Abstract
As the primary vector of Lyme disease spirochetes and several other medically significant pathogens, Ixodes scapularis presents a threat to public health in the United States. The incidence of Lyme disease is growing rapidly in upper midwestern states, particularly Michigan, Minnesota, and Wisconsin.The probability of a tick bite, acarological risk, is affected by the phenology of host-seeking I. scapularis. Phenology has been well-studied in northeastern states, but not in the Upper Midwest. We conducted biweekly drag sampling across 4 woodland sites in Minnesota between April and November from 2015 to 2017. The majority of ticks collected were I. scapularis (82%). Adults were active throughout our entire 8-month collection season, with sporadic activity during the summer, larger peaks in activity observed in April, and less consistent and lower peaks observed in October. Nymphs were most active from May through August, with continuing low-level activity in October, and peak activity most commonly observed in June. The observed nymphal peak corresponded with the typical peak in reported human Lyme disease and anaplasmosis cases.These findings are consistent with previous studies from the Upper Midwest and highlight a risk of human exposure to I. scapularis at least from April through November. This information may aid in communicating the seasonality of acarological risk for those living in Minnesota and other upper midwestern states as well as being relevant to the assessment of the ecoepidemiology of Lyme disease and the modeling of transmission dynamics.
Keywords: Ixodes scapularis, blacklegged tick, phenology, Minnesota, Lyme disease
Introduction
The blacklegged tick (Ixodes scapularis) and its associated pathogens pose an increasing public health threat in the United States, with established and expanding regional foci in the Northeast and Upper Midwest (Eisen et al. 2016, Eisen and Eisen 2018, Fleshman et al. 2021, 2022). Ixodes scapularis is a vector of 7 human pathogens that are known to circulate in the upper midwestern United States: Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) sensu stricto and Bo. mayonii (Spirochaetales: Spirochaetaceae) (Lyme disease), Bo. Miyamotoi (Spirochaetales: Spirochaetaceae) (Bo. miyamotoi disease), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae) (anaplasmosis), Ehrlichia muris eauclairensis (Rickettsiales: Ehrlichiaceae) (a form of ehrlichiosis), Babesia microti (Piroplasmida: Babesiidae) (babesiosis), and Powassan virus (a neuroinvasive viral disease) (Barbour et al. 2009, Pritt et al. 2011, 2016, Johnson et al. 2018b, Lehane et al. 2021, Fleshman et al. 2022). In order to define habitat types and geographic locations (e.g., counties) where persons are at risk for exposure to ticks and tickborne pathogens, several studies have explored changes in the distribution of I. scapularis, its associated pathogens, and acarological risk in the Upper Midwest (Lee et al. 2013, 2019, Robinson et al. 2015, Johnson et al. 2018a, 2018b, Foster et al. 2022). However, relatively few studies have been conducted in the region with the goal of describing the phenology of host-seeking I. scapularis, which provides information on when persons are at risk for tick bites (Neitzel et al. 1993, Brinkerhoff et al. 2011, Hamer et al. 2012, Diuk-Wasser et al. 2014, Stromdahl et al. 2014, Ogden et al. 2018).
The phenology of I. scapularis has been well-described in the northeastern United States, with an asynchronous pattern wherein nymphal activity precedes larval host-seeking. Peak host-seeking activity of nymphs is typically observed in early summer while peak host-seeking activity of larvae is most commonly observed in late summer (Piesman et al. 1987, Yuval and Spielman 1990, Ostfeld et al. 1996, Stafford et al. 1998, Falco et al. 1999, Simmons et al. 2015, Elias et al. 2020). Similar patterns have been observed in the Northeast for I. scapularis collected directly from humans through passive surveillance systems (Xu et al. 2016, Little et al. 2019, Rounsville et al. 2021). Ixodes scapularis nymphs are considered the most epidemiologically important life stage with adults also being capable of transmitting pathogens (Stafford et al. 1998, Pepin et al. 2012). The relative timing of nymphal and larval emergence is important as well as asynchronous peaks may promote the transmission of Bo. burgdorferi s.s., while synchronous peaks may increase the rate of co-feeding between life stages potentially facilitating the transmission of other pathogens (Ogden et al. 2007, Hassett and Thangamani 2021).
The phenology of questing I. scapularis in the Upper Midwest has received less attention by researchers than that in the Northeast, but I. scapularis phenology has generally been observed to be similar, with adults most active in the spring and fall and nymphs active in the early summer (Neitzel et al. 1993, Diuk-Wasser et al. 2014, Stromdahl et al. 2014, Ogden et al. 2018). Unlike northeastern I. scapularis populations, in the Upper Midwest, the larval peak has been observed to overlap with the nymphal peak in the early summer (Brinkerhoff et al. 2011, Hamer et al. 2012). Documenting when each life stage is questing for hosts aids in understanding seasonal variation in risk for tickborne diseases (Piesman et al. 1987, Moore et al. 2014, Lin et al. 2019). We documented the host-seeking activity of I. scapularis at 4 geographically distinct woodland sites throughout Minnesota in order to improve our understanding of the temporal risk for exposure to I. scapularis nymphs and adults. We also aimed to assess the interannual and geographic variability in host-seeking phenology and determine the relative timing of the activity peaks for larvae and nymphs.
Materials and Methods
Site Description
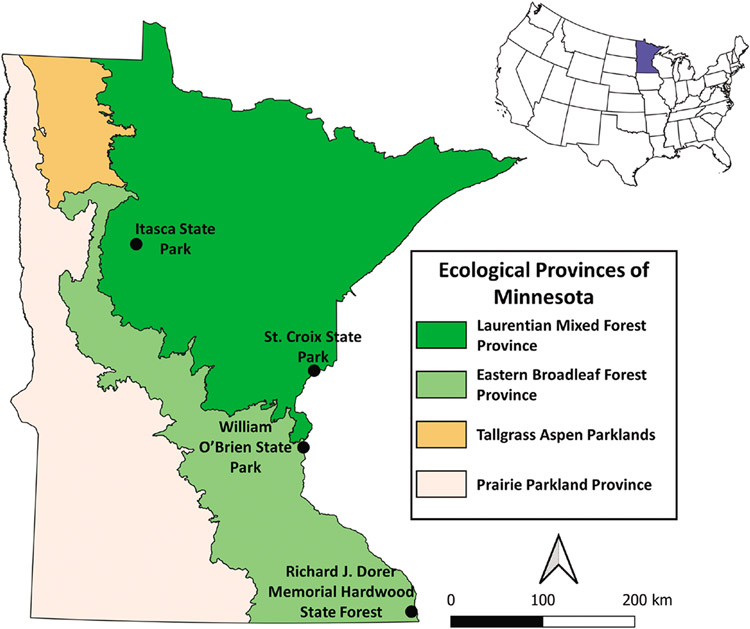
Four geographically distinct sites on public land in Minnesota were chosen to assess host-seeking phenology of I. scapularis. These 4 sites were distributed across a latitudinal gradient and included 2 sites within the eastern broadleaf forest (Richard J. Dorer Memorial Hardwood (RJDMH hereafter) State Forest and William O’Brien State Park) and 2 sites within the Laurentian mixed forest (Itasca and St. Croix State Parks) (Fig. 1). Specific tick sampling transects at each site were identified based on accessibility, land manager recommendations, and a priori knowledge of I. scapularis established populations. Vegetation at all 4 sites is primarily deciduous, oak-dominated forest with dense leaf litter and understory, with the exception of the site at Itasca State Park, which is better described as mixed forest with hardwoods and pines. Specific tick sampling transects at each site were identified based on suitable I. scapularis habitat (i.e., wooded and brushy mesic areas with at least 50% canopy coverage) (Ostfeld et al. 1995, Guerra et al. 2002, Lubelczyk et al. 2004), accessibility, and land manager recommendations. We also collected temperature and precipitation data between April 1st and November 30th for each year. Data were collected from NOAA weather stations nearby each site, USC00210018 (Itasca), USC00211198 (RJDMH), USC00210190 (St. Croix), and USC00218450 (William O’Brien).
Fig. 1.
Locations of the 4 collection sites in Minnesota. Minnesota is highlighted on the map of the lower 48 states.
Tick Collections
Distance-based drag sampling was performed in which 2 samplers pulled a modified 1 m2 white canvas cloth with weighted fingers over the ground to collect host-seeking I. scapularis (Siegel et al. 1991). In total, 750 m2 were sampled at each site on each sampling occasion. Samplers stopped every 15 m to inspect the drag cloths and their clothing. Samplers wore light colored clothing taped to closed toe boots. Ticks found on researchers were also counted towards the total. Ticks of all life stages collected were returned to the transect of collection after being identified in the field by trained researchers. Atypical ticks of all life stages were collected for morphologic identification to the species level using published taxonomic keys (Keirans and Litwak 1989, Durdens and Keirans 1996, Coley 2015). The species, life stage, and number of ticks collected were recorded for each 15 m section of the transects. Sites were sampled every 2 weeks from April through November in 2015, 2016, and 2017. Tick dragging was only performed under favorable weather conditions for tick activity (i.e., no rain or snow cover with ambient air temperatures ≥4 °C). Transects were marked with flagging tape to ensure researchers sampled the same area on each visit and between years. Minor deviations were made on rare occasions when the route was impassable (i.e., downed tree or flooding).
Data Analysis
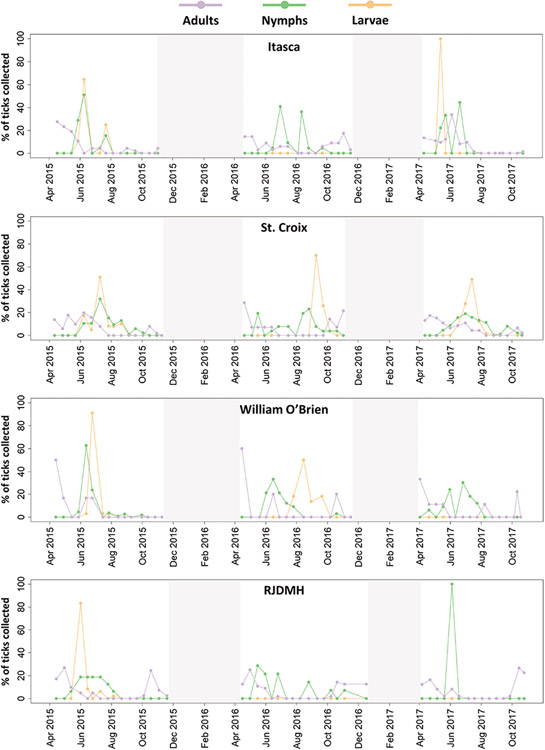
Peak densities for I. scapularis nymphs and adults were defined as the observed maximum number collected during our collection periods (April–November) per 750 m2 collection divided by year, site, and life stage. Onset and cessation of nymphal host-seeking activity was defined as the first and last occasion nymphs were collected per collection period. The distribution of host-seeking adults was bimodal. Therefore, onset and cessation of adult host-seeking was defined as the earliest and latest dates of activity first in the spring (April–June) and then in the fall (October–November) within each calendar year. The I. scapularis nymph data had exponential increases and decreases on each site for most years. Separate models were constructed during the increasing phases of the peaks as well as the decreasing phases. We used the output from the models between the first collection day to the peak (increasing) of each site and from those peaks to the last collection day (decreasing) to predict the dates between which activity peaks were at 25% and 50% of their maximums (Eisen et al. 2002). All data used for the linear models were log-transformed, with +1 added to account for zeros. This allowed our data to conform with the assumption of normality and account for the exponential nature of the data. Normality was visually assessed by examining quantile–quantile plots of the model residuals. We also compared the AIC values of models using transformed and untransformed datasets. The transformed data sets performed significantly better (ΔAIC > 2) for all models. These analyses were conducted for each site with all years (2015, 2016, 2017) combined. Data analyses were performed using the base package in R (v. 4.0.3) (R Core Team 2022). Our full dataset can be found as Supplemental Materials. In Fig. 2, density data are expressed as the percentage of ticks that are active per life stage at a given time point relative to the total numbers collected per life stage per year. This normalization of the data allowed us to directly compare phenology between life stages, years, and sites. We recognize that drag sampling does not accurately estimate larval abundance but expressing abundance as a percentage of totals collected provides reasonable estimates of seasonal host-seeking activity (Ginsberg et al. 2020).
Fig. 2.
Activity of I. scapularis adults (purple) nymphs (green), and larvae (orange) on each of the 4 collection sites (St. Croix, Itasca, RJDMH, William O’Brien) between 2015 and 2017. Data are presented as the percentage of ticks of each life stage collected within a season that were collected on each collection date. Shaded sections represent periods when no tick sampling occurred.
Results
Site Visits and Weather Data
Over the 3 yr of study, a total of 46 visits were made each to St. Croix and William O’Brien State Parks. There were 47 visits to RJDMH State Forest and 48 visits to Itasca State Park. A total of 3,273 ticks were collected and identified by staff on transects with the majority (82%) of ticks being identified as I. scapularis (41% larvae, 34% adults, and 24% nymphs). An additional 14% of ticks were identified to be Dermacentor variabilis (Acari: Ixodidae) (93% adults and 7% larvae) and 4% were D. albipictus, all of which were larvae. The number of I. scapularis found on transects varied by life stage, collection date, and site. The average density of adults and nymphs collected per visit across all sites and years was 0.64 adults per 100 m2 and 0.47 nymphs per 100 m2 (Table 1). There was limited variation, with mean minimum temperature varying by no more than 4.8 °C and mean maximum varied by no more than (3.0 °C). There was a north-to-south gradient in temperature, with Itasca being the coldest and RJDMH being the warmest. Average cumulative precipitation was similar (699–801 mm) across 3 sites (Itasca, RJDMH, William O’Brien), but was lower on Itasca at 500 mm. Overall, 2016 was a wetter year, with the difference being most no-table on Itasca and RJDMH.
Table 1.
Maximum and average densities (per 100 m2) and total number of I. scapularis adults and nymphs on each of the 4 sites located in Minnesota between 2015 and 2017
| Life stage |
Avg/max | Year | Itasca State Park |
St. Croix State Park |
William O’Brien State Park |
RJDMH State Forest |
All sites |
|---|---|---|---|---|---|---|---|
| Adults | Average (per visit) | 2015 | 0.78 | 0.88 | 0.27 | 0.60 | 0.64 |
| 2016 | 0.58 | 0.46 | 0.05 | 0.97 | 0.52 | ||
| 2017 | 0.92 | 1.04 | 0.14 | 1.01 | 0.77 | ||
| All Years | 0.76 | 0.79 | 0.15 | 0.84 | 0.64 | ||
| Maximum (per visit) | 2015 | 3.47 | 2.93 | 1.87 | 2.67 | 3.47 | |
| 2016 | 1.33 | 2.53 | 0.40 | 3.07 | 3.07 | ||
| 2017 | 4.27 | 2.27 | 0.67 | 3.60 | 4.27 | ||
| All Years | 4.27 | 2.93 | 1.87 | 3.60 | 4.27 | ||
| Total number of ticks collected | 2015 | 45 | 85 | 110 | 16 | 256 | |
| 2016 | 22 | 26 | 33 | 14 | 95 | ||
| 2017 | 9 | 265 | 33 | 2 | 309 | ||
| All Years | 76 | 376 | 176 | 32 | 660 | ||
| Nymphs | Average (per visit) | 2015 | 0.38 | 0.71 | 0.98 | 0.13 | 0.55 |
| 2016 | 0.18 | 0.23 | 0.29 | 0.12 | 0.20 | ||
| 2017 | 0.08 | 2.36 | 0.28 | 0.02 | 0.67 | ||
| All Years | 0.21 | 1.09 | 0.50 | 0.09 | 0.47 | ||
| Maximum (per visit) | 2015 | 3.07 | 3.60 | 9.20 | 0.40 | 9.20 | |
| 2016 | 1.20 | 0.80 | 1.47 | 0.53 | 1.47 | ||
| 2017 | 0.53 | 6.67 | 1.33 | 0.27 | 6.67 | ||
| All Years | 3.07 | 6.67 | 9.20 | 0.53 | 9.20 | ||
| Total number of ticks collected | 2015 | 47 | 51 | 6 | 41 | 145 | |
| 2016 | 34 | 14 | 5 | 56 | 109 | ||
| 2017 | 74 | 46 | 9 | 49 | 178 | ||
| All Years | 155 | 111 | 20 | 146 | 432 |
The “all years” column shows the densities of each site averaged across all 3 collection years.
Ixodes scapularis Activity
A peak in adult I. scapularis activity was generally observed in the spring, with a second smaller peak observed in the fall in some years. In some years, we did not observe a fall increase in host-seeking adult densities, as occurred at St. Croix in 2017 or Itasca in 2015, among others (Fig. 2). Adult I. scapularis were generally active across a broad range of dates, with the potential to be collected throughout the field season (April–November). The earliest collection of adults was on April 3, while the latest was on November 19. Nymphal questing activity was unimodal with most observations of peak activity occurring in between May and July (Table 2, Fig. 2), but they were often still collected in the fall through October. The earliest collection of nymphs was on April 21, while the latest was on November 3 (Table 2, Fig. 2). Our larval data are somewhat limited as activity was low or undetected in some years on several sites, specifically in Itasca and RJDMH in 2016 and 2017 as well as in William O’Brien in 2017. Among sites where activity was detected, the timing of peak larval host-seeking activity varied, generally occurring sometime between June and September. The timing of these peaks relative to those of I. scapularis nymphs also varied across sites and years, but often resulted in overlapping activity between nymphs and larvae (Fig. 2).
Table 2.
The dates when the first, last, and peak for I. scapularis adults and nymphs that were collected across sites and years during our collection period (April–November)
| Site | Year | First collection | Peak collection | Last collection |
|---|---|---|---|---|
| Adults | ||||
| Itasca | 2015 | April 17 | April 30/September 2 | November 2 |
| 2016 | April 22 | May 5/November 3 | November 17 | |
| 2017 | April 12 | June 7/October 25 | October 25 | |
| All Years | April 12 | June 7/November 3 | November 17 | |
| St. Croix | 2015 | April 11 | June 8/October 16 | October 30 |
| 2016 | April 20 | April 20/October 6 | November 2 | |
| 2017 | April 13 | April 13/October 13 | October 22 | |
| All Years | April 11 | June 8 /October 16 | November 2 | |
| William O’Brien | 2015 | April 13 | June 12/NA | June 24 |
| 2016 | April 15 | April 15/October 16 | October 19 | |
| 2017 | April 3 | April 3/October 12 | October 19 | |
| All Years | April 3 | April 3/October 12 | October 19 | |
| RJDMH | 2015 | April 14 | April 30/October 18 | November 19 |
| 2016 | April 16 | May 1/October 20 | November 17 | |
| 2017 | April 7 | April 23/October 16 | October 26 | |
| All Years | April 7 | May 1/October 16 | November 19 | |
| Nymphs | ||||
| Itasca | 2015 | May 28 | June 9 | July 22 |
| 2016 | June 16 | July 1 | September 22 | |
| 2017 | May 15 | June 22 | June 22 | |
| All Years | May 15 | June 9 | September 22 | |
| St. Croix | 2015 | June 8 | July 10 | October 3 |
| 2016 | May 17 | Aug 26 | October 20 | |
| 2017 | May 19 | July 2 | October 22 | |
| All Years | May 17 | July 2 | October 22 | |
| William O’Brien | 2015 | May 28 | June 12 | September 30 |
| 2016 | June 2 | June 16 | October 19 | |
| 2017 | April 21 | June 27 | July 25 | |
| All Years | April 21 | June 12 | October 19 | |
| RJDMH | 2015 | May 13 | June 15 | Aug 5 |
| 2016 | May 15 | May 15 | November 3 | |
| 2017 | June 5 | June 5 | June 5 | |
| All Years | May 13 | May 15 | November 3 |
There are 2 peak dates for adults showing a peak between April and July and a second peak between August and November. No adults were collected in the fall on William O’Brien in 2015, so the second adult peak is labeled “NA”. The “all years” rows show the earliest start and latest end for the season on each site, as well as the date of the highest peak density across all 3 years.
Generally, the increasing phase occurred more rapidly than the decreasing phase of the nymph activity peaks, with nymphs being collected in the fall through October, but rarely before May (Table 2, Fig. 2). This is reflected in the dates wherein nymphs are estimated to be at 25% and 50% of their peaks based upon our linear models (Table 3). Across sites and years, nymphs are estimated to be at 50% of their peak between June 2 and August 4, and 25% of their peak between May 14 and August 27 (Table 4). As with the observed peak densities, there is variation between sites and different years.
Table 3.
The output from the linear models for the “increasing” (early season) and “decreasing” (late season) phases of I. scapularis nymphal host-seeking activity across all years within each site (St. Croix, Itasca, RJDMH, William O’Brien)
| Site | Intercept increase/decrease | Slope increase/decrease | r2 increase/decrease | df increase/decrease | P-value increase/decrease |
|---|---|---|---|---|---|
| Itasca | 2.53/2.63 | 0.02/−0.01 | 0.337/0.263 | 14/30 | 0.018/0.003 |
| St. Croix | −2.06/8.59 | 0.02/−0.03 | 0.284/0.611 | 22/21 | 0.007/<0.001 |
| William O’Brien | −1.65/5.91 | 0.02/−0.02 | 0.467/0.603 | 15/27 | 0.003/<0.001 |
| RJDMH | −2.57/1.74 | 0.02/−0.01 | 0.661/0.254 | 13/29 | <0.001/0.004 |
All data were log-transformed to conform with the assumptions of normality and homoscedasticity.
Table 4.
Estimated start and end dates between which I. scapularis nymphal densities will be at 25 and 50%of their peak based upon the linear models for the “increasing” (early season) and “decreasing” (late season) phases
| Site | 25% | 50% | Dates of recorded peak density |
|---|---|---|---|
| Itasca | June 2–August 16 | June 23–July 12 | 2015: June 9 |
| 2016: July 1 | |||
| 2017: June 22 | |||
| St. Croix | June 25–August 27 | Jul 18–August 4 | 2015: July 10 |
| 2016: August 26 | |||
| 2017: July 2 | |||
| William O’Brien | May 14–August 27 | Jun 2–July 24 | 2015: June 12 |
| 2016: June 16 | |||
| 2017: June 27 | |||
| RJDMH | May 18–August 6 | Jun 5–July 11 | 2015: June 15 |
| 2016: May 16 | |||
| 2017: June 5 |
The 25% peak represent moderate seasonal risk for bites from I. scapularis nymphs and 50% of the peak represents high risk. Other dates represent lower, but a still present risk, of tick bites.
Dermacentor variabilis Activity
Dermacentor variabilis adults were active primarily in May and June across all sites and years. The earliest they were detected at any site was April 13 with the latest collection being on August 21. We did not have sufficient data for D. variabilis larvae and nymphs to provide any information regarding their activity peaks.
Discussion
We documented the seasonal host-seeking activity of I. scapularis by life stage in 4 field sites across Minnesota. Generally, in the context of Lyme disease in the eastern United States, I. scapularis nymphs are considered the most epidemiologically important life stage (Stafford et al. 1998, Pepin et al. 2012). Our data suggest that nymphs were active between May and August, with peak activity occurring in June and July. Of the confirmed Lyme disease cases reported in Minnesota, cases typically begin to be reported in May, peak in June, and continue into August (Moore et al. 2014). Specifically, of the 876 Lyme disease cases reported in 2018 with known illness onset dates, illness onsets peaked from June through August (MDH 2022). This indicates likely tick exposure in late May through July, which aligns with the nymphal activity peaks we observed. Some of the variability in tick densities observed both within and between sites may be due to variation in field conditions or the time of day for sampling (Schulze and Jordan 2003). It is also possible that the dates for the 25% and 50% activity peaks calculated using our linear models are slightly inaccurate, as it is possible that we missed the true peak of nymphal activity, which would shift the dates predicted by our models. Despite this, our estimated activity dates largely match those of the observed peaks (Table 4) and align with previous observations for nymph activity in the Upper Midwest (Neitzel et al. 1993, Diuk-Wasser et al. 2014, Stromdahl et al. 2014, Ogden et al. 2018).
The phenology of immature I. scapularis can play an important role in pathogen transmission dynamics, with Bo. burgdorferi s.s. being particularly notable, as hosts can remain capable of infecting ticks for long periods of time (Lindsay et al. 1997). If nymphs emerge early in the season, prior to larvae, they can infect a larger portion of the reservoir host population before the larvae feed, facilitating the transmission of Bo. burgdorferi s.s. between generations of I. scapularis (Ogden et al. 2007). This phenological pattern is common in northeastern I. scapularis populations (Carey et al. 1981, Wilson and Spielman 1985, Schulze et al. 1986). Here, as previously observed (Brinkerhoff et al. 2011, Hamer et al. 2012), our data suggest that the activity peaks for I. scapularis nymphs and larvae in the Upper Midwest can overlap. Despite this, the infection prevalence of Bo. burgdorferi in I. scapularis is similar in the Northeast (Prusinski et al. 2014, Feldman et al. 2015, Lehane et al. 2021) and Upper Midwest (Johnson et al. 2018a, Lehane et al. 2021). It has been hypothesized that asynchronous peaks for the activity of larvae and nymphs facilitate increased transmission of Bo. burgdorferi (Ogden et al. 2007) but it is possible that different strains of Bo. burgdorferi dominate depending upon the timing of the larval and nymphal activity peaks (Gatewood et al. 2012, Hamer et al. 2012,Arsnoe et al. 2015). It is also possible that the transmission of other pathogens, like Powassan virus, may be facilitated by co-feeding (Hassett and Thangamani 2021). Ultimately, more research is needed regarding the impact of larval and nymphal phenology on the transmission of different Bo. burgdorferi strains and other I. scapularis-associated pathogens, particularly considering the variability observed in larval densities when drag cloths are the collection instrument.
Synchronicity between the activity peaks of the 2 life stages varied by year and collection site. Larvae were not consistently detected across all 4 sites and years of study, despite other life stages being present. Our data from the 2 sites with sufficient larval collections to observe patterns suggest that the larval and nymphal activity peaks can be synchronous (e.g., Itasca in 2017), with little evidence for a late summer larval peak (Fig. 2), as is often observed in northeastern populations (Carey et al. 1981, Wilson and Spielman 1985, Schulze et al. 1986). Larvae and nymphs have been observed feeding concurrently on small mammals in the Upper Midwest (Caporale et al. 2005). Our larval data should be interpreted with caution as drag sampling is not the best method for estimating larval tick densities due to their questing height (Mejlon and Jaenson 1997), patchy distribution (Daniels and Fish 1990), and strong effect of weather conditions on their questing activity (Schulze et al. 1997, Ginsberg et al. 2020). It is also possible that other Ixodes species were included in our I. scapularis counts, as ticks were identified in the field without the use of a microscope, but previous work in Minnesota has shown that other Ixodes species are rarely collected (Johnson et al. 2018b). Despite these limitations, the overlapping activity of larvae and nymphs observed here matches previous observations of synchronous activity between the 2 life stages from the Upper Midwest (Brinkerhoff et al. 2011, Hamer et al. 2012).
While there was some variation between sites and years, adult I. scapularis host-seeking activity was generally bimodal with peak activity observed in the spring and a smaller peak in the fall. Sampling was not performed in the winter, so it is possible that adult I. scapularis were actively host-seeking when there was limited snow cover and temperatures were above the activity threshold temperature of 4 °C (Duffy and Campbell, 1994). There was no appreciable north-to-south pattern in activity across sites. There was also limited variation in temperature between years, so we cannot evaluate the effect of temperature on tick activity patterns. There was higher precipitation in 2016 on the Itasca and RJDMH sites, but we did not observe any consistent effect on I. scapularis activity. Dermacentor variabilis is an important vector for the agents of Rocky Mountain Spotted Fever (Rickettsia rickettsii) and tularemia (Francisella tularensis). Cases of both diseases are rare in Minnesota, but do occur occasionally. This species was not the focus of this study as their primary habitat of moist and humid forest-grassland ecotones (Sonenshine and Stout 1968, Sonenshine and Levy 1972) were not directly targeted, yet adult host-seeking D. variabilis were still encountered in our wooded sites in Minnesota. Their activity peaked in June, and few were collected after August. This adult tick activity coincided with adult I. scapularis activity in the spring and early summer, but we did not observe D. variabilis questing behavior in the fall.
Previous tick surveillance efforts in Minnesota have demonstrated that the range of I. scapularis is expanding and each of its 7 associated human pathogens have been detected in host-seeking nymphs and adults (Johnson et al. 2018b, Lehane et al. 2020). Our findings are consistent with previous studies (Brinkerhoff et al. 2011, Hamer et al. 2012) and highlight a risk of human exposure to I. scapularis at least from April through November. In areas where I. scapularis is emerging, the public may consider ticks a threat predominantly in the summer, although they are active throughout the spring, fall, and potentially the winter. The potential for exposure to infected I. scapularis throughout the spring, summer, and fall is important to relay in public health messaging so people can incorporate tick bite prevention measures and awareness into their daily routines throughout the year.
Supplementary Material
Acknowledgments
The authors would like to especially thank Franny Dorr and Molly Peterson (Minnesota Department of Health); Jeanne Minnerath (Saint Mary’s University of Minnesota); Sonia Kjos, Amy Prunuske, and Cole Fisher (University of Minnesota - Duluth); David Biesboer (University of Minnesota - Itasca Biological Field Station); and Fred Anderson, Kristofer Keller, Casey Kipping, Abigail Miller, and Tessa Whitemarsh (Washington County Department of Public Health and Environment). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of CDC or the Department of Health and Human Services.
Funding
This work was supported by the Centers for Disease Control and Prevention Emerging Infections Program Cooperative Agreement (U50CK000204, U50CK000490). There were no potential conflicts of interest throughout this study.
Footnotes
Supplementary Material
Supplementary material are available at Journal of Medical Entomology online.
References
- Arsnoe IM, Hickling GJ, Ginsberg HS, McElreath R, Tsao JI. Different populations of blacklegged tick nymphs exhibit differences in questing behavior that have implications for human Lyme disease risk. PLoS One. 2015:10(5):e0127450. 10.1371/journal.pone.0127450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, Tsao JI. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009:81(6):1120–1131. 10.4269/ajtmh.2009.09-0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkerhoff RJ, Folsom-O’Keefe CM, Streby HM, Bent SJ, Tsao K, Diuk-Wasser MA. Regional variation in immature Ixodes scapularis parasitism on North American songbirds: implications for transmission of the Lyme pathogen, Borrelia burgdorferi. J Med Entomol. 2011:48:422–428. [DOI] [PubMed] [Google Scholar]
- Caporale DA, Johnson CM, Millard BJ. Presence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) in southern Kettle Moraine State Forest, Wisconsin, and characterization of strain W97F51. J Med Entomol. 2005:42(3):457–472. 10.1093/jmedent/42.3.457 [DOI] [PubMed] [Google Scholar]
- Carey MG, Carey AB, Main AJ, Krinsky WL, Sprance HE. Ixodes dammini (Acari: Ixodidae) in forests in Connecticut. J Med Entomol. 1981:18(2):175–176. 10.1093/jmedent/18.2.175 [DOI] [PubMed] [Google Scholar]
- Coley K. Identification guide to larval stages of ticks of medical importance in the USA. University Honors Program Theses, Paper 110; 2015. [Google Scholar]
- Daniels TJ, Fish D. Spatial distribution and dispersal of unfed larval Ixodes dammini (Acari: Ixodidae) in southern New York. Environ Entomol. 1990:19(4):1029–1033. 10.1093/ee/19.4.1029 [DOI] [Google Scholar]
- Diuk-Wasser MA, Gatewood AG, Cortinas MR, Yaremych-Hamer S, Tsao J, Kitron U, Hickling G, Brownstein JS, Walker E, Piesman J, et al. Spatiotemporal patterns of host-seeking Ixodes scapularis nymphs (Acari: Ixodidae) in the United States. J Med Entomol. 2014:43:166–176. [DOI] [PubMed] [Google Scholar]
- Duffy DC, Campbell SR. Ambient air temperature as a predictor of activity of adult Ixodes scapularis (Acari: Ixodidae). J Med Ent. 1994:31(1):178–180. [DOI] [PubMed] [Google Scholar]
- Durden LA, Keirans JE. Nymphs of the genus Ixodes (Acari: Ixodidae) of the United States: taxonomy, identification. Key, distribution, hosts, and medical/veterinary importance. Annapolis (MD): Entomological Society of America; 1996. [Google Scholar]
- Eisen L, Eisen RJ, Lane RS. Seasonal activity patterns of Ixodes pacificus nymphs in relation to climatic conditions. Med Vet Entomol. 2002:16(3):235–244. 10.1046/j.1365-2915.2002.00372.x [DOI] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L. The blacklegged tick, Ixodes scapularis: an increasing public health concern. Trends Parasitol. 2018:34(4):295–309. 10.1016/j.pt.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Beard CB. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J Med Entomol. 2016:53(2):349–386. 10.1093/jme/tjv237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias SP, Maasch KA, Anderson NT, Rand PW, Lacombe EH, Robich RM, Lubelczyk CB, Smith RP. Decoupling of blacklegged tick abundance and Lyme disease incidence in Southern Maine, USA. J Med Entomol. 2020:57(3):755–765. 10.1093/jme/tjz218 [DOI] [PubMed] [Google Scholar]
- Falco RC, McKenna DF, Daniels TJ, Nadelman RB, Nowakowski J, Fish D, Wormser GP. Temporal relation between Ixodes scapularis abundance and risk for Lyme disease associated with erythema migrans. Am J Epidemiol. 1999:149(8):771–776. 10.1093/oxfordjournals.aje.a009886 [DOI] [PubMed] [Google Scholar]
- Feldman KA, Connally NP, Hojgaard A, Jones EH, White JL, Hinckley AF. Abundance and infection rates of Ixodes scapularis nymphs collected from residential properties in Lyme disease-endemic areas of Connecticut, Maryland, and New York. J Vector Ecol. 2015:40(1):198–201. 10.1111/jvec.12153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman AC, Foster E, Maes SE, Eisen RJ. Reported county-level distribution of seven human pathogens detected in host-seeking Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the contiguous United States. J Med Entomol. 2022;59(4):1328–1335. [DOI] [PubMed] [Google Scholar]
- Fleshman AC, Graham CB, Maes SE, Foster E, Eisen RJ. Reported county-level distribution of Lyme disease spirochetes, Borrelia burgdorferi sensu stricto and Borrelia mayonii (Spirochaetales: Spirochaetaceae), in host-seeking Ixodes scapularis and Ixodes pacificus ticks (Acari: Ixodidae) in the contiguous United States. J Med Entomol. 2021:58(3):1219–1233. 10.1093/jme/tjaa283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster E, Burtis J, Sidge JL, Tsao JI, Bjork J, Liu G, Neitzel DF, Lee X, Paskewitz S, Caporale D, et al. Inter-annual variation in prevalence of Borrelia burgdorferi sensu stricto and Anaplasma phagocytophilum in host-seeking Ixodes scapularis (Acari: Ixodidae) at long-term surveillance sites in the upper midwestern United States: Implications for public health practice. Ticks Tick Borne Dis. 2022:13(2):101886. 10.1016/j.ttbdis.2021.101886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood AG, Liebman KA, Vourc’h G, Bunikis J, Hamer SA, Corinas R, Melton F, Cislo P, Kirton U, Tsao J, et al. Climate and tick seasonality are predictors of Borrelia burgdorferi genotype distribution. Appl Environ Microbiol. 2012:75:2476–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HS, Rulison EL, Miller JL, Pang G, Arsnoe IM, Hickling GJ, Ogden NH, LeBrun RA, Tsao JI. Local abundance of Ixodes scapularis in forests: effects of environmental moisture, vegetation characteristics, and host abundance. Ticks Tick Borne Dis. 2020:11(1):101271. 10.1016/j.ttbdis.2019.101271 [DOI] [PubMed] [Google Scholar]
- Guerra M, Walker E, Jones C, Paskewitz S, Cortinas MR, Stancil A, Beck L, Bobo M, Kitron U. Predicting the risk of Lyme disease: habitat suitability for Ixodes scapularis in the north central United States. Emerg Infect Dis. 2002:8(3):289–297. 10.3201/eid0803.010166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer SA, Hickling GJ, Sidge JL, Walker ED, Tsao JI. Synchronous phenology of juvenile Ixodes scapularis, vertebrate host relationships, and associated patterns of Borrelia burgdorferi ribotypes in the Midwestern United States. Ticks Tick Borne Dis. 2012:3(2):65–74. 10.1016/j.ttbdis.2011.11.004 [DOI] [PubMed] [Google Scholar]
- Hassett EM, Thangamani S. Ecology of Powassan Virus in the United States. Microorganisms. 2021:9(11):2317. 10.3390/microorganisms9112317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TL, Boegler KA, Clark RJ, Delorey MJ, Bjork JK, Dorr FM, Schiffman EK, Neitzel DF, Monaghan AJ, Eisen RJ. An acarological risk model predicting the density and distribution of host-seeking Ixodes scapularis nymphs in Minnesota. Am J Trop Med. 2018a:98:1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TL, Graham CB, Maes SE, Hojgaard A, Fleshman A, Boegler KA, Delory MJ, Slater KS, Karpathy SE, Bjork JK, et al. Prevalence and distribution of seven human pathogens in host-seeking Ixodes scapularis (Acari: Ixodidae) nymphs in Minnesota, USA. Ticks Tick Borne Dis. 2018b:9(6):1499–1507. 10.1016/j.ttbdis.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirans JE, Litwak TR. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi river. J Med Entomol. 1989:26(5):435–448. 10.1093/jmedent/26.5.435 [DOI] [PubMed] [Google Scholar]
- Lee X, Hardy K, Johnson DH, Paskewitz SM. Hunter-killed deer surveillance to assess changes in the prevalence and distribution of Ixodes scapularis (Acari: Ixodidae) in Wisconsin. J Med Entomol. 2013:50(3):632–639. 10.1603/me12234 [DOI] [PubMed] [Google Scholar]
- Lee X, Murphy DS, Hoang Johnson D, Paskewitz SM. Passive animal surveillance to identify ticks in Wisconsin, 2011–2017. Insects. 2019:10(9):289. 10.3390/insects10090289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehane A, Maes SE, Graham CB, Jones E, Delorey M, Eisen RJ. Prevalence of single and coinfections of human pathogens in Ixodes ticks from five geographical regions in the United States, 2013–2019. Ticks Tick Borne Dis. 2021:12(2):101637. 10.1016/j.ttbdis.2020.101637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Shrestha S, Prusinski MA, White JL, Lukacik G, Smith M, Jianhai L, Backenson B. The effects of multiyear and seasonal weather factors on incidence of Lyme disease and its vector in New York State. Sci Total Environ. 2019:665:1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay LR, Barker IK, Surgeoner GA, McEwen SA, Campbell GD. Duration of Borrelia burgdorferi infectivity in white-footed mice for the tick vector Ixodes scapularis under laboratory and field conditions in Ontario. J Wildl Dis. 1997:33(4):766–775. 10.7589/0090-3558-33.4.766 [DOI] [PubMed] [Google Scholar]
- Little EA, Anderson JF, Stafford KC, Eisen L, Eisen RJ, Molaei G. Predicting spatiotemporal patterns of Lyme disease incidence from passively collected surveillance data for Borrelia burgdorferi sensu lato-infected Ixodes scapularis ticks. Ticks Tick Borne Dis. 2019:10(5):970–980. 10.1016/j.ttbdis.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubelczyk CB, Elias SP, Rand PW, Holman MS, Lacombe EH, Smith RP. Habitat associations of Ixodes scapularis (Acari: Ixodidae) in Maine. Environ Entomol. 2004:33(4):900–906. 10.1603/0046-225x-33.4.900 [DOI] [Google Scholar]
- MDH. Minnesota Department of Health. Lyme Disease Statistics. 2022. [accessed 2022 Jun 24]. https://www.health.state.mn.us/diseases/lyme/statistics.html#:~:text=In%202018%2C%20950%20confirmed%20Lyme,evidence%20of%20infection)%20were%20reported. [Google Scholar]
- Mejlon HA, Jaenson TG. Questing behaviour of Ixodes ricinus ticks (Acari: Ixodidae). Exp Appl Acarol. 1997:21:747–754. [DOI] [PubMed] [Google Scholar]
- Moore SM, Eisen RJ, Monaghan A, Mead P. Meteorological influences on the seasonality of Lyme disease in the United States. Am J Trop Med Hyg. 2014:90(3):486–496. 10.4269/ajtmh.13-0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neitzel DF, Jarnefeld JL, Sjogren RD. An Ixodes scapularis (deer tick) distribution study in the Minneapolis-St.Paul, Minnesota area. Soc Vect Ecol. 1993:18:67–73. [Google Scholar]
- Ogden NH, Bigras-Poulin M, O’Callaghan CJ, Barker IK, Kurtenbach K, Lindsay LR, Charron DF. Vector seasonality, host infection dynamics and fitness of pathogens transmitted by the tick Ixodes scapularis. Parasitology. 2007:134(Pt 2):209–227. 10.1017/S0031182006001417 [DOI] [PubMed] [Google Scholar]
- Ogden NH, Pang G, Ginsberg HS, Hickling GJ, Burke RL, Beati L, Tsao JI. Evidence for geographic variation in life-cycle processes affecting phenology of the Lyme disease vector Ixodes scapularis (Acari: Ixodidae) in the United States. J Med Entomol. 2018:55(6):1386–1401. 10.1093/jme/tjy104 [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, Cepeda OM, Hazler KR, Miller MC. Ecology of Lyme disease: habitat associations of ticks (Ixodes scapularis) in a rural landscape. Ecol Appl. 1995:5(2):353–361. 10.2307/1942027 [DOI] [Google Scholar]
- Ostfeld RS, Hazler KR, Cepeda OM. Temporal and spatial dynamics of Ixodes scapularis (Acari: Ixodidae) in a rural landscape. J Med Entomol. 1996:33(1):90–95. 10.1093/jmedent/33.1.90 [DOI] [PubMed] [Google Scholar]
- Pepin KM, Eisen RJ, Mead PS, Piesman J, Fish D, Hoen AG, Barbour AG, Hamer S, Diuk-Wasser MA. Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am J Trop Med. 2012:86:1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Mather TN, Dammin GJ, Telford SR, Lastavica CC, Spielman A. Seasonal variation of transmission risk of Lyme disease and human babesiosis. Am J Epidemiol. 1987:126(6):1187–1189. 10.1093/oxfordjournals.aje.a114757 [DOI] [PubMed] [Google Scholar]
- Pritt BS, Respicio-Kingry LB, Sloan LM, Schriefer ME, Replogle AJ, Bjork J, Liu G, Kingry LC, Mead PS, Neitzel DF, et al. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int J Syst Evol. 2016:66:4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Sloan LM, Johnson DKH, Munderloh UG, Paskewitz SM, McElroy KM, McFadden JD, Binnicker MJ, Neitzel DF, Liu G, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. NEJM. 2011:365:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusinski MA, Kokas JE, Hukey KT, Kogut SJ, Lee J, Backenson PB. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (acari: Ixodidae) collected from recreational lands in the Hudson Valley region, New York state. J Med Entomol. 2014:51(1):226–236. 10.1603/me13101 [DOI] [PubMed] [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2022. https://www.R-project.org/. [Google Scholar]
- Robinson SJ, Neitzel DF, Moen RA, Craft ME, Hamilton KE, Johnson LB, Mulla DJ, Munderloh UG, Redig PT, Smith KE, et al. Disease risk in a dynamic environment: the spread of tick-borne pathogens in Minnesota, USA. EcoHealth. 2015:12(1):152–163. 10.1007/s10393-014-0979-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsville TF, Dill GM, Bryant AM, Desjardins CC, Dill JF. Statewide passive surveillance of Ixodes scapularis and associated pathogens in Maine. Vector Borne Zoonotic Dis. 2021:21(6):406–412. 10.1089/vbz.2020.2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze TL, Bowen GS, Lakat MF, Parkin WE, Shisler JK. Seasonal abundance and hosts of Ixodes dammini (Acari: Ixodidae) and other ixodid ticks from an endemic Lyme disease focus in New Jersey, USA. J Med Entomol. 1986:23(1):105–109. 10.1093/jmedent/23.1.105 [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA. Meteorologically mediated diurnal questing of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. J Med Entomol. 2003:40(4):395–402. 10.1603/0022-2585-40.4.395 [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Hung RW. Biases associated with several sampling methods used to estimate abundance of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1997:34(6):615–623. 10.1093/jmedent/34.6.615 [DOI] [PubMed] [Google Scholar]
- Siegel JP, Kitron U, Bouseman JK. Spatial and temporal distribution of Ixodes dammini (Acari: Ixodidae) in a northwestern Illinois state park. J Med Entomol. 1991:28(1):101–104. 10.1093/jmedent/28.1.101 [DOI] [PubMed] [Google Scholar]
- Simmons TW, Shea J, Myers-Claypole MA, Kruise R, Hutchinson ML. Seasonal activity, density, and collection efficiency of the blacklegged tick (Ixodes scapularis) (Acari: Ixodidae) in Mid-Western Pennsylvania. J Med Entomol. 2015:52(6):1260–1269. 10.1093/jme/tjv132 [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, Levy GF. Ecology of the American dog tick, Dermacentor variabilis, in a study area in Virginia. 2. distribution in relation to vegetative types. Ann Entomol Soc. 1972:65:1175–1182. [Google Scholar]
- Sonenshine DE, Stout IJ. Use of old-field habitats by the American dog tick, Dermacentor variabilis. Ann Entomol Soc. 1968:61:679–686. [DOI] [PubMed] [Google Scholar]
- Stafford KC, Cartter ML, Magnarelli LA, Ertel SH, Mshar PA. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Microbiol. 1998:36(5):1240–1244. 10.1128/JCM.36.5.1240-1244.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromdahl E, Hamer S, Jenkins S, Sloan L, Williamson P, Foster E, Nadolny R, Elkins C, Vince M, Pritt B. Comparison of phenology and pathogen prevalence, including infection with the Ehrlichia muris-like (EML) agent, of Ixodes scapularis removed from soldiers in the midwestern and the northeastern United States over a 15 year period (1997-2012). Parasites Vectors. 2014:7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ML, Spielman A. Seasonal activity of immature Ixodes dammini (Acari: Ixodidae). J Med Entomol. 1985:22(4):408–414. 10.1093/jmedent/22.4.408 [DOI] [PubMed] [Google Scholar]
- Xu G, Mather TN, Hollingsworth CS, Rich SM. Passive surveillance of Ixodes scapularis (Say), their biting activity, and associated pathogens in Massachusetts. Vector Borne Zoonotic Dis. 2016:16(8):520–527. 10.1089/vbz.2015.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval B, Spielman A. Duration and regulation of the developmental cycle of Ixodes dammini (Acari: Ixodidae). J. Medical Entomol. 1990:27(2):196–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.