Abstract
Background:
Understanding the relationship between tobacco use and symptom burden may inform tobacco treatment interventions tailored to the needs of individuals with cancer.
Methods:
The study included 1409 adult cancer survivors from Wave 5 of the US Food and Drug Administration Population Assessment of Tobacco and Health (PATH) Study. A multivariate analysis of variance controlling for age, sex, and race/ethnicity assessed the association of cigarette smoking and vaping on cancer-related symptom burden (fatigue, pain, emotional problems) and quality of life (QoL). Generalized linear mixed models controlling for the same factors were used to assess associations among symptom burden, QoL, and quit-smoking intentions, quit-smoking likelihood, and past 12-month smoking quit attempts.
Results:
Weighted rates of current cigarette smoking and vaping were 14.21% and 2.88%, respectively. Current smoking was associated with greater fatigue (p < .0001; partial η2 = .02), pain (p < .0001; partial η2 = .08), emotional problems (p < .0001; partial η2 = .02), and worse QoL (p < .0001; partial η2 = .08). Current vaping was associated with greater fatigue (p = .001; partial η2 = .008), pain (p = .009; partial η2 = .005), and emotional problems (p = .04; partial η2 = .003), but not worse QoL (p = .17). Higher cancer symptom burden was not associated with reduced interest in quitting, likelihood of quitting, or odds of past year quit attempts (p > .05 for each).
Conclusions:
Among adults with cancer, current smoking and vaping were associated with greater symptom burden. Survivors’ interest in and intentions to quit smoking were not related to symptom burden. Future research should examine the role of tobacco cessation in improving symptom burden and QoL.
Keywords: cancer survivors, emotional distress, fatigue, pain, quality of life, tobacco use, vaping
INTRODUCTION
Cigarette smoking is a major modifiable risk factor for cancer incidence and a leading cause of preventable cancer death.1 Continued smoking after a diagnosis of cancer lowers survival rates,2–6 increases the likelihood of recurrence and second malignancies,3,5,7,8 and decreases the efficacy of cancer-directed therapy (surgery, radiation, chemotherapy),3,9–11 whereas quitting smoking postdiagnosis potentially improves these outcomes.12–14 Approximately one in four adults with cancer reports current cigarette smoking at the time of their diagnosis.15 Most patients quit or attempt to quit shortly after their diagnosis, but as many as 58% of individuals smoking at the time of diagnosis report continued smoking postdiagnosis.16,17 The high rates of continued smoking and failed quit attempts among patients with cancer indicate a dire need to improve existing tobacco treatments available to this population.
Extant research suggests that cigarette smoking increases total symptom burden (encompassing symptoms of cancer and side effects of cancer treatment, such as nausea, fatigue, sleep disturbance, pain, mood disturbance, and cognitive issues). Among a sample of 947 patients undergoing chemotherapy and/or radiation, current smoking was associated with significantly greater increases in total symptom burden during and 6 months after treatment.18 A recent study of 12,571 patients with cancer found that current smoking and former smoking were associated with 15% worse symptom burden and 13% more severe side effects compared with never smoking.19 With regard to specific symptoms, current smoking has been linked to greater pain intensity,20–23 greater fatigue,20,23,24 reduced appetite,24 shortness of breath,24 greater weight and hair loss,18 concentration problems, mood disturbance,18,23 and worse overall quality of life (QoL).24
Although informative in elucidating the relationship between symptom burden and tobacco use, these studies have several limitations. Nearly all studies involved fewer than 100 currently smoking patients, most were conducted in the context of active treatment at National Cancer Institute-designated comprehensive cancer centers, and none measured electronic cigarette (e-cigarette) use. Therefore, these samples may not reflect the broader population receiving care in community settings, who are long-term disease-free survivors, or who are users of multiple nicotine products. In addition, few studies have considered how symptom burden may be related to relevant patient attitudes and behaviors around smoking. Among cancer-related symptoms, psychological distress (especially depression and anxiety)25–27 and pain28 have been identified as potential barriers to attempting to quit and staying quit in individuals with cancer. Based primarily on studies conducted in noncancer populations, models of bidirectional influence have been posited to explain the relationship between greater physical symptoms, emotional distress, and smoking behavior. For example, individuals may smoke to cope with pain and discomfort, which may contribute to the worsening of painful conditions over time.21 Short-term changes in mood, physical symptoms, and energy are also common symptoms of nicotine withdrawal, and extant literature suggests that having elevated distress and physical symptoms before a quit attempt may undermine one’s willingness to quit or success with quitting behavior. For example, a longitudinal study of individuals with cancer found that patients smoked more on days when their pain was elevated and that those with higher average pain were less likely to make a quit attempt.28 Although findings regarding the impact of anxiety on quit success are mixed,25,27 several studies found that depression proneness and depressive symptoms are predictive of relapse among patients with cancer who are attempting to quit.25,26 However, less is known about the relationship between one of the most prevalent cancer-related symptoms (fatigue) and smoking-cessation attitudes and intentions, limiting current understanding of potential targets for tailored tobacco treatment.
The current study involves secondary analyses of Wave 5 of the US Food and Drug Administration Population Assessment of Tobacco and Health (PATH) study, a nationally representative sample gathered to produce detailed epidemiologic information on tobacco use.29 Our secondary analyses of PATH data explored the relationship between symptom burden, cigarette smoking, vaping, and intentions to quit smoking (including quit attempts) among cancer survivors. We hypothesized that: (1) current smoking and vaping would be associated with greater symptom burden (worse pain, fatigue, emotional problems) and QoL compared with former/never smoking or vaping status; and (2) greater symptom burden and worse QoL would be associated with lower interest in quitting smoking, likelihood of quitting, or past year quit attempts.
MATERIALS AND METHODS
Data source and inclusion criteria
Data were obtained from Wave 5 of the PATH study, a household-based, longitudinal, representative cohort study designed to provide epidemiologic data regarding patterns of tobacco use in the United States. All analyses used PATH-provided variable weights to ensure analyses were representative of the US population. The response rate for adult surveys in Wave 5 of the PATH study was 69.4% compared with 74.0% for Wave 1.30 Although the PATH data set contains data collected from over 45,000 youth and adults, the current analyses were limited to 1409 individuals aged 18 years or older who had a self-reported history of cancer. By using the National Cancer Institute definition of survivorship, in which anyone who has been diagnosed with cancer is considered a survivor regardless of treatment course,31 participants were considered cancer survivors and included in analyses if they responded yes to the question, Have you ever been told by a doctor or other health professional that you had cancer? in Wave 5 or in any of the previous waves of the PATH study. Data files and additional survey methodological details are available elsewhere.29,30 Briefly, participants were randomly recruited by home address and were sampled to include those with current, former, and never tobacco use statuses. Participants completed computer-assisted and audio-assisted surveys and received $35 for their participation; surveys were designed to take approximately 45 minutes. The PATH study was approved by the Westat Institutional Review Board.
Variables
Sociodemographic measures
Among the 1409 cancer survivors, we included the following participant characteristics self-reported from the survey: age, sex, race/ethnicity, educational attainment, and annual household income.
Tobacco use categories
We used the PATH study’s current established cigarette smoking variable, which is consistent with criteria from the National Health Interview Survey (NHIS).32 Participants were assigned current-smoking status if they smoked >100 cigarettes in their lifetime and reported currently smoking cigarettes every day or some days. Former-smoking status was assigned to those who had smoked ≥100 cigarettes in their lifetime but reported currently not smoking at all, and never-smoking status was assigned to those who smoked <100 cigarettes in their lifetime. Because of the low frequency of e-cigarette use/vaping in the sample, current established vaping was split into two groups rather than three: current (current some day or everyday use) versus not current (former and never use).
Cancer remission status
Participants were considered in remission if they reported a cancer diagnosis in a previous wave and responded affirmatively to the question, in the past, you told us that you had cancer: are you in remission for cancer? Participants were considered not in remission if they reported being diagnosed in the past 12 months in Wave 5 or if they endorsed a recurrence of cancer reported in a previous wave. The PATH study did not measure cancer stage/severity, the exact time since diagnosis or treatment, treatment status, or cancer treatments received, and access to cancer type data was restricted.
Symptom burden
PATH survey questions relevant to symptom burden included single-item assessments of fatigue, pain, and emotional problems adapted from validated questionnaires in the widely used PROMIS (Patient-Reported Outcomes Measurement Information System) suite of measures,29,33,34 as described below:
Fatigue
Patients were asked to rate their fatigue (feeling unrested or overly tired during the day, no matter how many hours of sleep you’ve had) on average over the past 7 days on a 5-point Likert scale from 1 (none) to 5 (very severe).
Pain
Participants were asked to rate their level of pain on average in the past 7 days on an 11-point Likert scale from 0 (no pain) to 10 (worst pain imaginable).
Emotional problems
Participants were asked to rate how often they have been bothered by emotional problems (such as feeling anxious, depressed, or irritable) in the past 7 days on a 5-point Likert scale from 1 (never) to 5 (very often).
Quality of life
Participants were asked to rate their QoL in general on a 5-point Likert scale from 1 (excellent) to 5 (poor), with higher scores indicating worse QoL.
Quit intentions, likelihood, and attempts
Those established as currently smoking cigarettes in the PATH study were asked the following questions, which were adapted from well established surveys, including the National Cancer Institute’s Tobacco Use Supplement to the Current Population Survey items on cigarette smoking (TUS-CPS) 29,35:
Level of interest in quitting cigarettes
Participants rated their interest level on a 10-point Likert scale from 1 (not at all interested) to 10 (extremely interested).
Likelihood of quitting cigarettes in the next 6 months
Participants were asked their likelihood of succeeding if they tried to quit smoking altogether in next 6 months, rated on a 4-point Likert scale from 1 (not at all likely) to 4 (very likely).
Quit attempts
Participants responded yes or no to the question; In the past 12 months, have you tried to quit smoking completely?
Statistical analyses
We used the Fay balanced repeated replication weighting procedures36 to assess the prevalence of sociodemographic variables and symptoms within the sample, and χ2 tests and linear regression were used to preliminarily evaluate differences in these characteristics by smoking status (current/former/never) that may be related to any primary findings.
We then assessed the effect of cigarette smoking and vaping status on symptom burden (fatigue, pain, emotional problems) and QoL using a multivariable analysis of variance controlling for key demographics and clinical characteristics (age, sex, race/ethnicity, remission status). Model residuals were evaluated for collinearity and multivariate normality using Q-Q plots. We used distribution-specific (Gaussian, multinomial, and binary) generalized linear mixed models controlling for the same demographics to assess associations between symptom burden (fatigue, pain, emotional problems), QoL, and quit-smoking intentions, quit-smoking likelihood, and past 12-month smoking quit attempts. Examination of variance inflation factors and correlations between symptom burden variables and QoL were not suggestive of multicollinearity. The Benjamini–Hochberg alpha correction procedure was used to decrease the false-discovery rate.37 The rate of missing data was low (from <1.00% to 2.88%) for all variables; therefore, analyses used all available cases.38 All analyses were conducted using SAS software (version 9.4; SAS Institute Inc.).
RESULTS
Sample characteristics
Characteristics of adult cancer survivors in Wave 5 of the PATH study by smoking status are presented in Table 1. Based on representative weights, an estimated 2.5 million adults with a history of cancer (14.21% of survivors) currently smoke cigarettes. A weighted 60.48% of survivors (N = 761) reported that their cancer was in remission, whereas 39.52% (N = 648) did not. Observed numbers and weighted percentages for each smoking status were as follows: current smoking (N = 451; 14.21%), former smoking (N = 478; 39.27%), and never smoking (N = 383; 35.77%). A weighted 2.88% (N = 87) reported current vaping, and 64.16% of those currently vaping reported current dual use (cigarettes and e-cigarettes). There were overall differences in sex, age group, race/ethnicity, education, income, e-cigarette use, and remission status by smoking status (see Table 1). Those currently smoking combustible tobacco (N = 451) on average scored 6.24 of 10 (95% CI, 5.91–6.57) in their self-reported degree of interest in quitting cigarettes and endorsed a high self-reported likelihood of quitting cigarette smoking in the next 6 months, scoring 3 of 4 on average, with a score of 4 indicating extremely likely to quit (mean score, 3.00; 95% CI, 2.83–3.17). Approximately two fifths (41.87%) of those continuing to smoke reported making a serious quit attempt in the past 12 months.
TABLE 1.
Demographic characteristics by smoking status.
| Weighted % or mean (95% CI) |
||||||
|---|---|---|---|---|---|---|
| Weighted % or mean: Overall sample | Current smoking, N = 451 | Former smoking, N = 478 | Never smoking, N = 383 | χ2 analysis or t-test | p a | |
|
| ||||||
| Age group, years | ||||||
| 18–24 | 0.89 | 15.77 (6.09–25.44) | 16.76 (4.82–28.68) | 67.48 (55.58–79.38) | 135.14 | < .0001*** |
| 25–34 | 2.82 | 51.07 (35.41–66.74) | 13.22 (6.47–19.96) | 35.71 (19.07–52.34) | ||
| 35–44 | 6.53 | 30.37 (20.46–40.29) | 22.30 (12.03–32.57) | 47.33 (33.56–61.10) | ||
| 45–54 | 10.67 | 28.29 (21.14–35.44) | 25.10 (15.89–34.31) | 46.60 (36.19–57.02) | ||
| 55–64 | 21.79 | 21.06 (15.44–26.67) | 38.56 (31.01–46.12) | 40.38 (32.33–48.43) | ||
| 65 or older | 57.30 | 7.77 (5.96–9.59) | 54.69 (48.74–60.64) | 37.53 (31.52–43.54) | ||
| Sex | ||||||
| Male | 43.96 | 13.28 (10.30–16.27) | 49.24 (43.26–55.22) | 37.47 (31.80–43.15) | 7.70 | .02 |
| Female | 55.95 | 18.03 (15.32–20.73) | 39.96 (34.52–45.39) | 42.01 (36.71–47.30) | ||
| Race/ethnicity | ||||||
| Non-Hispanic White | 81.96 | 14.69 (12.33–17.05) | 45.05 (40.83–49.26) | 40.27 (35.92–44.61) | 9.67 | .14 |
| Non-Hispanic Black/African American | 6.22 | 27.41 (18.25–36.58) | 36.81 (25.15–48.49) | 35.77 (23.92–47.60) | ||
| Hispanic, any race | 4.97 | 18.11 (9.14–27.09) | 29.90 (13.52–46.29) | 51.99 (34.94–69.03) | ||
| Otherb | 4.51 | 13.67 (2.22–25.11) | 46.70 (26.84–66.56) | 39.63 (19.67–59.59) | ||
| Education | ||||||
| Less than bachelors | 65.32 | 21.87 (18.91–24.82) | 47.84 (43.18–52.51) | 30.29 (25.40–35.18) | 102.81 | < .0001*** |
| Bachelors or beyond | 34.41 | 3.86 (2.49–5.22) | 36.68 (30.72–42.63) | 59.47 (53.50–65.44) | ||
| Income | ||||||
| <$50K | 46.76 | 24.26 (20.77–27.76) | 45.25 (40.35–50.15) | 30.49 (25.03–35.95) | 54.26 | <.0001*** |
| ≥$50K | 44.53 | 8.03 (5.94–10.11) | 42.65 (36.94–48.36) | 49.32 (43.91–54.73) | ||
| Remission status | ||||||
| Not in remission | 39.52 | 20.77 (17.21–24.33) | 40.83 (35.05–46.61) | 38.41 (32.44–44.37) | 9.75 | .008** |
| Remission | 60.48 | 12.71 (10.41–15.02) | 46.10 (40.66–51.54) | 41.18 (36.04–46.33) | ||
| Current E-cig use | ||||||
| Not current | 97.12 | 14.31 (12.26–16.36) | 44.52 (40.40–48.64) | 41.16 (37.06–45.26) | 92.67 | < .0001*** |
| Current | 2.88 | 64.16 (49.47–78.86) | 28.26 (14.32–42.41) | 7.57 (1.25–13.90) | ||
| Symptom burden scores | ||||||
| Fatiguec | 2.31 | 2.67 (2.52–2.82) | 2.31 (2.20–2.43) | 2.22 (2.12–2.32) | 4.92d | < .0001*** |
| Paine | 3.08 | 4.75 (4.38–5.12) | 3.11 (2.78–3.43) | 2.53 (2.18–2.89) | 8.58d | < .0001*** |
| Emotional problemsf | 2.15 | 2.64 (2.50–2.79) | 2.03 (1.90–2.16) | 2.12 (1.97–2.26) | 5.38d | < .0001*** |
| Quality of lifeg | 2.30 | 2.83 (2.69–2.96) | 2.35 (2.22–2.47) | 2.09 (1.96–2.21) | 7.84d | < .0001*** |
Note:
p < .01
p < .0001.
Abbreviations: CI, confidence interval; E-cig, electronic cigarette.
Result of χ2 analyses or t-tests.
The other category included non-Hispanic Asian, Native American, Pacific Islander, other race, and multiracial.
Average level of fatigue in past 7 days, from 1 (none) to 5 (very severe).
Comparing current versus never-smoking status.
Average level of pain in the past 7 days, from 0 (no pain) to 10 (worst pain imaginable).
Degree to which bothered by emotional problems in the past 7 days, from 1 (never) to 5 (very).
Self-rated quality of life, from 1 (excellent) to 5 (poor), with higher scores indicating worse quality of life.
Tobacco use and symptom burden
In multivariate analyses, current smoking was associated with the greatest overall symptom burden and worst QoL compared with former and never smoking, including greater fatigue (F[2,12] = 15.80; p < .0001; partial η2 = .02), greater pain (F[2,12] = 52.83; p < .0001; partial η2= .08), more frequent emotional problems (F[2,12] = 11.58; p < .0001; partial η2 = .02), and worse QoL (F[2,12] = 56.46; p < .0001; partial η2 = .08; Figure 1). Individuals who formerly smoked reported lower levels of symptom burden (fatigue, pain, emotional problems) and better QoL compared with those currently smoking but had higher overall symptom burden and lower QoL compared with those who never smoked. Specifically, individuals who formerly smoked reported similar levels of fatigue (mean difference, 0.13; 95% CI, 0.00–0.26; p = .06) and emotional problems (mean difference, 0.03; 95% CI, −0.13, 0.18; p = .74) compared with those who never smoked but had worse pain (mean difference, 0.84; 95% CI, 0.46–1.22; p < .0001) and QoL (mean difference, 0.30; 95% CI, 0.16–0.43; p < .0001; Figure 1).
FIGURE 1.
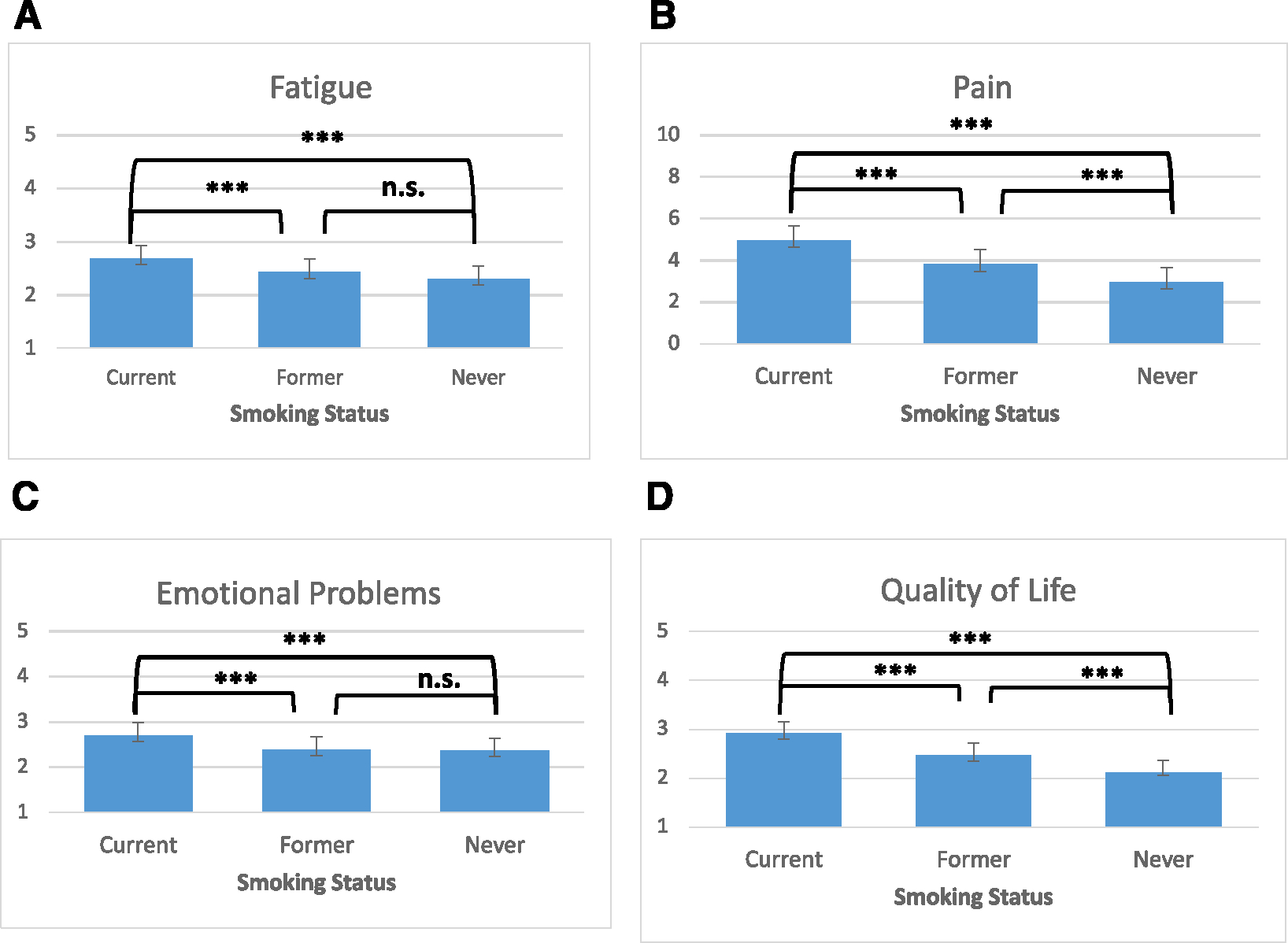
Symptom burden and quality of life by smoking status: least squared means with 95% CIs. ***p < .0001. (A) Average level of fatigue in the past 7 days ranged from 1 (none) to 5 (very severe). (B) Average level of pain in the past 7 days ranged from 0 (no pain) to 10 (worst pain imaginable). (C) Degree to which bothered by emotional problems in the past 7 days ranged from 1 (never) to 5 (very). (D) Self-rated QoL ranged from 1 (excellent) to 5 (poor), with higher scores indicating worse QoL. n.s. indicates not significant; QoL, quality of life.
Current vaping was also associated with greater fatigue (F[1,12] = 10.27; p = .001; partial η2 = .008), greater pain (F[1,12] = 6.86; p = .009; partial η2 = .005), and more frequent emotional problems (F[1,12] = 4.12; p = .04; partial η2 = .003), but not worse QoL (F[1,12] = 1.78; p = .17; Figure 2).
FIGURE 2.
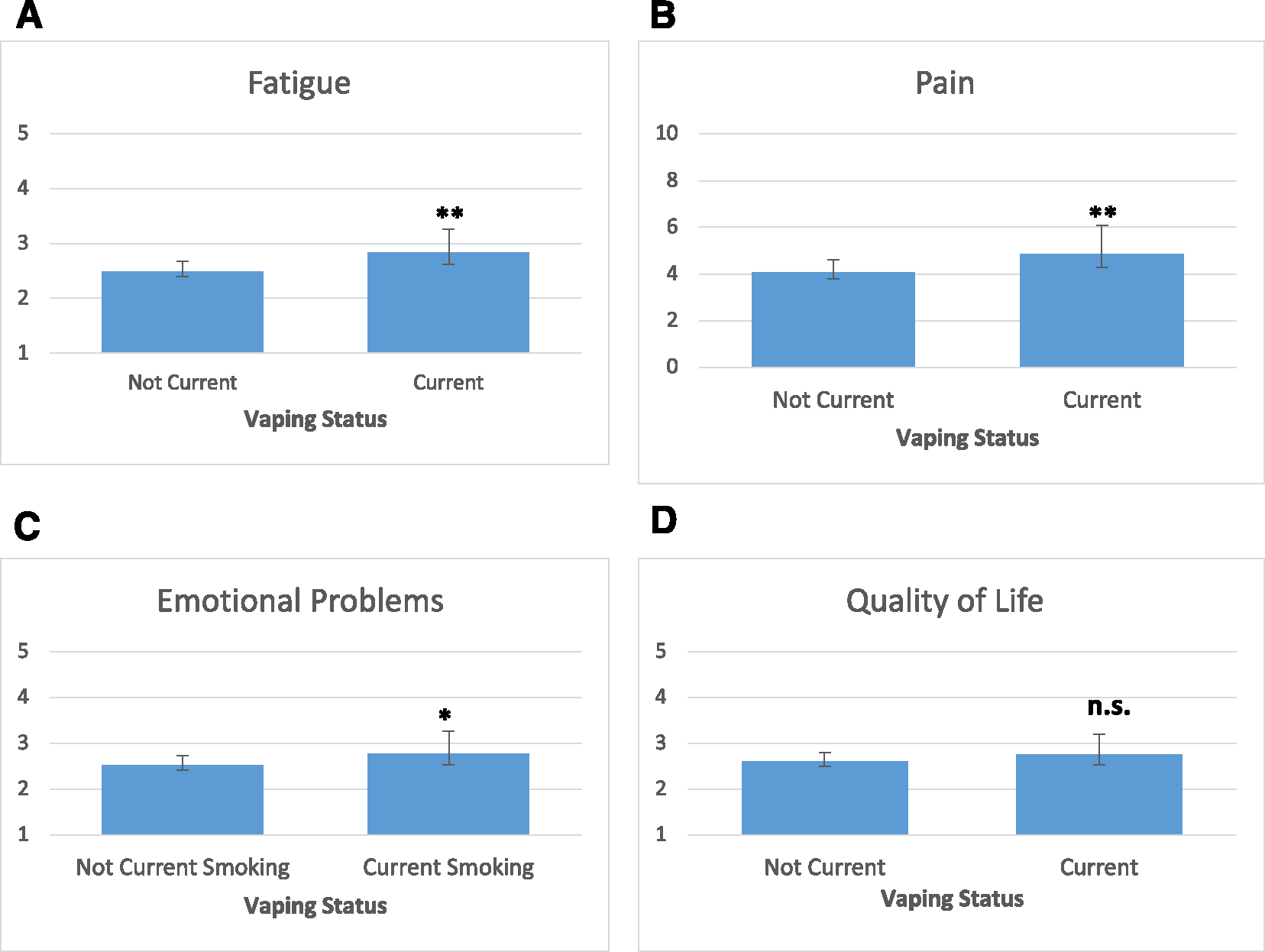
Symptom burden and quality of life by vaping status: least squared means with 95% CIs. *p < .05; **p < .01. (A) Average level of fatigue in the past 7 days ranged from 1 (none) to 5 (very severe). (B) Average level of pain in the past 7 days ranged from 0 (no pain) to 10 (worst pain imaginable). (C) Degree to which bothered by emotional problems in the past 7 days ranged from 1 (never) to 5 (very). (D) Self-rated QoL ranged from 1 (excellent) to 5 (poor), with higher scores indicating worse QoL. n.s. indicates not significant; QoL, quality of life.
Symptom burden and quit-smoking interest, likelihood, and attempts
Higher cancer symptom burden and worse QoL were not associated with lower interest in quitting smoking, reduced likelihood of quitting smoking in the next 6 months, or lower odds of having tried to quit smoking in the past 12 months among those currently smoking (adjusted p > .10; Table 2).
TABLE 2.
Symptom burden and quality of life predicting level of interest in quitting smoking, likelihood of quitting, and quit attempts (controlling for remission status, age, sex, and race/ethnicity).
| Predictor variable | Estimate (OR) | SE | t-test | Adj. p |
|---|---|---|---|---|
|
| ||||
| Level of interest in quitting smoking a | ||||
|
| ||||
| Fatigue | 0.006 | 0.03 | 0.20 | .85 |
| Pain | −0.002 | 0.009 | −0.022 | .85 |
| Emotional problems | 0.04 | 0.02 | 1.44 | .85 |
| Quality of life | −0.01 | 0.03 | −0.43 | .85 |
|
| ||||
| Likelihood of quitting smoking b | ||||
|
| ||||
| Fatigue | 0.06 | 0.24 | 0.25 | .84 |
| Pain | −0.05 | 0.06 | −0.75 | .84 |
| Emotional problems | −0.21 | 0.19 | −1.13 | .84 |
| Quality of life | 0.13 | 0.19 | 0.71 | .84 |
|
| ||||
| Past year quit attempt c | ||||
|
| ||||
| Fatigue | −0.09 (2.42) | 0.12 | −0.73 | .46 |
| Pain | 0.07 (3.61) | 0.04 | 1.94 | .27 |
| Emotional problems | −0.09 (2.33) | 0.10 | −0.89 | .46 |
| Quality of life | 0.19 (2.46) | 0.11 | 1.65 | .39 |
Abbreviations: Adj. p, Benjamini–Hochberg corrected p value; OR, odds ratio; SE, standard error.
Level of interest in quitting smoking from 1 (not at all interested) to 10 (extremely interested).
Likelihood of quitting cigarettes in the next 6 months from 1 (not at all likely) to 4 (very likely).
Past year quit attempt (did you try to stop completely in the last 12 months?): yes versus no.
DISCUSSION
By using a recently collected nationally representative US sample of 1409 cancer survivors, this study aligns with and extends previous research demonstrating that current smoking is associated with worse symptom burden and QoL among individuals with a history of cancer,19,24,39–41 and that individuals who formerly smoked report lower symptom burden and better QoL compared with those who currently smoke.40,41 Indeed, in the current study, although past tobacco use was associated with worse pain and QoL compared with never using tobacco, there was no difference in fatigue or emotional problems between survivors with never and former smoking statuses. Although multiple studies to date have found favorable symptom burden profiles for those with former compared with current smoking status, studies that carefully assess the temporal relationship between cancer symptoms and smoking cessation are needed to determine whether symptom burden among individuals with cancer improves over time after smoking abstinence or a reduction in tobacco exposure.
In addition, it is important to emphasize that nicotine products exist on a spectrum of potential harm (with combustible tobacco being by far the greatest contributor to morbidity and mortality) when interpreting results of this study. Findings that individuals who are currently vaping may experience worse symptom burden should be interpreted with caution given the cross-sectional design, small number of current e-cigarette users in the current sample, and the strong degree of overlap between current/former smoking status and current e-cigarette use within the sample. Given the small number of current e-cigarette users, our analyses included all current e-cigarette users regardless of current combustible tobacco use. It is therefore possible that greater past or current exposure to combustible cigarettes among those currently vaping accounts for the relationship between vaping and symptom burden because 64.16% of those currently vaping in this sample also reported current cigarette smoking. Given this overlap, it is also possible that individuals experiencing greater symptom burden may have switched to vaping to reduce smoking and improve deteriorating health. Future research with larger samples (especially e-cigarette users who never smoked combustible cigarettes), patient perspectives, and longitudinal designs should clarify the temporal relationship between combustible cigarette smoking history, e-cigarette use, and symptom burden.
Although testing potential mechanisms that link smoking and vaping to worse symptom burden was beyond the scope of the current study, there are numerous mechanisms that may explain this relationship, including established relationships between tobacco use and increased risk of chronic inflammation and comorbid health conditions (e.g., pulmonary, cardiovascular, and metabolic disease; neuropathy; arthritis), as well as disruption of sleep.42,43
Although more research is needed to make strong claims that reducing or quitting tobacco use improves cancer-related symptoms, this is an important area of future inquiry because potential symptom relief is a more proximal outcome than survival and thus may be especially compelling to patients. The possibility that reduced tobacco exposure could favorably influence cancer-related symptom burden does not appear to be well known. Indeed, many patients with cancer do not see a connection between smoking cessation and QoL improvement, and some oncology providers view smoking cessation as a lower priority compared with pain and stress relief or even as a direct impediment to treating these factors.44 A recent survey found that this trend is especially true in palliative treatment settings, where oncology care providers are less likely to believe that tobacco use negatively impacts treatment outcomes or to advise smokers to quit.45 Future studies should test whether framing smoking cessation as part of cancer symptom management enhances patient and clinician willingness to discuss and pursue smoking cessation. Regardless of their symptom burden, cancer survivors are generally motivated to quit46,47 and should be offered and provided with tobacco-cessation support.48 These findings directly contradict common clinical assumptions that patients are resistant to tobacco treatment or that treating tobacco use will induce significant discomfort.45
The results of this study must be considered within the context of its limitations. The cross-sectional design and lack of detailed information about prediagnosis functioning and QoL limit our ability to draw strong conclusions about the strength or directionality of the relationship between survivor or remission status, smoking, vaping, and symptom burden. The PATH data set also does not include cancer-specific information that has been correlated with symptom burden, including cancer type, treatment status, time since diagnosis or treatment, cancer stage or severity,49,50 or specific treatments received.19,49–51 It is possible that the strength of association between smoking and symptom burden differs by cancer type and stage, and we were not able to evaluate this in the current study. In addition, we were unable to determine whether the association between ever-smoking history and symptom burden may be partially explained by the finding that individuals who smoke are more likely to develop certain types of cancer (e.g., lung) associated with greater symptom burden, worse QoL, and worse survival.52,53 Indeed, we observed lower rates of self-reported cancer remission status among individuals currently smoking. Although the association between smoking status and greater symptom burden remained after controlling for cancer remission status, it is possible that individuals currently smoking had more severe disease in ways that were not captured with the basic remission status variable. The PATH study used patient self-report to assess cancer remission status, and although there is a precedent and pragmatic rationale for assessing cancer history through patient self-report, extant research suggests that self-report measures are more consistent with medical records for certain variables (cancer type and treatment categories) compared with others (pathologic results, recurrence, or remission).54–56 The lack of specificity in characterizing participants’ cancer history limits our understanding of the degree to which the symptoms observed were directly related individuals’ cancer diagnosis or treatment. Indeed, it is possible that, for certain participants (e.g., those diagnosed with early stage disease, those many years posttreatment), the symptoms are entirely unrelated to cancer history. Nevertheless, the associations found between smoking status, symptom burden, and QoL in the current study align with those observed among patients who were assessed during cancer care by Oswald and colleagues (2022) after controlling for cancer site, stage, and treatment history using clinical information abstracted from internal cancer center databases.19
Although the PATH study seeks to provide representative data with respect to the US population, the subset that we selected for the current study (comprised of individuals who reported a cancer history and had complete Wave 5 data on smoking, QoL, and symptom burden) was not representative with respect to race and ethnicity (only 6% Black/African American and 5% Hispanic), limiting the generalizability of our findings. Future research on cigarette and e-cigarette use among cancer populations should aim to have fully representative samples, especially because we observed that current-smoking status differed by key demographics (age group, sex, income, and education), indicating that continued smoking postdiagnosis may be both a product of and contributor to health disparities.
Another limitation of the current research is the use of single-item measures to assess cancer-related symptoms. Although there is substantial precedent for assessing fatigue, pain, emotional problems, and QoL using single-item measures,57–59 these measures generally have less clinical validity and reliability compared with established multi-item scales or symptom burden inventories.58,60 Although the size of the effects of current tobacco use on symptom burden and QoL were small (η2 < .09), and we do not have benchmarks to determine whether these differences are clinically significant, the effect was stable across multiple symptoms, suggesting the robust nature of these associations. Despite its limitations, the study also has numerous strengths, namely, its nationally representative sample and diversity in representation of smoking, vaping, cancer remission status, and (likely) cancer treatment setting. Therefore, these findings extend the existing research and point to areas for further study.
In summary, the results of this study suggest that current cigarette smoking and e-cigarette use are associated with worse symptom burden and that current smoking is associated with worse QoL, but greater symptom burden and worse QoL do not appear to reduce interest in or plans to quit smoking. Therefore, it is critically important that oncology providers assess tobacco use, offer tobacco-cessation support, and take ownership of the delivery of tobacco treatment to oncology patients.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (grants/contracts NIH-T32-HL144470, T32CA122061, and P30 CA138313) and by the National Institutes of Health and the US Food and Drug Administration Center for Tobacco Products (grant/contract U54DA036151). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Food and Drug Administration.
Funding information
National Institutes of Health, Grant/Award Numbers: NIH-T32-HL144470, P30 CA 138313, T32CA122061; National Institutes of Health and the US Food and Drug Administration Center for Tobacco Products, Grant/Award Number: U54DA036151
Footnotes
CONFLICT OF INTEREST STATEMENT
Lisa M. Fucito reports consulting fees from Imbrium Therapeutics outside the submitted work. Evan Graboyes reports grants/contracts and personal fees from Castle Biosciences outside the submitted work. Benjamin A. Toll reports that, in the past 3 years, he has testified on behalf of plaintiffs who have filed litigation against the tobacco industry. The remaining authors made no disclosures
REFERENCES
- 1.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31–54. 10.3322/caac.21440 [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services, Centers for Disease Control and Prevention (CDC), National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. Smoking Cessation: A Report of the Surgeon General; CDC; 2020. Accessed April 16, 2022. https://www.cdc.gov/tobacco/data_statistics/sgr/2020-smoking-cessation/index.html#full-report
- 3.Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013;132(2):401–410. doi: 10.1002/ijc.27617 [DOI] [PubMed] [Google Scholar]
- 5.Foerster B, Pozo C, Abufaraj M, et al. Association of smoking status with recurrence, metastasis, and mortality among patients with localized prostate cancer undergoing prostatectomy or radiotherapy: a systematic review and meta-analysis. JAMA Oncol. 2018;4(7):953–961. doi: 10.1001/jamaoncol.2018.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SH, Terrell JE, Bradford CR, et al. Does quitting smoking make a difference among newly diagnosed head and neck cancer patients? Nicotine Tob Res. 2016;18(12):2216–2224. doi: 10.1093/ntr/ntw189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawaguchi T, Matsumura A, Iuchi K, et al. Second primary cancers in patients with stage III non-small cell lung cancer successfully treated with chemo-radiotherapy. Jpn J Clin Oncol. 2006;36(1):7–11. doi: 10.1093/jjco/hyi208 [DOI] [PubMed] [Google Scholar]
- 8.Do KA, Johnson MM, Lee JJ, et al. Longitudinal study of smoking patterns in relation to the development of smoking-related secondary primary tumors in patients with upper aerodigestive tract malignancies. Cancer. 2004;101(12):2837–2842. doi: 10.1002/cncr.20714 [DOI] [PubMed] [Google Scholar]
- 9.Boeri L, Soligo M, Frank I, et al. Cigarette smoking is associated with adverse pathological response and increased disease recurrence amongst patients with muscle-invasive bladder cancer treated with cisplatin-based neoadjuvant chemotherapy and radical cystectomy: a single-centre experience. BJU Int. 2019;123(6):1011–1019. doi: 10.1111/bju.14612 [DOI] [PubMed] [Google Scholar]
- 10.Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328(3):159–163. doi: 10.1056/NEJM199301213280302 [DOI] [PubMed] [Google Scholar]
- 11.Sørensen LT, Hørby J, Friis E, Pilsgaard B, Jørgensen T. Smoking as a risk factor for wound healing and infection in breast cancer surgery. Eur J Surg Oncol. 2002;28(8):815–820. doi: 10.1053/EJSO.2002.1308 [DOI] [PubMed] [Google Scholar]
- 12.Hawari FI, Obeidat NA, Rimawi D, Jamal K. Smoking cessation care can translate to lower hazard of death in the short-run in cancer patients—a retrospective cohort study to demonstrate the value of smoking cessation services within the treatment phase of cancer. BMC Cancer. 2019;19(1):580. doi: 10.1186/s12885-019-5778-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnett TE, Lu Y, Gehr AW, Ghabach B, Ojha RP. Smoking cessation and survival among people diagnosed with non-metastatic cancer. BMC Cancer. 2020;20(1):726. doi: 10.1186/s12885-020-07213-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheikh M, Mukeriya A, Shangina O, Brennan P, Zaridze D. Postdiagnosis smoking cessation and reduced risk for lung cancer progression and mortality: a prospective cohort study. Ann Intern Med. 2021;174(9):1232–1239. doi: 10.7326/M21-0252 [DOI] [PubMed] [Google Scholar]
- 15.Talluri R, Fokom Domgue J, Gritz ER, Shete S. Assessment of trends in cigarette smoking cessation after cancer diagnosis among US adults, 2000 to 2017. JAMA Netw Open. 2020;3(8):e2012164. doi: 10.1001/jamanetworkopen.2020.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gritz ER, Talluri R, Fokom Domgue J, Tami-Maury I, Shete S. Smoking behaviors in survivors of smoking-related and non-smoking-related cancers. JAMA Netw Open. 2020;3(7):209072. doi: 10.1001/jamanetworkopen.2020.9072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox LS, Africano NL, Tercyak KP, Taylor KL. Nicotine dependence treatment for patients with cancer. Cancer. 2003;98(3):632–644. doi: 10.1002/cncr.11538 [DOI] [PubMed] [Google Scholar]
- 18.Peppone LJ, Mustian KM, Morrow GR, et al. The effect of cigarette smoking on cancer treatment-related side effects. Oncologist. 2011;16(12):1784–1792. doi: 10.1634/theoncologist.2011-0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oswald LB, Brownstein NC, Whiting J, et al. Smoking is related to worse cancer-related symptom burden. Oncologist. 2022;27(2):e176–e184. doi: 10.1093/oncolo/oyab029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniel M, Keefe FJ, Lyna P, et al. Persistent smoking after a diagnosis of lung cancer is associated with higher reported pain levels. J Pain. 2009;10(3):323–328. doi: 10.1016/j.jpain.2008.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ditre JW, Brandon TH, Zale EL, Meagher MM. Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychol Bull. 2011;137(6):1065–1093. doi: 10.1037/a0025544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YJ, Dev R, Reddy A, et al. Association between tobacco use, symptom expression, and alcohol and illicit drug use in advanced cancer patients. J Pain Symptom Manag. 2016;51(4):762–768. doi: 10.1016/j.jpainsymman.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 23.Novy DM, Lam C, Gritz ER, Hernandez M, Driver LC, Koyyalagunta D. Distinguishing features of cancer patients who smoke: pain, symptom burden, and risk for opioid misuse. J Pain. 2012;13(11): 1058–1067. doi: 10.1016/j.jpain.2012.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garces YI, Yang P, Parkinson J, et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126(6):1733–1741. doi: 10.1378/chest.126.6.1733 [DOI] [PubMed] [Google Scholar]
- 25.Guimond AJ, Croteau VA, Savard MH, Bernard P, Ivers H, Savard J. Predictors of smoking cessation and relapse in cancer patients and effect on psychological variables: an 18-month observational study. Ann Behav Med. 2017;51(1):117–127. doi: 10.1007/S12160-016-9834-4 [DOI] [PubMed] [Google Scholar]
- 26.Simmons VN, Litvin EB, Jacobsen PB, et al. Predictors of smoking relapse in patients with thoracic cancer or head and neck cancer. Cancer. 2013;119(7):1420–1427. doi: 10.1002/cncr.27880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Streck JM, Luberto CM, Muzikansky A, et al. Examining the effects of stress and psychological distress on smoking abstinence in cancer patients. Prev Med Rep. 2021;23:101402. doi: 10.1016/j.pmedr.2021101402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aigner CJ, Cinciripini PM, Anderson KO, Baum GP, Gritz ER, Lam CY. The association of pain with smoking and quit attempts in an electronic diary study of cancer patients trying to quit. Nicotine Tob Res. 2016;18(6):1449–1455. doi: 10.1093/ntr/ntv118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyland A, Ambrose BK, Conway KP, et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob Control. 2017;26(4):371–378. doi: 10.1136/tobaccocontrol-2016-052934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Department of Health and Human Services, National Institutes of Health, National Institute on Drug Abuse; US Department of Health and Human Services, Food and Drug Administration, Center for Tobacco Products. Population Assessment of Tobacco and Health (PATH) Study [United States] Public-Use Files (ICPSR 36498). InterUniversity Consortium for Political and Social Research [distributor]; 2022. doi: 10.3886/ICPSR36498.v17 [DOI] [Google Scholar]
- 31.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute (NCI). Definition of survivor. NCI Dictionary of Cancer Terms. NCI; 2021. Accessed June 27, 2022. doi:https://www.cancer.gov/publications/dictionaries/cancer-terms/def/survivor [Google Scholar]
- 32.US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. National Health Interview Survey: Adult Tobacco Use—Smoking Status Recodes. Accessed April 29, 2022. https://www.cdc.gov/nchs/nhis/tobacco/tobacco_recodes.htm [Google Scholar]
- 33.National Institutes of Health. PROMIS. Accessed January 13, 2023. https://www.healthmeasures.net/explore-measurement-systems/promis [Google Scholar]
- 34.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Institutes of Health, National Cancer Institute, Division of Cancer Control and Population Sciences, Behavioral Research Program. The Tobacco Use Supplement to the Current Population Survey. National Cancer Institute; 2022. Accessed January 13, 2023. https://cancercontrol.cancer.gov/brp/tcrb/tus-cps [Google Scholar]
- 36.Fay RE. Theory and application of replicate weighting for variance calculations. In: Section on Survey Research Methods, American Statistical Association, eds. Proceedings of the Section on Survey Research Methods. American Statistical Association; 1989:212–221. [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;57(1):289–300. doi: 10.1111/J.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 38.Bennett DA . How can I deal with missing data in my study? Aust N Z J Public Health. 2001;25(5):464–469. doi: 10.1111/J.1467-842X.2001.tb00294.x [DOI] [PubMed] [Google Scholar]
- 39.Peppone LJ, Mustian KM, Morrow GR, et al. The effect of cigarette smoking on cancer treatment–related side effects. Oncologist. 2011;16(12):1784–1792. doi: 10.1634/theoncologist.2011-0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ditre JW, Gonzalez BD, Simmons VN, Faul LA, Brandon TH, Jacobsen PB. Associations between pain and current smoking status among cancer patients. Pain. 2011;152(1):60–65. doi: 10.1016/j.pain.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baser S, Shannon VR, Eapen GA, et al. Smoking cessation after diagnosis of lung cancer is associated with a beneficial effect on performance status. Chest. 2006;130(6):1784–1790. doi: 10.1378/chest.130.6.1784 [DOI] [PubMed] [Google Scholar]
- 42.National Center for Chronic Disease and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention (US); 2014. [Google Scholar]
- 43.Gu F, Li XF, Xu JF, Gao GH, Wu YF, Zhou CC. Effect of nicotine dependence on quality of life and sleep quality in patients with lung cancer who continue to smoke after diagnosis. J Thorac Dis. 2018;10(5):2583–2589. doi: 10.21037/jtd.2018.05.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wells M, Aitchison P, Harris F, et al. Barriers and facilitators to smoking cessation in a cancer context: a qualitative study of patient, family and professional views. BMC Cancer. 2017;17(1):1–15. doi: 10.1186/s12885-017-3344-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derksen JWG, Warren GW, Jordan K, et al. European practice patterns and barriers to smoking cessation after a cancer diagnosis in the setting of curative versus palliative cancer treatment. Eur J Cancer. 2020;138:99–108. doi: 10.1016/j.ejca.2020.07.020 [DOI] [PubMed] [Google Scholar]
- 46.Conlon MSC, Santi SA, Meigs ML, Davidson SM, Saunders D. Cigarette-smoking characteristics and interest in cessation in patients with head-and-neck cancer. Curr Oncol. 2020;27(5):478–485. doi: 10.3747/co.27.6019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampson L, Papadakos J, Milne V, et al. Preferences for the provision of smoking cessation education among cancer patients. J Cancer Educ. 2016;33(1):7–11. doi: 10.1007/s13187-016-1035-0 [DOI] [PubMed] [Google Scholar]
- 48.Toll BA, Brandon TH, Gritz ER, Warren GW, Herbst RS. Assessing tobacco use by cancer patients and facilitating cessation: an American Association for Cancer Research policy statement. Clin Cancer Res. 2013;19(8):1941–1948. doi: 10.1158/1078-0432.CCR-13-0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kokkonen K, Tasmuth T, Lehto JT, et al. Cancer patients’ symptom burden and health-related quality of life (HRQoL) at tertiary cancer center from 2006 to 2013: a cross-sectional study. Anticancer Res. 2019;39(1):271–277. doi: 10.21873/anticanres.13107 [DOI] [PubMed] [Google Scholar]
- 50.Cleeland CS, Zhao F, Chang VT, et al. The symptom burden of cancer: evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer. 2013;119(24):4333–4340. doi: 10.1002/cncr.28376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogt J, Beyer F, Sistermanns J, et al. Symptom burden and palliative care needs of patients with incurable cancer at diagnosis and during the disease course. Oncologist. 2021;26(6):e1058–e1065. doi: 10.1002/onco.13751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bubis LD, Davis L, Mahar A, et al. Symptom Burden in the First Year After Cancer Diagnosis: an Analysis of Patient-Reported Outcomes. J Clin Oncol. 2018;36(11):1103–1111. doi: 10.1200/jco.2017.76.0876 [DOI] [PubMed] [Google Scholar]
- 53.Jensen RE, Potosky AL, Moinpour CM, et al. United States population-based estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017;35(17):1913–1920.doi: 10.1200/jco.2016.71.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Diamant AL, Thind A, Maly RC. Validity of self-reports of breast cancer treatment in low-income, medically underserved women with breast cancer. Breast Cancer Res Treat. 2010;119(3):745–751. doi: 10.1007/S10549-009-0447-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kool M, Bastiaannet E, Van de Velde CJH, Marang-van de Mheen PJ. Reliability of self-reported treatment data by patients with breast cancer compared with medical record data. Clin Breast Cancer. 2018;18(3):234–238. doi: 10.1016/j.clbc.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 56.Cowdery SP, Stuart AL, Pasco JA, Berk M, Campbell D, Williams LJ. Validity of self-reported cancer: comparison between self-report versus cancer registry records in the Geelong Osteoporosis Study. Cancer Epidemiol. 2020;68:101790. doi: 10.1016/j.canep.2020.101790 [DOI] [PubMed] [Google Scholar]
- 57.Bowling A Just one question: if one question works, why ask several? J Epidemiol Community Health. 2005;59(5):342–345. doi: 10.1136/jech.2004.021204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhi WI, Gentile D, Diller M, et al. Patient-reported outcomes of pain and related symptoms in integrative oncology practice and clinical research: evidence and recommendations. Oncology (Williston Park). 2021;35(1):35–41. doi: 10.46883/onc.2021.3501.0035 [DOI] [PubMed] [Google Scholar]
- 59.Jacobsen PB. Assessment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;2004(32):93–97. doi: 10.1093/jncimonographs/lgh010 [DOI] [PubMed] [Google Scholar]
- 60.Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32(17):1840–1850. doi: 10.1200/jco.2013.53.4495 [DOI] [PMC free article] [PubMed] [Google Scholar]


