Abstract
Background:
Renal ischemia and reperfusion (IR) contribute to perioperative acute kidney injury, and oxygen is a key regulator of this process. We hypothesized that oxygen administration during surgery and renal IR would impact postoperative kidney function and injury in mice.
Methods:
Mice were anesthetized, intubated, and mechanically ventilated with FiO2 0.10 (hypoxia), 0.21 (normoxia), 0.60 (moderate hyperoxia), or 1.00 (severe hyperoxia) during 67 minutes of renal IR or sham IR surgery. Additional mice were treated prior to IR or sham IR surgery with 50 mg/kg tempol, a superoxide scavenger. At 24 hours, mice were sacrificed, and blood and kidney collected. We assessed and compared kidney function and injury across groups by measuring blood urea nitrogen (BUN, primary endpoint), renal histological injury, renal expression of neutrophil gelatinase-associated lipocalin (NGAL), and renal heme oxygenase 1 (Ho-1), peroxisome proliferator-activated receptor gamma coactivator 1-α (Pgc1-α), and glutathione peroxidase 4 (Gpx-4) transcripts, to explore potential mechanisms of any effect of oxygen.
Results:
Hyperoxia and hypoxia during renal IR surgery decreased renal function and increased kidney injury compared to normoxia. Median (interquartile range) baseline BUN was 22.2 mg/dL (18.4–26.0), and 24 hours after IR surgery, BUN was 17.5 mg/dL (95% CI: 1.3 to 38.4; p=0.034) higher in moderate hyperoxia treated animals, 51.8 mg/dL (95% CI: 24.9 to 74.8; p<0.001) higher in severe hyperoxia treated animals, and 64.9 mg/dL (95% CI: 41.2 to 80.3; p<0.001) higher in hypoxia treated animals compared to animals treated with normoxia (p<0.001, overall effect of hyperoxia). Hyperoxia-induced injury but not hypoxia-induced injury was attenuated by pre-treatment with tempol. Histological injury scores, renal NGAL staining, and renal transcription of Ho-1 and suppression of Pgc1-α followed the same pattern as BUN, in relation to the effects of oxygen treatment.
Conclusions:
In this controlled preclinical study of oxygen treatment during renal IR surgery, hyperoxia and hypoxia impaired renal function, increased renal injury, and impacted expression of genes that affect mitochondrial biogenesis and antioxidant response. These results might have implications for patients during surgery when high concentrations of oxygen are frequently administered, especially in cases involving renal ischemia and reperfusion.
Introduction
Acute kidney injury (AKI) affects up to 25% of patients undergoing major surgery and increases cost, duration of hospitalization, dialysis, extrarenal organ injury, and death.1 AKI is the result of multiple perioperative events including deficient renal perfusion, reperfusion injury, oxidative damage, and inflammation.2 Oxygen is a fundamental mediator of these events, and both hypoxia and hyperoxia have distinct cellular consequences. For example, renal hypoxia results in mitochondrial dysfunction, metabolite accumulation, epithelial vacuolization, loss of brush border and cellular polarity, apoptosis, and necrosis.3–5 Reperfusion, in turn, increases production of reactive oxygen species (ROS) in mitochondria, oxidizes lipids and proteins, and induces inflammation, resulting in further cellular injury and renal dysfunction.6–8 Hyperoxic reperfusion may exacerbate these events.9,10 In addition, hyperoxia impairs endothelium-dependent and -independent vascular reactivity, dysregulating tissue perfusion.11 During major surgery, alterations in cardiac output, intravascular volume, and vascular resistance, venous congestion, hemorrhage, transfusion, and anesthetics all impact renal ischemia and reperfusion (IR) and may culminate in AKI. Thus, renal IR injury is common during major surgery, and oxygenation may affect this mechanism of kidney injury.
The ideal oxygen tension to limit IR injury during surgery remains unclear. This is reflected by a lack of consensus for intraoperative oxygen administration,12,13 and the use of supplemental oxygen is ubiquitous in major surgery. Patients receive a wide range of oxygen treatments during surgery, ranging from 21% to 100% oxygen. Overall, the effect of hyperoxia on perioperative organ injury is not fully understood. To explore this phenomenon, we tested the hypothesis that oxygen treatment during renal ischemia and reperfusion affects postoperative serum BUN, the primary outcome measure, and other markers of kidney injury in mice.
Methods
We administered four different fractions of inspired oxygen (FiO2) to mice during mechanical ventilation, surgery, renal ischemia, and renal reperfusion. We then measured renal function, renal injury, and renal transcriptomic changes in genes involved in antioxidant responses to injury and mitochondrial function. In addition, we treated a subgroup of animals with tempol, a superoxide dismutase mimetic, to test whether scavenging of superoxide impacts any effect of oxygen treatment.
Animals
All procedures involving animals were approved by the Vanderbilt Institutional Animal Care and Use Committee and adhere to guidelines set in the National Institutes of Health Guide for the Care and use of Laboratory Animals. Male FVB/NJ mice were obtained from Jackson Laboratories (Bar Harbor, Maine) and acclimated to their environment for at least 3 days prior to experimentation at 8 weeks of age. Mice were housed in institutional department of animal care facilities, provided standard chow and water ad libitum, and maintained on a 12-hour light/dark cycle.
Oxygen treatment and surgical model
On the day of surgery, mice were assigned to hypoxia (FiO2: 0.10), normoxia (FiO2: 0.21), moderate hyperoxia (FiO2: 0.60), or severe hyperoxia (FiO2: 1.00) treatment (Figure 1). We did not explicitly use a randomization scheme to assign groups to animals, but we minimized confounding by not having any particular order to the treatments and performing identical sets of measurements on each animal. To further limit confounding, all animals were housed in our lab group’s cage location at the core facility, and we used mice of identical genetic background, age, and weight. Mice were anesthetized with intraperitoneal 100 mg/kg ketamine and 10 mg/kg xylazine and provided analgesia with 1 mg/kg sustained release buprenorphine. The dorsal flanks were shaved and prepped for surgery. Mice were then intubated with a 20-gauge angiocath, connected to a small animal ventilator (CWE, Incorporated, Ardmore, Pennsylvania), and ventilated with volume control mode, tidal volume 0.25 mL, and respiratory rate 110 breaths per minute. Oxygen treatments were achieved using the Sechrist 3500 low flow air-oxygen mixer (Sechrist, Anaheim, CA) and tanks with 10%, 21%, and 100% oxygen. Mice were delivered their assigned FiO2 treatment for 67 minutes, including 7 minutes for renal dissection and clamping, 30 minutes of renal ischemia, and 30 minutes of reperfusion, before extubation to room air.
Figure 1:
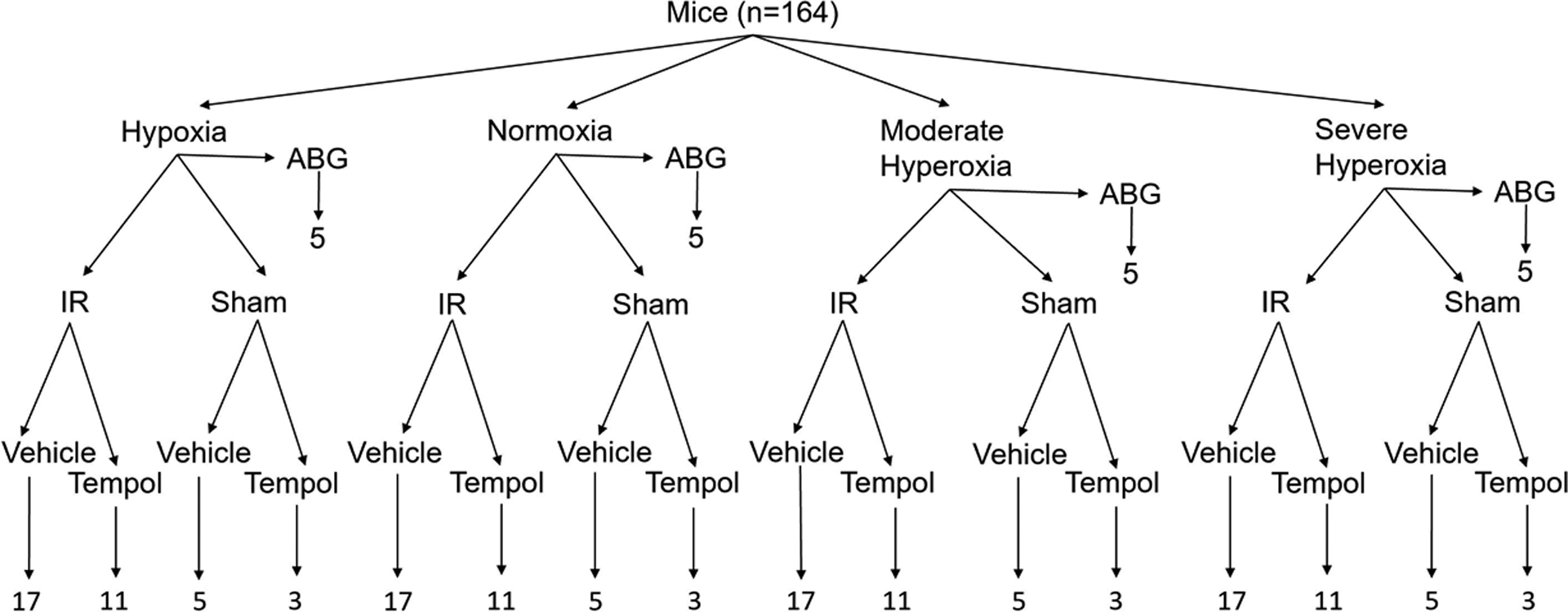
Experimental design matrix. One hundred and sixty-four mice were assigned to one of four oxygen treatment groups and underwent either arterial blood gas analysis, renal ischemia and reperfusion (IR) surgery, or sham IR surgery. Some mice were pre-treated with the superoxide scavenger tempol and others with vehicle.
We performed a unilateral nephrectomy and contralateral renal ischemia and reperfusion injury, as described previously.14 Briefly, through a midline incision, the right flank was incised and right kidney exposed, dissected, ligated, and discarded. The left flank was incised, and the hilum of the left kidney exposed. The renal artery and vein were then occluded with a nontraumatic vascular clamp for 30 minutes. After 30 minutes of ischemia, the clamp was released, the flank muscle closed, and skin approximated with wound clips. Following 30 minutes of reperfusion, all mice were extubated to room air and administered subcutaneous 0.5 mL saline for hydration, then actively warmed for 3 hours until fully recovered. Sham IR surgery mice received identical treatment, except no renal vascular clamp was applied.
We treated a subset of animals with the antioxidant tempol (50 mg/kg, approximately 0.25 mL, intraperitoneal and isolated from the kidney due to the dorsal approach for IR and retroperitoneal location of the kidney) ten minutes prior to ischemia to test the hypothesis that hyperoxia-induced injury is mediated at least in part by increased ROS. Tempol (Tokyo Chemical Industry, Tokyo, Japan) is a stable free-radical scavenger and superoxide dismutase mimetic.15
Twenty-four hours after reperfusion, mice were again anesthetized with intraperitoneal ketamine and xylazine. The chest was opened, and the animal euthanized via terminal exsanguination during cardiac puncture and blood collection. The renal artery, vein, and ureter were transected, and the IR kidney removed. Blood and kidney were immediately processed as described below.
To confirm the effect of FiO2 on arterial oxygenation, we measured arterial blood gas values in a separate cohort of mice. These mice were anesthetized, intubated, and mechanically ventilated as described above. After 15 minutes of mechanical ventilation, the chest was opened and arterial blood was aspirated from the left ventricle and oxygenation measured with a blood analyzer (iSTAT; CG4+ cartridge; Zoetis, Florham Park, NJ).
Renal function and semi-quantitative analysis of renal injury
Immediately after collection, blood was centrifuged for 10 minutes at 1000 G at −4 °C in a heparinized vial. Plasma was frozen in liquid nitrogen and stored at −80 °C until analysis. Plasma blood urea nitrogen (BUN), the primary efficacy endpoint of the study, was quantified with the enzymatic UV-kinetic initial rate method (Infinity Urea; Thermo Scientific, Middletown, VA).
The poles of the IR kidneys were removed, and a 2 mm thick axial section was cut and fixed in formalin phosphate acetate salt for 24 hours. Samples were then embedded in paraffin and cut into 5-μm sections for staining. Periodic acid Schiff (PAS) stain was used to highlight tissue architecture for tubular injury assessment. A treatment-blinded investigator scored PAS-stained kidneys on the basis of four categories 1) tubular vacuolization, 2) tubular dilatation, 3) presence of casts, and 4) loss of brush border. Each high-powered field was given a score of 0 if the abnormality represented <5% of the field, 1 if the abnormality was present in 5–25% of the field, 2 for 25–50%, 3 for 50–75%, and 4 for greater than 75%. Scores for each of the 4 components were averaged for a histological injury score with range 0–4.
We also performed immunohistochemical staining for neutrophil gelatinase associated lipocalin (NGAL), a marker of distal renal tubule damage.16 NGAL is a siderophore-chelating protein that is upregulated in renal tubule cells within hours of renal injury and is a marker of preclinical and clinical AKI.16,17 Non-overlapping cortical and outer medullary sections were assessed at 400x magnification in 10 randomly selected sections of each kidney, and the percentage of total kidney area stained in the cortex and outer medulla was quantified via threshold analysis using CellSens software by an investigator blinded to treatment group.
To explore pathways related to mitochondrial function and oxidative stress as potential mechanisms of oxygen treatment effects on kidney injury, we measured transcriptomic changes in heme oxygenase 1 (Ho-1), peroxisome proliferator-activated receptor gamma coactivator 1-α (Pgc1-α), and glutathione peroxidase 4 (Gpx-4) using quantitative reverse transcription polymerase chain reaction in kidneys collected from subsets of animals in each oxygen group. Ho-1 is an enzyme that converts pro-oxidant heme to biliverdin, carbon monoxide, and iron. The scavenging of free heme and generation of carbon monoxide reduce oxidative damage and inflammation.18 Hypoxia, oxidative stress, and inflammatory cytokines induce Ho-1 expression.19 Pgc1-α is a transcription factor that regulates mitochondrial gene expression and function, resulting in mitochondrial biogenesis, oxidative phosphorylation, and glucose utilization.20 Gpx-4 is an antioxidant enzyme that catalyzes the reduction of hydrogen peroxide to water while converting glutathione from the reduced to oxidized form and serves as a key regulator of ferroptosis, an iron-dependent pathway of cell death that can be induced by ROS.21
To measure these transcripts, RNA was extracted from renal tissue (RNeasy mini kit, Quiagen, Hilden, Germany), and cDNA was synthesized using the Bio-Rad iScript kit (Hercules, CA). Transcripts were analyzed using VWR PerfeCTa SYBR Green FastMix kit and Bio-Rad CFX Real Time PCR quantification system, and mRNA concentration was expressed using the ΔCt equation versus the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase. Relative expression levels of genes were quantified using Bio-Rad CFX Maestro software, version 1.1. Primer sequences are listed in Supplemental Table 1.
Statistical Analysis
All continuous measurements including BUN, histological injury score, immunohistochemical staining score, and relative quantification of gene expression are reported as median (interquartile range). Since we administered ordered doses of supplemental oxygen and expected an ordered response to oxygen treatment across the normoxia-moderate hyperoxia-severe hyperoxia spectrum, the Jonckheere-Terpstra test for trend was used to assess for a dose-dependent response to hyperoxia across normoxia, moderate hyperoxia, and severe hyperoxia groups. We did not expect an ordered response to oxygen treatment, however, across the hypoxia-normoxia-hyperoxia spectrum,22–24 a requisite of the Jonckheere-Terpstra test.25 We therefore separately compared hypoxia to normoxia treatment groups using the Mann-Whitney U-Test. Additional comparisons between two treatment groups were also quantified with the Mann-Whitney U-Test. Median differences with 95% confidence intervals between groups were calculated to assess effects of treatments. Fisher’s exact test was used to assess effects of oxygen treatment on survival rates. Nonparametric analysis methods were used throughout to maintain a conservative approach. Statistical analyses were performed with Stata software (version 17.0), and primary results were considered statistically significant when P <0.05.
Sample size was calculated based on pilot IR experiments in which the standard deviation of BUN in each oxygen group was ~20 mg/dL and previous studies that demonstrated that an acute increase of 20 mg/dL BUN leads to persistent renal dysfunction.26 To detect a BUN difference of 20 mg/dL between hyperoxia and normoxia treatment groups with a standard deviation of 20 mg/dL, a type I error rate of 5%, and 80% power, 17 mice per treatment group are needed. Variability was lower amongst animals in the sham surgery groups (standard deviation only 3 mg/dL in pilot animals). Five animals in each sham IR surgery oxygen group provided 80% power to detect the same difference in BUN. The study design and all analyses abided by ARRIVE guidelines.
Results
Oxygenation
The median (interquartile range) PaO2 was 40 mmHg (36–44) in the hypoxia group, 104 mmHg (94–112) in the normoxia group, 218 mmHg (211–222) in the moderate hyperoxia group, and 318 mmHg (270–401) in the severe hyperoxia group (p<0.001, effect of oxygen, Figure 2).
Figure 2:
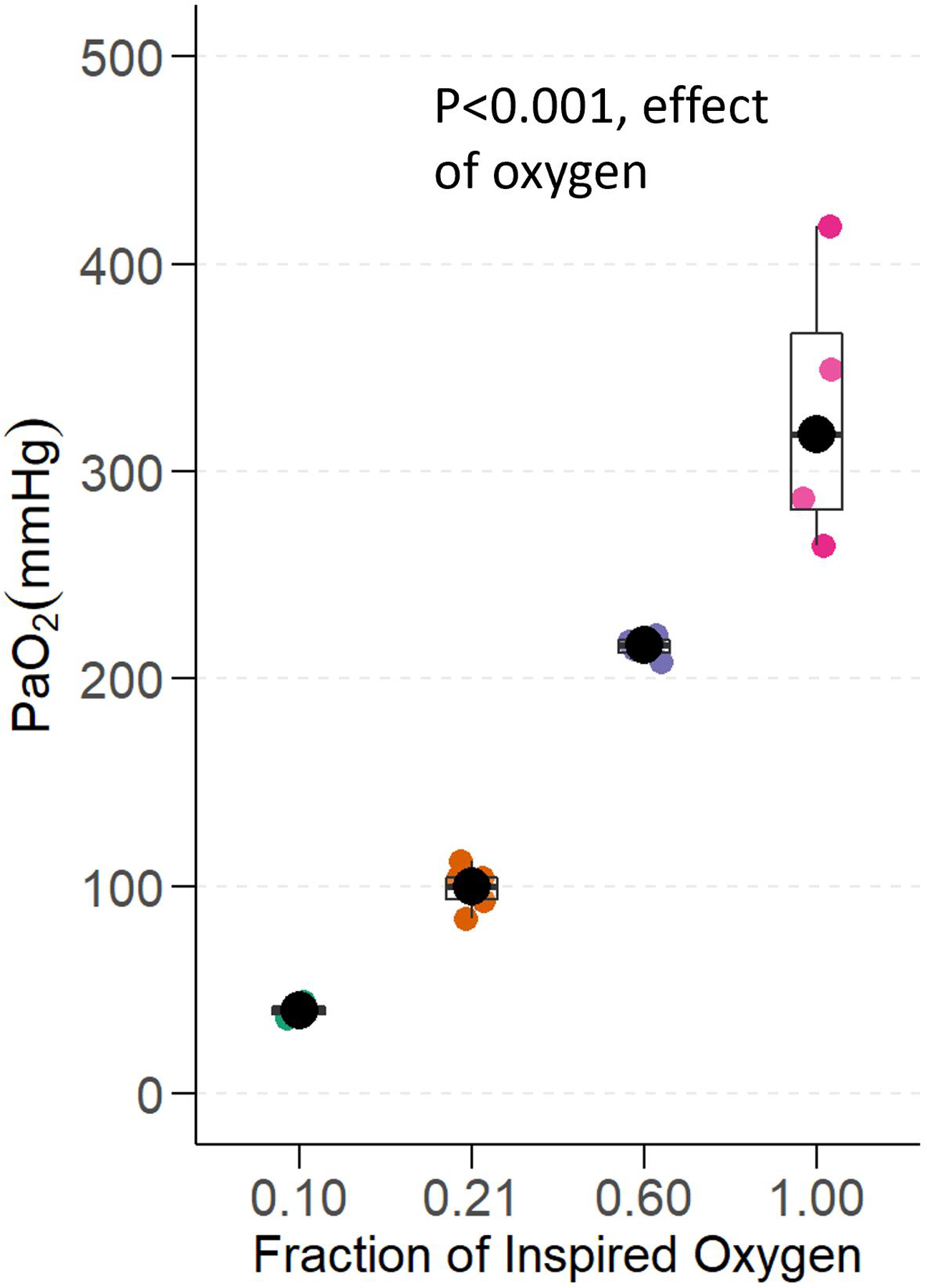
Oxygenation. Partial pressure of arterial oxygen (PaO2) in mice ventilated with hypoxia (FiO2 0.10), normoxia (FiO2 0.21), moderate hyperoxia (FiO2 0.60), and severe hyperoxia (FiO2 1.00). P value represents the effect of oxygen treatment on PaO2 (n=5/group).
Survival
Survival at 24 hours was 36 of 36 (100%) in all animals assigned to normoxia, 35 of 36 (97%) in animals assigned moderate hyperoxia, and 36 of 36 (100%) in animals severe hyperoxia. In the hypoxia groups, 16 of 36 mice (44%) died during mechanical ventilation or shortly thereafter (survival 20 of 36 [56%], p<0.001, effect of oxygen treatment on survival).
Renal Function
The median (interquartile range) baseline BUN was 22.2 mg/dL (18.4–26.0). Mice treated with hyperoxia or with hypoxia during renal IR surgery had greater BUN 24 hours after IR than mice treated with normoxia during renal IR surgery (Figure 3A, Supplemental Table 2). BUN was a median of 17.5 mg/dL (95% CI: 1.3 to 38.4; p=0.034) higher in mice treated with moderate hyperoxia and 51.8 mg/dL (24.9 to 74.8; p<0.001) higher in mice treated with severe hyperoxia than in mice treated with normoxia. There was also a significant dose effect of increasing hyperoxia on BUN (p<0.001, Jonckheere-Terpstra test). BUN was a median of 64.9 mg/dL (95% CI: 41.2 to 80.3; p<0.001) higher in mice treated with hypoxia compared to mice treated with normoxia.
Figure 3:
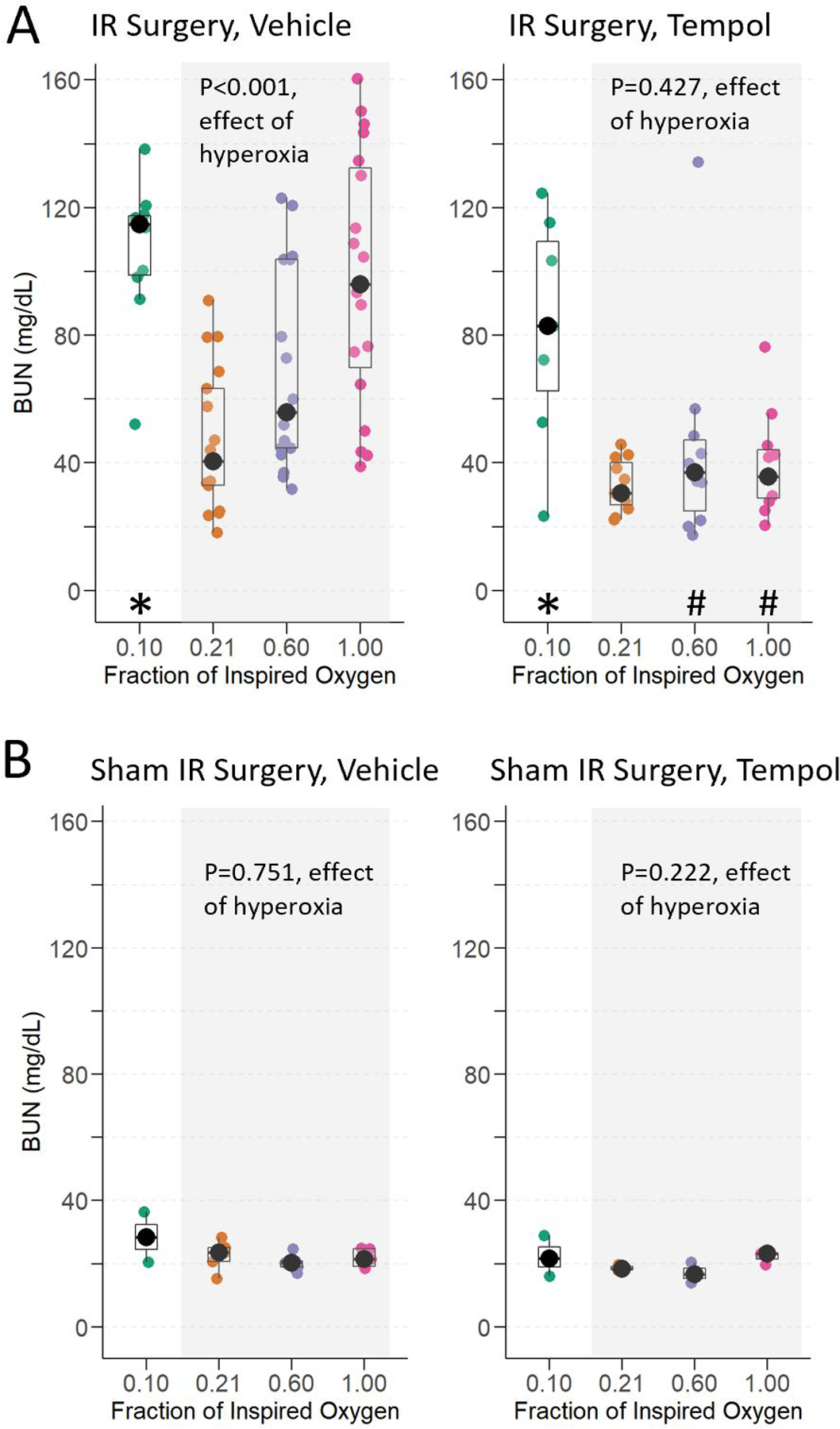
Renal Function. Blood urea nitrogen (BUN) at 24-hours in mice exposed to hypoxia, normoxia, moderate hyperoxia, and severe hyperoxia during renal IR surgery (A) and sham IR surgery (B). Effect of hyperoxia treatment determined by Jonckheere-Terpstra test for trend across normoxia, moderate hyperoxia, and severe hyperoxia groups. * indicates p <0.05 for comparison between animals administered hypoxia vs. normoxia and # indicates p<0.05 for comparison of tempol vs. vehicle within each oxygen treatment, determined by Mann-Whitney U-Test.
Conversely, 24 hours after sham IR surgery, renal function was preserved compared to baseline amongst all oxygen groups (Figure 3B). There was no effect of hyperoxia (p=0.751) or hypoxia (p=0.857) on BUN following sham IR surgery.
With tempol pre-treatment, the effect of increasing hyperoxia on BUN was lost (p=0.427, effect of hyperoxia, Figure 3). The hyperoxia tempol groups were not different from normoxia (median difference between moderate hyperoxia tempol and normoxia tempol: 4.4 mg/dL [95% CI: −18.1 to 8.2; p=0.605] and between severe hyperoxia tempol and normoxia tempol: 4.3 mg/dL [−14.5 to 5.1; p=0.411]). Additionally, with tempol treatment, the severe hyperoxia group was not different from the normoxia vehicle group (median difference 4.7 mg/dL [95% CI: −8.0 to 22.1]; p=0.547) (i.e., tempol protected renal function in mice subjected to hyperoxia during renal IR surgery). In contrast to tempol’s impact on the effect of hyperoxia on renal dysfunction, tempol did not alter the effect of hypoxia. The BUN in mice assigned hypoxia and tempol remained elevated (52.4 mg/dL higher [95% CI: 11.0 to 85.0]; p=0.006) compared to mice assigned normoxia and tempol and was not different from mice assigned hypoxia and vehicle (i.e., no tempol protection from effects of hypoxia on renal function). Renal function among the sham IR surgery groups was also not affected by tempol pre-treatment (p=0.222, effect of hyperoxia on BUN).
Histological Injury
Mice that received hyperoxia and hypoxia during renal IR surgery had worse renal histological injury 24 hours after IR surgery compared to mice that received normoxia during renal IR surgery (Figure 4A). Histological injury score was a median of 0.7 points (95% CI: 0.1 to 1.1; p=0.026) higher in mice treated with moderate hyperoxia and 1.2 points (95% CI: 0.5 to 1.8; p<0.001) higher in mice treated with severe hyperoxia compared to mice treated with normoxia. This reflected a significant dose effect (p<0.001, effect of increasing hyperoxia on histological injury score, Figure 4B). Hypoxia also increased renal histological injury versus normoxia (3.8 points [95% CI: 2.7 to 5.0; p<0.001]).
Figure 4:
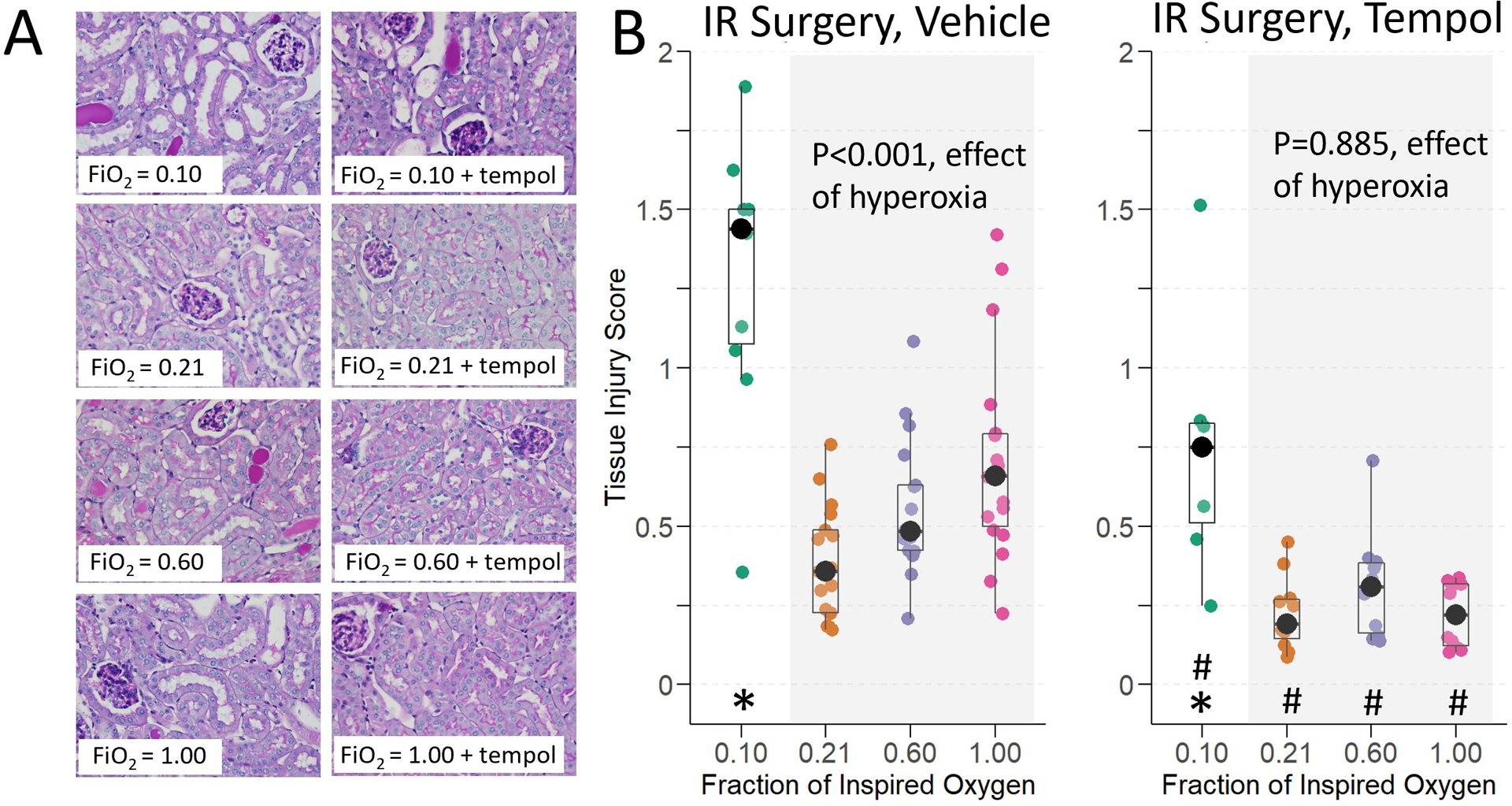
Histological injury at 24-hours in mice exposed to hypoxia, normoxia, moderate hyperoxia, and severe hyperoxia during renal IR surgery. A) Representative images of PAS-stained corticomedullary kidney sections at 400x magnification. B) Tissue injury score. Effect of hyperoxia treatment determined by Jonckheere-Terpstra test for trend across normoxia, moderate hyperoxia, and severe hyperoxia groups. * indicates p <0.05 for comparison between animals administered hypoxia vs. normoxia, and # indicates p<0.05 for comparison of tempol vs. vehicle within each oxygen treatment, determined by Mann-Whitney U-Test.
Histological injury was minimal in sham-operated animals, and there was no effect of hyperoxia on the injury score (p=0.907, effect of hyperoxia, Supplemental Figure 1A).
Pre-treatment with tempol reduced the degree of histological injury and eliminated the effect of increasing hyperoxia on histological injury (p=0.885, effect of hyperoxia among tempol IR treatment groups). In fact, tempol pre-treatment in mice assigned hyperoxia decreased histological injury a median of 0.6 (95% CI: 0.2 to 1.0; p=0.016) below that of mice assigned normoxia vehicle, evidence of tempol protection from hyperoxia during IR. Similar to BUN, histological injury score remained higher in mice assigned hypoxia tempol (median 1.9 points higher in [95% CI: 0.7 to 2.9]; p<0.001) compared to normoxia vehicle, evidence of no tempol protection from hypoxia during IR. Similar to mice treated with vehicle prior to sham renal IR surgery, there was no effect of oxygen exposure on histological injury in mice pre-treated with tempol prior to sham renal IR surgery (p=0.059, effect of hyperoxia and p=0.100, effect of hypoxia; Supplemental Figure 1A).
Immunohistochemical Injury Markers
At baseline, NGAL expression was low, comprising a median (interquartile range) of 0.49% (0.39–0.81) of the area of the kidney. Consistent with the effects of oxygen treatment on BUN and tissue injury scores, NGAL staining was greatest in the hypoxic and severe hyperoxic groups, oxygen treatment did not affect NGAL staining in mice exposed to sham IR surgery, and tempol treatment reduced NGAL in mice exposed to hyperoxia during renal IR surgery (Figure 5A). A dose effect of increasing hyperoxia during renal IR on increased NGAL staining was observed (p<0.001, effect of hyperoxia, Figure 5B). NGAL expression was also higher in IR surgery animals compared to sham IR surgery animals. In contrast to renal IR surgery experiments, hyperoxia decreased NGAL staining in sham IR surgery animals (p=0.030, effect of hyperoxia, Supplemental Figure 1B).
Figure 5:
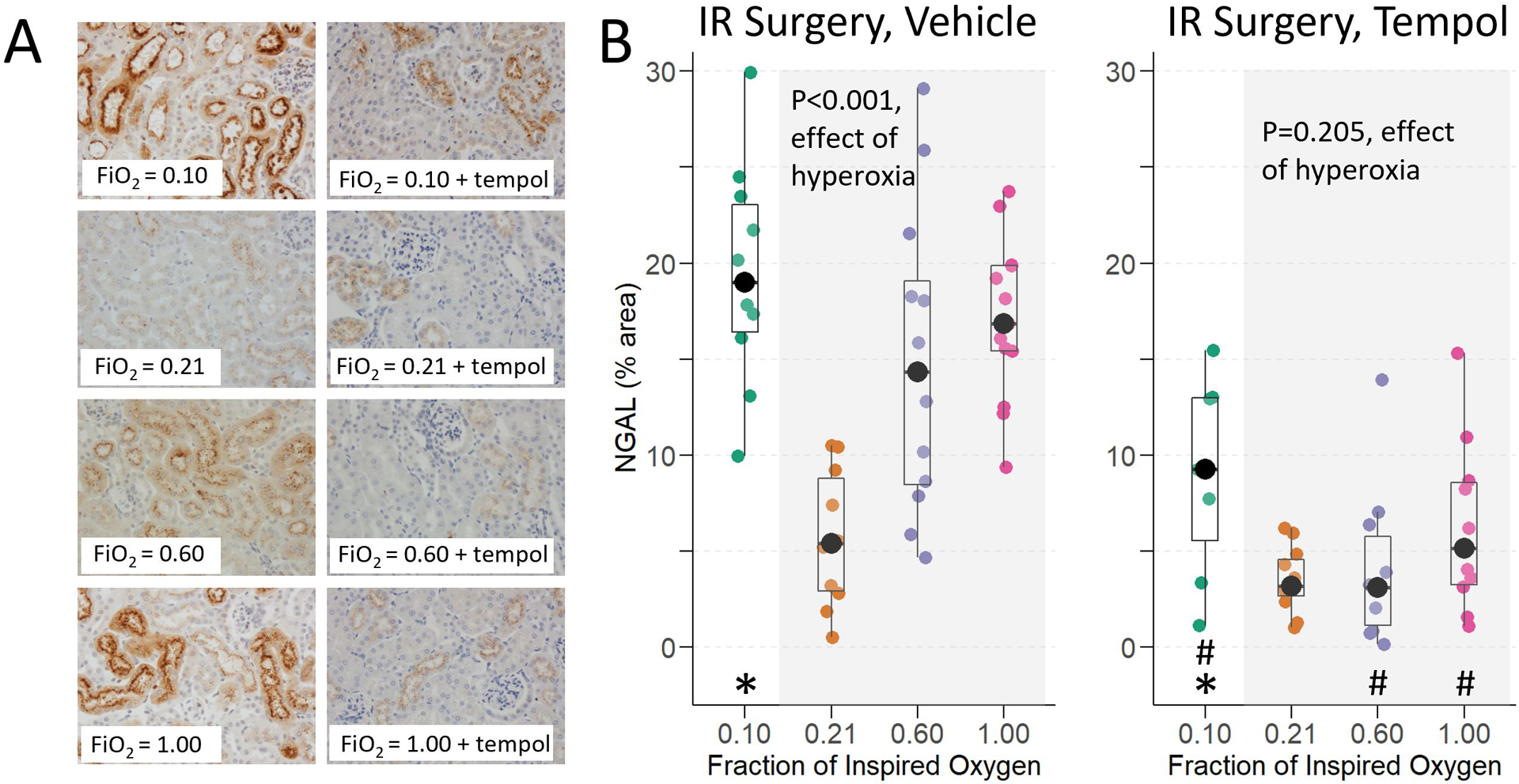
Neutrophil gelatinase associated lipocalin (NGAL) staining at 24 hours in mice exposed to hypoxia, normoxia, moderate hyperoxia, and severe hyperoxia during renal IR surgery. A) Representative images of immunohistochemical staining for NGAL. B) Threshold quantification of NGAL expression. Effect of hyperoxia treatment determined by Jonckheere-Terpstra test for trend across normoxia, moderate hyperoxia, and severe hyperoxia groups. * indicates p <0.05 for comparison between animals administered hypoxia vs. normoxia, and # indicates p<0.05 for comparison of tempol vs. vehicle within each oxygen treatment, determined by Mann-Whitney U-Test.
Tempol reduced NGAL staining in hyperoxic and hypoxic treated animals. Thus, tempol treatment eliminated the effect of hyperoxia on NGAL staining (p=0.205, effect of hyperoxia), and tempol treatment decreased NGAL staining in hypoxia treated animals a median of 9.9% (95% CI: 4.4 to 16.7; p=0.001) less than in hypoxia vehicle treated animals. This last finding is discordant from the lack of an effect of tempol on BUN or tissue injury in hypoxia treated animals. Tempol did not affect NGAL expression in the normoxia group (median difference 2.0% [95% CI: −1.0 to 5.6]; p=0.223, tempol normoxia vs. vehicle normoxia).
Expression of genes associated with antioxidant mechanisms and mitochondrial function
Hyperoxia and hypoxia, but not normoxia, induced renal Ho-1 expression, suppressed Pgc-1α expression, and did not affect Gpx-4 expression. Tempol attenuated hyperoxia- but not hypoxia-induced expression of Ho-1 and suppression of Pgc1-α mRNAs.
At baseline, Ho-1 expression was low (relative quantification [interquartile range]: 0.09 [0.08–0.11]). At 24-hours, Ho-1 was elevated in the hyperoxia groups, with a significant dose effect of hyperoxia on increased Ho-1 expression (p=0.006, effect of hyperoxia), and of hypoxia (p=0.017, effect of hypoxia) (Figure 6A). Tempol pre-treatment eliminated the dose-dependent effect of hyperoxia on Ho-1 induction (p=0.872, effect of hyperoxia) but not the effect of hypoxia on Ho-1 expression (p=0.002, hypoxia tempol vs. normoxia tempol). Ho-1 was much lower in sham IR surgery, and there was no effect of hyperoxia on Ho-1 expression in sham IR surgery animals (p=0.222, Supplemental Figure 2A).
Figure 6:
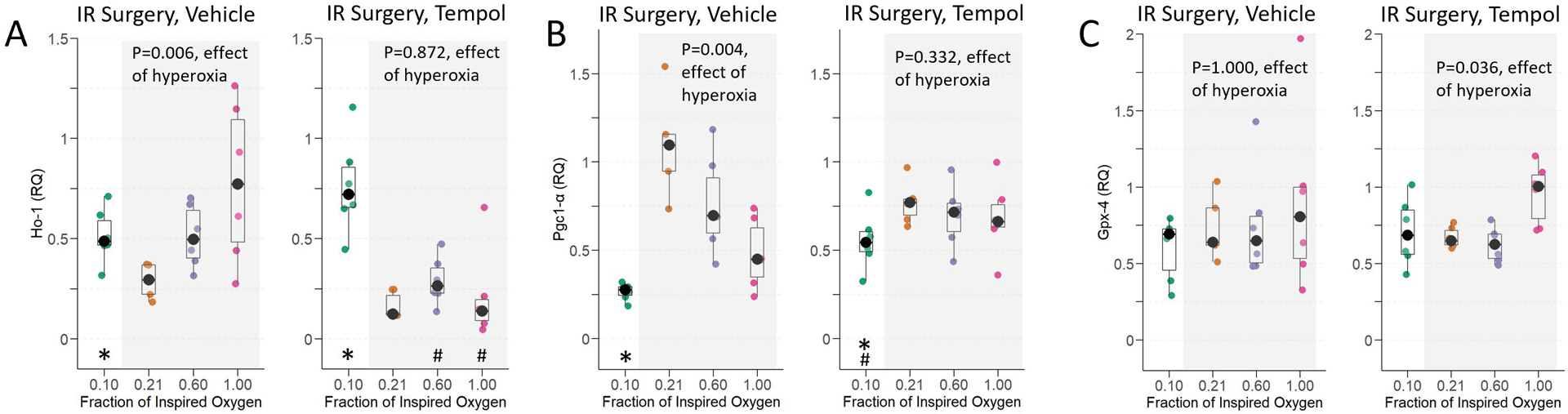
Renal Ho-1 (A), Pgc1-α (B), and Gpx-4 (C) expression at 24-hours in mice exposed to hypoxia, normoxia, moderate hyperoxia, and severe hyperoxia during renal IR surgery. Effect of hyperoxia treatment determined by Jonckheere-Terpstra test for trend across normoxia, moderate hyperoxia, and severe hyperoxia groups. * indicates p <0.05 for comparison between animals administered hypoxia vs. normoxia, and # indicates p<0.05 for comparison of tempol vs. vehicle within each oxygen treatment, determined by Mann-Whitney U-Test.
At baseline, kidneys expressed relatively high levels of Pgc1-α mRNA (relative quantification 1.52 [interquartile range: 1.47–1.78]). In animals that underwent IR surgery, the normoxia treated animals had the highest renal Pgc1-α expression, and both hyperoxia and hypoxia suppressed Pgc1-α (p=0.004, effect of hyperoxia and p=0.004, effect of hypoxia, Figure 6B). Tempol pre-treatment eliminated the effect of hyperoxia on Pgc1-α suppression (p=0.332, effect of hyperoxia). Following sham IR surgery, Pgc1-α expression remained relatively high, and there was no effect of hyperoxia on Pgc1-α expression (p=0.222, effect of hyperoxia, Supplemental Figure 2B).
Hyperoxia had no effect on the renal expression of Gpx-4 mRNA following renal IR surgery (p=1.000, effect of hyperoxia, Figure 6C) or following sham IR surgery (p=0.059, effect of hyperoxia, Supplemental Figure 2C).
Discussion
In this controlled preclinical study of oxygen treatment during renal IR surgery, hypoxia and hyperoxia impaired renal function, increased renal injury, and impacted mitochondrial and antioxidant gene expression compared to normoxia. Moreover, severe hyperoxia had a greater harmful effect on renal injury and function than moderate hyperoxia, consistent with an oxygen dose effect. Hyperoxia-induced renal injury but not hypoxia-induced renal injury was attenuated by pre-treatment with the superoxide scavenger tempol, supporting oxidative stress as a mechanism of hyperoxia- but not hypoxia-induced injury during renal IR.
Some prior preclinical studies have also investigated the effect of oxygen during IR on the kidney and on other organs.27–29 Zwemer and colleagues treated rabbits with 100% oxygen during surgery and renal ischemia and then 100% versus 21% oxygen during reperfusion.24 Rabbits treated with 100% oxygen during reperfusion had increased plasma BUN, serum creatinine, and histological injury compared to those treated with 21% oxygen. Additional oxygenation groups, markers of kidney injury, and potential mechanisms were not examined. In a non-survival renal IR surgical model study from our laboratory, renal concentrations of 4-hydroxynonenol, a marker of lipid peroxidation, were increased in mice spontaneously breathing 100% oxygen compared to in those mice breathing air.23 The non-survival nature of that study precluded investigation of renal function. The current study included multiple oxygenation groups, some markers of potential mechanisms, and also examined multiple sham renal IR surgery groups across the hypoxic to hyperoxic spectrum to best characterize the effect of oxygen with and without renal IR. Prior studies of tempol provided evidence that this antioxidant may reduce kidney injury following renal IR, although oxygen administration was not been controlled in these experiments, limiting investigation of the interactions between oxygen therapy and antioxidant treatment during renal IR injury.30,31 In the current study, the protective effect of tempol directly correlated with the extent of hyperoxia, suggesting that tempol’s action opposed that of hyperoxia. Hyperoxia may increase ROS, thus providing greater opportunity for tempol to affect an oxidative damage pathway of renal injury. Tempol pre-treatment markedly improved renal function in the severe hyperoxia group but not in the normoxia group and provided minimal protection in the hypoxic group. We included the hypoxic groups as positive controls, since renal hypoxia is known to impair renal function and damage kidneys, and to better assess the effect of normoxia in relation to both hypoxia and hyperoxia.5,32 Indeed, systemic hypoxia (FiO2 0.10) during renal IR surgery led to high intraoperative mortality and significant kidney injury in mice that survived. This effect was not attenuated by tempol, suggesting that excessive ROS production is not a primary mediator of hypoxia-induced renal injury.
Renal injury and damage correlated with increased Ho-1 mRNA expression and decreased Pgc1-α mRNA expression. Previous work has shown that ROS, free heme, and tissue ischemia contribute to Ho-1 transcription following clinical AKI.33 This is consistent with the findings that hyperoxia increased Ho-1 mRNA during renal IR as compared to normoxia and that tempol treatment reduced Ho-1 mRNA more in animals treated with hyperoxia than in those treated with normoxia or hypoxia. These findings are further supported by prior studies that demonstrated hyperoxic pre-conditioning and post-conditioning induction of Ho-1 and linked Ho-1 induction to renal protection.34 Conversely, transcription of Pgc1-α, a regulator of mitochondrial biogenesis and mitochondrial function, was decreased following renal IR surgery in animals with the greatest renal injury. Studies have shown that decreased mitochondrial biogenesis is associated with severity of kidney injury.8
This study may have relevance to patients who experience perioperative renal ischemia and reperfusion because these patients are often exposed to high concentrations of oxygen during surgery. Although several retrospective clinical studies have reported an association between intraoperative hyperoxia and postoperative AKI,35–37 clinical trials to date have found little evidence that intraoperative hyperoxia affects AKI.38,39 In the current study, oxygen treatment alone was insufficient to cause renal injury – there was no effect of oxygen treatment on renal injury among animals that received sham IR surgery. It is possible that in clinical studies, renal IR was less severe than in this preclinical model or that other injurious renal mechanisms may be involved.
We note additional limitations that impact translation to clinical care. First, as compared to young mice, humans undergoing major surgery and those at greatest risk for AKI are typically older and with preexisting cardiovascular and renal diseases that could alter the effects of oxygen on renal IR injury. In addition, FVB/NJ mice are an inbred strain that have phenotypic characteristics that may be useful for studying the mechanisms underlying kidney and cardiac disease. These characteristics may limit the generalizability of our findings even to out bred strains of mice or to other species. Second, our surgical model induced complete acute cessation of renal blood flow, whereas patients more commonly have periods of decreased renal perfusion coincident with intraoperative hypotension, hypovolemia, anemia, or venous congestion.40 The surgical model, however, provided a simple and reproducible insult to the kidney. Third, all endpoints were assessed 24 hours after IR surgery, which was sufficient to capture differences in both renal injury and function but limited the ability to determine the time course of renal injury, resolution, or persistence. Finally, the study was powered to detect differences in kidney function in animals exposed to hyperoxia vs. normoxia during renal IR. We did not adjust sample size for comparisons of multiple oxygen treatments, biomarkers, and expression of several genes, although statistical significance of these results was high. Analyses of secondary endpoints may be underpowered, specifically RNA transcriptome analysis.
This study demonstrated a consistent, deleterious dose-dependent effect of hyperoxia on kidney injury and function following renal IR surgery. Gene expression and the impact of the selective superoxide scavenger tempol on hypoxic, normoxic, and hyperoxic groups indicate that oxidative mechanisms of hyperoxia may be involved. These results may have implications for intraoperative oxygen administration during human surgery, especially when significant renal ischemia and reperfusion is expected.
Supplementary Material
Key Points Summary:
Question: Does intraoperative oxygen administration during renal ischemia and reperfusion affect kidney injury in mice?
Findings: Hypoxia and hyperoxia during renal ischemia and reperfusion worsened renal function and kidney injury compared to normoxia; and tempol, a superoxide scavenger, attenuated hyperoxia-induced, but not hypoxia-induced, kidney injury.
Meaning: Hyperoxic ventilation may exacerbate kidney injury in scenarios of renal ischemia and reperfusion.
Acknowledgements:
We acknowledge the Vanderbilt University Translational Pathology Shared Resource Core and the O’Brien Center Histology and Molecular Pathology Core for assistance with histology and immunostaining.
This work was funded a 2018 Burroughs Wellcome Fund Physician-Scientist Institutional Award to Vanderbilt University (MJK), the Vanderbilt Undergraduate Summer Research Program (TJN) and grant support from the National Institutes of Health (T32GM108554 [EHM], K23GM129662 and R01HL164909 [MGL], R01DK132844 and UC2DK126122 [MDC], and R01GM112871, R01HL157583, and R35GM145375 [FTB]
Glossary of Terms:
- AKI
acute kidney injury
- IR
ischemia and reperfusion
- ROS
reactive oxygen species
- FiO2
fraction of inspired oxygen
- PaO2
partial pressure of oxygen in arterial blood
- FVB/NJ mice
Friend Virus B NIH Jackson mice
- BUN
blood urea nitrogen
- NGAL
neutrophil gelatinase-associated lipocalin
- Ho-1
heme oxygenase 1
- Pgc1-α
peroxisome proliferator-activated receptor gamma coactivator 1-α
- Gpx-4
glutathione peroxidase 4
Footnotes
Conflicts of Interest: None
Contributor Information
Melissa J. Kimlinger, Vanderbilt University School of Medicine.
Tom J. No, Vanderbilt University
Eric H. Mace, Department of General Surgery, Vanderbilt University Medical Center.
Rachel D. Delgado, Department of Nephrology, Vanderbilt University Medical Center.
Marcos G. Lopez, Department of Anesthesiology, Vanderbilt University Medical Center.
Mark P. de Caestecker, Department of Nephrology, Vanderbilt University Medical Center.
Frederic T. Billings, IV, Department of Anesthesiology, Vanderbilt University Medical Center.
References:
- 1.O’Neal J, Shaw A, Billings F. Acute kidney injury following cardiac surgery: current understanding and future directions. Critical care (London, England). 07/04/2016 2016;20(1)doi: 10.1186/s13054-016-1352-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billings FT, Lopez MG, Shaw AD. The incidence, risk, presentation, pathophysiology, treatment, and effects of perioperative acute kidney injury. Canadian journal of anaesthesia. 2021 Mar 2021;68(3)doi: 10.1007/s12630-020-01894-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chouchani ET, Pell VR, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014/11/01 2014;515(7527):431–435. doi: 10.1038/nature13909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmago S, Shah SV, Walker PD, walkerpd@kidneybx.com. Meprin, a brush-border enzyme, plays an important role in hypoxic/ischemic acute renal tubular injury in rats1. Kidney International. 2002/03/01 2002;61(3):959–966. doi: 10.1046/j.1523-1755.2002.00209.x [DOI] [PubMed] [Google Scholar]
- 5.Khan S, Cleveland R, Koch C, Schelling J. Hypoxia induces renal tubular epithelial cell apoptosis in chronic renal disease. Laboratory investigation; a journal of technical methods and pathology. 1999 Sep 1999;79(9) [PubMed] [Google Scholar]
- 6.Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nature Medicine. 2011/11/01 2011;17(11):1391–1401. doi: 10.1038/nm.2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yellon D, Hausenloy D. Myocardial reperfusion injury. The New England journal of medicine. 09/13/2007 2007;357(11)doi: 10.1056/NEJMra071667 [DOI] [PubMed] [Google Scholar]
- 8.Plotnikov E, Kazachenko A, Vyssokikh M, et al. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney international. 2007 Dec 2007;72(12)doi: 10.1038/sj.ki.5002568 [DOI] [PubMed] [Google Scholar]
- 9.Fessel J, Flynn C, Robinson L, et al. Hyperoxia synergizes with mutant bone morphogenic protein receptor 2 to cause metabolic stress, oxidant injury, and pulmonary hypertension. American journal of respiratory cell and molecular biology. 2013 Nov 2013;49(5)doi: 10.1165/rcmb.2012-0463OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoner J, Clanton T, Aune S, Angelos M. O2 delivery and redox state are determinants of compartment-specific reactive O2 species in myocardial reperfusion. American journal of physiology Heart and circulatory physiology. 2007 Jan 2007;292(1)doi: 10.1152/ajpheart.00925.2006 [DOI] [PubMed] [Google Scholar]
- 11.Mace EH, Kimlinger MJ, No TJ, et al. Soluble guanylyl cyclase activation rescues hyperoxia-induced dysfunction of vascular relaxation. Shock. 08/16/2022 2022; In Pressdoi: 10.1097/SHK.0000000000001982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenk M, Van Aken H, Zarbock A. The New World Health Organization Recommendations on Perioperative Administration of Oxygen to Prevent Surgical Site Infections: A Dangerous Reductionist Approach? Anesthesia and analgesia. 2017 Aug 2017;125(2)doi: 10.1213/ANE.0000000000002256 [DOI] [PubMed] [Google Scholar]
- 13.Allegranzi B, Zayed B, Bischoff P, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. The Lancet Infectious diseases. 2016 Dec 2016;16(12)doi: 10.1016/S1473-3099(16)30402-9 [DOI] [PubMed] [Google Scholar]
- 14.Skrypnyk NI, Harris RC, de Caestecker MP. Ischemia-reperfusion model of acute kidney injury and post injury fibrosis in mice. J Vis Exp. Aug 9 2013;(78)doi: 10.3791/50495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilcox C Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacology & therapeutics. 2010 May 2010;126(2)doi: 10.1016/j.pharmthera.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt-Ott KM, Mori K, Li JY, et al. Dual Action of Neutrophil Gelatinase–Associated Lipocalin. 2007-02-01 2007;doi: 10.1681/ASN.2006080882 [DOI] [PubMed] [Google Scholar]
- 17.Devarajan P Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomarkers in medicine. 2010;4(2):265–280. doi: 10.2217/bmm.10.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nath K, Balla G, Vercellotti G, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. The Journal of clinical investigation. 1992 Jul 1992;90(1)doi: 10.1172/JCI115847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waza AA, Hamid Z, Ali S, Bhat SA, Bhat MA. A review on heme oxygenase-1 induction: is it a necessary evil. ReviewPaper. Inflammation Research. 2018-04-24 2018;67(7):579–588. doi:doi: 10.1007/s00011-018-1151-x [DOI] [PubMed] [Google Scholar]
- 20.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovascular research. 07/15/2008 2008;79(2)doi: 10.1093/cvr/cvn098 [DOI] [PubMed] [Google Scholar]
- 21.Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. OriginalPaper. Nature Cell Biology. 2014-11-17 2014;16(12):1180–1191. doi:doi: 10.1038/ncb3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. American journal of physiology Renal physiology. 12/01/2014 2014;307(11)doi: 10.1152/ajprenal.00425.2014 [DOI] [PubMed] [Google Scholar]
- 23.Kimlinger MJ, Mace EH, Harris RC, et al. Impact of inhaled oxygen on reactive oxygen species production and oxidative damage during spontaneous ventilation in a murine model of acute renal ischemia and reperfusion. Research Articles. https://esmedorg/MRA/mra. 2021-10-29 2021;9(10)doi:https://esmed.org/MRA/mra/article/view/2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zwemer CF, Shoemaker JL Jr, Hazard SW 3rd, Davis RE, Bartoletti AG, Phillips CL. Hyperoxic reperfusion exacerbates postischemic renal dysfunction. Surgery. Nov 2000;128(5):815–21. doi: 10.1067/msy.2000.109117 [DOI] [PubMed] [Google Scholar]
- 25.Jonckheere AR. A DISTRIBUTION-FREE k-SAMPLE TEST AGAINST ORDERED ALTERNATIVES. Biometrika. 1954;41(1–2):133–145. doi: 10.1093/biomet/41.1-2.133 [DOI] [Google Scholar]
- 26.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. OriginalPaper. Nature Medicine. 2010-05-02 2010;16(5):535–543. doi:doi: 10.1038/nm.2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujita M, Tsuruta R, Kaneko T, et al. Hyperoxia suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats: laboratory study. Shock. Sep 2010;34(3):299–305. doi: 10.1097/SHK.0b013e3181ceeeec [DOI] [PubMed] [Google Scholar]
- 28.Shin HK, Oka F, Kim JH, Atochin D, Huang PL, Ayata C. Endothelial dysfunction abrogates the efficacy of normobaric hyperoxia in stroke. J Neurosci. Nov 12 2014;34(46):15200–7. doi: 10.1523/jneurosci.1110-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zangl Q, University Hospital of the Ludwig- Maximilians-University DoA, Klinikum Grosshadern, Munich, Germany, Martignoni A, et al. Postoperative Hyperoxia (60%) Worsens Hepatic Injury in Mice. Anesthesiology. 2014;121(6):1217–1225. doi: 10.1097/ALN.0000000000000447 [DOI] [PubMed] [Google Scholar]
- 30.Aksu U, Ergin B, Bezemer R, et al. Scavenging reactive oxygen species using tempol in the acute phase of renal ischemia/reperfusion and its effects on kidney oxygenation and nitric oxide levels. OriginalPaper. Intensive Care Medicine Experimental. 2015-07-04 2015;3(1):1–10. doi:doi: 10.1186/s40635-015-0057-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang G, Wang Q, Zhou Q, et al. Protective Effect of Tempol on Acute Kidney Injury Through PI3K/Akt/Nrf2 Signaling Pathway. Kidney and Blood Pressure Research. 2022;41(2):129–138. doi: 10.1159/000443414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. research-article. https://doiorg/101152/ajprenal004252014. 2014. Dec 01 2014;doi: 10.1152/ajprenal.00425.2014 [DOI] [PubMed] [Google Scholar]
- 33.Billings FT, Yu C, Byrne JG, Petracek MR, Pretorius M. Heme Oxygenase-1 and Acute Kidney Injury following Cardiac Surgery. Cardiorenal Medicine. 2022;4(1):12–21. doi: 10.1159/000357871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pei J, Cai S, Song S, et al. Normobaric hyperoxia plays a protective role against renal ischemia-reperfusion injury by activating the Nrf2/HO-1 signaling pathway. Biochemical and biophysical research communications. 10/29/2020 2020;532(1)doi: 10.1016/j.bbrc.2020.07.004 [DOI] [PubMed] [Google Scholar]
- 35.Bae J, Kim J, Lee S, et al. Association Between Intraoperative Hyperoxia and Acute Kidney Injury After Cardiac Surgery: A Retrospective Observational Study. Journal of cardiothoracic and vascular anesthesia. 2021 Aug 2021;35(8)doi: 10.1053/j.jvca.2020.11.054 [DOI] [PubMed] [Google Scholar]
- 36.Shen Y, Weizhe R, Cao L, Jiang R, Xu X. Impact of partial pressure of oxygen trajectories on the incidence of acute kidney injury in patients undergoing cardiopulmonary bypass - Journal of Cardiology. Journal of Cardiology. 2022;79(4):545–550. doi:doi: 10.1016/j.jjcc.2021.10.028 [DOI] [PubMed] [Google Scholar]
- 37.McIlroy DR, Shotwell MS, Lopez MG, et al. Oxygen administration during surgery and postoperative organ injury: observational cohort study. BMJ (Clinical research ed). 11/30/2022 2022;379 doi: 10.1136/bmj-2022-070941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGuinness S, Parke R, Drummond K, et al. A Multicenter, Randomized, Controlled Phase IIb Trial of Avoidance of Hyperoxemia during Cardiopulmonary Bypass. Anesthesiology. 2016 Sep 2016;125(3)doi: 10.1097/ALN.0000000000001226 [DOI] [PubMed] [Google Scholar]
- 39.Smit B, Smulders Y, de Waard M, et al. Moderate hyperoxic versus near-physiological oxygen targets during and after coronary artery bypass surgery: a randomised controlled trial. Critical care (London, England). 03/10/2016 2016;20 doi: 10.1186/s13054-016-1240-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez M, Shotwell M, Morse J, et al. Intraoperative venous congestion and acute kidney injury in cardiac surgery: an observational cohort study. British journal of anaesthesia. 2021 Mar 2021;126(3)doi: 10.1016/j.bja.2020.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


