Abstract
Chronic stress can change how we learn and, thus, how we make decisions by promoting the formation of inflexible, potentially maladaptive, habits. Here we investigated the neuronal circuit mechanisms that enable this. Using a multifaceted approach in male and female mice, we reveal a dual pathway, amygdala-striatal, neuronal circuit architecture by which a recent history of chronic stress shapes learning to disrupt flexible goal-directed behavior in favor of inflexible habits. Chronic stress inhibits activity of basolateral amygdala projections to the dorsomedial striatum to impede the action-outcome learning that supports flexible, goal-directed decisions. Stress also increases activity in direct central amygdala projections to the dorsomedial striatum to promote the formation of rigid, inflexible habits. Thus, stress exerts opposing effects on two amygdala-striatal pathways to promote premature habit formation. These data provide neuronal circuit insights into how chronic stress shapes learning and decision making, and help understand how stress can lead to the disrupted decision making and pathological habits that characterize substance use disorders and other psychiatric conditions.
Keywords: learning, decision making, instrumental conditioning, basolateral amygdala, central amygdala, reward, striatum
When making a decision, we can use what we have learned about our actions and their outcomes to prospectively evaluate the consequences of our potential choices1–3. This goal-directed strategy supports our agency. It allows us to choose actions that lead to desirable consequences and avoid those that lead to outcomes that are not currently beneficial. This strategy is, thus, highly flexible to changing circumstances. But we don’t always think about the consequences of our behavior. Often this is fine. Such habits are a way for our brain to efficiently execute routine behaviors based on past success, without forethought of their consequences4,2,5–7. The brain balances goal-directed and habitual control to allow behavior to be adaptive when needed, yet efficient when appropriate8,9. But disrupted goal-directed control and overreliance on habit can cause inadequate consideration of consequences, inflexible behavior, a lower threshold for compulsivity, and disrupted decision making10–13. This can contribute to cognitive deficits in numerous diseases, including substance use disorder14–25, obsessive-compulsive disorder26–28, obesity12,21,29, schizophrenia30–32, depression31,33, anxiety34, and autism35. Chronic stress tips the balance of behavioral control towards habit36–60. Stress can change how we learn and, thus, how we make decisions, promoting inflexible habits. Because stress is a major predisposing factor for addiction and other psychiatric conditions61–66, understanding how stress promotes habit formation will illuminate one avenue of vulnerability for these conditions. Yet, despite importance for understanding adaptive and maladaptive behavior, little is known of the neuronal circuits that enable stress-potentiated habit formation.
Amygdala-striatal projections are potential candidate pathways by which stress could bias learning and behavioral control strategy. The dorsomedial striatum (DMS) is an evolutionarily conserved hub for the action-outcome learning that supports goal-directed behavioral control4,67–75. Suppression of DMS activity prevents goal-directed control and promotes inflexible habits70,76. The basolateral amygdala (BLA) is needed for goal-directed behavior77–79 and sends a direct excitatory projection to the DMS80–86. We know very little of the function of the BLA➔DMS pathway, though it is well-positioned to facilitate the action-outcome learning that supports goal-directed behavior. Conversely, the central amygdala (CeA) has been implicated in habit79. It sends a direct, likely inhibitory87,88, projection to the striatum80,81. No function for this newly identified CeA➔striatum pathway has yet to be reported but these projections are poised to oppose DMS function. Both the BLA and CeA are highly implicated in stress processing89–92. Thus, here we investigated whether chronic stress acts via these amygdala-striatal pathways to promote habit formation.
RESULTS
Chronic stress potentiates habit formation
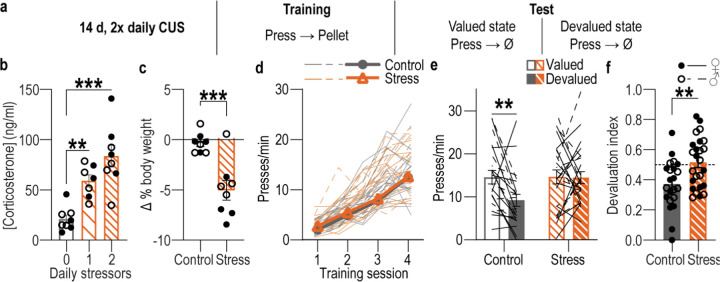
We first designed a behavioral procedure to model stress-potentiated habit formation in male and female mice (Figure 1a). Mice received 14 consecutive days of chronic mild unpredictable stress (CUS) including daily exposure to 2 of 6 stressors of variable duration at pseudorandom times and order: damp bedding (4–16 hrs), tilted cage (4–16 hr), white noise (80 db; 2–16 hr), continuous illumination during the dark phase (12 hr), physical restraint (2 hr), footshock (0.7-mA, 1-s, 5 shocks/10 min). We selected this stress protocol to model aspects of the repeated and varied nature of stress experienced by humans, including uncontrollable physical aversive events, disrupted sleep, and poor environmental conditions. Controls received equated handling. Demonstrating efficacy, serum corticosterone levels were elevated (Figure 1b) and body weight was reduced (Figure 1c) in stressed mice relative to controls. This procedure was intentionally mild to model low-level chronic stress. Accordingly, it did not cause major anxiety- or depression-like phenotypes in classic assays of such behavior (Figure 1-1). 24 hours following the last stressor, mice were trained to lever press to earn a food-pellet reward. We used a random-ratio schedule of reinforcement to encourage action-outcome learning and goal-directed behavioral control8,93–96. Mice were food-deprived for training and, thus, bodyweight did not differ between control and stressed mice during training (Supplemental Table 1). Control and stressed mice similarly acquired the instrumental behavior (Figure 1d). Thus, CUS does not cause general learning, motivational, or locomotor impairments. To evaluate behavioral control strategy, we used the gold-standard outcome-specific devaluation test2–4,7,67,97–100. Mice were given 90-min, non-contingent access to the food pellet earned during training to induce a sensory-specific satiety rendering that specific food pellet temporarily devalued. Lever pressing was assessed in a 5-min, non-reinforced probe test immediately following the prefeeding. Performance was compared to that following satiation on an alternate food pellet to control for general satiety (Valued state; test order counterbalanced). Both control and stressed mice consumed similar amounts during the prefeed (Supplemental Table 2), indicating that CUS did not alter food consumption. CUS also did not affect outcome discrimination or devaluation efficacy. Both groups rejected the devalued outcome in a post-probe consumption choice test (Supplemental Table 3). If subjects have learned the action-outcome relationship and are using this to support prospective consideration of action consequences for flexible, goal-directed behavioral control, they will reduce lever pressing when the outcome is devalued relative to the valued state. We saw this effect in control subjects (Figure 1e–f; see also Supplemental Figure 1-2 for data on entries into the food-delivery port). Behavior in stressed mice was insensitive to devaluation, indicating the lack of consideration of action consequences that marks inflexible habits4,7,8,67,97. Thus, a recent history of chronic stress causes premature habit formation.
Figure 1: A recent history of chronic stress potentiates habit formation.
(a) Procedure schematic. CUS, chronic unpredictable mild stress. Valued state = prefed on untrained food-pellet type to control for general satiety. Devalued state = prefed on trained food-pellet type to induce sensory-specific satiety devaluation. (b) Blood serum corticosterone concentrations 24 hr after completion of 14 d of 1 stressor/d, 2 stressors/d, or daily handling (Control). Stress: F(2, 20) = 17.35, P < 0.0001. Control: N = 8 (4 male), 1x CUS: N = 7 (3 male), 2x CUS: N = 8 (4 male). (c) Percent change (Δ) in body weight averaged across the first 10 d of CUS on ad libitum food. t14 = 4.50, P = 0.0005. N = 8/group (4 male). (d) Press rate across training following CUS (Stress) or daily handling control. Training: F(2.12, 95.32) = 168.20, P < 0.0001; Stress: F(1, 45) = 0.64, P = 0.43, Training x Stress: F(3, 135) = 0.15, P = 0.93. (e) Press rate during the subsequent devaluation probe test immediately following sensory-specific satiety devaluation of the trained pellet (Devalued) or an alternate pellet type (Valued). Stress x Value: F(1, 45) = 4.43, P = 0.04; Stress: F(1, 45) = 2.52, P = 0.12; Value: F(1, 45) = 4.88, P = 0.03. (f) Devaluation index [(Devalued condition presses)/(Valued condition presses + Devalued presses)]. t(45) = 2.99, P = 0.005. Control N = 22 (13 male), Stress N = 25 (12 male). Males = closed circles/solid lines, Females = open circles/dashed lines. **P <0.01, ***P < 0.001.
Chronic stress attenuates BLA➔DMS and engages CeA➔DMS pathway activity during learning.
We next confirmed the existence of direct BLA80–86 and CeA80,81 projections to dorsal striatum using both anterograde and retrograde tracing. We found that like the BLA, the CeA also directly projects the DMS (Supplemental Figure 2). We then characterized the activity of the BLA➔DMS and CeA➔DMS pathways during action-outcome learning and asked whether it is influenced by a recent history of chronic stress. We used fiber photometry to record fluorescent activity of the genetically encoded calcium indicator GCaMP8s expressed using an intersectional approach in either BLA or CeA neurons that project to the DMS (Figure 2a, b, d, j). Mice received 14 consecutive days of CUS or daily handling control prior to being trained to lever press to earn a food-pellet reward, as above (Figure 2c). Again, both control and stressed mice similarly acquired the instrumental behavior (Figure 2e, k, see also Figure 2-1 for food-port entry data). Fiber photometry (473 nm calcium-dependent, 415 nm isosbestic) recordings were made during each training session. BLA➔DMS neurons were robustly activated by the earned reward during learning (Figure 2f–i). Thus, the BLA➔DMS pathway is active when subjects are able to learn the rewarding consequence of their actions and form the action-outcome memories that support goal-directed behavioral control. This activity was absent in stressed mice (Figure 2g). Thus, recent chronic stress attenuates BLA➔DMS activity associated with action-outcome learning. Conversely, CeA➔DMS neurons are not robustly active during this form of instrumental learning, but chronic stress engages this pathway particularly around earned reward experience later in training (Figure 2l–o). Thus, chronic stress recruits the CeA➔DMS pathway to instrumental learning. We detected similar patterns in response to unpredicted rewards in both pathways (Figure 2-2). Both BLA➔DMS and CeA➔DMS projections were acutely activated by unpredicted footshock and this was not altered by stress (Figure 2-2), providing a positive control for our ability to detect signal in all groups. This also indicates that neither BLA➔DMS nor CeA➔DMS activity is valence-specific. Chronic stress did, however, reduce post-shock activity in the BLA➔DMS pathway, consistent with its effects on reward signals in this pathway. Chronic stress did not alter baseline spontaneous calcium activity in either pathway, indicating it does not generally increase or decrease excitability in these pathways (Figure 2-3). Together these data indicate that a recent history of chronic stress oppositely modulates BLA➔DMS and CeA➔DMS projections during learning, turning off learning-related activity in the BLA➔DMS pathway and instead engaging the CeA➔DMS pathway.
Figure 2: Chronic stress attenuates BLA➔DMS and engages CeA➔DMS pathway activity during instrumental learning.
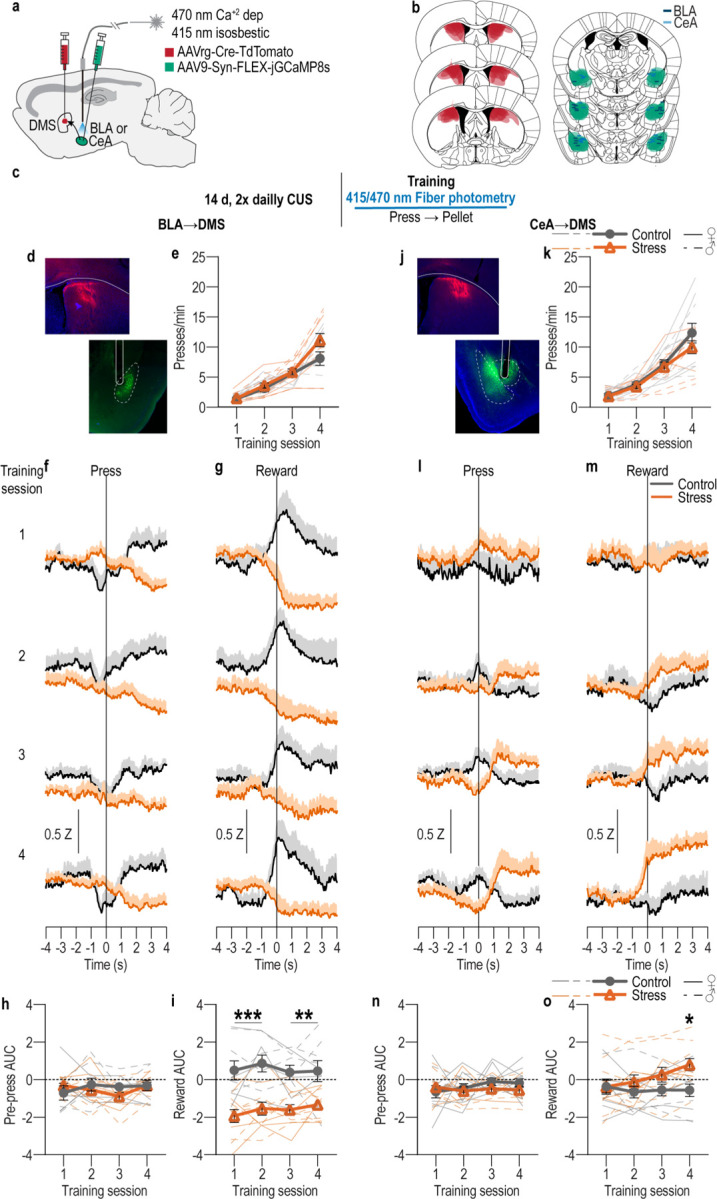
(a) Intersectional approach for fiber photometry calcium imaging of DMS-projecting BLA or CeA neurons. (b) Schematic representation of retrograde AAV-cre in DMS and cre-dependent GCaMP8s expression and optical fiber tips in BLA or CeA for all subjects. (c) Procedure schematic. CUS, chronic unpredictable stress. (d-i) Fiber photometry recordings of GCaMP8s in BLA➔DMS neurons during instrumental lever press – food-pellet reward learning. (d) Representative images of retro-cre expression in DMS and immunofluorescent staining of cre-dependent GCaMP8s expression and fiber placement in BLA. (e) Press rate across training following CUS (Stress) or daily handling control. Training: F(1.72, 32.66) = 81.40, P < 0.0001; Stress: F(1, 19) = 2.25, P = 0.15; Training x Stress: F(3, 57) = 3.19, P = 0.03. (f-g) Trial-averaged Z-scored Δf/F BLA➔DMS GCaMP8s fluorescence changes aligned to bout-initiating presses (f; first press of the session and first press after a reward collection) and reward collection (g; first food-port entry after reward delivery) across each training session. (h-i) Trial-averaged quantification of area under the BLA➔DMS GCaMP8s Z-scored ∆f/F curve (AUC) during the 3-s period prior to initiating presses (h; Training: F(2.49, 47.38) = 0.91, P = 0.43; Stress: F(1, 19) = 0.18, P = 0.68; Training x Stress: F(3, 57) = 1.77, P = 0.16) or following reward collection (i; Stress: F(1, 19) = 24.13, P < 0.0001; Training: F(2.41, 45.71) = 0.91, P = 0.43; Training x Stress: F(3, 57) = 0.78, P = 0.51) across training. Control N = 9 (4 male), Stress N = 12 (5 male). (j-o) Fiber photometry recordings of GCaMP8s in CeA➔DMS neurons during instrumental lever press – food-pellet reward learning. (j) Representative immunofluorescent image of retro-cre expression in DMS and cre-dependent GCaMP8s expression and fiber placement in CeA. (k) Press rate across training following CUS (Stress) or daily handling control. Training: F(1.51, 30.23) = 65.61, P < 0.0001; Stress: F(1, 20) = 1.106, P = 0.31; Training x Stress: F(3, 60) = 1.05, P = 0.38. (l-m) Trial-averaged Z-scored Δf/F CeA➔DMS GCaMP8s fluorescence changes aligned to bout-initiating presses (l) and reward collection (m) across each training session. (n-o) Trial-averaged quantification of CeA➔DMS GCaMP8s Z-scored ∆f/F AUC during the 3-s period prior to initiating presses (n; Training: F(2.36, 47.26) = 0.60, P = 0.58; Stress: F(1, 20) = 0.74, P = 0.40; Training x Stress: F(3, 60) = 0.92, P = 0.44) or following reward collection (o; Training x Stress: F(3, 60) = 4.51, P = 0.006; Training: F(2.34, 46.75) = 3.23, P = 0.04; Stress: F(1, 20) = 2.41, P = 0.14). Control N = 11 (6 male), Stress N = 11 (4 male). Males = closed circles/solid lines, Females = open circles/dashed lines. *P <0.05, **P <0.01, ***P < 0.001.
Suppression of BLA➔DMS pathway activity disrupts action-outcome learning and enables chronic stress to promote habit formation
We next reasoned that the stress-induced suppression of BLA➔DMS projection activity during action-outcome learning might promote premature habit formation. We tested this by asking whether activating BLA➔DMS projections during instrumental learning is sufficient to restore goal-directed behavioral control in stressed mice. Using an intersectional approach (Figure 3a), we expressed the excitatory designer receptor human M3 muscarinic receptor (hM3Dq) or fluorophore control in DMS-projecting BLA neurons (Figure 3b–c). Following 14 consecutive days of CUS or daily handling control, mice received instrumental training to lever press to earn food-pellet rewards (Figure 3d). Prior to each training session, mice received the hM3Dq ligand clozapine-N-oxide (CNO; 0.2 mg/kg101–104 i.p.) to activate BLA➔DMS projections during learning. Neither stress nor chemogenetic activation of BLA➔DMS projections altered acquisition of the instrumental lever-press behavior (Figure 3e; see also Figure 3-1 for food-port entry data). Whereas controls were sensitive to subsequent outcome devaluation, indicating action-outcome learning and goal-directed behavioral control, stressed mice were, again, insensitive to devaluation, indicating premature habit formation (Figure 3f–g). Chemogenetic activation of BLA➔DMS projections during learning restored normal action-outcome learning enabling goal-directed behavioral control, as evidenced by sensitivity to devaluation, in stressed mice (Figure 3f–g; see Figure 3-2 for effects of chemogenetic BLA➔DMS activation in handled controls). Thus, activation of BLA➔DMS projections during learning is sufficient to overcome the effect of prior chronic stress and restore action-outcome learning for flexible, goal-directed behavioral control.
Figure 3: Suppression of BLA➔DMS pathway activity disrupts action-outcome learning and enables chronic stress to promote habit formation.
(a-g) Chemogenetic activation of BLA➔DMS projections during post-stress instrumental learning. (a) Intersectional approach for chemogenetic activation of DMS-projecting BLA neurons. (b) Representative immunofluorescent images of retro-cre expression in DMS and cre-dependent hM3Dq expression in BLA. (c) Schematic representation of retro-cre in DMS and cre-dependent hM3Dq expression in BLA for all subjects. (d) Procedure schematic. CUS, chronic unpredictable stress. CNO, clozapine-N-oxide. Valued state = prefed on untrained food-pellet type to control for general satiety. Devalued state = prefed on trained food-pellet type to induce sensory-specific satiety devaluation. (e) Press rate across training following CUS (Stress) or daily handling control. Training: F(1.90, 53.30) = 60.55, P < 0.0001; Group: F(2, 28) = 1.05, P = 0.36; Training x Group: F(6, 84) = 0.80, P = 0.57. (f) Press rate during the subsequent devaluation probe test immediately following sensory-specific satiety devaluation of the trained pellet (Devalued) or an alternate pellet type (Valued). Value: F(1, 28) = 18.07, P = 0.0002; Group: F(2, 28) = 2.06, P = 0.15; Group x Value: F(2, 28) = 0.75, P = 0.48. (g) Devaluation index [(Devalued condition presses)/(Valued condition presses + Devalued presses)]. Group: F(2, 28) = 3.38, P = 0.048. Control mCherry N = 12 (7 male), Stress mCherry N = 9 (5 male), Stress hM3Dq N = 10 (5 male). (h-n) Optogenetic inactivation of BLA➔DMS projections at reward during instrumental learning. (h) Approach for optogenetic inhibition of BLA terminals in DMS. (i) Representative immunofluorescent images of Arch expression in BLA and optical fiber tip in the vicinity of Arch-expression BLA terminals in the DMS. (j) Schematic representation of Arch expression in BLA and approximate location of optical fiber tips in DMS for all subjects. (k) Procedure schematic. (l) Press rate across training. Training: F(1.70, 32.34) = 41.26, P < 0.0001; Virus: F(1, 19) = 2.43, P = 0.14; Training x Virus: F(3, 57) = 1.14, P = 0.34. (m) Press rate during the subsequent devaluation probe test immediately following sensory-specific satiety devaluation of the trained pellet (Devalued) or an alternate pellet type (Valued). Stress x Value: F(1, 19) = 14.35, P = 0.001; Stress: F(1, 19) = 1.43, P = 0.25; Value: F(1, 19) = 0.43, P = 0.52. (n) Devaluation index. t(19) = 5.03, P < 0.0001. GFP N = 10 (5 male), Arch N = 11 (5 male). Males = closed circles/solid lines, Females = open circles/dashed lines. *P <0.05, ***P <0.001.
Given that BLA➔DMS projections are activated by earned rewards during learning, we next reasoned that this activity might be critical for the action-outcome learning that supports goal-directed behavioral control. If this is true, then inhibiting reward-evoked BLA➔DMS activity should suppress action-outcome learning and prematurely promote habit formation in mice without a history of chronic stress. To test this, we used pathway-specific optogenetic inhibition of BLA➔DMS activity at the time of earned reward during action-outcome learning. We expressed the inhibitory opsin archaerhodopsin (Arch) or fluorophore control in the BLA and implanted optical fibers in the DMS in the vicinity of Arch-expressing BLA axons and terminals (Figure 3h–j). We used green light (532 nm, 10 mW, 5 s) to inhibit BLA terminals in the DMS during collection of each earned reward during instrumental lever press - food pellet training (Figure 3k). BLA➔DMS inhibition did not affect acquisition of the instrumental behavior (Figure 3l; see also Figure 3-1 for food-port entry data), but did disrupt action-outcome learning as evidenced by subsequent insensitivity to outcome devaluation at test (Figure 3m–n). BLA➔DMS inhibition was not inherently rewarding or aversive (Figure 3-3). Thus, BLA➔DMS projections are normally activated by rewarding events to enable the action-outcome learning that supports flexible, goal-directed behavior. Chronic stress turns off this activity to disrupt action-outcome learning and promote premature habit formation.
Chronic stress engages the CeA➔DMS pathway to promote habit formation
As opposed to the BLA➔DMS pathway, chronic stress engages the CeA➔DMS pathway during learning. We reasoned that this, too, may be a mechanism through which stress promotes premature habit formation. If this is true, then preventing the stress-induced increase in CeA➔DMS activity during learning should restore action-outcome learning and goal-directed behavioral control. We tested this in two ways. First, we used an intersectional approach (Figure 4a) to express the inhibitory designer receptor human M4 muscarinic receptor (hM4Di), or a fluorophore control in DMS-projecting CeA neurons (Figure 4b–c). Following 14 consecutive days of CUS or daily handling control, mice received instrumental training to lever press to earn food-pellet rewards (Figure 4d). Prior to each training session, mice received the hM4Di ligand clozapine-N-oxide (CNO; 2.0 mg/kg105–107 i.p.) to inactivate CeA➔DMS projections during learning. Neither stress nor chemogenetic inactivation of CeA➔DMS projections altered acquisition of the instrumental behavior (Figure 4e; see also Figure 4-1 for food-port entry data). At test, we again found evidence of goal-directed behavioral control, sensitivity to devaluation, in control subjects and potentiated habit formation, insensitivity to devaluation, in stressed subjects (Figure 4f–g). Chemogenetic inhibition of CeA➔DMS projection activity during learning restored action-outcome learning to enable goal-directed behavioral control, sensitivity to devaluation, in stressed mice (Figure 4f–g).
Figure 4: Chronic stress engages the CeA➔DMS pathway to promote habit formation.
(a-g) Chemogenetic inhibition of CeA➔DMS projections during post-stress instrumental learning. (a) Intersectional approach for chemogenetic inhibition of DMS-projecting CeA neurons. (b) Representative immunofluorescent images of retro-cre expression in DMS and cre-dependent hM4Di expression in CeA. (c) Schematic representation of retro-cre in DMS and cre-dependent hM4Di expression in CeA for all subjects. (d) Procedure schematic. CUS, chronic unpredictable stress. CNO, clozapine-N-oxide. Valued state = prefed on untrained food-pellet type to control for general satiety. Devalued state = prefed on trained food-pellet type to induce sensory-specific satiety devaluation. (e) Press rate across training following CUS (Stress) or daily handling control. Training: F(1.46, 42.19) = 12.57, P = 0.0002; Group: F(2, 29) = 0.23, P = 0.79; Training x Group: F(6, 87) = 0.59, P = 0.74. (f) Press rate during the subsequent devaluation probe test immediately following sensory-specific satiety devaluation of the trained pellet (Devalued) or an alternate pellet type (Valued). Group x Value: F(2, 29) = 5.85, P = 0.007; Value: F(1, 29) = 12.86, P = 0.001; Group: F(2, 29) = 2.65, P = 0.09. (g) Devaluation index [(Devalued condition presses)/(Valued condition presses + Devalued presses)]. Group: F(2, 29) = 3.59, P = 0.04. Control mCherry N = 12 (5 male), Stress mCherry N = 11 (5 male), Stress hM4Di N = 9 (4 male). (h-n) Optogenetic inactivation of CeA➔DMS projections at reward during post-stress learning. (h) Approach for optogenetic inhibition of CeA terminals in DMS. (i) Representative immunofluorescent images of Arch expression in CeA and optical fiber tip in the vicinity of Arch-expressing CeA terminals in the DMS. (j) Schematic representation of Arch expression in BLA and approximate location of optical fiber tips in DMS for all subjects. (k) Procedure schematic. (l) Press rate across training following CUS (Stress) or daily handling control. Training: F(1.80, 36.14) = 22.71, P < 0.0001; Group: F(2, 20) = 0.93, P = 0.41; Training x Group: F(6, 60) = 1.67, P = 0.34. (m) Press rate during the subsequent devaluation probe test immediately following sensory-specific satiety devaluation of the trained pellet (Devalued) or an alternate pellet type (Valued). Group x Value: F(2, 20) = 4.25, P = 0.03; Value: F(1, 20) = 13.66, P = 0.001; Group: F(2, 20) = 1.74, P = 0.20. (n) Devaluation index. Group: F(2, 20) = 7.81, P = 0.003. Control GFP N = 7 (4 male), Stress GFP N = 7 (6 male), Stress Arch N = 9 (5 male). (o-u) Optogenetic stimulation of CeA➔DMS projections at reward during learning following subthreshold chronic stress. (o) Intersectional approach for optogenetic stimulation of DMS-projecting CeA neurons. (p) Representative images of retro-cre expression in DMS and immunofluorescent staining of cre-dependent ChR2 expression in CeA. (q) Schematic representation of retro-cre in DMS and cre-dependent ChR2 expression in CeA for all subjects. (r) Procedure schematic. (s) Press rate across training following once/daily, 14 d CUS. Training: F(2.11, 33.69) = 76.17, P < 0.0001; Virus: F(1, 16) = 0.33, P = 0.57; Training x Virus: F(3, 48) = 0.19, P = 0.90. (t) Press rate during the subsequent devaluation probe test immediately following sensory-specific satiety devaluation of the trained pellet (Devalued) or an alternate pellet type (Valued). Virus x Value: F(1, 16) = 4.56, P = 0.049; Virus: F(1, 16) = 0.09, P = 0.76; Value: F(1, 16) = 0.004, P = 0.95. (u) Devaluation index. t(16) = 3.12, P = 0.007. GFP N = 8 (4 male), ChR2 N = 10 (6 male). Males = closed circles/solid lines, Females = open circles/dashed lines. ns = not significant. *P <0.05, **P < 0.01, ***P <0.001.
Chronic stress engages the CeA➔DMS pathway specifically at reward experience during learning. Therefore, we next asked whether this stress-induced CeA➔DMS reward response is necessary for premature habit formation, by optogenetically inhibiting CeA terminals in the DMS at the time of earned reward during learning in stressed mice. We expressed the inhibitory opsin Arch or fluorophore control in the CeA and implanted optical fibers in the DMS in the vicinity of Arch-expressing CeA axons and terminals (Figure 4h–j). Following 14 days of CUS or daily handling control, mice received instrumental training to lever press to earn food-pellet rewards (Figure 4k). We used green light (532 nm, 10 mW, 5 s) to inhibit CeA terminals in the DMS during the collection of each earned reward (Figure 4k). Neither stress nor optogenetic inhibition of CeA➔DMS projection activity in stressed subjects altered acquisition of the instrumental behavior (Figure 4l; see also Figure 4-1 for food-port entry data). Similar to chemogenetic inhibition, optogenetic inhibition of CeA➔DMS activity at reward during learning restored action-outcome learning to enable goal-directed behavioral control in stressed mice, as evidenced by sensitivity to devaluation (Figure 4m–n). Neither chemogenetic nor optogenetic inhibition of CeA➔DMS projections altered learning or subsequent behavioral control strategy in mice without a history of chronic stress (Figure 4-2). Thus, chronic stress engages CeA➔DMS projections during subsequent reward learning experience to promote premature habit formation.
We next used pathway-specific optogenetic stimulation to ask whether CeA➔DMS pathway activity at reward during learning is sufficient to promote premature habit formation. We used an intersectional approach (Figure 4a) to express the excitatory opsin Channelrhodopsin 2 (ChR2) or a fluorophore control in DMS-projecting CeA neurons and implanted optic fibers above the CeA (Figure 4o–q). We first used blue light (473 nm, 10 mW, 20 Hz, 25-ms pulse width, 2 s) to stimulate CeA➔DMS neurons during the collection of each earned reward during instrumental learning in mice without a history of chronic stress. This neither affected acquisition of the lever-press behavior, nor the action-outcome learning needed to support flexible, goal-directed behavioral control during the devaluation test (Figure 4-3). Thus, activation of the CeA➔DMS pathway during reward learning experience is not alone sufficient to disrupt action-outcome learning or promote habit formation. We next asked whether activation of CeA➔DMS projections is sufficient to amplify the effects of a less frequent prior chronic stress experience on habit formation. We repeated the experiment this time in mice with a history of once daily CUS for 14 consecutive days (Figure 4r). Again, neither CeA➔DMS activation nor stress altered acquisition of the instrumental behavior (Figure 4s; see Figure 4-1 for food-port entry data). The less frequent chronic stress was itself insufficient to cause premature habit formation, but when coupled with activation of CeA➔DMS projections at reward during learning did cause premature habit formation as evidenced by greater insensitivity to devaluation in subjects that received stimulation relative to those that did not (Figure 4t–u). Thus, activation of CeA➔DMS projections during learning is sufficient to amplify the effects of prior subthreshold chronic stress to promote habit formation. CeA➔DMS stimulation was not found to be inherently rewarding or aversive in either control or stressed subjects (Figure 4-4). Together, these data indicate that chronic stress recruits the CeA➔DMS pathway to subsequent learning to promote the premature formation of inflexible habits.
DISCUSSION
These data reveal a dual pathway neuronal circuit architecture by which a recent history of chronic stress shapes learning to disrupt flexible, goal-directed behavioral control in favor of inflexible habits. Both the BLA and CeA send direct projections to the DMS. The BLA➔DMS pathway is activated by rewarding events and this mediates the action-outcome learning that supports flexible, goal-directed control of reward-seeking behavior. Chronic stress attenuates this activity to promote premature habit formation. Conversely, the CeA➔DMS pathway is not robustly active during action-outcome learning, but stress recruits this pathway to promote premature habit formation. Thus, chronic stress promotes habit formation by altering the balance of BLA and CeA input to the DMS.
That the BLA➔DMS pathway mediates action-outcome learning is consistent with evidence that BLA lesion disrupts goal-directed behavior77–79 and BLA/DMS disconnection disrupts action-outcome learning82. Here we implicate direct BLA➔DMS projections and show that this pathway is activated by rewarding events so one can link those rewards to the actions that earned them and, subsequently, use those action-outcome memories to engage in the prospective consideration of action consequences needed for flexible decision making. These data are, however, inconsistent with evidence that BLA➔DMS ablation does not disrupt action-outcome learning108. Thus, temporally-specific manipulation was needed to reveal the function of this pathway in action-outcome learning. Chronic stress suppresses learning-related activity in BLA➔DMS projections. This was surprising because the BLA is, generally, hyperactive following chronic stress109–116 (c.f.117), but it indicates that chronic stress suppresses activity in the BLA pathway that is critical for the learning that supports flexible, goal-directed decision making. Given that the BLA➔DMS projection is predominately excitatory118–120, the BLA is poised to excite the DMS neuronal activity that mediates action-outcome learning70–72,121–125. Stress removes this influence to disrupt action-outcome learning and promote habit formation.
Like the BLA, we found that the CeA also targets the DMS. Unlike the BLA➔DMS pathway, we find that the CeA➔DMS pathway is not typically activated during action-outcome learning but is recruited by chronic stress to promote premature habit formation. This is consistent with evidence that the CeA is itself required for natural habit formation79. It is also consistent with evidence that stress increases CeA activity126–131. Here we show that a recent history of chronic stress causes CeA➔DMS projections to be activated by rewards during instrumental learning. This alters learning, disrupting the ability to engage in subsequent prospective consideration of action consequences in favor of inflexible, habitual behavioral control. Indeed, given that most CeA neurons are inhibitory88,132, the recruitment of this pathway is poised to attenuate the DMS activity needed for action-outcome learning70–72,121–123. Previous work has shown that the CeA works indirectly, likely via the midbrain133–136, with the dorsolateral striatum to regulate habit formation79,137. Thus, the CeA may promote habit learning through both direct and indirect pathways to the striatum. Whether stress acts via multiple CeA outputs to promote habit is a ripe question for future investigation. Interestingly, activation of the CeA➔DMS pathway alone was not sufficient to recapitulate the effects of stress to promote habit formation. Rather CeA➔DMS activation was sufficient to tip the balance towards habit following a subthreshold mild chronic stress experience. Thus, stress exposure may prime the CeA➔DMS pathway to be recruited during subsequent learning. Additionally, CeA➔DMS activation may work along with a confluence of disruptions, likely to the BLA➔DMS pathway, but also to cortical inputs to DMS60,123,138 and perhaps CeA outputs to midbrain133,134, to promote habit formation.
These data support a parallel model139 of amygdala function whereby, via distinct outputs to the DMS, the amygdala actively gates the nature of learning to regulate the balance of behavioral control strategies. Unlike valence-processing models of amygdala function140,141, the data here indicate that BLA and CeA projections to DMS are unlikely to convey simple positive or negative valence, but rather differentially shape the content of learning. Chronic stress flips the balance of BLA and CeA inputs to the DMS to promote the formation of rigid, inflexible habits.
The discoveries here open the door to many important future questions. One is how prior chronic stress affects learning-associated BLA➔DMS and CeA➔DMS activity. That chronic stress occurred before training and did not alter spontaneous activity in either pathway, suggests that it may lay down neuroplastic changes in these pathways that become influential during subsequent learning opportunities. How such changes occur is a big and important question for future research. An equally substantial next question is how these pathways influence downstream DMS activity to promote habit formation. Indeed, DMS neuronal activity, especially plasticity in dopamine D1 receptor-expressing neurons121, is critical for the action-outcome learning that supports goal-directed behavior70–72,123 and when suppressed promotes inflexible habits70,76. A reasonable speculation is that the excitatory BLA➔DMS pathway promotes downstream learning-related activity in DMS to support action-outcome learning and that the inhibitory CeA➔DMS pathway dampens such activity to encourage habit formation. Amygdala-striatal inputs may coordinate in this regard with corticostriatal inputs, known to be important for supporting action-outcome learning22,60,142. The BLA➔DMS and CeA➔DMS pathways mediate the influence of stress on the learning that supports behavioral control strategies. Manipulations restricted to learning were sufficient to rescue normal goal-directed behavioral control following chronic stress. Whether these pathways similarly contribute to the expression of behavioral control strategies is also a ripe question for future investigation. Both amygdala subregions and DMS participate in drug-seeking137,143–148 and active avoidance behavior149. The central amygdala is particularly implicated in compulsive drug seeking and drug seeking after extended use, dependence and withdrawal, or stress137,147,150,151. Thus, more broadly, our results indicate chronic stress is poised to oppositely modulate BLA➔DMS and CeA➔DMS pathways to promote maladaptive drug-seeking and/or avoidance habits.
Adaptive decision making often requires understanding your agency in a situation. Knowing that your actions can produce desirable or undesirable consequences and using this to make thoughtful, deliberate, goal-directed decisions. Chronic stress can disrupt such agency and promote inflexible, habitual control over behavior. We found that stress does this with a one-two punch to the brain. Chronic stress dials down the BLA➔DMS pathway activity needed to learn the association between an action and its consequence to enable flexible, well-informed decisions. It also dials up activity in the CeA➔DMS pathway, causing the formation of rigid, inflexible habits. These data provide neuronal circuit insights into how chronic stress shapes how we learn and, thus, how we decide. This helps understand how stress can lead to the disrupted decision making and pathological habits that characterize substance use disorders and mental illness.
METHODS
See Supplemental Table 5 for key reagents.
Subjects
Male and female wildtype C57/Bl6J mice (Jackson Laboratories, Bar Harbor, ME) aged 9–12 weeks old at the time of surgery served as subjects. Rabies tracing was conducted with Drd1a-Cre and Adora2A-Cre transgenic mice bred in house and aged 8–16 weeks at the time of surgery. Mice were housed in a temperature (68–79 °F) and humidity (30–70%) regulated vivarium on 12:12 hour reverse dark/light cycle (lights off at 7 AM). Behavioral experiments were performed during the dark phase. Mice were group housed in same-sex groups of 3–4 mice/cage prior to onset of behavioral experiments and subsequently single-housed for the remainder of the experiment to facilitate food deprivation and preserve implants. Unless noted below, mice were provided with food (standard rodent chow, Lab Diet, St. Louis, MO) and water ad libitum in the home cage. Mice were handled for 3–5 days prior to the start of behavioral training for each experiment. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the UCLA Institutional Animal Care and Use Committee.
Surgery
Mice were anesthetized with isoflurane (3% induction, 1% maintenance), and positioned in a digital stereotaxic frame (Kopf, Tujunga, CA). Subcutaneous Rimadyl (Carprofen; 5 mg/kg; Zoetis, Parsippany, NJ) was given pre-operatively for analgesia and anti-inflammatory purposes. Small cranial holes (1–2 mm2) were drilled, through which virus or fluorescent tracers were delivered via a guide cannula (DMS: 28 ga, BLA/CeA: 33 ga), PlasticsOne, Roanoke, VA) connected to a 1-mL syringe (Hamilton Company, Reno, NV) by intramedic polyethylene tubing (BD; Franklin Lakes, NJ) and controlled by a syringe pump (Harvard Apparatus, Holliston, MA). Coordinates (from Bregma) were determined by mouse brain reference atlas152 and were as follows: CeA, AP −1.2, ML ±2.8, DV −4.6 mm; BLA, AP −1.5, ML ±3.2, DV −5.0 mm; DMS, AP +0.2, ML ±1.8, DV −2.65 mm. Virus or tracers were infused at a rate of 0.1 µL/min and cannulae were left in place for at least 10 min post-injection. For injection-only surgeries, the skin was re-closed with Vetbond tissue adhesive (3M, Saint Paul, MN). For surgeries requiring fiber-optic cannulae, fibers were placed 0.3 mm above the target region for optogenetic experiments and at the infusion site for fiber photometry experiments, secured to the skull using RelyX Unicem Universal Self-Adhesive Resin (3M) and a head cap was created using C&B Metabond quick adhesive cement system (Parkell Inc., Brentwood, NY), followed by opaque dental cement (Lang Dental Manufacturing, Wheeling, IL). After surgery, mice were kept on a heating pad maintained at 35 °C for 1 hour and then single-housed in a new homecage for recovery and monitoring. Mice received chow containing the antibiotic TMS for 7 days following surgery to prevent infection, after which they were returned to standard rodent chow. Specific surgical details for each experiment are described below. In all cases, surgery occurred prior to the onset of stress and/or behavioral training.
Chronic mild unpredictable stress
The chronic mild unpredictable stress (CUS) procedure was modified from60,153–156. Mice assigned to the CUS group were exposed to 2 stressors/day (foot shock, physical restraint, tilted cage, white noise, continuous illumination, or damp bedding) for 14 days in a pseudorandomized manner at variable time onset and for varying durations between 2 and 16 hours. Control subjects received equated daily handling in the vivarium by the same experimenter administering the CUS. CUS was administered in a separate, enclosed laboratory space distinct from both the vivarium and behavioral testing rooms. CUS mice had home-cage nesting material removed for the duration of the CUS exposure157. Mice were transported to the stress space in individual 16-oz clear polyethylene containers and on a dedicated transport cart and placed into individual cages in the stress space. Stress efficacy was assessed by daily body weight measurements158. Sub-threshold CUS exposure was identical to CUS except mice received only 1 stressor/day.
Stressors
Footshock:
Subjects were placed in the conditioning chamber for 2 min to acclimate and then exposed to 5, 2–3 s, 0.7-mA footshocks with a variable intertrial interval averaging 60 sec (30–90 sec range). The footshock chamber had a similar grid floor to the behavioral testing chambers (described below) but was otherwise distinct in wall shape (round), pattern (monochrome polka dot), lack of bedding, scent (75% ethanol), and lighting (off). The chambers also lacked food ports and levers. Chambers were cleaned with 75% ethanol between subjects.
Physical Restraint:
Subjects were immobilized in modified 50-mL polypropylene conical tubes with 4 air holes per side, 1 at the top, and 1 in the cap for the tail (10 total). Mice were scruffed and placed inside the conical tube for 2 hours in their stress cage.
Tilted cage:
Stress cages were placed on chocks to tilt each cage at approximately a 45-degree angle for 6 – 16 hours.
White noise:
100-db white noise was played in the stress space for all CUS mice for a duration of 6 – 16 hours.
Continuous illumination:
Overhead lights were turned on during the dark phase of the light cycle (7PM – 7AM).
Damp bedding:
~200 mL of water was mixed with the stress cage corncob bedding. Mice were placed in their stress cage with this damp bedding for 6 – 16 hours. Mice were returned to a new home cage with clean, dry bedding afterwards.
Corticosterone ELISA
N = 11 male and N = 12 female mice were used for corticosterone measurements of blood serum after exposure to 0, 1, or 2 stressors per day for 14 day. Measurements were taken 24 hours after the final stress exposure. Mice were decapitated and trunk blood was collected in 1.7-mL sample tubes on ice. Tubes were centrifuged at 2000 g for 10 min at 4 °C. Clear supernatant was collected and placed in new 1.7-mL sample tubes and frozen at −20 °C. Samples were diluted 1:40 in sample dilution buffer. Serum corticosterone levels were assessed using a Corticosterone ELISA kit as directed (Enzo Biosciences; Farmingdale, NY) and quantified on a microplate reader (Molecular Devices, San Jose, CA).
Behavioral procedures
Instrumental conditioning and tests
Instrumental conditioning procedures were adapted from our prior work97.
Apparatus.
Training took place in Med Associates wide mouse conditioning chambers (East Fairfield, VT) housed within sound- and light-attenuating boxes. Each chamber had metal grid floors and contained a retractable lever to the left of a recessed food-delivery port (magazine) on the front wall. A photobeam entry detector was positioned at the entry to the food port. Each chamber was equipped with 2 pellet dispensers to deliver either 20-mg grain or chocolate-flavored purified pellets (Bio-Serv, Frenchtown, NJ) into the food port. A fan mounted to the outer chamber provided ventilation and external noise reduction. A 3-watt, 24-volt house light mounted on the top of the back wall opposite the food port provided illumination. To monitor subject behavior, monochrome digital cameras (Med Associates) were positioned over top of the conditioning chambers. For optogenetic manipulations, chambers were outfitted with an Intensity Division Fiberoptic Rotary Joint (Doric Lenses, Quebec, QC, Canada) connecting the output fiber optic patch cords to a 473-nm or 593-nm laser (Dragon Lasers, ChangChun, JiLin, China) positioned outside of the chamber.
Food deprivation.
3 – 5 days prior to the start of behavioral training, mice were food-deprived to maintain 85%−90% of their free-feeding body weight. Mice were given 1.5 – 3.0 g of their home chow at the same time daily at least 2 hours after training sessions. For experiments involving CUS, food deprivation began during the last 3 days of the stress procedure. Owing to food deprivation, body weights did not differ between groups at the start or end of training (see Supplemental Table 1).
Outcome pre-exposure.
To familiarize subjects with the food pellet that would become the instrumental outcome, mice were given 1 session of outcome pre-exposure. Mice were placed in a clean, empty cage and allowed to consume 20 – 30 of the food pellets from a metal cup. If any pellets remained, they were placed in the home cage overnight for consumption.
Magazine conditioning.
Mice received 1 session of training in the operant chamber to learn where to receive the food pellets (20-mg grain or chocolate-purified pellets). Mice received 20 – 30 non-contingent pellet deliveries from the food port with a fixed 60-s intertrial interval.
Instrumental conditioning.
Mice received 4 sessions (1 session/day consecutively), minimum, of instrumental conditioning in which lever presses earned delivery of a single food pellet. Earned pellet type (grain or chocolate) was counterbalanced across subjects within each group of each experiment. Each session began with the illumination of the house light and extension of the lever, and ended with the retraction of the lever and turning off of the house light. Sessions ended after the total available outcomes (20 or 30, as noted for each experiment below) had been earned or a maximum time limit (20 or 30 min, as noted below) had been reached. In all cases, training began on a fixed-ratio 1 schedule (FR-1), in which each action was reinforced with one food pellet outcome. Once mice completed 2 sessions in which they achieved 80% performance criteria (earned 80% of the max outcomes), the reinforcement schedule was escalated to either random interval (RI) or random ratio (RR) as described for each experiment below. For the RI protocol, mice received 1 session on an RI-15 s schedule then 2 – 3 sessions on the final RI-30 s schedule (variable average 15-s or 30-s interval must elapse following a reinforcer for another press to be reinforced). Subjects on the RR protocol received 1 session each of RR-2, RR-5, and RR-10 schedule of reinforcement (variable press requirement average of 2, 5, or 10 presses to earn the food pellet).
Alternate outcome exposures.
To equate exposure of the non-trained pellet, all mice were given non-contingent access to same number of the alternate food pellets (e.g., chocolate pellets if grain pellets served as the training outcome) as the earned pellet type in a different context (clear plexiglass cage) a minimum of 2 hours before or after (alternated daily) each RI or RR instrumental training session.
Sensory-specific satiety outcome devaluation test.
Testing began 24 hr after the final instrumental conditioning session. Mice were given 1 – 1.5 hours access to either 4 g of the food pellets previously earned by lever pressing (Devalued condition) or 4 g of the non-trained pellets to control for general satiety (Valued condition). The remaining pellets were weighed following prefeeding to measure total consumption. Consumption did not significantly differ between the Devalued v. Valued conditions for any experiment (Supplemental Table 2). Immediately after this prefeeding, lever pressing was assessed during a brief, 5-min, non-reinforced probe test. Following the probe test, mice were given a 10-min consumption choice test with simultaneous access to 1 g of both pellet types to ensure rejection of the devalued outcome. In all cases, mice consumed less of the prefed pellet than non-prefed pellet, indicating successful sensory-specific satiety devaluation (Supplemental Table 3). 24 hr after the first devaluation test, mice received 1 session of instrumental retraining on the final reinforcement schedule (RI30 or RR10), followed the next day by a second devaluation test in which they were prefed the opposite food pellet. Thus, each mouse was tested in both the Valued and Devalued conditions, with test order counterbalanced across subjects within each group for each experiment.
Real-time place preference/avoidance test
Procedure was conducted as described previously159. Mice were habituated to a 2-sided opaque plexiglass chamber (20 × 42 × 27 cm) for 10 min, during which their baseline preference for the left or right side of the chamber was measured. During the first 10-min test session, one side of the chamber was assigned to the light-delivery side (counterbalanced across subjects within each group). Mice were placed in the non-stimulation side to start the experiment. Light (Dragon Laser; Changchun, China) was delivered upon entry into the light-paired side and continued until the subject exited that side (optical stimulation: 473 nm, 5-ms pulse width, 20 Hz, ~8–10 mW at fiber tip; optical inhibition: 593 nm, continuous, ~8–10 mW). Mice then received a second test, identical to the first, in which the opposite side of the chamber served as the light-paired side. Sessions were video-recorded using a CCD camera. This camera interfaced with Biobserve software (Biobserve GmbH, Germany) and a Pulse Pal (Sanworks, Rochester, NY), to track subject position in real time and trigger laser delivery. The apparatus was cleaned with 75% ethanol after each session. Distance traveled, movement velocity, and time spent in each chamber was generated by Biobserve software post-session. Time spent in laser-paired chamber was compared between groups to assess preference or aversion of laser delivery.
Open-field test
Procedure was conducted as described previously159. Mice were placed in an opaque plexiglass arena (34 × 34 × 34 cm) for a single 10-min session. Sessions were video recorded using a CCD camera interfaced with Anymaze (Stoelting Co., Wood Dale, IL) software, which was used to track subject position in real time. Center region was defined as the innermost third of the floor area. Brightness above the OFT was ~70 lux. The apparatus was cleaned with 75% ethanol after each subject. Distance traveled, movement velocity, and time spent in either center or surrounding outer area was generated by Biobserve software and compared between groups.
Elevated plus maze
Procedure was conducted as described previously160. The dimensions of the elevated plus maze (EPM) arms were 30 cm x 7 cm, and the height of the closed arm walls was 20 cm. The maze was 65 cm elevated from the floor and was placed in the center of the behavior room away from other stimuli. Brightness above the EPM was ~70 lux. For the 10-min EPM test, mice were placed in the center of EPM facing a closed arm. Each session was video recorded using a CCD camera, which interfaced with Anymaze software to track subject location in real time. The apparatus was cleaned with 75% ethanol after each session. Distance traveled, movement velocity, and time spent in the center, open arms, or closed arms was generated by Biobserve software and compared between groups.
Light/dark emergence test
The dark side of a 2-chamber apparatus was made of black opaque plexiglass and completely enclosed except for a small entry through the middle divider. The light side was made of white opaque plexiglass and was open to the light above. Brightness in the light chamber was ~70 lux. Mice were placed in the open portion of the apparatus to initiate a 10-min session. Each session was video recorded using a CCD camera, which interfaced with Anymaze software to track subject location. The apparatus was cleaned with 75% ethanol after each session. Distance traveled, movement velocity, and time spent in the light chamber was generated by Biobserve software and compared between groups.
Sucrose-preference test
Mice first received habituation to 2 standard home-cage water bottles filled with water in the home cage for 16 hours. Subsequently, one water bottle was replaced with a bottle of 10% sucrose. Bottles were left in place for 24 hours and weighed before and after placement. Bottle positions were switched for another 24-hour period and subsequently weighed again. Amount of sucrose and water consumed, as well as a ratio of the two, during the 48-hour period was compared between groups.
Progressive ratio test
Mice were trained on the instrumental training protocol above to a reinforcement schedule of RR-10. They were then given a progressive ratio test in which the number of lever presses required to receive a pellet increased by 4 with each reinforcer delivered (e.g., 1, 5, 9, 13, 17, 21 etc.). The session ended after >5-min break in pressing or maximum duration of 4 hr. Session duration, rewards delivered, total presses, and the break point (last completed press requirement) were collected and compared between groups.
Tracing
Anterograde tracing of CeA neurons was performed as previously described161. Male (N = 2) and female (N = 2) naïve mice were infused bilaterally with the anterograde tracer AAV8-Syn-mCherry (Addgene, Watertown, MA) in the CeA (0.2 µL). Virus was allowed to express for 4 weeks, following which mice were perfused and histology was processed as described below to identify fluorescently labeled fibers in the dorsal striatum.
For retrograde tracing of DMS-projecting amygdala neurons, male (N = 2) and female (N = 2) naïve mice were infused with Fluorogold (Sigma, St. Louis, MO; 4% in sterile saline) in the DMS (0.2 µL). Virus was allowed to express for 5 days, following which mice were perfused and histology was processed as described below to identify fluorescently labeled cell bodies in CeA and BLA.
For retrograde tracing of monosynaptic inputs onto Drd1a+ or A2A+ DMS neurons, male (N = 4) and female (N = 4) Drd1a-cre or male (N = 3) and female (N = 2) Adora2A-cre naïve mice were infused with 0.3 µL AAV2-hSyn-FLEX-TVA-P2A-eGFP-2A-oG (Salk Gene Transfer, Targeting and Therapeutics Facility) in the DMS. Three weeks later, mice were infused with 0.3 µL EnvA G-deleted Rabies-mCherry at the same DMS coordinates. Mice were perfused 1 week later and tissue was processed as described below to identify monosynaptically-labeled inputs in CeA and BLA. 4 Drd1a-cre and 1 Adora2A-cre subjects were removed due to starter virus spillover in the BNST.
Fiber photometry calcium imaging of CeA➔DMS or BLA➔DMS projections during instrumental learning following CUS
Male and female (CeA➔DMS: N = 38, 14 male; BLA➔DMS: N = 34, 13 male) naïve mice were used in this experiment to monitor calcium fluctuations in CeA➔DMS and BLA➔DMS projections during instrumental conditioning after CUS. 18 subjects with off-target viral expression and/or fiber location were excluded from the dataset. 4 subjects were excluded for loss of optic fibers/headcaps. 4 subjects were excluded for missing recording data from one session. 3 subjects that did not complete instrumental conditioning were also excluded. Mice were randomly assigned to Virus and Stress groups. At surgery, mice received unilateral infusion (left/right hemisphere counterbalanced across subjects within each group) of a retrogradely trafficked AAV encoding cre-recombinase (AAVrg-Syn-Cre-P2A-dTomato, Addgene) into the DMS (0.3 µl) and of an AAV encoding the cre-dependent genetically encoded calcium indicator GCaMP8s (AAV9-Syn-FLEX-GcAMP8s-GFP, Addgene) into either the CeA or BLA (0.1–0.2 µl). Optic fiber cannulae (5.0-mm length (BLA) or 4.6 mm (CeA), 200-µm diameter, 0.37 NA, Inper, Hangzhou, China) were implanted over the GCaMP infusion site for calcium imaging at cell bodies. Mice were given 1 – 2 weeks to recover post-surgery, followed by 14 consecutive days of twice/daily CUS or daily handling as described above. Mice were habituated to restraint during the final 3 days of the CUS/handling period. 24 hours after the final CUS exposure, mice began instrumental conditioning as described above. Each session began with a 3-minute baseline period prior to the start of the instrumental session for assessment of changes in baseline calcium activity. After completion of FR-1, mice received 1 session each of training on an RR-2, RR-5, and RR-10 reinforcement schedule (max 20 outcomes/20 min/session). We chose an RR reinforcement schedule for this experiment because it tends to promote action-outcome learning and goal-directed behavioral control94,95,100,123 and would, thus, make it more difficult for prior CUS to induce habits, increasing the robustness of the results.
Fiber photometry was used to image bulk calcium activity in CeA➔DMS or BLA➔DMS neurons for 3-min prior to and throughout each instrumental conditioning session using a commercial fiber photometry system (Neurophotometrics Ltd., San Diego, CA). Two light-emitting LEDs (470 nm: Ca2+-dependent GCaMP fluorescence; 415 nm: autofluorescence, motion artifact, Ca2+-independent GCaMP fluorescence) were reflected off dichroic mirrors and coupled via a patch cord (200 µm; 0.37 NA, Inper) to the implanted optical fiber. The intensity of excitation light was adjusted to ∼100 µW at the tip of the patch cord. Fluorescence emission was passed through a 535-nm bandpass filter and focused onto the complementary metal-oxide semiconductor (CMOS) camera sensor through a tube lens. Samples were collected at 20 Hz interleaved between the 415 nm and 470 nm excitation channels using a custom Bonsai workflow. Time stamps of task events were collected simultaneously through an additional synchronized camera aimed at the Med Associates interface, which sent light pulses coincident with task events (onset, press, entry, reward). Signals were saved using Bonsai software and exported to MATLAB (MathWorks, Natick, MA) for analysis.
To assess the response to appetitive and aversive stimuli and provide a positive signal control, fiber photometry measurements were made during subsequent non-contingent reward and footshock sessions. In the first session, mice received 10 non-contingent food-pellet deliveries with a variable 60-s intertrial interval. 24 hours later, they received a session of 5, 2-s, 0.7mA footshocks with a variable 60-s intertrial interval. Calcium signal was aligned to reward collection or shock onset using timestamps collected as above. Mice were then perfused and brain tissue was processed with standard histology procedures described below to assess viral expression location/spread and fiber location.
Fiber photometry analysis
Data were pre-processed using a custom-written pipeline in MATLAB (MathWorks, Natick, MA) as previously162. The 415 nm and 470 nm signals were fit using an exponential curve. Change in fluorescence (ΔF/F) at each time point was calculated by subtracting the fitted 415 nm signal from the 470 nm signal and normalizing to the fitted 415 nm data [(470-fitted 415)/fitted 415)]. The ΔF/F data was Z-scored to the average of the whole session [(ΔF/F - mean ΔF/F)/std(ΔF/F)]. Z-scored traces were then aligned to behavioral event timestamps throughout each session. Area under the curve (AUC) was calculated for each individual aligned trace within each session using a trapezoidal function. We use the 3-s period prior to initiating presses to quantify activity related to the initiation of actions. We used the 3-s period following reward collection to quantify activity related to the earned outcome and unpredicted reward. We used the 1-s period following shock onset to quantify acute shock responses and the 2-s post-shock period to quantify activity following the shock. Quantifications and signal aligned to events were averaged across trials within a session and compared across sessions and between groups. Spontaneous activity was recorded during a 3-minute baseline period in the instrumental training context prior to each training session. Calcium events were identified as described previously163. First, we fitted the isosbestic channel to the 470 nm signal using an exponential function then subtracted the isosbestic trace from the calcium trace to remove calcium-independent artifacts. We defined a series of sliding-moving windows (15-s window, 1-second step) along the trace in which we filtered out high-amplitude events (greater than 2x the median of the 15-s window) and calculated the median absolute deviation of the resultant trace. Calcium transients with local maxima greater than 2 times above the median absolute deviation were selected as events. These events were used to calculate spontaneous event frequency and amplitude for BLA➔DMS and CeA➔DMS pathways.
Chemogenetic activation of BLA➔DMS projections during instrumental learning following CUS
Male and female (mCherry N = 28, 14 male; hM3Dq: N = 26, 16 male) naïve mice were used in this experiment to assess whether activation of BLA➔DMS projections during learning is sufficient to rescue action-outcome learning for goal-directed behavioral control in subjects with a history of CUS. 15 subjects with off-target viral expression and 2 subjects that did not complete instrumental conditioning were excluded from the dataset. Mice were randomly assigned to Viral and Stress groups. At surgery, all mice received bilateral infusion of the retrogradely-trafficked canine-adenovirus encoding cre-recombinase (CAV2-Cre-GFP; Plateforme de Vectorologie de Montpellier, Montpellier, France) into the DMS (0.3 µl) and AAV encoding the cre-inducible excitatory designer receptor human M3 muscarinic receptor (hM3Dq; AAV2-Syn-DIO-hM3Dq-mCherry; Addgene, Watertown, MA) or fluorophore control (AAV2-Syn-DIO-mCherry; Addgene) into the BLA (0.1–0.2 µl). Mice were given 1–2 weeks to recover post-surgery, followed by 14 consecutive days of twice/daily CUS or daily handling, as described above. Mice were habituated to i.p. injections during the final 3 days of the CUS/handling period. 24 hours after the final CUS exposure, mice began instrumental conditioning, as described above. All subjects received an intraperitoneal (i.p.) injection of clozapine-n-oxide (water soluble CNO; 0.2 mg/kg101–104,164; Hello Bio, Princeton, NJ) 30 min prior to each instrumental conditioning session. Upon completion of FR-1 (80% max rewards delivered), mice received 1 training session the RI-15s reinforcement schedule following by 2 sessions on an RI-30s schedule (max 30 outcomes/30 min/session). We chose an RI schedule of reinforcement for this experiment because it tends to promote habit formation 94,95,100,123 and, thus, would make it more difficult to neurobiologically prevent stress-potentiated habit, increasing the robustness of the results. Following training mice received a counterbalanced pair of sensory-specific satiety outcome-specific devaluation tests, as above. No CNO was given on test days. CNO was given prior to the retraining session (RI-30s) in between tests. After instrumental training and testing, mice were perfused and brain tissue was processed using standard histology procedures described below to assess viral expression location and spread.
Optogenetic inhibition of BLA➔DMS projections during instrumental learning
Male and female (eYFP N = 20, 10 male; Arch: N = 20, 10 male) naïve mice were used in this experiment to assess the necessity of BLA➔DMS projection activity at outcome experience during training for the action-outcome learning that supports goal-directed behavioral control. 13 subjects with off-target viral expression or fiber location and 6 subjects that did not complete instrumental conditioning were excluded from the dataset. Mice were randomly assigned to Viral group. At surgery, mice received bilateral infusion of an AAV encoding the inhibitory opsin archaerhodopsin (AAVDJ-Syn-eArch-eYFP, Stanford Vector Core) or fluorophore control (AAV8-Syn-GFP; Addgene) into the BLA (0.1 – 0.2 µl). Optic fiber cannulae (2.4-mm length, 100-µm diameter, 0.22 NA, Inper) were implanted over the DMS. Mice were given 3 weeks to recover and allow for viral expression. Mice were habituated to restraint for attaching optical fibers for 3 days immediately prior to instrumental conditioning. During instrumental conditioning, mice were tethered to a 100-µm diameter optic fiber bifurcated patchcord (Inper) attached to a 593-nm laser (Dragon Laser) via a rotary joint. Mice were habituated to the tether during the magazine training session, but no laser was delivered. Beginning with the first FR-1 session, all subjects received laser delivery during reward collection (first magazine entry after reward delivery; 5-s pulse, 8–10 mW). After completion of FR-1, mice received 1 session each of instrumental conditioning on an RR-2, RR-5, and RR-10 reinforcement schedule (max 20 outcomes/20 min/session). We chose an RR schedule of reinforcement for this experiment because tends to promote action-outcome learning and goal-directed behavioral control 94,95,100,123 and, thus, would make it more difficult to neurobiologically induce habit formation, increasing the robustness of the results. Following training mice received a counterbalanced set of sensory-specific satiety outcome-specific devaluation tests, as above. Mice were tethered but no laser was delivered on test days. Mice received laser as in training during the intervening retraining session. After instrumental training and testing, mice were tested in the RTPP test as described above. Mice were then perfused and brain tissue was processed with standard histology procedures described below to assess viral expression location/spread and fiber placement.
Chemogenetic inactivation of CeA➔DMS projections during instrumental learning following CUS
Male and female (mCherry N = 32, 16 male; hM4Di: N = 32, 16 male) naïve mice were used in this experiment to assess the necessity of CeA➔DMS projection activity during learning for stress-potentiated habit formation. 16 subjects with off-target viral expression and 3 subjects that did not complete instrumental conditioning were excluded from the dataset. Mice were randomly assigned to Viral and Stress groups. At surgery, all mice received bilateral infusion of the retrogradely-trafficked CAV encoding cre-recombinase (CAV2-Cre-GFP; Plateforme de Vectorologie de Montpellier) into the DMS (0.3 µl) and AAV encoding the cre-inducible inhibitory designer receptor human M4 muscarinic receptor (hM4DGi; AAV2-Syn-DIO-hM4Di-mCherry; Addgene) or fluorophore control (AAV2-Syn-DIO-mCherry; Addgene) into the CeA (0.1–0.2 µl). Mice were given 1–2 weeks to recover post-surgery, followed by 14 consecutive days of twice/daily CUS or daily handling as described above. Mice were habituated to i.p. injections during the final 3 days of the CUS/handling period. 24 hours after the final CUS exposure, mice began instrumental conditioning as described above. All subjects received an intraperitoneal (i.p.) injection of CNO (2 mg/kg106,107,164,165; Hello Bio) 30 min prior to each instrumental conditioning session. Upon completion of FR-1, mice received 1 session of training on an RI-15s reinforcement schedule followed by 2 sessions on the RI-30s schedule (max 30 outcomes/30 min/session). We chose an RI reinforcement schedule for this experiment because it tends to promote habit formation 94,95,100,123 and, thus, would make it more difficult to neurobiologically prevent stress-potentiated habit, increasing the robustness of the results. Following training, mice received a counterbalanced pair of sensory-specific satiety outcome-specific devaluation tests, as above. No CNO was given on test days. CNO was given prior to the retraining session. After instrumental training and testing, mice were then perfused and brain tissue was processed with standard histology procedures described below to assess viral expression location and spread.
Optogenetic inactivation of CeA➔DMS projections during instrumental learning following CUS
Male and female (control, GFP N = 18, 11 male; control, Arch: N = 26, 12 male) naïve mice were used in this experiment to assess the necessity of CeA➔DMS projection activity at outcome experience during learning for stress-potentiated habit formation. 10 subjects with off-target viral expression or fiber location and 2 subjects that did not complete instrumental conditioning were excluded from the dataset. Mice were randomly assigned to Virus and Stress groups. At surgery, mice received bilateral infusion an AAV encoding the inhibitory opsin Arch (AAVDJ-Syn-eArch-eYFP, Stanford Vector Core) or fluorophore control (AAV8-Syn-GFP; Addgene) into the CeA (0.1–0.2 µl). Optic fiber cannulae (2.4-mm length, 100-µm diameter, 0.22 NA, Inper) were implanted over the DMS. Mice were given 1 – 2 weeks to recover post-surgery, followed by 14 consecutive days of twice/daily CUS or daily handling as described above. Mice were habituated to restraint for attaching optical fibers during the final 3 days of the CUS/handling period. 24 hours after the final CUS exposure, mice began instrumental conditioning as described above. During instrumental conditioning, mice were tethered to a 100-µm diameter optic fiber bifurcated patchcord (Inper) attached to a 593-nm laser (Dragon Laser) via a rotary joint. Mice were habituated to the tether during the magazine training session, but no laser was delivered. Beginning with the first FR-1 session, all subjects received laser delivery during reward collection, (first magazine entry after reward delivery; 5-s pulse, 8–10 mW). After completion of FR-1, mice received 1 training session on an RI-15s reinforcement schedule and 2 training sessions on the RI-30s schedule (max 20 outcomes/20 min/session). We chose an RI reinforcement schedule for this experiment because it tends to promote habit formation 94,95,100,123 and, thus, would make it more difficult to neurobiologically prevent stress-potentiated habit, increasing the robustness of the results. Following training, mice received a counterbalanced set of sensory-specific satiety outcome-specific devaluation tests, as above. Mice were tethered but no laser was delivered on test days. Mice received laser as in training during the intervening retraining session. After instrumental training and testing, mice were tested in the RTPP test as described above. Mice were then perfused and brain tissue was processed with standard histology procedures described below to assess viral expression location/spread and fiber placement.
Optogenetic activation of CeA➔DMS projections during instrumental learning
Male and female (GFP N = 18, 9 male; ChR2: N = 20, 12 male) naïve mice were used in this experiment to assess whether CeA➔DMS projection activation at outcome experience during learning is sufficient to promote habit formation. 11 subjects with off-target viral expression or fiber location and 4 subjects who did not complete instrumental conditioning were excluded from the dataset. Mice were randomly assigned to Virus group. Given the low density of CeA➔DMS projections, we choose to activate DMS-projecting CeA cell bodies. At surgery, mice received bilateral infusion of a retrogradely trafficked AAV encoding cre-recombinase (AAVrg-Syn-Cre-P2A-dTomato, Addgene) into the DMS (0.3 µl) and AAV encoding the cre-inducible excitatory opsin channelrhodopsin 2 (ChR2; AAV8-Syn-DIO-ChR2-eYFP, Stanford Vector Core) or fluorophore control (AAV8-Syn-DIO-eYFP, Stanford Vector Core) into the CeA (0.1–0.2 µl). Optic fiber cannulae (4.4-mm length, 100-µm diameter, 0.22 NA, Inper) were implanted over the CeA. Mice were given 3 weeks to recover and allow for viral expression. Mice were habituated to restraint for 3 days prior to instrumental conditioning. During instrumental conditioning, mice were tethered to a 100-µm diameter optic fiber bifurcated patchcord (Inper) attached to a 473-nm laser (Dragon Laser) via a rotary joint. Mice were habituated to the tether during the magazine training session, but no laser was delivered. Beginning with the first FR-1 session, all subjects received laser delivery during reward collection (first magazine entry after reward delivery; 2-s duration, 20 Hz, 5-ms pulse width, 8–10 mW). After completion of FR-1, mice received 1 day each of training on an RR-2, RR-5, and RR-10 reinforcement schedule (max 20 outcomes/20 min/session). We chose an RR schedule of reinforcement for this experiment because it tends to promote action-outcome learning and goal-directed behavioral control 94,95,100,123 and, thus, would make it more difficult to neurobiologically induce habit formation, increasing the robustness of the results. Following training mice received a counterbalanced set of sensory-specific satiety outcome-specific devaluation tests, as above. Mice were tethered but no laser was delivered on test days. Mice received laser as in training during the intervening retraining session. After instrumental training and testing, mice were tested in the RTPP test, as described above. Mice were then perfused and brain tissue was processed with standard histology procedures described below to assess viral expression location/spread and fiber placement.
Optogenetic activation of CeA➔DMS projections during instrumental learning following sub-threshold CUS
Male and female (GFP N = 12, 5 male; ChR2: N = 18, 8 male) naïve mice were used in this experiment to assess whether CeA➔DMS projection activation at outcome experience during learning is sufficient to promote habit formation in mice with a history of less-frequent CUS (subthreshold for promoting habit formation). 9 subjects with off-target viral expression or fiber location and 3 subjects who did not complete instrumental conditioning were excluded from the dataset. Mice were randomly assigned to viral groups. Similar to optogenetic activation of CeA➔DMS neurons in control mice, we chose to target cell bodies with this approach. At surgery, mice received bilateral infusion of a retrogradely trafficked AAV encoding cre-recombinase (AAVrg-Syn-Cre-P2A-dTomato, Addgene) into the DMS (0.3 µl) and AAV encoding the cre-inducible excitatory opsin ChR2 (AAV8-Syn-DIO-ChR2-eYFP, Stanford Vector Core) or fluorophore control (AAV8-Syn-DIO-eYFP, Stanford Vector Core) into the CeA (0.1–0.2 µl). Optic fiber cannulae (4.4-mm length, 100-µm diameter, 0.22 NA, Inper) were implanted over the CeA. Mice were given 1 – 2 weeks to recover post-surgery, followed by 14 consecutive days of once/daily CUS or daily handling as described above. Mice were habituated to restraint for attaching optical fibers during the final 3 days of the subthreshold CUS/handling period. 24 hours after the final stress exposure, mice began instrumental conditioning, as described above. During instrumental conditioning, mice were tethered to a 100-µm diameter optic fiber bifurcated patchcord (Inper) attached to a 473-nm laser (Dragon Laser) via a rotary joint. Mice were habituated to the tether during the magazine training session, but no laser was delivered. Beginning with the first FR-1 session, all subjects received laser delivery during reward collection, (first magazine entry after reward delivery; 2-s duration, 20 Hz, 5-ms pulse width, 8–10 mW). After completion of FR-1, mice received 1 day each of training on an RR-2, RR-5, and RR-10 reinforcement schedule (max 20 outcomes/20 min/session). Following training mice received a counterbalanced set of sensory-specific satiety outcome-specific devaluation tests, as above. Mice were tethered but no laser was delivered on test days. Mice received laser during the intervening retraining session. After instrumental training and testing, mice were tested in the RTPP test, as described above. Mice were then perfused and brain tissue was processed with standard histology procedures described below to assess viral expression location/spread and fiber placement.
Immunohistochemistry
Mice were anesthetized with isoflurane and transcardially perfused with ice-cold PBS followed by cold 4% paraformaldehyde. The brains were removed, post-fixed in 4% paraformaldehyde, then cryoprotected in 30% sucrose in PBS. 30-µm coronal slices were taken on a cryostat and collected in PBS. Immunohistochemical analysis was performed as described previously162,166–168. Briefly, floating sections were blocked for 1 hour at room temperature in blocking solution (3% normal goat serum (NGS, Jackson ImmunoResearch Laboratories), 0.3% Triton X-100 (Fisher)) in PBS and then incubated overnight with gentle agitation at 4 °C in blocking solution plus 1:1000 dilution primary antibody (chicken anti-GFP polyclonal, Abcam; rabbit anti-dsRed polyclonal, Takara Bio). Sections were then incubated covered with gentle agitation for 2 hours at room temperature in blocking solution plus 1:500 dilution secondary antibody (goat anti-rabbit IgG Alexafluor 594 conjugate; goat anti-chicken IgG Alexafluor 488 conjugate, Invitrogen). All sections were washed 3 times for 5 min each in PBS before and after each incubation step and mounted on slides using ProLong Gold antifade reagent with DAPI (Invitrogen). All images were acquired using a Keyence (BZ-X710) microscope with 4X, 10X, and 20X objectives (CFI Plan Apo), CCD camera, and BZ-X Analyze software and a Zeiss Confocal LSM with 2.5X and 20X objectives and Zeiss ZEN (blue edition) image acquisition software.
Statistical Analysis
Datasets were analyzed by 2-tailed t-tests, or 1-, 2-, or 3-way repeated-measures analysis of variance (ANOVA), as appropriate (GraphPad Prism, GraphPad, San Diego, CA; SPSS, IBM, Chicago, IL). Some datasets were slightly non-normally distributed. For these datasets statistical tests were also run on the normally-distributed log-transformed data and the results were highly consistent (Supplemental Table 4). We opted to use parametric statistics for consistency across experiments and given evidence that ANOVA is robust to slight non-normality169,170. Bonferroni post hoc tests corrected for multiple comparisons were performed to clarify statistical interactions. Greenhouse-Geisser correction was applied to mitigate the influence of unequal variance between conditions. Alpha levels were set at P < 0.05.
Sex as a biological variable
For the initial behavioral finding, sex was included as a factor in the ANOVA and found to not significantly account for variance (No main effect of Sex on lever pressing acquisition: F(1, 43) = 0.43, P = 0.51, devaluation test press rate: F(1,43) = 0.60, P = 0.44, or devaluation index: F(1,43) = 0.04, P = 0.84). Therefore, data from male and female mice was combined for analyses. For subsequent experiments, male and female mice were used in approximately equal numbers, but the N per sex was underpowered to examine sex differences. Sex was therefore not included as a factor in statistical analyses, though individual data points are visually disaggregated by sex.
Rigor and reproducibility
Group sizes were estimated based on prior work with this behavioral task171 and to ensure counterbalancing of virus, stress, pellet type, and devaluation test order. Investigators were not blinded to viral or stress group because they were required to administer infusions and stress exposure. All behaviors were scored using automated software (Med Associates). Each experiment included at least 1 replication cohort and cohorts were balanced by Viral group, Stress group, and hemisphere (for photometry recordings and tracing) prior to the start of the experiment. Investigators were blinded to group when performing histological validation and determining exclusions based on viral spread or mistargeted implant.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by NIH R01DA046679 (KMW), NIH T32DA024635 (JRG), NIH F32DA056201 (JRG), A.P. Giannini Fellowship (JRG), NIH TL4GM118977 (NP), NIH R01MH119089 (AA), and the Staglin Center for Behavior and Brain Sciences. UCLA Behavioral Testing Core provided space and behavioral testing equipment for the open field, elevated plus maze and light-dark emergence test.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors have no biomedical financial interests or potential conflicts of interest to declare.
Data and code availability
All data that support the findings of this study are available from the corresponding author upon request. Custom-written MATLAB code is accessible via Dryad repository172 and available from the corresponding author upon request.
REFERENCES
- 1.Wassum K. M. Amygdala-cortical collaboration in reward learning and decision making. Elife 11 (2022). 10.7554/eLife.80926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balleine B. W. The Meaning of Behavior: Discriminating Reflex and Volition in the Brain. Neuron 104, 47–62 (2019). 10.1016/j.neuron.2019.09.024 [DOI] [PubMed] [Google Scholar]
- 3.Balleine B. W. & Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 37, 407–419 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Malvaez M. & Wassum K. Regulation of habit formation in the dorsal striatum. Current Opinion in Behavioral Sciences 20, 67–74 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graybiel A. M. Habits, rituals, and the evaluative brain. Annu Rev Neurosci 31, 359–387 (2008). 10.1146/annurev.neuro.29.051605.112851 [DOI] [PubMed] [Google Scholar]
- 6.Adams C. D. Variations in the sensitivity of instrumental responding to reinforcer devaluation. Quarterly Journal of Experimental Psychology 34, 77–98 (1982). [Google Scholar]
- 7.Smith K. S. & Graybiel A. M. Habit formation. Dialogues Clin Neurosci 18, 33–43 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickinson A. Actions and Habits: the development of behavioural autonomy. Philosphical Transactions of the Royal Society of London B308, 67–78 (1985). [Google Scholar]
- 9.Dolan R. J. & Dayan P. Goals and habits in the brain. Neuron 80, 312–325 (2013). 10.1016/j.neuron.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redish A. D., Jensen S. & Johnson A. A unified framework for addiction: vulnerabilities in the decision process. Behav Brain Sci 31, 415–437; discussion 437–487 (2008). 10.1017/S0140525X0800472X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandaele Y. & Ahmed S. H. Habit, choice, and addiction. Neuropsychopharmacology 46, 689–698 (2021). 10.1038/s41386-020-00899-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voon V. et al. Disorders of compulsivity: a common bias towards learning habits. Mol Psychiatry 20, 345–352 (2015). 10.1038/mp.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillan C. M., Robbins T. W., Sahakian B. J., van den Heuvel O. A. & van Wingen G. The role of habit in compulsivity. Eur Neuropsychopharmacol 26, 828–840 (2016). 10.1016/j.euroneuro.2015.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbit L. H. & Janak P. H. Habitual Alcohol Seeking: Neural Bases and Possible Relations to Alcohol Use Disorders. Alcohol Clin Exp Res (2016). 10.1111/acer.13094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostlund S. B. & Balleine B. W. On habits and addiction: An associative analysis of compulsive drug seeking. Drug Discov Today Dis Models 5, 235–245 (2008). 10.1016/j.ddmod.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zapata A., Minney V. L. & Shippenberg T. S. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci 30, 15457–15463 (2010). 10.1523/JNEUROSCI.4072-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogarth L. & Chase H. W. Parallel goal-directed and habitual control of human drug-seeking: Implications for dependence vulnerability. J Exp Psychol Anim Behav Process (2011). [DOI] [PubMed] [Google Scholar]
- 18.Belin D., Belin-Rauscent A., Murray J. E. & Everitt B. J. Addiction: failure of control over maladaptive incentive habits. Curr Opin Neurobiol (2013). 10.1016/j.conb.2013.01.025 [DOI] [PubMed] [Google Scholar]
- 19.Hogarth L., Balleine B. W., Corbit L. H. & Killcross S. Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Ann N Y Acad Sci 1282, 12–24 (2013). 10.1111/j.1749-6632.2012.06768.x [DOI] [PubMed] [Google Scholar]
- 20.Leblanc K. H., Maidment N. T. & Ostlund S. B. Repeated cocaine exposure facilitates the expression of incentive motivation and induces habitual control in rats. PLoS One 8, e61355 (2013). 10.1371/journal.pone.0061355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furlong T. M., Jayaweera H. K., Balleine B. W. & Corbit L. H. Binge-like consumption of a palatable food accelerates habitual control of behavior and is dependent on activation of the dorsolateral striatum. J Neurosci 34, 5012–5022 (2014). 10.1523/JNEUROSCI.3707-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Renteria R., Baltz E. T. & Gremel C. M. Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat Commun 9, 211 (2018). 10.1038/s41467-017-02615-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogarth L., Attwood A. S., Bate H. A. & Munafò M. R. Acute alcohol impairs human goal-directed action. Biol Psychol 90, 154–160 (2012). 10.1016/j.biopsycho.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 24.Ray L. A. et al. Capturing habitualness of drinking and smoking behavior in humans. Drug Alcohol Depend 207, 107738 (2020). 10.1016/j.drugalcdep.2019.107738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groman S. M., Massi B., Mathias S. R., Lee D. & Taylor J. R. Model-Free and Model-Based Influences in Addiction-Related Behaviors. Biol Psychiatry 85, 936–945 (2019). 10.1016/j.biopsych.2018.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillan C. M. et al. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry 168, 718–726 (2011). 10.1176/appi.ajp.2011.10071062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillan C. M. et al. Enhanced avoidance habits in obsessive-compulsive disorder. Biol Psychiatry 75, 631–638 (2014). 10.1016/j.biopsych.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaghi M. M. et al. Action-Outcome Knowledge Dissociates From Behavior in Obsessive-Compulsive Disorder Following Contingency Degradation. Biol Psychiatry Cogn Neurosci Neuroimaging 4, 200–209 (2019). 10.1016/j.bpsc.2018.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horstmann A. et al. Slave to habit? Obesity is associated with decreased behavioural sensitivity to reward devaluation. Appetite 87, 175–183 (2015). 10.1016/j.appet.2014.12.212 [DOI] [PubMed] [Google Scholar]
- 30.Morris R. W., Cyrzon C., Green M. J., Le Pelley M. E. & Balleine B. W. Impairments in action-outcome learning in schizophrenia. Transl Psychiatry 8, 54 (2018). 10.1038/s41398-018-0103-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffiths K. R., Morris R. W. & Balleine B. W. Translational studies of goal-directed action as a framework for classifying deficits across psychiatric disorders. Front Syst Neurosci 8, 101 (2014). 10.3389/fnsys.2014.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris R. W., Quail S., Griffiths K. R., Green M. J. & Balleine B. W. Corticostriatal control of goal-directed action is impaired in schizophrenia. Biol Psychiatry 77, 187–195 (2015). 10.1016/j.biopsych.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 33.Byrne K. A., Six S. G. & Willis H. C. Examining the effect of depressive symptoms on habit formation and habit-breaking. J Behav Ther Exp Psychiatry 73, 101676 (2021). 10.1016/j.jbtep.2021.101676 [DOI] [PubMed] [Google Scholar]
- 34.Alvares G. A., Balleine B. W. & Guastella A. J. Impairments in goal-directed actions predict treatment response to cognitive-behavioral therapy in social anxiety disorder. PLoS One 9, e94778 (2014). 10.1371/journal.pone.0094778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvares G. A., Balleine B. W., Whittle L. & Guastella A. J. Reduced goal-directed action control in autism spectrum disorder. Autism Res (2016). 10.1002/aur.1613 [DOI] [PubMed] [Google Scholar]
- 36.Soares J. M. et al. Stress-induced changes in human decision-making are reversible. Transl Psychiatry 2, e131 (2012). 10.1038/tp.2012.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwabe L. & Wolf O. T. Stress-induced modulation of instrumental behavior: from goal-directed to habitual control of action. Behav Brain Res 219, 321–328 (2011). 10.1016/j.bbr.2010.12.038 [DOI] [PubMed] [Google Scholar]
- 38.Schwabe L. & Wolf O. T. Stress prompts habit behavior in humans. J Neurosci 29, 7191–7198 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwabe L., Dickinson A. & Wolf O. T. Stress, habits, and drug addiction: a psychoneuroendocrinological perspective. Exp Clin Psychopharmacol 19, 53–63 (2011). 10.1037/a0022212 [DOI] [PubMed] [Google Scholar]
- 40.Fournier M., d’Arripe-Longueville F. & Radel R. Effects of psychosocial stress on the goal-directed and habit memory systems during learning and later execution. Psychoneuroendocrinology 77, 275–283 (2017). 10.1016/j.psyneuen.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 41.Pool E. R. et al. Determining the effects of training duration on the behavioral expression of habitual control in humans: a multilaboratory investigation. Learn Mem 29, 16–28 (2022). 10.1101/lm.053413.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedel E. et al. How Accumulated Real Life Stress Experience and Cognitive Speed Interact on Decision-Making Processes. Front Hum Neurosci 11, 302 (2017). 10.3389/fnhum.2017.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meier J. K., Staresina B. P. & Schwabe L. Stress diminishes outcome but enhances response representations during instrumental learning. Elife 11 (2022). 10.7554/eLife.67517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quaedflieg C. W. E. M., Stoffregen H., Sebalo I. & Smeets T. Stress-induced impairment in goal-directed instrumental behaviour is moderated by baseline working memory. Neurobiol Learn Mem 158, 42–49 (2019). 10.1016/j.nlm.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 45.Kalia V. & Knauft K. Emotion regulation strategies modulate the effect of adverse childhood experiences on perceived chronic stress with implications for cognitive flexibility. PLoS One 15, e0235412 (2020). 10.1371/journal.pone.0235412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon A. L., Patterson T. K. & Knowlton B. J. Early-life stress is associated with a preponderance of habitual responding in a novel instrumental avoidance learning paradigm. Neurobiol Learn Mem 175, 107316 (2020). 10.1016/j.nlm.2020.107316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patterson T. K., Craske M. G. & Knowlton B. J. Enhanced Avoidance Habits in Relation to History of Early-Life Stress. Front Psychol 10, 1876 (2019). 10.3389/fpsyg.2019.01876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leong K. C. & Packard M. G. Exposure to predator odor influences the relative use of multiple memory systems: role of basolateral amygdala. Neurobiol Learn Mem 109, 56–61 (2014). 10.1016/j.nlm.2013.11.015 [DOI] [PubMed] [Google Scholar]
- 49.Schwabe L., Dalm S., Schächinger H. & Oitzl M. S. Chronic stress modulates the use of spatial and stimulus-response learning strategies in mice and man. Neurobiol Learn Mem 90, 495–503 (2008). 10.1016/j.nlm.2008.07.015 [DOI] [PubMed] [Google Scholar]
- 50.Sadowski R. N., Jackson G. R., Wieczorek L. & Gold P. E. Effects of stress, corticosterone, and epinephrine administration on learning in place and response tasks. Behav Brain Res 205, 19–25 (2009). 10.1016/j.bbr.2009.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grissom E. M. et al. Learning strategy is influenced by trait anxiety and early rearing conditions in prepubertal male, but not prepubertal female rats. Neurobiol Learn Mem 98, 174–181 (2012). 10.1016/j.nlm.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 52.Goodman J. & McIntyre C. K. Impaired Spatial Memory and Enhanced Habit Memory in a Rat Model of Post-traumatic Stress Disorder. Front Pharmacol 8, 663 (2017). 10.3389/fphar.2017.00663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lormant F. et al. Training level reveals a dynamic dialogue between stress and memory systems in birds. Behav Brain Res 408, 113280 (2021). 10.1016/j.bbr.2021.113280 [DOI] [PubMed] [Google Scholar]
- 54.Packard M. G. & Gabriele A. Peripheral anxiogenic drug injections differentially affect cognitive and habit memory: role of basolateral amygdala. Neuroscience 164, 457–462 (2009). 10.1016/j.neuroscience.2009.07.054 [DOI] [PubMed] [Google Scholar]
- 55.Packard M. G. & Wingard J. C. Amygdala and “emotional” modulation of the relative use of multiple memory systems. Neurobiol Learn Mem 82, 243–252 (2004). 10.1016/j.nlm.2004.06.008 [DOI] [PubMed] [Google Scholar]
- 56.Gourley S. L. et al. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc Natl Acad Sci U S A 109, 20714–20719 (2012). 10.1073/pnas.1208342109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dieterich A. et al. Chronic corticosterone administration induces negative valence and impairs positive valence behaviors in mice. Transl Psychiatry 9, 337 (2019). 10.1038/s41398-019-0674-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alizamini M. M. et al. Corticotropin-releasing factor receptor 1 in infralimbic cortex modulates social stress-altered decision-making. Prog Neuropsychopharmacol Biol Psychiatry 116, 110523 (2022). 10.1016/j.pnpbp.2022.110523 [DOI] [PubMed] [Google Scholar]
- 59.Hinton E. A., Li D. C., Allen A. G. & Gourley S. L. Social Isolation in Adolescence Disrupts Cortical Development and Goal-Dependent Decision-Making in Adulthood, Despite Social Reintegration. eNeuro 6 (2019). 10.1523/ENEURO.0318-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dias-Ferreira E. et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science 325, 621–625 (2009). 10.1126/science.1171203 [DOI] [PubMed] [Google Scholar]
- 61.Koob G. F. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 27, 232–243 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Agid O., Kohn Y. & Lerer B. Environmental stress and psychiatric illness. Biomed Pharmacother 54, 135–141 (2000). 10.1016/S0753-3322(00)89046-0 [DOI] [PubMed] [Google Scholar]
- 63.Baumeister D., Lightman S. L. & Pariante C. M. The Interface of Stress and the HPA Axis in Behavioural Phenotypes of Mental Illness. Curr Top Behav Neurosci 18, 13–24 (2014). 10.1007/7854_2014_304 [DOI] [PubMed] [Google Scholar]
- 64.María-Ríos C. E. & Morrow J. D. Mechanisms of Shared Vulnerability to Post-traumatic Stress Disorder and Substance Use Disorders. Front Behav Neurosci 14, 6 (2020). 10.3389/fnbeh.2020.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brady K. T. & Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry 162, 1483–1493 (2005). 10.1176/appi.ajp.162.8.1483 [DOI] [PubMed] [Google Scholar]
- 66.Duffing T. M., Greiner S. G., Mathias C. W. & Dougherty D. M. Stress, substance abuse, and addiction. Curr Top Behav Neurosci 18, 237–263 (2014). 10.1007/7854_2014_276 [DOI] [PubMed] [Google Scholar]
- 67.Malvaez M. Neural substrates of habit. J Neurosci Res (2019). 10.1002/jnr.24552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balleine B. W. & O’Doherty J. P. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35, 48–69 (2010). 10.1038/npp.2009.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balleine B. W., Delgado M. R. & Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci 27, 8161–8165 (2007). 10.1523/JNEUROSCI.1554-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yin H. H., Knowlton B. J. & Balleine B. W. Blockade of NMDA receptors in the dorsomedial striatum prevents action-outcome learning in instrumental conditioning. Eur J Neurosci 22, 505–512 (2005). [DOI] [PubMed] [Google Scholar]
- 71.Corbit L. H. & Janak P. H. Posterior dorsomedial striatum is critical for both selective instrumental and Pavlovian reward learning. Eur J Neurosci 31, 1312–1321 (2010). 10.1111/j.1460-9568.2010.07153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lex B. & Hauber W. The role of dopamine in the prelimbic cortex and the dorsomedial striatum in instrumental conditioning. Cereb Cortex 20, 873–883 (2010). 10.1093/cercor/bhp151 [DOI] [PubMed] [Google Scholar]
- 73.Liljeholm M., Tricomi E., O’Doherty J. P. & Balleine B. W. Neural correlates of instrumental contingency learning: differential effects of action-reward conjunction and disjunction. J Neurosci 31, 2474–2480 (2011). 10.1523/JNEUROSCI.3354-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McNamee D., Liljeholm M., Zika O. & O’Doherty J. P. Characterizing the associative content of brain structures involved in habitual and goal-directed actions in humans: a multivariate FMRI study. J Neurosci 35, 3764–3771 (2015). 10.1523/JNEUROSCI.4677-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Hare J., Calakos N. & Yin H. H. Recent Insights into Corticostriatal Circuit Mechanisms underlying Habits: Invited review for Current Opinions in Behavioral Sciences. Curr Opin Behav Sci 20, 40–46 (2018). 10.1016/j.cobeha.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yin H. H., Ostlund S. B., Knowlton B. J. & Balleine B. W. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci 22, 513–523 (2005). [DOI] [PubMed] [Google Scholar]
- 77.Balleine B. W., Killcross A. S. & Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci 23, 666–675 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ostlund S. B. & Balleine B. W. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J Neurosci 28, 4398–4405 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lingawi N. W. & Balleine B. W. Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits. J Neurosci 32, 1073–1081 (2012). 10.1523/JNEUROSCI.4806-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pan W. X., Mao T. & Dudman J. T. Inputs to the dorsal striatum of the mouse reflect the parallel circuit architecture of the forebrain. Front Neuroanat 4, 147 (2010). 10.3389/fnana.2010.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wall N. R., De La Parra M., Callaway E. M. & Kreitzer A. C. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron 79, 347–360 (2013). 10.1016/j.neuron.2013.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corbit L. H., Leung B. K. & Balleine B. W. The role of the amygdala-striatal pathway in the acquisition and performance of goal-directed instrumental actions. J Neurosci 33, 17682–17690 (2013). 10.1523/JNEUROSCI.3271-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelley A. E., Domesick V. B. & Nauta W. J. The amygdalostriatal projection in the rat--an anatomical study by anterograde and retrograde tracing methods. Neuroscience 7, 615–630 (1982). [DOI] [PubMed] [Google Scholar]
- 84.Kita H. & Kitai S. T. Amygdaloid projections to the frontal cortex and the striatum in the rat. J Comp Neurol 298, 40–49 (1990). 10.1002/cne.902980104 [DOI] [PubMed] [Google Scholar]
- 85.McDonald A. J. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience 44, 15–33 (1991). [DOI] [PubMed] [Google Scholar]
- 86.McDonald A. J. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience 44, 1–14 (1991). [DOI] [PubMed] [Google Scholar]
- 87.Swanson L. W. & Petrovich G. D. What is the amygdala? Trends Neurosci 21, 323–331 (1998). [DOI] [PubMed] [Google Scholar]
- 88.Ehrlich I. et al. Amygdala inhibitory circuits and the control of fear memory. Neuron 62, 757–771 (2009). 10.1016/j.neuron.2009.05.026 [DOI] [PubMed] [Google Scholar]
- 89.Gilpin N. W., Herman M. A. & Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry 77, 859–869 (2015). 10.1016/j.biopsych.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moscarello J. M. & Penzo M. A. The central nucleus of the amygdala and the construction of defensive modes across the threat-imminence continuum. Nat Neurosci 25, 999–1008 (2022). 10.1038/s41593-022-01130-5 [DOI] [PubMed] [Google Scholar]
- 91.Roozendaal B., McEwen B. S. & Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci 10, 423–433 (2009). 10.1038/nrn2651 [DOI] [PubMed] [Google Scholar]
- 92.Zhang W. H., Zhang J. Y., Holmes A. & Pan B. X. Amygdala Circuit Substrates for Stress Adaptation and Adversity. Biol Psychiatry 89, 847–856 (2021). 10.1016/j.biopsych.2020.12.026 [DOI] [PubMed] [Google Scholar]
- 93.Wassum K. M., Cely I. C., Maidment N. T. & Balleine B. W. Disruption of endogenous opioid activity during instrumental learning enhances habit acquisition. Neuroscience 163, 770–780 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hilário M. R., Clouse E., Yin H. H. & Costa R. M. Endocannabinoid signaling is critical for habit formation. Front Integr Neurosci 1, 6 (2007). 10.3389/neuro.07.006.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dickinson A. D., Nicholas J. & Adams C. D. The effect of the instrumental training contingency on susceptibility to reinforcer devaluation. Quarterly Journal of Experimental Psychology 35.1, 35–51 (1983). [Google Scholar]
- 96.Garr E., Padovan-Hernandez Y., Janak P. H. & Delamater A. R. Maintained goal-directed control with overtraining on ratio schedules. Learn Mem 28, 435–439 (2021). 10.1101/lm.053472.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Malvaez M. et al. Habits Are Negatively Regulated by Histone Deacetylase 3 in the Dorsal Striatum. Biol Psychiatry (2018). 10.1016/j.biopsych.2018.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dickinson A. & Balleine B. W. (1994).
- 99.Robbins T. W. & Costa R. M. Habits. Curr Biol 27, R1200–R1206 (2017). 10.1016/j.cub.2017.09.060 [DOI] [PubMed] [Google Scholar]
- 100.Adams C. D. & Dickinson A. Instrumental responding following reinforcer devaluation. The Quarterly Journal of Experimental Psychology 33, 109–121 (1981). [Google Scholar]
- 101.Alexander G. M. et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–39 (2009). 10.1016/j.neuron.2009.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vazey E. M. & Aston-Jones G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci U S A 111, 3859–3864 (2014). 10.1073/pnas.1310025111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qiu M. H., Chen M. C., Fuller P. M. & Lu J. Stimulation of the Pontine Parabrachial Nucleus Promotes Wakefulness via Extra-thalamic Forebrain Circuit Nodes. Curr Biol 26, 2301–2312 (2016). 10.1016/j.cub.2016.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu H. et al. Cre-dependent DREADD (Designer Receptors Exclusively Activated by Designer Drugs) mice. Genesis 54, 439–446 (2016). 10.1002/dvg.22949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pomrenze M. B. et al. A Transgenic Rat for Investigating the Anatomy and Function of Corticotrophin Releasing Factor Circuits. Front Neurosci 9, 487 (2015). 10.3389/fnins.2015.00487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tipps M., Marron Fernandez de Velasco E., Schaeffer A. & Wickman K. Inhibition of Pyramidal Neurons in the Basal Amygdala Promotes Fear Learning. eNeuro 5 (2018). 10.1523/ENEURO.0272-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tuscher J. J., Taxier L. R., Fortress A. M. & Frick K. M. Chemogenetic inactivation of the dorsal hippocampus and medial prefrontal cortex, individually and concurrently, impairs object recognition and spatial memory consolidation in female mice. Neurobiol Learn Mem 156, 103–116 (2018). 10.1016/j.nlm.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fisher S. D., Ferguson L. A., Bertran-Gonzalez J. & Balleine B. W. Amygdala-Cortical Control of Striatal Plasticity Drives the Acquisition of Goal-Directed Action. Curr Biol (2020). 10.1016/j.cub.2020.08.090 [DOI] [PubMed] [Google Scholar]
- 109.Ugolini A., Sokal D. M., Arban R. & Large C. H. CRF1 receptor activation increases the response of neurons in the basolateral nucleus of the amygdala to afferent stimulation. Front Behav Neurosci 2, 2 (2008). 10.3389/neuro.08.002.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Z. P. et al. Chronic stress impairs GABAergic control of amygdala through suppressing the tonic GABAA receptor currents. Mol Brain 7, 32 (2014). 10.1186/1756-6606-7-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosenkranz J. A., Venheim E. R. & Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry 67, 1128–1136 (2010). 10.1016/j.biopsych.2010.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hetzel A. & Rosenkranz J. A. Distinct effects of repeated restraint stress on basolateral amygdala neuronal membrane properties in resilient adolescent and adult rats. Neuropsychopharmacology 39, 2114–2130 (2014). 10.1038/npp.2014.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rau A. R., Chappell A. M., Butler T. R., Ariwodola O. J. & Weiner J. L. Increased Basolateral Amygdala Pyramidal Cell Excitability May Contribute to the Anxiogenic Phenotype Induced by Chronic Early-Life Stress. J Neurosci 35, 9730–9740 (2015). 10.1523/JNEUROSCI.0384-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sharp B. M. Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction. Transl Psychiatry 7, e1194 (2017). 10.1038/tp.2017.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Masneuf S. et al. Glutamatergic mechanisms associated with stress-induced amygdala excitability and anxiety-related behavior. Neuropharmacology 85, 190–197 (2014). 10.1016/j.neuropharm.2014.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lowery-Gionta E. G. et al. Chronic stress dysregulates amygdalar output to the prefrontal cortex. Neuropharmacology 139, 68–75 (2018). 10.1016/j.neuropharm.2018.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Blume S. R., Padival M., Urban J. H. & Rosenkranz J. A. Disruptive effects of repeated stress on basolateral amygdala neurons and fear behavior across the estrous cycle in rats. Sci Rep 9, 12292 (2019). 10.1038/s41598-019-48683-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McDonald A. J. Projection neurons of the basolateral amygdala: a correlative Golgi and retrograde tract tracing study. Brain Res Bull 28, 179–185 (1992). [DOI] [PubMed] [Google Scholar]
- 119.Hunnicutt B. J. et al. A comprehensive excitatory input map of the striatum reveals novel functional organization. Elife 5 (2016). 10.7554/eLife.19103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mulder A. B., Hodenpijl M. G. & Lopes da Silva F. H. Electrophysiology of the hippocampal and amygdaloid projections to the nucleus accumbens of the rat: convergence, segregation, and interaction of inputs. J Neurosci 18, 5095–5102 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shan Q., Ge M., Christie M. J. & Balleine B. W. The acquisition of goal-directed actions generates opposing plasticity in direct and indirect pathways in dorsomedial striatum. J Neurosci 34, 9196–9201 (2014). 10.1523/JNEUROSCI.0313-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peak J., Chieng B., Hart G. & Balleine B. W. Striatal direct and indirect pathway neurons differentially control the encoding and updating of goal-directed learning. Elife 9 (2020). 10.7554/eLife.58544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gremel C. M. & Costa R. M. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun 4, 2264 (2013). 10.1038/ncomms3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vandaele Y., Ottenheimer D. J. & Janak P. H. Dorsomedial Striatal Activity Tracks Completion of Behavioral Sequences in Rats. eNeuro 8 (2021). 10.1523/ENEURO.0279-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lobo M. K. & Nestler E. J. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat 5, 41 (2011). 10.3389/fnana.2011.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Partridge J. G. et al. Stress increases GABAergic neurotransmission in CRF neurons of the central amygdala and bed nucleus stria terminalis. Neuropharmacology 107, 239–250 (2016). 10.1016/j.neuropharm.2016.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hoffman A. N., Lorson N. G., Sanabria F., Foster Olive M. & Conrad C. D. Chronic stress disrupts fear extinction and enhances amygdala and hippocampal Fos expression in an animal model of post-traumatic stress disorder. Neurobiol Learn Mem 112, 139–147 (2014). 10.1016/j.nlm.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Patel S., Cravatt B. F. & Hillard C. J. Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacology 30, 497–507 (2005). 10.1038/sj.npp.1300535 [DOI] [PubMed] [Google Scholar]
- 129.He F., Ai H., Wang M., Wang X. & Geng X. Altered Neuronal Activity in the Central Nucleus of the Amygdala Induced by Restraint Water-Immersion Stress in Rats. Neurosci Bull 34, 1067–1076 (2018). 10.1007/s12264-018-0282-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Giovanniello J. et al. A Central Amygdala-Globus Pallidus Circuit Conveys Unconditioned Stimulus-Related Information and Controls Fear Learning. J Neurosci 40, 9043–9054 (2020). 10.1523/JNEUROSCI.2090-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang T. et al. Plastic and stimulus-specific coding of salient events in the central amygdala. Nature 616, 510–519 (2023). 10.1038/s41586-023-05910-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Y. et al. Multimodal mapping of cell types and projections in the central nucleus of the amygdala. Elife 12 (2023). 10.7554/eLife.84262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu J. et al. Differential efferent projections of GABAergic neurons in the basolateral and central nucleus of amygdala in mice. Neurosci Lett 745, 135621 (2021). 10.1016/j.neulet.2020.135621 [DOI] [PubMed] [Google Scholar]
- 134.Sah P., Faber E. S., Lopez De Armentia M. & Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev 83, 803–834 (2003). 10.1152/physrev.00002.2003 [DOI] [PubMed] [Google Scholar]
- 135.Lee H. J., Gallagher M. & Holland P. C. The central amygdala projection to the substantia nigra reflects prediction error information in appetitive conditioning. Learn Mem 17, 531–538 (2010). 10.1101/lm.1889510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kong M. S. & Zweifel L. S. Central amygdala circuits in valence and salience processing. Behav Brain Res 410, 113355 (2021). 10.1016/j.bbr.2021.113355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Murray J. E. et al. Basolateral and central amygdala differentially recruit and maintain dorsolateral striatum-dependent cocaine-seeking habits. Nat Commun 6, 10088 (2015). 10.1038/ncomms10088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Siemsen B. M., Franco D. & Lobo M. K. Corticostriatal contributions to dysregulated motivated behaviors in stress, depression, and substance use disorders. Neurosci Res (2022). 10.1016/j.neures.2022.12.014 [DOI] [PubMed] [Google Scholar]
- 139.Balleine B. W. & Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci 29, 272–279 (2006). [DOI] [PubMed] [Google Scholar]
- 140.Namburi P. et al. A circuit mechanism for differentiating positive and negative associations. Nature 520, 675–678 (2015). 10.1038/nature14366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tye K. M. Neural Circuit Motifs in Valence Processing. Neuron 100, 436–452 (2018). 10.1016/j.neuron.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gremel C. M. et al. Endocannabinoid Modulation of Orbitostriatal Circuits Gates Habit Formation. Neuron 90, 1312–1324 (2016). 10.1016/j.neuron.2016.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Whitelaw R. B., Markou A., Robbins T. W. & Everitt B. J. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology (Berl) 127, 213–224 (1996). [PubMed] [Google Scholar]
- 144.Alderson H. L., Robbins T. W. & Everitt B. J. The effects of excitotoxic lesions of the basolateral amygdala on the acquisition of heroin-seeking behaviour in rats. Psychopharmacology (Berl) 153, 111–119 (2000). [DOI] [PubMed] [Google Scholar]
- 145.Belin-Rauscent A., Everitt B. J. & Belin D. Intrastriatal shifts mediate the transition from drug-seeking actions to habits. Biol Psychiatry 72, 343–345 (2012). 10.1016/j.biopsych.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 146.Murray J. E., Belin D. & Everitt B. J. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology 37, 2456–2466 (2012). 10.1038/npp.2012.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Roberts A. J., Cole M. & Koob G. F. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res 20, 1289–1298 (1996). [DOI] [PubMed] [Google Scholar]
- 148.Corbit L. H., Nie H. & Janak P. H. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol Psychiatry 72, 389–395 (2012). 10.1016/j.biopsych.2012.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wendler E. et al. The roles of the nucleus accumbens core, dorsomedial striatum, and dorsolateral striatum in learning: performance and extinction of Pavlovian fear-conditioned responses and instrumental avoidance responses. Neurobiol Learn Mem 109, 27–36 (2014). 10.1016/j.nlm.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 150.Weera M. M., Schreiber A. L., Avegno E. M. & Gilpin N. W. The role of central amygdala corticotropin-releasing factor in predator odor stress-induced avoidance behavior and escalated alcohol drinking in rats. Neuropharmacology 166, 107979 (2020). 10.1016/j.neuropharm.2020.107979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Marti-Prats L. et al. Baclofen decreases compulsive alcohol drinking in rats characterized by reduced levels of GAT-3 in the central amygdala. Addict Biol 26, e13011 (2021). 10.1111/adb.13011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Franklin K. B. J. & Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3rd Edition edn, (Elsevier, 2008). [Google Scholar]
- 153.Tye K. M. et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541 (2013). 10.1038/nature11740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cerniauskas I. et al. Chronic Stress Induces Activity, Synaptic, and Transcriptional Remodeling of the Lateral Habenula Associated with Deficits in Motivated Behaviors. Neuron 104, 899–915.e898 (2019). 10.1016/j.neuron.2019.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Monteiro S. et al. An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front Psychiatry 6, 6 (2015). 10.3389/fpsyt.2015.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bavley C. C., Fischer D. K., Rizzo B. K. & Rajadhyaksha A. M. Ca. Neurobiol Stress 7, 27–37 (2017). 10.1016/j.ynstr.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Freymann J., Tsai P. P., Stelzer H. D., Mischke R. & Hackbarth H. Impact of bedding volume on physiological and behavioural parameters in laboratory mice. Lab Anim 51, 601–612 (2017). 10.1177/0023677217694400 [DOI] [PubMed] [Google Scholar]
- 158.Pałucha-Poniewiera A., Podkowa K., Rafało-Ulińska A., Brański P. & Burnat G. The influence of the duration of chronic unpredictable mild stress on the behavioural responses of C57BL/6J mice. Behav Pharmacol 31, 574–582 (2020). 10.1097/FBP.0000000000000564 [DOI] [PubMed] [Google Scholar]
- 159.La-Vu M. Q. et al. Sparse genetically defined neurons refine the canonical role of periaqueductal gray columnar organization. Elife 11 (2022). 10.7554/eLife.77115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Reis F. M. et al. Dorsal periaqueductal gray ensembles represent approach and avoidance states. Elife 10 (2021). 10.7554/eLife.64934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lichtenberg N. T. et al. The Medial Orbitofrontal Cortex-Basolateral Amygdala Circuit Regulates the Influence of Reward Cues on Adaptive Behavior and Choice. J Neurosci 41, 7267–7277 (2021). 10.1523/JNEUROSCI.0901-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sias A. et al. A bidirectional corticoamygdala circuit for the encoding and retrieval of detailed reward memories. eLife 10 (2021). 10.7554/eLife.68617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sherathiya V. N., Schaid M. D., Seiler J. L., Lopez G. C. & Lerner T. N. GuPPy, a Python toolbox for the analysis of fiber photometry data. Sci Rep 11, 24212 (2021). 10.1038/s41598-021-03626-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Roth B. L. DREADDs for Neuroscientists. Neuron 89, 683–694 (2016). 10.1016/j.neuron.2016.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pomrenze M. B. et al. A Corticotropin Releasing Factor Network in the Extended Amygdala for Anxiety. J Neurosci 39, 1030–1043 (2019). 10.1523/JNEUROSCI.2143-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Malvaez M., Shieh C., Murphy M. D., Greenfield V. Y. & Wassum K. M. Distinct cortical–amygdala projections drive reward value encoding and retrieval. Nature Neuroscience (2019). 10.1038/s41593-019-0374-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Collins A. L. et al. Nucleus Accumbens Cholinergic Interneurons Oppose Cue-Motivated Behavior. Biol Psychiatry (2019). 10.1016/j.biopsych.2019.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lichtenberg N. T. et al. The medial orbitofrontal cortex - basolateral amygdala circuit regulates the influence of reward cues on adaptive behavior and choice. J Neurosci (2021). 10.1523/JNEUROSCI.0901-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schmider E., Ziegler M., Danay E., Beyer L. & Bühner M. Is it really robust? Reinvestigating the robustness of ANOVA against violations of the normal distribution assumption. Methodology : European journal of research methods for the behavioral & social sciences 6, 147–151 (2010). [Google Scholar]
- 170.Knief U. & Forstmeier W. Violating the normality assumption may be the lesser of two evils. Behav Res Methods 53, 2576–2590 (2021). 10.3758/s13428-021-01587-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Malvaez M. et al. Habits are negatively regulated by HDAC3 in the dorsal striatum. bioRxiv (2017). 10.1101/153734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wassum K. et al. (Dryad, 2021).
- 173.Mineur Y. S., Belzung C. & Crusio W. E. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res 175, 43–50 (2006). 10.1016/j.bbr.2006.07.029 [DOI] [PubMed] [Google Scholar]
- 174.Fang X. et al. Chronic unpredictable stress induces depression-related behaviors by suppressing AgRP neuron activity. Mol Psychiatry 26, 2299–2315 (2021). 10.1038/s41380-020-01004-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gong S. et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci 27, 9817–9823 (2007). 10.1523/JNEUROSCI.2707-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Valjent E., Bertran-Gonzalez J., Hervé D., Fisone G. & Girault J. A. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci 32, 538–547 (2009). 10.1016/j.tins.2009.06.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.