Abstract
Regulation of directed axon guidance and branching during development is essential for the generation of neuronal networks. However, the molecular mechanisms that underlie interstitial axon branching in the mammalian brain remain unresolved. Here, we investigate interstitial axon branching in vivo using an approach for precise labeling of layer 2/3 callosal projection neurons (CPNs), allowing for quantitative analysis of axonal morphology at high acuity and also manipulation of gene expression in well-defined temporal windows. We find that the GSK3β serine/threonine kinase promotes interstitial axon branching in layer 2/3 CPNs by releasing MAP1B-mediated inhibition of axon branching. Further, we find that the tubulin tyrosination cycle is a key downstream component of GSK3β/MAP1B signaling. We propose that MAP1B functions as a brake on axon branching that can be released by GSK3β activation, regulating the tubulin code and thereby playing an integral role in sculpting cortical neuron axon morphology.
Keywords: Interstitial axon branching, cortical neuron development, microtubules, intracellular signaling
INTRODUCTION
The cerebral cortex is a six-layered brain structure that is essential for sensory processing, motor skills, learning and memory, and thought. The establishment of functional cortical connectivity requires newly born neurons to migrate into appropriate layers during embryogenesis and then elaborate complex and unique axon and dendrite branching patterns. This results in laminar-specific organization of interstitial axon branches and also the elaboration of select intra- and inter-areal cortical connections. Cortical excitatory projection neurons possess long axons and connect to target neurons in other cortical and subcortical regions.1 Since a single neuron often projects to multiple targets, both extensive and precise axon branching patterns are required to provide distinct inputs to specific brain regions.2,3 Given the complexity of axon trajectories, precise regulation of collateral axon branch formation is vital for generation of a functional brain connectome.4 However, the intracellular signaling pathways, receptors, cell surface molecules and extracellular ligands that regulate laminar-specific interstitial axon branching in the cortex are poorly understood.
Two major types of axonal branching are growth cone splitting and interstitial (collateral) branching.5,6 Though growth cone splitting is typical for peripheral neurons, a significant fraction of cortical neuron axon branches are generated by interstitial branching: a new axon collateral growing from a stable, previously established, axon shaft.4,7 It can take several days until a daughter branch is formed from a primary axon. For example, axons of excitatory layer 2/3 callosal projection neurons (CPNs) develop from postnatal day 0 (P0) in mice, however their first collateral branches begin to be established starting at ~P3 in the ipsilateral cortex, a time when the growth cone of the primary axon has already crossed the CNS midline.8 Therefore, cytoskeletal components within the axon shaft must be locally rearranged at the site of the axonal branch point, followed by the stabilization of newly formed microtubules (MTs) that support the growth of the nascent branch.7 Much previous work in vitro has identified components of axon branching in cortical neurons and also dorsal root ganglion (DRG) neurons, and a major challenge is to relate these observations to in vivo axon branching.9–11 The factors that contribute to regulation of axon collateral branching in vitro include different MT-associated proteins (MAPs) (MAP7, MAP1B), MT severing proteins spastin and katanin, molecules that regulate actin (drebrin, Arp2) and various enzymes (including GSK3β). For example, in cultured DRG neurons, the MT-associated protein 7 (MAP7) prevents nascent axon branches from retracting, while the MT-associated protein 1B (MAP1B) restricts axon branch initiation.12,13 In addition, mitochondria and the endoplasmic reticulum localize to the base of developing axonal branches and contribute to their stabilization.14,15 However, the molecular mechanisms that regulate interstitial axon branching in vivo remain to be elucidated.
The underlying molecular basis of central nervous system (CNS) interstitial axon branching has been studied in vivo in Drosophila, Xenopus, and Zebrafish models,16–21 owing in part to available genetic labeling strategies that allow for precise and dynamic visualization of individual neurons. However, translation of these observations to mammals is challenging since the experimental approaches that allow for studying mouse neuronal connectivity in a quantitative fashion at the single cell level are limited.22 Currently, some of the most promising tools include certain viral-based labeling strategies which, however, are not ideal for developmental studies due to the long period of time (days to weeks) necessary for viral-mediated gene expression.23,24 Further, the immense diversity of neuronal subtypes in the mammalian CNS, including subtypes with unique morphologies and distinct neural process trajectories, makes the identification of the molecular pathways that direct unique axon branching patterns in individual neuronal subtypes in vivo challenging.
We have recently developed tools11 that overcome many of these difficulties, allowing for temporally-controlled targeting of small populations of layer 2/3 CPNs and quantitative assessment of axon branching patterns. Importantly, most layer 2/3 CPNs have a highly conserved bimodal interstitial branching pattern; their axons form no, or very few, primary interstitial axon branches within layer 4 and only branch robustly in layer 5, where they form ~6 – 8 primary interstitial branches.8 The few layer 2/3 CPN interstitial axon branches that do form in layer 4 rarely extend within this layer and instead grow apically or basally to innervate layer 2/3 or layer 5, respectively. Taken together, layer 2/3 CPNs in the mouse cortex provide a useful model system to uncover molecular mechanisms that regulate interstitial axon branching in the mammalian CNS.
Here, we show that activation of the serine threonine kinase GSK3β induces excessive branching in layer 2/3 CPNs, and we identify one of its targets, MAP1B, as a cell-autonomous branch restricting factor. In addition, we find that GSK3β/MAP1B signaling regulates the tyrosination/detyrosination cycle of α-tubulin which in turn increases or decreases the probability of generating interstitial axon branches in layer 2/3 CPNs. Together, these data describe a cell-autonomous molecular pathway that regulates interstitial axon branching in mammalian cortical neurons.
RESULTS
Activation of GSK3β in excitatory cortical neurons promotes interstitial axon branching
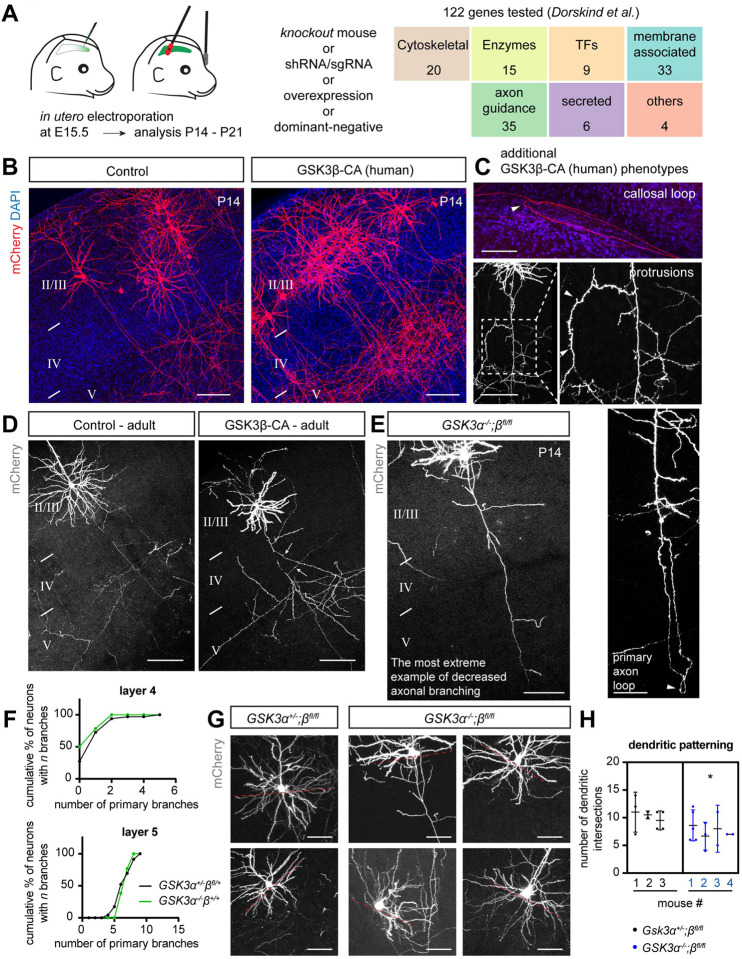
To investigate the molecular mechanisms that govern interstitial axon branching in neocortical neurons, we recently performed an in utero electroporation-based candidate screen to assess the functions of various receptors, signaling molecules, and cytoskeletal proteins in layer 2/3 CPNs11 (Fig EV1A). One of the strongest phenotypes we observed resulted from overexpression of human constitutively-active GSK3β kinase, which harbors the S9A mutation (GSK3β-CA25). Axonal morphologies of these neurons were significantly altered (Figs EV1B and C); we observed increased interstitial branching in layer 4, extensive looping and twisting of aberrant axon branches in the cortex, altered axon trajectories within the corpus callosum, and generation of shorter protrusions along layer 2/3 axons. This initial observation was surprising since previous in vitro studies using DRG neurons, and also in vivo studies on peripheral nerves, showed an opposite effect of GSK3β activity on axon branching.26–30 These studies suggested that inhibition of GSK3β activity increases the probability of axonal branch formation, however, they were not specifically focused on interstitial axon branching. Given that growth cone splitting is a major mode of peripheral nerve axon branching,5 our current results suggest that GSK3β may regulate axon branching in a context-dependent manner. Furthermore, morphological analysis of cultured hippocampal neurons isolated from Gsk3β−/− mice did not show changes in neurite numbers.28
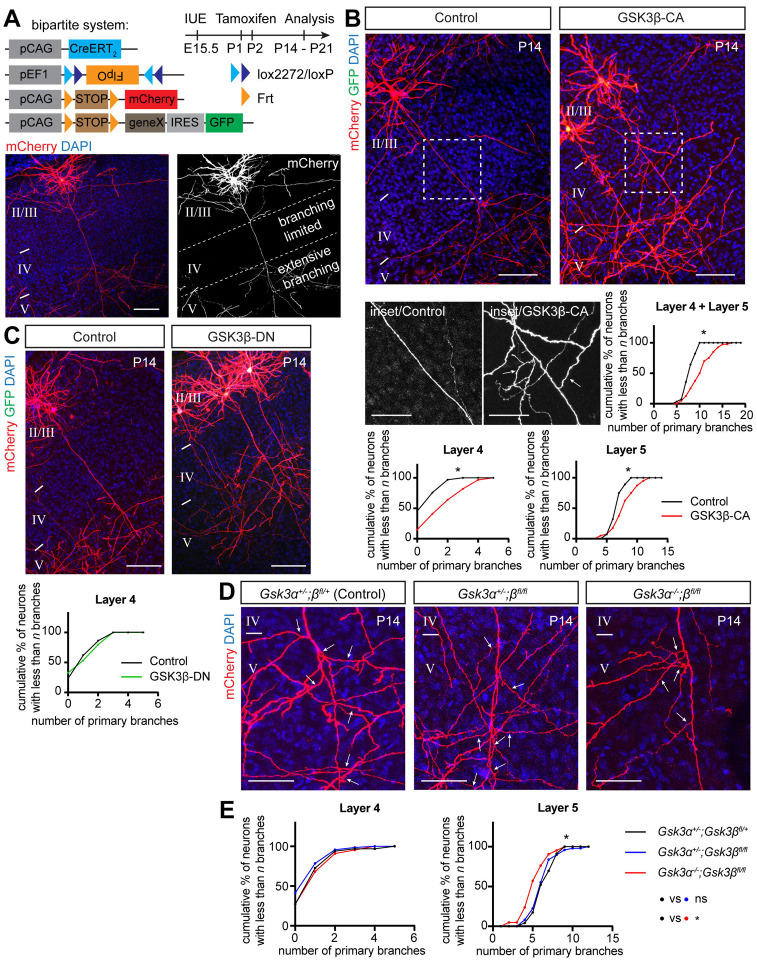
To investigate GSK3β in the context of neocortical collateral axon branching, we prepared a new mouse GSK3β-CA mutant construct and overexpressed it in layer 2/3 CPNs using our recently developed tamoxifen-dependent bipartite system that allows for sparse (~1–100 neurons/brain) and robust neuronal labeling along with the capacity to perform genetic manipulations via in utero electroporation11 (Fig 1A). This approach permits examination of individual axon morphologies and quantitative morphological assessments at a single cell resolution. Indeed, overexpression of mouse GSK3β-CA (using a GFP-expressing and FlpO-dependent pCAG-FSF-GSK3β-CA-IRES-GFP plasmid; see Methods and Table EV1 for experimental details) at embryonic day 15.5 (E15.5) in layer 2/3 CPNs resulted in a phenotype comparable to our original observations with human GSK3β11 (Fig 1B). We quantified layer 2/3 neuron primary axon collaterals in layers 4 and 5 at postnatal day 14 (P14) and found their numbers were significantly increased in comparison to control brains, displayed here in cumulative frequency plots (Fig 1B). These plots represent relative cumulative distribution (percentages) of neurons according to the extent of their interstitial axon branching; neurons with more branches shift these curves to the right. Data points represent number of interstitial branches determined for all neurons analyzed across all individual animals. For clarity, all data are also presented in the form of scatter plots depicting absolute number of axon branches in individual neurons from individual animals, together with the mean number of branches and standard deviations (Source Data 1 – 5; also, see Fig 1 Legend and Materials and Methods for fitting data to the Mixed Effects Model and the statistical tests that were applied to these data to determine significance). In addition, we analyzed the effect of GSK3β-CA expression at E15.5 in 3-month old mice and found results similar to those we observed at P14 (Fig EV1D), indicating that these ectopic branches become a stable component of cortical axon projections.
Figure 1. Activation of GSK3β in layer 2/3 callosal projection neurons promotes interstitial axon branching.
(A)Bipartite system for sparse gene expression and neuronal labeling in the layer 2/3 CPNs. Plasmids (pCAG-CreERT2, pEF1-Flex-FlpO, pCAG-Frt-STOP-Frt-mCherry, optionally pCAG-Frt-STOP-Frt-geneX-IRES-GFP) were in utero electroporated at E15.5 and tamoxifen was administered into the milk spot of the P1 and P2 pups. Brains were analyzed at P14 or P21 (note a time stamp in all images). An example of an individual sparsely labeled layer 2/3 neuron is shown in lower panels. The DAPI signal was used to identify individual cortical layers (II/III, IV and V) in all experiments. See Table EV1 for detailed information about plasmids used in all experiments. Scale bars: 100 μm.
(B) Overexpression of constitutively active GSK3β induces interstitial axon branching in layer 2/3 CPNs. Confocal images of representative neurons are shown, with insets from axonal segments passing through layer 4 (arrows, ectopic interstitial branches). Primary interstitial axon branches were quantified in layers 4 and 5. Data are presented as a relative cumulative distribution of neurons with the indicated number of axonal branches. A curve shifted to the right represents increased branching and vice versa. The same graphical representation is used throughout all following figures. See Source Data for detailed branching quantification for every sample in the form of scatter dot plots. For the description of statistics, see Materials/Methods. Data were fitted into the Mixed Effects Model72 and analyzed with either a t-test or a one-way ANOVA. Table EV1 contains absolute p values for all comparisons. * p<0.05, t-test. Scale bars: 100 μm, 50 μm (inset).
(C) Overexpression of dominant-negative GSK3β does not lead to changes in interstitial axon branching. Branches were quantified in layer 4, and data analysis is the same as in panel B. Scale bars: 100 μm.
(D, E) GSK3α and GSK3β are necessary for the generation of interstitial axon branches. Cell-autonomous deletion of Gsk3β (using pCAG-CreERT2, pEF1-Flex-FlpO, pCAG-Frt-STOP-Frt-mCherry plasmids) does not influence axonal branching, however removal of both Gsk3α and Gsk3β leads to a decrease in the number of primary interstitial axon branches in cortical layer 5. The insets show axonal segments passing through layer 5 (arrows, interstitial branches). Data analysis and presentation is the same as in panel A. * p<0.05, ANOVA with post hoc Dunnet’s test. Scale bars: 50 μm.
Next, we asked if GSK3β enzymatic activity is required for the regulation of collateral axon branching by overexpressing catalytically inactive (dominant negative) GSK3β, which harbors the K85R mutation (GSK3β-DN31). We did not observe increased branching in layer 4 following GSK3β-DN overexpression (Fig 1C), indicating that GSK3β kinase activity is sufficient to induce layer 2/3 CPN ectopic collateral axon formation in layer 4. To test if GSK3β is necessary for interstitial axon branching, we utilized conditional Gsk3βfl/fl mice32 to delete GSK3β specifically in layer 2/3 CPNs using IUE and our bipartite labeling and expression system (Fig 1D). We performed these GSK3βfl/fl IUE-mediated single neuron knock-out experiments in Gsk3α−/− and Gsk3α+/− genetic backgrounds to account for GSK3α compensatory effects. Our results showed no changes in collateral axon branching across cortical layers in a Gsk3β knockout layer 2/3 neurons but revealed significant decreases in the number of primary layer 5 collateral axon branches in Gsk3α/β double knockouts (Figs 1D and E; Fig EV1E). We also noted that in GSK3α−/− mutants there were no changes in axon branching across cortical layers (Fig EV1F). These results show that both GSK3α and GSK3β kinases are positive regulators of layer 2/3 CPN interstitial axon branching. In line with previous findings,32 we also observed that the patterning of basal dendrites is disrupted in Gsk3α/β double knockouts (Figs 1G and H).
Taken together, these results in cortical excitatory neurons in vivo reveal an unexpected and previously unrecognized role for GSK3β kinase in the regulation of interstitial axon branching. Activation of GSK3β is sufficient to promote interstitial axon branching by layer 2/3 CPNs, and both GSK3α and GSK3β are necessary for development of collateral axon branches.
MAP1B restricts layer 2/3 CPN axon interstitial branching
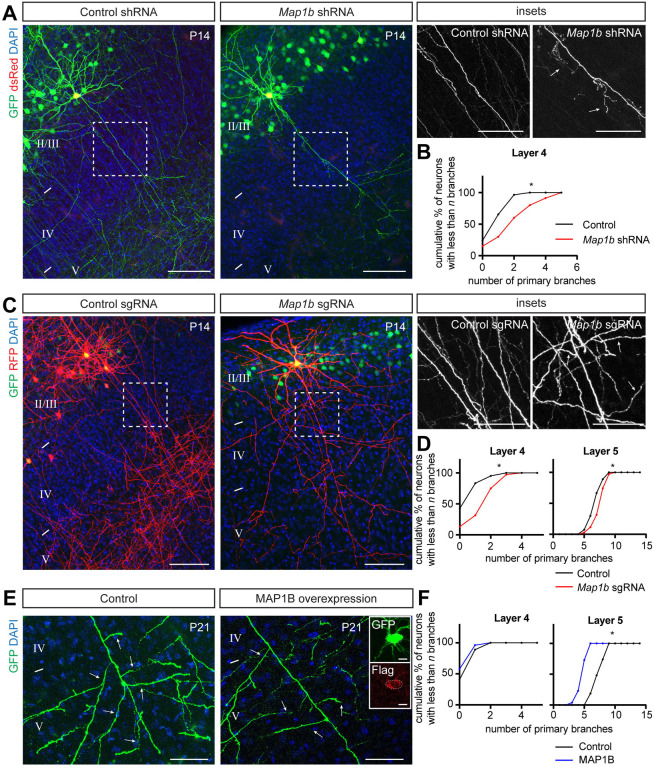
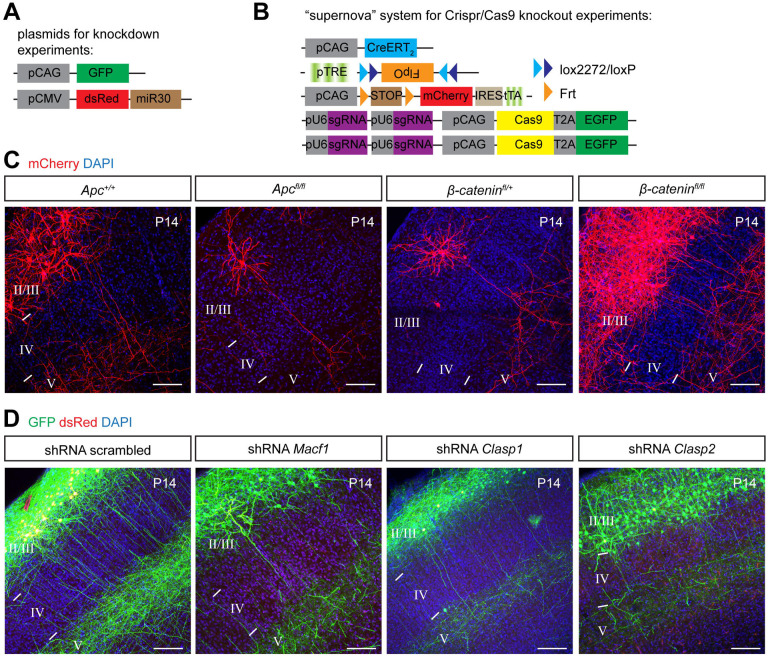
What are the downstream targets that GSK3β regulates to mediate interstitial axon branching in layer 2/3 excitatory neurons? GSK3β serves a hub for more than 100 molecules,33 including regulators of gene expression, protein synthesis, cell cycle regulation, neuronal differentiation and regulation of cell morphology through modification of the cytoskeleton. Since we find that GSK3β-CA overexpression leads to neuronal process morphological phenotypes, we focused on known GSK3β phosphorylation targets with the capacity to influence neuronal cytoskeletal dynamics. These include members of both canonical and non-canonical Wnt signaling pathways, such as MAP1B, MACF1, CLASP1, CLASP2, APC and β-catenin, all of which can be phosphorylated by GSK3β.33 We utilized either conditional knockout mice (Apcfl/fl, β-cateninfl/fl)34,35 or previously characterized shRNAs36–38 that we cloned into the pPrime-dsRed-miR30 vector (Fig EV2A) to enable direct control of expression in individual targeted layer 2/3 neurons. From this survey we found that knockdown of MAP1B in layer 2/3 CPNs causes ectopic collateral axon branching in layer 4 (Figs 2A and B), suggesting that MAP1B acts to restrict axon branching. We did not observe any changes in interstitial axon branching for any of the other candidate genes we tested (Figs EV2C and D). In addition to these shRNA-mediated loss-of-function (LOF) experiments, we validated our MAP1B results using a CRISPR/Cas9 approach (Figure EV2B). IUE using Cas9 and sgRNAs that recognize four different exons within the Map1b locus resulted in similar ectopic interstitial axon branching patterns in layer 4 and, in addition, ectopic interstitial axon branching in layer 5 (Figs 2C and D).
Figure 2. MAP1B restricts layer 2/3 CPN axon interstitial branching.
(A, B) Knockdown of Map1b using shRNA IUE leads to the generation of aberrant interstitial axon branches in layer 4 (arrows, right insets). Data analysis and presentation as in Figure 1. * p<0.05, t-test. Scale bars: 100 μm, 50 μm (insets).
(C, D) Knockout of Map1b using CRISPR/Cas9 IUE leads to the generation of aberrant interstitial axon branches in cortical layers 4 and 5 (arrows, insets on right). Data analysis and presentation is the same as in Figure 1. * p<0.05, t-test. Scale bars: 100 μm, 50 μm (insets).
(E, F) Gain-of-function (GOF) experiments with a full-length Flag-tagged MAP1B leads to reduction of interstitial branching in layer 5 (arrows). Inset on the right shows GFP and Flag signals in the cell body of analyzed neurons. Data analysis and presentation as in Figure 1. * p<0.05, t-test. Scale bars: 50 μm, 10 μm (inset on right).
We next investigated the localization of MAP1B in developing layer 2/3 axons. We used the recently developed targeted knock-in with two guides (TKIT) method for endogenous protein tagging mediated by CRISPR/Cas9 mutagenesis39 (Fig EV3A). We designed two guide RNAs directed towards the 5’ and 3’ introns of the targeted Map1b exon and provided a donor exon sequence fused to GFP with a short linker. TKIT plasmids (pX330 carrying two guides and Cas9 and pMini carrying the donor sequence) were electroporated in utero at E15.5 together with an mCherry expression plasmid to label targeted neurons. As a proof of concept, we tagged endogenous actin and observed robust localization of GFP in dendritic spines, well known actin-rich structures (Fig EV3B). Using this approach, we observed that MAP1B is highly expressed at P4 (the time when interstitial axon branching begins in layer 2/3 CPNs8) and localized in the cytoplasm of dendrites and axons, as well as in growing axon collaterals and distal axons in the corpus callosum (Fig EV3C). Similar results were found after tagging endogenous GSK3β (Fig EV3D).
Since our endogenous tagging showed that MAP1B is not restricted to any axonal compartment, we next asked if its overexpression can inhibit collateral axon branching. We overexpressed full length C-terminal Flag-tagged MAP1B in layer 2/3 CPNs using our bipartite system and quantified collateral axon branching in layer 5. Indeed, electroporated neurons showed a ~30% reduction in the number of primary intestinal axonal branches in layer 5 (Figs 2E and F) compared to control neurons. There were no changes in axon branching in layer 4, as expected (Fig 2F). Together, these data show that MAP1B is expressed in developing layer 2/3 cortical axons and restricts interstitial axon branching of these CPNs.
MAP1B acts downstream of GSK3β in axon collateral branching regulation
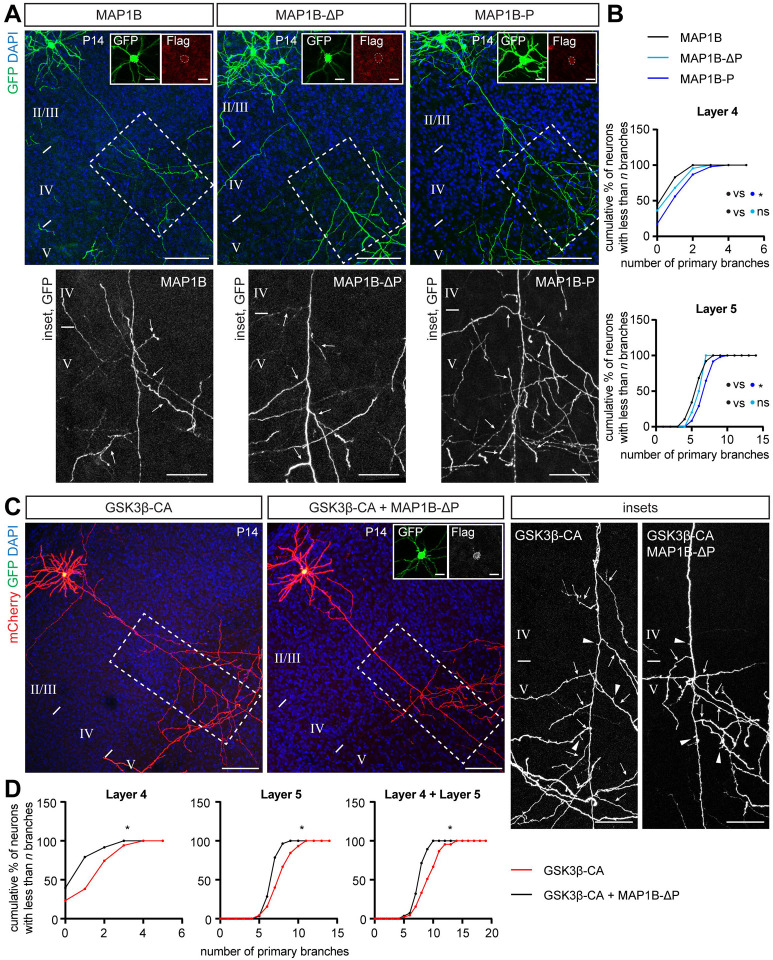
MAP1B is phosphorylated by multiple kinases including GSK3β, Dyrk1a and casein kinase 1 (CK1).40 There are at least three residues within the MAP1B microtubule-association domain that are recognized by GSK3β: two unprimed (S1260, T1265) and one (S1391) that is primed by Dyrk1a phosphorylation at S1395.41 MAP1B phosphorylated at all of these sites is known as “mode I phosphorylated MAP1B.” Interestingly, previous experiments showed that MAP1B is poorly phosphorylated when overexpressed alone (e.g. without a GSK3β kinase) in heterologous non-neuronal cells in vitro.42 Since our data suggest that overexpression of MAP1B restricts axon branching (Fig 2E), we hypothesized that this effect is mediated by non-phosphorylated (naïve) MAP1B. We therefore asked whether GSK3β phosphorylation of MAP1B is necessary for collateral axon branching by taking advantage of point mutations in MAP1B phosphoresidues. We prepared a dephosphomimetic (non-phosphorylatable) MAP1B mutant harboring the mutations S1260A, T1265A and S1391A (referred to here as MAP1B-ΔP), and also a phosphomimetic MAP1B mutant harboring S1260D, T1265D and S1391D mutations (referred to here as MAP1B-P). We tested the effects of these MAP1B mutants on layer 2/3 CPN interstitial branching compared to overexpression of naïve MAP1B. We found that neurons expressing MAP1B-ΔP had a similar number of interstitial axon branches in layers 4 and 5 as neurons expressing wild-type MAP1B (Figs 3A and B). On the other hand, overexpression of the MAP1B-P phosphomimetic gain-of-function mutant protein led to a significant increase in interstitial axon branching in layers 4 and 5, as compared to wild-type MAP1B (Figs 3A and B), indicating that phosphorylated MAP1B promotes interstitial axon branching. Direct comparison with control WT neurons showed that phosphomimetic MAP1B-P induces ectopic axon branches in layer 4 (Fig EV3E). Thus, our results gained from GSK3β and MAP1B manipulations suggest that GSK3β is a MAP1B activating enzyme. Therefore, we hypothesized that preventing MAP1B phosphorylation should rescue the GSK3β-CA phenotype. We overexpressed either GSK3β-CA alone or together with our dephosphomimetic MAP1B-ΔP construct and quantified the number of primary axon branches in layer 4 and layer 5. Indeed, we found that that overexpression of MAP1B-ΔP is epistatic to GSK3β-induced ectopic interstitial axon branching (Figs 3C and D). This suppression was not complete, since we detected the formation of small protrusions along some axons despite the presence of MAP1B-ΔP (Fig 3C, arrowheads), suggesting that another substrate of GSK3β may be involved in this process. In line with this notion, our data show that overexpression of MAP1B-P does not fully phenocopy the GSK3β-CA phenotype and leads to increased branching in layer 4, but not in layer 5, as compared to WT neurons (Fig EV3E). Alternatively, it is also possible that the maximal level of MAP1B-P that can be obtained using IUE does not meet the threshold necessary for promoting ectopic branching in layer 5. Taken together, these results show that MAP1B restricts interstitial axon branching in its non-phosphorylated state, and that these axonal branch-restrictive properties of MAP1B (a ‘MAP1B brake’ on interstitial axon branches) are released following GSK3β phosphorylation.
Figure 3. Branch-restrictive properties of MAP1B are released following GSK3β phosphorylation.
(A, B) Overexpression of Flag-tagged MAP1B, dephosphomimetic MAP1B mutant (MAP1B S1260A, T1265A, S1391A, referred to here as MAP1B-ΔP) and phosphomimetic MAP1B mutant (MAP1B S1260D, T1265D, S1391D, referred as MAP1B-P) in layer 2/3 CPNs. Insets show details of neuronal cell bodies with GFP and Flag signals. Insets at bottom show interstitial axon branches forming in layer 5 (arrows). MAP1B-ΔP has a similar effect on branching as wild-type MAP1B, while MAP1B-P increases the number of axonal branches in comparison with wild-type MAP1B. Data analysis and presentation as in Figure 1. * p<0.05, ANOVA with post hoc Dunnet’s test. Scale bars: 100 μm, 50 μm (insets at bottom), 10 μm (cell body insets).
(C, D) MAP1B-ΔP rescues the GSK3β-CA phenotype. Overexpression of GSK3β-CA together with dephosphomimetic MAP1B-ΔP reduces the overall number of interstitial axon branches compared to GSK3β-CA alone. Inset shows GFP and Flag signals in the neuronal cell body. Insets on right show segments of layer 4 and 5 with arrows marking interstitial axon branches. Note that short protrusions (axon segments shorter than 30 μm) are formed under both conditions (arrowheads). Data analysis and presentation as in Figure 1. * p<0.05, t-test. Scale bars: 100 μm, 50 μm (insets), 10 μm (cell body insets).
α-tubulin tyrosination promotes collateral axon branching
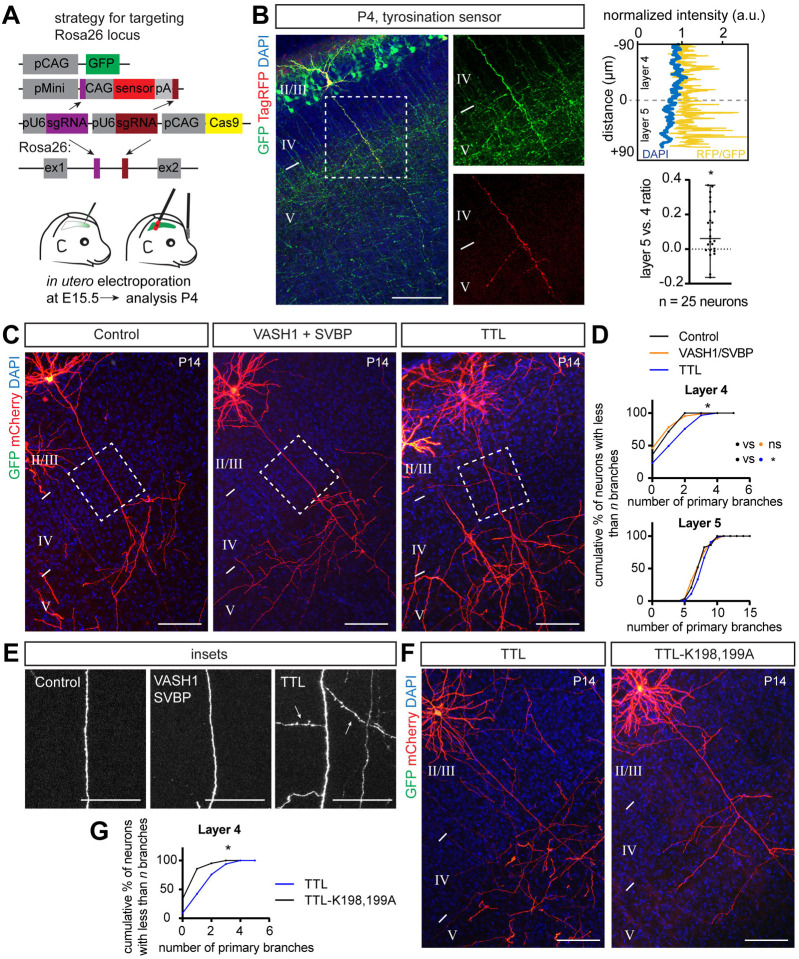
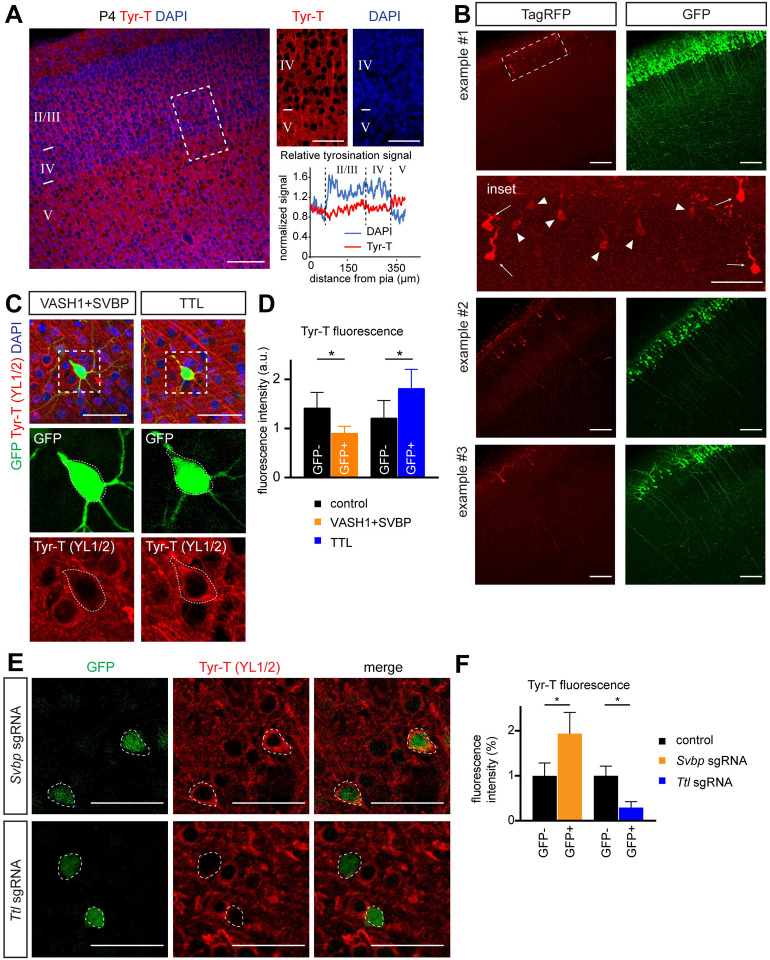
Our data show that GSK3β promotes interstitial axon branching through phosphorylation of MAP1B, while naïve dephosphorylated MAP1B restricts it. Next, we investigated the link between GSK3β-mediated MAP1B phosphorylation and cytoskeletal changes that could regulate collateral axon branching. Shifting the balance of MAP1B towards its phosphorylated form increases the ratio of tyrosinated:detyrosinated MTs,42 and our results suggest that MAP1B phosphorylation underlies the formation of axon collaterals. Therefore, we hypothesized that promotion of interstitial axon branching requires higher levels of tyrosinated MTs, in contrast to increased levels of detyrosinated MTs that would restrict branching. This further suggests that total tyrosinated MT levels will be higher in layer 2/3 axons as they pass through layer 5, where interstitial axon branching occurs, as compared to layer 4, where it does not. We first tested this idea by immunostaining cortical sections using an antibody that specifically recognizes tyrosinated tubulins (see Methods). We found this antibody indeed labeled neuropil in layer 5 more strongly than in layer 4 in the P4 cortex (Fig EV4A). To gain a more precise assessment of tyrosinated MT distribution, we used a recently designed fluorescent TagRFP-based sensor (Tyr-T sensor), which preferentially binds to tyrosinated tubulin43 and expressed it in individual layer 2/3 CPNs to directly measure MT tyrosination. We used the CRISPR/Cas9-based TKIT method combined with plasmid delivery by in utero electroporation, as described above, employing CRISPR guides to target the endogenous mouse Rosa26 locus and also providing a donor plasmid that encodes the Tyr-T sensor sequence (Fig 4A). We co-electroporated a GFP-expressing construct to identify IUE-targeted regions in the cortex, harvested the brains at P4, and analyzed tyrosinated MT distribution in individual neurons as the ratio of TagRFP to GFP fluorescence (neuronal compartments with high concentrations of tyrosinated MTs will have a stronger TagRFP signal).
Figure 4. α-tubulin tyrosination promotes collateral axon branching.
(A) Strategy for analysis of tyrosinated α-tubulin distribution using a Tag-RFP sensor that recognizes tyrosinated tubulin and a CRISPR/Cas9 knockin approach (see main text for sensor details). Plasmids were delivered at E15.5 via in utero electroporation, the sensor was expressed from the Rosa26 locus following targeted insertion, and the tyrosination signal was analyzed at P4.
(B) An example of tyrosinated tubulin sensor distribution along layer 2/3 CPN axons. The Tag-RFP signal in layer 4 and layer 5 was normalized to co-electroporated GFP and is presented as a normalized intensity. A sample trace is shown at the top right, where the blue line denotes the DAPI signal and the yellow line denotes normalized RFP/GFP intensity. Bottom right: RFP/GFP intensity in axons passing through layer 4 and layer 5 was compared using a Wilcoxon Matched-pairs test and is presented as a layer 5/layer 4 ratio (individual values together with mean ± min/max are plotted). * p<0.05. Scale bar: 100 μm.
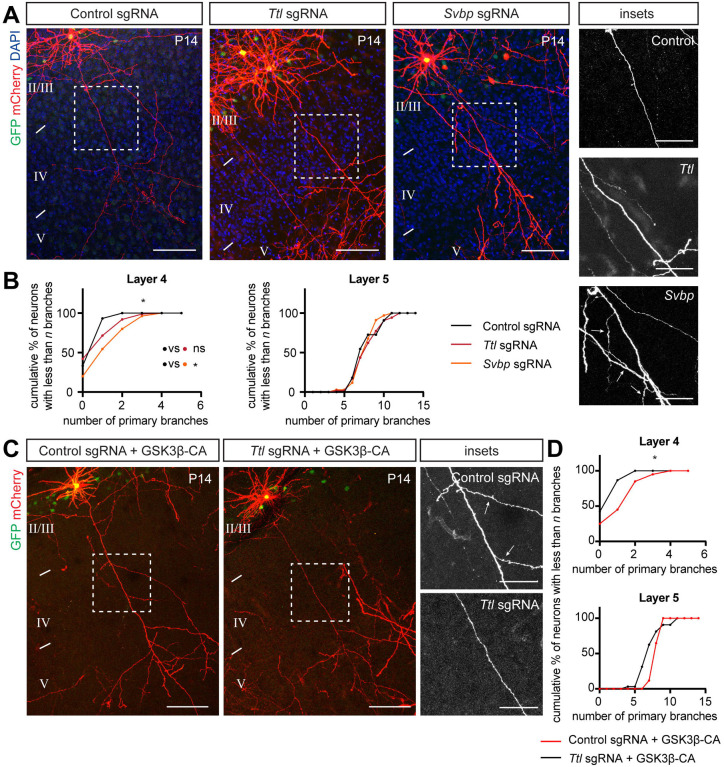
(C) Gain of function experiments with VASH1 and SVBP (to promote detyrosination) and TTL (to promote tyrosination) show that TTL overexpression is sufficient to promote interstitial axon branching. Promoting detyrosination did not influence interstitial axon branch formation. Scale bars: 100 μm.
(D) Quantification of the effect of VASH1/SVBP and TTL overexpression on interstitial axon branching. Data analysis and presentation as in Figure 1. * p<0.05, ANOVA with post hoc Dunnet’s test.
(E) Insets from (C) highlight axon segments within layer 4. Note ectopic branches formed in layer 4 following TTL overexpression (arrows). Scale bars: 50 μm.
(F, G) Overexpression of a dominant-negative TTL (TTL-K198,199A) does not lead to the formation of ectopic interstitial axon branches in layer 2/3 CPNs, indicating that enzymatic activity of TTL is necessary to for this process. Data analysis and presentation as in Figure 1. * p<0.05, t-test. Scale bars: 100 μm.
Initial examination of the TagRFP+ fluorescence signal in layer 2/3 cortical excitatory neuron cell bodies revealed a bimodal distribution of fluorescence intensities, likely reflecting recombination of either a single, or both, Rosa26 alleles (Fig EV4B). We did not observe TagRFP+ signal in control animals that were electroporated with donor plasmids but no CRISPR guides (data not shown). Next, we measured the fluorescence intensity of the sensor along individual layer 2/3 axons in layer 4 and layer 5 at P4. Only neurons with brighter cell bodies showed detectable sensor fluorescence in axons, and we focused, therefore, on these for our analyses. The TagRFP+ signal was normalized to the GFP signal from the same axonal segment to calculate the RFP/GFP ratio. Comparing this parameter between layer 4 and layer 5 in individual layer 2/3 neuron axons revealed a significant increase in the RFP/GFP ratio in layer 2/3 CPN axons within layer 5, as compared to axon segments within layer 4 (Fig 4B); this indicates that the distribution of tyrosinated tubulin in the axons of layer 2/3 CPNs is biased towards layer 5. Thus, these results show a positive correlation between the MT tyrosination signal and the layer-specific position of layer 2/3 CPN axon segments in the cortical wall, favoring tyrosinated MTs in the axonal segment that is normally enriched in interstitial axon branches.
We next addressed the functional relationship between tubulin tyrosination and interstitial axon branching. Using our bipartite system, we overexpressed either full length TTL (tyrosine tubulin ligase) or detyrosinating enzymes (VASH1 or VASH2) together with SVBP (small vasohibin binding protein). SVBP acts as a chaperon and cofactor for VASH1 and VASH2.44 These approaches led to expected changes in the level of tyrosinated tubulin: a decrease in MT tyrosination with VASH1/SVBP and an increase in MT tyrosination with TTL, as revealed by immunolabeling of slices derived from experimental cortices (Figs EV4C and 4D). Quantification of interstitial axon branching showed that overexpression of TTL increases interstitial axon branching in layers 4 and 5, while VASH1/SVBP and VASH2/SVBP had no effect (Figs 4C – E; VASH2/SVBP not shown). This effect of TTL is likely mediated by its enzymatic activity since overexpression of dominant-negative TTL mutant (TTL-DN, harboring K198A and D200A mutations45,46) in layer 2/3 CPNs significantly decreased branching in layer 4 as compared to naïve TTL overexpression (Figs 4F and G). These results suggest that increasing the level of tubulin tyrosination is sufficient to promote interstitial axon branching in cortical neurons.
Disrupting the tubulin tyrosination cycle causes aberrant interstitial axon branching
The role of the tubulin tyrosination cycle in interstitial axon branching is unexplored.47 Previous experiments have focused on analysis of axon outgrowth in immature neuronal cultures.48,49 However, contrasting results have been obtained from Ttl−/− neurons and Svbp−/− neurons. Embryonic hippocampal or precerebellar neurons isolated from embryonic Ttl−/− mice show an increased axon growth rate and neurite branching after 2 days in vitro (DIV2).48,50 Similarly, embryonic hippocampal neurons isolated from Svbp−/− mice display more neurite branches in vitro at DIV2.44 Ttl−/− mice die within a day after birth, and so analysis of interstitial axon branching in vivo in these mice is not possible.48 Neuronal morphology in Svbp−/− or the recently generated Matcap−/− mice, which both lack detyrosinated microtubules, has not yet been investigated.51,52 Our results from gain-of-function experiments targeting the tubulin tyrosination cycle suggest that increasing tubulin tyrosination promotes interstitial axon branching.
To address the necessity of tubulin tyrosination for interstitial axon branching, we used a CRISPR mutagenesis strategy to generate mutations that result in the deletion of enzymes that modulate the tubulin tyrosination cycle in vivo in individual layer 2/3 neurons. We designed CRISPR guides targeting the first four exons of Ttl or the first three exons of Svbp, respectively. Control staining with an antibody against tyrosinated MTs confirmed the efficiency of this strategy at the level of single neurons (Figs EV4E and F). Deletion of Ttl, which shifts the tyrosination/detyrosination ratio toward detyrosination, did not lead to increased or decreased axon branching, although we noted a marked increase in dendritic branching in targeted neurons (Fig 5A). However, deletion of Svbp, which favors tyrosination, resulted in an increase in layer 2/3 CPN interstitial axon branching in layer 4, but not in layer 5 (Figs 5A and B). We also noted a mild defect in neuronal migration of mutated cells (data not shown), which is in line with previous observations.44 Further, analysis of Svbp−/− mice showed that the depletion of detyrosinated tubulin is incomplete in these mutants,52 suggesting the existence of additional enzyme(s) with detyrosinating activity. Indeed, a recent study identified the MATCAP protein, which also removes tyrosine residues from tubulin.51 Therefore, we simultaneously deleted both Svbp and Matcap, however this manipulation did not lead to a further increase in interstitial axon branching since we found a phenotype comparable to that observed following Svbp deletion (Figs EV5A – C). Nevertheless, our loss-of-function experiments suggest that disruption of the tyrosination/detyrosination ratio causes aberrant interstitial axon branching in layer 2/3 CPNs.
Figure 5. Disrupting the tubulin tyrosination cycle causes aberrant interstitial axon branching.
(A, B) Loss-of-function of enzymes regulating tyrosination/detyrosination cycle using a CRISPR/Cas9 approach. Svbp deletion increased the number of primary axon branches in layer 4. Ttl deletion did not affect interstitial axon branching. Insets on right show ectopic axon branches in axons passing through layer 4 following removal of Svbp (arrows). Data analysis and presentation as in Figure 1. * p<0.05, ANOVA with post hoc Dunnet’s test. Scale bars: 100 μm, 50 μm (insets).
(C, D) TTL acts downstream from GSK3β. Removal of Ttl via CRISPR/Cas9 in neurons overexpressing GSK3β-CA rescues GSK3β-CA-induced ectopic branching (arrows in insets on right). Data analysis and presentation as in Figure 1. * p<0.05, t-test. Scale bars: 100 μm, 50 μm (insets).
GSK3β/MAP1B signaling regulates interstitial axon branching through modification of tubulin tyrosination
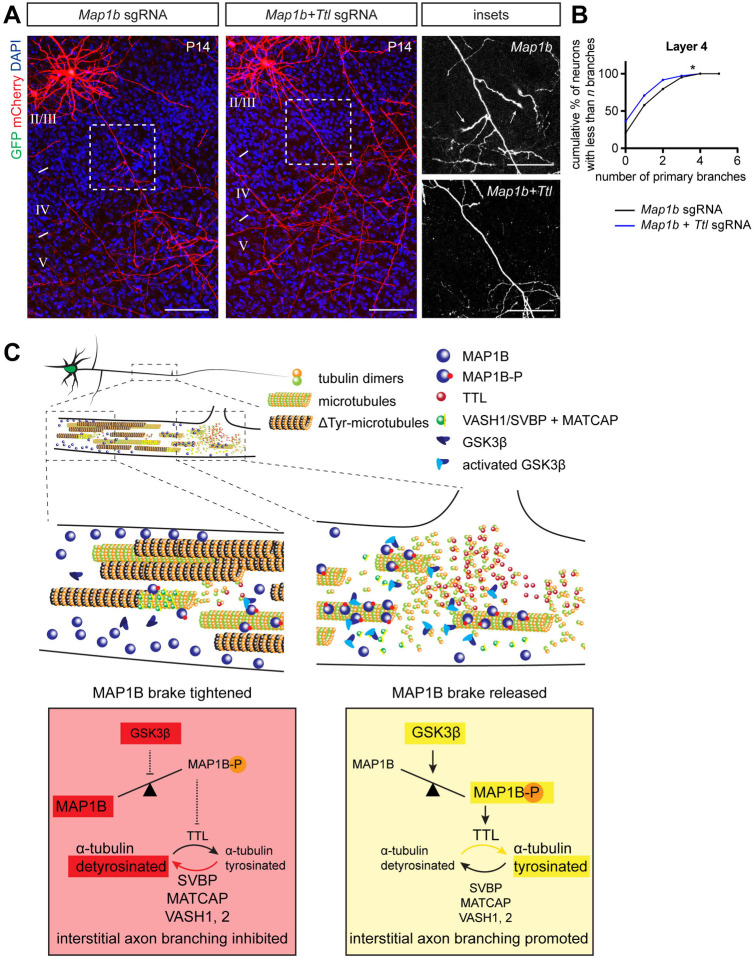
Since we find that GSK3β signaling regulates interstitial axon branching by releasing an inhibitory MAP1B brake on axon branching, resulting in the promotion of tubulin tyrosination, we next asked if there is indeed a link between GSK3β activity and tubulin tyrosination. If alteration of the tubulin tyrosination/detyrosination cycle occurs downstream of a MAP1B brake on the regulation of axon branching, activation of GSK3β should promote tyrosination, and blocking tyrosination via TTL deletion should prevent formation of GSK3β-induced interstitial axon branches. To test these ideas, we overexpressed GSK3β-CA together with CRISPR/Cas9 plasmids targeting TTL. As a control, GSK3β-CA was co-electroporated with control CRISPR/Cas9 constructs. We found that removing TTL suppresses aberrant GSK3β-CA-induced interstitial axon branch formation in layer 4 and layer 5 (Figs 5C and D). Next, we investigated the interaction between the MAP1B brake on interstitial axon branching and tyrosination using CRIPSR-mediated loss-of-function. We deleted either Map1b alone (control), or together with Ttl to inhibit tyrosination (Fig 6A). These manipulations showed that preventing tubulin tyrosination normalizes ectopic interstitial axon branching induced by MAP1B deficiency (Fig 6B). Taken together, these results show that TTL acts downstream from GSK3β/MAP1B signaling axis, demonstrating how regulated posttranslational MT modifications (the tubulin code) contribute to stereotypic patterns of CPN interstitial axon branching.
Figure 6. GSK3β/MAP1B regulates interstitial axon branching through modification of tubulin tyrosination.
(A, B) TTL acts downstream from MAP1B. Removal of Ttl via CRISPR/Cas9 in neurons overexpressing GSK3β-CA rescues ectopic interstitial axon branching induced by Map1b deletion (arrows in insets on right). Data analysis and presentation as in Figure 1. * p<0.05, t-test. Scale bars: 100 μm, 50 μm (insets).
(C) The intracellular signaling pathway that regulates the formation of interstitial axon branches in cortical neurons. Our results suggest the following model: MAP1B functions a brake on branching (left, red panel), which can be released following GSK3β-mediated phosphorylation (right, yellow panel). Subsequently, the ratio of tyrosinated/detyrosinated tubulin shifts towards the tyrosinated form, promoting the formation of interstitial axon branches.
DISCUSSION
Interstitial axon branching is a fundamental phenomenon, allowing CNS neurons to connect to multiple targets that are spatially distinct. In the neocortex, laminar-specific interstitial axon branching provides a scaffold for the elaboration of select cortical circuitry. Defining molecular mechanisms that regulate interstitial axon branching in the CNS remains a major unresolved question. Here, we use recently developed robust methods to study axonal morphology in vivo in mammalian cortical neurons,11 and we quantitatively analyze axonal morphology of thousands of neurons at a single-cell resolution to reveal cytosolic regulators of interstitial axon branching in mammalian layer 2/3 CPNs. A major finding from our experiments is that the MT binding protein MAP1B acts to restrict interstitial CPN axon branching in vivo, and this brake on branching can be released following GSK3β-mediated phosphorylation. Subsequently, the ratio of tyrosinated/detyrosinated tubulin shifts towards the tyrosinated form and promotes formation of interstitial axon branches (see Fig 6C for a graphical summary). These conclusions are supported by the following: 1) GSK3β loss-of-function and gain-of-function experiments show that GSK3α and β isoforms are necessary, and GSK3β is sufficient, for interstitial axon branching; 2) MAP1B loss-of-function, overexpression and mutagenesis experiments show that naïve unphosphorylated MAP1B restricts interstitial branching while phosphorylated MAP1B promotes it; 3) Genetic manipulations of the tubulin tyrosination cycle reveal that increasing the ratio of tyrosinated to detyrosinated α-tubulin promotes interstitial axon branching; and 4) Decreasing tyrosination using CRISPR-mediated loss-function prevents excessive formation of interstitial axon branches induced by GSK3β gain-of-function or MAP1B loss-of-function. Together, these observations reveal an intracellular signaling pathway that cell-autonomously regulates interstitial axon branching in mammalian cortical neurons.
The identification of signaling pathways that regulate interstitial axon branching so as to regulate cortical laminar-specific innervation has been elusive, though there are multiple signaling molecules known to direct cortical neuronal axon pathfinding and axon extension in cortical neuron subtypes. These include classical axon guidance cues, morphogens, and their receptors (reviewed in:4,7,9,10,53,54). In addition, cytosolic signaling molecules, including Ankyrin-B,55 are capable of refining the formation of terminal contralateral CPN axon branching patterns, and LKB1-NUAK1 kinases regulate terminal CPN axon branching by presynaptic capture of mitochondria.14 Further, Wnk kinases, in both Drosophila mechanosensory neurons and mammalian CPNs, regulate terminal axon branching.20 However, these signaling molecules and many others, including a wide range of signaling molecules we have assessed using approaches described elsewhere11 and as part of this present study (data not shown), do not appear to function in the regulation of interstitial axon branches that arise de novo from the axon shaft. To the best of our knowledge, there are currently very few mouse mutants that display specific defects in cortical interstitial axon branching such as ectopic interstitial ipsilateral axon branching of layer 2/3 CPNs in layer 411. Further, this stereotypic interstitial branching pattern is likely to arise from the effects of extracellular cues, incorporated within the extracellular matrix or on the surface of cells in layer 4 and 5, that are yet to be discovered.
There are several important considerations when investigating interstitial axon branching. These include distinguishing interstitial axon branching from among the various types of axon branching described to date that include: growth cone splitting, terminal axon branching, and terminal axon branching coupled to synaptogenesis which can be influenced by neural activity.7,9 Current labeling techniques are limited with respect to their ability to allow for precise analysis of interstitial axon branching in the developing mammalian brain. The evaluation of cortical axon branching often relies on quantification of fluorescence intensities from axonally-expressed fluorophores in large numbers of neurons, which is limited with regard to defining precise levels of interstitial axon branching.20,56,57 Extensive analyses of axon branching in various in vitro systems have been very informative.12,58–60 However, the interpretation of results gleaned from dissociated neurons in vitro is complicated since interstitial axon branching in vivo is spatially regulated to allow for laminar-specific innervation by subsets of CPNs, so it is important to complement these studies with in vivo assessments of factors that regulate axon branching.
A central conclusion from this study is that activation of GSK3β can induce interstitial axonal branching. GSK3β has been previously implicated in regulating various aspects of neuronal development, including cortical excitatory neuron migration, neuronal polarization, axon outgrowth and neurotrophin-induced axon branching.61 To study the role of GSK3β in interstitial axon branching, we employed a labeling approach, which we have termed here a “bipartite system”.11 This made it possible to recombine the Gsk3β floxed allele in post migratory neurons, allowing us to expand upon previous work which reported severe cortical neuron migration defects and early postnatal lethality in Gsk3α−/−Gsk3βfl/fl:Neurod6-Cre mutants.32 Our data suggest that GSK3β is locally inhibited in layer 2/3 CPN axons within layer 4, resulting in ectopic interstitial axon branches. In line with previous findings,28,32 we also observe some redundancy between the GSK3 isoforms α and β – we find that only deletion of both (using Gsk3α−/−;Gsk3βfl/fl mice) inhibits layer 2/3 CPN interstitial axon branching in vivo in layer 5.
We have identified MAP1B, a microtubule-binding protein known to predominantly bind to MT polymer shafts, in our search for downstream GSK3β targets that regulate interstitial axon branching.62 MAP1B regulates neuronal polarity and axon development, but its role in interstitial axon branching has not been quantitatively assessed in vivo.63–65 However, previous work indicates that MAP1B restricts axon branching in vitro in regenerating DRG axons,13 and our observations are in line with these findings. Interestingly, we found that other candidate GSK3β substrate molecules, including APC, MACF1 and CLASPs, which belong to the group of plus-end tracking proteins (+TIPs) and thus bind to the growing end of MTs,66 do not appear to regulate layer 2/3 CPN interstitial axon branching. Given MAP1B binding preferences, we speculate that this GSK3β/MAP1B pathway is important for local changes in axon MT dynamics and thus is involved during the initial phase of interstitial axon branch formation.7 In addition, we find that GSK3β-mediated phosphorylation of MAP1B is also necessary for collateral axon branch formation since overexpression of dephosphomimetic MAP1B mutant prevented GSK3β-induced axon branching. This suggests that MAP1B should be preferentially phosphorylated in axon segments in layer 5. Interestingly, enrichment of mode I phosphorylated MAP1B has indeed been observed in layer 5 axons in developing mouse cortex.67 Thus, we propose a ‘MAP1B brake’ model in which a brake on the formation of interstitial axon branches is released by GSK3β-mediated MAP1B phosphorylation.
Previous work shows that MAP1B regulates microtubule dynamics through modulation of tubulin tyrosination cycle; phosphorylated MAP1B causes a nearly complete loss of detyrosinated microtubules.42 Tubulin detyrosination, the removal of C-terminal tyrosine from the α-tubulin isoform, can be reversed by tyrosination. The tyrosination/detyrosination cycle is one feature of the tubulin code, which includes multiple post-translational tubulin modifications, each of which is capable of exerting distinct effects on cellular functions.47,68 For example, it has been recently shown that the tubulin glutamylases TTLL6 and TTLL11 regulate motor axon development in Zebrafish.21 However, the function of the tyrosination cycle in regulating neuronal morphology has not been extensively tested in vivo. Our in vivo loss-of-function and gain-of-function experiments utilizing tyrosination cycle enzymes show that shifting the ratio towards tyrosinated MTs is sufficient to induce interstitial axon branching in layer 2/3 CPNs. Ttl loss of function rescues GSK3β-CA-induced ectopic axon branching, indicating that the tyrosinating enzyme TTL acts downstream from GSK3β. Together, our results show that formation of CPN axon collateral branches is regulated by the tubulin code.
How the MAP1B brake and tubulin tyrosination cycle are linked remains to be determined. We observed that TTL overexpression does not increase branching in neurons overexpressing MAP1B (data not shown), although we find that TTL acts downstream of MAP1B. Thus, we hypothesize that GSK3β-mediated phosphorylation of MAP1B could increase its binding to microtubule polymer shafts, as opposed to binding actin. This idea is supported by the observation that dephosphorylated MAP1B binds to actin69, and this would inhibit physical association between MTs and detyrosinating VASH/SVBP complexes. As a result, microtubules would remain tyrosinated, be more dynamic and subject to polymerizing/depolymerizing events more often, and so would lead to axon branch formation. To investigate how tyrosinated MTs affect interstitial axon branching we tested the involvement of the p60 catalytic subunit of Katanin, a MT severing protein known to preferentially act on tyrosinated MTs.70 However, we did not find evidence that p60 Katanin is sufficient or necessary to modulate CPN interstitial axon branching in vivo (data not shown). This could reflect the need for p60 Katanin at levels we were not able to achieve in our experiments, or functional redundancy among multiple MT severing proteins (including Katanin-like 1 or Spastin).
Our results uncover an intracellular signaling pathway that cell-autonomously regulates interstitial axon branching in CPNs during neural development. However, the stereotypic laminar-specific patterning of interstitial axon branches in the neocortex likely reflects unknown instructive and/or permissive cues that act locally in cortical layers 4 and 5. The activity of GSK3β is tightly controlled by Protein Kinase B and PTEN,71 and so it is possible that these enzymes serve as a link between a cell surface receptor and GSK3β. The identity of cell surface proteins capable of transmitting laminar-specific extracellular signals that regulate GSK3β/MAP1B/MT tyrosination-mediated interstitial axon branching is an important next step in understanding how cortical circuits are established during development.
In conclusion, using methodologies that allow for robust, sparse labeling and precise spatiotemporal genetic manipulation of layer 2/3 CPNs we have identified molecular regulators of interstitial axon branching. We propose a model whereby a MAP1B brake restricts interstitial axon branching until it is released by GSK3β phosphorylation, allowing for regulated generation of a pool of tyrosinated microtubules in the axonal shaft. These observations are an important first step in understanding the generation of stereotypical laminar-specific CPN axon branches and may be generally applicable to multiple populations of cortical excitatory projection neurons.
MATERIALS AND METHODS
Resource availability
All reagents generated in this study are available upon request. Source data are provided with this paper. A list of reagents used in the study is provided in the Table EV2. All raw microscopy data and analyses related to this paper will be deposited at the Johns Hopkins Research Data Repository that is managed by Johns Hopkins Data Services (JHDS). This repository provides public access to these data through an established platform supported by storage and preservation practices that follow the Open Archival Information System reference model. Deposited data will be given a standard data citation and persistence identifier (DOI). Data are archived under a memorandum of understanding renewed every 5 years with the PI’s consent.
Animals
Experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH. The animal protocol was approved by the Animal Care and Use Committees of the Johns Hopkins University School of Medicine. Timed pregnant CD1 wild-type females were obtained from Charles River Laboratory. The day of birth was designated as P0. Mice of both sexes were used in all experiments and were housed in a 12:12 light-dark (LD) cycle. Gsk3α−/− and Gsk3βfl/+ mutant mice were gifts from Feng-Quan Zhou,32 Apcfl/fl mice were gift from dr. Bart Williams34 and β-cateninfl/fl mice were gift from Jeremy Nathans.35
Plasmids
All plasmids used in this study are listed in the Key Resources Table. Plasmids sued for the bipartite labeling method were as described.11 Supernova plasmids are as described.73 Plasmids used for knockdown experiments are as described.74 MAP1B was amplified from a GFP-MAP1B-expressing plasmid (a gift from Phillip Gordon-Weeks, Addgene# 4439641), the N-terminal GFP sequence was removed and the DNA sequence encoding the Flag epitope was added in frame at the C-terminus. In addition, we introduced a silent mutation to create an AgeI restriction site, simplifying the cloning of phospho-mutant constructs. These were generated by PCR mutagenesis of the core portion of MAP1B (between the endogenous HindIII site and a new AgeI site) and included phosphomimetic (S1260D, T1265D, S1391D) and dephosphomimetic (S1260A, T1265A, S1391A) mutations. Coding DNA sequences for GSK3β, TTL, SVBP, VASH1 and VASH2 were amplified from mouse cortical P5 cDNA. The TTL-DN plasmid was generated by PCR mutagenesis and contained K198A and D200A mutations.46 Sequences of TKIT donor plasmids were amplified from mouse genomic DNA and GFP with a short linker placed in frame at the 5’ or 3’ end, in line with the amino-terminal or carboxy-terminal tagging. Polycistronic CRISPR plasmids pX33075 and pX45876 were optimized so that one vector contained two U6 promotors with two sgRNA sequences.39 Guide RNA sequences were chosen as described.77,78 The DNA sequence encoding the TagRFP-T_A1aY1 Tyr-T sensor was amplified from Addgene plasmid #158751 (a gift from Minhajuddin Sirajuddin43) and cloned into the pMini donor plasmid together with the CAG promoter and bGH polyA signal. In all cases, multiple fragments were assembled together using a HiFi NEBuilder kit. All new constructs were verified by restriction reaction and sequencing. For the purpose of in utero electroporations, endotoxin-free plasmid DNA was prepared using NucleoBond® Xtra Midi EF kit.
Surgeries
In utero electroporations were performed as described.8 Briefly, timed pregnant E15.5 mice were anesthetized with isoflurane and placed on a heating pad. The incision site was shaved, cleaned, and local anesthetic (Bupivacain) was applied subcutaneously. A small laparotomy was performed, and the embryos were removed and rinsed with warm sterile PBS. The lateral ventricles were then injected with small volumes (approximately 0.5 – 1 μl) of endotoxin-free DNA solutions in PBS with Fast Green dye. Embryos were electroplated with gene paddles (Harvard Apparatus) using a BTX square pulse electroporator (parameters: 40 V, three 50-ms pulses with a 950-ms interval). Animals were placed on a heating pad to recover and pain was further controlled by subcutaneous injection of Buprenorphine. All procedures were conducted in accordance to our IUCAC-approved protocol.
Tamoxifen injections
10x stock solution was prepared as follows: Tamoxifen (10 mg) was dissolved in 250 μl Ethanol and the solution was mixed with 750 μl Corn Oil. Stock solution was stored at 4°C up to 10 days. 1x tamoxifen solution was prepared by dissolving the stock solution in Corn Oil, resulting in 1 mg/ml final concentration. Approximately 30 μl of 1x Tamoxifen was applied intragastrically (milk spot injection) at P1 and P2.
Brain processing and immunostaining
Animals were trans-cardially perfused with a modified PHEM buffer (27mM PIPES, 25 mM HEPES, 5 mM EGTA, 0.47 mM MgCl2, pH = 6.9) followed by ice-cold 4% PFA/PHEM with 5% sucrose and 0.1% Triton-X100. Brains were isolated and post-fixed overnight at 4°C. Then, all samples were washed multiple times in PBS, sliced coronally (250 μm sections) and incubated in a blocking buffer for at least 30 minutes at the room temperature (3% BSA with 0.3% Triton-X100 and 0.02% Sodium Azide in PBS, filtered). Brain sections were incubated with primary antibodies dissolved in a blocking buffer with 5% normal goat serum (NGS) overnight at 4°C. Slices were then washed three times (each round at least one hour, room temperature) in PBST (PBS with 0.1% Triton-X100) and incubated in secondary antibodies and DAPI dissolved in PBST overnight at 4°C. Following another three washes, slices were mounted on a glass slides using a Fluorogel/DABCO.
Analysis of tyrosination levels in neurons after genetic manipulations of the tubulin tyrosination cycle was performed as follows: mice (at P4 – P7) were perfused and brains isolated as described before. 100 μm thick slices were prepared and immunostained as described above. We used a rat YL1/2 antibody that specifically recognizes tyrosinated MTs. Tyrosination fluorescence signals were measured in ImageJ in GFP+ (electroporated) neurons and compared to fluorescence of neighboring GFP-negative neurons from the same slice.
Analysis of tubulin tyrosination in individual axons expressing the TagRFP sensor (Figure 4A) was performed as follows: mice (P4) were perfused and brains isolated as described before. 100 μm thick slices were prepared and immunostained as described above with antibodies directed against GFP and RFP. The sensor’s fluorescence was measured in ImageJ: we traced the axon passing through layer 4 and layer 5 and along the same trace measured the ratio of the RFP/GFP. Layer 5 and layer 4 ratios were compared using a Wilcoxon Matched-pairs test.
Microscopy
Slices were imaged with inverted or upright Zeiss 700 confocal microscopes using a 20x objective (PlanApochromat 20x/0.8). Imaging parameters were as follows: px size 0.31×0.31 μm (2048×2048 px resolution at 0.5 zoom, total image size was 640 × 640 μm), optical sections 1.8 μm, z-stack 1.5 μm. 50–70 z-stack images were taken per neuron, which usually covered the main axon extending from the cell body to the border between layers 5 and 6. Dendritic spines shown in Figure S3B were captured using 63x oil-immersion objective (Plan-Apochromat 63x/1.4 Oil DIC).
Quantification and Statistical analysis
Confocal image stacks were manually analyzed in ImageJ. First, we defined the borders between layers 4 and 5 according to the DAPI signal. Primary interstitial axon branches elaborated from the main axon were quantified in the layer 4 and 5. For neurons in which the main axon in layer 5 was missing (due to sectioning) only layer 4 branches were counted, and vice versa. Orientation of basal dendrites (Fig EV1G) was measured as follows: a line at the base of analyzed neuron was drawn (in right angles to the apicobasal neuron orientation) and the number of dendritic intersections with the line was quantified. The numbers of analyzed neurons/animals are listed in the Table EV1. Branching data were statistically analyzed by fitting a mixed effects model72 as implemented in GraphPad Prism 9.5.0. This mixed model uses a compound symmetry covariance matrix, and is fit using Restricted (Residual) Maximum Likelihood (REML). Significance was determined with either a t-test or a one-way ANOVA with a post-hoc Dunnet’s test. Distribution of tyrosination signal in axons (Fig 4B) was analyzed with Wilcoxon matched-pairs test. All p values are reported in the Table EV1.
Extended Data
Figure EV1: GSK3βregulates neuronal morphology in layer 2/3 CPNs. (related to Figure 1).
(A) Overview of original candidate molecule screen11 for identification of CPN interstitial axon branching regulators.
(B) Overexpression of human constitutively active GSK3β (GSK3β-CA) induces ectopic interstitial axon branching in layer 2/3 CPNs. Scale bars: 100 μm.
(C) Additional changes in axonal morphology induced by human GSK3β-CA include axonal loops and spiny protrusions along axons (arrowheads). Scale bars: 100 μm (including callosal loop and protrusions, right), 50 μm (primary axon loop, bottom right).
(D) GSK3β-CA-induced interstitial axon branching (arrows) in layer 2/3 CPNs persist until adulthood. Scale bars: 100 μm.
(E) Example of a severe loss of interstitial axon branches following removal of both Gsk3α and Gsk3β isoforms in the mouse (related to Fig 1D). Scale bars: 100 μm.
(F) Quantification of axon interstitial branching in GSK3α−/−;β+/+ mutant mice. Controls are the same as those shown in Fig 1D.
(G) Basal dendrite patterning in layer 2/3 CPNs is regulated by GSK3 isoforms. Red dotted line (placed at right angle to the apicobasal orientation of a neuron) highlights the region where dendritic intersections were calculated. Scale bars: 50 μm.
(H) Quantification of dendritic intersections from (G). * p<0.05, t-test.
Figure EV2: Candidate survey for GSK3β targets that regulate interstitial axon branching. (related to Figure 2).
(A) Experimental approach for shRNA-mediated knockdown experiments.
(B) Experimental approach for CRISPR/Cas9-mediated knockout experiments.
(C) Removal of Apc or β-catenin using conditional knockout mice does not influence interstitial axon branching in layer 4. Scale bars: 100 μm.
(D) Knockdown of Macf1, Clasp1 or Clasp2 does not influence interstitial axon branching in layer 4. Scale bars: 100 μm.
Figure EV3: Analysis of the GSK3β target MAP1B subcellular localization in cortical neurons using CRISPR/Cas9 endogenous tagging. (related to Figure 2 and Figure 3).
(A) Experimental design for endogenous tagging experiments using CRISPR/Cas9 knockin approach.
(B) Example of endogenous GFP-β-actin tagging. Note accumulation of GFP in actin-rich dendritic spines (dendritic spines, right). Scale bars: 100 μm (left), 10 μm (dendritic spines).
(C–D) Representative images showing endogenous tagging of Map1b and Gsk3β at P4 or P14 cortices. Note strong expression of MAP1B, which fills entire neuron, including the most distal axonal compartments in the corpus callosum (panel C, bottom). Insets in C and D show axonal expression. Scale bars: 100 μm.
(E) Overexpression of MAP1B-P leads to ectopic interstitial axon branching in layer 4. For this analysis, control neurons from Fig 2E were used.
Figure EV4: Tyrosination of α-tubulin in S1 neocortex under normal and experimental conditions. (related to Figure 4).
(A) Immunostaining of P4 cortex with an antibody directed against tyrosinated tubulin (Tyr-T, YL1/2 clone). Note sharp increase in the Tyr-T signal between layer 4 and 5. The tyrosination signal in layers 2 – 5 was normalized to the signal in cortical layer 1. Scale bar: 100 μm, 50 μm (insets).
(B) Examples of a fluorescence intensity distributions after targeting Rosa26 locus via the TKIT approach (see main text). In the detail for the example #1, note that a small population of neurons expresses high levels of TagRFP (arrows), while a larger population of neurons expresses lower levels of TagRFP (arrowheads). Scale bar: 100 μm, 50 μm (inset).
(C, D) Immunostaining of Tyr-T after TTL and VASH1/SVBP overexpression experiments. Note lack of a tyrosination signal following VASH1/SVBP overexpression and an increase in the tyrosination signal after TTL overexpression. Data are presented as mean ± st.d. * p<0.05, t-test. Scale bar: 50 μm.
(E) Immunostaining of Tyr-T following CRISPR knockdown. Note decrease of the tyrosination signal after TTL deletion and an increase in the signal after SVBP deletion. GFP labels electroporated cells that harbor the pX458 plasmid (cells with a dashed contour). Scale bar: 50 μm.
(F) Quantification of the tyrosination signal in targeted (GFP+) cells, normalized to GFP- cells from the same slices. Data are presented as mean ± st.d. * p<0.05, t-test.
Figure EV5: Disrupting the tubulin/tyrosination cycle causes aberrant interstitial axon branching.(related to Figure 5).
(A, B) Simultaneous removal of Svbp and Matcap promotes interstitial axon branching to the same extent as Svbp deletion alone. Data analysis and presentation are as in Figure 1. * p<0.05, t-test. Scale bars: 100 μm.
(C) Comparison of Svbp knockdown with combined Svbp+Matcap knockdown. t-test. Scale bars: 100 μm.
Supplementary Material
Source Data 1: Complete data for Figure 1 and Fig EV1
(A) Complete data from interstitial axon branching analysis in mice overexpressing GSK3β-CA, related to Fig 1B. For this plot and every subsequent plot, each dot represents a single neuron. Each column represents a single animal; mean ± SD is plotted.
(B) Complete data from interstitial axon branching analysis in mice overexpressing GSK3β-DN, related to Fig 1C.
(C) Complete data from interstitial axon branching analysis in Gsk3α and Gsk3β knockout mice, related to Fig 1E & Fig EV1F.
(D) Complete data from the original screen: interstitial axon branching quantification in mice overexpressing human GSK3β-CA, related to Fig EV1B.
(E) Complete data from interstitial axon branching analysis in adult mice overexpressing GSK3β-CA, related to Fig EV1D.
Source Data 2: Complete data for Figure 2
(A) Complete data from interstitial axon branching analysis in mice with Map1b shRNA-mediated knockdown, related to Fig 2A.
(B) Complete data from interstitial axon branching analysis in mice with Map1b sgRNA-mediated knockdown, related to Fig 2C.
(C) Complete data from interstitial axon branching analysis in mice overexpressing naïve MAP1B, related to Fig 2E.
Source Data 3: Complete data for Figure 3
(A) Complete data from interstitial axon branching analysis in mice overexpressing naïve MAP1B, MAP1B-ΔP and MAP1B-P, related to Fig 3A
(B) Complete data from GSK3β-MAP1B rescue experiment, related to Fig 3C.
Source Data 4: Complete data for Figure 4
(A) Complete data from interstitial axon branching analysis in mice overexpressing TTL or VASH1/SVBP, related to Fig 4C.
(B) Complete data from interstitial axon branching analysis in mice overexpressing TTL or TTL-DN, related to Fig 4F.
Source Data 5: Complete data for Figure 5, Figure EV5 and Figure 6.
(A) Complete data from interstitial axon branching analysis in mice with sgRNA-mediated knockdown of Ttl and Svbp, related to Fig 5A.
(B) Complete data from GSK3β-TTL rescue experiment, related to Fig 5C.
(C) Complete data from combined Svbp and Matcap sgRNA-mediated knockdown, related to Fig EV5A.
(D) Complete data from MAP1B-TTL interaction experiment, related to Fig 6A.
Table EV1.
Table presents mouse strain/mouse lines, plasmids used for in utero electroporations, age of analyses, number of animals and neurons quantified, statistical tests, p values, and details of the post-hoc tests (where applicable).
Table EV2.
Oligonucleotide sequences related to all experiments in this study.
HIGHLIGHTS.
GSK3β activation induces excessive interstitial axon branching in excitatory cortical neurons
MAP1B, acting as a brake, is a downstream effector of GSK3β-mediated axon branching
MAP1B inhibition of axon branching is released by GSK3β phosphorylation
GSK3β/MAP1B regulation of interstitial axon branching is through modification of the tubulin code
ACKNOWLEDGMENTS
We thank members of the Kolodkin laboratory for thoughtful input on this work. We also thank Ulrich Mueller, Feng-Quan Zhou and Takanari Inoue for comments on the manuscript, and Jeremy Nathans, Feng-Quan Zhou and Bart Williams for mouse reagents. We also thank Jay Vaidya for consultation on statistics. This work was supported in part by the Howard Hughes Medical Institute (ALK), the CMM Graduate Training Program at The Johns Hopkins University School of Medicine–T32-GM008752 (JD), the Kavli Neuroscience Discovery Institute at Johns Hopkins (SS), and an EMBO Postdoctoral Fellowship no. 364–2021 (JZ).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests. RH currently serves as a Director of Neuroscience at Prilenia Therapeutics. JD currently works at Novartis.
REFERENCES
- 1.Winnubst J., Bas E., Ferreira T.A., Wu Z., Economo M.N., Edson P., Arthur B.J., Bruns C., Rokicki K., Schauder D., et al. (2019). Reconstruction of 1,000 Projection Neurons Reveals New Cell Types and Organization of Long-Range Connectivity in the Mouse Brain. Cell 179, 268–281.e13. 10.1016/j.cell.2019.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chédotal A., and Richards L.J. (2010). Wiring the brain: the biology of neuronal guidance. Cold Spring Harb. Perspect. Biol. 2, a001917. 10.1101/cshperspect.a001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greig L.C., Woodworth M.B., Galazo M.J., Padmanabhan H., and Macklis J.D. (2013). Molecular logic of neocortical projection neuron specification, development and diversity. Nat. Rev. Neurosci. 14, 755–769. 10.1038/nrn3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armijo-Weingart L., and Gallo G. (2017). It takes a village to raise a branch: Cellular mechanisms of the initiation of axon collateral branches. Mol. Cell. Neurosci. 84, 36–47. 10.1016/j.mcn.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson D.A., and Ma L. (2011). Developmental regulation of axon branching in the vertebrate nervous system. Development 138, 183–195. 10.1242/dev.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kornack D.R., and Giger R.J. (2005). Probing microtubule +TIPs: regulation of axon branching. Curr. Opin. Neurobiol. 15, 58–66. 10.1016/j.conb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Kalil K., and Dent E.W. (2014). Branch management: mechanisms of axon branching in the developing vertebrate CNS. Nat. Rev. Neurosci. 15, 7–18. 10.1038/nrn3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hand R.A., Khalid S., Tam E., and Kolodkin A.L. (2015). Axon Dynamics during Neocortical Laminar Innervation. Cell Rep. 12, 172–182. 10.1016/j.celrep.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorskind J.M., and Kolodkin A.L. (2021). Revisiting and refining roles of neural guidance cues in circuit assembly. Curr. Opin. Neurobiol. 66, 10–21. 10.1016/j.conb.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoersting A.-K., and Schmucker D. (2021). Axonal branch patterning and neuronal shape diversity: roles in developmental circuit assembly: Axonal branch patterning and neuronal shape diversity in developmental circuit assembly. Curr. Opin. Neurobiol. 66, 158–165. 10.1016/j.conb.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Dorskind J.M., Sudarsanam S., Hand R.A., Ziak J., Amoah-Dankwah M., Guzman-Clavel L., Soto-Vargas J.L., and Kolodkin A.L. (2023). Drebrin Regulates Collateral Axon Branching in Cortical Layer II/III Somatosensory Neurons. 2023.06.21.545958. 10.1101/2023.06.21.545958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tymanskyj S.R., Yang B., Falnikar A., Lepore A.C., and Ma L. (2017). MAP7 Regulates Axon Collateral Branch Development in Dorsal Root Ganglion Neurons. J. Neurosci. 37, 1648–1661. 10.1523/JNEUROSCI.326016.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnat M., Benassy M.-N., Vincensini L., Soares S., Fassier C., Propst F., Andrieux A., von Boxberg Y., and Nothias F. (2016). The GSK3-MAP1B pathway controls neurite branching and microtubule dynamics. Mol. Cell. Neurosci. 72, 9–21. 10.1016/j.mcn.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Courchet J., Lewis T.L., Lee S., Courchet V., Liou D.-Y., Aizawa S., and Polleux F. (2013). Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell 153, 1510–1525. 10.1016/j.cell.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spillane M., Ketschek A., Merianda T.T., Twiss J.L., and Gallo G. (2013). Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Rep. 5, 1564–1575. 10.1016/j.celrep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantallops I., Haas K., and Cline H.T. (2000). Postsynaptic CPG15 promotes synaptic maturation and presynaptic axon arbor elaboration in vivo. Nat. Neurosci. 3, 1004–1011. 10.1038/79823. [DOI] [PubMed] [Google Scholar]
- 17.Kim M.J., Liu I.-H., Song Y., Lee J.-A., Halfter W., Balice-Gordon R.J., Linney E., and Cole G.J. (2007). Agrin is required for posterior development and motor axon outgrowth and branching in embryonic zebrafish. Glycobiology 17, 231–247. 10.1093/glycob/cwl069. [DOI] [PubMed] [Google Scholar]
- 18.Drinjakovic J., Jung H., Campbell D.S., Strochlic L., Dwivedy A., and Holt C.E. (2010). E3 Ligase Nedd4 Promotes Axon Branching by Downregulating PTEN. Neuron 65, 341–357. 10.1016/j.neuron.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urwyler O., Izadifar A., Vandenbogaerde S., Sachse S., Misbaer A., and Schmucker D. (2019). Branchrestricted localization of phosphatase Prl-1 specifies axonal synaptogenesis domains. Science 364, eaau9952. 10.1126/science.aau9952. [DOI] [PubMed] [Google Scholar]
- 20.Izadifar A., Courchet J., Virga D.M., Verreet T., Hamilton S., Ayaz D., Misbaer A., Vandenbogaerde S., Monteiro L., Petrovic M., et al. (2021). Axon morphogenesis and maintenance require an evolutionary conserved safeguard function of Wnk kinases antagonizing Sarm and Axed. Neuron 109, 2864–2883.e8. 10.1016/j.neuron.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Martin D.T., Jardin N., Giudicelli F., Gasmi L., Vougny J., Haumaître C., Nicol X., Janke C., Fassier C., and Hazan J. (2022). A key role for p60-Katanin in axon navigation is conditioned by the tubulin polyglutamylase TTLL6. 2022.01.20.477127. 10.1101/2022.01.20.477127. [DOI] [Google Scholar]
- 22.Fenlon L.R., and Richards L.J. (2015). Contralateral targeting of the corpus callosum in normal and pathological brain function. Trends Neurosci. 38, 264–272. 10.1016/j.tins.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Economo M.N., Winnubst J., Bas E., Ferreira T.A., and Chandrashekar J. (2019). Single-neuron axonal reconstruction: The search for a wiring diagram of the brain. J. Comp. Neurol. 527, 2190–2199. 10.1002/cne.24674. [DOI] [PubMed] [Google Scholar]
- 24.Ueda H.R., Dodt H.-U., Osten P., Economo M.N., Chandrashekar J., and Keller P.J. (2020). Whole-Brain Profiling of Cells and Circuits in Mammals by Tissue Clearing and Light-Sheet Microscopy. Neuron 106, 369–387. 10.1016/j.neuron.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eldar-Finkelman H., Argast G.M., Foord O., Fischer E.H., and Krebs E.G. (1996). Expression and characterization of glycogen synthase kinase-3 mutants and their effect on glycogen synthase activity in intact cells. Proc. Natl. Acad. Sci. U. S. A. 93, 10228–10233. 10.1073/pnas.93.19.10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokuoka H., Yoshida T., Matsuda N., and Mishina M. (2002). Regulation by glycogen synthase kinase-3beta of the arborization field and maturation of retinotectal projection in zebrafish. J. Neurosci. Off. J. Soc. Neurosci. 22, 10324–10332. 10.1523/JNEUROSCI.22-23-10324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou F.-Q., Zhou J., Dedhar S., Wu Y.-H., and Snider W.D. (2004). NGF-Induced Axon Growth Is Mediated by Localized Inactivation of GSK-3β and Functions of the Microtubule Plus End Binding Protein APC. Neuron 42, 897–912. 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Kim W.-Y., Zhou F.-Q., Zhou J., Yokota Y., Wang Y.-M., Yoshimura T., Kaibuchi K., Woodgett J.R., Anton E.S., and Snider W.D. (2006). Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron 52, 981–996. 10.1016/j.neuron.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Z., Wang Z., Gu Y., Feil R., Hofmann F., and Ma L. (2009). Regulate axon branching by the cyclic GMP pathway via inhibition of glycogen synthase kinase 3 in dorsal root ganglion sensory neurons. J. Neurosci. Off. J. Soc. Neurosci. 29, 1350–1360. 10.1523/JNEUROSCI.3770-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilimoria P.M., de la Torre-Ubieta L., Ikeuchi Y., Becker E.B.E., Reiner O., and Bonni A. (2010). A JIP3regulated GSK3β/DCX signaling pathway restricts axon branching. J. Neurosci. Off. J. Soc. Neurosci. 30, 16766–16776. 10.1523/JNEUROSCI.1362-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun H., Jiang Y., Yu Q., Luo C., and Zou J. (2008). Effect of mutation K85R on GSK-3beta: Molecular dynamics simulation. Biochem. Biophys. Res. Commun. 377, 962–965. 10.1016/j.bbrc.2008.10.096. [DOI] [PubMed] [Google Scholar]
- 32.Morgan-Smith M., Wu Y., Zhu X., Pringle J., and Snider W.D. (2014). GSK-3 signaling in developing cortical neurons is essential for radial migration and dendritic orientation. eLife 3, e02663. 10.7554/eLife.02663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland C. (2011). What Are the bona fide GSK3 Substrates? Int. J. Alzheimers Dis. 2011, 505607. 10.4061/2011/505607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruxvoort K.J., Charbonneau H.M., Giambernardi T.A., Goolsby J.C., Qian C.-N., Zylstra C.R., Robinson D.R., Roy-Burman P., Shaw A.K., Buckner-Berghuis B.D., et al. (2007). Inactivation of Apc in the mouse prostate causes prostate carcinoma. Cancer Res. 67, 2490–2496. 10.1158/0008-5472.CAN-06-3028. [DOI] [PubMed] [Google Scholar]
- 35.Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D.H., McMahon A.P., Sommer L., Boussadia O., and Kemler R. (2001). Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264. 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 36.Benoist M., Palenzuela R., Rozas C., Rojas P., Tortosa E., Morales B., González-Billault C., Ávila J., and Esteban J.A. (2013). MAP1B-dependent Rac activation is required for AMPA receptor endocytosis during long-term depression. EMBO J. 32, 2287–2299. 10.1038/emboj.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ka M., Jung E.-M., Mueller U., and Kim W.-Y. (2014). MACF1 regulates the migration of pyramidal neurons via microtubule dynamics and GSK-3 signaling. Dev. Biol. 395, 4–18. 10.1016/j.ydbio.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mimori-Kiyosue Y., Grigoriev I., Lansbergen G., Sasaki H., Matsui C., Severin F., Galjart N., Grosveld F., Vorobjev I., Tsukita S., et al. (2005). CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J. Cell Biol. 168, 141–153. 10.1083/jcb.200405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang H., Bygrave A.M., Roth R.H., Johnson R.C., and Huganir R.L. (2021). An optimized CRISPR/Cas9 approach for precise genome editing in neurons. eLife 10, e65202. 10.7554/eLife.65202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villarroel-Campos D., and Gonzalez-Billault C. (2014). The MAP1B case: An old MAP that is new again. Dev. Neurobiol. 74, 953–971. 10.1002/dneu.22178. [DOI] [PubMed] [Google Scholar]
- 41.Scales T.M.E., Lin S., Kraus M., Goold R.G., and Gordon-Weeks P.R. (2009). Nonprimed and DYRK1A-primed GSK3 beta-phosphorylation sites on MAP1B regulate microtubule dynamics in growing axons. J. Cell Sci. 122, 2424–2435. 10.1242/jcs.040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goold R.G., Owen R., and Gordon-Weeks P.R. (1999). Glycogen synthase kinase 3beta phosphorylation of microtubule-associated protein 1B regulates the stability of microtubules in growth cones. J. Cell Sci. 112, 3373–3384. 10.1242/jcs.112.19.3373. [DOI] [PubMed] [Google Scholar]
- 43.Kesarwani S., Lama P., Chandra A., Reddy P.P., Jijumon A.S., Bodakuntla S., Rao B.M., Janke C., Das R., and Sirajuddin M. (2020). Genetically encoded live-cell sensor for tyrosinated microtubules. J. Cell Biol. 219, e201912107. 10.1083/jcb.201912107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aillaud C., Bosc C., Peris L., Bosson A., Heemeryck P., Van Dijk J., Le Friec J., Boulan B., Vossier F., Sanman L.E., et al. (2017). Vasohibins/SVBP are tubulin carboxypeptidases (TCPs) that regulate neuron differentiation. Science 358, 1448–1453. 10.1126/science.aao4165. [DOI] [PubMed] [Google Scholar]
- 45.Prota A.E., Magiera M.M., Kuijpers M., Bargsten K., Frey D., Wieser M., Jaussi R., Hoogenraad C.C., Kammerer R.A., Janke C., et al. (2013). Structural basis of tubulin tyrosination by tubulin tyrosine ligase. J. Cell Biol. 200, 259–270. 10.1083/jcb.201211017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szyk A., Deaconescu A.M., Piszczek G., and Roll-Mecak A. (2011). Tubulin tyrosine ligase structure reveals adaptation of an ancient fold to bind and modify tubulin. Nat. Struct. Mol. Biol. 18, 1250–1258. 10.1038/nsmb.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanyal C., Pietsch N., Ramirez Rios S., Peris L., Carrier L., and Moutin M.-J. (2023). The detyrosination/re-tyrosination cycle of tubulin and its role and dysfunction in neurons and cardiomyocytes. Semin. Cell Dev. Biol. 137, 46–62. 10.1016/j.semcdb.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Erck C., Peris L., Andrieux A., Meissirel C., Gruber A.D., Vernet M., Schweitzer A., Saoudi Y., Pointu H., Bosc C., et al. (2005). A vital role of tubulin-tyrosine-ligase for neuronal organization. Proc. Natl. Acad. Sci. 102, 7853–7858. 10.1073/pnas.0409626102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iqbal Z., Tawamie H., Ba W., Reis A., Halak B.A., Sticht H., Uebe S., Kasri N.N., Riazuddin S., van Bokhoven H., et al. (2019). Loss of function of SVBP leads to autosomal recessive intellectual disability, microcephaly, ataxia, and hypotonia. Genet. Med. Off. J. Am. Coll. Med. Genet. 21, 1790–1796. 10.1038/s41436-018-0415-8. [DOI] [PubMed] [Google Scholar]
- 50.Marcos S., Moreau J., Backer S., Job D., Andrieux A., and Bloch-Gallego E. (2009). Tubulin Tyrosination Is Required for the Proper Organization and Pathfinding of the Growth Cone. PLOS ONE 4, e5405. 10.1371/journal.pone.0005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landskron L., Bak J., Adamopoulos A., Kaplani K., Moraiti M., van den Hengel L.G., Song J.-Y., Bleijerveld O.B., Nieuwenhuis J., Heidebrecht T., et al. (2022). Posttranslational modification of microtubules by the MATCAP detyrosinase. Science 376, eabn6020. 10.1126/science.abn6020. [DOI] [PubMed] [Google Scholar]
- 52.Pagnamenta A.T., Heemeryck P., Martin H.C., Bosc C., Peris L., Uszynski I., Gory-Fauré S., Couly S., Deshpande C., Siddiqui A., et al. (2019). Defective tubulin detyrosination causes structural brain abnormalities with cognitive deficiency in humans and mice. Hum. Mol. Genet. 28, 3391–3405. 10.1093/hmg/ddz186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanderhaeghen P., and Polleux F. (2023). Developmental mechanisms underlying the evolution of human cortical circuits. Nat. Rev. Neurosci. 24, 213–232. 10.1038/s41583-023-00675-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bradke F. (2022). Mechanisms of Axon Growth and Regeneration: Moving between Development and Disease. J. Neurosci. Off. J. Soc. Neurosci. 42, 8393–8405. 10.1523/JNEUROSCI.1131-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Creighton B.A., Afriyie S., Ajit D., Casingal C.R., Voos K.M., Reger J., Burch A.M., Dyne E., Bay J., Huang J.K., et al. (2021). Giant ankyrin-B mediates transduction of axon guidance and collateral branch pruning factor sema 3A. eLife 10, e69815. 10.7554/eLife.69815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De León Reyes N.S., Mederos S., Varela I., Weiss L.A., Perea G., Galazo M.J., and Nieto M. (2019). Transient callosal projections of L4 neurons are eliminated for the acquisition of local connectivity. Nat. Commun. 10, 4549. 10.1038/s41467-019-12495-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suárez R., Fenlon L.R., Marek R., Avitan L., Sah P., Goodhill G.J., and Richards L.J. (2014). Balanced Interhemispheric Cortical Activity Is Required for Correct Targeting of the Corpus Callosum. Neuron 82, 1289–1298. 10.1016/j.neuron.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 58.Ketschek A., Spillane M., Dun X.-P., Hardy H., Chilton J., and Gallo G. (2016). Drebrin coordinates the actin and microtubule cytoskeleton during the initiation of axon collateral branches. Dev. Neurobiol. 76, 1092–1110. 10.1002/dneu.22377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spillane M., Ketschek A., Jones S.L., Korobova F., Marsick B., Lanier L., Svitkina T., and Gallo G. (2011). The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia. Dev. Neurobiol. 71, 747–758. 10.1002/dneu.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu W., Qiang L., Solowska J.M., Karabay A., Korulu S., and Baas P.W. (2008). The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol. Biol. Cell 19, 1485–1498. 10.1091/mbc.e07-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim Y.T., Hur E.-M., Snider W.D., and Zhou F.-Q. (2011). Role of GSK3 Signaling in Neuronal Morphogenesis. Front. Mol. Neurosci. 4, 48. 10.3389/fnmol.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bodakuntla S., Jijumon A.S., Villablanca C., Gonzalez-Billault C., and Janke C. (2019). Microtubule-Associated Proteins: Structuring the Cytoskeleton. Trends Cell Biol. 29, 804–819. 10.1016/j.tcb.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Dajas-Bailador F., Bonev B., Garcez P., Stanley P., Guillemot F., and Papalopulu N. (2012). microRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat. Neurosci. 15, 697–699. 10.1038/nn.3082. [DOI] [PubMed] [Google Scholar]
- 64.Meixner A., Haverkamp S., Wässle H., Führer S., Thalhammer J., Kropf N., Bittner R.E., Lassmann H., Wiche G., and Propst F. (2000). Map1b Is Required for Axon Guidance and Is Involved in the Development of the Central and Peripheral Nervous System. J. Cell Biol. 151, 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonzalez-Billault C., Avila J., and Cáceres A. (2001). Evidence for the Role of MAP1B in Axon Formation. Mol. Biol. Cell 12, 2087–2098. 10.1091/mbc.12.7.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akhmanova A., and Hoogenraad C.C. (2005). Microtubule plus-end-tracking proteins: mechanisms and functions. Curr. Opin. Cell Biol. 17, 47–54. 10.1016/j.ceb.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Majewska B., and Skangiel-Kramska J. (2000). Phosphorylated MAP-1B isoforms in the developing mouse barrel cortex. Int. J. Dev. Neurosci. 18, 113–119. 10.1016/S0736-5748(99)00070-2. [DOI] [PubMed] [Google Scholar]
- 68.Janke C., and Magiera M.M. (2020). The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 21, 307–326. 10.1038/s41580-020-0214-3. [DOI] [PubMed] [Google Scholar]
- 69.Pedrotti B., and Islam K. (1996). Dephosphorylated but not phosphorylated microtubule associated protein MAP1B binds to microfilaments. FEBS Lett. 388, 131–133. 10.1016/0014-5793(96)00520-0. [DOI] [PubMed] [Google Scholar]
- 70.Szczesna E., Zehr E.A., Cummings S.W., Szyk A., Mahalingan K.K., Li Y., and Roll-Mecak A. (2022). Combinatorial and antagonistic effects of tubulin glutamylation and glycylation on katanin microtubule severing. Dev. Cell 57, 2497–2513.e6. 10.1016/j.devcel.2022.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beurel E., Grieco S.F., and Jope R.S. (2015). Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol. Ther. 0, 114–131. 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maxwell S.E., Delaney H.D., and Kelley K. (2018). Designing Experiments and Analyzing Data: A Model Comparison Perspective 3rd ed. (New York: Routledge.). [Google Scholar]
- 73.Luo W., Mizuno H., Iwata R., Nakazawa S., Yasuda K., Itohara S., and Iwasato T. (2016). Supernova: A Versatile Vector System for Single-Cell Labeling and Gene Function Studies in vivo. Sci. Rep. 6, 35747. 10.1038/srep35747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stegmeier F., Hu G., Rickles R.J., Hannon G.J., and Elledge S.J. (2005). A lentiviral microRNA-based system for single-copy polymerase II-regulated RNA interference in mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 102, 13212–13217. 10.1073/pnas.0506306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A., et al. (2013). Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 339, 819–823. 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ran F.A., Hsu P.D., Wright J., Agarwala V., Scott D.A., and Zhang F. (2013). Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308. 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doench J.G., Fusi N., Sullender M., Hegde M., Vaimberg E.W., Donovan K.F., Smith I., Tothova Z., Wilen C., Orchard R., et al. (2016). Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191. 10.1038/nbt.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morgens D.W., Wainberg M., Boyle E.A., Ursu O., Araya C.L., Tsui C.K., Haney M.S., Hess G.T., Han K., Jeng E.E., et al. (2017). Genome-scale measurement of off-target activity using Cas9 toxicity in high-throughput screens. Nat. Commun. 8, 15178. 10.1038/ncomms15178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Source Data 1: Complete data for Figure 1 and Fig EV1
(A) Complete data from interstitial axon branching analysis in mice overexpressing GSK3β-CA, related to Fig 1B. For this plot and every subsequent plot, each dot represents a single neuron. Each column represents a single animal; mean ± SD is plotted.
(B) Complete data from interstitial axon branching analysis in mice overexpressing GSK3β-DN, related to Fig 1C.
(C) Complete data from interstitial axon branching analysis in Gsk3α and Gsk3β knockout mice, related to Fig 1E & Fig EV1F.
(D) Complete data from the original screen: interstitial axon branching quantification in mice overexpressing human GSK3β-CA, related to Fig EV1B.
(E) Complete data from interstitial axon branching analysis in adult mice overexpressing GSK3β-CA, related to Fig EV1D.
Source Data 2: Complete data for Figure 2
(A) Complete data from interstitial axon branching analysis in mice with Map1b shRNA-mediated knockdown, related to Fig 2A.
(B) Complete data from interstitial axon branching analysis in mice with Map1b sgRNA-mediated knockdown, related to Fig 2C.
(C) Complete data from interstitial axon branching analysis in mice overexpressing naïve MAP1B, related to Fig 2E.
Source Data 3: Complete data for Figure 3
(A) Complete data from interstitial axon branching analysis in mice overexpressing naïve MAP1B, MAP1B-ΔP and MAP1B-P, related to Fig 3A
(B) Complete data from GSK3β-MAP1B rescue experiment, related to Fig 3C.
Source Data 4: Complete data for Figure 4
(A) Complete data from interstitial axon branching analysis in mice overexpressing TTL or VASH1/SVBP, related to Fig 4C.
(B) Complete data from interstitial axon branching analysis in mice overexpressing TTL or TTL-DN, related to Fig 4F.
Source Data 5: Complete data for Figure 5, Figure EV5 and Figure 6.
(A) Complete data from interstitial axon branching analysis in mice with sgRNA-mediated knockdown of Ttl and Svbp, related to Fig 5A.
(B) Complete data from GSK3β-TTL rescue experiment, related to Fig 5C.
(C) Complete data from combined Svbp and Matcap sgRNA-mediated knockdown, related to Fig EV5A.
(D) Complete data from MAP1B-TTL interaction experiment, related to Fig 6A.
Table EV1.
Table presents mouse strain/mouse lines, plasmids used for in utero electroporations, age of analyses, number of animals and neurons quantified, statistical tests, p values, and details of the post-hoc tests (where applicable).
Table EV2.
Oligonucleotide sequences related to all experiments in this study.