Abstract
Why individuals have negative consequences following stress is a complex phenomenon that is dictated by individual factors, the timing of stress within the lifespan, and when the consequences are measured. Women who undergo adverse childhood experiences are at risk for lasting biological consequences, including affective and stress dysregulation. We have shown that pubertal adversity is associated with a blunted glucocorticoid response within the hypothalamic-pituitary-adrenal axis in both peripartum humans and mice. In mice, we examined puberty-stress reprogramming in the paraventricular nucleus (PVN) of the hypothalamus, which initiates the HPA axis response. We found that pubertal stress led to an upregulation of six immediate early genes (IEGs) in the PVN of adult, pregnant mice. Separately, we showed that the pregnancy-associated hormone allopregnanolone is necessary and sufficient to produce the blunted stress response phenotype in pubertally stressed mice. Here, we examined the response of the IEGs in the PVN to the primary disruption of pubertal stress in early adolescence and to the secondary disruption of increased allopregnanolone in pregnancy. We found that in adult female, but not male, mice previously stressed during puberty, intra-PVN allopregnanolone was sufficient to recapitulate the pubertal stress associated baseline IEG expression profile. We also examined baseline IEG expression during adolescence, where we found that IEGs have sex-specific developmental trajectories that were disrupted by pubertal stress. Altogether, these data establish that IEGs can act as a key molecular switch that leads to increased vulnerability to negative outcomes in adult, pubertally stressed animals. Understanding how the factors that produce vulnerability combine throughout the lifespan will further our understanding of the etiology of negative outcomes and will help guide both the nature and timing of potential treatments.
Keywords: stress, sex differences, puberty, transcriptome, hypothalamus, allopregnanolone
Introduction
Risk for neuropsychiatric disorders in adulthood is increased by exposure to adverse childhood experiences (ACEs) (1,2). Women who are exposed to ACEs during puberty are at the greatest risk for neuropsychiatric disorders across the lifespan (3–5) and suffer unique physiological and biological symptoms that are not shared by those who experienced adversity in childhood or adulthood (6–8). We previously established that adversity during puberty in female mice mimicked pubertal ACEs in women, significantly blunting their stress reactivity only during the peripartum window (9). Risk of peripartum depression and anxiety are elevated in women with high adversity and are associated with long-term negative outcomes for both mother and baby (10). Although we know that multiple experiences such as pubertal stress and pregnancy compound over the lifespan to influence risk for negative outcomes, we have limited understanding of the developmental trajectory of the biological programming that underlies increasing risk.
We have shown lasting effects of stress during puberty on the physiology and behavior of female mice and humans (8,9,11,12). To understand the mechanisms underlying the lasting effects of pubertal stress, our focus has been on the paraventricular nucleus of the hypothalamus (PVN), both a key brain region that regulates the hypothalamic-pituitary-adrenal (HPA) stress axis response and a region we have previously identified to have disruption of both the epigenome and transcriptome following pubertal stress (9,11). It has been of great interest to scientists and clinicians to understand the molecular underpinnings of the lasting effects of exposure to stress or trauma early in life (13–15). Here, we leveraged our pubertal stress-associated molecular signature, where six immediate early genes (IEGs) were permissively expressed at baseline in the PVN of pregnant females, to provide insight into whether early life stress-induced molecular changes are present as an immediate consequence of pubertal stress or are apparent only in adulthood. In addition to the IEG signature, we have shown that allopregnanolone may be the part of pregnancy that is responsible for unmasking the latent molecular programming. However, whether allopregnanolone and the IEGs are linked is unknown. Although IEGs are associated with neural activation and plasticity after exposure to stimuli, IEG mRNA and proteins are canonically very low to undetectable at baseline (16–18). So, their increased expression at baseline in pregnant, pubertally stressed females suggests a potential mechanistic role in influencing how the PVN is poised to respond to stimuli. Further, pubertal stress occurs much earlier in the lifespan compared to when we examine physiology and the transcriptional state of the brain (early adolescence vs adulthood). We also examined whether IEGs are the primary programming of pubertal stress by addressing whether baseline IEG expression is dynamic during puberty and whether any potential change is influenced by pubertal stress.
Therefore, we examined the response of the IEGs in the PVN to the primary disruption of pubertal stress in early adolescence and to the secondary disruption of increased allopregnanolone in pregnancy. We hypothesized that if the IEGs are a molecular trigger of the negative consequences we have observed in pubertally stressed, pregnant mice and humans, we would observe increased responsiveness of these genes in the PVN of pubertally stressed mice in both developmental phases.
Methods
Animals
All mice were virgin, in house mixed strain C57BL/6:129 (N = 143) (9,11,12). Some mice were heterozygous Crh-IRES-Cre;Ai14, capable of expressing tdTomato in corticotropin releasing factor cells, although the transgenic background was not utilized in this study (19,20). All mice were kept on a 12-hour light-dark cycle and food and water were available ad libitum. All procedures were approved by the West Virginia University Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Pubertal stress
Starting on postnatal day (PN) 21, mice underwent two weeks of chronic variable stress (CVS) (9,11,12). The stressors began within the first hour of lights on (between 8:00 – 9:00 am) during the daily nadir of circadian corticosterone levels. CVS lasted two hours each day, excluding the acute restraint, which was only 15 minutes in length. Two stressors were randomly assigned each day and varied between three different sensory modalities: tactile (wet bedding, wire mesh, no bedding, marbles, multiple cage changes, 15-minute acute restraint stress), auditory (white noise, owl screech), and olfactory (70% ethanol, puma odor [1:200 2-Phenethylamine, CAS 64–04-0, in mineral oil]). Mice experienced rotating combinations of stressors (tactile and auditory, tactile and olfactory, or auditory and olfactory), combinations of which also rotated between specific stressors in each category. For Experiment 1, mice in the CVS group were weaned into singly housed cages at the beginning of stress (PN21) and were pair-housed with a same-sex, same-stress littermate at the end of the 14 days of stress (PN34). Control mice were weaned on PN28 into same-sex, pair-housed cages. They were left undisturbed until adulthood (10–18 weeks). For Experiment 2, mice in the CVS group were weaned into singly housed cages at the beginning of stress (PN21). They were left undisturbed for at least 24 hours after the last stressor and prior to brain collection, which occurred at PN28 or PN35. Control mice collected at PN21 and PN28 were removed directly from the litter at the time of collection. Control mice used for PN35 collations were weaned on PN28 into same-sex, pair-housed cages.
Experiment 1: pharmacological manipulation of allopregnanolone in adulthood
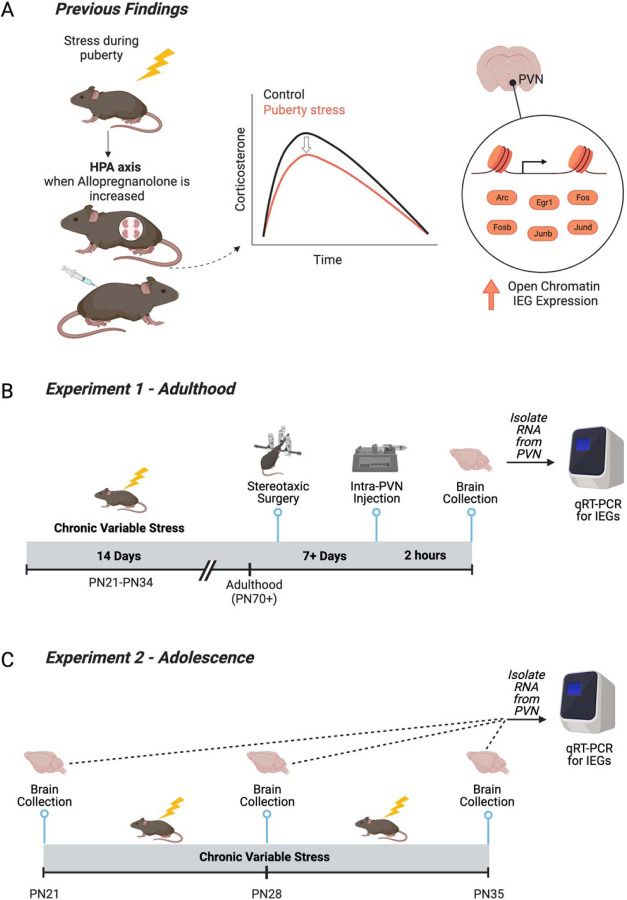
We previously observed pubertal stress-associated physiological, transcriptomic, and chromatin outcomes in adulthood when outcomes were examined under conditions of high allopregnanolone (pregnancy or peripheral allopregnanolone treatment, Figure 1A). Here we sought to mechanistically link allopregnanolone to the transcriptomic outcomes, a phenotype where six immediate early genes (IEGs) are permissively expressed in the PVN at baseline conditions. (Figure 1B). To test a direct role for allopregnanolone in the IEG phenotype in the PVN, adult male and female mice were treated with allopregnanolone (100 ng per side of PVN) or vehicle (20% weight/volume 2-Hydroxypropyl-β-cyclodextrin in water). Allopregnanolone was suspended in vehicle at 100ng/200nL for intra-PVN administration. It was prepared in advance and stored at −20°C.
Figure 1. Relevant prior findings and experimental timelines for the current study.
(A) We previously established that chronic variable stress (CVS) during puberty leads to a blunted hypothalamic-pituitary-adrenal axis in adulthood only when allopregnanolone is present at high levels in the brain, including during pregnancy and under allopregnanolone treatment (9,11). We identified the paraventricular nucleus of the hypothalamus (PVN) as a key tissue of the HPA axis that was reprogrammed by pubertal stress. Pubertal stress and pregnancy combined to lead to an altered chromatin landscape, including an increase in the number of open chromatin sites, and permissive expression of six immediate early genes (IEGs) at baseline. These findings suggest that IEGs play a mechanistic role in the puberty-stress induced phenotype that is apparent during times of dynamic change in allopregnanolone, including pregnancy. (B) Here, we directly tested the relationship between allopregnanolone within the PVN and the puberty stress associated IEG expression. Female and male mice were exposed to CVS from PN21–34. In adulthood, mice were implanted with a double-barrel cannula aimed at the PVN. Following recovery, mice were injected with either vehicle or allopregnanolone. Two hours later, brains were collected and processed for the measurement of gene expression. (C) We further examined a potential mechanistic role for IEG expression during the pubertal stress window. Brains were collected in baseline, non-stimulated conditions from female and male mice at either at PN21 (Control only), PN28 (Control and CVS), or PN35 (Control and CVS). Brains from CVS mice were collected 24 hours after exposure to the last stressor.
In adulthood (age 10–18 weeks), female (N = 26) and male (N = 19) mice underwent stereotaxic cannulation to selectively target the PVN. Mice were anesthetized using 4% isoflurane in an induction chamber and immediately connected to the surgery setup, where they received constant isoflurane (0.5%-2.5%). In addition, local analgesic carprofen (SC, 5 mg/kg) and anesthetics bupivacaine (at incision site, 1.5 mg/kg) and lidocaine (at incision site, 0.5 mg/kg) were administered before beginning the procedure. A double-barreled cannula (0.6mm center-to-center placing, 4mm cut below pedestal, P1 Technologies) was implanted in the brain at the PVN relative to bregma (−0.3mm medial/lateral, −0.85mm anterior/posterior) and lowered 3.8mm below the skull. Dental cement was used to fill the incision and create a headcap around the cannula, securing it to the skull. Mice were monitored for five consecutive days and received peripheral carprofen (SC, 5 mg/kg) for the first three days post-surgery. Following recovery (7–22 days), 200nL of allopregnanolone (100 ng/200 nl) or 200 nl vehicle was simultaneously infused into each side of the double-barreled cannula using two Hamilton Microsyringes and Dual Syringe Nanoliter Pump over a 1 minute period. The needle was left in place for an additional 1 minute to allow the infusion to diffuse away from the needle. Mice were briefly restrained to accomplish the insertion and removal of the injection needle. The dose and timing have been shown effective in altering behavior in prior studies (21,22). Mice were euthanized two hours later via cervical dislocation. Brains were frozen on dry ice and stored at −80°C until use.
Experiment 2: Influence of CVS on IEG expression during adolescence
Brains were collected from female and male mice at one of three time points: PN21, PN28, or PN35. At PN28 and PN35, brains were either from Control, unstressed mice or CVS mice 24 hours after the last stressor. Sample sizes started at N=10 per group, although there was some attrition (Table S1). Mice were anesthetized with vaporized Isoflurane and brains were collected under baseline (non-stimulated) conditions. Brains were frozen on dry ice and stored at −80°C until use.
PVN collection and analysis
Brains were cryosectioned to collect precise samples of the PVN. Two, 300 um slices were taken from the brain at the anatomic location of the PVN using Paxinos and Franklin’s stereotaxic coordinates (23) for all brains, except those from PN21 animals, for which we utilized two, 250 um slices. Pre-chilled 1mm biopsy tissue punchers were used to remove the PVN from each slice. PVN biopsies were ejected into a clean, pre-chilled 1.5 mL tube and stored at −80°C until use.
Verification of accurate cannula placement for Experiment 1 was made by inspecting the 300um slices of each sample for clear indications that the cannula and infusion needles had passed through the tissue. Further confirmation of accurate PVN location was completed by taking 10um slices before and after the region. The sections were stained using neutral red to verify anterior and posterior sites around the PVN. Samples were omitted from the study if the injection site fell outside of this region or neutral red staining revealed incorrect cryosectioning of the PVN (1 male and 1 female, Table S1).
Gene expression
We examined the expression of the six target IEGs (Fos, Fosb, Arc, Egr1, Junb, and Jund). RNA was isolated from PVN tissue using the RNeasy Mini Kit (Qiagen) following the manufacturer’s instructions. The quality of isolated RNA was assessed using a NanoDrop and Qubit RNA High Sensitivity Kit. The Applied Biosystems High-Capacity cDNA Reverse Transcriptase Kit was used to generate cDNA, and the quality of cDNA was assessed on a NanoDrop.
Gene expression was measured using the QuantStudio 5 quantitative real-time PCR (qRT-PCR) system. All gene expression assays were prepared with TaqMan Fast Advanced Master Mix and probes for the target genes and reference gene Gapdh (Table S2). Each reaction was run in triplicate using an equal volume of cDNA and Gapdh was run on each plate. Relative gene expression was calculated using the ΔΔCt method.
Statistical analyses
For experiment 1, gene expression data were analyzed by a 3-way ANOVA with sex, stress, and treatment (vehicle vs. allopregnanolone) as factors. Interactions were further investigated by 2-way ANOVAs (stress x treatment) within sex. For experiment 2, gene expression data were analyzed using several approaches: independent samples t-test for baseline sex differences at PN21, linear regression for trajectory of gene expression from PN21 to PN35, and 3-way ANOVA with sex, stress, and age (P28, PN35) as factors. Fisher’s least significant difference post-hoc tests were utilized if appropriate. Data were considered outliers and excluded from analysis if they were ± two standard deviations from the mean. Sample sizes for all groups are provided in the Supplement (Table S1). All analyses were performed in Prism (GraphPad) with an alpha level of p < 0.05.
Results
Intra-PVN allopregnanolone is sufficient to recapitulate the pubertal stress associated IEG expression profile in adult females.
We have previously shown that pubertal stress (CVS) resulted in a blunted corticosterone response to acute restraint stress in adult mice during pregnancy or with allopregnanolone treatment (Figure 1A). Further, we identified a distinct epigenetic phenotype in the PVN in pregnant, pubertally stressed females, such that the chromatin landscape was open and there was permissive expression of six IEGs at baseline. Here, we tested whether it is the increased levels of allopregnanolone present in pregnancy that interact with prior pubertal stress experience to increase baseline IEG expression in the PVN (Figure 2A).
Figure 2. Intra-PVN allopregnanolone interacted with pubertal stress and sex to alter baseline immediate early gene expression.
(A) Adult female and male mice that had been exposed to pubertal stress or not were cannulated so that vehicle or allopregnanolone could be microinjected into the PVN. Two hours after microinjection, brains were collected in non-stimulated conditions. The PVN was isolated, RNA was isolated, and qRT-PCR was performed for six puberty-stress associated IEGs (Arc, Egr1, Fos, Fosb, Junb, Jund). (B) Heat map showing expression relative to Control Vehicle females within each gene. Relative expression was calculated using the ∆∆Ct method. Intra-PVN Allopregnanolone generally led to a decrease in IEG expression in Control females. In contrast, CVS females responded with an increase in IEG expression. (C) In contrast to females, males generally had less expression of all IEGs, except for Jund. IEG expression in males was less sensitive to either pubertal stress or intra-PVN Allopregnanolone. (D-F) When IEGs were examined within sex, there was a significant interaction between pubertal stress (CVS) and intra-PVN allopregnanolone for Arc, Egr1, and Fos. This expression recapitulates previous findings in pregnant females, where pubertally stressed females with high allopregnanolone levels in the PVN had increased IEG expression at baseline. CVS = chronic variable stress, IEG = immediate early gene, PVN = paraventricular nucleus of the PVN. *p < 0.05 on Fisher’s LSD post-hoc test.
Adult female and male mice that had either been exposed to CVS or not received an intra-PVN injection of allopregnanolone or vehicle. In five of the six IEGs (Arc, Egr1, Fos, Junb, Jund), there was an effect of sex on baseline gene expression (Table S3). There was no effect of sex, stress, or allopregnanolone on the expression of Fosb (Figure S1). Our findings in vehicle-treated mice are consistent with our previous work, such that there were no significant differences between Control and CVS vehicle-treated adult mice (Figure 2B,C). The heatmap shows that males generally have decreased expression relative to vehicle treated female Controls, and the expression in males is less dynamic than in females in response to pubertal stress or intra-PVN allopregnanolone. This is consistent with our previous findings showing that males are less vulnerable to the effects of pubertal stress in the PVN and on the HPA axis than females.
Because there was an effect of sex on IEG expression, we examined the relationship between pubertal stress and intra-PVN allopregnanolone within sex. In males, there were no significant effects of pubertal stress or intra-PVN allopregnanolone on any of the IEGs (p > 0.05). However, in females, there was a significant interaction between pubertal stress and intra-PVN allopregnanolone on baseline gene expression for Arc (Figure 2D, F(1, 18) = 8.68, p = 0.008), Egr1 (Figure 2E, F 1, 18) = 7.54, p = 0.01), and Fos (Figure 2F, F(1, 18) = 4.84, p = 0.04). For these genes, post-hoc testing revealed the same thing. Under allopregnanolone treatment, the gene expression for Control and CVS females diverged, such that Control females had significantly decreased gene expression relative to Control Vehicle females (Arc p = 0.01, Egr1 p = 0.03, Fos p = 0.02) and relative to CVS Allopregnanolone females (Arc p = 0.001, Egr1 p = 0.002, Fos p = 0.03). The same non-significant pattern was observed in the expression of Fosb, Junb, and Jund (Figure S1). Thus, acute allopregnanolone delivered into the PVN of adult females recapitulates the phenotype that we have previously observed in the brain of late pregnant females, such that pubertally stressed females have increased baseline IEG expression relative to control females. These findings are specific to females, as adult males show no significant effect of pubertal stress or intra-PVN allopregnanolone on baseline IEG expression.
IEGs have sex-specific developmental trajectories in adolescence that are disrupted by chronic pubertal stress.
To further understand the potential mechanistic role for the baseline IEG expression patterns in the lasting consequences of pubertal stress, we measured gene expression in the PVN during the age range when CVS is applied (PN21–35, Figure 3A). We then examined whether each gene had any type of variability in baseline expression between the three ages examined (PN21, PN28, PN35) in Control animals. Linear regression analysis showed that there was a significant slope in the trajectory from PN21 to PN35 for Arc (F(1,47) = 6.58, p = 0.01) and Jund (F(1,50) = 6.04, p = 0.02) in Controls (Figure 3B, Figure S2). For Arc, exposure to pubertal stress led to a flattening of the gene expression trajectory, with CVS mice having no significant slope in the trajectory from PN21 to PN35 (p > 0.05, Table S4). For Egr1, Fos, and Fosb, there was no change in trajectory for expression from PN21 to PN35 in Controls (p > 0.05, Table S4). However, exposure to pubertal stress led to a significant decrease in gene expression from PN21 to PN35 in CVS mice for Egr1 (F(1,53) = 5.90, p = 0.02), Fos (F (1,50) = 59.83, p < 0.0001), Fosb (F (1,51) = 18.57, p < 0.0001), and Jund (F (1,52) = 7.83, p = 0.01). We performed further testing to confirm that the regression lines significantly differed between Control and CVS mice. A test of differences in slope or elevation showed that Control and CVS mice had significantly different slopes of expression through the early adolescent window for Egr1, Fos, Fosb, Junb, and Jund. Some of the effects on the trajectory of expression were more pronounced in one sex (Figure 3C).
Figure 3. Baseline immediate early genes expression was dynamic during early adolescence and was altered by pubertal stress.
(A) Brains were collected from young female and male mice that were exposed to pubertal stress or not at three time points. These time points represent prior to starting CVS (PN21), after 1 week of CVS (PN28), and after two weeks of CVS (PN35). Brains were collected 24 hours after the prior stressor and in non-stimulated conditions. The PVN was dissected, RNA was isolated, and qRT-PCR was performed for six puberty-stress associated immediate early genes (IEG; Arc, Egr1, Fos, Fosb, Junb, Jund). (B) Heat map depicting the direction of the slope of the linear regression line calculated from PN21 to PN35 for gene expression in the PVN of Control (female and male) and CVS (female and male) mice. The heat map shows that CVS disrupts the trajectories of each of the six IEGs, and always by decreasing gene expression. (C) There was a sex difference in the impact of pubertal stress on the trajectory of baseline IEG expression during adolescence for several genes. (B,C) Asterisks indicate a slope of the regression line from PN21-PN35 is significantly non-zero, while pound signs indicate that the slopes are significantly different between the groups within a gene. (D,E) Some IEGs were influenced in an interactive way by age, stress, and pubertal stress. (D) Egr1 displayed a dynamic change throughout the pubertal stress window in Control mice in which females and males showed an opposite pattern of expression from PN21 to PN35. (E) The experience of pubertal stress interacted with age and sex to determine Egr1 expression in the PVN. (F,G) Some IEGs were strongly influenced by pubertal stress, like Fos. (F) In Control mice, females and males show little change during the PN21-PN35 age window. (F,G) However, pubertal stress led to a significant decrease in Fos expression at both PN28 and PN35 in female and male mice. CVS = chronic variable stress, IEG = immediate early gene, PN = postnatal day, PVN = paraventricular nucleus of the PVN. (B-C) *p < 0.05 for non-zero slope following linear regression; #p < 0.05 for significantly different slopes between groups but within gene (D-G) *p < 0.05 for main effect of CVS or for interaction terms following 3-way ANOVA analysis.
At PN21, there were no differences between females and males in baseline gene expression (Table S5). Due to the unbalanced design that results from the inclusion of PN21 pre-stress gene expression in an ANOVA, we analyzed gene expression in PN28 and PN35 mice. Gene expression was calculated relative to that of PN21 Controls. For four of the six IEGs, there was a significant interaction between age and sex (Fosb, Junb, Jund) or a main effect of age (Egr1) on gene expression in the PVN (Table S6). This effect of age provides further support that, even in non-stimulated, baseline conditions, IEG expression in the PVN has a dynamic developmental trajectory throughout early adolescence.
IEG expression was altered by exposure to pubertal CVS. Gene expression was examined either after 1 week (PN28) or the complete 2 weeks (PN35) of CVS. As brains were collected 24 hours after the final stressor, these findings represent non-stimulated, baseline levels of gene expression following chronic stress. For four of the six genes, there was a significant effect of stress (Fos, Fosb, Junb) or interaction between stress, sex, and age (Egr1) on gene expression in the PVN (Table S6, Figure S3).
Overall, these analyses show that IEGs were influenced in an interactive way by age, stress, and pubertal stress. For example, Egr1 displayed a dynamic change throughout the pubertal stress window in Control mice in which females and males showed an opposite pattern of expression from PN21 to PN35 (Figure 3D–E). Some IEGs, like Fos, were strongly influenced by pubertal stress (Figure 3F–G). Thus, for all genes, pubertal stress altered the trajectory of normative baseline gene expression during the two-week window during which CVS is applied (PN21 – PN35).
Discussion
Why individuals have negative consequences following stress is a complex phenomenon that is dictated by individual factors (e.g., genetics, sex, resilience), when in the lifespan the stress is experienced (e.g., childhood, adolescence), and when the consequences are measured, both proximity to the stressful event and timepoint within the lifespan (24–29). Understanding how these factors combine will further our understanding of the etiology of negative outcomes and will help guide both the nature and timing of potential treatments. For women, who are more likely than men to suffer from affective disorders, risk factors include experiencing stressful or traumatic events around the onset of puberty and experiencing later times of hormonal change such as pregnancy and aging (30,31). We have previously shown that in both humans and mice, adversity experienced around the onset of puberty led to a blunted hypothalamic-pituitary-adrenal (HPA) stress response during the peripartum window and increased reporting of postpartum depressive symptoms in humans (9). We have used this translationally-relevant mouse model to investigate the mechanisms underlying the lasting effect of pubertal stress on the HPA axis response during the peripartum window. Our previous work identified latent epigenetic changes in the paraventricular nucleus of the hypothalamus (PVN) that may be uncovered by the increased allopregnanolone levels during pregnancy (11). Here, we expand on those findings to directly link allopregnanolone action in the PVN to the molecular changes associated with pubertal stress.
Our previous transcriptomic analysis of the PVN revealed increases in the baseline expression of six immediately early genes (IEGs) in pregnant females as a result of pubertal adversity (9). Here, we utilized PVN-specific pharmacological manipulations to address whether allopregnanolone alone and delivered acutely is sufficient to induce an increase in baseline IEG expression in pubertally stressed mice. In adult female mice, a single dose of allopregnanolone administered into the PVN recapitulated the increased IEG expression only in pubertally stressed females. Allopregnanolone is a neurosteroid metabolite of progesterone that is present at high levels in the brain during pregnancy. It binds to several receptors, including extrasynaptic GABA-A, pregnane X receptors, and progesterone receptors (32). Notably, the function of allopregnanolone as an FDA approved treatment for postpartum depression, under the names Brexanolone and Zuranolone, is attributed to the functions of allopregnanolone at the GABA-A receptor (33–35). In our studies, allopregnanolone does not act to reduce risk for negative outcomes in prenatal mice. One potentially meaningful difference between the models that established allopregnanolone as an effective postpartum depression treatment are that those do not involve early life stress and our model does. Future work will need to resolve whether individuals who have a history of preconception adversity are likely to fully benefit from neurosteroid-based antidepressants. It is possible that the effect of allopregnanolone in altering the transcriptome in response to pubertal stress is working via different mechanisms, including the transcription-regulating pregnane X receptor (36).
These findings provide further evidence that increased baseline IEG expression is a key molecular switch that may be ‘wired’ by pubertal stress and leads to the vulnerability of an altered HPA axis in adulthood, during which allopregnanolone can ‘flip the switch’ to uncover the vulnerability. However, whether this molecular switch is wired immediately after pubertal stress, at postnatal day 35, or whether it develops in the weeks between PN35 and adulthood, was unknown. Understanding the first steps in the molecular cascade that result in negative outcomes are typically identified in adulthood will lead to early interventions and preventative approaches for neuropsychiatric disorders (37). Therefore, we examined IEG expression in the PVN during the window of pubertal stress in females and males. First, we examined developmental changes in gene expression in female and male mice. Overall, baseline IEG expression in the PVN was dynamic during the window when we apply pubertal stress. Males and females did not differ in gene expression at PN21, but often showed different trajectories of gene expression when examined 1 week (PN28) or 2 weeks (PN35) later. These data show that IEG expression in the PVN is not static. We found that pubertal stress altered IEG expression during this adolescent window. In general, the effect of pubertal stress was to decrease baseline IEG expression in both females and males.
Future studies will resolve these findings in a cell-type specific manner. In our current and past studies, we have examined the complete PVN. While limited work has addressed cell-type specific outcomes in the hypothalamus following adolescent stress, there is work from early prenatal stress that informs future cell-type specific studies. HPA axis activation is dictated by the signaling of corticotropin-releasing factor (CRF), glutamatergic, and GABA cells. Limited bedding and nesting produced alterations to the transcriptome of CRF cells in 1.5 week old mice. Single-cell sequencing of CRF cells in the early postnatal period provides evidence for enhanced sensitivity of CRF cells to the stress of limited bedding and nesting (38). This single-cell sequencing experiment showed that, although GABA neurons were not analyzed, it was CRF cells that co-expressed glutamate, and not GABA, that showed sensitivity to this early life adversity. This is consistent with prior work showing increased synaptic glutamatergic drive onto neonatal PVN CRF neurons following limited nesting (39). This study also showed that mice exposed to limited nesting had altered sensitivity to allopregnanolone. It is possible that what we and others are finding is a mechanism wherein CRF cells are being tuned by both glutamatergic and GABAergic systems, and that early life and adolescent stressors impinge upon both neurotransmitter systems to lead to vulnerability to negative outcomes later in life.
Another future goal will be to merge this cell-type specificity with tracking of the same cells throughout the lifespan, which will overcome our current limitation of examining adolescent and adult outcomes in different animals. Recent work has shown that a stress exposure early, in the form of maternal separation, led to the prolonged sensitivity of a set of cells in the nucleus accumbens to stressors in adulthood (40). Using a transgenic strategy in mice to permanently mark cells that had increased Arc expression in response to early life stress, Balouek et al showed that neurons tagged by maternal separation were the same cells reactivated by stress later in life and that blocking their activation ameliorated the negative behavioral consequences. Interestingly, it was males that were sensitive to the adult manipulations. This is in contrast to our study and others conducted in adolescence, where females tend to be more vulnerable to lasting negative outcomes.
While our model considers times in the lifespan when females are more vulnerable than males, we included male mice in all experiments here. Consistent with our previous work showing that adult females are more vulnerable to the lasting effects of pubertal stress, we found that IEG expression in males is less sensitive in adulthood than it is in females. This contrasts with our finding that adolescent males display changes in IEG expression the PVN in response to pubertal stress during the stress window. Future studies will resolve why the molecular responsiveness in the PVN of males does not also result in negative outcomes in adulthood and will consider other brain regions and behavioral outcomes that might provide evidence of negative plasticity in males. Altogether, our study and others conducted recently continue to lend support for to the literature on sex differences in vulnerability to early life stress (41–44). Ongoing studies will continue to refine our knowledge on the molecular underpinnings of how sex and early life stress interact to produce vulnerability to negative outcomes.
Few studies have examined the expression levels of immediate early genes in non-stimulated conditions, as they are expressed at low levels at baseline and are canonically associated with stimulus-induced expression (16–18). Studies where either IEG mRNA or protein have been measured have been focused on the impact of neonatal experiences. For instance, embryonic rearing conditions of chick eggs causes lateralization of spontaneous (baseline) c-Fos expression in several brain regions (45). Another study that examined the regulators of Arc expression in response to acute immobilization stress showed that blocking brain-derived neurotrophic factor reduces baseline Arc expression in the neocortex, which gives insight into molecules that may participate in the lasting effects that we have observed in our model (46). In our model, four of the six IEGs are from the Jun and Fos family of proteins, which dimerize to form the AP-1 complex in order to regulate transcription (47). The AP-1 complex interacts with the glucocorticoid receptor, such that AP-1 binding is required in the recruitment of GR (48). It is possible that the lasting HPA axis physiological plasticity observed in pregnant, pubertally stressed females is driven by alterations to the AP-1 complex that subsequently impact glucocorticoid signaling.
There are competing hypotheses for the way in which multiple stressors throughout the lifespan interact to influence risk for negative outcomes. Some findings support a mismatch theory, where organisms that experience early life stress are well suited to future stressful environments and appear resilient (49). Our prior results and new findings support the idea of cumulative stress, such that a ‘second hit’ challenge results in an increased risk for negative outcomes, also termed an increased allostatic load (50). However, when humans and animals experience that ‘second hit’ of challenge, whether another exposure to stress later in life or to physiological changes such as pregnancy, it has not been clear what the molecular agents of that ‘hit’ are. We have identified that allopregnanolone is a molecular agent of that ‘second hit’ of risk and that it interacts directly with IEGs to influence their baseline expression (Figure 4). This interaction at baseline could lead to differences in how the cells in the PVN respond to stimuli and underlie the blunted HPA axis response and altered behavior we have previously observed.
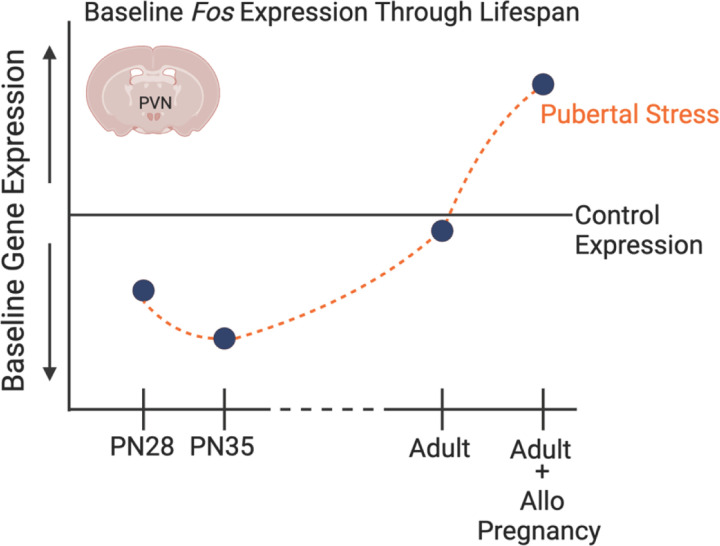
Figure 4. Schematic exemplifying our findings on the influence of pubertal stress on the transcriptome of the developing and adult hypothalamus.
Through prior and current findings, we have demonstrated that a set of immediate early genes in the paraventricular nucleus of the hypothalamus is sensitive to pubertal stress (chronic variable stress from postnatal days 21–34) both during the stress exposure and in adulthood. Of note, all IEG expression differences were measured in non-stimulated, baseline conditions, and so may represent how the PVN is poised to respond to stimuli. Given here is the example of Fos, which is decreased during the pubertal stress window relative to Controls. Whether adult mice are pregnant or are exposed to allopregnanolone, there is an increase in baseline IEG expression. This suggests that the PVN is poised to respond differently, which we have observed in the form of a blunted physiological response to restraint in adulthood. The new findings that baseline IEG expression is altered provide support for a role of IEGs in the mechanism underlying the lasting effect of pubertal stress on the PVN.
We have taken a developmental approach to the question of how stress during puberty impacts an individual through the lifespan, wherein we are generating a trajectory of molecular, physiological, and behavioral outcomes both during early life stress and into adulthood. Indeed, our findings show that a key molecular switch for the negative adult outcomes after pubertal stress – the permissive expression of a set of IEGs - is plastic, displaying normative changes throughout development, and that stress during puberty results in a disruption of baseline expression that is detectable in adolescence. These studies provide novel insight into the mechanisms underlying female-relevant risk for stress dysregulation, a central endophenotype of affective disorders. Understanding the early signs and mechanisms of negative outcomes like stress dysregulation and affective dysfunction that, both in humans and animal models, are generally identified and treated in adulthood, will lead to early interventions, preventative approaches, and better outcomes for mental health.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R00 HD091376 (KEM) and start-up funds to KEM. KNG and JMM were additionally funded by the West Virginia University Summer Undergraduate Research Experience program.
Footnotes
Disclosure Statement
The authors report no conflicts of interest.
Data Availability
Data will be publicly available via the Open Science Framework repository upon publication.
References
- 1.Merrick MT, Ports KA, Ford DC, Afifi TO, Gershoff ET, Grogan-Kaylor A (2017): Unpacking the impact of adverse childhood experiences on adult mental health. Child Abuse Negl 69: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SD, Lu W, Mueser KT, Jankowski MK, Cournos F (2007): Correlates of Adverse Childhood Events Among Adults With Schizophrenia Spectrum Disorders. Psychiatr Serv 58: 245–253. [DOI] [PubMed] [Google Scholar]
- 3.Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF (2004): Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 82: 217–225. [DOI] [PubMed] [Google Scholar]
- 4.Janssen I, Krabbendam L, Bak M, Hanssen M, Vollebergh W, de Graaf R, van Os J (2004): Childhood abuse as a risk factor for psychotic experiences. Acta Psychiatr Scand 109: 38–45. [DOI] [PubMed] [Google Scholar]
- 5.Heim C, Shugart M, Craighead WE, Nemeroff CB (2010): Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol 52: 671–690. [DOI] [PubMed] [Google Scholar]
- 6.Leenarts LEW, Vermeiren RRJM, van de Ven PM, Lodewijks HPB, Doreleijers TAH, Lindauer RJL (2013): Relationships Between Interpersonal Trauma, Symptoms of Posttraumatic Stress Disorder, and Other Mental Health Problems in Girls in Compulsory Residential Care. J Trauma Stress 26: 526–529. [DOI] [PubMed] [Google Scholar]
- 7.Jaffee SR (2017): Child Maltreatment and Risk for Psychopathology in Childhood and Adulthood. Annu Rev Clin Psychol 13: 525–551. [DOI] [PubMed] [Google Scholar]
- 8.Morrison KE, Stenson AF, Marx-Rattner R, Carter S, Michopoulos V, Gillespie CF, et al. (2022): Developmental Timing of Trauma in Women Predicts Unique Extracellular Vesicle Proteome Signatures. Biol Psychiatry 91: 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison KE, Epperson CN, Sammel MD, Ewing G, Podcasy JS, Hantsoo L, et al. (2017): Preadolescent Adversity Programs a Disrupted Maternal Stress Reactivity in Humans and Mice. Biol Psychiatry 81: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slomian J, Honvo G, Emonts P, Reginster J-Y, Bruyère O (2019): Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Womens Health Lond Engl 15. 10.1177/1745506519844044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison KE, Cole AB, Kane PJ, Meadows VE, Thompson SM, Bale TL (2020): Pubertal adversity alters chromatin dynamics and stress circuitry in the pregnant brain. Neuropsychopharmacology 45: 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison KE, Narasimhan S, Fein E, Bale TL (2016): Peripubertal Stress With Social Support Promotes Resilience in the Face of Aging. Endocrinology 157: 2002–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis EP, McCormack K, Arora H, Sharpe D, Short AK, Bachevalier J, et al. (2022): Early life exposure to unpredictable parental sensory signals shapes cognitive development across three species. Front Behav Neurosci 16: 960262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER (2013): The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 38: 1858–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warhaftig G, Almeida D, Turecki G (2023): Early life adversity across different cell-types in the brain. Neurosci Biobehav Rev 148: 105113. [DOI] [PubMed] [Google Scholar]
- 16.Gallo FT, Katche C, Morici JF, Medina JH, Weisstaub NV (2018): Immediate Early Genes, Memory and Psychiatric Disorders: Focus on c-Fos, Egr1 and Arc. Front Behav Neurosci 12. Retrieved September 19, 2023, from 10.3389/fnbeh.2018.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes P, Lawlor P, Druganow M (1992): Basal expression of Fos, Fos-related, Jun, and Krox 24 proteins in rat hippocampus. Mol Brain Res 13: 355–357. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Cadahía B, Drobic B, Davie JR (2011): Activation and function of immediate-early genes in the nervous system. Biochem Cell Biol Biochim Biol Cell 89: 61–73. [DOI] [PubMed] [Google Scholar]
- 19.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. (2010): A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, et al. (2011): A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71: 995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabinowitz A, Cohen SJ, Finn DA, Stackman RW (2014): The neurosteroid allopregnanolone impairs object memory and contextual fear memory in male C57BL/6J mice. Horm Behav 66: 238–246. [DOI] [PubMed] [Google Scholar]
- 22.Engin E, Treit D (2007): The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: the amygdala, medial prefrontal cortex, or hippocampus. Behav Pharmacol 18: 461. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G, Franklin KBJ (2019): Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates. Academic Press. [Google Scholar]
- 24.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. (2014): Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci 111: 16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison KE, Epperson CN, Bale TL (2020): Chapter 6 - Sex differences in the programming of stress resilience. In: Chen A, editor. Stress Resilience. Academic Press, pp 81–94. [Google Scholar]
- 26.Rincón-Cortés M, Herman JP, Lupien S, Maguire J, Shansky RM (2019): Stress: Influence of sex, reproductive status and gender. Neurobiol Stress 10: 100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peña CJ, Nestler EJ, Bagot RC (2019): Environmental Programming of Susceptibility and Resilience to Stress in Adulthood in Male Mice. Front Behav Neurosci 13. Retrieved September 22, 2023, from 10.3389/fnbeh.2019.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feder A, Fred-Torres S, Southwick SM, Charney DS (2019): The Biology of Human Resilience: Opportunities for Enhancing Resilience Across the Life Span. Biol Psychiatry 86: 443–453. [DOI] [PubMed] [Google Scholar]
- 29.Boyce WT, Levitt P, Martinez FD, McEwen BS, Shonkoff JP (2021): Genes, Environments, and Time: The Biology of Adversity and Resilience. Pediatrics 147: e20201651. [DOI] [PubMed] [Google Scholar]
- 30.Morrison KE (2020): Animal models built for women’s brain health: Progress and potential. Front Neuroendocrinol 59: 100872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Bowen A, Bowen R, Balbuena L, Feng C, Bally J, Muhajarine N (2019): Mood instability during pregnancy and postpartum: a systematic review. Arch Womens Ment Health. 10.1007/s00737-019-00956-6 [DOI] [PubMed] [Google Scholar]
- 32.Walton N, Maguire J (2019): Allopregnanolone-based treatments for postpartum depression: Why/how do they work? Neurobiol Stress 11: 100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison KE, Cole AB, Thompson SM, Bale TL (2019): Brexanolone for the treatment of patients with postpartum depression. Drugs Today 55: 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deligiannidis KM, Meltzer-Brody S, Maximos B, Peeper EQ, Freeman M, Lasser R, et al. (2023): Zuranolone for the Treatment of Postpartum Depression. Am J Psychiatry 180: 668–675. [DOI] [PubMed] [Google Scholar]
- 35.Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. (2018): Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Lond Engl 392: 1058–1070. [DOI] [PubMed] [Google Scholar]
- 36.Frye CA, Koonce CJ, Walf AA (2014): Novel receptor targets for production and action of allopregnanolone in the central nervous system: a focus on pregnane xenobiotic receptor. Front Cell Neurosci 8: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uhlhaas PJ, Davey CG, Mehta UM, Shah J, Torous J, Allen NB, et al. (2023): Towards a youth mental health paradigm: a perspective and roadmap. Mol Psychiatry 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Short AK, Thai CW, Chen Y, Kamei N, Pham AL, Birnie MT, et al. (2023): Single-Cell Transcriptional Changes in Hypothalamic Corticotropin-Releasing Factor–Expressing Neurons After Early-Life Adversity Inform Enduring Alterations in Vulnerabilities to Stress. Biol Psychiatry Glob Open Sci 3: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunn BG, Cunningham L, Cooper MA, Corteen NL, Seifi M, Swinny JD, et al. (2013): Dysfunctional Astrocytic and Synaptic Regulation of Hypothalamic Glutamatergic Transmission in a Mouse Model of Early-Life Adversity: Relevance to Neurosteroids and Programming of the Stress Response. J Neurosci 33: 19534–19554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balouek J-A, Mclain CA, Minerva AR, Rashford RL, Bennett SN, Rogers FD, Peña CJ (2023): Reactivation of Early-Life Stress-Sensitive Neuronal Ensembles Contributes to Lifelong Stress Hypersensitivity. J Neurosci 43: 5996–6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009): Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10: 434–445. [DOI] [PubMed] [Google Scholar]
- 42.Sandman CA, Glynn LM, Davis EP (2013): Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res 75: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim DR, Bale TL, Epperson CN (2015): Prenatal programming of mental illness: current understanding of relationship and mechanisms. Curr Psychiatry Rep 17: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Connor TG, Heron J, Golding J, Beveridge M, Glover V (2002): Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry J Ment Sci 180: 502–508. [DOI] [PubMed] [Google Scholar]
- 45.Lorenzi E, Mayer U, Rosa-Salva O, Morandi-Raikova A, Vallortigara G (2019): Spontaneous and light-induced lateralization of immediate early genes expression in domestic chicks. Behav Brain Res 368: 111905. [DOI] [PubMed] [Google Scholar]
- 46.Benekareddy M, Nair AR, Dias BG, Suri D, Autry AE, Monteggia LM, Vaidya VA (2013): Induction of the plasticity-associated immediate early gene Arc by stress and hallucinogens: role of brain-derived neurotrophic factor. Int J Neuropsychopharmacol 16: 405–415. [DOI] [PubMed] [Google Scholar]
- 47.Hai T, Curran T (1991): Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A 88: 3720–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biddie SC, John S, Sabo PJ, Thurman RE, Johnson TA, Schiltz RL, et al. (2011): Transcription Factor AP1 Potentiates Chromatin Accessibility and Glucocorticoid Receptor Binding. Mol Cell 43: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nederhof E, Schmidt MV (2012): Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol Behav 106: 691–700. [DOI] [PubMed] [Google Scholar]
- 50.Kuhn M, Scharfenort R, Schümann D, Schiele MA, Münsterkötter AL, Deckert J, et al. (2016): Mismatch or allostatic load? Timing of life adversity differentially shapes gray matter volume and anxious temperament. Soc Cogn Affect Neurosci 11: 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.