Abstract
A recently developed diaminotriazine derivative [O,O′-bis(1,2-dihydro-2,2-tetramethylene-4,6-diamino-S-triazin-1-yl)-1,6-hexanediol dihydrochloride; T-46; SIPI 1029] was examined for activity against African trypanosomes in in vitro and in vivo model systems. In vitro, SIPI 1029 was 50% inhibitory for growth of bloodstream trypomastigotes of four strains of Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense at 0.15 to 2.15 nM (50% inhibitory concentrations). In in vivo mouse laboratory models of T. b. rhodesiense clinical isolate infections, SIPI 1029 was curative for 12 of 13 isolates at ≤10 mg/kg of body weight/day for 3 days. In eight infections, a single dose was ≥60% curative, and in six of these, a dose of ≤5 mg/kg was sufficient for ≥60% cure rates. A number of these isolates were resistant to the standard trypanocide melarsoprol (Arsobal) and/or the diamidines diminazene aceturate (Berenil) and pentamidine. SIPI 1029 was also curative in combination with dl-α-difluoromethylornithine (Ornidyl) in a T. b. brucei central nervous system model infection. Some evidence of toxicity was found in dosage regimens of 10 mg/kg/day for 2 or 3 days in which deaths were observed in 6 of 65 animals given this dosage regimen. The activity of SIPI 1029 in this study indicates that this class of compounds (diaminotriazines) should be explored as leads for new human and veterinary trypanocides.
African trypanosomiasis has continued to disrupt human life, animal husbandry, and wildlife in over 10,000,000 km2 of sub-Saharan Africa (13, 17). Recent civil strife with resulting breakdown of the medical infrastructure has resulted in new outbreaks in the Sudan, Zaire, and Rwanda (14, 17). Routine treatment with pentamidine, diminazene aceturate (Berenil), and melarsoprol (Arsobal) (Fig. 1) for over 50 years has resulted in development of resistance to all three agents (13), while dl-α-difluoromethylornithine (DFMO; Ornidyl), the only recently approved trypanocide, has seen limited use because of cost and sporadic activity against East African disease (16, 17). It is therefore important that new, low-cost agents be developed for both human and animal diseases.
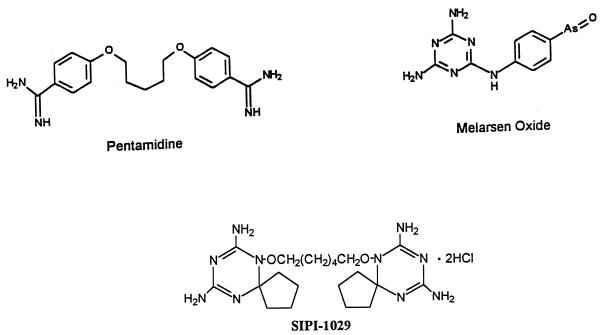
FIG. 1.
Structures of SIPI 1029, pentamidine, and melarsen oxide.
Triazine derivatives have appeared in the literature as active agents against malaria (Clociguanil [11]) and African trypanosomes (bis-triazine analogs; Trypanosoma brucei rhodesiense [15] and Trypanosoma congolense [12]). Recently, the Shanghai Institute of Pharmaceutical Industry has synthesized a series of triazine derivatives and found that one of them, O,O′-bis(1,2-dihydro-2,2-tetramethylene-4,6-diamino-S-triazin-1-yl)-1,6-hexanediol dihydrochloride (SIPI 1029 [Fig. 1]), was curative of experimental Trypanosoma evansi infections in mice, rats, buffalo, and cattle (19).
The present study was undertaken to examine the efficacy of SIPI 1029 in vitro and in vivo against experimental infections by African trypanosomes by using isolates of Trypanosoma brucei brucei, infective of domestic animals, and T. b. rhodesiense, infective of domestic animals, wildlife, and humans.
MATERIALS AND METHODS
Trypanosome strains.
T. b. brucei Lab 110 EATRO is a continuously passaged isolate used by our laboratory and others in many studies (1). Most T. b. rhodesiense strains used are clinical isolates obtained from the Kenya Trypanosomiasis Research Institute (KETRI) through A. R. Njogu. Their drug sensitivities have been described elsewhere (2, 4). T. b. rhodesiense KETRI 243 As-10-3 is a clone of KETRI 243 which is highly resistant to melamine-based arsenicals, pentamidine, and diminazene aceturate (2). Two T. b. rhodesiense isolates were obtained from the American Type Culture Collection: 30119 (EATRO 105, Uganda 1959) and 30027 (Wellcome CT, 1934).
A central nervous system (CNS) model infection was used; the TREU 667 isolate of T. b. brucei was obtained from F. W. Jennings, University of Glasgow (10).
In vitro growth inhibition studies.
Bloodstream trypomastigote forms were cultured in HMI-18 medium (9), with 20% horse serum and 1 μM hypoxanthine (2). Drug studies were done in duplicate (24-well plates, 1 ml of medium/well) over 48 h, with one-half the volume of the wells being changed daily. Cultures were incubated at 37°C in 4% CO2–air and counted with a Z1 Coulter Counter. Fifty percent inhibitory concentrations (IC50s) were determined from semilog plots.
Animals.
Female Swiss Webster mice (20 to 25 g) were purchased from Ace Animals, Inc., Boyertown, Pa.
Drug studies.
All of the above isolates except TREU 667 produce an acute parasitemia which kills the animals in 3 to 10 days. For acute infections, groups of five animals were used, with animals infected intraperitoneally with 2.5 × 105 trypanosomes. Drug studies were begun 24 h postinfection. Intraperitoneal dosing was used throughout the study. Animals were checked weekly for parasites in tail vein blood. Animals surviving >30 days beyond the deaths of untreated controls, with no parasites in their blood, were considered cured (4).
The TREU 667 model infection was used to gauge activity against late-stage CNS infection (10). This model has been used by us previously to detect activity of new agents alone and in combination with other agents (1, 2, 5). Briefly, mice (groups of five) were infected with 104 parasites and the infection was allowed to develop for 21 days, when treatment was begun. After treatment ended, animals were checked weekly for tail vein blood parasitemia, and those positive for parasitemia were removed from cages and sacrificed. Animals were considered cured upon surviving 180 days after the end of treatment with no peripheral blood parasites. One control group was always included per experiment, in which animals were treated with a single 40-mg/kg-of-body-weight dose of diminazene aceturate. This initially cleared the blood but not the CNS of parasites, with the parasites eventually repopulating the blood (10).
In these studies, DFMO was administered at 2% in the drinking water for 14 days as part of combination studies with SIPI 1029. In our laboratory, animals consumed an average of 5 ml/day for a dose rate of 5 g/kg of body weight per day.
Chemicals.
SIPI 1029 was provided by the Shanghai Institute of Pharmaceutical Industry. Diminazene aceturate was purchased from Sigma Chemical (St. Louis, Mo.). DFMO was purchased from Ilex Oncology, San Antonio, Tex. Melarsen oxide was a gift of Rhone Merieux, Toulouse, France.
RESULTS
In vitro studies.
SIPI 1029 was tested for activity against one strain of T. b. brucei and three strains of T. b. rhodesiense in a standard in vitro screen (Table 1). IC50s were compared to those obtained for melarsen oxide, a standard clinical trypanocide. SIPI 1029 had IC50s of 0.15 to 2.15 nM, with values for the KETRI isolates at <1 nM. The IC50 for SIPI 1029 was about equal to that of melarsen oxide for arsenical-sensitive T. b. brucei but 20- to 160-fold lower for resistant T. b. rhodesiense.
TABLE 1.
IC50s for SIPI 1029 and the clinical trypanocide melarsen oxide against growth of bloodstream-form trypanosomes in vitroa
| Strain | IC50 (nM)
|
|
|---|---|---|
| SIPI 1029 | Melarsen oxide | |
| T. b. brucei Lab 110 EATRO | 2.15 | 4.4 |
| T. b. rhodesiense | ||
| KETRI 243 | 0.6 | 13.0 |
| KETRI 269 | 0.6 | 33.0 |
| KETRI 243 As-10-3 | 0.15 | 24.0 |
Activity of SIPI 1029 against acute model infections.
SIPI 1029 was initially tested for activity against an acute model infection strain, T. b. brucei Lab 110 EATRO (Table 2). This agent was extremely active at a dose range of 0.5 to 10 mg/kg/day for 3 days, with 100% cure rates at 0.5, 1.0, 2.5, and 5 mg/kg. Single doses in the same range were also effective, yielding a dose-response curve with the 2.5-mg/kg dose having a 60% cure rate and the 10-mg/kg dose having a 100% cure rate. In the 10-mg/kg group, treated for 3 days, one animal was found dead at day 2, and this was attributed to possible toxicity.
TABLE 2.
Activity of SIPI 1029 versus T. b. brucei in a standard model infectiona
| Dose (mg/kg/day) | MSD (days) | No. cured/no. total | % Cured |
|---|---|---|---|
| Control | 4.0 | 0/5 | 0 |
| 3-day dosing | |||
| 0.5 | 5/5 | 100 | |
| 1.0 | 5/5 | 100 | |
| 2.5 | 5/5 | 100 | |
| 5.0 | 5/5 | 100 | |
| 10.0 | 2.0 (T) | 4/5 | 80 |
| 1-day dosing | |||
| 0.5 | 9.8 | 1/5 | 20 |
| 1.0 | 8.7 | 2/5 | 40 |
| 2.5 | 12.5 | 3/5 | 60 |
| 5.0 | 3.0 | 4/5 | 80 |
| 10.0 | 5/5 | 100 |
Groups of five mice (20-g females) were infected with 2.5 × 105 trypanosomes, and the infections were allowed to develop for 24 h prior to treatment. SIPI 1029 was dissolved in distilled water and injected once daily (intraperitoneally) for 1 or 3 days. Animals surviving >30 days beyond the deaths of the untreated controls with no trypanosomes in tail vein blood smears were considered cured. MSD, mean survival in days exclusive of cured animals. (T), possible toxicity.
SIPI 1029 was then tested with 13 clinical isolates of T. b. rhodesiense. Doses used were 0.5, 1, 2.5, 5, and 10 mg/kg/day for 1 or 3 days. In all of these infections, a dose-response curve was obtained, with the lowest curative dose presented in Table 3. We obtained cure rates of ≥60% with SIPI 1029 against 12 of the 13 isolates tested (Table 3). In eight of the infections, a single dose was curative, and in six of the infections, a dose of ≥5 mg/kg was sufficient for ≥60% cures; 100% cure rates were obtained with six isolates.
TABLE 3.
Summary of SIPI 1029 activity against T. b. rhodesiense clinical isolates in model infectionsa
| Isolate | Lowest curative dose (mg/kg/day) | Duration of treatment (days) | No. cured/no. total | % Cured |
|---|---|---|---|---|
| KETRI 2538 | 1.0 | 3 | 4/5 | 80 |
| 5.0 | 1 | 5/5 | 100 | |
| KETRI 243 | 5.0 | 3 | 4/5 | 80 |
| KETRI 243 As-10-3 | 10.0 | 3b | 0/5 | 0 |
| KETRI 269 | 10.0 | 3 | 3/5 | 60 |
| KETRI 2636 | 5.0 | 3 | 5/5 | 100 |
| 10.0 | 1 | 4/5 | 80 | |
| KETRI 1992 | 5.0 | 3 | 4/5 | 80 |
| KETRI 2002 | 0.5 | 3 | 5/5 | 100 |
| 2.5 | 1 | 5/5 | 100 | |
| KETRI 2285 | 0.5 | 3 | 3/5 | 60 |
| 2.5 | 1 | 4/5 | 80 | |
| KETRI 2545 | 5.0 | 3 | 4/5 | 80 |
| 10.0 | 1 | 3/5 | 60 | |
| KETRI 2708 | 5.0 | 3 | 4/5 | 80 |
| KETRI 2772 | 1.0 | 3 | 5/5 | 100 |
| 2.5 | 1 | 5/5 | 100 | |
| EATRO 105 (ATCC 30119) | 0.5 | 3 | 5/5 | 100 |
| 0.5 | 1 | 3/5 | 60 | |
| Wellcome CT (ATCC 30027) | 0.5 | 3 | 5/5 | 100 |
| 2.5 | 1 | 3/5 | 60 |
These data summarize dose-response studies with each isolate using standard procedures as for Table 2 and as described in Materials and Methods. Doses used were 0.5, 1, 2.5, 5, and 10 mg/kg once daily for 1 day and for 3 days. The lowest doses resulting in ≥60% cures (three of five animals) for 1-day and 3-day dosing are presented. The absence of data for 1-day dosing indicates that there was no effective single dose ≤10 mg/kg for that strain.
Highest dose tested, not curative. For KETRI 243 As-10-3, SIPI 1029 was not curative with any dose tested, up to 10 mg/kg/day for 3 days.
An experiment was designed to use T. b. brucei Lab 110 EATRO to examine the efficacy of SIPI 1029 in a delayed-treatment regimen. In this experiment, mice were infected (2.5 × 105 trypanosomes) and parasites were allowed to multiply for 24, 48, or 72 h before treatment was begun, leading to initial parasite densities of 1.5 × 107/ml, 2.5 × 107/ml, and 2.53 × 108/ml, respectively (averages of counts for five control animals). Animals were dosed at 1, 5, or 10 mg/kg/day for 3 days. Complete cures (five of five animals) were obtained in all groups at 24 and 48 h. The most interesting results were found with the 72-h group. At a dose of 1 mg/kg/day, 40% of these animals were cured, while at 5 and 10 mg/kg/day, 80 and 60% cure rates, respectively, were obtained. In this acute infection, deaths routinely occur in untreated controls at 72 to 96 h, with circulating parasite densities of 108 to 109/ml.
CNS infection.
SIPI 1029 was also examined for trypanocidal activity in the TREU 667 CNS model infection (Table 4). In these experiments, SIPI 1029 was given at a dose range of 0.5 to 10 mg/kg alone and in combination with 2% DFMO in the drinking water for 14 days. This is a noncurative dose of DFMO, but we have found it to be synergistic with suramin and other agents in curing CNS model infections (1, 2, 5). In these experiments, SIPI 1029 was not curative when used alone at up to 10 mg/kg for 3 days or at 5 mg/kg for 7 days. In combination with a 14-day course of DFMO, 33 to 50% cure rates were obtained (experiment 1, 2.5 and 5 mg/kg for 7 days and 10 mg/kg for 3 days). When dosing was delayed until the midpoint of the DFMO regimen (day 7), an 80% cure rate with 10 mg/kg for 3 days was obtained (experiment 1). In experiment 2, significant cure rates were also obtained at 5 mg/kg for 7 days (60%) or at 10 mg/kg for 3 days (75%) when SIPI 1029 was started at the end of the 14-day dosage regimen.
TABLE 4.
Activity of SIPI 1029 and DFMO in the T. b. brucei TREU 667 CNS infectiona
| Treatment | Dose (mg/kg/day or %) | Duration of treatment (days) | Avg day of relapse | No. cured/no. total (%) |
|---|---|---|---|---|
| Expt 1 | ||||
| Diminazene aceturate | 40 | 1 | 16.2 | 0/5 |
| SIPI 1029 | 10b | 3 | 21.5 | 0/5 |
| DFMO | 2% DW | 14 | 27 | 0/5 |
| 2% DFMO for 14 days plus SIPI 1029 | 2.5 | 7 | 44.3 | 2/5 (40) |
| 5 | 7 | 47.5 | 1/3 (33) | |
| 10 | 3 | 112 | 2/4 (50) | |
| 2.5 | 7 (day 7–13) | 64.0 | 1/5 (20) | |
| 5 | 7 (day 7–13) | 55.5 | 3/5 (60) | |
| 10 | 3 (day 7–10) | 78 | 4/5 (80) | |
| Expt 2 | ||||
| Diminazene aceturate | 40 | 1 | 35 | 0/5 |
| DFMO | 2% DW | 14 | 33 | 0/5 |
| SIPI 1029 | 10b | 3 | 22 | 0/3 |
| 2% DFMO for 14 days plus SIPI 1029 | 2.5 | 7 | 63.2 | 0/5 |
| 5 | 7 | 54.5 | 3/5 (60) | |
| 10 | 3 | 46.3 | 0/3 | |
| 2.5 | 7 (day 7–13) | 49.4 | 1/5 (20) | |
| 2% DFMO for 14 days plus SIPI 1029 | 5 | 7 (day 7–13) | 44 | 1/3 (33) |
| 10 | 3 (day 7–10) | 44 | 2/4 (50) | |
| 2.5 | 7 (day 14–20) | 72 | 1/5 (20) | |
| 5 | 7 (day 14–20) | 58 | 3/5 (60) | |
| 10 | 3 (day 14–16) | 53 | 3/4 (75) | |
| DFMO | 2% DW | 7 | 16 | 0/5 |
| 2% DFMO for 7 days plus SIPI 1029 | 10 | 3 (day 7–13) | 41 | 0/4 |
Groups of five mice were infected with 10,000 trypanosomes, and the infection was allowed to progress for 21 days, when treatment commenced (day 0). Animals were examined weekly for parasites in tail vein blood smears. Those recrudescing were removed from cages; those surviving ≥180 days beyond the recrudescence of diminazene aceturate controls, with no bloodstream parasitemia, were considered cured. In these experiments, DFMO was given at 2% in the drinking water (DW) for 7 or 14 days (day 0 to 6 or 0 to 13) and SIPI 1029 was given intraperitoneally at a single injection daily for 3 or 7 days, starting on day 0. In some groups, SIPI 1029 was given sequentially after DFMO treatment ended (day 7 to 13 or day 14 to 20) or starting at the midpoint of a 14-day dose (day 7 to 13). Animals dying within 5 days of starting treatment, without completing it, were not considered in the final total for that group.
None of the dosage regimens of SIPI 1029 below 10 mg/kg/day was effective in extending the day of relapse or curing.
DISCUSSION
The results presented in the current study indicate that SIPI 1029 is curative (≥60%) in both acute and CNS model trypanosome infections and is effective in animals with a heavy parasite burden (e.g., >108/ml). This agent was ≥60% curative for the 1 T. b. brucei isolate and 12 of 13 T. b. rhodesiense isolates studied. Several of the KETRI isolates are resistant to standard trypanocides: KETRI 243 As-10-3 (arsenicals, diminazene aceturate, and pentamidine), 269 (DFMO and pentamidine), 1992 (arsenicals and pentamidine), 2636 (pentamidine), and 2708 (arsenicals). KETRI 243 As-10-3 is completely refractory to melarsen oxide, melarsoprol, and pentamidine and highly refractory to diminazene aceturate (2). This strain was the only one tested for which SIPI 1029 was not curative, although the survival time of animals doubled with a 10-mg/kg dose for 3 days (data not shown).
We examined the toxicity of SIPI 1029 since, at the highest dosage regimen tested, 10 mg/kg/day for 3 days, several animals in separate experiments died after the second or third injection. In these groups, the remainder of the animals were cured (usually an 80% cure rate resulted). In a previous study, SIPI 1029 had a median (50%) effective dose in mice of 0.28 mg/kg and a median (50%) lethal dose of 9.8 mg/kg (18). The present study indicates that, in a single-dose regimen for Lab 110 EATRO, the 50% effective dose was approximately 1.75 mg/kg. On the basis of the observed toxicity with two or three daily doses of 10 mg/kg, we initiated a small-scale toxicity test in which groups of three animals were dosed with 5, 10, or 15 mg/kg intraperitoneally for 1 day or 3 days. All animals receiving 5- and 10-mg/kg doses survived; however, all animals receiving 15 mg/kg died, indicating that, at 10 mg/kg, infected mice do not tolerate SIPI 1029 as well as do uninfected animals and that this dose is on the borderline for acute toxicity.
The mode of action of this agent is not clearly known. In the previous study (19), SIPI 1029, like diminazene aceturate, inhibited incorporation of [3H]hypoxanthine into DNA in T. evansi, with an IC50 of 1.33 μg/ml (compared with diminazene aceturate IC50 of 1.73 μg/ml [17]). In vitro, it was growth inhibitory at low (10−9 M) concentrations, while in in vitro lysis tests, incubation with 100 μM SIPI 1029 resulted in nearly complete lysis of bloodstream forms within 30 min at 37°C (data not shown). Since DFMO, as well as several diamidines and a recently developed trypanocidal agent (CGP 40215), inhibits polyamine metabolism (2, 6), we also examined SIPI 1029 for inhibition of trypanosome ornithine decarboxylase, S-adenosylmethionine (AdoMet) synthetase, and AdoMet decarboxylase (3). SIPI 1029 was not inhibitory to ornithine decarboxylase or AdoMet synthetase at up to 500 μM but inhibited AdoMet decarboxylase with an IC50 of 38 μM. Further studies will be directed towards determining whether reduction of polyamine content is contributory toward its mode of action and whether the parasite concentrates this agent through adenosine or AdoMet transporters (8).
Collectively, the data presented indicate that diaminotriazine derivatives are trypanocidal for a wide range of T. b. rhodesiense isolates, some of which are resistant to standard trypanocides. In a previous study with 100 T. evansi-infected cattle and buffalo, SIPI 1029 had a cure rate of 94% at single doses of 0.5 to 1.5 mg/kg (19). These studies provide the basis for a reexamination of this class of agents as novel human and veterinary trypanocides.
ACKNOWLEDGMENTS
This work was supported by the United Nations Development Program/World Bank/World Health Organization Special Program for Research and Training in Tropical Disease (950594 to C.J.B., 970306 to W.Z.) and grant AI 17340 from the National Institutes of Health (to C.J.B.).
We thank Angela German, Elvis Rosero, and Karen Sanabria for technical assistance.
REFERENCES
- 1.Bacchi C J, Berens R L, Nathan H C, Klein R S, Elegbe I A, Rao K V B, McCann P P, Marr J J. Synergism between 9-deazainosine and difluoromethylornithine in treatment of experimental African trypanosomiasis. Antimicrob Agents Chemother. 1987;31:1406–1413. doi: 10.1128/aac.31.9.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacchi C J, Brun R, Croft S L, Alicea K, Buhler Y. In vivo trypanocidal activities of new S-adenosylmethionine decarboxylase inhibitors. Antimicrob Agents Chemother. 1996;40:1448–1453. doi: 10.1128/aac.40.6.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacchi C J, Garofalo J, Ciminelli M A, Rattendi D, Goldberg B, McCann P P, Yarlett N. Resistance to dl-α-difluoromethylornithine by clinical isolates of Trypanosoma brucei rhodesiense: role of S-adenosylmethionine. Biochem Pharmacol. 1993;46:471–481. doi: 10.1016/0006-2952(93)90524-z. [DOI] [PubMed] [Google Scholar]
- 4.Bacchi C J, Nathan H C, Livingston T, Valladares G, Saric M, Sayer P D, Njogu A R, Clarkson A B., Jr Differential susceptibility to dl-α-difluoromethylornithine in clinical isolates of Trypanosoma brucei rhodesiense. Antimicrob Agents Chemother. 1990;34:1183–1188. doi: 10.1128/aac.34.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacchi C J, Nathan H C, Yarlett N, Goldberg B, McCann P P, Sjoerdsma A, Clarkson A B., Jr Combination chemotherapy of drug-resistant Trypanosoma brucei rhodesiense infections in mice using dl-α-difluoromethylornithine and standard trypanocides. Antimicrob Agents Chemother. 1994;38:563–569. doi: 10.1128/aac.38.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitonti A J, Dumont J A, McCann P P. Characterization of Trypanosoma brucei brucei S-adenosyl-l-methionine decarboxylase and its inhibition by Berenil, pentamidine and methylglyoxal bis(guanylhydrazone) Biochem J. 1986;237:685–689. doi: 10.1042/bj2370685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brun R, Buhler Y, Sandmeier U, Kaminsky R, Bacchi C J, Rattendi D, Lane S, Croft S L, Snowdon D, Yardley V, Caravatti G, Frei J, Stanek J, Mett H. In vitro trypanocidal activities of new S-adenosylmethionine decarboxylase inhibitors. Antimicrob Agents Chemother. 1996;40:1442–1447. doi: 10.1128/aac.40.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg B, Yarlett N, Sufrin J, Lloyd D, Bacchi C J. A unique transporter of S-adenosylmethionine in African trypanosomes. FASEB J. 1997;11:256–260. doi: 10.1096/fasebj.11.4.9068614. [DOI] [PubMed] [Google Scholar]
- 9.Hirumi H, Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol. 1989;75:985–989. [PubMed] [Google Scholar]
- 10.Jennings F W, Gray A R. Relapsed parasitemia following chemotherapy of chronic Trypanosoma brucei infections in mice and its relationship to cerebral trypanosomes. Contrib Microbiol Immunol. 1983;7:147–154. [PubMed] [Google Scholar]
- 11.Knight D J, Peters W. The antimalarial activity of N-benzyloxydihydrotriazines. I. The activity of clociguanil (BRL50216) against rodent malaria, and studies on its mode of action. Ann Trop Med Parasitol. 1980;40:393–404. [PubMed] [Google Scholar]
- 12.Knight D J, Ponsford R J. Trypanocidal activity and prophylaxis evaluation of a series of bis-oxydihydrotriazines in mice. Ann Trop Med Parasitol. 1982;76:589–594. doi: 10.1080/00034983.1982.11687588. [DOI] [PubMed] [Google Scholar]
- 13.Kuzoe F. Current situation of African trypanosomiasis. Acta Trop. 1993;54:153–162. doi: 10.1016/0001-706x(93)90089-t. [DOI] [PubMed] [Google Scholar]
- 14.McKinley J C., Jr Deadly epidemic emerges in Sudan. N Y Times. 1997;1997(18 July):A1. [Google Scholar]
- 15.Turner W R, Werbel L M. Novel bis-(1,6-dihydro-6,6-dimethyl-1,3,5-triazine-2,4-diamines) as antitrypanosomal agents. J Med Chem. 1985;28:1728–1740. doi: 10.1021/jm00149a032. [DOI] [PubMed] [Google Scholar]
- 16.Van Nieuwenhove, S. 1992. Advances in sleeping sickness therapy. Ann. Soc. Belge Med. Trop. 72(Suppl. 1):39–51. [PubMed]
- 17.World Health Organization. Tropical disease research. Twelfth programme report. Geneva, Switzerland: World Health Organization; 1995. [Google Scholar]
- 18.Zhang C, Shen J, Zheng R J, Fang M B, Zhou W C, Zhang X P. Effect of T-46 in vitro on incorporation of [3H]hypoxanthine into nucleic acid of Trypanosoma evansi. Chin J Vet Parasitol. 1993;1:10–12. [Google Scholar]
- 19.Zhou W C, Xin Z M, Zhang X P, Shen J, Qiu Q P. Synthesis and antiprotozoal activities of some new triazine derivatives including a new antitrypanosomal agent, SIPI-1029. Acta Pharm Sin. 1996;31:823–830. [PubMed] [Google Scholar]