Abstract
Background:
Despite evidence that low muscle increases the risk of chemotoxicity, most chemotherapies are dosed on body surface area without considering body composition. Among 178 patients with colon cancer, we assessed muscle and adipose tissue with multiple techniques and examined their associations with relative dose intensity (RDI) and adverse events.
Methods:
We estimated 1) cross-sectional skeletal muscle area (SMA) and total adipose tissue (TAT) area at L3 from computed tomography (CT); 2) appendicular lean mass (ALM) and total body fat (TBF) mass from dual-energy X-ray absorptiometry (DXA); and 3) total body skeletal muscle mass using D3-creatine (D3Cr) dilution. We standardized each measurement by its sex-specific standard deviation (SD). The primary outcome was reduced RDI (RDI <85%). The secondary outcome was the number of moderate and severe adverse events during each cycle of chemotherapy. We estimated the associations of muscle and adipose tissue measurements (per SD increase) with reduced RDI using logistic regression and adverse events using generalized estimating equations for repeated measures.
Results:
Higher CT SMA and DXA ALM were significantly associated with lower risk of reduced RDI (odds ratios: 0.56 [0.38, 0.81] for CT SMA; 0.56 [0.37, 0.84] for DXA ALM). No measurements of muscle or adipose tissue were associated with adverse events.
Conclusions:
More muscle was associated with improved chemotherapy completion among patients with colon cancer, whereas muscle and adipose tissue were not associated with adverse events.
Impact:
Considering body composition may help personalize dosing for colon cancer chemotherapy by identifying patients at risk for poor chemotherapy outcomes.
Keywords: Colon Cancer, Body Composition, Computed Tomography, Dual-Energy X-Ray, D3-creatine, Chemotherapy
INTRODUCTION
Adjuvant chemotherapy is the standard of care for patients with stage III colon cancer and recommended for patients with stage II colon cancer at high risk of recurrence (1,2). Several chemotherapeutic agents such as 5-fluorouracil, capecitabine, leucovorin, and oxaliplatin and their combinations are commonly used to treat colon cancer (1,2). For each patient, the doses of chemotherapeutic agents are calculated according to body surface area, which is derived from height and weight (3). However, this strategy does not consider muscle and adipose tissue, which can alter the pharmacokinetics of these agents, affect their volume of distribution and effectiveness, and increase the likelihood of adverse events (4,5). In light of this, the American Society for Clinical Oncology (ASCO) has recently updated the guideline on “Appropriate Systemic Therapy Dosing for Obese Adult Patients With Cancer” and called for additional research on the impact of sarcopenia and other measurements of body composition on optimal antineoplastic dosing (6).
Due to advances in imaging and biochemical technologies, there are multiple, validated techniques for assessing muscle and adipose tissue. Computed tomography (CT) imaging performed for colon cancer staging and surveillance is a reliable tool: cross-sectional skeletal muscle area (SMA) and total adipose tissue (TAT) area at the third lumbar vertebra (L3) correlate well with the whole-body volumes of muscle and adipose tissue on magnetic resonance imaging (MRI), respectively (7). Dual-Energy X-Ray Absorptiometry (DXA) is another accurate method to quantify lean body mass (LBM) and total body fat (TBF) mass (8). Since DXA LBM includes muscle, organs, and other tissues, appendicular lean mass (ALM; a surrogate of limb muscles) is commonly used given its high correlation with the whole-body volume of muscle (9). In addition, there is a growing interest in applying the deuterated-creatine (D3-creatine) dilution method to cancer patients, which can provide an accurate estimate of total body skeletal muscle mass via measuring the whole-body creatine pool size (10). D3-creatine (D3Cr) muscle mass also strongly correlates with the whole-body volume of muscle, but the results from this technique have not been previously reported in any cancer population. Since these techniques reflect different constructs of muscle and adipose tissue, a study in colon cancer patients undertaking all three techniques can additionally inform the impact of different body composition measurements on chemotherapy outcomes.
Relative dose intensity (RDI), the ratio of the delivered dose intensity to planned dose intensity, is a summary measure that reflects dose delay, reduction, and discontinuation of chemotherapy treatment (11). RDI <85% is considered as a clinically significant deviation from standard chemotherapy (12), and the decrease of RDI was reported to be associated with early recurrence and mortality survival among patients with colorectal cancer (CRC) (13,14). An important reason for reduced RDI is the occurrence of adverse events during each cycle of chemotherapy, which is very common in chemotherapy treatment (15). As patients with higher RDI receive more chemotherapy dose, we hypothesize that patients with better chemotherapy completion may experience similar or even more adverse events (1,2). In sum, considering RDI and adverse events is important to inform the associations of body composition and chemotherapy effectiveness.
Among patients with nonmetastatic colon cancer enrolled in a resistance training trial, we assessed their muscle (CT SMA, DXA ALM, and D3Cr muscle mass) and adipose tissue (CT TAT and DXA TBF) at enrollment (prior to or shortly after chemotherapy initiation). We examined the associations of body composition measurements with reduced RDI and the number of moderate and severe adverse events. To our knowledge, this is the first study to use multiple techniques (particularly D3-creatine for total body skeletal muscle mass) to assess body composition in relation to RDI and adverse events among a cancer population.
MATERIALS AND METHODS
Data Source and Study Population
FOcus on Reducing dose-limiting toxicities in Colon cancer with resistance Exercise (FORCE) was an NCI-sponsored multicenter clinical trial conducted in 181 patients with stage II or III colon cancer after curative resection (ClinicalTrials.gov Identifier: NCT03291951) (16). Patients were randomized to the intervention group (resistance training) or the control group (usual care) to examine the differences between RDI and number of moderate and severe adverse events during chemotherapy. As previously described (16), the intervention consisted of home-based prescribed resistance exercise under the supervision of nationally certified exercise professionals. Patients were eligible to enroll before the initiation of their third chemotherapy cycle. For this study, we combined all patients together as one cohort and adjusted for randomization arm in the analysis. Institutional review board approval was obtained from all participating institutions (Kaiser Permanente Northern California, Dana-Farber Cancer Institute, and Penn State Cancer Institute), and all participants provided written, informed consent.
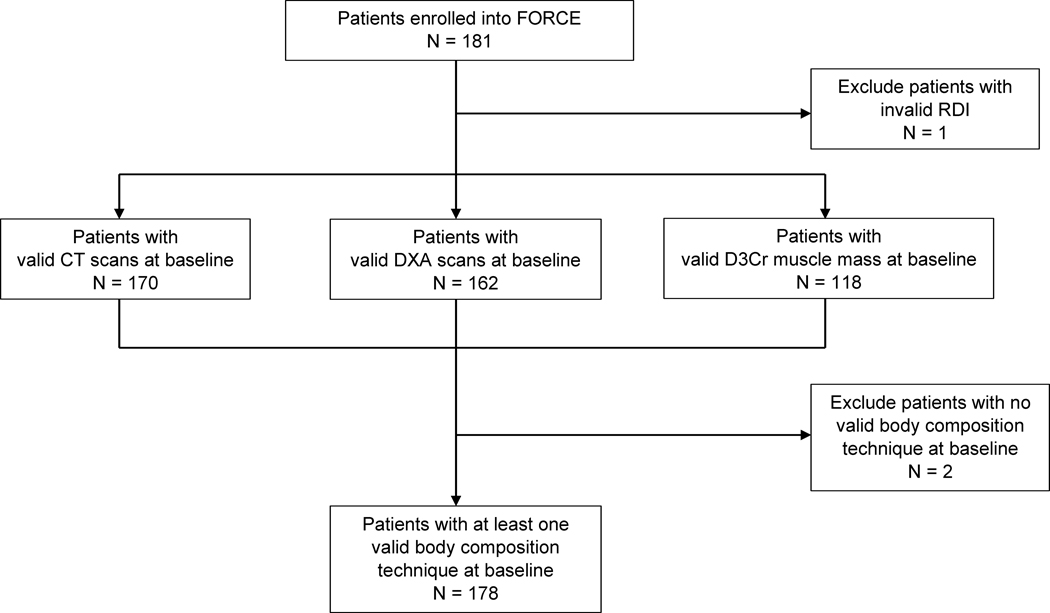
Computed tomography (CT) scans performed for colon cancer diagnosis were extracted from clinical imaging archives. Dual-energy X-ray absorptiometry (DXA) was conducted at the enrollment visit for body composition assessment. D3-creatine dilution was assessed as part of an ancillary study requiring additional consent, and an oral dose of D3-creatine was provided at the enrollment visit for patients who agreed to participate. Patients with at least one available body composition technique were combined as the final population (N = 178; Figure 1). Although not every patient undertook all three techniques, patients in each CT (N = 170), DXA (N = 162), and D3Cr (N=118) group were comparable to the final population (N = 178; Supplementary Table 1).
Figure 1.
The Flow Diagram of Patients in FORCE for Analysis of Body Composition, Relative Dose Intensity, and Adverse Events
Abbreviations: CT, computed tomography; D3Cr, D3-creatine; DXA, Dual X-Ray Absorptiometry; FORCE, FOcus on Reducing Dose-Limiting Toxicities in Colon Cancer with Resistance Exercise; RDI, relative dose intensity.
Muscle
To assess CT SMA at L3, a single research assistant segmented muscle from other tissues using anatomic knowledge and the muscle-specific Hounsfield unit range (−29 HU, 150 HU) on SliceOmatic Software (TomoVision Inc., Magog, Canada) (17). Our prior studies suggested high reliability for CT SMA assessment (coefficient of variation [CV]: 1.2%) (17). CT scans were performed at a median of 8.9 (interquartile: [6.4, 11.4]) weeks before study enrollment.
To assess DXA ALM, certified operators performed the scans using well-calibrated DXA machines in the total body scanning mode. We derived DXA ALM by subtracting adipose tissue mass and bone mass from the total mass of arms and legs. DXA assessment was performed at a median of 1 (interquartile: [0, 7]) day after study enrollment.
To assess D3Cr muscle mass, we provided each patient with a single, oral dose of 60mg D3-creatine at the FORCE enrollment visit. Between 3 and 6 days following the oral dose, patients collected their second voids of morning urine after an overnight fast and kept samples frozen until overnight shipment to a central laboratory facility (storage temperature: −20 °C). Samples were then delivered to University of California, Berkeley (Berkeley, CA) to analyze D3-creatinine enrichment. D3Cr muscle mass was calculated using the algorithm of D3Cr spillage correction as previously described (10,18). Prior studies suggest high reliability (CV: <10%) and robustness of D3Cr assessment to the variation of renal function and hydration status (10,18). D3-creatine assessment was performed at a median of 4 (interquartile: [0, 4]) days after study enrollment.
Adipose Tissue
From the CT scans, the same research staff member also segmented TAT (a sum of visceral, subcutaneous, and intermuscular adipose tissue at L3) using anatomic knowledge and adipose-tissue-specific Hounsfield unit ranges (17). From the DXA scans, certified operators also recorded DXA TBF from DXA machines (16). Prior studies suggested high reliability for CT and DXA in adipose tissue assessment (17,19).
Outcomes
The primary outcome was reduced RDI (RDI <85%) of the regimen, which was defined as the average RDI of chemotherapeutic agents (5-fluorouracil, capecitabine, and oxaliplatin). The RDI of each agent was calculated using the Weycker method (11):
Given that DXA and D3-creatine dilution were assessed at enrollment, which was shortly before randomization (resistance training vs. usual care) but could occur after chemotherapy initiation (16), the starting time point of RDI calculation was selected from the first chemotherapy cycle after the intervention randomization.
The secondary outcome was the number of moderate and severe adverse events that patients reported during each cycle of chemotherapy. To collect the information of adverse events, we would email or mail a questionnaire for every time when a chemotherapy appointment was scheduled. The questionnaire was modified based on Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) (15) and included nine common adverse events induced by colon cancer chemotherapy: nausea, vomiting, diarrhea, shortness of breath, hand-foot syndrome, numbness or tingling of hands or feet, pain, muscle aches, and fatigue (16). The collection of patient-reported toxicities (PRO-CTCAE), rather than physician-reported toxicities (CTCAE), was due to the primary goal of FORCE: this trial was focused on reducing patient-reported symptomatic toxicities through resistance training. Moderate and severe adverse events were defined as PRO-CTCAE grade ≥2 and summed at each cycle of chemotherapy. The median number of self-reported questionnaires that patients completed over the course of the study was 7 (range: 1–14).
Patient Characteristics
We collected patient characteristics through electronic medical records and physical exams at the FORCE enrollment visit: age (years), sex (men, women), race and ethnicity (Asian, Black, Hispanic, non-Hispanic White, and Others), stage (II, III), body mass index (BMI; derived from weight/height2]), BSA (m2), 5-fluorouracil per BSA (mg/m2), capecitabine per BSA (mg/m2), and oxaliplatin per BSA (mg/m2).
Statistical Analysis
We compared patients’ characteristics by RDI status using the t-test for continuous variables and the χ2 test for categorical variables. We standardized each measurement of body composition by its sex-specific standard deviation (SD) (Supplementary Table 2). We estimated the associations of each body composition measurement (per SD increase) with reduced RDI using logistic regression, and the relative changes (%) in the number of moderate and severe adverse events using negative binomial generalized estimating equations (GEE) for repeated measures at each cycle of chemotherapy. The outcome of clinically reduced RDI was dichotomized as <85% (outcome event = 1) vs. ≥85% (outcome event = 0). The relative change (%) was defined as the absolute difference of the number of adverse events divided by the previous number of adverse events, when the body composition measurement increased by 1 SD (20). Both models were adjusted for age, sex, height, regimen, and randomization arm; and GEE models were additionally adjusted for treatment duration (weeks).
For sensitivity analysis, first, we examined if the findings remained similar after applying common methods of scaling body composition measurements to body size (21,22). Rather than using absolute quantity, we scaled these body composition measurements: CT SMA, DXA ALM, CT TAT, and DXA TBF were divided by height squared (m2) (21), whereas D3Cr muscle mass was divided body weight (kg) (22). Second, beyond the regimen, we estimated the associations of body composition with reduced RDI separately for each agent (e.g., 5-fluorouracil vs. capecitabine vs. oxaliplatin). Third, rather than counting from the first cycle after randomization, we calculated an alternative RDI by counting from the first cycle when patients started chemotherapy (rather than from randomization as in our main analyses) and estimated its associations with body composition accordingly.
A priori we examined the associations of each body composition measurement with RDI and adverse events separately by sex, given that men and women differ in quantity and distribution of muscle mass and adipose tissue. For stratification analysis, we included the interaction term between sex and each body composition measurement into its corresponding fully adjusted model.
We conducted analyses using SAS statistical software, version 9.4 (SAS Institute, Inc) and R, version 4.2.0 (R Foundation for Statistical Computing). Statistical significance was defined as P <0.05 or 95% confidence interval (CI) excluding 1.0 (for odds ratios [OR]) or 0 (for relative changes [%]).
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request.
RESULTS
Of 178 patients, the mean (standard deviation [SD]) age was 55.2 (12.8) years, 94 (52.8%) were men, 116 (65.2%) were non-Hispanic White, and 155 (87.1%) were stage III (Table 1). Eighty-seven (48.9%) experienced reduced RDI (RDI <85%). Of 165 patients self-reporting nine adverse events, 148 (89.7%) reported at least one moderate and severe adverse events during chemotherapy. The mean (SD) number of adverse events were 1.1 (1.5) for the first cycle and 2.1 (2.3) for the last cycle. Compared to patients with RDI ≥85%, patients with reduced RDI were more likely to be women and had lower BMI, BSA, oxaliplatin per BSA, CT SMA, and DXA ALM (Table 1).
Table 1.
Patient Characteristics Among 178 Patients with Nonmetastatic Colon Cancer by RDI Status (<85 vs. ≥85%)
| Characteristicsa | All (N = 178) | RDI <85% (N = 87) | RDI ≥85% (N = 91) | P-valueb |
|---|---|---|---|---|
|
| ||||
| Age (years) | 55.2 (12.8) | 55.6 (13.5) | 54.8 (12.2) | 0.66 |
| Sex | 0.02 | |||
| Men | 94 (52.8) | 38 (43.7) | 56 (61.5) | |
| Women | 84 (47.2) | 49 (56.3) | 35 (38.5) | |
| Race | 0.89 | |||
| Asian | 22 (12.4) | 12 (13.8) | 10 (11.0) | |
| Black | 13 (7.3) | 5 (5.7) | 8 (8.8) | |
| Hispanic | 18 (10.1) | 8 (9.2) | 10 (11.0) | |
| Non-Hispanic White | 116 (65.2) | 57 (65.5) | 59 (64.8) | |
| Othersc | 9 (5.1) | 8 (9.2) | 4 (4.4) | |
| Stage | 0.22 | |||
| II | 23 (12.9) | 15 (16.1) | 9 (9.89) | |
| III | 155 (87.1) | 78 (83.9) | 77 (90.6) | |
| BMI (kg/m2) | 27.4 (5.6) | 26.4 (5.6) | 28.4 (5.4) | 0.02 |
| BMI Categories | 0.04 | |||
| Underweight (<18.5 kg/m2) | 1 (0.6) | 1 (1.1) | 0 (0) | |
| Normal (18.5– 24.9 kg/m2) | 68 (38.2) | 41 (47.1) | 27 (29.7) | |
| Overweight (25–29.9 kg/m2) | 55 (30.9) | 24 (27.6) | 31 (34.1) | |
| Obese (≥30 kg/m2) | 54 (30.3) | 21 (24.1) | 33 (36.3) | |
| BSA (m2) | 1.9 (0.3) | 1.9 (0.2) | 2.0 (0.3) | 0.02 |
| 5-fluorouracil per BSA (mg/m2) | 2,732.4 (334.3) | 2,706.8 (421.3) | 2,769.9 (122.4) | 0.34 |
| Capecitabine per BSA (mg/m2) | 1,884.7 (238.4) | 1,900.7 (244.1) | 1,874.7 (236.7) | 0.64 |
| Oxaliplatin per BSA (mg/m2) | 101.1 (21.9) | 97.2 (19.9) | 104.6 (23.2) | 0.03 |
| CT SMA (cm2) | 144.3 (39.1) | 132.3 (35.0) | 154.8 (39.6) | <0.001 |
| DXA ALM (kg) | 20.9 (5.6) | 19.5 (5.5) | 22.1 (5.3) | 0.002 |
| D3Cr Muscle (kg) | 26.7 (7.5) | 25.5 (7.4) | 27.8 (7.4) | 0.10 |
| CT TAT (cm2) | 364.7 (198.9) | 343.9 (197.3) | 382.8 (199.6) | 0.21 |
| DXA TBF (kg) | 27.2 (10.0) | 26.2 (9.7) | 28.1 (10.2) | 0.23 |
Abbreviations: ALM, appendicular lean mass; BMI, body mass index; BSA, body surface area; CT, computed tomography; DXA, dual-energy X-ray absorptiometry; D3Cr, D3-creatine; SMA, skeletal muscle area; TAT, total adipose tissue; TBF, total body fat.
Continuous variables were presented as mean (standard deviation) and categorical variables as number (%). Percentages may not add up to 100% because of rounding.
P-values were calculated using the t-test for continuous variables and the χ2 test for categorical variables. If the assumption of the χ2 test was violated, the Fisher exact test was used instead.
Patients who did not self-identify as Asian, Black, Hispanic, or non-Hispanic White were classified as others for race.
RDI and Adverse Events
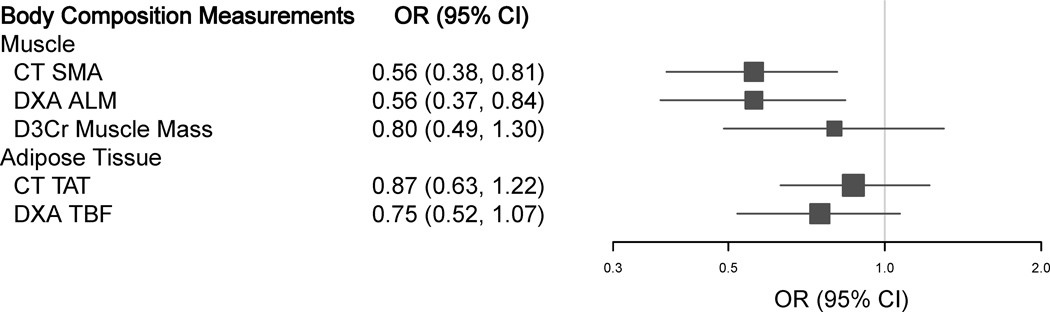
One SD increase in CT SMA and DXA ALM were significantly associated with lower risk of reduced RDI (OR: 0.56 [0.38, 0.81] for CT SMA and 0.56 [0.37, 0.84] for DXA ALM). An inverse (non-significant) association was also observed per SD increase in D3Cr muscle mass (OR: 0.80 [0.49, 1.30]), CT TAT (OR: 0.87 [0.63, 1.22]), and DXA TBF (OR: 0.75 [0.52, 1.07]), but confidence intervals were wide (Table 2 and Figure 2).
Table 2.
The Adjusted and Unadjusted Associations of Body Composition Measurements (Per SD Increase) with the Odds Ratios for Reduced RDI (RDI <85%)
| Body Composition Measurements | Sample Size | Eventsa | Reduced RDI |
|
|---|---|---|---|---|
| Unadjusted | Adjustedb | |||
|
| ||||
| Muscle | ||||
| CT SMA | 170 | 79 | 0.76 (0.57, 1.03) | 0.56 (0.38, 0.81) |
| DXA ALM | 162 | 76 | 0.66 (0.49, 0.89) | 0.56 (0.37, 0.84) |
| D3Cr Muscle Mass | 118 | 56 | 1.09 (0.80, 1.49) | 0.80 (0.49, 1.30) |
| Adipose Tissue | ||||
| CT TAT | 170 | 79 | 0.83 (0.61, 1.13) | 0.87 (0.63, 1.22) |
| DXA TBF | 162 | 76 | 0.75 (0.54, 1.03) | 0.75 (0.52, 1.07) |
Abbreviations: ALM, appendicular lean mass; CI, confidence interval; CT, computed tomography; DXA, dual-energy X-ray absorptiometry; D3Cr, D3-creatine; RDI, relative dose intensity; SD, standard deviation; SMA, skeletal muscle area; TAT, total adipose tissue; TBF, total body fat.
Events refer to the number of reduced RDI.
Adjusted for age (years), sex (men, women), height (cm), regimen (FOLFOX/5FU-LV, CAPOX/CAPE), and randomization arm (resistance training, usual care).
Figure 2.
The Adjusteda Associations of Body Composition Measurements (Per SD Increase) with Reduced RDI
Abbreviations: ALM, appendicular lean mass; CI, confidence interval; CT, computed tomography; DXA, dual-energy X-ray absorptiometry; D3Cr, D3-creatine; RDI, relative dose intensity; OR, odds ratio; SD, standard deviation; SMA, skeletal muscle area; TAT, total adipose tissue; TBF, total body fat.
a Adjusted for age (years), sex (men, women), height (cm), regimen (FOLFOX/5FU-LV, CAPOX/CAPE), and randomization arm (resistance training, usual care).
More muscle or adipose tissue was not significantly associated with the number of moderate and severe adverse events (Table 3 and Figure 3): relative changes in the number of adverse events were 9.7% (−8.2%, 31.1%) for CT SMA, 9.0% (−7.0%, 27.8%) for DXA ALM, −1.5% (−18.5%, 19.1%) for D3Cr muscle mass, 16.0% (−0.4%, 35.0%) for CT TAT, and 15.1% (−1.4%, 34.3%) for DXA TBF.
Table 3.
The Adjusted and Unadjusted Associations of Body Composition Measurements (Per SD Increase) with the Relative Changes (%) in the Number of Moderate and Severe Adverse Events
| Body Composition Measurements | Sample Size | Occurrence Number (%) | Event Number at First Cyclea | Event Number at Last Cyclea | Relative Changes(%) |
|
|---|---|---|---|---|---|---|
| Unadjusted | Adjustedb | |||||
|
| ||||||
| Muscle | ||||||
| CT SMA | 157 | 140 (89.2%) | 1.1 (1.5) | 2.0 (2.4) | 11.0 (−3.4, 27.6) | 9.7 (−8.2, 31.1) |
| DXA ALM | 156 | 139 (89.1%) | 1.1 (1.5) | 2.0 (2.4) | 6.3 (−7.3, 22.0) | 9.0 (−7.0, 27.8) |
| D3Cr Muscle Mass | 113 | 97 (85.8%) | 0.9 (1.5) | 1.7 (2.0) | 2.2 (−9.4, 15.3) | -1.5 (−18.5, 19.1) |
| Adipose Tissue | ||||||
| CT TAT | 157 | 140 (89.2%) | 1.1 (1.5) | 2.0 (2.4) | 13.9 (−1.6, 31.8) | 16.0 (−0.4, 35.0) |
| DXA TBF | 156 | 139 (89.1%) | 1.1 (1.5) | 2.0 (2.4) | 13.6 (−1.9, 31.6) | 15.1 (−1.4, 34.3) |
Abbreviations: ALM, appendicular lean mass; CI, confidence interval; CT, computed tomography; DXA, dual-energy X-ray absorptiometry; D3Cr, D3-creatine; SD, standard deviation; SMA, skeletal muscle area; TAT, total adipose tissue; TBF, total body fat.
Event number refers to the mean (SD) number of moderate and severe adverse events.
Adjusted for age (years), sex (men, women), height (cm), regimen (FOLFOX/5FU-LV, CAPOX/CAPE), randomization arm (resistance training, usual care), and treatment duration (weeks).
Figure 3.
The Adjusteda Associations of Body Composition Measurements (Per SD Increase) with the Relative Changes (%) in the Number of Moderate and Severe Adverse Events
Abbreviations: ALM, appendicular lean mass; CI, confidence interval; CT, computed tomography; DXA, dual-energy X-ray absorptiometry; D3Cr, D3-creatine; SD, standard deviation; SMA, skeletal muscle area; TAT, total adipose tissue; TBF, total body fat.
a Adjusted for age (years), sex (men, women), height (cm), regimen (FOLFOX/5FU-LV, CAPOX/CAPE), randomization arm (resistance training, usual care), and treatment duration (weeks).
Unadjusted associations for RDI and adverse events were also included in the Tables 2 and 3 and generally aligned with the adjusted findings (Figures 2 and 3).
Sensitivity Analysis
Overall, the associations of body composition with RDI and adverse events remained similar (Supplementary Tables 3-5). For chemotherapy agents (Supplementary Table 4), associations were similar in magnitude and direction but may differ in statistical significance for capecitabine vs. 5-fluorouracil and oxaliplatin. For example, the associations of CT SMA with reduced RDI were not significant for capecitabine (OR: 0.64 [0.36, 1.12]), but significant for 5-fluorouracil (OR: 0.46 [0.27, 0.78]) and oxaliplatin (OR: 0.62 [0.43, 0.89]). This was very likely due to the differences of sample size for capecitabine (N = 74) vs. 5-fluorouracil (N = 96) and oxaliplatin (N = 155).
Stratification Analysis
Overall, the associations of body composition with RDI and adverse events did not differ by sex (Supplementary Table 6). For adverse events, we observed 1) only one significant interaction with sex (PInteraction = 0.03 for DXA ALM), which could be a chance finding; and 2) the directions of associations of muscle measurements were generally positive for men vs. negative for women. While the results of this secondary outcome and stratified analysis should be interpreted cautiously, this difference may be due to the larger body surface area and absolute and proportional quantities of muscle in men vs. women. This could have led to greater total chemotherapy dose in men and therefore a positive association with the number of adverse events.
DISCUSSION
To our knowledge, this is the first study to use multiple techniques to assess body composition in relation to RDI and adverse events in a cancer population. We found that higher CT SMA and DXA ALM were significantly associated with lower risk of reduced RDI (RDI <85%). However, no measurements of muscle or adipose tissue were significantly associated with the number of moderate and severe adverse events. Overall, these associations did not differ by sex.
Reduced RDI is a summary measure of dose delay, reduction, and discontinuation to signal the chemotherapy completion (11). Prior studies in CRC have investigated the associations of body composition with dose delay, reduction, and discontinuation (23–30), but few studies reported the associations of body composition with RDI among patients with colorectal or other gastrointestinal cancers. If reported, CT scans were most frequently used since they were available opportunistically as part of clinical care. Using 533 patients with colon cancer at stage II/III undertaking FOLFOX (a combination of leucovorin, 5-fluorouracil, and oxaliplatin), we previously reported that higher CT SMA was associated, but not significantly, with lower risk of reduced RDI (OR: 0.46 [0.21, 1.02]) (23). The findings remained similar for stratified chemotherapy agents: the ORs were 0.45 (0.20, 1.01) for 5-fluorouracil and 0.82 (0.40, 1.69) for oxaliplatin (23). Another study included 188 patients with gastric cancer at stage II/III undertaking fluoropyrimidine-based chemotherapy and found that patients with higher psoas muscle index (≥3.2 cm2/m2) had higher RDI than those with lower psoas muscle index (<3.2 cm2/m2): 72.0% vs. 51.6% (P = 0.02). However, this analysis was unadjusted for potential confounders such as age (31). No studies reported the association of adipose tissue with RDI among patients with CRC. One study investigated BMI, a surrogate of total body adiposity and found that average RDI of FOLFOX did not differ by BMI groups (32). Overall, our findings were consistent with the small number of previous studies addressing this topic: more muscle is associated with improved chemotherapy completion. The insignificant association between D3Cr muscle mass and reduced RDI (OR: 0.80 [0.49, 1.30]) was likely due to the smaller sample size of the D3Cr group (N = 119) compared to the groups of CT (n = 170) and DXA (N = 162). Such findings also aligned with our observation that women were more likely to have reduced RDI (Table 1), since women typically have less muscle than men (33). Thus, in this analysis, we considered sex differences in body composition and standardized body composition measurements by their sex-specific standard deviations. In addition to muscle assessment, our study is the first to directly assess adipose tissue and report that higher CT TAT and DXA TBF were not associated with reduced RDI: these findings added to the previous literature using BMI only.
Regarding body composition and adverse events in CRC, there were seven studies looking into CT SMA (26,27,29,30,34–36) and one study looking into CT TAT (30). To our knowledge, no prior study reported the associations of DXA ALM, D3Cr muscle mass, or DXA TBF with adverse events. Instead of using the number of moderate and severe events, prior studies focused on the occurrence of any severe event (grade ≥3) or any dose-limiting toxicity (a result of adverse events leading to dose delay, reduction, and discontinuation). Higher CT SMA was inconsistently associated with the occurrence of severe adverse events or dose limiting toxicities (26,27,29,30,34–36), and some studies with small sample size reported ORs with wide confidence intervals such as 12.99 (1.25, 134.80) and 13.55 (1.08, 169.31) (34,35). The only study examining CT TAT reported no association with dose limiting toxicities (30). In line with previous studies, we found that muscle and adipose tissue were not significantly associated with self-reported moderate or severe adverse events. Adding rigor to our approach, we repeatedly measured adverse events, assessed muscle and adipose tissue with multiple techniques, and adjusted for potential confounders in statistical analyses.
Since most chemotherapeutic agents have narrow therapeutic windows (37), it is important to individualize chemotherapy dosing. Most chemotherapy is dosed on BSA, given the belief that patients have different volumes of distribution and metabolizing capacities if they differ in body size (4). However, it is well-known that body composition often differs between patients with the same BSA (38), and body compartments including muscle and adipose tissue may have different impact on pharmacokinetics. For example, skeletal muscle accounts for 40% of body mass and is 75% water (39), which makes it the largest body compartment for the distribution of hydrophilic chemotherapy agents and many drugs to treat colon cancer (such as 5-fluorouracil) are hydrophilic (40). In this study, we found that more muscle was significantly associated with lower risk of reduced RDI, particularly for 5-fluorouracil (OR: 0.46 [0.27, 0.78] for CT SMA and 0.47 [0.26, 0.84] for DXA ALM) (41). Since patients with more muscle may have improved chemotherapy completion, one may hypothesize that patients with more muscle should have lower numbers of moderate and severe advents. However, adverse events are very common in the chemotherapy treatment and increase with duration of chemotherapy (42). In this study, we found that 89.7% of patients reported at least one adverse event during chemotherapy (similar to ~90% reported by prior studies (43,44)) and patients with more muscle safely endured more chemotherapy doses without more adverse events. For example, more muscle may attenuate chemotherapy-induced inflammatory responses that can contribute to neuropathy and other adverse events (45), whereas low muscle is an important indicator for frailty and accelerated biological age that have been frequently linked to increased risk of chemotherapy intolerance and adverse events (46,47). Increasing evidence suggests incorporating biomarkers of frailty and biological age may help personalize cancer treatment and supportive care (48,49), which further supports the importance of examining the role of muscle in chemotherapy dosing, completion, and adverse events.
As for adipose tissue, it was previously commonplace for oncologists to cap the chemotherapy dose for obese patients with high BSA (e.g., >2 m2) (50). However, the latest ASCO guideline suggested full dosing, given that obese patients tolerate chemotherapy as well as nonobese patients (6). In line with this latest suggestion, our findings suggested that increased adiposity (CT TAT and DXA TBF) was not associated with unfavorable chemotherapy outcomes. One possible explanation is that most chemotherapeutic agents are partly distributed through adipose tissue unless they have weak lipophilicity (51). For example, while 5-fluorouracil and oxaliplatin are often considered as hydrophilic, they also have moderate lipophilicity, especially for lipophilic derivatives of 5-fluorouracil and oxaliplatin (52,53); and capecitabine is generally considered as a lipophilic chemotherapeutic agent (51). Thus, chemotherapy dosing should consider both adipose tissue and pharmacokinetics of specific drugs.
Clinical Impact
Our findings have the potential to inform clinical practice. Using multiple imaging and biochemical techniques, we assessed muscle and adipose tissue among patients with colon cancer and investigated their associations with chemotherapy completion and adverse events. We found that more muscle was associated with improved chemotherapy completion, whereas neither muscle nor adipose tissue was associated with adverse events. While most chemotherapies are dosed on body surface area without considering body composition, these findings suggest that patients with increasing pre-chemotherapy muscle (as measured by 1 SD increase of CT SMA or DXA ALM) may tolerate treatment better and therefore are less likely to experience reduced RDI (RDI <85%). Future studies should investigate clinical thresholds of muscle, below which can identify patients at higher risk of reduced RDI (RDI <85%).
Strengths and Limitations
This study has several strengths including multiple techniques to assess muscle and adipose tissue and a variety of robust statistical and sensitivity analyses. However, several limitations should be noted. First, the sample size is relatively small, and a larger study is needed to generate more precise estimates of the impact of body composition on colon cancer chemotherapy outcomes. Second, this study focused on body composition at study enrollment: longitudinal studies with repeated body composition assessments are needed to confirm these findings. Third, each assessment may be subject to measurement error, which can weaken the associations. For example, multiple types of clinical CT and research DXA scanners were used across sites, which may lead to minor differences in the quantification of muscle and adiposity. Fourth, we assessed body composition using a single manually segmented CT slice, rather than over a larger field of view or at multiple anatomic landmarks. However, single-slice abdominal measurements of muscle and adipose tissue are considered a reference method for body composition, and they correlate well with the whole-body volumes from MRI (7). Fifth, we did not have information regarding adverse events for chemotherapy cycles prior to study enrollment. Sixth, our study population was derived from an intervention trial, which may be younger and have better physical function than the general colon cancer population.
In conclusion, more muscle was associated with improved chemotherapy completion among patients with nonmetastatic colon cancer. No measurements of muscle or adipose tissue were significantly associated with percent changes in the number of self-reported moderate and severe adverse events over time following enrollment into the FORCE study. Considering body composition in colon cancer treatment may help personalize chemotherapy dosing and identify patients at high risk of reduced dose intensity.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Cancer Institute of the National Institutes of Health: R01CA240394 (PI: E.M. Cespedes Feliciano) and R01CA206196 (MPI: B.J. Caan, J.A. Meyerhardt, and K.H. Schmitz). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest:
Dr. Jeffrey A. Meyerhardt has served as an advisor/consultant to Merck Pharmaceutical and COTA Healthcare.
REFERENCES
- 1.Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw 2018;16(4):359–69 doi 10.6004/jnccn.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baxter NN, Kennedy EB, Bergsland E, Berlin J, George TJ, Gill S, et al. Adjuvant Therapy for Stage II Colon Cancer: ASCO Guideline Update. J Clin Oncol 2022;40(8):892–910 doi 10.1200/JCO.21.02538. [DOI] [PubMed] [Google Scholar]
- 3.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 1987;317(17):1098 doi 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 4.Hopkins JJ, Sawyer MB. A review of body composition and pharmacokinetics in oncology. Expert Rev Clin Pharmacol 2017;10(9):947–56 doi 10.1080/17512433.2017.1347503. [DOI] [PubMed] [Google Scholar]
- 5.Silvestris N, Argentiero A, Natalicchio A, D’Oronzo S, Beretta GD, Acquati S, et al. Antineoplastic dosing in overweight and obese cancer patients: an Associazione Italiana Oncologia Medica (AIOM)/Associazione Medici Diabetologi (AMD)/Societa Italiana Endocrinologia (SIE)/Societa Italiana Farmacologia (SIF) multidisciplinary consensus position paper. ESMO Open 2021;6(3):100153 doi 10.1016/j.esmoop.2021.100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griggs JJ, Bohlke K, Balaban EP, Dignam JJ, Hall ET, Harvey RD, et al. Appropriate Systemic Therapy Dosing for Obese Adult Patients With Cancer: ASCO Guideline Update. J Clin Oncol 2021;39(18):2037–48 doi 10.1200/JCO.21.00471. [DOI] [PubMed] [Google Scholar]
- 7.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97(6):2333–8 doi 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 8.Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 2018;9(2):269–78 doi 10.1002/jcsm.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr 2002;76(2):378–83 doi 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- 10.Clark RV, Walker AC, O’Connor-Semmes RL, Leonard MS, Miller RR, Stimpson SA, et al. Total body skeletal muscle mass: estimation by creatine (methyl-d3) dilution in humans. J Appl Physiol (1985) 2014;116(12):1605–13 doi 10.1152/japplphysiol.00045.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weycker D, Barron R, Edelsberg J, Kartashov A, Lyman GH. Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res Treat 2012;133(1):301–10 doi 10.1007/s10549-011-1949-5. [DOI] [PubMed] [Google Scholar]
- 12.Nielson CM, Bylsma LC, Fryzek JP, Saad HA, Crawford J. Relative Dose Intensity of Chemotherapy and Survival in Patients with Advanced Stage Solid Tumor Cancer: A Systematic Review and Meta-Analysis. Oncologist 2021;26(9):e1609–e18 doi 10.1002/onco.13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aspinall SL, Good CB, Zhao X, Cunningham FE, Heron BB, Geraci M, et al. Adjuvant chemotherapy for stage III colon cancer: relative dose intensity and survival among veterans. BMC cancer 2015;15(1):1–13 doi 10.1186/s12885-015-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Żok J, Bieńkowski M, Radecka B, Korniluk J, Adamowicz K, Duchnowska R. Impact of relative dose intensity of oxaliplatin in adjuvant therapy among stage III colon cancer patients on early recurrence: a retrospective cohort study. BMC cancer 2021;21(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 2014;106(9) doi 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caan BJ, Meyerhardt JA, Brown JC, Campbell KL, Cespedes Feliciano EM, Lee C, et al. Recruitment strategies and design considerations in a trial of resistance training to prevent dose-limiting toxicities in colon cancer patients undergoing chemotherapy. Contemp Clin Trials 2021;101:106242 doi 10.1016/j.cct.2020.106242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, et al. Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev 2017;26(7):1008–15 doi 10.1158/1055-9965.EPI-17-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shankaran M, Czerwieniec G, Fessler C, Wong PA, Killion S, Turner SM, et al. Dilution of oral D3 -Creatine to measure creatine pool size and estimate skeletal muscle mass: development of a correction algorithm. J Cachexia Sarcopenia Muscle 2018;9(3):540–6 doi 10.1002/jcsm.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borga M, West J, Bell JD, Harvey NC, Romu T, Heymsfield SB, et al. Advanced body composition assessment: from body mass index to body composition profiling. J Investig Med 2018;66(5):1–9 doi 10.1136/jim-2018-000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Törnqvist L, Vartia P, Vartia YO. How should relative changes be measured? Am Stat 1985;39(1):43–6. [Google Scholar]
- 21.Cheng E, Kirley J, Cespedes Feliciano EM, Caan BJ. Adiposity and cancer survival: a systematic review and meta-analysis. Cancer Causes Control 2022;33(10):1219–46 doi 10.1007/s10552-022-01613-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orwoll ES, Blackwell T, Cummings SR, Cauley JA, Lane NE, Hoffman AR, et al. CT Muscle Density, D3Cr Muscle Mass, and Body Fat Associations With Physical Performance, Mobility Outcomes, and Mortality Risk in Older Men. J Gerontol A Biol Sci Med Sci 2022;77(4):790–9 doi 10.1093/gerona/glab266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cespedes Feliciano EM, Lee VS, Prado CM, Meyerhardt JA, Alexeeff S, Kroenke CH, et al. Muscle mass at the time of diagnosis of nonmetastatic colon cancer and early discontinuation of chemotherapy, delays, and dose reductions on adjuvant FOLFOX: The C-SCANS study. Cancer 2017;123(24):4868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JC, Meyerhardt JA, Cespedes Feliciano EM, Cheng E, Caan BJ. The association of abdominal adiposity with premature discontinuation of postoperative chemotherapy in colon cancer. Clin Nutr 2022;41(7):1600–4 doi 10.1016/j.clnu.2022.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali R, Baracos VE, Sawyer MB, Bianchi L, Roberts S, Assenat E, et al. Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med 2016;5(4):607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, den Braver NR, Berkhof J, Langius JA, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol 2016;34(12):1339–44. [DOI] [PubMed] [Google Scholar]
- 27.Kurk S, Peeters P, Stellato R, Dorresteijn B, de Jong P, Jourdan M, et al. Skeletal muscle mass loss and dose-limiting toxicities in metastatic colorectal cancer patients. J Cachexia Sarcopenia Muscle 2019;10(4):803–13 doi 10.1002/jcsm.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5-fluorouracil–based chemotherapy toxicity. Clin Cancer Res 2007;13(11):3264–8. [DOI] [PubMed] [Google Scholar]
- 29.da Silva Dias D, Machado M, Trabulo C, Gosalbez B, Ravasco P. Impact of Body Composition on Prognosis and Dose-Limiting Toxicities on Metastatic Colorectal Cancer. Front Nutr 2021;8:671547 doi 10.3389/fnut.2021.671547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plas RL, van Norren K, van Baar H, van Aller C, de Bakker M, Botros N, et al. Side-effects related to adjuvant CAPOX treatment for colorectal cancer are associated with intermuscular fat area, not with total skeletal muscle or fat, a retrospective observational study. JCSM Clin Rep 2018;3(1):1–13. [Google Scholar]
- 31.Fujita S, Sakuramoto S, Matsui K, Ebara G, Nishibeppu K, Oya S, et al. Relative dose intensity and 1-year psoas muscle index reduction rate as prognostic factors in gastric cancer patients with postoperative adjuvant chemotherapy. Int J Clin Oncol 2022:1–11. [DOI] [PubMed] [Google Scholar]
- 32.Suibhne SN, King F, Treacy V, Kennedy J, Collins I, Weidmann A. Toxicity and relative dose intensity (RDI) of FOLFOX 6 chemotherapy in patients of differing body mass index treated for colorectal cancer. Eur J Hosp Pharm 2012;19(2):238–9 doi 10.1136/ejhpharm-2012-000074.403. [DOI] [Google Scholar]
- 33.Janssen I, Heymsfield SB, Wang Z, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. Journal of applied physiology 2000. [DOI] [PubMed] [Google Scholar]
- 34.Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer 2014;66(4):583–9. [DOI] [PubMed] [Google Scholar]
- 35.Gökyer A, Küçükarda A, Köstek O, Hacıoğlu M, Sunal B, Demircan N, et al. Relation between sarcopenia and dose-limiting toxicity in patients with metastatic colorectal cancer who received regorafenib. Clin Transl Oncol 2019;21(11):1518–23. [DOI] [PubMed] [Google Scholar]
- 36.Jung HW, Kim JW, Kim JY, Kim SW, Yang HK, Lee JW, et al. Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support Care Cancer 2015;23(3):687–94 doi 10.1007/s00520-014-2418-6. [DOI] [PubMed] [Google Scholar]
- 37.Hottinger M, Liang BA. Deficiencies of the FDA in evaluating generic formulations: addressing narrow therapeutic index drugs. Am J Law Med 2012;38(4):667–89 doi 10.1177/009885881203800403. [DOI] [PubMed] [Google Scholar]
- 38.Hopkins JJ, Sawyer MB. Interactions of lean soft-tissue and chemotherapy toxicities in patients receiving anti-cancer treatments. Cancer Chemother Pharmacol 2018;82(1):1–29 doi 10.1007/s00280-018-3614-8. [DOI] [PubMed] [Google Scholar]
- 39.Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int 2015;96(3):183–95 doi 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- 40.Lin TF, Yeh SH. Thermosensitive Interfacial Migration of 5-FU in the Microenvironment of Pluronic Block Copolymers. Polymers (Basel) 2021;13(16) doi 10.3390/polym13162705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knikman JE, Rosing H, Guchelaar HJ, Cats A, Beijnen JH. A review of the bioanalytical methods for the quantitative determination of capecitabine and its metabolites in biological matrices. Biomed Chromatogr 2020;34(1):e4732 doi 10.1002/bmc.4732. [DOI] [PubMed] [Google Scholar]
- 42.Ingrand I, Defossez G, Lafay-Chebassier C, Chavant F, Ferru A, Ingrand P, et al. Serious adverse effects occurring after chemotherapy: A general cancer registry-based incidence survey. Br J Clin Pharmacol 2020;86(4):711–22 doi 10.1111/bcp.14159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearce A, Haas M, Viney R, Pearson SA, Haywood P, Brown C, et al. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS One 2017;12(10):e0184360 doi 10.1371/journal.pone.0184360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyerhardt JA, Shi Q, Fuchs CS, Meyer J, Niedzwiecki D, Zemla T, et al. Effect of Celecoxib vs Placebo Added to Standard Adjuvant Therapy on Disease-Free Survival Among Patients With Stage III Colon Cancer: The CALGB/SWOG 80702 (Alliance) Randomized Clinical Trial. JAMA 2021;325(13):1277–86 doi 10.1001/jama.2021.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cata JP, Weng HR, Lee BN, Reuben JM, Dougherty PM. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol 2006;72(3):151–69. [PubMed] [Google Scholar]
- 46.Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin 2017;67(5):362–77 doi 10.3322/caac.21406. [DOI] [PubMed] [Google Scholar]
- 47.Ho YW, Tang WR, Chen SY, Lee SH, Chen JS, Hung YS, et al. Association of frailty and chemotherapy-related adverse outcomes in geriatric patients with cancer: a pilot observational study in Taiwan. Aging (Albany NY) 2021;13(21):24192–204 doi 10.18632/aging.203673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hägg S, Jylhävä J. Should we invest in biological age predictors to treat colorectal cancer in older adults? Eur J Surg Oncol 2020;46(3):316–20 doi 10.1016/j.ejso.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Mandelblatt JS, Ahles TA, Lippman ME, Isaacs C, Adams-Campbell L, Saykin AJ, et al. Applying a Life Course Biological Age Framework to Improving the Care of Individuals With Adult Cancers: Review and Research Recommendations. JAMA Oncol 2021;7(11):1692–9 doi 10.1001/jamaoncol.2021.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powis G, Reece P, Ahmann DL, Ingle JN. Effect of body weight on the pharmacokinetics of cyclophosphamide in breast cancer patients. Cancer Chemother Pharmacol 1987;20(3):219–22 doi 10.1007/BF00570489. [DOI] [PubMed] [Google Scholar]
- 51.Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet 2010;49(2):71–87 doi 10.2165/11318100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 52.Lollo G, Matha K, Bocchiardo M, Bejaud J, Marigo I, Virgone-Carlotta A, et al. Drug delivery to tumours using a novel 5-FU derivative encapsulated into lipid nanocapsules. J Drug Target 2019;27(5–6):634–45 doi 10.1080/1061186X.2018.1547733. [DOI] [PubMed] [Google Scholar]
- 53.Abu Ammar A, Raveendran R, Gibson D, Nassar T, Benita S. A Lipophilic Pt(IV) Oxaliplatin Derivative Enhances Antitumor Activity. J Med Chem 2016;59(19):9035–46 doi 10.1021/acs.jmedchem.6b00955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request.