Abstract
Background:
Circadian disruption is a potential risk factor for advanced prostate cancer, and light at night (LAN) exposure may disrupt circadian rhythms. We evaluated whether outdoor LAN increases the risk of prostate cancer.
Methods:
We prospectively followed 49,148 participants in the Health Professionals Follow-up Study from 1986 through 2016. We estimated baseline and cumulative time-varying outdoor LAN with ~1 km2 resolution using data from the US Defense Meteorological Satellite Program’s Operational Linescan System, which was assigned to participants’ geocoded addresses. Participants reside in all 50 US states and reported a work or home address. We used multivariable Cox models to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the association between outdoor LAN and risk of overall (7,175 cases) and fatal (915 cases) prostate cancer adjusting for individual and contextual factors.
Results:
There was no association between the interquartile range increase in cumulative LAN and total (HR:1.02, 95% CI 0.98, 1.06) or fatal (HR: 1.05, 95% CI: 0.96, 1.15) prostate cancer in adjusted models. However, there was a positive association between baseline LAN and total prostate cancer among non-movers (HR: 1.06, 95% CI:1.00,1.14) including among highly screened participants (HR: 1.11, 95% CI:1.01,1.23).
Conclusions:
There was a suggestive positive association between baseline outdoor LAN and total prostate cancer. Additional studies with different measures of outdoor LAN and in more diverse populations are necessary.
Impact:
To our knowledge, this is the first longitudinal cohort study exploring the relationship between outdoor LAN and prostate cancer.
Introduction
Prostate cancer is the most commonly diagnosed non-cutaneous cancer and the second leading cause of cancer death in men living in the United States(1). Despite its contribution to cancer burden, there are few established risk factors for prostate cancer, particularly potentially modifiable factors(2). Night shift work, via its influence on circadian rhythms, is considered probably carcinogenic to humans (Group 2A) by the World Health Organization(3,4). Multiple studies have reported associations between measures of circadian disruption and prostate cancer(5–9). Specifically, sleep problems, germline variants in circadian genes, and lower melatonin levels were associated with an increased risk of advanced prostate cancer in cohorts based in Iceland and the United States(5–7,10).
Circadian rhythms are behavioral, mental, and physical changes that lead to daily oscillations of biochemical and physiologic processes controlled by the suprachiasmatic nucleus, an internal clock comprised of 20,000 nerve cells located in the hypothalamus, that receive direct input from the eyes. Light plays a central role in regulating circadian rhythms through its direct input to the suprachiasmatic nucleus, it inhibits the production of the sleep promoting hormone melatonin, which has anticarcinogenic properties, and deregulates circadian genes involved in cancer pathways(11–13). In animal models, light at night can disrupt circadian rhythms and increases tumor growth across malignancies(13–16).
Artificial light at night is ubiquitous in modern societies and is a potentially modifiable factor. The amount of outdoor light at night (LAN) on the Earth’s surface has increased over time(17). To date, investigators have found a suggestive increase in breast cancer risk associated with higher outdoor LAN(18–24). Considering the impact of light on circadian rhythms, the association between different measures of circadian disruption and prostate cancer, and the suggestive results of breast cancer and outdoor LAN, outdoor LAN may be a risk factor for prostate cancer, another sex hormone-dependent cancer. The epidemiological data on outdoor LAN and prostate cancer risk is limited(22,25–27).
In this study, we examined the association between outdoor LAN and prostate cancer risk in a nationwide prospective cohort of health professionals. One of the challenges in defining prostate cancer etiology is its biologic and clinical heterogeneity. Moreover, routine screening with prostate-specific antigen (PSA) has led to increased detection and diagnosis of asymptomatic, non-aggressive prostate cancer in health-conscious individuals, but the increased likelihood of diagnosis in these populations may not correspond to an increased risk of disease nor adverse disease-related outcomes(28–31). As such, there is a need to investigate risk factors for more aggressive disease and to carefully account for PSA screening. We hypothesized that participants living in locations of greater outdoor LAN have an increased risk of prostate cancer, particularly fatal prostate cancer.
Materials and Methods
Study population
We conducted this study in the Health Professionals Follow-up Study (HPFS), an ongoing cohort of 51,529 health professionals aged 40–75 years at enrollment in 1986 who identified as men. Participants completed questionnaires to provide information on demographics, lifestyle factors, medical history, and health outcomes including prostate cancer at baseline and every two years thereafter. Geocoded addresses were available from questionnaire mailing records from 1988 through 2016 and were located throughout the United States, representing all 50 states. Participants were asked if the listed mailing address was their home, work, or other address on the 1988 questionnaire. We used the 1988 address for 1986 to begin follow-up in 1986. The follow-up rate at each two-year cycle exceeded 90% and mortality follow-up was over 98%(32).
We excluded participants who had a history of cancer prior to enrollment (other than nonmelanoma skin cancer) (N=2,076), were missing data on outdoor LAN (N=267), died before the return of the 1986 questionnaire (N=2), or had a missing date of birth (N=36). The study protocol was approved by the institutional review board of Harvard T.H. Chan School of Public Health and those of participating cancer registries as required.
Prostate cancer ascertainment
Incident prostate cancer diagnoses were first reported by participants on biennial questionnaires. Study personnel obtained and reviewed medical records, pathology reports, and a physician questionnaire for each reported case to confirm the diagnosis, and to extract information on pathology and clinical features of the tumor. If records were unavailable, diagnoses were confirmed via linkage to state tumor registries. Deaths were identified by reports from next of kin, postal service, and the National Death Index. We obtained data on treatment, disease progression, and metastases through biennial disease-specific questionnaires.
Our main analyses focused on the incidence of total or fatal prostate cancer, and as a secondary outcome, lethal prostate cancer. Total prostate cancer was defined as any diagnosis of prostate cancer, fatal as death from prostate cancer as the primary cause, and lethal as death with prostate cancer as the primary cause of death or development of metastasis at diagnosis (stage M1) or during follow-up. The event time for all outcomes was date of first diagnosis of prostate cancer.
Outdoor LAN ascertainment
Average annual outdoor LAN was derived from satellite imagery data from the US Defense Meteorological Satellite Program’s (DMSP’s) Operational Linescan System, which is maintained by the National Oceanic and Atmospheric Administration’s (NOAA’s) Earth Observation Group(33). These annual averages were calculated after excluding sun and moon luminance, outer quarters of satellite swath, clouds, glare, fires, and atmospheric lightning. The imagery data has a resolution of 1 km2. We used the DMSP Global Radiance Calibrated Nighttime Lights high-dynamic range data because previous studies showed that the low dynamic range data did not accurately reflect differences in LAN across urban areas(34). We used interannual calibration coefficients by NOAA to derive exposure estimates to ensure comparability across years and satellites(35). These high-dynamic data were available for 1996, 1999, 2000, 2002, 2004, 2005, and 2010. We assigned an outdoor LAN value to each participant address – home, work, or other - over follow-up, addresses and LAN values were updated every two years. For addresses between 1988 and 1998, we assigned the LAN value based on data from 1996. We assigned the LAN value in 1988 to the 1986 time period. For addresses in 1998, we assigned the LAN value based on data from 1999. For addresses after 1998, we assigned exposure based on the most recent past LAN measure. We calculated cumulative average outdoor LAN for each participant at each biennial questionnaire, accounting for changes in participant addresses and LAN over time. As a secondary exposure, we examined the association between baseline LAN and each prostate cancer outcome.
Covariates
All covariates were chosen a priori based on confounding variables identified in prior research(2) and Directed Acyclic Graphs (DAGs)(36). Information on covariates was obtained on biennial follow-up questionnaires in HPFS. We adjusted for time-invariant covariates including race (white, non-white), height (≤ 68, 68>70,70>72, >72 inches), and family history of prostate cancer. Time-varying covariates were updated every two years at each biennial questionnaire and included: PSA screening in the prior time period (lagged by two-year interval), PSA screening intensity (reported PSA screening in over half of prior questionnaires, lagged by two-year interval), smoking status (never, current, former), quintiles of physical activity (MET-hours/week), body mass index, (BMI - underweight, normal, overweight, obese), quintiles of population density derived from the United States Decennial Census (1990, 2000, 2010), with places with a population of more than 1,000 people per square mile defined as urban, and quintiles of neighborhood socioeconomic status (nSES). NSES was assessed using a composite score derived in HPFS(37). Data used to generate the nSES score were obtained from the United States Decennial Census (1990, 2000, 2010) and the American Community Survey (2006–2010) and linked to participants’ addresses. The nSES score includes census tract level variables for educational attainment (% over 25 with college or higher education), income (median family income), wealth (median family home value, % families receiving interest dividends or rent income, % occupied housing units), employment status (% population 16 + years old unemployed), and racial composition (% White, % Black, % foreign-born). We calculated a summary index of nSES by z-scaling each component measure and then summing across the nine indicators.
Statistical analysis
Person-time was accrued from the date of return of the baseline questionnaire in 1986 to the date of diagnosis of incident prostate cancer, date of death, or end of follow-up (January 1, 2017), whichever came first. We used Cox proportional hazards models with calendar time on study as the time scale and stratified by age and questionnaire cycle to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between outdoor LAN and each of the three prostate cancer endpoints. The proportional hazards assumption was assessed by including interaction terms between time and our exposure; there was no evidence of nonproportionality. We tested for nonlinear relationships between LAN and each outcome using splines. We examined the possibly non-linear relation between outdoor LAN and the hazard ratio of total and fatal prostate cancer non-parametrically with restricted cubic splines(38). Tests for non-linearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. As no evidence of non-linearity was observed, we modeled LAN continuously, scaled to an interquartile range (IQR) increase (Supplementary Figure 1).
Multivariable-adjusted models for total prostate cancer were adjusted for race, height, family history of prostate cancer, reported PSA screening in the prior questionnaire cycle, PSA screening intensity, neighborhood SES, and population density. Models for fatal prostate cancer were additionally adjusted for smoking status, physical activity, and BMI. Since outdoor LAN may have greater relevance at home residence, we also repeated our main analysis among the subset of men who reported a home address at baseline.
To assess sensitivity to the choice of etiologic window used for our outdoor LAN measure, we conducted a secondary analysis looking at the relationship between baseline outdoor LAN and total and fatal prostate cancer among men who did not move over the follow-up period and reported a home address at baseline. We also evaluated potential effect modification by population density (≥1,000 people/mi2 vs <1,000 people/mi2), neighborhood SES (below vs above the median nSES index), and PSA screening intensity (reported PSA screening in more than 50% of the prior questionnaires vs not) using likelihood ratio tests and reported stratified models.
Data Availability
The data generated in this study are available upon request from the corresponding author.
Results
After exclusions, our analytic sample included 49,148 participants, with 7,175 total and 915 fatal prostate cancer cases over 1,125,157 person-years of follow-up. Maps of participant locations and outdoor LAN in 2010 (Figure 1) display the geographic extent and exposure distribution in this study. The age-adjusted characteristics of the study population are shown in Table 1. The average age of the participants over follow up was 64.2 years, and 90.7% were white. Men who lived in areas with the highest quintile of outdoor LAN were less physically active, less likely to be white, and less likely to have a PSA test. They also lived in areas with a higher neighborhood SES and higher population density compared to participants in the lowest quintile. In 1988, 34.7% of the participants reported a home address, 43% reported an office address, 1.6% reported other, and 20.7% did not report the type of address (Table 1).
Figure 1:
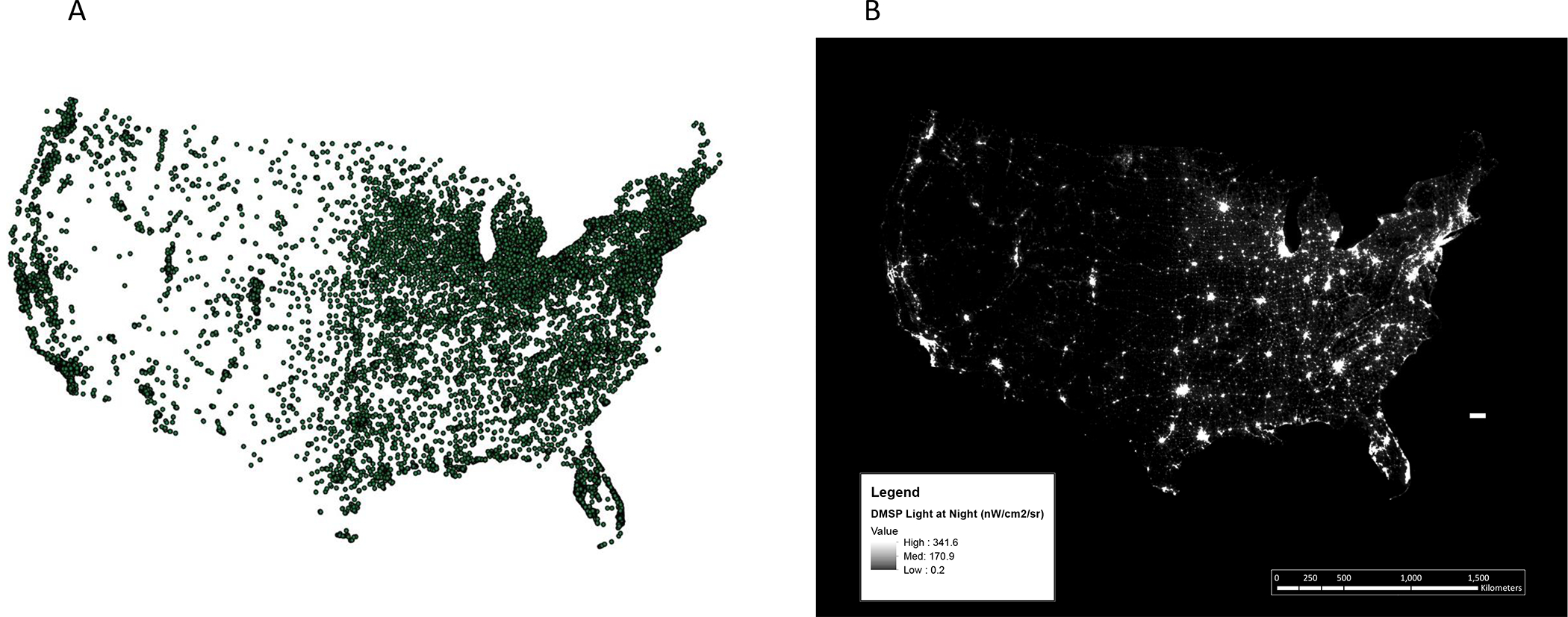
Participant locations and outdoor LAN in the United States (A) Locations of addresses of HPFS participants in 1988 and (B) 2010 U.S. Defense Metereological Satellite Program’s (DMSP’s) Operational Linescan System (OLS) light at night (LAN) data in nanowatts per centimeter squared per streradian
Table 1:
Age-standardized characteristics by quintile of cumulative outdoor light at night (LAN) among participants in the Health Professionals Follow-up Study from 1986 to 2016
| Cumulative Outdoor Light at Night (nW/cm2/sr) | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Total | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 |
|
| ||||||
| Participants, no. | 49,148 | 11,982 | 14,009 | 14,958 | 14,811 | 12,257 |
| Age (years)* | 64.2 (11.3) | 64 (11.4) | 64.4 (11.3) | 64.6 (11.2) | 64.4 (11.2) | 63.7 (11.2) |
| Cumulative LAN (nW/cm2/sr) | 37.3 (32.6) | 5.7 (2.7) | 15.5 (3.4) | 29.4 (4.4) | 47.1 (6.1) | 88.6 (31.2) |
| Baseline LAN (1986) (nW/cm2/sr) | 39.9 (35.7) | 6.3 (4.1) | 16.9 (8.8) | 31.8 (12.1) | 50.5 (15) | 93.4 (34.7) |
| Height (inches) | 70.2 (2.8) | 70.3 (2.7) | 70.3 (2.7) | 70.2 (2.8) | 70.1 (2.8) | 69.9 (2.9) |
| BMI (kg/m2) | 26.1 (3.8) | 26.3 (3.9) | 26.1 (3.8) | 26.1 (3.7) | 26 (3.8) | 25.9 (3.8) |
| Physical activity (MET-hour/week) | 32.2 (28.7) | 33.3 (30.6) | 32.9 (29.3) | 32.8 (28.8) | 31.8 (27.9) | 30.2 (26.4) |
| White, % | 90.7 | 92.9 | 92.4 | 91.1 | 89.9 | 87.2 |
| Smoking status | ||||||
| - Never, % | 56.0 | 54.9 | 55.4 | 56.0 | 56.7 | 57.0 |
| - Former, % | 38.8 | 39.2 | 39.4 | 39.1 | 38.5 | 38.0 |
| - Current, % | 5.2 | 5.9 | 5.2 | 4.9 | 4.8 | 5.0 |
| Family history of prostate cancer, % | 11.9 | 12.7 | 11.8 | 11.8 | 12.1 | 11.0 |
| Ever had PSA test, % | 85.8 | 85.0 | 86.9 | 86.7 | 86.4 | 84.1 |
| Had PSA test in prior period, % | 30.3 | 29.8 | 31.4 | 32.2 | 31.2 | 26.9 |
| PSA test on at least half of all questionnaires, % | 30.5 | 28.9 | 31.8 | 32.7 | 32.0 | 26.7 |
| Address type (1988) | ||||||
| - Home, % | 34.7 | 48.7 | 38.4 | 33.4 | 29.1 | 23.8 |
| - Office, % | 43.0 | 29.3 | 39.9 | 44.8 | 48.6 | 52.7 |
| - Other, % | 1.6 | 2.5 | 2.2 | 1.5 | 1.1 | 0.8 |
| - Not reported, % | 20.7 | 19.5 | 19.5 | 20.3 | 21.2 | 22.6 |
| Address type (2008) | ||||||
| - Office, % | 15.9 | 12.3 | 14.9 | 15.9 | 18.0 | 18.9 |
| - Home, % | 67.8 | 71.4 | 69.9 | 67.3 | 65.9 | 63.8 |
| - Not reported, % | 15.7 | 14.8 | 14.7 | 16.2 | 15.7 | 17.0 |
| - Both, % | 0.6 | 1.5 | 0.5 | 0.5 | 0.3 | 0.3 |
| Population density, (People/mile2) | 3,948.9 (9,416.1) | 531.76(966.8) | 1,766.3 (2,017.5) | 2,908.1 (2,930.7) | 4,172.9 (4,543) | 10,368.8 (187,121) |
| Neighborhood SES | 0 (3.8) | −2.4 (2.9) | −0.5 (3.4) | 0.6 (3.7) | 1 (3.6) | 1.5 (3.8) |
| Urban (≥ 1,000 people/miles2), % | 66.4 | 15.2 | 54.6 | 78.2 | 89.3 | 95.1 |
| Census region | ||||||
| - Northeast, % | 21.6 | 21.2 | 22.5 | 22.2 | 18.8 | 23.5 |
| - Midwest, % | 26.3 | 30.7 | 23.8 | 22.8 | 26.0 | 28.0 |
| - South, % | 28.9 | 29.3 | 31.7 | 30.4 | 28.7 | 24.4 |
| - West, % | 23.2 | 18.8 | 22.0 | 24.6 | 26.5 | 24.0 |
Values are means(SD) or medians(Q25, Q75) for continuous variables; percentages or ns or both for categorical variables, and are standardized to the age distribution of the study population.
Value is not age adjusted
In the analysis in the full cohort, there was no statistically significant association between cumulative average LAN and total (HR:1.02, 95% CI 0.98, 1.06) or fatal (HR: 1.05, 95% CI: 0.96, 1.15) prostate cancer in fully adjusted models (Table 2). Results were similar when restricted to participants who reported a home address at baseline (N=17, 983) (Table 2). An interquartile increase in baseline LAN was associated with a 6% increase in the hazard of total prostate cancer (HR: 1.06, 95% CI:1.00,1.14) in the subset of participants who reported a home address and who did not move over follow-up (N=15,132). There was no clear association with fatal prostate cancer (HR: 0.94, 95% CI: 0.79, 1.11) in this group (Table 2). Results for lethal prostate cancer were similar to those for fatal prostate cancer (Supplementary Table 1).
Table 2.
Hazard ratios and 95% confidence intervals of the association between outdoor light at night (LAN) and prostate cancer risk in HPFS (1986–2016)
| Total population (n=49,148) | ||||||
|---|---|---|---|---|---|---|
| Total | Fatal | |||||
| Exposure | Cases (PYs) | aHR (95% CI) | bHR (95% CI) | Cases (PYs) | aHR (95% CI) | cHR (95% CI) |
| dCumulative LAN | 7,175(1,125,157) | 0.99 (0.97, 1.02) | 1.02 (0.98, 1.06) | 915 (1,131,169) | 1.05 (0.97, 1.13) | 1.05 (0.96, 1.15) |
| e Home addresses (n=17,983) | ||||||
| dCumulative LAN | 2,847(389,614) | 1.01 (0.97, 1.06) | 1.04 (0.98, 1.10) | 409 (391,913) | 0.99 (0.89, 1.11) | 0.98 (0.85, 1.14) |
| dBaseline LAN in nonmovers (N=15,132) | 2,348 (326,942) | 1.04 (0.99, 1.09) | 1.06 (1.00, 1.14) | 359 (328,813) | 0.98 (0.86, 1.10) | 0.94 (0.79, 1.11) |
Age, calendar time
Age, race, PSA screening, family history of prostate cancer, height, neighborhood SES, population density
Age, race, PSA screening, family history of prostate cancer, height, neighborhood SES, population density, physical activity, body mass index (BMI), smoking
per IQR increase
Men who reported having a home address in 1988
There was no evidence of effect modification by urbanicity or neighborhood SES for fatal or total prostate cancer in the whole cohort or in those who reported a home address at baseline (Table 3). In the whole cohort, there was a slight positive association between cumulative average outdoor LAN and fatal prostate cancer in those who were not highly screened (HR:1.07, 95% CI:0.97, 1.19), while there was no clear evidence for an association between outdoor LAN and fatal prostate cancer among those who were highly screened (HR: 0.95, 95% CI:0.77, 1.19). However, this finding was not statistically significant (p-het=0.17). In the subset of the cohort that reported a home address at baseline, there was an association with total prostate cancer among those who were more frequently PSA screened (HR:1.11, 95% CI:1.01,1.23), but not in those less frequently screened (HR:1.03, 95% CI:0.94,1.12) (p-het=0.08). We observed similar results for lethal as fatal prostate cancer (Supplementary Table 2).
Table 3:
Hazard ratios and 95% confidence intervals for the association between outdoor light at night (LAN) and prostate cancer incidence in HPFS 1986–2016, stratified by PSA screening intensity, urbanicity, and neighborhood socioeconomic status (nSES)
| Total prostate cancer | Fatal prostate cancer | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Model | aHR (95% CI) | bHR (95% CI) | Phet | aHR (95% CI) | cHR (95% CI) | Phet |
|
| ||||||
| Stratified by PSA screening intensity | ||||||
|
| ||||||
| Cumulative LAN in the total population | ||||||
|
| ||||||
| Total population (N=49,148) | Cases (PYs)=7,175 (1,125,157) | Cases (PYs)=915 (1,131,169) | ||||
| eLow PSA screening intensity | 0.99 (0.95, 1.02) | 1.01 (0.97, 1.06) | 0.47 | 1.08 (0.99, 1.17) | 1.07 (0.97, 1.19) | 0.17 |
| fHigh PSA screening intensity | 1.01 (0.97, 1.06) | 1.03 (0.97, 1.09) | 0.94 (0.78, 1.12) | 0.95 (0.77, 1.19) | ||
|
| ||||||
| Baseline LAN in those who reported a home address | ||||||
|
| ||||||
| d Nonmovers (N=15,132) | Cases (PYs)=2,348 (326, 942) | Cases (PYs)= 359 (328,813) | ||||
| eLow PSA screening intensity | 1.00 (0.94, 1.07) | 1.03 (0.94, 1.12) | 0.08 | 1.00 (0.87, 1.16) | 0.93 (0.76, 1.14) | 0.73 |
| fHigh PSA screening intensity | 1.09 (1.01, 1.17) | 1.11 (1.01, 1.23) | 0.88 (0.67, 1.14) | 1.01 (0.72, 1.42) | ||
|
| ||||||
| Stratified by Urbanicity (urban: >1,000 people/mile 2 , nonurban: <1,000 people/mile 2 ) | ||||||
|
| ||||||
| Cumulative LAN in the total population | ||||||
|
| ||||||
| Total population (N=49,148) | Cases (PYs)=7,175 (1,125,157) | Cases (PYs)=915 (1,131,169) | ||||
| Urban | 0.99 (0.96, 1.03) | 1.02 (0.98, 1.06) | 0.67 | 1.06 (0.97, 1.16) | 1.06 (0.95, 1.17) | 0.94 |
| Nonurban | 1.07 (0.98, 1.16) | 1.04 (0.94, 1.13) | 1.06 (0.80, 1.39) | 1.03 (0.77, 1.37) | ||
|
| ||||||
| Baseline LAN in those who reported a home address | ||||||
|
| ||||||
| dNonmovers (N=15,132) | Cases (PYs)=2,348 (326, 942) | Cases (PYs)= 359 (328,813) | ||||
| Urban | 1.04 (0.98, 1.10) | 1.06 (0.99, 1.14) | 0.91 | 0.95 (0.81, 1.11) | 0.93 (0.78, 1.12) | 0.80 |
| Nonurban | 1.10 (0.89, 1.37) | 1.04 (0.82, 1.32) | 0.73 (0.38, 1.40) | 0.74 (0.38, 1.43) | ||
|
| ||||||
| Stratified by neighborhood SES at the median | ||||||
|
| ||||||
| Cumulative LAN in the total population | ||||||
|
| ||||||
| Total population (N=49,148) | Cases (PYs)= 7,175 (1,125,157) | Cases (PYs)= 915 (1,131,169) | ||||
| Low | 1.01 (0.96, 1.06) | 1.03 (0.97, 1.10) | 0.32 | 1.05 (0.93, 1.20) | 1.03 (0.88, 1.21) | 0.66 |
| High | 0.98 (0.94, 1.02) | 1.01 (0.97, 1.06) | 1.04 (0.94, 1.15) | 1.07 (0.95, 1.20) | ||
|
| ||||||
| Baseline LAN in those who reported a home address | ||||||
|
| ||||||
| dNonmovers (N=15,132) | Cases (PYs)= 2,348 (326, 942) | Cases (PYs)= 359 (328,813) | ||||
| Low | 1.05 (0.97, 1.15) | 1.08 (0.96, 1.21) | 0.53 | 0.85 (0.66, 1.10) | 0.69 (0.48, 1.01) | 0.27 |
| High | 1.01 (0.94, 1.07) | 1.06 (0.98, 1.15) | 1.00 (0.85, 1.19) | 1.07 (0.87, 1.31) | ||
Adjusted for age and calendar time
Adjusted for age, calendar time, race, PSA screening, family history of prostate cancer, height, neighborhood SES
Adjusted for age, calendar time, race, PSA screening, family history of prostate cancer, height, population density, physical activity, body mass index (BMI), smoking
Men who reported having a home address in 1988 and did not move over follow-up
Reported a PSA test in less than 50% of questionnaires in prior time periods
Reported a PSA test in 50% or more of questionnaires in prior time periods
Discussion
In this large prospective study with 30 years of follow-up, we did not find an association between cumulative average outdoor LAN during follow-up and overall prostate cancer. However, we found a small increased risk of total prostate cancer associated with higher baseline outdoor LAN among participants who reported a home address at baseline and who did not move over follow-up. These findings are consistent with the hypothesis that outdoor LAN has a delayed effect on carcinogenesis. It is also possible that the home address at baseline better captures the participants’ exposure to LAN in the evening so there is less exposure misclassification than in the whole cohort. However, this latter explanation is less likely since we did not observe an association between cumulative average outdoor LAN and prostate cancer among those who reported a home address at baseline.
There is limited research looking at the relationship between outdoor LAN and prostate cancer, but our findings align with prior literature. An ecological study found a positive association between country level exposure to outdoor LAN and age-standardized prostate cancer rates(25,27). A district-level ecological study in South Korea found a positive association between outdoor LAN and prostate cancer(26). A case-control study conducted in Spain found an inverse association between outdoor LAN in the visual spectrum and prostate cancer risk overall(22). This study also found an increased odds of overall prostate cancer with higher levels of self-reported indoor LAN and outdoor LAN in the blue light spectrum(22). Light in the blue spectrum is more biologically relevant, decreasing melatonin levels and in turn is more likely to impact carcinogenesis, particularly of hormone-dependent cancers(39–41). DMSP-OLS outdoor LAN data available in HPFS only includes information on light intensity.
Light at night may increase prostate cancer susceptibility via multiple biological mechanisms. Exposure to light inhibits the release of melatonin, a hormone released by the pineal gland that has anti-carcinogenic properties, its suppression increases the risk of carcinogenesis. Another potential mechanism is that light at night is a general stressor that weakens the immune system and acts as an endocrine disruptor. Extended hours of light during nighttime allows people to participate in nighttime activities, which can disrupt the circadian rhythm on a daily basis and increase the risk of hormone-dependent diseases, including prostate cancer(42). Further, studies have found that circadian genes regulate cancer pathways such as proliferation, DNA damage response, metabolism, and apoptosis(43,44).
In HPFS some participants reported their work addresses instead of their home address, which could introduce non-differential measurement error in assessing outdoor LAN if participants’ outdoor LAN exposure occurs in the home setting. However, many people tend to live close to their work and if they work long hours, exposure to outdoor LAN at work could also be of importance. We were able to distinguish between home and work addresses, and findings for cumulative average outdoor LAN were similar to the whole cohort when restricted to individuals who reported a home address at baseline. There is also potential for misclassification of light at night since we are using satellite-based exposures with a resolution of 1km2, which may be an imprecise estimate of individual exposure to outdoor light at night(45). Although having a personal exposure level of these environmental exposures would help further elucidate prostate cancer etiology, collecting personal level data on these exposures is more costly and challenging to measure over long time periods. Ambient exposure metrics allow us to include a larger sample size as well as long-term exposure information, which is essential for studying cancer etiology. Further, there is potential for residual confounding due to the high correlation between outdoor LAN and urbanicity. Areas with higher levels of outdoor LAN are more likely to be urban and there is greater access to care in urban areas, so the positive association we see between baseline outdoor LAN and prostate cancer could be explained by this residual confounding. However, our study population consists of health professionals with similar access to care and we adjusted for PSA screening and population density, so residual confounding is unlikely to explain our results.
A potential limitation is that the DMSP-OLS data has low spatial resolution compared to other measurements now available, including satellite images from VIIRS-DNB as well as photos taken by astronauts from the international space station(21,22). This lower resolution could lead to measurement error and limited power to examine this relationship(46). Further, we were not able to differentiate spectral bands or study the impact of blue light in prostate carcinogenesis, which has the greatest impact on melatonin production and was found to be associated with greater prostate cancer risk(22). However, these alternate exposure measures were not available prior to 2011 when the majority of the cases in HPFS were diagnosed. Using DMSP-OLS data allowed us to assess the long-term, time-varying impact of outdoor LAN, which is the most relevant for the slow process of carcinogenesis and the long latency observed in prostate cancer specifically.
Our study had some strengths to consider. This was a large prospective cohort study with address data on the individual level. Given updated information on addresses, our study included time-varying outdoor LAN measurements, as well as detailed and time-varying data on important prostate cancer risk factors and geographic correlates of outdoor LAN. The cohort included a large number of participants and prostate cancer cases, and high cohort retention over follow-up. Also, HPFS participants live throughout the United States, making our analysis representative of the large geographic differences in amount of outdoor LAN in the country. Biennial questionnaires collected detailed information on the history of prostate cancer screening, this detailed information on PSA screening allowed us to address potential diagnostic bias by adjusting for two measures of PSA screening and to conduct analyses stratified by screening intensity. We were able to study clinically relevant endpoints of fatal and lethal prostate cancer. This is important given the clinical heterogeneity in prostate cancer, which poses a challenge in studying prostate cancer etiology(29). Further, more aggressive forms of prostate cancer appear to have a different set of risk factors(2,28).
In summary, we did not find an association between cumulative average outdoor LAN and prostate cancer risk overall but did observe a positive association between baseline LAN and total prostate cancer in the population that reported a home address at baseline and did not move over follow-up. Future studies should examine this relationship in more racially and socioeconomically diverse populations as there are large disparities in both light pollution exposure and prostate cancer outcomes in the United States, with Black men more likely to live in areas with high levels of light pollution and experiencing higher prostate cancer incidence and mortality than white men(30,47–49). Further, it would be important to explore this relationship with other measures of outdoor LAN that have higher resolution and in which spectral bands can be distinguished (e.g. VIIRS and photos from the international space station).
Supplementary Material
Acknowledgements
We thank the participants and study personnel of the Health Professionals Follow-up Study for providing the detailed data used in this study. The authors would like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Central registries may also be supported by state agencies, universities, and cancer centers. Participating central cancer registries include the following: Alabama, Alaska, Arizona, Arkansas, California, Delaware, Colorado, Connecticut, Florida, Georgia, Hawaii, Idaho, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Seattle SEER Registry, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, Wyoming. The authors assume full responsibility for analyses and interpretation of these data. We are grateful for administrative support from the Channing Division of Network Medicine, Department of Medicine, Brigham & Women’s Hospital and Harvard Medical School, Boston, MA. The Health Professionals Follow-up Study is supported by National Cancer Institute grant U01 CA167552. This project was supported by an administrative supplement to the U01 and by R01 CA202690. I.M.C. was supported by National Cancer Institute grant T32 CA009001. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest
COI: SCM reports employment with Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and stock ownership of Merck & Co., Inc., Rahway, NJ, USA. LAM has received research funding from Astra Zeneca and Janssen, serves on the EAB for Convergent Therapeutics, and has consulted with Bayer Pharmaceuticals. None of these are related to this project. The other authors declare no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022. Jan;72(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Pernar CH, Ebot EM, Wilson KM, Mucci LA. The Epidemiology of Prostate Cancer. Cold Spring Harb Perspect Med. 2018;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straif K, Baan R, Grosse Y, Secretan B, Ghissassi F El, Bouvard V, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8(12):1065. [DOI] [PubMed] [Google Scholar]
- 4.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans., International Agency for Research on Cancer. Night Shift Work: IARC Monographs on the Identification of Carcinogenic Hazards to Humans Volume 124. 2020. 371 p. [Google Scholar]
- 5.Sigurdardottir LG, Valdimarsdottir UA, Fall K, Rider JR, Lockley SW, Schernhammer E, et al. Circadian disruption, sleep loss, and prostate cancer risk: A systematic review of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markt SC, Flynn-Evans EE, Valdimarsdottir UA, Sigurdardottir LG, Tamimi RM, Batista JL, et al. Sleep duration and disruption and prostate cancer risk: A 23-year prospective study. Cancer Epidemiol Biomarkers Prev. 2016;25(2):302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sigurdardottir LG, Markt SC, Rider JR, Haneuse S, Fall K, Schernhammer ES, et al. Urinary melatonin levels, sleep disruption, and risk of prostate cancer in elderly men. Eur Urol. 2015;67(2):191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wendeu-Foyet MG, Menegaux F. Circadian Disruption and Prostate Cancer Risk: An Updated Review of Epidemiological Evidences. Cancer Epidemiol Biomarkers Prev. 2017;26(7):985–91. [DOI] [PubMed] [Google Scholar]
- 9.Wendeu-Foyet MG, Bayon V, Cénée S, Trétarre B, Rébillard X, Cancel-Tassin G, et al. Night work and prostate cancer risk: Results from the EPICAP Study. Occup Environ Med. 2018. Aug 1;75(8):573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigurdardottir LG, Valdimarsdottir UA, Mucci LA, Fall K, Rider JR, Schernhammer E, et al. Sleep disruption among older men and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(5):872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anisimov VN. The light-dark regimen and cancer development. Neuro-endocrinology Lett. 2002;23 Suppl 2:28–36. [PubMed] [Google Scholar]
- 12.Anisimov VN, Baturin DA, Popovich IG, Zabezhinski MA, Manton KG, Semenchenko AV, et al. EFFECT OF EXPOSURE TO LIGHT-AT-NIGHT ON LIFE SPAN AND SPONTANEOUS CARCINOGENESIS IN FEMALE CBA MICE. Int J Cancer [Internet]. 2004;111:475–9. Available from: www.interscience. [DOI] [PubMed] [Google Scholar]
- 13.Stevens RG, Blask DE, Brainard GC, Hansen J, Lockley SW, Provencio I, et al. Meeting report: The role of environmental lighting and circadian disruption in cancer and other diseases. In: Environmental Health Perspectives. 2007. p. 1357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jasser SA, Blask DE, Brainard GC. Light during darkness and cancer: Relationships in circadian photoreception and tumor biology [Internet]. Vol. 17, Cancer Causes and Control. Springer; 2006. [cited 2020 Dec 2]. p. 515–23. Available from: 10.1007/s10552-005-9013-6 [DOI] [PubMed] [Google Scholar]
- 15.Haim A, Zubida AE. Artificial light at night: Melatonin as a mediator between the environment and epigenome. Philos Trans R Soc B Biol Sci. 2015;370(1667). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blask DE, Dauchy RT, Dauchy EM, Mao L, Hill SM. Light Exposure at Night Disrupts Host/Cancer Circadian Regulatory Dynamics: Impact on the Warburg Effect, Lipid Signaling and Tumor Growth Prevention. PLoS One [Internet]. 2014. [cited 2020 Dec 2];9(8):102776. Available from: www.nih.gov [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyba CCM, Kuester T, De Miguel AS, Baugh K, Jechow A, Hölker F, et al. Artificially lit surface of Earth at night increasing in radiance and extent. Sci Adv. 2017;3(11):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurley S, Goldberg D, Nelson D, Hertz A, Horn-Ross PL, Bernstein L, et al. Light at night and breast cancer risk among california teachers. Epidemiology. 2014;25(5):697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James P, Bertrand KA, Hart JE, Schernhammer ES, Tamimi RM, Laden F. Outdoor light at night and breast cancer incidence in the nurses’ health study II. Environ Health Perspect. 2017;125(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamphar H, Kocifaj M, Limón-Romero J, Paredes-Tavares J, Chakameh SD, Mego M, et al. Light pollution as a factor in breast and prostate cancer. Sci Total Environ. 2022. Feb 1;806:150918. [DOI] [PubMed] [Google Scholar]
- 21.Rybnikova NA, Portnov BA. Outdoor light and breast cancer incidence: a comparative analysis of DMSP and VIIRS-DNB satellite data. Int J Remote Sens. 2017;38(21):5952–61. [Google Scholar]
- 22.Garcia-Saenz A, Sánchez de Miguel A, Espinosa A, Valentin A, Aragonés N, Llorca J, et al. Evaluating the Association between Artificial Light-at-Night Exposure and Breast and Prostate Cancer Risk in Spain (MCC-Spain Study). Environ Health Perspect. 2018;126(4):47011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens RG, Blask DE, Brainard GC, Hansen J, Lockley SW, Provencio I, et al. Meeting Report: The Role of Environmental Lighting and Circadian Disruption in Cancer and Other Diseases. Environ Health Perspect. 2007;115(9):1357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urbano T, Vinceti M, Wise LA, Filippini T. Light at night and risk of breast cancer: a systematic review and dose–response meta-analysis. Int J Health Geogr [Internet]. 2021. Dec 1 [cited 2023 Jan 10];20(1):44. Available from: /pmc/articles/PMC8520294/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kloog I, Haim A, Stevens RG, Portnov BA. Global co-distribution of light at night (LAN) and cancers of prostate, colon, and lung in men. Chronobiol Int. 2009;26(1):108–25. [DOI] [PubMed] [Google Scholar]
- 26.Kim KY, Lee E, Kim YJ, Kim J. The association between artificial light at night and prostate cancer in Gwangju City and South Jeolla Province of South Korea. Chronobiol Int [Internet]. 2017;34(2):203–11. Available from: 10.1080/07420528.2016.1259241 [DOI] [PubMed] [Google Scholar]
- 27.Rybnikova NA, Haim A, Portnov BA. Is prostate cancer incidence worldwide linked to artificial light at night exposures? Review of earlier findings and analysis of current trends. Arch Environ Occup Heal. 2017;72(2):111–22. [DOI] [PubMed] [Google Scholar]
- 28.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121(7):1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahn JL, Giovannucci EL, Stampfer MJ, Jahn J. The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the Prostate-specific Antigen-era Part I: Prevalence of Asymptomatic Prostate Cancer at Autopsy and Random Biopsy. UICC Int J Cancer IJC. 2015;137:2795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebbeck TR. Prostate cancer disparities by race and ethnicity: From nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kensler KH, Rebbeck TR. Cancer progress and priorities: Prostate cancer. Cancer Epidemiol Biomarkers Prev. 2020;29(2):267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rich-Edwards JW, Corsano KA, Stampfer MJ. A BRIEF ORIGINAL CONTRIBUTION Test of the National Death Index and Equifax Nationwide Death Search [Internet]. Vol. 140, American Journal of Epidemiology. 1994. Available from: https://academic.oup.com/aje/article/140/11/1016/179736 [DOI] [PubMed] [Google Scholar]
- 33.NOAA/NGDC - Earth Observation Group [Internet]. [cited 2022 Jul 12]. Available from: https://ngdc.noaa.gov/eog/dmsp.html
- 34.Hurley S, Nelson DO, Garcia E, Gunier R, Hertz A, Reynolds P. A cross-sectional analysis of light at night, neighborhood sociodemographics and urinary 6-sulfatoxymelatonin concentrations: Implications for the conduct of health studies. Int J Health Geogr [Internet]. 2013;12(1):1. Available from: International Journal of Health Geographics; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NOAA/NGDC - Earth Observation Group - Defense Meteorological Satellite Progam, Boulder [Internet]. [cited 2023 Jan 10]. Available from: https://ngdc.noaa.gov/eog/dmsp/download_radcal.html
- 36.Greenland S, Pearl J, Robins JM. Causal Diagrams for Epidemiologic Research. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 37.DeVille N V, Iyer HS, Holland, Isabel Bhupathiraju S, Chai B, James P, Kawachi I, et al. Neighborhood Socioeconomic Status and Mortality in the Nurses’ Health Study (NHS) and the Nurses’ Health Study II (NHSII). Environ Epidemiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med [Internet]. 1989. May 1 [cited 2023 Jan 11];8(5):551–61. Available from: 10.1002/sim.4780080504 [DOI] [PubMed] [Google Scholar]
- 39.Cajochen C, Mü M, Kobialka S, Krä K, Steiner R, Oelhafen P, et al. High Sensitivity of Human Melatonin, Alertness, Thermoregulation, and Heart Rate to Short Wavelength Light. 2005; Available from: http://www. [DOI] [PubMed]
- 40.Aubé M, Roby J, Kocifaj M. Evaluating Potential Spectral Impacts of Various Artificial Lights on Melatonin Suppression, Photosynthesis, and Star Visibility. PLoS One [Internet]. 2013;8(7):67798. Available from: http://www.fqrnt.gouv.qc.ca/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens RG, Zhu Y. Opinion piece Electric light, particularly at night, disrupts human circadian rhythmicity: is that a problem? Available from: 10.1098/rstb.2014.0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haim A, Portnov BA. Light pollution as a new risk factor for human breast and prostate cancers. Light Pollution as a New Risk Factor for Human Breast and Prostate Cancers. 2013. 1–168 p. [Google Scholar]
- 43.Gu F, Zhang H, Hyland PL, Berndt S, Gapstur SM, Wheeler W, et al. Inherited variation in circadian rhythm genes and risks of prostate cancer and three other cancer sites in combined cancer consortia. Int J Cancer [Internet]. 2017. Nov 1 [cited 2020 Dec 2];141(9):1794–802. Available from: 10.1002/ijc.30883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Markt SC, Valdimarsdottir UA, Shui IM, Sigurdardottir LG, Rider JR, Tamimi RM, et al. Circadian clock genes and risk of fatal prostate cancer. Cancer Causes Control [Internet]. 2015. Jan 1 [cited 2020 Dec 2];26(1):25–33. Available from: 10.1007/s10552-014-0478-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huss A, van Wel L, Bogaards L, Vrijkotte T, Wolf L, Hoek G, et al. Shedding some light in the dark—A comparison of personal measurements with satellite-based estimates of exposure to light at night among children in the Netherlands. Environ Health Perspect. 2019;127(6):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McIsaac MA, Sanders E, Kuester T, Aronson KJ, Kyba CCM. The impact of image resolution on power, bias, and confounding. Environ Epidemiol. 2021;5(2):e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nadybal SM, Collins TW, Grineski SE. Light pollution inequities in the continental United States: A distributive environmental justice analysis. Environ Res [Internet]. 2020;189(June):109959. Available from: 10.1016/j.envres.2020.109959 [DOI] [PubMed] [Google Scholar]
- 48.Tessum CW, Apte JS, Goodkind AL, Muller NZ, Mullins KA, Paolella DA, et al. Inequity in consumption of goods and services adds to racial–ethnic disparities in air pollution exposure. Proc Natl Acad Sci [Internet]. 2019. Mar 26 [cited 2020 Dec 1];116(13):6001–6. Available from: https://www.pnas.org/content/116/13/6001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krieger N, Feldman JM, Kim R, Waterman PD. Cancer incidence and multilevel measures of residential economic and racial segregation for cancer registries. JNCI Cancer Spectr. 2018;2(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.


