Abstract
Mitral valve leaflets and chordae have been shown to contain different amounts and proportions of glycosaminoglycans (GAGs) and proteoglycans (PGs) corresponding to in vivo normal or diseased cyclic strain patterns. To understand the effect of cyclic strains on GAG/PG synthesis by valvular interstitial cells (VICs) isolated from valve leaflet and chordae separately, porcine VICs were seeded within collagen gels and alternately stretched or relaxed for 24 hours periods for one week in a custom-designed tissue engineering bioreactor. We found cyclic-stretch-induced upregulation of total GAGs and of individual GAG classes secreted into the culture medium. Leaflet cells showed a delayed response to stretching compared to chordal cells, but altered the proportions of various GAG classes they secreted during the culture duration. Decorin and biglycan PGs were slightly responsive to stretch. We demonstrated that mechanical stretch and relaxation conditions reversibly regulate GAG and PG production in a novel 3D model of valve tissues. This is the first study using cyclic strains to modulate GAG/PG synthesis by valve cells and our results may have implications for the remodeling of the mitral valve as well as other tissues.
Keywords: heart valves, mechanical stretch, tissue engineering, extracellular matrix, collagen gels
INTRODUCTION
The organization and remodeling of extracellular matrix (ECM) is partially controlled by mechanical stresses and strains. Glycosaminoglycans (GAGs) and proteoglycans (PGs) are essential ECM components in mitral valve tissues and have been shown to be segregated according to the type of loading that distinct regions (leaflet or chordae) experience.10 GAGs and PGs regulate and perform a variety of biological and biophysical functions during the growth, remodeling and pathogenesis of tissues.18,29 Glycosaminoglycans consist of linear chains of disaccharides that bind to the core protein of proteoglycans. The abundance of particular GAGs and PGs can vary according to different biological needs of the tissues.16 For example, the dermatan sulfate (mostly 4-sulfated) PGs decorin and biglycan regulate the formation and orientation of collagen fibrils and hence tissue tensile strength,7,32 whereas the GAG hyaluronan (HA), which does not covalently bind to a core protein, entraps large amounts of water to create a swelling force.37 Correspondingly, previous research on mitral valves has shown that dermatan 4-sulfate and small leucine-rich PGs are prominent in valve regions experiencing cyclic tension (chordae), while GAGs HA and chondroitin 6-sulfate and the PG versican are abundantly present in regions of cyclic compression (such as the leaflet free edges).10 Furthermore, in diseased conditions such as myxomatous degeneration, valves are subjected to altered tissue stress23 and contain more GAGs and PGs than normal.11,12 Hence, leaflet and chordal regions of the mitral valve experience distinct cyclic strains and during pathological conditions the magnitude of these strains changes, as do the amounts of GAGs and PGs.
These observations suggest that mechanical strains may regulate GAG and PG synthesis by valvular interstitial cells (VICs), which have been characterized as myofibroblasts.34 There are several studies that have shown that various mechanical strains such as centrifugal forces,15,28 shear forces8,9 and cyclic tensile strains25-27 can modulate GAG and PG synthesis; cyclic mechanical strains are the most relevant to the in vivo loading of valve cells. In addition, an elegant investigation of fibroblasts seeded on collagen gels subjected to repeated cycles of static stretch and relaxation showed that collagen XII synthesis was increased by tensile strains in a repeatable and reversible manner.35 A combination of cyclic stretch and relaxation conditions in repeated cycles, therefore, offers a precise method to study strain-induced ECM production by VICs. The response of valve cells to pressure and shear forces has been characterized in organ culture,38,39 but only two studies published to date have examined the effect of cyclic mechanical strains in 2D valvular cell culture; these latter investigations, however, did not measure GAGs and PGs.21,36 Determining how GAG and PG synthsis responds to mechanical strains is important since these ECM molecules are altered extensively during myxomatous valve degeneration. Recently, we showed that static strains induce the synthesis of specific GAGs by VICs in 3D cultures.14 We now report a cyclic strain based analysis in which the 3D cultures were stretched in a custom designed bioreactor. Many different types of scaffolds and cell environments have been used to investigate the effects of mechanical stress and strain conditions on various cell types. Here, we have seeded VICs cells within a collagen matrix since collagen is the most abundant protein in mitral valve tissue.22 We believe that this approach will provide a more biologically and anatomically appropriate 3D model for studying the regulation of ECM deposition than growing VICs in monolayers, since it will mimic the in vivo environment of the VICs,.20,31 Indeed, VICs seeded within collagen gels have been shown to retain their native phenotype and secrete GAGs and PGs that are normally present in heart valves.5,30,33 Therefore, the purpose of this study was to determine if the GAG and PG production by mitral VICs cultured in 3D collagen gels is inducible by cyclic stretch, reversibly regulated and dependent upon cell loading in vivo (leaflet vs. chordae).
METHODS
Cell Culture
Primary cultures of VICs were dissociated from porcine mitral valve leaflets and chordae, obtained from an abattoir. Valves were first washed extensively with sterile phosphate buffered saline and then placed in serum-free Dulbecco’s modified Eagle medium (DMEM, Mediatech, Herndon, VA), containing 2 mg/mL collagenase type II (Worthington, Lakewood, NJ), within an incubated shaker for 20 min (140 rpm, 37°C). Afterwards, the loosened endothelial cells were removed by swabbing the valve surface with a cotton swab and the chordae tendineae were separated from the leaflet. The leaflet and chordae were minced and digested in serum-free DMEM containing 1 mg/mL collagenase type III and 0.1 mg/mL hyaluronidase (both from Worthington) for 4 h in an incubated shaker (140 rpm, 37°C). The resulting cell suspension was filtered through a sterile 70 μm cell strainer (BD Falcon, San Jose, CA) and cultured in DMEM:F12 (1:1, containing low glucose with HEPES, Mediatech) with 10% bovine growth serum (BGS, HyClone, Logan, UT) and 1% antibiotic-antimycotic solution (Mediatech). The culture was incubated in a humidified atmosphere of 95% air/ 5% CO2 at 37°C with changes of medium every 48 hours. The cells were split in a 1:3 ratio when they became 90% confluent. Separate leaflet and chordal cell cultures between passages 5-9 were used to prepare the collagen gels.
Device Assembly and Mold Preparation
A custom-designed motorized stretching device (Fig. 1) was used for dynamic loading of the cell-seeded collagen gels. The components of the device were built in the machine shop at Rice University and at a rapid prototyping company (Laser Reproductions, Columbus, OH). The device consisted of an aluminum base unit, culture lid, and stretching cam. The stretching holders connected to the collagen gel inside the culture lid at one end, passed through a lubricated thin tube in the lid, and connected to a roller bearing that moved around the stretching cam. The stretching cam was shaped as a 4-sinusoid waveform superimposed around the circumference of a circle that stretched the cell-seeded collagen gel four times with each cam rotation. The cam radii and , initial collagen gel construct length , angle around the perimeter of the cam and percentage strain were related by the equation
FIGURE 1.

Photograph of cyclic stretching device showing various components as indicated.
A 24 V DC gearhead motor (Barber Colman, Rockford, IL), located within the aluminum base unit, rotated the stretching cam. The entire bioreactor fit easily within an incubator. The motor was controlled by an external power supply (Jameco Electronics, Belmont, CA) that was located outside the incubator. The cells-seeded gels were contained within a cross-shaped silicone rubber mold that fit snugly within the culture lid (Fig. 1). This mold and the anchors for the collagen gel were described previously.14 All bioreactor components were either autoclaved or wiped clean with 70% ethanol prior to use.
Cell Seeding in Collagen Gels
To mimic the 3D aspect of the cells’ native tissue environment, the VICs were seeded within collagen gels as previously described.14 Briefly, rat tail collagen type I (BD Biosciences, Bedford, MA) at a concentration of 2.28 mg/mL was mixed with 1 million cells per mL of total volume. After the collagen gel was poured, incubated, and began to contract (1-2 hours), 2-3 mL of regular DMEM:F12 medium with serum was added. As the collagen gel contracted, it was anchored only by the 4 mesh holders at the periphery of the well, and did not stick to the walls or base of the silicone rubber mold. Twenty-four hours after pouring the gel, the “pre-conditioning” culture medium was removed and stored (−20°C) and the culture was replenished with fresh medium. The collagen gel was then subjected to 10% strain at 1.167 Hz for 24 hours, then no cyclic stretch (relaxation) for 24 hours; this cycle of stretching and relaxation was repeated 2 more times. Conditioned media samples were removed and stored after each stretching or relaxation period, for a total of 7 days, before the collagen gels were collected for biochemical analysis. Four collagen gels were prepared using leaflet VICs and 7 using chordal VICs. Several additional collagen gels were prepared using chordal cells, since these were initially found to be the most responsive to cyclic strain. Two (2) of these additional collagen gels were subjected to continuous cyclic stretching for 7 days. Eight (8) of these additional collagen gels were prepared and subjected to only partial culture periods in order to measure the GAGs and PGs retained within the collagen gels at these timepoints. These partial culture periods were 24 hours of stretching (S), 24 hours of stretch followed by 24 hours of relaxation SR), SRS, and SRSR. Two (2) collagen gels were prepared for each partial culture experiment.
Image Analysis for Contraction Assessment
A digital image of the collagen gel was taken at the time of each medium change. The area of the collagen gel was digitally measured using Image-Pro software (Media Cybernetics, Silver Spring, MD) and the percentage contraction was calculated relative to the original mold size.14
Glycosaminoglycan Analysis
Fluorophore–assisted carbohydrate electrophoresis (FACE) was used to analyze the different GAG classes (chondroitin/dermatan sulfates and HA).14 Briefly, intact collagen gels were weighed to measure the wet weight, lyophilized overnight, and then re-weighed to calculate percentage hydration. Samples were then dissociated with proteinase-K (10 mg/mL in ultrapure water, 60°C, 2 hours, EMD Pharmaceutical, Durham, NC); conditioned medium aliquots were similarly digested with proteinase-K (50 μg per mL). The medium samples (in addition to a control volume of fresh medium) were then subjected to ion exchange purification to remove the glucose from the medium samples and isolate the GAG chains. A 50% Q-Sepharose bead slurry (Amersham Pharmacia Biotech, Uppsala, Sweden) was added to the medium samples (0.24 mL per mL of original volume of medium) and washed with 40 column volumes of 7 mol/L urea buffer. Finally, the bound purified GAGs were eluted with 4 column volumes of 7 mol/L urea buffer containing 3 mol/L NaCl. Each medium and gel sample was analyzed using FACE after digestion with chondroitinase ABC and ACII (Associates of Cape Cod, Falmouth, MA) to cleave the GAG chains into disaccharides exactly as described previously.10,14 FACE gels were imaged with a Kodak Gel Logic 100 imaging system (Kodak, Eastman, MA) and analyzed using Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD). Specific GAGs were identified by corresponding bands in a disaccharide standard lane and quantified by comparison to a fluorescence standard curve. Total GAGs were calculated as the sum of various classes of GAGs.
Proteoglycan Measurement
To identify the core proteins of the PGs, gel samples were extracted using 4 mol/L guanidine HCl with protease inhibitors overnight at 4°C using previously reported techniques.10,17 The extracted samples were dialyzed into 7 mol/L urea (pH 7.5). Dialyzed gel samples and conditioned medium samples were then purified by ion exchange using Q-Sepharose beads. The purified PGs were ethanol-precipitated (95% ethanol, 1.3% potassium acetate, −20°C) from solution and digested with chondroitinase ABC to remove the GAG chains from the core proteins. The samples were vacuum dried, dissolved in SDS buffer at 100°C, separated on a 4-12% SDS-polyacrylamide gel, and then either visualized by Coomassie blue staining or blotted for western analyses. The nitrocellulose membrane was blotted with antibodies for specific PGs (anti-decorin LF-122 and anti-biglycan LF-104, courtesy of Larry Fisher, NIH; anti-versican 2-B-1, Associates of Cape Cod).3,19
Proteoglycan Immunostaining
Immunohistochemistry was performed to demonstrate the presence of the PGs within the collagen gel. A representative gel was fixed with 10% formalin while still constrained, then paraffin embedded and sectioned en face into 10 μm sections. Tissue slides were processed through a series of graded alcohols and hydrated to water. All slides were pretreated with chondroitinase ABC (200 mU/ml, 1 hour) to remove the GAG chains from the PG core proteins. Sections were blocked with 10% goat-serum (Sigma, St. Louis, MO), then incubated with primary antibodies against PGs for overnight at 4°C. The rabbit anti-porcine decorin (LF-122, dilution 1:500) and biglycan antibodies (LF-104, dilution 1:500) were generously provided by Larry Fisher, Ph.D., NIH.3 After rinsing in PBS, biotinylated secondary antibodies were applied (goat anti-rabbit IgG, Jackson Immunoresearch Inc., West Grove, PA) for 1 hour at room temperature. Postive staining was demonstrated by a chromogen reaction using Vectastain Elite ABC and diaminobenzidine kits (Vector Laboratories, Burlingame, CA).
DNA and Collagen Analysis
Cell and collagen density within the cell-seeded collagen gels were monitored by the Hoechst dye DNA assay24 and a Sirius red collagen assay, respectively. The DNA assay was performed on aliquots of the proteinase-K digested collagen gel samples, as prepared for GAG analysis.11 The Sircol sirius red assay (Biocolor, Ireland, UK) for collagen was performed on collagen gel samples dissolved in pepsin (1 mg/mL in 0.5 mol/L acetic acid, keeping the ratio of pepsin to sample wet weight as 1:10) overnight at room temperature.
Data Analysis
Differences in specific GAG classes were compared between each 24-hour period of stretching and relaxation for leaflet and chordal cells. Total GAGs present in the conditioned medium were calculated by subtracting GAGs present in the fresh medium. The proportions of specific GAGs (relative to total GAGs) were also determined for each day. Proteoglycan band intensities on days 2-7 were normalized to the band intensity of the same PG from day 1 (preconditioning). Band intensities on each electrophoresis gel and western blot were analyzed in duplicate and then averaged. Data was expressed as mean ± standard deviation. Two-way ANOVAs were performed to compare the different 24-hour periods and leaflet vs. chordal groups. Post-hoc Tukey tests were used to examine period-to-period differences. Paired t-tests were performed to compare GAGs secreted during sequential stretching and relaxation periods. The level of significance was chosen as α=0.05.
RESULTS
Collagen Gel Contraction
Gel contraction increased over the seven-day culture period. The collagen gels contracted faster during the first 2-3 days and more slowly afterwards. The maximum contraction at the end of 7 days was found to be 50%. The total percentage that a given gel contracted was slightly dependent on cell lines but the overall trend was the same (Fig. 2). The gels contracted more during relaxation periods as compared to stretching periods; this response was repeated with each cycle of stretching and relaxation. There was no significant difference in total percentage contraction between gels seeded with leaflet cells and chordal cells.
FIGURE 2.

Representative contraction profile of collagen gel seeded with mitral chordal cells over 7 day period. Data represented as mean ± standard deviation. PC- preconditioning, S- stretching, R- relaxation.
Total GAGs Secreted into Conditioned Medium
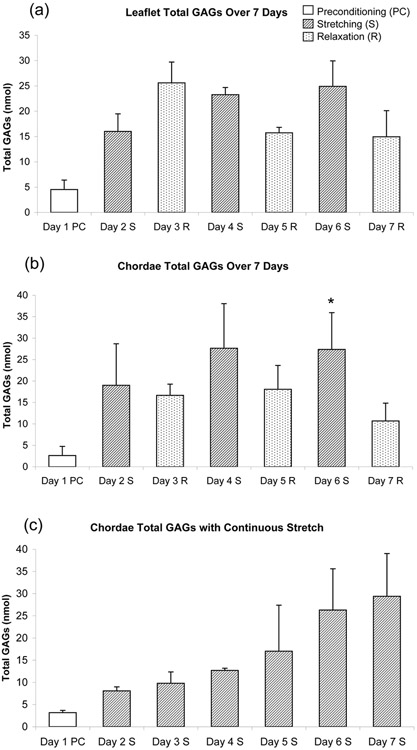
GAGs produced by the cells were either retained within the collagen gels or secreted into the surrounding medium. In our analysis only GAGs secreted into the medium over each 24-hour period of stretching or relaxation were measured. Culture medium from both leaflet and chordal cell-seeded collagen gels tended to show an up-regulation of total GAGs secreted during periods of stretching compared to periods of relaxation (p<0.02, chordal days 6 S vs. day 7 R, Fig. 3a,b). Although chordal cells demonstrated this pattern starting with the first stretch-relax periods, leaflet cells showed this repetitive response only for the last 2 cycles of stretching and relaxation. A significant difference between stretching and relaxation was found for chordal cells when the total GAGs released during each cycle were averaged together (p<0.004, Fig. 4). A similar trend was found for leaflet cells when only the last 2 cycles of stretching and relaxation were considered (p<0.004). The reproducibility of the analysis of bands on FACE gels was found to be within 7%. In the collagen gels continuously stretched for the entire culture duration (Fig. 3c), the amount of total GAGs secreted into the culture medium gradually increased over time.
FIGURE 3.
Total GAGs secreted into the medium of collagen gels seeded with cells isolated from regions of (a) mitral leaflet (n=4) and (b) mitral chordae (n=4). (c) Total GAGs secreted into the medium of continuously stretched collagen gels seeded with chordal cells (n=2). Data represented as mean ± standard deviation. *p<0.02 vs. Day 7 R.
FIGURE 4.

Total GAGs secreted into the medium combining cycles of stretching and relaxation (a) leaflet VICs (2 cycles, n=4) (b) chordal VICs (3 cycles, n=4). Data represented as mean ± standard deviation.
Specific GAG Classes Secreted into the Medium
The most common GAG classes found in heart valves are HA and unsulfated, 4-sulfated and 6-sulfated chondroitin/dermatan (0-S, 4-S and 6-S).12 We found all four of these GAGs in the medium of collagen gels; HA and 4-S GAGs were the most prominent GAGs secreted into the medium whereas 0-S and 6-S were present in lesser amounts. All four GAG classes tended to be upregulated during stretching periods compared to relaxation periods for chordal cells (Fig. 5), with significant differences found for HA (p<0.05, day 6 S vs. days 5 and 7 R) and 6-S (p<0.03, day 7 R vs. day 6 S). In general, the various GAG classes secreted by leaflet cells were also slightly upregulated with stretching (compared to relaxation) but only after 1 stretch-relax cycle (Fig. 6). When GAGs secreted with each stretch-relax cycle were averaged (3 cycles for chordal and 2 cycles for leaflet), the differences between stretching and relaxation periods were particularly evident. In the four collagen gels seeded with chordal cells, all GAG classes except 0-S were more abundant in stretching periods (p<0.008), whereas in the four gels seeded with leaflet cells only HA (p<0.04) and 4-S (p<0.03) were more abundant. When all VICs (leaflet and chordae, total n=8) were grouped together for combined ANOVA, HA (p<0.004), 6-S (p<0.01) and 4-S (p<0.01) were all upregulated during stretching periods.
FIGURE 5.
Various GAG classes secreted in to the medium of collagen gels seeded with mitral chordal cells (n=4). Data represented as mean ± standard deviation. *p<0.05 vs. Day 6 S, †p<0.03 vs. Day 7 R.
FIGURE 6.
Various GAG classes secreted in to the medium of collagen gels seeded with mitral leaflet cells (n=4). Data represented as mean ± standard deviation.
There were also general differences found between the medium surrounding collagen gels seeded with chordal cells (n=4) and leaflet cells (n=4). Compared to chordal cells, leaflet cells secreted more HA during relaxation periods (p<0.02, averaged cycles) and less 6-S during stretching periods (p<0.001, averaged cycles). Overall, secretion of 6-S GAGs by leaflet cells was higher than for chordal cells even when all stretching and relaxation cycles were averaged together (p<0.001). Continuous, as opposed to intermittent, stretching of collagen gels increased the absolute amounts of each of the individual GAG classes over the duration of culture, but these increases were consistent with the trend in total secreted GAGs noted in Figure 3c.
Proportions of Various GAG Classes
Proportions of GAG classes relative to total GAGs secreted into the medium for each cycle were different for leaflet and chordal cells (Fig. 7). Although there appears to be an up-regulation of total GAGs produced during stretching compared to relaxation (Fig. 3), the overall composition of GAGs secreted into the medium demonstrated some notable trends. The proportions of HA and 6-S decreased (both p<0.04) and 4-S increased (p<0.05) over the entire culture duration irrespective of stretching or relaxation for collagen gels seeded with leaflet cells. For collagen gels seeded with chordal cells, a decrease in proportions of 6-S (p<0.05) over the culture duration was the only significant trend; the proportions of all other GAGs remained steady. Compared to chordal cells, leaflet cells secreted higher proportions of HA (p<0.002) and 4-S (p<0.03), and lower proportions of 6-S (p<0.001) when all stretching and relaxation cycles were averaged together. Interestingly, the continuously stretched collagen gels showed that the secreted GAGs contained a very high proportion of HA (50-75%) compared to the intermittently stretched and relaxed gels (15-30%).
FIGURE 7.

Proportions of various GAGs in the medium of collagen gels seeded with cells isolated from (a) leaflet (n=4), *p<0.04 vs. Days 5-7, †p<0.04 vs. Days 3-7, ‡p<0.05 vs. Days 5-7 and (b) chordae (n=4), *p<0.04 vs. Days 4-7, †p<0.05 vs. Day 3 R. Data represented as mean ± standard deviation.
Proteoglycans
Proteoglycans are another major component of ECM that are secreted by valve cells. We analyzed decorin, biglycan and versican, three PGs previously found in heart valve tissues.10 All three PGs were present in the conditioned medium of collagen gels seeded with either leaflet or chordal cells. Overall, PGs secreted during stretching and relaxation showed promising trends but many of these never attained statistical significance. PGs secreted by chordal cells generally increased with each stretching cycle compared to relaxation (Fig. 8), although the only statistically significant difference was found for biglycan secretion by chordal cells (p=0.035). Similar to GAG secretion, leaflet cells also showed, albeit weakly, delayed responsiveness for PG secretion (stretch-relax pattern apparent after 1 cycle only).
FIGURE 8.
Decorin, biglycan and versican present in the medium of collagen gels seeded with leaflet and chordal cells. PG band intensities on western blots were normalized to preconditioning (Day 1 PC). Data represented as mean ± standard deviation. The only statistically significant difference (p=0.035) was found for biglycan secretion by chordal cells by combining the cycles of stretching and relaxation.
ECM and Cells in Collagen Gels After Stretching Protocol
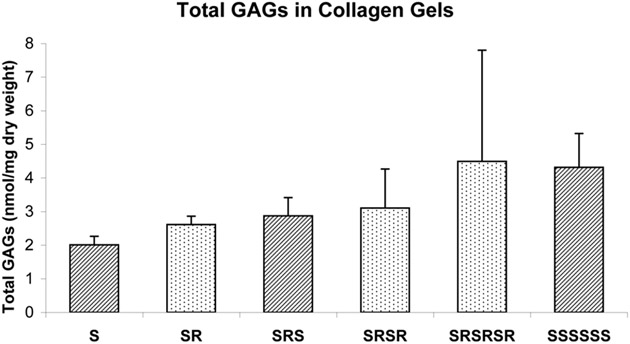
The identities and abundances of GAG classes, PGs, collagen and DNA within the collagen gels were measured and normalized to dry gel weight at the end of stretching and relaxation protocol. The amount of total GAGs retained within the chordal cell-seeded collagen gels gradually increased with each additional 24 hour cycle, whether stretching or relaxation occurred (Figure 9). However, the proportions of various classed of retained GAGs remained consistent during the various stretching protocols, as found in the changes of culture medium from the chordal cell-seeded collagen gels. After 7 days, the amount of total GAGs retained within the continuously stretched collagen gels was comparable to the final amount retained within the intermittently stretched and relaxed gels. Greater proportions of 4-S and 6-S GAGs were found in the collagen gels (94.2±7.3% of total GAGs, n=17) compared to conditioned medium (63.2±6.3% of total GAGs, n=40, p<0.0001).
FIGURE 9.
Total GAGs present within collagen gels (seeded with chordal cells only, n=2 except SRSRSR (n=4)) after the stretching protocol. Data represented as mean ± standard deviation. S- stretching, R- relaxation.
The amounts of PGs in the collagen gels did not show any specific trends though they too increased in abundance over time (data not shown). Interestingly, at the end of the culture period, the amount of PGs within the continuously stretched gels was lower than in the intermittently stretched and relaxed gels (data not shown), even some of the gels subjected to a partial culture period (i.e., SRS, SRSR). The presence of the PGs decorin and biglycan within the collagen gels was confirmed by immunostaining, which showed that these PGs were evenly distributed throughout the gel (Figure 10).
FIGURE 10.
Images of collagen gels immunohistochemically stained for decorin and biglycan. Control was prepared without primary antibodies. Scale bar represents 200 μm.
The collagen content remained almost the same after the 7-day period, compared to initial amounts used for collagen gel preparation, for both chordal and leaflet collagen gels. However, DNA content in collagen gels was reduced to nearly 50% at the end of 7 days for both leaflet (from 308±30 μg to 140±26 μg) and chordal cells (from 392±8 μg to 180±76 μg).
DISCUSSION
In this study, we used a novel custom-designed bioreactor to perform the first characterization of GAG and PG secretion by VICs subjected to cyclic strains. We found that VICs from both leaflet and chordae secrete abundant GAGs (in similar total magnitudes) into the culture medium of collagen gels, confirming our previous static tension model.10 Repetitive stretching and relaxation periods demonstrated that these cells respond reversibly to mechanical strains. Mitral chordal VICs showed stretching-induced upregulation of total GAGs secreted into the medium followed by a relaxation-induced decrease in GAGs. Mitral leaflet VICs showed the same trends as chordal VICs but it was only apparent after at least one cycle. This same delay of upregulation with stretching was also observed for the secretion of the PGs decorin and biglycan. This interesting difference between leaflet and chordal VICs may be due to a more dramatic dissimilarity between their in vivo conditions and our 3D model, or it may be due to inherent phenotypic differences between the cell types. Chordae tendineae in the mitral valve normally experience uniaxial tension and undergo dramatic remodeling (both mechanically and biochemically) in pathological conditions.11,12 Also, research in our lab has shown that mitral chordal cells have approximately 50% higher levels of smooth muscle α-actin expression than leaflet cells.4 Based on findings in this study, we propose that VICs from chordae may be particularly responsive to mechanical strains.
The various GAG classes were also reversibly modulated by cyclic stretch. The most abundant GAG class found in the medium was 4-S; this GAG is associated with the PGs decorin and biglycan and is the most abundant GAG (representing approximately 50% of total GAGs) in native chordae, which experience tension during the cyclic closure of the valve.11,12 All GAG types (except 0-S) had increased secretion during stretching although this trend was only apparent in the leaflet 3D cultures after a delay (similar to total GAGs). In contrast to the trends displayed by the GAG amounts, the proportions of various GAG classes did not show specific upregulation and downregulation patterns during stretching and relaxation periods. This trend of increase of GAG secretion during stretching but finding no change in GAG composition is supported by reports in other cell lines, such as endothelial cells subjected to shear stresses.8 Our finding of a statistically significant increase in the amount of 6-S GAGs during stretching is supported by the similar findings of Leung et al., who tested arterial smooth muscle cells in 3D collagen constructs.26
Because 4-S and 6-S GAGs tend to be found on different PGs that are predominantly located in tensile and compressive loading regions of the mitral valve,10 respectively, the relative abundance of 4-S and 6-S is an indicator of the type of loading experienced. For leaflet cells, the proportions of HA and 6-S decreased and 4-S increased during the 7 day period irrespective of stretching and relaxation. For chordal cells, the proportions of 6-S decreased each day. Therefore, the ratio of 4-S to 6-S for both leaflet and chordal cells increased with each day regardless of cyclic stretching or relaxation conditions. Interestingly, this 4-S/6-S ratio was much higher for leaflet cells than chordal cells because over time the leaflet cells secreted more 4-S GAGs into the medium compared to 6-S GAGs. We speculate that application of cyclic tensile strains caused the leaflet cells to reverse their in vivo GAG synthesis pattern (which is normally more 6-S and less 4-S in native leaflet tissues).10 The interesting finding that the proportion of HA in the continuously stretched collagen gels was much higher than the HA proportion in the stretched and relaxed gels underscores that further investigation is needed regarding the mechanically stimulated regulation of HA synthesis and degradation within native and engineered heart valves. Overall, this study provides guidance for constructing engineered tissues in which a tensile environment will induce specific GAG or PG production.
These data have also provided an in vitro confirmation that leaflet VICs will alter their proportional secretion of GAG classes when exposed to cyclic tension. This trend would support the remodeling of the myxomatous mitral valve leaflets that is believed to occur following surgical repair (when normal leaflet loading is restored) as well as the fibrotic-type remodeling found in mitral valves in heart failure (when their tensile loading increases). The finding that chordal cells responded the most rapidly to cyclic strains may be related to reports that myxomatous degeneration affects chordae with greater severity (i.e., matrix changes and loss of mechanical integrity) than this process affects leaflets.1,2,11,12 The ability of the leaflet cells to adapt gradually to the tensile strain environment by producing different GAG classes may explain why the leaflet tissues (in contrast to chordae) are less prone to rupture during myxomatous degeneration. Although the actual conditions of myxomatous disease are far more complex than the variables have been investigated here, our data can be interpreted to draw several conclusions that are relevant to in vivo and pathological conditions.
This is the first study to apply cyclic strains to valve cells to modulate GAG and PG synthesis, yet our results are quite consistent with previous investigations of other cell types.6 In a time-dependent study, cyclic strains applied to SMCs induced GAG/PG synthesis within 24 hours,25 which supports our selection of 24-hour periods of stretching and relaxation. That same study reported that cyclic strain increased the abundance of chondroitin/dermatan sulfate GAGs and the PGs versican and biglycan in the medium and cell layer but decreased decorin synthesis; clearly all GAGs and PGs are not mechanically regulated in the same manner, which befits their diverse biological roles. Biaxial cyclic strains applied to SMCs cultured on 2D elastic membranes also increased the expression of syndecan-4, a cell surface PG.27 Collagen XII secretion by fibroblasts has been shown to accumulate in the medium of statically stretched collagen gels after 24 hours; whereas static relaxation for another 24 hours decreased secretion.35 This response was repeatable for up to 3 cycles of stretching and relaxation, which has excellent agreement with our findings. Hence, this study demonstrated that the valve cells (especially chordal) are quickly responsive to cyclic mechanical strains and that response is reversible and repeatable.
Limitations to this study include the large standard deviations in the data, which we attribute to the variability that arises in the analysis of bands on FACE gels and western blots. Also, the significant loss of cells during the culture duration may have caused slight variability in ECM secretion between different collagen gels; however, these cell results are comparable to other reports of cell-seeded collagen gels.40 The reduced cell number in our engineered tissues suggests that many of these cells underwent apoptosis during the culture period, as reported in another time-dependent study.13,40 It appears that the sample size that was able to detect differences in GAGs is not sufficient to detect changes in PGs, which may be due to the less quantitative methodologies of blot analysis. The levels of all PGs increased during stretching and relaxation periods compared to preconditioning; this increase limited our ability to detect changes in versican secretion since the magnitude of the versican levels, even after normalizing to day 1, were highly variable between gels. Future investigations on the time-dependent ECM synthesis and turnover by VICs within collagen gels may provide more mechanistic information. Finally, our 3D model used the same biaxial construct format for both chordal and leaflet cells even though these cells experience different strain patterns in vivo. In addition, the magnitude of stretch experienced by the embedded cells may have varied somewhat across the different regions of the gel, due to its geometry. However, our rationale was that providing the same collagen gel design conditions (gel volume, medium volume, cell number) for both leaflet and chordal cells would permit the most direct comparison of the effect of mechanical strains. Moreover, our previous studies on VIC-seeded collagen gels grown under static tension showed that regional differences in GAG and PG production across the collagen gel were smaller than the differences caused by the presence or absence of strain,13,14 which provided motivation for the stretching regimens used in this study.
CONCLUSIONS
In conclusion, we have shown that GAG secretion by VICs seeded within 3D collagen gels is dependent on the presence or absence of cyclic strains. GAG secretion was significantly upregulated during cyclic stretch and downregulated during relaxation. The proportional composition of distinct GAG classes remained fairly consistent for chordal cells but tended to change for leaflet cells, which reflects the leaflet VICs’ adaptation to high cyclic tensile strains. We have also shown that the chordal cells respond to cyclic strains more rapidly than leaflet cells, which is an exciting finding given that chordal regions undergo most dramatic changes during myxomatous degeneration. Overall, this study improves our understanding of the role of mechanical strains on GAG and PG synthesis in the remodeling of mitral valve as well as other native and engineered tissues. This study also provides valuable information for tissue engineering research and applications in which mechanical strains can be used to regulate ECM synthesis and organization.
ACKNOWLEDGEMENT
This project was funded by the Whitaker Foundation, the Rice University Brown Foundation Undergraduate Research Internship program, and the American Heart Association Ohio Valley Affiliate Summer Undergraduate Research Fellowship.
REFERENCES
- 1.Barber JE, Kasper FK, Ratliff NB, Cosgrove DM, Griffin BP and Vesely I. Mechanical properties of myxomatous mitral valves. J Thorac Cardiovasc Surg 122:955–962, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Barber JE, Ratliff NB, Cosgrove DM 3rd, Griffin BP and Vesely I. Myxomatous mitral valve chordae. I: Mechanical properties. J Heart Valve Dis 10:320–324, 2001. [PubMed] [Google Scholar]
- 3.Bianco P, Fisher LW, Young MF, Termine JD and Robey PG. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem 38:1549–1563, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Blevins TL, Peterson SB, Lee EL, Bailey AM, Frederick JD, Huynh TN, Gupta V and Grande-Allen KJ. Mitral valvular interstitial cells demonstrate regional, adhesional, and synthetic heterogeneity. Cells Tissues Organs 187:113–122, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butcher JT and Nerem RM. Porcine aortic valve interstitial cells in three-dimensional culture: Comparison of phenotype with aortic smooth muscle cells. J Heart Valve Dis 13:478–485; discussion 485-476, 2004. [PubMed] [Google Scholar]
- 6.Chiquet M, Matthisson M, Koch M, Tannheimer M and Chiquet-Ehrismann R. Regulation of extracellular matrix synthesis by mechanical stress. Biochem Cell Biol 74:737–744, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P and Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic ehlers-danlos-like changes in bone and other connective tissues. J Bone Miner Res 17:1180–1189, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Elhadj S, Mousa SA and Forsten-Williams K. Chronic pulsatile shear stress impacts synthesis of proteoglycans by endothelial cells: Effect on platelet aggregation and coagulation. J Cell Biochem 86:239–250, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD and Vink H. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 290:H458–452, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Grande-Allen KJ, Calabro A, Gupta V, Wight TN, Hascall VC and Vesely I. Glycosaminoglycans and proteoglycans in normal mitral valve leaflets and chordae: Association with regions of tensile and compressive loading. Glycobiology 14:621–633, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Grande-Allen KJ, Griffin BP, Calabro A, Ratliff NB, Cosgrove DM 3rd and Vesely I. Myxomatous mitral valve chordae. II: Selective elevation of glycosaminoglycan content. J Heart Valve Dis 10:325–332; discussion 332-323, 2001. [PubMed] [Google Scholar]
- 12.Grande-Allen KJ, Griffin BP, Ratliff NB, Cosgrove DM and Vesely I. Glycosaminoglycan profiles of myxomatous mitral leaflets and chordae parallel the severity of mechanical alterations. J Am Coll Cardiol 42:271–277, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Gupta V, Werdenberg JA, Mendez JS and Grande-Allen KJ. Influence of strain on proteoglycan synthesis by valvular interstitial cells in three-dimensional culture. Acta Biomaterialia 4:88–96, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Gupta V, Werdenberg JA, Blevins TL and Grande-Allen KJ. Synthesis of glycosaminoglycans in differently loaded regions of collagen gels seeded with valvular interstitial cells. Tissue Eng 13:41–49, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hamada M, Kusuyama Y, Nishio I, Ura M, Takeda J, Hano T and Masuyama Y. Effect of centrifugal force and catecholamines on glycosaminoglycans synthesis of vascular smooth muscle cells in culture. Atherosclerosis 83:147–153, 1990. [DOI] [PubMed] [Google Scholar]
- 16.Hardingham TE and Fosang AJ. Proteoglycans: Many forms and many functions. Faseb J 6:861–870, 1992. [PubMed] [Google Scholar]
- 17.Hascall VC, Calabro A, Midura RJ and Yanagishita M. Isolation and characterization of proteoglycans. Methods Enzymol 230:390–417, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Iozzo RV. Matrix proteoglycans: From molecular design to cellular function. Annu Rev Biochem 67:609–652, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Isogai Z, Shinomura T, Yamakawa N, Takeuchi J, Tsuji T, Heinegard D and Kimata K. 2B1 antigen characteristically expressed on extracellular matrices of human malignant tumors is a large chondroitin sulfate proteoglycan, PG-M/versican. Cancer Res 56:3902–3908, 1996. [PubMed] [Google Scholar]
- 20.Kanda K and Matsuda T. Mechanical stress-induced orientation and ultrastructural change of smooth muscle cells cultured in three-dimensional collagen lattices. Cell Transplant 3:481–492, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Ku CH, Johnson PH, Batten P, Sarathchandra P, Chambers RC, Taylor PM, Yacoub MH and Chester AH. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res 71:548–556, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Kunzelman KS, Cochran RP, Murphree SS, Ring WS, Verrier ED and Eberhart RC. Differential collagen distribution in the mitral valve and its influence on biomechanical behaviour. J Heart Valve Dis 2:236–244, 1993. [PubMed] [Google Scholar]
- 23.Kunzelman KS, Quick DW and Cochran RP. Altered collagen concentration in mitral valve leaflets: Biochemical and finite element analysis. Ann Thorac Surg 66:S198–205, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Labarca C and Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem 102:344–352, 1980. [DOI] [PubMed] [Google Scholar]
- 25.Lee RT, Yamamoto C, Feng Y, Potter-Perigo S, Briggs WH, Landschulz KT, Turi TG, Thompson JF, Libby P and Wight TN. Mechanical strain induces specific changes in the synthesis and organization of proteoglycans by vascular smooth muscle cells. J Biol Chem 276:13847–13851, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Leung DY, Glagov S and Mathews MB. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science 191:475–477, 1976. [DOI] [PubMed] [Google Scholar]
- 27.Li L and Chaikof EL. Mechanical stress regulates syndecan-4 expression and redistribution in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 22:61–68, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Merrilees MJ, Merrilees MA, Birnbaum PS, Scott PJ and Flint MH. The effect of centrifugal force on glycosaminoglycan production by aortic smooth muscle cells in culture. Atherosclerosis 27:259–264, 1977. [DOI] [PubMed] [Google Scholar]
- 29.Raman R, Sasisekharan V and Sasisekharan R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem Biol 12:267–277, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Rothenburger M, Volker W, Vischer P, Glasmacher B, Scheld HH and Deiwick M. Ultrastructure of proteoglycans in tissue-engineered cardiovascular structures. Tissue Eng 8:1049–1056, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Scherberich A and Beretz A. Culture of vascular cells in tridimensional (3-D) collagen: A methodological review. Therapie 55:35–41, 2000. [PubMed] [Google Scholar]
- 32.Schonherr E, Hausser H, Beavan L and Kresse H. Decorin-type I collagen interaction. Presence of separate core protein-binding domains. J Biol Chem 270:8877–8883, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Taylor PM, Allen SP, Dreger SA and Yacoub MH. Human cardiac valve interstitial cells in collagen sponge: A biological three-dimensional matrix for tissue engineering. J Heart Valve Dis 11:298–306; discussion 306–297, 2002. [PubMed] [Google Scholar]
- 34.Taylor PM, Batten P, Brand NJ, Thomas PS and Yacoub MH. The cardiac valve interstitial cell. Int J Biochem Cell Biol 35:113–118, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Trachslin J, Koch M and Chiquet M. Rapid and reversible regulation of collagen XII expression by changes in tensile stress. Exp Cell Res 247:320–328, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Warnock JN, Burgess SC, Shack A and Yoganathan AP. Differential immediate-early gene responses to elevated pressure in porcine aortic valve interstitial cells. J Heart Valve Dis 15:34–41; discussion 42, 2006. [PubMed] [Google Scholar]
- 38.Xing Y, He Z, Warnock JN, Hilbert SL and Yoganathan AP. Effects of constant static pressure on the biological properties of porcine aortic valve leaflets. Ann Biomed Eng 32:555–562, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Xing Y, Warnock JN, He Z, Hilbert SL and Yoganathan AP. Cyclic pressure affects the biological properties of porcine aortic valve leaflets in a magnitude and frequency dependent manner. Ann Biomed Eng 32:1461–1470, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Zhu YK, Umino T, Liu XD, Wang HJ, Romberger DJ, Spurzem JR and Rennard SI. Contraction of fibroblast-containing collagen gels: Initial collagen concentration regulates the degree of contraction and cell survival. In Vitro Cell Dev Biol Anim 37:10–16, 2001. [DOI] [PubMed] [Google Scholar]