Abstract
Objective:
This randomized comparative effectiveness trial evaluated a novel insomnia treatment using Acceptance and Commitment Therapy (ACT) among women veterans. Participants received either the Acceptance and the Behavioral Changes to treat Insomnia (ABC-I) or Cognitive-Behavioral Therapy for Insomnia (CBT-I). Primary objectives were to determine whether ABC-I was non-inferior to CBT-I in improving sleep and to test whether ABC-I resulted in higher treatment completion and adherence versus CBT-I.
Method:
149 women veterans with insomnia disorder (mean age 48.0 years) received ABC-I or CBT-I. Main sleep outcomes were Insomnia Severity Index (ISI), Pittsburgh Sleep Quality Index (PSQI), and sleep efficiency (SE) by actigraphy (objective) and sleep diary (subjective). Measures were collected at baseline, immediate post-treatment and 3-month post-treatment follow-up. Treatment completion and adherence were assessed during the interventions.
Results:
Both interventions improved all sleep outcomes from baseline to immediate post-treatment and 3-month post-treatment follow-up. At immediate post-treatment, ABC-I was statically non-inferior for sleep diary SE and objective SE, but non-inferiority was not statistically confirmed for ISI or PSQI total scores. At 3-month post-treatment follow-up, ABC-I was non-inferior for all 4 key outcome variables. There was not a statistically significant difference between the number of participants who discontinued CBT-I (11%) versus ABC-I (18%; p = .248) before completing treatment. ABC-I was superior to CBT-I for some adherence metrics.
Conclusions:
Overall, ABC-I was similar in effectiveness compared to CBT-I for treatment of insomnia and may improve adherence to some behavioral elements of treatment.
Keywords: Sleep, Insomnia, Women, Veterans, Cognitive Behavioral Therapy, Acceptance and Commitment Therapy
Up to 30% of the general population experiences insomnia symptoms in their lifetime (Buysse et al., 2008; Schutte-Rodin et al., 2008; Taylor et al., 2007), and women, military veterans, and those with comorbid medical and psychiatric conditions are disproportionally impacted by insomnia (Edinger et al., 2008; Kessler et al., 2011; Walker, 2004; Zammit et al., 1999). Insomnia contributes to lost productivity, psychological distress, medical morbidity, and mortality risk (Reynolds & Ebben, 2017). Given the prevalence and impact of insomnia, connecting patients to effective treatment is of the utmost importance. The first-line recommended treatment for insomnia disorder, Cognitive-Behavioral Therapy for Insomnia (CBT-I), has been shown to improve insomnia symptoms for most patients, including those with comorbid conditions (Edinger et al., 2021; Mysliwiec et al., 2020; Qaseem et al., 2016; Wu et al., 2015). CBT-I is a brief psychotherapy (typically 4–8 sessions) that helps patients change problematic thoughts and behaviors contributing to poor sleep and typically involves two core behavioral therapies: stimulus control and sleep restriction therapy (Edinger et al., 2021; Miller et al., 2014). In CBT-I, these are typically combined with sleep hygiene education, cognitive therapy and relaxation/counter-arousal strategies. VA has a national program to disseminate CBT-I through a provider training program, the goal of which is to increase access to this care for all veterans using VA healthcare (Manber et al., 2012).
Despite the body of research demonstrating the efficacy and effectiveness of CBT-I, challenges with treatment remain (Ong et al., 2008). Specifically, adherence is a challenge for some patients, with the most extreme version of non-adherence being withdrawal from treatment (Bouchard et al., 2003). Many of the same patient groups disproportionately impacted by insomnia symptoms (e.g., patients with comorbidities) also experience greater difficulty completing CBT-I (van de Laar et al., 2015). There are several factors that may contribute to non-adherence and premature treatment discontinuation in CBT-I including: 1) the counterintuitive treatment recommendations associated with sleep restriction and stimulus control approaches, 2) discouragement and reduced motivation to adhere to recommendations and complete treatment, and 3) difficulty changing sleep-related thoughts, and 4) cognitive arousal resulting from challenging maladaptive thoughts about sleep. These factors can lead to secondary distress (Koffel et al., 2018), exacerbating concerns about sleep problems and sustaining beliefs about one’s inability to intervene on sleep problems (i.e., meta-cognitions) which can further exacerbate insomnia symptoms (Galbiati et al., 2021).
Due to these potential challenges to CBT-I adherence, research has begun to explore the utility of mindfulness and acceptance-based approaches in the treatment of insomnia (Dalrymple et al., 2010; Ong et al., 2012). The “third wave” behavioral therapy that best represents this model is Acceptance and Commitment Therapy (ACT) (Hayes et al., 1999; Hayes et al., 2012). Within the ACT model, therapeutic change occurs by changing the relationship one has with “dysfunctional thoughts” through contacting the present moment and, based on what that situation affords, acting in accordance with one’s chosen values (Hayes et al., 1999). This process is referred to as psychological flexibility and there are interrelated middle-level processes which promote psychological flexibility: mindfulness (being in the present moment), acceptance of personal experiences and experiential willingness (i.e., embracing current experience and giving up the need to control or change private experiences), cognitive defusion (i.e., non-identification with thoughts), and commitment to “act” in accordance with one’s chosen values. This framework has the potential to reduce the secondary distress that can manifest during CBT-I.
In some current ACT-based interventions for insomnia, these elements are incorporated into treatment that includes established behavioral components of insomnia treatment (e.g., sleep restriction and stimulus control) (Paulos-Guarnieri et al., 2022). Theoretically, ACT-based approaches may improve adherence to recommendations through multiple mechanisms. First, psychological flexibility predicts reduced insomnia severity and fewer avoidance behaviors that are linked to poor sleep (Hall et al., 2007; McCracken et al., 2011). Second, orientation to values and committed actions may help individuals to see beyond the initial discomfort associated with insomnia treatment recommendations. Lastly, acceptance and defusion techniques may reduce counterproductive sleep effort and secondary distress from sleep-related thoughts (Dalrymple et al., 2010; Hertenstein et al., 2014; Lundh, 2005; Ong et al., 2012).
Only a small number of studies have examined the effectiveness of ACT in improving insomnia symptoms. A recent systematic review of 19 ACT studies showed that ACT interventions improved sleep duration, sleep quality, and sleep-related cognitive/emotional processes (Lappalainen et al., 2019; Zakiei & Khazaie, 2019; Zakiei et al., 2021). Although only three of the studies had insomnia symptoms as a primary outcome (Salari et al., 2020). There is also evidence that ACT for insomnia improves proposed mechanisms of change including reducing experiential avoidance and dysfunctional beliefs and attitudes about sleep (Lappalainen et al., 2019; Zakiei et al., 2021). Despite these promising preliminary findings, no studies have examined the impact of ACT for insomnia as compared to “gold standard” CBT-I, and none have evaluated the impact of ACT on adherence to behavioral recommendations. Identifying approaches beyond CBT-I may offer patients and providers an alternative when CBT-I is not indicated or is ineffective, and the goal of our current work is to expand the options for behavioral (non-medication) treatment options for insomnia in patient groups who experience challenges in completion of evidence-based psychotherapy interventions, such as women veterans (Eftekhari et al., 2020).
The current study was a randomized comparative effectiveness trial, designed to compare a novel ACT-based treatment program (Acceptance and the Behavioral Changes to treat Insomnia [ABC-I]) to CBT-I in women veterans with insomnia disorder. ABC-I is a 5-week manualized program which integrates key components of CBT-I with components of ACT to achieve sleep improvement similar to CBT-I and increase retention in treatment. The objectives were to 1) compare the effectiveness of the ABC-I program in improving insomnia symptoms and sleep quality from baseline to immediate post-treatment and from baseline to 3-months post-treatment follow-up compared to CBT-I, and 2) compare discontinuation rates and adherence to behavioral recommendations between ABC-I and a similarly-structured CBT-I program. We hypothesized that ABC-I would be non-inferior to CBT-I in improving insomnia and sleep quality. We also examined whether the ABC-I program would be superior to CBT-I in terms of treatment completion rates and adherence to the behavioral components of the interventions (i.e., sleep restriction, stimulus control and sleep hygiene recommendations). We also evaluated change in select intervention process measures including sleep hygiene behaviors, dysfunctional thoughts, and psychological flexibility from baseline to immediate post-treatment and 3-month post-treatment follow-up.
Methods
Recruitment and Participants
The sampling frame included women veterans who were current users of a large urban VA healthcare system (i.e., had at least 1 visit in the previous six months) who lived within a 50-mile catchment area. Between October 2014 and June 2017, 12,225 postal surveys were mailed to this sampling frame. This previously described postal survey (Martin et al., 2017) contained items addressing the diagnostic criteria for insomnia disorder (Medicine, 2005), and was used to identify women veterans with insomnia symptoms for further screening. In total, 2,669 women veterans returned a completed survey. Among respondents, 1,776 (66.5%) endorsed at least one symptom of poor sleep at least 3 times per week with daytime consequences, lasting for more than 3 months, and were therefore potentially eligible. Further telephone screening was attempted with 1,372 survey respondents who met these criteria in order to identify conditions that would make study participation difficult.
In total, we reached 793 individuals by phone, of whom 286 refused to complete the screening. Among the 507 who completing screening, 22 were ineligible. Exclusions during telephone screening included lack of transportation to the medical center, unstable housing, and self-described significant health or emotional problems that would make it difficult to participate in a research trial. Of the 485 who were eligible, 347 agreed to participate and were enrolled in the study. See Figure 1 and Supplementary Methods). Among enrolled participants, 334 completed a pre-treatment baseline assessment. Prior to randomization, baseline assessments were reviewed by a study psychologist and a study physician to make a final determination about diagnosis of insomnia disorder (based on DSM-5 (Association, 2013)) and appropriateness for behavioral treatment of insomnia. A total of 175 participants, were excluded prior to randomization (see Supplementary Methods), most commonly due to severe psychiatric illness or other contraindication for sleep restriction therapy (n=49; i.e., unstable/severe symptoms, bipolar disorder, or active substance use identified from administration of the Mini-International Neuropsychiatric Interview (MINI (Sheehan et al., 1998)), untreated severe sleep apnea (n=36 identified from overnight home sleep apnea testing), or not meeting diagnostic criteria for insomnia disorder (n=26). See Supplementary Methods for a full description of the MINI and overnight home sleep apnea testing, and Supplementary Table S10 for a summary of diagnoses based on the MINI assessment. In addition, 10 individuals withdrew prior to being randomized. In total, 149 women were randomized (74 to the ABC-I group and 75 to the CBT-I group). Baseline characteristics of the ABC-I and CBT-I groups are shown in Table 1 and Supplementary Table S1.
Figure 1:
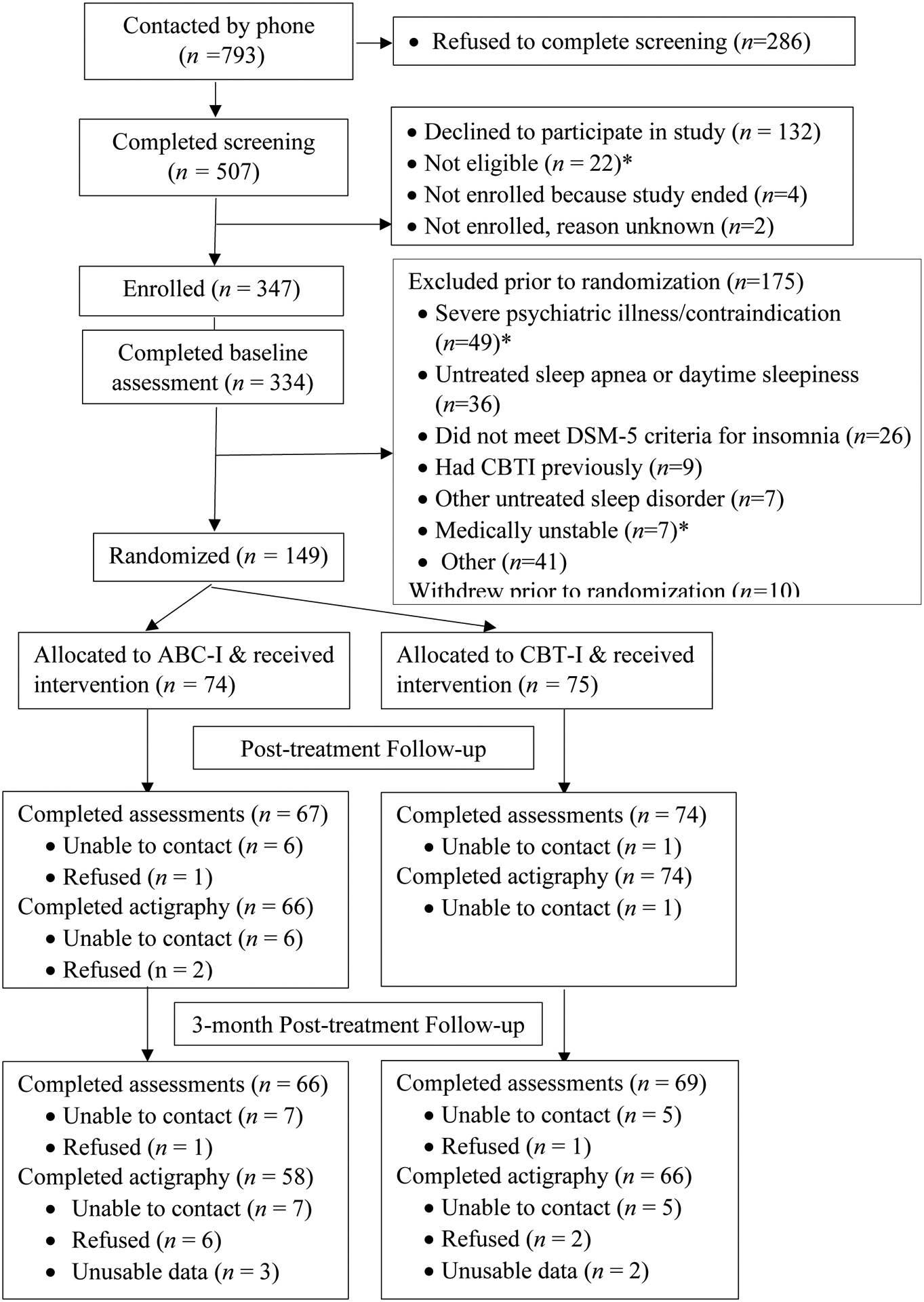
CONSORT Flow Diagram (*additional details in supplementary materials)
Table 1:
Baseline Demographic, Sleep and Other Characteristics of Study Sample
| Variable | Overall N = 149 Mean (SD) or Number (%) |
ABC-I N = 74 Mean (SD) or Number (%) |
CBT-I N = 75 Mean (SD) or Number (%) |
p-valueb |
|---|---|---|---|---|
| Age, in years | 48.0 (13.2) | 48.3 (13.0) | 47.8 (13.5) | 0.819 |
| Years of Education | 16.3 (2.7) | 16.1 (2.5) | 16.4 (2.9) | 0.611 |
| Racea | ||||
| White/Caucasian | 66 (44.3%) | 35 (47.3%) | 31 (41.3%) | 0.511 |
| African American/Black | 49 (32.9%) | 21 (28.4%) | 28 (37.3%) | 0.296 |
| American Indian/Alaska Native | 9 (6.0%) | 9 (12.2%) | 0 (0.0%) | 0.001 |
| Asian American | 6 (4.0%) | 4 (5.4%) | 2 (2.7%) | 0.442 |
| Native Hawaiian/Pacific Islander | 2 (1.3%) | 0 (0.0%) | 2 (2.7%) | 0.497 |
| Other | 9 (6.0%) | 7 (9.5%) | 2 (2.7%) | 0.098 |
| Ethnicity | ||||
| Hispanic/Latina | 34 (22.8%) | 17 (23.0%) | 17 (22.7%) | 1.000 |
| Non-Hispanic/Latina | 115 (77.2%) | 57 (77.0%) | 58 (77.3%) | |
| Marital Status | ||||
| Married or living as married | 54 (38.0%) | 23 (31.9%) | 31 (44.3%) | 0.035 |
| Divorced | 34 (23.9%) | 23 (31.9%) | 11 (15.7%) | |
| Separated | 4 (2.8%) | 0 (0.0%) | 4 (5.7%) | |
| Widowed | 6 (4.2%) | 4 (5.6%) | 2 (2.9%) | |
| Single or never married | 44 (31.0%) | 22 (30.6%) | 22 (31.4%) | |
| Sexual orientation | ||||
| Heterosexual or straight | 117 (82.4%) | 63 (87.5%) | 54 (77.1%) | 0.129 |
| Gay or lesbian | 20 (14.1%) | 6 (8.3%) | 14 (20.0%) | |
| Bisexual | 5 (3.5%) | 3 (4.2%) | 2 (2.9%) | |
| PTSD Checklist (PCL-5) | 25.8 (21.3) | 26.3 (21.8) | 25.3 (21.0) | 0.784 |
| Patient Health Questionnaire (PHQ-9) | 10.4 (5.5) | 10.6 (5.7) | 10.3 (5.4) | 0.763 |
| Generalized Anxiety Disorder (GAD) | 9.7 (5.7) | 8.9 (5.6) | 10.4 (5.6) | 0.104 |
Participants could check all that applied.
p-value is from an independent samples t-test or Fisher’s exact test.
Procedure
The VA Greater Los Angeles Healthcare System’s Institutional Review Board reviewed and approved the study (NCT02076165). Participants provided written informed consent prior to initiation of study activities. Recruitment began in October 2014 and continued through June 2017. Follow-up data collection began in February 2015 and was completed in February 2018. Enrolled participants completed a pre-intervention baseline assessment consisting of three visits. Ninety percent of participants completed these visits in 21 days or less. During visit 1, informed consent was obtained, followed by administration of a questionnaire to obtain demographic and baseline health status information. Participants were sent home with a home sleep apnea testing (HSAT) device (WatchPAT 100, Itamar Medical, Ltd.) to wear overnight for one night to determine the presence and severity of sleep apnea. During visit 2, participants returned the HSAT device and were administered questionnaires to evaluate mental health symptoms. Participants were then provided with an actigraph (Actiwatch Spectrum, Minimitter/Respironics, Bend, OR) to wear at home for 8 days/nights and a sleep diary to complete during that 8-day period. During visit 3, participants returned the actigraph and sleep diary and were administered self-report sleep measures. All questionnaires and measures were administered by research staff in interview format.
Participants who completed the baseline assessment, met all inclusion/exclusion criteria and agreed to continue were randomized 1:1 to ABC-I or CBT-I. Prior to study commencement, a randomization sequence was created by the study statistician using Stata 13.1 statistical software. A senior research staff member not involved in enrollment, assessment or intervention prepared opaque sequentially numbered envelopes and implemented the random allocation sequence. Participants and assessment research staff were blinded to group assignment.
Follow-up assessments were completed immediately after the 5-week intervention (immediate post-treatment assessment) and again three months later (3-month post-treatment assessment). These follow-up assessments were similar in structure to visits 2–3 of the baseline assessment. Participants who completed the baseline, immediate post-treatment and 3-month post-treatment follow-up assessments were compensated up to $275 for their travel and time.
Outcomes Measures
The key outcome measures included commonly used patient-reported sleep questionnaires, sleep diaries and objective metrics based on wrist actigraphy, and were collected at baseline, immediate post-treatment, and 3-month post-treatment follow-up assessments.
Insomnia symptom severity (primary study outcome) was measured with the Insomnia Severity Index (ISI) (Bastien et al., 2001). The ISI includes seven Likert-type scales with scores from 0 (not at all) to 4 (very much). Total summed scores range from 0 to 28, with higher scores indicating greater insomnia severity. This is the main outcome measure used in clinical implementation of CBT-I in VA. Sleep quality and disturbances were measured with the Pittsburgh Sleep Quality Index (PSQI), a 19-item questionnaire that assesses seven sleep components (sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction) (Buysse et al., 1989). In addition to calculating total score (range 0–21, higher scores indicating worse sleep quality), a 3-factor scoring model was used (sleep efficiency, perceived sleep quality, and daily disturbance)(Cole et al., 2006) and is presented in the Supplementary Materials.
Sleep efficiency is defined as the percent of time asleep out of total time in bed. This was measured subjectively from the sleep diary. Higher values indicate greater (i.e., better) sleep efficiency. The sleep diary was based on the Consensus Sleep Diary and was adapted for use in the current study using a cognitive interviewing process (Carney et al., 2012). Time asleep was calculated as the average time in bed over the week minus the average time awake while in bed for each participant. Total time in bed was calculated as the average get-up time minus the average go-to-bed time. Sleep efficiency was calculated as the percent time asleep while in bed.
Objective sleep efficiency was measured with wrist actigraphy (Philips Actiwatch Spectrum, Bend, OR), worn on the nondominant wrist for 8 consecutive days/nights. Activity levels were collected in 1-minute epochs. Following accepted protocols and guidelines (Ancoli-Israel et al., 2015; Smith et al., 2018a, 2018b), the major sleep period was determined using corresponding sleep diary bedtime and rise times to determine the “rest” intervals. Using these intervals, sleep efficiency (SE) was calculated automatically by the Actiware software (v. 6.0.8) utilizing default sleep scoring algorithms with medium threshold settings.
Adherence to behavioral recommendations was captured in the daily sleep diary and compared to the recommendations documented by the study interventionist in the structured session notes. Detailed methods and calculations are shown in Supplementary Materials. For both treatments, participants’ daily adherence with bedtime and rise times was determined by comparing the target times determined during the session with the times recorded by the participant in the daily sleep diary. Although there is no universally-accepted definition of sleep schedule adherence (Mellor et al., 2022), we used prior research on the topic to develop study-specific criteria (Matthews et al., 2012). Bedtime was coded as adherent if it was no more than 15 minutes earlier than the target time (otherwise it was not adherent). Rise time was coded as adherent if it was no more than 15 minutes later than the target time (otherwise it was not adherent). Totals were computed by person and week, calculating 1) the total number of bedtimes, 2) the number of adherent bedtimes, 3) the total number of rise times, and 4) the number of adherent rise times. We then calculated, by person and week, the proportion of adherent bedtimes, and the proportion of adherent rise times. For each sleep hygiene and stimulus control recommendation that a participant received, we calculated the number of possible adherence days during each week of the treatment (i.e., number of days between the date recommendation was given and last sleep diary tracking day). We then summed the number of days a participant marked as following the recommendation in their daily sleep diary (see Supplementary Methods) and divided this by the total possible adherence days to get a proportion for each recommendation. The proportion adherence was averaged over all recommendations provided in a given week for each participant.
Demographic and Health-related Measures
Sociodemographic and health-related information were collected at baseline. Age in years was calculated using birth date and enrollment date. Participants reported their identified race/ethnicity, sexual orientation, number of years of education, annual household income, relationship status, and employment status. Medical and psychiatric comorbidities were collected from a checklist based on two longitudinal women’s health studies (Selim et al., 2004; Sowers et al., 2000) and input from experts in women’s health (EMY and DLW).
Mental Health Measures
The PTSD checklist for DSM-5 (PCL-5) was used to assess post-traumatic stress disorder (PTSD) (Blevins et al., 2015). The PCL-5 is a 20-item self-report measure that assesses the 20 DSM-5 symptoms of PTSD with a score range from 0–80 (higher scores indicate more symptoms). Prior to administering the PCL-5, the interviewer asked the participant 5 questions to assess whether she met DSM-5 criterion A (witnessed or experienced a life-threatening event) (Association, 2013). Only participants meeting criterion A were administered the PCL-5.
Depressive symptom severity was measured with the 9-item Patient Health Questionnaire (PHQ-9) (Kroenke et al., 2001). The items align to the DSM-IV diagnostic criteria for depressive disorders. The Generalized Anxiety Disorder-7 (GAD-7), another component of the PRIME-MD suite of evaluation tools, was used to screen for Generalized Anxiety Disorder (GAD). The GAD-7 is a 7-item scale (scores range from 0 to 27), with a cut off score of ≥10 yielding both sensitivity and specificity above .80 for GAD (Spitzer et al., 2006).
Intervention Process Measures
Three measures were completed by participants at the first and last treatment sessions and at 3-month post-treatment follow-up to evaluate possible processes through which the interventions could improve sleep. The 7-item Acceptance and Action Questionnaire-II (AAQ-II) assessed psychological flexibility and experiential avoidance (higher scores suggest less psychological flexibility and more avoidance) (Bond et al., 2011; Hayes et al., 2000). The Dysfunctional Beliefs and Attitudes about Sleep-10 (DBAS-10) (Edinger & Wohlgemuth, 2001) was used to examine beliefs about insomnia (lower scores are indicative of fewer negative beliefs about insomnia). The Sleep Hygiene Index (SHI) was used to measure sleep hygiene behaviors (where lower scores are associated with better sleep hygiene practices) (Mastin et al., 2006).
Interventions
The 5-week ABC-I and CBT-I interventions are described in Figure 2, Supplementary Methods and Supplemental Figures S1 and S2. Since treatment completion was a main study outcome, procedures for contacting participants after randomization were standardized. Each participant received one reminder telephone call the day before each session. Study staff made three attempts to reach the participant by telephone if they did not attend, and if a participant did not respond, one letter asking them to reschedule the session was sent. To monitor treatment Fidelity, sessions were audio-recorded, and treatment fidelity was assessed by review of recordings for a 10% random sample of participants (45 ABC-I and 40 CBT-I sessions). The rater (one of the other study interventionists) used a structured fidelity rating form on which the degree to which each topic within each session was covered was categorized as: 3=Therapist skillfully and thoroughly explained all of the concepts related to this content; 2=Therapist skillfully and thoroughly explained the majority of the concepts related to this content, and 1=Therapist explained less than half of the concepts related to this content. The proportion of each score (1, 2 or 3) was then computed and compared for each of the two treatments (see Supplementary Methods).
Figure 2.
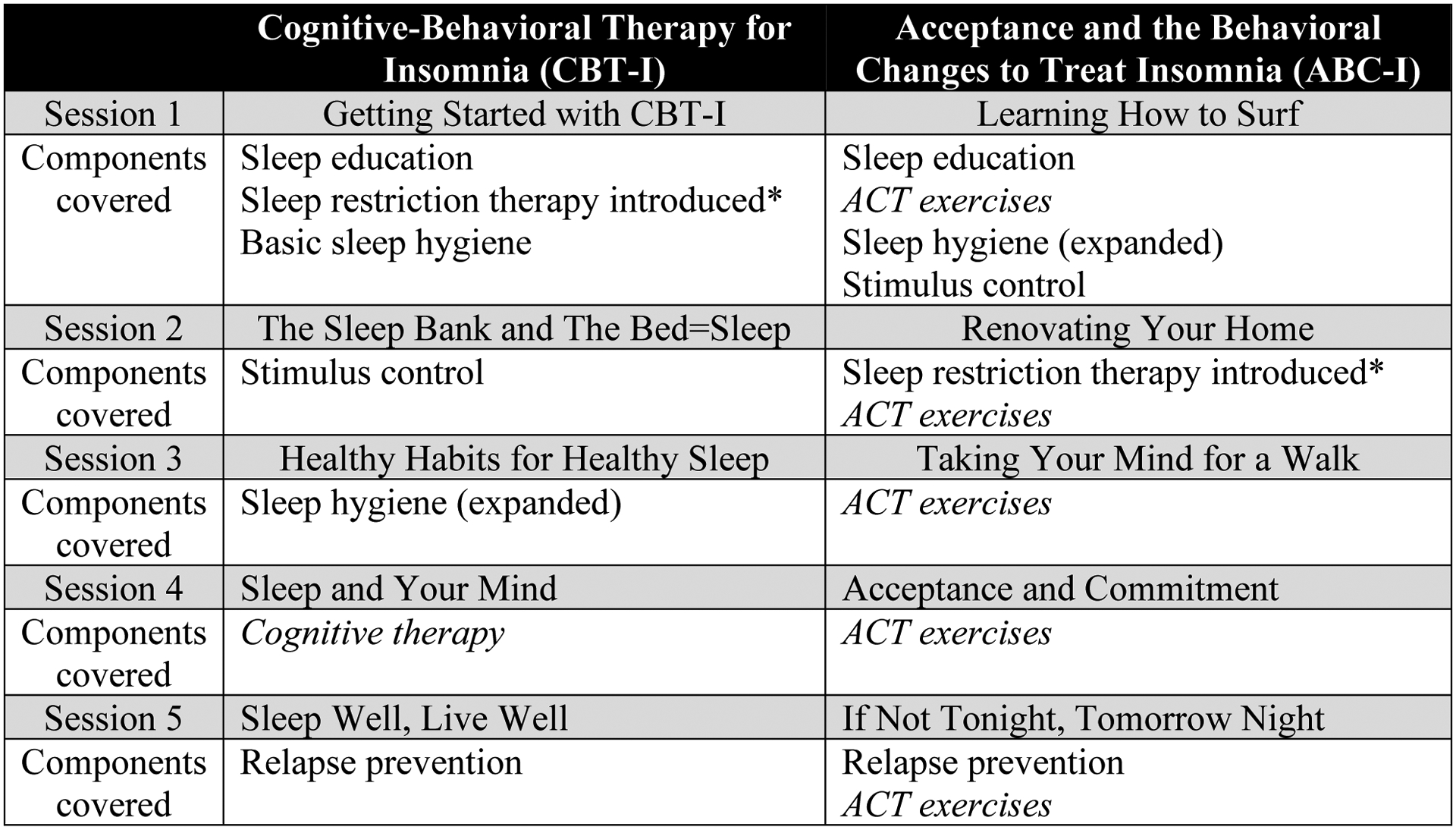
Components of Each Session for the Cognitive-Behavioral Therapy for Insomnia (CBT-I) and Acceptance and the Behavioral Changes to Treat Insomnia (ABC-I) Interventions.
Notes: After introduction of sleep restriction therapy, the sleep schedule was adjusted at subsequent sessions using the sleep diary. For other components, recommendations were reviewed at the next session. Additional details about each session are shown in Supplementary Figures S1 and S2.
Data Analysis
Data preparation and power computations were performed using Stata version 17.1 (StataCorp, 2021) and are described in Supplementary Methods. Baseline measures for ABC-I and CBT-I groups were compared using independent samples t-tests for continuous measures and Fisher’s Exact tests for nominal measures.
Non-Inferiority of ABC-I versus CBT-I in improving insomnia symptoms
We used a two-level mixed-effects analysis modeling each outcome as a function of treatment group (ABC-I, CBT-I) and time (as a factor variable with three levels -- baseline, immediate post-treatment, and 3-months post-treatment), with time as level 1 and person as level 2. The residuals were estimated using an unstructured covariance matrix. Models were estimated using the mixed command (StataCorp, 2021). To describe the pattern of results, we estimated, for each outcome, the mean and 90% confidence interval (CI), for each of the six cells formed by crossing group (ABC-I vs. CBT-I) and time (baseline, immediate post-treatment, and 3-month post-treatment follow-up). Estimates were computed using the margins command (StataCorp, 2021).
To measure the impact of ABC-I vs. CBT-I on change in outcomes 1) from baseline to immediate post-treatment and 2) from baseline to 3-month post-treatment follow-up, two planned interaction contrasts were tested that crossed treatment group with time. The first interaction contrast applied the coefficients “−1 1 0” to time, yielding a test contrasting ABC-I vs. CBT-I by the contrast of immediate post-treatment vs. baseline. The second interaction contrast applied the coefficients “−1 0 1” to time, yielding a test contrasting ABC-I vs. CBT-I by the contrast of 3-month post-treatment vs. baseline. The resulting parameter estimates from such interaction contrasts are often called a Difference in Difference (which we abbreviate as DiD). The interaction contrasts were computed using the margins command (StataCorp, 2021).
The rationale and final non-inferiority margins (Walker & Nowacki, 2010) are described in Supplementary Methods. In short, the non-inferiority margin was based on a-priori information when available; otherwise the non-inferiority margin was specified as 4/10ths of the baseline standard deviation (that is, an effect size of d=0.40). For outcomes where higher values represent poorer sleep quality, the one sided null and alternative hypotheses are Ho: DiD ≥ δ; Ha: DiD < δ. For outcomes where higher values indicate better sleep quality, the one sided null and alternative hypotheses are Ho: DiD ≤ -δ; Ha: DiD < -δ. Using the parameter estimates and standard errors from the interaction contrasts from the mixed model, these null hypotheses were tested using one sided Wald Z-tests (Liu, 2016) with alpha=0.05 (one sided). We computed 90% confidence intervals for each DiD (to mirror the one sided, alpha=0.05, significance tests.
Treatment Discontinuation Rates in ABC-I versus CBT-I
We hypothesized that participants who received ABC-I program would be less likely to discontinue treatment than participants who received CBT-I. Participants were coded as completing treatment (attended 5 session) or discontinuing treatment (attended 4 or fewer sessions). To compare the percent who discontinued treatment between groups, a two tailed Fisher’s exact test was computed using the tabulate command with the exact option using Stata.
Adherence with Recommendations in ABC-I versus CBT-I
Three variables were used to assess adherence to recommendations using proportions; bedtime adherence, rise time adherence, and adherence to other behavioral recommendations. Similar to prior research on adherence to bedtime and rise time (Agnew et al., 2021; Matthews et al., 2012), for each week, we computed the proportion of adherent bedtimes (and proportion of adherent rise times), then compared these proportions between the ABC-I and CBT-I groups using fractional logistic regression with the fracreg command in Stata (StataCorp, 2021). Since there is no prior literature upon which to base our methods for adherence to other recommendations, we extrapolated the proportion adherence to recommendations using sleep diary documentation (described in detail in Supplementary Methods). Again, the proportions were compared between the ABC-I and CBT-I group for each week using fractional logistic regression with the fracreg command in Stata.
Analyses of Intervention Process Measures
Exploratory analyses examined change in the three intervention process measures (AAQ, DBAS-10 and SHI) between baseline and immediate post-treatment and between baseline and 3-month post-treatment follow-up for both treatment groups using mixed models ANOVA.
Results
Sociodemographic variable descriptive statistics for participants are included in Table 1. Table 2 shows descriptive statistics (Mean and SD) for the main study outcomes by treatment group at baseline, immediate post-treatment, and 3-month post-treatment follow-up.
Table 2:
Sample Statistics for Main Outcome Variables by Treatment Group and Time Point.
| Overall N = 149 |
ABC-I N = 74 |
CBT-I N = 75 |
||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | p-valuea | |
| Baseline | ||||
| Insomnia Severity Index (ISI), total score | 14.4 (5.0) | 14.2 (4.8) | 14.6 (5.1) | 0.638 |
| Pittsburgh Sleep Quality Index (PSQI), total score | 10.7 (3.7) | 10.3 (3.4) | 11.1 (4.0) | 0.209 |
| Sleep efficiency (SE) by sleep diary, % | 77.8 (13.2) | 77.9 (12.4) | 77.7 (13.9) | 0.932 |
| Sleep efficiency (SE), by actigraphy, % | 80.8 (8.2) | 80.3 (9.0) | 81.3 (7.4) | 0.456 |
| Immediate Post-Treatment | ||||
| Insomnia Severity Index (ISI), total score | 5.4 (4.9) | 5.5 (4.8) | 5.4 (5.0) | |
| Pittsburgh Sleep Quality Index (PSQI),total score | 5.1 (3.4) | 5.2 (3.3) | 5.0 (3.5) | |
| Sleep efficiency (SE) by sleep diary, % | 91.2 (7.2) | 91.3 (5.8) | 91.2 (8.3) | |
| Sleep efficiency (SE), by actigraphy, % | 82.9 (6.7) | 82.8 (6.9) | 83.0 (6.6) | |
| 3-Month Post-Treatment Follow-up | ||||
| Insomnia Severity Index (ISI), total score | 6.5 (5.7) | 6.0 (4.9) | 6.9 (6.4) | |
| Pittsburgh Sleep Quality Index (PSQI),total score | 5.9 (4.2) | 5.5 (3.7) | 6.4 (4.6) | |
| Sleep efficiency (SE) by sleep diary, % | 89.8 (7.5) | 90.1 (7.3) | 89.6 (7.7) | |
| Sleep efficiency (SE), by actigraphy, % | 81.7 (7.0) | 81.6 (7.7) | 81.7 (6.5) | |
p-value comparing groups at baseline using an independent samples t-test.
Non-Inferiority of ABC-I to CBT-I
Table 3 shows the estimated means and 90% confidence intervals (computed from the mixed model) for the primary outcomes by group (ABC-I, CBT-I) and time (Baseline, immediate post-treatment, and 3-months post-treatment). Table 4 shows the analyses of the tests of non-inferiority for the seven primary outcomes, showing the DiD, 90% CI, and the one-sided test of non-inferiority at immediate post-treatment and at 3-month post-treatment follow-up. At post-treatment, ABC-I was non-inferior for 3 out of 4 outcome variables (ISI, sleep diary sleep efficiency, and objective sleep efficiency from actigraphy; p’s<.048), but non-inferiority was not established for PSQI total score at immediate post-treatment. However, at 3-month post-treatment follow-up, ABC-I was non-inferior to CBT-I for all 4 of the key outcome variables (ISI, PSQI, sleep diary sleep efficiency and objective sleep efficiency; p’s<.028). Figure 3 shows the DiD and 90% CI immediate post-treatment and 3-month post-treatment follow up for the main sleep outcomes (PSQI subscales shown in Supplementary Figure S5).
Table 3:
Estimated Means (From Mixed Model) of Sleep Outcomes at Each Time Point (ABC-I = 74, CBT-I = 75)
| Outcome | Group | Baseline | Post-Treatment | 3-Month Follow- up |
|---|---|---|---|---|
| Mean (90% CI) | Mean (90% CI) | Mean (90% CI) | ||
| Insomnia Severity Index (ISI) total score | ABC-I | 14.2 (13.2, 15.1) | 5.5 (4.5, 6.5) | 5.9 (4.8, 7.0) |
| CBT-I | 14.6 (13.6, 15.5) | 5.4 (4.5, 6.3) | 6.9 (5.8, 8.0) | |
| Pittsburgh Sleep Quality Index (PSQI) total score | ABC-I | 10.3 (9.6, 11.0) | 5.2 (4.6, 5.9) | 5.4 (4.5, 6.2) |
| CBT-I | 11.1 (10.4, 11.8) | 5.1 (4.4, 5.7) | 6.3 (5.6, 7.2) | |
| Sleep efficiency (SE) by sleep diary, % | ABC-I | 77.6 (75.1, 80.1) | 91.5 (90.0, 92.9) | 90.3 (88.8, 91.9) |
| CBT-I | 77.8 (75.2, 80.3) | 91.2 (89.9, 92.6) | 89.8 (88.3, 91.2) | |
| Sleep efficiency (SE), by actigraphy, % | ABC-I | 80.3 (78.7, 81.9) | 82.6 (91.3, 84.0) | 81.6 (80.1, 83.1) |
| CBT-I | 81.3 (79.8, 82.9) | 82.9 (81.7, 84.2) | 81.5 (80.1, 82.9) |
Table 4.
Non-inferiority Analyses of Sleep Outcomes (ABC-I =74, CBT-I = 75)
| Outcome | Non-Inferiority Marginc (NIM) | Change Baseline to Immediate Post-Treatment | Change Baseline to 3-months post-treatment | |||
|---|---|---|---|---|---|---|
| Difference in Difference (DiD): Mean (90% CI) | One sided-test of non-inferiority (z, p-value) | Difference in Difference (DiD): Mean (90% CI) | One sided-test of non-inferiority (z, p-value) | |||
| Insomnia Severity Index (ISI)a | 1.98 | 0.50 (−0.98, 1.98) | z = −1.64, p = 0.050 | −0.57 (−2.32, 1.17) | z = −2.41, p = 0.008 | |
| Pittsburgh Sleep Quality Index (PSQI), total scorea | 1.48 | 0.90 (−0.15, 1.94) | z = −0.92, p = 0.180 | −0.27 (−1.47, 0.93) | z = −2.40, p = 0.008 | |
| Sleep efficiency (SE) diary, %b | −5.27 | 0.41 (−2.92, 3.74) | z = 2.81, p = 0.002 | 0.76 (−2.72, 4.24) | z = 2.85, p = 0.002 | |
| Sleep efficiency (SE) actigraphy, %b | −3.27 | 0.68 (−1.21, 2.57) | z = 3.43,p < 0.001 | 1.11 (−1.14, 3.36) | z = 3.20, p = 0.001 | |
Notes: Higher values reflect worse sleep; non-inferiority test is left-sided;
Higher values reflect better sleep; non-inferiority test is right-sided. See Supplementary Methods and Table S3 for information on selection of non-inferiority margins. CBT-I: Cognitive-behavioral therapy for insomnia; ABC-I Acceptance and the behavioral changes to treat insomnia.
Figure 3.
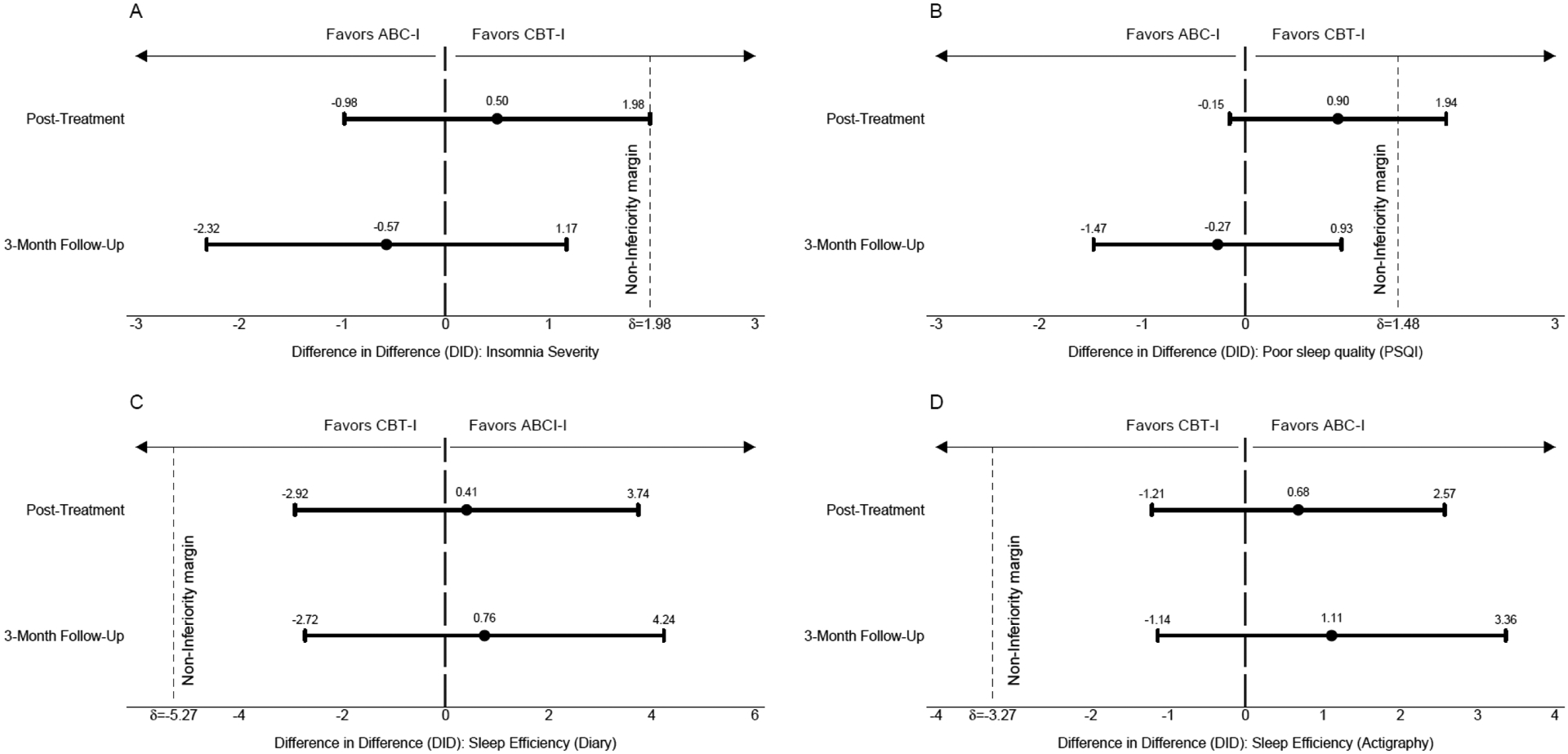
Non-inferiority outcomes: Difference in Difference (DiD) with 90% Confidence Intervals for Sleep Outcomes
Notes: Panel A: Insomnia Severity Index (ISI; p<.05 for non-inferiority) total score; Panel B Pittsburgh Sleep Quality Index (PSQI) total score (n.s.); Panel C: Sleep Efficiency based on Sleep Diary (p<.05 for non-inferiority); Panel D: Sleep Efficiency based on Wrist Actigraphy (p<.05 for non-inferiority). Non-inferiority margin (δ) depicted as dotted line on x-axis. CBT-I: Cognitive-behavioral therapy for insomnia; ABC-I Acceptance and the behavioral changes to treat insomnia.
Clinical Significance of ISI improvements
Using published response and remission threshold criteria, we evaluated the clinical significance of the changes in the ISI across time for each treatment group (Morin et al., 2011). ISI scores at immediate post-treatment and 3-month post-treatment follow-up were compared to baseline values -- improvements exceeding seven points were categorized as responding to treatment. ISI scores less than or equal to seven were categorized as indicating insomnia remission. (Missing values were categorized as showing neither response to treatment nor remission from insomnia.) Table 5 shows response and remission rates by treatment group at immediate post-treatment and 3-month post-treatment follow-up. A Z test of independent proportions compared the proportion responding to treatment for ABC-I versus CBT-I, finding no significant difference at immediate post-treatment (z = −0.688; p = 0.49) or at 3-month post-treatment follow-up (z = 0.04; p = 0.968). Similarly, there was no significant difference between groups in the proportion showing remission at immediate post-treatment (z = 0.752; p = 0.496) or at 3-month post-treatment follow-up (z = 0.267, p = 0.532). The pattern of results did not differ when the assessment-response only group was analyzed.
Table 5:
Response and Remission Rates for ABC-I and CBT-I groups using change in Insomnia Severity Index (ISI) scores.
| Response | ||||
|---|---|---|---|---|
| Post-Treatment | 3-month follow-up | |||
| ITT n/N (%) |
Assessment- Response Only n/N (%) |
ITT n/N (%) |
Assessment- Response Only n/N (%) |
|
| ABC-I | 40/74 (54.1) | 40/67 (59.7) | 36/74 (48.7) | 36/66 (54.6) |
| CBT-I | 49/75 (65.3) | 49/74 (66.2) | 36/75 (48.0) | 36/69 (52.2) |
| Remission | ||||
| Post-Treatment | 3-month follow-up | |||
| ITT n/N (%) |
Assessment- Response Only n/N (%) |
ITT n/N (%) |
Assessment- Response Only n/N (%) |
|
| ABC-I | 47/74 (63.5) | 47/67 (70.2) | 49/74 (66.2) | 49/66 (74.2) |
| CBT-I | 56/75 (74.7) | 56/74 (75.7) | 42/75 (56.0) | 42/69 (60.9) |
Notes: Response: Change of > 7 points on the ISI at post-treatment/follow-up, relative to baseline. Remission: ISI score ≤ 7 at post-treatment/follow-up. ITT: Analyzes all randomized cases -- missing cases coded as “No” for Remission and “No” for Response. Assessment-Response Only: Analyzes only cases with valid data. ABC-I Acceptance and the behavioral changes to treat insomnia. CBT-I: Cognitive-behavioral therapy for insomnia.
Treatment Discontinuation Rates in ABC-I versus CBT-I
There was not a statistically significant difference in rates of treatment completion between the ABC-I group (85.1%) versus the CBT-I group (92.0%, p = .208). Among the 17 participants who did not complete the intervention, nine attended only one session (5 in the ABC-I group, 4 in the CBT-I group), four attended two sessions (3 ABC-I, 1 CBT-I), one attended three sessions (ABC-I), and three attended 4 sessions (2 ABC-I, 1 CBT-I). The most common reason given for nonattendance was a change in work schedule (5 participants). Other reasons included dislike of aspects of the intervention (e.g., homework assignments, sleep diaries n=3), moving (n=1), traveling (n=1), unable to drive (n=1), and family responsibilities (n=1). Six participants’ reasons for nonattendance were unknown.
There were no significant differences in the duration of treatment sessions, or the total amount of time spent in treatment with an average of 45.2 (SD 1.1) and 42.3 (SD 1.0) minutes in the ABC-I and CBT-I sessions, respectively (p = 0.06). The mean number of face-to-face intervention hours over the 5 weeks (totaled within each participant) was 3.8 (SD 0.1) and 3.5 (SD 0.1) hours in the ABC-I and CBT-I groups, respectively (p = .06).
Adherence with Behavioral Recommendations in ABC-I versus CBT-I
Bedtime and rise time
Participant adherence with the prescribed sleep schedule during the intervention period is shown in Figure 4. Average adherence to the prescribed bedtime was greater than 80% after week 1 and both groups increased bedtime adherence over the treatment period. There were no significant differences between groups. Average adherence with the prescribed rise time was slightly lower than for bedtime, with the ABC-I group having better adherence during the second week (p = 0.028).
Figure 4.
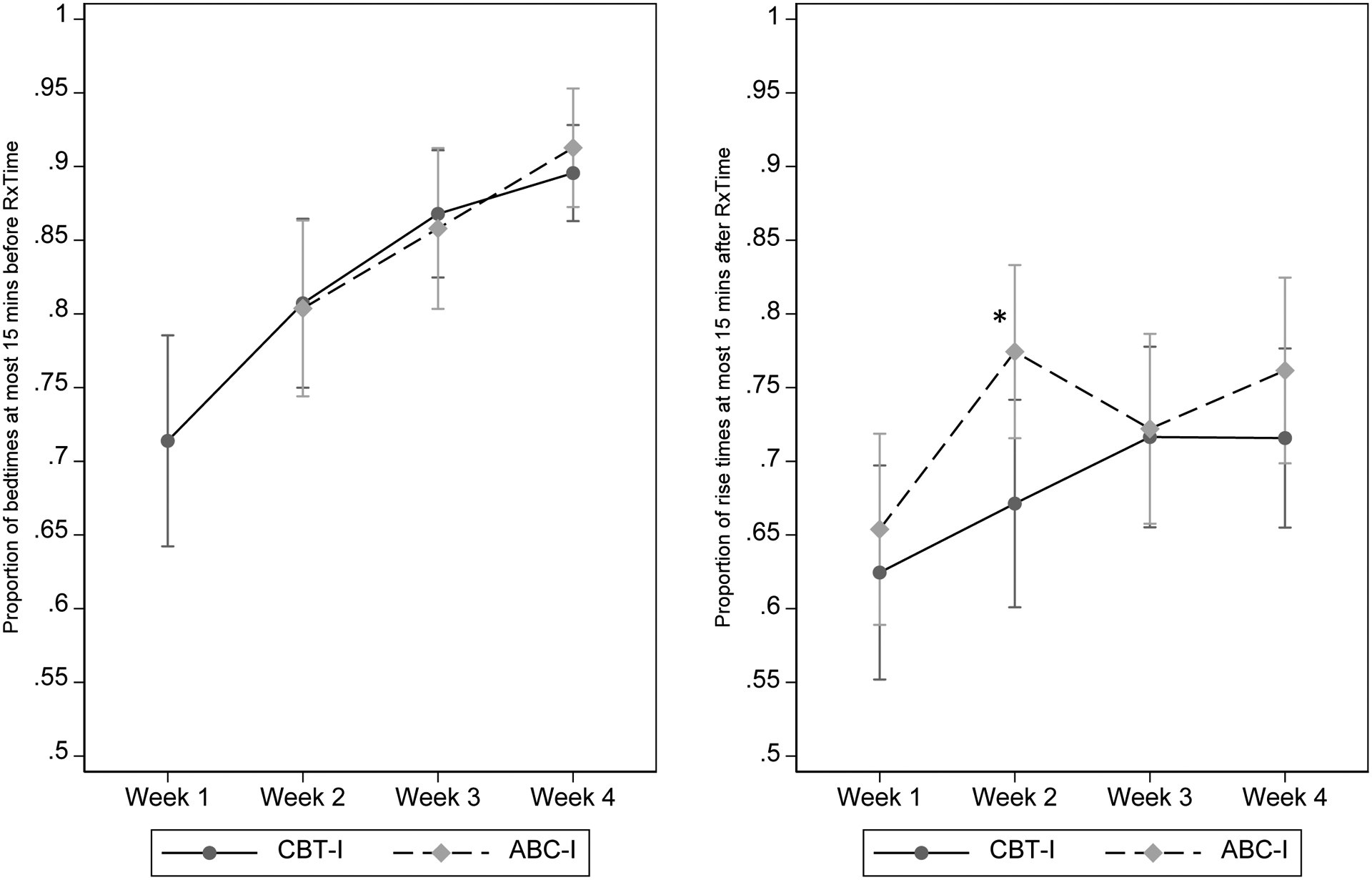
Adherence to Recommended Bedtime (left panel) and Rise Time (right panel)
Notes: No bedtime recommendation was given for ABC-I in week 1. For week 2 bedtime, adherence was significantly higher for ABC-I compared to CBT-I (*p<.05). No other differences were statistically significant. CBT-I: Cognitive-behavioral therapy for insomnia; ABC-I Acceptance and the behavioral changes to treat insomnia.
Behavioral recommendations
The mean number of behavioral recommendations that participants were asked to track during weeks 2 through 4 was 2.4 (range 0 to 4) in the ABC-I group and 3.3 (range 0 to 5) in the CBT-I group. Adherence with the weekly behavioral recommendations is shown in Figure 5. Weekly comparisons show that the ABC-I group had significantly better adherence compared to the CBT-I group for weeks 2–4 (no recommendations given to the CBT-I group at session 1).
Figure 5.
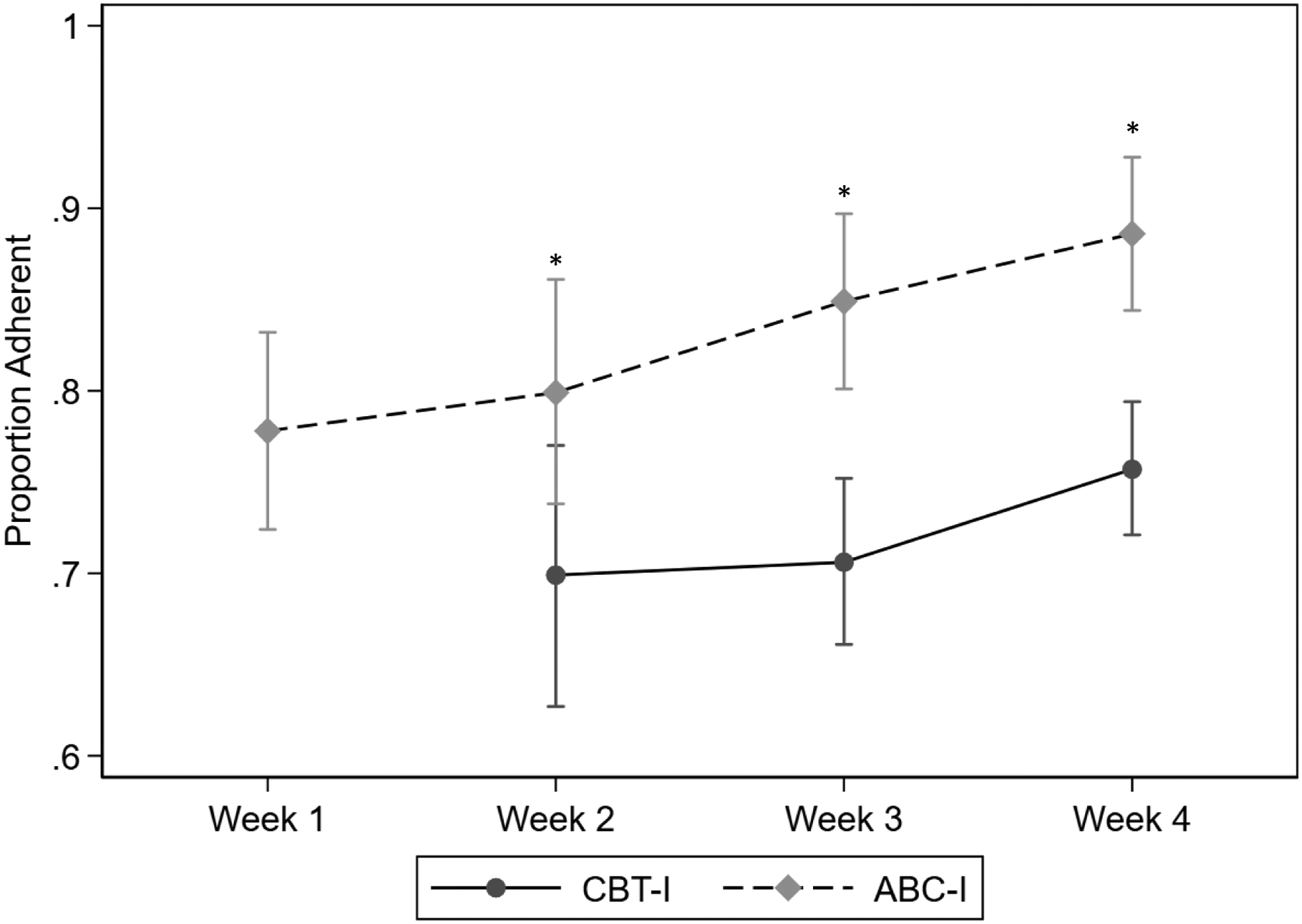
Adherence to Sleep Hygiene and Stimulus Control Recommendations at Each Session, expressed as a proportion of opportunities to adhere to recommendations
Notes: Participants documented their adherence to each recommendation in their daily sleep dairy (see Supplementary Figures S3 and S4). The overall proportion was calcualted for all recommendations given each week. No stimulus control or sleep hygiene recommendations were given in Session 1 for CBT-I. Adherence was higher for ABC-I compared to CBT-I for weeks 2–4 (*p’s<.05). CBT-I: Cognitive behavioral therapy for insomnia; ABC-I acceptance and behavioral changes ot treat insomnia.
Treatment Fidelity
Overall, treatment fidelity ratings were acceptable (see Supplementary Methods and Results). Ninety-eight percent of ABC-I and CBT-I session topics were covered, and raters founds only two instances of contamination across the ABC-I (1 suggestion for thought restructuring) and CBT-I (1 mention of an ACT metaphor) treatments.
Process Measures
Within group comparisons showed that SHI, DBAS, and AAQ improved significantly from baseline to post-treatment and from baseline to 3-month post-treatment follow-up for both ABC-I and CBT-I groups (see Supplemental Table S8). There were no between group differences in SHI scores at either timepoint. DBAS scores were significantly lower for the CBT-I group compared to the ABC-I group at post-treatment, but not at 3-month follow-up. AAQ scores improved more (i.e., greater reductions) in the ABC-I group compared to the CBT-I group at 3-month post-treatment follow-up, but not at immediate post-treatment (see Supplemental Table S9).
Discussion
This was the first comparative effectiveness trial of CBT-I versus ABC-I that included sleep, treatment dropout, and treatment adherence as main outcome variables. Findings from the current study indicate that ABC-I is an effective treatment and is comparable to the current first-line recommended treatment for insomnia, CBT-I. Both treatments were shown to improve all sleep outcome variables relative to pre-treatment baseline values. The effects of ABC-I were non-inferior to the effects of CBT-I on diary-based sleep efficiency and objective sleep efficiency (from actigraphy) at immediate post-treatment. While the effects of ABC-I were not definitively non-inferior to the effects of CBT-I at immediate post-treatment on ISI or PSQI total score non-inferiority was confirmed at the 3-months primary outcome timepoint for all sleep outcome variables. These differences may be, in part, because sleep restriction therapy was started in Session 1 for CBT-I but Session 2 for ABC-I. Since sleep restriction therapy requires adjustments to the sleep schedule, full benefit may not have been achieved by the end of treatment; however, it does appear that some participants in the CBT-I arm who achieved “remission” at immediate post-treatment did not sustain this level of benefit at the 3-month post-treatment follow-up visit. These findings suggest that ABC-I promotes sustained behavior change without specifically challenging maladaptive thoughts. Findings are consistent with the ACT model, which theorizes that identification of values early in treatment promotes committed actions and early adherence.
Findings partially supported our hypothesis that ACT components would improve treatment completion and adherence among participants assigned to the ABC-I condition compared to participants assigned to the CBT-I condition. Most participants in both treatment arms completed all sessions and there was no significant difference in treatment discontinuation, potentially due to a ceiling effect. Given the highly structured nature of this clinical trial, follow-up and rescheduling efforts were part of the research protocol in both groups and followed an “ideal” standard of care. This, in addition to exclusion of participants with significant illness or mental health-related conditions, likely contributed to higher completion rates compared to rates observed in clinical settings. Future implementation trials should compare the delivery of ABC-I and CBT-I in clinical settings and monitor differences in treatment dropout rates accordingly. Also of note, treatment components were not introduced in the same order in ABC-I and CBT-I. These differences emerged during our development of the ABC-I intervention program. Based on participant and therapist feedback, we elected to introduce sleep restriction therapy in Session 2, after participants had completed the ACT-based exercises in Session 1. We elected to maintain a more standard CBT-I protocol in which sleep restriction is generally introduced in the first session.
While low and comparable dropout rates were observed in the current study, participants assigned to the ABC-I condition showed significantly better adherence to treatment recommendations compared to participants assigned to the CBT-I condition. Again, identification of values and corresponding committed actions may have contributed to increased motivation and adherence to behavioral recommendations within the ABC-I condition. Although our study was not designed to fully elucidate mechanisms of action, reductions in experiential avoidance and increases in psychological flexibility, specifically defusion from sleep-related meta-cognitions, may have further improved treatment adherence within the ABC-I condition compared to the CBT-I condition. It is also possible that the ACT-I intervention is particularly suited for individuals with high sleep-related arousal. In addition, it was not possible to look at individual behavioral recommendations to see if adherence was better for some versus others (e.g., were participants more likely to adhere to basic sleep hygiene like reducing caffeine than moving non-sleep activities out of the bedroom as part of stimulus control) as each participant received personalized recommendations and the treatments components were interwoven across sessions.
This study had multiple strengths. To our knowledge, this was the first comparative effectiveness trial of CBT-I and ABC-I in a diverse sample of women veterans. This study not only compared the impact of both treatments on sleep outcomes variables, but also compared treatment adherence and completion rates and explored possible intervention process measures as well. The population from which the current sample was recruited is highly relevant to the study questions, with many participants reporting medical and psychiatric comorbidities (i.e., known barriers to CBT-I adherence, see Supplementary Tables S2 and S10). This study included a comprehensive assessment battery of well-established measures and methods. Several limitations should be also noted. While women veterans are a relevant population to current study questions, findings may not generalize to patients outside of the women veteran population. Generalizability may also be affected by other factors, including education level of the sample. To the extent possible, we limited exclusion of participants with significant illness or mental health-related conditions to situations in which CBT-I would need significant modification within clinical care (e.g., sleep restriction therapy is generally contra-indicated in patients with a history of mania) as the treatment duration was standardized within the study, and we did not exclude participants with significant psychiatric symptoms of PTSD, depression or anxiety. For ethical reasons, we also excluded individuals with a recent suicide attempt or daily suicidal ideation as sufficient resources and support could not be provided within the study. As a result of these limitations, testing the ABC-I program in clinical settings where modifications are sometimes made to the behavioral components is needed prior to widespread use of the intervention program. Finally, we conducted multiple statistical tests to evaluate our findings, and in an effort to balance the risk of type I and type II errors, we did not implement an experiment-wide type I error reducing approach; rather, we reviewed the pattern of our findings, which showed 10 out of 14 tests of non-inferiority (p<.05) reached statistical significance. We have interpreted the pattern of our results rather than the results of any one test.
Our findings have important implications for patient care. ABC-I may offer a comparable alternative to patients who find cognitive restructuring distressing or not affirming and to patients who experience difficulty adhering to insomnia treatment recommendations (e.g., changing their sleep schedule). We hypothesize that the focus on behavior change occurs after treatment engagement is linked to patient values, and the open discussion of accepting thoughts (ACT) may be less distressing than challenging long-held beliefs about sleep (CBT). ABC-I also expands the array of evidence-based techniques available to sleep medicine providers to utilize in their clinical practice. For example, providers who are already trained in the delivery of ACT can leverage existing clinical skillsets to treat insomnia, potentially increasing the availability of insomnia treatments to patients who are in need of care.
Supplementary Material
Public health statement:
ABC-I represents a viable alternative to CBT-I for women veterans, resulting in similar benefits in improved sleep 3 months after treatment with better adherence to some behavioral elements of treatment. This allows providers to choose the intervention that best fits the clinical context and may provide an alternative for patients who do not achieve benefit from CBT-I.
Funding:
This project was funded by VA/HSR&D IIR-HX002300 (PI: Martin). Dr. Martin is supported by a VA HSR&D Research Career Scientist Award RCS 20-191 and NIH/NHLBI K24HL143055. Drs. Carlson, Kelly, Saldana and May were supported by the VA Office of Academic Affiliations through the Women’s Health (Carlson), Advanced Fellowship in Geriatrics (Kelly) and Behavioral Sleep Medicine/Primary Care Mental Health Psychology Residency (Saldana and May) programs. Dr. Carlson is also supported by VA HSR&D CDA 20-227. Dr. Kelly is also supported by NIH/NHLBI K23 HL157754. Dr. Yano is supported by a VA HSR&D Senior Research Career Scientist Award RCS 05-195. Support was also provided by the VA Greater Los Angeles Geriatric Research, Education and Clinical Center.
Footnotes
Clinical Trials Registration: NCT02076165
References
- Agnew S, Vallières A, Hamilton A, McCrory S, Nikolic M, Kyle SD, Fleming L, & Crawford MR (2021). Adherence to Cognitive Behavior Therapy for Insomnia: An Updated Systematic Review. Sleep Med Clin, 16(1), 155–202. 10.1016/j.jsmc.2020.11.002 [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L, Liu L, Meltzer LJ, Sadeh A, Spira AP, Taylor D, Owens J, & Smith M (2015). The SBSM Guide to actigraphy monitoring: clinical and research applications. Journal of Behavioral Sleep Medicine, 13(Suppl. 1), S4–S38. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disoders Fifth Edition (DSM-5).
- Bastien CH, Vallières A, & Morin CM (2001). Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine, 2(4), 297–307. [DOI] [PubMed] [Google Scholar]
- Blevins CA, Weathers FW, Davis MT, Witte TK, & Domino JL (2015). The posttraumatic stress disorder checklist for DSM‐5 (PCL‐5): Development and initial psychometric evaluation. Journal of Traumatic Stress, 28(6), 489–498. [DOI] [PubMed] [Google Scholar]
- Bond FW, Hayes SC, Baer RA, Carpenter KM, Guenole N, Orcutt HK, Waltz T, & Zettle RD (2011). Preliminary psychometric properties of the Acceptance and Action Questionnaire–II: A revised measure of psychological inflexibility and experiential avoidance. Behavior Therapy, 42(4), 676–688. [DOI] [PubMed] [Google Scholar]
- Bouchard S, Bastien C, & Morin CM (2003). Self-efficacy and adherence to cognitive-behavioral treatment of insomnia. Behavioral Sleep Medicine, 1(4), 187–199. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, & Rössler W (2008). Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep, 31(4), 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF III, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, & Morin CM (2012). The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep, 35(2), 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, & Irwin MR (2006). Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep, 29(1), 112–116. [DOI] [PubMed] [Google Scholar]
- Dalrymple KL, Fiorentino L, Politi MC, & Posner D (2010). Incorporating principles from acceptance and commitment therapy into cognitive-behavioral therapy for insomnia: A case example. Journal of Contemporary Psychotherapy, 40(4), 209–217. [Google Scholar]
- Edinger JD, Arnedt JT, Bertisch SM, Carney CE, Harrington JJ, Lichstein KL, Sateia MJ, Troxel WM, Zhou ES, Kazmi U, Heald JL, & Martin JL (2021). Behavioral and psychological treatments for chronic insomnia disorder in adults: An American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med, 17(2), 255–262. 10.5664/jcsm.8986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger JD, Means MK, Carney CE, & Krystal AD (2008). Psychomotor performance deficits and their relation to prior nights’ sleep among individuals with primary insomnia. Sleep, 31(5), 599–607. 10.1093/sleep/31.5.599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger JD, & Wohlgemuth WK (2001). Psychometric comparsions of the standard and abbreviated DBAS-10 versions of the dysfunctional beliefs and attitudes about sleep questionnaire. Sleep Medicine, 2, 493–500. [DOI] [PubMed] [Google Scholar]
- Eftekhari A, Crowley JJ, Mackintosh MA, & Rosen CS (2020). Predicting treatment dropout among veterans receiving prolonged exposure therapy. Psychol Trauma, 12(4), 405–412. 10.1037/tra0000484 [DOI] [PubMed] [Google Scholar]
- Galbiati A, Sforza M, Scarpellino A, Salibba A, Leitner C, D’Este G, Mombelli S, Ferini-Strambi L, & Castronovo V (2021). “Thinking About Thinking” in Insomnia Disorder: The Effect of Cognitive-Behavioral Therapy for Insomnia on Sleep-Related Metacognition. Frontiers in Psychology, 3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M, Thayer JF, Germain A, Moul D, Vasko R, Puhl M, Miewald J, & Buysse DJ (2007). Psychological stress is associated with heightened physiological arousal during NREM sleep in primary insomnia. Behavioral Sleep Medicine, 5(3), 178–193. [DOI] [PubMed] [Google Scholar]
- Hayes S, Bissett R, Strosahl K, Wilson K, Pistorello J, Dykstra T, & Mccurry S (2000). Psychometric properties of the Acceptance and Action Questionnaire (AAQ). Unpublished manuscript. [Google Scholar]
- Hayes S, Strosahl K, & Wilson K (1999). Acceptance and commitment therapy: Understanding and treating human suffering. New York: Guilford. [Google Scholar]
- Hayes S, Strosahl K, & Wilson K (2012). Acceptance and commitment therapy: The process and practice of mindful change. New York, NY, US. In: Guilford Press. 10.1177/0011000012460836. [DOI] [Google Scholar]
- Hertenstein E, Thiel N, Lüking M, Külz AK, Schramm E, Baglioni C, Spiegelhalder K, Riemann D, & Nissen C (2014). Quality of life improvements after acceptance and commitment therapy in nonresponders to cognitive behavioral therapy for primary insomnia. Psychotherapy and Psychosomatics, 83(6), 371–373. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Coulouvrat C, Hajak G, Roth T, Shahly V, Shillington AC, Stephenson JJ, & Walsh JK (2011). Insomnia and the performance of US workers: Results from the America insomnia survey. Sleep, 34(9), 1161–1171. 10.5665/sleep.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffel E, Bramoweth AD, & Ulmer CS (2018). Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): a narrative review. Journal of General Internal Medicine, 33(6), 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ‐9: validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen P, Langrial S, Oinas-Kukkonen H, Muotka J, & Lappalainen R (2019). ACT for sleep-internet-delivered self-help ACT for sub-clinical and clinical insomnia: A randomized controlled trial. Journal of Contextual Behavioral Science, 12, 119–127. [Google Scholar]
- Liu X (2016). Methods and Applications of Longitudinal Data Analysis. Elsevier, Inc. [Google Scholar]
- Lundh L-G (2005). The Role of Acceptance and Mindfulness in the Treatment ofInsomnia.
- Manber R, Carney C, Edinger J, Epstein D, Friedman L, Haynes PL, Karlin BE, Pigeon W, Sieburn AT, & Trockel M (2012). Dissemination of CBT-I to the non-sleep specialist: Protocol development and training issues. Journal of Clinical Sleep Medicine, 8(2), 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Schweizer CA, Hughes JM, Fung CH, Dzierzewski JM, Washington DL, Kramer BJ, Jouldjian S, Mitchell MN, & Josephson KR (2017). Estimated prevalence of insomnia among women veterans: Results of a postal survey. Women’s Health Issues, 27(3), 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastin DF, Bryson J, & Corwyn R (2006). Assessment of sleep hygiene using the Sleep Hygiene Index. Journal of Behavioral Medicine, 29(3), 223–227. [DOI] [PubMed] [Google Scholar]
- Matthews EE, Schmiege SJ, Cook PF, Berger AM, & Aloia MS (2012). Adherence to cognitive behavioral therapy for insomnia (CBT-I) among women following primary breast cancer treatment: A pilot study. Behavioral Sleep Medicine, 10(3), 217–229. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Williams JL, & Tang NK (2011). Psychological flexibility may reduce insomnia in persons with chronic pain: A preliminary retrospective study. Pain Medicine, 12(6), 904–912. [DOI] [PubMed] [Google Scholar]
- Medicine, A. A. o. S. (2005). I. Insomnia. In The International Classification of Sleep Disorders (2 ed., pp. 1–31). American Academy of Sleep Medicine. (Reprinted from In File) [Google Scholar]
- Mellor A, Kavaliotis E, Mascaro L, & Drummond SPA (2022). Approaches to the assessment of adherence to CBT-I, predictors of adherence, and the association of adherence to outcomes: A systematic review. Sleep Med Rev, 63, 101620. 10.1016/j.smrv.2022.101620 [DOI] [PubMed] [Google Scholar]
- Miller CB, Espie CA, Epstein DR, Friedman L, Morin CM, Pigeon WR, Spielman AJ, & Kyle SD (2014). The evidence base of sleep restriction therapy for treating insomnia disorder. Sleep Med Rev, 18(5), 415–424. 10.1016/j.smrv.2014.01.006 [DOI] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Bélanger L, & Ivers H (2011). The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep, 34(5), 601–608. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysliwiec V, Martin JL, Ulmer CS, Chowdhuri S, Brock MS, Spevak C, & Sall J (2020). The Management of Chronic Insomnia Disorder and Obstructive Sleep Apnea: Synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med, 172(5), 325–336. 10.7326/m19-3575 [DOI] [PubMed] [Google Scholar]
- Ong JC, Kuo TF, & Manber R (2008). Who is at risk for dropout from group cognitive-behavior therapy for insomnia? Journal of Psychosomatic Research, 64(4), 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong JC, Ulmer CS, & Manber R (2012). Improving sleep with mindfulness and acceptance: A metacognitive model of insomnia. Behaviour Research and Therapy, 50(11), 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulos-Guarnieri L, Linares IMP, & El Rafihi-Ferreira R (2022). Evidence and characteristics of Acceptance and Commitment Therapy (ACT)-based interventions for insomnia: A systematic review of randomized and non-randomized trials. Journal of Contextual Behavioral Science, 23, 1–14. [Google Scholar]
- Qaseem A, Kansagara D, Forciea MA, Cooke M, & Denberg TD (2016). Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med, 165(2), 125–133. 10.7326/m15-2175 [DOI] [PubMed] [Google Scholar]
- Reynolds SA, & Ebben MR (2017). The Cost of Insomnia and the Benefit of Increased Access to Evidence-Based Treatment: Cognitive Behavioral Therapy for Insomnia. Sleep Medicine Clinics, 12(1), 39–46. [DOI] [PubMed] [Google Scholar]
- Salari N, Khazaie H, Hosseinian-Far A, Khaledi-Paveh B, Ghasemi H, Mohammadi M, & Shohaimi S (2020). The effect of acceptance and commitment therapy on insomnia and sleep quality: A systematic review. BMC Neurology, 20(1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, & Sateia M (2008). Clinical guideline for the evaluation and management of chronic insomnia in adults. Journal of Clinical Sleep Medicine, 4(5), 487–504. [PMC free article] [PubMed] [Google Scholar]
- Selim AJ, Fincke G, Ren XS, Lee A, Rogers WH, Miller DR, Skinner KM, Linzer M, & Kazis LE (2004). Comorbidity assessments based on patient report: Results from the Veterans Health Study. Journal of Ambulatory Care Management, 27(281), 295. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan H, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, & Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(suppl 20), 22–33. [PubMed] [Google Scholar]
- Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, & Carden KA (2018a). Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med, 14(7), 1231–1237. 10.5664/jcsm.7230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, & Carden KA (2018b). Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J Clin Sleep Med, 14(7), 1209–1230. 10.5664/jcsm.7228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold E, Greendale G, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, & Kelsey J (2000). SWAN: A Multicenter, Multiethnic, Community-Based Cohort Study of Women and the Menopausal Transition. Sybil L. Crawford [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, & Löwe B (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. [DOI] [PubMed] [Google Scholar]
- StataCorp. (2021). Stata Statistical Software. In (Version 17) StataCorp LLC. [Google Scholar]
- Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, & Bush AJ (2007). Comorbidity of chronic insomnia with medical problems. Sleep, 30(2), 213–218. [DOI] [PubMed] [Google Scholar]
- van de Laar M, Pevernagie D, van Mierlo P, & Overeem S (2015). Psychiatric comorbidity and aspects of cognitive coping negatively predict outcome in cognitive behavioral treatment of psychophysiological insomnia. Behavioral Sleep Medicine, 13(2), 140–156. [DOI] [PubMed] [Google Scholar]
- Walker DA (2004). Cognitive-behavioural thearpy for depression in a person with Alzheimer’s dementia. Behavioural and Cognitive Psychotherapy, 32, 495–500. (In File) [Google Scholar]
- Walker E, & Nowacki AS (2010). Understanding equivalence and noninferiority testing. Journal of General Internal Medicine, 26(2), 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JQ, Appleman ER, Salazar RD, & Ong JC (2015). Cognitive Behavioral Therapy for Insomnia Comorbid With Psychiatric and Medical Conditions: A Meta-analysis. JAMA Intern Med, 175(9), 1461–1472. 10.1001/jamainternmed.2015.3006 [DOI] [PubMed] [Google Scholar]
- Zakiei A, & Khazaie H (2019). The effectiveness of acceptance and commitment therapy on insomnia patients (A single-arm trial plan). Journal of Turkish Sleep Medicine-Turk Uyku Tibbi Dergisi, 6(3), 65–73. [Google Scholar]
- Zakiei A, Khazaie H, Rostampour M, Lemola S, Esmaeili M, Dürsteler K, Brühl AB, Sadeghi-Bahmani D, & Brand S (2021). Acceptance and commitment therapy (ACT) improves sleep quality, experiential avoidance, and emotion regulation in individuals with insomnia—results from a randomized interventional study. Life, 11(2), 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit GK, Weiner J, Damato N, Sillup GP, & McMillan CA (1999). Quality of life in people with insomnia. Sleep, 22(Suppl 2), S379–S385. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


