Abstract
Solitary fibrous tumor (SFT) has been increasingly reported in various anatomic sites. However, it is still extremely rare in the pancreas. Herein, we present the first series of primary pancreatic SFTs. Nine cases of primary pancreatic SFTs were analyzed. The mean age was 60 years (36-76) with no sex predilection. Six tumors were in the head, three were in the tail. On imaging studies, tumors were described as a hypervascular mass, two revealed cystic areas, and three were favored to be neuroendocrine tumors. On biopsy, two cases were diagnosed as atypical spindle cell tumor; one was misdiagnosed as suspicious for sarcoma, and another case as metastatic renal cell carcinoma. Two were diagnosed as low-grade sarcoma and low-grade stromal tumor on frozen sections. Grossly, tumors were well-demarcated with a median size of 4 cm (0.9-15). Microscopically, they were composed of ovoid to spindle tumor cells with no significant mitotic activity and were arranged in alternating hypercellular and hypocellular areas. Staghorn-like vessels and entrapped pancreatic parenchyma were also detected within all tumors. Tumor cells revealed diffuse/strong nuclear STAT6 expression in seven of eight, CD34 in seven of nine, and bcl-2 in four of four tested cases. One tested tumor harbored NAB2-STAT6 fusion. Eight patients with available follow up data were free of disease at a mean follow-up of 76 months (3-189). SFT should be considered in the differential diagnoses of mesenchymal neoplasms of the pancreas. Immunohistochemical nuclear STAT6 expression is a characteristic feature of SFT. Primary pancreatic SFTs seem to have favorable biological behavior in our series.
Keywords: Solitary fibrous tumor, pancreas, pancreatic, mesenchymal tumor
INTRODUCTION
Solitary fibrous tumor (SFT) is a rare mesenchymal neoplasm with variable histology and biological behavior. Even though pleura is the most site of common origin, SFT may arise in any anatomic site including abdominal organs. Extra-pleural SFTs occur in adults without sex predilection. They are either discovered incidentally or present with non-specific symptoms (1, 2). Rarely, they cause paraneoplastic hypoinsulinaemic hypoglycemia due to insulin-like growth factor production (Doege-Potter syndrome) (3–8). Macroscopically, these tumors are often well circumscribed, multinodular, and firm. Morphologically, they are characterized with patternless distribution of ovoid to spindle-shaped tumor cells, stromal hyalinization and hemangiopericytoma-like vessels (1, 2). They harbor NAB2-STAT6 gene fusion (9, 10), which leads to highly specific nuclear STAT6 expression by immunohistochemical staining (11). Although the vast majority of SFTs have a benign prognosis, about 10% may behave aggressively with local recurrences or distant metastasis. As per the current (2019) WHO, features related with aggressive behavior include older patient age, larger tumor size, high cellularity, cytological atypia, >4 mitosis/2 mm2, hemorrhage, necrosis and sarcomatous transformation (2).
Pancreas is an exceedingly rare localization for SFT. The first primary pancreatic SFT case was described in 1999 by Lüttges et al (12). Since then, only single case reports have been described in the literature (12–44). In this study, we present the first series of primary pancreatic SFTs, with the aim of further defining their clinicopathologic features and challenging differential diagnoses.
MATERIALS AND METHODS
Surgical pathology databases of the authors’ institutions were searched for cases with a diagnosis of primary pancreatic solitary fibrous tumor. Available gross photographs, descriptions and histologic sections were reevaluated to confirm the diagnosis and further characterize the morphologic and immunohistochemical findings. Available medical records including radiology reports were reviewed to obtain clinical data including age, sex, presenting symptoms, tumor location, presence of prior biopsy or frozen section, surgical procedure type and outcome.
For all cases, the following histopathological information was recorded: tumor size; lymph node status; surgical margin status; growth pattern (as infiltrative or expansile); degree of cellularity (as low, moderate, high) and pleomorphism (as low, moderate, high); mitotic rate (by counting 10 field at x400 on an Olympus microscope=0.45mm2); presence of necrosis, hemorrhage, myxoid changes and entrapped pancreatic parenchyma. Prognostic risk stratifications were determined by applying three different classification system: WHO 2019 criteria, Pasquali’s recurrence risk model (Supplemental Table 1) and Demicco’s modified four-variable risk stratification model for development of metastasis (Supplemental Table 2) (2, 45, 46).
A representative formalin-fixed paraffin-embedded tissue section of the cases, for which a paraffin block was available, was immunolabeled using the standard avidin-biotin peroxidase method with STAT6 (EP325, Cell Marque) antibody and nuclear staining was accepted as positive result. Results of other immunohistochemical and as well as electron microscopy studies were recorded from the original pathology reports.
One case, for which additional material was available, was subjected to a custom targeted, RNA-based panel (MSK-Fusion) that utilizes Archer Anchored Multiplex PCR technology and next-generation sequencing to detect gene fusions in 62 genes (including NAB2 and STAT6) known to be involved in chromosomal rearrangements (47–49). This custom assay has been validated and approved for the clinical use at Memorial Sloan Kettering Cancer Center by the New York State Department of Health Clinical Laboratory Evaluation Program.
RESULTS
We identified nine cases all of which were surgical resections.
Clinical Features
The mean patient age was 60 years (range, 36-76). Five patients were male and four were female. Four patients presented with abdominal pain, one of these patients also had weight loss, and another patient presented with back pain. The other three tumors were detected incidentally during work-up for other conditions. None of the patients presented with hypoglycemia. One patient had experienced acute pancreatitis 6 years before diagnosis of SFT.
An incidental intestinal phenotype ampullary adenocarcinoma (1 cm in greatest dimension, pT1) was found synchronously in one patient. Another patient had two separate incidental well differentiated WHO 2019-Grade 1 pancreatic neuroendocrine tumors (2 cm and 0.8 cm in greatest dimensions), and a neuroendocrine microadenoma (0.2 cm). Three patients had history of other neoplastic conditions including meningioma, renal cell carcinoma, breast carcinoma and cutaneous basal cell carcinoma.
Two of the eight tumors with available imaging results were described as a cystic lesion, while the others were described as a well circumscribed solid mass. Tumors were described as a hypervascular mass showing persistent enhancement on delayed phase imaging of dynamic CT or MRI studies (Figure 1). Three were favored to be neuroendocrine tumors. Other radiologic differential diagnoses included solid pseudopapillary neoplasm, inflammatory conditions, and cystic malignancies.
Figure 1:
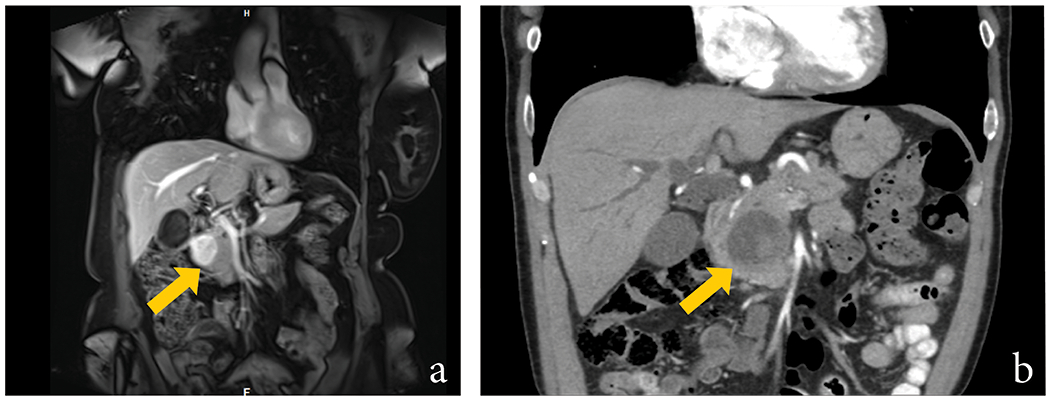
Coronal MRI of Case #3 demonstrated a hypointense solid mass (arrow) centered in the head of the pancreas. No dilatation of the main pancreatic duct was noted. A neuroendocrine tumor was favored (A). Coronal CT image of Case #4 revealed a heterogeneous cystic lesion (arrow) with central solid component in the pancreatic head, with biliary ductal dilatation. The lesion was reported as “consistent with a cystic malignancy” (B).
One case had a biopsy diagnosis of “atypical spindle cell tumor, suspicious for sarcoma” and it was diagnosed as “spindle cell proliferation, favor low grade sarcoma” on frozen section. Another case was favored to be “low grade stromal tumor” on frozen section. Fine needle aspiration of another case was interpreted as metastatic renal carcinoma based on immunohistochemical PAX8 expression and patient’s history of renal cell carcinoma.
None of the patients received neoadjuvant chemotherapy. All patients underwent surgical resection (six pancreaticoduodenectomy, three distal pancreatectomy).
Clinical features of the patients are summarized in Table 1.
Table 1:
Clinicopathologic Features of Our Cases
| Case | Age, Sex | Symptom(s) | Radiologic Findings / Differential Diagnosis | Location | Size (cm) | Cellularity, Pleomorphism, Necrosis, Mitotic Count | Immunohistochemical Findings | Outcomes, Follow up (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 72, M | Abdominal pain | Solid hypervascular mass / PanNET, focal pancreatitis | Head | 5 | Low, low, no, 3/10 HPF |
Positive: STAT6, bcl-2, vimentin Negative: CD34, CD117, ALK, SMA, CMA, desmin, S100, AE1/AE3, CAM5.2, ER, PR, inhibin |
NED, 17 |
| 2 | 76, F | Back pain | Solid hypervascular mass / PanNET, less likely a thrombosed splenic artery aneurysm | Tail | 4 | Moderate, low, no, 0/10 HPF |
Positive: STAT6, bcl-2, focal SMA, focal CMA, focal ER, focal inhibin Negative: CD34, desmin, PR |
NED, 138 |
| 3 | 65, F | Incidental | Solid hypervascular mass / PanNET | Head | 4 | Low, low, no, 0/10 HPF |
Positive: STAT6, CD34 Negative: SMA, desmin, S100, ERG, CD31, CDK4, MDM2, pan cytokeratin |
NED, 42 |
| 4 | 56, M | Incidental | Cystic mass with solid component / Cystic malignancies | Head | 3.7 | Low, low, no, 0/10 HPF |
Positive: STAT6, CD34 Negative: CD117, CDK4, MDM2, ER, PR |
NED, 47 |
| 5 | 36, F | Abdominal pain | Complex cystic mass / Inflammatory conditions, SPN, mucinous neoplasms | Tail | 2.7 | Low, low, no, 0/10 HPF |
Positive: STAT6, CD34, SMA Negative: Desmin, S100 |
NED, 189 |
| 6 | 55, F | Abdominal pain | Solid hypervascular mass / Non-ductal neoplasms | Head | 3 | Low, low, no, 0/10 HPF |
Positive: STAT6, CD34, calponin Negative: CD117, DOG1, ALK, SMA, MSA, desmin, S100 |
NED, 107 |
| 7 | 57, M | Incidental | Solid hypervascular mass / N/A | Head | 0.9 | Low, low, no, 0/10 HPF |
Positive: CD34, PAX8, PAX2 Negative: CD117, DOG1, S100, pan cytokeratin, synaptophysin, chromogranin A |
NED, 3 |
| 8 | 56, M | Abdominal pain, weight loss | Solid hypervascular mass / N/A | Head | 15 | Moderate, low, focal, 1/10 HPF |
Positive: CD34, bcl-2 Negative: CD117, DOG1, SMA, CMA, desmin, S100, CD99, AE1/AE3 Non-contributory: STAT6 |
NED, 62 |
| 9 | 70, M | N/A | N/A | Tail | 13 | Low, low, no, <1/10 HPF |
Positive: STAT6, CD34, bcl-2, CD99 Negative: CD117, DOG1, SMA, desmin, S100, HMB45, CD31, ERG, EMA, Claudin, MUC4, synaptophysin, chromogranin A, ER, inhibin, WT1, calretinin |
N/A |
PanNET: Pancreatic neuroendocrine tumor, SPN: Solid pseudopapillary tumor, PD: Pancreaticoduodenectomy/Whipple procedure, DP: Distal pancreatectomy, SMA: Smooth muscle actin, CMA: Common muscle actin, ER: Estrogen receptor, PR: Progesterone receptor, MSA: Muscle specific actin, EMA: Epithelial membrane antigen, NED: No evidence of disease, m: months, N/A: Not available.
Pathologic Features
Six tumors were in the head of the pancreas, and three were in the tail. Grossly, tumor size varied from 0.9 to 15 cm (median, 4 cm). Tumors were described as well-demarcated, firm, solid lesions with white-tan cut surfaces. Three tumors revealed cystic degeneration (Figure 2).
Figure 2:
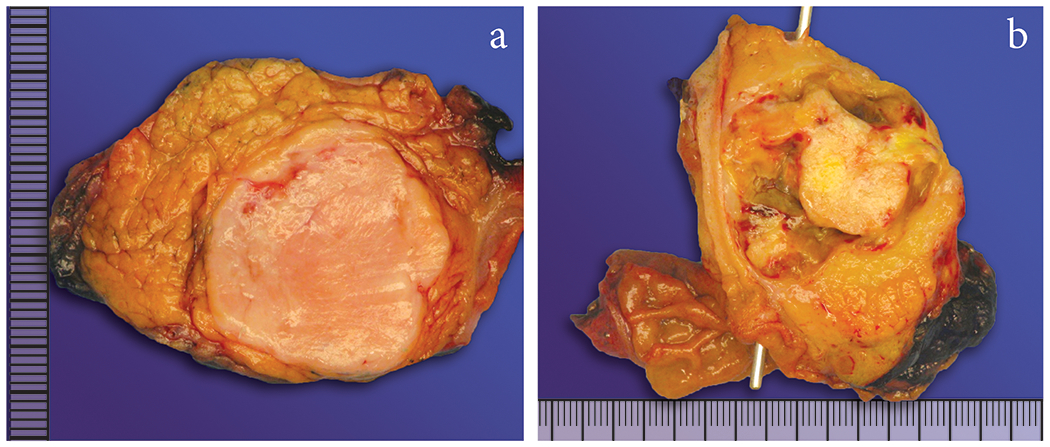
The tumors were well-circumscribed, white/tan, solid nodules (A), one of the reasons why these tumors were diagnosed as NETs or SPN on imaging. Two tumors also showed cystic degeneration (B).
Microscopically, sections revealed relatively well circumscribed tumors with lobular appearance and expansile borders (Figure 3), although, in some areas, extension to adjacent pancreatic parenchyma was noted. Moreover, on close examination entrapped pancreatic parenchyma was seen both at the periphery and within the tumor in all cases (Figure 4). In general, the tumors were characterized by so-called patternless pattern and staghorn-like vessels (Figure 5). Some areas were extremely hypocellular, others were hypercellular and abrupt transition between these hypocellular and hypercellular areas were common (Figure 6). Loose stroma and myxoid change were seen in all cases. One case had histiocyte accumulations in degenerative areas. Only one case (Case #8) revealed focal necrosis. On high magnification, the tumors were composed of cytologically bland, ovoid to spindle shaped tumor cells (Figure 7). Significant cytologic atypia was not identified in any of the cases and none of the tumors were mitotically very active (no mitosis or ≤ 3 mitotic figures per 10 HPFs in 9 or 9 cases).
Figure 3:
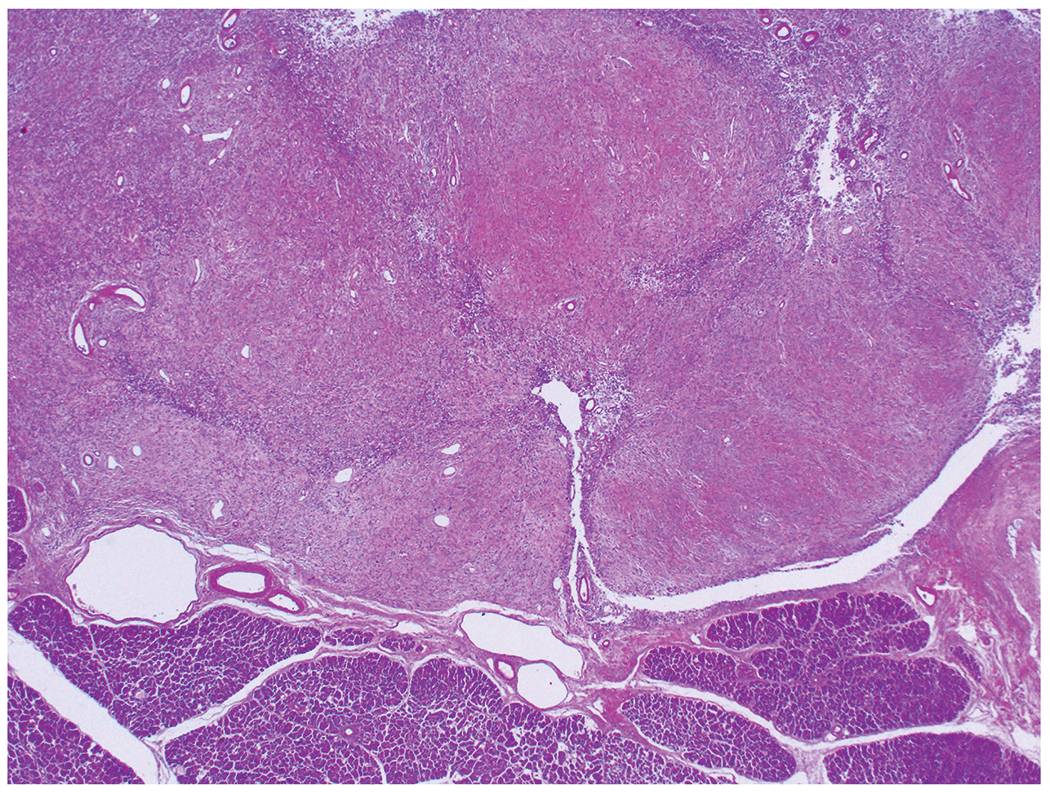
The tumors are relatively well circumscribed with lobular appearance and expansile borders.
Figure 4:
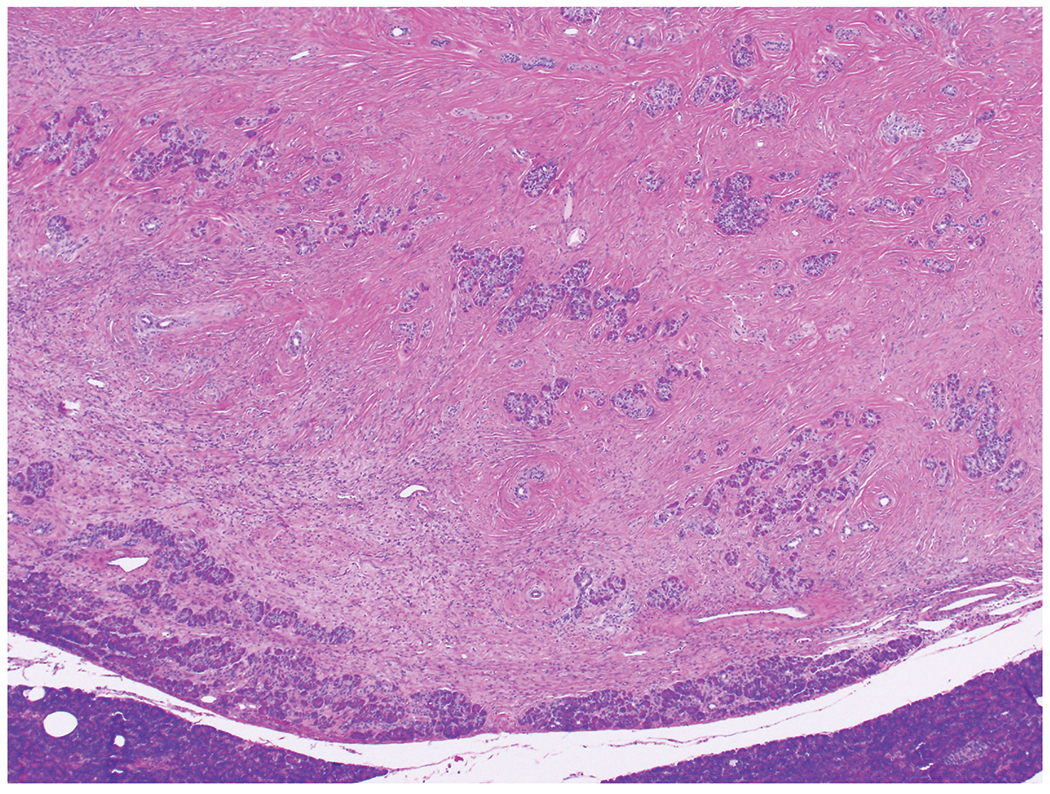
Although solitary fibrous tumor has expansile borders, there is entrapped pancreatic parenchyma both at the periphery of the tumor and within the tumor.
Figure 5:
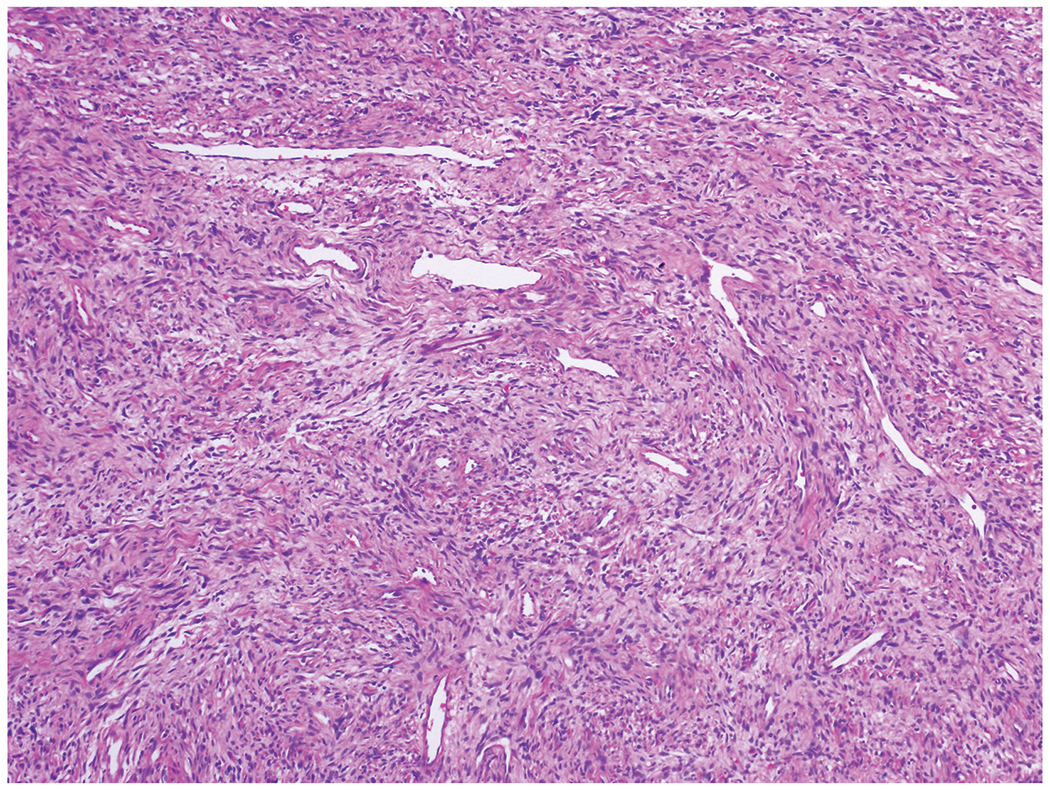
Solitary fibrous tumors reveal so-called patternles pattern and staghorn-like vessels.
Figure 6:
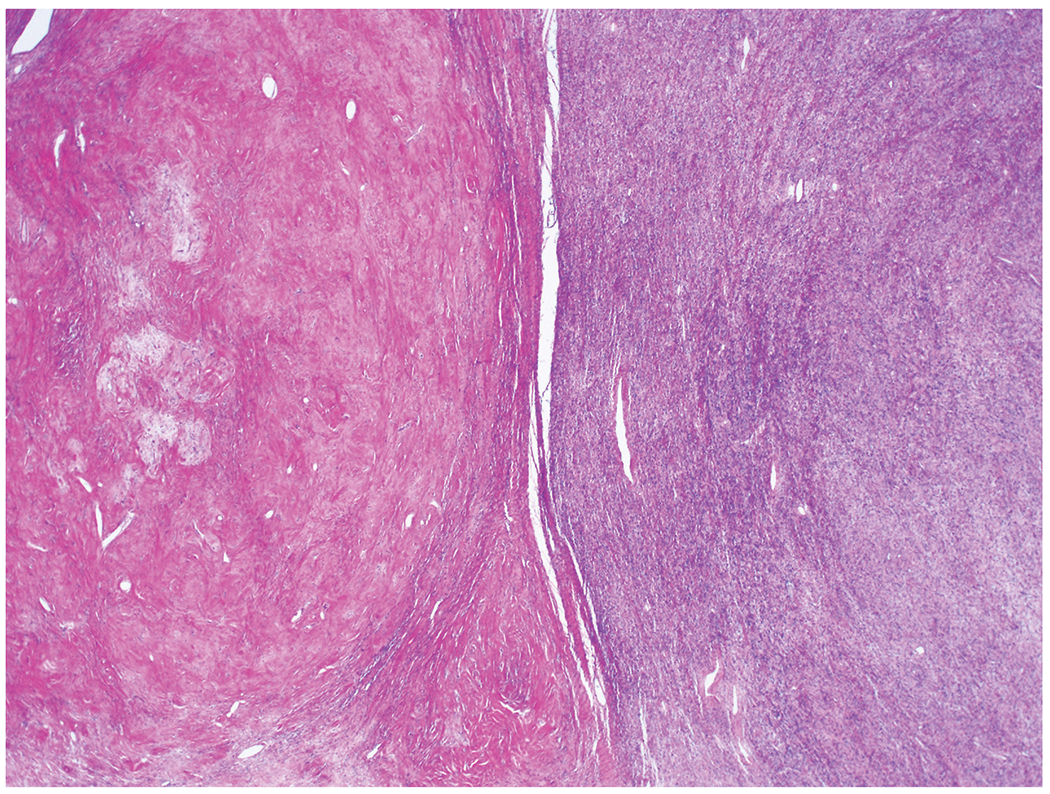
Some foci are extremely hypocellular, others are hypercellular and abrupt transition between thesehypocellular and hypercellular areas is common.
Figure 7:
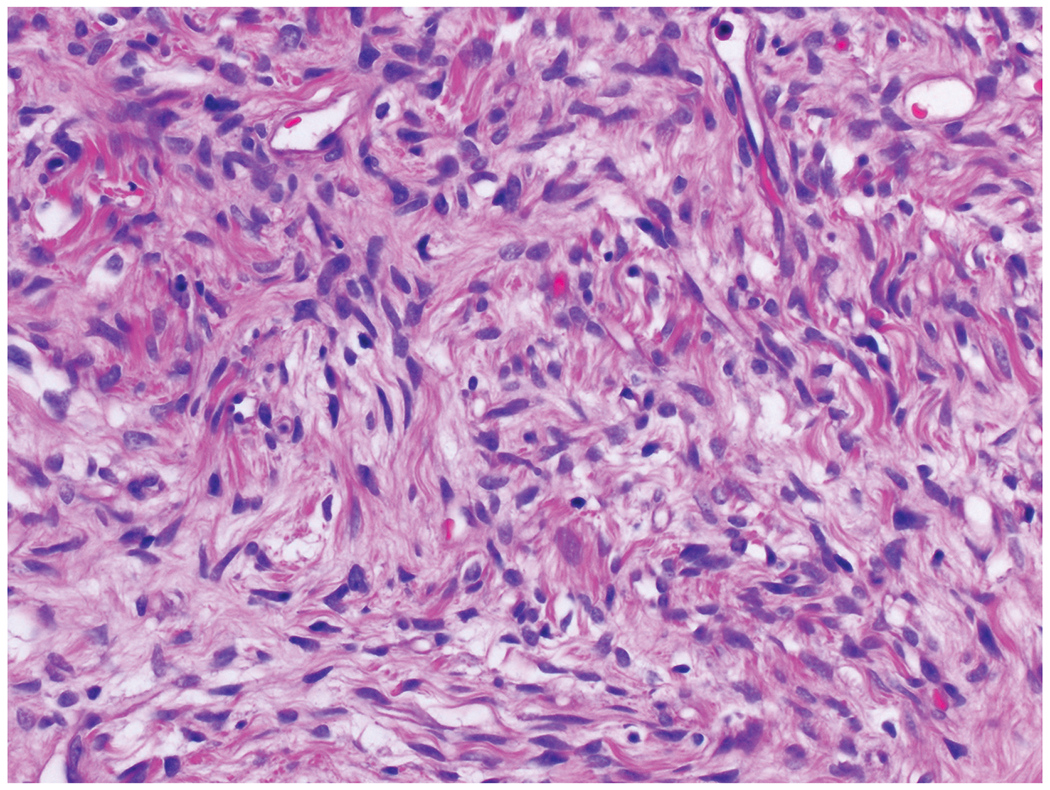
On high magnification, the tumors are composed of cytologically bland, ovoid to spindle shaped cells.
Surgical margins were free of the tumor in all cases. No lymph node metastasis was detected, although the case with synchronous ampullary adenocarcinoma revealed metastatic ampullary adenocarcinoma in one of five lymph nodes (pN1).
Immunohistochemically, seven of eight tested tumors revealed nuclear STAT6 expression (Figure 8a); in the 8th case, STAT6 staining was non-contributory as the tumor was no longer present on the immunohistochemistry slide. However, the tumor had classic morphological features of SFT and labeled with bcl-2 and CD34, and was negative for AE1/AE3, CD117, DOG1, smooth muscle actin, desmin, S100, and CD99. Seven (7/9, 78%) tumors were positive for CD34 (Figure 8b) and four (4/4, 100%) tumors were positive for bcl-2.
Figure 8:
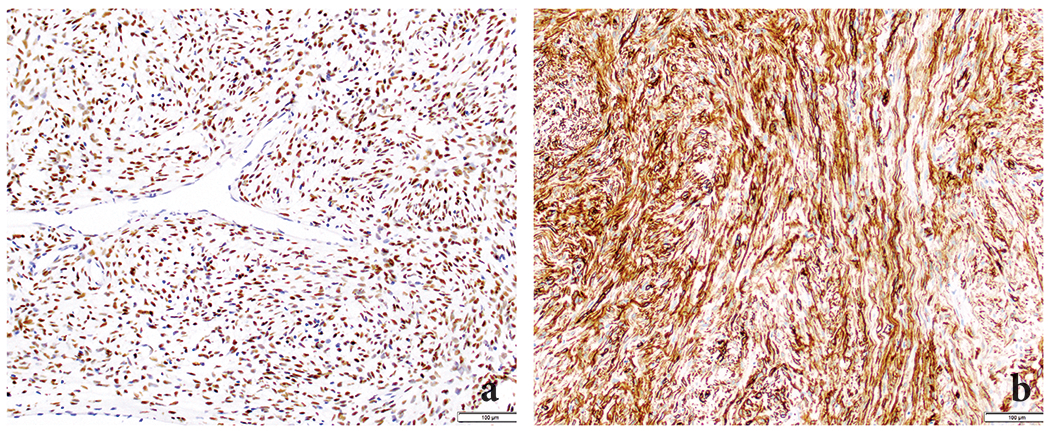
Immunohistochemically, all tumors revealed STAT6 (nuclear) (A) and CD34 (B) expression.
Electron microscopy reported to demonstrate fusiform and spindle shaped fibroblast-like tumor cells, with a moderately developed rough endoplasmic reticulum, surrounded by collagen fibers in the only tumor tested (Case #1), supporting the diagnosis of SFT.
MSK-Fusion assay revealed NAB2-STAT6 fusion (NAB2 exon 4 [NM_005967] and STAT6 exon 2 [NM_001178078]) in the only tumor tested (Case #3).
Pathologic features of the tumors are summarized in Table 1.
Risk Stratification
None of the cases were considered as malignant:
Based on the current (2019) WHO criteria, the tumors are not expected to behave aggressively as none revealed high cellularity, cytological atypia, >4 mitosis/2 mm2, hemorrhage, or sarcomatous transformation. Only one case (Case #8) revealed focal necrosis (2).
As per Pasquali’s SFT recurrence risk model (Supplemental Table 1), seven cases are classified as having very low risk for recurrence, and two (Case #2 and case #8) were classified as having low risk due to moderate cellularity (45).
As per Demicco’s modified four-variable risk stratification model (Supplemental Table 2), eight cases are classified as having low risk of metastasis, and one case (Case #8) is classified as having intermediate risk due to ≥55 age and ≥15 cm tumor size (46).
Clinical Outcomes
Follow-up information was available for eight patients (89%). None of the patients received adjuvant therapy and all patients with available survival data are alive with no evidence of disease, with a mean follow-up of 76 months (median, 55 months; range, 3 to 189 months).
Literature Analysis
When all the cases in the literature are combined with our cohort (n=44), the following clinicopathologic characteristics are elucidated: All patients were adults except for one pediatric patient who was diagnosed at the age of 14 months. Mean age was 55 years (range, 14 months – 82 years). There was no sex predilection (F/M=1). Most of the patients were asymptomatic (n=19, 44%) or presented with abdominal pain (n=15, 35%); other symptoms/findings include jaundice (n=4, 9%), abdominal discomfort (n=3, 7%), back pain (n=2, 5%), weight loss (n=2, 5%), and hypoglycemia (n=1, 2%). The tumor sizes ranged from 0.9 cm to 18.5 cm (median, 4 cm). Tumors involved head/neck/uncinate process of the pancreas (n=25, 57%) more frequently than body/tail (n=19, 43%). Due to their demarcated round nature, radiologically, the first differential diagnosis was of non-ductal tumors, especially pancreatic well differentiated neuroendocrine tumor, followed by solid pseudopapillary neoplasm and less likely gastrointestinal stromal tumor. All but one patient were surgically treated with different procedures based on tumor location, including enucleation, pancreaticoduodenectomy, or distal pancreatectomy. For one patient, surgery was not possible due to co-morbidities. The clinicopathologic features of all reported cases are summarized in Tables 2 and 3.
Table 2:
Summary of Primary Pancreatic SFTs Reported in the Literature
| Author, year | Age, Sex | Symptom(s) | Radiologic/clinic differential diagnoses | Location, procedure | Size (cm) | Reported malignancy criteria | Reported diagnosis | Immunohistochemical findings | Outcomes, Follow up (months) |
|---|---|---|---|---|---|---|---|---|---|
| Lüttges, 1999 [12] | 50, F | Incidental | PanNET | Body, DP | 5.5 | Moderate pleomorphism, no mitosis or necrosis | SFT |
Positive: CD34, bcl-2, CD99, vimentin Negative: SMA, S100, NSE, cytokeratin, synaptophysin, chromogranin A, insulin, p53 |
Alive, RF, 20 |
| Chatti, 2006 [13] | 41, M | Abdominal pain | PanNET | Body, Enucleation | 13 | Necrosis, no atypia or mitosis | SFT |
Positive: CD34, bcl-2, focal CD99, focal CD117, focal SMA, vimentin Negative: S100, cytokeratin, EMA |
Death on postoperative 3rd day due to complications |
| Gardini, 2007 [14] | 62, F | Abdominal pain | PanNET | Head, PD | 3 | N/A | SFT |
Positive: CD34, bcl-2, CD99, focal SMA, vimentin Negative: CD117, desmin, S100 |
Alive, RF, 16 |
| Miyamoto, 2007 [15] | 41, F | Abdominal pain | PanNET | Neck, Enucleation | 2 | No atypia, mitosis, or necrosis | SFT |
Positive: CD34, bcl-2 Negative: CD117, SMA, desmin, S100, AE1/AE3, CAM5.2 |
Alive, RF, 7 |
| Kwon, 2008 [16] | 54, M | Incidental | PanNET, SPN, GIST, neurogenic tumor | Body, Median segmentectomy | 7.6 | No atypia | SFT |
Positive: CD34, CD99, vimentin Negative: CD117, S100, cytokeratin |
Alive, RF, 88 |
| Srinivasan, 2008 [17] | 78, F | Back pain, weight loss | PanNET | Body, DP | 5 | No atypia or necrosis, <1/10 HPF mitosis | SFT |
Positive: focal CD34, bcl-2, CD99, vimentin Negative: CD117, CD10, SMA, desmin, S100, CAM5.2, synaptophysin, chromogranin A |
Alive, RF, 7 |
| Chetty, 2009 [18] | 67, F | Incidental | PanNET | Uncinate process, PD | 2.6 | No atypia, mitosis, or necrosis | SFT |
Positive: CD34, bcl-2, CD99 Negative: CD117, SMA, desmin, MSA, S100, AE1/AE3, CAM5.2, synaptophysin, chromogranin A, beta-catenin |
Alive, RF, 6 |
| Ishiwatari, 2009 [19] | 58, F | Incidental | PanNET with cystic changes | Head, PD | 3 | Atypia (low), 0/10 HPF mitosis, focal necrosis | SFT |
Positive: CD34, bcl-2 Negative: CD99, CD117, SMA, S100, AE1/AE3, EMA, synaptophysin, chromogranin A |
Alive, RF, 42 |
| Sugawara, 2010 [20] | 55, F | Incidental | N/A | Head, PD | 7 | N/A | SFT |
Positive: CD34 Negative: CD117, SMA, S100, ALK, cytokeratin |
N/A |
| Azadi, 2012 [21] | 57, M | Incidental | N/A | Tail, DP | 3.1 | No atypia or necrosis, low mitotic index | SFT |
Positive: CD34, bcl-2 Negative: CD117, desmin, myogenin, AE1/AE3 |
N/A |
| dos Santos, 2012 [22] | 40, F | Incidental | N/A | Body, Partial pancreatectomy | 3 | No atypia, mitosis, or necrosis | SFT |
Positive: CD34, beta-catenin Negative: CD117, SMA, desmin, S100 cytokeratin, EMA |
N/A |
| Tasdemir, 2012 [23] | 24, F | Epigastric pain | Mesenchymal tumor | Head, Enucleation | 18.5 | 1-2/10 HPF mitoses | SFT |
Positive: CD34, focal bcl-2, vimentin, beta-catenin Negative: Desmin, S100, cytokeratin |
Alive, RF, 3 |
| van der Vorst, 2012 [24] | 67, F | Abdominal pain | PanNET | Uncinate process, Enucleation | 2.8 | No atypia, mitosis, or necrosis | SFT |
Positive: CD34, bcl-2, CD99 Negative: CD117, beta-catenin |
N/A |
| Yamanashi, 2012 [25] | 50, M | Incidental | PanNEC | Tail, DP | 10, 3.5, 3 | Intrapancreatic metastases, mild atypia, >2/HPF mitoses, necrosis, hypercellularity | Malignant SFT |
Positive: CD34, bcl-2, vimentin Negative: CD117, cytokeratin, synaptophysin, chromogranin A |
Alive, Local recurrence after postoperative 21 months, 32 |
| Chen, 2013 [26] | 49, F | Abdominal pain, distension | PanNET, SPN | Head, PD | 13 | Necrosis | SFT |
Positive: CD34, bcl-2, CD68, MSA Negative: CD117, CD99, SMA, desmin, S100, cytokeratin |
Alive, RF, 30 |
| Hwang, 2014 [27] | 53, F | Incidental | PanNET, SPN | Head, Partial head resection | 5.2 | N/A | SFT |
Positive: CD34, bcl-2, CD99, CD10, SMA, ER, PR Negative: CD117, DOG1, caldesmon, desmin, S100, AE1/AE3, EMA |
Alive, RF, 6 |
| Kim, 2014 [28] | 52, F | Incidental | PanNET, SPN, pancreatic cancer | Tail, DP | 2 | No atypia | SFT |
Positive: Focal CD34, bcl-2 Negative: CD117, SMA, desmin, S100 |
Alive, RF, 12 |
| Han, 2015 [29] | 77, F | Jaundice | PanNET | Head, Any surgery was not performed due to patient’s surgical history | 1.5 | No atypia, mitosis, or necrosis | SFT |
Positive: CD34, CD99 Negative: CD117, S100 |
Alive, PF with residual tumor, 10 |
| Baxter, 2015 [30] | 58, F | Abdominal pain | PanNET, GIST, sarcoma, SPN, SFT, mass forming pancreatitis | Head, PD | 3.5 | No mitosis or necrosis | SFT |
Positive: CD34, bcl-2, focal beta-catenin, focal CD99 Negative: CD117, SMA, desmin, S100, Melan-A, HMB45, AE1/AE3, CAM5.2, EMA, synaptophysin, chromogranin A, CD56, PR |
Alive, RF, 24 |
| Estrella, 2015 [31] | 52, F | Jaundice | PanNET | Head, PD | 15 | Sarcomatous component, nuclear pleomorphism, marked cellularity, up to 17/10 HPF mitoses, necrosis | Malignant SFT |
Positive: CD34, bcl-2, and p53 and p16 in malignant areas Negative: CD117, CD99, synaptophysin, chromogranin A |
Alive, RF, 40 |
| Murakami, 2016 [32] | 82, M | Hypertension, hypercortisolism, hypokalemia, edema | PanNET with ectopic secretion of ACTH | Tail, DP | 6 | N/A | SFT |
Positive: STAT6, CD34, bcl-2, focal NSE, focal ACTH, focal POMC Negative: synaptophysin, chromogranin A |
Death after postoperative 4th month due to sepsis |
| Spasevska, 2016 [33] | 47, M | Epigastric pain, jaundice | Cystadenocarcinoma | Head, PD | 3.5 | Minimal pleomorphism and atypia, 1-2/10 HPF mitoses, no necrosis | SFT |
Positive: CD34, bcl-2, CD99, vimentin, focal beta-catenin, focal actin Negative: CD117, caldesmon, desmin, S100, cytokeratin, EMA |
Death after postoperative 1st week due to complications |
| Zhang, 2016 [34] | 63, M | Incidental | PanNET | Body/tail, DP | 3 | Marked focal atypia, necrosis, obvious mitosis, hypercellularity | Malignant SFT |
Positive: CD34, CD99, CD117, SMA, NSE, vimentin. Ki67 index >30%. Negative: F-VII, AE1/AE3, EMA, synaptophysin, chromogranin A, gastrin, VIP, somatostatin |
Multiple metastases within postoperative 6 months, Death after postoperative 10th month |
| 46, M | Abdominal pain | Chronic pancreatitis | Body/tail, DP | 4 | N/A | SFT |
Positive: CD34, bcl-2, CD99, SMA, focal CD68 Negative: CD117, DOG1, caldesmon, desmin, S100, ALK, CD31, CD21, CD35, AE1/AE3 |
Alive, RF, N/A | |
| 43, M | Abdominal discomfort | Castleman’s disease | Body/tail, Excision | 4 | N/A | SFT |
Positive: Focal CD34 Negative: CD117, SMA, desmin, S100 |
Alive, RF, N/A | |
| Paramythiotis, 2016 [35] | 55, M | Incidental | PanNET, SPN, GIST, SFT | Body, Resection | 3.6 | Rare mitosis, necrosis | SFT |
Positive: CD34, CD99, Bcl-2, vimentin, focal S100 Negative: CD117, SMA, desmin, cytokeratin, EMA |
Alive, RF, 12 |
| Clare, 2017 [36] | 39, F | Incidental | PanNET, SPN | Head, PD | 2.2 | 6/10 HPF mitoses, no necrosis | Malignant SFT |
Positive: STAT6, CD34, bcl-2, variable expression of AE1/AE3, CAM5.2 Negative: CD117, SMA, desmin, S100, MNF116, CK7, CK20, CDX2, synaptophysin, chromogranin A, ER |
Alive, RF, 1 |
| D’Amico, 2017 [37] | 52, M | Incidental | PanNET | Body, Enucleation | 2 | 1/10 HPF mitosis | SFT |
Positive: STAT6, CD34 Negative: N/A |
Alive, RF, 24 |
| Oana, 2017 [38] | 73, M | Abdominal discomfort | PanNET, GIST, ACC | Head, Partial pancreatectomy | 6.5 | No mitosis or necrosis | SFT |
Positive: CD34, bcl-2 Negative: CD117, SMA, desmin, S100, cytokeratin |
Alive, RF, 36 |
| Sheng, 2017 [39] | 14 m, M | Obstructive jaundice | N/A | Head, PD | 2 | Duodenal invasion, mild to moderate pleomorphism, 2-5/10 HPF mitoses in hypercellular area, no necrosis | SFT with low grade malignancy |
Positive: CD34, focal SMA, vimentin Negative: Bcl-2, CD117, CD99, desmin, myogenin, S100, ALK, cytokeratin, EMA, synaptophysin, chromogranin A, CD56 |
Alive, RF, 12 |
| Afzal, 2020 [40] | 43, M | Incidental | PanNET, GIST | Head/neck, PD | 12.7 | Moderate pleomorphism in hypercellular area, no increased mitosis, no necrosis | SFT |
Positive: STAT6, focal CD34, bcl-2, CD99, CD117 Negative: DOG1, SMA, desmin, S100, SOX10, ERG, CD31, pancytokeratin, synaptophysin, beta-catenin |
N/A |
| Geng, 2020 [41] | 48, M | Hypoglycemia | N/A | Body, DP, liver metastasectomy | 6.5 | Liver metastases, pleomorphism, 4-5/10 HPF mitoses, focal necrosis | Malignant SFT |
Positive: STAT6, CD34, bcl-2, CD31, D2-40 Negative: CD117, SMA, desmin, S100, GFAP |
Alive, PF with residual liver tumor, 6 |
| Li, 2020 [42] | 61, M | Abdominal pain | Pancreatic cancer, GIST | Body, DP | 11 | Splenic vein invasion, 25/10 HPF mitoses in hypercellular area | Malignant SFT |
Positive: STAT6, CD34, focal cytokeratin, focal EMA. Ki67 index; 20%. Negative: CD117, DOG1, desmin, S100, TLE-1, beta-catenin, CD21, CD35, CD23 |
Alive, RF, 4 |
| Taguchi, 2020 [43] | 60, M | Palpable mass | PanNEC, GIST, SFT | Head, PD | 8 | Venous invasion, duodenal invasion, 12/10 HPF mitoses, necrosis, hypercellularity | Malignant SFT |
Positive: STAT6 (weak), CD34, bcl-2, focal AE1/AE3, vimentin Negative: CD117, DOG1, SMA, desmin, S100, synaptophysin, chromogranin A |
Alive, RF, 12 |
| Liu, 2022 [44] | 54, F | Incidental | Benign or low-grade malignant pancreatic tumor | Head, Duodenum-preserving pancreatic head resection | 3.1 | No atypia or necrosis, 0-2/10 HPF mitosis | SFT |
Positive: STAT6, CD34, CD99 Negative: CD117, SMA, desmin, S100, EMA, cyclin-D1 |
Alive, RF, 6 |
PanNET: Pancreatic neuroendocrine tumor, SPN: Solid pseudopapillary tumor, GIST: Gastrointestinal stromal tumor, PanNEC: Pancreatic neuroendocrine carcinoma, ACTH: Adrenocorticotropic hormone, ACC: Acinar cell carcinoma, PD: Pancreaticoduodenectomy/Whipple procedure, DP: Distal pancreatectomy, SMA: Smooth muscle actin, NSE: Neuron specific enolase, MSA: Muscle specific actin, POMC: proopiomelanocortin, VIP: Vasoactive intestinal polypeptide, GFAP: Glial fibrillary acidic protein, RF: Recurrence-free, PF: Progression-free, m: months, N/A: Not available.
Table 3:
Comparison of Our Series and the Literature
| Our Series | Literature | |
|---|---|---|
| Mean age (years) | 60 | 54 |
| Male / Female | 5 / 4 | 17 / 18 |
| Symptoms | Abdominal pain (n=4), back pain (n=1), weight loss (n=1) | Abdominal/epigastric pain (n=10), jaundice (n=4), abdominal discomfort/distension (n=3), back pain (n=1), weight loss (n=1), palpable mass (n=1) |
| Head-neck / Body-tail | 6 / 3 | 19 / 16 |
| Median tumor size (cm) | 4 | 4 |
| Benign / Malignant | 9 / 0 | 28 / 7 |
| Mean follow-up (months) | 76 | 17 |
| Status | All are alive with NED | 23 are alive with no evidence of disease 2 are alive with residual disease 1 is alive with local recurrence 2 died due to post-operative complications 1 died due to malignant metastatic SFT 1 died due to sepsis |
DISCUSSION
Even though primary mesenchymal tumors of the pancreas are rare, various mesenchymal tumor types arising from pancreatic stromal tissue have been reported, including inflammatory myofibroblastic tumor, desmoid tumor, GIST, lymphangioma, cavernous hemangioma, angiomyolipoma, schwannoma, ganglioneuroma, leiomyosarcoma, rhabdomyosarcoma, liposarcoma, angiosarcoma, Ewing sarcoma/PNET, undifferentiated/unclassified sarcoma (28, 34, 50). However, experience with solitary fibrous tumor (SFT) is very limited. Our current understanding of this tumor is mainly based on individual case reports (12–44). In this study we analyzed nine cases.
Our findings, in combination with the previously published cases, reveal that most primary pancreatic SFTs occur in older adults (mean age, 55 years) without any sex predilection. The patients are either asymptomatic or present with non-specific findings such as abdominal pain or back pain. Jaundice is rare. The tumors are usually located in the head/neck with a median size of 4 cm. Due to their well circumscribed nature, clinically they are frequently diagnosed as pancreatic well differentiated neuroendocrine tumors.
Gross appearance of mesenchymal tumors is not distinctive either and mimic not only non-ductal epithelial tumors but also each other. Microscopically, SFTs may have variable histology. Therefore, distinguishing SFT from the other mesenchymal tumors, which show spindle-cell morphology could be even more challenging. Main microscopic differential diagnoses include desmoid tumor/fibromatosis, inflammatory myofibroblastic tumor, schwannoma, gastrointestinal stromal tumor, and leiomyoma. Needless to mention, melanoma should always be considered in the differential diagnoses of spindle cell lesions (2). Of note, although pancreatic well differentiated neuroendocrine tumor is considered as the main presurgical differential of SFT, microscopically they are usually easier to distinguish due to their more cellular epithelial nature and unique chromatin pattern. When in doubt, immunohistochemical staining with neuroendocrine markers such as chromogranin A and synaptophysin would be helpful (51).
In contrast to well circumscribed nature of the SFTs, desmoid tumors are infiltrative and are characterized with long fascicles of spindle or stellate cells. Although prominent vasculature is present, there are no staghorn-like vessels. Similarly, inflammatory myofibroblastic tumors are composed of loose fascicles of uniform, plump spindle cells. There is also inflammatory infiltrate, predominantly composed of lymphocytes and plasma cells, a feature not common in SFTs. Schwannomas reveal loose fascicles of spindle cells with eosinophilic cytoplasm and tapering nuclei. When present, characteristic Antoni A and B areas are helpful for establishing the right diagnosis. Gastrointestinal stromal tumors seen in the pancreas are usually spindle cell type, closely mimicking SFTs. Similarly, leiomyomas are characterized with long intersecting fascicles of spindle cells.
Fortunately, immunohistochemical studies are helpful for tumor classification. Although, bcl-2, CD34, and CD99 antibodies have been widely used for SFT diagnosis, these non-specific stains are not only positive in other tumors but can also be negative in SFTs. In our series, while bcl-2 expression was observed in all stained cases, CD34 was negative in 22%. However, after the discovery of NAB2-STAT6 fusion as the hallmark of SFTs (9, 10), subsequent studies have demonstrated that STAT6 is a reliable immunohistochemical marker for detecting this genetic alteration with very high sensitivity and specificity regardless of anatomic site and morphological features (52–56). Like these studies, all our cases revealed nuclear STAT6 expression, including the two CD34 negative cases. Of course it should be kept in mind that STAT6 may be positive in a small subset of dedifferentiated liposarcomas, most likely due to close location of STAT6 and MDM2 on chromosome 12 (52, 54). However, presence of well differentiated liposarcoma component and positive MDM2 and CDK4 staining can be helpful to distinct dedifferentiated liposarcoma from SFT.
In contrast to SFTs, desmoid tumors are characterized with nuclear β-catenin expression due to CTNNB1 gene activating mutations and may reveal SMA and/or desmin labeling (57). Inflammatory myofibroblastic tumors harbor ALK or ROS1 fusions and are characterized with corresponding ALK or ROS1 expression (58). They are also usually positive for SMA, desmin and less frequently keratin. Although schwannomas may express CD34, unlike SFTs, they are also positive for S100, GFAP and nestin (59). Similarly, GISTs may express CD34. However, since most GISTs harbor activating mutations of KIT or PDGFR, they also label with CD117 and/or DOG1 (60). Leiomyomas are positive for SMA, desmin, caldesmon, and calponin (61). Finally, melanomas are positive for S100, HMB-45, Melan-A and SOX10 (62).
In addition to these well-known entities, the most challenging differential diagnosis in the pancreas is sclerosing epithelioid mesenchymal tumor, a recently described novel entity specific to the pancreas (63). Similar to SFTs, these tumors are also well circumscribed and solid, and the density of neoplastic cells is significantly different throughout the tumor. The neoplastic cells also exhibit variable morphology. Spindle cells with irregular, hyperchromatic nuclei closely mimic SFT. However, presence of epithelioid cells containing scant cytoplasm and round to oval nuclei with open chromatin and lack of staghorn-like vessels are helpful features. More importantly, sclerosing epithelioid mesenchymal tumors are only positive for vimentin, CD99, keratin (CK18) and extensive molecular testing failed to identify any specific mutation or fusion in these tumors, although they have a distinct methylation profile (63).
While most of SFTs have favorable course, about 10% of the cases reported to act aggressively with local recurrence or distant metastasis (1, 2). Although various risk stratification models have been proposed to predict the behavior of SFTs, there is still uncertainty for the most useful assessment method in daily practice. The most used parameters include patient’s age, anatomical site, tumor size, mitotic count, cellularity, pleomorphism, hemorrhage/necrosis, surgical margins status and presence of sarcomatous transformation/dedifferentiation, (45, 46, 64–71). Subjectivity, lack of reproducibility and absence of absolute cut-off values for the assessment of cellularity, cellular atypia, and necrosis cause difficulties to determine malignancy potential of tumor. Also, tumors originated from different anatomic sites and the use of different outcome measures have led to inconsistencies between studies (3, 45, 46, 72, 73). In the current (2019) WHO Classification, older patient age, larger tumor size, presence of hypercellularity, cytologic atypia, mitotic count >4 per 2 mm2, hemorrhage, necrosis and sarcomatous transformation are described as the features related with aggressive behavior (2). In the largest study including 243 extra-pleural and non-meningeal SFTs, Pasquali et al. reported that mitotic activity, hypercellularity and pleomorphism correlate with recurrence and interestingly, larger tumor size is a better prognostic feature (45). Based on these, they developed a SFT recurrence scoring system that has very low-, low-, intermediate- and high-risk categories (with 100%, 88.9%, 65.9% and 52.6% disease-free survival rates at 5 years, respectively) (45). Subsequently, Demicco et al. by scoring patient age, tumor size, mitotic count and tumor necrosis but not cellularity and pleomorphism, categorized non-meningeal SFTs as having low-risk, intermediate-risk and high-risk (with no metastasis at 10 years, 10% risk of metastasis at 10 years and 73% risk of metastasis at 5 years, respectively) (46). None of our nine SFTs were considered malignant based on WHO malignancy criteria, while eight revealed low-risk and one (Case #8) revealed intermediate-risk for metastasis as per Demicco’s model. The patient with intermediate-risk SFT is alive with no evidence of disease at 62 months follow-up. However, among previously published primary pancreatic SFTs, there are seven cases that were reported as a malignant SFT (25, 31, 34, 36, 41–43). These tumors were characterized with marked cellularity (reported in 5 out of 7 cases), nuclear pleomorphism and/or atypia (reported in 4 of 7 cases), necrosis (reported in 5 out of 7 cases) and frequent (up to 25 per 10 HPF) mitoses (reported in all 7 cases). Only one of these patients developed multiple metastases within 6 months and succumbed to the disease 10 months after surgery. However, this patient’s tumor is reported to be positive for CD34 and CD117 (34). Therefore, the diagnosis appears to be questionable. One patient presented with multiple liver and bone metastases at diagnosis and is alive with disease at 6 months follow up (41); another one with intra-pancreatic metastases at the time of the diagnosis developed local recurrence after postoperative 21 months is alive at 32 months follow up (25). The other four patients, including one with sarcomatous component in the tumor, are alive with no evidence of disease at 1, 4, 12 and 40 months follow up (31, 36, 42, 43).
The clinicopathologic features of all reported cases are summarized in Tables 2 and 3.
Of note, recently, it has been suggested that hypoglycemia is also a high risk for unfavorable prognosis (3). In our study, none of the cases were found hypoglycemic. However, one of the known primary pancreatic SFTs presented with recurrent incidences of hypoglycemia without history of any endocrine disease (41). As mentioned above, the patient was eventually diagnosed with a malignant SFT of the pancreas with metastases to the liver and bone as well as Doege-Potter syndrome.
In addition, recent studies suggest that presence of TERT promoter mutation and/or P53 mutation in SFTs may be associated with malignant behavior (74–77). Demicco et al. also reported that TERT promoter mutations correlate with older age, larger tumor size, presence of necrosis and development of metastasis, but they have no impact on overall survival and disease-specific survival. As such, TERT promoter mutation status provides no additional information to predict of tumor behavior in low- and high-risk tumors. However, they may be used to identify the cases that have higher risk of metastasis in intermediate-risk group (78). At any rate, there is no TERT mutation studies in primary pancreatic SFTs in the literature.
In conclusion, SFTs form demarcated round tumors in the pancreas creating the impression of non-ductal tumors, especially well differentiated neuroendocrine tumors radiologically. Microscopically, SFT should be considered in the differential diagnosis of spindle cell neoplasms identified in the pancreas. The alternating cellularity, staghorn-like vessels, the intermixing of tumor cells with entrapped pancreatic parenchyma, and characteristic immunophenotype (nuclear STAT6 expression) are helpful features. Although primary pancreatic SFTs tend to have a favorable prognosis, marked cellularity, nuclear pleomorphism, necrosis and high mitotic activity are risk factors for aggressive behavior.
Supplementary Material
Source of funding:
This work was funded in part by the Melamed Family Foundation and by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. Jiaqi Shi is supported in part by the National Cancer Institute of the National Institutes of Health under award number R37CA262209.
Footnotes
Conflict of interest: None declared.
This study was presented in part at the annual meeting of the United States and Canadian Academy of Pathology in Los Angeles, CA, in March 2020.
REFERENCES
- 1.Demicco EG, Fritchie KJ, Han A Solitary fibrous tumour. In: WHO Classification of Tumours Editorial Board, ed. WHO Classification of Tumours: Soft Tissue and Bone Tumours. Lyon, France: IARC Press; 2020:104–108. [Google Scholar]
- 2.Fritchie KJ, Hornick JL, Rossi S Solitary fibrous tumour. In: WHO Classification of Tumours Editorial Board, ed. WHO Classification of Tumours: Digestive System Tumours. Lyon, France: IARC Press; 2019:448–449. [Google Scholar]
- 3.Yamada Y, Kohashi K, Kinoshita I, et al. Clinicopathological review of solitary fibrous tumors: Dedifferentiation is a major cause of patient death. Virchows Archiv. 2019;475:467–477. [DOI] [PubMed] [Google Scholar]
- 4.Roy T, Burns M, Overly D, et al. Solitary fibrous tumor of the pleura with hypoglycemia: the Doege-Potter syndrome. The Journal of the Kentucky Medical Association. 1992;90:557–560. [PubMed] [Google Scholar]
- 5.Strøm E, Skjørten F, Aarseth L, et al. Solitary fibrous tumor of the pleura: an immunohistochemical, electron microscopic and tissue culture study of a tumor producing insulin-like growth factor I in a patient with hypoglycemia. Pathology-Research and Practice. 1991;187:109–113. [DOI] [PubMed] [Google Scholar]
- 6.Fukasawa Y, Takada A, Tateno M, et al. Solitary fibrous tumor of the pleura causing recurrent hypoglycemia by secretion of insulin-like growth factor II. Pathology international. 1998;48:47–52. [DOI] [PubMed] [Google Scholar]
- 7.Wakami K, Tateyama H, Kawashima H, et al. Solitary fibrous tumor of the uterus producing high-molecular-weight insulin-like growth factor II and associated with hypoglycemia. International journal of gynecological pathology. 2005;24:79–84. [PubMed] [Google Scholar]
- 8.Aviel G, Doviner V, Pollak-Dresner R, et al. Metastatic Solitary Fibrous Tumor to the Pancreas Causing Non-islet Cell Tumor Hypoglycemia. Isr Med Assoc J. 2020;22:119–121. [PubMed] [Google Scholar]
- 9.Chmielecki J, Crago AM, Rosenberg M, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nature genetics. 2013;45:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson DR, Wu Y-M, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nature genetics. 2013;45:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schweizer L, Koelsche C, Sahm F, et al. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta neuropathologica. 2013;125:651–658. [DOI] [PubMed] [Google Scholar]
- 12.Lüttges J, Mentzel T, Hübner G, et al. Solitary fibrous tumour of the pancreas: a new member of the small group of mesenchymal pancreatic tumours. Virchows Archiv. 1999;435:37–42. [DOI] [PubMed] [Google Scholar]
- 13.Chatti K, Nouira K, Ben Reguigua M, et al. [Solitary fibrous tumor of the pancreas. A case report]. Gastroenterol Clin Biol. 2006;30:317–319. [DOI] [PubMed] [Google Scholar]
- 14.Gardini A, Dubini A, Saragoni L, et al. [Benign solitary fibrous tumor of the pancreas: a rare location of extra-pleural fibrous tumor. Single case report and review of the literature]. Pathologica. 2007;99:15–18. [PubMed] [Google Scholar]
- 15.Miyamoto H, Molena DA, Schoeniger LO, et al. Solitary fibrous tumor of the pancreas: a case report. International Journal of Surgical Pathology. 2007;15:311–314. [DOI] [PubMed] [Google Scholar]
- 16.Kwon H-J, Byun JH, Kang J, et al. Solitary fibrous tumor of the pancreas: imaging findings. Korean journal of radiology. 2008;9:S48–S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasan VD, Wayne JD, Rao MS, et al. Solitary fibrous tumor of the pancreas: case report with cytologic and surgical pathology correlation and review of the literature. J Pancreas. 2008;9:526–530. [PubMed] [Google Scholar]
- 18.Chetty R, Jain R, Serra S Solitary fibrous tumor of the pancreas. Annals of diagnostic pathology. 2009;13:339–343. [DOI] [PubMed] [Google Scholar]
- 19.Ishiwatari H, Hayashi T, Yoshida M, et al. [A case of solitary fibrous tumor of the pancreas]. Nihon Shokakibyo Gakkai Zasshi. 2009;106:1078–1085. [PubMed] [Google Scholar]
- 20.Sugawara Y, Sakai S, Aono S, et al. Solitary fibrous tumor of the pancreas. Japanese journal of radiology. 2010;28:479–482. [DOI] [PubMed] [Google Scholar]
- 21.Azadi J, Subhawong A, Durand DJ. F-18 FDG PET/CT and Tc-99m sulfur colloid SPECT imaging in the diagnosis and treatment of a case of dual solitary fibrous tumors of the retroperitoneum and pancreas. J Radiol Case Rep. 2012;6:32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.dos Santos LAM, dos Santos VM, Oliveira OCG, et al. Solitary fibrous tumour of the pancreas: a case report. Anales del sistema sanitario de Navarra; 2012:133–136. [DOI] [PubMed] [Google Scholar]
- 23.Tasdemir A, Soyuer I, Yurci A, et al. A huge solitary fibrous tumor localized in the pancreas: a young women. JOP Journal of the Pancreas. 2012;13:304–307. [PubMed] [Google Scholar]
- 24.van der Vorst JR, Vahrmeijer AL, Hutteman M, et al. Near-infrared fluorescence imaging of a solitary fibrous tumor of the pancreas using methylene blue. World journal of gastrointestinal surgery. 2012;4:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamanashi T, Toriumi F, Yahagi M, et al. A case of primary malignant solitary fibrous tumor of the pancreas. Jpn J Gastroenterol Surg. 2012;45:961–969. [Google Scholar]
- 26.Chen J-w, Tao L, Liu H-b, et al. A solitary fibrous tumor in the pancreas. Chinese medical journal. 2013;126:1388–1389. [PubMed] [Google Scholar]
- 27.Hwang JD, Kim JW, Chang JC. Imaging findings of a solitary fibrous tumor in pancreas: a case report. Journal of the Korean Society of Radiology. 2014;70:53–57. [Google Scholar]
- 28.Kim JY, Song JS, Park H, et al. Primary mesenchymal tumors of the pancreas: single-center experience over 16 years. Pancreas. 2014;43:959–968. [DOI] [PubMed] [Google Scholar]
- 29.Han SH, Baek YH, Han S-y, et al. Solitary fibrous tumor of the pancreas: A case report and review of the literature. The Korean Journal of Medicine. 2015;88:293–298. [Google Scholar]
- 30.Baxter AR, Newman E, Hajdu CH. Solitary fibrous tumor of the pancreas. Journal of surgical case reports. 2015;2015:rjv144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estrella JS, Wang H, Bhosale PR, et al. Malignant solitary fibrous tumor of the pancreas. Pancreas. 2015;44:988–994. [DOI] [PubMed] [Google Scholar]
- 32.Murakami K, Nakamura Y, Felizola SJ, et al. Pancreatic solitary fibrous tumor causing ectopic adrenocorticotropic hormone syndrome. Molecular and cellular endocrinology. 2016;436:268–273. [DOI] [PubMed] [Google Scholar]
- 33.Spasevska L, Janevska V, Janevski V, et al. Solitary fibrous tumor of the pancreas: a case report and review of the literature. prilozi. 2016;37:115–120. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Yu S, Wang W, et al. Primary mesenchymal tumors of the pancreas in a single center over 15 years. Oncology letters. 2016;12:4027–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paramythiotis D, Kofina K, Bangeas P, et al. Solitary fibrous tumor of the pancreas: Case report and review of the literature. World journal of gastrointestinal surgery. 2016;8:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clare F, Russell D, Rocha FG. Malignant solitary fibrous tumor of the pancreas. J Pancreas. 2017;18:159–162. [Google Scholar]
- 37.D’Amico FE, Ruffolo C, Romano M, et al. Rare neoplasm mimicking neuoroendocrine pancreatic tumor: a case report of solitary fibrous tumor with review of the literature. Anticancer research. 2017;37:3093–3097. [DOI] [PubMed] [Google Scholar]
- 38.Oana S, Matsuda N, Sibata S, et al. A case of a “wandering” mobile solitary fibrous tumor occurring in the pancreas. Clinical journal of gastroenterology. 2017;10:535–540. [DOI] [PubMed] [Google Scholar]
- 39.Sheng Q, Xu W, Liu J, et al. Pancreatic solitary fibrous tumor in a toddler managed by pancreaticoduodenectomy: a case report and review of the literature. OncoTargets and therapy. 2017;10:1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Afzal A, Maldonado-Vital M, Khan S, et al. Solitary Fibrous Tumor of Pancreas With Unusual Features: A Case Report. Cureus. 2020;12:e10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geng H, Ye Y, Jin Y, et al. Malignant solitary fibrous tumor of the pancreas with systemic metastasis: A case report and review of the literature. World J Clin Cases. 2020;8:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Li J, Xiong Y, et al. Atypical/malignant solitary fibrous tumor of the pancreas with spleen vein invasion: Case report and literature review. Medicine (Baltimore). 2020;99:e19783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taguchi Y, Hara T, Tamura H, et al. Malignant solitary fibrous tumor of the pancreas: a case report. Surg Case Rep. 2020;6:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W, Wu S, Cai Y, et al. Total laparoscopic duodenum-preserving pancreatic head resection for solitary fibrous tumor: The first case report. Asian J Surg. 2022;45:651–652. [DOI] [PubMed] [Google Scholar]
- 45.Pasquali S, Gronchi A, Strauss D, et al. Resectable extra-pleural and extra-meningeal solitary fibrous tumours: a multi-centre prognostic study. European Journal of Surgical Oncology (EJSO). 2016;42:1064–1070. [DOI] [PubMed] [Google Scholar]
- 46.Demicco EG, Wagner MJ, Maki RG, et al. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Modern Pathology. 2017;30:1433. [DOI] [PubMed] [Google Scholar]
- 47.Benayed R, Offin M, Mullaney K, et al. High Yield of RNA Sequencing for Targetable Kinase Fusions in Lung Adenocarcinomas with No Mitogenic Driver Alteration Detected by DNA Sequencing and Low Tumor Mutation Burden. Clin Cancer Res. 2019;25:4712–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20:1479–1484. [DOI] [PubMed] [Google Scholar]
- 50.Askan G, Basturk O. Mesenchymal Tumors Involving the Pancreas: A Clinicopathologic Analysis and Review of the Literature. Turk Patoloji Derg. 2022;38:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chetty R, Serra S. Spindle cell pancreatic endocrine tumor associated with Cushing’s syndrome. Endocrine pathology. 2005;16:145–151. [DOI] [PubMed] [Google Scholar]
- 52.Doyle LA, Vivero M, Fletcher CD, et al. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Modern Pathology. 2014;27:390. [DOI] [PubMed] [Google Scholar]
- 53.Cheah AL, Billings SD, Goldblum JR, et al. STAT6 rabbit monoclonal antibody is a robust diagnostic tool for the distinction of solitary fibrous tumour from its mimics. Pathology. 2014;46:389–395. [DOI] [PubMed] [Google Scholar]
- 54.Demicco EG, Harms PW, Patel RM, et al. Extensive survey of STAT6 expression in a large series of mesenchymal tumors. American journal of clinical pathology. 2015;143:672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshida A, Tsuta K, Ohno M, et al. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. The American journal of surgical pathology. 2014;38:552–559. [DOI] [PubMed] [Google Scholar]
- 56.Saeed O, Zhang S, Cheng L, et al. STAT6 Expression in Solitary Fibrous Tumor and Histologic Mimics: a Single Institution Experience. Applied immunohistochemistry & molecular morphology: AIMM. 2019. [DOI] [PubMed] [Google Scholar]
- 57.Fritchie KJ, Hornick JL, Rossi S Desmoid fibromatosis. In: WHO Classification of Tumours Editorial Board, ed. WHO Classification of Tumours: Digestive System Tumours. Lyon, France: IARC Press; 2019:446–447. [Google Scholar]
- 58.Fritchie KJ, Hornick JL, Rossi S Inflammatory myofibroblastic tumour. In: WHO Classification of Tumours Editorial Board, ed. WHO Classification of Tumours: Digestive System Tumours. Lyon, France: IARC Press; 2019:444–445. [Google Scholar]
- 59.Antonescu CR, , Hornick, Schwannoma JL In: WHO Classification of Tumours Editorial Board, ed. WHO Classification of Tumours: Digestive System Tumours. Lyon, France: IARC Press; 2019:477–478. [Google Scholar]
- 60.Dei Tos APH, J.L., Miettinen M Gastrointestinal stromal tumour. In: WHO Classification of Tumours Editorial Board, ed. WHO Classification of Tumours: Digestive System Tumours. Lyon, France: IARC Press; 2019:439–443. [Google Scholar]
- 61.Dry SM, Kumarasinghe MP . Leiomyoma. In: WHO Classification of Tumours Editorial Board, ed. WHO Classification of Tumours: Digestive System Tumours. Lyon, France: IARC Press; 2019:456–457. [Google Scholar]
- 62.Scolyer RA, Prieto VG Mucosal melanoma of the digestive system. In: WHO Classification of Tumours Editorial Board, ed. WHO Classification of Tumours: Digestive System Tumours. Lyon, France: IARC Press; 2019:502–503. [Google Scholar]
- 63.Basturk O, Weigelt B, Adsay V, et al. Sclerosing epithelioid mesenchymal neoplasm of the pancreas–a proposed new entity. Modern Pathology. 2020;33:456–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. The American journal of surgical pathology. 1989;13:640–658. [DOI] [PubMed] [Google Scholar]
- 65.Enzinger FM, Smith BH. Hemangiopericytoma: an analysis of 106 cases. Human pathology. 1976;7:61–82. [DOI] [PubMed] [Google Scholar]
- 66.Vallat-Decouvelaere A-V, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. The American journal of surgical pathology. 1998;22:1501–1511. [DOI] [PubMed] [Google Scholar]
- 67.Mosquera J-M, Fletcher CD. Expanding the spectrum of malignant progression in solitary fibrous tumors: a study of 8 cases with a discrete anaplastic component—is this dedifferentiated SFT? The American journal of surgical pathology. 2009;33:1314–1321. [DOI] [PubMed] [Google Scholar]
- 68.Kim JM, Choi Y-L, Kim YJ, et al. Comparison and evaluation of risk factors for meningeal, pleural, and extrapleural solitary fibrous tumors: A clinicopathological study of 92 cases confirmed by STAT6 immunohistochemical staining. Pathology-Research and Practice. 2017;213:619–625. [DOI] [PubMed] [Google Scholar]
- 69.Tapias LF, Mino-Kenudson M, Lee H, et al. Risk factor analysis for the recurrence of resected solitary fibrous tumours of the pleura: a 33-year experience and proposal for a scoring system. European Journal of Cardio-Thoracic Surgery. 2012;44:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salas S, Resseguier N, Blay J-Y, et al. Prediction of local and metastatic recurrence in solitary fibrous tumor: construction of a risk calculator in a multicenter cohort from the French Sarcoma Group (FSG) database. Annals of oncology. 2017;28:1979–1987. [DOI] [PubMed] [Google Scholar]
- 71.Gold JS, Antonescu CR, Hajdu C, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94:1057–1068. [PubMed] [Google Scholar]
- 72.van Houdt WJ, Westerveld CM, Vrijenhoek JE, et al. Prognosis of solitary fibrous tumors: a multicenter study. Annals of surgical oncology. 2013;20:4090–4095. [DOI] [PubMed] [Google Scholar]
- 73.Wilky BA, Montgomery EA, Guzzetta AA, et al. Extrathoracic location and “borderline” histology are associated with recurrence of solitary fibrous tumors after surgical resection. Annals of surgical oncology. 2013;20:4080–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park HK, Yu DB, Sung M, et al. Molecular changes in solitary fibrous tumor progression. Journal of Molecular Medicine. 2019;97:1413–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akaike K, Kurisaki-Arakawa A, Hara K, et al. Distinct clinicopathological features of NAB2-STAT6 fusion gene variants in solitary fibrous tumor with emphasis on the acquisition of highly malignant potential. Human pathology. 2015;46:347–356. [DOI] [PubMed] [Google Scholar]
- 76.Bahrami A, Lee S, Schaefer I-M, et al. TERT promoter mutations and prognosis in solitary fibrous tumor. Modern Pathology. 2016;29:1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Machado I, Morales GN, Cruz J, et al. Solitary fibrous tumor: a case series identifying pathological adverse factors—implications for risk stratification and classification. Virchows Archiv. 2019:1–11. [DOI] [PubMed] [Google Scholar]
- 78.Demicco EG, Wani K, Ingram D, et al. TERT promoter mutations in solitary fibrous tumour. Histopathology. 2018;73:843–851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


