Abstract
Background:
Frail older adults may be less likely to receive guideline-directed medical therapy (GDMT)—renin-angiotensin blockers, beta-blockers, and mineralocorticoid receptor antagonists—for heart failure with reduced ejection fraction (HFrEF). We aimed to examine the uptake of angiotensin receptor neprilysin inhibitor (ARNI) and GDMT in frail older adults with HFrEF.
Methods:
Using 2015–2019 Medicare data, we estimated the proportion of beneficiaries with HFrEF receiving ARNI and GDMT each year by frailty status, defined by a claims-based frailty index. Logistic regression was used to identify clinical characteristics associated with ARNI initiation. Cox proportional hazards regression was used to examine the association of GDMT use in 2015 and death or heart failure hospitalization in 2016–2019.
Results:
Among 147,506–180,386 beneficiaries with HFrEF (mean age: 77 years; 27% women; 42.6–49.1% frail) in 2015–2019, the proportion of patients receiving ARNI increased in both non-frail (0.4% to 16.4%) and frail (0.3% to 13.7%) patients (p for yearly-trend-by-frailty=0.970). Among those not receiving a renin-angiotensin system blocker, patients with age≥85 years (odds ratio [95% CI], 0.89 [0.80–0.99]), dementia (0.88 [0.81–0.96]), and frailty (0.87 [0.81–0.94]) were less likely to initiate ARNI. The proportion of patients receiving all 3 GDMT classes increased in non-frail patients (22.0% to 27.0%) but changed minimally in frail patients (19.6% to 21.8%). Regardless of frailty status, treatment with at least 1 class of GDMT was associated with lower death or heart failure hospitalization compared to no GDMT medications (hazard ratio [95% CI], 0.94 [0.91–0.97], 0.92 [0.89–0.94], 0.94 [0.91–0.97] for 1, 2, and 3 classes, respectively).
Conclusions:
Our results suggest an evidence-practice gap in the use of ARNI and GDMT in Medicare beneficiaries with HFrEF, particularly those with frailty. Efforts to narrow this gap are needed to reduce the burden of HFrEF in older adults.
Keywords: angiotensin receptor neprilysin inhibitor, heart failure with reduced ejection fraction, guideline-directed medical therapy, frailty
INTRODUCTION
Heart failure is a leading cause of death and hospitalization in Medicare population. Almost half of heart failure patients are frail,1,2 a clinical state that is characterized by decline in physiologic function and increased vulnerability to adverse health events.3 Older adults with heart failure and frailty have poorer quality of life, more hospitalizations, and greater risk of death compared to non-frail patients.1,2 But clinicians are generally less likely to prescribe new drugs or evidence-based therapy to frail patients who are eligible because of the concerns for adverse drug events.2,4,5
In July 2015 the U.S. Food and Drug Administration (FDA) approved sacubitril/valsartan, an angiotensin receptor neprilysin inhibitor (ARNI), for symptomatic heart failure (New York Heart Association Class II-IV) with reduced ejection fraction (HFrEF).6 Based on the PARADIGM-HF trial,7 the American College of Cardiology/American Heart Association/Heart Failure Society of America recommends renin-angiotensin system inhibition with an ARNI, angiotensin converting enzyme (ACE) inhibitor, or angiotensin receptor blocker (ARB) for the treatment of HFrEF as a component of the guideline-directed medical therapy (GDMT).8–10 Although clinical trials show consistent benefits of GDMT such as dapagliflozin or spironolactone in heart failure patients who are frail5,11 the uptake of ARNI in older adults with heart failure and frailty has not been well examined.
The objective of this study was to assess trends in ARNI uptake and GDMT use since the approval of ARNI in Medicare beneficiaries with HFrEF. We hypothesized that frailty was associated with lower use of ARNI and GDMT and that lower utilization of GDMT was associated with an increased risk of death and heart failure hospitalization.
METHODS
Data Sources and Study Overview
This analysis of Medicare claims data was approved by the Institutional Review Board at Brigham and Women’s Hospital, Boston, Massachusetts. Waiver of informed consent was obtained. We analyzed inpatient, outpatient, skilled nursing facility, home health, carrier, and durable medical equipment claims in 2014–2019 to measure clinical characteristics and outcomes. Medication use was captured from part D prescription drug event claims. Vital status and date of death were obtained from Medicare Beneficiary Summary Files. We conducted 1) drug utilization analysis of ARNI and GDMT and 2) clinical outcome analysis by GDMT status. Each analysis is described separately.
Drug Utilization Analysis of ARNI and GDMT
a. Study Population:
We identified fee-for-service Medicare beneficiaries who were ≥66 years old and had prevalent HFrEF as of January 1 of each calendar year (“index date”) from 2015 through 2019. We defined HFrEF by applying the Chronic Conditions Data Warehouse algorithm for heart failure12,13 and a validated claims-based algorithm for reduced ejection fraction14 to the 1-year claims data before the index date. This algorithm has a positive predictive value of 73% for detecting an ejection fraction less than 45%.14 Beneficiaries were excluded from each calendar year cohort if they 1) did not have continuous enrollment in Medicare parts A, B, and D throughout the previous year; 2) had end-stage renal disease or received dialysis in the previous year (because ARNI is contraindicated in an estimated glomerular filtration rate below 30 ml per minute per 1.73 m2 of body-surface area); or 3) did not survive the entire calendar year.
b. Use of Heart Failure Medication and GDMT:
Heart failure medication use was defined as any prescription fill in part D claims in a given calendar year for ARNI, ACE inhibitor, ARB, evidence-based beta-blockers (bisoprolol, carvedilol, or metoprolol), mineralocorticoid receptor antagonists (MRA) (spironolactone and eplerenone), hydralazine and isosorbide dinitrate, and loop diuretics. GDMT was defined as filling of a prescription for drugs in the following 3 classes within a given year: 1) an ARNI, ACE inhibitor, or ARB; 2) a beta-blocker; or 3) a MRA.8–10 Sodium-glucose cotransporter-2 inhibitors were not considered because they had not been approved for heart failure during our study period.
c. Measurements of Clinical Characteristics:
We considered the following covariates available in claims data on the index date: age (65–74, 75–84, or ≥85 years), sex, race (Black, Other [Hispanic, Asian, and Other], and White as reported in the Medicare Beneficiary Summary Files), dual eligibility for Medicare and Medicaid, and selected chronic conditions defined using the Chronic Conditions Data Warehouse algorithms15 (Alzheimer’s disease and related dementias [ADRD], anemia, atrial fibrillation, cancer, chronic kidney disease [CKD], chronic obstructive pulmonary disease [COPD], depression, diabetes, hip or pelvic fracture, hypertension, myocardial infarction, osteoporosis, rheumatoid arthritis or osteoarthritis, and stroke or transient ischemic attack). The Gagne combined comorbidity score16,17 and the Kim claims-based frailty index (CFI) were calculated from claims data in the previous year.18–20 We chose to use the Gagne combined comorbidity score because it predicted mortality in older adults better than the Charlson and Elixhauser measures.17,21 The CFI estimates a deficit-accumulation frailty index (range 0 to 1; greater values indicate more severe frailty). Both a cut-point of 0.2022 or 0.2518,19 have been used previously to define frailty. In this analysis, we used ≥0.20 to ensure an adequate number of patients by frailty status. The CFI has a correlation of 0.59 with a clinical deficit-accumulation frailty index and C statistics of 0.78 for frailty phenotype and 0.84 for having severe activity of daily living disability.23 As surrogate measures of heart failure severity, we measured having a cardiology visit within the past 30 days, ≥2 heart failure hospitalizations within the past year, and heart failure hospitalization within the past 30 days before the index date.
d. Statistical analysis:
To identify clinical characteristics associated with the initiation of an ARNI versus an ACE inhibitor or ARB, we fitted multivariable logistic regression that includes the above-listed covariates among patients who did not receive any drug from ARNI, ACE inhibitor, or ARB in the previous year. We calculated the proportion of patients with HFrEF receiving heart failure medications and GDMT by patients’ frailty status in each year. We tested whether there was a temporal trend by modeling the receipt of a heart failure medication class or GDMT as a function of year (continuous variable), frailty status (binary), and their interaction term, adjusting for all the clinical characteristics mentioned above, using generalized estimating equation logistic regression with exchangeable correlation structure to account for correlation within the same individuals. We repeated the analysis by sex and race.
Clinical Outcome Analysis by GDMT Status
a. Study Population:
We included fee-for-service beneficiaries ≥66 years old with prevalent HFrEF as of January 1, 2016 (“index date”), and used the information on prescription fills from part D claims between January 1, 2015, and December 31, 2015, to define GDMT status. Those who did not have continuous enrollment in Medicare part A, B, and D throughout 2015 were excluded. Beneficiaries were followed from January 1, 2016, until December 31, 2019, for death and heart failure hospitalization.
b. Clinical Outcomes and Follow-Up Period:
The study outcome was a composite endpoint of all-cause death or heart failure hospitalization. Vital status and date of death were obtained from Medicare Beneficiary Summary File. Heart failure hospitalization was defined from inpatient claims with heart failure diagnosis in the primary position. In the clinical outcome analysis, patients were followed from January 1, 2016, until the earliest occurrence of the outcome, disenrollment from Medicare part A and B, or December 31, 2019.
c. Statistical Analysis:
We estimated the incidence rates of death or heart failure hospitalization by the number of GDMT classes that beneficiaries received between January 1, 2015, and December 31, 2015. Cox proportional hazards regression was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the clinical outcomes associated with the number of GDMT class received. We adjusted for age, sex, race, dual eligibility, chronic conditions listed above, Gagne combined comorbidity index, frailty, cardiology visit within the past 30 days, ≥2 heart failure hospitalizations within the past year, and heart failure hospitalization within the past 30 days. We also repeated the analysis separately for frail or non-frail groups. Analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC). A 2-sided p-value <0.05 was considered statistically significant.
RESULTS
Characteristics of Study Population from 2015 to 2019
We identified Medicare beneficiaries with HFrEF on January 1st each year from 147,506 in 2015 to 180,386 in 2019 (Table 1). The mean age (standard deviation) of the population was 77 (SD 7.1) years and 27% were women. The racial composition was 10% Black, 6% Other, and 84% White. There was an upward trend in Gagne comorbidity index [mean (SD): 5.0 (2.8) in 2015 to 6.2 (3.1) in 2019], prevalence of CKD (54.5% in 2015 to 68.5% in 2019), depression (33.2% to 36.8%), and arthritis (60.7% to 64.8%); there was a downward trend in the prevalence of myocardial infarction (21.1% to 20.5%), stroke or transient ischemic attack (23.4% to 22.6%), and frailty (47.2% to 42.6%).
Table 1.
Characteristics of Medicare Beneficiaries with Heart Failure with Reduced Ejection Fraction, 2015–2019
| Characteristics | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|
| Sample size, n | 180386 | 157058 | 149325 | 147782 | 147506 |
| Age, years, mean (SD) | 76.7 (7.1) | 76.7 (7.0) | 76.6 (7.1) | 76.6 (7.1) | 76.5 (7.0) |
| Male, % | 73.3 | 73.0 | 72.9 | 73.3 | 74.3 |
| Race, % | |||||
| White | 85.1 | 84.1 | 83.6 | 83.8 | 84.0 |
| Black | 9.9 | 10.7 | 10.8 | 10.3 | 10.0 |
| Other | 4.9 | 5.3 | 5.6 | 5.9 | 6.1 |
| Dual eligibility, % | 19.4 | 19.5 | 19.4 | 18.5 | 17.7 |
| Gagne comorbidity index, mean (SD) | 5.0 (2.8) | 5.7 (2.9) | 6.1 (3.1) | 6.2 (3.1) | 6.2 (3.1) |
| Cardiovascular comorbidities, % | |||||
| Atrial fibrillation | 54.2 | 55.6 | 55.7 | 55.7 | 55.8 |
| Myocardial infarction | 21.1 | 21.1 | 20.9 | 20.7 | 20.5 |
| Hypertension | 97.0 | 97.4 | 97.4 | 97.3 | 97.1 |
| Stroke or transient ischemic attack | 23.4 | 23.8 | 23.5 | 23.2 | 22.6 |
| Non-cardiovascular comorbidities, % | |||||
| ADRD | 15.2 | 15.9 | 16.7 | 17.1 | 16.9 |
| Anemia | 70.6 | 72.1 | 71.7 | 71.1 | 70.3 |
| Cancer | 19.0 | 19.3 | 19.2 | 19.2 | 19.0 |
| Chronic kidney disease | 54.5 | 60.3 | 65.7 | 67.7 | 68.5 |
| COPD | 47.7 | 48.6 | 48.2 | 47.2 | 46.1 |
| Depression | 33.2 | 34.7 | 35.8 | 36.4 | 36.8 |
| Diabetes | 57.3 | 63.1 | 64.2 | 63.2 | 62.2 |
| Frailty | 47.2 | 49.1 | 43.6 | 43.3 | 42.6 |
| Hip or pelvic fracture | 3.5 | 3.5 | 3.5 | 3.6 | 3.5 |
| Osteoporosis | 14.3 | 14.4 | 14.3 | 14.0 | 13.8 |
| Rheumatoid arthritis or osteoarthritis | 60.7 | 62.2 | 63.5 | 64.3 | 64.8 |
Abbreviations: ADRD, Alzheimer’s disease and related dementia; COPD, chronic obstructive pulmonary disease; SD, standard deviation.
Characteristics Associated with ARNI Initiation (vs ACE Inhibitors or ARBs)
Among the beneficiaries who were not treated with ACE inhibitors, ARBs, or ARNI in the previous year, more patients were treated with an ACE inhibitor or ARB than ARNI (33,868 vs 4,547) (Table 2). Characteristics associated with reduced odds of ARNI initiation were age ≥85 years (odds ratio [95% CI], 0.89 [0.80–0.99]), dual eligibility (0.76 [0.70–0.83]), higher comorbidity score (0.96 [0.95–0.98] per 1-point increase), presence of ADRD (0.88 [0.81–0.96]), frailty (0.87 [0.81–0.94]), hip or pelvic fracture (0.83 [0.70–0.98]), and heart failure hospitalization within the prior 30 days (0.62 [0.56–0.70]). Conversely, male sex (1.27 [1.17–1.39]), anemia (1.10 [1.01–1.20]), CKD (1.12 [1.03–1.23]), COPD (1.09 [1.01–1.16]), cardiology visit within the prior 30 days (1.25 [1.16–1.34]), ≥2 heart failure hospitalizations in the previous year (1.41 [1.27–1.56]), and use of MRAs (1.68 [1.57–1.80]), isosorbide dinitrate (1.37 [1.17–1.60]), and loop diuretics (1.51 [1.38–1.65]) were associated with increased odds of ARNI initiation. When we replaced frailty status by frailty index in the multivariable logistic regression, ARNI initiation was associated with an OR [95%CI] of 0.71 [0.66, 0.78] per 0.1-point increase in frailty index. (Supplementary Table S1)
Table 2.
Characteristics Associated with Receipt of Angiotensin Receptor Neprilysin Inhibitor in Medicare Beneficiaries with Heart Failure with Reduced Ejection Fraction
| Characteristics | Initiation of ARNI (vs ACE Inhibitor or ARB)a |
||
|---|---|---|---|
| ARNI | ACE Inhibitor or ARB | OR (95% CI) | |
| Sample size, n | 4547 | 33868 | |
| Age category, % | |||
| 65 to <75 years old | 43.5 | 44.5 | Reference |
| 75 to <85 years old | 43.2 | 39.9 | 1.08 (1.01, 1.16) |
| ≥85 years old | 13.3 | 15.7 | 0.89 (0.80, 0.99) |
| Male, % | 79.5 | 75.8 | 1.27 (1.17, 1.39) |
| Race, % | |||
| White | 82.5 | 80.9 | Reference |
| Black | 11.4 | 12.7 | 0.98 (0.88, 1.09) |
| Other | 6.1 | 6.5 | 1.02 (0.89, 1.17) |
| Dual eligibility, % | 17.4 | 23.8 | 0.76 (0.70, 0.83) |
| Gagne comorbidity index, mean (SD) | 7.2 (3.3) | 7.3 (3.5) | 0.96 (0.95, 0.98) |
| Cardiovascular comorbidities, % | |||
| Atrial fibrillation | 64.6 | 60.4 | 1.07 (1.00, 1.15) |
| Myocardial infarction | 27.2 | 29.0 | 0.98 (0.91, 1.05) |
| Hypertension | 98.0 | 98.3 | 0.81 (0.63, 1.03) |
| Stroke or transient ischemic attack | 27.1 | 30.3 | 0.94 (0.88, 1.02) |
| Non-cardiovascular comorbidities, % | |||
| ADRD | 20.8 | 25.4 | 0.88 (0.81, 0.96) |
| Anemia | 80.5 | 80.3 | 1.10 (1.01, 1.20) |
| Cancer | 19.8 | 20.3 | 1.01 (0.93, 1.09) |
| Chronic kidney disease | 78.7 | 76.2 | 1.12 (1.03, 1.23) |
| COPD | 58.4 | 58.0 | 1.09 (1.01, 1.16) |
| Depression | 42.4 | 44.5 | 1.02 (0.95, 1.09) |
| Diabetes | 66.7 | 68.5 | 0.93 (0.87, 1.00) |
| Frailty | 53.2 | 59.8 | 0.87 (0.81, 0.94) |
| Hip or pelvic fracture | 3.8 | 5.1 | 0.83 (0.70, 0.98) |
| Osteoporosis | 13.6 | 15.5 | 1.01 (0.91, 1.11) |
| Rheumatoid arthritis or osteoarthritis | 69.3 | 68.3 | 1.07 (0.99, 1.15) |
| Cardiovascular care, % | |||
| Cardiology visit within 30 days | 72.5 | 68.2 | 1.25 (1.16, 1.34) |
| HF hospitalization ≥2 in prior year | 15.8 | 14.3 | 1.41 (1.27, 1.56) |
| HF hospitalization within 30 days | 10.1 | 12.4 | 0.62 (0.56, 0.70) |
| Other heart failure medications, % | |||
| Evidence-based beta-blockers | 88.9 | 87.7 | 1.03 (0.93, 1.14) |
| Mineralocorticoid receptor antagonists | 43.1 | 29.8 | 1.68 (1.57, 1.80) |
| Hydralazine | 12.5 | 12.1 | 0.95 (0.85, 1.06) |
| Isosorbide dinitrate | 5.7 | 4.4 | 1.37 (1.17, 1.60) |
| Loop diuretics | 82.3 | 74.4 | 1.51 (1.38, 1.65) |
Abbreviations: ACE, angiotensin converting enzyme; ADRD, Alzheimer’s disease and related dementia; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HF, heart failure; OR, odds ratio; SD, standard deviation.
Initiators were defined as beneficiaries who filled the prescription of ARNI vs ACE inhibitors or ARB without prior fills of any drug in ARNI, ACE inhibitor, or ARB classes in the previous year.
Trends of Heart Failure Medication and GDMT Use by Frailty Status, Sex, and Race
From 2015 to 2019, the proportion of beneficiaries with HFrEF treated with ACE inhibitors decreased (non-frail: 59.3% to 46.1%; frail: 53.2% to 40.8%), while the proportion treated with ARNI (non-frail: 0.4% to 16.4%; frail: 0.3% to 13.7%) and MRA (non-frail: 28.4% to 34.4%; frail: 28.3% to 31.6%) increased (Table 3 and Figure 1A). The rates of use for ARB, beta-blockers, hydralazine and isosorbide dinitrate, and loop diuretics changed minimally, although some trends were statistically significant due to large sample sizes. After adjusting for clinical characteristics of the study population, the difference in trends of ARNI use was not statistically significant by frailty (p for yearly-trend-by-frailty=0.940). The proportion of patients receiving all 3 classes of GDMT increased more in those without frailty (22.0% to 27.0%) than in those with frailty (19.6% to 21.8%) (p for yearly-trend-by-frailty<0.001) (Table 3 and Figure 1B).
Table 3.
Trend of Use of Heart Failure Medications in Patients with Heart Failure and Reduced Ejection Fraction, 2015 to 2019, by Frailty
| Medications | 2015 | 2016 | 2017 | 2018 | 2019 | P-value for yearly trendb | P for yearly trend by frailtyb |
|---|---|---|---|---|---|---|---|
| ARNI | |||||||
| Frail | 0.3% | 3.3% | 7.4% | 10.7% | 13.7% | <0.001 | 0.940 |
| Non-frail | 0.4% | 3.9% | 8.6% | 12.5% | 16.4% | <0.001 | |
| ACE inhibitors | |||||||
| Frail | 53.2% | 50.9% | 47.0% | 43.6% | 40.8% | <0.001 | 0.001 |
| Non-frail | 59.3% | 57.5% | 52.9% | 49.4% | 46.1% | <0.001 | |
| ARB | |||||||
| Frail | 21.6% | 22.8% | 22.3% | 22.6% | 21.8% | 0.051 | 0.979 |
| Non-frail | 24.0% | 25.3% | 25.0% | 24.8% | 23.7% | <0.001 | |
| MRA | |||||||
| Frail | 28.4% | 30.4% | 31.0% | 31.2% | 31.6% | <0.001 | <0.001 |
| Non-frail | 28.3% | 31.6% | 33.1% | 33.5% | 34.4% | <0.001 | |
| Evidence-based beta-blockersa | |||||||
| Frail | 85.4% | 86.7% | 87.1% | 87.2% | 86.8% | <0.001 | <0.001 |
| Non-frail | 86.4% | 88.0% | 88.8% | 88.9% | 88.9% | <0.001 | |
| Hydralazine + Isosorbide dinitrate | |||||||
| Frail | 2.2% | 2.4% | 2.6% | 2.5% | 2.2% | <0.001 | 0.588 |
| Non-frail | 1.1% | 1.3% | 1.5% | 1.4% | 1.4% | 0.005 | |
| Loop diuretics | |||||||
| Frail | 73.6% | 76.7% | 77.0% | 76.3% | 75.6% | 0.477 | <0.001 |
| Non-frail | 60.2% | 65.2% | 66.2% | 65.4% | 64.0% | 0.033 | |
| All 3 of ARNI/ACE inhibitors/ARB + beta-blockers + MRA | |||||||
| Frail | 19.6% | 21.3% | 21.6% | 21.8% | 21.8% | <0.001 | <0.001 |
| Non-frail | 22.0% | 24.8% | 25.9% | 26.4% | 27.0% | <0.001 | |
| 2 of ARNI/ACE inhibitors/ARB + beta-blockers + MRA | |||||||
| Frail | 51.1% | 50.6% | 50.1% | 50.0% | 49.4% | 0.011 | 0.017 |
| Non-frail | 55.1% | 54.5% | 53.6% | 53.2% | 52.5% | <0.001 | |
| 1 of ARNI/ACE inhibitors/ARB + beta-blockers + MRA | |||||||
| Frail | 24.1% | 23.4% | 23.8% | 23.5% | 24.0% | <0.001 | <0.001 |
| Non-frail | 18.7% | 17.3% | 17.4% | 17.2% | 17.3% | <0.001 | |
| None of ARNI/ACE inhibitors/ARB + beta-blockers + MRA | |||||||
| Frail | 5.2% | 4.7% | 4.6% | 4.6% | 4.8% | <0.001 | <0.001 |
| Non-frail | 4.2% | 3.4% | 3.2% | 3.2% | 3.2% | <0.001 | |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor neprilysin inhibitor; MRA, mineralocorticoid receptor antagonists.
Evidence-based beta-blockers include carvedilol, metoprolol succinate, and bisoprolol.
The receipt of a heart failure medication class or GDMT was modeled as a function of year (continuous variable), frailty status (binary), and their interaction term, adjusting for age, sex, race, dual eligibility, Alzheimer’s disease and related dementias, anemia, atrial fibrillation, cancer, chronic kidney disease, chronic obstructive pulmonary disease, depression, diabetes, hip or pelvic fracture, hypertension, myocardial infarction, osteoporosis, rheumatoid arthritis or osteoarthritis, and stroke or transient ischemic attack, Gagne combined comorbidity index, cardiology visit within the past 30 days, ≥2 heart failure hospitalizations within the past year, and heart failure hospitalization within the past 30 days, using generalized estimating equation logistic regression with exchangeable correlation structure to account for correlation within the same individuals.
Figure 1. Temporal Trends of Heart Failure Medications and Guideline-Directed Medical Therapy in Medicare Beneficiaries with Heart Failure with Reduced Ejection Fraction, 2015–2019, by Frailtya.
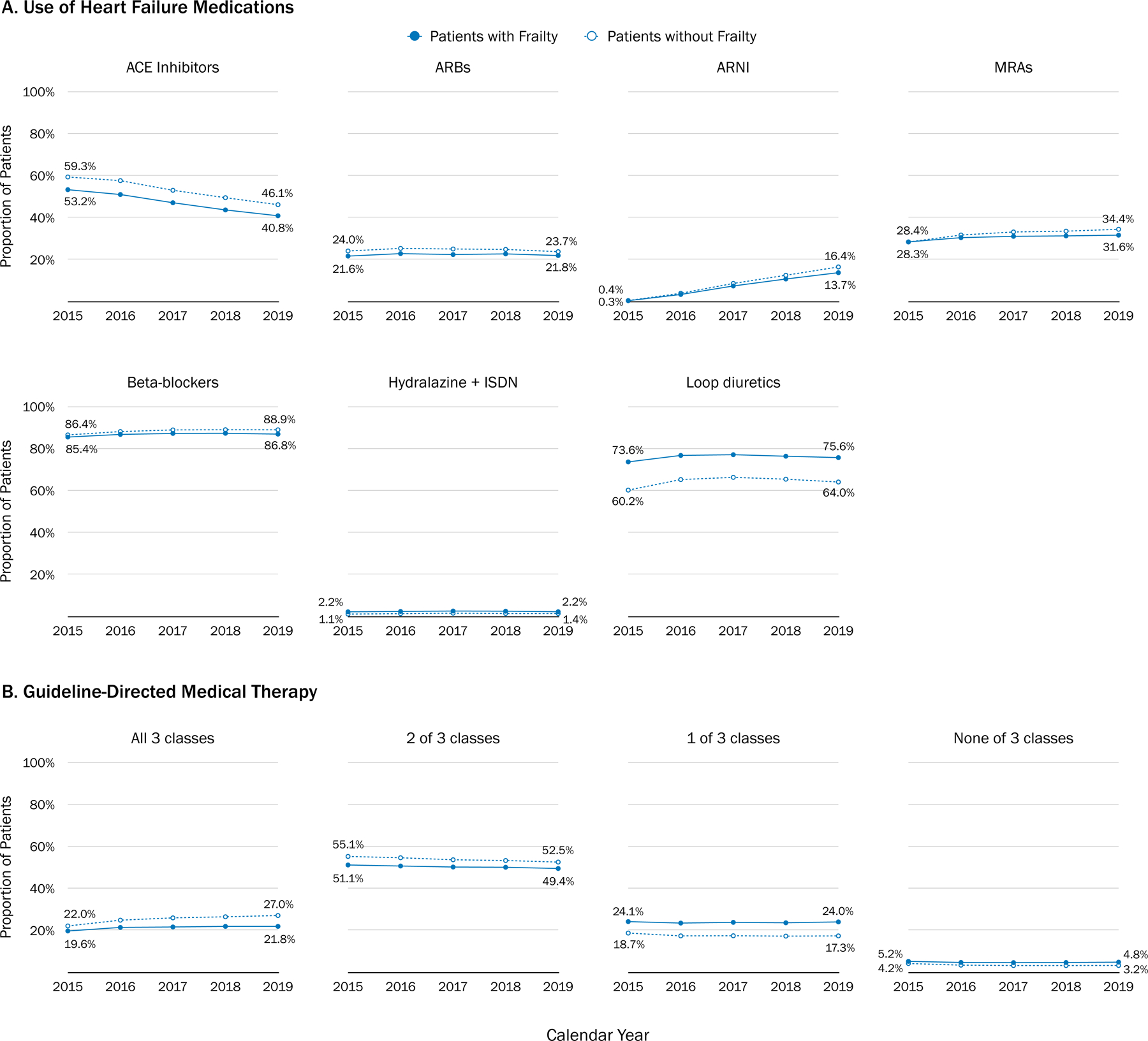
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; ISDN, isosorbide dinitrate; MRA, mineralocorticoid receptor antagonist.
a Figure 1 shows the proportion of Medicare beneficiaries with heart failure and reduced ejection fraction receiving heart failure medications and guideline-directed medical therapy (GDMT) by patients’ frailty status in each year. Heart failure medication use was defined as any prescription fill in part D claims in a given calendar year for ARNI, ACE inhibitor, ARB, evidence-based beta-blockers (bisoprolol, carvedilol, or metoprolol), MRA (spironolactone and eplerenone), hydralazine and ISDN, and loop diuretics. GDMT was defined as filling of a prescription for drugs in the following 3 classes within a given year: 1) an ARNI, ACE inhibitor, or ARB; 2) a beta-blocker; or 3) a MRA.
Women had a lower uptake of ARNI than men (men: 0.4% to 15.8%; women: 0.3% to 13.8%), although the difference in trends was not statistically significant by sex after adjusting for clinical characteristics (p=0.36). In fact, a higher proportion of women had all 3 classes of GDMT compared with men (men: 18.9% to 22.9%; women: 26.5% to 30.3%) (p for yearly-trend-by-sex<0.001) (Supplementary Table S2 and Figure S4). Patients of Black and Other races had a higher uptake of ARNI than White patients (Black: 0.4% to 16.3%; Other race: 0.5% to 17.4%; White: 0.4% to 15.0%), but the difference not statistically significant by race after multivariable adjustment (p=0.19). A higher proportion of Black patients (24.6% to 27.3%) received all 3 GDMT classes compared with patients of White (20.6% to 24.5%) and other race (19.8% to 24.6%) (p for yearly-trend-by-race<0.001) (Supplementary Table S3 and Figure S5).
Use of GDMT and Rate of Death or Heart Failure Hospitalization Over 4 Years
The incidence rate (per 100 person-years) of death or heart failure hospitalization was 22.8, 22.3, 18.3, and 18.9 for those who received none, 1, 2, and 3 GDMT drug classes, respectively (Table 4 and Supplementary Figure S6A). The corresponding multivariable-adjusted HRs (95% CI) were 0.94 (0.91, 0.97), 0.92 (0.89, 0.94), and 0.94 (0.91, 0.97) for the groups receiving 1, 2, and 3 classes. The mortality rate (per 100 person-years) was 20.1, 19.3, 14.9, and 14.7 for those who received none, 1, 2, and 3 GDMT drug classes, respectively (Table 4 and Supplementary Figure S6B). The multivariable-adjusted HRs (95% CI) were 0.94 (0.91, 0.97), 0.89 (0.86, 0.91), and 0.88 (0.86, 0.91) for the groups receiving 1, 2, and 3 classes, respectively. The mortality rates were higher in those with frailty than those without, but receiving more GDMT medication classes was associated with lower rates of the clinical outcomes in both non-frail and frail groups (Table 4).
Table 4.
Use of Guideline-Directed Medical Therapy and Rate of Death or Heart Failure Hospitalization Over 4 Years
| GDMT Usea |
Sample Size | Outcome: Death or HF hospitalization |
Outcome: Death |
||||
|---|---|---|---|---|---|---|---|
| No of Events | Rate (per 100 PY) | Adjusted HR (95% CI) |
No of Events | Rate (per 100 PY) | Adjusted HR (95% CI) |
||
| Total Population | |||||||
| 0 classes | 5970 | 2933 | 22.8 | Reference | 2727 | 20.1 | Reference |
| 1 class | 30028 | 15034 | 22.3 | 0.94 (0.91, 0.97)b | 13771 | 19.3 | 0.94 (0.91, 0.97)b |
| 2 classes | 78302 | 34686 | 18.3 | 0.92 (0.89, 0.94)b | 30278 | 14.9 | 0.89 (0.86, 0.91)b |
| 3 classes | 32699 | 14976 | 18.9 | 0.94 (0.91, 0.97)b | 12649 | 14.7 | 0.88 (0.86, 0.91)b |
|
| |||||||
| Frail | |||||||
|
| |||||||
| 0 classes | 3324 | 1927 | 29.4 | Reference | 1824 | 26.3 | Reference |
| 1 class | 16896 | 9867 | 28.4 | 0.95 (0.91, 0.99)c | 9269 | 25.2 | 0.95 (0.91, 0.99)c |
| 2 classes | 37213 | 20138 | 24.7 | 0.92 (0.89, 0.96)c | 18176 | 20.6 | 0.89 (0.85, 0.92)c |
| 3 classes | 15002 | 8270 | 25.3 | 0.95 (0.91, 0.99)c | 7217 | 19.9 | 0.89 (0.85, 0.92)c |
|
| |||||||
| Non-Frail | |||||||
|
| |||||||
| 0 classes | 2646 | 1006 | 15.9 | Reference | 903 | 13.6 | Reference |
| 1 class | 13132 | 5167 | 15.8 | 0.93 (0.89. 0.98)c | 4502 | 13.0 | 0.92 (0.88, 0.97)c |
| 2 classes | 41089 | 14548 | 13.4 | 0.91 (0.87, 0.95)c | 12102 | 10.5 | 0.89 (0.85, 0.93)c |
| 3 classes | 17697 | 6706 | 14.5 | 0.92 (0.88, 0.96)c | 5432 | 10.9 | 0.88 (0.84, 0.92)c |
Abbreviations: CI, confidence interval; GDMT, guideline-directed medical therapy; HF, heart failure; HR, hazard ratio; PY, person-years.
GDMT use is defined as filling a prescription for the following 3 drug classes between January 1, 2015, and December 31, 2015: 1) an ARNI, ACE inhibitor, or ARB; 2) an evidence-based beta-blocker; or 3) a mineralocorticoid receptor antagonist.
The model was adjusted for age, sex, race, dual eligibility, Alzheimer’s disease and related dementias, anemia, atrial fibrillation, cancer, chronic kidney disease, chronic obstructive pulmonary disease, depression, diabetes, hip or pelvic fracture, hypertension, myocardial infarction, osteoporosis, rheumatoid arthritis or osteoarthritis, and stroke or transient ischemic attack, Gagne combined comorbidity index, frailty, cardiology visit within the past 30 days, ≥2 heart failure hospitalizations within the past year, and heart failure hospitalization within the past 30 days.
The model was adjusted for all above variables except for frailty.
DISCUSSION
We found that ARNI use in Medicare beneficiaries with HFrEF steadily increased while ACE inhibitors use gradually decreased following FDA approval for ARNI in July 2015. However, patients over 85 years old, dual eligibility, higher comorbidity burden, ADRD, frailty, hip and pelvic fracture, and heart failure hospitalization within 30 days were less likely to receive ARNI in favor of an ACE inhibitors or ARB. In addition, patients with frailty were less likely to receive GDMT-class medications and more likely to receive hydralazine and isosorbide dinitrate, and loop diuretics. Regardless of frailty status, patients receiving at least 1 class of GDMT was associated with lower heart failure hospitalizations and all-cause mortality than those receiving no GDMT medications over 4 years.
The prevalence of frailty measured using CFI in our study population was 42.6–49.1%, which is consistent with previous studies based on clinical assessments.1,2 Despite high burden and poor clinical outcomes of patients with heart failure and frailty,24 clinical trial evidence on the outcomes of these patients is scarce.2,25 The PARADIGM-HF trial, which included few patients 85 years or older (1.44% of the study population) and did not assess frailty at baseline,32 provide little information on how ARNI works in older patients with frailty. This may have contributed to the lower rate of initiation of ARNI found in our study, particularly those with age 85 years or older and frailty. Whether ARNI provides similar benefits to older adults with and without frailty remains to be elucidated. However, recent studies suggest that ARNI is effective and safe in patients aged ≥75 years.30–32 These findings are expected to encourage more use of ARNI in eligible older HFrEF patients. We also found lower utilization of ARNI in HFrEF patients with ADRD. There is some concern that the neprilysin inhibition might slow the degrading of amyloid plaques and worsen dementia.26 Although a retrospective cohort study showed similar Mini-Mental State Examination scores in patients with at least 3-month use of ARNI compared to controls,27 long-term cognitive effects of ARNI remain unknown.28 An ongoing phase III randomized controlled trial (NCT 02884206) is being conducted to address this concern.29
In our study, about 73% Medicare beneficiaries with HFrEF were male. This is consistent with previous studies that found 71% male in the Change the Management of Patients with Heart Failure (CHAMP-HF) registry30 and 78% male in PARADIGM-HF trial.7 Some studies show that ARNI is less initiated in women, although the benefits of ARNI are similar between women and men.31,32 Our study also found a slightly lower ARNI utilization in women. In addition, we found that ARNI uptake was the highest in patients of other race, followed by patients of Black and White race. However, the difference by sex and race in the uptake of ARNI was no longer statistically significant after adjusting for the differences in clinical characteristics of the study population over time. A recent analysis of contemporary data from the CHAMP-HF registry also found that sex and race were not independently associated with use of ARNI after adjusting for clinical characteristics.30
Recent studies show that HFrEF patients who are frail are less likely to achieve optimal GDMT.33,34 Our study found that patients with HFrEF who were frail were less likely to receive GDMT and more likely to receive hydralazine and isosorbide, and diuretics. Because the ACC/AHA recommends hydralazine and isosorbide for people with HFrEF who cannot be given ACE inhibitors or ARBs,35 higher hydralazine and isosorbide use in the frail HFrEF population could reflect contraindications to ACE inhibitors or ARBs, such as chronic renal insufficiency. Similarly, Golwala et al found that chronic renal insufficiency was one of the most important predictor of hydralazine and isosorbide use in adults with HFrEF, and that these patients are less likely prescribed ACE inhibitors.36 On the other hand, higher diuretic use in older adults with HFrEF and frailty may be due to more advanced HFrEF.35
Clinicians may be reluctant to introduce new GDMT in patients who are frail because of the concern that these medications might have a less favorable risk-benefit profile in this population. Because greater severity in frailty is associated with higher risks of heart failure hospitalization and mortality,34,37,38 GDMT may be more beneficial in patients with frailty. The TOPCAT trial has demonstrated that the benefit of spironolactone in heart failure with preserved ejection fraction (HFpEF) was not attenuated by frailty.11 The effectiveness of ARNI in the PARAGON-HF trial37 and the effectiveness of dapagliflozin in the DELIVER trial5 were greater among patients with HFpEF and frailty. A secondary analysis of the REHAB-HF trial demonstrated that patients with acute decompensated heart failure and frailty had more improvement in physical function from a multicomponent rehabilitation.38 We found lower rates of death or heart failure hospitalization in both frail and non-frail patients with HFrEF who used at least 1 GDMT compared with those who did not receive any GDMT. Further research on the effectiveness and safety of ARNI versus an ACE inhibitors or ARB in older patients with HFrEF and frailty is warranted.
Limitations
We did not have information on ejection fraction to define HFrEF, clinical frailty assessment, and laboratory test results that may affect the number of eligible patients for ARNI or other GDMT medication classes. As a result, our estimates of ARNI and GDMT utilization may have been inaccurate. In addition, our clinical outcome analysis was based on the GDMT medication class prescription fill in a 1-year assessment period, without accounting for the duration or dose of GDMT medication use, which may result in exposure misclassification. Because patients receiving all 3 GDMT classes might have more severe or long-standing heart failure or better access to health care, unmeasured confounding cannot be excluded. Lastly, our data do not reflect practice patterns under the latest guideline, which now recommends ARNI for HFpEF and sodium-glucose cotransporter-2 inhibitors, regardless of diabetes status.10 Nonetheless, our study provides data on the latest trends in ARNI uptake and GDMT use in a representative population of older adults with HFrEF.
CONCLUSIONS
Our study found that use of ARNI and GDMT remains low in Medicare beneficiaries with HFrEF over 2015–2019, particularly among those with frailty. However, GDMT use was associated with lower rates of mortality and heart failure hospitalizations regardless of frailty status. Our results call for effort to narrow the evidence-practice gap to optimize pharmacological management in older adults with HFrEF and frailty.
Supplementary Material
Supplementary Table S1. Characteristics of ARNI Initiators vs ACE Inhibitors/ARB Initiators for Heart Failure with Reduced Ejection, Replacing Frailty (binary) with Frailty Index
Supplementary Table S2. Trend of Heart Failure Medications in Patients with Heart Failure with Reduced Ejection Fraction, 2015 to 2019, by Sex
Supplementary Table S3. Trend of Heart Failure Medications in Patients with Heart Failure with Reduced Ejection Fraction, 2015 to 2019, by Race
Supplementary Figure S4. Temporal Trends of Heart Failure Medications and Guideline-Directed Medical Therapy in Medicare Beneficiaries with Heart Failure with Reduced Ejection Fraction, 2015–2019, by Sexa
Supplementary Figure S5. Temporal Trends of Heart Failure Medications and Guideline-Directed Medical Therapy in Medicare Beneficiaries with Heart Failure with Reduced Ejection Fraction, 2015–2019, by Racea
Supplementary Figure S6. Use of Guideline-Directed Medical Therapy and Kaplan-Meier Curves of Heart Failure Hospitalization-Free and Overall Survival Over 4 Years
Key Points:
In patients with HFrEF, patients with age over 85 years, dementia, and frailty were less likely to start ARNI.
Use ARNI and GDMT remains low in Medicare beneficiaries with HFrEF in 2015–2019, particularly among those with frailty.
Those who received at least 1 class of GDMT were associated with a lower rate of death or heart failure hospitalization than those who did not receive any GDMT.
Why does this matter?
The slow uptake and low utilization of guideline-directed medical therapy in frail older adults call for an innovative care model to optimize heart failure management in this population.
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG062713 and K24AG073527. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding:
Funding for this project was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG062713 and K24AG073527 (to Dr. Kim) and K08AG055670 (to Dr. Patorno) from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: Preliminary results were presented at the Gerontological Society of America 2021 annual scientific meeting, and was published in supplement at doi: 10.1093/geroni/igab046.805. An abstract of this work was presented at the 2023 American College of Cardiology meeting.
Conflict of Interest: Dr. Kim reports grants from the National Institute on Aging on unrelated work and personal fees from Alosa Health and VillageMD. Dr. Ko reports grant 5K23HL151903–01 receives investigator-initiated research grant from Boston Scientific Corporation on unrelated work and a consulting fee from Eagle Pharmaceutical. Dr. Patorno reports research grants from the Patient Centered Outcomes Research Institute (DB-2020C2–20326) and the Food and Drug Administration (5U01FD007213). She is investigator of research grant to the Brigham and Women’s Hospital from Boehringer Ingelheim, not related to the topic of this work. Other authors have no conflict of interest.
Sponsor’s Role: The institutions listed had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
REFERENCES
- 1.Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: A systematic review and meta-analysis. Int J Cardiol Jun 1 2017;236:283–289. doi: 10.1016/j.ijcard.2017.01.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dewan P, Jackson A, Jhund PS, et al. The prevalence and importance of frailty in heart failure with reduced ejection fraction - an analysis of PARADIGM-HF and ATMOSPHERE. Eur J Heart Fail Nov 2020;22(11):2123–2133. doi: 10.1002/ejhf.1832 [DOI] [PubMed] [Google Scholar]
- 3.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet Mar 2 2013;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sze S, Pellicori P, Zhang J, Weston J, Squire IB, Clark AL. Effect of frailty on treatment, hospitalisation and death in patients with chronic heart failure. Clin Res Cardiol Jan 5 2021;doi: 10.1007/s00392-020-01792-w [DOI] [PMC free article] [PubMed]
- 5.Butt JH, Jhund PS, Belohlavek J, et al. Efficacy and Safety of Dapagliflozin According to Frailty in Patients With Heart Failure: A Prespecified Analysis of the DELIVER Trial. Circulation Oct 18 2022;146(16):1210–1224. doi: 10.1161/CIRCULATIONAHA.122.061754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Entresto Fala L. (Sacubitril/Valsartan): First-in-Class Angiotensin Receptor Neprilysin Inhibitor FDA Approved for Patients with Heart Failure. Am Health Drug Benefits Sep 2015;8(6):330–4. [PMC free article] [PubMed] [Google Scholar]
- 7.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med Sep 11 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol Sep 27 2016;68(13):1476–1488. doi: 10.1016/j.jacc.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 9.Maddox TM, Januzzi JL, Allen LA, et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction. Journal of the American College of Cardiology 2021;77(6):772–810. doi:doi: 10.1016/j.jacc.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 10.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation May 3 2022;145(18):e895–e1032. doi: 10.1161/cir.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 11.Sanders NA, Supiano MA, Lewis EF, et al. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail Nov 2018;20(11):1570–1577. doi: 10.1002/ejhf.1308 [DOI] [PubMed] [Google Scholar]
- 12.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care May 2005;43(5):480–5. doi: 10.1097/01.mlr.0000160417.39497.a9 [DOI] [PubMed] [Google Scholar]
- 13.Rector TS, Wickstrom SL, Shah M, et al. Specificity and sensitivity of claims-based algorithms for identifying members of Medicare+Choice health plans that have chronic medical conditions. Health Serv Res Dec 2004;39(6 Pt 1):1839–57. doi: 10.1111/j.1475-6773.2004.00321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai RJ, Lin KJ, Patorno E, et al. Development and Preliminary Validation of a Medicare Claims-Based Model to Predict Left Ventricular Ejection Fraction Class in Patients With Heart Failure. Circ Cardiovasc Qual Outcomes Dec 2018;11(12):e004700. doi: 10.1161/CIRCOUTCOMES.118.004700 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Medicare and Medicaid Services. Chronic conditions data warehouse Accessed 06.03, 2021. https://www2.ccwdata.org/web/guest/condition-categories
- 16.Sun JW, Rogers JR, Her Q, et al. Adaptation and Validation of the Combined Comorbidity Score for ICD-10-CM. Med Care Dec 2017;55(12):1046–1051. doi: 10.1097/MLR.0000000000000824 [DOI] [PubMed] [Google Scholar]
- 17.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol Jul 2011;64(7):749–59. doi: 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci Jun 14 2018;73(7):980–987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DH, Glynn RJ, Avorn J, et al. Validation of a Claims-Based Frailty Index Against Physical Performance and Adverse Health Outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci Jul 12 2019;74(8):1271–1276. doi: 10.1093/gerona/gly197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gautam N, Bessette L, Pawar A, Levin R, Kim DH. Updating International Classification of Diseases 9th Revision to 10th Revision of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci Jun 14 2021;76(7):1316–1317. doi: 10.1093/gerona/glaa150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider C, Aubert CE, Del Giovane C, et al. Comparison of 6 Mortality Risk Scores for Prediction of 1-Year Mortality Risk in Older Adults With Multimorbidity. JAMA Netw Open Jul 1 2022;5(7):e2223911. doi: 10.1001/jamanetworkopen.2022.23911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orkaby AR, Nussbaum L, Ho YL, et al. The Burden of Frailty Among U.S. Veterans and Its Association With Mortality, 2002–2012. J Gerontol A Biol Sci Med Sci Jul 12 2019;74(8):1257–1264. doi: 10.1093/gerona/gly232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DH, Patorno E, Pawar A, Lee H, Schneeweiss S, Glynn RJ. Measuring Frailty in Administrative Claims Data: Comparative Performance of Four Claims-Based Frailty Measures in the U.S. Medicare Data. J Gerontol A Biol Sci Med Sci May 22 2020;75(6):1120–1125. doi: 10.1093/gerona/glz224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandey A, Kitzman D, Reeves G. Frailty Is Intertwined With Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. JACC Heart Fail Dec 2019;7(12):1001–1011. doi: 10.1016/j.jchf.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butrous H, Hummel SL. Heart Failure in Older Adults. Can J Cardiol Sep 2016;32(9):1140–7. doi: 10.1016/j.cjca.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doehner W. Dementia and the heart failure patient. Eur Heart J Suppl Dec 2019;21(Suppl L):L28–L31. doi: 10.1093/eurheartj/suz242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Vecchis R, Ariano C, Di Biase G, Noutsias M. Cognitive performance of patients with chronic heart failure on sacubitril/valsartan : A retrospective cohort study. Herz Sep 2019;44(6):534–540. Kognitive Leistungsfahigkeit unter Sacubitril/Valsartan bei Patienten mit chronischer Herzinsuffizienz : Eine retrospektive Kohortenstudie. doi: 10.1007/s00059-018-4683-5 [DOI] [PubMed] [Google Scholar]
- 28.Poorgolizadeh E, Homayouni Moghadam F, Dormiani K, Rezaei N, Nasr-Esfahani MH. Do neprilysin inhibitors walk the line? Heart ameliorative but brain threatening! European journal of pharmacology Mar 5 2021;894:173851. doi: 10.1016/j.ejphar.2021.173851 [DOI] [PubMed] [Google Scholar]
- 29.Galo J, Celli D, Colombo R. Effect of Sacubitril/Valsartan on Neurocognitive Function: Current Status and Future Directions. Am J Cardiovasc Drugs May 2021;21(3):267–270. doi: 10.1007/s40256-020-00445-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greene SJ, Butler J, Albert NM, et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol Jul 24 2018;72(4):351–366. doi: 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 31.Regitz-Zagrosek V. Sex and Gender Differences in Heart Failure. Int J Heart Fail Jul 2020;2(3):157–181. doi: 10.36628/ijhf.2020.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swaraj S, Kozor R, Arnott C, Di Bartolo BA, G AF. Heart Failure with Reduced Ejection Fraction-Does Sex Matter? Curr Heart Fail Rep Dec 2021;18(6):345–352. doi: 10.1007/s11897-021-00533-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamada T, Kubo T, Kawai K, et al. Frailty interferes with the guideline-directed medical therapy in heart failure patients with reduced ejection fraction. ESC Heart Fail Feb 2023;10(1):223–233. doi: 10.1002/ehf2.14163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan MS, Segar MW, Usman MS, et al. Frailty, Guideline-Directed Medical Therapy, and Outcomes in HFrEF: From the GUIDE-IT Trial. JACC Heart Fail Apr 2022;10(4):266–275. doi: 10.1016/j.jchf.2021.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol Oct 15 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 36.Golwala HB, Thadani U, Liang L, et al. Use of hydralazine-isosorbide dinitrate combination in African American and other race/ethnic group patients with heart failure and reduced left ventricular ejection fraction. J Am Heart Assoc Aug 21 2013;2(4):e000214. doi: 10.1161/JAHA.113.000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butt JH, Dewan P, Jhund PS, et al. Sacubitril/Valsartan and Frailty in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol Sep 20 2022;80(12):1130–1143. doi: 10.1016/j.jacc.2022.06.037 [DOI] [PubMed] [Google Scholar]
- 38.Pandey A, Kitzman DW, Nelson MB, et al. Frailty and Effects of a Multidomain Physical Rehabilitation Intervention Among Older Patients Hospitalized for Acute Heart Failure: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol Feb 1 2023;8(2):167–176. doi: 10.1001/jamacardio.2022.4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Characteristics of ARNI Initiators vs ACE Inhibitors/ARB Initiators for Heart Failure with Reduced Ejection, Replacing Frailty (binary) with Frailty Index
Supplementary Table S2. Trend of Heart Failure Medications in Patients with Heart Failure with Reduced Ejection Fraction, 2015 to 2019, by Sex
Supplementary Table S3. Trend of Heart Failure Medications in Patients with Heart Failure with Reduced Ejection Fraction, 2015 to 2019, by Race
Supplementary Figure S4. Temporal Trends of Heart Failure Medications and Guideline-Directed Medical Therapy in Medicare Beneficiaries with Heart Failure with Reduced Ejection Fraction, 2015–2019, by Sexa
Supplementary Figure S5. Temporal Trends of Heart Failure Medications and Guideline-Directed Medical Therapy in Medicare Beneficiaries with Heart Failure with Reduced Ejection Fraction, 2015–2019, by Racea
Supplementary Figure S6. Use of Guideline-Directed Medical Therapy and Kaplan-Meier Curves of Heart Failure Hospitalization-Free and Overall Survival Over 4 Years


