Abstract
Objectives:
To describe population rate of hysterectomy for benign disease in the United States, including geographic variation across states and Hospital Service Areas (HSAs; areas defined by common patient flows to healthcare facilities).
Design:
Cross-sectional study
Setting:
Four US states including 322 HSAs
Population:
316,052 cases of hysterectomy from 2012-2016
Methods:
We compiled annual hysterectomy cases, merged female populations, and adjusted for reported rates of prior hysterectomy. We assessed small-area variation and created multilevel Poisson regression models.
Main Outcome Measures:
Prior-hysterectomy-adjusted population rates of hysterectomy for benign disease
Results:
The annual population rate of hysterectomy for benign disease was 49 per 10,000 hysterectomy-eligible residents, declining slightly over time, mostly among reproductive-age populations. Rates peaked among residents ages 40-49, and declined with increasing age, apart from an increase with universal coverage at age 65. We found large differences in age-standardized population rates of hysterectomy across states (range 42.2-69.0), and HSAs (range: overall 12.9-106.3; 25th-75th percentile 44.0-64.9). Among the non-elderly population, those with government-sponsored insurance had greater variation than those with private insurance (coefficient of variation 0.61 vs 0.32). Proportions of minimally invasive procedures were similar across states (71.0%-74.8%) but varied greatly across HSAs (27%-96%). In regression models, HSA population characteristics explained 31.8% of observed variation in annual rates. Higher local proportions of government-sponsored insurance and non-White race were associated with lower population rates.
Conclusions:
We found substantial variation in rate and route of hysterectomy for benign disease in the US. Local population characteristics explained less than 1/3 of observed variation.
Keywords: Epidemiology: General Gynaecology, Gynaecological Surgery: General, Health Services Research, Minimal Access Surgery, Statistics: Epidemiological Surveys
Introduction
Hysterectomy is one of the most frequently performed major surgical procedures.1 Most hysterectomies are performed for benign indications (e.g. endometriosis, fibroids, pain, or prolapse), for which effective, less invasive management alternatives are often available.2,3 International data have shown recent declines in the annual population rate of hysterectomy in Australia,4 Denmark,5 Israel,6 the Netherlands,7 Switzerland,8 and the UK.9 These studies found significant small-area geographic variation, with rates varying 2-3 fold, and sizable discrepancies in utilization of minimally invasive techniques.4–9 Small-area variation in surgical utilization is thought to be primarily driven by differences in provider practice.10
The United States (US) has a larger, more dispersed population, as well as less centralized or nationalized health insurance and care delivery systems. In the US, inpatient hysterectomy utilization declined 36% between 2002 and 2010, from 681,000 to 433,000 cases annually,11 though in part due to growing outpatient utilization, with 100,000-200,000 cases in 2011.2,12 The population rate of hysterectomy, and geographic variation therein, have not been well described in the US, likely due to the limited availability of population-based data sources in the more fragmented healthcare system.13
For our study, we combined population-based data on inpatient and outpatient surgery for a subset of four US states, with published descriptive data on local populations, and survey data to adjust of population totals for prior hysterectomy. Our primary objective was to estimate the population rate of hysterectomy for benign disease in the US, assessing for geographic variation in rate and route of surgery. Secondly, we sought to use regression models to estimate the role of local population characteristics in explaining observed variation in hysterectomy rates, and the residual unexplained variation that is likely primarily related to differences in patient preferences for surgery, provider practice, and disparities in access to care.
Methods
Study Data
This was a cross-sectional study of hysterectomy for benign disease. See Appendix S1 for additional details throughout. We utilized US Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP) State Inpatient Database (SID) and State Ambulatory Surgery and Services Database (SASD) files.14,15 Together, the SID and SASD capture nearly 100% of surgeries performed annually in a given state, and both report ZIP code of patient residence. We compiled data from Kentucky, Maryland, Florida, and North Carolina for the years 2012-2016. These four states were selected as being regionally related, while representing some diversity in population demographic characteristics.
We merged data from four sources for geographic analysis. (1) Publicly available crosswalk files from the Dartmouth Atlas were used to compile data from ZIP codes to Hospital Service Areas (HSAs).16 HSAs are non-overlapping compilations of geographically linked ZIP codes defined by common flows of patients residing in those ZIP codes to common healthcare providers. HSAs describe regional hubs of care, and they are commonly used for analyses of small-area variation in healthcare.17 (2) State-level estimates of adult (age >18) female population totals by single-year age, and race (White, Black, other/multiple; self-reported) were accessed from the US Census for years 2012-2016.18 (3) Small-area and state population totals and demographics were drawn from Social Determinants of Health Database (SDOHD) for 2012-2016.19,20 (4) We utilized the Behavioral Risk Factor Surveillance System (BRFSS), a large, nationally representative survey of the US population,21 to estimate population proportions with prior hysterectomy (by state, age, race, and year) and adjust the denominator population totals to those still eligible for hysterectomy, using a similar technique to previously published works.22,23
Sample Selection
For our patient sample, we selected for hysterectomy by International Classification of Diseases, 9th (2012 to 3rd quarter 2015) and 10th Revisions (4th quarter 2015-2016) code from SID files, or Common Procedural Terminology code from SASD files (Table S1). We first limited this sample to patients ages ≥18, and excluded hysterectomies indicated for cancer, or with pregnancy/obstetrics diagnoses (13.3% of initial sample; Table S2). We next limited the sample based on patient ZIP code of patient residence. For state-level analyses of population rates of surgery, we excluded patients residing outside of the state of surgery. For HSA-level analyses, we limited to patients residing in one of 322 HSAs with healthcare utilization based primarily in one of the four included states, as these patients would be expected to predominantly seek care from facilities represented in included files. Of note, some HSA boundaries cross state lines, leading to small differences in the included cases for HSA vs. state analyses.
Study Variables
Characteristics of hysterectomy cases were derived from SID and SASD data files, and included age (18-44, 45-64, 65-79, 80+), race/ethnicity (White, Black, or other/multiple race, and Hispanic or non-Hispanic ethnicity, as reported by facility to HCUP), insurance coverage (any private, Medicare only, any Medicaid, other, uninsured), Charlson comorbidity score (count of 17 comorbidities predicting mortality risk, by diagnosis codes),24 quartile of residential ZIP code median income (1 to 4 in increasing income, as reported to HCUP), facility type (inpatient vs. ambulatory surgery center), surgical approach (open, laparoscopic, or vaginal), surgery type (supracervical vs. total), visit charges, and primary indication for surgery (Table S3).
HSA population characteristics were derived from SDOHD data files, and included proportion of HSA population by age (18-44, 45-64, 65-79, 80+), race (White, Black, other/multiple), Hispanic ethnicity, insurance coverage (any private, Medicare only, any Medicaid, other, uninsured), income in relation to Federal Poverty Level (<100% FPL, 100-124%, 125-199%, ≥200%), unemployment, and lack of high school degree or equivalent education, as well as characteristics of population density (per square mile), and metro/rural rating (ranging to 1 to 10 for core metropolitan to rural; Economic Research Service Rural-Urban Community Area rating).
Study Outcomes
Our primary outcome was the population rate of hysterectomy per 10,000 hysterectomy-eligible adult residents. The numerator was calculated as the sum of hysterectomies performed for adult (age ≥18) patients residing in the given area (HSA, state) annually, while the denominator was calculated as the total population of adult (age ≥18) female residents without prior hysterectomy living in the respective area in that year. The ratios were then multiplied by 10,000 to report annual rates per 10,000 hysterectomy-eligible residents, overall and in subgroups. We performed direct age-standardization for overall and state-specific rates. We also considered the outcome of proportion of hysterectomies performed by route (open, laparoscopic, or vaginal).
Statistical Analysis
For analysis of small-area variation by HSA, we averaged overall and subgroup rates over the 5 included years to minimize the impact of random year-to-year variations. We described small-area variation with three commonly used measures: coefficient of variation (CoV; mean/standard deviation), interquartile ratio (IQR; 75th percentile/25th percentile), and truncated extremal quotient (EQ5-95; 95th percentile/5th percentile).10 We assessed visually for variation with Violin plots of HSA hysterectomy rates, overall and by subgroup, and of HSA hysterectomy route proportions. As expected small-area variation differs with the overall rate and underlying population distribution, there is no accepted null hypothesis or method of statistical significance testing.25
We explored the role of observable HSA population characteristics in explaining the observed small-area variation in population rate of hysterectomy using a similar technique to that of a recent study of hysterectomy in Switzerland.8 We created multilevel Poisson regression models of annual prior-hysterectomy-adjusted population rate of hysterectomy by HSA, with HSA set as a random intercept. The initial model controlled only for year, to account for changes over time. We then added controls for an a priori selected set of observable HSA population characteristics (age, race, ethnicity, insurance, income, education, and density) that may impact variation in hysterectomy rates. We assessed for the extent to which included variables explained variation across HSAs by comparing the variance in the random intercept in the initial and adjusted models.8
We considered statistical significance by p<0.05. STROBE guidelines for observational research were followed.26 All analyses were conducted with STATA v15.1 (Statacorp, College Station, TX, USA). The Duke University IRB considered this study exempt from review. Figures were created with STATA or Microsoft PowerPoint/Excel v14.7.7 (Microsoft Corporation, Redmond, WA, USA).
Results
We identified 317,644 cases of hysterectomy for non-obstetric, benign indications performed in adults between 2012 and 2016 in Kentucky, Maryland, Florida, and North Carolina (Figure S1). After adjusting population totals to the adult female population without prior hysterectomy using a multivariable linear model including age, race, state, and year (Table S4), the annual hysterectomy-eligible population across the four states was approximately 12.5 million residents.
The four included states represented a spectrum of diversity in HSA populations (Table S5), and hysterectomy cases (Tables S6). Florida HSAs were highly concentrated with those ages 65+ (25.8% vs. 18.0%-21.9% by population; 12.1% vs. 5.6-8.2% of hysterectomies), and of Hispanic ethnicity (16.4% vs. 2.2%-8.8% by HSA population; 13.5% vs. 2.9-7.8% of hysterectomies). Kentucky HSAs had the highest concentration of White residents (93.2% vs. 55.8-80.4% of population; 94.5% of hysterectomies vs. 60.2-76.9%) and the lowest hysterectomy-eligible population (15,346 vs. 30,008-60,294 per HSA). Maryland HSAs averaged only 10.1% of the population having income below the Federal Poverty Level (FPL; vs. 16.2-22.1%), and higher rates of private coverage (66.2% by population, 73.9% of hysterectomies) and lower rates of uninsurance (9.6%) compared with other states (50.5-51.8%, 13.9-17.7% respectively).
Clinical characteristics of hysterectomy cases by HSA are presented in Table S7. North Carolina and Kentucky HSAs tended to contain smaller populations and lower absolute hysterectomy case numbers, and they were more likely to be performed outpatient. While there was notable variation across HSAs in the distribution of route of hysterectomy, open surgery rates by state ranged only from 25.1% to 29.8%. There was greater use of vaginal hysterectomy in North Carolina (21.7% vs. 11.2-14.1% in other states). Indications for surgery were generally similar across states, with abnormal uterine bleeding, polyp, and fibroids collectively comprising the primary indication in over 50% of cases. Kentucky HSAs had a higher proportion of cases for pelvic pain or endometriosis compared with other states (21.2% vs. 7.2-12.1%), and a lower proportion of surgery for fibroids (20.9% vs. 32.0-40.5%).
The mean annual population rate of hysterectomy for benign disease across the 322 included HSAs was 49.3 per 10,000 hysterectomy eligible residents, declining slightly from 51.0 in 2012 to 47.9 in 2016. Prior-hysterectomy-adjusted population rates of in-state residents using state population totals were similar (49.1 per 10,000 overall; declining from 50.6 in 2012 to 47.7 in 2016; Figure S2).
Annual state population rates varied starkly by age (Figure 1), with rates near 0 for ages 18-24, peaking with annual rates >100 per 10,000 hysterectomy-eligible residents ages 40-49. We observed the greatest variation in age-specific rates by state and race among these middle-ages, with hysterectomy-eligible Kentucky residents ages 40-44 undergoing surgery at a rate of 159.5 per 10,000, versus 99.7, 120.6, and 124.8 in Maryland, Florida, and North Carolina, respectively. Hysterectomy-eligible residents of non-White race in these middle age groups also had higher rates (169 per 10,000 hysterectomy-eligible Black residents vs. 106 for White race). Although population rates of hysterectomy for benign disease showed sharp decline after age 50, we observed an increased rate among hysterectomy-eligible residents ages 65-69 (40.8 per 10,000) after Medicare eligibility, versus those ages 60-64 (33.8 per 10,000).
Figure 1.
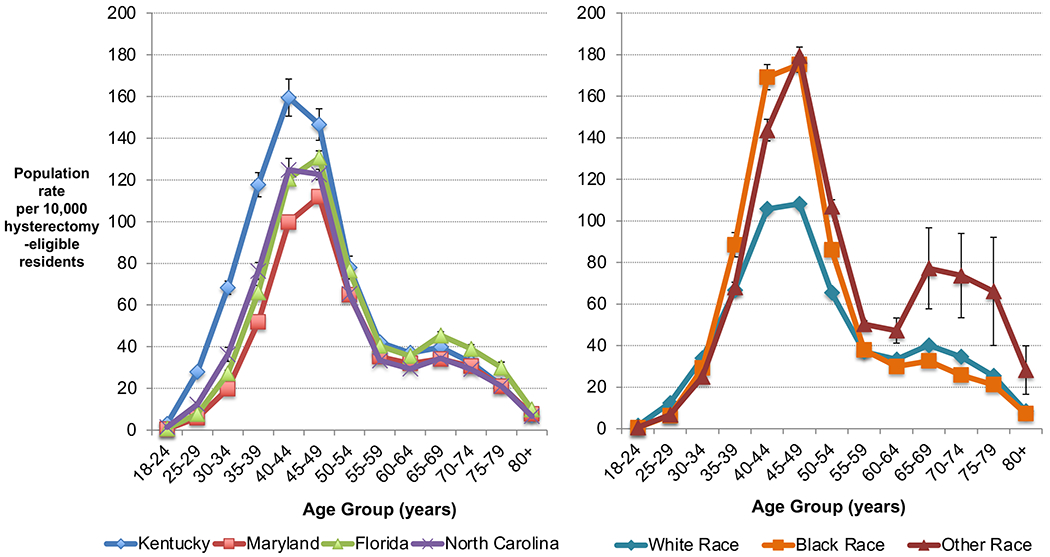
Annual population rate of hysterectomy for benign disease per 10,000 hysterectomy-eligible residents by age, race, and state, Kentucky, Maryland, Florida, and North Carolina, 2012-2016. Estimates averaged over included years, with error bars representing standard deviation. Excludes obstetric cases and ages <18.
Prior-hysterectomy-adjusted female population rates of hysterectomy for benign disease showed notable variation across HSAs, even when averaged over the five included years (Table S8, Figure 2), with an overall range of 12.9 to 106.3 per 10,000 hysterectomy-eligible residents (range 44.0-64.9 for 25th-75th percentile). Age-standardized population rates varied across the four states (ranging from 42.2 per 10,000 hysterectomy-eligible residents in Maryland to 69.0 in Kentucky). This variation across states is notable considering inability to estimate HSA-specific rates of prior hysterectomy. However, variation across HSAs was similar within each state (CoV 0.23-0.27; IQR 1.34-1.41; EQ5-95 1.96-2.60). Variation was notably lower in the 45-64 age range compared to older and younger ages (CoV 0.25 vs 0.42, 0.39, and 0.81 for ages 18-44, 65-79, and 80+). While there was some visual regional grouping of similar rates for adjacent HSAs on maps, there were not clear patterns, with some sharp discrepancies for adjacent HSAs (see Figure 3). The population with Medicare coverage alone (excluding most Medicare recipients owning supplemental coverage) exhibited high annual rates (100.3 per 10,000 hysterectomy-eligible residents), while the uninsured had low rates of surgery (9.6 per 10,000). The Medicaid population had higher variation (CoV 0.61, IQR 2.41; EQ5-95 6.50) than those with private coverage (CoV 0.32; IQR 1.57, EQ5-95 3.09). In a sensitivity analysis without the adjustment for prior hysterectomy, estimates of variation were consistent with the primary analysis (Table S9).
Figure 2.
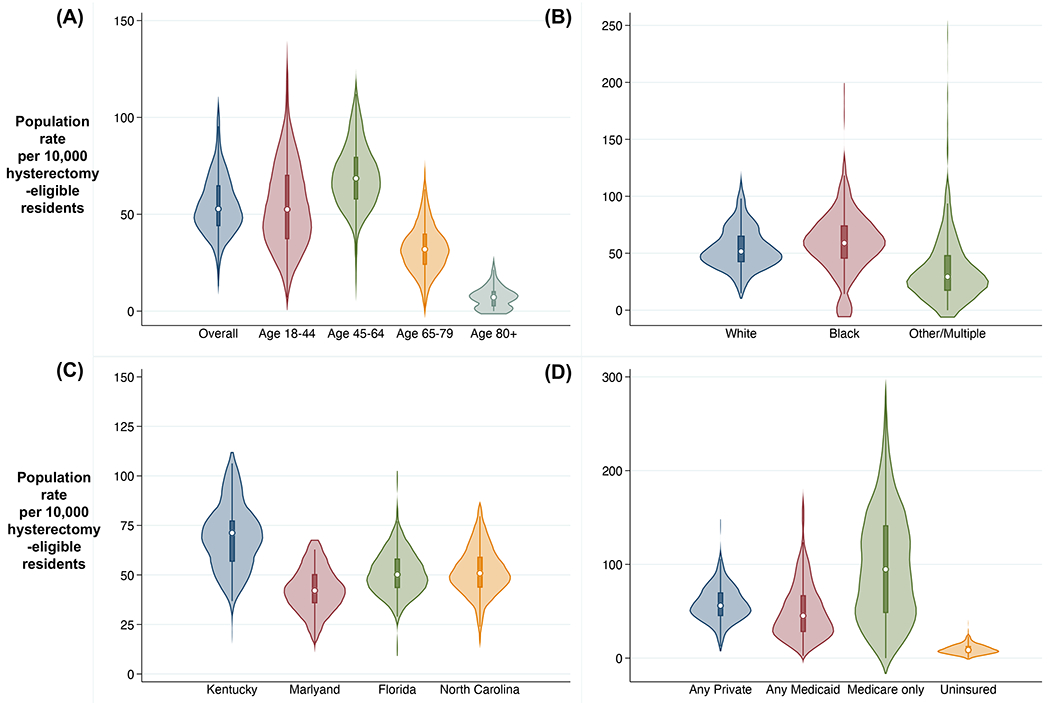
Violin plots depicting small-area variation in annual population rate of hysterectomy for benign disease per 10,000 hysterectomy-eligible residents across Hospital Service Areas, (A) by age, (B) race, (C) state, and (D) insurance, Kentucky, Maryland, Florida, and North Carolina, 2012-2016. Plotted areas represent kernel density frequency distribution of population rate estimates for individual Hospital Service Area mean rates over included years, with central dot marking median and box marking interquartile range (25th percentile to 75th percentile) for the distribution; excludes obstetric case and ages <18.
Figure 3.
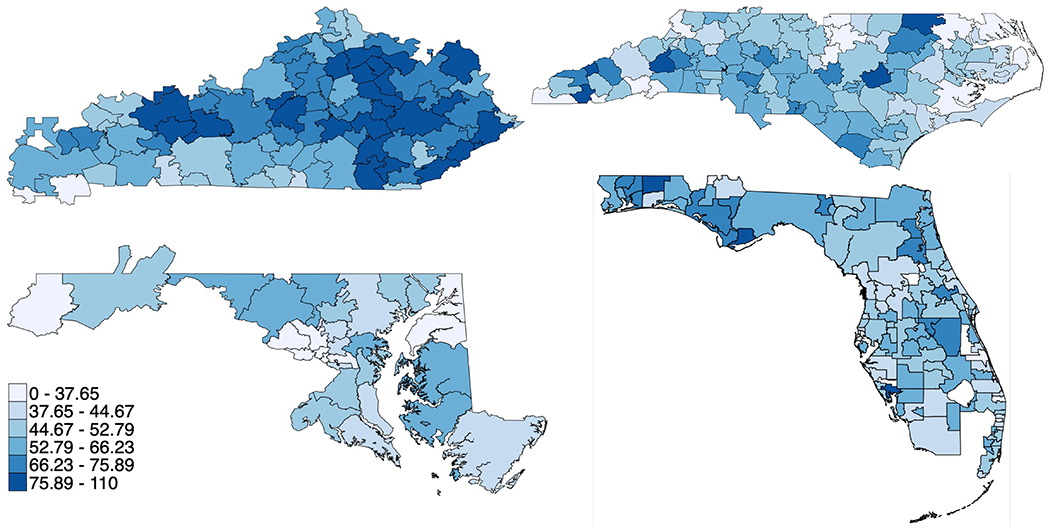
Maps depicting geographic small-area variation in annual population rate of hysterectomy for benign disease per 10,000 hysterectomy-eligible residents across Hospital Service Areas in Kentucky (top left), Maryland (bottom left), North Carolina (top right), and Florida (bottom right) with darker colors for higher rates (bottom 10%ile, 10-25%ile, 25-50%ile, 50-75%ile, 75-90%ile, and top 10%ile); excludes obstetric cases and ages <18.
We also identified significant variation in route of hysterectomy (Figure 4). The share of hysterectomy for benign disease performed with minimally invasive techniques ranged from 26.5% to 95.6% across HSAs (67%-82% 25th-75th percentile). Vaginal hysterectomy represented <5% of cases in 15 HSAs, but this approach was utilized in less than one third of cases in 14 HSAs (12 in North Carolina). Although the mean proportion of hysterectomies performed open was 26%, there were 37 HSAs (11%) with over 40% open cases.
Figure 4.
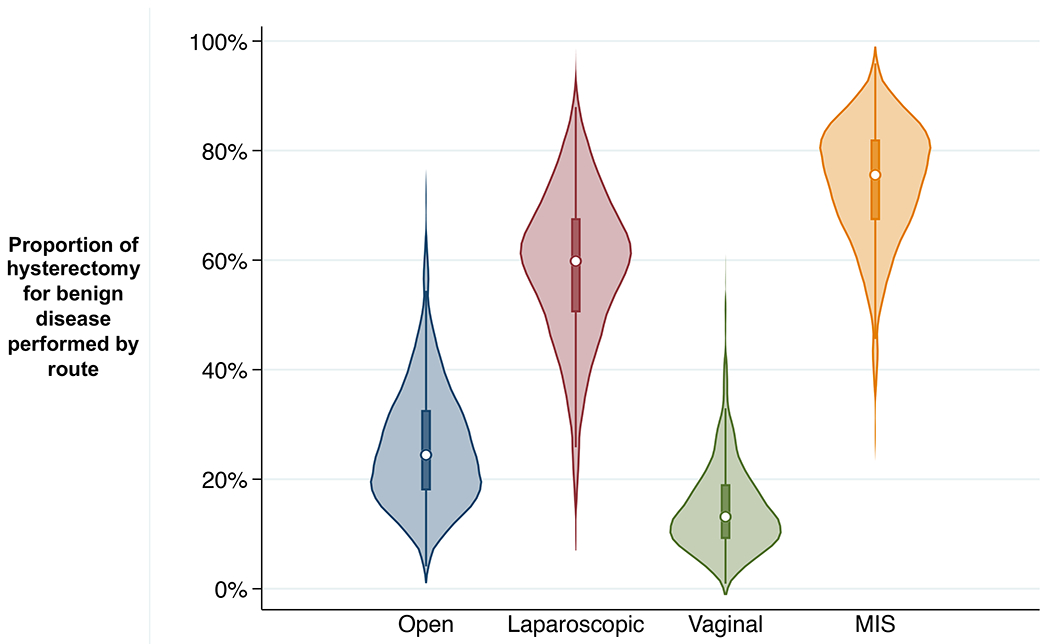
Small-area variation across Hospital Service Areas in the percent of cases of hysterectomy for benign disease performed by route: open, laparoscopic, vaginal, and minimally invasive surgery (MIS; laparoscopic or vaginal), Kentucky, Maryland, Florida, and North Carolina, 2012-2016. Plotted areas represent kernel density frequency distribution of population rate estimates for individual Hospital Service Area mean rates over included years, with central dot marking median and box marking interquartile range (25th percentile to 75th percentile) for the distribution; excludes obstetric case and ages <18.
In Poisson regression assessing the role of HSA population characteristics in explaining small-area variation (Table S10), we found that adding controls for HSA demographics explained 15.8% of the observed variation. Layering controls for other observable factors explained another 16.0% of the variation, meaning that 68.2% of the small-area variation in prior-hysterectomy-adjusted population rate of hysterectomy for benign disease remained unexplained by observable local population characteristics. These characteristics played the greatest role in explaining variation in the reproductive-aged population (53%). Despite higher race-specific hysterectomy population rates for non-White residents (Table S10, Figure 1), in the adjusted regression model, HSAs with higher proportion of the population with Black race showed lower overall local population rates. Higher population proportions with Hispanic ethnicity and private insurance, and more rural HSAs were also associated with lower population rates.
Discussion
Main Findings
We present a novel analysis of population rates of hysterectomy for benign disease in the US, using a sample of four regionally located but diverse states, and including correction of population denominators for prior hysterectomy. We found the population rate of hysterectomy for benign disease to be approximately 50 in 10,000 hysterectomy-eligible residents annually, declining slightly between 2012 and 2016, primarily among reproductive-aged residents. We identified significant variation in the annual population rate of hysterectomy for benign disease, across states (range 41-65 per 10,000 hysterectomy-eligible residents) and HSAs (range 13-106). Similarly, we observed notable variation in share of minimally invasive procedures across HSAs (ranging from 26-96%) with greatest variation in use of vaginal approach.
Strengths and Limitations
The strength of this study is in its unique combination of multiple data sources, including population-based inpatient and outpatient surgical data, geographic contextual data, and population data for correction for prior hysterectomy. More comprehensive data sources for this kind of analysis in the US are unavailable. National Inpatient Sample is comprehensive and nationally inclusive, but lacks geographic identifiers for small area analysis, with cases only specified to regional groupings of states. Claims data sources sometimes contain more specific geographic identifiers but are not comprehensive or representative of the overall population in a geographic area. Our study was limited to four states, but these states were selected to contain diverse populations in terms of race/ethnicity, insurance, and income. Our analysis approach of adjusting for prior hysterectomy is underutilized in studies of population rate of surgery, particularly for such a common procedure.
However, results from these states are not necessarily generalizable to other US states. We were also limited in the accuracy of hysterectomy-eligible population estimates. While we used population-based survey data and multivariable models to generate age, race, and state-specific estimates of rates of prior hysterectomy, these estimates are not perfectly representative of all subpopulations, and the geographic specificity of these estimates was limited to the state, rather than HSA level. Therefore, some of the variation in hysterectomy-eligible population rates could be due to small area variation in prior hysterectomy. We did perform a sensitivity analysis without adjustment for prior hysterectomy, and found similar measures of variation, though this measure has the same limitation of being impacted by unmeasured variation in prior hysterectomy. However, the four states demonstrated significant variation in rates despite state-specific estimates of prior-hysterectomy rates. Our data is missing cases of hysterectomy for residents performed at out-of-state facilities, and therefore may slightly underestimate population rates. However, out-of-state residents represented <4% of hysterectomies from included states, and HSA borders were designed by patient flows to healthcare facilities, thereby minimizing issues of border crossing. Lastly, changes in procedure coding could affect our data, though no concerning trends were observed.
Interpretation
Our estimate of overall population rate of hysterectomy for benign disease appears higher than estimates for other geographic states/countries (49 per 10,000 vs. 34 in California27, 35 in Austrailia,4 18 in Denmark,5 23 in Israel,6 17 in the Netherlands,7 30 in Switzerland8). Although none of these prior studies corrected for prior hysterectomy, our estimate without this correction of 42 per 10,000 adult female residents remains higher, indicating overall greater utilization of benign hysterectomy in the US versus comparable countries. The only corrected studies we identified, estimated a similar population rates of 48 per 10,000 hysterectomy-eligible residents in Western Australia,28 and 47 in North Carolina,23 using similar data to our study. It is unclear if higher utilization in the US is related to demographic factors, practice differences, or some combination thereof. Observed small-area variation in hysterectomy rates across HSAs was similar to prior estimates from other countries.5–8 as well as US studies of small areas of Massachusetts,29 and reproductive-age residents of North Carolina counties.30 Since the first descriptions of small-area variation 50 years ago by Wennberg and colleagues,31 we have made little progress in reducing it.
In addition to adding adjustment for prior hysterectomy, our study sought to assess the contribution of local demographic factors to the variation, which few studies, particularly of hysterectomy, have attempted previously. We found that local observable HSA population demographics explained only 32% of variation overall. A similar study of Swiss HSAs found that 36% of the variation could be explained by observable factors,8 including a control for local concentration of gynecologists, which we lacked for our study. Importantly, we cannot determine whether the observed unexplained variation represents overutilization, underutilization, or a combination. Some unexplained variation may be warranted due to unobservable differences in distributions of gynecologic disease, or patient preference for this elective surgery. However, it is likely that, to some extent, this variation is unwarranted, either due to differences in local practice in the threshold for surgery, or due to geographic, racial, or socioeconomic disparities in access.
Graduates of trainee programs tend to most often practice regionally around the areas in which they trained,32 which may explain some variation across states. For example, the higher rate of surgery in Kentucky may indicate a lower local threshold for surgery, and the higher share of vaginal approach in North Carolina may indicate greater training in this technique. We also noted an increase in population rates with universal coverage eligibility at age 65 via Medicare, despite a general decline in rates after age 50. The transition to Medicare eligibility has been associated with greater healthcare utilization in general,33 and improvements in cancer screening rates in particular.34 This could represent pent-up demand from symptomatic but underinsured residents under the age of 65, and indicates the possibility of an undesired role of access, patient cost, or provider reimbursement in influencing the utilization of surgical management.
The decision to pursue hysterectomy for benign indications is a complicated choice influenced by both patient and provider factors. While our study indicates that a significant portion of variation cannot be explained by observable patient demographic factors, further research is needed to elucidate the unobserved drivers. Novel data sources are needed to assess the role of prevalence and severity of gynecologic disease for indication-specific study, as well as data on provider factors such as OB/GYN concentration, local practice patterns, and access to surgical care. We also observed interesting findings by race, with race-specific higher population rates of hysterectomy for benign disease among Black residents, but trends towards lower overall population rates in HSAs with higher proportion of Black residents in the population. This may be in part due to differing age distributions by race. Further work is needed to understand and minimize the role of race in healthcare utilization.
Conclusion
Hysterectomy for benign disease is a largely elective procedure with significant geographic variation in utilization, both in rate and route of surgery. A substantial proportion of this variation cannot be explained by differences in local population characteristics. While some of the unexplained variation may be attributable to differences in patient preferences for surgery, it is likely that differences in provider practice and disparate access to care are also unobserved drivers of variation in this study, which are unwarranted medically, and understudied in general. While our study is limited to the decentralized health system of the United States, similar trends have been observed in other countries and health systems. Across global health systems, we should design pathways that lead to valuing patient preferences, create equitable access, and minimize perverse incentives related to cost and reimbursement.35,36 Further work is needed to better understand the drivers of variation, particularly from the provider side, and to optimize the utilization of hysterectomy in benign gynecologic disease.
Supplementary Material
ACKNOWLEDGEMENTS
We have no named acknowledgements to report.
FUNDING
BBA received a grant from the Charles B. Hammond Research Fund, Duke University School of Medicine, Durham, NC as primary study funding. HAM was supported by early career funding from the National Institutes of Health (BIRCWH K12HD043446), unrelated to this work. APL was supported by a grant (NCI R37 CA248470-01) unrelated to this work. ERM was supported by grants P50-HS023418, GB10416-154701, 5R01-MD011680-02, unrelated to this work.
Funding
This study was funded by a grant from the Charles B. Hammond Research Fund at the Duke University School of Medicine. This funding did not inlcude external peer review. The funding body had no input on research methods or manuscript drafting.
Footnotes
DISCLOSURE OF INTERESTS
The authors report no conflicts of interest or financial interests.
DETAILS OF ETHICS APPROVAL
This study was granted exemption from full review by the Duke University Institutional Review Board, as it involved no identifiable patient data.
REFERENCES
- 1.Elixhauser A, Andrews RM. Profile of inpatient operating room procedures in US hospitals in 2007. Arch Surg. 2010. Dec 20;145(12):1201–8. [DOI] [PubMed] [Google Scholar]
- 2.Moore BJ, Steiner CA, Davis PH, Stocks C, Barrett ML. Trends in Hysterectomies and Oophorectomies in Hospital Inpatient and Ambulatory Settings, 2005-2013. HCUP Statistical Brief #214. [Internet]. Rockville, MD; 2016. [PubMed] [Google Scholar]
- 3.Papadopoulos MS, Tolikas AC, Miliaras DE. Hysterectomy—Current Methods and Alternatives for Benign Indications. Obstet Gynecol Int. 2010;2010:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill EL, Graham ML, Shelley JM. Hysterectomy trends in Australia - between 2000/01 and 2004/05. Aust New Zeal J Obstet Gynaecol. 2010. Apr 1;50(2):153–8. [DOI] [PubMed] [Google Scholar]
- 5.Lykke R, Blaakær J, Ottesen B, Gimbel H. Hysterectomy in Denmark 1977–2011: Changes in rate, indications, and hospitalization. Eur J Obstet Gynecol Reprod Biol. 2013. Dec 1;171(2):333–8. [DOI] [PubMed] [Google Scholar]
- 6.Lauterbach R, Joseph M, Haklai Z, Gil L, Lowenstein L. Geographic variation of hysterectomy rates in the Israeli health care system during the years 2007–2016. Isr J Health Policy Res. 2019. Jul 16;8(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanstede MMF, Burger MJ, Timmermans A, Burger MPM. Regional and temporal variation in hysterectomy rates and surgical routes for benign diseases in the Netherlands. Acta Obstet Gynecol Scand. 2012. Feb 1;91(2):220–5. [DOI] [PubMed] [Google Scholar]
- 8.Stoller N, Wertli MM, Zaugg TM, Haynes AG, Chiolero A, Rodondi N, et al. Regional variation of hysterectomy for benign uterine diseases in Switzerland. Garzon S, editor. PLoS One. 2020. May 14;15(5):e0233082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madhvani K, Curnow T, Carpenter T. Route of hysterectomy: a retrospective, cohort study in English NHS Hospitals from 2011 to 2017. BJOG An Int J Obstet Gynaecol. 2019. May 30;126(6):795–802. [DOI] [PubMed] [Google Scholar]
- 10.Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Vol. 382, The Lancet. Lancet Publishing Group; 2013. p. 1121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright JD, Herzog TJ, Tsui J, Ananth CV., Lewin SN, Lu YS, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 Pt 1):233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen SL, Ajao MO, Clark NV., Vitonis AF, Einarsson JI. Outpatient Hysterectomy Volume in the United States. Obstet Gynecol. 2017. Jul 1;130(1):130–7. [DOI] [PubMed] [Google Scholar]
- 13.Alluri RK, Leland H, Heckmann N. Surgical research using national databases [Internet]. Vol. 4, Annals of Translational Medicine. AME Publishing Company; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agency for Healthcare Research and Quality. State Inpatient Database [Internet]. Healthcare Cost and Utilization Project (HCUP). Available from: https://www.hcup-us.ahrq.gov/db/state/siddbdocumentation.jsp [PubMed] [Google Scholar]
- 15.Agency for Healthcare Research and Quality. State Ambulatory Surgery Database [Internet]. Healthcare Cost and Utilization Project (HCUP). Available from: https://www.hcup-us.ahrq.gov/db/state/sasddbdocumentation.jsp [PubMed] [Google Scholar]
- 16.The Dartmouth Atlas. Supplemental Data [Internet]. 2021. Available from: https://data.dartmouthatlas.org/supplemental/
- 17.Wennberg JE, Cooper MM, Birkmeyer JD, Bronner KK, Bubolz TA, Fisher EF, et al. The Dartmouth Atlas of Health Care 1998 [Internet]. Chicago; 1998. [Google Scholar]
- 18.US Census Bureau. State Population Characteristics: 2010-2019 [Internet]. 2020. Available from: https://www.census.gov/data/tables/time-series/demo/popest/2010s-state-detail.html
- 19.Agency for Healthcare Research and Quality. Social Determinants of Health Database (Beta Version) [Internet]. 2020. Available from: https://www.ahrq.gov/sdoh/data-analytics/sdoh-data.html
- 20.US Census Bureau. American Community Survey (ACS) [Internet]. Available from: https://www.census.gov/programs-surveys/acs
- 21.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System [Internet]. 2020. Available from: https://www.cdc.gov/brfss/index.html
- 22.Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017. May 15;123(6):1044–50. [DOI] [PubMed] [Google Scholar]
- 23.Gartner DR, Delamater PL, Hummer RA, Lund JL, Pence BW, Robinson WR. Integrating Surveillance Data to Estimate Race/Ethnicity-specific Hysterectomy Inequalities among Reproductive-aged Women: Who’s at Risk? Epidemiology. 2020. May 1;31(3):385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived from ICD-9-CCM administrative data. Med Care. 2002. Aug;40(8):675–85. [DOI] [PubMed] [Google Scholar]
- 25.Diehr P, Cain K, Connell F, Volinn E. What is too much variation? The null hypothesis in small-area analysis. Health Serv Res. 1990. Feb;24(6):741–71. [PMC free article] [PubMed] [Google Scholar]
- 26.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Vol. 147, Annals of Internal Medicine. American College of Physicians; 2007. p. 573–7. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson GF, Shaber RE, Armstrong MA, Hung YY. Hysterectomy Rates for Benign Indications. Obstet Gynecol. 2006. Jun;107(6):1278–83. [DOI] [PubMed] [Google Scholar]
- 28.Spilsbury K, Semmens J, Hammond I, Bolck A. Persistent high rates of hysterectomy in Western Australia: a population-based study of 83 000 procedures over 23 years. BJOG An Int J Obstet Gynaecol. 2006. Jul 1;113(7):804–9. [DOI] [PubMed] [Google Scholar]
- 29.Haas S, Acker D, Donahue C, Katz ME. Variation in hysterectomy rates across small geographic areas of Massachusetts. Am J Obstet Gynecol. 1993. Jul 1;169(1):150–4. [DOI] [PubMed] [Google Scholar]
- 30.Gartner DR, Doll KM, Hummer RA, Robinson WR. Contemporary Geographic Variation and Sociodemographic Correlates of Hysterectomy Rates among Reproductive-Age Women. South Med J. 2018. Oct 1;111(10):585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wennberg J, Gittelsohn A. Small area variations in health care delivery. Science. 1973;182(4117):1102–8. [DOI] [PubMed] [Google Scholar]
- 32.Dorner FH, Burr RM, Tucker SL. The geographic relationships between physicians’ residency sites and the locations of their first practices. Acad Med. 1991;66(9):540–4. [PubMed] [Google Scholar]
- 33.McWilliams JM, Meara E, Zaslavsky AM, Ayanian JZ. Use of Health Services by Previously Uninsured Medicare Beneficiaries. N Engl J Med. 2007. Jul 12;357(2):143–53. [DOI] [PubMed] [Google Scholar]
- 34.Meyer CP, Allard CB, Sammon JD, Hanske J, McNabb-Baltar J, Goldberg JE, et al. The impact of Medicare eligibility on cancer screening behaviors. Prev Med (Baltim). 2016. Apr 1;85:47–52. [DOI] [PubMed] [Google Scholar]
- 35.Wennberg JE. Dealing with medical practice variations: A proposal for action. Health Aff. 1984. Jul 24;3(2):6–32. [DOI] [PubMed] [Google Scholar]
- 36.Wennberg JE. Time to tackle unwarranted variations in practice. BMJ. 2011. Mar 17;342(7799):687–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


