubule lattice and away from the adjacent protofilament to the right, while the corresponding secondary heterodimers clash with the adjacent protofilament to the left (Figure 6B and C). Inspection of the interdimer region reveals further differences. When the four tubulin chains in the ASU are aligned to the microtubule at the primary β-subunit, the secondary β-subunit is shifted both towards the lumen and the minus-end of the microtubule by 10.5 Å (the other two subunits are shifted by smaller distances) (Figure 6E). Similarly, when aligned at the primary α-subunit the secondary α-subunit is shifted towards the lumen and the minus-end by 16.5 Å (again, the other two subunits are shifted by smaller distances) (Figure 6D). These shifts are due to both the reversal of curvature and the perpendicular orientation between the microtubule and the ring, and to the shift from a helical arrangement (involving a rise) in the former to a circular and in-register one in the latter. Together, these observations suggest that formation of the Rev-tubulin complex does not involve a simple peeling away of two adjacent protofilaments from the microtubule but rather involves rearrangement of tubulin heterodimers.
Figure 6. Structural relationship between rings and microtubules.
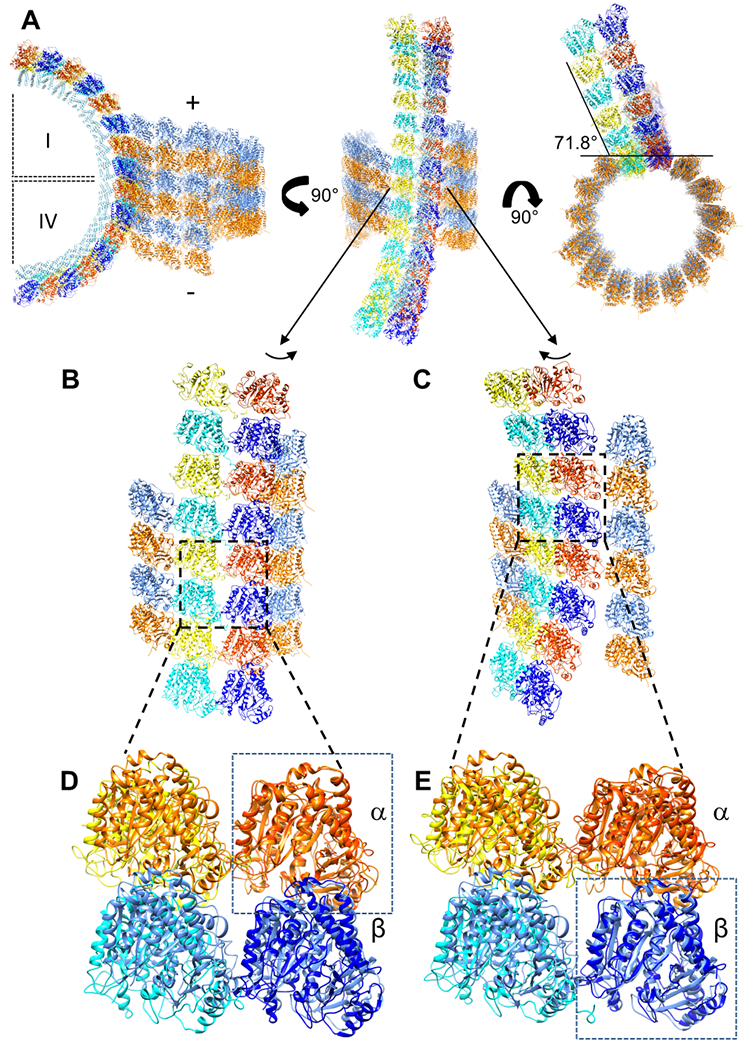
(A) The Rev-tubulin ring-complex aligned to the inner microtubule of the KLP10A-tubulin complex (PDB: 6B0C), as viewed in three orthogonal orientations. Left, the first (I) quadrant of the ring complex as positioned by alignment of a primary β-subunit with a β-subunit of the microtubule (representing a complex formed at the plus-end), and the fourth (IV) quadrant of the ring complex as positioned by alignment of a primary α-subunit with an α-subunit of the microtubule (representing a complex formed at the minus-end). Middle, the model as viewed from the center of the ring. Right, the model as viewed towards the microtubule plus-end. Microtubule α- and β-tubulin are shown in orange and blue, respectively. Primary ring α- and β-tubulin, red orange and dark blue, respectively. Secondary ring α- and β-tubulin, yellow and cyan, respectively. (B) View similar to (A, middle) but turned slightly to show clashes with the protofilament to the left. Boxed area is enlarged in (D). (C) As in (B) but turned slightly to show the subunits oriented away from the protofilament to the right. Boxed area is enlarged in (E). (D) The four tubulin subunits of the ASU, and four tubulin subunits from the microtubule lattice, with the primary α-subunit and the microtubule α-subunit aligned (boxed). (E) As in (D) but with the primary β-subunit and the microtubule β-subunit aligned (boxed).
Conformational changes in tubulin subunits lead to loosening of lateral interactions
In MTs the lateral association of protofilaments is normally mediated by the interaction of the M-loops (S7-H9 loop) of one subunit with the H1-S2 and H2-S3 loops of the adjacent subunit, for both the α- and β-subunits 57–59. Straight tubulin structures show that both the β-tubulin Y281 and α-tubulin H-283 are consistently buried at the interfaces, pointing towards the adjacent monomer, suggesting key roles for these residues in the lateral interactions 37,60.
Alignment of the α- and β-subunits of the inner straight microtubule of PDB: 6B0C with their counterparts in the current ring structure shows that the M-loops have undergone a reorientation (Figure 7A). In the primary β-subunit M-loop, the hydroxyl group of Y281 moves 14.1 Å away from the adjacent subunit and in the secondary β-subunit it moves away by 13.9 Å (Figure 7B). Loss of this interaction by mutation of β281 was shown to be sufficient to reduce dimer association dwell time and negatively affect microtubule growth 61. In the primary and secondary α-subunit M-loops, the reorientation of H283 is less pronounced (5.8 Å and 9.3 Å, respectively) (Figure 7C). The H1-S2 and H2-S3 loops do not change much relative to those in the microtubule (Figure 7A). In both cases the sidechains point away from the adjacent tubulin subunit. Together, these conformational changes in the M-loops likely contribute to the different lateral associations of the heterodimers in rings as compared to the microtubule, and to depolymerization of the latter. Further, they may explain why the Rev-tubulin complexes do not stack onto one another. Such M-loop conformational changes can be seen in other curved tubulin structures such as Stathmin-tubulin (PDB: 3RYC 62), Kin-13-tubulin (PDB: 6BBN 42), and Cryptophycin-tubulin (PDB: 7LXB 38) complexes where the changes in β-tubulin are even more pronounced than in the case of Rev-tubulin complexes (Figure S8).
Figure 7. Conformational changes in tubulin subunits lead to loosening of lateral interactions.
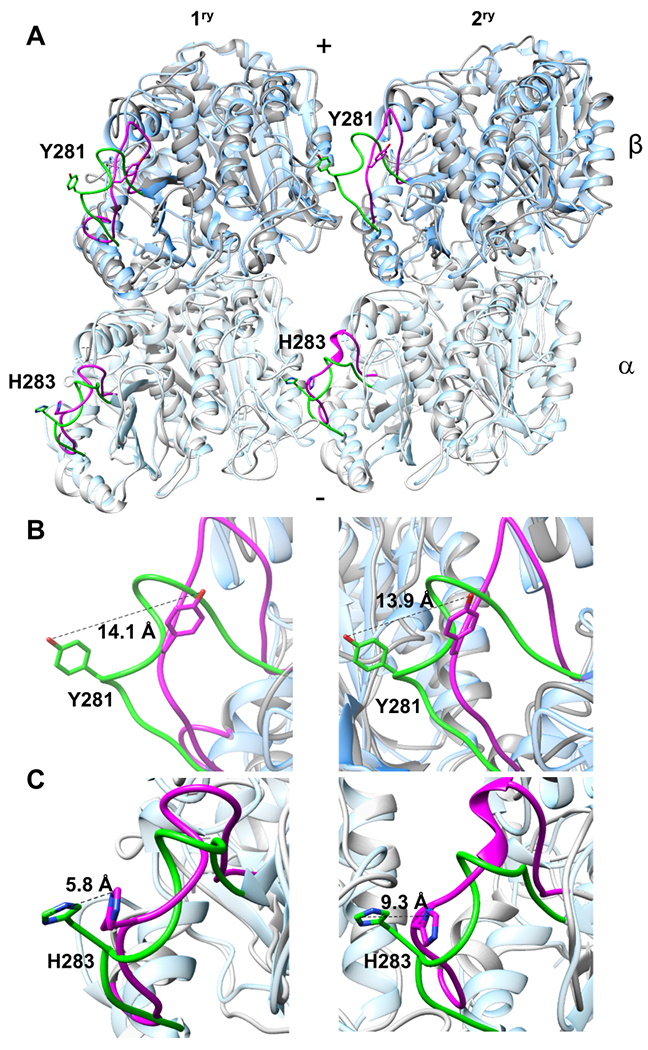
(A) Alignment of a straight microtubule (PDB: 6EVY) α- and β-tubulin heterodimers with Rev-tubulin ring complex α- and β-heterodimers for both the primary and secondary rings. The two adjacent heterodimers are viewed from the outside (the luminal side of a microtubule). M-loops of straight microtubule are shown in green and Rev-tubulin are shown in magenta. The sidechains of α-H283 and β-Y281 are shown. (B) Close-up views of Rev-primary β-tubulin M-loop, left; and Rev-secondary β-tubulin M-loop, right aligned to straight microtubule. (C) Close-up views of Rev-primary α-tubulin M-loop, left; and Rev-secondary α-tubulin M-loop, right aligned to straight microtubule. Distances between straight and curved (ring) β-Y281 and α-H283 are shown. Whereas in a microtubule, where the M-loops on both the α- and β-subunits are oriented towards the adjacent subunits, in the Rev-tubulin ring-complex they are oriented away, in both the primary and secondary rings, suggesting a weakening of the lateral interactions.
Rev depolymerizes tubulin in cells
Previously we have shown that exogenous Rev inhibits aster microtubule formation in Xenopus egg extracts, indicating that the interaction occurs in the presence of cellular constituents including RNAs and proteins 22. Here we expressed Rev within the cells to see if it retains its depolymerization activity in intact cells where cellular nucleic acids and Rev binding partners such as Crm1/RanGTP, Impβ, B23 etc. exist. For this purpose, we chose two different cell types: Astrocytes and HeLa cells. Astrocytes are specialized glial cells with distinct functional and morphological characteristics in different parts of the brain. They are involved in maintaining several complex processes needed for a healthy central nervous system. Astrocytes are susceptible to HIV-1 infection and may serve as a reservoir for the virus 63. In addition, a possible link between astrocyte infection and HIV-associated dementia has been suggested 63. Interestingly, an earlier study found abundant expression of HIV-1 Nef and Rev proteins in the astrocytes of HIV-1 patients with dementia 64.
Our results clearly show that in both types of cells Rev can localize to the cytoplasm, nucleus, and nucleolus and that there are no significant differences between the two in terms of Rev distribution (Figure 8). In both cell types Rev was able to depolymerize the MTs, indicating that Rev’s action on tubulin is not cell-type specific in vitro. Tubulin depolymerization can be observed by shortening the tubulin from the cell periphery towards the nucleus and formation of tubulin speckles. In some cases, holes in the tubulin network could be observed. Notably, some of our micrographs show the tubulin that was not readily depolymerized by Rev was acetylated tubulin. As acetylation is known to stabilize tubulin our results suggest that Rev might preferentially depolymerize the less stable unmodified tubulin (Figure 8). This is similar to what has been observed for other MT depolymerization agents such as nocodazole and colchicine where MTs higher resistance to these agents contained more acetylated α-tubulin than the rest of cellular microtubules 65. A comparison between nocadazole depolymerized tubulin and Rev depolymerized tubulin are comparable even though we observed a stronger effect with nocodazole (Figure 9). In some of the Rev transfected cells we observed that the acetylated tubulin bundled around the nucleus and in some cases accumulated at the nuclear membrane periphery.
Figure 8. Confocal microscopy images depicting tubulin depolymerization.
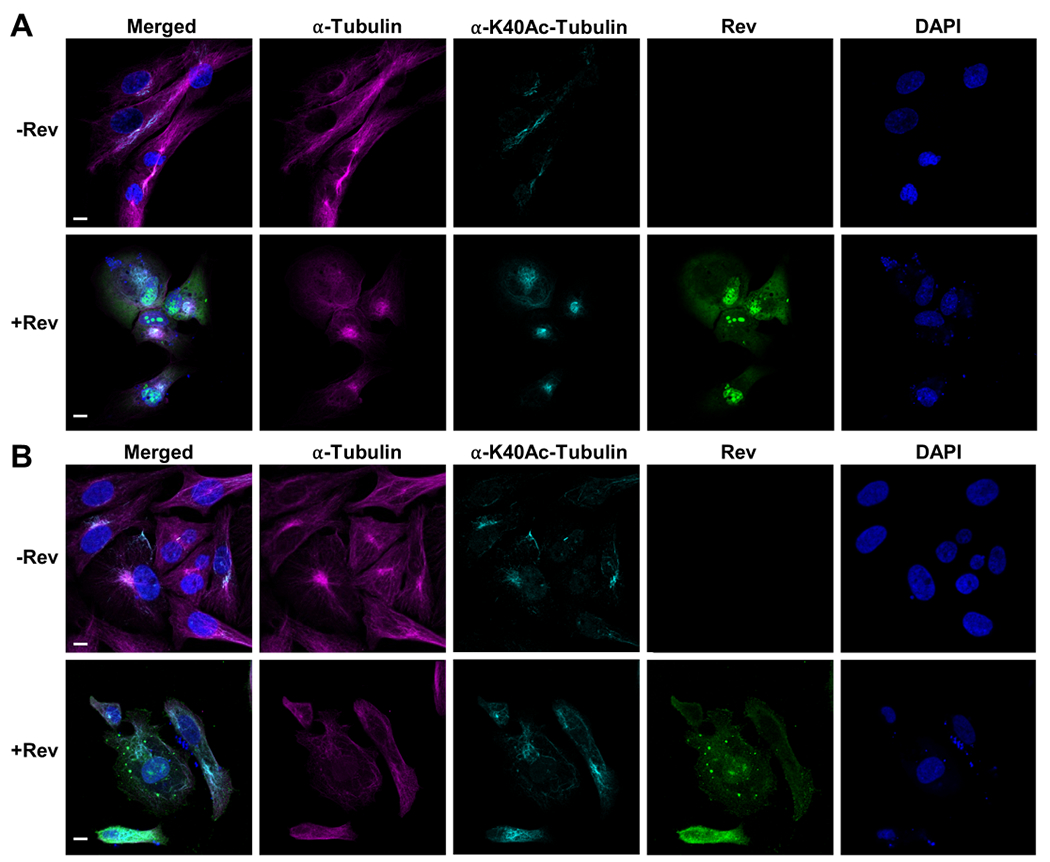
Tubulin depolymerization in astrocytes (A) and HeLa (B) cells. Top panels show control cells, bottom panels show Rev-expressing cells. From left to right: merged, α-tubulin (magenta) detected by antibodies against α-tubulin, acetylated α-tubulin (cyan) detected by antibodies against α-tubulin K40Ac antibodies, Rev (green) detected by anti-FLAG M2 antibodies and nuclei stained with DAPI. See materials and methods section for additional details. Scale bars, 10 μm.
Figure 9. Comparison of tubulin depolymerization by nocodazole and Rev.
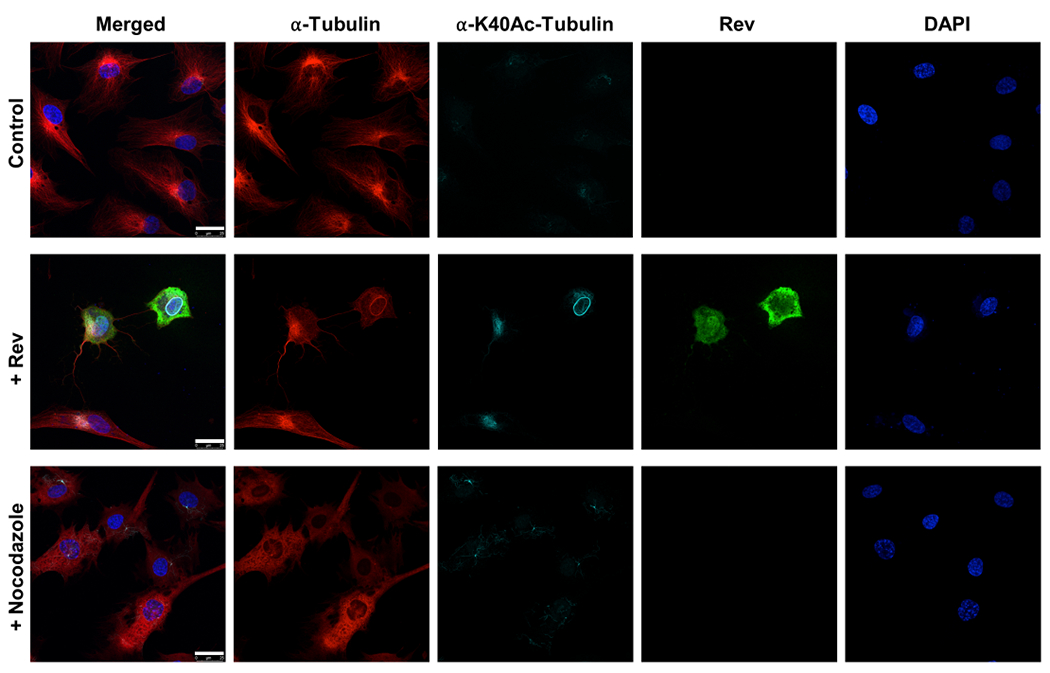
Tubulin depolymerization in astrocytes. Top panel shows control cells, middle panel shows Rev transfected cells and bottom panel shows 10 μM nocodazole treated cells. From left to right: merged, α-tubulin (magenta) detected by antibodies against α-tubulin, acetylated α-tubulin (cyan) detected by antibodies against α-tubulin K40Ac antibodies, Rev (green) detected by anti-FLAG M2 antibodies and nuclei stained with DAPI. Scale bars, 25 μm.
DISCUSSION
Viruses depend on many of the metabolic functions of their hosts to enable their own replication. HIV-1 has a small genome that codes for only 15 proteins and while the primary functions of these proteins are now largely known it has become clear that they also play multiple additional roles. As one of the key regulatory proteins of HIV-1, the Rev interactome has been studied by immuno-precipitation and mass spectrometry by several groups identifying up to 250 potential host interactors 66–68. Among these, 27 have been verified by at least two different groups, which suggests that Rev has some unexplored roles in the viral replication cycle 28. However, Rev-host protein interaction studies either by biochemical or structural means have remained very limited. Our structural studies and in vitro tubulin depolymerization experiments show a specific Rev-tubulin interaction. In vivo, this might occur in the cytoplasm at perinuclear MTOCs when Rev dissociates from the nuclear export complex. Given the fact that Rev expression does not induce apoptosis immediately, i.e. within 16h-24h post transfection, it can be assumed that Rev only regulates MT dynamics without permanently altering the organization of the MT network. One of the roles of Rev may be to control the spindle assembly during mitosis akin to the Kin13s. Supporting this theory, Rev is redistributed during mitosis in a similar way as nucleolar proteins such as B23 which becomes associated with the mitotic spindle and interacts with the minus-ends of MTs 69,70. There are other examples of viral proteins that interact and depolymerize tubulin during the viral life cycle such as rotavirus NSP2 71 and rabies virus M protein 72. NSP2 was shown to bind tubulin acidic regions through its positively charged grooves and induce a rearrangement of the MT network. On the other hand, M protein induced depolymerization of tubulin increases viral RNA synthesis.
To date all the Rev structures showed it in complex with RNA or antibodies, or only Rev in the form of tubular polymers. In all such helical lattices, the Rev dimers were arranged with the obtuse angle between the H2 helices oriented outwards and the long axis of the dimer aligned almost parallel to the long axis of the filament. The six-start helical lattice was continuously variable, giving rise to non-polar tubes with diameters varying between 114 Å and 144 Å. The arrangement of oligomerized Rev in the Rev-tubulin structure described here is different from all others reported to date. In the current structure, the Rev monomers are arranged in a single ring with the H2 helices facing outwards; in other words, in a helix with zero rise and with a much greater diameter than in the Rev tubes (~470 Å vs 130 Å). It appears that the inherently highly curved helical Rev polymer associates with a straight protofilament in microtubules (or slightly curved heterodimers in solution; soluble tubulin dimers are thought to have an angle of ~6° between the subunits) to form a ring-like assembly involving a compromise between the inherent curvature of each partner. Rev adapts to these exigencies, a planar arrangement as well as a polar partner with two different chains, by adopting different angles (ranging from 117° - 127°) between the monomers (unlike in helical assemblies where for a given lattice the angles are all the same). The adjacent tubulin ring is held through charge interactions between the basic H2 helices of alternate Rev monomers and the acidic CTT of the α- and β-tubulins intercalated between them. Further lateral association of the rings is prevented by reorientation of the M-loops of both tubulin rings. Our structure does not give clues whether Rev interacts with the primary and secondary ring sequentially or simultaneously. On the other hand, given the fact that the CTTs extend on the outside of the MTs they may act as sensors where Rev makes its first point of contact.
Rev is much smaller than, and structurally different from, the Kin13s. It only shares ~25-27% sequence identity with the structurally most similar α-helical H4 and the neck domain of Kin13s such as KLP10A, Kif2A, Kif2B, MCAK, etc. On the other hand, a complex of six Rev monomers makes contact with the primary ring of tubulin over an area, and with a predicted interaction energy, comparable to that of a single Kin13 targeting similar locations on tubulin. Also, Rev like the depolymerizing kinesins, can break down microtubules from both ends. Unlike the Kin13s, Rev has no ATPase cycle to undergo repeated engagement with tubulin but there may be no need to have evolved this function given that the interaction probably occurs late in infection. Alternatively, Rev can disengage from tubulin by binding to another cytoplasmic partner such as Impβ.
The Rev-tubulin complexes described here were obtained in the absence of other cellular components and likely represent intermediate particles that are “locked in” by in vitro conditions. A number of proteins have been observed to assemble as spirals (KLP10A), bracelets (Kin-13), sleeves (hematoma upregulated protein, HURP), and rings (Dam1) around microtubules73. Kin-13 (in humans there are four types) localizes to different places in the cell including the spindle poles, and centromeres and kinetochores, where it regulates spindle assembly, dynamics, chromosome attachment, and segregation 51. It is also interesting to note that the Rev-tubulin rings described here have the same diameter (ca. 470 Å) as the Kin-13-tubulin spirals assembled around a 15-pf microtubule (PDB: 6B0C). Rev-tubulin rings modeled onto such microtubules in an analogous manner fit essentially perfectly (Figure S9). It is unclear how the Rev CTDs in such a ring, not resolved in our or any other structure so far, might interact with a microtubule. It may be that they interact with MAPs such as the kinesin-like KID, which is highly homologous to Rev/Rex effector binding protein (REBP). It has been shown that Rev binds to REBP via its CTD 74, suggesting that, plausibly, a Rev-tubulin ring may assemble on a microtubule under the influence of KID or other MAP. REBP also binds to HTLV-1 Rex and to Equine infectious anemia (EIAV) Rev, suggesting this may be a common mechanism.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Paul T. Wingfield (wingfiep@mail.nih.gov).
Materials availability
This study did not generate new unique reagents.
Data and code avaibility
All data generated or analyzed during this study are included in the manuscript and supporting files. Rev-tubulin map and model have been deposited in the Electron Microscopy Data Bank, https://www.ebi.ac.uk/pdbe/emdb/ (accession number: EMD-26257), and in the Protein Data Bank, https://www.ebi.ac.uk/pdbe/ (PDB ID: 7U0F).
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
For protein expression in bacteria E. coli BL21(DE3) cells were used. The bacteria were transformed with pET11a-Rev-1-116 plasmid containing the full length HIV-1 Rev gene. Cells were initially grown in Luria Broth (LB) medium containing 0.1 mg/ml ampicillin at 37 °C and induced with 1 mM IPTG.
For protein expression in mammalian cells human astrocytes and HeLa cells were used. The cells were transfected with Rev- pcDNA3.1+/C-DYK plasmid containing the full length HIV-1 Rev gene with a C-terminal FLAG tag. Expression and/or purification were carried out as described in method details.
METHOD DETAILS
Expression and purification of Rev
Rev was purified as previously described 36. Briefly, untagged Rev subcloned into expression plasmid pET11a-Rev-1-116 was expressed in E. coli BL21(DE3) cells (New England Biolabs) in Luria broth supplemented with 0.1 mg/ml ampicillin in a 2 L fermenter (Sartorius Stedim) operated at 37°C. Cells were induced for 2 hours by the addition of IPTG to a final concentration of 1 mM. The cells were collected by centrifugation at 5000 rpm for 20 min at 4 °C using a Beckman Coulter Avanti J30I refrigerated centrifuge (Beckman Coulter). The cells were suspended in 50 mM Tris, pH 8.0, 0.1 M NaCl, 1 mM EDTA and 2 M urea supplemented with cOmplete EDTA free protease inhibitor cocktail (Roche) then lysed by two passes through a French Press at 12,000 psi with one minute of sonication after each pass to reduce viscosity. The material was clarified by centrifugation in a JA-14 rotor at 13,000 rpm for two hours at 4 °C and then fractionated by DEAE Sepharose anion exchange resin (Cytiva). Flow-through fractions were pooled, subjected to a 40% ammonium sulfate cut, and gel filtered on Superdex 75 (GE Healthcare) equilibrated with 50 mM Tris, pH 8.0, 0.1 M NaCl, 1 mM EDTA and 8 M urea. Just before the experiments Rev was refolded by dialysis against 50 mM NaH2PO4, 500 mM (NH4)2SO4, 150 mM NaCl, 50 mM Na-citrate, pH 7.0 and then dialyzed against 20 mM HEPES, 100 mM NaCl, 50 mM Na-citrate, pH 7.0.
Preparation of taxol stabilized microtubules
Porcine brain tubulin was purchased from Cytoskeleton (cat # T238P-A). Lyophilized tubulin was resuspended in 80 mM PIPES pH 7.0, 2 mM MgCl2, 0.5 mM EGTA, 5% glycerol and 1 mM GTP to a concentration of 5 mg/ml. In order to obtain stabilized tubulin filaments taxol was added to a final concentration of 10 μM. The tubulin-taxol mix was incubated at 37°C for 45 min for tubulin polymerization.
Preparation of Rev-tubulin rings for cryo-EM
Refolded Rev (as described above) was added to resuspended tubulin to a molar stoichiometric ratio of 4:1 and the final sample was diluted to 0.5 mg/ml in 80 mM PIPES pH 7.0, 0.5 mM EGTA, 1 mM GTP and 2 mM MgCl2. The protein mix was then incubated at room temperature overnight to ensure the highest amount of ring formation.
Subtilisin treatment of tubulin and taxol stabilized microtubules
Subtilisin treatment of tubulin and microtubules were carried out as described before 87. Briefly, a fresh solution of subtisilin was prepared in 10 mM HEPES, pH 7.5, 50 mM NaCl to 10 mg/ml. Then subtilisin was added to 5 mg/ml tubulin or taxol stabilized microtubules to a final concentration of 250 μg/ml and incubated for 60 min at 30°C. Tubulin digestion was stopped by addition of 1 mM PMSF.
Negative staining
400 mesh copper grids with carbon film (Electron Microscopy Sciences) were glow discharged for 45 s using a home-made instrument. 3 μl of 0.5 mg/ml samples were directly pipetted on the grids. After 60 s the sample was dried briefly by blotting the grid on a Whatman #1 filter paper (Whatman). The grids were washed three times by swiping the grids briefly through the surfaces of 100 μl cell culture grade water drops pipetted onto glass slides and blotting them onto the filter paper after each swipe. Then 3 μl of 1% uranyl acetate was pipetted directly to the grids and the grids were incubated for 60 s. After the incubation the grids were blotted one last time and air dried for 2-3 min. Grids were screened and imaged using a Tecnai T12 120 kV Transmission Electron Microscope (ThermoFisher).
Sample preparation and data collection
4 μl of the samples at 0.5 mg/ml were applied to Quantifoil R1.2/1.3 300 mesh Cu grids coated with 2 nm ultrathin carbon (Quantifoil) which were previously plasma-cleaned for 10 s with an argon-oxygen mixture (25% oxygen) in a model 1020 plasma cleaner (Fischione). Using a Leica EM GP plunger (Leica Microsystems), excess liquid was blotted 4 s after a 60 s wait time (95% humidity, 20°C) and grids were flash frozen in liquid ethane.
Data collection was performed with a Titan Krios G3 cryo-electron microscope (ThermoFisher) operated at 300 kV at the MICEF (NIH). Micrographs were recorded as dose-fractionated movies with a Gatan K2 Summit direct electron detector operated in counting mode at a nominal magnification of x130,000 (calibrated pixel size of 1.06 Å). The total dose for each exposure was 73 e−/Å2 where the total exposure time was 10 s fractionated into 40 frames with 0.25 s exposure time for each frame. The nominal defocus range used was −0.5 to −2.5 μm. 2605 movies were acquired using SerialEM 77.
Cryo-EM image processing
The movies were aligned using Bsoft 78. Contrast transfer function estimations were carried out using CTFFIND-4.1 79 in RELION 3.1 80. A total of 91719 particles were hand picked from 2605 micrographs using the manual picking feature of RELION-3.1. The selected particles were transferred to cisTEM 81 for further processing. The particles were composed of rings with 3 different sizes, 28-mer, 30-mer and 32-mer. Initially we performed a 2D classification with 100 classes to increase the sample separation. Since the ring sizes were different and some views allowed us to count the number of subunits we initially picked classes that had similar ring sizes and/or numbers of subunits. This initial 2D classification revealed that the majority of the rings were 30-mer rings. We performed another round of 2D classification with 70 classes to filter out smaller or larger rings and once again we picked the classes based on ring size and quality of the 2D classes. We then performed one more round of 2D classification with 50 classes. With the selected particles we generated an ab initio in cisTEM in C1 (for verification of true symmetry and confirmation of the 30-mer ring majority in selected particles). Then we generated another ab initio model first in C1 and then we aligned the symmetry axes to C15 in subsequent iterations (C15 ab initio). For the refinement we used the C15 ab initio model as the reference. During refinement we also performed a 3D classification with 3 classes and we picked the class with the highest resolution, which had a final number of 34534 particles. A C15 symmetry was applied during auto refinement iterations. For final refinement a 60 Å low-pass filtered full mask of the tubulin rings and the Rev ring was created with soft edges using the RELION mask creation tool. A final round of manual refinement was carried out using the RELION generated mask. The resolution calculation was performed in cisTEM and was based on the volume-corrected Fourier Shell Correlation (part-FSC) at the 0.143 criterion. A detailed scheme of cryo-EM work flow is shown in Supplementary Figure 4. Local resolution was calculated using ResMap 82. Cryo-EM data collection, refinement, and validation statistics are given in Table S1. MolProbity was used for structure validation 86.
Cryo-EM model building and refinement
Model building into the cryo-EM maps was initiated by fitting the previously solved X-ray crystal structure of tubulin (PDB ID: 6XER, 75) and Rev (PDB ID: 5DHV, 33) into the maps using UCSF Chimera 83 and real-space refinement in Phenix 84. The resulting structure was rebuilt in Coot 85 followed by multiple cycles of real-space refinement in Phenix. The final map was sharpened using Phenix.
Expression of Rev in mammalian cells
Rev (1-116) was subcloned into the pcDNA3.1+/C-DYK plasmid (GenScript) which contains a C-terminus Flag Tag. For microscopy ~ 1 x 105 astrocytes or HeLa cells (90% confluency) were plated to μ-Slide 4 well ibiTreat slides in Opti-MEM (Gibco) without antibiotics or FBS. To each well 500 ng of plasmid DNA complexed with 1 μl Lipofectamine 3000 reagent (Invitrogen) in 50 μl Opti-MEM was added and the slides were incubated for 24 h at 37°C in a Forma Series II water jacketed CO2 incubator (Thermo Fisher) before imaging.
Fluorescence microscopy imaging
Transfection media was removed from each well and the cells were washed with PBS 3 times at room temperature. The cells then were fixed with freshly prepared 2% paraformaldehyde for 15 min. The fixed cells were rinsed 3 times with PBS for 5 min. The cells were blocked for 1 h in 5% BSA and 0.2% Triton X-100 in PBS. After blocking, the cells were incubated with the following antibodies overnight at 4°C: Alexa Fluor 555 anti-α-tubulin antibody (Abcam cat # ab275113), Alexa Fluor 647-conjugated anti-acetylated α-tubulin antibody (K40Ac; Abcam cat # ab218591) and a mouse anti-FLAG M2 (Sigma, cat # F1804-50UG). Next day, the cells were rinsed 3 times with PBS for 5 min. The cells were then incubated with goat anti-mouse IgG (H+L) Alexa Flour Plus 488 secondary antibody (Invitrogen cat # A32723) for 1 h at room temperature. Finally, cells were rinsed 3 times with PBS for 5 min. DAPI was added to a final concentration of 5 μg/ml in the second rinse for nuclear staining. After the final rinse the cells were imaged with fluorescence microscopy. Imaging was performed using either a Zeiss LSM780 confocal system driven by the Zeiss ZEN Black software or a Leica TCS SP8 X confocal system driven by the Leica LAS X software. Micrographs acquired on the Zeiss system were obtained employing a Plan Apochromatic 63X/1.4NA oil immersion lens (Zeiss) with pinhole size set to 1.0 Airy Units (AU). Fluorescence emission was captured sequencially to avoid crosstalk between channels using a diode 405 nm laser to excite the DAPI, a 488 nm Argon ion laser to excite the Alexa Fluor Plus 488 secondary antibody, a 561 nm Helium-Neon ion laser to excite the Alexa Fluor 555-conjugated anti-α-tubulin antibody and a 633 nm Helium-Neon ion laser to excite the Alexa Fluor 647-conjugated anti-acetylated α-tubulin. Images acquired on the Leica system were taken with a HC Plan Apochromatic CS2 63X/1.4NA oil immersion lens (Leica) with pinhole set to 1 AU. Fluorescence emission was captured sequencially to avoid crosstalk between channels using a solid state 405 nm laser to excite the DAPI and employing a White Light Laser (Leica) with excitation wavelengths set at: 488 nm to excite the Alexa Fluor Plus 488 secondary antibody, 555 nm to excite the Alexa Fluor 555-conjugated anti-α-tubulin antibody and at 647 nm to excite the Alexa Fluor 647-conjugated anti-acetylated α-tubulin. Micrographs (1,024 pixels wide) were exported as .czi or .lif files, converted into .Ims files and then processed using Imaris 9.8 image analysis software (Oxford Instruments) to linearly adjust intensity levels for each individual channel, when needed.
Molecular Dynamics Simulations
The initial model was obtained from the solved cryo-EM structure and consisted of 4 tubulin heterodimers and 10 Rev monomers. MD simulations were carried out using the Desmond-Maestro simulation package (Schrödinger Release 2021-4). The model was prepared by Protein Preparation Wizard in Maestro Schrödinger and the optimized potentials for liquid simulation force field (OPLS_2005) parameters were used in restraint minimization and system building 88. The pre-defined TIP3P 89 water model was used to build the system, which could act as water molecules and these are constructed in the orthorhombic periodic boundary conditions at the distances of 20 Å units. The charge of the complex was electrically neutralized with counter Na+/Cl− ions and the salt concentration was adjusted to 0.15 M NaCl. The complex was subjected to the minimization protocol based on the steepest descend method, then heated at 0-300 K with the annealing steps of 2000 and the time steps of 0.001 ps. The system normalized in an equilibrium state at 1000 steps with the time step of 0.001 ps. The NPT ensemble with 300 °K, and a pressure of 1 bar was applied in the run using the Nose-Hoover method 90. The simulation was performed three times for 100 ns, the energy recording was done at 1.2 ns and the trajectory sampling was done at an interval of 40 ps. The short-range coulombic interactions were analyzed using a cutoff value of 9.0 Å using the short-range method. The smooth particle mesh Ewald method was used for handling long-range coulombic interactions. The stability of MD simulation was monitored by RMSD of the protein atom positions in time. Hydrogen-bond and salt-bridge formation was analyzed using Visual Molecular Dynamics (VMD) 76.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical data in Table S1 calculated for the cryo-EM structures were obtained from the outputs of Phenix, MolProbity and the wwwPDB oneDep validation server (https://validate-rcsb-2.wwpdb.org).
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rev-Fab | Paul T. Wingfield 33 | Rev-Fab |
| Alexa Fluor 555 anti-α-tubulin antibody | Abcam | Cat# ab275113 |
| Alexa Fluor 647-conjugated anti-acetylated α-tubulin antibody | Abcam | Cat# ab218591 |
| Mouse anti-FLAG M2 | Sigma-Aldrich | Cat# F1804-50UG |
| Goat anti-mouse IgG (H+L) Alexa Flour Plus 488 secondary antibody | Invitrogen | Cat# A32723 |
| Bacterial and virus strains | ||
| E. coli BL21 (DE3) | New England Biolabs | Cat# C2527H |
| Biological samples | ||
| Chemicals, peptides, and recombinant proteins | ||
| Porcine brain tubulin | Cytoskeleton | Cat# T238P-A |
| Rev (1-116) | Paul T. Wingfield 31 | Rev (1-116) |
| cOmplete EDTA free protease inhibitor cocktail | Roche | Cat# 11873580001 |
| Nocodazole | Sigma-Aldrich | Cat# M1404-2MG |
| Taxol (paclitaxel) | Invitrogen | Cat# P3456 |
| DAPI staining solution | Abcam | Cat# ab228549 |
| Lipofectamine 3000 | Invitrogen | Cat# L3000015 |
| Opti-MEM | Gibco | Cat# 31985070 |
| Subtilisin | Hampton Research Proti-Ace kit | Cat# HR2-429 |
| GTP | Millipore Sigma | Cat# 10106399001 |
| Critical commercial assays | ||
| Deposited data | ||
| Rev-tubulin complex structure | This study | PDB: 7U0F |
| Rev-tubulin complex structure | This study | EMDB: EMD-26257 |
| Tubulin structure | Chen et al. 75 | PDB: 6XER |
| Rev structure | DiMattia et al. 33 | PDB: 5DHV |
| Kinesin structure | Tan et al. 41 Trofimova et al. 42 Benoit et al. 37 |
PDB: 3EDL, 6BBN, and 6B0C |
| Tubulin-stathmin structure | Prota et al. 39,40 Waight et al. 43 Nawrotek et al. 62 |
PDB: 4I4T, 5IYZ, 5LXT, 3RYC |
| Straight tubulin structure | Manka et al. 60 | PDB: 6EVY |
| Tubulin-cryptophycin | Eren et al. 38 | PDB: 7LXB, 7M18, and 7M20 |
| Experimental models: Cell lines | ||
| Human astrocytes | ScienCell | Cat# 1800 |
| Human HeLa cells | Millipore Sigma | Cat# 93021013-1VL |
| Experimental models: Organisms/strains | ||
| Oligonucleotides | ||
| Recombinant DNA | ||
| pET11a-Rev-1-116 | Paul T. Wingfield (Genscript) | plasmid # 1978 |
| Rev- pcDNA3.1+/C-DYK | Paul T. Wingfield (Genscript) | plasmid # U156AGE070 |
| Software and algorithms | ||
| Desmond-Maestro | Schrödinger, Inc. | https://www.schrodinger.com/products/desmond |
| Visual Molecular Dynamics (VMD) | Humphrey et al. 76 | https://www.ks.uiuc.edu/Research/vmd |
| SerialEM | Mastronarde et al. 77 | https://bio3d.colorado.edu/SerialEM/download.html |
| Bsoft | Heymann 78 | https://sbgrid.org/software/titles/bsoft |
| CTFFIND-4.1 | Rohou et al. 79 | https://grigoriefflab.umassmed.edu/ctffind4 |
| RELION 3.1 | Scheres 80 | https://www3.mrc-lmb.cam.ac.uk/relion/index.php/Main_Page |
| cisTEM | Grant et al. 81 | https://cistem.org |
| ResMap | Kucukelbir et al. 82 | https://sbgrid.org/software/titles/resmap |
| Chimera | Pettersen et al. 83 | https://www.cgl.ucsf.edu/chimera/ |
| Phenix | Adams et al. 84 | https://phenix-online.org |
| Coot | Emsley et al. 85 | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| MolProbity | Williams et al. 86 | http://molprobity.biochem.duke.edu |
| Zeiss ZEN Black | Zeiss | https://www.zeiss.com/microscopy/en/products/software/zeisszen.html |
| Leica LAS X | Leica | https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/ |
| Imaris 9.8 image analysis | Oxford Instruments | https://imaris.oxinst.com/versions/9-8 |
| Other | ||
| Tecnai T12 120 kV Transmission Electron Microscope | ThermoFisher | NA |
| 400 mesh copper grids with carbon film | Electron Microscopy Sciences | Cat# CF400-Cu-50 |
| Quantifoil R1.2/1.3 300 mesh Cu grids coated with 2 nm ultrathin carbon | Quantifoil | Cat# Q46868 |
| 1020 plasma cleaner | Fischione | https://www.fischione.com/products/contamination-solutions/model-1020-plasma-cleaner |
| Leica EM GP plunger | Leica Microsystems | https://www.leica-microsystems.com/products/sample-preparation-for-electron-microscopy/p/leica-em-gp2/ |
| Titan Krios G3 cryo-electron microscope | ThermoFisher | NA |
| Zeiss LSM780 confocal system | Zeiss | https://www.zeiss.com/microscopy/en/products/light-microscopes/confocal-microscopes.html |
| Leica TCS SP8 X confocal system | Leica | https://www.leica-microsystems.com/products/confocal-microscopes/p/leica-tcs-sp8-x/ |
| Superdex 75 prep grade | GE Healthcare | Cat# 17-1044-01 |
| DEAE Sepharose anion exchange resin | Cytiva | Cat# 17070901 |
| Beckman Coulter Avanti J30I refrigerated centrifuge | Beckman Coulter | NA |
Highlights.
cryo-EM structure of Rev-tubulin complex shows an asymmetric ring formation
HIV-1 Rev depolymerizes microtubules from both ends in a kinesin-like manner
Rev selectively disrupts the microtubule cytoskeleton in astrocytes and Hela cells
First visualization of the α, β-tubulin C-terminal tails (CTTs)
Acknowledgments
This work utilized the NIH Multi-Institute Cryo-EM Facility (MICEF) and the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov). The authors thank Huaibin Wang for technical support on the NIH MICEF Titan Krios Electron Microscope. This research was supported by the Intramural Research Program of the NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases and and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no conflicts of interest with the contents of this article.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
REFERENCES
- 1.Goodson HV, and Jonasson EM (2018). Microtubules and Microtubule-Associated Proteins. Cold Spring Harb Perspect Biol 10. 10.1101/cshperspect.a022608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cote RH, and Borisy GG (1981). Head-to-tail polymerization of microtubules in vitro. J Mol Biol 150, 577–599. 10.1016/0022-2836(81)90382-x. [DOI] [PubMed] [Google Scholar]
- 3.Mitchison TJ (1993). Localization of an exchangeable GTP binding site at the plus end of microtubules. Science 261, 1044–1047. 10.1126/science.8102497. [DOI] [PubMed] [Google Scholar]
- 4.Kaverina I, and Straube A (2011). Regulation of cell migration by dynamic microtubules. Semin Cell Dev Biol 22, 968–974. 10.1016/j.semcdb.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li R, and Gundersen GG (2008). Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol 9, 860–873. 10.1038/nrm2522. [DOI] [PubMed] [Google Scholar]
- 6.Howard J, and Hyman AA (2009). Growth, fluctuation and switching at microtubule plus ends. Nat Rev Mol Cell Biol 10, 569–574. 10.1038/nrm2713. [DOI] [PubMed] [Google Scholar]
- 7.Mandelkow E, and Mandelkow EM (1995). Microtubules and microtubule-associated proteins. Curr Opin Cell Biol 7, 72–81. 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 8.Vale RD, and Milligan RA (2000). The way things move: looking under the hood of molecular motor proteins. Science 288, 88–95. 10.1126/science.288.5463.88. [DOI] [PubMed] [Google Scholar]
- 9.Carnes SK, and Aiken C (2019). Host proteins involve in microtuble-dependent HIV-1 intracellular transport and uncoating. Future Virology 14. 10.2217/fvl-2019-0004. [DOI] [Google Scholar]
- 10.Dharan A, and Campbell EM (2018). Role of Microtubules and Microtubule-Associated Proteins in HIV-1 Infection. J Virol 92. 10.1128/JVI.00085-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naghavi MH (2021). HIV-1 capsid exploitation of the host microtubule cytoskeleton during early infection. Retrovirology 18, 19. 10.1186/s12977-021-00563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietrantoni G, Ibarra-Karmy R, and Arriagada G (2020). Microtubule Retrograde Motors and Their Role in Retroviral Transport. Viruses 12. 10.3390/v12040483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D, Wang M, Zhou S, and Zhou Q (2002). HIV-1 Tat targets microtubules to induce apoptosis, a process promoted by the pro-apoptotic Bcl-2 relative Bim. EMBO J 21, 6801–6810. 10.1093/emboj/cdf683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Mareuil J, Carre M, Barbier P, Campbell GR, Lancelot S, Opi S, Esquieu D, Watkins JD, Prevot C, Braguer D, et al. (2005). HIV-1 Tat protein enhances microtubule polymerization. Retrovirology 2, 5. 10.1186/1742-4690-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giacca M. (2005). HIV-1 Tat, apoptosis and the mitochondria: a tubulin link? Retrovirology 2, 7. 10.1186/1742-4690-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huo L, Li D, Sun L, Liu M, Shi X, Sun X, Li J, Dong B, Dong X, and Zhou J (2011). Tat acetylation regulates its actions on microtubule dynamics and apoptosis in T lymphocytes. J Pathol 223, 28–36. 10.1002/path.2768. [DOI] [PubMed] [Google Scholar]
- 17.Butler TR, Smith KJ, Self RL, Braden BB, and Prendergast MA (2011). Neurodegenerative effects of recombinant HIV-1 Tat(1-86) are associated with inhibition of microtubule formation and oxidative stress-related reductions in microtubule-associated protein-2(a,b). Neurochem Res 36, 819–828. 10.1007/s11064-011-0409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faller EM, Sugden SM, McVey MJ, Kakal JA, and MacPherson PA (2010). Soluble HIV Tat protein removes the IL-7 receptor alpha-chain from the surface of resting CD8 T cells and targets it for degradation. J Immunol 185, 2854–2866. 10.4049/jimmunol.0902207. [DOI] [PubMed] [Google Scholar]
- 19.Ajasin D, and Eugenin EA (2020). HIV-1 Tat: Role in Bystander Toxicity. Front Cell Infect Microbiol 10, 61. 10.3389/fcimb.2020.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagashev A, and Sawaya BE (2013). Roles and functions of HIV-1 Tat protein in the CNS: an overview. Virol J 10, 358. 10.1186/1743-422X-10-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark E, Nava B, and Caputi M (2017). Tat is a multifunctional viral protein that modulates cellular gene expression and functions. Oncotarget 8, 27569–27581. 10.18632/oncotarget.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watts NR, Sackett DL, Ward RD, Miller MW, Wingfield PT, Stahl SS, and Steven AC (2000). HIV-1 rev depolymerizes microtubules to form stable bilayered rings. J Cell Biol 150, 349–360. 10.1083/jcb.150.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson PEH, Dzhivhuho G, Rekosh D, and Hammarskjold ML (2020). Sequence and Functional Variation in the HIV-1 Rev Regulatory Axis. Curr HIV Res 18, 85–98. 10.2174/1570162X18666200106112842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollard VW, and Malim MH (1998). The HIV-1 Rev protein. Annu Rev Microbiol 52, 491–532. 10.1146/annurev.micro.52.1.491. [DOI] [PubMed] [Google Scholar]
- 25.Rausch JW, and Le Grice SF (2015). HIV Rev Assembly on the Rev Response Element (RRE): A Structural Perspective. Viruses 7, 3053–3075. 10.3390/v7062760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suhasini M, and Reddy TR (2009). Cellular proteins and HIV-1 Rev function. Curr HIV Res 7, 91–100. 10.2174/157016209787048474. [DOI] [PubMed] [Google Scholar]
- 27.Vercruysse T, and Daelemans D (2013). HIV-1 Rev multimerization: mechanism and insights. Curr HIV Res 11, 623–634. 10.2174/1570162x12666140307094603. [DOI] [PubMed] [Google Scholar]
- 28.Truman CT, Jarvelin A, Davis I, and Castello A (2020). HIV Rev-isited. Open Biol 10, 200320. 10.1098/rsob.200320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daugherty MD, Liu B, and Frankel AD (2010). Structural basis for cooperative RNA binding and export complex assembly by HIV Rev. Nat Struct Mol Biol 17, 1337–1342. 10.1038/nsmb.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dearborn AD, Eren E, Watts NR, Palmer IW, Kaufman JD, Steven AC, and Wingfield PT (2018). Structure of an RNA Aptamer that Can Inhibit HIV-1 by Blocking Rev-Cognate RNA (RRE) Binding and Rev-Rev Association. Structure 26, 1187–1195 e1184. 10.1016/j.str.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiMattia MA, Watts NR, Stahl SJ, Rader C, Wingfield PT, Stuart DI, Steven AC, and Grimes JM (2010). Implications of the HIV-1 Rev dimer structure at 3.2 A resolution for multimeric binding to the Rev response element. Proc Natl Acad Sci U S A 107, 5810–5814. 10.1073/pnas.0914946107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayaraman B, Crosby DC, Homer C, Ribeiro I, Mavor D, and Frankel AD (2014). RNA-directed remodeling of the HIV-1 protein Rev orchestrates assembly of the Rev-Rev response element complex. Elife 3, e04120. 10.7554/eLife.04120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiMattia MA, Watts NR, Cheng N, Huang R, Heymann JB, Grimes JM, Wingfield PT, Stuart DI, and Steven AC (2016). The Structure of HIV-1 Rev Filaments Suggests a Bilateral Model for Rev-RRE Assembly. Structure 24, 1068–1080. 10.1016/j.str.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watts NR, Eren E, Zhuang X, Wang YX, Steven AC, and Wingfield PT (2018). A new HIV-1 Rev structure optimizes interaction with target RNA (RRE) for nuclear export. J Struct Biol 203, 102–108. 10.1016/j.jsb.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watts NR, Misra M, Wingfield PT, Stahl SJ, Cheng N, Trus BL, Steven AC, and Williams RW (1998). Three-dimensional structure of HIV-1 Rev protein filaments. J Struct Biol 121, 41–52. 10.1006/jsbi.1998.3964. [DOI] [PubMed] [Google Scholar]
- 36.Wingfield PT, Stahl SJ, Payton MA, Venkatesan S, Misra M, and Steven AC (1991). HIV-1 Rev expressed in recombinant Escherichia coli: purification, polymerization, and conformational properties. Biochemistry 30, 7527–7534. 10.1021/bi00244a023. [DOI] [PubMed] [Google Scholar]
- 37.Benoit M, Asenjo AB, and Sosa H (2018). Cryo-EM reveals the structural basis of microtubule depolymerization by kinesin-13s. Nat Commun 9, 1662. 10.1038/s41467-018-04044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eren E, Watts NR, Sackett DL, and Wingfield PT (2021). Conformational changes in tubulin upon binding cryptophycin-52 reveal its mechanism of action. J Biol Chem 297, 101138. 10.1016/j.jbc.2021.101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prota AE, Bargsten K, Redondo-Horcajo M, Smith AB 3rd, Yang CH, McDaid HM, Paterson I, Horwitz SB, Fernando Diaz J, and Steinmetz MO (2017). Structural Basis of Microtubule Stabilization by Discodermolide. Chembiochem 18, 905–909. 10.1002/cbic.201600696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prota AE, Bargsten K, Zurwerra D, Field JJ, Diaz JF, Altmann KH, and Steinmetz MO (2013). Molecular mechanism of action of microtubule-stabilizing anticancer agents. Science 339, 587–590. 10.1126/science.1230582. [DOI] [PubMed] [Google Scholar]
- 41.Tan D, Rice WJ, and Sosa H (2008). Structure of the kinesin13-microtubule ring complex. Structure 16, 1732–1739. 10.1016/j.str.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trofimova D, Paydar M, Zara A, Talje L, Kwok BH, and Allingham JS (2018). Ternary complex of Kif2A-bound tandem tubulin heterodimers represents a kinesin-13-mediated microtubule depolymerization reaction intermediate. Nat Commun 9, 2628. 10.1038/s41467-018-05025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waight AB, Bargsten K, Doronina S, Steinmetz MO, Sussman D, and Prota AE (2016). Structural Basis of Microtubule Destabilization by Potent Auristatin Anti-Mitotics. PLoS One 11, e0160890. 10.1371/journal.pone.0160890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Battiste JL, Mao H, Rao NS, Tan R, Muhandiram DR, Kay LE, Frankel AD, and Williamson JR (1996). Alpha helix-RNA major groove recognition in an HIV-1 rev peptide-RRE RNA complex. Science 273, 1547–1551. 10.1126/science.273.5281.1547. [DOI] [PubMed] [Google Scholar]
- 45.Kjems J, Calnan BJ, Frankel AD, and Sharp PA (1992). Specific binding of a basic peptide from HIV-1 Rev. EMBO J 11, 1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cochrane A, Murley LL, Gao M, Wong R, Clayton K, Brufatto N, Canadien V, Mamelak D, Chen T, Richards D, et al. (2009). Stable complex formation between HIV Rev and the nucleosome assembly protein, NAP1, affects Rev function. Virology 388, 103–111. 10.1016/j.virol.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Fankhauser C, Izaurralde E, Adachi Y, Wingfield P, and Laemmli UK (1991). Specific complex of human immunodeficiency virus type 1 rev and nucleolar B23 proteins: dissociation by the Rev response element. Mol Cell Biol 11, 2567–2575. 10.1128/mcb.11.5.2567-2575.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spittler D, Indorato RL, Boeri Erba E, Delaforge E, Signor L, Harris SJ, Garcia-Saez I, Palencia A, Gabel F, Blackledge M, et al. (2022). Binding stoichiometry and structural model of the HIV-1 Rev/importin beta complex. Life Sci Alliance 5. 10.26508/lsa.202201431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roll-Mecak A. (2015). Intrinsically disordered tubulin tails: complex tuners of microtubule functions? Semin Cell Dev Biol 37, 11–19. 10.1016/j.semcdb.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hertzer KM, and Walczak CE (2008). The C-termini of tubulin and the specific geometry of tubulin substrates influence the depolymerization activity of MCAK. Cell Cycle 7, 2727–2737. 10.4161/cc.7.17.6590. [DOI] [PubMed] [Google Scholar]
- 51.Ems-McClung SC, and Walczak CE (2010). Kinesin-13s in mitosis: Key players in the spatial and temporal organization of spindle microtubules. Semin Cell Dev Biol 21, 276–282. 10.1016/j.semcdb.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunter AW, Caplow M, Coy DL, Hancock WO, Diez S, Wordeman L, and Howard J (2003). The kinesin-related protein MCAK is a microtubule depolymerase that forms an ATP-hydrolyzing complex at microtubule ends. Mol Cell 11, 445–457. 10.1016/s1097-2765(03)00049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hertzer KM, Ems-McClung SC, Kline-Smith SL, Lipkin TG, Gilbert SP, and Walczak CE (2006). Full-length dimeric MCAK is a more efficient microtubule depolymerase than minimal domain monomeric MCAK. Mol Biol Cell 17, 700–710. 10.1091/mbc.e05-08-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moores CA, Yu M, Guo J, Beraud C, Sakowicz R, and Milligan RA (2002). A mechanism for microtubule depolymerization by KinI kinesins. Mol Cell 9, 903–909. 10.1016/s1097-2765(02)00503-8. [DOI] [PubMed] [Google Scholar]
- 55.Moores CA, Cooper J, Wagenbach M, Ovechkina Y, Wordeman L, and Milligan RA (2006). The role of the kinesin-13 neck in microtubule depolymerization. Cell Cycle 5, 1812–1815. 10.4161/cc.5.16.3134. [DOI] [PubMed] [Google Scholar]
- 56.Ogawa T, Nitta R, Okada Y, and Hirokawa N (2004). A common mechanism for microtubule destabilizers-M type kinesins stabilize curling of the protofilament using the class-specific neck and loops. Cell 116, 591–602. 10.1016/s0092-8674(04)00129-1. [DOI] [PubMed] [Google Scholar]
- 57.Keskin O, Durell SR, Bahar I, Jernigan RL, and Covell DG (2002). Relating molecular flexibility to function: a case study of tubulin. Biophys J 83, 663–680. 10.1016/S0006-3495(02)75199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lowe J, Li H, Downing KH, and Nogales E (2001). Refined structure of alpha beta-tubulin at 3.5 A resolution. J Mol Biol 313, 1045–1057. 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 59.Nogales E, Whittaker M, Milligan RA, and Downing KH (1999). High-resolution model of the microtubule. Cell 96, 79–88. 10.1016/s0092-8674(00)80961-7. [DOI] [PubMed] [Google Scholar]
- 60.Manka SW, and Moores CA (2018). The role of tubulin-tubulin lattice contacts in the mechanism of microtubule dynamic instability. Nat Struct Mol Biol 25, 607–615. 10.1038/s41594-018-0087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mickolajczyk KJ, Geyer EA, Kim T, Rice LM, and Hancock WO (2019). Direct observation of individual tubulin dimers binding to growing microtubules. Proc Natl Acad Sci U S A 116, 7314–7322. 10.1073/pnas.1815823116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nawrotek A, Knossow M, and Gigant B (2011). The determinants that govern microtubule assembly from the atomic structure of GTP-tubulin. J Mol Biol 412, 35–42. 10.1016/j.jmb.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 63.Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Vesselingh SL, and Purcell DF (2003). Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res 1, 463–473. 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- 64.Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapasalo H, and Krohn K (1995). Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS 9, 1001–1008. 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Piperno G, LeDizet M, and Chang XJ (1987). Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol 104, 289–302. 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arizala JAC, Chomchan P, Li H, Moore R, Ge H, Ouellet DL, and Rossi JJ (2019). Identification of Nucleolar Factors During HIV-1 Replication Through Rev Immunoprecipitation and Mass Spectrometry. J Vis Exp. 10.3791/59329. [DOI] [PubMed] [Google Scholar]
- 67.Jager S, Cimermancic P, Gulbahce N, Johnson JR, McGovern KE, Clarke SC, Shales M, Mercenne G, Pache L, Li K, et al. (2011). Global landscape of HIV-human protein complexes. Nature 481, 365–370. 10.1038/nature10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naji S, Ambrus G, Cimermancic P, Reyes JR, Johnson JR, Filbrandt R, Huber MD, Vesely P, Krogan NJ, Yates JR 3rd, et al. (2012). Host cell interactome of HIV-1 Rev includes RNA helicases involved in multiple facets of virus production. Mol Cell Proteomics 11, M111 015313. 10.1074/mcp.M111.015313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dundr M, Leno GH, Lewis N, Rekosh D, Hammarskjoid ML, and Olson MO (1996). Location of the HIV-1 Rev protein during mitosis: inactivation of the nuclear export signal alters the pathway for postmitotic reentry into nucleoli. J Cell Sci 109 (Pt 9), 2239–2251. [DOI] [PubMed] [Google Scholar]
- 70.Zatsepina OV, Rousselet A, Chan PK, Olson MO, Jordan EG, and Bornens M (1999). The nucleolar phosphoprotein B23 redistributes in part to the spindle poles during mitosis. J Cell Sci 112 (Pt 4), 455–466. [DOI] [PubMed] [Google Scholar]
- 71.Martin D, Duarte M, Lepault J, and Poncet D (2010). Sequestration of free tubulin molecules by the viral protein NSP2 induces microtubule depolymerization during rotavirus infection. J Virol 84, 2522–2532. 10.1128/JVI.01883-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zan J, Liu S, Sun DN, Mo KK, Yan Y, Liu J, Hu BL, Gu JY, Liao M, and Zhou JY (2017). Rabies Virus Infection Induces Microtubule Depolymerization to Facilitate Viral RNA Synthesis by Upregulating HDAC6. Front Cell Infect Microbiol 7, 146. 10.3389/fcimb.2017.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davis TN, and Wordeman L (2007). Rings, bracelets, sleeves, and chevrons: new structures of kinetochore proteins. Trends Cell Biol 17, 377–382. 10.1016/j.tcb.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Venkatesh LK, Gettemeier T, and Chinnadurai G (2003). A nuclear kinesin-like protein interacts with and stimulates the activity of the leucine-rich nuclear export signal of the human immunodeficiency virus type 1 rev protein. J Virol 77, 7236–7243. 10.1128/jvi.77.13.7236-7243.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen H, Deng S, Albadari N, Yun MK, Zhang S, Li Y, Ma D, Parke DN, Yang L, Seagroves TN, et al. (2021). Design, Synthesis, and Biological Evaluation of Stable Colchicine-Binding Site Tubulin Inhibitors 6-Aryl-2-benzoyl-pyridines as Potential Anticancer Agents. J Med Chem 64, 12049–12074. 10.1021/acs.jmedchem.1c00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Humphrey W, Dalke A, and Schulten K (1996). VMD: visual molecular dynamics. J Mol Graph 14, 33–38, 27-38. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 77.Mastronarde DN (2005). Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol 152, 36–51. 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 78.Heymann JB (2001). Bsoft: image and molecular processing in electron microscopy. J Struct Biol 133, 156–169. 10.1006/jsbi.2001.4339. [DOI] [PubMed] [Google Scholar]
- 79.Rohou A, and Grigorieff N (2015). CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J Struct Biol 192, 216–221. 10.1016/j.jsb.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scheres SH (2012). RELION: implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol 180, 519–530. 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grant T, Rohou A, and Grigorieff N (2018). cisTEM, user-friendly software for single-particle image processing. Elife 7. 10.7554/eLife.35383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kucukelbir A, Sigworth FJ, and Tagare HD (2014). Quantifying the local resolution of cryo-EM density maps. Nat Methods 11, 63–65. 10.1038/nmeth.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, and Ferrin TE (2004). UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 84.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221. 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Emsley P, Lohkamp B, Scott WG, and Cowtan K (2010). Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66, 486–501. 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williams CJ, Headd JJ, Moriarty NW, Prisant MG, Videau LL, Deis LN, Verma V, Keedy DA, Hintze BJ, Chen VB, et al. (2018). MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci 27, 293–315. 10.1002/pro.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saoudi Y, Paintrand I, Multigner L, and Job D (1995). Stabilization and bundling of subtilisin-treated microtubules induced by microtubule associated proteins. J Cell Sci 108 (Pt 1), 357–367. 10.1242/jcs.108.1.357. [DOI] [PubMed] [Google Scholar]
- 88.Banks JL, Beard HS, Cao Y, Cho AE, Damm W, Farid R, Felts AK, Halgren TA, Mainz DT, Maple JR, et al. (2005). Integrated Modeling Program, Applied Chemical Theory (IMPACT). J Comput Chem 26, 1752–1780. 10.1002/jcc.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jorgensen WLC,J; Madura JD; Impey RW, Klein ML (1983). Comparison of simple potential functions for simulating liquid water. J. Chem. Phys 79, 926–935. [Google Scholar]
- 90.Nosé SA (1984). A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys 81, 511–519. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


