Abstract
In human vascular anatomy, blood flows from the heart to organs and tissues through a hierarchical vascular tree, consisting of large arteries that branch into arterioles and further into capillaries where gas and nutrient exchange occur. Engineering a complete, integrated vascular hierarchy possessing vessels large enough to suture, strong enough to withstand hemodynamic forces, and a branching structure to permit immediate perfusion of a fluidic circuit across scales would be transformative for regenerative medicine, enabling the translation of engineered tissues of clinically-relevant size, and perhaps whole organs. How close are we to solving this biological plumbing problem? In this review, we highlight advances in engineered vasculature at individual scales and focus on recent strategies to integrate across scales.
Keywords: hierarchical, vasculature, capillary, arteriole, artery
The need for hierarchical vasculature
Regenerative medicine (RM) (see Glossary) has captivated the attention of physicians, scientists, and patients alike, with the hope that engineered tissues and organs may become reality for individuals afflicted with traumatic injuries, birth defects, or awaiting an organ transplant. In the US alone there are more than 100,000 people on the organ transplant list, with a new person added every 9 minutesi. Groundbreaking developments, including the generation of induced pluripotent stem cells (iPSC), decellularized organs, 3D bioprinting, the FDA approval of engineered skin and cartilage products, and clinical trials (CT) for hollow organs and vessels, have furthered the field towards the brink of new therapies for nearly every solid tissue and organ in the body. Despite numerous advancements, off-the-shelf organs to end the organ shortage and remain more promise than reality. To fulfill the promise of RM, the grand challenge to engineer a fully interconnected, perfusable, hierarchical vascular tree must be solved.
The native vascular hierarchy consists of arteries, arterioles, capillaries, venules, and veins. Blood flows from the heart through the large caliber arteries to branching smaller arterioles and finally through the capillary beds [1, 2]. Nutrient and gas exchange occurs primarily in the capillary beds, along with the collection of waste products that are transported back through the venous system to the heart. Each of these vessels has unique anatomy to support the physiological function of the cardiovascular system (Fig. 1, Box 1).
Figure 1. The native vascular hierarchy and methods for engineering the individual components.
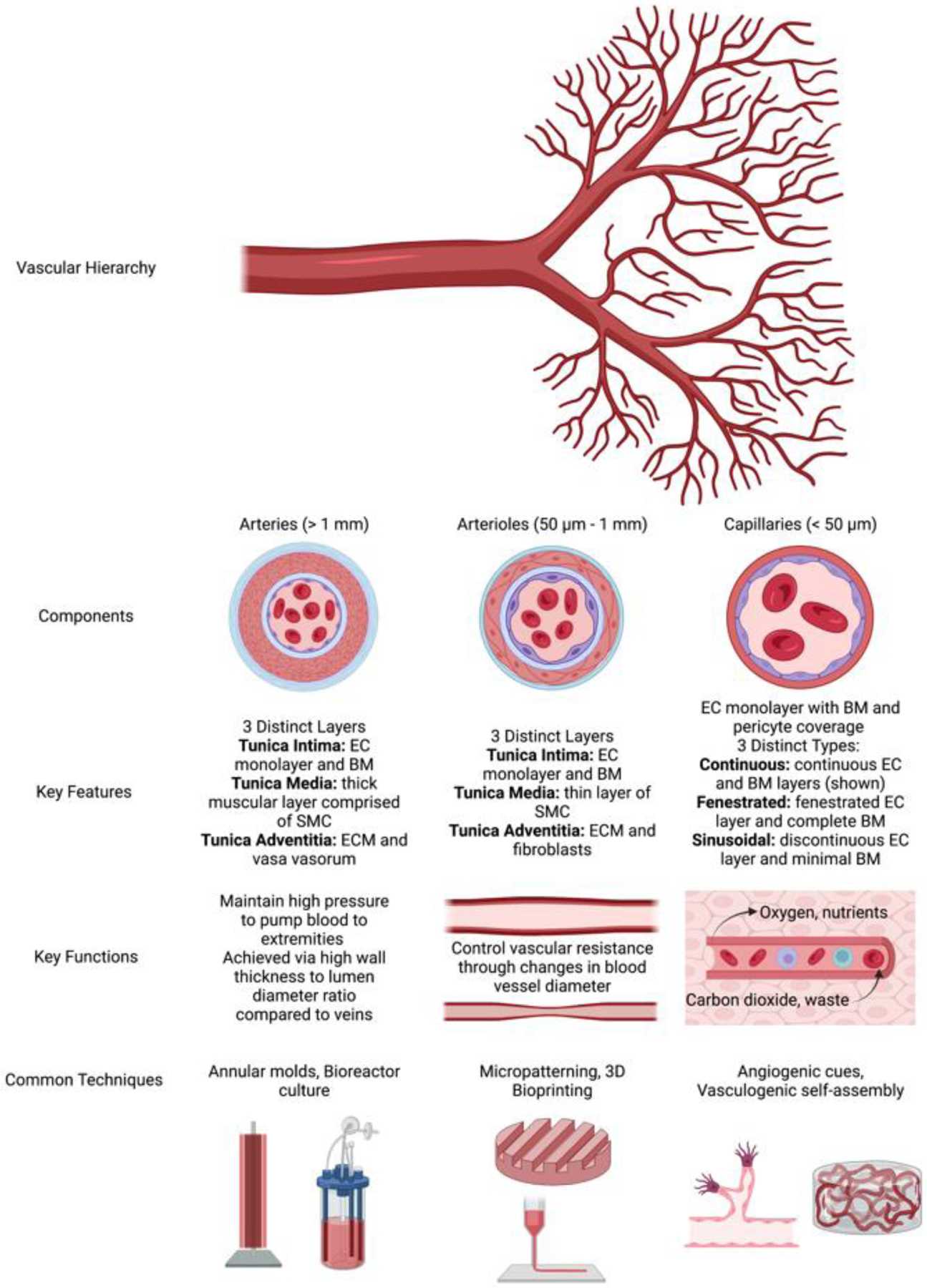
The human vascular system is a complex hierarchical network with five main vessel types: arteries and arterioles that carry oxygenated blood away from the heart, veins and venules that carry deoxygenated blood toward the heart, and capillaries that bridge the two sides and support diffusion within tissues. Vessels that carry oxygenated blood away from the heart have been a primary focus of the tissue engineering field, and are the main focus of this review. Arteries branch into smaller arterioles which branch further into a complex network of capillaries. Each vessel type has a unique structure, function, and set of engineering fabrication strategies. Abbreviations: EC – endothelial cell, BM – basement membrane, SMC – smooth muscle cell, and ECM – extracellular matrix. Figure created using BioRender.com.
Box 1. Native vascular hierarchy.
Vessels of all calibers have a tunica intima comprised of a single endothelial monolayer and a basement membrane (BM). The smallest vessels in the body, the capillaries, facilitate transport of oxygen, nutrients, and other small molecules and typically only have a single discontinuous layer of supportive pericytes beyond the tunica intima. These pericytes are critical for blood vessel formation, maintenance, and barrier function [100]. Pericytes are also characterized by alpha-smooth muscle actin (a-SMA) expression, which plays a role in maintaining the contractile tone of these blood vessels. There are three main categories of capillaries - continuous, fenestrated, and sinusoid - characterized by their different functions and their different endothelial and pericyte phenotypes. Continuous capillaries are characterized by tight cell-cell junctions permissive only to the transport of water, gases, and some small molecules. The endothelial cell (EC) monolayer and BM are completely intact. Fenestrated capillaries maintain tight cell-cell junctions, but also contain small pores between some cells and within the BM to allow the transport of larger molecules through the vessel wall. These capillaries are prevalent in tissues that require significant exchange between the blood and the tissues, such as the kidney and small intestine, responsible for filtration of waste products and absorption of nutrients from food, respectively. Finally, sinusoid capillaries are the leakiest. These capillaries lack tight cell-cell junctions leading to gaps in the endothelial monolayer permissive to the transport of very large molecules and some cells such as new blood cells and immune cells. In addition to gaps in the endothelial monolayer, these vessels have gaps in the BM layer and decreased pericyte coverage compared to that of continuous and fenestrated capillaries. Sinusoid capillaries are prevalent in the liver and bone marrow.
Arterioles, the next caliber of vessels, have a thicker vessel wall composed of 1–5 layers of smooth muscle cells (SMC), extracellular matrix (ECM), and adventitia [101]. The smooth muscle layers of these vessels are responsible for changes in diameter, and therefore blood flow, in response to tissues’ needs for nutrients and oxygen. While the SMC enact changes in arteriole diameter, EC in the intimal layer sense and respond to shear stresses and inflammatory cues by secreting vasoactive compounds to signal the SMC to contract or relax, thereby regulating arteriole diameter. A critical result of this ability to change vessel diameter is dilation in response to both tissue hypoxia and high metabolic activity in downstream parenchymal tissues, both of which necessitate increased blood flow to the tissues. Venules, the venous vessels at this scale, only have one layer of SMCs and connective tissue in the tunica media and the tunica adventitia layer blends with the surrounding tissue. Venules have a larger lumen and thinner wall compared to arterioles due to the lower blood pressure in the venous system.
Arteries, the largest caliber vessels, have a much thicker tunica media and tunica adventitia to support large volumes of blood flow and maintain blood pressure. Muscular arteries (e.g., femoral artery, brachial artery) have a thick tunica media composed of many layers of SMC and a thinner tunica adventitia compared to elastic arteries (e.g., aorta, pulmonary artery), which have a few layers of SMC and many layers of elastin, which allows for a constant maintenance of vessel pressure. The adventitial layer of these vessels is composed of supportive connective tissue and contains small capillaries, called the vasa vasorum, to supply nutrients to cells within thick vessel walls. Veins have a much thinner wall compared to arteries due to the lower pressure within this part of the vascular system. Unlike arteries, veins contain valves to prevent backflow of blood in the low-pressure environment where blood is traveling against gravity. The vein wall is composed of a few layers of SMC and a thick supportive adventitia composed of connective tissues. The adventitia layer is the thickest layer of the vein wall and contains large amounts of collagen fibers as well as sympathetic nerves. Although venous vasculature is also critical to engineering functional tissues and solid organs, the primary focus of this review is the arterial vasculature.
Complementary to the blood vascular system, the lymphatic system is essential for fluid homeostasis, transport, and the immune system. Lymph travels through lymphatic vessels (which have many similarities to blood vessels) and carries white blood cells and destroyed/damaged bacteria, viruses, and cells back to the bloodstream. The lymphatic vascular system is a unidirectional transport system. Blind-ended lymphatic vessels in the tissues reabsorb tissue metabolites and excess interstitial arterial fluid and filter these products before returning the fluid to the blood circulation. These blind ended lymphatics, often called lymphatic capillaries, are thin walled and highly permeable vessels that transport fluid from the tissues to the larger collecting lymphatic vessels. Although not the focus of this review, the native lymphatic vascular system and recent advances to engineer it are highlighted in other recent reviews and studies [39, 40, 102–106].
Despite the hierarchical nature of the cardiovascular system, and the multiscale damage caused by cardiovascular diseases, many clinical interventions typically focus on a single scale. For example, synthetic vascular grafts used to treat peripheral artery disease (PAD) bypass around a blocked artery to restore blood flow. With the advent of tissue engineering (TE), new biological grafts are being explored. Newer RM strategies have focused on injection of pro-angiogenic factors or cells to the downstream ischemic site to generate new blood vessels and reestablish capillary blood flow. These therapeutic angiogenesis strategies focus on revascularizing the necrotic tissues, but alone do not address the blocked artery which created the initial ischemia. To fully mitigate disease symptoms and progression, there is a significant, unmet need to restore perfusion across scales from capillaries to arteries.
Hierarchical vasculature can potentially meet this need, and is similarly needed to engineer functional organs for transplant. Clinical successes in TE have been achieved in thin (e.g., skin) [3], tubular (e.g., urethra) [4], or hollow (e.g., bladder) [5] tissues and organs. However, solid organ engineering has not yet reached clinical success due in part to the need for extensive vasculature to enable oxygenation throughout the entire thickness beyond diffusion limitations of ~200 μm [6]. To engineer, and eventually transplant, more complex tissues and/or solid organs for clinical applications, a hierarchical vascular blood supply is needed. In this review, we highlight select recent advances in engineering vasculature at individual scales, studies that have integrated two or more scales into a primitive hierarchy, key anatomical features required for hierarchical and organ-specific vasculature, and key barriers to the translation of hierarchical vasculature. We conclude with our vision for furthering the engineering of hierarchical vascular constructs for clinical utility.
Engineering the vascular tree
For this review, capillaries are considered <50 μm in diameter, mesoscale vessels are considered 50–1000 μm, and vascular grafts are considered >1 mm.
Tissue engineered vascular grafts
Since the 1950s, synthetic materials, such as Dacron, expanded polytetrafluoroethylene, and polyurethane [7], have been used as vascular grafts for repair of large diameter vessels. These medical devices have high patency rates (>90%) for large diameter vessel applications (e.g., in the treatment of aortic aneurysms), but are not well-suited for small diameter (< 6 mm) applications due in part to compliance mismatch with native vessels [8]. For smaller diameter applications, including coronary artery bypass grafting (CABG), autologous vessels such as the saphenous vein are typically used. However, autologous vessels vary in quality due to patient age, cardiovascular health, and other comorbidities. Vein grafts also present potential compliance mismatch complications when used in arterial applications. To overcome these limitations, tissue engineered vascular grafts (TEVG) have been a major focus of the TE/RM community for nearly 30 years.
TEVG fabrication typically combines vascular cells with tubular scaffolds composed of natural and/or synthetic hydrogels, degradable polymers, or decellularized human and/or animal tissues. TEVG are often subjected to mechanical loading in perfusion bioreactors, as physiologic loading regimens significantly improve their mechanical properties by inducing new matrix deposition [9–11]. While recent progress manufacturing TEVG has been more extensively summarized in other reviews [12–14], several fabrication techniques are also relevant for fabricating hierarchical vasculature. These include tubular/annular molding [15], cell sheets [16], electrospinning [17, 18], decellularization [19], and 3D bioprinting [20–23].
A recent study described a stepwise molding technique to fabricate TEVG with three distinct layers to mimic the tunica intima, tunica media, and tunica adventitia (Fig. 2A) [24]. The complete TEVG attained properties similar to a native carotid artery. A similar three-layer vascular graft was fabricated by a unique drop-on-demand 3D printing technique where each layer was comprised of merged microdroplets [25]. The intima layer was fabricated by printing droplets of dissolvable gelatin containing a high density of EC. The media layer was fabricated by printing droplets of an SMC containing fibrinogen ink and a thrombin-based ink integrated to create a thin layer of SMC containing fibrin around the inner gelatin layer. Then a hybrid fibrin-collagen hydrogel containing fibroblasts was cast around the 3D printed object to fabricate the adventitia layer. Finally, the sacrificial gelatin was dissolved leaving behind the ECs lining the inside of the media layer yielding the intima. The authors showed that both printed and cast cells remained in the distinct layers. Of note, they were able to successfully print fibrin hydrogels, a typically challenging material to print, with this novel printing technique.
Figure 2. Fabrication strategies for each scale of the vascular tree.

Schematic examples of distinct strategies for forming vasculature ranging from arteries to capillaries. (A-C) Fabrication of artery-scale grafts has been achieved using an annular molding technique with (A) liquid prepolymer solutions containing cells, (B) cell suspensions alone, or (C) sheets of decellularized ECM. (A) One method involves injection of a liquid hydrogel precursor into an annular mold; following crosslinking of the hydrogel, the inner mandrel is removed leaving an open lumen. A multistep process applying this method repeatedly has been used to fabricate multilayered TEVG. Abbreviations – SMC: smooth muscle cells. EC: endothelial cells. ASC: adipose-derived stem cells. (B) Another method involves adding human stromal cell suspensions into microfabricated agarose annular molds, which facilitate self-assembly of the cells into tissue rings over time in culture. Multiple tissue rings can then be stacked onto a mandrel in direct contact with each other to enable fusion into an integrated vascular graft. (C) A third multistep process to fabricate vascular grafts involves wrapping SIS material around a mandrel, followed by crosslinking and then drying under vacuum. Grafts fabricated via this method were subsequently functionalized with heparin and vascular endothelial growth factor (VEGF). Abbreviations – SIS: Small intestinal submucosa. EDC: 1-ethyl-3-(3-dimethylaminopropyl) NHS: N-hydroxysuccinimide. SH: SIS + heparin graft. VEGF: vascular endothelial growth factor. SVH: SIS + heparin + VEGF graft. Cell sheets and electrospinning typically use a mandrel, with sheets of cells and ECM wrapped around a mandrel or electrospun polymer collected around a mandrel, yielding a lumen upon mandrel removal. (D-F) Methods to fabricate mesoscale vasculature employ (D) 3D printing of sacrificial glass or (E, F) removable solid templates – spring (E) or needle (F). (D) 3D printed sacrificial carbohydrate glass lattices were encapsulated in extracellular matrix and then incubated in saline to dissolve, leaving behind mesoscale channels which were then endothelialized to create mesovessels. Abbreviations – Par VP: parallel vascular patch, Grid VP: grid vascular patch, Sm_D VP: small diameter vascular patch, EP: endothelial patch, AP: acellular patch). (E) Another fabrication strategy involved hydrogel crosslinking around a spring, followed by removal of the spring to create a spiral shaped channel. The channel was seeded with EC via a perfusion seeding process. In the lower panel, a cylinder of parenchymal cells was patterned within the spiral shape. (F) A single linear mesovessel was fabricated within a collagen hydrogel through sacrificial needle templating. Removal of the needle yielded a single linear channel which was seeded with mesenchymal stem cells (MSC) and endothelial cells (EC) in a dual step seeding process. Representative images depict the vessel post seeding. (G-I) Capillary-scale structures have been fabricated via (G) monocultures of cells with angiogenic factors to elicit vasculogenesis-like self-assembly, (H) co-culture of cells which undergo vasculogenesis-like self-assembly, or (I) angiogenic sprouting from existing blood vessels. Panels (A), (B), (C), (D), (E), (F), and (G) reproduced with permission from [24], [26], [28], [37], [43], [41], and [50], respectively. Panels (H) and (I) created using BioRender.com.
Not all TEVG approaches fully replicate the three distinct layers of native arteries; simpler constructs containing only single layer organization have advanced to preclinical and CT. One approach fabricated tissue rings from various types of stromal cells (SC), using an annular agarose mold [26, 27]. Multiple rings were then merged to form a full graft (Fig. 2B). Polymer cuffs were incorporated at the ends of the vessel to facilitate anastomosis. Other studies implanted single-layer acellular grafts into rodent models, showing successful perfusion and the maintenance of patency achieved by recruiting host cells to populate the grafts. One such study used an annular mold and naturally derived small intestinal submucosa, which was rolled around a mandrel and subsequently functionalized with heparin and vascular endothelial growth factor (VEGF) (Fig. 2C) [28]. These acellular TEVG were implanted as arterial interpositional grafts in the descending aorta of adult mice with 60% graft patency. The cellularization of VEGF-functionalized grafts correlated well with native arterial anatomy with CD144 EC marker expression on the lumenal side and α-SMA SC marker expression in the wall of the graft. Another study used a similar methodology of rolling decellularized bovine pericardial ECM integrated with poly(propylene fumarate) around a mandrel to create a biohybrid vascular graft with circumferential stresses and suture retention strengths comparable to those of native porcine arteries [29]. Two such constructs implanted as interpositional grafts in the rat abdominal aorta remained patent throughout a two-week study with evidence of host cell recruitment.
Cell-seeded TEVG have also been fabricated with both natural and synthetic biodegradable matrices. One study seeded aortic EC into the lumens of collagen grafts and implanted them interpositionally in the femoral artery of inbred Lewis rats [30]. One week after implantation, 40% of the grafts remained patent and expressed endothelial markers on the lumenal surface and the outside of the graft. In this study, an endothelial lining was required to prevent thrombosis, as none of the grafts seeded with EC that subsequently detached from the surface after surgical handling remained thrombus free. However, this may be animal model or material specific as other studies, as noted in the preceding paragraph, have shown somewhat surprising success without lumenal seeding when host cells populate and endothelialize the graft. In addition to EC linings, heparin coating, incorporation of elastin, and increased surface roughness/topography have shown antithrombogenic effects [31]. Another study implanted TEVG fabricated from biodegradable synthetic materials seeded with iPSC-SMC into the abdominal aorta of nude rats [32]. Grafts cultured for 8 weeks under pulsatile radial stress exhibited burst pressures and suture retention strengths comparable to human saphenous veins. Their high mechanical strength prevented rupture and deformation during a one-month in vivo study. The implanted grafts remained cellularized and showed evidence of collagen deposition and host cell recruitment.
Excitingly, studies showing long-term patency of TEVG in large animal and human studies are now leading to commercialization efforts. Building on the success of their TEVG in baboons, sheep, and pigs, one such effort to commercialize allografts is being led by a start-up called Vascudyne. Their approach involves first seeding fibrin-based TEVG with human dermal fibroblasts, followed by culture in a pulsed flow/mechanical stretch bioreactor to attain suitable tissue mechanical properties and then decellularization prior to implantation [33]. The burst pressures and suture retention strengths were comparable to those of vessels commonly used for CABG. When implanted in baboons, patency rates were 83% at 3 months and 60% at 6 months. Grafts at both timepoints exhibited significant SC recruitment within all regions of the graft and host EC recruitment within the lumen. The grafts exhibited extensive remodeling, no signs of calcification or aneurysm, and only minimal inflammatory responses, all positive indications for future clinical testing.
A similar strategy involving decellularization of TEVG is being commercialized by Humacyte, a start-up that has significantly advanced their human acellular vessels (HAV) through multiple CT. In a pivotal study, suture strengths and burst pressures of HAV were comparable to, or exceeded, that of the human internal mammary artery. Grafts underwent significant remodeling and host cellular infiltration, eventually developing into a native vessel mimetic with three distinct layers [34]. Originally a single layer graft, a neoadventitial layer formed around the graft after implantation, along with lumenal endothelialization representative of an intima (Fig. 3A). As early as 16 weeks, capillaries mimicking the vasa vasorum were observed within the adventitial layer, and complete endothelialization of the lumen was observed at 44 weeks. Humacyte has reported positive progress in the development of their grafts for CABGii, PAD (CT NCT02887859), and the treatment of traumatic injuries (CT NCT03005418 & [35]).
Figure 3. Engineered vasculature connects to the host yielding perfused vasculature in vivo.

(A) Engineered large caliber decellularized vascular grafts (HAV: human acellular vessels) support cellular infiltration and remodeling upon transplantation as shown by fluorescent images of explanted grafts. HAV are fabricated by first seeding human vascular cells onto degradable polyglycolic acid tubular scaffolds, subjecting constructs to radial strain via a pulsatile flow bioreactor during a maturation period of 8 weeks, and then subsequently decellularizing them before implantation as an HAV. A neoadventitia with a vasa vasorum was observed as early as 16 weeks (top left) while an endothelialized lumen was observed at 44 weeks (bottom). Abbreviations – a: adventitia, m: media. (B) Parallel patterned mesovessels restored blood flow to ischemic tissues. The Par VP (schematic shown in Fig. 2D) inosculated with the host upon implantation at the ischemic site, yielding perfusion of ischemic limbs by day 5 as shown by Laser Doppler Perfusion Imaging. (C, D) Microvascular and (E) hierarchical micro-mesovascular constructs connected to host tissues yielded host blood-perfused vessels within engineered tissues. (C) ECFC and MPC were injected into an ischemic injury site one day after femoral artery and vein ligation. Implanted human self-assembled vessels (red) and hydrogel-infiltrating mouse vessels (green) were perfused with a lectin via tail vein injection to stain species-specific EC and identify only perfused vessels. There is evidence of chimeric vasculature in both red stained vessels. White arrows point to perfused vessels at day 14 after injection of cells at the site. Scale bar – 50 μm. Abbreviations – ECFC: endothelial colony forming cells, MPC: myeloid progenitor cells. (D) Human EC and bone marrow-derived MSC within a fibrin matrix were co-injected into the subcuteanous space on the dorsal surface of immunocompromised mice. By day 7, injected cells self-assembled into microvessels that inosculated with the host vasculature. The green stain for human CD31 identifies human vessels, while the red stain indicates the fluorescent dextran. In this image, the red stain is found within the lumens of human cell-derived capillaries, indicating a functional, non-leaky micrvasculature. Scale bar – 20 μm. Abbreviations – EC: endothelial cell, BMSC: bone marrow derived MSC. (E) Hierarchical meso-microvascular implants were evaluated in a subcutaneous mouse model. Human vessels stained green, host vessels stained blue, and dextran indicated in red. Representative image showing inosculation between host and implanted vasculature that is perfused via host blood. Scale bar – 50 μm. Panel (A) reprinted from [34] with permission from AAAS. Panels (B), (C), (D), and (E) reproduced with permission from [37], [58], [107], and [64], respectively.
While initial efforts in the creation of TEVG focused on biological mimicry of native vascular anatomy, recent progress with acellular grafts suggests two questions. How critical is it that TEVG be comprised of three distinct layers representing the tunica intima, media, and adventitia prior to implantation? Can function be satisfied without complete structural mimicry? Many of the studies described above showed successful patency, perfusion, and remodeling by the host without fully mimicking the native architecture of arteries when initially implanted in vivo. Single layer grafts and grafts lacking an antithrombogenic EC lining showed substantial success. Therefore, at this scale, function may be achieved with more simplistic structures that mature into native-like vessels following post-implantation remodeling, potentially providing an easier route to clinical translation. Relying on host remodeling, rather than trying to fully prescribe the anatomical architecture, may also be applicable for creating a more complete hierarchical vasculature.
Mesovascular tissue engineering
Vascular engineering at the mesoscale has similarly focused on both acellular and cellular tissue constructs. One simple approach incorporates acellular mesoscale channels within engineered tissues to increase the surface area for diffusion [36]; in some studies, these channels were seeded with EC to fabricate mesovessels comparable to the size of native arterioles (Fig. 2D, AP vs. Par VP) [37]. Similar to studies fabricating single-layer TEVG, some strategies to fabricate mesovessels have been fabricated with a single EC layer mimicking the arteriole intima layer but have not necessarily incorporated the smooth muscle layers characteristic of native arterioles.
Acellular channels have been used to better integrate biomaterial implants with host vasculature. Host cells and vessels can migrate through empty channels and populate the interior more easily than having to traverse an entire solid implant. However, this technique has had varying success in different in vivo models. One study examining implants with or without channels in a subcutaneous model observed increased tissue ingrowth and vessel perfusion in channeled implants compared to unchanneled bulk implants [38]. In another study, acellular channels alone failed to restore limb perfusion in a hindlimb ischemia model [37]. However, mesovessels seeded with EC were spatially patterned into parallel and grid-like orientations (Fig. 2D), with the former arrangement able to effectively restore limb perfusion (Fig. 3B).
Efforts to engineer the mesovasculature have examined a range of vascular morphologies (Fig. 2D–F), including single linear channels, uniquely shaped and branched channels, and multilayered gridded structures. Studies have investigated both blood and lymphatic (Box 1) [39, 40] vasculature at this scale. One study fabricated single blood vessels within collagen hydrogels with varying ratios of supportive MSC to EC, where the MSC were intended to function as SMC in vivo [41] (Fig. 2F). Vessels surrounded by a greater number of MSC exhibited greater barrier function characterized by lower permeability to fluorescent tracers. These types of models have been used to study changes resulting from flow and/or pressure, cell-cell interactions, response to inflammatory cues, and other pathophysiological conditions.
New 3D bioprinting technologies have also enabled the fabrication of more complex channel geometries, including curved, zigzagging, and winding patterns. One group used coaxial printing to fabricate unique shaped channels from a decellularized ECM bioink and examined EC morphology, permeability, platelet adhesion, and inflammatory response [42]. These vessels additionally supported pump-driven perfusion throughout the duration of culture, a distinction over traditional polydimethylsiloxane (PDMS)-based microfluidic devices which often rely on gravity-driven flow. Another unique technique enabled the fabrication of a 3D spiral shaped vessel using a spring to template the vessel (Fig. 2E), rather than a needle traditionally used for single linear vessels (Fig. 2F) [43]. This model was used to assess endothelial flow responses in a curved geometry more replicative of in vivo anatomy, with increased cell proliferation observed in spiral channels compared to straight channels despite similar morphologic response to various flow rates.
Compared to the large numbers of studies and impressive translational progress on macrovasculature, very few studies have focused on mesovasculatue, with even fewer advancing to preclinical studies. Most focus on using these vessels as models of vascular pathophysiology and/or for drug testing applications. Furthermore, only one of the above studies incorporated supportive SC, even though SMC in the tunica media of native arterioles play critical roles in physiologic functions by regulating vascular resistance and vessel diameter as blood flows from larger arteries into smaller capillaries (Fig. 1). The replication of both the tunica intima and the tunica media will likely be critical to the proper functioning of mesovasculature, but it is not yet clear if such complexity needs to be fully prescribed a priori. Much like studies with TEVG have shown for macroscale vessels, morphogenetic adaptation may take over to remodel crude mesoscale conduits into bona fide arterioles, especially when subjected to blood flow and host cells upon transplantation. There is also a need to better understand the integration of engineered vasculature across scales. The native vascular hierarchy is a continuum, with a gradual tapering of vessel sizes as they branch, dictated in part by flow-mediated mechanotransduction. Reproducing this hierarchical and biomechanical continuum represents a significant challenge and opportunity.
Engineering capillary networks
The primary methodologies for engineering capillaries seek to replicate vasculogenesis (Fig. 2G, H), and/or angiogenesis (Fig. 2I). Both processes often rely on supportive SC [44], which regulate the development of vessel networks through the secretion of growth factors and take on a perivascular phenotype to promote maturation and proper function of the microvasculature [45–47]. Matrix composition, stiffness, and degradability also significantly impact these processes.
Despite the critical roles of SC in microvasculature, cell-based vascularization strategies involving multiple cell types may complicate regulatory approval and clinical translation, which has prompted focus on acellular approaches or those using EC alone [48]. One such study fabricated a hydrogel composed of a collagen and norbornene-modified hyaluronic acid interpenetrating network (IPN) [49]. The degree of vascular formation, the number of branch points, and vessel lumenization were dependent on the IPN stiffness, which was modified by increasing the crosslinking. Another recent study reported hydrogels containing dynamic crosslinks supported EC self-assembly into vessel-like networks [50]. Vascular assembly depended on cellular contractility to promote integrin recruitment and focal adhesion development. Another paper investigated neutral-swelling synthetic PEG hydrogels which yielded enhanced capillary morphogenesis compared to controls, though minimal lumen formation occurred [51].
While the aforementioned studies successfully engineered microvasculature without SC, their presence and tissue-specific origins have also been shown to influence microvascular network formation, lumenization, and function in both natural and synthetic hydrogels. For example, recent work revealed that fibroblasts from either lung or skin supported a greater degree of vessel formation than bone marrow SC in co-culture models of vasculogenic self-assembly [52]. This mirrors a prior study in a fibrin-based sprouting angiogenesis model system, which demonstrated that SC identity affects the mechanism of fibrinolysis during vascular morphogenesis [53]. Another recent study involving SC focused on endothelial network formation in animal-component-free constructs under serum-free conditions, potentially providing a more reproducible route for clinical translation and regulatory approval [54].
While in vitro studies are useful to investigate the mechanisms underlying the formation of microvascular networks, in vivo investigations are critical for vascularization strategies to progress to clinical translation. They shed light on inosculation between host and engineered vasculature and the ability to restore physiologic vascular function in regenerative and disease models. A recent study investigated modular microbead constructs and showed that prevascularization catalyzed the formation of new microvasculature at the implant site [55]. A key element of microvascular engraftment and inosculation is the host inflammatory response. Utilizing a subcutaneous model, another group found that prevascularized networks failed to inosculate with host vessels upon transplantation when neutrophils were first depleted in the host [56]. Vascularization was recovered when neutrophils were transferred to mice. Such insights suggest vascularized constructs may perform differently in immune-deficient patient populations. Other studies have shown that cells suspended in hydrogels capable of assembly into microvascular networks salvaged limbs, prevented necrosis, and restored blood perfusion compared to EC-only or acellular hydrogel controls [57, 58], with one observing similar effects using prevascularized tissue constructs. These hindlimb ischemia models bear some resemblance to human PAD and serve as a key step towards progressing cell-based therapies to the clinic. In an ischemic stroke model, a study of integrin binding in engineered hydrogels showed that scaffolds capable of supporting α3/α5β1 integrin binding led to increased angiogenesis at the stroke site, yielding non-tortuous, non-leaky vasculature by day 10 post stroke [59].
Though several studies have advanced to animal models focused on ischemic injuries, cell-based approaches to restore perfusion have not yet been successfully translated into human patients, despite many CT using MSC or EC alone. There are many possible reasons, including poor cell survival upon delivery into an ischemic tissue as well as issues of cell sourcing and the underlying health status of the patient. Approaches containing both EC and SC in hydrogel delivery vehicles have not progressed into CT, to the best of our knowledge. However, in our view, cell-based microvasculature is unlikely to be effective as a treatment alone for a disease that begins with an arterial blockage (e.g., PAD, stroke), unless the blockage is also cleared and/or the occluded vessel is stented open. Similarly, stenting or vascular grafting alone, without microvasculature, may not fully restore perfusion to the tissue if the function of the existing capillary beds has been compromised by underlying disease [60]. An additional challenge is the creation of perfusable vasculature, which has had greater success on the macro- and meso-scale compared to the micro-scale. Given these challenges, vascular integration across scales may be required in the treatment of ischemic conditions, as we expect it will be for engineering functional solid organs.
Engineering multiscale hierarchical vasculature
While many studies have engineered vasculature at single scales, progress towards integrated hierarchical vasculature has been limited. As described above, each vascular scale often involves different fabrication techniques, which presents a challenge to engineering a complete vascular tree. Initial efforts focused on integrating capillary-artery and capillary-arteriole vascular networks, with more recent efforts to create three-scale hierarchies spanning capillary-arteriole-artery.
Several groups have engineered capillary-mesoscale hierarchical vasculature, mostly within microfluidic chips. A common approach involves templating larger mesoscale channels with acupuncture needles [61, 62] or with a sacrificial ink via 3D bioprinting [63], followed by seeding the lumens with EC. Capillaries are then formed via vasculogenic assembly of co-cultured SC and EC between the channels. An important distinction between these studies is the presence of flow within the system and how the fluidic circuit is established. Samples have been cultured statically [61], under oscillatory flow applied via a rocker plate [62], or under unidirectional flow via a syringe pump [63]. Such microphysiological systems identify key parameters for engineering functional hierarchies, enable fundamental studies to assess vascular morphogenesis, perfusion, and permeability, and have been touted as innovative platforms to evaluate and screen drugs and/or biologics. However, their small size and encasing within glass/PDMS chambers limits their potential as transplantable tissue constructs. Two studies engineering capillary-arteriole hierarchies created larger tissue implants and evaluated their hierarchical structure versus capillary-only implants in a subcutaneous mouse model [64] and rat infarct model [65]. In the subcutaneous model, host vessel infiltration into hierarchical implants and blood perfusion increased compared to capillary only implants (Fig. 3E). In the ischemia model, increased numbers of perfused vessels and increased density of transplanted cardiomyocytes were observed in hierarchical implants. One of these capillary-mesoscale vascular systems, the AngioChip, featured capillaries that sprouted from 100–500 μm mesovessels, supported parenchymal cell function, and was successfully integrated with host vasculature via both an artery-to-artery and an artery-to-vein surgical anastomosis aided by surgical cuffs and tissue glue (Fig. 4B) [66].
Figure 4. Surgically anastomosed grafts with structurally complex hierarchical architecture perfuse and integrate with host tissues.

(A) A two-scale artery-capillary hierarchy surgically connected to host arteries (top) showed evidence of blood perfusion in capillaries formed within a cell-laden bioprinted hydrogel. In this approach, a polymeric macrovessel with holes along its length was embedded within a 3D printed collagen hydrogel containing cells capable of undergoing vasculogenesis. Following implantation in vivo, cells in the gel formed nascent microvessels, which deposited BM protein laminin (middle), were supported by perivascular SC expressing αSMA (bottom), and infiltrated the macrovessel scaffold through the holes. (B) Engineered devices containing meso-microvascular hierarchies surgically anastomosed with host arteries yielded functional perfusion as seen by the flow of blood through the device. Shown here is the AngioChip, a mechanically tunable scaffold containing a perfusable, 3D branched vessel network capable of supporting parenchymal cells. (C) In another study, rat femoral tissue containing the femoral artery and vein with the associated branching arterioles were first isolated, and then combined with EC and cardiomyocyte cell sheets cultured on top of the explanted vascular tissue, yielding an integrated three-scale hierarchy after perfusion culture. In this study, the artery and vein pair were connected to a syringe pump in vitro and could be sutured into existing circulation upon transplantation into a second animal. Surgically anastomosed three-scale vascular hierarchies yielded significantly greater functional perfusion than hierarchies that were not surgically anastomosed, or microvascular only cell sheets. The panel shows bioluminescent perfusion with significant signal shown in the anastomosed group and limited signal in the other two groups. Panels (A), (B), and (C) reproduced and adapted with permission from [68] [66], and [69], respectively.
Though less physiologic initially, capillary-artery hierarchical vasculature has also been investigated. These approaches have the advantage of direct surgical anastomosis of the artery in vivo to provide immediate perfusion to interconnected capillaries within the engineered hierarchy. One study investigated sprouting between two segments of isolated artery/vein ex vivo, tested with both rodent and human vessels [67]. Vessel explants were connected by sprouted capillaries within 21 days and successfully perfused. In the presence of hierarchical vasculature, co-cultured cardiomyocytes exhibited a lower excitation threshold and more organized sarcomeres compared to controls. A promising recent study described the fabrication of similar capillary-artery hierarchical vasculature by integrating vasculogenic self-assembly and 3D printing [68]. The artery-like conduit was fabricated from a synthetic polymer containing small pores along its length while the capillary-like network was fabricated via vasculogenic self-assembly within 3D printed methacrylated collagen. The polymeric artery-like structure was seeded with EC which sprouted through the pores to integrate with capillaries in the surrounding hydrogel. In an in vivo rat femoral artery ligation model, host vasculature infiltrated the hierarchical construct, with many vessels invested with α-SMA-expressing mural cells (Fig. 4A). The entire vascular construct was perfused via the host blood circulation.
Few studies have attempted to engineer a complete vascular hierarchy containing capillaries, arterioles, and arteries. Two distinct strategies have been explored: explanted functional vasculature integrated with tissue engineered microvasculature or a fully tissue-engineered structure. As an example of the former, one group explanted rat femoral tissue containing an artery-vein bundle and the surrounding branching arterioles, and then cultured this vasculature with EC-containing cardiac cell sheets [69]. In the presence of growth factors, EC in the cell sheets assembled into vessels and inosculated with the explanted vascular bed, creating an integrated vascular hierarchy capable of supporting parenchymal cells. When implanted ectopically in vivo, the surgically anastomosed hierarchical vasculature integrated with the rat’s blood circulation to a significantly greater degree than capillaries within cell sheets alone or non-surgically anastomosed hierarchies (Fig. 4C). As an example of the latter approach, a recent study tissue engineered a complete hierarchy by employing vasculogenic self-assembly, needle templating, and annular molding to fabricate a three-scale hierarchy containing a TEVG, four 1 mm diameter arterioles and a self-assembled capillary network [70]. Although this hierarchical construct was not evaluated in vivo, the hydrogel and cell components used were all human-derived, suggesting a clearer path towards clinical translation of this promising approach.
As mentioned above, 3D bioprinting is quickly emerging as a new technology to engineer hierarchical vasculature, organ models, and full-scale organs [71, 72]. A projection stereolithography-based printing approach, named SLATE (Stereolithographic Apparatus for Tissue Engineering), was able to generate multiscale vascular tissue constructs resembling alveolar morphology and a grid-like structure capable of supporting co-transplanted parenchymal liver cells in vivo [73]. Another approach utilized a shear-thinning microbead slurry support bath to bioprint multiscale vasculature from MRI and models of the human heart [22]. This technique, named FRESH (Freeform Reversible Embedding of Suspended Hydrogels), enabled high-resolution printing of soft hydrogel-based materials with micron-scale precision. Another approach used high density cell aggregates (organoids) capable of forming functional microtissues as a support bath for extrusion-based bioprinting [23]. This approach, termed SWIFT (Sacrificial Writing into Functional Tissue), enabled the successful printing of multiscale vascular tissue capable of supporting the perfusion and function of engineered cardiac muscle tissue. Another unique printing technique is the use of cell-only bioinks without the support of hydrogel materials [74], which could be applied as a means to facilitate the interconnection of vasculature across different scales. Despite these promising strides, bioprinted constructs containing hierarchical vasculature, with macroscale vessels large enough for surgical anastomosis integrated with meso- and microscale vessels capable of supporting the immediate perfusion of embedded parenchymal cells, have not yet been realized, to the best of our knowledge.
Replication of anatomical vasculature and key barriers to translation
As engineered tissues progress towards the clinic, replication of physiologic vascular function is critical. But is complete replication of vascular anatomy a priori also required? At the arterial scale, simpler TEVG remain functional upon implantation, despite not fully replicating all anatomical features. At the microvascular scale, tissue-specific capillary density and permeability are critical to the normal function of native tissues. With respect to the former, skeletal muscle and cardiac muscle have densities of ~500–1500 capillaries/mm2 [75] and ~2000–4000 capillaries/mm2 [76], respectively. However, current approaches have largely ignored these tissue-specific differences, and typically yield ~100–200 vessels/mm2 in varying in vivo models [55, 77]. Though these engineered tissues may function in vitro and provide therapeutic benefit in small animal models, strategies to achieve physiologic capillary densities will be necessary for the proper functionality of human-scale engineered tissues and organs. With respect to the latter, physiologic control of permeability is a key tissue-specific function of the vasculature [78, 79]. For example, brain microvasculature (continuous) is highly impermeable to constitute the blood-brain barrier (BBB) [80], while kidney microvasculature (fenestrated) is leakier to facilitate nutrient exchange and glomerular filtration. Vascular permeability is tightly correlated with endothelial phenotype. Whether specialized EC will be required to create vasculature with tissue-specific permeabilities remains unclear. It’s also possible that EC may have some degree of phenotypic plasticity [81–83], enabling them to adapt to distinct tissue-specific microenvironments. Additionally, both organotypic EC [84] and SC [61, 83] exhibit differences both transcriptomically and functionally [83], which may be critical for translation of engineered tissues.
More broadly, the question of cell source remains a significant challenge for vascularizing engineered tissues. Human umbilical vein EC (HUVEC) have been widely used as a model EC type. They readily and reproducibly form robust microvasculature both in vitro and in vivo, but their applicability for clinical applications is often questioned, in part due to their venous origins. Other cell sources, including endothelial colony forming cells (ECFC) [85–87] and EC derived from induced pluripotent stem cells (iPSC-EC) [88], may be more translatable.
An important consideration when evaluating alternative cell sources for vascularization is a lack of standardization with respect to assays used to evaluate EC phenotypes and functions. The Matrigel cord assay, though widely-used, involves EC cultured in 2D on top of Matrigel and yields cord-like structures that typically lack hollow lumens and form via a process that does not require ECM proteolysis. Studies directly comparing the potential of iPSC-EC and HUVEC have yielded different results, depending on the functional outcome measured in a specific assay [88–91]. In one study, HUVEC and iPSC-EC were compared in an in vitro sprouting angiogenesis assay, in which EC coated on microcarrier beads were evaluated for their ability to undergo vascular morphogenesis in the presence of supportive SC, and in vivo [90, 92]. While HUVEC reliably sprouted and formed vessels, iPSC-EC sprouted to significantly lesser degrees and sometimes did not sprout at all. iPSC-EC fared better at forming capillaries in vivo, albeit still to a lesser degree than HUVEC [92]. Another head-to-head comparison of HUVEC and iPSC-derived human brain microvascular endothelial cells (BMEC) evaluated the ability of the EC to form a monolayer on the channel wall and assessed vascular permeability and the expression of junctional markers in a linear mesovessel model [89]. In this model, EC proliferate and migrate to form a confluent monolayer and develop tight cell-cell junctions to maintain good barrier function, a critical feature of the BBB. The iPSC-derived BMEC had ~100-fold lower permeability to 3 kDa dextran than HUVEC on day 1, and also expressed and properly localized multiple junctional proteins in static and perfused conditions. These studies suggest a need to standardize assays evaluating EC for multiple functions before adopting a specific population for clinical translation.
Despite iPSC-derived cells being hailed as a potentially autologous cell source for clinical translation, a recent study found that 72% of skin cell-derived iPSC and 18% of blood-derived iPSC at one cell bank may harbor genetic mutations [93]. Some of these mutations were detected in genes implicated in cancer. These mutations were not previously identified because the testing required for banking cells is less stringent than the clinical standard of practice. Therefore, despite the increased interest in using iPSC to generate cells for therapies and engineered tissues, many significant challenges must be overcome before these cells represent a clinically viable cell source.
Another important consideration with all EC sources is the age of the donor. Most cells purchased or isolated by labs are from young, healthy adults and/or children. Results generated using these cells may not readily translate to autologous cell therapies for older adults with cardiovascular disease or other comorbidities. In addition, both the sex and ancestral background of the source may influence how cells respond to different stimuli used in creating engineered tissue constructs [94, 95]. Understanding the influence of patient-specific differences may therefore be an additional consideration when fabricating engineered tissues and organs that represent equitable clinical solutions.
Concluding remarks and future perspectives
TE has progressed considerably towards transplantable tissues and whole organs over the last three decades; however, the number of FDA-approved tissue-engineered products remains somewhat underwhelming. The need for hierarchical vasculature is a critical limiting factor, if not THE limiting factor, in realizing the potential of this field. Many examples cited herein provide proof-of-principle techniques to engineer vasculature at individual scales. However, integrating these techniques (or creating new ones) to engineer hierarchical vasculature will be essential to manufacture functional implantable tissues and organs.
While this “vascularization challenge” has long been recognized, it remains unsolved. The advent of new biofabrication techniques over the last 15–20 years, along with dedicated funding streams, triggered a pivot towards microfluidic lab- and organ-on-chip systems for drug development and as model systems for development and disease. The recent passage of the FDA Modernization Act to utilize such systems in lieu of small animal models is especially exciting and will undoubtedly lead to more widespread adoption [96, 97]. These systems are useful for mechanistic investigations of vessel development in healthy and diseased states as well, but their small sizes often allow cells within to be sustained by diffusive transport alone.
To solve this grand challenge of the field of TE, perhaps it is time for a vascularization “moon shot.” The technical breadth required to solve a problem of this scope is immense, and will necessitate collaboration between scientists, engineers, and clinicians with complementary skill sets. Fundamental and technical gaps exist, including the need to better understand vascular biology at each scale, the requirements for tissue- and organ-specific vascular form and function, and the technologies to fabricate large constructs, perfuse them, and maintain their viability and sterility over prolonged periods of time.
The maturation of existing methodologies and the emergence of new technologies has the community poised to achieve this long-standing goal. Recent advancements in bioprinting have yielded remarkable progress to fabricate vasculature of multiple scales within a single construct. However, like most emergent technologies, the promotion has so far outpaced the reality. More progress has been made in the development of instrumentation and bioinks than large living tissue constructs. Cell viability and function in bioprinted constructs, following either extrusion and/or photocrosslinking, and the stability of printed architectures also remain concerns [98]. In addition, moral and ethical concerns have been raised over the widespread use of these technologies [99]. While these (and many other) critical hurdles need to be overcome, and many questions remain unanswered (see Outstanding questions), we are cautiously optimistic the vascularization challenge will not be cited as the biggest hurdle to the clinical translation of engineered solid tissues and organs in 20 years, as it has been for the last 20 years!
Outstanding Questions.
What cell sources are most appropriate for engineering hierarchical vasculature? Are pluripotent (iPSC-EC) or other adult EC sources suitable for clinical translation? Are distinct populations of cells needed for macro-, meso-, and microvasculature? Should they be autologous or allogeneic? Can tolerance to allogeneic cells in vessels be realistically achieved?
How will donor age, comorbidities, sex, and ancestry influence clinical translation and regulatory approval?
What level of anatomical replication and mimicry is required for full physiologic functionality and restoration of blood flow in vivo? Can simpler structures that only partially replicate anatomy initiate morphogenetic programs to achieve full functionality of a complete vascular hierarchy?
To what degree does engineered vasculature need to be tissue-specific? Can parenchymal cells influence vascular phenotype, and vice-a-versa, to achieve tissue- and organ-specificity?
How will multicellular constructs with proper spatial patterning and organization of both parenchymal and stromal components be aseptically manufactured and maintained? Media formulations beneficial to vascular development often do not support development and function of parenchymal cells, and vice versa.
How will human testing and clinical trials proceed for hierarchical vasculature and whole solid organs? Will multiple clinical trials be needed to test hierarchical vascular tissue constructs in different locations within the body and for different diseases? How will these results be compared? Whole organ testing will undoubtably be affected by the end-stage disease often associated with the need for organ transplant, so how will these differences be accounted for in clinical assessment of engineered organs?
Highlights.
Vasculature within the human body is arranged in a hierarchical manner beginning with large macro-vessels that taper into smaller meso-vessels and finally into the smallest microvessels.
Replicating this hierarchical vasculature within engineered tissue constructs is a critical hurdle for the clinical translation of engineered tissues and organs.
A major limitation in the development of hierarchical vasculature is the distinct and diverse fabrication strategies required for creating vasculature at each scale.
New advances in fabrication strategies, particularly 3D bioprinting, are enabling the engineering of a multiscale vascular hierarchy within a single 3D tissue construct.
Multidisciplinary collaboration and cooperation between experts in academia, industry, and government will be essential to address this grand challenge and further propel hierarchical vasculature toward clinical and commercial success.
Acknowledgements
The authors gratefully acknowledge support from the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01-HL085339 (AJP). EAM was partially supported by the Tissue Engineering and Regeneration Training Program at the University of Michigan (T32-DE007057) and the Cellular Biotechnology Training Program at the University of Michigan (T32-GM008353). NEF was partially supported by the Tissue Engineering and Regeneration Training Program at the University of Michigan (T32-DE007057) and by the Rackham Merit Fellowship at UM. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
- Anastomosis
the connection and integration of two unique blood vessel structures via surgical manipulation and suturing.
- Angiogenesis
the formation of new blood vessels from existing blood vessels.
- Basement membrane (BM)
a dense layer of ECM proteins lining the abluminal side of blood vessels providing support to the vessel. The BM is comprised mostly of collagens and laminins, and are a rich source of signaling molecules.
- Biofabrication
the use of biological materials for constructing tissues and organs.
- Biomaterial
a natural or synthetic material compatible with cells and suitable for engineering tissues and organs.
- Endothelial cell (EC)
the cells that line the inner vascular wall of all blood vessels in the human body and have organ specific phenotype and function.
- Endothelial colony forming cell (ECFC)
blood circulating ECs derived from the bone marrow that serve as a more translatable EC source.
- Extracellular matrix (ECM)
the network of proteins and macromolecules that provides structural and biochemical support to cells.
- Human umbilical vein endothelial cell (HUVEC)
ECs harvested from human umbilical veins that serve as a model EC source in vascular tissue engineering applications.
- Hydrogel
a crosslinked network of natural proteins, polysaccharides, and/or synthetic polymers capable of holding large amounts of water.
- Induced pluripotent stem cells (iPSC)
embryonic-like cells capable of being programmed into any type of human cells.
- Induced pluripotent stem cell derived endothelial cell (iPSC-EC)
endothelial cells derived from iPSC.
- Inosculation
the connection and integration of two unique vascular structures via natural growth and connection (e.g., two capillaries connecting within a gel, or a host capillary connecting to an implanted capillary in an in vivo model).
- Mandrel
a cylindrical rod around which hydrogels or other materials can be gelled or wrapped around to fabricate a vascular graft.
- Perfusion
the passage of fluid through a hollow tubular system (e.g., the circulatory system).
- Prevascularization
the culture of vascular cells within a tissue construct to form vessels prior to implantation.
- Regenerative medicine (RM)
a field that integrates biology, medicine, and engineering to create biomimetic tissues towards the repair and replacement of damaged tissues and organs in the human body.
- Smooth muscle cell (SMC)
the principal cell type in the media layer of blood vessels, particularly arteries and arterioles. These cells maintain blood vessel wall integrity and support remodeling.
- Stromal cell (SC)
supportive cells within the connective tissue of any organ that support the development and function of the parenchymal cells of that organ. In vascular applications, these are typically stem cells, fibroblasts, and pericytes that support vessel development, maturation, and function.
- Tunica adventitia
the outer layer of the blood vessel wall consisting primarily of connective tissue.
- Tunica intima
the innermost layer of the blood vessel wall composed of a monolayer of endothelial cells that line the lumen of the blood vessel and basement membrane proteins or loose connective tissue.
- Tunica media
the middle layer of the blood vessel wall composed primarily of concentrically arranged smooth muscle cells, and in some cases elastic tissue.
- Vasculogenesis
the de novo formation of new blood vessels from cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
None are declared by the authors.
References
- 1.Semenza GL (2007) Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J Cell Biochem 102, 840–847 [DOI] [PubMed] [Google Scholar]
- 2.Ouarne M, et al. (2021) From remodeling to quiescence: The transformation of the vascular network. Cells Dev 168, 203735. [DOI] [PubMed] [Google Scholar]
- 3.Sierra-Sanchez A, et al. (2021) Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. NPJ Regen Med 6, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rashidbenam Z, et al. (2019) Overview of Urethral Reconstruction by Tissue Engineering: Current Strategies, Clinical Status and Future Direction. Tissue Eng Regen Med 16, 365–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horst M, et al. (2019) Tissue Engineering in Pediatric Bladder Reconstruction-The Road to Success. Front Pediatr 7, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RK, et al. (2005) Engineering vascularized tissue. Nat Biotechnol 23, 821–823 [DOI] [PubMed] [Google Scholar]
- 7.Brewster L, Brey EM, Greisler HP (2007) Blood Vessels. In Principles of Tissue Engineering (Third edn), pp. 569–584, Academic Press [Google Scholar]
- 8.Stewart SF and Lyman DJ (1992) Effects of a vascular graft/natural artery compliance mismatch on pulsatile flow. J Biomech 25, 297–310 [DOI] [PubMed] [Google Scholar]
- 9.Udelsman BV, et al. (2014) Characterization of evolving biomechanical properties of tissue engineered vascular grafts in the arterial circulation. J Biomech 47, 2070–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt JB and Tranquillo RT (2016) Cyclic Stretch and Perfusion Bioreactor for Conditioning Large Diameter Engineered Tissue Tubes. Ann Biomed Eng 44, 1785–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn MS, et al. (2007) Physiologic pulsatile flow bioreactor conditioning of poly(ethylene glycol)-based tissue engineered vascular grafts. Ann Biomed Eng 35, 190–200 [DOI] [PubMed] [Google Scholar]
- 12.Shi X, et al. (2021) Human iPS Cell-derived Tissue Engineered Vascular Graft: Recent Advances and Future Directions. Stem Cell Rev Rep 17, 862–877 [DOI] [PubMed] [Google Scholar]
- 13.Ong CS, et al. (2017) Tissue engineered vascular grafts: current state of the field. Expert Rev Med Devices 14, 383–392 [DOI] [PubMed] [Google Scholar]
- 14.Fleischer S, et al. (2020) From Arteries to Capillaries: Approaches to Engineering Human Vasculature. Advanced Functional Materials 30, 1910811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syedain Z, et al. (2016) Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nat Commun 7, 12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing Q, et al. (2017) Aligned Nanofibrous Cell-Derived Extracellular Matrix for Anisotropic Vascular Graft Construction. Adv Healthc Mater 6, 1601333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott MB, et al. (2019) Regenerative and durable small-diameter graft as an arterial conduit. Proc Natl Acad Sci U S A 116, 12710–12719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radakovic D, et al. (2017) A multilayered electrospun graft as vascular access for hemodialysis. PLoS One 12, e0185916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gui L, et al. (2009) Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng Part A 15, 2665–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, et al. (2022) Microfluidic bioprinting of tough hydrogel-based vascular conduits for functional blood vessels. Sci Adv 8, eabq6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolati F, et al. (2014) In vitro evaluation of carbon-nanotube-reinforced bioprintable vascular conduits. Nanotechnology 25, 145101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee A, et al. (2019) 3D bioprinting of collagen to rebuild components of the human heart. Science 365, 482–487 [DOI] [PubMed] [Google Scholar]
- 23.Skylar-Scott MA, et al. (2019) Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci Adv 5, eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helms F, et al. (2021) A 3-Layered Bioartificial Blood Vessel with Physiological Wall Architecture Generated by Mechanical Stimulation. Ann Biomed Eng 49, 2066–2079 [DOI] [PubMed] [Google Scholar]
- 25.Schoneberg J, et al. (2018) Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci Rep 8, 10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strobel HA, et al. (2018) Assembly of Tissue-Engineered Blood Vessels with Spatially Controlled Heterogeneities. Tissue Eng Part A 24, 1492–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strobel HA, et al. (2018) Fabrication of Custom Agarose Wells for Cell Seeding and Tissue Ring Self-assembly Using 3D-Printed Molds. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith RJ Jr., et al. (2019) Implantation of VEGF-functionalized cell-free vascular grafts: regenerative and immunological response. FASEB J 33, 5089–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimicata M, et al. (2020) Assessment of decellularized pericardial extracellular matrix and poly(propylene fumarate) biohybrid for small-diameter vascular graft applications. Acta Biomater 110, 68–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, et al. (2020) Evaluation of 1-mm-diameter endothelialized dense collagen tubes in vascular microsurgery. J Biomed Mater Res B Appl Biomater 108, 2441–2449 [DOI] [PubMed] [Google Scholar]
- 31.Radke D, et al. (2018) Tissue Engineering at the Blood-Contacting Surface: A Review of Challenges and Strategies in Vascular Graft Development. Advanced Healthcare Materials 7, 1701461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo J, et al. (2020) Tissue-Engineered Vascular Grafts with Advanced Mechanical Strength from Human iPSCs. Cell Stem Cell 26, 251–261 e258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Syedain ZH, et al. (2017) A completely biological “off-the-shelf” arteriovenous graft that recellularizes in baboons. Sci Transl Med 9, eaan4209. [DOI] [PubMed] [Google Scholar]
- 34.Kirkton RD, et al. (2019) Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med 11, eaau6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutowski P, et al. (2020) Arterial reconstruction with human bioengineered acellular blood vessels in patients with peripheral arterial disease. J Vasc Surg 72, 1247–1258 [DOI] [PubMed] [Google Scholar]
- 36.Lim KS, et al. (2019) Microchannels in Development, Survival, and Vascularisation of Tissue Analogues for Regenerative Medicine. Trends Biotechnol 37, 1189–1201 [DOI] [PubMed] [Google Scholar]
- 37.Mirabella T, et al. (2017) 3D-printed vascular networks direct therapeutic angiogenesis in ischaemia. Nat Biomed Eng 1, 0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang F, et al. (2020) Microchannels Are an Architectural Cue That Promotes Integration and Vascularization of Silk Biomaterials in Vivo. ACS Biomater Sci Eng 6, 1476–1486 [DOI] [PubMed] [Google Scholar]
- 39.Gong MM, et al. (2019) Human organotypic lymphatic vessel model elucidates microenvironment-dependent signaling and barrier function. Biomaterials 214, 119225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson RL, et al. (2018) Design principles for lymphatic drainage of fluid and solutes from collagen scaffolds. J Biomed Mater Res A 106, 106–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alimperti S, et al. (2017) Three-dimensional biomimetic vascular model reveals a RhoA, Rac1, and N-cadherin balance in mural cell-endothelial cell-regulated barrier function. Proc Natl Acad Sci U S A 114, 8758–8763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao G, et al. (2018) Coaxial Cell Printing of Freestanding, Perfusable, and Functional In Vitro Vascular Models for Recapitulation of Native Vascular Endothelium Pathophysiology. Adv Healthc Mater 7, e1801102. [DOI] [PubMed] [Google Scholar]
- 43.Mandrycky C, et al. (2020) 3D curvature-instructed endothelial flow response and tissue vascularization. Sci Adv 6, eabb3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S, et al. (2013) Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13, 1489–1500 [DOI] [PubMed] [Google Scholar]
- 45.Meijer EM, et al. (2022) Implementation of Pericytes in Vascular Regeneration Strategies. Tissue Eng Part B Rev 28, 1–21 [DOI] [PubMed] [Google Scholar]
- 46.Warren E and Gerecht S (2023) BEYOND THE ENDOTHELIUM: THE ROLE OF MURAL CELLS IN VASCULAR BIOLOGY: In vitro systems to study endothelial/pericyte cell interactions. Vasc Biol 5, e220021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holm A, et al. (2018) Microvascular Mural Cell Organotypic Heterogeneity and Functional Plasticity. Trends Cell Biol 28, 302–316 [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, et al. (2022) Interstitial flow promotes the formation of functional microvascular networks in vitro through upregulation of matrix metalloproteinase-2. Adv Funct Mater 32, 2206767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crosby CO, et al. (2021) Phototunable interpenetrating polymer network hydrogels to stimulate the vasculogenesis of stem cell-derived endothelial progenitors. Acta Biomater 122, 133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei Z, et al. (2020) Hydrogel Network Dynamics Regulate Vascular Morphogenesis. Cell Stem Cell 27, 798–812 e796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown A, et al. (2020) Engineering PEG-based hydrogels to foster efficient endothelial network formation in free-swelling and confined microenvironments. Biomaterials 243, 119921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beamish JA, et al. (2019) Deciphering the relative roles of matrix metalloproteinase- and plasmin-mediated matrix degradation during capillary morphogenesis using engineered hydrogels. J Biomed Mater Res B Appl Biomater 107, 2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghajar CM, et al. (2010) Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms. Exp Cell Res 316, 813–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andree B, et al. (2019) Formation of three-dimensional tubular endothelial cell networks under defined serum-free cell culture conditions in human collagen hydrogels. Sci Rep 9, 5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friend NE, et al. (2020) Injectable pre-cultured tissue modules catalyze the formation of extensive functional microvasculature in vivo. Sci Rep 10, 15562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin RZ, et al. (2017) Host non-inflammatory neutrophils mediate the engraftment of bioengineered vascular networks. Nat Biomed Eng 1, 0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bang S, Tahk D, Choi YH, Lee S, Lim J, Lee S-R, Kim B-S, Kim HN, Hwang NS, & Jeon NL (2021) 3D Microphysiological System-Inspired Scalable Vascularized Tissue Constructs for Regenerative Medicine. Advanced Functional Materials 32, 2105475 [Google Scholar]
- 58.Kang KT, et al. (2017) Endothelial colony forming cells and mesenchymal progenitor cells form blood vessels and increase blood flow in ischemic muscle. Sci Rep 7, 770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li S, et al. (2017) Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat Mater 16, 953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ziegler T, et al. (2019) Atherosclerosis and the Capillary Network; Pathophysiology and Potential Therapeutic Strategies. Cells 9, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Margolis EA, et al. (2021) Stromal cell identity modulates vascular morphogenesis in a microvasculature-on-a-chip platform. Lab Chip 21, 1150–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song HG, et al. (2020) Transient Support from Fibroblasts is Sufficient to Drive Functional Vascularization in Engineered Tissues. Adv Funct Mater 30, 2003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee VK, et al. (2014) Generation of Multi-Scale Vascular Network System within 3D Hydrogel using 3D Bio-Printing Technology. Cell Mol Bioeng 7, 460–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Debbi L, et al. (2022) Integrating engineered macro vessels with self-assembled capillaries in 3D implantable tissue for promoting vascular integration in-vivo. Biomaterials 280, 121286. [DOI] [PubMed] [Google Scholar]
- 65.Redd MA, et al. (2019) Patterned human microvascular grafts enable rapid vascularization and increase perfusion in infarcted rat hearts. Nat Commun 10, 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang B, et al. (2016) Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater 15, 669–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiu LL, et al. (2012) Perfusable branching microvessel bed for vascularization of engineered tissues. Proc Natl Acad Sci U S A 109, E3414–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szklanny AA, et al. (2021) 3D Bioprinting of Engineered Tissue Flaps with Hierarchical Vessel Networks (VesselNet) for Direct Host-To-Implant Perfusion. Adv Mater 33, e2102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sekine H, et al. (2013) In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun 4, 1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Helms F, et al. (2022) An encapsulated fibrin-based bioartificial tissue construct with integrated macrovessels, microchannels, and capillary tubes. Biotechnol Bioeng 119, 2239–2249 [DOI] [PubMed] [Google Scholar]
- 71.Lee H, et al. (2021) Freeform 3D printing of vascularized tissues: Challenges and strategies. J Tissue Eng 12, 20417314211057236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, et al. (2021) Recent advances in 3D bioprinting of vascularized tissues. Materials & Design 199, 109398 [Google Scholar]
- 73.Grigoryan B, et al. (2019) Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364, 458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeon O, et al. (2019) Individual cell-only bioink and photocurable supporting medium for 3D printing and generation of engineered tissues with complex geometries. Mater Horiz 6, 16251631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGuire BJ and Secomb TW (2003) Estimation of capillary density in human skeletal muscle based on maximal oxygen consumption rates. Am J Physiol Heart Circ Physiol 285, H2382–2391 [DOI] [PubMed] [Google Scholar]
- 76.Rakusan K, et al. (1992) Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation 86, 38–46 [DOI] [PubMed] [Google Scholar]
- 77.Chuang CH, et al. (2018) Comparison of covalently and physically cross-linked collagen hydrogels on mediating vascular network formation for engineering adipose tissue. Artif Cells Nanomed Biotechnol 46, S434–S447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bang S, et al. (2017) A Low Permeability Microfluidic Blood-Brain Barrier Platform with Direct Contact between Perfusable Vascular Network and Astrocytes. Sci Rep 7, 8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X, et al. (2016) Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip 16, 282–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Linville RM, et al. (2019) Human iPSC-derived blood-brain barrier microvessels: validation of barrier function and endothelial cell behavior. Biomaterials 190–191, 24–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pasut A, et al. (2021) Endothelial cell plasticity at the single-cell level. Angiogenesis 24, 311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao G, et al. (2021) Single-Cell Transcriptomics Reveals Endothelial Plasticity During Diabetic Atherogenesis. Front Cell Dev Biol 9, 689469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Curtis MB, et al. (2022) Organotypic stromal cells impact endothelial cell transcriptome in 3D microvessel networks. Scientific Reports 12, 20434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gifre-Renom L, et al. (2022) Organ-Specific Endothelial Cell Differentiation and Impact of Microenvironmental Cues on Endothelial Heterogeneity. International Journal of Molecular Sciences 23, 1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peters EB (2018) Endothelial Progenitor Cells for the Vascularization of Engineered Tissues. Tissue Eng Part B Rev 24, 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Melero-Martin JM (2022) Human Endothelial Colony-Forming Cells. Cold Spring Harb Perspect Med 12, a041154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen YC, et al. (2012) Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels. Adv Funct Mater 22, 2027–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Macklin BL, et al. (2022) Intrinsic epigenetic control of angiogenesis in induced pluripotent stem cell-derived endothelium regulates vascular regeneration. NPJ Regen Med 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Faley SL, et al. (2019) iPSC-Derived Brain Endothelium Exhibits Stable, Long-Term Barrier Function in Perfused Hydrogel Scaffolds. Stem Cell Reports 12, 474–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bezenah JR, et al. (2018) Evaluating the potential of endothelial cells derived from human induced pluripotent stem cells to form microvascular networks in 3D cultures. Sci Rep 8, 2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hajal C, et al. (2022) Engineered human blood-brain barrier microfluidic model for vascular permeability analyses. Nat Protoc 17, 95–128 [DOI] [PubMed] [Google Scholar]
- 92.Bezenah JR, et al. (2019) Assessing the ability of human endothelial cells derived from induced-pluripotent stem cells to form functional microvasculature in vivo. Biotechnol Bioeng 116, 415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rouhani FJ, et al. (2022) Substantial somatic genomic variation and selection for BCOR mutations in human induced pluripotent stem cells. Nat Genet 54, 1406–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.James BD and Allen JB (2021) Sex-Specific Response to Combinations of Shear Stress and Substrate Stiffness by Endothelial Cells In Vitro. Adv Healthc Mater 10, e2100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ryan H, et al. (2022) Ancestral Background Is Underreported in Regenerative Engineering. Regen Eng Transl Med 8, 499–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ingber DE (2022) Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat Rev Genet 23, 467–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nahle Z (2022) A proof-of-concept study poised to remodel the drug development process: Liver-Chip solutions for lead optimization and predictive toxicology. Front Med Technol 4, 1053588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jayasinghe SN (2017) Thoughts on Scaffolds. Adv Biosyst 1, e1700067. [DOI] [PubMed] [Google Scholar]
- 99.Pavlovich S (2016) Should Society Encourage The Development Of 3D Printing, Particularly 3D Bioprinting Of Tissues And Organs? International Journal of Scientific & Technology Research 5, 41–46 [Google Scholar]
- 100.Grant MA, Karsan A (2018) The Blood Vessel Wall. In Hematology (Seventh edn), pp. 1843–1856, Elsevier [Google Scholar]
- 101.Martinez-Lemus LA (2012) The dynamic structure of arterioles. Basic Clin Pharmacol Toxicol 110, 5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chan KLS, et al. (2014) Crosslinking of collagen scaffolds promotes blood and lymphatic vascular stability. J Biomed Mater Res A 102, 3186–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Price GM, et al. (2008) Effect of cyclic AMP on barrier function of human lymphatic microvascular tubes. Microvasc Res 76, 46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oliver G (2004) Lymphatic vasculature development. Nature Reviews Immunology 4, 3545. [DOI] [PubMed] [Google Scholar]
- 105.Oliver G, et al. (2020) The Lymphatic Vasculature in the 21(st) Century: Novel Functional Roles in Homeostasis and Disease. Cell 182, 270–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Petrova TV and Koh GY (2017) Organ-specific lymphatic vasculature: From development to pathophysiology. Journal of Experimental Medicine 215, 35–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grainger SJ, et al. (2013) Stromal cell identity influences the in vivo functionality of engineered capillary networks formed by co-delivery of endothelial cells and stromal cells. Tissue Eng Part A 19, 1209–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]


