Abstract
Neurons are remarkably long-lived, non-dividing cells that must maintain their functional features (e.g., electrical properties, chemical signaling) for extended periods of time – decades in humans. How neurons accomplish this incredible feat is poorly understood. Here, we review recent advances, primarily in the nematode C. elegans, that have enhanced our understanding of the molecular mechanisms that enable post-mitotic neurons to maintain their functionality across different life stages. We begin with “terminal selectors” - transcription factors necessary for the establishment and maintenance of neuronal identity. We highlight new findings on five terminal selectors (CHE-1 [Glass], UNC-3 [Collier/Ebf1-4], LIN-39 [Scr/Dfd/Hox4-5], UNC-86 [Acj6/Brn3a–c], AST-1 [Etv1/ER81]) from different transcription factor families (ZNF, COE, HOX, POU, ETS). We compare the functions of these factors in specific neuron types of C. elegans with the actions of their orthologs in other invertebrate (D. melanogaster) and vertebrate (M. musculus) systems, highlighting remarkable functional conservation. Finally, we reflect on recent findings implicating chromatin-modifying proteins, such as histone methyltransferases and Polycomb proteins, in the control of neuronal terminal identity. Altogether, these new studies on transcription factors and chromatin modifiers not only shed light on the fundamental problem of neuronal identity maintenance, but also outline mechanistic principles of gene regulation that may operate in other long-lived, post-mitotic cell types.
Keywords: neuronal identity, maintenance, transcription factors, terminal selectors, chromatin-modifying proteins, C. elegans, D. melanogaster, Mus musculus
1. INTRODUCTION
Human neurons must acquire during development and maintain, for decades of post-natal life, their functional features: e.g., neurotransmitter (NT) biosynthesis, electrical activity, and signaling properties1. Understanding how these non-dividing (post-mitotic) cells accomplish this remarkable feat can provide critical insights into the etiology, diagnosis, and treatment of developmental and degenerative disorders of the nervous system. Extensive research over the past few decades has made seminal contributions to our understanding of the molecular mechanisms underlying neurogenesis. However, the focus on early development has left poorly explored the molecular mechanisms underlying the last steps of neuronal differentiation, during which post-mitotic neurons acquire their functional features1. Further, and perhaps most importantly, the mechanisms that ensure maintenance of such features throughout post-natal life remain largely unknown; these mechanisms are the focus of this review.
During the final steps of neuronal differentiation, distinct neuron types must express neuron type-specific gene batteries of terminal identity genes (often called “effector genes”). These encode proteins that determine the functional properties of a neuron throughout its lifetime2, e.g., enzymes and transporters necessary for NT biosynthesis, ion channels that determine the resting potential of a neuron, neuropeptides and other signaling proteins (Figures 1–2)2. Altogether, these proteins form a distinct molecular signature for each neuron type that defines its terminal identity and function throughout life. Therefore, the challenge of understanding how neurons acquire and maintain their functional features lies in understanding how the expression of these terminal identity genes is regulated over time in specific neuron types. Are the mechanisms used for initial establishment (during development) and maintenance (throughout life) of neuron type-specific terminal identity genes distinct, or do establishment and maintenance rely upon the same mechanism? Addressing this question impacts the fields of developmental neuroscience and stem cell research, where it remains an outstanding challenge to generate mature and specialized neuron types in vitro, suitable for disease modeling and drug screening.
Figure 1. Terminal selectors of neuronal identity.
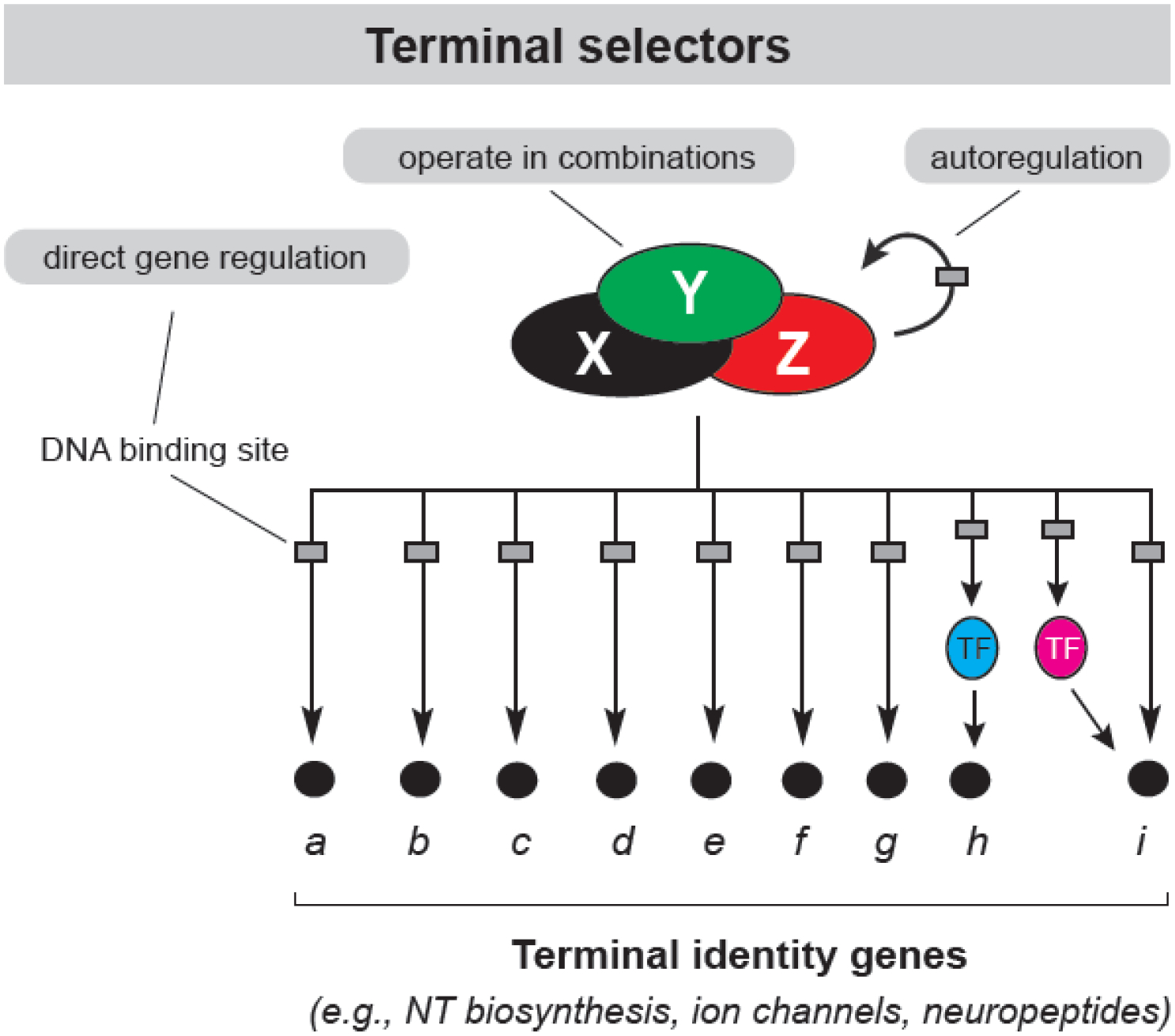
Terminal selector transcription factors can operate alone, or in combinations, to establish and maintain neuronal terminal identity. They directly regulate expression of terminal identity genes and intermediary transcription factors through binding at the cis-regulatory regions of these genes. Continuous terminal selector expression, required for neuronal identity maintenance, is ensured through direct positive autoregulation.
Figure 2. Terminal selector CHE-1 9 controls ASE identity.
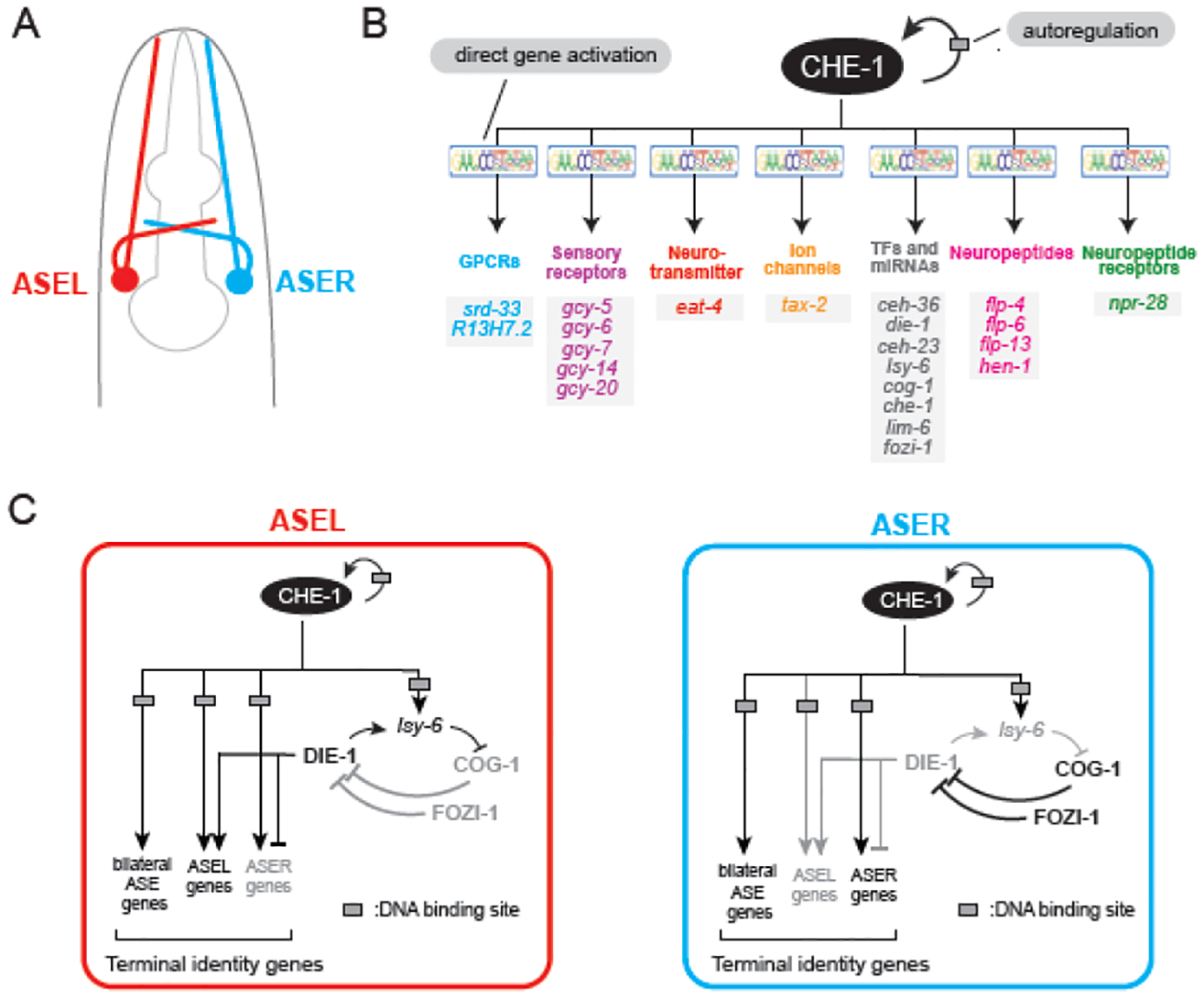
A. Schematic of ASE chemosensory neurons in the C. elegans head. B. CHE-1 directly promotes terminal identity gene and intermediary transcription factor expression through binding at its cognate site in the cis-regulatory regions of these genes. Continuous CHE-1 expression is ensured through autoregulation. C. ASE subtype diversification via a bistable feedback loop. CHE-1 specifies both ASEL and ASER subtype-specific fates through activation of a downstream bistable feedback loop. Initiation of ASER fate by the transcription factor COG-1 (later maintained by FOZI-1) is antagonized by DIE-1 (via the miRNA lsy-6) in the ASEL.
In this review, we primarily focus on the nervous system of the nematode Caenorhabditis elegans, where recent studies have advanced our understanding of the molecular principles that maintain neuronal terminal identity. To relate to readers outside the C. elegans community, we also compare C. elegans studies with findings reported in Drosophila and mice. Due to space constraints, we only cover C. elegans studies in a comprehensive manner.
1.1. Terminal selectors establish and maintain neuronal identity
The nematode C. elegans is a powerful system to study the problem of neuronal identity; it offers an unparalleled resource of available terminal identity markers that label with single-cell resolution each of the 118 neuron types that make up its nervous system3,4. Studies conducted with these markers, along with powerful genetics and behavioral analysis, led to the discovery of “terminal selectors”, a distinct class of regulators of neuronal identity. Terminal selectors are transcription factors with continuous expression, from development through adulthood, in specific neuron types4–6. They can act in combinations to establish neuronal terminal identity during development. Mechanistically, terminal selectors bind directly to the cis-regulatory regions of neuron type-specific terminal identity genes (e.g., NT receptors, ion channels, neuropeptides) and activate their transcription (Figure 1).
Terminal selectors have been described to date for 111 of the 118 C. elegans neuron types4,7. Beyond C. elegans, terminal selectors have been identified in the nervous system of fruit flies (Drosophila), cnidarians (Nematostella vectensis), marine chordates (Ciona intestinalis), and mice (Mus musculus)8, suggesting a deeply conserved role for this type of transcriptional regulators. While the essential role of terminal selectors in establishing neuronal terminal identity during development is well-attested, their involvement in maintaining neuronal identity in later life remains poorly examined.
Here, we provide an update on recent work demonstrating a continuous requirement for different terminal selectors in specific neuron types, solidifying their critical roles not only in establishing neuronal terminal identity during development, but also in maintaining it during later life stages. We focus on the best characterized examples of C. elegans terminal selectors: CHE-1 (Glass) in head sensory neurons, UNC-3 (Collier/Ebf) and LIN-39 (HOX) in nerve cord MNs, AST-1 (ETS) in dopamine neurons, and UNC-86 (POU) in sensory neurons (Table 1). Further, we highlight evolutionarily conserved functions of these terminal selectors in Drosophila and mouse neurons, complementing a previous review article on this subject1 (Table 1). Due to space constraints, we do not cover emerging studies suggesting a broader role for terminal selectors in the adult, i.e., they are not only required to maintain neuronal identity, but also maintain axonal architecture and connectivity11,12. Finally, we go beyond transcription factorbased mechanisms and highlight recent exciting work on how chromatin-modifying proteins control neuronal terminal identity.
Table 1.
Transcription factors and chromatin modifiers necessary for maintenance of neuron type identity
| Transcription or chromatin factor | Neuron type | Literature |
|---|---|---|
| C. elegans | ||
| CHE-1 (C2H2 Zn finger) | ASEL/R gustatory neurons | 16,17 |
| DIE-1 (C2H2 Zn finger) | ASEL subtype identity | 25 |
| LSY-12 (MYST-type histone acetyltransferase) | ASEL subtype identity | 25 |
| UNC-3 (EBF) | ventral nerve cord cholinergic MNs | 31,39,63 |
| CFI-1 (ARID) | ventral nerve cord cholinergic MNs | 39,40 |
| BNC-1 (Zn finger) | cholinergic MN subtype (VA, VB) | 42 |
| MAB-9 (T-box) | cholinergic MN subtype (DA, DB) | 42 |
| UNC-4 (paired-type homeobox)/UNC-37 (Groucho-like?) complex | cholinergic MN subtype (DA) | 115 |
| LIN-39 | midbody cholinergic MNs | 32,63 |
| UNC-86 (POU) | PLM glutamatergic touch sensory neurons | |
| UNC-86 (POU) | PVD glutamatergic sensory neuron | 88 |
| UNC-86 (POU) | PHC glutamatergic sensory neurons | 88 |
| UNC-86 (POU) | PLN cholinergic sensory neurons | 88 |
| UNC-86 (POU) | PQR peptidergic sensory neuron | 88 |
| UNC-86 (POU) | HSN serotonergic MN | 88 |
| UNC-86 (POU) | BDU peptidergic interneurons | 88 |
| UNC-86 (POU) | URB cholinergic interneuron | 88 |
| UNC-86 (POU) | URX cholinergic sensory neuron and ring interneuron | 88 |
| UNC-86 (POU) | NSM serotonergic pharyngeal neuron | 88 |
| AST-1 (ETS), UNC-86 (POU), SEM-4 (Spalt-type Zn finger), EGL-46 (Insm-type Zn finger), EGL-18 (GATA factor) | HSN serotonergic MN | 86 |
| CEH-43 (Dlx-type homeodomain)/AST-1 (ETS) | All dopaminergic neurons | 93,94 |
| CTBP-1 | AIA interneuron | 116 |
| LAG-1 | ADF serotonergic chemosensory neuron | 117 |
| mig-32 and spat-3 (PRC1 component) | AIY interneurons | 109 |
| D. melanogaster | ||
| Ubx | thoracic dopaminergic neurons | 80 |
| Ubx and abdA | abdominal leucokinergic neurons | 81 |
| Dfd | feeding MNs | 82 |
| M. musculus | ||
| Hoxc8 | brachial MNs | 74 |
| Hoxa5 | phrenic motor column neurons | 79 |
| Hoxa5 | brainstem neurons | 77 |
| BRN3A (POU) | medial habenula glutamateric and cholinergic neurons | 88 |
| CBP and p300 (KAT3) | excitatory hippocampal neurons | 112,113 |
| Ezh2 (PRC2 component) | cerebellar Purkinje cells, medium spiny neurons | 106 |
| Eed (PRC2 component) | midbrain dopaminergic neurons | 105 |
| Pet-1 | Hindbrain serotonin neurons | 46,47 |
2. Lessons from the terminal selector CHE-19 in chemosensory neurons
The ASE chemosensory neurons in the C. elegans head have been a prime model to obtain mechanistic insights on the control of neuronal terminal identity. The ASE neurons are a bilateral pair, with one neuron on the left (ASEL) and another on the right (ASER), critical for sensing water-soluble chemical cues. ASEL and ASER demonstrate morphological symmetry, and share many molecular features, including terminal identity genes that code for neuropeptides, ion channels, and signaling molecules (Figure 2A). However, ASEL and ASER also show molecular and functional asymmetries13; while ASEL detects one set of salt cues (e.g., sodium and lithium) and expresses specific transmembrane receptors (e.g., gcy7/guanylate cyclase), ASER detects a separate set of cues (e.g., potassium and chloride) and expresses another set of transmembrane receptors (ex. gcy-5/guanylate cyclase). Hence, they are classified into separate subtypes, ASEL and ASER (Figure 2A–B).
2.1. CHE-1 is required to establish and maintain ASE neuron identity
The che-1 (chemotaxis-defective) gene encodes a C2H2 zinc-finger transcription factor selectively expressed in both ASE neurons14,15. CHE-1 (Glass in Drosophila) is required for ASE identity; in che-1 null mutant animals, ASE neurons are normally generated but fail to acquire their terminal identity. Expression of ASE-shared and subtype-specific terminal identity genes (e.g., ion channels, guanylate cyclases, neuropeptide receptors) is lost. CHE-1 is also sufficient for ASE gene expression; expression of CHE-1 in other neuron classes causes ectopic expression of ASE terminal identity genes15. CHE-1 directly regulates these genes by binding at their cis-regulatory regions via its cognate binding site14 (Figure 2B). The expression of che-1 in post-mitotic ASE neurons is continuous, suggesting a role for CHE-1 in maintenance of ASE identity in the adult14,16. Indeed, temporally controlled CHE-1 depletion through RNAi or auxin-inducible degradation after ASE birth showed that CHE-1 is continuously required to maintain ASE identity and chemotactic behavior16,17. Hence, CHE-1 acts as a terminal selector of ASE terminal identity.
2.2. Positive autoregulation of che-1 is required to establish and maintain ASE neuron identity
A key feature of terminal selectors is their maintained expression in specific neuron types, often achieved through positive autoregulation5,6. Expression of che-1 in ASE neurons is initiated, but not maintained, by the nuclear receptor NHR-67/Tailless/TLX18. Two pieces of evidence suggest CHE-1 employs direct positive autoregulation after initiation. First, che-1 reporter expression is lost in ASE neurons of che-1 mutant animals14. Second, CRISPR/Cas9-mediated mutation of the CHE-1 binding site in the endogenous che-1 locus led to a striking decrease of che-1 expression in adult ASE neurons19. Importantly, this manipulation impacted the expression of ASE terminal identity genes and led to chemosensory defects in adults. Altogether, direct positive autoregulation of che-1 is required to maintain ASE terminal identity during late developmental and adult stages. Interestingly, Glass (CHE-1 ortholog) also acts as a terminal selector in sensory neurons (photoreceptors) of the Drosophila retina. Like che-1, Glass positively autoregulates and controls the expression of terminal identity genes in photoreceptors20,21. Hence, the terminal selector function of che-1 appears conserved from C. elegans to Drosophila, albeit in a different neuron type.
Positively autoregulating genes must be continuously expressed above a critical threshold to ensure maintenance of their autoregulation17,19. In the case of CHE-1, transient depletion of CHE-1 protein caused a permanent loss of CHE-1 expression, ASE identity, and chemotactic behavior17. This suggests additional mechanisms must be in place to maintain CHE-1 positive autoregulation. One possible mechanism is preferential recruitment of CHE-1 to its own promoter relative to its target gene promoters. In silico modeling, informed by in vivo quantification of mRNA levels and protein half-life, demonstrated that during depletion of CHE-1, levels of target gene transcripts decreased, whereas che-1 transcript levels did not. Taken together, these data suggest a model where CHE-1 binding at target gene promoters provides a reservoir of CHE-1 protein that can then be preferentially recruited to the che-1 locus upon a transient drop in CHE-1 levels. This “target gene reservoir buffering model” may constitute a general mechanism for stable maintenance of cell identity17.
2.3. A bistable feedback loop downstream of CHE-1 diversifies ASE neurons
Since che-1 acts as a terminal selector in both ASE neuron subtypes (ASEL/R), how are the unique identities of ASEL and ASER established and maintained? A gene regulatory network downstream of CHE-1 composed of miRNAs and transcription factors is responsible for ASE subtype diversification (Figure 2C)13,22–24. One key transcription factor within this network is the C2H2 zinc-finger transcription factor DIE-113. Initially identified in a genetic screen, DIE-1 is required for appropriate ASE subtype specification. DIE-1 functions to simultaneously promote ASEL and repress ASER identity; in die-1 genetic null mutant animals, ASEL is transformed to an ASER identity. Importantly, post-developmental removal of die-1 via RNAi also led to an ASEL-to-ASER identity conversion, indicating that die-1 is required continuously to maintain ASEL identity22,25.
Restriction of die-1 expression to ASEL is initially ensured by the homeobox gene cog-1/GTX/Nkx6, and maintained by the zinc finger transcription factor fozi-122. In turn, die-1 functions through the miRNA lsy-6 to repress cog-1 from being expressed in the ASEL13,24. Altogether, die-1, lsy-6, and cog-1 form a double-negative feedback loop downstream of the terminal selector CHE-1. This loop ensures ASE neurons adopt one of two possible identities (ASEL or ASER), thereby ensuring their diversification (Figure 2C). Feedback loops like the one described here are referred to as “bistable” because they determine that a system (e.g., ASE neurons) stably exists in one of two possible states26,27.
2.4. The MYST histone acetyltransferase complex is required to initiate and maintain ASE subtype identity
Intriguingly, a forward genetic screen for genes involved in ASEL/ASER specification identified lsy-12 (laterally symmetric), which encodes a chromatin-modifying enzyme28. Specifically, lsy-12 is an MYST-type histone acetyltransferase (HAT) of the MOZ/MORF subfamily25. Animals carrying a mutant allele of lsy-12 display the ASEL-to-ASER conversion phenotype, and post-developmental elimination of LSY-12 function causes a similar phenotype, demonstrating that LSY-12 is continuously required to maintain ASEL identity25. The similarity between DIE-1 and LSY-12 in the maintenance of ASEL neuron identity suggests a model in which DIE-1, a sequence-specific transcription factor, recruits LSY-12, a MYST-type HAT, to the cis-regulatory regions of ASEL-specific terminal identity genes, thereby ensuring local histone acetylation, and thus transcriptional activation, of ASEL genes25. Taken together, the continuous requirement of DIE-1 and LSY-12 exemplifies the need of both transcription factors and chromatin-modifying proteins for the control of neuronal identity maintenance.
3. Lessons from UNC-3 (Collier/Ebf) in cholinergic motor neurons
In the C. elegans ventral nerve cord, a structure analogous to the vertebrate spinal cord, there are 8 different classes (or subtypes) of motor neurons (MNs)29. Five subtypes of cholinergic (DA, DB, VA, VB, AS) and two subtypes of GABAergic (DD, VD) MNs control locomotion, whereas one cholinergic subtype30 is essential for egg laying (Figure 3A). At least one terminal selector has been identified to date for each of these subtypes: UNC-3 (Collier/Ebf) controls DA, DB, VA, VB, and AS identity31, LIN-39 (Scr/Dfd/Hox4-5) controls VC identity32, and UNC-30 (PITX) controls DD and VD identity33,34. Below, we highlight recent studies on UNC-3 that provide mechanistic insights into how terminal selectors maintain neuronal identity throughout life.
Figure 3: UNC-3 is a terminal selector of cholinergic MN identity.
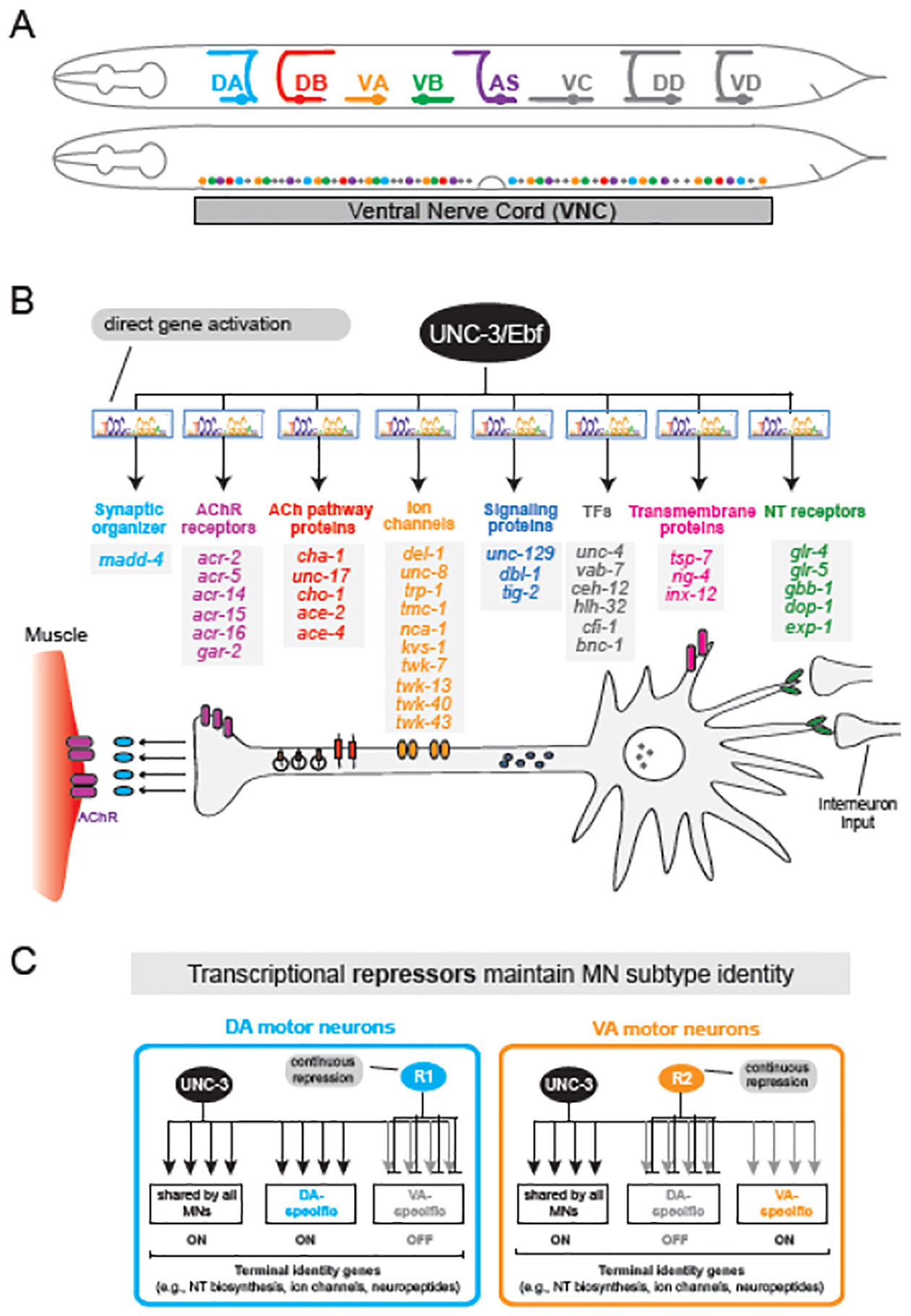
A. Five (colored) cholinergic MN subtypes used for C. elegans locomotion. The 50 cells that comprise these subtypes intermingle along the ventral nerve cord. B. UNC-3/Ebf activates, via direct binding at its cognate site (COE motif) in cis-regulatory regions, a diverse suite of genes essential for cholinergic MN function. Schematic modified from114 C. UNC-3 broadly activates both shared and subtype-specific terminal identity genes for cholinergic MNs. Subtype diversity is established and maintained by various subtype-specific repressor proteins (R1, R2) that antagonize UNC-3’s ability to activate terminal identity genes.
3.1. The terminal selector UNC-3 (Collier/Ebf) establishes cholinergic MN identity during development
The unc-3 gene, named after the uncoordinated locomotion phenotype its mutation causes, encodes the sole C. elegans orthologue of the COE (Collier/Olf/Ebf) transcription factor family35,36. UNC-3 is expressed in neurons of the head, tail, and ventral nerve cord31,37. In five subtypes of cholinergic MNs (DA, DB, VA, VB, AS), UNC-3 promotes the expression of dozens of terminal identity genes (e.g., NT biosynthesis components, NT receptors, ion channels, neuropeptides)31,38,39 (Figure 3B). Notably, these MNs are normally generated in unc-3 mutants, but fail to acquire their terminal identity. UNC-3 is also sufficient to promote cholinergic MN identity, as its forced expression in non-cholinergic neurons induced the ectopic expression of MN markers31. Two major pieces of evidence suggest UNC-3 binds directly to activate its target genes. First, ChIP-sequencing for UNC-3 revealed hundreds of terminal identity genes as putative direct targets, with UNC-3 binding occurring in their promoters and/or enhancers39. Second, mutagenesis experiments of UNC-3’s cognate DNA binding sites (COE motifs) in target genes resulted in unc-3 mutant-like phenotypes31,39,40. In summary, UNC-3 acts directly to establish cholinergic MN identity.
3.2. UNC-3 (Collier/Ebf) maintains cholinergic MN identity
The continuous expression of UNC-3 in cholinergic MNs throughout adulthood suggests UNC-3 is required at both early and later stages of life to control MN identity31. While UNC-3’s importance in promoting terminal identity gene expression in early stages has been demonstrated in animals carrying unc-3 null alleles31,38, its continuous requirement in maintaining that expression was only recently shown. Using an auxin-inducible degradation41 method for UNC-3 depletion selectively in adult cholinergic MNs, two recent studies reported adult-specific loss of expression of various UNC-3 targets genes39,42. Altogether, the terminal selector UNC-3 (Collier/Ebf) initiates during development and maintains throughout life the expression of numerous terminal identity genes in cholinergic MNs (Figure 3B), thus ensuring locomotory function in C. elegans.
3.3. Transcriptional repressors antagonize UNC-3 to generate and maintain MN diversity
Neurons are classified into types and subtypes based on molecular, morphological and functional criteria. Like the ASEL/R neurons discussed above (Figure 2C), the five cholinergic subtypes of nerve cord MNs (DA, DB, VA, VB, and AS) also provide an ideal model to study how neuronal subtype identities are established and maintained (Figure 3C)31,42. The terminal selector UNC-3 not only initiates and maintains the expression of terminal identity genes shared by all five subtypes (e.g., genes coding for acetylcholine biosynthesis components), but also of genes specific to certain subtypes (e.g., ion channels, NT receptors, neuropeptides) (Figure 3B). What prevents UNC-3 from activating these subtype-specific terminal identity genes more broadly, i.e., in all five MN subtypes?
Two recent studies pointed out that the answer lies in transcriptional repressor proteins, present in unique combinations in each MN subtype40,42. These repressors bind directly to the cis-regulatory regions of UNC-3 target genes and prevent their broad activation by UNC-340,42 (Figure 3C). For example, the combinatorial repressor activity of C2H2 Zinc finger transcription factor BNC-1 (Disco/BNC1–2) and Prd-type homeodomain protein UNC-4 (UNCX) defines VA subtype identity (Figure 3C). Similarly, the transcription factors MAB-9 (TBX20), VAB-7 (Eve/EVX), CFI-1 (Dead ringer/ARID3) and UNC-55 (COUP) also repress UNC-3 target gene expression in specific MN subtypes40,42. These repressor proteins are also continuously expressed in adult MNs, so they may be continuously required to antagonize UNC-3. Indeed, post-embryonic depletion of BNC-1 or MAB-9 with the auxin-inducible degradation system shows that repressors are continuously required to maintain MN subtype terminal identity40,42. Altogether, MN subtype identities are shaped by unique combinations of repressor proteins that continuously antagonize the terminal selector UNC-3. This strategy may constitute a general principle of neuron subtype diversification, as suggested by studies in C. elegans sensory neurons43, as well as in mouse midbrain dopaminergic neurons44 and retina photoreceptors45.
3.4. UNC-3 function in MNs is modular across different life stages
The continuous requirement of transcriptional repressors in C. elegans MNs prompts the question of how repressor expression is maintained throughout life. Unlike terminal selectors, these repressors do not positively autoregulate39,42. Instead, the terminal selector UNC-3 is specifically required to maintain repressor expression in MNs during late larval and adult stages. This maintenance-only requirement contrasts with UNC-3’s critical role in both initiating and maintaining the expression of terminal identity genes (Figure 3B). Hence, the suite of UNC-3 target genes in C. elegans MNs is partially modified over time because UNC-3 gains new targets (e.g., transcriptional repressors) specifically during late larval and adult stages. This phenomenon was recently described as “temporal modularity” in terminal selector function39.
Current evidence suggests that such modularity is beneficial for generating and maintaining neuronal subtype diversity. By expanding the pool of its target genes in late development to include transcriptional repressors, UNC-3 has an additional means of regulating individual subtype identities. Besides acting directly to activate and maintain expression of subtype-specific terminal identity genes, UNC-3 can also indirectly repress alternate identity genes by maintaining repressor gene expression (Figure 3C).
Temporal modularity has also been observed in mouse serotonergic neurons, where the terminal selector Pet-1 changes its transcriptional targets over time46. Pet-1 is also required to maintain chromatin accessibility during serotonergic neuron maturation, suggesting open chromatin in post-mitotic neurons requires continuous regulatory input by a terminal selector47. The cases of UNC-3 and Pet-1 showcase how the same terminal selector controls different target genes over time to establish and maintain neuron type identity. A recent study in embryonic stem cell (ESC)-derived MNs reported an additional mechanism: maintenance of expression of the same terminal identity genes is achieved via transient enhancers activated by stage-specific combinations of TFs48. Altogether, these studies point out that the transcriptional programs that maintain neuronal identity are more dynamic than previously thought and require further investigation across different life stages.
3.5. The terminal selector function of UNC-3 is conserved across species
Accumulating evidence suggests that the terminal selector function of UNC-3 is conserved across species. In the chordate Ciona intestinalis, UNC-3 ortholog COE is both necessary and sufficient for cholinergic MN identity31. In mice, UNC-3 orthologs Ebf1 and Ebf2 are respectively expressed in MNs of the hypaxial and medial columns of the developing spinal cord. Ebf2 is required for proper differentiation of a subset of these neurons49. While Ebf1’s function remains unknown, it remains highly expressed in spinal MNs of adult mice50. Mouse Ebf1 and Ebf2, and C. intestinalis COE, demonstrate functional equivalence to UNC-3, as each is able to compensate for loss of unc-3 in C. elegans31,49.
UNC-3 orthologs also control neuronal terminal identity beyond MNs. In C. intestinalis, COE controls the terminal identity of dorsal interneurons at the motor ganglion (analogous to vertebrate spinal cord)51, and in mice, EBF transcription factors (along with Lhx2 and LDB1) are required for the terminal differentiation of olfactory neurons52,53,54,55. Importantly, mutations in the human UNC-3 ortholog EBF3 cause a neurodevelopmental disorder characterized by developmental delay, ataxia and intellectual disability56,57. Future studies are needed to determine whether these clinical manifestations are due to loss of EBF3 terminal selector function in human neurons.
4. Hox proteins function as terminal selectors in C. elegans MNs
The highly conserved family of Hox transcription factors has been primarily associated with early development roles, such as anterior-posterior patterning of the embryo and the nervous system58,59. An overwhelming body of work in invertebrate and vertebrate models firmly attests that Hox gene function is essential for cell survival, proliferation, specification and migration. However, recent studies in the C. elegans nervous system have uncovered non-canonical roles for Hox genes during later stages of nervous system development and adult life, broadening the functional repertoire of this fundamental family of transcription factors beyond early patterning. Below, we highlight findings demonstrating a continuous requirement for Hox gene activity in the context of post-mitotic neurons in C. elegans, Drosophila and mice.
4.1. C. elegans Hox genes are continuously expressed in MNs
Like in other organisms, Hox genes in C. elegans are chromosomally clustered. In total, there are six Hox genes (ceh-13/Lab/Hox1, lin-39/Scr/Dfd/Hox4-5, mab-5/Antp/Hox6-8, egl-5/AbdB/Hox9-13, nob-1/Abd-B/Hox9-13, and php-3/Abd-B/Hox9-13)60, five of which exhibit spatial collinearity, i.e., the order of Hox expression domains along the rostro-caudal axis corresponds to their order on the chromosome (only ceh-13 breaks the collinearity rule)61,62. Hox genes in C. elegans are expressed in various cell types of a specific region along the rostro-caudal axis of embryos and larvae60. In addition to their critical roles during early patterning, recent C. elegans studies in touch and MNs uncovered that Hox genes are necessary for the final steps of neuronal development, i.e., the control of terminal identity7,32,63–69. To date, Hox involvement in maintaining neuron identity in the adult has only been demonstrated in MNs; therefore, we focus our discussion on this cell type.
The C. elegans ventral nerve cord is composed of various subtypes of cholinergic MNs (DA, DB, VA, VB, VC, AS) (Figure 3A). Each subtype contains multiple neurons (DA = 9 cells, DB = 7, VA = 12, VB = 11, AS = 11, VC = 6), and their cell bodies intermingle along the A-P axis (Figure 3A). Four of the six C. elegans Hox genes are expressed continuously, from development through adulthood, in specific groups of MNs. The anterior Hox ceh-13 (Lab/Hox1) is not spatially restricted, and is expressed in most MNs along the rostro-caudal axis of the nerve cord (Figure 4A). On the other hand, the midbody Hox gene lin-39 (Scr/Dfd/Hox4-5) is selectively expressed in midbody MNs, mab-5 (Antp/Hox6-8) in MNs of the posterior half of the cord, and the posterior Hox gene egl-5 (Abd-B/Hox9-13) in the most posterior members of the DA and VA subtypes (DA9, VA12) (Figure 4A). The continuous expression of these four Hox genes, from embryonic through adult stages32,63,66, suggests they may function as terminal selectors to establish MN terminal identity during development and maintain it in the adult.
Figure 4: Hox factors collaborate with terminal selectors to control MN identity along the rostro-caudal axis in C. elegans and mice.
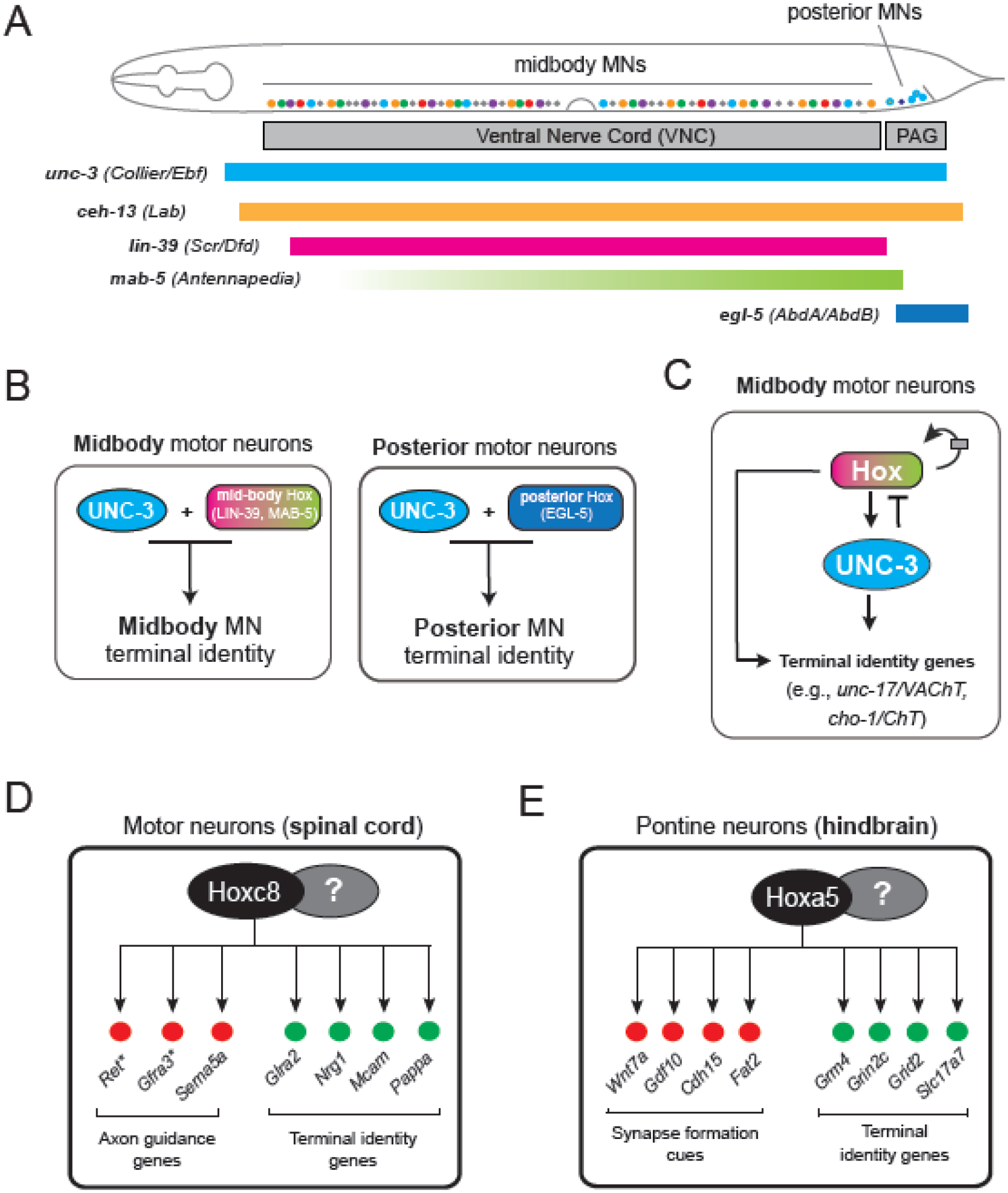
A. Four of the six C. elegans Hox genes are expressed continuously from development through adulthood in specific groups of MNs, depending on their positions in the ventral nerve cord. Hox factors thus intersect with UNC-3 in a region-specific manner. B. Distinct Hox genes and UNC-3 collaborate in a region-specific manner to establish and maintain the spatial identities of MNs along the rostro-caudal axis of the C. elegans nerve cord. C. Hox factors and UNC-3 collaborate to regulate terminal identity genes in a feed-forward-loop (FFL): both Hox and UNC-3 directly activate terminal identity genes; Hox directly activates UNC-3 expression; Hox expression is amplified by positive autoregulation and limited by negative feedback from UNC-3. This interaction may serve to buffer terminal identity gene expression against fluctuations in the levels of either activator. Panels A-C modified from60 D. Hoxc8 is required to maintain MN terminal identity in the mouse spinal cord. E. Hoxa5 is needed in the mouse brainstem to maintain neuronal terminal identity and function.
4.2. Hox genes collaborate with UNC-3 (Collier/Ebf) to establish MN terminal identity
The terminal selector unc-3 (Collier/Ebf) is expressed in all neurons of the DA, DB, VA, VB, and AS subtypes, but the expression of Hox genes (lin-39, mab-5 and egl-5) is spatially restricted, intersecting with unc-3 in a region-specific manner (Figure 4A). Genetic analyses revealed that lin-39 and mab-5 are required, like unc-3, in midbody MNs to establish the expression of various terminal identity genes (e.g., unc-17/VAChT, cho-1/ChT, del-1/ENAC)32,63,66. Similarly, egl-5 is required in posterior (rostral) MNs, activating itr-1/ITPR, glr-4/GRIK4, mig-13/LRP12, and flp-18/FMRF66. These findings point to an intersectional strategy controlling cholinergic MN terminal identity: distinct Hox genes and UNC-3 collaborate in a region-specific manner to establish the spatial (regional) identity of MNs along the A-P axis of the C. elegans nerve cord (Figure 4B).
A similar strategy may control the identity of spinal MNs in mice. Isl1, responsible for establishing spinal MN terminal identity70, is not spatially restricted in its expression. Distinct Hox genes, however, are expressed in different MN subtypes located at different regions along the rostro-caudal axis of the mouse spinal cord59. Future studies are needed to determine whether Isl1 and distinct Hox genes collaborate, in a region-specific manner, to establish and maintain MN terminal identity.
4.3. The midbody Hox gene lin-39 (Scr/Dfd/Hox4-5) functions as a terminal selector of cholinergic MN identity
Although mab-5 and egl-5 are involved in the establishment of terminal identity of midbody and posterior MNs, respectively66, whether they are continuously required remains untested (Figure 4B). However, accumulating evidence strongly suggests that lin-39 (Scr/Dfd/Hox4-5) acts as a terminal selector of cholinergic MN identity in the midbody region of the C. elegans ventral nerve cord. First, LIN-39 ChIP-sequencing revealed that LIN-39, like UNC-3, binds directly at its terminal identity gene targets. Mutational analysis of LIN-39 binding sequences upstream of these loci confirmed they are functionally important32,63, indicating LIN-39 regulates terminal identity genes via direct binding at their cis-regulatory regions. Second, like UNC-3, LIN-39 is continuously required to maintain the terminal identity of midbody MNs. Temporally controlled depletion of LIN-39 in adult C. elegans led to reduced expression of various MN terminal identity markers, indicating LIN-39 is not only necessary for establishment, but also maintenance, of MN identity32,63. Altogether, LIN-39 acts as a bona fide terminal selector that works with UNC-3 to establish and maintain cholinergic MN identity in the midbody region (Figure 4B–C). The function of LIN-39 as a terminal selector extends to other MNs that do not express unc-3, i.e., the cholinergic VC neurons that control egg-laying32, suggesting widespread employment by other MN subtypes of a Hox-based strategy for the control of their terminal identity.
4.4. Homeostatic control of Hox gene expression in adult MNs
The terminal selector lin-39 positively autoregulates its expression. Transgenic reporters of lin-39 fail to be expressed in MNs of lin-39 null mutant animals32, and LIN-39 binds directly to its own locus, suggesting it autoregulates at the transcriptional level in MNs (Figure 4C). Consistent with this idea, CRISPR/Cas9-mediated disruption of LIN-39 binding sites in the lin-39 locus decreased lin-39 mRNA and protein levels. This manipulation also reduced expression of terminal identity genes (e.g., cho-1/ChT) in MNs. Importantly, inducible degradation of LIN-39 in adult MNs led to decreased lin-39 expression, suggesting that this Hox gene is continuously required to maintain its own expression32.
Hox expression levels must be tightly controlled to avoid detrimental effects during tissue patterning71,72. In the C. elegans nerve cord, low levels of LIN-39 lead to a failure to maintain expression of terminal identity genes in MNs, whereas high LIN-39 levels result in locomotion defects63. Hence, lin-39 expression levels must be tightly controlled in MNs. This is achieved through a two-component principle where Hox positive autoregulation is counterbalanced by negative UNC-3 feedback32: UNC-3 direct binding to the lin-39 locus limits lin-39 mRNA levels, suggesting a direct negative effect on lin-39 transcription.
Thus, Hox gene expression in adult C. elegans MNs is under homeostatic control, with positive autoregulation counterbalanced by negative UNC-3 feedback (Figure 4C). Because Hox genes are expressed in adult neurons of the fly, mouse and human, homoeostatic mechanisms may be a conserved strategy to control Hox expression and therefore neuronal identity across metazoan nervous systems.
4.5. Hox and UNC-3 operate in a feed-forward loop to ensure maintenance of cholinergic MN identity
Recent work revealed an additional role for Hox in the control of C. elegans MN identity: lin-39 controls expression of the terminal selector unc-3 (Figure 4C). LIN-39 binds extensively at the unc-3 locus, and mutational analysis combined with quantification of unc-3 mRNA levels in lin-39 mutants strongly suggest that LIN-39 acts directly to activate unc-3 transcription32. In summary, the Hox gene lin-39 acts directly in MNs to control transcription both of terminal identity genes and of unc-3, which in turn also activates terminal identity genes, creating a feed-forward loop (FFL) (Figure 4C). This FFL may be viewed as a form of “redundancy engineering” to ensure robust and continuous expression of terminal identity genes, as their protein products are critical for neuronal function (e.g., neurotransmitter receptors, ion channels). For example, if fluctuations in unc-3 expression levels occur, terminal identity gene expression in MNs will not be significantly affected due to the presence of LIN-39. Similarly, a transient decrease in the expression of the upstream activator (LIN-39) will not immediately cause a corresponding decrease in the transcription of its target genes due to the presence of the intermediate activator (UNC-3). Thus, the LIN-39-UNC-3 FFL may operate in C. elegans MNs throughout life to ensure robust levels of terminal identity gene expression.
4.6. Continuous requirement for Hox gene activity in fly and mouse neurons
Like in C. elegans, Drosophila and mouse Hox genes are also expressed in the nervous system during late developmental and adult stages73–79. Emerging evidence indicates that sustained Hox expression is required for maintaining neuron terminal identity in these systems as well, dovetailing C. elegans Hox studies.
One of the Drosophila Hox genes, Ultrabithorax (Ubx), remains expressed in adult fly cholinergic, glutamatergic, and dopaminergic neurons. RNAi-mediated knockdown of Ubx in adult Drosophila causes a decrease in tyrosine hydroxylase (TH) and SLC ion channel expression in dopaminergic neurons and also impacted flight muscle activity80, revealing a post-developmental role for Ubx in maintaining dopaminergic neuron identity. Other known examples of Hox involvement in maintaining neuronal identity include: (a) Ubx, abdominal-A (abd-A) and Abdominal-B (Abd-B), which are continuously required to maintain the identities of peptidergic abdominal neurons81, and (b) Deformed (Dfd), which is required for feeding motor unit formation from its initial specification to the establishment of active synapses82.
In mice, RNA-sequencing of isolated spinal MNs at early post-natal stages revealed sustained expression of multiple Hox genes in brachial (Hoxc4, Hoxa5, Hoxc5, Hoxa6, Hoxc6, Hoxa7, Hoxc8), thoracic (Hoxd9), and lumbar (Hoxa10, Hoxc10, Hoxa11) MNs74. MN-specific removal of Hoxc8 gene activity at early and late developmental stages resulted in significant downregulation of various terminal identity genes (Nrg1, Mcam, Pappa) and motor defects74, indicating that Hoxc8 is continuously required for MN terminal identity and function (Figure 4D). A similar continuous requirement has been shown for Hox5 genes in mouse spinal MNs responsible for breathing79. Lastly, tamoxifen-inducible depletion of Hoxa5 in the brainstem at postnatal stages resulted in downregulation of multiples terminal identity genes (e.g., Slc17a7/VGLUT1, and glutamate receptor subunits Grm4, Grin2c, Grid2) required for synaptic transmission and plasticity77, demonstrating a continuous requirement for Hoxa5 in the postnatal brainstem (Figure 4E). Thus, accumulating evidence, from C. elegans to flies and mammals, support the idea that Hox genes are continuously required to maintain the identity and functionality of post-mitotic neurons.
5. POU homeodomain transcription factors establish and maintain neuronal identity in worms, flies, and mice
The POU family of transcription factors, named after its founding members (Pit1, Oct1, UNC-86), all share a homeodomain and a distinct POU domain, both essential for DNA binding83. Here, we highlight recent evidence for POU-family proteins as important players in maintaining neuronal identity. For a comprehensive review on POU proteins in nervous system development, we refer the reader to a recent article by Leyva-Díaz et al.84.
The C. elegans POU ortholog UNC-86 is known to prevent lineage reiterations during early development85. In addition, UNC-86 acts as a terminal selector in diverse classes of neurons (13 classes of sensory neurons, 7 of interneurons, and 4 of MNs)84. In each class, UNC-86 activates a distinct battery of terminal identity genes by cooperating with class-specific transcription factors. For example, UNC-86 collaborates with at least five transcription factors from different families to establish the identity of the HSN MN in the midbody86, whereas it partners with CFI-1 (ARID3) to establish sensory neuron (IL2) identity in the head40,87. unc-86 expression is also post-developmentally maintained via autoregulation. Post-embryonic removal of UNC-86 does not affect neuronal survival, but does cause loss of neurotransmitter identity in specific neuron classes: glutamatergic touch neurons (PLM) and two other sensory neuron classes (PVD, PHC), cholinergic neuron classes (URB, URX), serotonergic neuron classes (NSM, HSN), and peptidergic sensory neurons (PQR, BDU)88.
Beyond C. elegans, the function of UNC-86 in controlling neuron terminal identity is conserved in flies and mammals. The fly ortholog of UNC-86, Acj6, is predominantly expressed in sensory neurons, and remains expressed in those neurons throughout life89. Its function is well studied in olfactory neurons, where a single olfactory receptor (OR) gene must be expressed for odor discrimination. Acj6 is necessary for OR gene activation, but also for preventing ectopic OR expression90. In the sea anemone Nematostella vectensis, the UNC-86 ortholog NvPOU4 is expressed in neural cell types, such as cnidocytes and sensory neurons, where it is also required for terminal differentiation91.
In mice, the orthologs of UNC-86, Brn3a, Brn3b, and Brn3c, are mostly expressed in sensory neurons (e.g., auditory, somatosensory)92. Brn3a function has been examined in mouse medial habenular (MH) neurons, where its expression persists throughout adulthood88. When Brn3a is specifically depleted in adults, 50% of all MH neurons lose their glutamatergic identity (assessed by loss of VGluT1), and 50% of cholinergic ventral MH neurons lose their cholinergic identity (loss of ChAT). Other terminal identity features, such as ion channels and neuropeptides, are also lost, indicating that Brn3a is required to maintain various terminal identity features in adult MH neurons88.
6. Control of dopaminergic neuron identity in C. elegans and mice
The eight dopaminergic neurons of C. elegans (ADEL/R, CEPDL/R, CEPV/R, PDEL/R) are used for mechano-sensation. They are defined by the co-expression of five terminal identity genes encoding proteins necessary for dopamine biosynthesis (cat-2/TH, cat-4/GTPCH, bas-1/AAAD, cat-1/VMAT, and dat-1/DAT). Through a combination of unbiased genetic screens and candidate gene approaches, the ETS-type factor AST-1, the Dlx/Distalless ortholog CEH-43, and the Pbx ortholog CEH-20 were identified as terminal selectors93,94. They bind directly to the cis-regulatory regions of dopamine biosynthesis genes and co-regulate their expression. AST-1 acts in all eight dopaminergic neurons, whereas CEH-43 acts primarily in ADE and CEP neurons, and CEH-20 acts in PDE neurons. Hence, the activity of AST-1 intersects with CEH-43 and CEH-20 in distinct types of dopaminergic neurons, forming neuron type-specific combinations of terminal selectors. All three factors are also continuously expressed in adult C. elegans dopaminergic neurons93,94. Transient rescue assays showed that ast-1 gene activity is continuously required to maintain the expression of dopamine biosynthesis genes94. Similarly, animals carrying a hypomorphic allele of ceh-43 showed a progressive loss of dopaminergic marker expression93.
Interestingly, the mouse orthologs of AST-1 (Etv1/ER81), CEH-43 (DLX2) and CEH-20 (PBX1) are also critical for the terminal differentiation of dopamine neurons in the mouse olfactory bulb94–98. Both Etv1/ER81 and PBX1 are known to directly bind at least one dopamine pathway gene (Th) to promote its expression96,98, and are also continuously expressed in olfactory bulb dopaminergic neurons throughout life, suggesting a conserved role for the C. elegans terminal selectors AST-1 and CEH-20 in maintenance of dopamine neuron identity.
7. Emerging roles for chromatin-modifying proteins in neuronal terminal identity
In addition to transcription factors, post-translational modifications of histones are critical for gene expression because they affect chromatin structure99. Polycomb group (PcG) proteins, as well as histone methyltransferases and acetyltransferases, are key families of evolutionarily conserved proteins, whose activity affects chromatin structure. We highlight here recent findings implicating these proteins in the establishment and/or maintenance of neuronal identity in C. elegans and mice.
7.1. Polycomb proteins control neuronal terminal identity
Polycomb group (PcG) proteins play evolutionarily conserved roles in gene regulation during development100,101. Polycomb-mediated gene regulation is provided by two multi-protein complexes, known as Polycomb repressive complex 1 (PRC1) and 2 (PRC2). PRC2 methylates histone H3 at lysine 27 (H3K27me3), a histone mark that leads to chromatin compaction at genes targeted for repression102–104. In mice, conditional deletion of the PRC2 component Embryonic ectoderm development (Eed) in postmitotic midbrain dopaminergic neurons resulted in a loss of H3K27me3, followed by a progressive activation of genes associated with alternate fates (e.g., Gata6, Foxg1, Dlx), as well as a progressive loss of terminal identity gene expression105. Importantly, this genetic manipulation did not affect dopaminergic neuron survival, but it did lead to behavioral deficits characteristic of Parkinson’s disease. Eed deletion in mouse serotonergic neurons yielded similar results105. Interestingly, a separate study showed that PRC2 is required to maintain silencing of death-promoting genes in medium spiny neurons and Purkinje cells, uncovering a key role for PRC2 in protecting neurons against degeneration106. Although these two studies did not employ adult-specific PRC2 inactivation, the findings nevertheless strongly suggest that PRC2 is required to maintain the identity and function of dopaminergic and serotonergic neurons in the adult mouse brain.
PRC1 can control gene expression positively or negatively. During mouse spinal MN development, genetic removal of a PRC1 component (Ring1) leads to increased chromatin accessibility and ectopic expression of various genes, suggesting MN development relies on PRC1-mediated gene repression107. However, recent data suggest that PRC1 can also play a role in gene activation103,108. In C. elegans, PRC1 affects the terminal identity of head interneurons (AIY) by controlling the expression levels of two terminal selector genes (ttx-3, ceh-10)109. Genetic removal of PRC1 factors has no effect on the initiation of these genes, but causes a progressive loss of expression in late larval and adult stages. PRC1 plays a similar role in C. elegans dopaminergic neurons (ADE)109. Altogether, this indicates that PRC1 factors are needed to ensure maintenance of terminal selector gene expression, a key step for maintenance of neuronal identity.
7.2. Histone methyltransferases and acetyltransferases involved in neuronal terminal identity
Accumulating evidence in C. elegans suggests that histone methylation is necessary for establishment of neuronal terminal identity. In the context of nerve cord MNs, mutations in H3K9 histone methyltransferases met-2 (SETDB1) and set-25 (SUV39H2) result in ectopic expression of terminal identity genes and transcription factors40,110. These studies provided genetic evidence that H3K9 methylation, the epigenetic hallmark of gene repression111, is necessary for MN terminal identity. On the other hand, recent work in the adult mouse brain showed that acetylation of lysine 27 in H3 (H3K27), a mark associated with active transcription, is necessary to maintain the terminal identity of forebrain and hippocampal neurons112. Combined elimination in adult forebrain neurons of CBP and p300, two proteins with a lysine acetyltransferase catalytic domain, led to decreased H3K27 acetylation and downregulation of neuronal identity genes, as well as a rapidly progressing ataxia phenotype. Similarly, combined depletion of CBP and p300 in excitatory hippocampal neurons resulted in reduced expression of terminal identity genes112,113. These findings demonstrate that CBP and p300 are required in mice to maintain excitatory neuron identity by preserving acetylation levels at neuron type-specific genes. Taken together, these recent studies in C. elegans and mice provide early evidence that post-translational modifications of histones (H3K9, H3K27) are essential for neuronal terminal identity.
8. OPEN QUESTIONS
This review highlights the functions of terminal selector-type transcription factors and chromatin modifiers in the control of neuronal terminal identity in C. elegans. Studies on fly and mouse orthologs of these C. elegans proteins uncovered deep functional conservation. Overwhelming evidence supports the idea that terminal selectors are not only required to establish during development, but also maintain throughout adult life, the identity of specific neuron types. Hence, initial establishment (during development) and maintenance (throughout life) of neuron type-specific identity relies upon the activity of the same transcription factors. However, a handful of studies showed that terminal selectors can also switch target genes – in the same neuron type – across different life stages12,39,46. Overall, the transcriptional mechanisms that maintain neuronal identity are likely more dynamic than previously thought, and future studies on chromatin accessibility are needed.
More recent studies indicate that chromatin modifiers are also necessary for the establishment of neuronal terminal identity, but whether they are actively required for its maintenance remains poorly understood. An unresolved issue relates to the classic problem of specificity. Since chromatin modifiers are expressed broadly (often ubiquitously) and act on DNA in a non-sequence-specific manner, how do they exert such specific effects on gene expression in specific neuron types? One to-be-tested hypothesis is that specificity arises through interactions with terminal selectors, which do bind specific DNA sequences and can potentially recruit chromatin factors to the cis-regulatory region of terminal identity genes, enabling their activation or repression in a neuron type-specific manner. Further work is also needed to test another clinically relevant hypothesis, originally formulated by Deneris and Hobert1, i.e., disruption of transcriptional programs that maintain neuronal identity can cause neurological conditions. Indeed, human mutations in terminal selectors, chromatin factors and their effector target genes are linked to neuropsychiatric and neurodegenerative conditions1. Exciting new findings in mammalian serotonergic neurons further support this hypothesis; terminal selectors Pet-1 and Lmxb1 are required, in the adult, to protect long-distance axons and synapses against slow degeneration11,12.
Acknowledgements
We would like to thank two members of the Kratsios lab (Jayson J Smith and Filipe Marques) for helpful discussions and edits pertaining to this manuscript. We apologize to colleagues whose work is not listed in Table 1 due to space constraints. This work was supported by the following NIH grants: T32 GM139782 to H.D, T32 HD055164 to M.P, and R01 NS116365 and R01 NS118078 to P.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of Interest
The authors declare no interests or relationships - financial, personal, or otherwise - that influence or could influence the work reported in this paper.
REFERENCES
- 1.Deneris ES & Hobert O Maintenance of postmitotic neuronal cell identity. Nat Neurosci 17, 899–907, doi: 10.1038/nn.3731 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobert O Regulation of terminal differentiation programs in the nervous system. Annu Rev Cell Dev Biol 27, 681–696, doi: 10.1146/annurev-cellbio-092910-154226 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Hobert O, Glenwinkel L & White J Revisiting Neuronal Cell Type Classification in Caenorhabditis elegans. Curr Biol 26, R1197–R1203, doi: 10.1016/j.cub.2016.10.027 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Hobert O A map of terminal regulators of neuronal identity in Caenorhabditis elegans. Wiley Interdiscip Rev Dev Biol 5, 474–498, doi: 10.1002/wdev.233 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobert O Regulatory logic of neuronal diversity: terminal selector genes and selector motifs. Proc Natl Acad Sci U S A 105, 20067–20071, doi:0806070105 [pii] 10.1073/pnas.0806070105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hobert O Terminal Selectors of Neuronal Identity. Curr Top Dev Biol 116, 455–475, doi: 10.1016/bs.ctdb.2015.12.007 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Reilly MB et al. Widespread employment of conserved C. elegans homeobox genes in neuronal identity specification. PLoS Genet 18, e1010372, doi: 10.1371/journal.pgen.1010372 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobert O & Kratsios P Neuronal identity control by terminal selectors in worms, flies, and chordates. Curr Opin Neurobiol 56, 97–105, doi: 10.1016/j.conb.2018.12.006 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Heinz S et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38, 576–589, doi: 10.1016/j.molcel.2010.05.004 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoxha E et al. Motor dysfunction and cerebellar Purkinje cell firing impairment in Ebf2 null mice. Mol Cell Neurosci 52, 51–61, doi:S1044–7431(12)00178–9 [pii] 10.1016/j.mcn.2012.09.002 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Donovan LJ et al. Lmx1b is required at multiple stages to build expansive serotonergic axon architectures. Elife 8, doi: 10.7554/eLife.48788 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitt MM et al. An adult-stage transcriptional program for survival of serotonergic connectivity. Cell Rep 39, 110711, doi: 10.1016/j.celrep.2022.110711 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang S, Johnston RJ Jr., Frokjaer-Jensen C, Lockery S & Hobert O MicroRNAs act sequentially and asymmetrically to control chemosensory laterality in the nematode. Nature 430, 785–789, doi: 10.1038/nature02752 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Etchberger JF et al. The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuron. Genes Dev 21, 1653–1674, doi: 10.1101/gad.1560107 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchida O, Nakano H, Koga M & Ohshima Y The C. elegans che-1 gene encodes a zinc finger transcription factor required for specification of the ASE chemosensory neurons. Development 130, 1215–1224, doi: 10.1242/dev.00341 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Etchberger JF, Flowers EB, Poole RJ, Bashllari E & Hobert O Cis-regulatory mechanisms of left/right asymmetric neuron-subtype specification in C. elegans. Development 136, 147–160, doi: 10.1242/dev.030064 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traets JJ, van der Burght SN, Rademakers S, Jansen G & van Zon JS Mechanism of life-long maintenance of neuron identity despite molecular fluctuations. Elife 10, doi: 10.7554/eLife.66955 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarin S, Antonio C, Tursun B & Hobert O The C. elegans Tailless/TLX transcription factor nhr-67 controls neuronal identity and left/right asymmetric fate diversification. Development 136, 2933–2944, doi: 10.1242/dev.040204 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leyva-Diaz E & Hobert O Transcription factor autoregulation is required for acquisition and maintenance of neuronal identity. Development 146, doi: 10.1242/dev.177378 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernardo-Garcia FJ, Fritsch C & Sprecher SG The transcription factor Glass links eye field specification with photoreceptor differentiation in Drosophila. Development 143, 1413–1423, doi: 10.1242/dev.128801 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Bernardo-Garcia FJ, Humberg TH, Fritsch C & Sprecher SG Successive requirement of Glass and Hazy for photoreceptor specification and maintenance in Drosophila. Fly (Austin) 11, 112–120, doi: 10.1080/19336934.2016.1244591 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochella L et al. Two distinct types of neuronal asymmetries are controlled by the Caenorhabditis elegans zinc finger transcription factor die-1. Genes Dev 28, 34–43, doi: 10.1101/gad.233643.113 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobert O Development of left/right asymmetry in the Caenorhabditis elegans nervous system: from zygote to postmitotic neuron. Genesis 52, 528–543, doi: 10.1002/dvg.22747 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Johnston RJ & Hobert O A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature 426, 845–849, doi: 10.1038/nature02255 (2003). [DOI] [PubMed] [Google Scholar]
- 25.O’Meara MM, Zhang F & Hobert O Maintenance of neuronal laterality in Caenorhabditis elegans through MYST histone acetyltransferase complex components LSY-12, LSY-13 and LIN-49. Genetics 186, 1497–1502, doi: 10.1534/genetics.110.123661 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrell JE Jr. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol 14, 140–148, doi: 10.1016/s0955-0674(02)00314-9 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Johnston RJ Jr., Chang S, Etchberger JF, Ortiz CO & Hobert O MicroRNAs acting in a double-negative feedback loop to control a neuronal cell fate decision. Proc Natl Acad Sci U S A 102, 12449–12454, doi: 10.1073/pnas.0505530102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarin S et al. Genetic screens for Caenorhabditis elegans mutants defective in left/right asymmetric neuronal fate specification. Genetics 176, 2109–2130, doi: 10.1534/genetics.107.075648 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Stetina SE, Treinin M & Miller DM 3rd. The motor circuit. Int Rev Neurobiol 69, 125–167, doi:S0074–7742(05)69005–8 [pii] 10.1016/S0074-7742(05)69005-8 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Loots GG et al. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res 15, 928–935, doi:gr.3437105 [pii] 10.1101/gr.3437105 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kratsios P, Stolfi A, Levine M & Hobert O Coordinated regulation of cholinergic motor neuron traits through a conserved terminal selector gene. Nat Neurosci 15, 205214, doi:nn.2989 [pii] 10.1038/nn.2989 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng W, Destain H, Smith JJ & Kratsios P Maintenance of neurotransmitter identity by Hox proteins through a homeostatic mechanism. Nat Commun 13, 6097, doi: 10.1038/s41467-022-33781-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eastman C, Horvitz HR & Jin Y Coordinated transcriptional regulation of the unc-25 glutamic acid decarboxylase and the unc-47 GABA vesicular transporter by the Caenorhabditis elegans UNC-30 homeodomain protein. J Neurosci 19, 6225–6234 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin Y, Hoskins R & Horvitz HR Control of type-D GABAergic neuron differentiation by C. elegans UNC-30 homeodomain protein. Nature 372, 780–783, doi: 10.1038/372780a0 (1994). [DOI] [PubMed] [Google Scholar]
- 35.Dubois L & Vincent A The COE--Collier/Olf1/EBF--transcription factors: structural conservation and diversity of developmental functions. Mech Dev 108, 3–12 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Prasad BC et al. unc-3, a gene required for axonal guidance in Caenorhabditis elegans, encodes a member of the O/E family of transcription factors. Development 125, 15611568 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Pereira L et al. A cellular and regulatory map of the cholinergic nervous system of C. elegans. Elife 4, doi: 10.7554/eLife.12432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y & Kratsios P Transgenic reporter analysis of ChIP-Seq-defined enhancers identifies novel target genes for the terminal selector UNC-3/Collier/Ebf. MicroPubl Biol 2021, doi: 10.17912/micropub.biology.000453 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y et al. Establishment and maintenance of motor neuron identity via temporal modularity in terminal selector function. Elife 9, doi: 10.7554/eLife.59464 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y et al. Cell context-dependent CFI-1/ARID3 functions control neuronal terminal differentiation. Cell Rep 42, 112220, doi: 10.1016/j.celrep.2023.112220 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Towbin BD et al. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell 150, 934–947, doi: 10.1016/j.cell.2012.06.051 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Kerk SY, Kratsios P, Hart M, Mourao R & Hobert O Diversification of C. elegans Motor Neuron Identity via Selective Effector Gene Repression. Neuron 93, 80–98, doi: 10.1016/j.neuron.2016.11.036 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cros C & Hobert O Caenorhabditis elegans sine oculis/SIX-type homeobox genes act as homeotic switches to define neuronal subtype identities. Proc Natl Acad Sci U S A 119, e2206817119, doi: 10.1073/pnas.2206817119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Salvio M et al. Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP. Nat Neurosci 13, 1481–1488, doi: 10.1038/nn.2661 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Peng GH, Ahmad O, Ahmad F, Liu J & Chen S The photoreceptor-specific nuclear receptor Nr2e3 interacts with Crx and exerts opposing effects on the transcription of rod versus cone genes. Hum Mol Genet 14, 747–764, doi: 10.1093/hmg/ddi070 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Wyler SC et al. Pet-1 Switches Transcriptional Targets Postnatally to Regulate Maturation of Serotonin Neuron Excitability. J Neurosci 36, 1758–1774, doi: 10.1523/JNEUROSCI.3798-15.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang XL, Spencer WC, Tabuchi N, Kitt MM & Deneris ES Reorganization of postmitotic neuronal chromatin accessibility for maturation of serotonergic identity. Elife 11, doi: 10.7554/eLife.75970 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhee HS et al. Expression of Terminal Effector Genes in Mammalian Neurons Is Maintained by a Dynamic Relay of Transient Enhancers. Neuron 92, 1252–1265, doi: 10.1016/j.neuron.2016.11.037 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Catela C et al. An ancient role for collier/Olf/Ebf (COE)-type transcription factors in axial motor neuron development. Neural Dev 14, 2, doi: 10.1186/s13064-018-0125-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel T et al. Transcriptional dynamics of murine motor neuron maturation in vivo and in vitro. Nat Commun 13, 5427, doi: 10.1038/s41467-022-33022-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Popsuj S & Stolfi A Ebf Activates Expression of a Cholinergic Locus in a Multipolar Motor Ganglion Interneuron Subtype in Ciona. Front Neurosci 15, 784649, doi: 10.3389/fnins.2021.784649 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang SS, Lewcock JW, Feinstein P, Mombaerts P & Reed RR Genetic disruptions of O/E2 and O/E3 genes reveal involvement in olfactory receptor neuron projection. Development 131, 1377–1388, doi: 10.1242/dev.01009 131/6/1377 [pii] (2004). [DOI] [PubMed] [Google Scholar]
- 53.Wang SS, Tsai RY & Reed RR The characterization of the Olf-1/EBF-like HLH transcription factor family: implications in olfactory gene regulation and neuronal development. J Neurosci 17, 4149–4158 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monahan K, Horta A & Lomvardas S LHX2- and LDB1-mediated trans interactions regulate olfactory receptor choice. Nature 565, 448–453, doi: 10.1038/s41586-018-0845-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monahan K et al. Cooperative interactions enable singular olfactory receptor expression in mouse olfactory neurons. Elife 6, doi: 10.7554/eLife.28620 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chao HT et al. A Syndromic Neurodevelopmental Disorder Caused by De Novo Variants in EBF3. Am J Hum Genet 100, 128–137, doi: 10.1016/j.ajhg.2016.11.018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sleven H et al. De Novo Mutations in EBF3 Cause a Neurodevelopmental Syndrome. Am J Hum Genet 100, 138–150, doi: 10.1016/j.ajhg.2016.11.020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mallo M, Wellik DM & Deschamps J Hox genes and regional patterning of the vertebrate body plan. Dev Biol 344, 7–15, doi: 10.1016/j.ydbio.2010.04.024 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Philippidou P & Dasen JS Hox genes: choreographers in neural development, architects of circuit organization. Neuron 80, 12–34, doi: 10.1016/j.neuron.2013.09.020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith JJ & Kratsios P Hox gene functions in the C. elegans nervous system: From early patterning to maintenance of neuronal identity. Semin Cell Dev Biol, doi: 10.1016/j.semcdb.2022.11.012 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aboobaker A & Blaxter M Hox gene evolution in nematodes: novelty conserved. Curr Opin Genet Dev 13, 593–598, doi: 10.1016/j.gde.2003.10.009 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Tihanyi B et al. The C. elegans Hox gene ceh-13 regulates cell migration and fusion in a non-colinear way. Implications for the early evolution of Hox clusters. BMC Dev Biol 10, 78, doi:1471–213X-10–78 [pii] 10.1186/1471-213X-10-78 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feng W et al. A terminal selector prevents a Hox transcriptional switch to safeguard motor neuron identity throughout life. Elife 9, doi: 10.7554/eLife.50065 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalis AK et al. Patterning of sexually dimorphic neurogenesis in the caenorhabditis elegans ventral cord by Hox and TALE homeodomain transcription factors. Dev Dyn 243, 159–171, doi: 10.1002/dvdy.24064 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Kalis AK et al. Hox proteins interact to pattern neuronal subtypes in Caenorhabditis elegans males. Genetics 220, doi: 10.1093/genetics/iyac010 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kratsios P et al. An intersectional gene regulatory strategy defines subclass diversity of C. elegans motor neurons. Elife 6, doi: 10.7554/eLife.25751 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng C, Diaz-Cuadros M & Chalfie M Hox Genes Promote Neuronal Subtype Diversification through Posterior Induction in Caenorhabditis elegans. Neuron 88, 514–527, doi: 10.1016/j.neuron.2015.09.049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng C, Jin FQ & Chalfie M Hox Proteins Act as Transcriptional Guarantors to Ensure Terminal Differentiation. Cell Rep 13, 1343–1352, doi: 10.1016/j.celrep.2015.10.044 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng C, Lee HMT & Pham K Nervous system-wide analysis of Hox regulation of terminal neuronal fate specification in Caenorhabditis elegans. PLoS Genet 18, e1010092, doi: 10.1371/journal.pgen.1010092 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cho HH et al. Isl1 directly controls a cholinergic neuronal identity in the developing forebrain and spinal cord by forming cell type-specific complexes. PLoS Genet 10, e1004280, doi: 10.1371/journal.pgen.1004280 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deschamps J et al. Initiation, establishment and maintenance of Hox gene expression patterns in the mouse. Int J Dev Biol 43, 635–650 (1999). [PubMed] [Google Scholar]
- 72.Svoboda LK et al. Overexpression of HOX genes is prevalent in Ewing sarcoma and is associated with altered epigenetic regulation of developmental transcription programs. Epigenetics 9, 1613–1625, doi: 10.4161/15592294.2014.988048 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allen AM et al. A single-cell transcriptomic atlas of the adult Drosophila ventral nerve cord. Elife 9, doi: 10.7554/eLife.54074 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Catela C, Chen Y, Weng Y, Wen K & Kratsios P Control of spinal motor neuron terminal differentiation through sustained Hoxc8 gene activity. Elife 11, doi: 10.7554/eLife.70766 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hutlet B et al. Systematic expression analysis of Hox genes at adulthood reveals novel patterns in the central nervous system. Brain Struct Funct 221, 1223–1243, doi: 10.1007/s00429-014-0965-8 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Lizen B et al. HOXA5 localization in postnatal and adult mouse brain is suggestive of regulatory roles in postmitotic neurons. J Comp Neurol 525, 1155–1175, doi: 10.1002/cne.24123 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Lizen B et al. Conditional Loss of Hoxa5 Function Early after Birth Impacts on Expression of Genes with Synaptic Function. Front Mol Neurosci 10, 369, doi: 10.3389/fnmol.2017.00369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maheshwari U et al. Postmitotic Hoxa5 Expression Specifies Pontine Neuron Positional Identity and Input Connectivity of Cortical Afferent Subsets. Cell Rep 31, 107767, doi: 10.1016/j.celrep.2020.107767 (2020). [DOI] [PubMed] [Google Scholar]
- 79.Philippidou P, Walsh CM, Aubin J, Jeannotte L & Dasen JS Sustained Hox5 gene activity is required for respiratory motor neuron development. Nat Neurosci 15, 1636–1644, doi: 10.1038/nn.3242 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raouf Issa A, J ACM, Padmanabhan A & Alonso CR A novel post-developmental role of the Hox genes underlies normal adult behavior. Proc Natl Acad Sci U S A 119, e2209531119, doi: 10.1073/pnas.2209531119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Estacio-Gomez A et al. Bithorax-complex genes sculpt the pattern of leucokinergic neurons in the Drosophila central nervous system. Development 140, 2139–2148, doi: 10.1242/dev.090423 (2013). [DOI] [PubMed] [Google Scholar]
- 82.Friedrich J et al. Hox Function Is Required for the Development and Maintenance of the Drosophila Feeding Motor Unit. Cell Rep 14, 850–860, doi: 10.1016/j.celrep.2015.12.077 (2016). [DOI] [PubMed] [Google Scholar]
- 83.Malik V, Zimmer D & Jauch R Diversity among POU transcription factors in chromatin recognition and cell fate reprogramming. Cell Mol Life Sci 75, 1587–1612, doi: 10.1007/s00018-018-2748-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leyva-Diaz E, Masoudi N, Serrano-Saiz E, Glenwinkel L & Hobert O Brn3/POUIV-type POU homeobox genes-Paradigmatic regulators of neuronal identity across phylogeny. Wiley Interdiscip Rev Dev Biol 9, e374, doi: 10.1002/wdev.374 (2020). [DOI] [PubMed] [Google Scholar]
- 85.Chalfie M, Horvitz HR & Sulston JE Mutations that lead to reiterations in the cell lineages of C. elegans. Cell 24, 59–69, doi: 10.1016/0092-8674(81)90501-8 (1981). [DOI] [PubMed] [Google Scholar]
- 86.Lloret-Fernandez C et al. A transcription factor collective defines the HSN serotonergic neuron regulatory landscape. Elife 7, doi: 10.7554/eLife.32785 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang F et al. The LIM and POU homeobox genes ttx-3 and unc-86 act as terminal selectors in distinct cholinergic and serotonergic neuron types. Development 141, 422–435, doi: 10.1242/dev.099721 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Serrano-Saiz E, Leyva-Diaz E, De La Cruz E & Hobert O BRN3-type POU Homeobox Genes Maintain the Identity of Mature Postmitotic Neurons in Nematodes and Mice. Curr Biol 28, 2813–2823 e2812, doi: 10.1016/j.cub.2018.06.045 (2018). [DOI] [PubMed] [Google Scholar]
- 89.Certel SJ, Clyne PJ, Carlson JR & Johnson WA Regulation of central neuron synaptic targeting by the Drosophila POU protein, Acj6. Development 127, 2395–2405, doi: 10.1242/dev.127.11.2395 (2000). [DOI] [PubMed] [Google Scholar]
- 90.Jafari S et al. Combinatorial activation and repression by seven transcription factors specify Drosophila odorant receptor expression. PLoS Biol 10, e1001280, doi: 10.1371/journal.pbio.1001280 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tourniere O et al. NvPOU4/Brain3 Functions as a Terminal Selector Gene in the Nervous System of the Cnidarian Nematostella vectensis. Cell Rep 30, 4473–4489 e4475, doi: 10.1016/j.celrep.2020.03.031 (2020). [DOI] [PubMed] [Google Scholar]
- 92.Badea TC et al. Combinatorial expression of Brn3 transcription factors in somatosensory neurons: genetic and morphologic analysis. J Neurosci 32, 995–1007, doi: 10.1523/JNEUROSCI.4755-11.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doitsidou M et al. A combinatorial regulatory signature controls terminal differentiation of the dopaminergic nervous system in C. elegans. Genes Dev 27, 1391–1405, doi: 10.1101/gad.217224.113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flames N & Hobert O Gene regulatory logic of dopamine neuron differentiation. Nature 458, 885–889, doi: 10.1038/nature07929 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brill MS et al. A dlx2- and pax6-dependent transcriptional code for periglomerular neuron specification in the adult olfactory bulb. J Neurosci 28, 6439–6452, doi: 10.1523/JNEUROSCI.0700-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cave JW et al. Differential regulation of dopaminergic gene expression by Er81. J Neurosci 30, 4717–4724, doi: 10.1523/JNEUROSCI.0419-10.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qiu M et al. Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes Dev 9, 2523–2538, doi: 10.1101/gad.9.20.2523 (1995). [DOI] [PubMed] [Google Scholar]
- 98.Remesal L et al. PBX1 acts as terminal selector for olfactory bulb dopaminergic neurons. Development 147, doi: 10.1242/dev.186841 (2020). [DOI] [PubMed] [Google Scholar]
- 99.Kishi Y & Gotoh Y Regulation of Chromatin Structure During Neural Development. Front Neurosci 12, 874, doi: 10.3389/fnins.2018.00874 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blackledge NP, Rose NR & Klose RJ Targeting Polycomb systems to regulate gene expression: modifications to a complex story. Nat Rev Mol Cell Biol 16, 643–649, doi: 10.1038/nrm4067 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gentile C & Kmita M Polycomb Repressive Complexes in Hox Gene Regulation: Silencing and Beyond: The Functional Dynamics of Polycomb Repressive Complexes in Hox Gene Regulation. Bioessays 42, e1900249, doi: 10.1002/bies.201900249 (2020). [DOI] [PubMed] [Google Scholar]
- 102.Margueron R & Reinberg D The Polycomb complex PRC2 and its mark in life. Nature 469, 343–349, doi: 10.1038/nature09784 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schuettengruber B, Bourbon HM, Di Croce L & Cavalli G Genome Regulation by Polycomb and Trithorax: 70 Years and Counting. Cell 171, 34–57, doi: 10.1016/j.cell.2017.08.002 (2017). [DOI] [PubMed] [Google Scholar]
- 104.Di Croce L & Helin K Transcriptional regulation by Polycomb group proteins. Nat Struct Mol Biol 20, 1147–1155, doi: 10.1038/nsmb.2669 (2013). [DOI] [PubMed] [Google Scholar]
- 105.Toskas K et al. PRC2-mediated repression is essential to maintain identity and function of differentiated dopaminergic and serotonergic neurons. Sci Adv 8, eabo1543, doi: 10.1126/sciadv.abo1543 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.von Schimmelmann M et al. Polycomb repressive complex 2 (PRC2) silences genes responsible for neurodegeneration. Nat Neurosci 19, 1321–1330, doi: 10.1038/nn.4360 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sawai A et al. PRC1 sustains the integrity of neural fate in the absence of PRC2 function. Elife 11, doi: 10.7554/eLife.72769 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cohen I, Bar C & Ezhkova E Activity of PRC1 and Histone H2AK119 Monoubiquitination: Revising Popular Misconceptions. Bioessays 42, e1900192, doi: 10.1002/bies.201900192 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bordet G, Couillault C, Soulavie F, Filippopoulou K & Bertrand V PRC1 chromatin factors strengthen the consistency of neuronal cell fate specification and maintenance in C. elegans. PLoS Genet 18, e1010209, doi: 10.1371/journal.pgen.1010209 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zheng C, Karimzadegan S, Chiang V & Chalfie M Histone methylation restrains the expression of subtype-specific genes during terminal neuronal differentiation in Caenorhabditis elegans. PLoS Genet 9, e1004017, doi: 10.1371/journal.pgen.1004017 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Padeken J, Methot SP & Gasser SM Establishment of H3K9-methylated heterochromatin and its functions in tissue differentiation and maintenance. Nat Rev Mol Cell Biol 23, 623–640, doi: 10.1038/s41580-022-00483-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lipinski M et al. KAT3-dependent acetylation of cell type-specific genes maintains neuronal identity in the adult mouse brain. Nat Commun 11, 2588, doi: 10.1038/s41467020-16246-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lipinski M et al. CBP Is Required for Establishing Adaptive Gene Programs in the Adult Mouse Brain. J Neurosci 42, 7984–8001, doi: 10.1523/JNEUROSCI.0970-22.2022 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Catela C & Kratsios P Transcriptional mechanisms of motor neuron development in vertebrates and invertebrates. Dev Biol, doi: 10.1016/j.ydbio.2019.08.022 (2019). [DOI] [PubMed] [Google Scholar]
- 115.Kurashina M et al. Sustained expression of unc-4 homeobox gene and unc-37/Groucho in postmitotic neurons specifies the spatial organization of the cholinergic synapses in C. elegans. Elife 10, doi: 10.7554/eLife.66011 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saul J, Hirose T & Horvitz HR The transcriptional corepressor CTBP-1 acts with the SOX family transcription factor EGL-13 to maintain AIA interneuron cell identity in Caenorhabditis elegans. Elife 11, doi: 10.7554/eLife.74557 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maicas M, Jimeno-Martin A, Millan-Trejo A, Alkema MJ & Flames N The transcription factor LAG-1/CSL plays a Notch-independent role in controlling terminal differentiation, fate maintenance, and plasticity of serotonergic chemosensory neurons. PLoS Biol 19, e3001334, doi: 10.1371/journal.pbio.3001334 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


