Abstract
Quinupristin-dalfopristin is a streptogramin antibiotic combination with activity against vancomycin-resistant Enterococcus faecium (VREF), but emergence of resistance has been recently reported. We studied the activity of quinupristin-dalfopristin against two clinical strains of VREF (12311 and 12366) in an in vitro pharmacodynamic model with simulated endocardial vegetations (SEVs) to determine the potential for resistance selection and possible strategies for prevention. Baseline MICs/minimal bactericidal concentrations (μg/ml) for quinupristin-dalfopristin, quinupristin, dalfopristin, and doxycycline were 0.25/2, 64/>512, 4/512, and 0.125/8 for VREF 12311 and 0.25/32, 128/>512, 2/128, and 0.25/16 for VREF 12366, respectively. Quinupristin-dalfopristin regimens had significantly less activity against VREF 12366 than VREF 12311. An 8-μg/ml simulated continuous infusion was the only bactericidal regimen with time to 99.9% killing = 90 hours. The combination of quinupristin-dalfopristin every 8 h with doxycycline resulted in more killing compared to either drug alone. Quinupristin-dalfopristin-resistant mutants (MICs, 4 μg/ml; resistance proportion, ∼4 × 10−4) emerged during the quinupristin-dalfopristin monotherapies for both VREF strains. Resistance was unstable in VREF 12311 and stable in VREF 12366. The 8-μg/ml continuous infusion or addition of doxycycline to quinupristin-dalfopristin prevented the emergence of resistance for both strains over the 96-h test period. These findings replicated the development of resistance reported in humans and emphasized bacterial factors (drug susceptibility, high inoculum, organism growth phase) and infectious conditions (penetration barriers) which could increase chances for clinical resistance. The combination of quinupristin-dalfopristin with doxycycline and the administration of quinupristin-dalfopristin as a high-dose continuous infusion warrant further study to determine their potential clinical utility.
Enterococci are commonly implicated pathogens in intra-abdominal infections and urinary tract infections and are the third-most-common cause of infective endocarditis (28). Until the last decade, clinical isolates of enterococci remained susceptible to glycopeptide antibiotics such as vancomycin or teicoplanin. After a first report in 1986, vancomycin-resistant Enterococcus faecium (VREF) has since spread dramatically around the world (12, 20, 23, 24, 40). Because infections caused by VREF are often resistant to nearly all available antibiotics, the search for alternatives has escalated rapidly.
Quinupristin-dalfopristin (RP 59500, Synercid) is a water-soluble, semisynthetic antibiotic combination derived from natural streptogramin compounds produced by Streptomyces pristinaespiralis (13). Quinupristin (RP 57669) is a group B streptogramin derived from pristinamycin IA, while dalfopristin (RP 54496) is a group A streptogramin derived from pristinamycin IIA (4, 13). Both drugs act by binding to the 23S RNA of the 50S ribosomal subunit to cause inhibition of protein synthesis via constriction of the nascent protein exit channel (3). Synergism occurs due to dalfopristin-induced conformation changes in the ribosome that improve quinupristin binding and result in a more stable drug-ribosome complex. Quinupristin-dalfopristin is bactericidal against most staphylococci and streptococci and is bacteriostatic or weakly bactericidal against most enterococci, including VREF (9, 13, 41).
The most common type of resistance to streptogramin antibiotics is associated with the erm gene family and is termed MLSB (macrolide, lincosamide, streptogramin group B) resistance (25). Resistance may be either constitutive or inducible and results in decreased quinupristin binding affinity to the ribosome (especially with constitutive MLSB resistance). Antibacterial activity usually is not appreciably decreased for the quinupristin-dalfopristin drug combination due to the synergistic effects of the two drugs (25). Other types of resistance to streptogramins also have been described, including enzymatic degradation, but the prevalence of isolates that possess inactivating enzymes is extremely low (25).
Quinupristin-dalfopristin is currently available under a compassionate use protocol for the treatment of VREF infections. Preliminary results from this study indicate a 65.4% clinical cure rate for patients with VREF infections (31). However, case reports and case series describing apparent quinupristin-dalfopristin-resistant VREF (provisional resistance breakpoint, 4 μg/ml) have recently emerged (14, 35, 36), and an overall resistance rate of 1.8% for 338 cases of VREF infection treated with quinupristin-dalfopristin has been reported (32).
Selection of stable and unstable VREF resistance to quinupristin-dalfopristin has been reported in vitro (29, 30, 39). In one study, stable quinupristin-dalfopristin resistance was selected for all 14 strains of E. faecium tested if the MICs for the mutant strain were ≥16 times the baseline MICs (30). In another study, resistant mutants of VREF were detected on 4 × MIC agar at a high frequency of 10−4 (39). Interestingly, combining quinupristin-dalfopristin with subinhibitory concentrations of doxycycline prevented the growth of quinupristin-dalfopristin-resistant VREF.
The in vivo and in vitro studies on quinupristin-dalfopristin-resistant VREF suggest that novel strategies should be investigated to reduce this threat, so as to help preserve the utility of this antimicrobial for the future. We studied the antibacterial activity of quinupristin-dalfopristin against VREF and the potential for the emergence of quinupristin-dalfopristin resistance during monotherapy regimens (dosing every 8 h or continuous infusions) and regimens involving combination with doxycycline in an in vitro pharmacodynamic model with simulated endocardial vegetations (SEVs). This model can accurately simulate in vivo antimicrobial pharmacokinetics, allows analysis of the effects of multiple antimicrobial doses, and provides a high bacterial inoculum in a sequestered site of infection, factors which would help contribute to the development of antimicrobial resistance.
(A portion of this research was presented at the 8th European Congress of Clinical Microbiology and Infectious Diseases, Lausanne, Switzerland, 28 May 1997.)
MATERIALS AND METHODS
Organisms.
Two clinical isolates of vancomycin-resistant E. faecium (12311 and 12366) obtained from the blood of patients at William Beaumont Hospital (Royal Oak, Mich.) were used in all investigations. VREF isolate 12311 was the pretreatment clinical isolate from a patient treated in the quinupristin-dalfopristin compassionate use program where resistance was later documented (14). VREF 12366 was an unrelated isolate that allowed comparison of activity and rates of resistance.
Antibiotics.
Quinupristin-dalfopristin (lots CB063235 and 9609410), quinupristin (lot P94122V), and dalfopristin (lot P95094) susceptibility-grade powders were obtained from Rhone-Poulenc Rorer (Collegeville, Pa., and France) and were reconstituted as outlined by the manufacturer. Doxycycline susceptibility-grade powder (lot 26H0213) was obtained from Sigma Chemical Company (St. Louis, Mo.). Stock solutions of each antibiotic were prepared on the first day of each experiment and stored at −70°C until use. Doses were thawed just prior to the scheduled administration times.
In vitro susceptibility testing.
The MICs and minimal bactericidal concentrations (MBCs) of quinupristin-dalfopristin, quinupristin, dalfopristin, doxycycline, and vancomycin were determined by microdilution methods with Mueller-Hinton broth supplemented with magnesium (12.5 mg/liter) and calcium (25 mg/liter) (SMHB; Difco Laboratories, Detroit, Mich.) and an inoculum of 5 × 105 CFU/ml following the guidelines of the National Committee for Clinical Laboratory Standards (33). The presence of an inoculum effect was tested by repeating the MIC and MBC determinations with a bacterial concentration of 5 × 107 CFU/ml. Agar dilution MICs were determined with Mueller-Hinton II agar (MHA, Difco). Expanded panels of erythromycin and clindamycin MICs were completed to determine the presence of cross-resistance to these related MLSB compounds.
Concentration time-kill curves.
Preliminary concentration time-kill curves were performed in duplicate with a starting inoculum of 106 CFU/ml. Three to five colonies from an overnight growth of VREF 12311 or 12366 on tryptic soy agar (TSA; Difco) plates were added to normal saline and adjusted as necessary to produce a 0.5 McFarland suspension of organisms. This suspension was diluted 1:10 with SMHB, and 0.8 ml was added to 7.2 ml of SMHB to provide the desired starting inoculum. Quinupristin-dalfopristin was added to provide a concentration of 6 μg/ml and was tested alone or in combination with doxycycline (4 μg/ml). Samples (100 μl) were taken at 0 h (inoculum control) and 2, 4, 8, and 24 h, serially diluted with cold normal saline, and plated in triplicate on TSA plates for determination of CFU/ml. For situations in which the first dilution was necessary for bacterial enumeration, samples were placed on a 0.45-μm-pore-size polysulfone filter (Gelman Sciences; Ann Arbor, Mich.) and washed with cold normal saline, and the filter was then applied aseptically to a TSA plate to minimize the potential effects of antibiotic carryover. Using these methods, we have previously determined our reliable limits of detection to be 100 CFU/ml (27).
Preparation of SEVs.
Concentrated organism suspensions (ca. 1010 CFU/ml) were prepared as previously described (22, 27). SEVs were made by combining 0.8 ml of human cryoprecipitate antihemolytic factor from volunteer donors (American National Red Cross, Detroit, Mich.), 0.1 ml of organism suspension (final inoculum, ∼109 CFU/g), 0.05 ml of aprotinin solution (2,000 kallikrein inhibitory units/ml, lot 66H7125; Sigma), and 0.05 ml of human platelet suspension (prepared by diluting 0.1 ml of platelet-rich plasma in 9.9 ml of 0.9% NaCl, yielding approximately 250,000 to 300,000 platelets per g of vegetation mass) in a sterile, siliconized 1.5-ml Eppendorf tube. A sterile monofilament line was inserted into the Eppendorf tube, and 0.1 ml of bovine thrombin solution (5,000 units/ml, lot R114A175; GenTrac, Inc., Middleton, Wis.), reconstituted with 5 ml of sterile 50-mmol calcium chloride solution, was added to the mixture. The resultant gelatinous mixture was removed from the Eppendorf tube with a sterile 21-gauge needle.
In vitro pharmacodynamic model with SEVs.
The in vitro pharmacodynamic model with SEVs has been previously described (22, 27). Quinupristin and dalfopristin were administered as a simultaneous 1-h infusion every 8 h with a programmable syringe pump (ATI Orion Research, Boston, Mass.) to simulate the peak concentrations obtained in humans after a dose of 7.5 mg/kg of body weight (ca. 2 μg/ml for quinupristin and ca. 6 μg/ml for dalfopristin) (5). Continuous infusions of 1.25 μg (low-dose continuous infusion) and 8 μg (high-dose continuous infusion) of quinupristin-dalfopristin per ml were simulated by bolusing the model to the desired concentration and then pumping in fresh SMHB containing a constant concentration of drug. The 1.25-μg/ml concentration approximated the 24-hour area under the concentration-time curve (AUC) value obtained from dosing of quinupristin-dalfopristin every 8 h (5). The 8-μg/ml concentration was chosen for the high-dose continuous infusion to represent the upper range of combined quinupristin-dalfopristin concentrations immediately following a 1-h, 7.5-mg/kg infusion (5). Doxycycline was given as a bolus to simulate a regimen of 200 mg every 24 h, yielding peak concentrations of 4 to 6 μg/ml (15). This regimen was chosen instead of the more commonly used regimen (100 mg every 12 h) to allow more efficient execution of the model, since human pharmacokinetic studies indicate that these two regimens produce serum concentration profiles that are not statistically different (15, 26). A peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, Ill.) was used to displace antibiotic-containing media with fresh SMHB to simulate the half-lives of dalfopristin (approximately 0.5 h), quinupristin (approximately 1 h), and doxycycline (approximately 19.5 h) (5, 15). During administration of these combination antimicrobial regimens, the central compartment elimination rate was set for the shortest half-life drug (dalfopristin); quinupristin and doxycycline were also administered into supplemental chambers to maintain their longer half-lives as previously described (6). The glass model apparatus was placed in a water bath and maintained at 37°C for the entire 96-h study period. Each experimental regimen was performed in duplicate in order to ensure reproducibility.
Pharmacokinetic analysis.
Samples (0.5 ml each) from the central compartment were obtained at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 8, and 24 h postinfusion for determination of antibiotic concentrations. For quinupristin and dalfopristin, samples were placed into tubes containing 0.12 ml of 0.25 N hydrochloric acid to ensure adequate drug stability and stored at −70°C until analysis (no later than 2 weeks from the sampling date). SEVs for pharmacokinetic analysis were removed from the models at 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 8, 24, 48, 72, and 96 h. The SEVs were weighed and placed in a 2-ml sterile capped vial prefilled with 3-mm-diameter glass beads and 1.0 ml of 1.25% trypsin solution (prepared by combining 1:250 trypsin powder [lot 26H71305; Sigma] with 0.9% sodium chloride solution). SEVs were homogenized by placing samples in a minibead beater grinder (Biospec Products, Bartlesville, Okla.) for 3 min, stabilized with 0.12 ml of 0.25 N hydrochloric acid, and stored at −70°C until analysis. Quinupristin, dalfopristin, and doxycycline concentrations were determined by standard agar diffusion microbioassay methods. Bacillus cereus (ATCC 11778) was used as the indicator organism for doxycycline. The correlation coefficient for this assay was consistently greater than 0.98; mean inter- and intraday coefficients of variation were 6.8 and 8% for the low (0.25 μg/ml) standard and 2.6 and 3.6% for the high (25 μg/ml) standard. Concentrations of quinupristin and dalfopristin were determined with indicator organisms possessing different patterns of susceptibility to the two compounds (16). Staphylococcus aureus HBD 511 (susceptible to quinupristin but resistant to dalfopristin via plasmid-mediated streptogramin A acetylase) was utilized as the indicator organism for quinupristin. To abolish any potential for synergy between quinupristin and dalfopristin in the samples, the assay was performed with antibiotic medium 2 (Difco) containing 20 μg of dalfopristin per ml. Staphylococcus epidermidis HBD 523 (susceptible to dalfopristin but resistant to quinupristin via constitutive expression of the erm MLSB resistance gene) was used as the indicator organism for dalfopristin. Antibiotic medium no. 5 (Difco) containing 20 μg of quinupristin per ml was used to prevent synergy from influencing dalfopristin zone sizes. Our limits of detection used for these assays were 0.1 μg/ml for quinupristin and 0.5 μg/ml for dalfopristin, which were close to those previously reported (16). The correlation coefficients for both assays were consistently >0.98. The mean inter- and intraday coefficients of variation for the quinupristin assay were 8.3 and 11.8% for the low (0.1 μg/ml) standard and 3.7 and 7.9% for the high (10 μg/ml) standard. The mean inter- and intraday coefficients of variation for the dalfopristin assay were 6.7 and 9.6% for the low (0.5 μg/ml) standard and 4.3 and 11.8% for the high (10 μg/ml) standard. Preparation of SEV standard samples with or without trypsin resulted in similar zone sizes. Pharmacokinetic parameters such as elimination half-lives, peak/trough concentrations, and AUC were determined with PKAnalyst software (Micromath, Salt Lake City, Utah).
Evaluation of quinupristin and dalfopristin stability in SMHB.
Both quinupristin and dalfopristin are known to be relatively unstable in non-acid-stabilized solutions. Excessively rapid degradation of one (or both) of these compounds could potentially affect the consistency of concentrations (and hence antibacterial activity) during the quinupristin-dalfopristin continuous infusion regimens. A large reservoir of drug-containing, room temperature SMHB (6 liters) was used during the overnight portion of these experiments, so drug degradation affecting experimental results was an issue. Degradation kinetics of quinupristin and dalfopristin were determined by repeated sampling of a known stock concentration of quinupristin-dalfopristin (8 μg/ml) in SMHB stored at room temperature or 37°C. Drug concentrations were determined by microbioassay as described above. The degradation half-lives of quinupristin at room temperature and 37°C were 107 ± 13 (mean ± standard deviation) and 49 ± 1 h, respectively; dalfopristin half-lives at room temperature and 37°C were 40 ± 3 and 20 ± 2 h.
Pharmacodynamic analysis.
Two to three SEVs were removed from each model at 0, 8, 24, 48, 72, and 96 h. SEVs were homogenized as described above and serially diluted with cold 0.9% saline, and 20-μl samples were placed in triplicate onto TSA plates. After incubation for 24 h at 37°C, the colonies were counted for bacterial enumeration (CFU/gram). Average bacterial densities (log10 CFU/gram) for the SEVs at each time point were plotted versus time to generate time-kill curves. The total reduction in the log10 CFU/gram over 96 h for each regimen was determined and compared with others. Bactericidal activity was defined as a reduction of ≥3 log10 CFU/g from the starting inoculum. Synergy was defined as an inoculum ≥2 log10 CFU/ml lower at 96 h than either antimicrobial regimen alone. Additivity was defined as a reduction in inoculum that was greater than either antimicrobial regimen alone. The time to achieve a 99.9% reduction in the starting inoculum was determined by linear regression (if R was ≥0.95) or by visual inspection of the time-kill curve line.
Detection of quinupristin-dalfopristin resistance.
To detect the emergence of resistance to quinupristin-dalfopristin during the different experimental dose regimens, samples (100 μl each) of homogenized SEVs taken at 0, 8, 24, 48, 72, and 96 h were spread onto MHA (Difco) containing quinupristin-dalfopristin at four and eight times the MIC for the original isolate. Because dalfopristin and quinupristin are unstable in agar, incubation for the standard 48-h time period could potentially result in erroneously elevated rates of quinupristin-dalfopristin resistance. A control strain of S. aureus (ATCC 29213) and an E. faecium strain (VREF 12366) were studied to determine the time limit of drug viability in agar and the incubation time limit for evaluation of resistance. Quinupristin-dalfopristin or dalfopristin alone was incorporated into MHA at a concentration of two times the MIC for the two strains tested. Three 20-μl-diameter spots of a 0.5 McFarland suspension of the control organisms were placed on drug-containing agar plates immediately after cooling (t = 0 h) and then at various time points after the agar preparation (t = 2, 4, 6, 8, 10, and 24 h). The agar plates used for each time point were stored at 37°C until inoculation of the organisms. The final time point at which no growth of organism was observed after 24 h of incubation was considered to be the incubation time limit for evaluation of quinupristin-dalfopristin resistance. As an additional test of drug stability in agar, organism suspensions were placed onto freshly prepared MHA plates containing either dalfopristin or quinupristin-dalfopristin (2 × MIC), incubated at 37°C, and then checked every 8 to 12 h until visible growth was observed. The last time point of no visible growth was considered the time limit of incubation. For both of these methods, growth considered as possibly related to drug degradation occurred after >48 h and <70 h of incubation. On the basis of these results, the standard incubation time of 48 h was considered valid for the evaluation of resistance.
Resistance plates were visually inspected for growth of resistant subpopulations after 24, 32, and 48 h of incubation. The number of colonies growing on drug-containing plates was divided by the number of organisms originally plated (the starting inoculum) to determine the frequency of resistance at each multiple of the MIC. MICs (quinupristin-dalfopristin, quinupristin, and dalfopristin) for resistant colonies were determined from direct colony samples from the antibiotic resistance plates as well as from colony samples from subcultures passed ≥10 times on antibiotic-free TSA plates (to evaluate resistance stability).
Evaluation of quinupristin-dalfopristin-resistant E. faecium.
Parent and quinupristin-dalfopristin-resistant strains of VREF recovered from the models were compared by genomic restriction analysis and pulsed-field gel electrophoresis. Organisms were grown in SMHB to the logarithmic growth phase. Cells were embedded in plugs of 0.75% low-melting-point agarose. Cell lysis was performed as previously described (38). Agarose plugs were digested overnight with either SmaI or SacII (New England BioLabs, Beverly, Mass.) and then placed into wells of a 1% agarose slab. Electrophoresis was done at 14°C with a CHEF-DR II system (Bio-Rad, Richmond, Calif.) with parameters of 6 V/cm and pulse times of 1 to 15 s for 10 h and 20 to 40 s for 8 h. Analysis of gels was done by comparison of pre- and postexposure isolate band patterns.
Statistical analysis.
Differences between the regimens in the change in log10 CFU/g from baseline were compared by two-way analysis of variance with Tukey’s test for multiple comparisons. For all tests, a P value of ≤0.05 was considered indicative of statistical significance. All statistical evaluations were performed with SPSS Statistical Software (release 6.1.3; SPSS, Inc., Chicago, Ill.).
RESULTS
Susceptibility testing and test tube time-kill studies.
The microdilution MICs and MBCs of quinupristin, dalfopristin, quinupristin-dalfopristin, doxycycline, and various other reference drugs for VREF 12311 and 12366 are shown in Table 1. The MICs and MBCs of quinupristin-dalfopristin did not change in the presence of a larger inoculum. Agar dilution MICs of quinupristin-dalfopristin and doxycycline were 0.5 and 0.25 μg/ml for both VREF 12311 and 12366. In the concentration time- kill curve studies, quinupristin-dalfopristin produced a 1.5 log10- CFU/ml reduction over 24 h for VREF 12366. Killing of VREF 12311 was more rapid and extensive; a reduction in inoculum of ≥4 log10 CFU/ml was obtained, and time to 99.9% killing was 13.5 h. Combination of quinupristin-dalfopristin with doxycycline had only a slight additive killing effect for VREF 12366 and no effect for VREF 12311.
TABLE 1.
Microtiter MICs and MBCs for VREF 12311 and 12366
| Antibiotic | MIC/MBC for:
|
|
|---|---|---|
| VREF 12311 | VREF 12366 | |
| Quinupristin-dalfopristin | 0.25/2 | 0.25/32 |
| Quinupristin | 64/>512 | 128/>512 |
| Dalfopristin | 4/512 | 2/128 |
| Doxycycline | 0.125/8 | 0.25/16 |
| Vancomycin | >1,024/>1,024 | 512/>1,024 |
| Erythromycin | >128/>128 | >128/>128 |
| Clindamycin | >128/>128 | >128/>128 |
Pharmacokinetics.
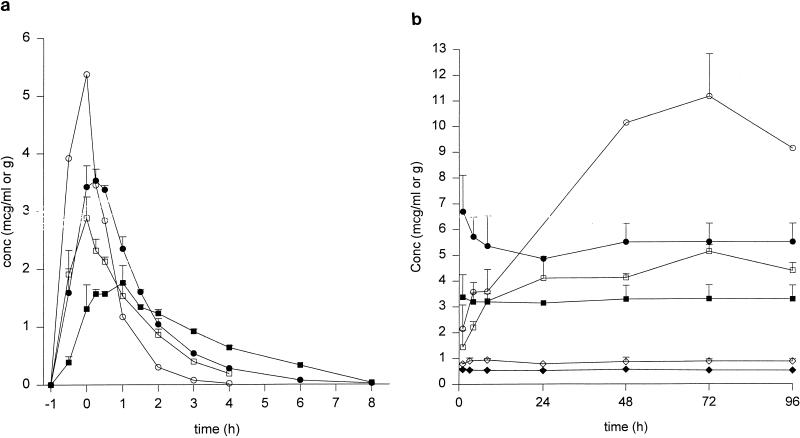
The central compartment pharmacokinetic parameters for quinupristin and dalfopristin and the concentrations in homogenized SEVs are summarized in Table 2. For doxycycline, peak and trough concentrations were 5.8 ± 0.2 and 2.5 ± 0.7 μg/ml; the elimination half-life was 18.1 ± 1.8 h. The concentration-time profiles for quinupristin and dalfopristin in the SEVs (expressed as micrograms per gram of homogenized SEV) compared to the central compartment (SMHB) concentrations over an 8-h dosing interval and during the continuous infusion regimens are represented in Fig. 1. For the dosing every 8 h, peak dalfopristin SEV concentrations were achieved approximately 0.25 h after the SMHB peak and were approximately 72% of the peak SMHB concentrations. The peak SEV concentration for quinupristin occurred at approximately 1 h postinfusion and was also approximately 72% of the SMHB concentration. Both quinupristin and dalfopristin tended to persist longer in the SEVs than in the central compartment. Elimination half-lives from the SEVs were approximately four times longer for quinupristin and approximately 2.5 times longer for dalfopristin than their respective central compartment elimination half-lives. Percent penetration into the SEVs (calculated as the AUCSEV over 8 h/ AUCSMHB over 8 h) was 240% for quinupristin and 124% for dalfopristin, indicating drug accumulation. For the high-dose continuous infusion, a gradual accumulation of both quinupristin and dalfopristin (to a higher extent) occurred over the 96-h experiment (Fig. 1b). For the low-dose continuous infusion regimen, all SEV concentrations were below the limits of detection of the microbioassay. This result was partially related to the twofold dilution step with trypsin solution that was necessary for SEV homogenization.
TABLE 2.
Summary of pharmacokinetic data (mean ± standard deviation) for quinupristin and dalfopristin in the in vitro models
| Drug and pharmacokinetic parameter | Mean ± SD results for indicated regimen
|
|||||
|---|---|---|---|---|---|---|
| Quinupristin-dalfopristin (q8h)
|
Continuous infusion
|
|||||
| Low dose
|
High dose
|
|||||
| Broth | SEVs | Broth | SEVs | Broth | SEVs | |
| Quinupristin | ||||||
| Peak (μg/ml or g) | 2.3 ± 0.6 | 1.6 ± 0.1 | 0.6 ± 0.1 | UD | 3.3 ± 0.5 | 4.6 ± 0.6 |
| Trough (μg/ml or g) | UDa | UD | NDb | ND | ND | ND |
| Half-life (h) | 0.9 ± 0.1 | 3.6 ± 0.5 | ND | ND | ND | ND |
| Kelim (h−1) | 0.8 ± 0.1 | 0.2 ± 0.0 | ND | ND | ND | ND |
| AUC24h (μg/ml/h) | 11.9 ± 1.5 | 28.6 ± 1.5 | 13.2 ± 1.0 | ND | 79.1 ± 12.9 | 108.2 ± 8.2 |
| Dalfopristin | ||||||
| Peak (μg/ml or g) | 5.3 ± 0.8 | 3.5 ± 0.2 | 0.9 ± 0.1 | UD | 5.9 ± 1.2 | 10.4 ± 1.4 |
| Trough (μg/ml or g) | UD | UD | ND | ND | ND | ND |
| Half-life (h) | 0.4 ± 0.0 | 1.1 ± 0.1 | ND | ND | ND | ND |
| Kelim (h−1) | 1.6 ± 0.1 | 0.7 ± 0.1 | ND | ND | ND | ND |
| AUC24h (μg/ml/h) | 20.3 ± 2.1 | 25.1 ± 1.8 | 20.4 ± 2.2 | ND | 121 ± 43.9 | 248.8 ± 6.3 |
UD, undetectable.
ND, not done.
FIG. 1.
Mean concentrations (conc) of quinupristin (Q; open square, broth; closed square, SEV) and dalfopristin (D; open circle, broth; closed circle, SEV) in broth and SEVs during the dosing regimens that took place every 8 h (a) and the high-dose (open circle, dalfopristin SEVs; closed circle, dalfopristin broth; open square, quinupristin SEVs; closed square, quinupristin broth) and low-dose (open diamond, dalfopristin broth; closed diamond, quinupristin broth) continuous infusion regimens (b). For the low-dose continuous infusion regimens, all drug concentrations in the SEVs were below the limits of detection. All data points represent mean values from at least four samples.
Pharmacodynamics.
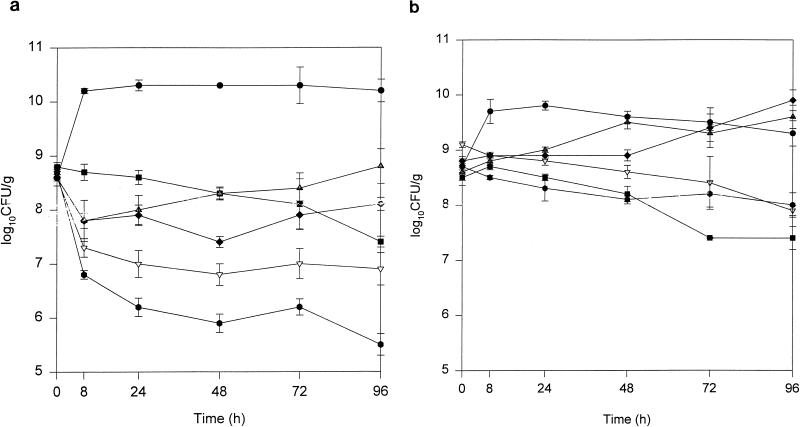
The changes in SEV bacterial density over 96 h and the residual inocula at 96 h are summarized in Tables 3 and 4 and Fig. 2. With the exception of the high-dose continuous-infusion regimen against VREF 12311, no regimen was bactericidal; the time to 99.9% killing for this regimen was approximately 90 h. All regimens tested were significantly better than growth control (P < 0.05). The high-dose continuous infusion resulted in a residual inoculum that was significantly lower than those for all other regimens (Table 3 and Fig. 2a). Although not synergistic, the combination of quinupristin-dalfopristin every 8 h with doxycycline resulted in a lower residual inoculum at 96 h compared to that for either regimen alone; this difference approached statistical significance (P = 0.06). Killing was observed primarily during the first 8 h of the experiments for all quinupristin-dalfopristin-containing regimens.
TABLE 3.
Residual bacterial inoculum (mean log10 CFU/g ± standard deviation) remaining after 96 h in the in vitro infection models for VREF 12311
| Regimen | Residual inoculum |
|---|---|
| Growth control | 10.2 ± 0.2 |
| Quinupristin-dalfopristin every 8 h | 8.7 ± 0.3a |
| Doxycycline | 7.4 ± 0.1a,d |
| Quinupristin-dalfopristin every 8 h + doxycycline | 6.9 ± 0.3a,c |
| Quinupristin-dalfopristin low-dose continuous infusion | 8.1 ± 0.1a,e |
| Quinupristin-dalfopristin high-dose continuous infusion | 5.5 ± 0.2b |
P < 0.05 versus growth control.
P < 0.05 versus all other regimens.
Significantly lower (P < 0.05) than doxycycline, quinupristin-dalfopristin every 8 h, and quinupristin-dalfopristin low-dose continuous infusion.
Significantly lower (P < 0.05) than quinupristin-dalfopristin every 8 h and quinupristin-dalfopristin low-dose continuous infusion.
Significantly lower (P < 0.05) than quinupristin-dalfopristin every 8 h.
TABLE 4.
Residual bacterial inoculum (mean log10 CFU/g ± standard deviation) remaining after 96 h in the in vitro infection models for VREF 12366
| Regimen | Residual inoculum |
|---|---|
| Growth control | 9.4 ± 0.2 |
| Quinupristin-dalfopristin every 8 h | 9.6 ± 0.2 |
| Doxycycline | 7.4 ± 0.2b,c,d |
| Quinupristin-dalfopristin every 8 h + doxycycline | 7.9 ± 0.1c,d |
| Quinupristin-dalfopristin low-dose continuous infusion | 10.0 ± 0.2a |
| Quinupristin-dalfopristin high-dose continuous infusion | 8.0 ± 0.2c,d |
Significantly higher (P < 0.05) than growth control.
Significantly lower (P < 0.05) than high-dose continuous infusion.
Significantly lower (P < 0.05) than growth control.
Significantly lower (P < 0.05) than quinupristin-dalfopristin every 8 h and quinupristin-dalfopristin low-dose continuous infusion.
FIG. 2.
Time-kill curves for quinupristin-dalfopristin every 8 h ( ), doxycycline (■), quinupristin-dalfopristin every 8 h plus doxycycline (▿), low-dose quinupristin-dalfopristin continuous infusion (⧫) and high-dose quinupristin-dalfopristin continuous infusion (
), doxycycline (■), quinupristin-dalfopristin every 8 h plus doxycycline (▿), low-dose quinupristin-dalfopristin continuous infusion (⧫) and high-dose quinupristin-dalfopristin continuous infusion ( ) versus VREF 12311 (a) and VREF 12366 (b). Each data point represents the mean log10 CFU/g (± standard deviation) from four to eight SEV samples. •, growth control.
) versus VREF 12311 (a) and VREF 12366 (b). Each data point represents the mean log10 CFU/g (± standard deviation) from four to eight SEV samples. •, growth control.
The activities of all quinupristin-dalfopristin-containing regimens were significantly less for VREF 12366 than for VREF 12311 (Table 4 and Fig. 2b). Residual inoculum at 96 h was similar to growth control for quinupristin-dalfopristin every 8 h or as a low-dose continuous infusion. Doxycycline administered alone had significantly lower residual inoculum at 96 h compared to those for the quinupristin-dalfopristin monotherapy regimens. The combination of doxycycline and quinupristin-dalfopristin every 8 h did not result in additive reductions in bacterial inoculum against VREF 12366.
Proportions of quinupristin-dalfopristin resistance in VREF.
The results of the resistance analyses are summarized in Table 5. Quinupristin-dalfopristin-resistant VREF (at eight times original MICs) was detected as early as 8 h during the monotherapy regimens with dosing every 8 h for both VREF 12311 and 12366. The proportion of resistance increased over 96 h to 4.4 × 10−4 for VREF 12311 and 3.8 × 10−4 for VREF 12366. The low-dose continuous infusion regimens delayed detectable resistance slightly (first detection at 24 h), but proportions from that time point onward were similar to those with dosing every 8 h (data not shown). High-dose continuous infusions prevented detectable quinupristin-dalfopristin-resistant VREF for both strains over the 96-h test period. The addition of doxycycline to quinupristin-dalfopristin given every 8 h completely prevented detectable resistance over the 96-h experiment for VREF 12366 and delayed time to detection of resistance to 96 h for VREF 12311 (final proportion, 3.4 × 10−7).
TABLE 5.
Proportion of quinupristin-dalfopristin-resistant subpopulations (at 8 × original MIC) for VREF 12311 and VREF 12366 in the in vitro pharmacodynamic models
| Time (h) | VREF 12311
|
VREF 12366
|
||||
|---|---|---|---|---|---|---|
| Q/D q8ha,b | Q/D q8h plus doxycycline | Q/D high-dose continuous infusion | Q/D q8hb | Q/D q8h plus doxycycline | Q/D high-dose continuous infusion | |
| 0 | <1 × 10−8.6 | <1 × 10−8.6 | <1 × 10−8.7 | <1 × 10−8.6 | <1 × 10−9.1 | <1 × 10−8.7 |
| 8 | 2.6 × 10−8 | <1 × 10−7.3 | <1 × 10−6.8 | 2.2 × 10−7 | <1 × 10−8.9 | <1 × 10−8.5 |
| 24 | 8.5 × 10−7 | <1 × 10−7 | <1 × 10−6.2 | 1.8 × 10−4 | <1 × 10−8.8 | <1 × 10−8.3 |
| 48 | 6.3 × 10−5 | <1 × 10−6.8 | <1 × 10−5.9 | 3.5 × 10−4 | <1 × 10−8.6 | <1 × 10−8.1 |
| 72 | 2.0 × 10−4 | <1 × 10−7 | <1 × 10−6.2 | 3.1 × 10−4 | <1 × 10−8.4 | <1 × 10−8.2 |
| 96 | 4.4 × 10−4 | 3.4 × 10−7 | <1 × 10−5.9 | 3.8 × 10−4 | <1 × 10−7.9 | <1 × 10−8.0 |
Q/D q8h, quinupristin-dalfopristin every 8 h.
Proportions of resistance for the low-dose continuous infusion were similar to the every-8-h regimen.
Susceptibility analysis of quinupristin-dalfopristin-resistant VREF.
The MICs and MBCs for the resistant isolates are summarized in Table 6. Isolate VREF 12311a was obtained directly from the 8 × MIC resistance plate from the model with quinupristin-dalfopristin given every 8 h. This isolate showed a 16-fold increase in the quinupristin-dalfopristin MIC and a 64-fold increase in the MBC. The dalfopristin MIC also increased 32-fold, while the quinupristin MIC and MBC remained relatively unchanged. This resistance was not entirely stable, as MICs for VREF 12311b (VREF 12311a passed >10 times on antibiotic-free media) decreased to only four times baseline; the quinupristin-dalfopristin MBC also reverted closer to baseline values. The dalfopristin MIC continued to be elevated, at 32 to 64 times baseline.
TABLE 6.
Quinupristin-dalfopristin MICs and MBCs for resistant VREF isolates recovered from the in vitro infection models
| Isolate | MIC/MBC (μg/ml) of:
|
||
|---|---|---|---|
| Quinupristin-dalfopristin | Quinupristin | Dalfopristin | |
| VREF 12311 (parent) | 0.25/2–4 | 64/>512 | 4/512 |
| VREF 12311A | 4/256 | 64/>512 | 128/>512 |
| VREF 12311B | 1/32 | 64/>512 | 128/>512 |
| VREF 12366 (parent) | 0.25/32 | 128/>512 | 2/128 |
| VREF 12366A | 4/>128 | 128/>512 | 128/>512 |
Unlike VREF 12311, resistant colonies from the quinupristin-dalfopristin monotherapy models versus VREF 12366 (VREF 12366a) showed a more stable elevation of the quinupristin-dalfopristin MIC (from 0.25 to 4 μg/ml), MBC (from 32 to 128 μg/ml) and the dalfopristin MIC (from 2 to 256 μg/ml). Pulsed-field gel electrophoresis analysis of the parent VREF strains and the quinupristin-dalfopristin-resistant mutants revealed only one noticeable band change in DNA fingerprint for VREF 12366a, indicating that the parents and mutants were likely from the same clone.
DISCUSSION
It has been difficult to apply in vitro quinupristin-dalfopristin data to the in vivo setting due to the distinct pharmacokinetic and pharmacodynamic profiles of each component and the rapid conversion of dalfopristin to an active metabolite (5). Studies indicate that quinupristin-dalfopristin MICs are similar over the range of expected in vivo ratios (8), but the potential impact of fluctuating antibiotic ratios on antibacterial activity needs to be considered, as discordant results between in vitro and in vivo activity have been noted for both staphylococci (16, 18) and enterococci (17). We were able to accurately simulate the in vivo pharmacokinetics of both quinupristin and dalfopristin (5) to study the effect of these important variables on antibacterial activity and resistance in the more controlled in vitro environment.
Penetration into the SEVs (as measured by AUC values) was greater for quinupristin than for dalfopristin. This observation was anticipated based on data from autoradiographic studies of experimental streptococcal vegetations that indicated rapid diffusion of quinupristin but slower dalfopristin diffusion (with a concentration gradient) (19). Although SEV concentrations obtained in our study were mean drug concentrations from homogenized samples, we believe that the lower degree of dalfopristin penetration suggests that similar concentration gradients existed. The SEVs appeared to act as a second compartment, and elimination half-lives were appreciably longer from this site of infection. Accumulation of both drugs in the SEVs was observed over 96 h in the high-dose continuous infusion regimens, a result which could have been related to the presence of proteins and bacteria in the SEVs (which could both bind the drugs). However, the increased concentrations of dalfopristin were probably not consistent throughout the entire SEV.
Both isolates used in our study were representative of typical VREF strains encountered in the clinical setting and displayed quinupristin-dalfopristin MICs that were below the MIC at which 50% of the isolates are inhibited for E. faecium (0.5 μg/ml) reported in a recent large surveillance study (21). The quinupristin-dalfopristin MBC for VREF 12366 (32 μg/ml) was much higher than that for VREF 12311 (2 μg/ml), a result which was probably related to its significantly lower killing in the infection models during all quinupristin-dalfopristin regimens. Substantial variation in quinupristin-dalfopristin MBCs for organisms for which MICs are similar has been reported previously (7, 10, 11) and these higher MBCs correlate with decreased antibacterial activity and shorter postantibiotic effect (1).
The bacteriostatic activity observed for quinupristin-dalfopristin in the infection models contrasted with the greater and more rapid antibacterial activity in the static test-tube kill curves. Differences between in vitro and in vivo activity have also been described for two strains of E. faecium with inducible MLSB resistance and one strain of MLSB-susceptible E. faecium (17), for which quinupristin-dalfopristin produced less killing in a rabbit enterococcal endocarditis infection model than would have been predicted from static kill curves. The rapid elimination of quinupristin-dalfopristin and the unequal penetration of the two components in the animal infection models and in our infection models likely produced decreased activity through transient states of inadequate synergy (17).
Other pharmacodynamic factors besides drug elimination and tissue penetration impacted antibacterial activity observed in the infection models. These models sequestered a high inoculum of bacteria, which reduces quinupristin-dalfopristin killing activity against enterococci and staphylococci (7, 10). The stationary growth phase of the organisms in the models also helped to decrease activity, as enterococci growing in logarithmic phase are much more susceptible to quinupristin-dalfopristin than organisms in stationary growth phase (10, 11). Bacterial killing in our models was most pronounced during the first 8 h of the experiments (coinciding with a brief logarithmic growth phase, Fig. 2), and only inhibitory activity occurred after organisms transitioned to stationary phase. Finally, the emergence of subpopulations for which quinupristin-dalfopristin MICs were higher also helped to decrease killing activity.
MLSB resistance is the most-common resistance mechanism impacting quinupristin-dalfopristin activity against gram-positive pathogens. MLSB resistance is inducible in nearly all enterococci, and all members of the MLSB family (including quinupristin) induce the production of ribosomal methylase in streptococci and enterococci (17, 37). Rates of in vitro selection of quinupristin-dalfopristin resistance are quite low for enterococci and staphylococci (≤10−8 to 10−9) (25). However, quinupristin-dalfopristin-resistant S. aureus has been recovered from a high-inoculum infection model (22), emphasizing the importance of large bacterial burdens during resistance selection. Selection of MLSB resistance usually does not change the in vitro quinupristin-dalfopristin susceptibility but it can significantly decrease in vivo activity (17, 25).
Resistance to dalfopristin and the group A streptogramin antibiotics is rare and not as well studied, but inactivation occurs via plasmid-mediated production of streptogramin A acetylase in both staphylococci and enterococci (2). In vitro selection of dalfopristin-resistant mutants of E. faecium (which are also resistant to quinupristin-dalfopristin) has occurred at low rates (≤10−8) (2, 17). Other reports suggest much higher rates of in vitro enterococcal quinupristin-dalfopristin resistance (29, 30, 39). In a study of 11 VREF and 3 VSEF strains, initial selection rates (at 4× MIC) were in the range of 10−4 to 10−6 (30). Quinupristin-dalfopristin resistance was unstable when isolates were grown on antibiotic-free blood agar in between passes, but uninterrupted sequential passes resulted in stable elevation of MICs (range, 4 to 512 μg/ml) for all 14 strains tested (30). The proportion of resistance obtained in our in vitro infection models supports (29, 30, 39) and contrasts with (17) the results from these previous studies. Although the inoculum densities were similar for our SEVs and for rabbit endocardial vegetations from which no resistant E. faecium isolates were recovered (17), the somewhat-larger mass of our SEVs likely increased the total bacterial burden and the statistical probability for resistance. Also, our VREF strains were much more resistant to quinupristin at baseline, which could increase the chances for selection of dalfopristin-resistant VREF.
Most of the in vitro investigations of quinupristin-dalfopristin resistance have used fixed concentrations of the drug in agar. The impact that fluctuating drug concentrations have on the development of quinupristin-dalfopristin resistance is unknown, but our results suggest that they increase resistance potential. The baseline SEVs had no detectable resistant subpopulations, but low levels of quinupristin-dalfopristin-resistant VREF could be detected as early as 8 h after the beginning of therapy. Proportions of resistant subpopulations were incrementally higher at each time point over the 96-h test period (final proportion at 8 × MIC, ca. 10−4). We expect that the repeated exposures to subtherapeutic and therapeutic drug concentrations at the site of the infection (Fig. 1) helped to select out for the resistant subpopulations. Interestingly, our findings for VREF 12311 agreed with those obtained for this isolate in vivo, as this strain was a pretreatment isolate from a patient with human immunodeficiency virus with bacteremia from whom a clonally similar strain was reisolated after 17 days of therapy with a quinupristin-dalfopristin MIC of 2 μg/ml (14).
We discovered two potential strategies that prevented quinupristin-dalfopristin resistance in VREF. Combination of quinupristin-dalfopristin with doxycycline completely prevented resistance in one strain and substantially reduced resistance in the other. Doxycycline might have exerted its protective effects simply by adequately penetrating the SEVs (34) and preventing the proliferation of VREF strains that were inadequately affected by quinupristin-dalfopristin. In a similar manner, ampicillin (which also homogeneously penetrates vegetation tissue) added to quinupristin-dalfopristin improved activity against enterococcal endocarditis compared to either drug alone even though there was no in vitro synergy (17). The potential contribution of doxycycline binding interactions with the bacterial ribosome also could explain its ability to prevent quinupristin-dalfopristin resistance, since subinhibitory concentrations were adequate to prevent selection of resistance (39). The ability of doxycycline to prevent resistance in tetracycline-resistant VREF needs further study, since many VREF strains are resistant to antibiotics in this class.
High-dose continuous infusions of quinupristin-dalfopristin also prevented quinupristin-dalfopristin resistance. These regimens provided continuously with high concentrations of quinupristin and dalfopristin in both the broth and the SEVs (>16× MIC), which appears to prevent the initial emergence of resistant subpopulations (30). The continued presence of dalfopristin may have been most important, as quinupristin-dalfopristin-resistant E. faecium appeared to be the result of dalfopristin resistance.
Our findings of resistance in both VREF strains with simulated human doses contrast with those obtained thus far in the emergency use and noncomparative phase III studies of quinupristin-dalfopristin for VREF infections in which emergence of VREF with decreased susceptibility to quinupristin-dalfopristin (MIC, ≥2 μg/ml) was low (2.4%) (31, 32). Differences between our infection models and human infections, such as the absence of active metabolites and immune factors, higher bacterial inoculum, and different penetration to the infection site, probably decreased activity and increased resistance in the models. However, patients in nearly all reported clinical cases of quinupristin-dalfopristin-resistant VREF have had a nidus that could promote a poor response, such as deep-seated infections or drainage catheters (35, 36). In the context of these data, the results from our infection model actually support the clinical experience with quinupristin-dalfopristin and emphasize the factors which could adversely impact treatment outcome.
In conclusion, the activity of quinupristin-dalfopristin against two strains of VREF and the emergence of resistance appeared to be influenced by many pharmacodynamic factors in an in vitro infection model. Resistance in both strains appeared to be related to alterations in dalfopristin susceptibility. The novel combination of quinupristin-dalfopristin with doxycycline or the administration of quinupristin-dalfopristin as a high-dose continuous infusion prevented or decreased resistance. Our findings in the infection models replicated those observed in humans and highlighted specific bacterial factors and infectious conditions that could result in an inadequate response to quinupristin-dalfopristin therapy.
ACKNOWLEDGMENTS
This research was supported by a grant from the Society of Infectious Diseases Pharmacists.
We also acknowledge LeeAnn Thal and Susan Donabedian for their valuable assistance with gel electrophoresis experiments and Philippe Moreillon for providing the bacterial strains for the quinupristin and dalfopristin microbioassays.
REFERENCES
- 1.Aeschlimann J R, Rebuck J, Rybak M J. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Pharmacodynamic analysis of quinupristin/dalfopristin (Q/D, Synercid) versus vancomycin-resistant Enterococcus faecium with differing MBCs using time-kill curve (KC) and postantibiotic effect (PAE) methods, abstr. E-136; p. 138. [Google Scholar]
- 2.Allignet J, El Solh N. Diversity among the gram-positive acetyltransferases inactivating streptogramin A and structurally related compounds and characterization of a new staphylococcal determinant, vatB. Antimicrob Agents Chemother. 1995;39:2027–2036. doi: 10.1128/aac.39.9.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aumercier, M., S. Boulhallab, M. L. Capmau, and F. Le Goffic. 1992. RP 59500: a proposed mechanism for its bactericidal activity. J. Antimicrob. Chemother. 30(Suppl. A):9–14. [DOI] [PubMed]
- 4.Barriere, J. C., D. H. Bouanchaud, J. M. Paris, et al. 1992. Antimicrobial activity against Staphylococcus aureus of semisynthetic injectable streptogramins: RP 59500 and related compounds. J. Antimicrob. Chemother. 30(Suppl. A):1–8. [DOI] [PubMed]
- 5.Bergeron, M., and G. Montay. 1997. The pharmacokinetics of quinupristin/dalfopristin in laboratory animals and humans. J. Antimicrob. Chemother. 39(Suppl. A):129–138. [DOI] [PubMed]
- 6.Blaser, J. In vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 25:57–68. [DOI] [PubMed]
- 7.Boswell, F. J., J. Sunderland, J. M. Andrews, and R. Wise. 1997. Time-kill kinetics of quinupristin/dalfopristin on Staphylococcus aureus with and without a raised MBC evaluated by two methods. J. Antimicrob. Chemother. 39(Suppl. A):29–32. [DOI] [PubMed]
- 8.Bouanchaud, D. H. 1992. In vitro and in vivo synergic activity and fractional inhibitory concentration (FIC) of the components of a semisynthetic streptogramin, RP 59500. J. Antimicrob. Chemother. 30(Suppl. A):95–99. [DOI] [PubMed]
- 9.Bouanchaud, D. H. 1997. In vitro and in vivo antibacterial activity of quinupristin/dalfopristin. J. Antimicrob. Chemother. 39(Suppl. A):15–21. [DOI] [PubMed]
- 10.Caron F, Gold H S, Wennersten C B, Farris M G, Moellering R C, Eliopoulos G M. Influence of erythromycin resistance, inoculum growth phase, and incubation time on assessment of the bactericidal activity of RP 59500 (quinupristin-dalfopristin) against vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother. 1997;41:2749–2753. doi: 10.1128/aac.41.12.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caron F, Gold H S, Wennersten C B, Moellering R C, Eliopoulos G M. Program and Abstracts of the Third International Conference on Macrolides, Azalides, and Streptogramins. Lisbon, Portugal. 1996. Role of growth phase and erythromycin susceptibility on the bactericidal effect of RP 59500 (quinupristin/dalfopristin) against vancomycin-resistant Enterococcus faecium, abstr. 6.17; p. 60. [Google Scholar]
- 12.Centers for Disease Control and Prevention. Nosocomial enterococci resistant to vancomycin—United States, 1989–1993. Morbid Mortal Weekly Rep. 1993;42:597–599. [PubMed] [Google Scholar]
- 13.Chant C, Rybak M J. Quinupristin/dalfopristin (RP 59500): a new streptogramin antibiotic. Ann Pharmacother. 1995;29:1022–1027. doi: 10.1177/106002809502901013. [DOI] [PubMed] [Google Scholar]
- 14.Chow J W, Donabedian S, Zervos M J. Emergence of increased resistance to quinupristin/dalfopristin during therapy for Enterococcus faecium bacteremia. Clin Infect Dis. 1997;24:90–91. doi: 10.1093/clinids/24.1.90. [DOI] [PubMed] [Google Scholar]
- 15.Cunha B A, Sibley C M, Ristuccia A M. Doxycycline. Ther Drug Monit. 1982;4:115–135. doi: 10.1097/00007691-198206000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Entenza J M, Drugeon H, Glauser M P, Moreillon P. Treatment of experimental endocarditis due to erythromycin-susceptible or -resistant methicillin-resistant Staphylococcus aureus with RP 59500. Antimicrob Agents Chemother. 1995;39:1419–1424. doi: 10.1128/aac.39.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fantin B, LeClercq R, Garry L, Carbon C. Influence of inducible cross-resistance to macrolides, lincosamides, and streptogramin B-type antibiotics in Enterococcus faecium on activity of quinupristin-dalfopristin in vitro and in rabbits with experimental endocarditis. Antimicrob Agents Chemother. 1997;41:931–935. doi: 10.1128/aac.41.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantin B, Leclercq R, Merle Y, et al. Critical influence of resistance to streptogramin B-type antibiotics on activity of RP 59500 (quinupristin/dalfopristin) in experimental endocarditis due to Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:400–405. doi: 10.1128/aac.39.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fantin B, Leclercq R, Ottaviani M, et al. In vivo activities and penetration of the two components of the streptogramin RP 59500 in cardiac vegetations of experimental endocarditis. Antimicrob Agents Chemother. 1994;38:432–437. doi: 10.1128/aac.38.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hospital Infection Control Practices Advisory Committee. Recommendations for preventing the spread of vancomycin resistance. Infect Control Hosp Epidemiol. 1995;16:105–113. doi: 10.1086/647066. [DOI] [PubMed] [Google Scholar]
- 21.Jones R N, Ballow C H, Biedenbach D, Schentag J J the SMART Study Group. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Streptogramin combination (quinupristin/dalfopristin) activity and spectrum from a 1997 study in over 200 medical centers: analysis for regional variations, co-resistances, and validity of proposed susceptibility breakpoints, abstr. E-134; p. 137. [Google Scholar]
- 22.Kang S L, Rybak M J. Pharmacodynamics of RP 59500 alone and in combination with vancomycin against Staphylococcus aureus in an in vitro infected fibrin clot model. Antimicrob Agents Chemother. 1995;39:1505–1511. doi: 10.1128/aac.39.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeClercq R, Courvalin P. Emerging problems with enterococcal infections. Curr Opin Infect Dis. 1996;9:115–119. [Google Scholar]
- 24.Leclerq R, Deroit E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988;319:157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- 25.Leclercq, R., L. Nantas, C. J. Soussy, and J. Duval. 1992. Activity of RP 59500, a new parenteral semisynthetic streptogramin against staphylococci with various mechanisms of resistance to macrolide-lincosamide-streptogramin antibiotics. J. Antimicrob. Chemother. 30(Suppl. A):67–76. [DOI] [PubMed]
- 26.Liebowitz B, Hakes J L, Cahn M M, et al. Doxycycline blood levels in normal subjects after intravenous and oral administration. Curr Ther Res Clin Exp. 1972;14:820–831. [PubMed] [Google Scholar]
- 27.McGrath B J, Kang S L, Kaatz G W, Rybak M J. Bactericidal activities of teicoplanin, vancomycin, and gentamicin alone and in combination against Staphylococcus aureus in an in vitro pharmacodynamic model of endocarditis. Antimicrob Agents Chemother. 1994;38:2034–2040. doi: 10.1128/aac.38.9.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Megran D W. Enterococcal endocarditis. Clin Infect Dis. 1992;15:63–71. doi: 10.1093/clinids/15.1.63. [DOI] [PubMed] [Google Scholar]
- 29.Millichap J, Ristow T, Bodner U, et al. Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Selection of Enterococcus faecium strains with stable and unstable resistance to RP 59500 using step-wise exposure in vitro, abstr. E-120; p. 106. [Google Scholar]
- 30.Millichap J, Ristow T A, Noskin G A, Peterson L R. Selection of Enterococcus faecium strains with stable and unstable resistance to the streptogramin RP 59500 using stepwise in vitro exposure. Diagn Microbiol Infect Dis. 1996;25:15–20. doi: 10.1016/0732-8893(96)00067-3. [DOI] [PubMed] [Google Scholar]
- 31.Moellering R L, Linden P K. Program and Abstracts of the 4th International Conference on the Macrolides, Azalides, Streptogramins, and Ketolides. Barcelona, Spain. 1998. Efficacy and safety of quinupristin/dalfopristin (Synercid) in the treatment of vancomycin-resistant Enterococcus faecium (VREF) infections, abstr. 2.14; p. 30. [Google Scholar]
- 32.Nadler H, Dowzicky M, Talbot G, Bompart F, Grote F. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Low rates of emerging resistance and superinfection in Synercid (quinupristin/dalfopristin, Q/D) treated patients during worldwide clinical program, abstr. E-136B; p. 5. [Google Scholar]
- 33.National Committee for Clinical Laboratory Standards. Approved standard. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Villanova, Pa: National Committee for Laboratory Standards; 1993. [Google Scholar]
- 34.Nicolau D P, Freeman C D, Nightingale C H, Coe C J, Quintilliani R. Minocycline versus vancomycin for treatment of experimental endocarditis caused by oxacillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:1515. doi: 10.1128/aac.38.7.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piper J, Furness K, Steele-Moore L, Ferris D. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Quinupristin/dalfopristin (Synercid) resistant vancomycin resistant Enterococcus faecium (SRVRE), abstr. C-80; p. 60. [Google Scholar]
- 36.Piper J, Steele-Moore L, Berg D. Abstracts of the 96th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1996. Acquired resistance to quinupristin/dalfopristin (Synercid®) during successful antimicrobial therapy of vancomycin-resistant enterococcus (VRE), abstr. A-109; p. 152. [Google Scholar]
- 37.Rosato A, Vicarini H, Leclercq R. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. High level cross-resistance to macrolides and lincomycin in streptococci and enterococci: constitutive or inducible expression of ribosomal methylase?, abstr. C-81; p. 49. [Google Scholar]
- 38.Smith C L, Warburton P E, Gaal A, Cantor C R. Analysis of genome organizations and rearrangements by pulsed field gradient gel electrophoresis. In: Setlow J K, Hollaender A, editors. Genetic engineering, principles and methods. New York, N.Y: Plenum Press; 1986. pp. 45–70. [Google Scholar]
- 39.Thal L A, Davison A, Chow J, Zervos M. Abstracts of the 34th Annual Meeting of the Infectious Diseases Society of America. New Orleans, La. 1996. In vitro evaluation of the development of Synercid® resistant mutants in Enterococcus faecium, abstr. 165; p. 66. [Google Scholar]
- 40.Uttley A H C, Collins C H, Naidoo J, et al. Vancomycin-resistant enterococci. Lancet. 1988;i:57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- 41.Williams, J. D., J. P. Maskell, A. C. Whiley, and A. M. Sefton. 1997. Comparative in-vitro activity of quinupristin/dalfopristin against Enterococcus spp. J. Antimicrob. Chemother. 39(Suppl. A):41–46. [DOI] [PubMed]