Abstract
Posttraumatic stress symptoms (PTSS) are associated with significant distress and impairment. Research has therefore focused on identifying neurobehavioral deficits that contribute to the pathophysiology of PTSS. One issue that has contributed to difficulty in identifying these deficits is the highly heterogeneous nature of PTSS. PTSS is comprised of four, factor analytically distinct dimensions of symptoms – re-experiencing, avoidance, hyperarousal, and negative cognitions and mood. It is therefore unlikely that there is one single mechanism that accounts for all of PTSS and elucidating neurobehavioral deficits associated with specific PTSS symptom dimensions may better inform clinical prevention and intervention efforts. Within the broader internalizing disorder literature, two key constructs that contribute to psychopathology are aberrant neural reactivity to threat and reward. However, the literature linking PTSS to these deficits is mixed, suggesting that aberrant neural reactivity to threat or reward may be specific to certain PTSS dimensions. In a sample of 51 trauma-exposed adults with a range PTSS, the present study therefore examined how the four dimensions of PTSS uniquely relate to two well-validated event-related potential (ERP) neural indices of threat and reward reactivity – the error-related negativity (ERN) and reward-related positivity (RewP), respectively. Results indicated that hyperarousal symptoms were associated with enhanced ERN, and enhanced RewP. In contrast, negative cognitions and mood symptoms were uniquely associated with a more blunted RewP. These results indicate that certain PTSS symptom dimensions have unique relations with neural indicators of threat and reward reactivity and may therefore have distinct pathophysiologies.
Keywords: Posttraumatic stress symptoms, Error-related negativity, Reward-related positivity
1. Introduction
Trauma exposure is highly common, and posttraumatic stress disorder (PTSD) is associated with significant impairment and distress, even at the subthreshold level (Gadermann et al., 2012; Zlotnick et al., 2002). It is therefore important to identify neurobehavioral deficits that may contribute to the development and maintenance of posttraumatic stress symptoms (PTSS). However, this effort is complicated by the heterogeneity of PTSS, which is evidenced by the wealth of factor analytic studies that have found PTSS to consist of four qualitatively different dimensions of symptoms: (1) re-experiencing, (2) avoidance, (3) negative cognitions and mood, and (4) hyperarousal symptoms (clusters B, C, D, and E, respectively; e.g., Elklit and Shevlin, 2007; Yufik and Simms, 2010). Given this, there are likely multiple PTSS profiles that are characterized by distinct neurobehavioral deficits. Examining the neurobehavioral correlates of specific PTSS dimensions could therefore lead to the dissemination of more individualized treatment, a goal that is in line with the National Institute of Mental Health’s (NIMH) Research Domain Criteria Initiative (RDoC; Cuthbert and Kozak, 2013).
Two neurobehavioral processes that may relate to specific PTSS dimensions are reactivity to threat and reward, as abnormalities in these processes have been implicated in the pathophysiology of various internalizing disorders that share core features with PTSS. For example, heightened defensive responding to threat is a core dysfunction implicated in panic disorder, a condition that shares elevated physiological arousal with the hyperarousal PTSS dimension (Gorka et al., In Press; Lieberman et al., 2016; Shankman et al., 2013). Meanwhile, blunted appetitive responding to reward has been evidenced in depression, a condition that shares anhedonia and low positive affect with the negative cognitions and mood PTSS dimension (Shankman et al., 2013). Of note is that heightened threat sensitivity has been found to be specific to panic and other fear disorders, relative to distress disorders such as depression (Gorka et al., In Press; Shankman et al., 2013). Likewise blunted reward sensitivity has been found to be specific to depression, relative to fear-based anxiety disorders (Shankman et al., 2013). Aberrant threat and reward responding therefore distinguishes distress and fear-based anxiety disorders. Given that PTSS includes unique symptom dimensions that overlap with both classes of disorders (Watson, 2005), it is possible that PTSS is characterized by aberrant threat and reward responding.
As might be expected given the heterogeneity of PTSS, there have been inconsistent findings regarding the association between post-traumatic stress and threat and reward responding. For instance, individuals with PTSD have been found to exhibit heightened (Grillon et al., 2009; Jovanovic et al., 2010; Morgan et al., 1995), comparable (Rabinak et al., 2013), and even blunted (Britton et al., 2005) defensive responding during the anticipation of threat, relative to individuals without PTSD. Likewise, PTSD has been associated with heightened (Myers et al., 2013), comparable (Casada and Roache, 2005; Van Rooij et al., 2015), blunted (Elman et al., 2009; Felmingham et al., 2014), appetitive responding to reward. These findings together highlight that PTSS, broadly and categorically defined is not necessarily characterized by aberrant threat and reward responding. However, specific subgroups or dimensions of PTSS may uniquely relate to blunted and/or enhanced threat and reward reactivity. In particular, blunted reward sensitivity may be specific to negative cognitions and mood PTSS given the abovementioned overlap in symptoms between depression and this PTSS dimension. In contrast, the overlap in symptoms between fear-based disorders and the hyperarousal PTSS dimension might suggest that heightened sensitivity to threat is specific to hyperarousal symptoms.
To date, there have been a few studies that have attempted to explore how specific PTSS dimensions relate to threat and reward responding. Grupe et al. (2016) found that hyperarousal and re-experiencing symptoms positively predicted neural reactivity to threat; however, Jovanovic et al. (2010) reported no association between startle potentiation to threat and any specific PTSS dimension. With regard to reward, Felmingham et al. (2014) and Elman et al. (2009) reported a negative association between emotional numbing symptoms of avoidance (e.g., anhedonia and restricted positive affect) and neural reactivity to reward, whereas Contractor et al. (2013) reported a positive association between avoidance symptoms and self-reported motivation for reward.
Taken together, there is some prior evidence to suggest that distinct PTSS symptom clusters map onto distinct neurobehavioral deficits. However, several key questions remain. First, the studies noted above all used DSM-IV defined PTSS, which were significantly revised for DSM-5 and restructured from three, to four clusters (a change based on numerous factor-analyses of the PTSD symptom structure; Elklit and Shevlin, 2007; Yufik and Simms, 2010). Therefore, it is presently unknown how threat and reward responding relate to the four, DSM-5 PTSS clusters that are currently referred to in clinical settings and have better psychometric properties than the prior versions. Second, no studies to our knowledge have examined how specific PTSS clusters relate to threat and reward responding in the same sample. It is therefore difficult to draw conclusions about the specificity of these neurobehavioral deficits to any particular PTSS dimension. Moreover, investigations of this question to date have focused on individuals with full syndromal PTSD. Thus, the range of PTSS within each symptom dimension may have been restricted, which could limit the detection of associations between these neurobehavioral constructs and PTSS dimensions. Focusing on only those individuals who are full syndromal PTSD also ignores individuals who are subthreshold – a group that is known to display functional impairment (Gadermann et al., 2012; Zlotnick et al., 2002).
Although there are multiple ways to elicit and measure neural responding to threat and reward, event-related potential (ERP) components provide well-validated indices of threat and reward responding, and have strong psychometric properties (Bress et al., 2015; Meyer et al., 2013). In particular, the error-related negativity (ERN) is a frontocentrally maximal negative-going deflection in the ERP waveform that occurs between 0 and 100 ms after the commission an error, which is a motivationally salient event that signals the potential for harm (i.e., threat) and therefore engages the defensive motivational system to take corrective action (Weinberg et al., 2015a,b). Greater ERN is indicative of greater defensive responding to threat (Weinberg et al., 2015a,b), and has been associated with multiple anxiety disorders and high trait anxiety (e.g., Hajcak and Simons, 2002; Weinberg et al., 2010). The reward-related positivity (RewP) is a frontocentrally maximal positive-going deflection in the ERP waveform that occurs between 200 and 250 ms after the receipt of reward (Proudfit, 2015). Greater RewP is indicative of greater appetitive responding to reward (Proudfit, 2015), and a blunted RewP has been shown to be associated with major depressive disorder (MDD), and high levels of depressive symptoms (Liu et al., 2014; Proudfit 2015; Weinberg et al., 2015a,b).
Despite the utility of these ERPs for studying internalizing psychopathology, and the growing literature on ERPs in PTSS (e.g., Lobo et al., 2014, 2015; Wessa et al., 2005) little is known about the ERN and RewP in PTSS. No studies to our knowledge have examined the RewP in PTSD, and only three studies have examined the ERN in relation to PTSD and reported no difference in ERN between individuals with PTSD and controls (Gorka et al., 2016; Rabinak et al., 2013; Swick et al., 2015). However, none of these ERN studies examined how the ERN relates to specific PTSS dimensions. The present study therefore examined how the DSM-5, four clusters of PTSS – re-experiencing, avoidance, negative cognitions and mood, and hyperarousal – relate to the ERN and RewP in a sample of trauma-exposed individuals. PTSS was defined dimensionally rather than categorically, to include the full PTSS spectrum. All participants completed two well-validated tasks designed to elicit the ERN and RewP. We hypothesized that greater hyerparousal symptoms (Cluster E) would predict greater ERN. We predicted that other dimensions of PTSS would not relate to ERN. We also predicted that greater negative cognitions and mood (Cluster D) symptoms would predict blunted RewP, but that other dimensions of PTSS would not relate to RewP.
2. Methods
2.1. Participants
Participants were recruited from the community as a part of a larger investigation on affective and physiological abnormalities across internalizing psychopathology. A variety of advertisements were used to recruit a clinically representative patient population with a range of internalizing disorders and symptoms. In line with the aims of the larger study, participants were included if they either (1) seeking treatment with pharmacotherapy (selective serotonin reuptake inhibitors/SSRIs) or cognitive behavioral therapy (CBT) for anxiety or depressive symptoms (i.e., patients), or (2) had no lifetime history of psychopathology (i.e., healthy controls). Participants were required to be between the ages of 18 and 65 years. Exclusion criteria included an inability to provide consent and read and write in English, a major active medical or neurological problem that could impact psychophysiological and brain function, lifetime history of mania or psychosis, any contraindication to receiving SSRIs, being already engaged in any form of psychiatric treatment, psychoactive medication use within the past four months, history of traumatic brain injury, left-handedness, and being pregnant.
The study took place at the University of Illinois-Chicago and was approved by the university Institutional Review Board. All participants provided written informed consent after review of the protocol. Participants completed a set of laboratory tasks and battery of questionnaires, which were administered in a counterbalance order to eliminate potential order effects. Participants received cash as payment for participation.
Of the 190 individuals who met inclusionary criteria for the larger study, 51 endorsed a trauma that met Criterion A for PTSD, as defined by DSM-5. Of those 51, 4 were missing ERN data and another 4 were missing RewP data due to technical issues or poor data quality (i.e., excessive artifact). Thus, the final sample for both sets of analyses (ERN and RewP) was 47 participants (ERN = 5 controls, 42 patients; RewP = 6 controls, 41 patients). See Table 1 for demographic and clinical characteristics of this sample. All data used in the current study was assessed prior to treatment, participants tested negative on a urine drug screen before ERP assessment, and none of the participants were taking psychoactive medications at the time of study entry.
Table 1.
Demographics and clinical characteristics.
| Demographics | |
| Age (years) | 28.10 (9.62) |
| Sex (% female) | 78.4% |
| Race/Ethnicity (%) | |
| Caucasian | 70.6% |
| African American | 11.8% |
| Asian | 15.7% |
| Other | 2% |
| Principal Diagnosis | |
| Generalized anxiety disorder | 35.3% |
| Social anxiety disorder | 7.8% |
| Panic disorder | 5.9% |
| Posttraumatic stress disorder | 9.8% |
| Dysthymia | 2.0% |
| Major depressive disorder | 27.5% |
| Current Diagnoses (Including Primary) | |
| Posttraumatic stress disorder | 35.3% |
| Generalized anxiety disorder | 70.6% |
| Social anxiety disorder | 45.1% |
| Panic disorder | 21.6% |
| Specific Phobia | 13.7% |
| Dysthymia | 2.0% |
| Major depressive disorder | 45.1% |
| Clinical Characteristics | |
| Lifetime posttraumatic stress disorder | 54.9% |
| Global Assessment of Functioning | 58.86 (10.69) |
| Mean # of Symptoms per PTSS Cluster | |
| Re-experiencing (Cluster B) | 7.96 (2.84) |
| Avoidance (Cluster C) | 3.47 (1.65) |
| Negative cognitions/mood (Cluster D) | 10.73 (3.81) |
| Hyperarousal (Cluster E) | 8.94 (2.53) |
| Mean Event-Related Potentials | |
| Mean difference between ERN and CRN | |
| Mean difference in RewP between |
Note. There are five symptoms in cluster B, two in cluster C, 7 in Cluster D, and 6 in Cluster E.
2.2. Measure of posttraumatic stress symptoms
Current and lifetime Axis-I psychopathology was assessed using a Structured Clinical Interview for DSM-5 Disorders (SCID-5; American Psychiatric Association, 2015). Of note is that the SCID-5 is a well-validated instrument that allows for the assessment of whether specific symptoms of DSM-defined disorders are absent, subthreshold, or threshold across a range of populations (i.e., individuals with and without a known history of psychopathology; First et al., 2014; Spitzer et al.,1992). The SCID-5 used in the present study was also modified (Shankman et al., Under Review) such that interview skip-outs were ignored and each and every symptom was given a dimensional rating (1 = not present, 2 = subthreshold, or 3 = present). Because no items were omitted, this allows for assessment of both categorical diagnoses (e.g., PTSD yes/no) and individual symptom severity. All research staff were trained to criterion on the SCID. After the evaluation, a consensus panel of at least 3 study staff/trained clinicians determined subjects0 eligibility and if there were co-occurring disorders, which was the principal disorder warranting treatment. Clinician-determined principal diagnosis was determined by the most severe and impairing symptoms from clinical interviews and self-reports.
With this SCID modification, the entire PTSD module, including each symptom, was administered to each participant. PTSS symptom cluster scores were therefore calculated by summing all the symptom items within each cluster. Based on the DSM-5, the clusters included re-experiencing (e.g., recurrent and intrusive distressing recollections of the event, Cluster B), avoidance (e.g., efforts to avoid reminders of the event, Cluster C), negative cognitions and mood (e.g., persistent negative emotional state, Cluster D), and hyperarousal (e.g., persistent symptoms of increased arousal, Cluster E). Given differences in total number of items between the different clusters, each of the four cluster dimensions were standardized using a Z-transformation prior to analyses. The overall PTSS dimension yielded from this modified SCID was found to exhibit significant retest reliability (current symptoms r = 0.87, p < 0.05; lifetime symptoms r = 0.85, p < 0.05) and acceptable Cronbach’s alpha values (0.89 and 0.94 for current and lifetime symptoms, respectively [Nunnally, 1978; Shankman et al., Under Review]). In the present sample, Clusters B, C, D, and E exhibited acceptable internal consistencies (Cronbach’s alphas ranging from 0.80 to 0.86 [Nunnally, 1978]).
2.3. Error and reward tasks
To elicit the ERN, participants completed a modified version of the flanker task (Eriksen and Eriksen, 1974). For each trial, participants viewed five horizontally aligned arrowheads. For half of the trials, arrows were compatible (“»»>” or “««<”) and for the other half of trials, the arrows were incompatible (“»<»” or “«>«”). Participants’ were instructed to respond as quickly and as accurately as possible to indicate the direction of the center arrow (left or right) by pressing either the left or right mouse button. Stimuli were presented for 200 ms, followed by a white fixation cross centrally presented on a black background. Participants were given up to 1800 ms after the offset of the arrows to respond; this was followed by an intertrial interval that varied randomly between 1000 and 2000 ms, during which participants again viewed a white fixation cross presented on a black background. The task was administered on a PentiumD class computer with a 19-in. monitor, using Presentation software (Neurobehavioral Systems, Inc. Albany, CA).
The task consisted of 11 blocks of 30 trials (330 trials in total), interspersed with self-timed breaks. To encourage both fast and accurate responding, participants received performance-based feedback at the end of each block. If accuracy was 75% correct or lower, the message “Please try to be more accurate” was presented; if accuracy was greater than 90%, the message, “Please try to respond faster” was displayed; in all other cases, participants saw the message, “You’re doing a great job”. A total of 30 practice trials were administered prior to beginning the task.
To elicit the RewP, participants completed the doors task, a well-validated guessing paradigm (Proudfit, 2015). For each trial, participants viewed an image of two doors and were instructed to select a door by clicking right or left. The doors remained on the screen until participants made a selection, after which point participants were presented with a fixation cross for 1000 ms. Participants were then presented with feedback in the form of a green upwards pointing arrow or red downwards pointing arrow, which indicated a correct or incorrect selection, respectively. Feedback remained in the screen for 2000 ms. Participants were told that for every correct guess they would win 80 cents, and for every incorrect guess they would lose 40 cents. Participants were presented with 30 win and 30 loss trials in a randomized order across the duration of the task. After receiving feedback, participants were presented with another fixation cross for 1500 ms. The message “Click for the next round” then appeared on the screen and remained until participants clicked to indicate that they were ready for the next trial.
2.4. EEG data recording and reduction
Continuous EEG was recorded during the task using an elastic cap and the ActiveTwo BioSemi system (BioSemi, Amsterdam, Netherlands). Thirty-four electrode sites (standard 32 channel setup, as well as FCz and Iz) were used, based on the 10/20 system. One electrode was also placed on each mastoid. The EEG signal was pre-amplified at the electrode to improve the signal-to-noise ratio. The data were digitized at 24-bit resolution with a Least Significant Bit (LSB) value of 31.25 nV and a sampling rate of 1024 Hz, using a low-pass fifth order sinc filter with a −3 dB cutoff point at 204.8 Hz. The voltage from each active electrode was referenced online with respect to a common mode sense active electrode producing a monopolar (non-differential) channel.
Off-line analyses were performed using Brain Vision Analyzer 2 software (Brain Products, Gilching, Germany). Data were rereferenced to the average of the two mastoids and high-pass (0.1 Hz) and low-pass (30 Hz) filtered. Eye blink and ocular corrections were performed using the method developed by Miller et al. (1988). Artifact analysis was used to identify a voltage step of more than 50.0 μV between sample points, a voltage difference of 300.0 μV within a trial, and a maximum voltage difference of less than 0.50 μV within 100 ms intervals. Trials were also visually inspected for remaining artifacts and rejected on a trial-to-trail basis.
For analysis of the ERN, data were segmented beginning 500 ms before each response onset and continuing for 1500 ms (i.e., for 1000 ms following the response). Baseline correction for each trial was performed using the 500 to 300 ms prior to response onset. The ERN and CRN were scored as the average activity on error and correct trials, respectively, from 0 to 100 ms after response at electrode Fz, where amplitude was maximal. Consistent with prior studies (e.g., Gorka et al., in press; Rabinak et al., 2013), for our analyses we calculated the ΔERN by subtracting the CRN from the ERN. More negative values for the difference score indicate greater reactivity to error relative to correct.
For analysis of the RewP, continuous EEG data were segmented beginning 100 ms before feedback onset and continuing for the 500 ms after onset (i.e., for 400 ms following the feedback). Baseline correction for each trial was performed using the 100 ms interval prior to feedback. ERPs were averaged across gain and loss trials, separately, and the RewP was scored as the mean amplitude 230–300 ms following feedback at a pooling of frontal sites (FCz and Fz), where the gain minus loss difference was maximal. Consistent with the ERN data analysis plan, for analyses we created a RewP difference score by subtracting average activity during losses from wins. More positive values for the difference score indicate greater reactivity to reward relative to loss.
2.5. Data analysis plan
To examine associations between PTSS clusters and ΔERN and RewP amplitude, we conducted two hierarchical linear regression analyses – one for ΔERN and one for RewP. Biological sex and principal DSM diagnoses1 were entered as covariates in Step 1. Consistent with prior studies of specific PTSS dimensions (e.g., Grupe et al., 2016), in order to evaluate the unique variance associated with each PTSS cluster, all four were entered simultaneously into Step 2. Of note, entering the PTSS clusters into the model simultaneously prevents potential mutual suppressor effects in which two correlated predictor variables (see Table 2) have the opposite effect on the criterion/outcome variable, causing the associations to be obscured when each subscale is examined separately (see Watson et al., 2013) (see Fig. 1).
Table 2.
Bivariate correlations of Posttraumatic Stress Symptom Dimensions.
| Re-experiencing | Avoidance | Negative Cog/Mood | Hyperarousal | |
|---|---|---|---|---|
| Re-experiencing | - | |||
| Avoidance | 0.61* | - | ||
| Negative Cog/Mood | 0.61* | 0.62* | - | |
| Hyperarousal | 0.56* | 0.48* | 0.61* | - |
p < 0.05.
Fig. 1.
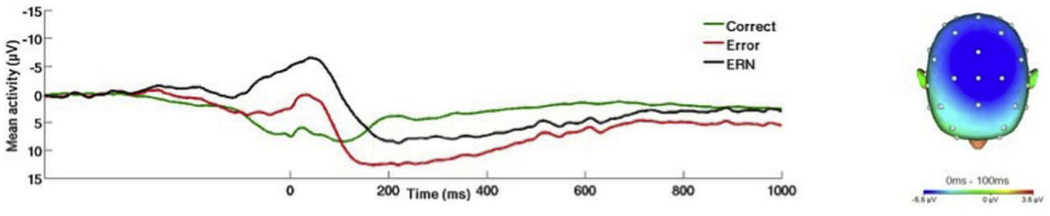
(Right) Scalp topographies depicting the error minus correct amplitude difference from 0 to 100 ms postresponse across all participants. (Left) Response-locked ERP waveforms at Fz showing and correct and error trial (and difference) waveforms across all participants.
3. Results
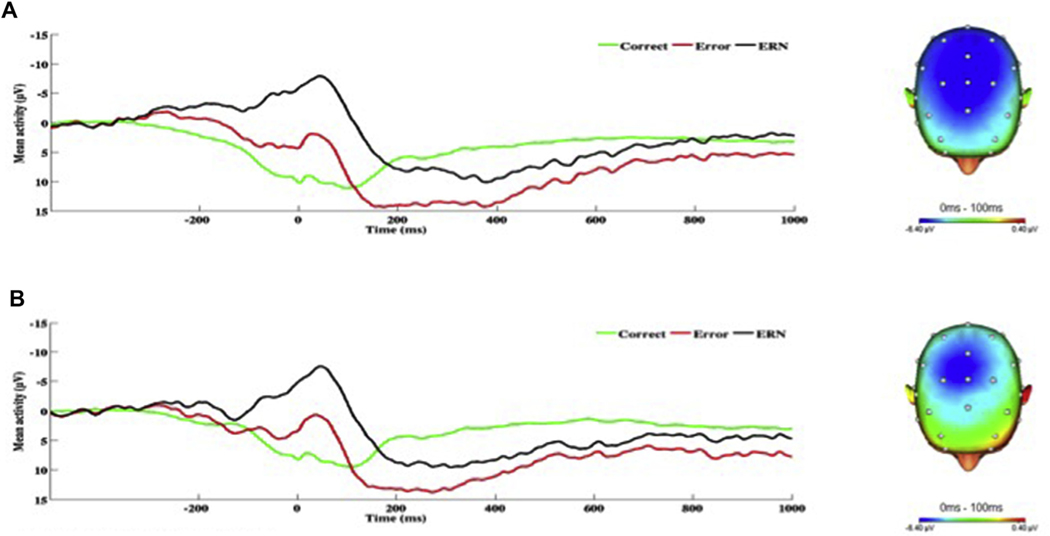
Across all participants, the flanker and doors tasks effectively elicited the ERN and RewP respectively. Task effects are presented in Figs. 1 and 2. Mean amplitude of the ΔERN was 5.20 (SD = 5.50), and mean amplitude of the RewP was 4.18 (SD = 7.06). For the flanker task, on average participants correctly responded on 81% (SD = 13.05) of trials. Mean reaction time for errors and correct responses was 308.84 ms (SD = 124.41) and 240.45 ms (SD = 118.40), respectively. The Results from the hierarchical linear regression analyses are presented in Table 3. With regard to the ΔERN, greater hyperarousal symptoms were associated with enhanced (i.e., more negative) ΔERN amplitude (see Fig. 3). There was no association between the ΔERN and re-experiencing, avoidance, or negative alternations in mood/cognition symptoms.
Fig. 2.
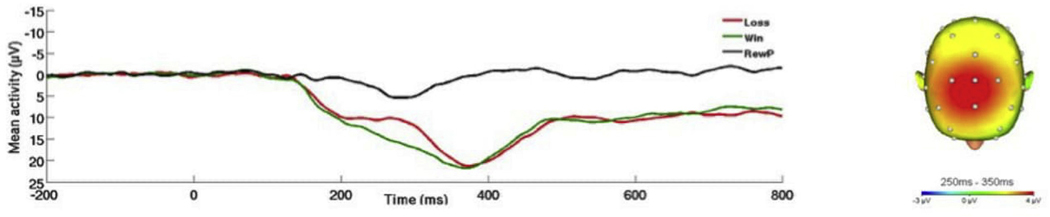
(Right) Scalp topographies depicting gain minus loss amplitude difference from 200 to 300 ms post feedback. (Left) Response-locked ERP waveforms pooled at FCz and Fz showing and gain and loss trial (and difference) waveforms across all participants.
Table 3.
Results from Linear Regression Analyses assessing the relation between specific PTSS clusters and ERPs
| B | t-score | R (ΔR2) | p | |
|---|---|---|---|---|
| Impact of PTSS on ERN | ||||
| Step 1 | 0.20 (0.04) | 0.42 | ||
| Sex | 0.16 | 0.10 | 0.32 | |
| Primary Diagnosis | 0.39 | 0.17 | 0.27 | |
| Step 2 | 0.48 (0.19) | 0.06 | ||
| Re-experiencing (Cluster B) | 0.08 | 0.42 | 0.67 | |
| Avoidance (Cluster C) | 0.37 | 1.98 | 0.06 | |
| Negative Mood/Cognition (Cluster D) | −0.18 | −0.91 | 0.37 | |
| Hyperarousal (Cluster E) | *−0.41 | 2.26 | 0.03 | |
| Impact of PTSS on RewP | ||||
| Step 1 | 0.18 (0.03) | 0.51 | ||
| Sex | −0.02 | −0.15 | 0.88 | |
| Primary Diagnosis | −0.18 | −1.14 | 0.26 | |
| Step 2 | 0.55 (0.27) | 0.01 | ||
| Re-experiencing (Cluster B) | 0.04 | 0.21 | 0.84 | |
| Avoidance (Cluster C) | −0.12 | −0.65 | 0.52 | |
| Negative Mood/Cognition (Cluster D) | *−0.53 | −2.68 | 0.01 | |
| Hyperarousal (Cluster E) | *.56 | 3.32 | 0.00 |
Note. PTSS = Posttraumatic Stress Symptoms; ERN = Error-related negativity; RewP = Reward Positivity.
p < 0.05.
Fig. 3.
On the left, response-locked ERP waveforms for correct and error trials, as well as the difference waves (error-related negativity; ΔERN) and on the right, topographic maps of activity (error minus correct) for individuals with a) high and b) low hyperarousal symptoms (defined by a median split for Cluster E).
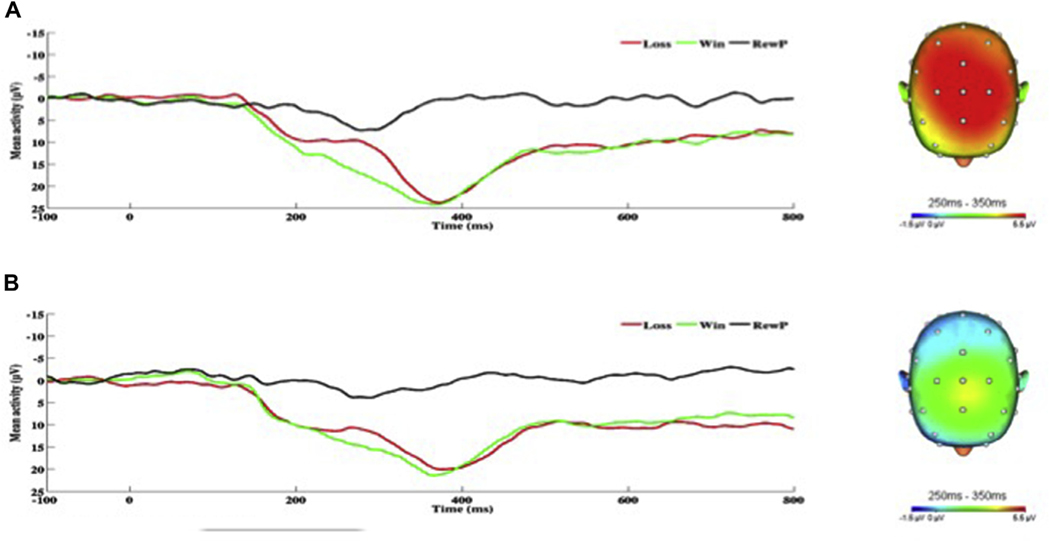
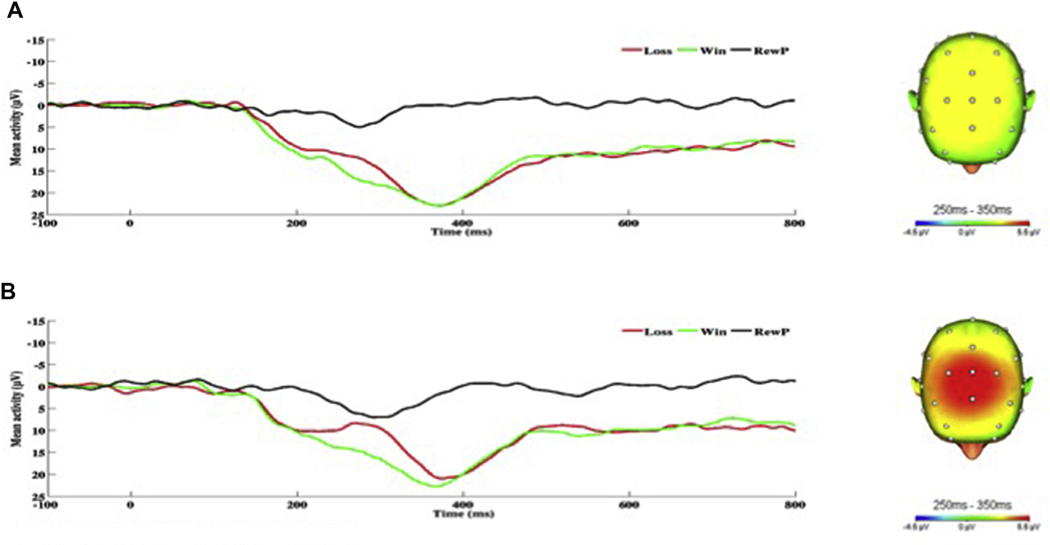
As for the RewP, greater hyperarousal symptoms were associated with greater RewP amplitude (see Fig. 4). In contrast, greater negative cognitions and mood symptoms were associated with reduced or blunted RewP amplitude (see Fig. 5). The RewP was not associated with re-experiencing symptoms, but there was a trend-level negative association between avoidance symptoms and RewP.
Fig. 4.
On the left, response-locked ERP waveforms for win and loss trials, as well as the difference waves (reward-related positivity; RewP) and on the right, topographic maps of activity (win minus loss) for individuals with a) high and b) low hyperarousal symptoms (defined by a median split for Cluster E).
Fig. 5.
On the left, response-locked ERP waveforms for win and loss trials, as well as the difference waves (reward-related positivity; RewP) and on the right, topographic maps of activity (win minus loss) for individuals with a) high and b) low negative cognition/mood symptoms (defined by a median split for Cluster D).
Lastly, to explore whether RewP or ERN were associated with the broad dimension of PTSS, as indexed by the z-scored sum of all PTSS symptoms, we conducted two hierarchical linear regression analyses to examine the relation between each ERP component and total current PTSS. Consistent with above analyses, gender and primary diagnosis were included as covariates. Neither the relation between ERN and total current PTSS (p = 0.57), nor the relation between RewP and total current PTSS (p = 0.66) were significant.
4. Discussion
Identifying neurobehavioral deficits that contribute to the pathophysiology of PTSD is critical for advancing prevention and treatment efforts. However, PTSS is highly heterogeneous (Galatzer-Levy and Bryant, 2013) and there are likely many PTSS phenotypes that are associated with distinct profiles of neurobehavioral abnormalities. Despite this, little is known about the neurobehavioral correlates of the four PTSS dimensions of re-experiencing, avoidance, negative cognitions and mood, and hyperarousal (APA, 2015). Results suggest that, as hypothesized, hyperarousal symptoms positively predicted greater ERN, and no other PTSS dimension was associated with ERN. Hyperarousal symptoms were also positively related to RewP. Meanwhile, greater negative cognitions and mood symptoms were associated with reduced RewP.
The current results indicate that hyperarousal PTSS (Cluster E) are associated with greater neural responding to errors/threat and reward, and suggest that individuals high in hyperarousal PTSS experience heightened reactivity to affective stimuli, regardless of valence (i.e., appetitive or aversive). Although not initially predicted, this finding is consistent with the core characteristics of hyperarousal symptomology. In particular, the PTSS dimension of hyperarousal is described as “marked alterations in arousal and reactivity” that can be characterized by “hypervigilance” (APA, 2015), and these symptoms are not denoted as being specific to threatening or negative situations. Greater ERN and RewP among those high in hyperarousal symptoms may therefore represent an enhanced preparedness of the defensive and appetitive motivational systems, respectively, which could contribute to chronic heightened arousal and excessive reactivity across situations of different valences.
Individuals with the propensity to experience excessive physiological responding to situations of different valences may also experience more intense emotional responses across contexts. That is, greater neural responding to threat and reward may contribute to mood instability that has been implicated in PTSS (Ehring and Quack, 2010). Within individuals high in hyperarousal PTSS, exaggerated reactivity to affective stimuli may be driven by greater reactivity of the anterior cingulate cortex (ACC). The ACC is a frontolimbic region that generates the ERN (Luu et al., 2004; Miltner et al., 2003), and is involved in appetitive responding (Bush et al., 2002; Shidara and Richmond, 2002). This region may therefore represent a neural treatment target for trauma-exposed individuals who are high in hyperarousal symptoms.
The results also revealed greater negative cognitive PTSS symptoms (Cluster D) were associated with a more blunted RewP. Cluster D is characterized by such symptoms as diminished interest in significant activities (i.e., anhedonia) and restricted positive affect, and so this finding is consistent with the broader literature. For instance, studies have shown that anhedonia and reduced positive affectivity are associated with a blunted RewP (Liu et al., 2014), and dampened reward reactivity more broadly (Pizzagalli et al., 2008). A blunted RewP has also been implicated in the onset of depression (Nelson et al., 2016) suggesting that abnormal reactivity to reward may be a key factor underlying the development of mood disorders. However, it is of note that among trauma-exposed individuals, blunted reactivity to rewards appears to contribute to chronically low mood (i.e., Cluster D symptoms) even when controlling for principal depressive diagnoses. Hypoactivity of the striatum, a region that is thought to generate the RewP (Carlson et al., 2011; Proudfit, 2015) and has been implicated in the processing of reward (Balleine et al., 2007; Delgado, 2007), may contribute to blunted reward sensitivity among individuals with negative cognitions and mood PTSS.
It is important to highlight that results from the present study revealed a positive correlation (r = 0.61) between negative cognition/mood and hyperarousal symptoms in the present study. This degree of overlap is somewhat higher than expected, given prior factor analytic studies (Elklit and Shevlin, 2007; Yufik and Simms, 2010). However, despite the relation between these symptom dimensions, negative cognition/mood and hyperarousal symptoms were found to exert opposite effects on psychophysiological measures. This pattern of results therefore suggests that Clusters D and E, although not entirely unique, are not wholly redundant constructs and that overlapping symptom clusters can relate to neurobiology in distinct ways.
Taken together, results from the present study suggest that the broader dimension of PTSS is not associated with aberrant threat or reward reactivity. Rather, specific PTSS dimensions may be associated with blunted reward responding or exaggerated reactivity to reward and threat. It is particularly noteworthy that negative cognitions and mood PTSS (Cluster D) was negatively associated with RewP, but hyperarousal PTSS (Cluster E) was positively associated with RewP. It is therefore possible that, depending on the profile of PTSS in a given sample, an investigation may yield a positive, negative, or null relation between broad PTSS and RewP. Thus, the present study’s results may help to clarify the mixed literature on threat and reward reactivity in PTSD.
That negative cognitions and mood PTSS was negatively associated with RewP, but hyperarousal PTSS was positively associated with RewP also suggests that the pathophysiology underlying these dimensions may differ. This pattern of results therefore highlights the possibility that specific PTSS profiles may be characterized by different neurobehavioral deficits and may therefore be best addressed by separate treatments. Given that participants were not required to have full syndromal PTSD, results also suggest that the occurrence of hyperarousal PTSS following a trauma may be associated with greater ERN and RewP, regardless of whether fullsyndromal PTSD is also present. Likewise, negative cognition and mood symptoms following a trauma may be associated with blunted RewP, regardless of whether full-syndromal PTSD is also present. Moreover, individuals in the present study had a range of co-occurring depressive and anxiety symptoms and the findings were observed independent of individuals’ primary DSM diagnoses. These results may therefore have broader implications and provide potential insight into the neurobiological constructs associated with distinct symptom profiles across a range of internalizing psychopathologies.
Although the present study had multiple strengths, such as the use of a clinically-representative treatment-seeking sample and two reliable, and well-validated neural indices of threat and reward responding, there were also several limitations that should be considered. First because individuals were not required to be DSM-5 criteria for PTSD, it is unclear whether the present findings would generalize to a sample with full syndromal illness (although in individuals with full PTSD, there may be a restriction of range in PTSS dimensions). Second, the sample included individuals with current anxiety and depressive disorders and although primary diagnosis was included as a covariate in our analyses, other comorbid psychopathologies may have impacted results. Given that a large proportion of individuals in the current study had cooccurring internalizing psychopathologies, future studies are needed to confirm whether the present findings generalize to other, less psychiatrically severe populations. Third, the sample size in the present study was moderate, and so a larger sample size may have revealed additional associations between specific PTSS dimensions and ERPs. Third, because ERPs were not assessed prior to trauma, it is presently unclear whether the neurobehavioral deficits observed in the current study were risk factors for or epiphenomena of these PTSS. Future studies should therefore explore this question using a longitudinal design. Fourth, the current study used the SCID-5 to capture PTSS, which is only one measure. It would be useful for future studies to replicate and validate the present findings using other dimensional assessments of PTSS symptoms such as the Clinician Administered PSTD Scale (CAPS-5; Weathers et al., 2013).
In sum, results indicated that hyperarousal PTSS (Cluster E) are associated with exaggerated threat and reward responding, whereas negative cognitions and mood PTSS (Cluster D) are associated with blunted reward responding. This study begins to address the heterogeneity within PTSS by linking specific symptom dimensions to neurobehavioral deficits. Specificity of associations between PTSS dimensions and neurobehavioral deficits may begin to highlight the potential for more individualized treatments among individuals experiencing PTSS.
Footnotes
Patterns of results for RewP and ERN were the same when principal diagnoses were removed from the models.
References
- American Psychiatric Association, 2015. Structured Clinical Interview for DSM-5 (SCID-5). Retrieved from: http://www.appi.org/products/structured-clinicalinterview-for-dsm-5scid-5/.
- Balleine BW, Delgado MR, Hikosaka O, 2007. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 27 (31), 8161–8165. 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Meyer A, Proudfit GH, 2015. The stability of the feedback negativity and its relationship with depression during childhood and adolescence. Dev. Psychopathol. 27 (4), 1285–1294. 10.1017/S0954579414001400. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I, 2005. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol. Psychiatry 57 (8), 832–840. 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR, 2002. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci. U. S. A. 99 (1), 523–528. 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi L, Harmon-Jones E, Hajcak G, 2011. Ventral striatal and medial prefrontal bold activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. NeuroImage 57 (4), 1608–1616. 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Casada JH, Roache JD, 2005. Behavioral inhibition and activation in posttraumatic stress disorder. J. Nerv. Ment. Dis. 193 (2), 102–109. 10.1097/01.nmd.0000152809.20938.37. [DOI] [PubMed] [Google Scholar]
- Contractor AA, Elhai JD, Ractliffe KC, Forbes D, 2013. PTSD’s underlying symptom dimensions and relations with behavioral inhibition and activation. J. Anxiety Disord. 27 (7), 645–651. 10.1016/j.janxdis.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Kozak MJ, 2013. Constructing constructs for psychopathology: the NIMH research domain criteria. J. Abnorm. Psychol. 122 (3), 928–937. 10.1037/a0034028. [DOI] [PubMed] [Google Scholar]
- Delgado MR, 2007. Reward-related responses in the human striatum. Ann. N. Y. Acad. Sci. 1104, 70–88. 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Ehring T, Quack D, 2010. Emotion regulation difficulties in trauma survivors: the role of trauma type and PTSD symptom severity. Behav. Ther. 41 (4), 587–598. 10.1016/j.beth.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Elklit A, Shevlin M, 2007. The structure of PTSD symptoms: a test of alternative models using confirmatory factor analysis. Br. J. Clin. Psychol. 46 (3), 299–313. 10.1348/014466506X171540. [DOI] [PubMed] [Google Scholar]
- Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK, 2009. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol. Psychiatry 66 (12), 1083–1090. 10.1016/j.biopsych.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen B, Eriksen C, 1974. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 16, 143–149. [Google Scholar]
- Felmingham KL, Falconer EM, Williams L, Kemp AH, Allen A, Peduto A, Bryant RA, 2014. Reduced amygdala and ventral striatal activity to happy faces in PTSD is associated with emotional numbing. PLoS One 9 (9), e103653. 10.1371/journal.pone.0103653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL, 2014. Structured Clinical Interview for DSM-5 Disorderseresearch Version (SCID-5-RV). American Psychiatric Association, Arlington. [Google Scholar]
- Gadermann AM, Alonso J, Vilagut G, Zaslavsky AM, Kessler RC, 2012. Comorbidity and disease burden in the national comorbidity survey replication (NCS-R). Depress. Anxiety 29 (9), 797–806. 10.1002/da.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy I, Bryant RA, 2013. 636,120 ways to have posttraumatic stress disorder. Perspect. Psychol. Sci. 8 (6), 651e662. 10.1177/1745691613504115. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Shankman SA, & Phan KL (In press). Startle potentiation to uncertain threat as a psychophysiological indicator of fear-based psychopathology: an examination across multiple internalizing disorders. J. Abnorm. Psychol.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, MacNamara A, Aase DM, Proescher E, Greenstein JE, Walters R, Phan KL, 2016. Impact of alcohol use disorder comorbidity on defensive reactivity to errors in veterans with posttraumatic stress disorder. Psychol. Addict. Behav. 10.1037/adb0000196. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M, 2009. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol. Psychiatry 66 (1), 47–53. 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Wielgosz J, Davidson RJ, Nitschke JB, 2016. Neurobiological correlates of distinct post-traumatic stress disorder symptom profiles during threat anticipation in combat veterans. Psychol. Med. 46 (9), 1885–1895. 10.1017/S0033291716000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Simons RF, 2002. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Res. 110, 63–72. 10.1016/S0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ, 2010. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress. Anxiety 27 (3), 244–251. 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman L, Gorka SM, Shankman SA, Phan KL, 2016. Impact of panic on psychophysiological and neural reactivity to unpredictable threat in depression and anxiety. Clin. Psychological Sci. 10.1177/2167702616666507. Ahead print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WH, Wang LZ, Shang HR, Shen Y, Li Z, Cheung EF, Chan RC, 2014. The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia 53, 213–220. 10.1016/j.neuropsychologia.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Lobo I, David IA, Figueira I, Campagnoli RR, Volchan E, Pereira MG, de Oliveira L, 2014. Brain reactivity to unpleasant stimuli is associated with severity of posttraumatic stress symptoms. Biol. Psychol. 103, 233–241. 10.1016/j.biopsycho.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Lobo I, Portugal LC, Figueira I, Volchan E, David I, Pereira MG, de Oliveira L, 2015. EEG correlates of the severity of posttraumatic stress symptoms: a systematic review of the dimensional PTSD literature. J. Affect. Disord. 183, 210–220. 10.1016/j.jad.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker D, Makeig S, 2004. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin. Neurophysiol. 115 (8), 1821–1835. 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Meyer A, Riesel A, Proudfit GH, 2013. Reliability of the ERN across multiple tasks as a function of increasing errors. Psychophysiology 50 (12), 1220–1225. 10.1111/psyp.12132. [DOI] [PubMed] [Google Scholar]
- Miller GA, Gratton G, Yee CM, 1988. Generalized implementation of an eye movement correction procedure. Psychophysiology 25, 241–243. [Google Scholar]
- Miltner WHR, Lemke U, Weiss T, Holroyd C, Scheffers MK, Coles MGH, 2003. Implementation of error-processing in the human anterior cingulate cortex: source analysis of the magnetic equivalent of the error-related negativity. Biol. Psychol. 64 (1–2), 157–166. 10.1016/S03010511(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Grillon C, Southwick SM, Davis M, Charney DS, 1995. Fear potentiated startle in posttraumatic stress disorder. Biol. Psychiatry 36 (6), 378–385. 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- Myers CE, Moustafa AA, Sheynin J, VanMeenen KM, Gilbertson MW, Orr SP, Servatius RJ, 2013. Learning to obtain reward, but not avoid punishment, is affected by presence of PTSD symptoms in male veterans: empirical data and computational model. PLoS One 8 (8), e72508. 10.1371/journal.pone.0072508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnally JC, 1978. Psychometric Theory, second ed. McGraw-Hill, New York. [Google Scholar]
- Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G, 2016. Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. Am. J. Psychiatry 173 (12), 1223–1230. 10.1176/appi.ajp.2016.15121524. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M, 2008. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J. Psychiatric Res. 43 (1), 76–87. 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH, 2015. The reward positivity: from basic research on reward to a biomarker for depression. Psychophysiology 52 (4), 449–459. 10.1111/psyp.12370. [DOI] [PubMed] [Google Scholar]
- Rabinak CA, Holman A, Angstadt M, Kennedy AE, Hajcak G, Phan KL, 2013. Neural response to errors in combat-exposed returning veterans with and without post traumatic stress disorder: a preliminary event-related potential study. Psychiatry Res. Neuroimaging 213 (1), 71–78. 10.1016/j.pscychresns.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Funkhouser CJ, Klein DN, Davila J, Lerner D, & Hee D. (Under review). Reliability and Validity of Dimensions of Psychopathology Assessed Using the Structured Clinical Interview for DSM-5 (SCID). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew E, Campbell ML, Altman SE, Gorka SM, 2013. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. J. Abnorm. Psychol. 122 (2), 322–338. 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidara M, Richmond BJ, 2002. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science 296 (5573), 1709–1711. 10.1126/science.1069504. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB, 1992. The structured clinical interview for DSM-III-R (SCID): I: history, rationale, and description. Archives general psychiatry 49 (8), 624–629. [DOI] [PubMed] [Google Scholar]
- Swick D, Honzel N, Turken U, 2015. Intact error monitoring in combat veterans with\post-traumatic stress disorder. Psychiatry Res. Neuroimaging 234 (2), 227–238. 10.1016/j.pscychresns.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooij SJH, Rademaker AR, Kennis M, Vink M, Kahn RS, Geuze E, 2015. Neural correlates of trauma-unrelated emotional processing in war veterans with PTSD. Psychol. Med. 45 (3), 575–587. 10.1017/S0033291714001706. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Chmielewski M, Kotov R, 2013. The value of suppressor effects in explicating the construct validity of symptom measures. Psychol. Assess. 25 (3), 929–941. 10.1037/a0032781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, 2005. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. J. Abnorm. Psychol. 114 (4), 522. 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, Keane TM, 2013. The Clinician-administered PTSD Scale for DSM-5 (CAPS-5). Interview available from: the National Center for PTSD; at. www.ptsd.va.gov. [Google Scholar]
- Weinberg A, Dieterich R, Riesel A, 2015a. Error-related brain activity in the age of RDoC: a review of the literature. Int. J. Psychophysiol. 98 (2), 276–299. 10.1016/j.ijpsycho.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Liu H, Proudfit GH, Shankman SA, 2015b. Blunted neural response to rewards as a vulnerability factor for depression: results from a family study. J. Abnorm. Psychol. 124, 878–889. 10.1037/abn0000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, Hajcak G, 2010. Increased error-related brain activity in generalized anxiety disorder. Biol. Psychol. 85 (3), 472–480. 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Wessa M, Karl A, Flor H, 2005. Central and peripheral psychophysiological responses to trauma-related cues in subclinical posttraumatic stress disorder: a pilot study. Exp. Brain Res. 167, 56–65. 10.1007/s00221-0050007-0. [DOI] [PubMed] [Google Scholar]
- Yufik T, Simms LJ, 2010. A meta-analytic investigation of the structure of posttraumatic stress disorder symptoms. J. Abnorm. Psychol. 119 (4), 764–776. 10.1037/a0020981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnick C, Franklin CL, Zimmerman M, 2002. Does “subthreshold” posttraumatic stress disorder have any clinical relevance? Compr. Psychiatry 43 (6), 413–419. 10.1053/comp.2002.35900. [DOI] [PubMed] [Google Scholar]