Abstract
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has led to significant global morbidity and mortality. A crucial viral protein, the non-structural protein 14 (nsp14), catalyzes the methylation of viral RNA and plays a critical role in viral genome replication and transcription. Due to the low mutation rate in the nsp region among various SARS-CoV-2 variants, nsp14 has emerged as a promising therapeutic target. However, discovering potential inhibitors remains a challenge. In this work, we introduce a computational pipeline for the rapid and efficient identification of potential nsp14 inhibitors by leveraging virtual screening and the NCI open compound collection, which contains 250,000 freely available molecules for researchers worldwide. The introduced pipeline provides a cost-effective and efficient approach for early-stage drug discovery by allowing researchers to evaluate promising molecules without incurring synthesis expenses. Our pipeline successfully identified seven promising candidates after experimentally validating only 40 compounds. Notably, we discovered NSC620333, a compound that exhibits a strong binding affinity to nsp14 with a dissociation constant of 427 ± 84 nM. In addition, we gained new insights into the structure and function of this protein through molecular dynamics simulations. We identified new conformational states of the protein and determined that residues Phe367, Tyr368, and Gln354 within the binding pocket serve as stabilizing residues for novel ligand interactions. We also found that metal coordination complexes are crucial for the overall function of the binding pocket. Lastly, we present the solved crystal structure of the nsp14-MTase complexed with SS148 (PDB:8BWU), a potent inhibitor of methyltransferase activity at the nanomolar level (IC50 value of 70 ± 6 nM). Our computational pipeline accurately predicted the binding pose of SS148, demonstrating its effectiveness and potential in accelerating drug discovery efforts against SARS-CoV-2 and other emerging viruses.
I. INTRODUCTION
The COVID-19 pandemic, caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has had a profound impact on global health, economies, and daily life. SARS-CoV-2 is a single-stranded, positive-sense RNA virus belonging to lineage B of the genus Beta-coronavirus in the Coronaviridae family [1]. Since its emergence in December 2019, it has led to a staggering number of infections and deaths worldwide, overwhelming healthcare systems and prompting unprecedented public health measures [2]. While the development of vaccines has been a significant step in combating the virus, the continued emergence of new variants highlights the ongoing need for effective antiviral treatments. Such treatments can play a crucial role in reducing the severity and impact of infections and informing future pandemic policy decisions [3]. The SARS-CoV-2 genome is notably large, containing nearly 30,000 base pairs and encoding 16 nonstructural proteins (nsps) that play vital roles in viral replication and transcription [4]. Because of this, nsps represent potential targets for therapeutics, and the availability of their structural data has facilitated the rapid development of antivirals [5]. One such target is non-structural protein 14 (nsp14), which has dual functions as an exonuclease (ExoN) and a methyltransferase (MTase). The ExoN function ensures replication fidelity through 3’–5’ proofreading, while the MTase function catalyzes N7-methylation of the guanosine triphosphate cap of the viral RNA, which is crucial for its stability and function [6, 7]. Due to its low mutation rate among different SARS-CoV-2 variants when compared to other viral proteins such as the spike protein [8], nsp14 is hypothesized to be essential for the function of the virus, and therefore it has emerged as a promising therapeutic target. This persistence increases the likelihood that inhibitors targeting nsp14 will remain effective against a broader range of evolving viral strains. Additionally, the amino acid sequence of SARS-CoV-2 nsp14 shows high homology with nsp14 from other coronaviruses, including SARS-CoV, MERS-CoV, and common human coronaviruses such as OC43, HKU1, 229E, and NL63 [8]. This sequence conservation suggests that inhibitors targeting nsp14 in SARS-CoV-2 could potentially also inhibit nsp14 MTase activity in other coronaviruses, which may have implications for the development of broad-spectrum antiviral drugs [9].
In this study, we developed a computational pipeline for rapid and efficient identification of potential nsp14 inhibitors. By leveraging virtual screening at various levels of accuracy and the NCI open compound collection [10, 11]—a database comprising 250,000 freely accessible molecules for researchers globally, we evaluated promising molecules without the expense and time typically associated with synthesis. This cost-effective and efficient strategy proves particularly valuable for early-stage hit identification, in cases where resources are constrained and time is of the essence. We utilized molecular docking [12, 13] and Molecular Mechanics Generalized Born Surface Area (MM/GBSA) calculations [14, 15] to gauge the strength of interaction between potential inhibitors and nsp14. Through these computational methodologies, we successfully identified a novel inhibitor for nsp14, with activity in the micromolar range, labelled NSC620333 (IC50 value of 5.3 ± 1.0 µM ), which was confirmed by orthogonal in vitro and in vivo assays. This inhibitor was unearthed through computational screening of the NCI open compound collection, with only the top 40 compounds undergoing experimental testing, highlighting the efficiency of our pipeline. Furthermore, to deepen our understanding of the protein structure and function, we conducted molecular dynamics (MD) simulations [16–18] exceeding 5 microseconds. These simulations unveiled previously unknown conformational states of nsp14, offering valuable insights into its overall function. Within the MTase lateral cavity, we identified flexible residues—specifically Phe367, Tyr368, and Gln354—that form stabilizing interactions with potential ligands. Our simulations also revealed that the two alpha-helices of the MTase domain undergo a significant shift/bend, corresponding to the opening and closing of the lateral cavity. Importantly, these conformational shifts were not observed in the absence of metal coordination complexes, emphasizing their crucial role in stabilizing interactions. Our MD simulations also shed light on the interaction of nsp14 with nsp10, a key process in viral replication and transcription. Nsp10 has been demonstrated to stimulate the ExoN and MTase activities of nsp14, making it a critical cofactor for the functionality of the protein [19]. In summary, our research showcases an advanced computational pipeline, built upon the robust capabilities of VirtualFlow 2.0 [20], specifically the VirtualFlow Unity (VFU) module. This component not only accommodates a wide spectrum of docking programs, callable through a Python pipeline, but also allows scoring at diverse levels of precision, thereby facilitating the rapid and proficient detection of prospective nsp14 inhibitors. This is achieved by integrating virtual screening strategies and harnessing the expansive NCI open compound collection. Through this methodology, we discovered NSC620333, a compound exhibiting strong binding affinity to nsp14, and gained fresh insights into the structure and function of the protein. Furthermore, we report the crystal structure of SS148, a potent inhibitor (IC50 value of 70 ± 6 nM) of methyltransferase activity, in complex with the nsp14-MTase domain [21, 22]. Our pipeline accurately predicted the binding pose of SS148, demonstrating its efficacy and potential for expediting drug discovery efforts against SARS-CoV-2 and other emerging viruses. By targeting the nsp14 protein, we aspire to contribute to the development of effective antiviral therapies against SARS-CoV-2 and its variants, and other coronaviruses as well. Our computational pipeline offers a cost-effective and efficient strategy for early-stage drug discovery, potentially providing significant assistance to researchers battling the rapidly mutating virus responsible for COVID-19 and aiding in the response to future disease outbreaks.
II. RESULTS
A. Employing Molecular Docking for identifying nsp14 inhibitors
The widespread use of structure-based drug discovery techniques has proven instrumental in the search for small molecules that can specifically target macromolecules. [23] The efficacy of molecular docking and free energy simulation methods is particularly noteworthy in assessing the binding strength between proteins and ligands. [24] These methods enabled us to identify and prioritize the most promising candidates, thereby facilitating subsequent experimental testing. Our aim was to identify inhibitors of the SARS-CoV-2 nsp14 MTase activity. To achieve this, we performed a computational screening of the Developmental Therapeutics Program (DTP) Open Compound Collection. [10, 11] Comprising approximately 250,000 molecules synthesized and tested for potential efficacy against cancer and acquired immunodeficiency syndrome, this collection offers a considerable resource for researchers. Importantly, these molecules are readily accessible for academic researchers, with the possibility to request up to 40 samples on a monthly basis.
We employed the Glide [25] SP docking program to target the MTase binding site, as depicted in Figure I. This approach allowed us to evaluate all the molecules in the ligand library. To enhance the reliability of our findings, we rigorously examined the top 1,000 molecules—those displaying the lowest docking scores—using the Glide XP setting to increase precision. Subsequently, we selected the top 100 performing molecules for further consideration, factoring in aspects such as diversity (see Methods: Docking V A, compound availability, and docking scores. In an effort to further refine our selection and improve the accuracy of binding prediction, we conducted a Molecular Mechanics Generalized Born Surface Area (MM/GBSA) analysis [14, 15] (see Methods: MM/GBSA Calculation V B). This step assisted us in narrowing down our list to the top 40 compounds, which we subsequently obtained from the NCI. Supplementary Table S2 lists the selected compounds, along with their corresponding docking scores and Molecular Mechanics Generalized Born Surface Area (MM/GBSA) binding affinities. Notably, NSC620333 stood out, showcasing the most promising results, as demonstrated by its lowest docking and MM/GBSA scores. In Figure 2, we provide the docked poses and 2D interaction diagrams of the three well performing inhibitors. These 2D interaction diagrams highlight the fact that the majority of residues in the binding pocket are hydrophobic or non-polar. Detailed information regarding the docking procedure and protein preparation can be found in the Methods section (see Methods: Docking V A).
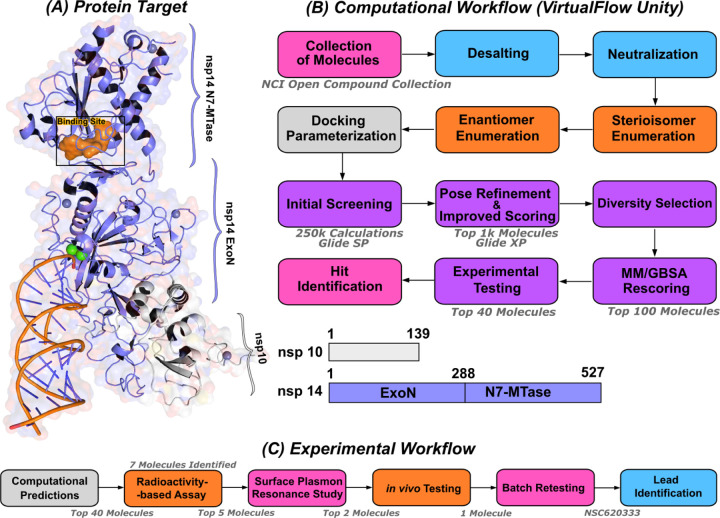
FIG. 1. Computational pipeline for identification of lead compounds.
(A) Depiction of the SARS-CoV-2 nsp14-MTase Protein Target and Computational Workflow. The protein target (PDB:7N0B), in complex with nsp10, is employed for the modeling process. The highlighted binding site (indicated in orange) within the MTase domain acts as the docking site for molecular interactions. (B) The computational workflow powered by VirtualFlow Unity (VFU), which processes SMILES strings from the NCI Open Compound Collections as input. The procedure includes desalting, neutralizing, and generating stereoisomers of the input molecule. Subsequently, all processed molecules from the database undergo docking onto the binding site using Glide-SP. The output docked compounds are ranked based on their docking scores, with the top 1,000 subjected to a more precise scoring algorithm, Glide-XP. A diverse subset of 100 molecules is chosen for an advanced binding energy estimation using Molecular Mechanics Generalized Born Surface Area (MM/GBSA). The top 40 of these compounds, ranked by their binding potential, are then selected for experimental evaluation. (C) The selected top-40 compounds are initially assessed using a radioactivity-based assay, yielding seven compounds with promising results. Subsequently, a detailed binding study is performed with the top-5 compounds utilizing surface plasmon resonance. The in-vivo evaluation follows, focusing on the top-2 compounds with SARS-CoV-2 infected Calu-3 cells. Ultimately, the compound identified as the most promising is re-tested after a custom synthesis process, leading not only to improved purity, but also confirming the identification of a lead compound.
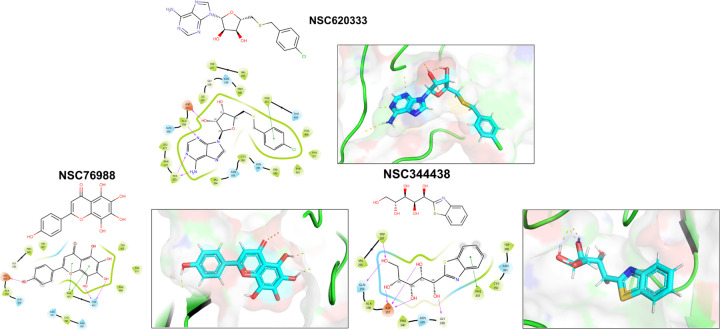
FIG. 2. Interaction Analysis of Identified MTase Inhibitors.
Presented are the 2D structural diagrams of experimentally validated compounds (top-3) found to inhibit nsp14 MTase activity. Alongside these structures, their respective 2D interaction diagrams, derived from docking studies, are displayed. The figure also depicts the putative 3D poses these compounds assume within nsp14’s lateral cavity.
B. Experimental Evaluation of Lead Compounds
In this study, we harnessed both the precision and accuracy of a state-of-the-art high-throughput radioactivity-based assay [21], to experimentally test the MTase activity of nsp14 under the influence of our selected compounds. Out of the 40 assessed compounds, seven manifested inhibitory properties (Table I). Among these, NSC76988 and NSC620333 emerged as particularly promising candidates, due to their relatively low IC50 values 6 and 5 µM, respectively, suggestive of a potent inhibitory effect. To further substantiate these findings and confirm the binding of these inhibitors to nsp14, we employed surface plasmon resonance (SPR). [26]. This technique was leveraged to investigate the binding interactions of the top four compounds from the radioactivity-based assay. Figure 2 provides detailed insight into these molecular interactions. It presents the 2D structural diagrams of the top four experimentally validated compounds that inhibit MTase activity. Alongside these are the 2D interaction diagrams derived from docking studies, and 3D poses showing how these compounds fit within the lateral cavity of nsp14. Interestingly, of these four inhibitors, NSC620333 had the lowest KD value of 544 ± 22 nM (cf. Figures3 and S1). This observation points to the potential of NSC620333 as an effective nsp14 inhibitor. Moreover, the results from batch re-testing with a purified sample of NSC620333 provide compelling reinforcement of this proposition, as depicted in Supplementary Figures S3 and S6.
TABLE I. High-throughput Screening Results of top 40 Compounds obtained for the Inhibition of nsp14 Methyltransferase (MTase) Activity.
This table summarizes the inhibitory effects of the top 40 compounds on nsp14 MTase activity as assessed by high-throughput screening. For each compound, data from two replicate experiments are included, revealing the inhibition percentages at a compound concentration of 100µM. The IC50 values, indicating the concentration at which half-maximal inhibition is achieved, are reported for the most promising compounds. The table also provides the observed quenching effects of each compound. The term ”ND” indicates that the value is not determined.
| Compound | nsp14 Inhibition % (100µM) | IC50 (µM) | Quenching effect % | |
|---|---|---|---|---|
|
| ||||
| Exp 1 | Exp 2 | |||
| NSC76988 | 100 | 99 | 6 | 8 |
| NSC620333 | 100 | 99 | 5 | 23 |
| NSC77131 | 99 | 99 | 9 | 52 |
| NSC400718 | 89 | 88 | 38 | 23 |
| NSC34443 | 86 | 81 | 57 | −7 |
| NSC630814 | 84 | 82 | ND | 75 |
| NSC102798 | 77 | 74 | ND | 61 |
| NSC4348 | 73 | 61 | 34 | 9 |
| NSC400937 | 73 | 66 | ND | 70 |
| NSC4624 | 65 | 47 | ND | 49 |
| NSC2269 | 64 | 60 | 27 | 1 |
| NSC255523 | 59 | 33 | ND | −7 |
| NSC670682 | 50 | 54 | ND | 43 |
| NSC99790 | 46 | 36 | ND | 33 |
| NSC646375 | 26 | 20 | ND | 1 |
| NSC27605 | 26 | 10 | ND | 2 |
| NSC107661 | 18 | 7 | ND | −2 |
| NSC293892 | 15 | 3 | ND | −5 |
| NSC44037 | 15 | 9 | ND | 8 |
| NSC77680 | 13 | 7 | ND | 21 |
| NSC80136 | 13 | 2 | ND | −3 |
| NSC114010 | 11 | −3 | ND | −2 |
| NSC268226 | 9 | 0 | ND | 3 |
| NSC163444 | 9 | 5 | ND | 2 |
| NSC377438 | 8 | −3 | ND | 17 |
| NSC131119 | 8 | 0 | ND | 14 |
| NSC39302 | 7 | −2 | ND | 12 |
| NSC317609 | 7 | 1 | ND | 8 |
| NSC232469 | 7 | −7 | ND | −4 |
| NSC655184 | 7 | −11 | ND | 5 |
| NSC92432 | 6 | −1 | ND | 8 |
| NSC137050 | 6 | −1 | ND | 3 |
| NSC158437 | 5 | 1 | ND | 15 |
| NSC613624 | 4 | −1 | ND | 6 |
| NSC60360 | 4 | −4 | ND | 10 |
| NSC330685 | 4 | 2 | ND | 1 |
| NSC54251 | 4 | −4 | ND | −1 |
| NSC313453 | 3 | −5 | ND | 5 |
| NSC132916 | −1 | −3 | ND | 3 |
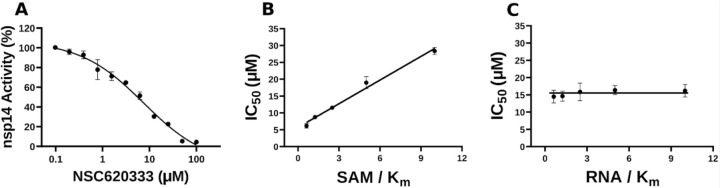
FIG. 3. Inhibition of nsp14 MTase activity by NSC620333.
(A) The IC50 value was determined for NSC620333 at Km of both SAM and RNA substrates (5.3 ± 1.0 µM, Hill Slope: − 0.9). The mechanism of action (MOA) of NSC620333 was also determined by IC50 determination for NSC620333 at (B) varying concentrations of SAM (from 0.15 to 2.5 µM ) at fixed concentration of RNA substrate (0.25 µM, 5x Km) and (C) varying concentrations of RNA substrate (from 31 to 500 nM ) at fixed concentration of SAM (1.25 µM, 5x Km). All values are presented as mean ± standard deviation of three independent experiments (n = 3). NSC620333 is a SAM competitive nsp14 inhibitor (B), and noncompetitive with respect to RNA substrate (C).
In the quest to further understand the specificity of NSC620333, we evaluated its inhibitory activity against a diverse panel of 33 human RNA-, DNA-, and protein-MTases (Supplementary Table S3). A critical part of our investigation focused on potential off-target effects within the host to ensure the safety and efficacy of the compounds. Our analyses revealed that NSC620333 selectively inhibited only human RNMT [27] (N7 guanosine RNA methyltransferase) and PRMT7 [28] (protein arginine methyltransferase 7), with IC50 values of 8.6 ± 1.3 and 7 ± 1.5 µM, respectively (Supplementary Figure S2). Notably, RNMT is involved in the process of mRNA capping, which is essential for maintaining mRNA stability, facilitating its export, and ensuring efficient translation [29]. In contrast, PRMT7 participates in the methylation of arginine residues on specific proteins, thereby impacting cellular processes such as gene transcription, DNA repair, and signal transduction [30]. Furthermore, to extrapolate our in vitro findings to a physiologically-relevant context, we tested NSC620333 and NSC76988 in an in vivo assay. This experiment was based on a lung epithelial SARS-CoV-2 infection model using Calu-3 cells. Encouragingly, both compounds demonstrated anti-SARS-CoV-2 activity (Supplementary Figure S5), providing supportive evidence for their potential as therapeutic agents. Taken together, the data from all three complementary assays converges towards a common conclusion: NSC620333 stands out as a potential therapeutic candidate against SARS-CoV-2. This compound exhibited a potent inhibitory effect, significant binding to nsp14, favorable selectivity, and in vivo efficacy. These promising attributes warrant further investigations into its potential for clinical application in SARS-CoV-2 treatment. On a separate note, it is interesting to observe that while no direct binding was detected between NSC76988 and nsp14, the compound displayed notable inhibitory effects. However, the mechanism of such inhibition is unclear.
C. MD Simulations of nsp14
Structural insights into nsp14 were obtained through molecular dynamics (MD) simulations performed under three different conditions: (i) the protein only, (ii) the protein in complex with NSC620333, and (iii) nsp14 in/out of complex with metal coordination complexes. Our findings are presented as follows:
1. Protein dynamics
Investigations into ligand binding within the MTase lateral cavity were conducted via numerous extended molecular dynamics simulations of the nsp14-nsp10 complex, all performed under a consistent thermal condition of 303.15 K (details in Methods: Molecular Dynamics Simulations V G). Throughout these simulations, we monitored the average motion of residues located within a 5-angstrom radius of the SS148 binding region, as depicted in Figure 4(A). A thorough evaluation of the complete motion of the associated residues is presented in Supplementary Figures S7. Interestingly, we found that residues Phe367, Tyr368, and Gln354 on average exhibited the most significant movement across all simulations. These residues displayed considerable stabilization when NSC620333 was present in the binding pocket. Specifically, the root-mean-square deviation (RMSD) movement of Phe367, Tyr368, and Gln354 reduced to 0.25, 0.29, and 0.26 angstroms respectively in the presence of NSC620333. Significantly, we observed a limited number of conformational changes in the nsp10 protein when it was complexed with nsp14 (see Figure 4(B) (Left)), indicating a high level of interaction stability between the two proteins based on our sampling. Figure 4(B) (Middle/Right) showcases the key residues involved in this interaction. Specifically, Phe24, Glu11, His85, Thr10, and Val26 were identified as the most crucial residues facilitating this interaction.
FIG. 4. Protein Conformation and Interaction Dynamics from Molecular Simulations.
(A) Bar charts presenting the mean displacement (expressed in angstroms) of amino acid residues within a 5-angstrom vicinity of the binding pocket, derived from 1-microsecond molecular dynamics simulations conducted at a stable temperature of 303.15 K. The error bars indicate the standard deviation of the mean, based on five distinct simulation runs. (B) (Left) A graphical representation of the most notable conformational shifts in nsp10, as determined through a series of MD-simulations involving nsp10–14, with only minor changes due to the consistent stability of the nsp10-nsp14 interaction. (Middle) Select residues at the nsp10 binding interface making the most significant contribution to the interaction with nsp16. (Right) Breakdown of individual residues of nsp10 with the highest free energy contributions (kcal/mol) to the overall binding free energy estimation of nsp10–14 interactions; the average contribution from a single residue is indicated by a dotted line.
2. Conformational Shifts
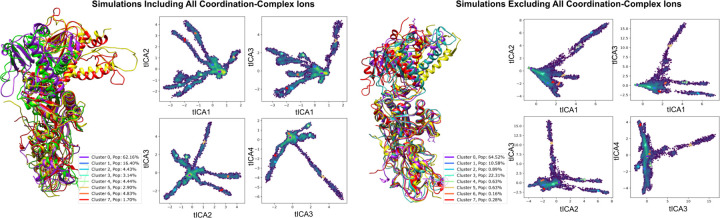
To gain a more comprehensive understanding of the conformational dynamics of the nsp14 complex, we conducted an in-depth analysis of the gathered molecular dynamics simulations [16]. This process involved amalgamating data from receptor-ligand simulations to scrutinize the specific dynamics of the MTase binding cavity and, in a separate analysis, the overall dynamics of the complex backbone across independent trajectories [31]. We employed time-lagged independent component analysis (tICA) to project pertinent trajectory features onto a lower-dimensionality landscape, which facilitated clustering and visualization of the sampled metastable states [32]. The features of the binding pocket were defined by pairwise distances between the heavy atoms of the NSC620333 ligand and the backbone α-carbons of 27 crucial residues within the MTase lateral cavity. The analysis of global dynamics similarly employed pairwise distances between α-carbons. The generated projections were clustered using the K-means algorithm [33], resulting in eight states that spanned the sampled landscape and were aligned for comparative visualization. In both instances, the cluster with the highest population (comprising approximately 60% of the frames) closely mirrored the anticipated native state, with one or two additional states constituting the majority of the remaining configurations. The visualization of the states underpinning global dynamics revealed that nsp10 and the exoribonuclease domain of nsp14 remained stable. In contrast, the MTase domain exhibited a bent configuration in a minor proportion of frames. The comparative analysis between trajectories both devoid of metal ions and those inclusive of metal ions yielded consistent overall dynamics (see Methods: Molecular Dynamics Simulations V G). However, a larger bend of the main alpha-helix MTase was observable in a more significant population for ion-inclusive trajectories, as evidenced by Cluster 5 and Cluster 7 in Figure 5 (4.6% vs. approximately 1%). This analysis showcases the influence of metal ion presence on the conformational flexibility of the MTase domain, shedding light on the critical role of coordination complex ions in regulating protein structure and dynamics.
FIG. 5. Analyzing Nsp14-nsp10 Conformational Variability through tICA Projections Influenced by Coordination Complexes.
This figure delineates the conformational differences in the nsp10-nsp14 complex under ion-devoid and ion-inclusive states, emphasizing structures from four representative cluster centers with high variance. Trajectories were derived from independent runs between 200 ns − 1 µs, focusing on pairwise distances between alternating backbone α-carbons to determine simulation features and enhance the sampling of diverse conformations.
3. Structural Insights into Inhibition
The application of tICA decomposition on protein-ligand interactions in the simulations identified a variety of binding poses for NSC620333 within the nsp14 MTase binding cavity. From this analysis, eight distinct clusters emerged, which were further refined into three primary states, each visualized in Figure 6. Detailed examination of these states facilitated the identification of specific interactions that contribute to the increased binding affinity of NSC620333, as supported by Wagner et al. [34]. Of particular interest were the hydrogen bonds observed to form from ASN538 to the purine group of the ligand, and the favorable lipophilic interactions of the chlorobenzene component of the ligand with the lipophilic subpocket, predominantly in Cluster 2 and Cluster 6 [35]. These interactions seem to play a crucial role in establishing a more stable binding configuration. Moreover, the varied poses of NSC620333 within the binding pocket further underscore the flexibility of the nsp14 MTase binding cavity, a factor that could be key in the design of effective therapeutic agents [36]. The presence of different binding modes, each with unique interaction profiles, may influence the ligand’s functional impact on the protein, potentially offering multiple pathways for pharmacological intervention [37].
FIG. 6. tICA Decomposition and Representative Structures of NSC620333 Alternative Binding Poses.
The figure illustrates the tICA decomposition and associated structures of various binding poses of NSC620333, derived from a combination of trajectories that incorporate unbiased molecular dynamics simulations. The trajectories were featurized by calculating pairwise distances between heavy ligand atoms and backbone α-carbons of key residues surrounding the binding site. Cluster 2 illustrates the prevalent metastable binding mode observed, while Cluster 0 and Cluster 6 depict twisted and inward binding modes, respectively.
III. CRYSTAL STRUCTURE OF NSP14 MTASE IN COMPLEX WITH SS148
To uncover the atomic details of the nsp14-SS148 interaction, we performed the crystallographic analysis of the nsp14/SS148 complex. We employed the fusion protein-assisted crystallization approach using the MTase domain of nsp14 (residues 300–527) N-terminally fused to a small crystallization tag TELSAM developed by Kottur et al. [38]. The nsp14 MTase-TELSAM/SS148 crystals belonged to the hexagonal P65 space group and diffracted to the 2.6 Å resolution. The structure of the nsp14 MTase-TELSAM/SS148 complex was subsequently solved by molecular replacement and further refined to good R factors and geometry, as summarized in Supplementary Table S4.
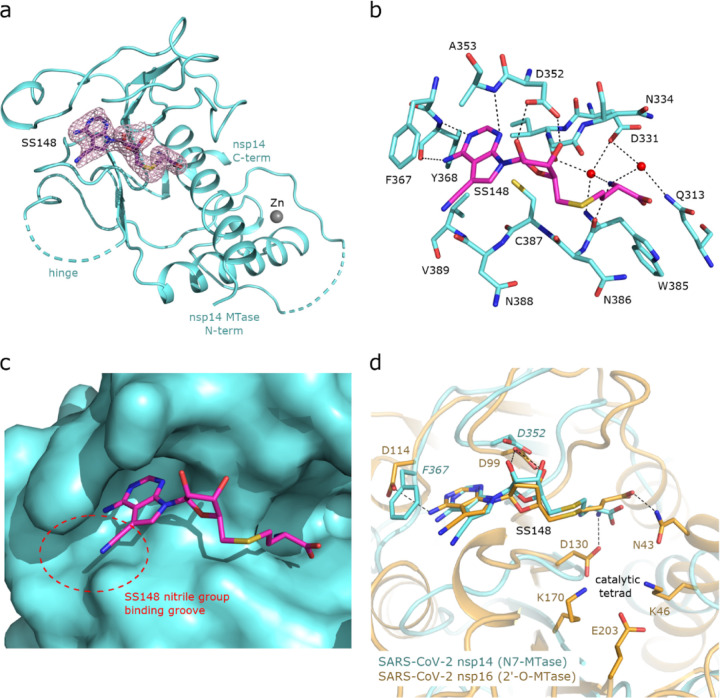
The SS148 ligand was bound to the SAM/SAH-binding site of nsp14 as expected (Fig 7a). The structure of SS148 is derived from SAH and thus, the mechanism of its interaction with nsp14 is also similar to SAH. The nsp14-SS148 interaction is mediated by multiple interactions including both direct (via Asp352, Ala353, Tyr368, and Trp385) and indirect water-mediated hydrogen bonds (via Gln313, Asp331, and Ile332) (Fig 7b). The amino acid moiety of SS148 binds to nsp14 amino acid residues Gln313, Trp385, and Asp331, while the central ribose-derived moiety of nsp14 interacts mainly with Asp352, and the basic adenine-derived moiety binds to Ala353 and Tyr368.
FIG. 7. Crystal structure of nsp14 MTase in complex with SS148.
(a), Overall view of the nsp14/SS148 complex. The protein backbone of the nsp14 MTase domain is shown in cartoon representation and colored in light blue. The SS148 ligand is shown in stick representation and colored according to elements: carbon, magenta; nitrogen, blue; oxygen, red; sulfur, yellow. The unbiased Fo-Fc omit map contoured at 2s is shown around the SS148 ligand. The TELSAM crystallization tag N-terminally fused to the nsp14 MTase domain is not shown. (b), Detailed view of the SS148 ligand binding site. The SS148 ligand and side chains of selected nsp14 amino acid residues are shown in stick representation, with carbon atoms colored according to the protein assignment and other elements colored as in (a). Water molecules are presented as red spheres; hydrogen atoms are not shown. Selected hydrogen bonds involved in the nsp14–SS148 interaction are depicted as dashed black lines. (c), SS148 ligand binding site with nsp14 shown in surface representation. The nitrile group binding groove on the surface of nsp14 potentially accepting larger substituents is highlighted with a red dashed circle. (d), Structural alignment of the SS148 binding sites of SARS-CoV-2 N7-MTase nsp14 and 2’-O-MTase nsp16. Protein backbones are shown in cartoon representation, while SS148 and side chains of selected residues are shown in stick representation. The carbon atoms of the nsp14/SS148 and nsp16/SS148 complexes are depicted in light blue and yellow, respectively; other elements are colored as in (a).
Compared to SAH, SS148 contains a nitrile group bound to the C7-position of the 7-deaza-adenine heterocyclic moiety. This nitrile group is bound to nsp14 in the groove formed by the nsp14 residues Phe367, Asn388, and Val389 (Fig 7c). Our study documents that introduction of substituents at the C7-position of this 7-deaza-adenine moiety, physically larger than the presented nitrile group, can be a promising strategy potentially leading to development of more potent and more specific nsp14 inhibitors.
SS148 is an inhibitor with dual activity against both SARS-CoV-2 N7-MTase nsp14 and 2’-O-MTase nsp16. Therefore, we compared the SS148 binding site of nsp14 with the previously crystallized SS148 binding site of nsp16 [22] (Fig 7d). Despite significant differences in the organization of the SS148 binding pockets in nsp14 and nsp16, we observed similar conformation of SS148. The conserved catalytic tetrad characteristic for 2’-O-MTases formed by Lys46, Asp130, Lys170, and Glu203 in nsp16 is not present in nsp14.
To judge the reliability of our computational pipeline, we performed re-docking of SS148 onto the nsp14 binding site. Our methodological approach was successful in accurately replicating the binding pose of the SS148 ligand within the nsp14 binding site, showcasing a striking alignment (RMSD 1.426Å) with the experimentally determined crystal structure (depicted in Supplementary Figure S4). The high degree of concordance between the computationally determined re-docking pose and the experimental crystal structure attests to the robustness of our docking pipeline methodology. The agreement between our computational predictions and experimental findings underscores the proficiency of our integrated approach and further substantiates the potential of SS148 as a promising dual inhibitor of SARS-CoV-2 MTases.
IV. CONCLUSION AND OUTLOOK
Our comprehensive study has provided valuable insights into the complex dynamics of the SARS-CoV-2 nsp14 protein and the therapeutic potential of compounds aimed at inhibiting it. In particular, we found that the compound NSC620333 demonstrates promising attributes as a therapeutic agent against SARS-CoV-2, primarily due to its potent inhibitory effects on the nsp14 Methyltransferase (MTase) activity and its substantial binding affinity to nsp14. The observed in-vivo efficacy and selectivity of this compound for development of therapeutics in treating COVID-19. In our study, we identified several critical residues within the MTase binding pocket of nsp14, and elucidated their dynamic behavior in the presence of potential inhibitors, such as NSC620333. Our findings lay a solid foundation for future drug design efforts, paving the way for the development of novel strategies to disrupt the nsp14 function. This could potentially lead to new therapeutic options for COVID-19.
Interestingly, we observed conformational changes in nsp14, particularly in the presence and absence of metal coordination complexes, which emphasizes the role of this metal ion in the structural dynamics of nsp14. The diverse binding poses of the NSC620333 ligand identified in our study highlight the necessity for a dynamic view of protein-ligand interactions. It suggests that a single static view of a protein-ligand complex is insufficient to capture the full complexity of these interactions, further emphasizing the value of molecular dynamics simulations in comprehending these systems. Our structural studies also revealed the binding mechanism of another potential inhibitor, SS148. By visualizing its binding mode and interactions with nsp14, we proposed that introducing substituents at the C7-position of the 7-deaza-adenine moiety could enhance the potency and specificity of nsp14 inhibitors. The crystal structure also revealed the differences and similarities in the SS148 binding site between nsp14 and nsp16, providing valuable structural insights for drug design.
While our results are promising, they represent only the initial steps in a much longer journey. Further studies are needed to fully understand the therapeutic potential of these compounds. Future work should focus on optimizing these lead compounds, assessing their pharmacokinetic and pharmacodynamic properties, and evaluating their safety and efficacy in pre-clinical and clinical trials. Furthermore, the mechanistic exploration of NSC76988 and its potential allosteric mode of inhibition should also be pursued. Overall, this study underscores the potential of computational methods in understanding the complexities of biological systems and guiding the development of new therapeutics. We hope that our findings will inspire and inform future research efforts towards the development of effective treatments for COVID-19 and potentially other related viral threats.
V. METHODS
A. Docking
The Cryo-EM structure of SARS-CoV-2 nsp10-nsp14 (PDB:7N0B [39]) was utilized as a reference for molecular docking. The receptor was prepared through Schrödinger’s protein preparation tool [40], which involved a series of critical steps. These steps included modeling missing residues, capping missing termini, removing water molecules, and assigning protonation states. The ligand binding site was identified by super-imposing SAM/SAH (PDB: 5C8S/5C8T [41]) onto the Cryo-EM structure. Upon completion of the overlay, a minimization was performed using the OPLS4 force field [42].
The process aimed to optimize the overall structure of the receptor-ligand complex, minimize potential clashes, and maximize binding interactions. For the molecular docking process, we employed Glide-SP and Glide-XP software [25], integrated within the VirtualFlow Unity [20] (VFU) pipeline (https://github.com/VirtualFlow/VFU). In the interest of scientific inclusivity, we provide a VFU setup that utilizes QuickVina [43] and Smina [44], two open-source docking programs.
The NCI Open Compound collection was procured from NCBI PubChem (https://pubchem.ncbi.nlm.nih.gov/) by selecting “DTP/NCI” as the Data Source. The provided isomeric smiles were employed as starting points for processing the initial 250,000 molecules. At the stage of identifying the top-100 molecules, the molecules were manually inspected to ensure correct stereochemistry.
Diversity for a set of molecules was calculated as:
The expression sim(X, Y ) computes the pairwise molecular similarity for all n structures calculated as the Tanimoto distance of the Morgan fingerprint (calculated with a radius of size 3 and a 2048 bit size) [45].
B. MM/GBSA Calculation
The AMBER ff14SB force field [46] and the General AMBER force field (GAFF2) [47] were employed for parameterizing proteins and ligands, respectively, in the complex systems. The antechamber module [48] was utilized to calculate the partial atomic charges using the AM1-BCC charge model for ligand molecules. To eliminate bad steric contacts, the systems were subjected to energy minimization with the steepest descent algorithm and conjugate gradient methods, without restraints. This process involved 2,500 iterations using the Sander MD engine [49]. The MM/GBSA energies were evaluated using the MMPBSA.py [14] script, which is part of the AmberTools21 package [50].
C. Protein Expression and Purification
The SARS-CoV-2 nsp14 MTase domain-encoding sequence (GenBank: YP_009725309.1, residues 300–527) was codon-optimized for expression in E. coli and synthesized by GeneArt (Thermo Scientific). The gene was cloned into a modified pRSFDuet vector containing an N-terminal hexahistidine (His6) purification tag, followed by a SUMO solubility and folding tag, and a TELSAM crystallization tag [38]. The plasmid was transformed into E. coli BL21 DE3 NiCo bacterial strain (New England Biolabs), and the protein was overexpressed using autoinduction ZY medium. Cells were collected by centrifugation and resuspended in lysis buffer (50 mM Tris, pH 8.0, 400 mM NaCl, 20 mM imidazole, 10 mM MgCl2, 10 µM ZnCl2, 3 mM β-mercaptoethanol, and 250U of DNA endonuclease DENERASE (c-LEcta)). The cells were then sonicated using the Q700 Sonicator instrument (QSonica). The lysate was subsequently cleared by centrifugation, and the supernatant was incubated with Ni-NTA agarose (Thermo Scientific), followed by extensive washing with lysis buffer. The protein was finally eluted using lysis buffer supplemented with 300 mM imidazole. Post-elution, the protein was treated with Ulp1 protease to cleave off the His6-SUMO tag. The nsp14 MTase-TELSAM protein was further purified by size exclusion chromatography using Superdex 200 16/600 (GE Healthcare) pre-equilibrated with size-exclusion buffer (25 mM Tris pH 8.3, 200 mM KCl, and 2 mM TCEP). Fractions containing the nsp14 MTase-TELSAM protein were concentrated to 4 mg/ml, flash-frozen, and stored at −80°C.
D. Crystallization and Crystallographic Analysis
Crystallization trials were conducted with a concentration of 4 mg/ml of the nsp14 MTase-TELSAM protein, supplemented with a twofold molar excess of the SS148 ligand. Crystals were observed to grow within two days at 18°C. This was achieved in a sitting drop composed of a 200 nl mixture of the protein and 200 nl of the mother liquor, which contained 100 mM bicine/Trizma pH 8.5; 10% w/v PEG 20,000; 20% v/v PEG MME 550; 20 mM D-glucose; 20 mM D-mannose; 20 mM D-galactose; 20 mM L-fucose; 20 mM D-xylose; 20 mM N-acetyl-D-glucosamine.
The crystallographic dataset was collected from a single crystal at the BL14.1 beamline, located at the BESSY II electron storage ring operated by Helmholtz-Zentrum Berlin [51]. Data integration and scaling were performed using XDS [52]. Molecular replacement was used to solve the structure of the nsp14 MTase-TELSAM/SS148 complex, with the nsp14 MTase-TELSAM/sinefungin complex structure serving as the search model (PDB entry 7TW9 [38]). Phaser from the Phenix package was used for the initial model [53, 54]. Model improvement was accomplished via automatic model refinement with Phenix.refine [55] and manual model building with Coot [56]. Statistics regarding data collection, structure solution, and refinement are provided in Supplementary Table S4. Structural figures were generated using PyMOL Molecular Graphics System v2.5 (Schrödinger, LLC) [57]. The atomic coordinates and structural factors have been deposited in the Protein Data Bank (https://www.rcsb.org) under the accession code 8BWU.
E. Radioactivity-based Assay Conditions
The assay conditions for nsp14 were as follows: The final concentrations in the reaction mixture were 1.5 nM for nsp14, 50 nM for Biotin-RNA (5’GpppACCCCCCCCC-Biotin 3’ ), and 250 nM for 3H-SAM. The incubation was carried out for 20 minutes at 23°C. The buffer used contained 20 mM Tris HCl at pH 7.5, 250 µM MgCl2, 5 mM DTT, and 0.01% Triton X-100, with an additional 5% DMSO.
F. SPR Conditions
For Surface Plasmon Resonance (SPR), the full-length biotinylated nsp14 was immobilized on the flow cell of a SA sensor chip in 1x HBS-EP buffer, achieving a response of 8000 RU. The assay was performed using the same buffer but with 2% DMSO added. Single-cycle kinetics were employed with a contact time of 60 seconds and a dissociation time of 120 seconds, at a flow rate of 75 µL/min. NSC620333 was tested at a concentration of 10 µM as the highest concentration, and a dilution factor of 0.25 was used to yield five different concentrations.
G. Molecular Dynamics Simulations
The crystal structure of nsp14 in complex with nsp10 was obtained from the Protein Data Bank (PDB) under the entry code 7N0B. All missing residues and variants relative to the wild-type were modeled using Schrodinger’s protein preparation tool. The RNA complex was removed for the purposes of our simulations. The system was solvated in a periodic cubic box containing TIP3P water with 150 mM KCl using CHARMM-GUI [58]. The CHARMM36m force field [59] with hydrogen mass repartitioning was employed, and input files were sourced directly from CHARMM-GUI. Molecular dynamics simulations were performed using the Gromacs 2020.3 software package [60]. The simulations employed a Langevin thermostat and a Nosé–Hoover Langevin piston barostat to maintain a pressure of 1 atm. Long-range interactions were treated using the particle mesh Ewald method, and non-bonded interactions were smoothed between 10 to 12 Å. The system underwent energy minimization for 1,500 steps, followed by equilibration to 303.15K with a timestep of 2 fs for 10 ns using the SHAKE and SETTLE algorithms [61]. Production simulations were conducted with a 4 fs timestep for a total of 1.0 µs, with five independent replicates. Simulations that included NSC620333 were parameterized using the Charmm General Force Field (CGenFF) framework [62]. Throughout the simulations, the root-mean-square deviation (RMSD) of the protein residues was calculated using the MDTraj package [63]. All our simulations can be downloaded from: https://drive.google.com/drive/folders/1tKiIsDxBbjZLCxceSoDXlJAczd4UgHIJ?usp=sharing.
H. Anti-SARS-CoV-2 and Cytotoxicity Assays in Calu-3 Cells
Anti-SARS-CoV-2 activity of NSC620333 and NSC76988 compounds were determined in the human lung adenocarcinoma cell line Calu-3 (ATCC, cat. no. HTB-55) using SARS-CoV-2 (isolate hCoV-19/Czech Republic/NRL_6632_2/2020). Briefly, two-fold eight serial dilutions of the compounds starting from 100 µM were added to 40, 000 Calu-3 cells seeded for 1 day in DMEM supplemented with 2% fetal bovine serum, 100 U of penicillin/mL and 100 µg of streptomycin/mL. After 1 h incubation at 37 °C, SARS-CoV-2 was added at a multiplicity of infection (MOI) of 0.03 IU/ml, incubated 3 days at 37 °C, and the virus-induced cytopathic effect (CPE) was quantified by formazan-based cell viability assay (XTT assay). XTT solution was added and after 4 h incubation, the absorbance at 450 nm was measured using the EnVision plate reader. After plotting the percentage cell viability versus log10 concentrations, the compound concentrations required to reduce viral cytopathic effect by 50% (EC50) were calculated by nonlinear regression. To determine compound cytotoxicity, Calu-3 cells were exposed to the same compound dilutions as above but without virus, and cell viability was determined using XTT assay. The compound concentrations causing 50% reduction in cell viability (CC50) were calculated similarly. Three-fold serial dilutions of Remdesivir from 10 µM served as a control in both experiments.
I. Assay Technique
The MTase activity of nsp14 was measured using a radiometric assay as described previously [21]. Chlorobenzothiophene 3 was tested at various concentrations ranging from 200 nM to 200 µM to determine the half-maximal inhibitory concentration (IC50) value. The final reaction mixture consisted of 1.5 nM nsp14, 250 nM 3H-SAM, and 50 nM RNA in buffer (20 mM Tris HCl, pH 7.5, 250 µM MgCl2, 5 mM DTT, and 0.01% Triton X-100). The reaction time was 20 min at 23°C. Data were fitted to the four-parameter logistic equation using GraphPad Prism 8.
J. Synthesis of SS148
The synthesis of SS148 (also referred to as CN-SAH in the literature) was carried out according to previously published procedures [64]. Comprehensive synthetic procedures and associated 1H and 13C -NMR data for SS148 can be found in the referenced literature.
Supplementary Material
ACKNOWLEDGEMENTS
AK.N. acknowledges funding from the Bio-X Stanford Interdisciplinary Graduate Fellowship (SGIF). Special thanks are extended to A.K. and Michael Bassik for their invaluable assistance in securing the SGIF funding. R.P. acknowledges funding through a Postdoc.Mobility fellowship by the Swiss National Science Foundation (SNSF, Project No. 191127). A.A.-G. thanks Dr. Anders G. Frøseth for his generous support. A.A.-G. also acknowledges the generous support of Natural Resources Canada and the Canada 150 Research Chairs program. M.F.D.H and V.A.V were supported in part by the National Institutes of Health grant 1R01GM123296. One part of the parameterization of simulations was performed on HPC resources supported in part by the National Science Foundation through major research instrumentation grant number 1625061 and by the US Army Research Laboratory under contract number W911NF-16-2-0189. The remaining parameterization was performed on the CB2RR cluster made possible through NIH Research Resource computer instrumentation grant S10-OD020095. S.S. is a Damon Runyon Quantitative Biology Fellow supported by the Damon Runyon Cancer Research Foundation (DRQ-14-22). This research was enabled in part by support provided by Calcul Québec (https://www.calculquebec.ca) and Compute Canada (www.computecanada.ca). HN acknowledges NVIDIA and Intel for funding. MD simulations for all compounds were performed on the Béluga supercomputer situated at the École de technologie supérieure in Montreal. We thank the Helmholtz-Zentrum Berlin für Materialien und Energie for the allocation of synchrotron radiation beamtime. This work was supported by the project National Institute Virology and Bacteriology (Programme EXCELES, Project No. LX22NPO5103) - Funded by the European Union - Next Generation EU. The research was also, in part, made possible thanks to funding provided to the University of Toronto’s Acceleration Consortium by the Canada First Research Excellence Fund (CFREF-2022-00042).
References
- [1].Paules Catharine I, Marston Hilary D, and Fauci Anthony S. Coronavirus infections—more than just the common cold. Jama, 323(8):707–708, 2020. [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization et al. The corona virus disease 2019 (covid-19). Brazilian Journal of Implantology and Health Sciences, 4(6):45–46, 2022. [Google Scholar]
- [3].Sharma Atul, Tiwari Swapnil, Deb Manas Kanti, and Marty Jean Louis. Severe acute respiratory syndrome coronavirus-2 (sars-cov-2): a global pandemic and treatment strategies. International journal of antimicrobial agents, 56(2):106054, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Naqvi Ahmad Abu Turab, Fatima Kisa, Mohammad Taj, Fatima Urooj, Singh Indrakant K, Singh Archana, Atif Shaikh Muhammad, Hariprasad Gururao, Hasan Gulam Mustafa, and Hassan Md Imtaiyaz. Insights into sars-cov-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1866(10):165878, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ng Teresa I, Correia Ivan, Seagal Jane, De-Goey David A, Schrimpf Michael R, Hardee David J, Noey Elizabeth L, and Kati Warren M. Antiviral drug discovery for the treatment of covid-19 infections. Viruses, 14(5):961, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bouvet Mickaël, Debarnot Claire, Imbert Isabelle, Selisko Barbara, Snijder Eric J, Canard Bruno, and Decroly Etienne. In vitro reconstitution of sars-coronavirus mrna cap methylation. PLoS pathogens, 6(4):e1000863, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen Yu, Cai Hui, Pan Ji’an, Xiang Nian, Tien Po, Ahola Tero, and Guo Deyin. Functional screen reveals sars coronavirus nonstructural protein nsp14 as a novel cap n7 methyltransferase. Proceedings of the National Academy of Sciences, 106(9):3484–3489, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Corman Victor M, Muth Doreen, Niemeyer Daniela, and Drosten Christian. Hosts and sources of endemic human coronaviruses. Advances in virus research, 100:163–188, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Delft Annette von, Hall Matthew D, Kwong Ann D, Purcell Lisa A, Saikatendu Kumar Singh, Schmitz Uli, Tallarico John A, and Lee Alpha A. Accelerating antiviral drug discovery: lessons from covid-19. Nature Reviews Drug Discovery, pages 1–19, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ihlenfeldt Wolf-Dietrich, Voigt Johannes H, Bienfait Bruno, Oellien Frank, and Nicklaus Marc C. Enhanced cactvs browser of the open nci database. Journal of chemical information and computer sciences, 42(1):46–57, 2002. [DOI] [PubMed] [Google Scholar]
- [11].Voigt Johannes H, Bienfait Bruno, Wang Shaomeng, and Nicklaus Marc C. Comparison of the nci open database with seven large chemical structural databases. Journal of chemical information and computer sciences, 41(3):702–712, 2001. [DOI] [PubMed] [Google Scholar]
- [12].Pagadala Nataraj S, Syed Khajamohiddin, and Tuszynski Jack. Software for molecular docking: a review. Biophysical reviews, 9:91–102, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nigam AkshatKumar, Pollice Robert, Tom Gary, Jorner Kjell, Thiede Luca A, Kundaje Anshul, and Aspuru-Guzik Alan. Tartarus: A benchmarking platform for realistic and practical inverse molecular design. arXiv preprint arXiv:2209.12487, 2022. [Google Scholar]
- [14].Miller Bill R III, Dwight McGee T Jr, Swails Jason M, Homeyer Nadine, Gohlke Holger, and Roitberg Adrian E. Mmpbsa. py: an efficient program for end-state free energy calculations. Journal of chemical theory and computation, 8(9):3314–3321, 2012. [DOI] [PubMed] [Google Scholar]
- [15].Ylilauri Mikko and Pentik¨:”ainen Olli T. Mmgbsa as a tool to understand the binding affinities of filamin–peptide interactions. Journal of chemical information and modeling, 53(10):2626–2633, 2013. [DOI] [PubMed] [Google Scholar]
- [16].Hollingsworth Scott A and Dror Ron O. Molecular dynamics simulation for all. Neuron, 99(6):1129–1143, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gelpi J. Hospital a, goñi r, orozco m. Molecular dynamics simulations: Advances and applications. Advances and Applications in Bioinformatics and Chemistry, 8:37–47, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Levin-Konigsberg Roni, Mitra Koushambi, Nigam AkshatKumar, Spees Kaitlyn, Hivare Pravin, Liu Katherine, Kundaje Anshul, Krishnan Yamuna, and Bassik Michael. Slc12a9 is a lysosome-detoxifying ammonium-chloride cotransporter. bioRxiv, pages 2023–05, 2023. [Google Scholar]
- [19].Ogando Natacha S, Zevenhoven-Dobbe Jessika C, Meer Yvonne van der, Bredenbeek Peter J, Posthuma Clara C, and Snijder Eric J. The enzymatic activity of the nsp14 exoribonuclease is critical for replication of mers-cov and sars-cov-2. Journal of virology, 94(23):10–1128, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gorgulla Christoph, Nigam AkshatKumar, Koop Matt, Cinaroglu Suleyman S, Secker Christopher, Haddadnia Mohammad, Kumar Abhishek, Malets Yehor, Hasson Alexander, Levin-Konigsberg Roni, et al. Virtualflow 2.0-the next generation drug discovery platform enabling adaptive screens of 69 billion molecules. bioRxiv, pages 2023–04, 2023. [Google Scholar]
- [21].Devkota Kanchan, Schapira Matthieu, Perveen Sumera, Yazdi Aliakbar Khalili, Li Fengling, Chau Irene, Ghiabi Pegah, Hajian Taraneh, Loppnau Peter, Bolotokova Albina, et al. Probing the sam binding site of sarscov-2 nsp14 in vitro using sam competitive inhibitors guides developing selective bisubstrate inhibitors. SLAS DISCOVERY: Advancing the Science of Drug Discovery, 26(9):1200–1211, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Klima Martin, Yazdi Aliakbar Khalili, Li Fengling, Chau Irene, Hajian Taraneh, Bolotokova Albina, Ümit Kaniskan H, Han Yulin, Wang Ke, Li Deyao, et al. Crystal structure of sars-cov-2 nsp10–nsp16 in complex with small molecule inhibitors, ss148 and wz16. Protein Science, 31(9):e4395, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schenone Monica, Dančík Vlado, Wagner Bridget K, and Clemons Paul A. Target identification and mechanism of action in chemical biology and drug discovery. Nature chemical biology, 9(4):232–240, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wan Shunzhou, Bhati Agastya P, Zasada Stefan J, and Coveney Peter V. Rapid, accurate, precise and reproducible ligand–protein binding free energy prediction. Interface Focus, 10(6):20200007, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Friesner Richard A, Banks Jay L, Murphy Robert B, Halgren Thomas A, Klicic Jasna J, Mainz Daniel T, Repasky Matthew P, Knoll Eric H, Shelley Mee, Perry Jason K, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. method and assessment of docking accuracy. Journal of medicinal chemistry, 47(7):1739–1749, 2004. [DOI] [PubMed] [Google Scholar]
- [26].Homola Jiří and Piliarik Marek. Surface plasmon resonance (SPR) sensors. Springer, 2006. [DOI] [PubMed] [Google Scholar]
- [27].Varshney Dhaval, Petit Alain-Pierre, Bueren-Calabuig Juan A, Jansen Chimed, Fletcher Dan A, Peggie Mark, Weidlich Simone, Scullion Paul, Pisliakov Andrei V, and Cowling Victoria H. Molecular basis of rna guanine-7 methyltransferase (rnmt) activation by ram. Nucleic acids research, 44(21):10423–10436, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jain Kanishk and Clarke Steven G. Prmt7 as a unique member of the protein arginine methyltransferase family: A review. Archives of biochemistry and biophysics, 665:36–45, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ramanathan Anand, Brett Robb G, and Chan Siu-Hong. mrna capping: biological functions and applications. Nucleic acids research, 44(16):7511–7526, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pal Sharmistha and Sif Saïd. Interplay between chromatin remodelers and protein arginine methyltransferases. Journal of cellular physiology, 213(2):306–315, 2007. [DOI] [PubMed] [Google Scholar]
- [31].Pande Vijay S, Beauchamp Kyle, and Bowman Gregory R. Everything you wanted to know about markov state models but were afraid to ask. Methods, 52(1):99–105, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Naritomi Yusuke and Fuchigami Sotaro. Slow dynamics in protein fluctuations revealed by time-structure based independent component analysis: the case of domain motions. The Journal of chemical physics, 134(6), 2011. [DOI] [PubMed] [Google Scholar]
- [33].Hartigan IA and Algoritm AS. 136: A k-means clustering algorithm/ja hartigan, ma wong. Journal of the Royal Statistical Society Series C (Applied Statistic), 28(1):100–108, 1979. [Google Scholar]
- [34].Wagner Jeffrey R, Lee Christopher T, Durrant Jacob D, Malmstrom Robert D, Feher Victoria A, and Amaro Rommie E. Emerging computational methods for the rational discovery of allosteric drugs. Chemical reviews, 116(11):6370–6390, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Andrews PR, Craik DJ, and Martin JL. Functional group contributions to drug-receptor interactions. Journal of medicinal chemistry, 27(12):1648–1657, 1984. [DOI] [PubMed] [Google Scholar]
- [36].Teague Simon J. Implications of protein flexibility for drug discovery. Nature reviews Drug discovery, 2(7):527–541, 2003. [DOI] [PubMed] [Google Scholar]
- [37].Li Huameng and Li Chenglong. Multiple ligand simultaneous docking: orchestrated dancing of ligands in binding sites of protein. Journal of computational chemistry, 31(10):2014–2022, 2010. [DOI] [PubMed] [Google Scholar]
- [38].Kottur Jithesh, Rechkoblit Olga, Quintana-Feliciano Richard, Sciaky Daniela, and Aggarwal Aneel K. High-resolution structures of the sars-cov-2 n7-methyltransferase inform therapeutic development. Nature Structural & Molecular Biology, 29(9):850–853, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liu Chang, Shi Wei, Becker Scott T, Schatz David G, Liu Bin, and Yang Yang. Structural basis of mismatch recognition by a sars-cov-2 proofreading enzyme. Science, 373(6559):1142–1146, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schrödinger Release. 2: Protein preparation wizard, epik, schrödinger, llc, new york, ny, 2021. Impact, Schrödinger, LLC, New York, NY, 2021. [Google Scholar]
- [41].Ma Yuanyuan, Wu Lijie, Shaw Neil, Gao Yan, Wang Jin, Sun Yuna, Lou Zhiyong, Yan Liming, Zhang Rongguang, and Rao Zihe. Structural basis and functional analysis of the sars coronavirus nsp14–nsp10 complex. Proceedings of the National Academy of Sciences, 112(30):9436–9441, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lu Chao, Wu Chuanjie, Ghoreishi Delaram, Chen Wei, Wang Lingle, Damm Wolfgang, Ross Gregory A, Dahlgren Markus K, Russell Ellery, Von Bargen Christopher D, et al. Opls4: Improving force field accuracy on challenging regimes of chemical space. Journal of chemical theory and computation, 17(7):4291–4300, 2021. [DOI] [PubMed] [Google Scholar]
- [43].Alhossary Amr, Handoko Stephanus Daniel, Mu Yuguang, and Kwoh Chee-Keong. Fast, accurate, and reliable molecular docking with quickvina 2. Bioinformatics, 31(13):2214–2216, 2015. [DOI] [PubMed] [Google Scholar]
- [44].Koes David Ryan, Baumgartner Matthew P, and Camacho Carlos J. Lessons learned in empirical scoring with smina from the csar 2011 benchmarking exercise. Journal of chemical information and modeling, 53(8):1893–1904, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rogers David and Hahn Mathew. Extended-connectivity fingerprints. Journal of chemical information and modeling, 50(5):742–754, 2010. [DOI] [PubMed] [Google Scholar]
- [46].Maier James A, Martinez Carmenza, Kasavajhala Koushik, Wickstrom Lauren, Hauser Kevin E, and Simmerling Carlos. ff14sb: improving the accuracy of protein side chain and backbone parameters from ff99sb. Journal of chemical theory and computation, 11(8):3696–3713, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang Junmei, Wolf Romain M, Caldwell James W, Kollman Peter A, and Case David A. Development and testing of a general amber force field. Journal of computational chemistry, 25(9):1157–1174, 2004. [DOI] [PubMed] [Google Scholar]
- [48].Wang Junmei, Wang Wei, Kollman Peter A, and Case David A. Antechamber: an accessory software package for molecular mechanical calculations. J. Am. Chem. Soc, 222(1), 2001. [Google Scholar]
- [49].Kaus Joseph W, Pierce Levi T, Walker Ross C, and Andrew McCammon J. Improving the efficiency of free energy calculations in the amber molecular dynamics package. Journal of chemical theory and computation, 9(9):4131–4139, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Case David A, Metin Aktulga H, Belfon Kellon, Ben-Shalom Ido, Brozell Scott R, Cerutti David S, Cheatham Thomas E III, Cruzeiro Vinícius Wilian D, Darden Tom A, Duke Robert E, et al. Amber 2021. University of California, San Francisco, 2021. [Google Scholar]
- [51].Mueller Uwe, Förster Ronald, Hellmig Michael, Huschmann Franziska U, Kastner Alexandra, Malecki Piotr, Pühringer Sandra, Röwer Martin, Sparta Karine, Steffien Michael, et al. The macromolecular crystallography beamlines at bessy ii of the helmholtz-zentrum berlin: Current status and perspectives. The European Physical Journal Plus, 130:1–10, 2015. [Google Scholar]
- [52].Kabsch Wolfgang. xds. Acta Crystallographica Section D: Biological Crystallography, 66(2):125–132, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].McCoy Airlie J, Grosse-Kunstleve Ralf W, Adams Paul D, Winn Martyn D, Storoni Laurent C, and Read Randy J. Phaser crystallographic software. Journal of applied crystallography, 40(4):658–674, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Liebschner Dorothee, Afonine Pavel V, Baker Matthew L, Bunkóczi Gábor, Chen Vincent B, Croll Tristan I, Hintze Bradley, Hung L-W, Jain Swati, McCoy Airlie J, et al. Macromolecular structure determination using x-rays, neutrons and electrons: recent developments in phenix. Acta Crystallographica Section D: Structural Biology, 75(10):861–877, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Afonine Pavel V, Grosse-Kunstleve Ralf W, Echols Nathaniel, Headd Jeffrey J, Moriarty Nigel W, Mustyakimov Marat, Terwilliger Thomas C, Urzhumtsev Alexandre, Zwart Peter H, and Adams Paul D. Towards automated crystallographic structure refinement with phenix. refine. Acta Crystallographica Section D: Biological Crystallography, 68(4):352–367, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Emsley Paul and Cowtan Kevin. Coot: model-building tools for molecular graphics. Acta crystallographica section D: biological crystallography, 60(12):2126–2132, 2004. [DOI] [PubMed] [Google Scholar]
- [57].DeLano Warren L. The pymol molecular graphics system. http://www.pymol.org/, 2002.
- [58].Jo Sunhwan, Kim Taehoon, Iyer Vidyashankara G, and Wonpil Im. Charmm-gui: a web-based graphical user interface for charmm. Journal of computational chemistry, 29(11):1859–1865, 2008. [DOI] [PubMed] [Google Scholar]
- [59].Huang Jing, Rauscher Sarah, Nawrocki Grzegorz, Ran Ting, Feig Michael, De Groot Bert L, Grubmüller Helmut, and MacKerell Alexander D Jr. Charmm36m: an improved force field for folded and intrinsically disordered proteins. Nature methods, 14(1):71–73, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Spoel David Van Der, Lindahl Erik, Hess Berk, Groenhof Gerrit, Mark Alan E, and Berendsen Herman JC. Gromacs: fast, flexible, and free. Journal of computational chemistry, 26(16):1701–1718, 2005. [DOI] [PubMed] [Google Scholar]
- [61].Miyamoto Shuichi and Kollman Peter A. Settle: An analytical version of the shake and rattle algorithm for rigid water models. Journal of computational chemistry, 13(8):952–962, 1992. [Google Scholar]
- [62].Vanommeslaeghe Kenno and MacKerell Alexander D Jr. Automation of the charmm general force field (cgenff) i: bond perception and atom typing. Journal of chemical information and modeling, 52(12):3144–3154, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].McGibbon Robert T, Beauchamp Kyle A, Harrigan Matthew P, Klein Christoph, Swails Jason M, Hernández Carlos X, Schwantes Christian R, Wang Lee-Ping, Lane Thomas J, and Pande Vijay S. Mdtraj: a modern open library for the analysis of molecular dynamics trajectories. Biophysical journal, 109(8):1528–1532, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Spurr Sophie S, Bayle Elliott D, Yu Wenyu, Li Fengling, Tempel Wolfram, Vedadi Masoud, Schapira Matthieu, and Fish Paul V. New small molecule inhibitors of histone methyl transferase dot1l with a nitrile as a non-traditional replacement for heavy halogen atoms. Bioorganic & Medicinal Chemistry Letters, 26(18):4518–4522, 2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.