SUMMARY
Zinc is an essential micronutrient required for all domains of life. Cells maintain zinc homeostasis using a network of transporters, buffers, and transcription factors. Zinc is required for mammalian cell proliferation, and zinc homeostasis is remodeled during the cell cycle, but whether labile zinc changes in naturally cycling cells has not been established. We use genetically encoded fluorescent reporters, long-term time-lapse imaging, and computational tools to track labile zinc over the cell cycle in response to changes in growth media zinc and knockdown of the zinc-regulatory transcription factor MTF-1. Cells experience a pulse of labile zinc in early G1, whose magnitude varies with zinc in growth media. Knockdown of MTF-1 increases labile zinc and the zinc pulse. Our results suggest that cells need a minimum zinc pulse to proliferate and that if labile zinc levels are too high, cells pause proliferation until labile cellular zinc is lowered.
In brief
Rakshit and Holtzen et al. use fluorescent reporters, live cell imaging, and computational tools to show that asynchronously cycling human cells experience an increase in labile zinc in early G1. Perturbation of zinc homeostasis by manipulating the media or knocking down MTF-1 reveals that cells require an optimal zinc pulse to proliferate.
Graphical abstract

INTRODUCTION
Zinc (Zn2+) is the second most abundant transition metal ion in mammals and is an essential micronutrient crucial for growth, cell differentiation, and cell proliferation.1–3 At the molecular level, Zn2+ is an essential cofactor for over 2,500 proteins4 and plays fundamental roles in metabolism,5–7 DNA synthesis,8–10 and transcriptional regulation.11,12 It is now well established that Zn2+ is required for cell proliferation.1,10 Early work by Chesters et al. suggested that Zn2+ is necessary for the G1-to-S transition13 and for DNA synthesis in S-phase.8,14 However, subsequent studies contradicted these conclusions, finding that after cells cross the restriction point in G1, they do not need Zn2+ until the G2/M transition.15 The contradictory findings of these early studies may have been confounded by long-term incubation with high concentrations of Zn2+ chelators, drugs, and cell synchronization, which often activates stress signaling pathways.16,17 More recently, Lo et al. showed that conditions of mild Zn2+ deficiency cause cells to either exit the cell cycle and go quiescent or stall in S-phase.10 Such studies suggest that Zn2+ may be required during multiple phases of the cell cycle, yet an open question is whether labile Zn2+ itself varies over the course of the cell cycle and, if so, how proteins responsible for preserving Zn2+ homeostasis might influence Zn2+ dynamics.
Mammalian cells use an elegant system of highly-coordinated proteins to control cellular uptake, organelle distribution, and elimination of excess Zn2+ across the plasma membrane to maintain intracellular Zn2+ homeostasis.18–21 Transporters such as ZIP (Slc39a1–14) and ZnT (Slc30a1–10) are responsible for the import and export of Zn2+ across organelle and plasma membranes.18,19,21 Metallothioneins (MTs) buffer intracellular Zn2+ and help maintain the labile Zn2+ pool.21,22 Finally, the metal regulatory transcription factor-1 (MTF-1) regulates labile Zn2+ levels by adjusting the expression of the exporters ZnT1 and ZnT2 and the cellular buffer MT.23,24 Multiple studies have suggested that disruption of zinc-regulatory proteins can attenuate proliferation.25–27 For example, downregulation of MT-2A and knockdown of zinc transporters ZnT1, ZnT6, and Zip11 reduce proliferation of MCF-7 breast cancer cells26 and Capan-1 pancreatic cancer cells,27 respectively. Furthermore, studies have shown that the levels and localization of zinc-regulatory proteins, particularly MT, vary in a cell cycle-dependent manner. MT levels are 3- to 5-fold higher in proliferating human Chang hepatocytes (CCl-13).28 In HT-29 cells, MT levels oscillate during the cell cycle, reaching a maximum in late G1 phase and at the G1/S transition.29 Finally, MT was shown to translocate to the nucleus when cells transition from quiescence to G1 and again early in S-phase.29 Combined, these studies suggest that mammalian cells remodel Zn2+ homeostasis to meet different Zn2+ requirements during different phases of the cell cycle.
While mammalian cells contain hundreds of micromolar total Zn2+, most of it is bound to proteins such that the labile pool is on the order of hundreds of picomolar.30–32 To our knowledge, there have only been two studies that have explored whether Zn2+ levels change during the mammalian cell cycle. The first study used X-ray fluorescence microscopy (XRFM) to show that total cellular Zn2+ increases over 3-fold in mitotic cells,33 perhaps to help daughter cells prepare for Zn2+ requirements. XRFM is powerful for revealing a spatial map of total (but not labile) zinc in fixed, frozen, or air-dried cells. In the second study, Li and Maret used the fluorescent reporter FluoZin-3 to measure labile Zn2+ in a population of PC-12 cells in a cuvette after release from serum starvation.34 Their study showed two peaks in Zn2+ at 3 and 12 h after release from synchronization. These peaks map roughly to early G1 and the G1/S transition in PC-12 cells, although an independent measurement of cell cycle was not carried out in this study. These studies represent an important first step in revealing fluctuations in the Zn2+ during the cell cycle. However, there are also limitations to these studies. The XRFM study focused on naturally cycling cells, but the technique is not compatible with real-time measurement in live cells. On the other hand, the FluoZin-3 study measured labile Zn2+ in live cells, but it relied on bulk analysis of serum-starved cells, which masks the heterogeneity inherent in individual cells and disrupts their natural cell cycle tempo.
Here, we overcome limitations associated with previous studies using high-throughput long-term imaging and computational single-cell image analysis to track individual cells in a naturally cycling population. We use a genetically encoded fluorescent Zn2+ sensor to measure labile Zn2+ levels in the cytosol and nucleus of asynchronously cycling cells expressing a fluorescent histone 2B marker to aid in cell tracking and mitosis detection. We identified a labile Zn2+ pulse immediately following mitosis that lasts for 2 h into early G1 phase. The magnitude of the Zn2+ pulse can be modulated by the total Zn2+ in the media and knockdown of the Zn2+-regulatory transcription factor MTF-1. Knockdown of MTF-1 also results in increased cytosolic labile Zn2+ and impaired reestablishment of homeostasis after elevation of Zn2+ in the growth media. Our results show that a Zn2+ pulse that is too low or too high is correlated with impaired proliferation, suggesting that maintaining Zn2+ levels and dynamics is an important part of cell cycle regulation.
RESULTS
Genetically encoded FRET-based sensor ZapCV2 illuminates intracellular Zn2+ dynamics
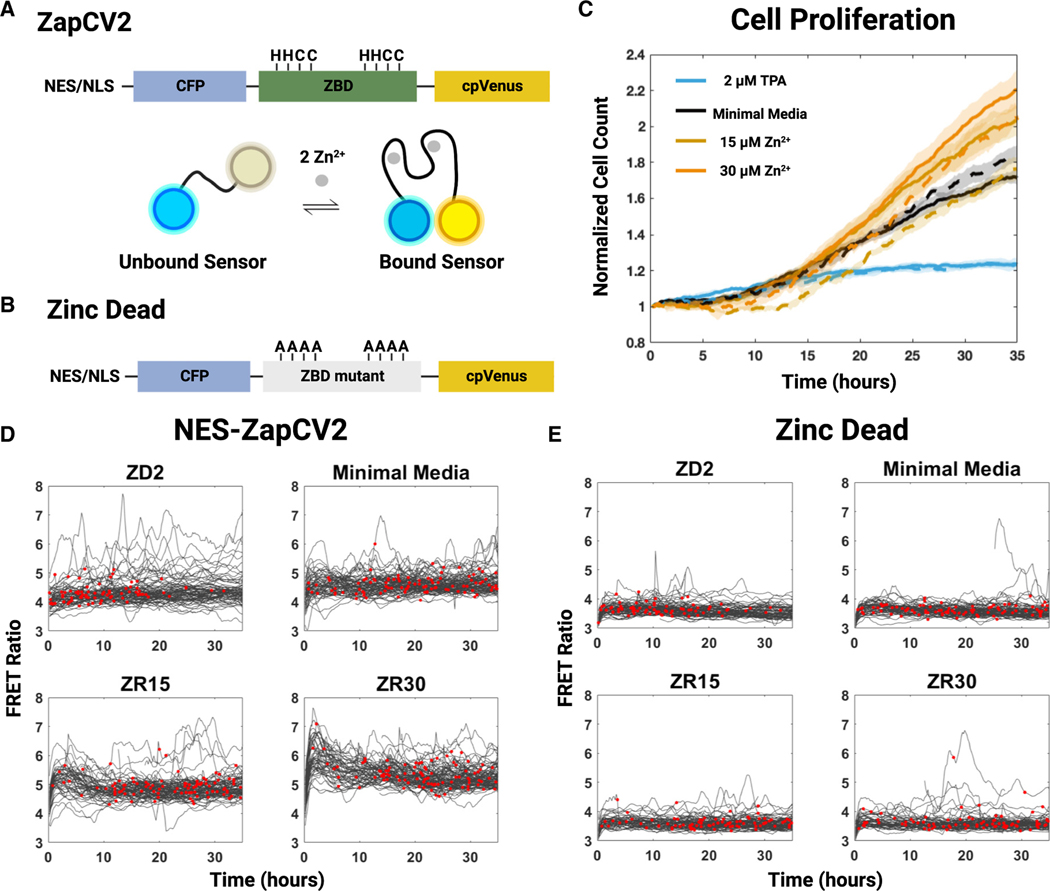
Previous work has established zinc as essential for cell proliferation and that withdrawal of zinc ions (Zn2+) induces quiescence in MCF10a cells.10 We sought to recapitulate these observations in naturally cycling MCF10a cells expressing the genetically encoded cytosolic zinc sensor ZapCV210,35 to examine whether there are changes in the labile zinc pool over the cell cycle. The ZapCV2 sensor is composed of a zinc-binding domain sandwiched between enhanced cyan fluorescent protein (ECFP) and a circularly permuted (cp) Venus (Figure 1A). The sensor can be targeted to the cytosol with a nuclear exclusion signal (NES-ZapCV2) or the nucleus with a nuclear localization signal (NLS-ZapCV2). Zn2+ binding induces a conformational change, increasing the Fö rster resonance energy transfer (FRET) from ECFP to cpVenus. The FRET/CFP ratio is proportional to the labile Zn2+ concentration in the compartment to which the sensor is targeted. We also developed a mutant ZapCV2 sensor in which all cysteine and histidine residues in the zinc-binding domain were replaced with alanine, abolishing Zn2+ binding (Figure 1B). This “zinc-dead” sensor does not respond to perturbations of the labile Zn2+ pool (Figure S1).
Figure 1. Zn2+ levels influence cell proliferation over time.
(A and B) Diagram of the ZapCV2 zinc-binding and zinc-dead sensors. Zinc-binding residues and alanine mutants are labeled. Illustration of sensor binding to zinc. Zinc binding induces a conformational change, which leads to increased FRET.
(C) Proliferation of cells expressing the cytosolic zinc sensor ZapCV2 (solid line) and zinc-dead sensor (dashed line) in different medium conditions over 35 h. Each line represents the average of four wells normalized to initial plating density. Shaded areas represent standard deviation.
(D and E) Single-cell FRET ratios of MCF10a cells expressing either NES-ZapCV2 or zinc-dead sensor. Red dots indicate a mitosis event. Each plotted trace must contain at least one mitosis event. 100 random traces across four wells are plotted in each condition. Zinc conditions: ZD3 (MM + 3 μM TPA), ZD2 (MM + 2 μM TPA), MM (minimal media), ZR15 (MM + 15 μM ZnCl2), ZR30 (MM + 30 μM ZnCl2), ZR50 (MM + 50 μM ZnCl2).
First, we sought to identify the global effects of Zn2+ availability on proliferation. We treated cells with media containing several different concentrations of Zn2+ and monitored proliferation over multiple days. Our minimal media (MM) contains 1.46 μM total Zn2+, which is enough to sustain proliferation.10 We then added 15 or 30 μM ZnCl2 for a zinc-rich (ZR) condition (ZR15, ZR30) or 2 μM tris(2-pyridylmethyl) amine (TPA) for a zinc-deficient (ZD2) condition. We have previously shown that the ZD2 condition lowers labile zinc to ~1 pM and that the ZR30 condition increases the labile zinc to 210 pM.10 We imaged asynchronously cycling cells in each condition for multiple days. These time-lapse images were segmented and tracked via the H2B-mCherry nuclear marker using an automated live cell tracking pipeline EllipTrack.36 After data extraction, we counted the number of segmented ellipses in each frame and normalized this count to the first frame. We observed that the ZD2 condition showed reduced proliferation when compared with MM (Figure 1C), consistent with previous observations of zinc deficiency impairing proliferation.10 To differentiate loss of proliferative ability from loss of tracking due to cell death, we plotted mitosis events and the FRET ratio of all conditions. Mitosis events dramatically decreased after 15 h in ZD2 media without significant loss of single-cell tracks, confirming that this observation is indeed due to induction of quiescence and not cell death (Figures 1D and 1E). The loss of proliferation is not due to off-target effects of TPA since the addition of equimolar ZnCl2/ TPA did not inhibit proliferation (Figure S2). The high-zinc conditions (ZR15, ZR30) showed enhanced proliferation and an increased FRET ratio, indicating increased labile cytosolic Zn2+. Finally, cells expressing the zinc-dead sensor showed a lower FRET ratio that did not change with Zn2+ in the media, and these cells showed a similar overall pattern of proliferation (Figures 1C and 1E). These data confirm that Zn2+ levels influence both cell proliferation and labile cytosolic Zn2+ and that sensor expression does not perturb normal cellular proliferation.
There is a Zn2+ pulse after mitosis that correlates with both nutritional and labile Zn2+
The single-cell FRET traces in Figure 1D suggest that the labile Zn2+ pool may fluctuate over the cell cycle. However, since the population is asynchronously cycling, it is difficult to evaluate changes in Zn2+ at specific cell cycle phases. In order to extract salient information about Zn2+ dynamics during the cell cycle, we aligned single-cell traces in silico to mitosis events detected by the cell tracking software. In silico alignment revealed that the FRET ratio, and hence the average labile Zn2+, is correlated with the amount of Zn2+ in the growth media (ZD2 has the lowest FRET ratio, ZR30 has the highest resting FRET ratio) (Figure 2A). The zinc-dead sensor did not show the commensurate FRET ratio increase with increased media Zn2+ (Figure 2B).
Figure 2. Tracking of labile zinc over the cell cycle reveals zinc dynamics around mitosis that is modulated by nutritional zinc.
(A and B) Single-cell traces of cells expressing either NES-ZapCV2 or the NES-zinc-dead sensor after alignment to mitosis are represented in gray (n = 100 randomly selected cells per plot). Average FRET ratios of these traces are overlaid in color.
(C) Averaged FRET ratio of NES-ZapCV2 and H2B-mCherry intensity in a 10 h window around mitosis. Resting FRET is calculated by averaging ten frames (4 h–2 h) approaching mitosis. Peak FRET is the maximum value within ten frames (2 h) of mitosis.
(D) Plot as shown in (C) with cells expressing the NES-zinc-dead sensor.
(E and F) Values of resting FRET (R) and peak FRET (P) in both zinc-binding and zinc-dead sensors. Mean values are shown as black bars. Each dot represents and individual cell track (n > 100 for each condition, one of three biological replicates is shown). Quantification of FRET change (ΔFRET) between resting and peak is shown below each group.
Close analysis of Zn2+ dynamics around mitosis reveals a peak in FRET ratio that persists for 1.5–2 h into G1 and occurs immediately following the spike in H2B-mCherry that denotes a mitosis event (Figure 2C). There is a slight FRET change in the zinc-dead sensor, but this does not correlate with the amount of Zn2+ in the media (Figure 2D). To define the change in FRET ratio at the peak of the pulse, we calculated the average FRET ratio of the track 2 h approaching mitosis, referred to as the “resting FRET ratio (R),” and the maximum value within the first 2 h of mitosis, referred to as the “peak FRET ratio (P)” (Figure 2C). The peak FRET ratio was positively correlated with both the labile Zn2+ pool and nutritional Zn2+ (Figure 2E). Additionally, the difference between the peak and resting FRET ratios increased with higher Zn2+. In contrast, the zinc-dead sensor did not show an increase in resting or peak FRET ratio in differential Zn2+ conditions (Figure 2F). Together, these results indicate that nutritional Zn2+ influences labile Zn2+ levels and the magnitude of the Zn2+ pulse after mitosis.
We also examined the labile Zn2+ pool in the nucleus with a nuclear localized zinc sensor (NLS-ZapCV2). Figure S2 shows fluorescence images of NES- and NLS-ZapCV2, along with H2B-mCherry. Figure S2 also shows that nuclear Zn2+ undergoes a transient increase immediately following mitosis, and the magnitude of the pulse correlates with labile Zn2+ and the amount of Zn2+ in the media. The nuclear localized zinc-dead sensor did not show the same rise in FRET ratio, confirming that the FRET ratio increase corresponds to an increase in labile nuclear Zn2+. We also observed that the Zn2+ pulse peaked earlier when the concentration of labile Zn2+ and Zn2+ in the media increased (Figures S3A and S3C). In contrast, the cytosolic and nuclear zinc-dead sensor did not show a peak shift with increased Zn2+, indicating that this shift is due to Zn2+ dynamics after mitosis and not volumetric changes during mitosis.
MTF-1 knockdown results in higher levels of labile Zn2+
After identifying a pulse of labile Zn2+ early in G1, we set out to determine whether proteins involved in zinc homeostasis could alter the labile Zn2+ pool and Zn2+ dynamics. We speculated that the metal regulatory transcription factor MTF-1 could play an active role in changing Zn2+ dynamics over the course of the cell cycle. MTF-1 is a Zn2+-binding transcription factor that binds to metal-responsive elements in the genome to promote transcription of target genes such as MTs and ZnT1 that are responsible for sequestering and exporting excess Zn2+ to maintain zinc homeostasis, respectively.23,24 Knockdown of MTF-1 would be expected to alter the buffering capacity of cells in elevated Zn2+ by interfering with the induction of MT expression.24
To determine how knockdown of MTF-1 affected cell proliferation and the labile Zn2+ pool, we developed stable cell lines using two lentiviral constructs encoding short hairpin RNAs (shRNAs), one against mtf1 to knock down the endogenous protein (MTF-1 KD) and another with non-specific scrambled shRNA as control (Scr Ctrl). Western blot analysis of MTF-1 expression in these cell lines showed a significant decrease in MTF-1 in KD cells compared with Scr Ctrl and wild-type (WT) cells (Figure 3A; MTF-1/α-tubulin protein level: 1 [WT], 0.96 [Scr Ctrl], and 0.55 [MTF-1 KD]; *p < 0.05 for Scr Ctrl vs. KD). As expected, downstream expression of MTs is not induced in KD cells upon treatment with 40 μM ZnCl2 for 48 h, whereas Scr Ctrl cells successfully induce MTs in high Zn2+ (Figure 3A). We also noticed that MTF-1 KD decreased basal levels of MT, even in the absence of excess Zn2+, as has been previously reported.24 Next, we performed a cell proliferation assay on KD and Scr Ctrl cells with ZD and ZR media for 48 h. The same number of cells were plated on day 0, and the number of viable cells was determined after 48 h with CellTiter-Glo. This assay revealed that the number of viable cells in both cell lines was less in ZD media compared with MM media, consistent with previous work10; however, there was no difference between the two cell lines. On the other hand, there were significantly less MTF-1 KD cells compared with Scr Ctrl in elevated Zn2+ (ZR30, *p = 0.011; ZR50 ****p < 0.0001; Figure 3B). This result was confirmed by fluorescence image analysis from long-term time-lapse images of cells grown for 24 vs. 48 h in differential zinc media (Figure 3C). Taken together, these results demonstrate that KD of MTF-1 lowers basal levels of MT and renders cells unable to induce MT expression in elevated zinc. Further, MTF-1 KD compromises cellular proliferation in high Zn2+.
Figure 3. MTF-1 knockdown results in less cell proliferation under high nutritional Zn2+ condition.
(A) Western blots for MTF-1 and MT in MCF10a wild-type (WT), scrambled control (Scr Ctrl), and MTF-1 knockdown (KD) cells show lower MTF-1 expression in KD cells compared with WT and Scr Ctrl cells. MT levels are higher in Scr Ctrl cells than MTF-1 KD cells upon treatment with 40 μM Zn2+ for 48 h compared with the no zinc condition. Duplicates were run per condition. Blots for WT and Scr Ctrl cells were run on a single gel, whereas blot for MTF-1 KD cells was run on a separate gel.
(B) CellTiter-Glo proliferation assay was performed on Scr Ctrl and MTF-1 KD cells grown for 48 h in different medium conditions. Cells were plated at a similar density in four biological replicates (n = 4) and technical triplicates. Intensity data were normalized to intensity of Scr Ctrl cells in MM condition. Each dot represents the mean of four technical replicates, and plot shows mean ± SEM of all biological replicates. Statistical analyses was performed using two-way ANOVA with Tukey-Kramer test (*p < 0.05, ****p < 0.0001).
(C) Fluorescence images of Scr Ctrl and MTF-1 KD cells grown in MM, ZR30, and ZR50 media for 24 and 48 h shows lower proliferation in KD cells with ZR30 and ZR50 compared with Scr Ctrl cells. Scale bar: 100 μm.
To determine whether KD of MTF-1 alters the intracellular cytosolic labile Zn2+ pool, we used the NES-ZapCV2 sensor and in situ calibrations to quantify the concentration of labile Zn2+ in the cytosol. Briefly, Scr Ctrl and MTF-1 KD cells were grown in MM and ZR50 media for 24 h, followed by an in situ calibration and conversion of FRET ratios to Zn2+ concentrations, as described in the STAR Methods. MTF-1 KD significantly increases the labile Zn2+ pool (Figure 4). For Scr Ctrl cells grown in MM, the median cytosolic [Znrest] was 0.29 nM, and 50% of the values were between 0.17 and 0.45 nM (quartile 1 [Q1] and Q3, respectively; Figures 4A and 4C). We noted that there was a broad, non-normal distribution of [Znrest] values from individual cells, so we report the median and the Q1–Q3 quartile range and use a non-parametric unpaired two-tailed Mann Whitney test as a statistical test. The value of 0.29 nM agrees with prior studies of cytosolic [Znrest] in mammalian cells.10,30,34,37 In comparison, MTF-1 KD cells grown in MM exhibited a significantly higher median [Znrest] of 9.67 nM and Q1–Q3 of 6.31–17.53 nM, demonstrating that MTF-1 plays an important role in maintaining resting Zn2+ levels in cells.
Figure 4. MTF-1 KD cells have higher resting zinc than Scr Ctrl cells.
FRET calibration experiments on MCF10a Scr Ctrl and MTF-1 KD cells stably expressing H2B-HaloTag and NES-ZapCV2 FRET sensor were carried out to quantify the labile zinc pool.
(A and B) Scatterplots showing a direct comparison of cytosolic resting Zn2+ [] in Scr Ctrl and MTF-1 KD cells grown for 24 h in MM and ZR50 media, respectively. Fractional saturation (FS) and dynamic range (DR) of the sensor were calculated, and [Zn2+] was quantified from each experiment as described in the STAR Methods section (Figures S1 and S4). Each dot on the scatterplot represents [] of a single cell plotted on a log scale. Only values within FS: 0–1 and DR: 1.35–1.65 ranges were considered for calculating []. For statistical analysis, cells were pooled from two biological replicates for Scr Ctrl cells in MM (131 cells) and ZR50 (200 cells) and for MTF-1 KD cells in MM (193 cells) and ZR50 (82 cells). Statistical analyses were performed using non-parametric unpaired two-tailed Mann Whitney test (with 99% confidence level) (n = 3 biological replicates, ****p ≤ 0.0001).
(C and D) Cumulative fractional distribution plots of cytosolic [] for Scr Ctrl cells grown in MM and ZR50 and for MTF-1 KD cells grown in MM and ZR50 conditions, respectively. Values along the x axis are truncated for better display of distribution of data points.
When cells were grown in elevated Zn2+ (ZR50), labile Zn2+ increased. As shown in Figures 4B and 4C, MTF-1 KD cells had significantly higher [Znrest] compared with Scr Ctrl cells. The corresponding median cytosolic Zn2+ in Scr Ctrl was 9.62 nM compared with 48.5 nM for MTF-1 KD. The Q1–Q3 ranges were 4.50–21.13 and 12.09–284.96 nM, respectively, for Scr Ctrl and MTF-1 KD. We note that the fractional saturation values for ZapCV2 in Scr Ctrl cells increased from 0.25 ± 0.08 (in MM) to 0.70 ± 0.09 (in ZR50), as depicted in Figure S4. On the other hand, MTF-1 KD cells increased from 0.64 ± 0.11 in MM to 0.77 ± 0.15 in ZR50. In the ZR50 condition, the sensor is close to the saturation limit, and hence labile Zn2+ may be underestimated. These results demonstrate that MTF-1 is critical for allowing cells to regulate cytosolic Zn2+ levels in response to increasing Zn2+ in the media.
Depletion of MTF-1 changes how cells respond to Zn2+ perturbation
Given that MTF-1 KD elevated cytosolic Zn2+, we wanted to define how MTF-1 KD affected the ability of cells to respond to Zn2+ perturbation. Cells were grown in MM for 24 h, followed by the addition of ZnCl2 or TPA. Addition of 2 or 3 μM TPA (ZD2 and ZD3, respectively) led to a rapid decrease in the FRET ratio of NES-ZapCV2, indicating a decrease in labile Zn2+. There were no significant differences between the MTF-1 KD cells or the Scr Ctrl in the ZD conditions. This result was expected since MTF-1 generally regulates the cellular response to high Zn2+. When Scr Ctrl cells were exposed to elevated Zn2+ (ZR15, ZR30, ZR50), there was a rise in labile Zn2+ lasting approximately 2 h, followed by a decrease, where Zn2+ approaches the initial resting level before the media change. (Figures 5A and S5). Depletion of MTF-1 impairs the cell’s ability to reestablish homeostasis after Zn2+ elevation (Figures 5B and S5).
Figure 5. Depletion of MTF-1 changes labile zinc dynamics after perturbation of available media zinc.
(A and B) Mean traces of FRET ratio before and after zinc perturbation in MTF-1 KD or Scr Ctrl cells. Cells were grown in MM, then changed to different zinc media (indicated by vertical dotted line). One representative biological replicate is shown, and the remaining biological replicates are shown in Figure S5.
(C) Parameters used to identify changes in free zinc dynamics after perturbation. Maximum FRET, ending FRET, and are calculated from 20 subsampled mean FRET traces.
(D) Maximum FRET after media change between different zinc levels and MTF-1 status.
(E) Time to FRET maximum after media change.
(F) Time for FRET ratio trace to decay to half the maximum value after media change ().
(G) Ending FRET ratio after media change. Ending FRET is calculated by taking the average of the final 30 time points of the mean FRET traces.
(H) Difference in cytosolic zinc at end of timelapse. Difference calculated by subtracting the cytosolic zinc at the end of the timelapse from the cytosolic zinc level before the media change. Comparisons conducted with one-way ANOVA, with follow-up t tests, which are plotted. *p < 0.05, ****p < 0.0001. NS indicates p >0.05. Figure created with BioRender. Cells in this experiment were expressing NES-ZapCV2, H2B-HaloTag, and either a Scr shRNA or an MTF-1 KD shRNA. Cells were plated with similar density in two biological replicates (n = 2) across five wells in each replicate.
To quantify aberrations in how cells handle high Zn2+ upon depletion of MTF-1, we took subsamples of 100–300 single-cell FRET ratio traces and averaged these FRET ratio traces to get a subsampled mean FRET trace (Figure 5C). From this trace, we extracted several parameters to describe the response curves. These included the maximum FRET ratio after the media change (FRET max), time at which this maximum is reached (tmax), time for the FRET ratio curve to decay to half of the maximum value (t1/2), ending FRET ratio at the end of the timelapse, and the change in the concentration of Zn2+ (compared with baseline) at 24 h. This process was repeated 20 times for different randomly subsampled mean FRET ratio traces.
We identified several interesting patterns in Zn2+ dynamics after Zn2+ perturbation. We found that cells deficient in MTF-1 reached a higher maximum FRET ratio after media Zn2+ increase (Figure 5D), likely due to decreased buffering capacity and decreased ability to lower cytosolic Zn2+ via ZnT1. Generally, the time to the FRET ratio maximum was not significantly different between MTF-1 KD and Scr Ctrl cells (Figure 5E) except for the ZR50 condition, where it took longer to reach FRET max (6 vs. 2 h). The decay of the FRET signal after media change took significantly longer in MTF-1 KD cells in high-Zn2+ conditions (Figure 5F). As a consequence, 24 h after media change, the MTF-1 KD cells displayed a higher FRET ratio and hence a higher Zn2+ concentration than prior to perturbation (Figures 5G and 5H). Combined, these results indicate that MTF-1 is necessary for cells to efficiently restore homeostasis after elevation of Zn2+.
MTF-1 KD compromises proliferation in high Zn2+
Given that MTF-1 plays an important role in regulating the cellular response to Zn2+ elevation, we set out to determine whether MTF-1 KD affected Zn2+ dynamics during the cell cycle. We carried out long-term imaging on Scr Ctrl and MTF-1 KD cells treated with MM and low-zinc and high-zinc media and tracked asynchronously cycling cells for multiple days. As shown in Figure 6, the Scr Ctrl and MTF-1 KD cells proliferated similarly in the MM condition, similar to our bulk results in Figure 3B. The Scr Ctrl cells showed lower proliferation in ZD2 and ZD3 and higher proliferation in ZR15 and ZR30 (Figure 6A and 6C), similar to the WT MCF10a cells in Figure 1C and previous studies.10 Finally, Scr Ctrl cells showed decreased proliferation in the ZR50 condition, suggesting that if Zn2+ levels are too high, then proliferation is impaired (Figure 6A). MTF-1 KD cells displayed a dose-dependent decrease in proliferation with increasing zinc in the media (Figure 6B), indicating that MTF-1 is essential for maintaining a balance of Zn2+ that supports proliferation. When we plotted the single-cell FRET tracks as a function of time and followed mitosis events, we observed several trends. As expected, mitosis events stopped within 15 h for cells grown in ZD2 and ZD3. In addition, we found that mitoses are generally distributed throughout the imaging window in both KD and Scr Ctrl cells in MM and ZR conditions (Figures 6C, 6D, and S6). The one exception is that, for MTF-1 KD cells in ZR50, there are very few mitosis events within the first 10 h of the timelapse. Overall, the number of mitosis events in MTF-1 KD cells is lower in all zinc conditions than Scr Ctrl cells, further indicating that mis-regulated Zn2+ homeostasis in MTF-1 KD cells results in perturbed cell proliferation. Finally, the number of mitosis events is decreased for Scr Ctrl ZR50 and all MTF-1KD ZR conditions, indicating that if Zn2+ levels get too high, then cells are unable to undergo mitosis.
Figure 6. MTF-1 KD compromises cell proliferation in high Zn2+.
(A and B) Proliferation of MCF10a Scr Ctrl and MTF-1 KD cells expressing NES-ZapCV2 and H2B-HaloTag in different zinc conditions over 48 h. Each line represents the average of four wells normalized to initial plating density. Shaded areas represent standard deviation.
(C and D) Histograms show the number of mitosis events of MCF10a Scr Ctrl and MTF-1 KD cells expressing NES-ZapCV2 over time. Scr Ctrl or MTF-1 KD cells were plated with similar cell density in two biological replicates (n = 2) across five wells in each replicate.
MTF-1 KD alters the Zn2+ pulse following mitosis
Aligning the single-cell FRET tracks to mitosis permits more detailed analysis of how MTF-1 KD affects the Zn2+ pulse at mitosis (Figure S6). All conditions show a zinc pulse immediately following mitosis, and the magnitude of the pulse correlates with the amount of Zn2+ in the media and in the cytosol. We categorized the mitosis events into early, mid, and late depending on their occurrence with respect to media change (at time t = 0). Because it takes more than 20 h for MTF-1 KD cells to establish a steady-state level of Zn2+ after perturbation, we focused on late mitosis events to compare the Zn2+ pulse between Scr Ctrl and MTF-1 KD (Figure S6). As expected, the magnitude of the FRET ratio peak increases with increasing Zn2+ in the media and in the cytosol. In elevated Zn2+, MTF-1 KD cells reach a significantly higher FRET ratio during the Zn2+ pulse compared with Scr Ctrl cells (Figures 7A and 7B). To compare the magnitude of the Zn2+ pulse, we converted the FRET ratio to Zn2+ concentration, revealing that in MM, in Scr Ctrl cells, there is an 83 increase in Zn2+ (197–1628 pM), while in MM, in MTF-1 KD cells, there is a 33 increase in Zn2+ (448–1,398 pM) (Table S2). In ZR conditions, the concentration of Zn2+ at the peak of the pulse reaches nM levels (39 nM for Scr Ctrl in ZR15 up to a high of 88 nM for MTF-1 KD in ZR50). Overall, MTF-1 KD cells show a higher concentration of Zn2+ at rest and a higher concentration of Zn2+ at the peak of the pulse (Figures 7C and 7D; Table S2). The peak FRET after mitosis shifts earlier as Zn2+ increases (Figure 7A, 7B, and S3E).
Figure 7. MTF-1 KD increases the magnitude of the Zn2+ pulse and alters Zn2+ dynamics following mitosis.
(A and B) Average FRET ratios of all traces aligned to mitosis showing late mitosis events (from the last 120–200 frames into the time series). Horizontal dashed line refers to the resting FRET ratio before mitosis, and vertical dashed line denotes alignment to mitosis (t = 0).
(C and D) Corresponding conversion of average FRET ratio to [Zn2+] from all the late mitosis events considering a mean DR of 1.55 for ZapCV2 sensor expressed in Scr Ctrl and MTF-1 KD cells. The dashed line denotes the resting to [Zn2+] level (n = 2, two biological replicates across five wells in each replicate).
High Zn2+ does not induce apoptosis
High Zn2+ has been shown to induce apoptosis,38 so it is possible that reduced cell numbers in high Zn2+ result from cell death as opposed to reduced proliferation. To evaluate this possibility, we performed an Annexin-V apoptosis assay. We observed that upon treatment with 6 μM camptothecin, a DNA-damaging drug, for 12 h, ~50% of cells were apoptotic (combined early and late apoptosis). However, the majority of Scr Ctrl and KD cells grown in MM, ZR15, and ZR30 for 48 h were non-apoptotic (~2%; Figure S7). Only 5.3% ± 2.5% MTF-1 KD cells were apoptotic in ZR50 media at 48 h, indicating that while apoptosis is slightly increased in ZR50 media in MTF-1 KD cells, the amount of apoptosis is not sufficient to explain the decreased proliferation of cells under this condition.
Cells emerging from serum starvation show different dynamics of labile Zn2+ compared with normally cycling cells
There are many reports suggesting that cell synchronization techniques such as serum starvation or contact inhibition induce stress in cells, altering their ability to proliferate like asynchronously cycling cells.16,17,39 For example, in one study, contact inhibition and serum starvation increase replication stress.39 We subjected cells expressing H2B-HaloTag, NES-ZapCV2, and DHB-mCherry, a sensor of CDK2 activity and commonly used tool to track cell cycle progression,40 to serum starvation to test whether the dynamics of labile Zn2+ are the same for naturally cycling cells and cells reentering the cell cycle following serum starvation (Figure S8A). After serum starvation for 48 h, we found that cells were not synchronized in G0 but did exist in a mixed population (Figure S8B). To extract populations of tracks that progressed similarly over time, we clustered the populations using k-medoid clustering, with dynamic time warping as the similarity function. We identified four clusters of cell behavior after serum starvation (Figure S8C). Serum starvation induced quiescence, as defined by low CDK2 activity, in only 30% of cells (Figures S8B and S8C). After serum resupply, the mitosis events of this population were far less synchronized, indicating that although these cells were ostensibly paused in G0, they did not reenter the cell cycle synchronously. 30% of cells had persistently high CDK2 activity. This population of cells was the most synchronized and divided robustly at 10 and again at 25 h after resupply. Using the FRET traces from this population, we detected an increase in labile Zn2+ following the synchronized mitosis events even though the pulse was spread due to temporal noise in the mitoses. After aligning FRET traces to the first and second waves of mitoses, we were able to resolve the Zn2+ pulse in early G1 (Figure S8D). Labile Zn2+ elevation was difficult to discern in all other clusters of cells, but the pulse was visible when the mitosis events were aligned (Figure S8E). Together, these data demonstrate that it is difficult to resolve Zn2+ dynamics in cells emerging from serum starvation due to the lack of synchronized reentry into the cell cycle. Therefore, live cell imaging is preferable to synchronization when studying cell cycle dynamics, and orthogonal confirmation of cell cycle status is imperative to define cell cycle state.
DISCUSSION
Successful completion of the eukaryotic cell cycle involves carefully coordinated temporal changes in major signaling modulators such as proteins, transcription factors, and even labile metal ions.41–44 Based on our studies of Zn2+ and cell proliferation,10 we have demonstrated that Zn2+ is one of the many key players necessary for successful completion of cell division. In mammalian cells, cytosolic labile Zn2+ is in the picomolar range,30–32 while human serum contains 12–15 μM Zn2+.45 However, the cytosolic Zn2+ pool is not static but can change in response to different stimuli (e.g., activation of mast cells by immunoglobulin G [IgG] stimulation46), signaling events (e.g., Ca2+ dynamics32,47–49), and environmental perturbations (e.g., changes in the Zn2+ concentration in extracellular media37). In this article, we set out to determine whether labile Zn2+ changes over the course of the cell cycle. Using fluorescent reporters and long-term live cell imaging, coupled with quantitative computational analysis, we measured both cytosolic and nuclear Zn2+ in a heterogeneous asynchronously cycling population for the first time. We report a Zn2+ pulse immediately following mitosis that lasts for 2 h into early G1 phase; cytosolic Zn2+ rises from 197 to 1,628 pM at the zinc peak in MM. The magnitude of the Zn2+ pulse is correlated with the amount of labile Zn2+, which in turn correlates with the total amount of Zn2+ in media. In moderate Zn2+-deficient conditions, Zn2+ rises from 1 to 3 pM, and proliferation is compromised.
There are two previous studies that specifically measured Zn2+ during the cell cycle. The first was an XFRM study on fixed cells that revealed that total zinc increases 3-fold in mitotic cells.33 The second was a direct measurement of labile Zn2+ using the FluoZin-3 AM fluorescent probe, which showed broad increases in Zn2+ in a bulk population of cells at 3 and 12 h after release from serum starvation.34 These time frames were inferred to correlate with early G1 and the G1/S transition. To compare our results to this previous work, we examined Zn2+ dynamics when serum was resupplied to cells that had been starved of serum for 48 h. Using a CDK2 cell cycle reporter,40 our results that reveal serum starvation does not lead to synchronization of the population in G0, an observation that has been seen in previously for PC12 cells.50 When the dynamics of individual cell tracks as a function of time were clustered, we observed four populations. The two most prevalent populations of cells (accounting for 67% of the population) show broad peaks in zinc dynamics, similar to what was observed in the study by Li and Maret. However, due to temporal noise and the incomplete induction of quiescence in serum starvation, it is not possible to map Zn2+ dynamics to specific cell cycle phases based solely on time since serum resupply. This suggests the Li and Maret study was flawed in its assignment of the broad Zn2+ peaks to G1 and G1/S. Our results reveal the necessity of single-cell measurements and rigorous assignment of cell cycle phase for identifying how labile Zn2+ changes over the course of the cell cycle.
We also showed that the zinc-regulatory protein MTF-1 affects the magnitude of this Zn2+ pulse. Notably, cells with too low or too high of a Zn2+ pulse show compromised proliferation, suggesting that maintaining Zn2+ levels and dynamics is important for cell cycle regulation. We speculate that cells need to meet or exceed the Zn2+ pulse requirement during mitosis to fulfill daughter cell Zn2+ requirements in early G1 and that cells possess a mechanism of remodeling of labile Zn2+ in daughter cells. In support of this idea, MT and ZnT1 have been previously shown to be necessary in proliferating cells.25–29 Further, MT has been shown to translocate to the nucleus as cells transition from quiescence to G1 transition and again early in S-phase of the cell cycle,29 and the MT concentration increases in late G1 phase and at the G1/S transition.29 However, as discussed below, the Zn2+ pulse still exists when MTF-1 is knocked down, and MT levels are dramatically decreased, so there are likely to be additional Zn2+-regulatory proteins necessary for remodeling Zn2+ during the cell cycle.
MTF-1 is the Zn2+-regulatory transcription factor that mediates the high-Zn2+ response by tuning expression of ZnT1 and MT. KD of MTF-1 decreased resting levels of MT, prevented the induction of MT in elevated Zn2+, and significantly increased resting cytosolic labile Zn2+. Our results are consistent with previous studies and also add new details related to the role of MTF-1 in Zn2+ homeostasis. Previous work has shown that homozygous MTF-1−/− knockout embryos show a 4- to 6-fold reduction of ZnT1 mRNA and undetectable MT mRNA.24 Additionally, steady-state levels of ZnT1 and MT-I mRNAs were significantly reduced in MTF-1 knockout mouse embryonic fibroblasts (MEFs) compared with WT MEFs, and 100 μM Zn2+ did not increase the level of ZnT1 or MT.24 Thus, it is clear that MTF-1 plays an important role not just in modulating MT and ZnT1 expression in response to high Zn2+ but also in regulating basal levels of these proteins. Our work shows that MTF-1 also regulates basal levels of Zn2+. The 33-fold increase in labile Zn2+ in MM conditions could result from decreased buffering capacity due to decreased MT levels, decreased export due to decreased ZnT1 levels, or both. As expected, KD of MTF-1 compromised cells’ ability to respond to elevated Zn2+, indicating that both MT buffering and ZnT1 export are important for allowing cells to restore homeostasis after Zn2+ elevation. It is important to note that our shRNA did not completely knock down MTF-1, and so residual MTF-1 could contribute to zinc regulation. However, other factors such as zinc transporters may become increasingly important when the cellular buffering capacity decreases.
Given the role of MTF-1 in helping cells maintain Zn2+ homeostasis, we examined how MTF-1 KD affected Zn2+ dynamics during the cell cycle. Cells with decreased MTF-1 levels, and hence decreased MT expression, still experienced a Zn2+ pulse after mitosis. The main consequence of MTF-1 KD is increased concentration of Zn2+ at the pulse peak for cells in elevated Zn2+. In MM, Zn2+ increases to just over 1 nM in both Scr Ctrl and MTF-1 KD cells, but in ZR conditions, Zn2+ peaks at 3.9 nM for Scr Ctrl and 9.8 nM for MTF-1 KD (ZR15) and increases up to 46 nM for Scr Ctrl and 89 nM for MTF-1 KD in ZR50. While we do not know the source of Zn2+ that comprises the pulse, we speculate that it is likely being imported from external media given that the magnitude of the pulse tracks with Zn2+ in the media and that Zn2+ cannot be released from a cellular buffer in MTF-1 KD cells given that the buffering capacity is greatly diminished. Our defined media and MTF-1 KD experiments suggest that cells require a minimum Zn2+ pulse to proliferate but also that if Zn2+ levels are too high, cells cannot undergo mitosis. MTF-1 KD cells grown in ZR50 fail to proliferate in the early stages of the timelapse until free zinc drops below a FRET ratio of 6.4, or approximately 4 μM free Zn2+ (Figures 6B, 6D, and S6D). In addition, proliferation is compromised for MTF-1 KD in ZR30, as there are fewer mitosis events in this condition (Figure 6B). Scr Ctrl cells in ZR50 have a Zn2+ pulse of 46 nM and show reduced proliferation (Figure 6). The elevated levels of Zn2+ used in this study do not induce cell death but perhaps induce cells to enter a reversible quiescent state until cells can restore an appropriate labile level. This could explain the lag in mitosis events observed in MTF-1 KD grown in high-zinc conditions.
Using long-term live cell imaging, fluorescent sensors, and computational tools, we identified a transient increase in Zn2+ in early G1, which we have dubbed the “Zn2+ pulse.” This pulse scales with the concentration of zinc in media. Further, we characterized the effects of MTF-1 KD on resting labile Zn2+ and the Zn2+ pulse. KD of this key zinc-regulatory transcription factor increases both the resting labile Zn2+ and the magnitude of the Zn2+ pulse. Cells deficient in MTF-1 respond to high media Zn2+ by pausing cellular proliferation until labile Zn2+ decreases below 4 μM and proliferation can restart. These observations lay the groundwork for further study of zinc’s role in regulating cellular proliferation.
Limitations of the study
One of the biggest limitations of this study is that we do not definitively identify the source of the labile Zn2+ pulse. Our results suggest that it could result from influx since the magnitude of the labile pulse tracks with the amount of total zinc in the media, and it still exists in the absence of the MT buffer. However, additional studies, such as isotope tracer studies, would be required to determine if the Zn2+ pulse is coming from internal sources, such as organelles, the external zinc media, or both. While we identify MTF-1 as an important player in regulating zinc homeostasis and modulating the Zn2+ pulse, there are likely to be other players, notably zinc transporters involved in remodeling zinc homeostasis during the cell cycle. Finally, while our results show that cells require a certain concentration range of labile Zn2+ to undergo mitosis and the Zn2+ pulse is critical for proliferation, we still do not know the mechanism of how Zn2+ regulates cell cycle processes.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Amy Palmer (amy.palmer@colorado.edu).
Materials availability
Plasmids generated in this study have been deposited to Addgene. Reagents used in this study are mentioned in the key resource table.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| Anti-MTF1 (H-300) rabbit polyclonal primary | Santa Cruz Biotechnology | Cat# Sc-48775; RRID:AB_2235184 |
| Anti-MT rabbit (FL-61) polyclonal IgG primary | Santa Cruz Biotechnology | Cat# Sc-11377; RRID:AB_2146825 |
| Anti-α-Tubulin mouse monoclonal primary | Santa Cruz Biotechnology | Cat# Sc-5266; RRID:AB_627308 |
| Goat anti-mouse (H + L) IgG secondary | Novus Biotechnology | Cat# NB7539 |
| Goat anti-Rabbit (H + L) IgG secondary | Novus Biotechnology | Cat# NB7160 |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| Tris(2-pyridylmethyl) amine 98% (TPA) | Sigma-Aldrich | Cat# 723134 |
| Zinc chloride, anhydrous, 99.95% (metals basis) | Aifa Aesar | Cat# 87900 |
| Zinc chloride, 0.1 M solution | Sigma-Aldrich | Cat# 39059 |
| Chelex-100, sodium form | Sigma-Aldrich | Cat# C7901 |
| Nonidet P-40 | Sigma-Aldrich | Cat# 74385 |
| Sodium deoxycholate | Sigma-Aldrich | Cat# D6750 |
| Protease and Phosphatase Inhibitor Cocktail (PPi) | Thermo Scientific | Cat# 78441 |
| (S)-(+)-Camptothecin, ≥90% (HPLC), powder | Sigma-Aldrch | Cat# C9911 |
| Janelia Fluor 669 Halo-Tag dye (JF669) Campus | Laboratory of Luke Lavis, Janelia Research | N/A |
| DMEM/F12, HEPES | Thermo Fisher Scientific | Cat# 11330057 |
| Horse Serum, New Zealand origin | Thermo Scientific | Cat# 16050122 |
| Hydrocortisone | Sigma-Aldrich | Cat# H4001 |
| FiuoroBrite DMEM | Fisher | Cat# A18967–01 |
| Gibco EGF Recombinant Human Protein | Thermo Fisher Scientific | Cat# PHG0313 |
| Ham’s F12 phenol red | Sigma Alridch | Cat# N6658 |
| Cholera Toxin | Sigma-Aldrich | Cat# C8052 |
| Pen/Strep | Gibco | Cat# 15140–122 |
| 0.05% Trypsin-EDTA(1X) | Gibco | Cat# 25300–120 |
| Hoechst 33258 | Sigma-Aldrich | Cat# 861405 |
|
| ||
| Critical commercial assays | ||
|
| ||
| Annexin V Conjugates for apoptosis detection | ThermoFisher | Cat# A23204 |
| CellTiter-Glo® 2.0 Cell Viability Assay | Promega | Cat# G9241 |
| Amersham ECL Prime Western Blotting Detection Reagent | GE Heaithcare | Cat# RPN2232 |
| Pierce™ BCA Protein Assay Kit | Thermo | Cat# S-11791 |
|
| ||
| Experimentai modeis: Cell lines | ||
|
| ||
| Human: MCF10a | ATCC | Cat# CRL-10317; RRID:CVCL_0598 |
| Human: MCF10a + PB-NES ZapCV2 and | Lo. et ai.10; | N/A |
| PB-H2B- mCherry (stable) | PMID: 32014109 | |
| Human: MCF10a + PB-NLS ZapCV2 and PB-H2B- mCherry (stable) | This paper | N/A |
| Human: MCF10a + PB-NES ZapCV2 and PB-H2B-HaloTag + Scrambled mtfl- mCherry (stable) | This paper | N/A |
| Human: MCF10a + PB-NLS Zinc-dead and PB-H2B- mCherry (stable) | This paper | N/A |
| Human: MCF10a + PB-NES Zinc-dead and PB-H2B- mCherry (stable) | This paper | N/A |
| Human: MCF10a + PB-NES ZapCV2 and PB-H2B-HaloTag + shRNA mtfl knockdown-mCherry (stable) | This paper | N/A |
| Human: MCF10a + PB-NES ZapCV2 and PB-H2B-HaloTag + CSII-EF-DHB-mCherry | This paper | N/A |
|
| ||
| Recombinant DNA | ||
|
| ||
| Plasmid: PB-H2B-HaloTag (used for stable cell line generation) | Grimm et al.51; PMID: 28869757 | N/A |
| Plasmid: PB-H2B-mCherry (used for stable cell line generation) | Published in previous Palmer lab paper; PMID: 32014109 | N/A |
| Plasmid: PB-NES-ZapCV2 (used for stable cell line generation) | Published in previous Palmer lab paper; PMID:27959493 | N/A |
| Plasmid: PB-NLS-ZapCV2 (used for stable cell line generation) | This paper | Nuclear FRET sensor in Piggybac vector; NES sequence was replaced by NLS sequence; Submitted to Addgene |
| Plasmid: PB-NES-Zinc-dead (used for stable cell line generation) | This paper | Nuclear Zinc dead sensor in Piggybac vector; Zinc binding sequence is replaced by non-binding domain; Submitted to Addgene |
| Plasmid: PB-NLS-Zinc-dead (used for stable cell line generation) | This paper | Nuclear Zinc dead sensor in Piggybac vector; Zinc binding sequence is replaced by non-binding domain |
| Plasmid: MTF1.34 human shRNA (used for MTF-1 KD mCherry stable cell line generation) | This paper GeneCopoeia | HSH011543–34-LVRU6MP Plasmid containing shRNA targeted to MTF-1 gene; Target sequence: CCAACTCTGTCCTAACTAATA; Submitted to Addgene |
| Plasmid: Used for MTF-1 Scrambled - mCherry stable cell line generation | This paper GeneCopoeia | CSHCTR001-LVRU6MP Plasmid containing non-specific scrambled shRNA; Target sequence: N/A |
| Plasmid: CSII-EF-DHB-mCherry | Laboratory of Sabrina Spencer, CU Boulder | CDK2 kinase translocation Sensor |
|
| ||
| Software and algorithms | ||
|
| ||
| BD FACSDiva Version 8 | BD Biosciences | N/A |
| Adobe Illustrator CS | Adobe | N/A |
| MATLAB 2017a and VR2020b | Mathworks | N/A |
| Prism Version 9.2.0 | GraphPad | N/A |
| Cell segmentation and tracking pipeline | Elliptrack36 | PMID: 32755578 |
| Live-cell analysis pipeline | live-cell-zinc-sensor | https://doi.org/10.5281/zenodo.7968726 |
Data and code availability
The image analysis code is accessible at https://doi.org/10.5281/zenodo.7968726. Time-lapse imaging data used in this current study are not publicly available but are available from the corresponding author on request. Protocols are also available upon request. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Cell culture
MCF10a cells were procured from ATCC and maintained in full growth medium (FGM) which consists of DMEM/F12 medium (FGM) supplemented with 5% horse serum, 1% pen/strep antibiotics, 20 ng/mL EGF, 0.5 μg/mL hydrocortisone, 100 ng/mL cholera toxin, and 10 μg/mL insulin. Cells were passaged with 0.05% trypsin-EDTA solution. To remove excess Zn2+ from horse serum and insulin and to generate the defined minimal medium (MM), we incubated serum and insulin solutions with Chelex-100 for 12 h, followed by sterile filtration to remove Chelex-100 resin. Minimal media (MM) consisted of 50:50 Ham’s F12 phenol red free/FluoroBrite DMEM with 1.5% Chelex 100-treated horse serum, 10 μg/mL Chelex 100-treated insulin, 1% pen/strep antibiotics, 20 ng/mL EGF, 0.5 μg/mL hydrocortisone, and 100 ng/mL cholera toxin. We previously quantified the metal content of MM containing Chelex-100-treated serum to MM containing serum that was not treated with Chelex-100. The ICP-MS results are presented in Lo et al.2 in Figure 1–Supplement 1 and show that Chelex-treatment leads to a reduction in zinc (from 2.18 to 1.46 μM) and nickel (from 0.178 to 0.012 μM), but no significant changes in other metals. ZD media was generated by adding 2 or 3 μM TPA for ZD2 and ZD3, respectively. ZR media was generated by supplementing MM with 15 μM ZnCl2, 30 μM ZnCl2, and 50 μM ZnCl2 developed from a 0.1 M ZnCl2 solution, for ZR15, ZR30, and ZR50, respectively. Serum starvation media contains 50:50 Ham’s F12 phenol red free/FluoroBrite DMEM, 1% pen/strep antibiotics, 20 ng/mL EGF, 0.5 μg/mL hydrocortisone, and 100 ng/mL cholera toxin and 3% bovine serum albumin. For imaging experiments, cells were grown and imaged in MM, ZD, or ZR media, except in the serum starvation experiment, when they were grown and imaged in serum starvation or MM. All cells were grown in a humidified incubator at 37°C and 5% CO2. Cell lines were routinely tested and confirmed to be mycoplasma negative by PCR.
Plasmids and cell lines
We generated MCF10a cell lines expressing PB-H2B-mCherry or PB-H2B-HaloTag and one of the following zinc sensor constructs: PB-NES ZapCV2, PB-NLS ZapCV2, PB-NES ZapCV2 “zinc dead”, or PB-NLS ZapCV2 “zinc dead”. The NLS-ZapCV2 plasmid was generated by replacing NES sequence with NLS sequence (Table S1). The “zinc dead” sensors were created by replacing all cysteines and histidines in the zinc binding domain with alanine in (Table S1). Stable cell lines were generated using the PiggyBac transposase system. Briefly, plasmids were transfected via TransIT-LT1 (Mirus Bio), according to manufacturer’s instructions, using 0.5 mg plasmid of interest and 2 μg Super PiggyBac transposase by electroporation. Cells were selected with G418 (0.4 mg/mL for H2B plasmid) and blasticidin (0.004 mg/mL, for zinc sensor plasmid). MCF10a cells expressing NES-ZapCV2, H2B-HaloTag was virally transfected with CSII-EF lentiviral vector cloned with plasmid containing DHB (aa994–1087)40,50 fused to mCherry. MCF10a cell lines expressing MTF-1 KD shRNA or scrambled control shRNA were generated by viral transduction. Briefly, HEK293T cells were transiently transfected with plasmid containing either MTF1.34 human shRNA (5 μg) or scrambled shRNA (5 μg) and Lenti-X fourth-generation lentiviral packaging plasmids PsPAX2 (4.5μg), and PmD2.G (0.5 μg) in OptiMEM. This was followed by viral amplification in HEK293T cells for 48 h. Successful transfection was verified via mCherry fluorescence marker. Viral particles were harvested from HEK293T cells 48 h after transfection via filtering through 0.45 μm syringe PES (polyethersulfone) filters and the virus-containing media supplemented with polybrene (8 μg/mL) was added to MCF10a cells stably expressing NES-ZapCV2 and H2B-HaloTag and incubated for 48h. Stable cell lines used for imaging were generated by antibiotic selection (puromycin: MTF-1.34 human shRNA, scrambled shRNA). Validation of MTF-1 knockdown was established by Western blot. For cell cycle experiments, stable MTF-1 KD and Scr Ctrl cells were sorted on BD FACSAria Fusion with the following optics: CFP: Ex 445 nm, Em 470/15 nm; YFP: Ex 488 nm, Em 530/30 nm; and mCherry Ex 561 nm, Em 610/20 nm.
METHOD DETAILS
Western blot
MTF-1 and MT expression of MCF10a WT, Scr-Ctrl and MTF1-KD cells in MM and in 40 μM ZnCl2 was assessed via Western blotting. Cells were plated in 10 cm dishes in minimal media (MM) with a seeding density of 2 × 106. Cells were harvested at 75% confluency. For Zn2+ enrichment assay, media was replaced with MM containing 40 μM ZnCl2 after 24h and cells were harvested after 48h of Zn2+ treatment. Proteins from WT, Scr-Ctrl and MTF-1KD cells in MM and ZR conditions were isolated by lysing the cells in RIPA buffer (1% Nonidet P-40, 10% Sodium deoxycholate, 1 M Tris-HCl (pH 8.0), 5M NaCl, 10% SDS) with 1X PPi and quantified with a BCA (bicinchoninic acid) assay. Protein (30 μg) was separated using 4–20% gradient pre-cast SDS polyacrylamide gel (Mini-PROTEIN TGX precast gel, BIO-RAD, catalog no. 456–1095) in 1X Tris/Glycine Buffer (BIO-RAD, catalog no. 1610771) and transferred to a PVDF (polyvinylidene difluoride) membrane (Cytiva, catalog no. 10600023). The membrane was cut into three parts: top part, MTF-1 protein, ~115 kDa; middle part, α-Tubulin, 50 kDa; and bottom part MT proteins,15–38 KDa. Blots were then blocked with 5% milk in TBST (tris-buffered saline with 0.1% Tween 20) solution and treated with primary antibodies (1:500 anti MTF-1, 1:1000 anti-MT, 1:1000 anti-tubulin in 5% milk in TBST) for 1h at room temperature. After washing four times with TBST, the corresponding secondary antibody (goat anti mouse:1:20K in 5% milk in TBST; goat anti-rabbit 1:10K in 1% milk in TBST) was added to respective blots. Blots were exposed to Amersham ECL Prime Western Blotting Detection Reagent (Thermo Scientific) for 3 min to visualize the presence of bound antibodies and imaged on ImageQuant LAS4000 imager (GE Healthcare Life Sciences).
Live cell imaging
For long term live cell imaging, MCF10a WT cells expressing NES/NLS ZapCV2 and zinc dead sensor were counted with a Countess II Automated Cell Counter (Thermo Fisher Scientific, Waltham, MA) and plated at a density of 4000 cells/well for ZD, MM, ZR15, and ZR30 conditions in minimal medium for 24 h before imaging in glass bottom 96-well plates (P96–1.5H-N, Cellvis, Mountain View, CA). For the serum starvation experiment, MCF10a cells expressing NES-ZapCV2, H2B-HaloTag, and DHB-mCherry were plated at a density of 4000 cells/well in serum starvation media and allowed to synchronize for 48h before imaging. Scr Ctrl and MTF-1 KD cells were plated at a density of 3,500 cells/well for MM, ZR15, ZR30 and ZR50 conditions and plated at 4000 cells/well for ZD3, ZD2 condition. After plating the cells, the plate was kept undisturbed in the biosafety cabinet for 45 min to ensure cells settle at the center of wells. This starting density was chosen to avoid significant contact inhibition during the imaging period for ZD and ZR conditions. In all imaging experiments other than the media change experiment and serum starvation experiment, cells were imaged in MM and 2X media of each condition (ZD, MM, ZR) was added immediately prior to imaging. For media change experiment (Figure 5), cells were imaged in MM for 24h first and then 2X respective MM, ZR and ZD medium was added and imaging was continued for another 24 h. Cells expressing Halo-Tag construct were incubated with 5 nM JF669 Halo-Tag dye for 15 min and washed twice before imaging. For serum starvation experiment, cells were imaged in serum starvation media for 4 h, then the media was changed to MM for an additional 44h.
Images were collected using a Nikon Ti-E High Content Analysis inverted microscope with a Lumencor SPECTRA X light engine (Lumencor, Beaverton, OR) and Hamamatsu Orca FLASH-4.0 V2 cMOS camera (Hamamatsu, Japan). Images were collected in time lapse series every 12 min with a 10X air, 0.45 NA Plan Apo objective lens (Nikon Instruments, Melville, NY). During imaging, cells were in a controlled environmental chamber surrounding the microscope (Okolab Cage Incubator, Okolab USA INC, San Bruno, CA) at 37°C, 5% CO2 and 90% humidity. Filter sets (excitation = Ex, emission = Em) used for live-cell imaging were as follows: CFP Ex: 440, 455 dichroic, Em: 480/20, power 50, exposure 500 ms; YFP Ex: 508, 518 dichroic, Em: 540/21; CFP/YFP FRET Ex: 440, 455 dichroic, Em: 540/21, power 50, exposure 500 ms; mCherry Ex: 555, 597 dichroic, Em: 595/40, power 50, exposure 200 ms; and Cy5 Ex: 640, 640 dichroic, Em: 705/22, power 50, exposure 500 ms.
FRET sensor calibration and analysis
In situ calibrations involve measuring the resting FRET ratio (R) in individual cells followed by addition of the cell permeable Zn2+ chelator TPA which removes any Zn2+ bound to the sensor, resulting in the minimum FRET ratio (). Subsequently, the sensor is saturated by addition of excess Zn2+, saponin, and pyrithione to measure the maximum FRET ratio (). Sensor calibrations of MCF10a cells stably expressing PB-NES-ZapCV2 or PB-NES-dead-ZapCV2 were performed using the Nikon Ti-E HCA. Cells were grown for 24 h in either MM, or ZR (50 μM ZnCl2) media. Before starting the experiment, cells were incubated with 10 nM JF669-HaloTag dye for 15 min in phosphate free HEPES-buffered Hanks’ Balanced Salt Solution (HHBSS) buffer and washed thrice. Cells were then equilibrated at room temperature in phosphate free HHBSS buffer for 30 min. For collection of , cells were imaged for CFP-YFP FRET (power 20, 200 ms exposure) and CFP (power 20, 200 ms exposure) every 30 s for several min. To collect , 100 μM TPA in 1mL of phosphate-free HHBSS was added to the cells in 1mL of phosphate-free HHBSS (final concentration 50 μM TPA). Cells were imaged for 5 min at 30 s intervals. Cells were then washed three times with phosphate-, calcium-, and magnesium-free HHBSS pH 7.4 to remove TPA. Finally, cells were treated with 59.6 nM buffered Zn2+ solution, 0.001% saponin, and 750 nM pyrithione in phosphate-free HHBSS for collection of , as previously described.52 All experiments were performed in replicates (n = 3 biological replicates). The average and were calculated by averaging across the time points collected. The maximum FRET ratio achieved after Zn2+ addition was used as . Images were either background corrected by drawing a region of interest in a dark area of the image and subtracting the average fluorescence intensity of the background from the average intensity of each channel or the images were background subtracted and processed via a MATLAB pipeline.
FRET ratios for each cell were calculated with the following equation:
The dynamic range (DR) of the sensor in each condition was calculated as:
| (Equation 1) |
The fractional saturation (FS) of the sensor in each condition was calculated as:
| (Equation 2) |
Finally, Zn2+concentrations were estimated by the following equation:
| (Equation 3) |
where the dissociation constant and n = 0.29 (Hill coefficient) were reported previously.10
Calculation of [Zn2+] during the Zn2+ pulse
To estimate the mean free Zn2+ during the Zn2+ pulse, as well as the lower and upper limits of this estimate, we used Equation 3 where dissociation constant , Hill coefficient (n) = 0.29, minimum FRET ratio () is the FRET ratio of ZD3 treatment, and a dynamic range (DR) = 1.45, 1.55, 1.65 for estimating upper bound, mean, and lower bound of free zinc, respectively (Table S2). These numbers are based on the calibrations of ZapCV2 in Figure 3.
CellTiter-Glo luminescent viability assay
MCF10a Scr-Ctrl and MTF-1 KD cells stably expressing NES-ZapCV2 and H2B-HaloTag were plated at a density of 3500 cells/well in a glass bottom black 96 well plate in minimal media. After 24h, 100 mL media was removed from each well and replaced with 100 μL of 2X concentration of ZD3, ZD2, fresh MM, ZR15, ZR30 and ZR50 media. After 48h of incubation, 100 μL media was again removed and 100 μL of 1:1 solution of CellTiter-Glo cocktail A&B was added. The 96 well plate was shaken on a gel rocker for 30 min for proper mixing and luminescence was recorded in a SpectraMax iD3 plate reader. Background luminescence intensity was recorded from wells with no cells. To quantify the cell viability, the luminescence intensity was background subtracted and the mean luminescence was calculated from triplicate measurements. All results were normalized to the average luminescence intensity from Scr-Ctrl cells grown in minimal media.
Annexin V apoptosis assay
MCF10a WT, Scr-Ctrl and MTF-1 KD cells were plated in minimal media in 6 well plates at a similar density. After 24 h, Scr-Ctrl and MTF-1 KD cells were treated with ZR15, ZR30 or ZR50 and incubated for either 24 h or 48h. MCF10a WT cells were incubated with an apoptosis inducing agent, camptothecin (6 μM, positive control) for 12h. After incubation, the respective medias were transferred to properly labeled tubes which was then followed by PBS(1X) washes and trypsinization for 10 min. After trypsinization, the media was collected and the cell suspension was centrifuged for 5 min at 1000 rpm. Cell pellets from the positive control, MM and ZR conditions were treated with Hoechst 33258 (0.1 μg/mL in PBS) and Annexin V Alexa Fluor 647 apoptosis assay reagents for 30 min. After 30 min of incubation, cells were analyzed on a BD FACS Aria Fusion instrument and data were processed with BD FACSDiva v8 software (BD Biosciences, San Jose, CA). DAPI: Ex 355 nm, Em 470/15 nm, Alexa 647 nm: Ex 637 nm, Em 670/30 nm. Depending on the response with the positive control, a gate was selected which describes the P2 Annexin V positive population. The P2 population defines total apoptotic cells including early apoptotic (Q2) and late apoptotic cells (Q4) which was then used to determine the percentage of apoptotic cells in different conditions.
Image processing
Live-cell imaging experiments were analyzed using the EllipTrack cell tracking pipeline36 in MATLAB 2017a and 2020b on the institutional computing cluster. Briefly, EllipTrack requires several generalized parameters for cell segmentation, tracking and event identification. The advanced parameters were unchanged from the original code accessible on Github. The parameters that were finetuned are listed below.
Training data
Training datasets for the EllipTrack event predictions were made using at least 500 events across each time series for both H2B-Hal-oTag and H2B-mCherry.
Segmentation
We used a nuclear radius of 12 pixels as the average size of one cell. The ellipse that is fitted to this nucleus was required to be at least 25 pixels in area. Images were log transformed and a blob detection algorithm was used to identify nuclei with a blob threshold of −0.075. Objects were separated using watershed algorithm and ellipses were fitted to each object.
Prediction of events
Mitoses were inferred based on morphological properties of each object before and after a potential mitosis event. Migration and migration speed was inferred using a density-dependent migration speed from the training data.
Track linking
All tracks greater than 10 frames were kept, and tracks were allowed to skip at most two frames.
Signal extraction
Intensity information from nuclear localized reporters was extracted using the nuclear mask. The intensity of cytoplasmic sensors was calculated in a three-pixel wide cytosolic ring around the nuclear mask.
Data analysis
Mother and daughter tracks from the output structure of EllipTrack were linked together by at least one mitosis event. Tracks containing CFP, FRET (CFP excitation YFP emission), H2B intensity, and mitosis information were smoothed using a moving mean of a five-frame window, then fed into a custom pipeline. This pipeline is available at https://doi.org/10.5281/zenodo.7968726. The tracks were then processed as laid out below.
Proliferation
EllipTrack approximates nuclei as ellipses and uses these fitted ellipses for cell tracking. Since one MCF10a cell virtually always contains one nucleus, we used the number of ellipses at each frame as a proxy for cell count. To correct for plating density, the cell count of the entire time lapse was divided by the first frame.
FRET calculation
Since ZapCV2 is an FRET-based sensor, we calculated the FRET ratio in the following manner:
Where is the background-corrected intensity in the FRET channel, and is the background-corrected intensity of the donor channel. Tracks with an FRET ratio of greater than 8 or less than 3 at any point in the time-lapse were discarded from further analyses.
Average FRET ratio
To identify long-term changes in labile Zn2+ levels in cells cultured in media containing different amounts of Zn2+, we averaged the FRET ratio of single-cell tracks across the entire time-lapse. Cells were then aligned to mitosis using all mitosis events detected by EllipTrack in each condition. The average FRET ratio of these aligned tracks at each time point was plotted. The resting FRET ratio was calculated by taking the average FRET ratio of frame −20 to frame −10 relative to mitosis to avoid any fluctuations immediately before mitosis. The peak FRET ratio was calculated by taking the maximum FRET ratio of the ten frames after mitosis, including the mitosis frame. Data analysis was done in MATLAB and GraphPad Prism.
Serum starvation analysis
The cytoplasm/nucleus (C/N) ratio for the DHB-mCherry sensor was calculated by taking the background corrected signal in the cytosol and dividing it by the background corrected signal in the nucleus. Tracks had to exist for the full time-lapse in order to be considered for analysis. Tracks also had to have a CDK2 activity of between 0.2 and 2, as was determined by analyzing the dynamic range of this particular construct. Cells finally had to have signal from both the NES-ZapCV2 sensor and the DHB-mCherry sensor. Clustering of DHB-mCherry signals using k-medoids and pairwise dynamic time warping (DTW) was done in MATLAB using standard parameters. The median DHB-mCherry signal and the mean FRET signal were plotted along with the 95% confidence interval. Each track was aligned to either the first and second mitoses and the average FRET ratio was plotted accordingly.
QUANTIFICATION AND STATISTICAL ANALYSIS
All statistical analyses were done in GraphPad prism. Figures were created in either Biorender or Adobe Illustrator. Two-way ANOVA with Tukey-Kramer test was performed in Figure 3. Statistical analyses were performed on the medians using non-parametric unpaired two-tailed Mann Whitney test (with 99% confidence level) in Figure 4. All other statistical analyses were done using a one-way ANOVA followed by multiple t-tests. Multiple hypothesis correction was applied using the Dunn-Šidák method. Significance was determined using an alpha value of 0.05.
For Figures 5D–5F, analyses were conducted using subsampling, similar to bootstrapping. A sampling of 100–300 random tracks from each time lapse was passed through a function that averaged the tracks and extracted maximum FRET, time of maximum FRET, and steady-state FRET for this average track. This was done 20 times to generate 20 subsampled data points per condition.
Supplementary Material
Highlights.
Human cells experience a transient zinc pulse in early G1
The magnitude of the pulse tracks with the amount of zinc in the cytosol and media
Knockdown of MTF-1 decreases metallothionein and increases the zinc pulse
Cells pause proliferation until labile cellular zinc is in the optimum range
ACKNOWLEDGMENTS
We thank the University of Colorado Flow Cytometry Core, which performed cell sorting; the University of Colorado Biochemistry Cell Culture Core (specifically Theresa Nahreini) for assistance with cell culture; and the BioFrontiers Computing Core and BioFrontiers IT for providing High Performance Computing resources. We thank Dr. Luke Lavis (HHMI Janelia) for the JF669-HaloTag dye. We would like to acknowledge NIH Director’s Pioneer Award DP1-GM114863 (A.E.P.), NIGMS MIRA R35 GM139644 (A.E.P.), and Anna and John J. Sie Foundation Postdoctoral fellowship (M.N.L.) for generous financial support.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
INCLUSION AND DIVERSITY
One or more of the authors of this paper self-identifies as a gender minority in their field of research. One or more of the authors of this paper self-identifies as a member of the LGBTQIA+ community.
REFERENCES
- 1.MacDonald RS (2000). The role of zinc in growth and cell proliferation. J. Nutr 130, 1500s–1508s. 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- 2.Beyersmann D, and Haase H. (2001). Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 14, 331–341. 10.1023/a:1012905406548. [DOI] [PubMed] [Google Scholar]
- 3.Kambe T, Tsuji T, Hashimoto A, and Itsumura N. (2015). The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev 95, 749–784. 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 4.Andreini C, Banci L, Bertini I, and Rosato A. (2006). Counting the zinc-proteins encoded in the human genome. J. Proteome Res 5, 196–201. 10.1021/pr050361j. [DOI] [PubMed] [Google Scholar]
- 5.Severo JS, Morais JBS, Beserra JB, dos Santos LR, de Sousa Melo SR, de Sousa GS, de Matos Neto EM, Henriques GS, and do Nascimento Marreiro D. (2020). Role of zinc in zinc-a2-glycoprotein metabolism in obesity: a Review of literature. Biol. Trace Elem. Res 193, 81–88. 10.1007/s12011-019-01702-w. [DOI] [PubMed] [Google Scholar]
- 6.Olechnowicz J, Tinkov A, Skalny A, and Suliburska J. (2018). Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci 68, 19–31. 10.1007/s12576-017-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesters CGC, and Rolinson GN (1950). Role of zinc in metabolism. Nature 165, 851–852. 10.1038/165851b0. [DOI] [PubMed] [Google Scholar]
- 8.Chesters JK, and Boyne R. (1991). Nature of the Zn2+ requirement for DNA synthesis by 3T3 cells. Exp. Cell Res. 192, 631–634. 10.1016/0014-4827(91)90085-9. [DOI] [PubMed] [Google Scholar]
- 9.Rubin H. (1972). Inhibition of DNA synthesis in animal cells by ethylene diamine tetraacetate, and its reversal by zinc. Proc. Natl. Acad. Sci. USA 69, 712–716. 10.1073/pnas.69.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo MN, Damon LJ, Wei Tay J, Jia S, and Palmer AE (2020). Single cell analysis reveals multiple requirements for zinc in the mammalian cell cycle. Elife 9, e51107. 10.7554/eLife.51107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanford L, Carpenter MC, and Palmer AE (2019). Intracellular Zn(2+) transients modulate global gene expression in dissociated rat hippocampal neurons. Sci. Rep 9, 9411. 10.1038/s41598-019-45844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damon LJ, Aaron J, and Palmer AE (2022). Single molecule microscopy to profile the effect of zinc status on transcription factor dynamics. Sci. Rep 12, 17789. 10.1038/s41598-022-22634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chesters JK, Petrie L, and Vint H. (1989). Specificity and timing of the Zn2+ requirement for DNA synthesis by 3T3 cells. Exp. Cell Res. 184, 499–508. 10.1016/0014-4827(89)90347-9. [DOI] [PubMed] [Google Scholar]
- 14.Chesters JK, Petrie L, and Travis AJ (1990). A requirement for Zn2+ for the induction of thymidine kinase but not ornithine decarboxylase in 3T3 cells stimulated from quiescence. Biochem. J 272, 525–527. 10.1042/bj2720525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chesters JK, and Petrie L. (1999). A possible role for cyclins in the zinc requirements during G1 and G2 phases of the cell cycle. J. Nutr. Biochem 10, 279–290. 10.1016/S0955-2863(99)00009-1. [DOI] [PubMed] [Google Scholar]
- 16.Min M, and Spencer SL (2019). Spontaneously slow-cycling subpopulations of human cells originate from activation of stress-response pathways. PLoS Biol. 17, e3000178. 10.1371/journal.pbio.3000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matson JP, and Cook JG (2017). Cell cycle proliferation decisions: the impact of single cell analyses. FEBS J. 284, 362–375. 10.1111/febs.13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kambe T, Taylor KM, and Fu D. (2021). Zinc transporters and their functional integration in mammalian cells. J. Biol. Chem 296, 100320. 10.1016/j.jbc.2021.100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hara T, Takeda TA, Takagishi T, Fukue K, Kambe T, and Fukada T. (2017). Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. J. Physiol. Sci 67, 283–301. 10.1007/s12576-017-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krężel A, and Maret W. (2017). The functions of metamorphic metallothioneins in zinc and copper metabolism. Int. J. Mol. Sci 18, 1237. 10.3390/ijms18061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colvin RA, Holmes WR, Fontaine CP, and Maret W. (2010). Cytosolic zinc buffering and muffling: their role in intracellularzinc homeostasis. Metallomics 2, 306–317. 10.1039/b926662c. [DOI] [PubMed] [Google Scholar]
- 22.Krężel A, and Maret W. (2021). The bioinorganic chemistry of mammalian metallothioneins. Chem. Rev 121, 14594–14648. 10.1021/acs.chemrev.1c00371. [DOI] [PubMed] [Google Scholar]
- 23.Hardyman JEJ, Tyson J, Jackson KA, Aldridge C, Cockell SJ, Wakeling LA, Valentine RA, and Ford D. (2016). Zinc sensing by metal-responsive transcription factor 1 (MTF1) controls metallothionein and ZnT1 expression to buffer the sensitivity of the transcriptome response to zinc. Metallomics 8, 337–343. 10.1039/c5mt00305a. [DOI] [PubMed] [Google Scholar]
- 24.Langmade SJ, Ravindra R, Daniels PJ, and Andrews GK (2000). The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J. Biol. Chem 275, 34803–34809. 10.1074/jbc.m007339200. [DOI] [PubMed] [Google Scholar]
- 25.Ji L, Zhao G, Zhang P, Huo W, Dong P, Watari H, Jia L, Pfeffer LM, Yue J, and Zheng J. (2018). Knockout of MTF1 inhibits the epithelial to mesenchymal transition in ovarian cancer cells. J. Cancer 9, 4578–4585. 10.7150/jca.28040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim D, Jocelyn KMX, Yip GWC, and Bay BH (2009). Silencing the Metallothionein-2A gene inhibits cell cycle progression from G1- to S-phase involving ATM and cdc25A signaling in breast cancer cells. Cancer Lett. 276, 109–117. 10.1016/j.canlet.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 27.Zhu B, Huo R, Zhi Q, Zhan M, Chen X, and Hua ZC (2021). Increased expression of zinc transporter ZIP4, ZIP11, ZnT1, and ZnT6 predicts poor prognosis in pancreatic cancer. J. Trace Elem. Med. Biol 65, 126734. 10.1016/j.jtemb.2021.126734. [DOI] [PubMed] [Google Scholar]
- 28.Studer R, Vogt CP, Cavigelli M, Hunziker PE, and Kä gi JH (1997). Metallothionein accretion in human hepatic cells is linked to cellular proliferation. Biochem. J 328 (Pt 1), 63–67. 10.1042/bj3280063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagel WW, and Vallee BL (1995). Cell cycle regulation of metallothionein in human colonic cancer cells. Proc. Natl. Acad. Sci. USA 92, 579–583. 10.1073/pnas.92.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinkenborg JL, Nicolson TJ, Bellomo EA, Koay MS, Rutter GA, and Merkx M. (2009). Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat. Methods 6, 737–740. 10.1038/nmeth.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin Y, Miranda JG, Stoddard CI, Dean KM, Galati DF, and Palmer AE (2013). Direct comparison of a genetically encoded sensor and small molecule indicator: implications for quantification of cytosolic Zn(2+). ACS Chem. Biol 8, 2366–2371. 10.1021/cb4003859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin Y, Dittmer PJ, Park JG, Jansen KB, and Palmer AE (2011). Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc. Natl. Acad. Sci. USA 108, 7351–7356. 10.1073/pnas.1015686108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McRae R, Lai B, and Fahrni CJ (2013). Subcellular redistribution and mitotic inheritance of transition metals in proliferating mouse fibroblast cells. Metallomics 5, 52–61. 10.1039/c2mt20176c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, and Maret W. (2009). Transient fluctuations of intracellular zinc ions in cell proliferation. Exp. Cell Res 315, 2463–2470. 10.1016/j.yexcr.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Fiedler BL, Van Buskirk S, Carter KP, Qin Y, Carpenter MC, Palmer AE, and Jimenez R. (2017). Droplet microfluidic flow cytometer for sorting on transient cellular responses of genetically-encoded sensors. Anal. Chem 89, 711–719. 10.1021/acs.analchem.6b03235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian C, Yang C, and Spencer SL (2020). EllipTrack: a global-local cell-tracking pipeline for 2D fluorescence time-lapse microscopy. Cell Rep. 32, 107984. 10.1016/j.celrep.2020.107984. [DOI] [PubMed] [Google Scholar]
- 37.Anson KJ, Corbet GA, and Palmer AE (2021). Zn(2+) influx activates ERK and Akt signaling pathways. Proc. Natl. Acad. Sci. USA 118, e2015786118. 10.1073/pnas.2015786118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Zhang S, and Li SJ (2013). Zn2+ induces apoptosis in human highly metastatic SHG-44 glioma cells, through inhibiting activity of the voltage-gated proton channel Hv1. Biochem. Biophys. Res. Commun 438, 312–317. 10.1016/j.bbrc.2013.07.067. [DOI] [PubMed] [Google Scholar]
- 39.Matson JP, House AM, Grant GD, Wu H, Perez J, and Cook JG (2019). Intrinsic checkpoint deficiency during cell cycle re-entry from quiescence. J. Cell Biol. 218, 2169–2184. 10.1083/jcb.201902143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spencer SL, Cappell SD, Tsai F-C, Overton KW, Wang CL, and Meyer T. (2013). The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell 155, 369–383. 10.1016/j.cell.2013.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews HK, Bertoli C, and de Bruin RAM (2022). Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 23, 74–88. 10.1038/s41580-021-00404-3. [DOI] [PubMed] [Google Scholar]
- 42.Sun L, Chai Y, Hannigan R, Bhogaraju VK, and Machaca K. (2007). Zinc regulates the ability of Cdc25C to activate MPF/cdk1. J. Cell. Physiol 213, 98–104. 10.1002/jcp.21090. [DOI] [PubMed] [Google Scholar]
- 43.Rhind N, and Russell P. (2012). Signaling pathways that regulate cell division. Cold Spring Harbor Perspect. Biol 4, a005942. 10.1101/cshperspect.a005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan HX, Xiong Y, and Guan KL (2013). Nutrient sensing, metabolism, and cell growth control. Mol. Cell 49, 379–387. 10.1016/j.molcel.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hess SY (2017). National risk of zinc deficiency as estimated by national surveys. Food Nutr. Bull 38, 3–17. 10.1177/0379572116689000. [DOI] [PubMed] [Google Scholar]
- 46.Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, and Hirano T. (2007). Zinc is a novel intracellular second messenger. J. Cell Biol 177, 637–645. 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanford L, and Palmer AE (2020). Dissociated hippocampal neurons exhibit distinct Zn(2+) dynamics in a stimulation-method-dependent manner. ACS Chem. Neurosci 11, 508–514. 10.1021/acschemneuro.0c00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, Woodruff TK, and O’Halloran TV (2011). Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem. Biol 6, 716–723. 10.1021/cb200084y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C, Maslar D, Minckley TF, LeJeune KD, and Qin Y. (2021). Spontaneous, synchronous zinc spikes oscillate with neural excitability and calcium spikes in primary hippocampal neuron culture. J. Neurochem 157, 1838–1849. 10.1111/jnc.15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hahn AT, Jones JT, and Meyer T. (2009). Quantitative analysis of cell cycle phase durations and PC12 differentiation using fluorescent biosensors. Cell Cycle 8, 1044–1052. 10.4161/cc.8.7.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grimm JB, Muthusamy AK, Liang Y, Brown TA, Lemon WC, Patel R, Lu R, Macklin JJ, Keller PJ, Ji N, and Lavis LD (2017). A general method to fine-tune fluorophores for live-cell and in vivo imaging. Nat. Methods 14, 987–994. 10.1038/nmeth.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carter KP, Carpenter MC, Fiedler B, Jimenez R, and Palmer AE (2017). Critical comparison of FRET-sensor functionality in the cytosol and endoplasmic reticulum and implications for quantification of ions. Anal. Chem 89, 9601–9608. 10.1021/acs.analchem.7b02933 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The image analysis code is accessible at https://doi.org/10.5281/zenodo.7968726. Time-lapse imaging data used in this current study are not publicly available but are available from the corresponding author on request. Protocols are also available upon request. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.