Summary
Background
Despite the importance of accurate and rapid assessment of hydration status in patients with acute diarrhoea, no validated tools exist to help clinicians assess dehydration severity in older children and adults. The aim of this study is to validate a clinical decision support tool (CDST) and a simplified score for dehydration severity in older children and adults with acute diarrhoea (both developed during the NIRUDAK study) and compare their accuracy and reliability with current WHO guidelines.
Methods
A random sample of patients aged 5 years or older presenting with diarrhoea to the icddr,b Dhaka Hospital in Bangladesh between Jan 30 and Dec 13, 2022 were included in this prospective cohort study. Patients with fewer than three loose stools per day, more than 7 days of symptoms, previous enrolment in the study, or a diagnosis other than acute gastroenteritis were excluded. Patients were weighed on arrival and assessed separately by two nurses using both our novel clinical tools and WHO guidelines. Patients were weighed every 4 h to determine their percent weight change with rehydration, our criterion standard for dehydration. Accuracy for the diagnosis of dehydration category (none, some, or severe) was assessed using the ordinal c-index (ORC). Reliability was assessed by comparing the prediction of severe dehydration from each nurse’s independent assessment using the intraclass correlation coefficient (ICC).
Findings
1580 patients were included in our primary analysis, of whom 921 (58·3%) were female and 659 (41·7%) male. The ORC was 0·74 (95% CI 0·71–0·77) for the CDST, 0·75 (0·71–0·78) for the simplified score, and 0·64 (0·61–0·67) for the WHO guidelines. The ICC was 0·98 (95% CI 0·97–0·98) for the CDST, 0·94 (0·93–0·95) for the simplified score, and 0·56 (0·52–0·60) for the WHO guidelines.
Interpretation
Use of our CDST or simplified score by clinicians could reduce undertreatment and overtreatment of older children and adults with acute diarrhoea, potentially reducing morbidity and mortality for this common disease.
Funding
US National Institutes of Health.
Introduction
Diarrhoea is the eighth leading cause of death globally and the fifth leading cause of death in low-income countries.1 Despite considerable progress in reducing early childhood deaths over the past 50 years, diarrhoea morbidity and mortality remains high in older children and adults with 5·7 billion cases and 1·1 million deaths globally in 2019.1 In many countries, epidemics of diarrhoeal disease are becoming more frequent due to the effects of climate change, conflict, and mass displacement.2 WHO warned of an “unprecedented” surge in cholera outbreaks in 2022, with epidemics reported in 30 countries and an overall case fatality ratio of nearly 2%—twice the acceptable limit and the highest in a decade.2 Although diarrhoea incidence varies by age and region, most adults and older children will experience at least one episode of diarrhoea annually.3 The vast majority of these episodes will follow a relatively benign course; however, approximately 5% of episodes in older children and adults, or about 285 million cases each year, will lead to moderate or severe disease requiring medical management.4 Evidence suggests that older people can be especially susceptible to diarrhoea morbidity and mortality.5
Although antibiotics can be useful in specific situations, appropriate rehydration remains the most important management strategy for acute diarrhoea in all populations.6,7 As the severity of dehydration from acute diarrhoea can vary widely, accurately assessing and managing dehydration remains the most crucial step in preventing morbidity and mortality.7-9 Patients with severe dehydration require immediate resuscitation with intravenous fluids in a hospital to prevent hypovolaemic shock, electrolyte derangements, and death; those with mild to moderate dehydration can be safely managed in an ambulatory setting with oral rehydration solution alone; and those with no dehydration require only guidance for continued oral intake and expectant management at home.7,10 Accurate assessment of dehydration status can thus improve the cost-effectiveness of diarrhoea management by reducing intravenous fluid and hospital bed usage while also reducing the morbidity and mortality that results from both overtreatment and undertreatment.
Despite the importance of accurate and rapid assessment of dehydration status in patients with acute diarrhoea, no validated tools exist to help clinicians assess dehydration severity in older children and adults. Although the Clinical Dehydration Scale has been validated in high-resource settings and the DeHydration: Assessing Kids Accurately (DHAKA) score has been validated in low-resource settings for children younger than 5 years, neither has been studied in older children or adults.11-14 Currently, the WHO Integrated Management of Adolescent and Adult Illness (IMAI) guidelines include an algorithm for determining the severity of dehydration in patients aged 5 years or older with acute diarrhoea, but this algorithm was adapted nearly verbatim from the WHO Integrated Management of Childhood Illness (IMCI) guidelines for children younger than 5 years and has never been validated in older children or adults.7 Clearly, differences in both adult physiology and diarrhoea aetiology might compromise the accuracy of any clinical diagnostic models developed for use in young children.15,16
In response, the recent Novel, Innovative Research for Understanding Dehydration in Adults and Kids (NIRUDAK, or “dehydrated” in Bangla) study empirically derived two new clinical diagnostic models (the full NIRUDAK and simplified NIRDAK models) for dehydration severity in patients aged 5 years or older with acute diarrhoea (appendix 2 p 2).17 Along with our previously validated DHAKA score for young children,13 these NIRUDAK models were incorporated into a mobile health (mHealth) clinical decision support tool (CDST) named FluidCalc to help frontline clinicians provide more appropriate, evidence-based management of this common yet deadly disease (appendix 2 p 2).18 The FluidCalc CDST also incorporates models to predict the specific fluid deficit of each patient and provides individualised guidance on fluid resuscitation. Finally, a numerical score was developed based on the simplified NIRUDAK model that could be implemented without the need for a smartphone (table 1). The primary aims of this study are to temporally validate this FluidCalc CDST and the simplified NIRUDAK score in patients aged 5 years or older with acute diarrhoea and compare their accuracy and reliability with the WHO IMAI algorithm. A secondary aim is to assess the usability of the FluidCalc CDST by providers.
Table 1:
14-point simplified NIRUDAK score
| Points | |
|---|---|
| Skin pinch | |
| Rapid | 0 |
| Slow | 2 |
| Very slow | 4 |
| Eye level | |
| Normal | 0 |
| Sunken | 2 |
| Respiration depth | |
| Normal | 0 |
| Deep | 2 |
| Urine output | |
| Normal | 0 |
| Decreased or dark | 1 |
| Minimal or none | 2 |
| Radial pulse | |
| Strong | 0 |
| Decreased | 1 |
| Absent | 4 |
Suggested scoring: <4=no dehydration, 4–6=some dehydration, >6=severe dehydration.
Methods
Study design and participants
Data were collected from Jan 30 to Dec 13, 2022, in a prospective cohort study of patients presenting to the icddr,b Dhaka Hospital, which provides free clinical services to about 200 000 patients annually from a catchment area of over 17 million people in Bangladesh.19 A random sample of patients aged 5 years or older presenting with acute diarrhoea were enrolled, regardless of illness severity. Patients with fewer than three loose stools per day, more than 7 days of symptoms, previous enrolment in the study, or a cause for diarrhoea other than acute gastroenteritis (COVID-19, inflammatory bowel disease, etc) were excluded.
Ethical approval for this study (NIRUDAK Study) was obtained from Rhode Island Hospital Institutional Review Board (1764819) and the icddr,b Ethical Review Committee (PR-21048).
Procedures
Study staff randomly selected patients for screening on arrival to Dhaka Hospital 24 h per day, 7 days per week by pulling either white (patient not selected for screening) or coloured (patient selected for screening) marbles from a black pouch as each patient arrived. The ratio of coloured marbles to white marbles in the black pouch ranged from 1:5 to 1:1 and was regularly adjusted during the course of the study to maintain a steady enrolment of 5–10 patients per day in order to avoid compromising the quality of data collection. If the patients randomly selected for screening met study eligibility criteria, research staff provided the patient or their parent or guardian with information about the goals, risks, and benefits of the study and obtained written consent in Bangla. For children aged 11–17 years, verbal or written assent was also obtained in addition to consent from their parent or guardian. Patients who could not read provided verbal consent and a thumb stamp on the consent form in lieu of their signature. Verbal or written consent was obtained for all enrolled patients.
After informed consent, patients were immediately weighed to the nearest 0·1 kg using an electronic Seca 952 chair or Seca 984 bed scale (Seca; Hamburg, Germany). Two study nurses, masked to each other’s clinical assessments, then assessed every enrolled patient using the full NIRUDAK model, the simplified NIRUDAK model, and the WHO IMAI algorithm (all signs for each model were recorded both on paper forms and entered into the mHealth CDST loaded on an Android phone by each nurse). Nurses then recorded the predicted dehydration category and fluid deficit for each model. Sex was self-reported by study participants, with “male” and “female” options provided on the case report forms. Appendix 2 provides detailed information on each model (appendix 2 p 2) and on the assessment methods for each clinical sign or symptom (appendix 2 pp 3-4; also included as information tabs within the FluidCalc CDST for quick provider reference).
Social and demographic information was also obtained from either the patient or their parent or guardian. After this initial assessment, all patients were managed according to standard icddr,b protocols by non-research study clinicians who did not have access to the CDST recommendations. Patients were weighed every 4 h on the same scale as they were initially weighed to determine their post-hydration stable weight. The CDST was used only at the patient’s initial assessment. CDST recommendations did not determine fluid administration at any point during the patient’s stay. Those who did not achieve a stable weight before discharge were called daily for up to 10 days until their diarrhoea resolved, then asked to return for a final weight check.
Study nurses also provided written consent before the start of the study and contributed their own data throughout the study period. Study nurses completed a demographic questionnaire (appendix 2 p 9) at the start of the study. In addition, they completed an anonymous, written survey immediately before and after their study training (see appendix 2 p 10) in order to evaluate the impact of training on the perceived usability of the mHealth CDST by providers. Nurses also completed this same survey after evaluating 10, 25, and 50 patients using the mHealth CDST in order to assess changes in its usability over time as nurses gained greater exposure to it. Completion of these surveys by the study nurses was both voluntary and anonymous.
Outcomes
Categorical variables were described using frequencies with percentages. Continuous variables with normal distribution were presented as means with SD or medians with IQR. Percent weight change with rehydration was the primary outcome and used as the criterion standard for dehydration severity, as it correlates almost perfectly with percent volume loss and has been recommended as the most practical criterion standard for assessing percent dehydration in paediatric, adult, and older patients by several review articles.11,20-22 Patients with dehydration will rapidly gain weight as they are rehydrated until they achieve their pre-illness weight, or stable weight, at which point they will stop gaining weight as their kidneys diurese excess fluid. For each patient, we calculated the primary outcome measure of dehydration category using the following formula:
For patients who did not achieve a stable weight before discharge, their final post-illness weight was used instead of their stable weight. We then categorised patients as having severe (>9%) dehydration, some (3–9%) dehydration, or no (<3%) dehydration based on current standards from the literature.13 Total fluid deficit on arrival for each patient was calculated by using the formula: fluid deficit=percent dehydration × stable weight
Statistical analysis
The CDST-based full NIRUDAK model, the simplified NIRUDAK score, and the WHO IMAI algorithm were assessed for their accuracy and reliability. Using the first nurse’s assessment, model discrimination (accuracy) for the prediction of dehydration category against the true dehydration category was calculated using the area under the receiver-operating characteristic (ROC) curve (AUC) for binary models and the similar ordinal c-index (ORC) for ordinal models (where 0·5 is no better than random chance and 1·0 represents a perfect model).23 We used bootstrapping with 1000 replicates to calculate a p value for comparison of the ORC across models. A p value of less than 0·05 was considered statistically significant. The accuracy of the CDST and the WHO algorithm for predicting the volume deficit of each patient on arrival was also calculated using the root mean squared error (RMSE) of each prediction, with greater RMSE suggesting lower accuracy. Of note, the WHO algorithm assigns a total fluid deficit of 100 cc/kg to all patients it classifies with severe dehydration and 75 cc/kg to all patients it classifies with some dehydration. The reliability was assessed by comparing the prediction of severe dehydration from each nurse’s independent assessment using the intraclass correlation coefficient (ICC).24 Test characteristics for the overall models (using probability cut-points along the ROC curves for the NIRUDAK models determined a priori by clinician focus groups in Bangladesh) and for individual clinical signs were also summarised.18 All statistical analyses were performed using R version 4.2.2.
The FluidCalc CDST architecture was based on a previously developed CDST that digitised WHO diarrhoeal disease management guidelines.25 The input page is configured with questions specific to the model(s) selected in the settings menu. The output page provides an assessment of percentage dehydration with an animated graph of the calculated fluid deficit, the recommended fluid rate for rehydration, and additional medication recommendations (ie, antibiotics and zinc; appendix 2 p 2). Agreement was calculated by comparing the data entered in the paper case report forms with that entered into the CDST by study nurses and uploaded to the server. Survey results were aggregated and summarised using percentages.
We used the methods described by Snell and colleagues for calculating the sample size for the validation of a clinical prediction model.26 Using the standard deviation of the linear predictor of 0·9 for the full NIRUDAK model and the 13% prevalence of severe dehydration found in our derivation study, a confidence width of plus or minus 0·04 for the validated model AUC, and a maximum loss to follow-up of 4%, a target enrolment of 1200 patients was planned for the study.17 Due to a lower-than-expected prevalence of severe dehydration in our study, the targeted sample was later expanded to 1600 before analysis began.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
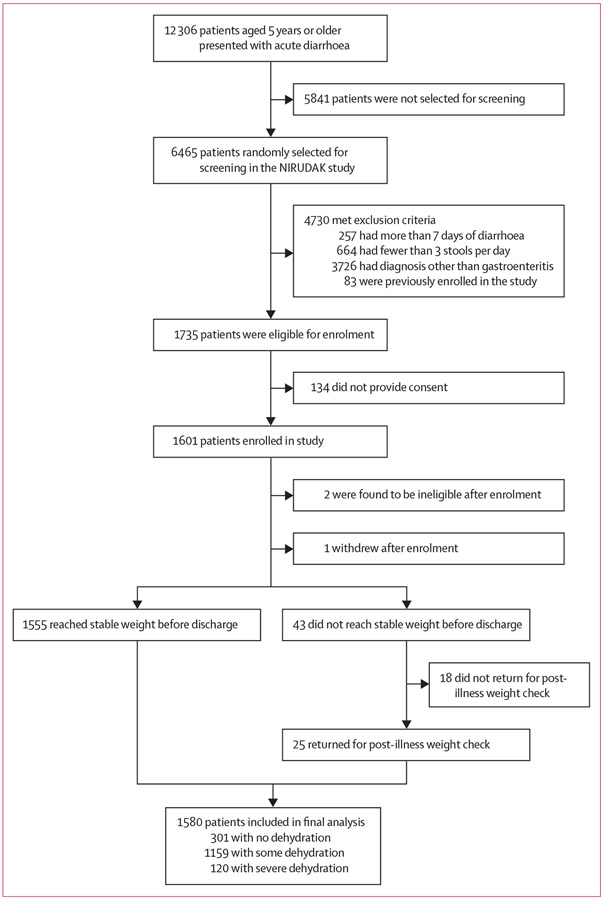
6465 patients were randomly selected for screening, of whom 1735 (26·8%) initially met eligibility criteria, 1601 (92·3%) provided consent, and 1580 (98·7%) had sufficient data for analysis (figure 1). Of these, 120 (7·6%) had severe dehydration by our criterion standard, 1159 (73·4%) had some dehydration, and 301 (19·1%) had no dehydration. The median age of participants was 28·0 years (IQR 21·0–39·0); 921 (58·3%) were female and 659 (41·7%) male. Table 2 provides sociodemographic and clinical characteristics of participants.
Figure 1:
Patient enrolment
Table 2:
Population demographics and baseline characteristics
| Overall (n=1580) | |
|---|---|
| Sociodemographic variables | |
| Age, years | |
| 5–10 | 10 (0·6%) |
| 11–20 | 358 (22·7%) |
| 21–30 | 563 (35·6%) |
| 31–40 | 351 (22·2%) |
| 41–50 | 176 (11·1%) |
| 51–60 | 89 (5·6%) |
| >60 | 33 (2·1%) |
| Sex | |
| Female | 921 (58·3%) |
| Male | 659 (41·7%) |
| Pregnancy status* | |
| Yes | 21 (1·3%) |
| No | 900 (57·0%) |
| Not applicable | 659 (41·7%) |
| Monthly household income, US$ | |
| First quintile: 0–92·47 | 441 (27·9%) |
| Second quintile: 92·48–138·71 | 497 (31·5%) |
| Third quintile: 138·72–147·96 | 40 (2·5%) |
| Fourth quintile: 147·97–184·94 | 303 (19·2%) |
| Fifth quintile: 184·95–924·72 | 299 (18·9%) |
| Years of patient education† | 5·0 (2·0–9·0) |
| Home location | |
| Urban | 1159 (73·4%) |
| Rural or suburban | 421 (26·6%) |
| Time between arrival and final weighing | 18 h 9 min (17 h 7 min) |
| Clinical variables | |
| Nutritional status (MUAC)‡ | |
| Severe acute malnutrition | 10 (0·63%) |
| Moderate acute malnutrition | 160 (10·1%) |
| No acute malnutrition | 1410 (89·2%) |
| Hours of diarrhoea | 14 (10·0–24·0) |
| Episodes of diarrhea | 22·9 (9·2) |
| Presence of watery stool§ | 1443 (91·3%) |
| Presence of bloody stool | 6 (0·4%) |
| Outcome ¶ | |
| Dehydration category | |
| Severe dehydration | 120 (7·6%) |
| Some dehydration | 1159 (73·4%) |
| No dehydration | 301 (19·1%) |
| Fluid deficit, L | 2·64 (1·5) |
Data are n (%), mean (SD), or median (IQR). MUAC=mid-upper arm circumference.
Pregnancy status is self-reported.
For patients younger than 16 years, mothers’ education is used.
For patients aged 5–9 years, severe acute malnutrition was defined as a MUAC measurement <135 mm, moderate acute malnutrition was 135–145 mm, and no acute malnutrition was >145 mm. For patients aged 10–14 years, severe acute malnutrition was defined as a MUAC measurement <160 mm, moderate acute malnutrition was 160–185 mm, and no acute malnutrition was >185 mm. For patients aged 15 years or older, severe acute malnutrition was defined as a MUAC measurement <185 mm, moderate acute malnutrition was 185–210 mm, and no acute malnutrition was >210 mm.27
Defined as clear or rice water colour.
Outcome measurements are based on our criterion standard of percent weight change; a tabulation of dehydration classification based on each model is provided in appendix 2 (p 4).
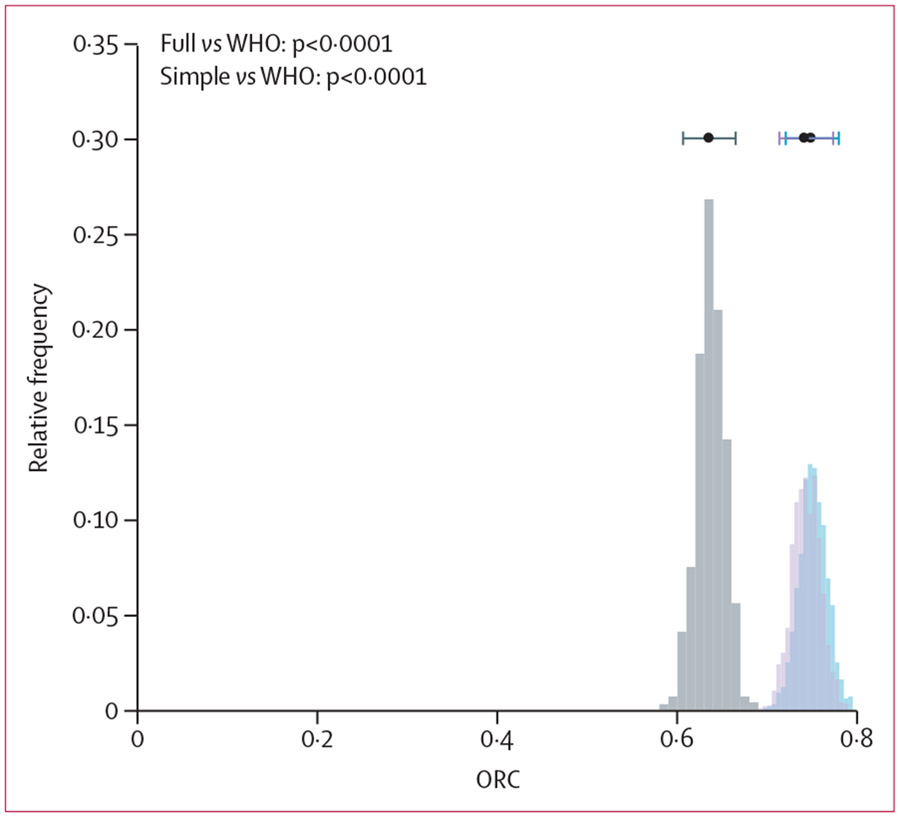
The CDST-based full NIRUDAK model had an ORC of 0·74 (95% CI 0·71–0·77), the simplified NIRUDAK score had an ORC of 0·75 (0·71–0·78), and the WHO algorithm had an ORC of 0·64 (0·61–0·67). As shown in figure 2, the ORC for both the full NIRUDAK model and the simplified NIRUDAK score were significantly greater than for the WHO algorithm when compared over 1000 bootstrap iterations (p<0·0001).
Figure 2: Comparison of ORC computed for NIRUDAK full model, NIRUDAK simplified model, and WHO IMAI algorithm.
Each histogram shows the distribution of ORC values derived from 1000 bootstrap samples. The bar at the top of each histogram gives the mean ORC and a 95% CI derived from the bootstraps. The grey bars signify the WHO IMAI algorithm, the light purple bars signify the full NIRUDAK model, and the blue bars signify the simplified NIRUDAK model; the dark purple colour denotes where the light purple and blue bars overlap. IMAI=Integrated Management of Adult and Adolescent Illnesses. ORC=ordinal c-index.
In a binary analysis that evaluated each diagnostic tool’s ability to discriminate between severe dehydration and the absence of severe dehydration, the CDST-based full NIRUDAK model had an AUC of 0·74 (95% CI 0·70–0·79), the simplified NIRUDAK score had an AUC of 0·74 (0·70–0·79), and the WHO algorithm had an AUC of 0·64 (0·60–0·68; see ROC curves in appendix 2 p 6). The CDST had a sensitivity of 0·82 and specificity of 0·52 for detecting severe dehydration, the simplified NIRUDAK score had a sensitivity of 0·83 and specificity of 0·52, and the WHO algorithm had a sensitivity of 0·77 and specificity of 0·51.
In a binary analysis that evaluated each diagnostic tool’s ability to discriminate between any dehydration (some or severe) and no dehydration, the CDST-based full NIRUDAK model had an AUC of 0·69 (95% CI 0·65–0·72), the simplified NIRUDAK score had an AUC of 0·69 (0·66–0·72), and the WHO algorithm had an AUC of 0·56 (0·54–0·59; see ROC curves in appendix 2 p 6). The CDST had a sensitivity of 0·86 and specificity of 0·35 for detecting any dehydration, the simplified NIRUDAK score had a sensitivity of 0·90 and specificity of 0·30, and the WHO algorithm had a sensitivity of 0·93 and specificity of 0·19. The test characteristics of the individual clinical variables included in each of these tools are shown in appendix 2 (p 7).
The FluidCalc CDST had a smaller (more accurate) RMSE of 1·68 L (95% CI 1·62–1·74), for predicting the total fluid deficit for each patient using the full NIRUDAK model and 1·60 L (1·54–1·65) using the simplified model, compared with 2·40 L (2·30–2·43) for the WHO algorithm (see scatterplot in appendix 2 p 8).
The median time between the nursing assessments for each patient was 6 min (IQR 6–7) with a range of 2–15 min. The CDST-based full NIRUDAK model had an ICC of 0·98 (95% CI 0·97–0·98) for detecting severe dehydration, the simplified NIRUDAK score had an ICC of 0·94 (0·93–0·95), and the WHO algorithm had an ICC of 0·56 (0·52–0·60).
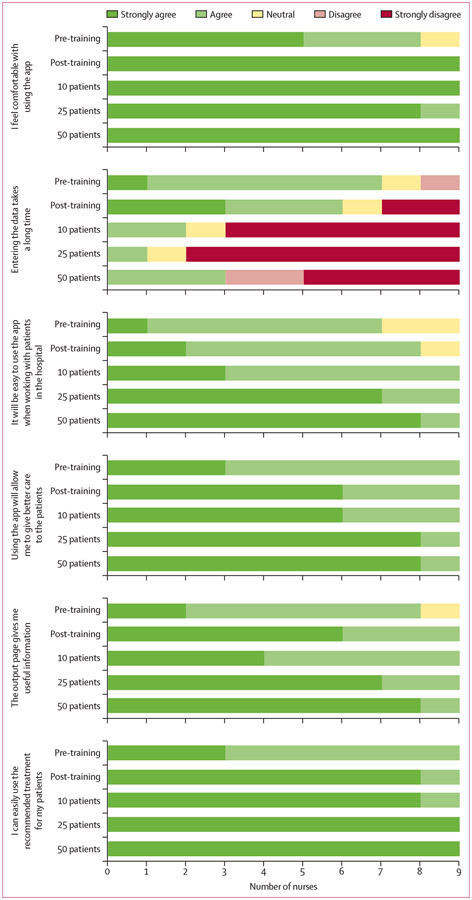
A total of nine nurses, ranging in age from 18 to 34 years, consented and completed all surveys. The level of nursing experience for all participants ranged from 1 to 4 years. All nurses had experience with using a smartphone in the past, with a range of 2 to 9 years of smartphone experience. The mean time to complete mHealth CDST data entry and obtain a fluid recommendation based on the full NIRUDAK model was 59 s for each patient. Comparing provider data entered into the mHealth CDST with that recorded in the paper case report forms demonstrated 99·8% agreement. Overall, the surveys revealed that receptiveness to use of the CDST was high at the start of the study and increased with usage (figure 3).
Figure 3:
Results of usability surveys showing the number of nurses agreeing or disagreeing with each statement based on their level of experience with the mobile health clinical decision support tool
Discussion
This study provides the first temporal validation of clinical diagnostic tools empirically derived for the assessment and management of dehydration in adults and children aged 5 years or older with acute diarrhoea. In particular, this study has temporally validated the full NIRUDAK model, integrated into an mHealth CDST, and a simplified NIRUDAK model, which can be applied using the mHealth CDST or as a paper-based numerical score. This study also demonstrates that each of these tools predicts a patient’s dehydration status with greater accuracy and reliability than the current WHO IMAI algorithm.
Although diarrhoea is well recognised as a major cause of disability and death in children younger than 5 years, it also remains a major cause of disability and death in older children and adults, but less attention has been paid to its assessment and management in this population.1,3,4 Of the 5·7 billion cases of diarrhoea each year in older children and adults, 5%, or 285 million cases, will lead to moderate or severe disease requiring medical management; 0·05%, or 2·85 million cases, will progress to severe dehydration requiring hospitalisation and intravenous fluids; and 1·1 million cases will result in death.1,3,4 Based on these estimates of diarrhoea frequency, if shown to be generalisable beyond Bangladesh and applied universally, our new tools would detect an additional 142 500 to 171 000 patients with severe dehydration each year that would be missed by the WHO IMAI algorithm, while preventing the overtreatment of 627 million to 912 million patients each year without any dehydration.1,3,4
The current WHO IMAI guidelines, which constitute the standard of care for diarrhoea management in most low-income and middle-income countries, were taken almost verbatim from the previously developed WHO IMCI guidelines without any empirical studies to show that they were valid in older children and adults. Moreover, the dehydration assessment algorithm within the diarrhoea care section of both the IMCI and IMAI guidelines has not been updated in over 20 years, despite a preponderance of new data suggesting that they do not provide the most accurate or reliable assessment of dehydration status in patients of any age.7,13,17,28
While all three of these tools include clinical signs in common, they also have important differences. In particular, two of the four signs used in the WHO algorithm have serious limitations. Previous studies have found mental status to be an accurate predictor of dehydration severity in children younger than 5 years.13 However, previous research has demonstrated that in adults and older children, reduced mental status lacks sensitivity for detecting dehydration compared with other clinical signs and symptoms.17 Thirst, on the other hand, has not been shown to be an accurate or reliable predictor of dehydration status in either children or adults.13,17 The low reliability of thirst likely contributed to the overall decreased reliability of the WHO algorithm in this study. Both the mHealth CDST and simplified NIRUDAK score would be considered to have excellent reliability, while the WHO IMAI algorithm does not even meet the threshold for good reliability.29
The two other clinical signs included in the WHO IMAI algorithm, skin turgor and sunken eyes, do appear to have reasonable accuracy and reliability in this validation study and are included in both the full and simplified versions of the NIRUDAK model. Although few previous studies have explored these clinical signs as predictors of dehydration outside of the paediatric population, their underlying pathophysiology in adults is likely similar to that in children. As noted by McGee and colleagues, the protein elastin, which is responsible for skin recoil, is affected by moisture content, so that a small decrease in water volume leads to a large increase in recoil time, which can be measured by pinching the skin and counting the seconds for it to return to its normal position.22 With regard to sunken eyes, cellular dehydration and interstitial space dehydration are presumed to be the underlying cause, although no specific studies could be found on the pathogenesis of this finding.22 Similarly, while elastin deteriorates with age, no previous studies have evaluated whether age moderates the effect of dehydration on skin recoil.22 In the derivation study for the NIRUDAK models, however, skin pinch performed similarly well as a predictor of dehydration in all age groups, including in patients older than 60 years.17
The accuracy and reliability of the full and simplified NIRUDAK models were similar in this validation study, so clinicians can confidently use the simplified NIRUDAK score if a smartphone is not available. When available, however, use of the FluidCalc CDST has some benefits. It can reduce calculation error by allowing clinicians to select clinical signs from a drop-down menu instead of adding up points manually to obtain a numerical score as they assess a patient. In addition, unlike both the numerical score and the WHO algorithm, which only predict the category of dehydration, the FluidCalc CDST provides a prediction of the estimated volume deficit of each patient, allowing a more individualised approach to fluid resuscitation for patients with some or severe dehydration. Finally, the FluidCalc CDST provides specific guidance to clinicians, including clear descriptions and videos of how to assess each clinical sign, specific instructions for clinicians on millilitres per hour or drops per minute that can be used for initial fluid management, and recommendations on when patients should be transferred to higher levels of care.
This study enrolled patients presenting to a single health facility in Bangladesh, which might not be representative of all health facilities globally. Previous research, however, has found the most common causes of diarrhoea for patients presenting to the icddr,b Dhaka Hospital to be similar to those for patients presenting to other facilities in Bangladesh and worldwide.5,15,30 Due to its strong reputation and free provision of care, icddr,b draws patients from both urban Dhaka and surrounding suburban and rural communities, as well as from all levels of income and education (table 2).19 Although icddr,b is a private research hospital, only a small percentage of its patients are transferred from other health-care facilities, so the patients enrolled in this study are more similar to those presenting to primary care facilities than those presenting to referral hospitals. Data were not collected on antibiotic use before arrival, which could have affected diarrhoea severity. While clinical nurses at icddr,b are likely to be more skilled in assessing dehydration, the research nurses hired for this study came from outside the icddr,b clinical nursing pool and were selected to be representative of general practice nurses in other low-resource settings. These nurses received training in usage of the FluidCalc CDST before the start of the study, although the app also includes similar provider training videos. Research nurses were paid for their work collecting data for this study, which might have affected their opinions regarding the usability of the mHealth CDST, although completion of the individual surveys by the research nurses at various time points was both voluntary and anonymous. This study was powered to evaluate the accuracy of each model in the full population of patients aged 5 years or older with acute diarrhoea. Future studies will be required to evaluate their accuracy in specific subgroups of patients, including patients with acute malnutrition.
This study presents the first temporal validation of an mHealth CDST and numerical clinical score for use in determining dehydration severity and management in patients aged 5 years or older with acute diarrhoea. It has also shown both these clinical diagnostic tools to be more accurate and reliable than the current WHO IMAI guidelines. These novel tools might be able to improve care for acute diarrhoea in older children and adults in low-resource settings, as well as during outbreaks of cholera and other diarrhoeal diseases where rapid and accurate triage and resuscitation of patients remains crucial.
Supplementary Material
Research in context.
Evidence before this study
The WHO Integrated Management of Adult and Adolescent Illnesses (IMAI) guidelines have served as the standard of care for assessment of dehydration in patients aged 5 years or older with acute diarrhoea since 2004, but to our knowledge no previous studies have validated their accuracy or reliability in this population. We systematically reviewed the scientific literature to identify studies published from inception up to June 18, 2023, addressing the assessment of dehydration in adults with diarrhoea. We searched PubMed, Cochrane Libraries, and Google Scholar to identify all published and unpublished trials in English using combinations of the following search terms: “dehydration”, “validation”, “dehydration assessment”, “diarrhoea” OR “gastroenteritis”, “adults” OR “elderly”, “clinical diagnostic models”. Additional studies were identified by hand searching references from included studies. Our literature search identified only our two NIRUDAK clinical prediction models for dehydration (one for dehydration category and one for percentage dehydration or fluid deficit) that have been derived using data from prospective cohorts of patients aged 5 years or older against a valid criterion standard, neither of which have been prospectively temporally validated.
Added value of this study
This study presents the first temporal validation of a clinical decision support tool (CDST) and simplified score for assessing dehydration in patients aged 5 years or older with acute diarrhoea. In this study, we temporally validated a novel CDST incorporating our recently developed full NIRUDAK model and a simplified NIRUDAK score in a new population of patients and compared their performance with the WHO IMAI algorithm for dehydration assessment. Our novel clinical tools were found to be significantly more accurate and reliable than the WHO IMAI algorithm.
Implications of all the available evidence
Use of our CDST and simplified clinical score by frontline clinicians could result in a considerable reduction in both undertreatment and overtreatment of patients aged 5 years or older with acute diarrhoea, potentially reducing the current mortality of 1·1 million deaths annually in this population. Further validation studies in other populations outside Bangladesh with different types of providers (community health workers, pharmacists, etc) should be prioritised to establish the generalisability of these new clinical diagnostic tools in patients worldwide.
Acknowledgments
Funding for data collection was provided through grants from the US National Institutes of Health (NIH) National Institute for Diabetes and Diarrheal and Kidney Diseases (NIDDK; Federal Identifier: DK116163). MAB was supported by a grant from the NIH National Institute on Drug Abuse (NIDA; Federal Identifier: T32DA013911). We thank all study participants and study staff at icddr,b’s Dhaka Hospital for their help and support. We are also grateful for the insights and dedication from the software development team at BeeHyv Software Solutions (Wilmington, DE; Hyderabad, India). There is no intellectual property claimed with respect to the software and the intent is to disseminate the software within the scope of a not-for-profit model.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Adam C Levine, Department of Emergency Medicine, Warren Alpert Medical School, Brown University, Providence, RI, USA.
Monique Gainey, Department of Emergency Medicine, Rhode Island Hospital, Providence, RI, USA.
Kexin Qu, Department of Biostatistics, School of Public Health, Brown University, Providence, RI, USA.
Sabiha Nasrin, Nutrition and Clinical Services Division, International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), Dhaka, Bangladesh.
Mohsena Bint-E Sharif, Nutrition and Clinical Services Division, International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), Dhaka, Bangladesh.
Syada S Noor, Nutrition and Clinical Services Division, International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), Dhaka, Bangladesh.
Meagan A Barry, Department of Emergency Medicine, Warren Alpert Medical School, Brown University, Providence, RI, USA.
Stephanie C Garbern, Department of Emergency Medicine, Warren Alpert Medical School, Brown University, Providence, RI, USA.
Christopher H Schmid, Department of Biostatistics, School of Public Health, Brown University, Providence, RI, USA.
Rochelle K Rosen, Department of Behavioral and Social Sciences, School of Public Health, Brown University, Providence, RI, USA.
Eric J Nelson, Departments of Pediatrics and Environmental and Global Health, Emerging Pathogens Institute, University of Florida, Gainesville, FL, USA.
Nur H Alam, Nutrition and Clinical Services Division, International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), Dhaka, Bangladesh.
Data sharing
The de-identified datasets, along with the corresponding data dictionary that defines each field in the set, are freely available with no restrictions via the Open Science Framework and can be accessed and downloaded at https://osf.io/pncms/. The original and updated versions of the mHealth CDST app used for this study can be downloaded for free at https://osf.io/CUX4V/ and https://osf.io/KEP3A/.
References
- 1.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations. UN News Global Perspective Human Stories. “2022 year in health: new Ebola and cholera outbreaks, mpox emergency, COVID-19 ‘not over’. https://news.un.org/en/story/2022/12/1131967 (accessed Jan 13, 2023).
- 3.Walker CL, Black RE. Diarrhoea morbidity and mortality in older children, adolescents, and adults. Epidemiol Infect 2010; 138: 1215–26. [DOI] [PubMed] [Google Scholar]
- 4.Lamberti LM, Fischer Walker CL, Black RE. Systematic review of diarrhea duration and severity in children and adults in low- and middle-income countries. BMC Public Health 2012; 12: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faruque ASG, Malek MA, Khan AI, Huq S, Salam MA, Sack DA. Diarrhoea in elderly people: aetiology, and clinical characteristics. Scand J Infect Dis 2004; 36: 204–08. [DOI] [PubMed] [Google Scholar]
- 6.World Gastroenterology Organisation. Acute diarrhea in adults and children: a global perspective. February, 2012. https://www.worldgastroenterology.org/guidelines/acute-diarrhea/acute-diarrhea-english (accessed Jan 15, 2023). [Google Scholar]
- 7.WHO. IMAI district clinician manual: hospital care for adolescents and adults. http://apps.who.int/iris/bitstream/10665/77751/3/9789241548290_Vol2_eng.pdf (accessed Jan 13, 2023).
- 8.Akech S, Ayieko P, Gathara D, et al. Risk factors for mortality and effect of correct fluid prescription in children with diarrhoea and dehydration without severe acute malnutrition admitted to Kenyan hospitals: an observational, association study. Lancet Child Adolesc Health 2018; 2: 516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Sharkawy AM, Virdee A, Wahab A, et al. Dehydration and clinical outcome in hospitalised older adults: a cohort study. Eur Geriatr Med 2017; 8: 22–29. [Google Scholar]
- 10.Fonseca BK, Holdgate A, Craig JC. Enteral vs intravenous rehydration therapy for children with gastroenteritis: a meta-analysis of randomized controlled trials. Arch Pediatr Adolesc Med 2004; 158: 483–90. [DOI] [PubMed] [Google Scholar]
- 11.Gorelick MH, Shaw KN, Murphy KO. Validity and reliability of clinical signs in the diagnosis of dehydration in children. Pediatrics 1997; 99: E6. [DOI] [PubMed] [Google Scholar]
- 12.Gravel J, Manzano S, Guimont C, Lacroix L, Gervaix A, Bailey B. Validation multicentrique du score clinique de déshydratation pédiatrique. Arch Pediatr 2010; 17: 1645–51. [DOI] [PubMed] [Google Scholar]
- 13.Levine AC, Glavis-Bloom J, Modi P, et al. External validation of the DHAKA score and comparison with the current IMCI algorithm for the assessment of dehydration in children with diarrhoea: a prospective cohort study. Lancet Glob Health 2016; 4: e744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St Jean DT, Chilyabanyama ON, Bosomprah S, et al. Development of a diarrhoea severity scoring scale in a passive health facility-based surveillance system. PLoS One 2022; 17: e0272981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer Walker CL, Sack D, Black RE. Etiology of diarrhea in older children, adolescents and adults: a systematic review. PLoS Negl Trop Dis 2010; 4: e768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382: 209–22. [DOI] [PubMed] [Google Scholar]
- 17.Levine AC, Barry MA, Gainey M, et al. Derivation of the first clinical diagnostic models for dehydration severity in patients over five years with acute diarrhea. PLoS Negl Trop Dis 2021; 15: e0009266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen RK, Garbern SC, Gainey M, et al. Designing a novel clinician decision support tool for the management of acute diarrhea in Bangladesh: formative qualitative study. JMIR Human Factors 2022; 9: e33325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamjid RM, Khan AKMTI. Icddr,b Annual Report 2021: Solving public health problems through innovative scientific research. Dec 20, 2022. https://www.icddrb.org/dmdocuments/Annual%20Report%202021_29Dec2022.pdf (accessed Jan 10, 2023). [Google Scholar]
- 20.Hooper L, Abdelhamid A, Attreed NJ, et al. Clinical symptoms, signs and tests for identification of impending and current water-loss dehydration in older people. Cochrane Database Syst Rev 2015; 2015: CD009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steiner MJ, DeWalt DABJ, Byerley JS. Is this child dehydrated? JAMA 2004; 291: 2746–54. [DOI] [PubMed] [Google Scholar]
- 22.McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA 1999; 281: 1022–29. [DOI] [PubMed] [Google Scholar]
- 23.Van Calster B, Van Belle V, Vergouwe Y, Steyerberg EW. Discrimination ability of prediction models for ordinal outcomes: relationships between existing measures and a new measure. Biom J 2012; 54: 674–85. [DOI] [PubMed] [Google Scholar]
- 24.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016; 15: 155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan AI, Mack JA, Salimuzzaman M, et al. Electronic decision support and diarrhoeal disease guideline adherence (mHDM): a cluster randomised controlled trial. Lancet Digit Health 2020; 2: e250–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snell KIE, Archer L, Ensor J, et al. External validation of clinical prediction models: simulation-based sample size calculations were more reliable than rules-of-thumb. J Clin Epidemiol 2021; 135: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cashin K, Oot L. Guide to anthropometry: a practical tool for program planners, managers, and implementers. May, 2018. https://www.fantaproject.org/tools/anthropometry-guide (accessed June 1, 2023). [Google Scholar]
- 28.Falszewska A, Szajewska H, Dziechciarz P. Diagnostic accuracy of three clinical dehydration scales: a systematic review. Arch Dis Child 2018; 103: 383–88. [DOI] [PubMed] [Google Scholar]
- 29.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. [PubMed] [Google Scholar]
- 30.Ferdous F, Ahmed S, Farzana FD, et al. Aetiologies of diarrhoea in adults from urban and rural treatment facilities in Bangladesh. Epidemiol Infect 2015; 143: 1377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The de-identified datasets, along with the corresponding data dictionary that defines each field in the set, are freely available with no restrictions via the Open Science Framework and can be accessed and downloaded at https://osf.io/pncms/. The original and updated versions of the mHealth CDST app used for this study can be downloaded for free at https://osf.io/CUX4V/ and https://osf.io/KEP3A/.