Abstract
Introduction:
The aim of this cross-sectional study was to investigate maxillomandibular morphology in hyperdivergent and hypodivergent individuals, using 3D surface models generated by cone-beam computed tomography (CBCT).
Methods:
The sample consisted of 60 CBCTs (30 males, 30 females) patients aged 12–30 years, divided into two groups comprising hyperdivergent (≥35°) and hypodivergent (≤30°) individuals, according to the mandibular plane (MP) angle. Multiplanar reconstructions were used to mark the landmarks, and 3D surface models were created to evaluate structures of the maxillomandibular complex, including condyle, ramus, symphysis and palatal height. Intergroup comparisons were performed by independent t-test. Pearson's correlation test was used (P < .05) to evaluate the correlation of the MP angle with the angles and linear measurements of other structures.
Results:
Significant differences were found between the groups regarding condylar width, ramus height, condylar plus ramus height, mandibular length, gonial angle, palatal plane angle and palatal-mandibular angle. No differences (P > .05) were found for the condylar height, symphysis inclination angle or palatal height. Correlations (P < .05) were found between the MP angle and structures of the maxillomandibular complex.
Conclusions:
Hyperdivergent (MP ≥ 35°) and hypodivergent (MP ≤ 30°) individuals present different skeletal morphology regarding condylar width, ramus height, condylar plus ramus height, mandibular length, gonial angle, palatal plane angle and palatal-mandibular angle. There is a significant correlation between MP angle and morphological structures such as condyle, ramus, symphysis, palatal plane angle and palatal-mandibular angle.
Keywords: 3D models, computed tomography, maxillomandibular morphology, vertical pattern growth
1 ∣. INTRODUCTION
The normal growth of the facial skull complex is related to the growth of several smaller skeletal units that exert an important influence on this process.1,2 Morphological changes at specific points in structures such as the mandible, the maxilla, or the alveolar bone may lead to skeletal changes in vertical dimensions of the face, and to vertical malocclusions.3,4
Genetic studies support the multifactorial heredity of craniofacial structures. However, this characteristic can be modified by countless environmental influences such as habits, excessive muscle strength and mechanisms that control growth, especially in areas of important development, such as the condyle.5-7 These factors may introduce morphological variations that induce skeletal disharmony and/or malocclusion.8,9
Individuals with a higher mandibular plane (MP) angle are called hyperdivergent, and tend to have a short ramus, increased anterior facial height and open bite. Individuals with a smaller MP angle are called hypodivergent, and often have a long ramus, decreased anterior facial height and tendency toward deep bite.5,7,10
Hyperdivergent individuals with deep bite, and hypodivergent individuals with open bite, are observed relatively often in orthodontic clinics. Dental compensations often mask these skeletal discrepancies.6 Knowing the intrinsic morphological characteristics of individuals with vertical alterations is extremely valuable, since vertical discrepancies have a marked influence on the prognosis and planning of orthodontic treatment.11-13 Although 2D cephalometric studies have evaluated these vertical facial patterns,1-8 3D studies are needed to evaluate the maxillomandibular morphology in individuals with different vertical growth patterns.
It is important to evaluate these characteristics with new tools that can appropriately apply the new methodologies to perform these evaluations. To this extent, cone beam computed tomography (CBCT) can be used to conduct an effective evaluation of the structures of the craniofacial complex three-dimensionally. Studies have shown the effectiveness of evaluations performed by 3D surface models constructed from CBCT, thus confirming it as an innovative tool with high accuracy, which enables measurements to be conducted in different spatial planes.14-22
The aim of this study was to investigate maxillomandibular morphology in hyperdivergent and hypodivergent individuals using 3D surface models.
2 ∣. MATERIALS AND METHODS
This cross-sectional study included individuals with a mean age of 16 years (ranging from 12 to 30 years of age). The data collected were obtained from the Department of Orthodontics of the Federal University of Rio de Janeiro, Brazil, for clinical purposes. This study was approved by the University of Rio de Janeiro institutional review board (# 2227593). The sample consisted of 60 CBCTs (30 of men and 30 of women of the southwest area of Brazil). The study population age ranged from 12 to 30 years old. The CBCTs were acquired between 2014 and 2016. The group was divided into two groups, 30 with a high MP angle (≥35°) and 30 with a small MP angle (≤30°).
The MP angle was used as a reference23 to define the skeletal morphology, and to separate the groups. Sample calculation was based on a previous study,24,25 and considered a power of 80%, α of 0.05, clinical difference to be detected of 1.5 mm, and standard deviation of 2.01 mm (condyle height), thus indicating that 30 patients would be needed per group.
Bias was avoided by not considering overbite in the present study, since the objective was to identify variations of skeletal origin between the groups, regardless of dental malocclusion (deep bite or open bite). The CBCTs were selected using the following inclusion criteria: (a) high MP angle (≥35°) and small MP angle (≤30°), (b) all permanent teeth present (except the third molar), (c) no previous orthodontic treatment, (d) dental Class I or II, and (e) no evident facial asymmetry. The facial asymmetry was checked by the clinical evaluation cards, and the skeletal asymmetry, by Dolphin Imaging® software.26
The exclusion criteria were: (a) dental Class III, (b) history of trauma, (c) surgery involving the craniofacial complex, (d) temporomandibular dysfunction, (e) syndromes, (f) mandibular pathology and (g) CBCT-related artefacts or noise.
ITK-SNAP version 2.2 (www.itksnap.org) open-source software was used to convert the DICOM files into GIPL (guys image processing lab) files and to build segmentations of the grey level images. Then 3D volumetric maps (segmentations) were plotted of the cranial base, maxilla and mandible. The semi-automatic software segmentation procedures were performed using active contour methods to compute anatomical structures based on the intensity of the grey level of the images and their limits. After obtaining semi-automatic segmentation, each scanned image was adjusted by using the threshold tool, considering that ITK-SNAP allows the user to edit these contours interactively.
The purpose of segmentation in this study was to construct 3D surface models that would enable head orientation and perform quantitative measurements and 3D assessments. It is noteworthy to mention that, despite efforts to standardize the position of a patient's head during image acquisition, this orientation alone is not enough to standardize the measurements. The surface models were oriented using the tool transforms in Slicer open-source software version 4.4 (https://www.slicer.org), based on the three planes (axial, coronal and sagittal), as described in a previous study.17 A fixed 3D coordinate system was displayed by the 3D Slicer software, which served as a reference to guide all the 3D models. The 3D models were oriented using the same system to ultimately obtain a standardized head orientation.
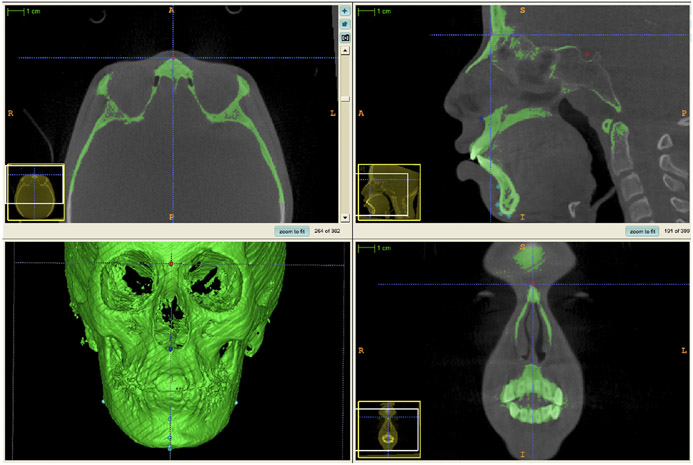
The landmarks and the morphological structures evaluated are described in Table 1. The landmarks were pre-labelled using ITK-SNAP, as initially proposed by Ruellas et al.18 All multiplanar cross-sections (sagittal, axial and coronal planes), as well as 3D image reconstruction and overall label opacity, were used to enable better identification of the landmarks (Figure 1). The three cross-sectional views (zoomed-in) display the Nasion pre-labeling (red dot that will generate a 3D surface model for measurements). For example, for the axial view, the Nasion was landmarked as the most anterior point in the center of the frontonasal suture (right–left); for the sagittal view, it was considered the most anterior point in the center of the suture (antero-posteriorly); for the coronal view, it was placed in the center of the suture (right–left and inferior-superiorly). The landmarks were marked in different colours to avoid mistakes during measurement: the landmark for the cranial base is pinpointed in red, that of the maxilla is in dark blue, and that of the mandible, in light blue (Figure 2).
TABLE 1.
Descriptive analysis and independent T test results of groups for linear measurements (mm) and difference between groups.
| Hyperdivergent |
Hypodivergent |
||
|---|---|---|---|
| Group | MP ≥ 35° | MP ≤ 30° | Difference |
| Condylar height (mm) | 6.60 (±0.84) | 6.94 (±1.18) | 0.34 (mm) |
| Ramus height (mm) | 44.35 (±4.45) | 49.88 (±5.58) | 5.53 (mm) * |
| Condylar plus ramus height (mm) | 50.98 (±4.85) | 56.77 (±5.97) | 5.79 (mm) * |
| Condylar width (mm) | 15.65 (±1.68) | 17.45 (±3.50) | 1.80 (mm) * |
| Symphysis width (mm) | 16.07 (±2.15) | 16.75 (±1.53) | 0.68 (mm) |
| Mandibular length (Go-Me) (mm) | 57.94 (±7.79) | 66.73 (±5.16) | 8.79 (mm) * |
| Palatal height (mm) | 40.23 (±3.45) | 41.22 (±2.76) | 0.99 (mm) |
| Mandibular plane angle (°) | 36.37 (±3.09) | 26.46 (±3.36) | 9.91 (°)* |
| Gonial angle (°) | 123.45 (±3.96) | 118.66 (±5.10) | 4.79 (°)* |
| Symphysis inclination angle (°) | 80.06 (±5.94) | 81.39 (±5.49) | 1.33 (°) |
| Palatal plane angle (°) | 8.70 (±3.26) | 6.68 (±3.03) | 2.02 (°)* |
| Palatal-mandibular angle (°) | 32.16 (±4.42) | 23.84 (±3.87) | 8.32 (°)* |
Note. Standard deviation values (±SD).
P < .05% means a significant statistical difference.
FIGURE 1.
ITK-SNAP Software screen showing multiplanar cross-sections (axial, sagittal and coronal) and the 3D model. The three cross-sectional views and the 3D model were used as references to place the landmark.
FIGURE 2.
3D surface model using ITK-SNAP. The landmarks were marked in different colours: light blue for the mandible ((A)—lateral view and (B)—posterior view), dark blue for the maxilla, and red for the cranial base, to avoid making mistakes during measurement (C).
The linear and angular variables analysed are described below:
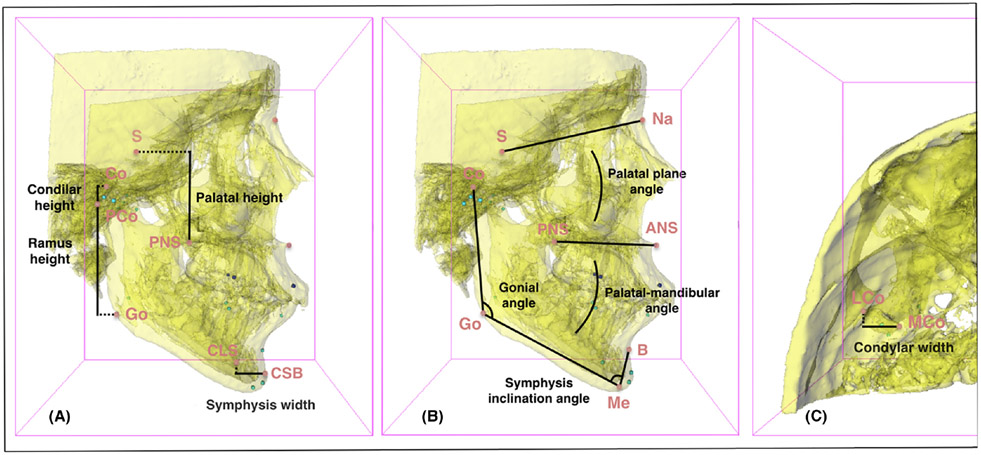
Condylar height (mm)—Ddistance between Co (most upper condyle point) and PCo (most posterior condyle point) landmarks. (Right and left sides).
Ramus height (mm)—distance between PCo (most posterior condyle point) and Go (gonion point) landmarks. (Right and left sides).
Condylar plus ramus height (mm)—distance between Co (most upper condyle point) and Go (gonion point) landmarks. (Right and left sides).
Condylar width (mm)—distance between Co (most upper condyle point) and Go (gonion point) landmarks. (Right and left sides).
Symphysis width (mm)—distance between CSB (most convex point of symphysis buccal curvature) and CSL (most convex point of symphysis lingual curvature) landmarks.
Mandibular length (mm)—distance between GoM (mean of left and right gonion points) and Me (menton point) landmarks.
Palatal height (mm)—distance between S (sella point) and PNS (posterior nasal spine point) landmarks.
Mandibular plane angle (°)—angle formed by S-N (sella and nasion points) and GoM-Gn (mean of left and right gonion points and gnathion point) lines.
Gonial angle (°)—angle formed by Co-Go (the most superior condyle [Co] point and the gonion [Go] point) and Go-Me (gonion and menton points) lines. (Right and left sides).
Symphysis inclination angle (°)—angle formed by GoM-Me (mean of left and right gonion point and menton point) and Me-B (menton and B points) lines.
Palatal plane angle (°)—a ngle formed by S-N (sella and nasion points) and ANS-PNS (anterior and posterior spine nasal points) lines.
Palatal-mandibular angle (°)—a ngle formed by ANS-PNS (anterior and posterior spine nasal points) and GoM-Me (mean of left and right gonion points and menton point) lines.
The measurements were performed using the Q3DC tool (Quantification of 3D Components) in the 3D Slicer software (Figure 3). After selecting the landmarks and choosing the desired measurement, the software automatically calculates the distances between the projections of 3D reference points in the x, y and z coordinate system. The tool evaluates each plane of the 3D space, generates vertical, transversal and anteroposterior measurements, as well as the 3D distance (Euclidean distance that refers to the smallest distance between two points), even when the points are on different planes (Figure 3).
FIGURE 3.
Linear and angular measurements made by the 3D Slicer software using the Q3DC tool. Lateral views ((A) and (C)—Linear measurements, and (B)—Angular measurements) and superior view (C) show projections of 3D reference landmarks in all dimensions (vertical, transverse and anteroposterior).
Calibration of the examiner and reliability of the measurements were assessed in 20% of the samples (measured twice at 1-week intervals), using the intraclass correlation coefficient (ICC = 0.96).
2.1 ∣. Statistical analysis
Statistical analysis was performed with the SPSS software program (version 18; SPSS). Normality and homogeneity were determined with the Shapiro–Wilk and Levene tests. Once the normal distribution and homogeneity of the results were certified, an independent t-test was used to make the intergroup comparisons (hyperdivergent and hypodivergent). The Pearson correlation test was applied to establish the correlational relationships between the MP angle and the other morphological characteristics evaluated. The level of significance was set at 5%.
3 ∣. RESULTS
Individuals with no evident asymmetry were selected for this study. Bilateral measurements were performed on the right and left sides to confirm that there was no statistically significant difference between the right and left sides. Then, the mean was calculated and used to make the bilateral measurements. The individuals selected for this study came from Brazil's Southwest.
Table 1 shows the means and standard deviations of linear (mm) and angular (°) measurements performed between the groups. The results showed that there was no difference (0.34 mm) in condylar height between the two groups. The hypodivergent group showed significantly higher values of ramus height (5.53 mm), condylar plus ramus height (5.79 mm), condyle width (1.80 mm), and mandibular length (8.79 mm), compared with the hyperdivergent group. The hyperdivergent group had significantly larger angular measurements in the gonial angle (4.79°), palatal plane angle (2.02°) and palatal-mandibular angle (8.32°), compared with the hypodivergent group. No statistical difference was found in the symphysis inclination angle (1.33°).
Table 2 shows the correlation between the MP angle and the other characteristics evaluated, among all the individuals. A significative correlation (P = .05) was observed between the MP angle and the ramus height (P = .001), the ramus plus condylar height (P = .002), the condylar width (P < .001), the symphysis width (P = .021), the mandibular length (P < .001), the gonial angle (P < .001), the symphysis inclination angle (P = .026), the palatal plane angle (P = .012) and the mandibular palatal plane angle (P < .001).
TABLE 2.
Pearson correlation test showing relationship between 3D MP angle with other evaluated characteristics (n = 60).
| MP angle | |
|---|---|
| Condylar height (mm) | |
| r | −.112 |
| P value | .393 |
| Ramus height (mm) | |
| r | −.404* |
| P value | .001 |
| Condylar plus ramus height (mm) | |
| r | −.387* |
| P value | .002 |
| Condylar width (mm) | |
| r | −.469* |
| P value | <.001 |
| Symphysis width (mm) | |
| r | −.298* |
| P value | .021 |
| Mandibular length (Go-Me) (mm) | |
| r | −.438* |
| P value | <.001 |
| Palatal heigth (mm) | |
| r | −.208 |
| P value | .110 |
| Gonial angle (°) | |
| r | .557* |
| P value | <.001 |
| Symphysis inclination angle (°) | |
| r | −.287* |
| P value | .026 |
| Palatal plane angle (°) | |
| r | .323* |
| P value | .012 |
| Palatal-mandibular angle(°) | |
| r | .783* |
| P value | <.001 |
Statistically significant difference at α = .05%.
4 ∣. DISCUSSION
This was the first study that evaluated morphological characteristics of individuals with different vertical growth patterns using 3D surface models. The 3D evaluations in this study allowed better visualization of structures, and greater accuracy in performing measurements on different planes.17,18 Multiplanar cross-sections were used to enter the landmarks in ITK-SNAP software in order to avoid possible inaccuracies. Measurements of the 3D models were performed only after obtaining the segmentations with prelabeled landmarks.18 This ensured greater precision, since the software executes a coordinate system in the x, y, z axes, thus offering more reliable information regarding location of spatial structures.17 Although some measurements used in our study are similar to the conventional cephalometry, the measurements obtained using the Q3DC tool from Slicer software are truly 3D measurements. The software allows performing measurements among landmarks that are on different planes of the space. Measurements obtained by the projection of landmarks in the same plane of space were not used.
The mean values of condylar height were slightly higher (P > .05) in the hypodivergent group (6.94 ± 1.18 mm), compared with the hyperdivergent group (6.60 ± 0.84 mm). In a similar study, Celik et al.27 evaluated condylar height, ramus height and condylar plus ramus height in hypodivergent, normal divergent and hyperdivergent individuals, using CBCT. Similar to our results, the authors found no statistically significant differences in condylar height among the groups. Our results for ramus height and condylar plus ramus height were consistent with those of the cited study.27 The hypodivergent group had higher values (P < .05) for ramus height and condylar plus ramus height than the hyperdivergent group. The authors27 of the cited study also reported that they found the highest and lowest values of ramus in the hypodivergent group. In our study, the highest value of ramus was found in the hypodivergent group (74.4 mm), and the lowest was found in the hyperdivergent group (43.4 mm).
Assessing the total condyle height was a limitation since it was difficult to standardize the landmark on the condylar neck. For this reason, the same methodology to evaluate the condylar height as that described by Habets et al.25 and other authors was used in the present study.28,29 Accordingly, the condylar height was defined as the vertical distance between the highest and most posterior point of the condyle. However, the present study did not evaluate the total height of the condyle head. This may have been one reason why the condylar height difference was not so evident, in as much as the condyle is already a small structure, and the measurement performed was smaller than its total height.
The condylar width in hypodivergent individuals (17.45 ± 3.50 mm) was significantly larger than in hyperdivergent individuals (15.65 ± 1.68 mm). Björk1 reported that hypodivergent individuals exhibit predominantly vertical condylar growth. Since the condyle is an area of intense growth of the mandible, there may also be greater growth in the lateral-medial direction in hypodivergent individuals. Another factor that may explain this finding is the difference in muscle strength between hyperdivergent and hypodivergent individuals.30-32 The hyperdivergent skeletal pattern is associated with low muscle activity.32
Bakke et al.31 reported an inverse relationship between vertical skeletal morphology and masseter length. Masticatory muscles have a strong influence on vertical maxillofacial morphology and occlusal strength.32-34 Thus, the larger condyle width in hypodivergent individuals may be related to a stronger condylar structure needed to support greater masticatory forces in these individuals.
We can speculate that the MP and gonial angles may open when facial sutures and alveolar growth are greater than condylar growth, thus promoting a backward rotation of the mandible. Conversely, if condylar growth is greater than the growth of facial sutures and alveolar bone, there will be a forward rotation of the mandible, thus decreasing the MP and gonial angles.1 Our study corroborates these findings. Smaller condylar plus ramus height (P < 0.05) in the hyperdivergent group versus the hypodivergent group may have influenced MP and gonial angles. There is a statistically significant correlation between condylar plus ramus height and MP angle: the smaller the condylar plus ramus height, the greater the MP angle.
Although the present study did not find statistically significant differences between the symphysis width and the symphysis inclination angle between the two groups, a correlation (P < 0.05) among MP angle, symphysis width and symphysis inclination angle were found. This indicates that the greater the MP angle, the thinner the symphysis, and the smaller the symphysis inclination angle. We believe that the reason there was no statistically significant difference between symphysis width and symphysis inclination is because overbite was not considered. This is because the objective of this study was to evaluate skeletal characteristics independent of dental characteristics. Consequently, the type of malocclusion may have influenced alveolar bone remodelling, and also affected symphysis shape and inclination.
The palatal plane angle was significantly higher in the hyperdivergent group (8.70 ± 3.26°) versus the hypodivergent group (6.68 ± 3.03°). There was a strong correlation between MP angle and palatal plane angle: the greater the MP angle, the greater the palatal plane angle. Nahoum et al.35 found different results for the palatal plane angle (measured in relation to the sella-nasion), namely that it was smaller in hyperdivergent individuals, suggesting that there was an upward inclination of the anterior palatal plane. The authors cited by Nahoum et al.35 believe that this upward inclination of the palatal plane decreases the upper facial height, thus increasing the lower facial height, the downward mandibular rotation, and the MP angle.
The palatal height in the posterior region (S-ENP) of the hypodivergent group (41.22 ± 2.76 mm) was slightly higher than that of the hyperdivergent group (40.23 ± 3.45 mm), but with no significant difference. The results showed an inversely moderate but not significant correlation (P > .05) between the MP angle and the palatal height, suggesting that the greater the MP angle, the lower the palatal height.
An interesting finding regarded the palatal-mandibular angle. Although this study did not evaluate dental patterns, such as open bite and deep bite, in either group, it did find that the hypodivergent group (23.84 ± 3.87) presented a significantly lower palatal-mandibular angle than the hyperdivergent group (32.16 ± 4.42).
Although it was not our main goal (due to the sample size) to further divide the two groups (hyperdivergent and hypodivergent groups) into four subgroups relative to MP angle values, we did so to enable evaluation of the maxillomandibular morphology in greater detail. The hyperdivergent group was divided into subgroups with a MP ≥ 38° (13 individuals) and a MP 37°–35° (17 individuals). The hypodivergent group was divided into subgroups with a MP 30°–27° (17 individuals) and MP ≤ 26° (13 individuals). The highest MP angle was 44°, and the lowest was 17°. The ANOVA/Tukey test was used to perform comparisons among the four subgroups (hyperdivergent and hypodivergent).
Table 3 shows the means and standard deviations of linear (mm) and angular (°) measurements performed among the four subgroups. Similar to the findings for the two main groups, the results for the condylar height showed no difference among the four subgroups. However, there were statistically significant differences for the lowest hypodivergent group (MP ≤ 26°), compared with the highest hyperdivergent group (MP ≥ 38°), regarding ramus height (6.41 mm), condylar plus ramus height (6.47 mm), condyle width (2.36 mm), symphysis width (2.02) and mandibular length (10.3 mm). The highest hyperdivergent group (MP ≥ 38°) had significantly larger angular measurements in the gonial angle (7.13°), palatal plane angle (2.32°) and palatal-mandibular angle (12.4°), compared with the lowest hypodivergent group (MP ≤ 26°).
TABLE 3.
Descriptive analysis and ANOVA/Tukey test results for linear (mm) and angular (°) measurements, and difference among groups
| Hyperdivergent |
Hypodivergent |
||||
|---|---|---|---|---|---|
| Group | ≥38° | 37°–35° | 30°–27° | ≤26° | Difference, ≥38° and ≥26° |
| Condylar height (mm) | 6.52 (±0.67) | 6.67 (±0.96) | 7.12 (±1.28) | 6.71 (±1.04) | 0.19 |
| Ramus height (mm) | 44.53 (±4.98)a | 44.22 (±4.15)a | 49.08 (±6.51)ab | 50.94 (±4.07)b | 6.41* |
| Condylar plus ramus height (mm) | 51.06 (±5.12)a | 50.91 (±4.78)a | 56.18 (±7.07)ab | 57.53 (±4.29)b | 6.47* |
| Condylar width (mm) | 15.26 (±1.90)a | 15.92 (±1.47)ab | 17.31 (±1.75)b | 17.62 (±2.07)b | 2.36* |
| Symphysis width (mm) | 15.33 (±1.99)a | 16.33 (±2.16)ab | 16.29 (±1.59)ab | 17.35 (±1.25)b | 2.02* |
| Mandibular length (Go-Me) (mm) | 57.39 (±3.34)a | 58.36 (±10.06)a | 65.99 (±6.01)b | 67.69 (±3.80)b | 10.03* |
| Palatal height (mm) | 40.22 (±2.92) | 40.24 (±3.98) | 40.88(±2.69) | 41.67 (±2.89) | 1.45* |
| Gonial angle (°) | 123.42 (±3.84)b | 123.47 (±4.16)b | 120.47 (±5.57)ab | 116.29 (±4.92)a | 7.13* |
| Symphysis inclination angle (°) | 80.55 (±5.90) | 89.69 (±6.12) | 79.80 (±5.57) | 83.47 (±4.82) | 2.92* |
| Palatal plane angle (°) | 8.21 (±3.92)ab | 9.08 (±2.73)b | 7.28 (±3.18)ab | 5.89 (±2.75)a | 2.32* |
| Palatal-mandibular angle (°) | 34.80 (±4.82)c | 30.14 (±2.83)b | 24.94 (±3.24)a | 22.40 (±4.26)a | 12.4* |
Note. Standard deviation values (±SD). Each column presents independent result for ANOVA/Turkey test. Different letters indicate statistically significant difference at α 0.05%.
P < .05% means a significant statistical difference.
The more extreme subgroups (MP ≥ 38° and MP ≤ 26°) were found to be different in almost all the analyses, and the intermediate subgroups (MP 37°–35° and MP 30°–27°) presented smaller skeletal differences in almost all the analyses for the maxilla and mandible, to the point that their values were closer to normal.1 These findings are important because they may influence the decision regarding orthodontic biomechanics for vertical control, considering that small variations of the MP angle did not produce significant morphological differences between the groups. Caution must be exercised in interpreting the results of the present study because of the sample size of the subgroups. Further characterization of mild and severe vertical skeletal facial features would benefit from future studies with larger samples, so that these features can be subdivided and compared using control groups.
Our study corroborates previous findings and emphasizes the need for correct diagnostic and biomechanical planning for vertical control, particularly based on measurements that show important clinical differences, such as condylar and ramus height, mandibular length, gonial angle, palatal plane and palatal-mandibular angle. These characteristics should be associated with other facial and dental characteristics to guide the diagnosis and treatment plan, such as active or passive lip seal, open bite or deep overbite tendency, and increased or decreased lower third of the face.
5 ∣. CONCLUSIONS
Hyperdivergent (≥35°) and hypodivergent (≤30°) individuals present characteristically different skeletal morphology.
Hypodivergent individuals present greater condylar width, ramus height, condylar plus ramus height and mandibular length, lower gonial angle and smaller palatal plane angle and palatal-mandibular angle than hyperdivergent individuals.
There are significant correlations between MP angle and maxillomandibular morphology of the condyles, ramus, symphysis, gonial angle, palatal plane angle and palatal-mandibular angle.
ACKNOWLEDGEMENTS
We thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), a Brazilian governmental agency, for the financial support provided.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare that are relevant to the content of this article.
DATA AVAILABILITY STATEMENT
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Björk A. Prediction of mandibular growth rotation. Am J Orthod. 1969;55:585–599. [DOI] [PubMed] [Google Scholar]
- 2.Skieller VB, Björk A, Linde-Hansen T. Prediction of mandibular growth rotation evaluated from a longitudinal implant sample. Am J Orthod. 1984;86:359–370. [DOI] [PubMed] [Google Scholar]
- 3.Ricketts R. Evolution of mandibular growth concepts in orthodontic science. Proc Found Orthod Res. 1971;1–10. [PubMed] [Google Scholar]
- 4.Steiner C. Cephalometrics for you and me. Am J Orthod. 1953;39:720–755. [Google Scholar]
- 5.Isaacson RJ, Speidel TM, Worms FW. Extreme variation in vertical facial growth and associated variation in skeletal and dental relations. Angle Orthod. 1971;41:219–229. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen L. Vertical malocclusions: etiology, development, diagnosis and some aspects of treatment. Angle Orthod. 1991;61(4):247–260. [DOI] [PubMed] [Google Scholar]
- 7.Cangialosi TJ. Skeletal morphologic features of anterior open bite. Am J Orthod. 1984;85:28–36. [DOI] [PubMed] [Google Scholar]
- 8.Nanda SK. Patterns of vertical growth in the face. Am J Orthod Dentofacial Orthop. 1988;93:103–106. [DOI] [PubMed] [Google Scholar]
- 9.Lai J, Ghosh J, Nanda RS. Effect of orthodontic therapy on the facial profile in long and short vertical facial patterns. Am J Orthod Dentofacial Orthop. 2000;118:505–513. [DOI] [PubMed] [Google Scholar]
- 10.Blanchette ME, Nanda RS, Currier GF, Ghosh J, Nanda SK. A longitudinal cephalometric study of the soft tissue profile of short- and long-face syndromes from 7 to 17 years. Am J Orthod Dentofacial Orthop. 1996;109:116–131. [DOI] [PubMed] [Google Scholar]
- 11.Swasty D, Lee J, Huang JC, et al. Cross-sectional human mandibular morphology as assessed in vivo by cone-beam computed tomography in patients with different vertical facial dimensions. Am J Orthod Dentofacial Orthop. 2011;139:377–389. [DOI] [PubMed] [Google Scholar]
- 12.Ha Y, Park YS, Lee SP. Do long-faced subjects really have a long anterior face? A longitudinal study. Am J Orthod Dentofacial Orthop. 2014;145:799–806. [DOI] [PubMed] [Google Scholar]
- 13.Sassouni V, Nanda S. Analysis of dentofacial vertical proportions. Am J Orthod. 1964;50:801–823. [Google Scholar]
- 14.Baumgaertel S, Palomo JM, Palomo L, Hans MG. Reliability and accuracy of cone-beam computed tomography dental measurements. Am J Orthod Dentofacial Orthop. 2009;136(1):19–25. [DOI] [PubMed] [Google Scholar]
- 15.Moreira CR, Sales MA, Lopes PM, Cavalcanti MG. Assessment of linear and angular measurements on three-dimensional cone-beam computed tomographic images. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:430–436. [DOI] [PubMed] [Google Scholar]
- 16.Cevidanes LHS, Heymann G, Cornelis MA, Declerck HJ, Tulloch C. Superimposition of 3-dimensional cone-beam computed tomography models of growing patients. Am J Orthod Dentofacial Orthop. 2009;136:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruellas ACO, Tonello C, Gomes LR, et al. Common 3-dimensional coordinate system for assessment of directional changes. Am J Orthod Dentofacial Orthop. 2016;149:645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruellas ACO, Ghislanzoni LTH, Gomes MR, et al. Comparison and reproducibility of 2 regions of reference for maxillary regional registration with cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2016;149:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruellas ACO, Yatabe MS, Souki BQ, et al. 3D mandibular superimposition: comparison of regions of reference for voxel-based registration. PLoS One. 2016;11(6):e0157625. doi: 10.1371/journal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olbrisch C, Santander P, Moser N, Klenke D, Meyer-Marcotty P, Quast A. Three-dimensional mandibular characteristics in skeletal malocclusion: a cross-sectional study. Journal of Orofac Orthop. 2022;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santander P, Quast A, Olbrisch C, et al. Comprehensive 3D analysis of condylar morphology in adults with different skeletal patterns–a cross-sectional study. Head Face Med. 2020;16(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shetty SR, Al-Bayatti S, AlKawas S, et al. Analysis of the volumetric asymmetry of the mandibular condyles using CBCT. Int Dent J. 2022;72(6):797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schudy FF. The control of vertical overbite in clinical orthodontics. Angle Orthod. 1968;38:19–39. [DOI] [PubMed] [Google Scholar]
- 24.Pandis N. Sample calculations for comparison of 2 means. Am J Orthod Dentofacial Orthop. 2012;141:519–521. [DOI] [PubMed] [Google Scholar]
- 25.Habets LLMH, Bezuur JN, Naeiji M, Hansson TL. The Orthopantomogram®, an aid in diagnosis of temporomandibular joint problems. J Oral Rehabil. 1988;15(5):465–471. [DOI] [PubMed] [Google Scholar]
- 26.Young NM, Sherathiya K, Gutierrez L, et al. Facial surface morphology predicts variation in internal skeletal shape. Am J Orthod Dentofacial Orthop. 2016;149:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celik S, Celikoglu M, Buyuk SK, Sekerci AE. Mandibular vertical asymmetry in adult orthodontic patients with different vertical growth patterns: a cone beam computed tomography study. Angle Orthod. 2016;86:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Taki A, Ahmed MH, Ghani HA, Al KF. Impact of different malocclusion types on the vertical mandibular asymmetry in young adult sample. Eur J Dent. 2015;9(3):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasimoglu Y, Tuna EB, Rahimi B, Marsan G, Gencay K. Condylar asymmetry in different occlusion types. Cranio. 2015;33:10–14. [DOI] [PubMed] [Google Scholar]
- 30.Gionhaku N, Lowe AA. Relationship between jaw muscle volume and craniofacial form. J Dent Res. 1989;68:805–809. [DOI] [PubMed] [Google Scholar]
- 31.Bakke M, Tuxetv A, Vilmann P, Jensen BR, Vilmann A, Toft M. Ultrasound image of human masseter muscle related to bite force, electromyography, facial morphology, and occlusal factors. Scand J Dent Res. 1992;100:164–171. [DOI] [PubMed] [Google Scholar]
- 32.Nair R, Deguchi T, Li X, Katashiba S, Chan YH. Quantitative analysis of the maxilla and the mandible in hyper- and hypodivergent skeletal class II pattern. Orthod Craniofac Res. 2009;12:9–13. [DOI] [PubMed] [Google Scholar]
- 33.Bresin A. Effects of masticatory muscle function and bite-raising on mandibular morphology in the growing rat. Swed Dent J Suppl. 2001;150:1–49. [PubMed] [Google Scholar]
- 34.Garcıa-Morales P, Buschang PH, Throckmorton GS, English JD. Maximum bite force, muscle efficiency and mechanical advantage in children with vertical growth patterns. Eur J Orthod. 2003;25:265–272. [DOI] [PubMed] [Google Scholar]
- 35.Nahoum HI. Vertical proportions and the palatal plane in anterior open bite. Am J Orthod. 1971;59:273–282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.