Abstract
Cystic Fibrosis (CF) is a genetic disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Due to the distribution of the CFTR protein, CF presents with a heterogeneous phenotype. Men with CF may present with infertility due to congenital abnormalities of the vas deferens. In addition, they may experience testosterone deficiency. Today, they can father biological children with assisted reproductive technologies. We reviewed the current literature on the pathophysiology of these conditions, describe interventions that allow men with CF to conceive biological children, and provide recommendations for management of CF patients with reproductive health concerns.
Keywords: Cystic Fibrosis, Congenital Bilateral Absence of the Vas Deferens (CBAVD), Infertility, Assisted Reproductive Technology (ART)
Introduction
Cystic Fibrosis (CF) is a genetic condition that is characterized by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene located on chromosome 7.1 CFTR plays a crucial role in regulating secretion viscosities in several organs including the lungs, pancreas, liver, and reproductive organs which leads to a variety of clinical manifestations, including pulmonary disease, pancreatitis, and infertility.1 According to the Cystic Fibrosis Foundation, there are close to 40,000 CF patients in the United States and 1000 new cases each year.2 The life expectancy of CF patients has risen from 32 years old in the 1990s to 46 years old or more in 2020 with the use of new therapies such as CFTR modulator drugs.3 As patients are living longer, improving their quality of life and reproductive potential has now become a more pressing concern for patients, their families, and their physicians. The majority of CF patients face infertility due to obstructive causes, mainly from congenital absence of the vas deferens (CAVD - CBAVD when bilateral and CUAVD when unilateral).4 Here, we aim to describe the impact of CF on male infertility and hypogonadism, outline recent advances in management that can be utilized to deliver high-quality care in CF sexual and reproductive health, and provide recommendations for optimal management of the sexual and reproductive concerns in this population. In this article, we focus solely on those with a CF phenotype to increase awareness of the broader reproductive and sexual health concerns in this population besides the well-described sequelae of the disease of infertility.
Pathophysiology of Infertility in Men with Cystic Fibrosis
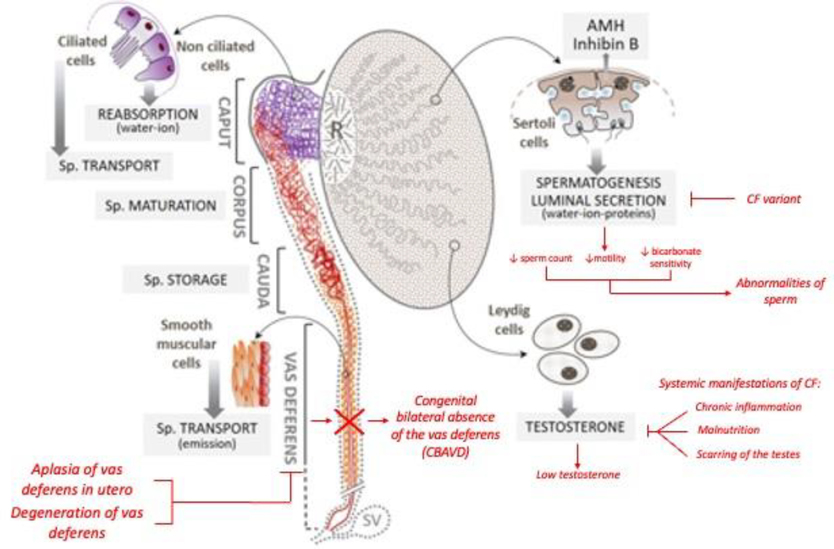
Infertility in men with cystic fibrosis (CF) is almost universal, with around 98% of the male CF population presenting with some form of male factor infertility.4,5 This is most commonly due to obstructive azoospermia (OA), where blockage of the vas deferens prevents sperm from passing out of the body during the process of ejaculation regardless of normal sperm production.6 While the absence of vas deferens bilaterally is the principal identifiable cause of infertility in men with CF, it is not the only gross anatomical male reproductive tract abnormality associated with disease as defects in other mesonephric duct derivatives may be seen alongside CBAVD, such as absence of the seminal vesicles and epididymis (Figure 1).6 Functionally, these present as reduced ejaculate volume (<1 mL), acidic semen pH, undetectable or low fructose concentrations (normal: >25 μM), and reduced α-glucosidase and carnitine concentrations.7,8
Figure 1.
Impact of CF variants on the male reproductive system with black text indicating normal function and red text indicating pathophysiology. Adapted from Bieth et al 2021.
The relationship between CFTR mutations and CBAVD is not entirely consistent across various studies, possibly due to differences in scanning methods and ethnic disparities.9 However, recent research confirmed that isolated CBAVD (iCBAVD) shares a common genetic origin with CF, with F508del, 5T, and R117H being the most frequently observed mutations.10 In a meta-analysis by Yu et al., it was found that 78% of CBAVD patients had at least one CFTR gene mutation, with 46% having two mutations and 28% having only one.10 Attardo et al. proposed that men with CBAVD should be considered carriers of at least one CFTR gene mutation, unless the entire gene has been analyzed and mutations have been excluded.5
Recent developments in our understanding of the tissue specific expression and regulation of CFTR have provided insight as to why CAVD is so widespread in men with CF. Post transcriptional modification of mRNA, such as alternative splicing, are less efficient in the vas deferens epithelia than in respiratory epithelia or other organ system tissues commonly affected in CF which potentially explains the subgroup of men carrying CFTR mutations whose only clinical manifestation is CBAVD.11 There exist two prevailing theories on how altered CFTR functions lead to abnormalities of the male reproductive tract: first, of agenesis, and second, of degeneration.
Theory of Disorder of Embryogenesis
The first theory is that of agenesis, where an early disorder of organogenesis leads to an absence of vas deferens development and disturbance in the development of other mesonephric derivatives.6 This is supported by the significant proportion of men with CAVD also having renal agenesis which is most frequently seen in men with CUAVD and concurrent renal abnormalities.9 More specifically, it has been proposed that in individuals with CAVD, a single mesonephric duct defect was produced before, or at the time of, the formation of the ureteral bud during fetal development leading to CUAVD and renal agenesis on the same side.6,9 This hypothesis is opposed by recent investigation, and less commonly cited as a possible cause of CAVD in CF. Arguments against this hypothesis include the low proportion of CFTR variant carrying patients with CAVD without renal agenesis. Additional support against this theory is the discovery that male fetuses with CF of gestational ages 12–22 weeks had morphologically normal reproductive tracts.12
Theory of Degeneration
The hypothesis of a degenerative process leading to CAVD has become a common and more popular theory to explain the CBAVD in men with CF. Believed to be related to a CFTR mutation induced loss of water-ion-PH homeostasis across the intraluminal epithelium of the vas deferens and epididymis, a gradual increase in the viscosity of the luminal fluid in utero leads to thickened mucous that cannot pass through the developing vas deferens.9 This impassable mucosal state leaves the lumen obstructed which is believed to induce progressive involution of the developing vas deferens.6,7,13 In addition to an increase in the fluid viscosity and slower flow rate in a coiled epididymis lumen, the observations of CF male fetuses with a normal-looking tract suggest that CF CAVD is not due to the complete absence of the vas deferens, but rather a pathophysiological process that begins in the second or third trimester of pregnancy and continues after birth.9
With the advent of new genetic and animal studies, support for this hypothesis as the cause of CBAVD in CF patients has increased. For example, Murine CFTR knockouts have demonstrated diminished levels of Wnt9b gene expression and ZO-1–associated nucleic acid binding proteins, both of which play a role in normal differentiation of the vas deferens.14,15 Research has revealed that neonatal rats without CFTR gene expression exhibit notable structural changes in certain segments of their epididymal duct and vas deferens.16 These changes include incomplete development of the periductal smooth muscle and irregularities in the surrounding epithelium.16 In the animal model, these conditions worsen quickly after birth, resulting in an underdeveloped epididymis and collapsed vas deferens.16 Eventually, the vas deferens disappears or becomes atretic in fully mature CFTR knockout rats.9 Additionally, a strong association between CBAVD and altered CFTR and SLC9A3 (a NaCl/HCO3- exchanger key in maintaining the pH and ion gradient in conjunction with CFTR in the vas deferens) suggests a potential molecular interplay.14 Further, SLC9A3 expression has been shown to be dependent on CFTR expression, which means that in tissues deficient in functional CFTR there may also be a deficiency of this exchanger.15
Despite recent evolution in our understanding of why the vas deferens are almost universally absent in CF, the true causal pathologic process remains unproven. Though current evidence favors the theory of a degenerative process, there is also evidence to support that luminal obstruction alone may not explain it in its entirety and the reality may lie in some combination of the two prevailing theories.
Abnormalities in Spermatogenesis
Infertility amongst individuals with CF may be more complicated than just the previously described anomalies of the vas deferens as CFTR has also been shown to play a role in spermatogenesis.17 However, the relationship between CFTR mutations and spermatogenesis or sperm function has been a subject of controversy in the field.7 Recent research has shown that CFTR protein is involved in multiple processes beyond ion transport, including spermatogenesis and sperm capacitation, and may impact various reproductive processes through different signaling pathways.7 While the majority of men do appear to have normal spermatogenesis, some studies find that during sperm retrieval men with CF have lower sperm concentration, decreased sperm motility, decreased bicarbonate sensitivity, and ultimately lower fertilization rates.18 Van der Ven et al demonstrated that among a group of 127 unrelated males, CF variant carrying men were more likely to demonstrate infertility or subfertility on semen analysis including 14.3% of men with at least one CF mutation presenting with azoospermia.19 An additional study of 93 men with CF phenotype showed varied qualitative and quantitative defects.
Hypogonadism in Men with CF
Hypogonadism, or the decreased production of sex hormones such as testosterone (T), is a common problem in men with CF.20 The prevalence of low T in men with CF is still uncertain, but is reported to be 25%–88% of men with CF which is higher than the prevalence of low T in the general population estimated to be 2.1–12.8%.20,21 T is the primary male sex hormone, and its deficiency can cause a range of physical and psychological symptoms such as low libido, erectile dysfunction, decreased muscle mass and bone density, low energy levels, and cognitive and mood changes.6 In CF, low T can occur due to a variety of factors, including hypergonadotropic hypogonadism caused by chronic inflammation or scarring of the testes or hypogonadotropic hypogonadism caused by a lack of maturation of the hypothalamic-pituitary-gonadal (HPG) axis. In CF men, this HPG axis dysfunction may result from chronic disease and low body fat.22 Moreover, certain medications used to treat CF, such as glucocorticoids, can interfere with steroid hormone production.6
Testosterone plays a crucial role in muscle development and maintenance, and low levels can lead to decreased muscle mass and strength. In CF patients who are already at risk of osteoporosis and muscle wasting, low T can exacerbate these musculoskeletal complications. According to clinical guidelines, the Cystic Fibrosis Foundation recommends considering screening for hypogonadism after a DXA scan Z-score is found to be between −1 and −2.3,23A low Bone Mineral Density (BMD) score (less than or equal to −2.0 standard deviations below mean using Dual-energy x-ray absorptiometry (DXA)) has been found in 9%–32% of children and adolescents with CF which increases their susceptibility to fractures, disability, and pain.24 A cross-sectional study conducted by Leifke et al. excluding those taking glucocorticoids, concluded that low testosterone was significantly correlated with low BMD (r=0.32; P<0.05) in CF males.20 Despite this, in our anecdotal experience we find that few CF men are offered testing for low T or offered referral to a specialist for further management of the sequelae of low T.
In the general population, testosterone replacement therapy (TRT) in hypogonadal men has been shown to be an effective treatment option for men with hypogonadism. TRT has been found to significantly improve libido, erectile function, sexual satisfaction, and increase muscle bone density, and decrease the risk of osteoporosis, leading to improved physical performance and decreased risk of falls and fractures.25 However, there is a lack of studies regarding benefits of TRT in CF males. Therefore, Cystic Fibrosis Foundation makes an emphasis on the importance of individualized therapy and personalized treatment plans that are tailored to the unique needs and goals of each individual.3
It is important to note the negative impacts that TT can have on sperm production including leading to azoospermia, therefore, cryopreservation of sperm may be a suitable option for men with CF and low testosterone (T) who may want to have children in the future but begin treatment with TT.26 Additional treatment options for patients desiring of fertility may include clomiphene citrate, enclomiphene citrate, or human chorionic gonadotropin (hCG).27,28 These management options remain off-label uses of these medications and require an in depth discussion about their use in this population with a physician trained in the field.
ART for Men with Cystic Fibrosis
Assisted Reproductive Technology (ART) has been a long-standing form of fertility treatment for those unable to conceive naturally. In vitro fertilization (IVF) allows conception to occur in the laboratory with an embryo then being implanted into the uterus. The advent of Intracytoplasmic Sperm Injection (ICSI), involving injection of a single sperm into an egg, delivered an alternative to conventional IVF, in which multiple sperm and an egg are incubated.29 Thus, men with CF who cannot conceive naturally have multiple options to achieve pregnancy.
Procedures for Sperm Retrieval
Four methods exist for sperm retrieval in men with CF shown in Table 1.30 It is important to note that the source of sperm (epididymal vs testicular) does not impact ART outcomes, and both can be used for IVF and ICSI in CF patients.31 In cases of CBAVD where only the caput of the epididymis is present, the limited space available for extraction must be taken into consideration. TESE in combination with ICSI has expanded the options available to infertility providers serving couples with CBAVD, and therefore may be a suitable option for men with CF who present with an atretic epidydimis.32 Overall, the choice of sperm retrieval method in CBAVD patients depends on various factors such as cost, availability of expertise, patient preference, and clinical indications.33 As mentioned previously, sperm cryopreservation may be an option for men with CF with low T, therefore the ideal method of sperm extraction must come from a comprehensive and patient-centered discussion based on each individual’s goals of care.
Table 1.
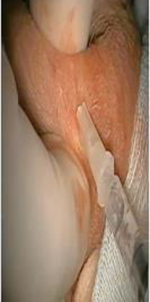
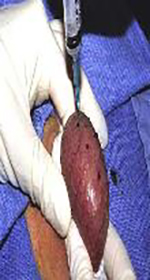
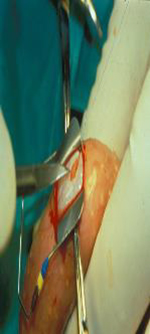
Sperm extraction options for men with CF. Images courtesy of Ranjith Ramasamy, MD.
| Technique | Surgical Approach | Source | Advantages | Disadvantages | Suitable for CF Patients | Photograph of Technique |
|---|---|---|---|---|---|---|
| Percutaneous Epididymal Sperm Aspiration (PESA) | Percutaneous | Epididymis | Outpatient procedure with local anesthesia Rapid patient recovery | 25% unsuccessful therefore requiring TESE or TESA | Yes |
|
| Testicular Sperm Aspiration (TESA) | Percutaneous | Testicular | Outpatient procedure with local anesthesia Rapid patient recovery | Minimal tissue obtained; usually insufficient for cryopreservation Lower motility than PESA | Yes |
|
| Testicular Sperm Extraction (TESE) | Open | Testicular | Highest yield in OA Large volume of sperm suitable for cryopreservation | Invasive Requires general anesthesia May require microsurgical training and microscope (micro-TESE) | Yes |
|
| Microepididymal Sperm Aspiration (MESA) | Open | Epididymis | High yield in OA Large volume of sperm suitable for cryopreservation | Invasive Requires general anesthesia Requires microsurgical training and microscope | Yes |
|
A retrospective study conducted by McCallum et al. investigated the outcomes of MESA (Microepidydimal Sperm Aspiration) in men with the F508del CFTR mutation. The authors reported a fertilization rate of 75% and a live birth rate per cycle of 43.75%.34 These results were corroborated by Persily et al who showed that MESA can be effective for men with CBAVD with fertilization and embryo transfer in 90% of cases, ongoing or delivered pregnancy rate of 46% per transfer and 42% per cycle, and live-birth rates of 53%.32 Additionally, a study of 23 men with CF who underwent sperm retrieval and IVF/ICSI found similar pregnancy per cycle, overall pregnancy, and live-birth rates (40%, 63%, and 47% respectively).17 These findings suggest that these techniques can be effective options for men with CBAVD and CF.32,35 Importantly, preimplantation testing of genetic conditions such as CF during the 8-cell stage is often recommended.36
Patient Provider Attitudes Regarding ART in Men with CF
Establishing a relationship between men with CF, their partners, and their providers is a necessary fundamental in providing the proper conversation about fertility. A systematic review reported reduced fertility awareness among men with CF and suggested that educational intervention is necessary.37 In particular, the mental health of men with CF is adversely affected by their concerns on CF associated infertility.38 A recent study that measured the impact of genetic counseling on the knowledge and perception of ART in CF participants indicated that low knowledge levels of ART exist within the population.39 This highlights that potential barriers exist for CF patients such as provider expertise and discourse surrounding sexual and reproductive health. Despite high importance to CF patients, they report feeling that their reproductive health concerns are of low importance during CF team visits perhaps due to provider discomfort or lack of knowledge of reproductive and sexual health topics and the traditional focus on pulmonary function and longevity rather than comprehensive care within these teams.38 Investigation of provider attitudes and perception of CF in urology teams may also be of importance in achieving comprehensive care for men with CF.
Urologist Recommendations for Management of Male Sexual and Reproductive Health Concerns in Men with CF
Given the increasing life expectancy of men with CF, we recommend referral of all adult men with CF to a urologist with expertise in sexual medicine and infertility for evaluation. To accomplish this, we recommend that CF care teams briefly discuss reproductive and sexual health concerns at each visit beginning in puberty through a brief questionnaire. This is especially important in patients who demonstrate a change in functional status that may reflect the sequelae of low T. Since there currently are no studies describing the optimal management of men with CF desiring fertility who are found to be hypogonadal and, given the nuances of individualized medical management of hormone deficiencies including alternatives to TT in this population, prompt referral to a urologist specializing in these topics is of high importance. Further, we recommend providing online resources and educational materials about the sexual and reproductive health concerns in CF team offices so that patients and their families can fully understand their options.
Conclusions
Here, we presented a review of the sexual and reproductive health concerns for patients with CF. With the advent of new treatments for CF, patients are living into adulthood, able to focus more on their overall quality of life and are considering their sexual and reproductive function. CF has numerous impacts on male sexual and reproductive health. Most well-known is the congenital absence of vas deferens leading to obstructive azoospermia, but patients with CF may also experience hypogonadism both of which may prevent these patients from conceiving naturally. Despite success with assisted reproductive technology to allow these patients to have biological children, there is work to be done to maximize collaboration between reproductive physicians and CF care teams to help CF patients reach their fertility and reproductive health goals.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen Q, Shen Y, Zheng J. A review of cystic fibrosis: Basic and clinical aspects. Anim Models Exp Med. 2021;4(3):220–232. doi: 10.1002/ame2.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CF Foundation Estimates Increase in CF Population | Cystic Fibrosis Foundation. Published July 28, 2022. Accessed June 2, 2023. https://www.cff.org/news/2022-07/cf-foundation-estimates-increase-cf-population
- 3.Cystic Fibrosis Foundation Patient Registry, 2020. Annual Data Report.
- 4.Chillón M, Casals T, Mercier B, et al. Mutations in the Cystic Fibrosis Gene in Patients with Congenital Absence of the Vas Deferens. N Engl J Med. 1995;332(22):1475–1480. doi: 10.1056/NEJM199506013322204 [DOI] [PubMed] [Google Scholar]
- 5.Attardo T, Vicari E, Mollica F, et al. Genetic, andrological and clinical characteristics of patients with congenital bilateral absence of the vas deferens. Int J Androl. 2001;24(2):73–79. doi: 10.1046/j.1365-2605.2001.00269.x [DOI] [PubMed] [Google Scholar]
- 6.Yoon JC, Casella JL, Litvin M, Dobs AS. Male reproductive health in cystic fibrosis. J Cyst Fibros. 2019;18:S105–S110. doi: 10.1016/j.jcf.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 7.de Souza DAS, Faucz FR, Pereira-Ferrari L, Sotomaior VS, Raskin S. Congenital bilateral absence of the vas deferens as an atypical form of cystic fibrosis: reproductive implications and genetic counseling. Andrology. 2018;6(1):127–135. doi: 10.1111/andr.12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boucher D Screening for cystic fibrosis transmembrane conductance regulator gene mutations in men included in an intracytoplasmic sperm injection programme. Mol Hum Reprod. 1999;5(6):587–593. doi: 10.1093/molehr/5.6.587 [DOI] [PubMed] [Google Scholar]
- 9.Bieth E, Hamdi SM, Mieusset R. Genetics of the congenital absence of the vas deferens. Hum Genet. 2021;140(1):59–76. doi: 10.1007/s00439-020-02122-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, Chen Z, Ni Y, Li Z. CFTR mutations in men with congenital bilateral absence of the vas deferens (CBAVD): a systemic review and meta-analysis. Hum Reprod. 2012;27(1):25–35. doi: 10.1093/humrep/der377 [DOI] [PubMed] [Google Scholar]
- 11.Mak V, Jarvi KA, Zielenski J, Durie P, Tsui LC. Higher Proportion of Intact Exon 9 CFTR mRNA in Nasal Epithelium Compared with Vas Deferens. Hum Mol Genet. 1997;6(12):2099–2107. doi: 10.1093/hmg/6.12.2099 [DOI] [PubMed] [Google Scholar]
- 12.Gaillard DA, Carré-Pigeon F, Lallemand A. Normal vas deferens in fetuses with cystic fibrosis. J Urol. 1997;158(4):1549–1552. [PubMed] [Google Scholar]
- 13.Patrizio P, Zielenski J. Congenital absence of the vas deferens: a mild form of cystic fibrosis. Mol Med Today. 1996;2(1):24–31. doi: 10.1016/1357-4310(96)88755-7 [DOI] [PubMed] [Google Scholar]
- 14.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9(2):283–292. doi: 10.1016/j.devcel.2005.05.016 [DOI] [PubMed] [Google Scholar]
- 15.Ruan YC, Wang Y, Da Silva N, et al. CFTR interacts with ZO-1 to regulate tight junction assembly and epithelial differentiation through the ZONAB pathway. J Cell Sci. 2014;127(Pt 20):4396–4408. doi: 10.1242/jcs.148098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plyler ZE, Birket SE, Schultz BD, et al. Non-obstructive vas deferens and epididymis loss in cystic fibrosis rats. Mech Dev. 2019;155:15–26. doi: 10.1016/j.mod.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBride JA, Kohn TP, Mazur DJ, Lipshultz LI, Coward RM. Sperm retrieval and intracytoplasmic sperm injection outcomes in men with cystic fibrosis disease versus congenital bilateral absence of the vas deferens. Asian J Androl. 2021;23(2):140–145. doi: 10.4103/aja.aja_48_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shteinberg M, Taylor-Cousar JL, Durieu I, Cohen-Cymberknoh M. Fertility and Pregnancy in Cystic Fibrosis. Chest. 2021;160(6):2051–2060. doi: 10.1016/j.chest.2021.07.024 [DOI] [PubMed] [Google Scholar]
- 19.van der Ven K, Messer L, van der Ven H, Jeyendran RS, Ober C. Cystic fibrosis mutation screening in healthy men with reduced sperm quality. Hum Reprod Oxf Engl. 1996;11(3):513–517. doi: 10.1093/humrep/11.3.513 [DOI] [PubMed] [Google Scholar]
- 20.Leifke E, Friemert M, Heilmann M, et al. Sex steroids and body composition in men with cystic fibrosis. Eur J Endocrinol. 2003;148(5):551–557. doi: 10.1530/eje.0.1480551 [DOI] [PubMed] [Google Scholar]
- 21.Zarotsky V, Huang MY, Carman W, et al. Systematic literature review of the risk factors, comorbidities, and consequences of hypogonadism in men. Andrology. 2014;2(6):819–834. doi: 10.1111/andr.274 [DOI] [PubMed] [Google Scholar]
- 22.Goldsweig B, Kaminski B, Sidhaye A, Blackman SM, Kelly A. Puberty in cystic fibrosis. J Cyst Fibros. 2019;18:S88–S94. doi: 10.1016/j.jcf.2019.08.013 [DOI] [PubMed] [Google Scholar]
- 23.Stalvey MS, Clines GA. Cystic fibrosis-related bone disease: insights into a growing problem. Curr Opin Endocrinol Diabetes Obes. 2013;20(6):547–552. doi: 10.1097/01.med.0000436191.87727.ec [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marson B, Valsalakumari C, Speight L, et al. P242 Bone density and testosterone levels in male cystic fibrosis patients. Thorax. 2011;66(Suppl 4):A166–A166. doi: 10.1136/thoraxjnl-2011-201054c.242 [DOI] [Google Scholar]
- 25.Bhasin S Testosterone replacement in aging men: an evidence-based patient-centric perspective. J Clin Invest. 2AD;131(4). doi: 10.1172/JCI146607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and Management of Testosterone Deficiency: AUA Guideline. J Urol. 2018;200(2):423–432. doi: 10.1016/j.juro.2018.03.115 [DOI] [PubMed] [Google Scholar]
- 27.Ide V, Vanderschueren D, Antonio L. Treatment of Men with Central Hypogonadism: Alternatives for Testosterone Replacement Therapy. Int J Mol Sci. 2020;22(1):21. doi: 10.3390/ijms22010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helo S, Ellen J, Mechlin C, et al. A Randomized Prospective Double-Blind Comparison Trial of Clomiphene Citrate and Anastrozole in Raising Testosterone in Hypogonadal Infertile Men. J Sex Med. 2015;12(8):1761–1769. doi: 10.1111/jsm.12944 [DOI] [PubMed] [Google Scholar]
- 29.Zheng D, Zeng L, Yang R, et al. Intracytoplasmic sperm injection (ICSI) versus conventional in vitro fertilisation (IVF) in couples with non-severe male infertility (NSMI-ICSI): protocol for a multicentre randomised controlled trial. BMJ Open. 2019;9(9):e030366. doi: 10.1136/bmjopen-2019-030366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naz Khan F, Mason K, Roe AH, Tangpricha V. CF and male health: Sexual and reproductive health, hypogonadism, and fertility. J Clin Transl Endocrinol. 2022;27:100288. doi: 10.1016/j.jcte.2021.100288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akerman JP, Hayon S, Coward RM. Sperm Extraction in Obstructive Azoospermia: What’s Next? Urol Clin North Am. 2020;47(2):147–155. doi: 10.1016/j.ucl.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 32.Persily JB, Vijay V, Najari BB. How do we counsel men with obstructive azoospermia due to CF mutations?—a review of treatment options and outcomes. Transl Androl Urol. 2021;10(3):1467–1478. doi: 10.21037/tau-19-681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher JS, Kim ED. Azoospermia: vasal agenesis. Asian J Androl. 2022;24(1):1–4. doi: 10.4103/aja.aja_113_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCallum TJ, Milunsky JM, Cunningham DL, Harris DH, Maher TA, Oates RD. Fertility in men with cystic fibrosis: an update on current surgical practices and outcomes. Chest. 2000;118(4):1059–1062. doi: 10.1378/chest.118.4.1059 [DOI] [PubMed] [Google Scholar]
- 35.Hubert D, Patrat C, Guibert J, et al. Results of assisted reproductive technique in men with cystic fibrosis. Hum Reprod Oxf Engl. 2006;21(5):1232–1236. doi: 10.1093/humrep/dei453 [DOI] [PubMed] [Google Scholar]
- 36.Keymolen K, Goossens V, De Rycke M, et al. Clinical outcome of preimplantation genetic diagnosis for cystic fibrosis: the Brussels’ experience. Eur J Hum Genet EJHG. 2007;15(7):752–758. doi: 10.1038/sj.ejhg.5201834 [DOI] [PubMed] [Google Scholar]
- 37.Pedro J, Brandão T, Schmidt L, Costa ME, Martins MV. What do people know about fertility? A systematic review on fertility awareness and its associated factors. Ups J Med Sci. 2018;123(2):71–81. doi: 10.1080/03009734.2018.1480186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke AR, Stransky OM, Bernard M, et al. Men’s sexual and reproductive health in cystic fibrosis in the era of highly effective modulator therapies-A qualitative study. J Cyst Fibros Off J Eur Cyst Fibros Soc. 2022;21(4):657–661. doi: 10.1016/j.jcf.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 39.Kushary S, Ali N, Spencer JB, Dokson J, Hunt WR. Assessment of a novel genetic counselling intervention to inform assisted reproductive technology treatments and other family-building options in adults with cystic fibrosis. Reprod Biomed Soc Online. 2021;13:37–45. doi: 10.1016/j.rbms.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]