Abstract
LY303,366 (LY) is a novel derivative of the echinocandin class of antifungal agents. The in vitro activities of LY, itraconazole (ITZ), and amphotericin B (AMB) were assessed against 60 Aspergillus isolates, including 35 isolates of A. fumigatus, eight isolates of A. terreus, eight isolates of A. flavus, eight isolates of A. niger and one isolate of A. nidulans. Four A. fumigatus isolates were resistant to ITZ. Susceptibility testing for all drugs was performed with a broth microdilution procedure. LY was tested in two media: antibiotic medium 3 (AM3) and Casitone with 2% glucose (CAS) with an inoculum of 2 × 103 spores/ml. ITZ and AMB were tested in RPMI 1640 with 2% glucose with an inoculum of 1 × 106 spores/ml. All tests were incubated at 37°C for 48 h. A novel end point was used to determine a minimal effective concentration (MEC) for LY, i.e., almost complete inhibition of growth save a few tiny spherical colonies attached to the microplate. MICs were measured for ITZ and AMB with a no-growth end point. Ranges and geometric mean (GM) MECs were from 0.0018 to >0.5 and 0.0039 mg/liter and from 0.0018 to >0.5 and 0.008 mg/liter for LY in AM3 and LY in CAS, respectively. Differences between species were apparent, with A. flavus being significantly less susceptible to LY than any other species tested with both media (P ≤ 0.05). Ranges and GM MICs were from 0.125 to >16 and 0.7 mg/liter for ITZ and from 0.25 to 16 and 1.78 mg/liter for AMB. Minimal fungicidal concentrations (MFCs) were also determined for all drugs. GM MFCs were 0.018, 0.09, 19.76, and 12.64 mg/liter for LY in AM3, LY in CAS, ITZ, and AMB, respectively. LY in AM3 and LY in CAS were fungicidal for 86.7 and 68% of isolates, respectively (98% killing). In comparison, ITZ and AMB were fungicidal for 35 and 70% of isolates, respectively (99.99% killing). A reproducibility study was performed on 20% of the isolates. For 12 isolates retested, the MEC or MIC was the same or was within 1 dilution of the original value for 11, 11, 10, and 9 isolates for LY in AM3, LY in CAS, ITZ, and AMB, respectively. In conclusion, LY seems to be a promising antifungal agent with excellent in vitro activity against Aspergillus spp.
Invasive aspergillosis is now one of the most common fungal infections found in immunocompromised patients (1) and is also one of the most fatal (2). Treatment of Aspergillus infections is still not ideal, and the two currently used antifungal drugs have a variety of associated problems. Amphotericin B (AMB) can cause serious side effects due to its toxicity and itraconazole (ITZ) is not always absorbed in high enough quantities to be therapeutic, especially in certain patient groups, e.g., AIDS patients.
The rise in serious fungal infection over the past decade has prompted the development of new antifungal agents with novel modes of action. LY303,366 (LY) is a semisynthetic derivative of a natural product class of antifungal agents belonging to the new class of drugs known as echinocandins. Echinocandins are noncompetitive inhibitors of (1,3)-β-d-glucan synthase which produces glucan polymers, a major component of the fungal cell wall (3). LY has been reported to have excellent activity against a wide range of fungal pathogens, including Aspergillus species (12) and Candida species (8, 10, 12).
In this study we evaluated the in vitro activity of LY against a variety of Aspergillus species and compared it with the activity of currently used antifungal agents, ITZ and AMB.
MATERIALS AND METHODS
Isolates.
Sixty Aspergillus isolates were used in the study, comprising 35 isolates of Aspergillus fumigatus, eight isolates of Aspergillus terreus, eight isolates of Aspergillus flavus, eight isolates of Aspergillus niger, and one isolate of Aspergillus nidulans. The isolates had been collected over a period of time in both the United States and the United Kingdom. This included four ITZ-resistant A. fumigatus isolates (4, 5) and one isolate resistant to AMB in vivo (11). The isolates were stored in 15% glycerol at −70°C until required for susceptibility testing. For susceptibility testing all isolates were grown on Sabouraud dextrose agar (Lab M, Bury, United Kingdom) at 30°C for 3 to 4 days.
Antifungal agents.
LY (Eli Lilly, Indianapolis, Ind.) was supplied as pure drug powder and was dissolved in dimethyl sulfoxide (Sigma, Dorset, United Kingdom) after adjusting for potency to a stock concentration of 1,280 mg/liter. ITZ (Janssen, Beerse, Belgium) was dissolved in 1:1 acetone–0.2 M HCl to a stock concentration of 1,280 mg/liter. The drug preparation was mixed vigorously and heated in a 60°C water bath until the drug had completely dissolved. AMB (Fungizone; Squibb, Middlesex, United Kingdom) was dissolved in water to a final concentration of 1,280 mg/liter after adjustment for sodium desoxycholate. All drugs were dispensed into small vials and stored in the dark at −20°C.
Preliminary work.
To evaluate the growth and end point determination with LY, four isolates were tested in several media with both broth micro- and macrodilution procedures. The broth macrodilution procedure was similar to the method used by Moore et al. (7). The media tested were RPMI 1640 (Sigma) buffered with morpholinopropanesulfonic acid (MOPS) (Sigma) and supplemented with 2% glucose (herein referred to as RPMI), antibiotic medium 3 supplemented with 2% glucose (Difco, Surrey, United Kingdom) (AM3), Casitone (Difco) supplemented with 2% glucose (CAS), and yeast nitrogen base (Difco) supplemented with 0.5% glucose (YNBG). The same batch of media was used throughout the study, including reproducibility tests. Two inocula were also evaluated: 1 × 106 conidia/ml and 2 × 103 conidia/ml.
After evaluation of this preliminary work, the method that was chosen to test LY was a broth microdilution method using two media, AM3 and CAS, and an inoculum size of 2 × 103 spores/ml.
Susceptibility testing.
Drug concentrations ranged from 0.0009 to 0.5 mg/liter for LY and from 0.03 to 16 mg/liter for ITZ and AMB. The medium that was used for ITZ and AMB tests was RPMI 1640. Previous in vivo and in vitro correlation work had determined that RPMI was the most suitable medium for ITZ (5).
A broth microdilution procedure was used for all drugs. Doubling dilutions were performed in the appropriate media in microtiter trays to produce the range of drug dilutions. Also included on the microtiter tray were a positive control containing 100 μl of medium and 100 μl of inoculum, a solvent control containing an amount of solvent equivalent to that of the highest drug concentration, and a negative control containing 200 μl of medium.
The inoculum was prepared by wetting a sterile loop into phosphate-buffered saline (Oxoid, Basingstoke, United Kingdom) with 0.05% Tween 80 (Fisons, Loughborough, United Kingdom) (PBS-Tween) and then transferring a loopful of Aspergillus conidia into sterile PBS-Tween. The conidial suspension was mixed vigorously to prevent clumping of the conidia and then was counted with a hemocytometer. The conidia were then diluted to a concentration of 2 × 103 conidia/ml for LY in both AM3 and CAS. For ITZ and AMB the conidium suspension was diluted with RPMI to a concentration of 106 conidia/ml, in accordance with prior work. A 100-μl volume of the inoculum was added to the drug dilution series, including the positive and solvent control, so that the final volume in the microtiter plates was 200 μl. Viability counts for all isolates were performed on horse blood agar to ensure that the correct inoculum had been calculated. The microtiter trays were incubated in a moist tray at 37°C for 48 h.
To establish reproducibility of the susceptibility tests, 20% of isolates were randomly chosen and were retested against all drugs.
MFCs.
For all drugs 100 μl was removed from all wells showing no growth and was plated on horse blood agar. The liquid was allowed to soak into the agar and the plate, once dry, was streaked with a sterile loop. This was to separate any spores that were present and to remove any spores from the drug. The plates were then incubated at 37°C for 48 h. For LY the minimal fungicidal concentration (MFC) was defined as the lowest drug concentration allowing two or fewer colonies to grow. This represented a killing of ≥98% of the original inoculum. For ITZ and AMB the MFC was defined as the lowest drug concentration allowing five or fewer colonies to grow. This represented a killing of ≥99.99% of the original inoculum.
End point determination.
A typical MIC end point is not seen when LY is tested against Aspergillus species. There is instead a transition from a homogeneous mat of long, thin hyphae to subspherical colonies, most being attached to the bottom of the microtiter well. Therefore, the minimal effective concentration (MEC) was considered to be the concentration in the first well to show the small subspherical colonies and no hyphal growth. For ITZ and AMB a no-growth end point was used.
Data analysis.
The differences between species were analyzed for each drug by a one-way analysis of variance with a Bonferroni correction for multiple comparisons (SPSS for Windows). A. nidulans was excluded from the statistical analysis because only one isolate of this species was being tested. For data analysis, MECs, MICs, and MFCs of >0.5 mg/liter were recorded as 1 mg/liter for LY and those of >16 mg/liter were recorded as 32 mg/liter for ITZ and AMB.
RESULTS
The preliminary study to assess the most appropriate susceptibility testing method for LY involved evaluating a variety of susceptibility methods, media, and inoculum sizes. The broth macrodilution method was found to be quite labor-intensive in comparison with the broth microdilution method, which was easier and quicker to perform. Also, growth of Aspergillus appeared only on the sides of the tubes and not in the media; hence, the tests were quite difficult to read. The broth microdilution method was therefore chosen in preference to the broth macrodilution method.
Two inoculum sizes were tested: 1 × 106 conidia/ml and 2 × 103 conidia/ml. No end point was found with the large inoculum, even after the range of drug dilutions was extended (0.0018 to 8 mg/liter). In comparison, the small inoculum showed a mass of hyphal growth gradually changing to small, subspherical colonies as the drug concentration increased. This end point correlated with in vivo in-house company data. Hence, the small inoculum (2 × 103/ml) was chosen for susceptibility testing.
Four media were initially tested with LY: AM3, RPMI, CAS, and YNBG. YNBG and RPMI showed poor growth when the small Aspergillus inoculum was used, and consequently these media were not chosen. The Aspergillus isolates were found to grow very well in both AM3 and CAS, producing a clear transition from hyphal growth to the formation of small colonies. Hence, AM3 and CAS were both chosen to test the in vitro activity of LY.
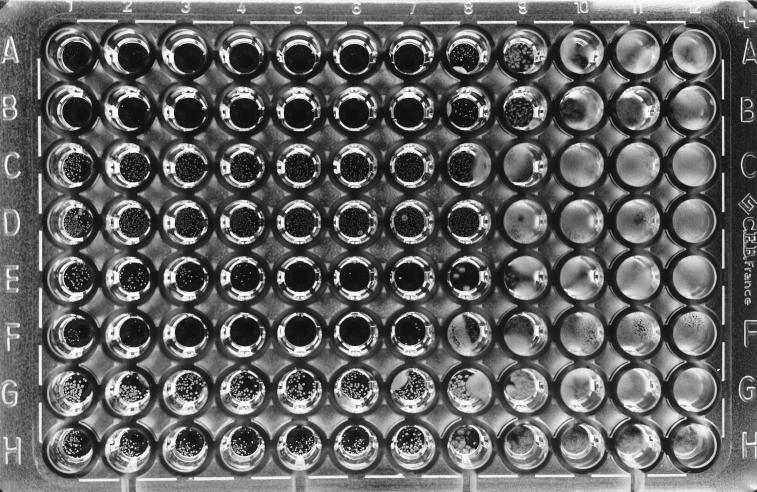
Figure 1 shows the type of growth that appears when Aspergillus is grown in the presence of LY. Two types of growth can be seen in the microtiter tray: hyphal growth in the lowest concentrations of LY, gradually changing to small, white colonies as the drug concentration increases. Differences in growth patterns between species are clearly seen in Fig. 2. A. terreus (Fig. 2, rows A and B) and A. niger (rows E and F) have only a small number of tiny colonies in the microplate wells, with a few wells having no visible growth at all. In comparison, the growth of A. fumigatus (Fig. 2, rows C and D) and A. flavus (rows G and H) is much greater and there are many more colonies present in the wells.
FIG. 1.
Detail of microtiter tray showing the transition between hyphal growth and small white colonies when LY is inoculated with Aspergillus species in CAS media. First and second rows, A. terreus; third and fourth rows, A. fumigatus; fifth row, A. niger. The highest drug concentrations are on the right.
FIG. 2.
Microtiter tray showing a range of drug dilutions of LY, from 0.009 to 0.5 mg/liter (wells 1 to 10), and positive and solvent controls (wells 12 and 11, respectively) in CAS media when inoculated with Aspergillus species. Rows A and B, A. terreus; rows C and D, A. fumigatus; rows E and F, A. niger; and rows G and H, A. flavus.
The range of MECs and MECs at which 50% (MEC50) and 90% (MEC90) of the isolates are inhibited for different species of Aspergillus tested against LY are shown in Table 1, together with the range of MICs and MIC50 and MIC90 for ITZ and AMB. LY in both AM3 and CAS was more active in vitro than ITZ or AMB. The MECs for LY in both media ranged from 0.0018 to >0.5 mg/liter, compared to MICs ranging from 0.125 to >16 mg/liter for ITZ and from 0.25 to 16 mg/liter for AMB. For MECs there were differences between species, with A. flavus being significantly less susceptible to LY in AM3 and CAS (P < 0.01). A. niger seemed to be much more susceptible than other species, with MICs being obtained for five of eight isolates in AM3 and for two of eight isolates in CAS (i.e., the well was clear and contained no subspherical colonies). There were no significant differences between the values obtained with the two media, AM3 and CAS, for testing LY, although AM3 values did tend to be always slightly lower than CAS values.
TABLE 1.
Range of MECs and MICs of drugs for different Aspergillus spp.
| Species (no. of isolates) | Antifungal agent | MEC or MIC (mg/liter)a
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| A. fumigatus (35) | LY in AM3 | 0.0018–0.015 | 0.0018 | 0.0075 |
| LY in CAS | 0.0018–0.015 | 0.0075 | 0.0075 | |
| ITZ | 0.25–>16 | 0.5 | 2 | |
| AMB | 0.5–2 | 2 | 2 | |
| A. terreus (8) | LY in AM3 | 0.0018–0.003 | 0.0018 | 0.003 |
| LY in CAS | 0.0075–0.003 | 0.003 | 0.0075 | |
| ITZ | 0.125–0.25 | 0.25 | 0.25 | |
| AMB | 4–8 | 4 | 8 | |
| A. flavus (8) | LY in AM3 | 0.015–>0.5 | 0.015 | 1 |
| LY in CAS | 0.0075–>0.5 | 0.03 | 1 | |
| ITZ | 0.5–8 | 0.5 | 4 | |
| AMB | 2–16 | 4 | 4 | |
| A. niger (8) | LY in AM3 | 0.0018–0.003 | 0.003 | 0.003 |
| LY in CAS | 0.0075–0.015 | 0.0075 | 0.0075 | |
| ITZ | 0.5–>16 | 1 | 8 | |
| AMB | 0.25–1 | 1 | 1 | |
| A. nidulans (1) | LY in AM3 | 0.003 | NCb | 0.003 |
| LY in CAS | 0.0075 | NC | 0.0075 | |
| ITZ | 0.125 | NC | 0.125 | |
| AMB | 2 | NC | 2 | |
| All isolates (60) | LY in AM3 | 0.0018–>0.5 | 0.003 | 0.015 |
| LY in CAS | 0.0018–>0.5 | 0.0075 | 0.015 | |
| ITZ | 0.125–>16 | 0.5 | 8 | |
| AMB | 0.25–16 | 2 | 4 | |
MEC50 and MEC90, for LY only; MIC50 and MIC90, for ITZ and AMB.
NC, not calculable.
The range of MFCs, MFC50, and MFC90 for LY in both AM3 and CAS, ITZ, and AMB are shown in Table 2. MFCs ranged from 0.0018 to >0.5 mg/liter for LY. There was a significant difference in LY MFCs between species (P = 0.003 for AM3 and CAS). Among isolates tested in AM3, A. flavus was significantly less susceptible than A. niger and A. terreus. In comparison, among isolates tested in CAS A. niger, A. fumigatus, and A. flavus were significantly less susceptible than A. terreus. The ratio between MFC and MEC was much higher for A. fumigatus and A. flavus than for other species. LY in both AM3 and CAS was fungicidal for 86.7 and 68% of isolates, respectively (98% killing). In contrast, the MFCs of ITZ and AMB were much higher. For ITZ, MFCs ranged from 2 to >16 mg/liter and, for AMB, MFCs ranged from 1 to >16 mg/liter. ITZ showed no significant differences from species to species (P = 0.141) and was fungicidal for 35% of isolates tested. In comparison, there were several significant differences between species (P = 0.0001) for AMB. A. terreus was significantly less susceptible than either A. fumigatus or A. niger. Also, A. flavus was significantly less susceptible than A. niger. Overall, AMB was fungicidal for 70% of the isolates tested.
TABLE 2.
Range of MFCs of antifungal agents for different Aspergillus spp.
| Species (no. of isolates) | Antifungal agent | MFC (mg/liter)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| A. fumigatus (35) | LY in AM3 | 0.0018–>0.5 | 0.015 | 1 |
| LY in CAS | 0.0018–>0.5 | 0.25 | 1 | |
| ITZ | 4–>16 | 16 | 16 | |
| AMB | 4–>16 | 8 | 16 | |
| A. terreus (8) | LY in AM3 | 0.0018–0.0075 | 0.003 | 0.003 |
| LY in CAS | 0.003–0.0075 | 0.003 | 0.0075 | |
| ITZ | 2–>16 | 8 | 16 | |
| AMB | >16 | 16 | 16 | |
| A. flavus (8) | LY in AM3 | 0.003–>0.5 | 0.015 | 0.5 |
| LY in CAS | 0.003–>0.5 | 0.5 | 0.5 | |
| ITZ | 2–>16 | 16 | 16 | |
| AMB | 16–>16 | 16 | 16 | |
| A. niger (8) | LY in AM3 | 0.0018–0.0075 | 0.003 | 0.003 |
| LY in CAS | 0.0075–>0.5 | 0.06 | 0.5 | |
| ITZ | 0.5–>16 | 16 | 16 | |
| AMB | 1–>16 | 16 | 16 | |
| A. nidulans (1) | LY in AM3 | 0.003 | NCa | 0.003 |
| LY in CAS | 0.003 | NC | 0.003 | |
| ITZ | 8 | NC | 8 | |
| AMB | 16 | NC | 16 | |
| All isolates (60) | LY in AM3 | 0.0018–>0.5 | 0.003 | 1 |
| LY in CAS | 0.0018–>0.5 | 0.125 | 1 | |
| ITZ | 2–>16 | 16 | 16 | |
| AMB | 1–>16 | 16 | 16 | |
NC, not calculable.
DISCUSSION
LY has been shown to have potent in vitro activity against a wide range of fungal pathogens, including Candida spp. (8, 10, 12) and Aspergillus spp. (12). Our in vitro studies with LY have shown similar results against Aspergillus spp., including ITZ-resistant isolates.
We found LY to have excellent activity against different species of Aspergillus and demonstrated that LY is more active at lower concentrations than either ITZ or AMB. One species of Aspergillus, A. flavus, was found to be slightly less susceptible than other species, and this was found to be statistically significant. It was also interesting that for some isolates of A. niger and A. terreus there were far fewer wells containing subspherical colonies and that in a small number of drug dilutions the wells were totally clear.
A typical MIC was not seen for Aspergillus spp. when they were tested against the echinocandin antifungal agent LY. Two distinct types of growth are apparent when Aspergillus is grown in the presence of LY. At the lowest concentrations of LY, a dense mat of hyphal growth completely covers the entire well. At higher concentrations of LY, wells containing purely white, subspherical colonies attached to the bottom of the microtiter plate were visible. Examination of these colonies (9) by electron microscopy has revealed that they appear to be cell wall-deficient microcolonies. However, subcultures in the absence of LY regain both their cell walls and their susceptibility to this drug (9). For the purposes of determining an inhibitory concentration that might be clinically useful, the transition from matted growth to microcolonies was taken as the first breakpoint and was recorded as the MEC. A second breakpoint of no growth at all was seen with some species of Aspergillus (i.e., A. niger and A. terreus). However, for other species of Aspergillus (i.e., A. fumigatus and A. flavus) initial MIC tests with the drug range extended up to 8 mg/liter still showed small colonies in the bottoms of the microtiter wells. One in vivo study with two A. fumigatus isolates showed that the MEC most closely correlates with the efficacy of LY in a murine model of invasive aspergillosis (11).
Phase I data from human volunteers following oral administration of LY showed concentrations in serum up to about 0.7 mg/liter and a half-life of 30 h (6). Such concentrations substantially exceed all the MECs and the majority of MFCs recorded here. Thus, LY has promising in vitro and in vivo activity against Aspergillus species and further investigation is warranted.
ACKNOWLEDGMENTS
We thank Eli Lilly and the Fungal Research Trust for their financial support of this research project.
REFERENCES
- 1.Bodey G P, Vartivarian S. Aspergillosis. Eur J Clin Microbiol Infect Dis. 1989;8:413–437. doi: 10.1007/BF01964057. [DOI] [PubMed] [Google Scholar]
- 2.Denning D W. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 3.Denning D W. Echinocandins and pneumocandins—a new antifungal class with a novel mode of action. J Antimicrob Chemother. 1997;40:611–613. doi: 10.1093/jac/40.5.611. [DOI] [PubMed] [Google Scholar]
- 4.Denning D W, Venkateswarlu K, Oakley K L, Anderson M J, Manning N J, Stevens D A, Warnock D W, Kelly S L. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1364–1368. doi: 10.1128/aac.41.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denning D W, Radford S A, Oakley K L, Hall L, Johnson E M, Warnock D W. Correlation between in vitro susceptibility testing to itraconazole and in vivo outcome of Aspergillus fumigatus infection. J Antimicrob Chemother. 1997;42:401–414. doi: 10.1093/jac/40.3.401. [DOI] [PubMed] [Google Scholar]
- 6.Lucas R, Desante K, Hatcher B, Hemingway J, Lachno R, Brooks S, Turik M. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. LY303366 single dose pharmacokinetics and safety in healthy male volunteers, abstr. F50; p. 108. [Google Scholar]
- 7.Moore C B, Law D, Denning D W. In vitro activity of the new triazole D0870 compared with amphotericin B and itraconazole against Aspergillus spp. J Antimicrob Chemother. 1993;32:831–836. doi: 10.1093/jac/32.6.831. [DOI] [PubMed] [Google Scholar]
- 8.Pfaller M A, Messer S A, Coffman S. In vitro susceptibilities of clinical yeast isolates to a new echinocandin derivative, LY303366, and other antifungal agents. Antimicrob Agents Chemother. 1997;41:763–766. doi: 10.1128/aac.41.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rennie R, Sand C, Sherburne R. Program and abstracts of the 13th Congress of the International Society for Human and Animal Mycology, Parma, Italy. 1997. Electron microscopic evidence of the effect of LY303366 on Aspergillus fumigatus, abstr. P451. [Google Scholar]
- 10.Uzun Ö, Kocagöz S, Çetinkaya Y, Arikan S, Ünal S. In vitro activity of a new echinocandin, LY303366, compared with those of amphotericin B and fluconazole against clinical yeast isolates. Antimicrob Agents Chemother. 1997;41:1156–1157. doi: 10.1128/aac.41.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verweij P E, Oakley K L, Morrissey J, Morrissey G, Denning D W. Efficacy of LY303366 against amphotericin B-susceptible and -resistant Aspergillus fumigatus in a murine model of invasive aspergillosis. Antimicrob Agents Chemother. 1998;42:873–878. doi: 10.1128/aac.42.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhanel G G, Karlowsky J A, Harding G A J, Balko T V, Zelenitsky S A, Friesen M, Kabani A, Turik M, Hoban D J. In vitro activity of a new semisynthetic echinocandin, LY-303366, against systemic isolates of Candida species, Cryptococcus neoformans, Blastomyces dermatitidis, and Aspergillus species. Antimicrob Agents Chemother. 1997;41:863–865. doi: 10.1128/aac.41.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]