Abstract
Objectives:
Resident synovial macrophages (RSM) provide immune sequestration of the joint space and are likely involved in initiation and perpetuation of the joint-specific immune response. We sought to identify RSM in synovial fluid (SF) and demonstrate migratory ability, in additional to functional changes that may perpetuate a chronic inflammatory response within joint spaces.
Methods:
We recruited human patients presenting with undifferentiated arthritis in multiple clinical settings. We used flow cytometry to identify mononuclear cells in peripheral blood and SF. We used a novel transwell migration assay with human ex-vivo synovium obtained intra-operatively to validate flow cytometry findings. We used single cell RNA-sequencing (scRNA-seq) to further identify macrophage/monocyte subsets. ELISA was used to evaluate the bone-resorption potential of SF.
Results:
We were able to identify a rare population of CD14dim, OPG+, ZO-1+ cells consistent with RSM in SF via flow cytometry. These cells were relatively enriched in the SF during infectious processes, but absolutely decreased compared to healthy controls. Similar putative RSM were identified using ex vivo migration assays when MCP-1 and LPS were used as migratory stimulus. scRNA-seq revealed a population consistent with RSM transcriptionally related to CD56+ cytotoxic dendritic cells and IDO+ M2 macrophages.
Conclusion:
We identified a rare cell population consistent with RSM, indicating these cells are likely migratory and able to initiate or coordinate both acute (septic) or chronic (autoimmune or inflammatory) arthritis. RSM analysis via scRNA-seq indicated these cells are M2 skewed, capable of antigen presentation, and have consistent functions in both septic and inflammatory arthritis.
Keywords: resident synovial macrophage, Type A synoviocyte, joint space immunoregulation, septic arthritis, inflammatory arthritis
Introduction:
Damage to the articular surface of joints resulting in arthritis may be secondary to infection, inflammation, and chronic or acute trauma. Different etiologies of arthritis result in unique local immune environments within the joint space. While healthy synovium delineates an immune-privileged space to which few circulating cells gain entry (1), there are significant numbers of immune cells in the synovial fluid (SF) of pathologic joints (2; 3; 4). This indicates the localized synovial immune response is coordinated by the synovium itself, which limits entry to the SF by actively sequestering inflammatory damage (5) versus allowing circulating immune cells to enter SF.
Synovium is primarily composed of two types of cells: Fibroblast-like Synoviocytes (FLS) and Resident Synovial Macrophages (RSMs), also known as Type A cells. FLS express MHC Class II and produce lubricating joint fluid, including hyaluronan (6). In pathologic settings, FLS are involved in joint inflammation and, ultimately, cartilage destruction (6). Conversely, RSMs compose approximately 10% of the synovium and have only been identified using tissue histology to date. RSMs are described as constitutively anti-inflammatory, as opposed to circulating monocytes which may either assist with sequestration of pathology or provide further momentum toward significant cellular collateral damage (5).
It was recently discovered that RSMs are derived from embryonic precursor cells and perpetuate through self-proliferation within synovium (7). Certain cellular surface receptors, chemokines, or structural proteins such as CD68, osteoprotegerin (OPG/TNFRSF11B), CX3CR1, ZO-1/TJP1, F11R/JAM-A/JAM-1/CD321, and Triggering Receptor Expressed on Myeloid cells 2 (TREM2) (8; 7) have all been proposed to identify these cells, yet it remains difficult to differentiate RSMs from macrophages recruited from circulation. RSMs have not been further evaluated in SF as it is unclear if they can migrate out of tissue. As inflammation dysregulates the tight junctions connecting these epithelial-like RSMs (7), it is possible that these cells could leave the synovium and participate in joint space immune responses.
Here, we used flow cytometry to describe a subset of CD14dimOPG+ZO-1+ M2 macrophages enriched in SF of pathologic joints consistent with previously published histological descriptions of RSMs. Ex vivo migration experiments validated migration from tissue. Single cell RNA sequencing (scRNA-seq) reveals these putative RSMs had dysregulated complement in settings of inflammatory arthritis, and a unique reactome signature involving threonine, niacin, and thiamine metabolism. This work is important in understanding how damage to the joint space is initiated and perpetuated both during infectious and inflammatory arthritis.
Materials and Methods:
Patient Recruitment:
These studies were approved by the Institutional Review Board (IRB) at the University of Iowa Hospitals and Clinics. For SF studies, we recruited patients under evaluation for septic arthritis. Exclusion criteria included significant joint trauma, joint surgery, or immunomodulatory medications. All patients provided a blood sample. Not all patients required or had successful arthrocentesis. SF samples were classified as Normal, Non-Inflammatory, Inflammatory, or Septic based on established guidelines (9). For synovium procurement, 6 patients >18 years of age receiving a scheduled, non-emergent, total joint replacement or resection arthroplasty secondary to infection were recruited. Synovium (knee) or pulvinar (hip) was sterilely obtained during normal operating protocol.
PBMC and SF Cell Sample Preparation:
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood over Ficoll-Paque PLUS (Fisherbrand). For SF samples, a 200 μl aliquot was centrifuged at 1000 RCF for 10 minutes and stored at −80°C for ELISA. The remainder of the SF was treated with bovine testes hyaluronidase (Sigma-Adritch) according to manufacturer’s SF clarification protocol. SF was then filtered through a 70μm nylon mesh strainer (Fisher Scientific), diluted to 10 mL with PBS, and centrifuged at 400 RCF for 10 minutes at room temperature. Supernatant was discarded. SF cells (SFCs) and PBMCs were counted and then cryopreserved in 90% FBS with 10% DMSO.
Transwell Migration Assay:
Synovium (knee) or pulvinar (hip) acquired in the operating room was transferred to the laboratory in PBS on ice. Whole synovium was washed twice in PBS, then sterilely dissected into 4x4 mm segments, and washed again. Segments were placed into 24-well transwell inserts with 5 μm pores (Corning), a size that should allow monocyte and macrophage migration, but prevent fibroblast migration (10). Bottom wells were treated with LPS (1, 10, or 100 μg/mL, Sigma-Aldrich) or MCP-1 (25 ng or 250 ng/mL, Fischer Scientific) in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. The inserts were then placed in the well, and enough media to cover the tissue was placed in the insert (approximately 200-400 μl). The transwell plates were incubated for 24 hours at 37°C and 5% CO2. At day 1, 2, 3, 5, and 7, changes to the synovium were compared to in vivo controls also obtained intra-operatively (Suppl. Figure 2). H&E staining was performed to evaluate changes to the synovial intimal and sub-intimal lining with MCP-1 and LPS treatment (Suppl. Figure 3) Validation for tissue survival was performed out to 7 days with normoxic (21% oxygen) and hyperoxic (50% oxygen) conditions and Caspace-3 immunohistochemistry (Suppl. Figure 4). After incubation, the cells in the bottom well media were counted, and a crystal violet assay was performed on cells adhered to the bottom of the well and the underside of the transwell per standard protocol. Migratory cells in solution were analyzed using flow cytometry.
Flow Cytometry Analysis:
Samples were plated in a 96-well round-bottom plate for single stain, unstained, patient test samples, and/or Fluorescence Minus One (FMO) controls. Controls were plated at 2x105 cells per well, and patient test samples at 1-2x106 cells per well. Antibodies are listed in Suppl. Table 1. For intracellular staining (OPG, CD68, F11R, TREM2, ZO-1 and RANKL), cells were fixed and permeabilized with Fixation Buffer and Intracellular Staining Perm Wash Buffer (BioLegend) or Cytofix/Cytoperm Fixation/Permeabilization Solution Kit (BD Bioscences). Final flow cytometry gating strategy for SFCs and PBMCs shown in Suppl. Figure 1. Flow cytometry was performed on a Cytek Aurora cytometer (Bethesda, MD). Analysis was performed using FlowJo (Ashland, OR) software.
Enzyme-linked Immunosorbent Assays (ELISA):
Human TIMP-1 (RAB0466-1KT), TGF-BETA (RAB0460-1KT), TRACP (RAB1755-1KT), OPG/TNFRSF11B (RAB0484-1KT), IFN GAMMA (RAB0222-1KT), TNF-ALPHA (RAB0476-1KT), MMP-9 (RAB0372-1KT) and BMP2 (RAB0028-1KT) ELISA kits were obtained from Millipore Sigma, and sRANKL (MBS262624) kit from MyBioSource. For TIMP-1 and MMP-9, SF was diluted 1:500, for sRANKL dilution was 1:100, and all others were diluted 1:20. TGF-β1 was activated and then neutralized per manufacturer’s protocol. Plates were read using a VERSAmax plate reader (Molecular Devices) and analyzed using MyAssays.com, Microsoft Excel, and GraphPad Prism 9.4.1.
Cell Sorting:
To prepare the highest quality sample for single cell RNA sequencing (scRNA-seq), patients who had the highest percentage of viable, non-neutrophil SFCs were selected, with 3 patients having septic arthritis, and 3 having inflammatory arthritis, regardless of crystal status. As SF in non-pathologic states lacks sufficient cellularity for scRNA-seq analysis, healthy patients were not included. SFCs were thawed in a 37°C water bath and diluted in 4 mL Fluorescence Activated Cell Sorting (FACS) buffer and centrifuged at 40°C at 1400 rpm. Supernatant was gently decanted, and cells were resuspended in 50 μL of ice cold FACS buffer before staining 1:1000 with DAPI and 5 μl/reaction of: CD244/APC (Clone C1.7), CD11b/BV695 (Clone ICRF44), CD66b/FITC (Clone G10F5) and CD56/PE (Clone 5.1H11) (BioLegend). Cells were incubated in the dark at 40°C for 30 minutes, washed once in ice cold FACS buffer, and resuspended in 50 μL of FACS buffer. Samples were then sorted on a Sony MA900 (San Jose, CA) with 100 μm sorting chip, sorting out CD66b+ and DAPI positive cells, then sorting on CD11b+, CD56+, or CD244+ positive cells into tubes containing cold PBS with 1% BSA. Cells were then counted for viability using trypan blue on a hemocytometer and concentrated according to 10X Chromium 3’ kit guidelines.
Single Cell RNA sequencing:
Cells were delivered to the Sequencing Core where RNA library generation was performed on a 10X Chromium Controller according to manufacturer’s guidelines. RNA libraries were then sent to NovoGene (Sacramento, CA) for sequencing. Analysis was performed in RStudio with R v4.2.2 using Seurat (11), ReactomeGSA (12) and EnhancedVolcano (13). MT-DNA percentage was limited to 15% during quality control analysis (14). The number of unique genes was set as 200 to 5000. A minimum of 3 cells were required to express each gene. Data was normalized using a global-scaling normalization method per Seurat with a scale factor of 10,000 with log transformation, then the data was integrated into a single database. A resolution of 0.4 was used to define clusters with dimensions set 1:30. Clusters were annotated by identifying top 10 gene expression in addition to expression of markers such as CD56, CD206, F11R, and CD68. Log2FC threshold was set to 0.5 for gene expression. Analysis was performed comparing septic arthritis to inflammatory arthritis. Adjusted p-values were used to determine significance of Differentially Expressed Genes (DEGs), with Log2FC threshold of 1.5 given the homogenous sample. For Conserved Markers, included genes had min.diff.pct set to 0.7, and min.pct to 0.25.
Statistics:
Descriptive statistics were used to compare patient demographics and underlying diagnoses. Statistical analysis was performed using GraphPad Prism v9.4.1. Flow Cytometry data was tabulated in FlowJo, and 2-way ANOVA with Tukey’s post-hoc correction was used to compare %parent or %total cells of control, inflammatory, and septic arthritis populations. Mann-Whitney U-tests were used to compare ELISA results between the 3 arthritis groups (control, inflammatory, and septic). DEG and Conserved gene analysis was performed in Seurat per individual cell cluster using adjusted p-values.
Results:
Patient Demographics:
To identify RSM in SF, 52 patients were recruited into the initial study. Of these, 32% of patients were female and 87% were Caucasian (Suppl. Table 2). SF was obtained from 36 of 52 patients. Eighteen patients were found to have septic arthritis based on SF analysis and final culture results, and 8 had inflammatory arthritis. Of the remainder, 10 were designated non-inflammatory (<2000 WBCs) or normal (<200 WBCs) (Suppl. Table 3).
Identification of SF Macrophages Consistent with RSM:
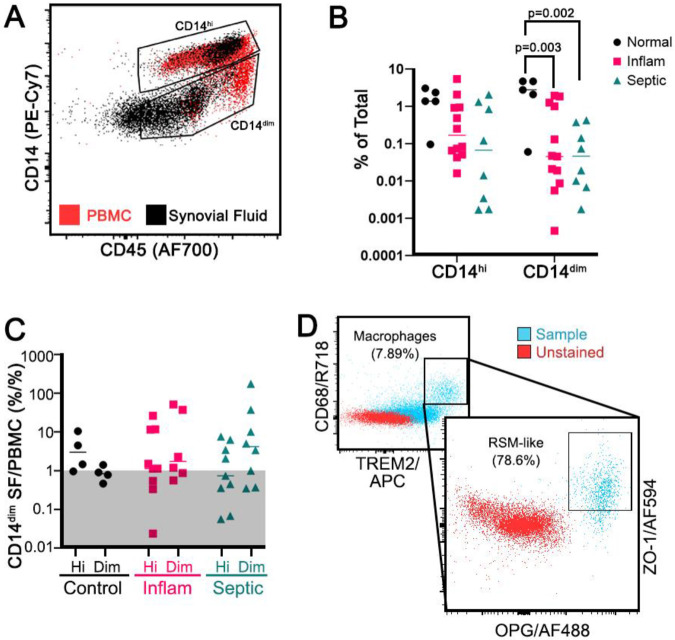
We identified a cell subset expressing myeloid marker CD11b, pan-macrophage marker CD68, anti-inflammatory macrophage marker TREM2 (15), Major Histocompatibility Complex marker Class II HLA-DR (16), CX3CR1 (7), OPG (16), and hematopoietic/macrophage adhesion marker CD45 (17). These cells were also negative for Receptor Activator of NF-kB Ligand/RANKL (16) and for the dendritic cell (DC) marker CD11c. There were two distinct subpopulations: a CD14hi and a CD14dim (Figure 1A). The CD45+CD14dim RSM-like population was found to have an absolute decrease in frequency in pathologic states compared to control SFCs (Figure 1B). However, it was also relatively enriched in SF compared to the PBMC fraction (Figure 1C). The Alive/CD45+CD14dim was then back-gated to better describe this population, first to evaluate the frequency of macrophages by CD68 and TREM2, then if macrophages also co-expressed RSM markers OPG and ZO-1 (Figure 1D). For patients with inflammatory arthritis, 1.96% of the CD14dim cells were macrophages, and of those macrophages, 34.35% were RSM-like by OPG and ZO-1 expression. For patients with septic arthritis, 0.92% of CD14dim cells were macrophages, and of those 9.97% were RSM-like.
Figure 1:
Resident Synovial Macrophage-like cells. Live, CD56−CD3−CD20−CD11c− TREM2+OPG+CD68+CD11b+HLA-DR+CX3CR1+ cells were identified with an enrichment of CD14dim cells in the SF compared to PBMCs (A, n=6 patients for preliminary evaluation for RSM cells). These CD14dim macrophages were decreased in absolute frequency in inflammatory and septic arthritis compared to controls (B, n=22 patients, 5-12 patients per group) but were relatively enriched in the SF of pathologic joints when SFCs were compared to PBMC, shown by ratio >1 (C, n=22 patients, 5-12 patients per group). Back-gating on the Alive/CD14dim population to identify M2 macrophages (D, inset), 78.6% were double positive for OPG and ZO-1 (D, representative patient with inflammatory arthritis). Two-way ANOVA with Tukey’s post-hoc correction.
Identification of RSM-like Cells Migrating from Intact Synovium:
We obtained synovium from patients undergoing total joint replacement or resection arthroplasty and placed tissue into transwells (Figure 2A). This model was validated out 24 hours to have a dose-dependent loss of intimal lining cells with MCP-1 and LPS stimulation (Suppl. Figure 3), and negligible apoptosis by Caspace-3 IHC at day 3 in standard culture conditions (Suppl. Figure 4). At 24 hours, the migratory synovial myeloid population (Live/CD56−/CD3−/CD11c−/CD20−/CD14+/CD11b+) in a representative patient sample contained OPG+, TREM-2+, ZO-1+, CX3CR1+, and F11R+ populations in the lower transwell media (Figure 2B). Sixty-five percent of CD11b+CD14dim cells migrating out of the synovium tissue were double positive for OPG and CX3CR1, and of those cells, 93.8% were double positive for tight junction markers F11R and ZO-1. However, we noted the CD14hi macrophages displayed the greatest increase in the proposed RSM markers (Figure 2B), and the relevance of CD14 dim versus high in the acute ex vivo setting requires further clarification. To evaluate co-expression of these RSM-specific markers, t-distributed Stochastic Neighbor Embedding (tSNE) plots were created. Of the synovial myeloid population described above, there was weak or low expression of all RSM-specific markers, but ZO-1 may be most specific to identifying migratory RSM in SF (Figure 2C, top right). Cells with markers of circulating immune subsets, including neutrophils (CD66b), NK cells (CD56), and T cells (CD3) were also present in the lower transwell chambers (Suppl. Figure 2). Though there were no significant differences found between treatments in this experiment, in all cases the stimuli resulted in decreased RSM-like cell migration compared to control, indicating these RSMs may remain active in the tissue, and migratory cells present in the control may be in response to tissue trauma, an effect countered by the stimuli. Cells found adhered to the underside of the transwell or in the bottom of the lower well were below the limit of detection by Crystal Violet assay (data not shown). Therefore, we conclude that RSMs can migrate out of the synovium, and ZO-1 is likely the most specific RSM marker expressed by myeloid cells, but tight junction markers in conjunction with M2 markers and OPG are necessary for identification.
Figure 2:
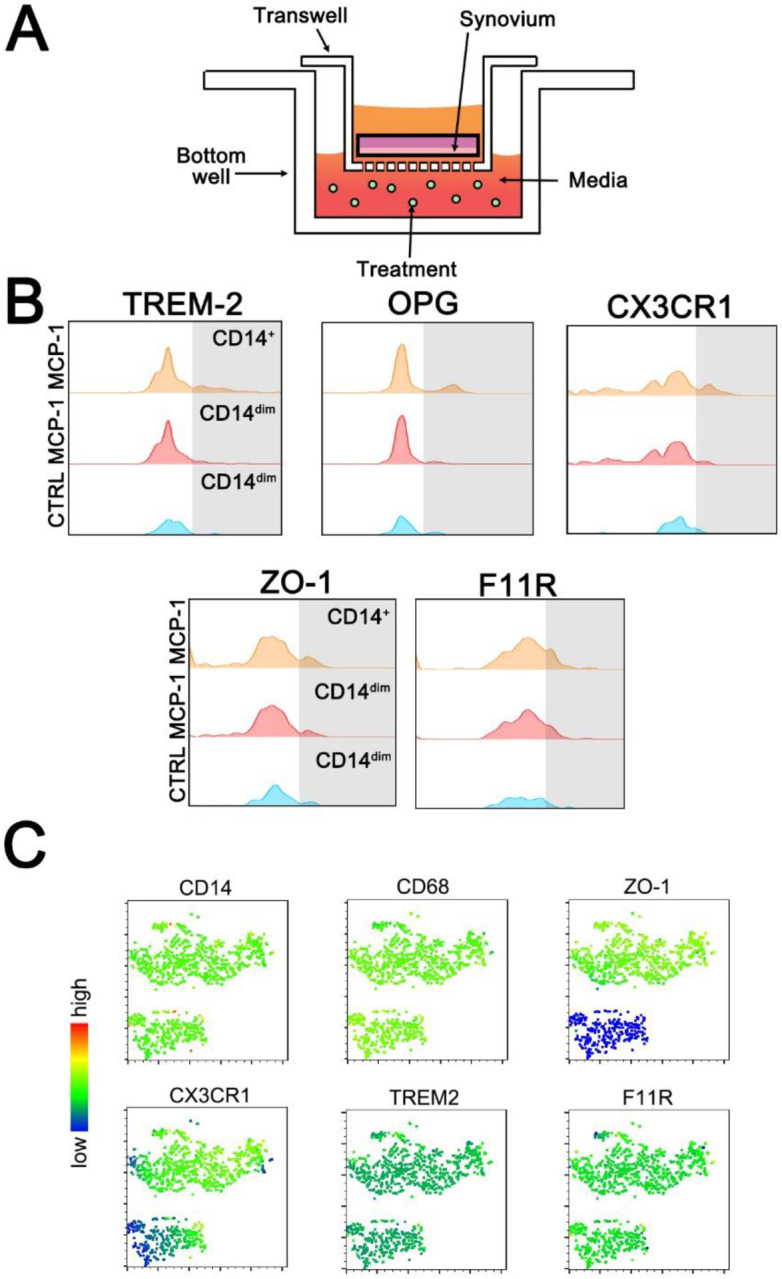
Transwell synovial cell migration assay. Schematic of the experimental set up (A). Resident synovial macrophage markers TREM-2+, OPG+, CX3CR1+, ZO-1+ and F11R+ were evaluated within the migratory monocyte/macrophage population (Alive/CD56−/CD3−/CD11c−/CD20−/CD14+/CD11b+) in a representative patient treated with 250 ng/mL of MCP-1 (B). tSNE plots of the same patient’s migratory myeloid cells demonstrating ZO-1 has the most delineation from other markers (C).
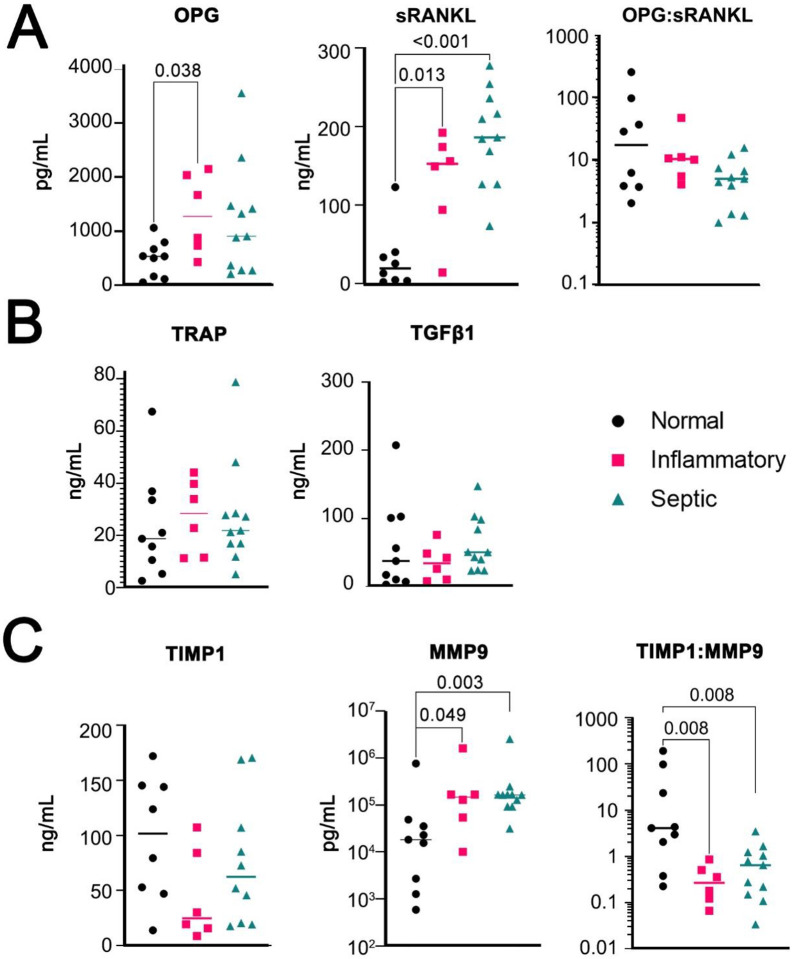
Evaluation of Pro and Anti-Resorptive Potential of SF:
SF supernatant was analyzed for cytokines implicated in bone and/or cartilage destruction. OPG is the decoy receptor for RANKL, which in turn is required for the formation of osteoclasts; the ratio between these two cytokines is an important method to evaluate bone maintenance versus destruction (Figure 3A) (18). The collagenase Tartrate Resistant Acid Phosphatase (TRAP), which is secreted by osteoclasts, had no observed change. Transforming Growth Factor Beta (TGF-β) not only inhibits osteoclastogenesis (19), but also stimulates osteoblasts (20) and further, it is stored in the latent phase within the extracellular matrix to be released during bone turnover (21; 22). Likewise, there was no significant difference between pathologic SF and control TGF-β levels (Figure 3B). We also tested Bone Morphogenic Protein 2 (BMP2), TNF-α and IFN-γ, however all were below the limit of detection (data not shown). Finally, Tissue Inhibitor of Matrix Metalloproteinases 1 (TIMP1) and Matrix Metallopeptidase 9 (MMP9) were evaluated (Figure 3C). TIMP1 is an inhibitor of MMPs. MMP9 is expressed by osteoclasts and is an important enzyme for bone remodeling (23). There was an increase in MMP9 in patients with inflammatory and septic arthritis, and a significantly decreased ratio of TIMP1:MMP9 in these patients as well. Therefore, there is increased potential for bone and cartilage damage in patients with inflammatory and septic arthritis due mainly to increased MMP9, which is possibly due to increased osteoclastogenesis secondary to increased RANKL, or increased synovial fibroblast production. As RSM-like cells were decreased in SF of septic arthritis patients that also had highest RANKL and MMP9, it is possible RSM provide a protective mechanism against bone and joint destruction. The specific cytokine production profile of RSM specifically will require further evaluation.
Figure 3:
ELISA results of SF supernatant protein concentration. OPG, sRANKL, and ratio of OPG:sRANKL (A). TRAP and TGF-β1, (B). Measures of TIMP1, MMP9, or ratio of TIMP1:MMP9. Normal (n=9), inflammatory (n=6), and septic (n=11). Multiple Mann-Whitney tests with FDR rate <0.05.
Identification of Rare Cell Subsets Using scRNA-seq:
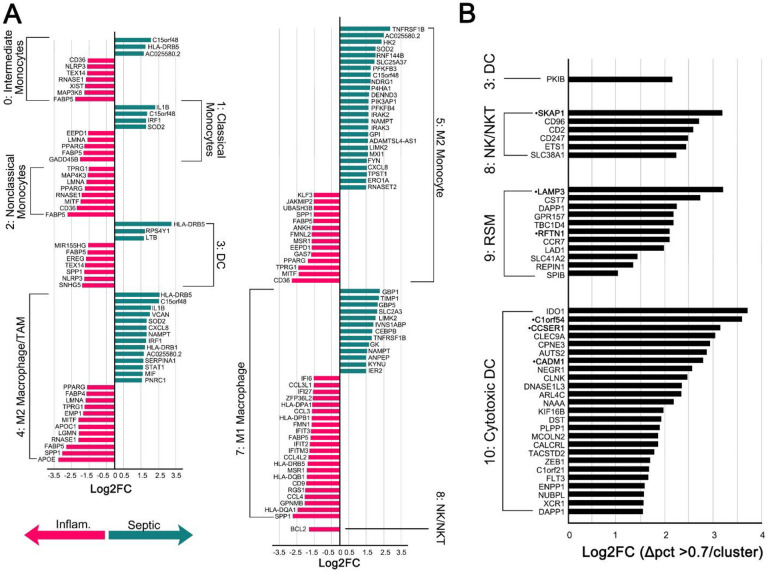
We analyzed sorted myeloid cells from patients with infectious and inflammatory arthritis for highly variable features, demonstrating a relevant focus of M1 and M2 functions (Suppl. Figure 6A). Cell subpopulations were clustered into 11 groups (Figure 4A) with manual annotation of the clusters based on top ten gene expression (Figure 4B). There were insufficient cell events to separately cluster NK and NKT cells, therefore they are represented in a single, though spatially separate, cluster.
Figure 4:
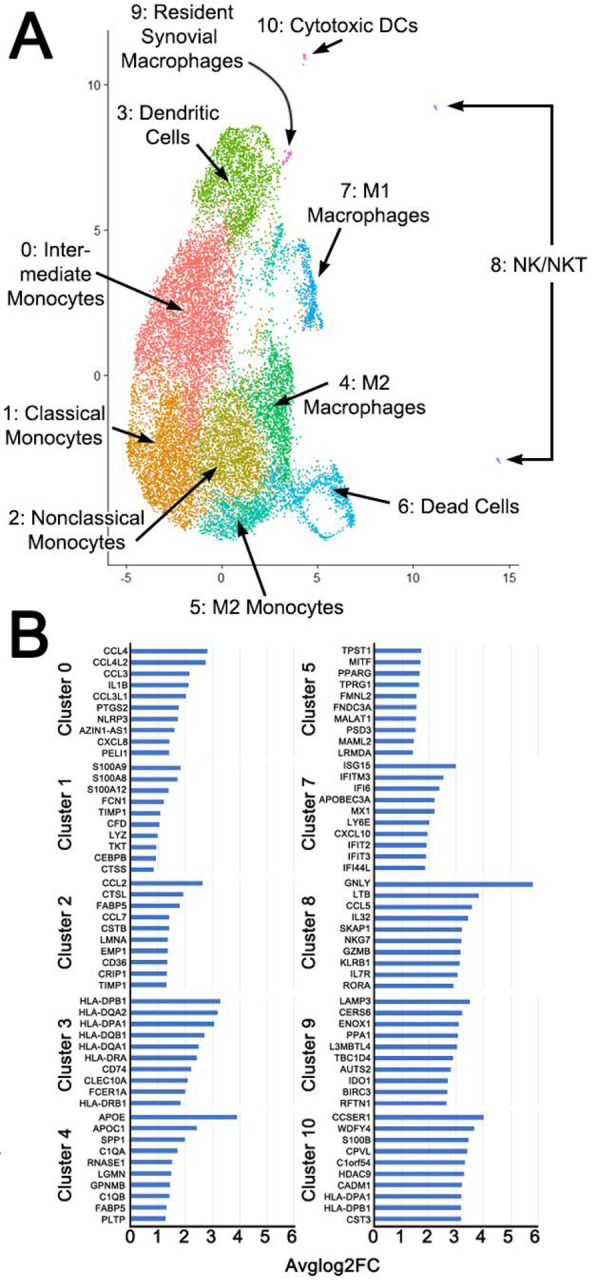
Unsupervised Cluster Analysis showing composite of 3 patients with inflammatory arthritis (A). Clusters were manually identified based on top ten expressed genes (B) in addition to classical markers. Analysis performed in R with resolution of 0.4 and dimensions 1:30. N = 3 patients per inflammatory and septic arthritis.
After assigning known and widely accepted monocyte/macrophage designations based on gene profiles, clusters 9 and 10 remained. Cluster 10 expressed NK-marker CD56, low CD68 (Suppl. Figure 6B), while also having high HLA expression (Figure 4B). However, Cluster 10 did not express any granzymes. Therefore, we putatively classified this cluster as cytotoxic DCs (24). Cluster 9 expressed F11R (7), CD68, and M2-marker IDO1 (25) (Figure 4B, Suppl. Figure 6B). It was also the only cluster with OPG expression, though this was not significant. This is putatively consistent with the RSM phenotype. ZO-1 was not expressed in any cluster. We also evaluated expression of resident macrophage transcription factor GATA6 (26), which was minimally expressed, but only in Cluster 3. This may indicate that RSMs are split between multiple clusters, as Cluster 3 was designated as DCs based on high expression of HLA—which is also consistent with sub-intimal RSM, but not intimal RSM (27). Based on PCA analysis (Suppl. Figure 6C), cytotoxic DCs were more closely related to NK/NKT cells than to RSM, and the cytotoxic DC and RSM populations may represent phases of differentiation of the same cell of origin, as both were distinct from the circulating monocyte/macrophage population. The plasticity of tissue macrophages, and ability to survive in new compartments has been advocated and challenged; the exact lineage remains unknown.
Differentially Expressed Genes (DEGs) were explored using a volcano plot (Suppl. Figure 6D) and a Log2FC threshold of 1.5. DEGs were statistically significant in Clusters 0-8 (Figure 5A). Transcripts highly upregulated in septic arthritis (and therefore down-regulated in inflammatory arthritis) among multiple clusters included HLA-DRB5, C15orf48, IL1B, AC025580.2, and SOD2. In inflammatory arthritis, common gene upregulation included PPARG, FABP5, CD36, NLRP3, SPP1, and MITF. M1 macrophages from the two different arthritis etiologies showed a distinctly different gene expression patterns with an upregulation of GBP1, GBP5, and TIMP1.
Figure 5:
Differentially Expressed Genes (DEGs) (A). Conserved genes where Log2FC > 1.5 and the change in percent expression in both septic arthritis and inflammatory arthritis was > 0.7 in each cluster compared to all other clusters. Gene names in •bold represent genes that were also in the top 10 expressed genes in Figure 4.
Conserved genes that remained highly expressed in both inflammatory and infectious arthritis were also examined, as these could represent novel targets in the treatment, or prevent the conversion of infectious to inflammatory arthritis. Conserved genes were only significant in DC, NK/NKT, RSM, and Cytotoxic DC clusters (Figure 5B, Suppl. Table 4). Nearly all genes were associated with cytolytic function, antigen presentation, M1/M2 polarity, or lysosomes.
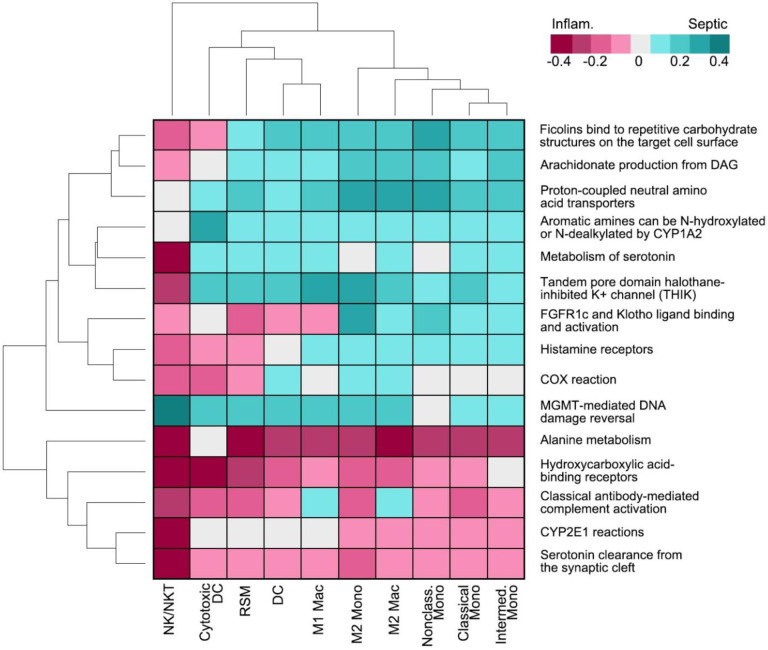
Gene Set Analysis (GSA) was then assessed to determine the overall, broad picture, of cell function and metabolism (Figure 6) with the 15 most upregulated or downregulated pathways, which identified complement, bone-derived FGF23 signaling, and COX signaling. Pathways specifically immune relevant, related to phagocytic potential, complement associated and adhesion related (Suppl. Figure 7) were also examined. In all cases except adhesion, Cytotoxic DC, NK/NKT, and RSMs populations shared a similar pattern of activity, indicating functional overlap. GSA was further performed specifically on the RSM cluster to identify the maximum changes in septic and inflammatory arthritis (Suppl. Figure 8), which identified threonine, pyridoxine, and thiamine metabolism.
Figure 6:
Top 15 most up or downregulated pathways using DEGs and ReactomeGSA.
Discussion:
RSMs are regulatory immune cells of the joint space that are historically identified using tissue histology. We identified a rare subset of SF macrophages identified by flow cytometry consistent with previously described RSMs, indicating migratory capacity especially during pathologies that dysregulate tight junctions. The role of these cells in SF has yet to be established.
Though the chemokine receptor CX3CR1 was found to be specific for murine RSM (1), we were unable to distinguish SFCs from peripheral monocytes using this marker. Likewise, HLA-DR expression was downregulated in the intimal-lining RSMs, but upregulated in sub-lining RSMs (8), and it is unclear if HLA-DR expression would assist in identification of RSM-like cells in the SF. We first identified putative RSMs as CD14dimOPG+ M2-macrophages, and while CD14dim monocytes have been described previously as non-classical and poorly phagocytic (28; 29), data is limited on CD14dim macrophages. CD14dim gingival macrophages were M2 and likely osteo-protective by high expression of IL-10 and TGF-β in the setting of gingivitis (30). To fully elucidate the utility of CX3CR1, HLA-DR, and CD14 in human SF macrophage subsets requires further work.
To determine whether our identified cells came from synovium or from circulation, we piloted a novel transwell migration assay with human ex vivo synovium that validated our flow cytometry findings. Therefore, we believe the CD68/TREM2/OPG/ZO-1/F11R myeloid cell fraction is the most representative of putative RSMs. This explant model could be used widely to study other synovial pathology.
We then utilized scRNA-seq to identify rare macrophage subpopulations. Markers identified using flow cytometry were not always identified in gene transcripts, including ZO-1. Instead we identified M2/Tumor Associated Macrophage markers such as IRF4 (31) and IDO1 (32) (Suppl. Figure 6B). While the putative RSM cluster resembled M2 macrophages, these cells also had a similar transcriptional signature to inflammatory NK/NKT and Cytotoxic DCs, indicating that RSMs may be capable of taking on an inflammatory and/or joint destructive phenotype in settings of chronic inflammation. This was demonstrated by the pro-inflammatory expression of CCR7 (Log2FC 2.25) and CD86 (Log2FC 0.92) in infectious settings.
To provide context to our findings, we compared our findings to scRNA-seq performed on synovium by other groups. Human MERTK+CD206+ RSMs were anti-inflammatory in patients in remission from rheumatoid arthritis (RA) (27). We found MERTK expressed highest in Cluster 5/M2 Monocytes (Log2FC 0.94), but it was not expressed in 9/RSM. Interestingly, MERTK−CD206− RSM indicated active RA (27). As all patients who received arthrocentesis and participated in the scRNA-seq were acutely symptomatic, a MERTK+CD206+ RSM profile was likely physiologically improbable. For CD206, this was expressed highest in Cluster 4/M2 Macrophages, which additionally expressed TREM2, FOLR2, and LYVE1 (27), though only TREM2 had Log2FC >0.5. Once RSMs exit synovial tissue to enter the SF, previously established profiles may no longer apply, and RSMs may be distributed amongst clusters rather than a discrete cluster.
Others found that RSMs arose from CSF1R+ interstitial macrophages (7). We found CSF1R expressed in clusters 3-6, and 8-10. It was also found that interstitial RSM expressed RETNLA, STMN1, and AQP1 (7). RETNLA and AQP1 transcripts were not identified in this study, while STMN1 was only expressed by Cluster 3/DCs, but this was not significant. Likewise, markers of tight junctions and cell polarity previously found included F11R, CLDN5, FAT4, and VANGL2. Of these, only F11R was identified, and expressed mainly in RSM and Cytotoxic DC clusters (Suppl. Figure 6B).
We identified LAMP3/CD208/DC-LAMP as the top gene expressed by Cluster 9/RSM, and while the exact function of LAMP3 has yet to be elucidated, it is likely to be involved with MHC Class II peptide presentation (34). LAMP3 is also traditionally considered a marker of mature DCs. DCs expressing LAMP3 are regulatory in nature and more enriched in draining lymph nodes rather than tumors (35). However, LAMP3 is upregulated by THP-1 macrophages with in vitro LPS stimulation (36), and is constitutively expressed in primary macrophages in multiple species (37). Further analysis of Cluster 9/RSM gene expression revealed high expression of ENOX1 (Figure 4), involved with reduction of oxygen to superoxide (38). Likewise, CERS6 contributes to mitochondrial dysfunction by promoting reactive oxygen species production in hepatocytes (39). IDO1 expression in macrophages has been associated with increased tumor immune cell infiltration (40) and tryptophan metabolism, the metabolites of which inhibit oxidative cell death (41). Together this indicates that these cells are likely to have potent generation of superoxide with inhibition of apoptosis, which may indicate perpetuation of chronic inflammation. Until these putative RSM can be compared to similar cells from healthy SF, the baseline role and function are unclear.
Given we believe these cells are capable of migratory function, markers of migration and extravasation were also examined. CADM1 was highly and conservatively expressed (Figure 5) and is strongly associated with TREM2+ tumor associated macrophages (42). ALCAM (Log2FC 0.73) is expressed by endothelial cells of the blood-brain barrier and migrating monocytes (43), which may be consistent with the relative immune privilege of the joint space. As ALCAM stabilizes tight junctions (44), this integrin may be important in the homeostasis of the joint space as maintained by RSM. PECAM1 (Log2FC −1.03) assists with leukocyte migration through tight junctions (45). Our data seems to suggest a differential regulation of tight junctions by macrophages depending on pathology.
As there were no DEGs identified in Cluster 9/RSM, GSA was performed to identify unique pathways and discovered threonine, pyridoxine, and thiamine metabolism (Suppl. Figure 8). The role of threonine catabolism is unclear in macrophages but is critically necessary for murine stem cell viability (46), which may be similar function to locally renewing macrophage populations. Pyridoxine suppresses IL-1β release through NLRP3 inhibition (47), while thiamine precursors have been found to both increase cellular glutathione stores and inhibit NF-kB translocation to the nucleus in microglial cells (48), the resident macrophages of the brain. Putative RSMs are upregulating anti-inflammatory pathways, but the question remains if the concurrent upregulation of complement and superoxide transcripts may supersede the protective mechanisms in place through B vitamins.
We also evaluated the protein levels of multiple bone-relevant cytokines and related this to cell populations in the joint space. The DEG TIMP1 was identified in Cluster 7/M1 Macrophage as highly upregulated in septic arthritis, with concurrent downregulation in inflammatory arthritis. As a 1:1 inhibitor of MMP9 (49), TIMP1 is likely protective in the setting of joint inflammation by preserving the extracellular matrix of cartilage and bone from enzymatic degradation. We observed a decrease in the stoichiometric ratio of TIMP1:MMP9 protein concentration in both infectious and inflammatory arthritis SF, which is concerning for MMP9 as a major cause of bone destruction in these pathologic states, and a potential therapeutic target. MMP9 was not differentially expressed, and MMP9 found by ELISA is likely from other cells, specifically fibroblast like synoviocytes and/or neutrophils (50), but possibly also osteoclasts (23). TIMP1 was expressed in Cluster 9/RSM (Log2FC −2.67) indicating RSM would not be the primary source of this in SF.
In conclusion, the profile of a subset of M2, likely osteoprotective macrophages in SF that can be stimulated to migrate out of synovial tissue ex vivo suggests these are equivalent to tissue resident macrophages. However, these putative RSM expressed transcripts heavily involved with antigen presentation (LAMP3), oxidative stress (ENOX1, CERS6, IDO1), and cell migration (ALCAM, PECAM). This suggests that in settings of infectious or inflammatory arthritis, these cells may perpetuate rather than attenuate inflammation, and could be involved in the transition to chronic symptomology once out of the normal synovial tissue niche. Further work will focus on the cytokine expression of these putative migratory RSM, and the interactions of RSM with other cells in the joint space, including T cells, B cells, and NK or NKT cells.
Supplementary Material
Acknowledgements:
The authors would like to thank Catherine Fairfield BSN, EM Research Coordinator, and Alex Peebles, Cameron Williams, Shannon Landers, Klaudia Golebiewski, Allison Herr, Noble Briggs, Vanko Bicar, Malea Pinckney, Heath Gibbs, Nicole Grossmann, Scott Tibbetts, Ike Appleton, Jay Miller, and Jacob Hampton with the Emergency Department Research Enroller Program (ED-REP) for their essential role in enrolling patients and collecting data for this project.
The authors would also like to thank Dr. Daniel Livorsi and the Division of Infectious Diseases for assistance with patient recruitment.
The data presented herein were obtained at the Flow Cytometry Facility, which is a Carver College of Medicine / Holden Comprehensive Cancer Center core research facility at the University of Iowa. The facility is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran's Administration Medical Center. Research reported in this publication was supported by: the National Center for Research Resources of the National Institutes of Health under Award Number 1 S10 OD034193-01; and the National Cancer Institute of the National Institutes of Health under Award Number P30CA086862
Sources of funding:
Cyndari: This project was supported by funding from the Herbert N. Hultgren Award (Wilderness Medicine Society), the University of Iowa Physician-Scientist Training Program, and the University of Iowa Department of Emergency Medicine.
References
- 1.Origin and function of synovial macrophage subsets during inflammatory joint disease. Culemann S, Grüneboom A, Krönke G. 2019, Adv Immunol, Vol. 143, pp. 75–98. [DOI] [PubMed] [Google Scholar]
- 2.Immune cell profiles in synovial fluid after anterior cruciate ligament and meniscus injuries. Kim-Wang S.Y., Holt A.G., McGowan A.M. et al. 280, 2021, Arthritis Res Ther, Vol. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Synovial fluid monocyte/macrophage subsets and their correlation to patient-reported outcomes in osteoarthritic patients: a cohort study. Gómez-Aristizábal A., Gandhi R., Mahomed N.N. et al. 26, 2019, Arthritis Res Ther, Vol. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.High percentages and activity of synovial fluid NK cells present in patients with advanced stage active Rheumatoid Arthritis. Yamin R, Berhani O, Peleg H, Aamar S, Stein N, Gamliel M, Hindi I, Scheiman-Elazary A, Gur C. 1, 2019, Sci Rep, Vol. 9, p. 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resident Macrophages Cloak Tissue Microlesions to Prevent Neutrophil-Driven Inflammatory Damage. Stefan Uderhardt, Martins Andrew J., Tsang John S., Lämmermann Tim, Germain Ronald N.,. 3, 2019, Cell, Vol. 177, pp. 541–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Bartok B, Firestein GS. 1, 2010, Immunol Rev, Vol. 233, pp. 233–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locally renewing resident synovial macrophages provide a protective barrier for the joint. Culemann S, et al. 7771, 2019, Nature, Vol. 572, pp. 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Critical Role of Synovial Tissue-Resident Macrophage and Fibroblast Subsets in the Persistence of Joint Inflammation. Kemble S, Croft AP. 2021, Front Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Synovial Fluid Tests: What Should Be Ordered? Shmerling RH, Delbanco TL, Tosteson ANA, Trentham DE. 8, 1990, JAMA, Vol. 264, pp. 1009–1014. [PubMed] [Google Scholar]
- 10.Corning Incorporated. Transwell Permeable Supports Selection and Use Guide. 2013. [Google Scholar]
- 11.Comprehensive Integration of Single-Cell Data. Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, III WMM, Hao Y, Stoeckius M, Smibert P, Satija R. 2019, Cell, Vol. 177, pp. 1888–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ReactomeGSA - Efficient Multi-Omics Comparative Pathway Analysis. Griss J, Viteri G, Sidiropoulos K, Nguyen V, Fabregat A, Hermjakob H. 2020, bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blighe K, Rana S, Lewis M. EnhancedVolcano: Publication-ready volcano plots with enhanced colouring and labeling. [Online] https://github.com/kevinblighe/EnhancedVolcano. [Google Scholar]
- 14.Systematic determination of the mitochondrial proportion in human and mice tissues for single-cell RNA-sequencing data quality control. Osorio D, Cai JJ. 7, 2021, Bioinformatics, Vol. 37, pp. 963–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trem-2 Promotes Emergence of Restorative Macrophages and Endothelial Cells During Recovery From Hepatic Tissue Damage. Inês Coelho, Nádia Duarte, André Barros, Paula Macedo Maria, Carlos Penha-Gonçalves. 2021, Frontiers in Immunology , Vol. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Synovial tissue macrophages: friend or foe? Kurowska-Stolarska M, Alivernini S. 2017, RMD Open, Vol. 3, p. e000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion. Roach Tamara, Salter Suzanne, Koval Michael et al. 6, 1997, Current Biology, Vol. 7, pp. 408–17. [DOI] [PubMed] [Google Scholar]
- 18.Biology of RANK, RANKL, and osteoprotegerin. Boyce BF, Xing L. Suppl 1, 2007, Arthritis Res Ther, Vol. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Interferon-Gamma-Mediated Osteoimmunology. Tang M, Tian L, Luo G, Yu X. 9, 2018, Front Immunol, Vol. 29, p. 1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Role of transforming growth factor-beta in bone remodeling. Bonewald LF, Mundy GR. 250, 1990, Clin Orthop Relat Res, pp. 261–76. [PubMed] [Google Scholar]
- 21.The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Hinz Boris. 2015, Matrix Biology, Vol. 47, pp. 54–65. [DOI] [PubMed] [Google Scholar]
- 22.TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Wu M, Chen G, Li YP. 4, 2016, Bone Res, Vol. 26, p. 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matrix metalloproteinases in bone development and pathology: current knowledge and potential clinical utility. Liang HPH, Xu J, Xue M, Jackson CJ. 2016, Metalloproteinases In Medicine, Vol. 3, pp. 93–102. [Google Scholar]
- 24.CD56 marks human dendritic cell subsets with cytotoxic potential. Roothans D, Smits E, Lion E, Tel J, Anguille S. 2, 2013, Oncoimmunology, Vol. 2, p. e23037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Critical role of synovial tissue-resident macrophage niche in joint homeostasis and suppression of chronic inflammation. Huang QQ, Doyle R, Chen SY, Sheng Q, Misharin AV, Mao Q, Winter DR, Pope RM. 2, 2021, Sci Adv, Vol. 7, p. eabd0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GATA6+ Peritoneal Resident Macrophage: The Immune Custodian in the Peritoneal Cavity. Jayakumar P, Laganson A, Deng M. 866993, 2022, Front Pharmacol, Vol. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Alivernini S., MacDonald L., Elmesmari A. et al. 2020, Nat Med, Vol. 26, pp. 1295–1306. [DOI] [PubMed] [Google Scholar]
- 28.Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Kapellos Theodore S., Bonaguro Lorenzo, Gemünd Ioanna, Reusch Nico, Saglam Adem, Hinkley Emily R., Schultze Joachim L. 2019, Frontiers in Immunology, Vol. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D'Cruz D, Casanova JL, Trouillet C, Geissmann F. 3, 2010, Immunity, Vol. 33, pp. 375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macrophage subset sensitivity to endotoxin tolerisation by Porphyromonas gingivalis. Foey AD, Crean S. 7, 2013, PLoS One, Vol. 8, p. e67955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Interferon-regulatory factors determine macrophage phenotype polarization. Günthner R, Anders HJ. 2013, Mediators Inflamm, p. 731023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The role of indoleamine 2,3-dioxygenase (IDO) in immune tolerance: focus on macrophage polarization of THP-1 cells. Wang XF, Wang HS, Wang H, Zhang F, Wang KF, Guo Q, Zhang G, Cai SH, Du J. 1-2, 2014, Cell Immunol, Vol. 289, pp. 42–8. [DOI] [PubMed] [Google Scholar]
- 33.TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Zhang F, Wang H, Wang X, Jiang G, Liu H, Zhang G, Wang H, Fang R, Bu X, Cai S, Du J. 32, 2016, Oncotarget, Vol. 7, pp. 52294–52306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vitamin D3 regulates LAMP3 expression in monocyte derived dendritic cells. Malaguarnera L., Marsullo A., Zorena K., Musumeci G., Di Rosa M.. 2017, Cellular Immunology, Vol. 311, pp. 13–21. [DOI] [PubMed] [Google Scholar]
- 35.Mature dendritic cells enriched in immunoregulatory molecules (mregDCs): A novel population in the tumour microenvironment and immunotherapy target. Li J, Zhou J, Huang H, Jiang J, Zhang T, Ni C. 2, 2023, Clin Transl Med, Vol. 13, p. e1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LAMP-3 (Lysosome-Associated Membrane Protein 3) Promotes the Intracellular Proliferation of Salmonella typhimurium. Lee EJ, Park KS, Jeon IS, Choi JW, Lee SJ, Choy HE, Song KD, Lee HK, Choi JK. 7, 2016, Mol Cells, Vol. 39, pp. 566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Characterisation and expression analysis of the rainbow trout (Oncorhynchus mykiss) homologue of the human dendritic cell marker CD208/lysosomal associated membrane protein 3,. Johansson Petronella, Corripio-Miyar Yolanda, Wang Tiehui, Collet Bertrand, Secombes Chris J., Zou Jun. 3-4, 2012, Developmental & Comparative Immunology, Vol. 37, pp. 402–413. [DOI] [PubMed] [Google Scholar]
- 38.Increased Expression of Ecto-NOX Disulfide-thiol Exchanger 1 (ENOX1) in Diabetic Mice Retina and its Involvement in Diabetic Retinopathy Development. YU-CHUEN HUANG, SHIH-PING LIU, SHIH-YIN CHEN, JANE-MING LIN, HUI-JU LIN, YU-JIE LEI, YEH-HAN WANG, WAN-TING HUANG, WEN-LING LIAO, FUU-JEN TSAI. 6, 2019, In Vivo, Vol. 33, pp. 1801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.CerS6 triggered by high glucose activating the TLR4/IKKβ pathway regulates ferroptosis of LO2 cells through mitochondrial oxidative stress. Li D, Tian L, Nan P, Zhang J, Zheng Y, Jia X, Gong Y, Wu Z. 2023, Mol Cell Endocrinol, Vol. 572, p. 111969. [DOI] [PubMed] [Google Scholar]
- 40.Evaluating the role of IDO1 macrophages in immunotherapy using scRNA-seq and bulk-seq in colorectal cancer. Xingwu Liu, Guanyu Yan, Boyang Xu, Han Yu, Yue An, Mingjun Sun. 2022, Frontiers in Immunology , Vol. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.IL4i1 and IDO1: Oxidases that control a tryptophan metabolic nexus in cancer. Zeitler L, Murray PJ. 6, 2023, J Biol Chem, Vol. 299, p. 104827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tissue-resident FOLR2+ macrophages associate with CD8+ T cell infiltration in human breast cancer. Ramos RN, Missolo-Koussou Yoann, Gerber-Ferder Yohan, et al. 7, 2022, Cell, Vol. 185, pp. 1189–1207. [DOI] [PubMed] [Google Scholar]
- 43.ALCAM (CD166) is involved in extravasation of monocytes rather than T cells across the blood-brain barrier. Lyck R, Lécuyer MA, Abadier M, Wyss CB, Matti C, Rosito M, Enzmann G, Zeis T, Michel L, García Martín AB, Sallusto F, Gosselet F, Deutsch U, Weiner JA, Schaeren-Wiemers N, Prat A, Engelhardt B. 8, 2017, J Cereb Blood Flow Metab, Vol. 37, pp. 2894–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dual role of ALCAM in neuroinflammation and blood-brain barrier homeostasis. Lécuyer MA, Saint-Laurent O, Bourbonnière L, Larouche S, Larochelle C, Michel L, Charabati M, Abadier M, Zandee S, Haghayegh Jahromi N, Gowing E, Pittet C, Lyck R, Engelhardt B, Prat A. 4, 2017, Proc Natl Acad Sci U S A, Vol. 114, pp. E524–E533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.PECAM-1: a multi-functional molecule in inflammation and vascular biology. Woodfin A, Voisin MB, Nourshargh S. 12, 2007, Arterioscler Thromb Vasc Biol, Vol. 27, pp. 2514–23. [DOI] [PubMed] [Google Scholar]
- 46.Dependence of Mouse Embryonic Stem Cells on Threonine Catabolism. Jian Wang et al. 2009, Science, Vol. 325, pp. 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vitamin B6 Prevents IL-1β Protein Production by Inhibiting NLRP3 Inflammasome Activation. Zhang P, Tsuchiya K, Kinoshita T, Kushiyama H, Suidasari S, Hatakeyama M, Imura H, Kato N, Suda T. 47, 2016, J Biol Chem, Vol. 291, pp. 24517–24527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benfotiamine Upregulates Antioxidative System in Activated BV-2 Microglia Cells. Bozic I., Savic D., Stevanovic I., Pekovic S., Nedeljkovic N., Lavrnja I. 9, 2015, Front. Cell. Neurosci, p. 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Structural characterisation of inhibitory and non-inhibitory MMP-9–TIMP-1 complexes and implications for regulatory mechanisms of MMP-9. Charzewski Ł, Krzyśko K.A. & Lesyng B. 2021, Sci Rep, Vol. 11, p. 13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. 6, 2013, Physiology (Bethesda), Vol. 28, pp. 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.