Abstract
Background and Objectives:
Over 25% of patients diagnosed with colorectal cancer (CRC) will develop colorectal liver metastases (CRLM). Controversy exists over the surgical management of these patients. This study aims to investigate the safety of a simultaneous surgical approach by stratifying patients based on procedure risk and operative approach.
Methods:
Using ACS-NSQIP (2016-2020), patients with CRC who underwent isolated colorectal, isolated hepatic, or simultaneous resections were identified. Colorectal and hepatic procedures were stratified by morbidity risk (high vs. low) and operative approach (open vs. minimally invasive). 30-day overall morbidity was compared between risk matched isolated and simultaneous resection groups.
Results:
65,417 patients were identified, with 1550 (2.4%) undergoing simultaneous resections. 1207 (77.9%) underwent a low-risk colorectal and low-risk liver resection. On multivariate analysis, there was no significant difference in overall morbidity between patients who had a simultaneous open high-risk colorectal/low-risk hepatic procedure compared to patients who had an isolated open high-risk colorectal procedure (OR, 1.19; 95% CI, 0.94-1.50; p=0.148). All other combinations of simultaneous procedures had statistically significant higher rates of morbidity than the isolated group.
Conclusions:
Simultaneous resection of colorectal and synchronous CRLM is associated with an increased risk of morbidity in most circumstances in a risk stratified analysis, although rates of readmission and reoperation were not increased. Minimally invasive surgical approaches may significantly mitigate this morbidity.
Keywords: minimally invasive surgery, colorectal cancer, hepatic metastases
INTRODUCTION
Colorectal cancer (CRC) is the third most common cause of cancer-related death in the United States [1]. Approximately 20-25% of patients present with metastatic disease at the time of colorectal cancer diagnosis [2]. For patients with resectable colorectal liver metastases (CRLM), surgical resection is the preferred treatment modality and offers excellent survival benefit compared to other stage 4 cancers, with a 5-year survival rate of approximately 38% and a median overall survival of 3.6 years [3]. Resection of CRLM can be performed simultaneously with the primary tumor or in a staged approach. Simultaneous resection offers potential benefits such as limiting the number of surgeries and anesthesia, quicker decrease of the tumor burden, and the potential to start adjuvant therapy earlier. Current literature is conflicting over whether simultaneous resection is safe or associated with an unacceptable increased risk of postoperative morbidity [4–6]. Although there is a general consensus that patients who undergo simultaneous procedures involving complex colorectal resections and multiple liver segments have increased risk of postoperative morbidity and mortality, it remains unclear if these findings are similarly observed in patients undergoing simultaneous procedures for less complex resections [4–6].
Moreover, recent advances in surgical techniques and operative approaches may help mitigate some of the morbidity traditionally associated with simultaneous hepatobiliary and colorectal surgery. Minimally invasive surgery (MIS) techniques offer the benefit of smaller incisions, possible decrease in postoperative pain, and shorter length of stay (LOS). With respect to simultaneous resection of CRLM and the primary tumor, an MIS approach has been associated with decreased LOS, blood loss, and hospital costs compared to an open approach, though these findings have only been reported in small case reports and single-center institutional studies [7,8].
This study aims to investigate these controversies by performing a procedure risk-stratified postoperative outcomes comparison between simultaneous and isolated colorectal and/or hepatic resections; and by investigating the impact of an MIS approach on outcomes using a large national dataset. We hypothesize that there may be safe combinations of colorectal and hepatic procedures that do not demonstrate increases in postoperative morbidity and mortality and that an MIS approach may mitigate some of the risks associated with a combined major liver and complex colorectal resection.
MATERIALS AND METHODS
Data Source
This was a retrospective analysis using the 2016–2020 American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database and its associated Colectomy, Proctectomy, and Hepatectomy procedure-targeted files. ACS-NSQIP is a nationally validated, risk-adjusted, outcomes-based database developed by surgeons that collects data on patients undergoing surgery from over 700 participating member hospitals of varying size and academic affiliation. This program allows for the prospective systematic data collection of more than 150 preoperative and intraoperative variables and has a 95% success rate with capturing variables related to 30-day postoperative morbidity and mortality [9,10]. This study was reviewed and approved by the Institutional Review Board of the Johns Hopkins University School of Medicine.
Study Population
Patients ≥18 years of age diagnosed with colorectal cancer or metastases from colorectal cancer who underwent either an isolated colorectal procedure, isolated hepatic procedure, or simultaneous colorectal and hepatic procedure were identified. Colon cancer diagnoses were identified using International Classification of Diseases, 9th, and 10th Revisions (ICD-9/10) codes. Colorectal and hepatic procedures were identified using Current Procedural Terminology (CPT) codes (Supplement A). Patients who underwent other major concurrent procedures, emergency procedures, or procedures for malignant bowel obstruction were excluded. Additionally, patients who were American Society of Anesthesiology (ASA) class V and/or had missing information on ASA classification and those who had a missing operative approach or one other than robotic, laparoscopic, or open were also excluded.
Baseline Characteristics of Patients
Demographic characteristics included age (<50, 50-59, 60-69, ≥ 70), sex, and race [white, black, other (includes American Indian, American Hawaiian, Asian, and other), unknown]. Age ranges were selected to have approximately evenly distributed groupings (~25% of patients) in the simultaneous resection cohort. Baseline clinical characteristics included ASA classification (I-II, III, IV), dependent functional status, obesity (Body Mass Index [BMI] ≥ 30), smoking status, diabetes, hypertension, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), pre-operative neoadjuvant chemotherapy within 90 days of surgery, immunosuppressive or steroid use within 30 days of surgery, procedure risk and operative approach. Operative approach (planned open, MIS) was categorized based on an “intention-to-treat” approach, with robotic and laparoscopic approaches forming a single MIS category.
Outcomes
The primary outcome was 30-day postoperative overall morbidity, defined as the occurrence of one or more of the following adverse events: wound infection, pneumonia, urinary tract infection (UTI), venous thromboembolism (VTE), cardiac complication, shock/sepsis, unplanned intubation, bleeding requiring transfusion, renal complication, on ventilator >48 hours, and organ/space surgical site infection (SSI). Secondary outcomes included serious morbidity, defined based on Clavien-Dindo class III-IV (cardiac or renal complications, shock/sepsis, unplanned intubation, on ventilator >48 hours, organ/space SSI, or reoperation) [11]. Other secondary outcomes were reoperation, 30-day postoperative mortality, hospital LOS, operative time, and 30-day postoperative readmission.
Procedure Risk Stratification of Patients
Patients were divided into high-risk or low-risk groups, based on the overall 30-day postoperative morbidity of the procedures performed. To determine the procedure risk stratification, CPT codes were grouped into 8 categories for colorectal procedures (right colectomy, left colectomy without diversion, left colectomy with diversion, abdominoperineal resection, total abdominal colectomy [TAC], low anterior resection [LAR], total proctocolectomy [TPC], partial proctectomy with low pelvic anastomosis) and 4 categories for hepatic procedures (partial hepatectomy, left hepatectomy, right hepatectomy, trisectionectomy) (Supplement A) [12]. These 12 procedure categories were each further divided into two groups based on operative approach [open and MIS (includes robotic and laparoscopic)]. Using the NSQIP database, overall 30-day postoperative morbidity (defined in the Outcomes section) for each of these 24 categories was calculated. Open colorectal, MIS colorectal, open hepatic, and MIS hepatic procedures were then separately ranked from lowest to highest morbidity (Figures 1, 2, and 3). In each group, operations were then divided into high-risk and low-risk groups based on a consensus among the authors after looking at demarcations in morbidity rates among procedures. After consolidation of the isolated colorectal and hepatic resections into risk groups based on approach, a Chi-squared test was performed to compare overall and serious morbidity between the two risk groups to ensure appropriate risk groupings. High risk colorectal procedures were defined those having ≥35% morbidity rate for open procedures and ≥25% morbidity rate for MIS procedures (due to the lower overall morbidity associated with MIS colorectal procedures). High risk hepatic procedures were defined those having ≥35% morbidity rate for open and MIS procedures.
Figure 1.
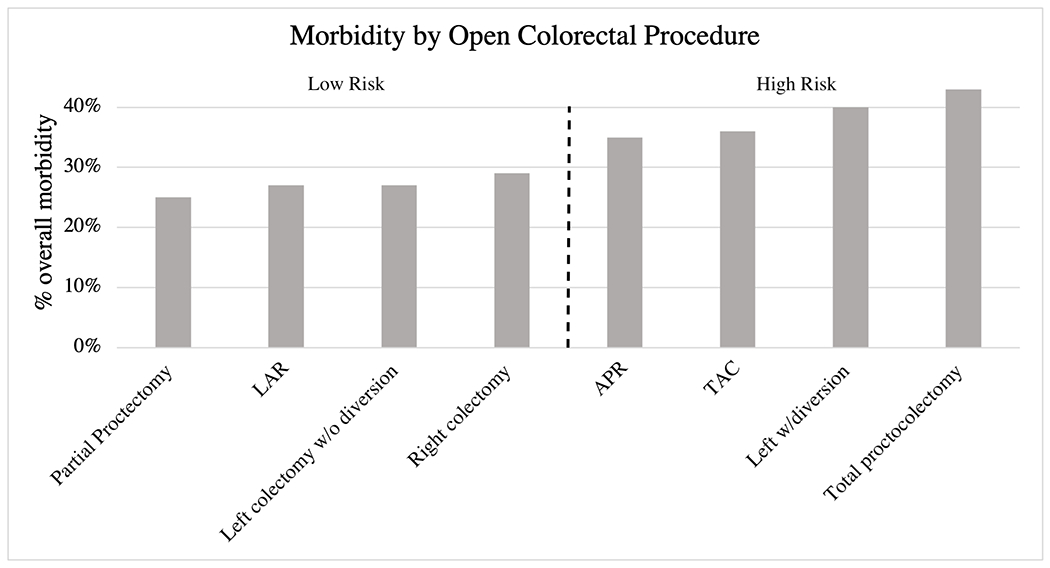
Overall morbidity by colorectal procedure for open approach. The dotted line denotes the procedures included in the high risk and low risk groups. Abbreviations: LAR, low anterior resection; APR, abdominoperineal resection; TAC, total abdominal colectomy; MIS, minimally invasive surgery (laparoscopic and robotic)
Figure 2.
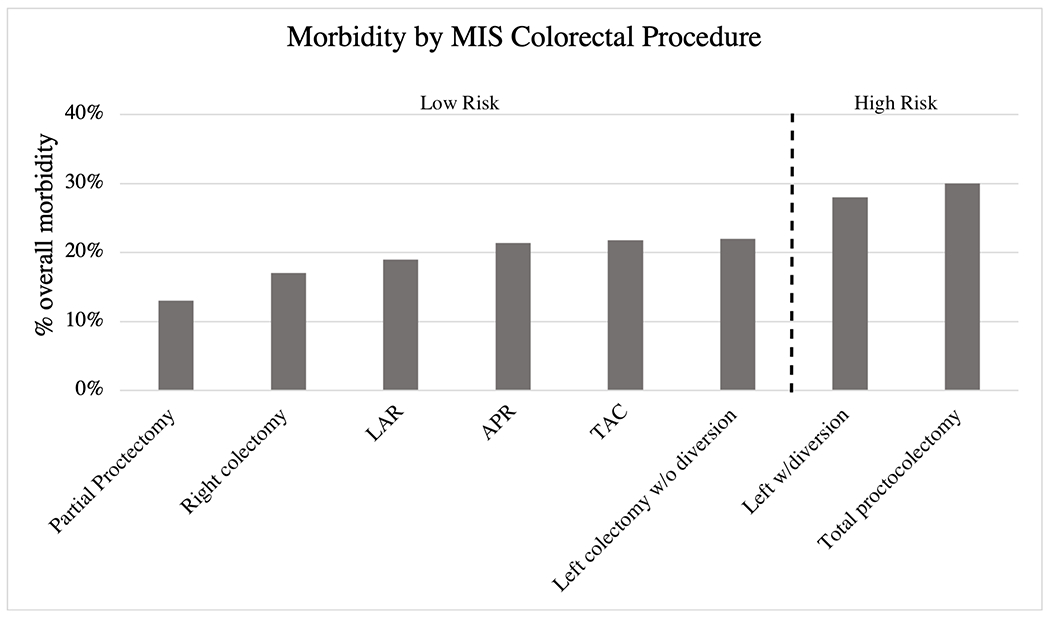
Overall morbidity by colorectal procedure for MIS approach. The dotted line denotes the procedures included in the high risk and low risk groups. Abbreviations: LAR, low anterior resection; APR, abdominoperineal resection; TAC, total abdominal colectomy; MIS, minimally invasive surgery (laparoscopic and robotic)
Figure 3.
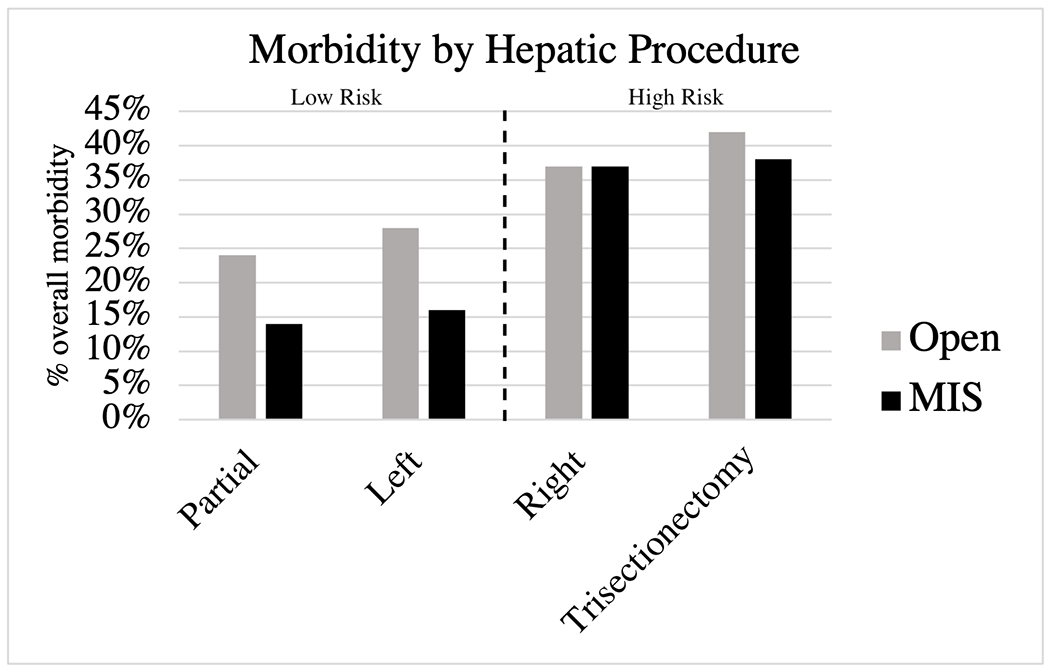
Overall morbidity by hepatic procedure for open and MIS approach. The dotted line denotes the procedures included in the high risk and low risk groups. Abbreviations: MIS, minimally invasive surgery (laparoscopic and robotic)
Statistical analysis
Baseline characteristics and postoperative outcomes were compared between patients who underwent simultaneous colorectal and liver resections and those who underwent isolated colorectal or hepatic resections. As a conservative approach, when comparing between the simultaneous and isolated groups, outcomes of the simultaneous group were compared to the isolated group with the higher overall morbidity. Final comparisons were made between groups with sufficient sample sizes and included the following: open high-risk colorectal/low-risk hepatic to open isolated high-risk colorectal, open low-risk colorectal/high-risk hepatic to open isolated high-risk hepatic, open low-risk colorectal/low-risk hepatic to open isolated low-risk colorectal, and MIS low-risk colorectal/low-risk hepatic to MIS isolated low-risk colorectal. Pearson’s Chi-squared test (or Fisher’s exact test, when appropriate) was used for categorical variables, and Kruskal-Wallis (or Mann-Whitney, when appropriate) test was used for continuous variables. Multivariable modified Poisson regression analysis was used to identify factors associated with overall morbidity, serious morbidity, and 30-day mortality while adjusting for all baseline characteristics listed in Table 1. Risk ratios (RR) were estimated. Statistical significance was indicated by p < 0.05. All statistical analyses were performed using Stata, version 17.0 (StataCorp, College Station, Texas, USA).
TABLE 1.
Demographic, Clinical, and Operative Characteristics.
| Characteristic, n (%) | Simultaneous 1550 (2.4) |
Isolated Colorectal 56,720 (86.7) |
Isolated Hepatic 7147 (10.9) |
|---|---|---|---|
| Age group, years | |||
| <50 | 383 (24.7) | 6866 (12.1) | 1425 (19.9) |
| 50-59 | 396 (25.6) | 12,221 (21.6) | 2015 (28.2) |
| 60-69 | 426 (27.5) | 15,206 (26.8) | 2174 (30.4) |
| ≥70 | 345 (22.3) | 22,427 (39.5) | 1533 (21.5) |
| Age, median (IQR) | 59 (50-68) | 66 (56-75) | 60 (51-68) |
| Sex | |||
| Male | 889 (57.4) | 31,314 (55.2) | 4083 (57.1) |
| Female | 661 (42.7) | 25,404 (44.8) | 3064 (42.9) |
| Race | |||
| White | 972 (62.8) | 36,948 (65.2) | 4533 (63.5) |
| Black | 157 (10.1) | 4733 (8.4) | 467 (6.5) |
| Other | 72 (4.7) | 3106 (5.5) | 317 (4.4) |
| Unknown | 348 (22.5) | 11,900 (21.0) | 1826 (25.6) |
| ASA classification | |||
| I-II | 331 (21.4) | 19,783 (34.9) | 1355 (19.0) |
| III | 1093 (70.5) | 33,227 (58.6) | 5172 (72.4) |
| IV | 126 (8.1) | 3710 (6.5) | 620 (8.7) |
| Dependent functional status | 22 (1.4) | 1294 (2.3) | 23 (0.4) |
| BMI ≥30 | 493 (31.9) | 19,993 (35.5) | 2498 (35.2) |
| Current smoking | 251 (16.2) | 7641 (13.5) | 920 (12.9) |
| Diabetes | 251 (16.2) | 10,956 (19.3) | 1078 (15.1) |
| Hypertension | 626 (40.4) | 29,266 (51.6) | 3054 (42.7) |
| History of severe COPD | 42 (2.7) | 2730 (4.8) | 177 (2.5) |
| History of CHF | 7 (0.5) | 711 (1.3) | 14 (0.2) |
| Preop chemotherapy | |||
| No | 521 (33.6) | 45475 (80.2) | 3035 (42.5) |
| Yes | 1021 (65.9) | 10616 (18.7) | 4072 (57.0) |
| Unknown | 8 (0.52) | 629 (1.11) | 40 (0.56) |
| Immunosuppressive therapy | 65 (4.2) | 1857 (3.3) | 227 (3.2) |
| Risk of procedure (high) | 343 (22.1) | 4685 (8.3) | 1690 (23.6) |
| Operative Approach | |||
| Open | 1239 (79.9) | 14,726 (26.0) | 5618 (78.6) |
| MIS | 311 (20.1) | 41,994 (74.0) | 1529 (21.4) |
Abbreviations: IQR; Interquartile Range; ASA, American Society of Anesthesiologists; BMI, Body Mass Index; CHF, Congestive Heart Failure; COPD, Chronic Obstructive Pulmonary Disease.
RESULTS
Overall Study Population
A total of 191,674 patients who underwent colorectal procedures from 2016-2020 were identified. After applying exclusion criteria, the final study cohort consisted of 65,417 patients, including 1,550 (2.4%) who underwent simultaneous resections, 56,720 (86.7%) who underwent isolated colorectal resections, and 7,147 (10.9%) who underwent isolated hepatic resections for colorectal liver metastases (Figure 4; Table 1).
Figure 4.
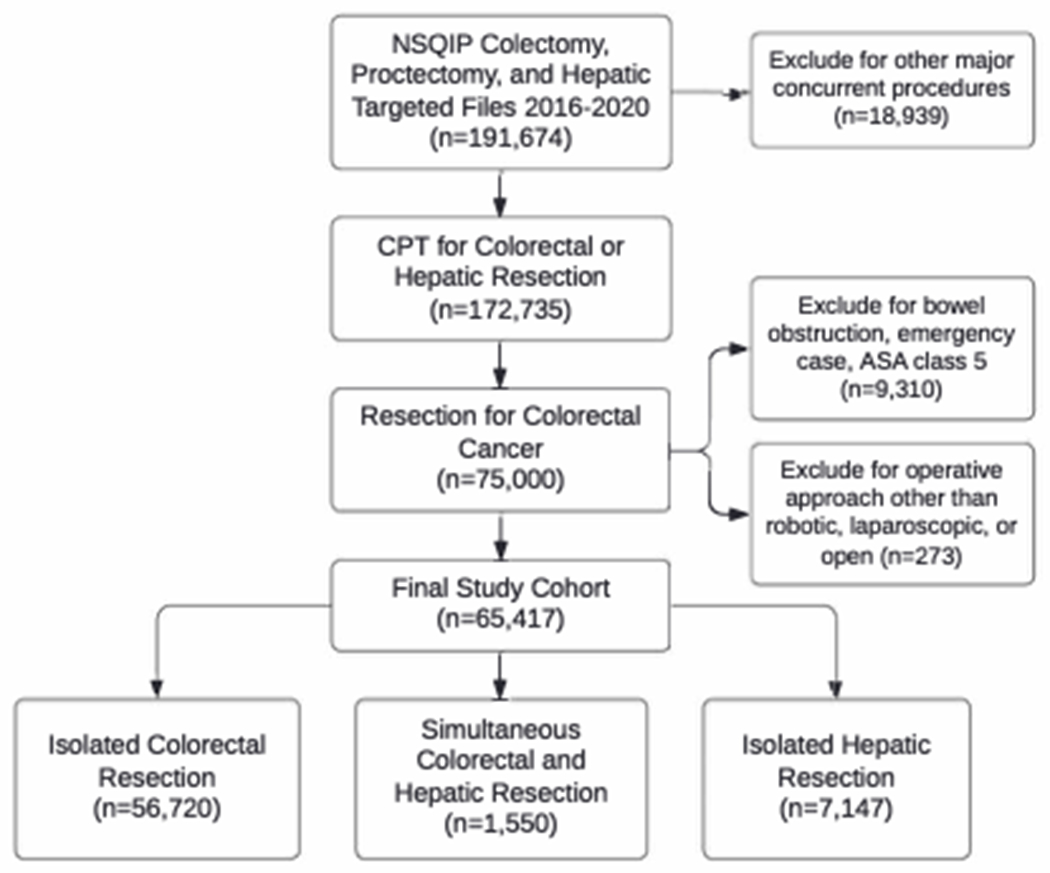
Flowchart of inclusion and exclusion criteria for our study population.
Definition of Colorectal Resection Risk Category Using Isolated Colectomy/Proctectomy Cohort
Of the 56,720 patients who underwent isolated colorectal procedures from 2016-2020, 20,727 patients (36.5%) underwent partial proctectomy with low pelvic anastomosis, 19,222 (33.9%) right colectomy, 5894 (10.4%) left colectomy without diversion, 5244 (9.2%) abdominoperineal resection (APR), 2043 (3.6%) left colectomy with diversion, 1586 (2.8%) low anterior resection (LAR), 1346 (2.4%) total abdominal colectomy (TAC), and 658 (1.2%) total proctocolectomy (TPC). Open colorectal procedures defined as low risk included: partial proctectomy with low pelvic anastomosis (morbidity rate 24.5%), LAR (26.8%), left colectomy without diversion (27.4%), and right colectomy (28.6%). High-risk open colorectal procedures included APR (morbidity rate 35.3%), TAC (35.6%), left colectomy with diversion (40.5%), and TPC (43.3%) (Figure 1; Supplement B). Rates of overall (37.6% vs. 26.9%, p<0.001) and serious morbidity (17.7% vs. 12.6%, p<0.001) differed between the high and low risk groups.
MIS colorectal procedures defined as low risk included: partial proctectomy with low pelvic anastomosis (morbidity rate 13.0%), right colectomy (17.2%), LAR (19.1%), APR (21.4%), TAC (21.8%), and left colectomy without diversion (22.1%). High-risk MIS colorectal procedures included left colectomy with diversion (morbidity rate 27.9%) and TPC (29.5%). (Figure 2; Supplement B). Rates of overall (28.4% vs. 16.2%, p<0.001) and serious morbidity (15.8% vs. 8.4%, p<0.001) differed between the high and low risk groups.
Following risk-stratification, we identified 11,446 patients (77.7%) who underwent open low-risk isolated colorectal procedures and 3,280 patients (22.3%) who underwent open high-risk isolated colorectal procedures. Among MIS procedures, 40,589 patients (96.7%) underwent MIS low-risk isolated colorectal procedures, and 1,405 (3.3%) underwent MIS high-risk isolated colorectal procedures.
Definition of Hepatic Resection Risk Category Using Isolated Hepatectomy Cohort
Of the 7147 patients who underwent isolated hepatectomy procedures from 2016-2020, 4975 patients (69.6%) underwent partial hepatectomy, 1122 (15.7%) right hepatectomy, 568 (7.9%) trisectionectomy, and 482 (6.7%) left hepatectomy. High risk procedures were defined those having ≥35% morbidity rate for open and MIS procedures. Open and MIS hepatectomies defined as low-risk procedures included partial hepatectomy and left hepatectomy (Figure 3; Supplement C). High-risk open and MIS hepatectomies included right hepatectomy and trisectionectomy. Rates of overall (open: 38.6% vs. 24.0%, p<0.001; MIS; 37.0% vs. 14.0%, p<0.001) and serious morbidity (open: 15.0% vs. 9.5%, p<0.001; MIS: 17.4% vs. 4.7%, p<0.001) differed between the high and low risk groups.
Following risk-stratification, we identified 4112 patients (73.2%) who underwent open low-risk isolated hepatic procedures and 1506 patients (26.8%) who underwent open high-risk isolated hepatic procedures. Among MIS procedures, 1345 patients (88.0%) underwent low-risk isolated hepatic procedures, and 184 (12.0%) underwent MIS high-risk isolated hepatic procedures.
Study Population of Simultaneous Resection Cohort
1550 patients (2.4%) underwent simultaneous resections for primary colorectal cancer with liver metastases (Table 1). Patients who had a simultaneous resection had a median age of 59 years. 21.4% had ASA class I-II, 70.5% had ASA class III, and 8.1% had ASA class IV. 31.9% were obese, 16.2% were current smokers, 40.4% had hypertension, and 65.9% had preoperative chemotherapy. 22.1% of patients had at least one high-risk procedure, and 79.9% underwent an open operative approach.
More specifically, among patients who had open simultaneous resections, 932 (75.2%) underwent low-risk colorectal/low-risk hepatic resections, 203 (16.4%) low-risk colorectal/high-risk hepatic resections, 87 (7.0%) high-risk colorectal/low-risk hepatic resections, and 17 (1.4%) high-risk colorectal/high-risk hepatic resections. Among patients who had MIS (n=311) simultaneous colorectal liver resections, 241 (16%) had laparoscopic resections and 70 (5%) had robotic resections. In the laparoscopic group 53 (n=22%) patients were converted to open, and in the robotic group 6 (9%) converted to open. In the simultaneous MIS group, 275 (88.4%) underwent low-risk colorectal/low-risk hepatic resections, 27 (8.7%) low-risk colorectal/high-risk hepatic resections, 8 (2.6%) high-risk colorectal/low-risk hepatic resections, and 1 (0.3%) high-risk colorectal/high-risk hepatic resections.
Compared to the isolated colorectal and isolated hepatic resection cohorts, the simultaneous resection cohort tended to be younger, have lower ASA class, fewer comorbidities, and higher rates of preoperative chemotherapy.
Unadjusted Outcomes for Simultaneous Resection Cohort Stratified by Colorectal and Hepatic Risk Groups
Open high-risk colorectal/low-risk hepatic simultaneous resection vs. Open high-risk isolated colorectal resection
The overall morbidity of patients who underwent open simultaneous high-risk colorectal/low-risk hepatic procedures (n=87) was comparable to those who underwent open isolated high-risk colorectal procedures (n=3,280) (46.0% vs. 37.6%, p=0.110) (Table 2; Supplement D). However, patients who underwent high-risk colorectal/low-risk hepatic procedures had significantly higher rates of bleeding requiring transfusion compared to the isolated high-risk colorectal group (29.9% vs. 18.7%, p=0.009). Hospital LOS (median 8 days, interquartile range (IQR) 6-11 vs. median 7 days, IQR 5-10, p=0.006) and operative time (median 359 minutes, IQR 284-446 vs. median 225 minutes, IQR 157-315, p<0.001) were also significantly longer for patients who had high-risk colorectal/low-risk hepatic synchronous procedures compared to those had isolated high-risk colorectal procedures. There were no differences in serious morbidity, readmission, reoperation, and mortality.
TABLE 2.
Primary and Secondary Outcomes Stratified by Procedure Risk
| n (%) | Overall morbiditya | Serious morbidityb | Mortality | Reoperation | LOS (days), median (IQR) | Readmission | Operative time, median (IQR) |
|---|---|---|---|---|---|---|---|
| Isolated | |||||||
| Open Low-Risk Colorectal | 3076 (26.9) | 1440 (12.6) | 230 (2.0) | 5446 (4.8) | 6 (4-8) | 1337 (11.7) | 149 (103-216) |
| Open High-Risk Colorectal | 1232 (37.6) | 579 (17.7) | 65 (2.0) | 208 (6.3) | 7 (5-10) | 479 (14.6) | 225 (157-315) |
| Open Low-Risk Liver | 985 (24.0) | 389 (9.5) | 27 (0.7) | 85 (2.1) | 5 (4-6) | 361 (8.8) | 204 (154-276) |
| Open High-Risk Liver | 581 (38.6) | 226 (15.0) | 16 (1.1) | 55 (3.7) | 6 (5-8) | 169 (11.2) | 259 (201-330) |
| MIS Low-Risk Colorectal | 6558 (16.2) | 3396 (8.4) | 297 (0.7) | 1608 (4.0) | 4 (3-6) | 3887 (9.6) | 189 (134-261) |
| MIS Low-Risk Liver | 188 (14.0) | 63 (4.7) | 297 (0.7) | 10 (0.07) | 3 (2-4) | 81 (6.0) | 180 9123-242) |
| Simultaneous | |||||||
| Open High/High | 8 (47.1) | 4 (23.5) | 0 (0) | 1 (5.9) | 9 (7-12) | 3 (17.7) | 355 (247-417) |
| Open High Colorectal/Low Liver | 40 (46.0) | 16 (18.4) | 1 (1.2) | 4 (4.6) | 8 (6-11)* | 8 (9.2) | 359 (284-446)* |
| Open Low Colorectal/High Liver | 103 (50.7)* | 52 (25.6)* | 7 (3.5)* | 14 (6.9)* | 7 (6-11)* | 33 (16.3)* | 325 (249-403)* |
| Open Low/Low | 320 (34.3)* | 165 (17.7)* | 9 (1.0)* | 53 (5.7) | 6 (5-9)* | 121 (13.0) | 288 (220-364)* |
| MIS Low/Low | 75 (27.3)* | 39 (14.2)* | 4 (1.5) | 11 (4.0) | 6 (4-8)* | 25 (9.1) | 322 9232-432)* |
Abbreviations: LOS, Length of Stay; IQR, Interquartile Range; MIS, Minimally Invasive Surgery
Overall morbidity: wound infection, pneumonia, urinary tract infection, VTE, cardiac complication, shock/sepsis, unplanned intubation, bleeding transfusion, renal complication, on ventilator >48 hours, organ space SSI.
Clavien-Dindo III-IV: cardiac complication, shock/sepsis, unplanned intubation, renal complication, on ventilator >48 hours, organ space SSI, and reoperation.
Denotes statistical significance with p < 0.05 when compared to the higher risk isolated procedure group for this outcome. Open high colorectal/high liver (n=17), MIS high colorectal/high liver (n=1), MIS high colorectal/low liver (n=8), MIS low colorectal/high liver (n=27) were not compared to isolated group due to small samples sizes.
Open low-risk colorectal/high-risk hepatic simultaneous resection vs. Open high-risk isolated hepatic resection
Patients who underwent open simultaneous low-risk colorectal/high-risk hepatic procedures (n=203) had significantly greater rates of overall morbidity compared to patients who underwent open isolated high-risk hepatic procedures (n=1506) (50.7% vs. 38.6%, p=0.001) (Table 2; Supplement E). More specifically simultaneous resection patients had higher rates of shock/sepsis (10.8% vs. 4.9%, p<0.001) and organ space SSI (20.7% vs. 8.6%, p<0.001) compared to the isolated hepatic resection group. Low-risk colorectal/high-risk hepatic simultaneous procedure patients also had significantly greater rates of serious morbidity (25.6% vs. 15.0%, p<0.001), readmission (16.3% vs. 11.2%, p=0.037), reoperation (6.9% vs. 3.7%, p=0.027), and mortality (3.5% vs. 1.1%, p=0.006). LOS (median 7 days, IQR 6-11 vs. median 6 days, IQR 5-8, p<0.001) and operative time (median 325 minutes, IQR 249-403 vs. median 259 minutes, IQR 201-330, p<0.001) were also longer in the simultaneous resection cohort.
Open low-risk colorectal/low-risk hepatic simultaneous resection vs. Open low-risk isolated colorectal resection
Patients who underwent open simultaneous low-risk colorectal/low-risk hepatic procedures had significantly greater rates of overall morbidity compared to patients who underwent open isolated low-risk colorectal procedures (34.3% vs. 26.9%, p<0.001) (Table 2; Supplement F). Rates of VTE (2.8% vs. 1.8%, p=0.027), shock/sepsis (6.3% vs. 4.5%, p=0.011), bleeding requiring transfusion (18.2% vs. 12.2%, p<0.001), and organ space SSI (10.3% vs. 5.3%, p<0.001) were more frequent in the low-risk colorectal/low-risk hepatic cohort compared to the isolated low-risk colorectal cohort.
Furthermore, the simultaneous resection group had higher rates of serious morbidity (17.7% vs. 12.6%, p<0.001) and longer LOS (median 6 days, IQR 5-9 vs. median 6 days, IQR 4-8, p<0.001) and operative time (median 288 minutes, IQR 220-364 vs. median 149 minutes, IQR 103-216, p<0.001). The mortality rate in the simultaneous group was 1.0% compared to 2.0% in the isolated low-risk colorectal group (p=0.026). There were no differences in readmission and reoperation rates.
MIS low-risk colorectal/low-risk hepatic simultaneous resection vs. MIS low-risk isolated colorectal resection
Similar to patients who underwent open low-risk colorectal/low-risk hepatic synchronous resections, patients who underwent MIS simultaneous low-risk colorectal/low-risk hepatic procedures had significantly greater rates of overall morbidity compared to patients who underwent MIS isolated low-risk colorectal procedures (27.3% vs. 16.2%, p<0.001) (Table 2; Supplement G). Rates of VTE (2.6% vs. 1.1%, p=0.029), bleeding requiring transfusion (12.7% vs. 6.2%, p<0.001), and organ space SSI (7.6% vs. 3.9%, p=0.002) were higher in the simultaneous cohort compared to the isolated low-risk colorectal cohort. Simultaneous resection patients also had higher rates of serious morbidity (14.2% vs. 8.4%, p=0.001) and longer LOS (median 6 days, IQR 4-8 vs. median 4 days, IQR 3-6, p<0.001) and operative time (median 322 minutes, IQR 232-432 vs. median 189 minutes, IQR 134-261, p<0.001). Readmission, reoperation, and mortality rates were comparable between the MIS low-risk colorectal/low-risk hepatic and MIS isolated low-risk colorectal groups.
Association between Risk-Stratified Procedure Type and 30-day Postoperative Morbidity
Multivariable modified Poisson regression analysis was performed to assess the association between risk-stratified procedure type and 30-day postoperative morbidity (Table 3). On adjusted analysis, patients who underwent open simultaneous high-risk colorectal/low-risk hepatic resections had comparable risk of postoperative morbidity when compared to patients who underwent open high-risk isolated colorectal resections (Incidence Rate Ratios [IRR] 1.19, 95% CI: [0.94-1.50], p=0.148). However, patients who underwent open simultaneous low-risk colorectal/high-risk hepatic procedures had 1.37 times the risk of having postoperative morbidity when compared to patients who underwent open high-risk isolated hepatic resections (IRR 1.37, 95% CI: [1.18-1.59], p<0.001).For patients who had open low-risk colorectal/low-risk hepatic procedures, they also had significantly increased risk of morbidity compared to patients who had open low-risk isolated colorectal resections (IRR 1.36, 95% CI: [1.23-1.50], p<0.001). This trend was observed similarly for patients undergoing MIS low-risk colorectal/low-risk hepatic procedures when compared to patients undergoing MIS low-risk isolated colorectal procedures (IRR 1.61, 95% CI: 1.32-1.95, p<0.001).
Table 3.
Multivariable Modified Poisson Regression Assessing the Association between the Procedure Type and Morbidity
| Unadjusted Analysis | Adjusted Analysis | |||
|---|---|---|---|---|
|
| ||||
| IRR (95% CI) | p | IRR (95% CI) | p | |
| Procedure type * | ||||
| Open high-risk isolated colorectal | Reference | Reference | ||
| Open high-risk colorectal/low-risk hepatic | 1.22 (0.97-1.54) | 0.088 | 1.19 (0.94-1.50) | 0.148 |
| Procedure type ** | ||||
| Open high-risk isolated hepatic | Reference | Reference | ||
| Open low-risk colorectal/high-risk hepatic | 1.32 (1.13-1.53) | <0.001 | 1.37 (1.18-1.59) | <0.001 |
| Procedure type ** | ||||
| Open low-risk isolated colorectal | Reference | Reference | ||
| Open low-risk colorectal/low-risk hepatic | 1.28 (1.16-1.40) | <0.001 | 1.36 (1.23-1.50) | <0.001 |
| Procedure type ** | ||||
| MIS low-risk isolated colorectal | Reference | Reference | ||
| MIS low-risk colorectal/low-risk hepatic | 1.69 (1.39-2.05) | <0.001 | 1.61 (1.32-1.95) | <0.001 |
Abbreviations: IRR, Incidence Rate Ratio; CI, Confidence Interval.
Adjusted for age, sex, ASA class, obesity, and COPD.
Adjusted for age, sex, race, ASA class, functional status, obesity, smoking status, COPD, CHF, and preoperative chemotherapy.
DISCUSSION
Advances in comprehensive cancer care have led to an increased number of patients presenting with resectable CRLM either at the time of initial diagnosis of CRC or at some point throughout the disease course. Further innovations in surgical techniques, particularly MIS, and the postoperative care of complex patients have increased questions and controversy surrounding the safety of simultaneous resection of colorectal cancer and synchronous CRLM. Questions that remain include the impact of the extent of colorectal and/or liver resection on postoperative morbidity and mortality, and the role of MIS approaches on these outcomes. To our knowledge, this study is one of only a few to use a risk stratified approach to examine the impact of simultaneous resections and the first to use the ACS-NSQIP to specifically examine the safety of simultaneous MIS colorectal and hepatic resections compared to isolated MIS procedures. Through a risk and approach stratified comparison we found that there are specific combinations of simultaneous procedures, for example an open high colorectal procedure with a low-risk hepatic procedure that do not lead to increased morbidity compared to an isolated procedure. Additionally, although there is an increase in morbidity with other combinations of procedures it is not additive, as an example an open low-risk colorectal with a low-risk liver resection only has a 10% increase in overall and 5% increase in serious morbidity compared to an isolated low-risk colorectal procedure. Utilizing an MIS approach to simultaneous procedures, specifically in low risk colorectal and low risk hepatic resections, can decrease overall morbidity by an average of 7% and serious morbidity by 3% compared to an open approach. Our findings highlight that ongoing discussions are needed to accurately identify patients who may be appropriate candidates for these combined approaches and evaluate patient preferences for a simultaneous vs. staged approach.
Careful selection of both patients and the combination of procedures is critical to decrease the morbidity and mortality associated with these complex operations. Our study sought to utilize a conservative approach in risk comparison. Instead of comparing the overall morbidity of the simultaneous procedure to an estimate of the cumulative risk of the two isolated procedures, we opted to compare to the higher risk of the two isolated procedures. Using this approach, we found that except for an open approach for a high-risk colorectal and low-risk hepatic combined procedure, all other combinations had increased overall morbidity compared to the isolated procedure group. However, these results should be interpreted with individual surgeon preference and patients in mind. For example, colorectal surgeons who practice at institutions with hepatobiliary surgeons comfortable with performing MIS can potentially decrease the overall and serious morbidity for patients undergoing a simultaneous approach. Additionally, surgeons can utilize the data presented here to counsel the patient more accurately on the outcomes of a staged approach versus a simultaneous approach and comment on specific complications that could occur. Given that patients may prefer to undergo one operation versus two, results from this study and additional granular data will help inform their decision-making. Importantly, some reports estimate that 16-35% of patients in the staged approach do not reach the second operation due to morbidity from the first procedure or progression of disease during this period [2].
One important finding is that in all simultaneous groups, except in the open low-risk colorectal procedure with a high-risk hepatic procedure combination, the percentage of patients who required a reoperation or readmission was not significantly higher than the isolated procedure groups. This finding is important in the context of multidisciplinary cancer care, as most of these patients will need to undergo adjuvant therapy. Two recent institutional studies have investigated the impact of simultaneous resections on long term oncological outcomes. Larsson et al. used a propensity matching of patients who underwent a staged vs simultaneous resection approach [13]. This study found that after matching, patients who underwent simultaneous resections had shorter LOS (11 vs. 16 days), fewer cancer recurrences, and no differences in disease-free survival or overall survival compared to those who underwent staged resections. Driedger et al. also used a single institution database of simultaneous resections and showed that increased surgical morbidity resulting in a delay or failure to receive planned adjuvant chemotherapy was associated with worse overall survival, with the impact being most evident in the major colorectal resection with a major hepatic resection group [4]. These studies highlight the importance of appropriate patient selection and preoperative counseling in order to select the most appropriate resection option for each patient to avoid delays in care.
Although a few studies have utilized the ACS-NSQIP to investigate the impact of simultaneous resections, ours is the first study to take operative approach into account when assessing combined procedure risk [12,14]. A recent publication by Snyder et al. found that all simultaneous resections were associated with significantly increased 30-day overall and procedure-specific postoperative morbidity; however, the authors of this study did not comment on the impact of a minimally invasive approach [6]. Moreover, the procedure risk stratification scheme was defined by the authors’ assessment of perceived procedural risk, rather than through morbidity data available from NSQIP, which was the approach performed by Shubert et al. that we adapted and refined for this study [12]. Such differences in categorizing procedure risk of simultaneous resections may result in different findings. Whereas prior studies concluded that all simultaneous procedures were associated with increased overall morbidity, by taking into account postoperative morbidity differences between open and MIS, our study demonstrates a more nuanced approach and shows that not all combinations of simultaneous procedures are associated with increased overall morbidity.
Indeed, minimally invasive surgery is now widely utilized in many colorectal procedures and offers the benefit of smaller incisions, decreased postoperative pain, and shorter LOS, with no differences in oncologic outcomes or mortality rates [15,16]. Though several case reports and small single-institutional studies on minimally invasive simultaneous colorectal and CRLM surgery have been published, this study is the first to our knowledge to report minimally invasive surgical outcomes using the ACS-NSQIP database [7,15–17]. In one systematic review on simultaneous laparoscopic resections, the authors included 12 studies for a total of 136 patients and found that compared to patients who underwent open simultaneous resections, MIS patients had shorter LOS and otherwise comparable operative times, postoperative morbidity, and long term oncologic outcomes [8]. Another systematic study investigating robotic-assisted simultaneous resections for patients with synchronous CRC and CRLM included 9 studies for a total of 29 patients and found that the majority of patients received a minor liver resection with an overall morbidity rate of 38% [7]. Although our study showed that the simultaneous MIS approach in a low-risk colorectal and low-risk hepatic procedure has a significantly higher morbidity rate compared to an isolated MIS colorectal procedure, this morbidity rate was nevertheless drastically lower compared to an open approach.
As such, the potential risk mitigation of simultaneous resections through a MIS approach is a topic that requires further investigation. In addition to having comparable oncologic outcomes and improved immediate postoperative outcomes, MIS is also less costly. A recent study found that in a propensity score matched analysis, minimally invasive liver surgery (MILS) was associated with lower hospital costs ($19,463 vs. $29,119) compared to an open liver resection [18]. Currently, MILS is limited to specialized centers, and concerns about the effectiveness and safety once it is disseminated beyond specialized centers is being actively investigated. Varley et al. utilized the National Cancer Database (NCDB) and found that even though overall liver resection (open and minimally invasive) 90-day mortality was lower at high-volume centers compared to low-volume centers, MILS was similar to open resection in both 90-day mortality and overall survival regardless of treatment center volume [19]. Our study was limited by the number of simultaneous MIS resections in the high-risk hepatic/low risk colorectal (n=27), low risk hepatic/high risk colorectal (n=17), and high-risk hepatic/high risk colorectal (n=1) groups and thus we were not able to compare morbidity between the simultaneous and isolated groups. As the utilization of an MIS approach in complex cases continues to expand it will be important to study the impact on morbidity in these procedure combinations.
This present study is not without limitations. ACS-NSQIP is a national, standardized, multi-institutional database that focuses on measuring surgical quality of care but does not include hospital-specific variables. Thus, the authors cannot comment on which centers are performing these simultaneous resections and whether they are in select regions of the U.S. or more widespread. The group of patients included in this analysis contained low numbers of patients in certain race (American Indian, Native Hawaiian, and Asian) and age (<50) categories and therefore limited more delated analysis of the potential impact of these factors on postoperative outcomes. Additionally, the dataset does not collect more granular cancer-related data beyond 30 days. As a result, the impact of morbidity after receiving adjuvant therapy, disease-free survival, and overall survival cannot be assessed. Due to limitations with case reporting in NSQIP the authors were not able to compare simultaneous resections to the combined risks of two individual procedures in a single patient. The authors believe that the approach presented in this paper is the most conservative for morbidity comparisons but acknowledge that future research could focus on these comparisons. Due to the limited number of simultaneous resections and particularly the limited number of MIS simultaneous resections, we did not report on procedure-specific complications such as ileus, anastomotic leak, and bile leak. These are important considerations that may add to morbidity and/or mortality in these simultaneous resections and should be investigated utilizing institutional datasets or in the future when a greater number of MIS simultaneous resections have been performed. Finally, we included both robotic and laparoscopic in the MIS group and analyzed these patients using an “intent-to-treat” approach for a more conservative analysis. Nuanced differences in outcomes between the two approaches may exist.
CONCLUSIONS
Controversy exists regarding the safety of simultaneous resections in CRC and CRLM. Our study showed that even in the most conversative comparative analysis (simultaneous vs. isolated procedure) there are some procedural combinations that can be performed without any increase in morbidity (i.e. high risk colon/low risk liver). Patients in our cohort who underwent simultaneous resections did not have increased rates of reoperation or readmission. Additionally, our study found that a laparoscopic or robotic approach to simultaneous resections mitigated some of the risk of the combined procedure. Results from this study can be utilized to more accurately counsel patients on expected outcomes and possible complications based on procedure combinations and surgical approach. This allows for a more precise, thorough, and patient centered discussion surrounding operative planning and the decision to perform an isolated or simultaneous resection. Lastly, the study adds to the growing body of literature reporting on the safety of minimally invasive surgical approaches in the simultaneous resection of CRC and CRLM in appropriate patients.
Supplementary Material
Synopsis for Table of Contents:
In an analysis of 1,550 patients from the NSQIP Database (2016-2020) resection of a colorectal primary tumor and synchronous colorectal liver metastases was associated with an increased risk of overall morbidity in most circumstances in a risk stratified analysis. Rates of readmission and reoperation were not increased. This increase in morbidity was not additive and minimally invasive surgical approaches mitigated this impact.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the role of the Johns Hopkins Surgery Center for Outcomes (JSCOR) for supporting this study.
Grant Support:
Shannon N. Radomski and Sophia Y. Chen received financial support from National Cancer Institute (NCI) Grant 5T32CA126607-12.
Other Funding Sources:
Mr. Edwin Lewis provided generous support of Dr. Efron’s Department of Surgery Research Fund. Mr. Pete and Teresa T. Nicholl provided generous support of Dr. Safar’s Department of Surgery Research Fund.
Footnotes
Disclosures:
Shannon N. Radomski: none declared
Sophia Y. Chen: none declared
Miloslawa Stem: none declared
Joy Z. Done: none declared
Bashar Safar: none declared
Jonathan E. Efron: none declared
Chady Atallah: none declared
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021; 71: 7–33. [DOI: 10.3322/caac.21654] [DOI] [PubMed] [Google Scholar]
- 2.Martin J, Petrillo A, Smyth EC, et al. Colorectal liver metastases: Current management and future perspectives. World J Clin Oncol 2020; 11: 761–808. [DOI: 10.5306/wjco.v11.i10.761] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor A, Primrose, Langeberg W, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol 2012; : 283. [DOI: 10.2147/CLEP.S34285] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driedger MR, Yamashita TS, Starlinger P, et al. Synchronous resection of colorectal cancer primary and liver metastases: an outcomes analysis. HPB 2021; 23: 1277–1284. [DOI: 10.1016/j.hpb.2021.01.002] [DOI] [PubMed] [Google Scholar]
- 5.Kleive D, Aas E, Angelsen J-H, et al. Simultaneous Resection of Primary Colorectal Cancer and Synchronous Liver Metastases: Contemporary Practice, Evidence and Knowledge Gaps. Oncol Ther 2021; 9: 111–120. [DOI: 10.1007/s40487-021-00148-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder RA, Hao S, Irish W, et al. Thirty-Day Morbidity after Simultaneous Resection of Colorectal Cancer and Colorectal Liver Metastasis: American College of Surgeons NSQIP Analysis. J Am Coll Surg 2020; 230: 617–627.e9. [DOI: 10.1016/j.jamcollsurg.2019.12.018] [DOI] [PubMed] [Google Scholar]
- 7.Machairas N, Dorovinis P, Kykalos S, et al. Simultaneous robotic-assisted resection of colorectal cancer and synchronous liver metastases: a systematic review. J Robot Surg 2021; 15: 841–848. [DOI: 10.1007/s11701-021-01213-8] [DOI] [PubMed] [Google Scholar]
- 8.Moris D, Tsilimigras DI, Machairas N, et al. Laparoscopic synchronous resection of colorectal cancer and liver metastases: A systematic review. J Surg Oncol 2019; 119: 30–39. [DOI: 10.1002/jso.25313] [DOI] [PubMed] [Google Scholar]
- 9.ACS NSQIP Participant Use Data File. ACS. Available from: https://www.facs.org/quality-programs/data-and-registries/acs-nsqip/participant-use-data-file/ [Google Scholar]
- 10.About ACS NSQIP. ACS. Available from: https://www.facs.org/quality-programs/data-and-registries/acs-nsqip/about-acs-nsqip/ [Google Scholar]
- 11.Dindo D, Demartines N, Clavien P-A. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg 2004; 240: 205–213. [DOI: 10.1097/01.sla.0000133083.54934.ae] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shubert CR, Habermann EB, Bergquist JR, et al. A NSQIP Review of Major Morbidity and Mortality of Synchronous Liver Resection for Colorectal Metastasis Stratified by Extent of Liver Resection and Type of Colorectal Resection. J Gastrointest Surg 2015; 19: 1982–1994. [DOI: 10.1007/s11605-015-2895-z] [DOI] [PubMed] [Google Scholar]
- 13.Larsson AL, Björnsson B, Jung B, et al. Simultaneous or staged resection of synchronous colorectal cancer liver metastases: a 13-year institutional follow-up. HPB 2022; 24: 1091–1099. [DOI: 10.1016/j.hpb.2021.11.019] [DOI] [PubMed] [Google Scholar]
- 14.Hamed OH, Bhayani NH, Ortenzi G, et al. Simultaneous colorectal and hepatic procedures for colorectal cancer result in increased morbidity but equivalent mortality compared with colorectal or hepatic procedures alone: outcomes from the National Surgical Quality Improvement Program. HPB 2013; 15: 695–702. [DOI: 10.1111/hpb.12031] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg 2018; 267: 199–207. [DOI: 10.1097/SLA.0000000000002353] [DOI] [PubMed] [Google Scholar]
- 16.Rahimli M, Perrakis A, Schellerer V, et al. Robotic and laparoscopic liver surgery for colorectal liver metastases: an experience from a German Academic Center. World J Surg Oncol 2020; 18: 333. [DOI: 10.1186/s12957-020-02113-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuirk M, Gachabayov M, Rojas A, et al. Simultaneous Robot Assisted Colon and Liver Resection for Metastatic Colon Cancer. JSLS J Soc Laparosc Robot Surg 2021; 25: e2020.00108. [DOI: 10.4293/JSLS.2020.00108] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei D, Johnston S, Patkar A, Buell JF. Comparison of clinical and economic outcomes between minimally invasive liver resection and open liver resection: a propensity-score matched analysis. HPB 2021; 23: 785–794. [DOI: 10.1016/j.hpb.2020.09.017] [DOI] [PubMed] [Google Scholar]
- 19.Varley PR, Tohme ST, Chidi AP, et al. Dissemination of Minimally Invasive Liver Resection for Primary Malignancy: Reevaluating Effectiveness. Ann Surg Oncol 2018; 25: 808–817. [DOI: 10.1245/s10434-017-6308-2] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


