Abstract
Background
Prelabour rupture of the membranes (PROM) at or near term (defined in this review as 36 weeks' gestation or beyond) increases the risk of infection for the woman and her baby. The routine use of antibiotics for women at the time of term PROM may reduce this risk. However, due to increasing problems with bacterial resistance and the risk of maternal anaphylaxis with antibiotic use, it is important to assess the evidence addressing risks and benefits in order to ensure judicious use of antibiotics. This review was undertaken to assess the balance of risks and benefits to the mother and infant of antibiotic prophylaxis for PROM at or near term.
Objectives
To assess the effects of antibiotics administered prophylactically to women with PROM at 36 weeks' gestation or beyond, on maternal, fetal and neonatal outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 July 2014).
Selection criteria
All randomised trials that compared outcomes for women and infants when antibiotics were administered prophylactically for prelabour rupture of the membranes at or near term, with outcomes for controls (placebo or no antibiotic).
Data collection and analysis
Two review authors independently extracted the data and assessed risk of bias in the included studies. Additional data were received from the investigators of included studies.
Main results
This update includes an additional two studies involving 1801 women, giving a total of four included studies of 2639 women. Whereas the previous version of this review showed a statistically significant reduction in endometritis with the use of antibiotics, no such effect was shown in this update (average risk ratio (RR) 0.34, 95% confidence interval (CI) 0.05 to 2.31). No differences were shown on the primary outcome measures of probable early‐onset neonatal sepsis (average RR 0.69, 95%; CI 0.21 to 2.33); definite early‐onset neonatal sepsis (average RR 0.57, 95% CI 0.08 to 4.26); maternal infectious morbidity (chorioamnionitis and/or endometritis) (average RR 0.48, 95% CI 0.20 to 1.15); stillbirth (RR 3.00, 95% CI 0.61 to 14.82); and perinatal mortality (RR 1.98, 95% CI 0.60 to 6.55), though the number of cases in the control group for these outcomes was low. There were no cases of neonatal mortality or serious maternal outcome in the studies assessed. Caesarean section was increased with the use of antibiotics (RR 1.33, 95% CI 1.09 to 1.61) as was duration of maternal stay in hospital (mean difference (MD) 0.06 days, 95% CI 0.01 to 0.11), largely owing to one study of 1640 women where repeat caesarean section, increased baseline hypertension and pre‐eclampsia were evident in the antibiotic group, despite random allocation and allocation concealment.
Subgroup analyses by timing of induction (early induction versus late induction) showed no difference in either probable or definite early‐onset neonatal sepsis in the early induction group (RR 1.47, 95% CI 0.80 to 2.70 and RR 1.29, 95% CI 0.48 to 3.44, respectively) or the late induction group (RR 0.14, 95% CI 0.02 to 1.13 and RR 0.16, 95% CI 0.02 to 1.34, respectively), although there were trends toward reduced probable and definite early‐onset neonatal sepsis in the late induction group. A test for subgroup differences confirmed a differential effect of the intervention on probable early‐onset neonatal sepsis between the subgroups (Chi² = 4.50, df = 1 (P = 0.03), I² = 77.8%). No difference in maternal infectious morbidity (chorioamnionitis and/or endometritis) was found in either subgroup, though again there was a trend towards reduced maternal infectious morbidly in the late induction group (average RR 0.34, 95% CI 0.08 to 1.47). No differences were shown in stillbirth or perinatal mortality. The quality of the evidence for the primary outcomes using GRADE was judged to be low to very low.
Authors' conclusions
This updated review demonstrates no convincing evidence of benefit for mothers or neonates from the routine use of antibiotics for PROM at or near term. We are unable to adequately assess the risk of short‐ and long‐term harms from the use of antibiotics due to the unavailability of data. Given the unmeasured potential adverse effects of antibiotic use, the potential for the development of resistant organisms, and the low risk of maternal infection in the control group, the routine use of antibiotics for PROM at or near term in the absence of confirmed maternal infection should be avoided.
Plain language summary
Antibiotics for rupture of membranes when a pregnant women is at or near term but not in labour
Background
Sometimes the protective bag of fluid around an unborn baby (the membranes) break when the baby is due without the onset of labour (regular uterine contractions). This is called PROM or prelabour rupture of the membranes. When this happens there is a risk of infection entering the womb (uterus) and affecting the mother and her baby. Newborn infections are rare but have the potential to cause serious harm requiring neonatal intensive care. Giving a pregnant woman antibiotics when she has PROM may reduce the risk of infections for the woman and her baby. Most women spontaneously start labour within 24 hours, so delaying induction of labour and waiting for spontaneous onset of labour (expectant management) may be a possibility. Another treatment for term PROM is to induce labour with oxytocin or prostaglandins. Women are often given antibiotics to prevent infection, but there are concerns about possible side‐effects of antibiotics, and that overuse of antibiotics can cause resistance to antibiotics so that they become less effective.
Our review questions
Do antibiotics given to women with PROM when they are at or near term (more than 36 weeks' gestation) but not in labour reduce the risk of infection for the baby and the mother? Are there adverse effects from the antibiotics?
What the studies showed
This review included four randomised controlled studies involving 2639 pregnant women at 36 weeks' gestation or more. The evidence showed that routine antibiotics for term PROM did not reduce the risk of infection for pregnant women or their babies when compared to the control group which received a placebo or no antibiotics. There was not enough strong evidence about other outcomes including death, allergic reactions for the woman or complications for the baby, which rarely occurred in the included studies. The quality of the evidence using GRADE was judged to be low to very low.
Overall
The conclusions from this review are limited by the low number of women who developed an infection across the studies overall. There is not enough information in this review to assess the possible side‐effects from the use of antibiotics for women or their infants, particularly for any possible long‐term harms. Because we do not know enough about side‐effects and because we did not find strong evidence of benefit from antibiotics, they should not be routinely used for pregnant women with ruptured membranes prior to labour at term, unless a woman shows signs of infection.
Summary of findings
Summary of findings for the main comparison. Any antibiotic compared with placebo or no antibiotic (subgrouped by timing of induction of labour) for prelabour rupture of membranes at or near term.
| Any antibiotic compared with no antibiotic (subgrouped by timing of induction of labour) for prelabour rupture of membranes at or near term | ||||||
| Population: pregnant women with prelabour rupture of membranes at or near term Settings: maternity hospitals in Spain, Egypt, Chile, Portugal Intervention: any antibiotic Comparison: placebo or no antibiotic | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Any antibiotic compared with no antibiotic (subgrouped by timing of induction of labour) | |||||
| Probable early‐onset neonatal sepsis ‐ Early induction of labour | Study population | RR 1.47 (0.8 to 2.7) | 1640 (1 study) | ⊕⊕⊝⊝ low1 | ||
| 21 per 1000 | 30 per 1000 (17 to 56) | |||||
| Moderate | ||||||
| 21 per 1000 | 31 per 1000 (17 to 57) | |||||
| Probable early‐onset neonatal sepsis ‐ Late induction of labour | Study population | RR 0.14 (0.02 to 1.13) | 838 (2 studies) | ⊕⊝⊝⊝ very low2,3 | ||
| 17 per 1000 | 2 per 1000 (0 to 19) | |||||
| Moderate | ||||||
| 10 per 1000 | 1 per 1000 (0 to 11) | |||||
| Definite early‐onset neonatal sepsis ‐ Early induction of labour | Study population | RR 1.29 (0.48 to 3.44) | 1640 (1 study) | ⊕⊕⊝⊝ low1 | ||
| 9 per 1000 | 11 per 1000 (4 to 29) | |||||
| Moderate | ||||||
| 9 per 1000 | 12 per 1000 (4 to 31) | |||||
| Definite early‐onset neonatal sepsis ‐ Late induction of labour | Study population | RR 0.16 (0.02 to 1.34) | 838 (2 studies) | ⊕⊝⊝⊝ very low2,3 | ||
| 15 per 1000 | 2 per 1000 (0 to 20) | |||||
| Moderate | ||||||
| 8 per 1000 | 1 per 1000 (0 to 11) | |||||
| Maternal infectious morbidity (chorioamnionitis and/or endometritis) ‐ Early induction of labour | Study population | RR 1.15 (0.64 to 2.08) | 1640 (1 study) | ⊕⊕⊝⊝ low1 | ||
| 24 per 1000 | 28 per 1000 (16 to 51) | |||||
| Moderate | ||||||
| 24 per 1000 | 28 per 1000 (15 to 50) | |||||
| Maternal infectious morbidity (chorioamnionitis and/or endometritis) ‐ Late induction of labour | Study population | RR 0.34 (0.08 to 1.47) | 838 (2 studies) | ⊕⊝⊝⊝ very low2,3 | ||
| 70 per 1000 | 24 per 1000 (6 to 103) | |||||
| Moderate | ||||||
| 109 per 1000 | 37 per 1000 (9 to 160) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Wide confidence interval crossing the line of no effect and less than the optimal information size. 2 Unclear and high risk of bias for allocation concealment. 3 Wide confidence interval crossing the line of no effect, few events and small sample size.
Summary of findings 2. Any antibiotic compared with placebo or no antibiotic for prelabour rupture of membranes at or near term.
| Any antibiotic compared with no antibiotic for prelabour rupture of membranes at or near term | ||||||
| Population: pregnant women with prelabour rupture of membranes at or near term Settings: maternity hospitals in Spain, Egypt, Chile, Portugal Intervention: any antibiotic Comparison: placebo or no antibiotic | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Any antibiotic compared with no antibiotic | |||||
| Stillbirth | Study population | RR 3 (0.61 to 14.82) | 1906 (3 studies) | ⊕⊕⊝⊝ low1 | ||
| 2 per 1000 | 6 per 1000 (1 to 31) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Perinatal mortality | Study population | RR 1.98 (0.60 to 6.55) | 2639 (4 studies) | ⊕⊕⊝⊝ low2 | ||
| 3 per 1000 | 6 per 1000 (2 to 20) | |||||
| Moderate | ||||||
| Neonatal mortality | See comment | See comment | Not estimable | 1906 (3 studies) | See comment | There were no cases of neonatal mortality in the trials assessed. |
| Serious maternal outcome | See comment | See comment | Not estimable | 1906 (3 studies) | See comment | There were no cases of serious maternal outcome in the trials assessed. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Wide confidence interval crossing the line of no effect, few events and small sample size. 2 Wide confidence interval crossing the line of no effect, few events.
Background
Description of the condition
Prelabour rupture of membranes (PROM) is defined as rupture of membranes prior to the onset of labour (Duff 1998). Approximately 8% of pregnant women at term experience PROM (Cammu 1990), but the decision as to how term PROM should be managed clinically remains controversial, and there is wide variation in practice with no clear consensus on what constitutes optimal treatment (Marowitz 2007). Although for the majority of women labour will start spontaneously within 24 hours following term PROM, up to 4% of women will not experience spontaneous onset of labour within seven days. While, traditionally, 'term' is defined as gestations of 37 to 41 weeks inclusive, for the purposes of this review infants born at 36 weeks' gestation have been included in the definition of term PROM, as many neonatal outcomes at these gestations are similar to those of gestations greater than 36 weeks (Marshall 2002; Neerhoff 1999).
Description of the intervention
Induction of labour is one strategy intended to reduce infectious morbidity associated with term PROM. There is some evidence that planned management (usually by induction) reduces the risk of infectious maternal morbidity and the number of infants requiring admission to neonatal intensive care (Dare 2006). On the basis of this evidence, clinicians may offer prompt induction with oxytocin or prostaglandins for term PROM (Hannah 1996; Mozurkewich 2006). Others utilise a policy of delayed induction of labour or expectant management (awaiting spontaneous onset of labour), although there remains much discussion regarding the optimum latent period for expectant management (Ezra 2004; Ingemarsson 1996). It is in situations where the latency period from membrane rupture to birth is prolonged that prelabour antibiotics may be beneficial. However, due to increasing problems with bacterial resistance (ACOG 2011; Edwards 2001; Lin 1999) and the rare but potentially life‐threatening risk of maternal anaphylaxis with antibiotic use (ACOG 2011; Heim 1991; Jao 2006), it is essential to ensure that antibiotics are used judiciously and when indicated.
How the intervention might work
The reasons for term PROM are not clearly understood. However, subclinical ascending infection is thought to be influential and has been detected in up to one‐third of women with term PROM (Romero 1992). There is also evidence of changes that happen within and between the cells of the fetal membranes (Moore 2006; Reti 2007). Despite the antibacterial properties of amniotic fluid, there is an increased risk of infection for the woman and her infant following term PROM (Newton 1993). Neonatal infections in the term population are rare occurrences (2% to 4%), but have the potential for causing mortality or serious morbidity (including the need for neonatal intensive care and mechanical ventilation). Prophylactic antibiotics can be effective against many (but not all) potential pathogens (ACOG 2011). Therefore, the routine use of antibiotics for women at the time of term PROM may reduce the risk of infection for the woman and her baby.
Why it is important to do this review
This review aims to address the balance of risks and benefits to the mother and infant of antibiotic prophylaxis for prelabour rupture of the membranes at or near term. The review will also explore the differential effects of antibiotics and induction of labour.
Although Group B Streptococcus (GBS) is the most common cause of serious neonatal infection in the first seven days of life, this review does not address the role of intrapartum antibiotic GBS prophylaxis as this is a separate clinical question from prelabour antibiotic usage. The role of intrapartum antibiotic prophylaxis for GBS is addressed in another Cochrane review (Ohlsson 2014).
Objectives
To assess the effects of antibiotics administered prophylactically to women with prelabour rupture of the membranes at 36 weeks' gestation or beyond, on maternal, fetal and neonatal outcomes.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials that compared outcomes for women and infants when antibiotics were administered prophylactically for prelabour rupture of the membranes at or near term, with outcomes for controls (placebo or no antibiotic). Studies using a quasi‐random allocation method and cross‐over design were excluded. Cluster‐randomised trials were eligible for inclusion.
Types of participants
Women with spontaneous rupture of the fetal membranes prior to the onset of regular uterine contractions at 36 weeks' gestation or beyond.
Types of interventions
Any antibiotics, administered as prophylaxis, by any route, to women at 36 weeks' gestation or beyond, with prelabour rupture of the membranes.
Types of outcome measures
In this review we aimed to examine the effect of prophylactic antibiotics on clinically important outcome measures of maternal and infant morbidity and mortality, and maternal adverse effects. We also explored cost effectiveness and health service utilisation.
Primary outcomes
Primary outcomes were chosen to be most representative of the clinically important measures of effectiveness and complications. We assessed the primary outcome of maternal infectious morbidity (chorioamnionitis and/or endometritis) collectively, and explored its individual components as secondary outcomes. Primary outcomes were:
probable early‐onset neonatal sepsis;
definite early‐onset neonatal sepsis;
maternal infectious morbidity (chorioamnionitis and/or endometritis);
stillbirth;
perinatal mortality;
neonatal mortality;
serious maternal outcome (defined as death, cardiac arrest, respiratory arrest, anaphylaxis, admission to intensive care unit).
Secondary outcomes
Secondary outcomes include other measures of morbidity and mortality, effectiveness, complications, health service utilisation and cost effectiveness.
Maternal outcomes
Chorioamnionitis (either suspected or proven);
endometritis;
caesarean section;
operative vaginal birth;
internal fetal monitoring;
epidural analgesia;
postpartum haemorrhage;
postpartum pyrexia;
postpartum septicaemia;
wound infection;
postpartum antibiotic usage;
breastfeeding on discharge from hospital;
adverse effects.
Neonatal outcomes
Neonatal meningitis;
neonatal pneumonia;
Apgar score less than seven at five minutes;
admission to neonatal special care nursery;
admission to neonatal intensive care unit;
antibiotic usage;
respiratory distress syndrome; and
use of mechanical ventilation.
Cost effectiveness
Health service utilisation
Duration of maternal stay in hospital (days);
duration of neonatal stay in hospital (days);
duration of neonatal stay in intensive care unit (days).
A priori subgroup analyses
Nulliparae;
early induction of labour (less than 12 hours from rupture of membranes);
late induction of labour (at 12 hours or greater from rupture of membranes).
Outcome definitions
Suspected or proven chorioamnionitis: uterine infection prior to birth of the baby diagnosed on clinical signs, including pyrexia with or without a positive culture result or haematological signs of infection.
Endometritis: clinical signs of uterine infection following labour and birth.
Maternal pyrexia: maternal temperature of 38 degrees Celsius or higher.
Postpartum septicaemia: maternal positive blood culture in the presence of pyrexia following birth of the baby.
Early onset sepsis: definite or probable infection within the first seven days of life.
Definite infection: positive culture from a normally sterile site.
Probable infection: clinical signs and blood count suggestive of infection and a possible causative organism identified (i.e. gastric aspirate, urine antigen).
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 July 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Searches carried out in the previous version of the review are listed in Appendix 1.
Data collection and analysis
For the methods used when assessing the studies identified in a previous version of this review, seeFlenady 2002.
For this update, the following methods were used for assessing the two studies that were identified as a result of the updated search. Where required, information pertaining to the two previous studies was updated according to methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
At least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We have assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We have assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention the participant received.
We assessed the methods as:
low risk of bias (e.g. blinding, and unlikely that the blinding could have been broken, or no blinding or incomplete blinding, but outcome unlikely to be influenced);
high risk of bias (e.g., no blinding, incomplete or broken blinding, and outcome likely to be influenced);
unclear risk of bias.
(5) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the study authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(6) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(7) Other bias (checking for bias due to problems not covered by (1) to (6) above)
We described for each included study any important concerns we had about other possible sources of bias.
Assessment of the body of evidence ‐ The GRADE approach
For this update we also evaluated the quality of the evidence using the GRADE approach (Schunemann 2009). The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for specific outcomes. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias. In this review we used the GRADE approach to assess the seven primary outcomes, as follows:
probable early‐onset neonatal sepsis;
definite early‐onset neonatal sepsis;
maternal infectious morbidity (chorioamnionitis and/or endometritis);
stillbirth;
perinatal mortality;
neonatal mortality;
serious maternal outcome (defined as death, cardiac arrest, respiratory arrest, anaphylaxis, admission to intensive care unit).
'Summary of findings' table
We used GRADE Profiler (GRADE 2008) to import data from Review Manager 5.3 (RevMan 2014) in order to create a 'Summary of findings’ table. A summary of the intervention effect and a measure of quality according to the GRADE approach is presented in the 'Summary of findings' table for each of the above outcomes. Due to high heterogeneity in the analysis of the first three primary outcomes (probable early‐onset neonatal sepsis; definite early‐onset neonatal sepsis; maternal infectious morbidity), a 'Summary of findings' table was produced presenting the results for these three outcomes subgrouped by timing of induction of labour (early induction versus late induction).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between studies. We planned to use the standardised mean difference (SMD) to combine studies that measured the same outcome, but used different methods. However, SMD was not used in this update.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review, but we may include trials of this type in future updates.
If cluster‐randomised trials are included in future reviews, we plan to include cluster‐randomised trials in the analyses along with individually‐randomised trials. Their sample sizes will be adjusted using the methods described in the Handbook (Higgins 2011) using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation units.
Other unit of analysis issues
Cross‐over trials
We excluded cross‐over designs as these are unlikely to be a valid study design for Pregnancy and Childbirth reviews.
Multiple pregnancies
We did not identify any eligible studies that included multiple pregnancies in this review update, but we may include studies of this type in future updates.
When multiple pregnancies are included in studies, wherever possible, analyses should be adjusted for clustering to take into account the non‐independence of babies from the same pregnancy (Gates 2004). Treating babies from multiple pregnancies as if they are independent, when they are more likely to have similar outcomes than babies from different pregnancies, will overestimate the sample size and give confidence intervals that are too narrow. Each woman can be considered a cluster in multiple pregnancy, with the number of individuals in the cluster being equal to the number of fetuses in her pregnancy. Analysis using cluster‐trial methods allows calculation of risk ratio and adjustment of confidence intervals. Usually this will mean that the confidence intervals become wider. Although this may make little difference to the conclusion of a trial, it avoids misleading results in those trials where the difference may be substantial.
If multiple pregnancies are included in future updates of this review, we will adjust for clustering in the analyses wherever possible, and to use the inverse variance method for adjusted analyses, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and in Yelland 2011.
Dealing with missing data
For included studies, levels of attrition were noted. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where studies were examining the same intervention, and the studies' populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between studies, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across studies was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between studies. If the average treatment effect was not clinically meaningful, we did not combine studies. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity among primary outcomes, we investigated it using subgroup analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
The following subgroup analyses were planned for the primary outcomes:
nulliparae;
early induction of labour (less than 12 hours from rupture of membranes);
late induction of labour (at 12 hours or greater from rupture of membranes).
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We did not conduct sensitivity analyses in this update due to the low number of included studies. In future updates we plan to carry out sensitivity analyses to explore the effect of risk of bias assessed by random sequence generation, concealment of allocation, and blinding of participants and personnel, with poor‐quality studies (including those assessed as high or unknown risk of bias) being excluded from the analyses in order to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
Eight studies in all were identified for possible inclusion in this review, with four studies being excluded for the reasons outlined below. This updated review includes an additional two studies involving 1801 women (Nabhan 2014; Passos 2012), giving a total of four included studies of 2639 women. The previous version included two studies and 838 women.
Included studies
Two of the included studies compared antibiotics versus no antibiotics (Cararach 1998; Passos 2012) and two compared antibiotics with placebo (Nabhan 2014; Ovalle 1998). Cararach 1998 received partial support from the Spanish Ministry of Health, Nabhan 2014 and Ovalle 1998 received in‐house support, and Passos 2012 received no funding. Declaration of conflicts of interest was provided for Nabhan 2014, Ovalle 1998 and Passos 2012, and all authors reported no conflicts.
Participants
The population of women in the included studies were homogenous. The gestation of women enrolled was 36 weeks or greater for Cararach 1998 and Nabhan 2014, 37 weeks or greater for Passos 2012, and 37 to 42 weeks for Ovalle 1998. All studies excluded women with multiple pregnancy and major obstetric complications and had stipulated criteria for diagnosis of membrane rupture.
Some differences were apparent in management protocols and types of antibiotics used as outlined below.
Management protocols
Cararach 1998 and Ovalle 1998 had systematic approaches to routine maternal cervicovaginal cultures on admission. Ovalle 1998 also conducted amniocentesis for culture of amniotic fluid.
Induction of labour was undertaken in all studies however, the timing of induction differed. Cararach 1998 employed a policy of induction of labour for all women not in labour after 12 hours of membrane rupture. In Ovalle 1998, induction of labour was undertaken within 24 hours of membrane rupture and in an unknown proportion an earlier threshold was used (details are unclear). In this study all women undergoing induction of labour were induced after 12 hours (late induction). Nabhan 2014 employed a policy of immediate induction, ensuring the latency between rupture of membranes and labour was less than 12 hours. In Passos 2012, whether induction of labour or caesarean section was performed was determined at the discretion of the attending physicians (no further details given). All studies used Intravenous oxytocin as the method of induction of labour.
All studies employed management protocols which involved attempts to avoid or minimise vaginal examinations. Routine antibiotic prophylaxis at the time of caesarean section was given in Cararach 1998, Nabhan 2014 and Passos 2012, but inconsistently in Ovalle 1998.
Nabhan 2014 reported that no Group B streptococcal cultures were taken and no routine intrapartum Group B streptococcal prophylaxis was given, as per the hospital protocol for spontaneous rupture of membranes. Group B streptococcal cultures were taken in Passos 2012 and women with a positive or unknown culture between 35 and 37 weeks were excluded from the study. Neither Cararach 1998 nor Ovalle 1998 included a description of a policy for prevention of neonatal early onset Group B streptococcal disease. Ovalle 1998 administered routine antibiotics to neonates of mothers with clinical chorioamnionitis or positive maternal admission cultures (Group B streptococcal, haemophilus influenzae or chlamydia trachomatis). As per hospital policy, Nabhan 2014 prescribed routine prophylactic antibiotics to all neonates where maternal risk factors were present. In Passos 2012, antibiotic therapy was initiated only following early‐onset neonatal infection, which was diagnosed, in this study, with or without a positive blood culture. Cararach 1998 did not describe the use of neonatal antibiotics for maternal risk factors.
Antibiotics
Cararach 1998 used intravenous ampicillin (or intramuscular erythromycin for women with penicillin allergy) with intramuscular gentamicin, Nabhan 2014 used parenteral ampicillin/sulbactam, Ovalle 1998 used intravenous cefuroxime and clindamycin for 48 hours then oral cefuroxime and clindamycin for a further 24 hours, and Passos 2012 used intravenous ampicillin with gentamicin.
Outcomes
The outcomes of maternal infectious morbidity (chorioamnionitis and/or endometritis) and early‐onset neonatal sepsis (probable and definite) were assessed in all included studies. Maternal infection (chorioamnionitis and/or endometritis) was well defined in all studies. All studies diagnosed early‐onset neonatal sepsis according to well defined clinical criteria, with or without positive blood culture. although no cases of neonatal morbidity were reported in Ovalle 1998.
Excluded studies
Four studies were excluded: Lebherz 1963 was excluded as the antibiotic (tetracycline) is now contraindicated in pregnancy and Brelje 1966 and Gordon 1974 because a quasi‐random method of allocation was employed. A further study was excluded because additional information on methods of randomisation and allocation to treatment was not available (Walss Rodriguez 1988).
Risk of bias in included studies
The quality of the included studies ranged from fair to high. Cararach 1998 was of low quality and Ovalle 1998 and Passos 2012 were of moderate quality. Nabhan 2014 was of very high quality, with low risk of bias on all domains.
Please see Figure 1 and Figure 2 for a summary of 'Risk of bias' assessments. Please refer to the table of Characteristics of included studies for details about each study.
1.
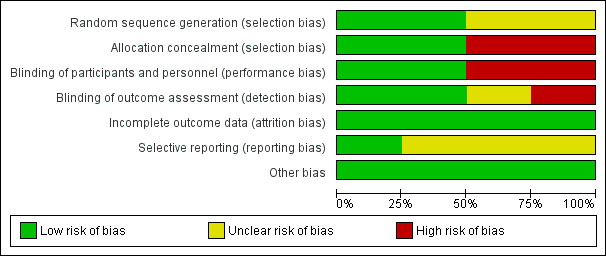
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
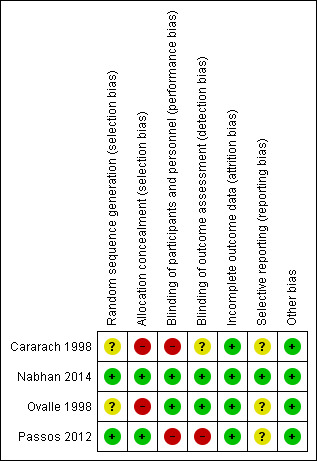
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Adequate sequence generation was achieved and judged as low risk for selection bias in two of the four studies (Nabhan 2014; Passos 2012), both of which used computer‐generated random number lists. Risk of bias was unclear in Cararach 1998 and Ovalle 1998. Cararach 1998 used a 'randomisation list in each participating centre' but provided no further details and Ovalle 1998 used a 'pre‐established allocation code' but, again, provided no further details. Allocation concealment was achieved in Nabhan 2014 and Passos 2012, but not in Cararach 1998 or Ovalle 1998.
Blinding
The intervention was blinded to participants and personnel in Ovalle 1998 and Nabhan 2014 and judged as low risk for performance bias owing to the use of a placebo for the control group. No placebo was used in Cararach 1998 or Passos 2012 and we therefore judged these studies as high risk for performance bias. Nabhan 2014 undertook blinded assessment of outcomes, as did Ovalle 1998 for all outcomes and Cararach 1998 for neonatal outcomes, with disclosure of allocation only in cases of neonatal sepsis. We judged the risk of detection bias as high for Passos 2012, who undertook only partial blinding of outcomes assessors (only one of the three outcome assessors was blind to group allocation during the process).
Incomplete outcome data
All studies reported an intention‐to‐treat analysis. Cararach 1998 and Ovalle 1998 reported complete follow‐up. Some attrition occurred in Nabhan 2014 due to incomplete data collection, and in Passos 2012 due to missing data and protocol violations, however, attrition was low and comparable between groups in both studies. All studies were judged as low risk for attrition bias.
Selective reporting
The potential for selective reporting was unclear in Cararach 1998, Ovalle 1998 and Passos 2012 as no protocols were available for review. A prospectively‐registered protocol was available for Nabhan 2014 and all outcomes were reported as expected (low risk for reporting bias).
Other potential sources of bias
We found no evidence of other bias in the studies.
Effects of interventions
Comparisons and subgroup analyses were undertaken as follows.
Comparison 1: any antibiotic compared with placebo or no antibiotic.
Comparison 2: any antibiotic compared with placebo or no antibiotic subgrouped by timing of induction of labour: early induction (less than 12 hours from rupture of membranes) versus late induction (at 12 hours or greater from rupture of membranes).
We were unable to conduct the remaining planned subgroup analysis due to unavailability of data.
Comparison 1. Any antibiotic compared with placebo or no antibiotic
The results of four studies comparing the use of antibiotics with no use of antibiotics for women with term prelabour rupture of membranes (PROM) (Cararach 1998; Nabhan 2014; Ovalle 1998; Passos 2012), involving a total of 2639 women, are included in this review.
Primary outcomes
Early‐onset neonatal sepsis (probable and definite)
A moderate degree of statistical heterogeneity was evident for both of these outcome measures. However, upon exploration of the possible reasons for the heterogeneity by examining clinical features of the studies, we considered an overall summary was meaningful using a random‐effects analysis. Comparing antibiotics to placebo or no antibiotics, no difference was shown in probable early‐onset neonatal sepsis (average risk ratio (RR) 0.69, 95% confidence interval (CI) 0.21 to 2.33; babies = 2639; studies = four; Tau² = 0.71; Chi² = 5.34, df = 2 (P = 0.07); I² = 63%) (Analysis 1.1), or definite early onset neonatal sepsis (average RR 0.57, 95% CI 0.08 to 4.26; babies = 2639; studies = four; Tau² = 1.51; Chi² = 3.14, df = 1 (P = 0.08); I² = 68%) (Analysis 1.2). Cases of definite early onset neonatal sepsis were low (less than 1% of all neonates), and there were no cases in two of the four studies (Ovalle 1998; Passos 2012).
1.1. Analysis.
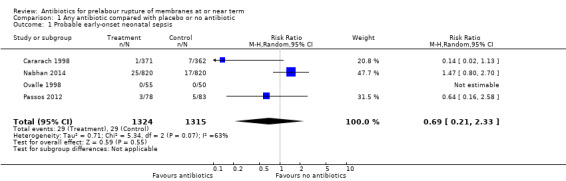
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 1 Probable early‐onset neonatal sepsis.
1.2. Analysis.
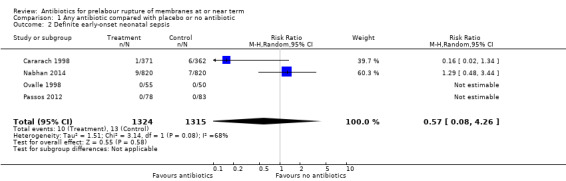
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 2 Definite early‐onset neonatal sepsis.
Maternal infectious morbidity (chorioamnionitis and/or endometritis) (Analysis 1.3)
1.3. Analysis.
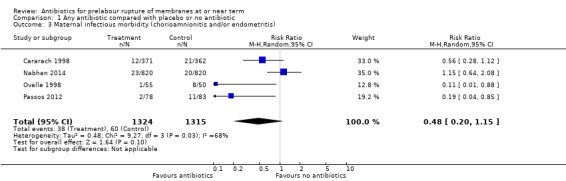
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 3 Maternal infectious morbidity (chorioamnionitis and/or endometritis).
A moderate degree of statistical heterogeneity was also evident for this outcome measure. Upon exploration of the possible reasons for the heterogeneity by examining clinical features of the studies, we again considered an overall summary was meaningful using a random‐effects analysis. No difference in maternal infectious morbidity (chorioamnionitis and/or endometritis) was shown (average RR 0.48, 95% CI 0.20 to 1.15; women = 2639; studies = four; Tau² = 0.48; Chi² = 9.27, df = 3 (P = 0.03); I² = 68%).
Stillbirth (Analysis 1.4)
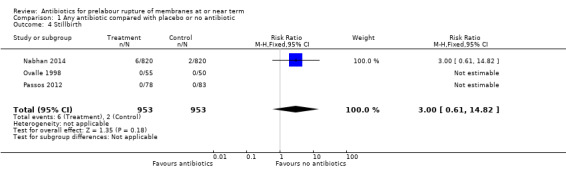
1.4. Analysis.
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 4 Stillbirth.
No difference in stillbirth was shown when comparing antibiotics with placebo or no antibiotics (RR 3.00, 95% CI 0.61 to 14.82; babies = 1906; studies = three). There were no cases of stillbirth in two of the three studies reporting this outcome (Ovalle 1998; Passos 2012).
Perinatal mortality (Analysis 1.5)
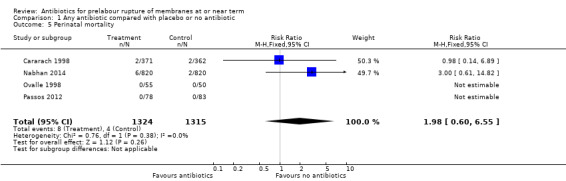
1.5. Analysis.
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 5 Perinatal mortality.
All studies reported data on perinatal mortality, and in two studies (Ovalle 1998; Passos 2012) there were no cases. Overall, no difference was detected (RR 1.98, 95% CI 0.60 to 6.55; babies = 2639; studies = four).
Neonatal mortality
There were no cases of neonatal mortality in three studies reporting this outcome (Nabhan 2014; Ovalle 1998; Passos 2012).
Serious maternal outcome (defined as death, cardiac arrest, respiratory arrest, anaphylaxis, admission to intensive care unit)
There were no cases of serious maternal outcome in the three trials reporting this outcome (Nabhan 2014; Ovalle 1998; Passos 2012).
Secondary outcomes
For the woman
Caesarean section was increased with the use of antibiotics when compared to placebo or no antibiotics (RR 1.33, 95% CI 1.09 to 1.61; number needed to treat to harm (NNTH) 20, 95% CI 11 to 74; women = 1906; studies = three (Analysis 1.10), as was duration of maternal stay in hospital (mean difference (MD) 0.06 days, 95% CI 0.01 to 0.11; women = 1906; studies = three) (Analysis 1.28).
1.10. Analysis.
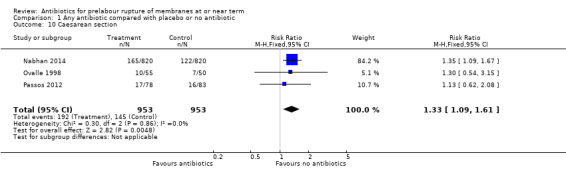
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 10 Caesarean section.
1.28. Analysis.
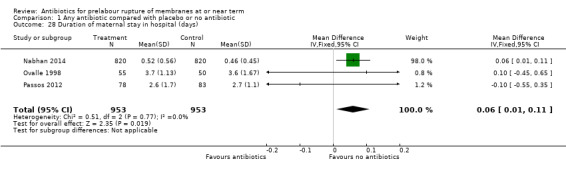
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 28 Duration of maternal stay in hospital (days).
Two studies comparing antibiotics with placebo reported data on postpartum antibiotic usage. Due to extreme heterogeneity (Tau² = 1.32; Chi² = 14.47, df = 1 (P = 0.0001); I² = 93%), we did not combine the data from these studies. One study of 1640 women (Nabhan 2014) reported no difference in postpartum antibiotic usage (RR 1.11, 95% CI 0.60 to 2.04), whereas the other study of 105 women (Ovalle 1998) reported an increase in postpartum antibiotic usage (RR 5.95, 95% CI 3.22 to 10.99, NNTH 2, 95% CI 1 to 3) (Analysis 1.18). The same two studies also reported data on postpartum pyrexia. Again, due to substantial heterogeneity (Tau² = 1.79; Chi² = 4.11, df = 1 (P = 0.04); I² = 76%) we did not combine these data. The Nabhan 2014 study reported no difference in postpartum pyrexia (RR 0.97, 95% CI 0.58 to 1.61), whereas the Ovalle 1998 study reported a reduction in postpartum pyrexia (RR 0.11, 95% CI 0.01 to 0.88; number needed to treat to benefit (NNTB) 7, 95% CI 8 to 53) (Analysis 1.15).
1.18. Analysis.
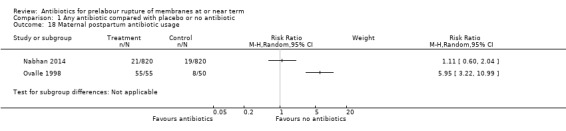
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 18 Maternal postpartum antibiotic usage.
1.15. Analysis.
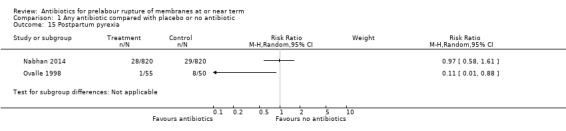
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 15 Postpartum pyrexia.
No differences were shown on the remaining secondary maternal outcomes as outlined below. For the outcomes of chorioamnionitis, endometritis, and postpartum haemorrhage, a moderate degree of statistical heterogeneity was evident, but upon exploration of the possible reasons for the heterogeneity by examining clinical features of the studies, we again considered an overall summary was meaningful using a random‐effects analysis:
chorioamnionitis (suspected or proven) (average RR 0.65, 95% CI 0.34 to 1.26; women = 2639; studies = four; Tau² = 0.17; Chi² = 5.07, df = 3 (P = 0.17); I² = 41%) (Analysis 1.8);
endometritis (average RR 0.34, 95% CI 0.05 to 2.31; women = 2639; studies = four; Tau² = 2.06; Chi² = 6.74, df = 3 (P = 0.08); I² = 55%) (Analysis 1.9);
operative vaginal birth (RR 0.95, 95% CI 0.63 to 1.44; women = 1906; studies = three) (Analysis 1.11);
internal fetal monitoring (no cases, two studies);
epidural analgesia (RR 1.00, 95% CI 0.98 to 1.02; women = 266; studies = two) (Analysis 1.13);
postpartum haemorrhage (average RR 1.11, 95% CI 0.14 to 8.93; women = 1906; studies = three; Tau² = 1.51; Chi² = 2.23, df = 1 (P = 0.14); I² = 55%) (Analysis 1.14);
postpartum septicaemia (no cases, three studies);
wound infection (RR 0.79, 95% CI 0.36 to 1.72; women = 1906; studies = three) (Analysis 1.17);
adverse effects (RR 2.93, 95% CI 0.12 to 71.63; women = 2639; studies = four) (Analysis 1.19).
1.8. Analysis.
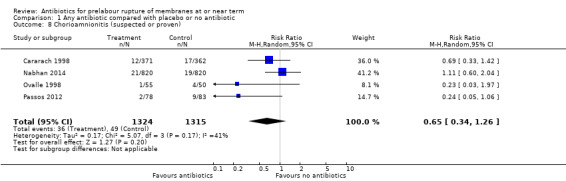
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 8 Chorioamnionitis (suspected or proven).
1.9. Analysis.
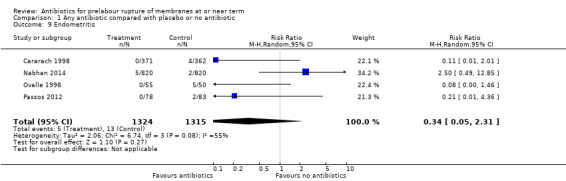
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 9 Endometritis.
1.11. Analysis.
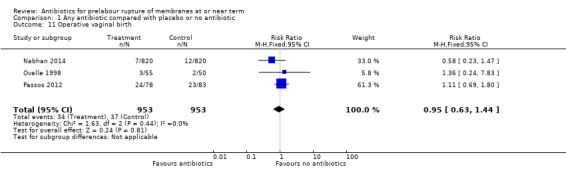
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 11 Operative vaginal birth.
1.13. Analysis.
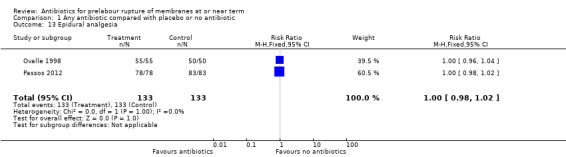
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 13 Epidural analgesia.
1.14. Analysis.
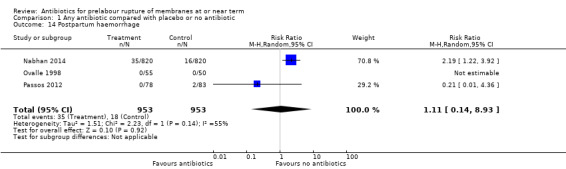
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 14 Postpartum haemorrhage.
1.17. Analysis.
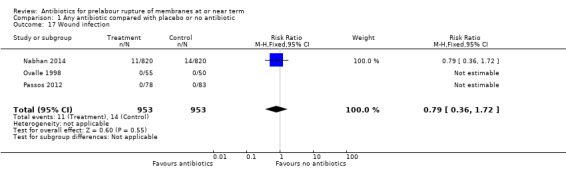
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 17 Wound infection.
1.19. Analysis.
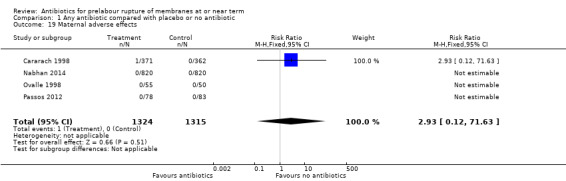
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 19 Maternal adverse effects.
No studies reported data on breastfeeding on discharge from hospital.
For the neonate
One study of 105 neonates (Ovalle 1998) reported reduced neonatal stay in hospital with the use of antibiotics (MD ‐0.90, 95% CI ‐1.34 to ‐0.46) (Analysis 1.29).
1.29. Analysis.
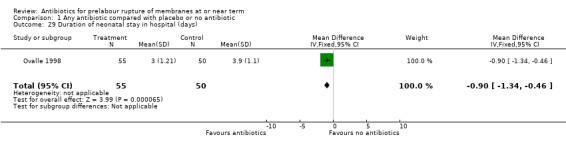
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 29 Duration of neonatal stay in hospital (days).
No differences were shown on the remaining secondary neonatal outcomes as outlined below. For the outcome of neonatal antibiotic usage a moderate degree of statistical heterogeneity was evident. Upon exploration of the possible reasons for the heterogeneity by examining clinical features of the studies, we considered an overall summary was meaningful using a random‐effects analysis:
neonatal meningitis (RR 0.33, 95% CI 0.03 to 3.11; babies = 2639; studies = four) (Analysis 1.20);
neonatal pneumonia (RR 0.33, 95% CI 0.01 to 7.96; babies = 2639; studies = four) (Analysis 1.21);
Apgar score less than seven at five minutes (RR 1.65, 95% CI 0.81 to 3.36; babies = 2639; studies = four) (Analysis 1.22);
admission to neonatal special care nursery (average RR 0.32, 95% CI 0.06 to 1.74; babies = 266; studies = two; Tau² = 0.77; Chi² = 1.97, df = 1 (P = 0.16); I² = 49%) (Analysis 1.23);
admission to neonatal intensive care unit (RR 1.23, 95% CI 0.82 to 1.85; babies = 1906; studies = three) (Analysis 1.24);
antibiotic usage (average RR 0.55, 95% CI 0.15 to 1.97; babies = 1906; studies = three; Tau² = 0.87; Chi² = 6.55, df = 2 (P = 0.04); I² = 69%) (Analysis 1.25);
respiratory distress syndrome (no cases, two studies);
use of mechanical ventilation (RR 1.21, 95% CI 0.50 to 2.91; babies = 2639; studies = four) (Analysis 1.27);
duration of neonatal stay in intensive care unit (MD 0.05 days, 95% CI ‐0.09 to 0.19; babies = 1640; studies = one) (Analysis 1.30).
1.20. Analysis.
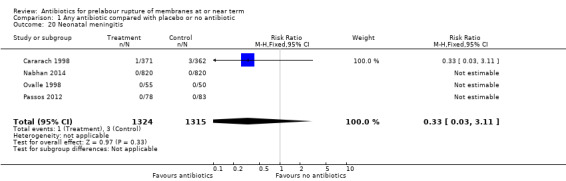
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 20 Neonatal meningitis.
1.21. Analysis.
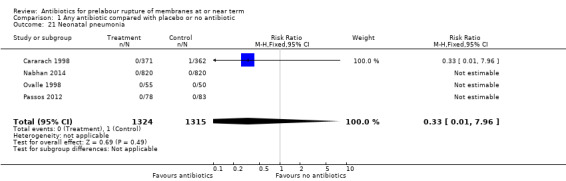
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 21 Neonatal pneumonia.
1.22. Analysis.
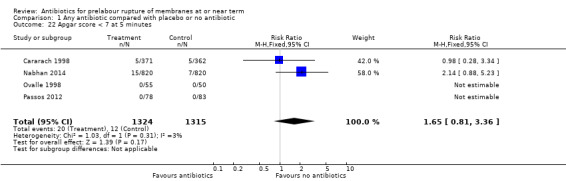
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 22 Apgar score < 7 at 5 minutes.
1.23. Analysis.
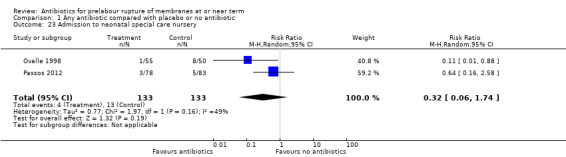
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 23 Admission to neonatal special care nursery.
1.24. Analysis.
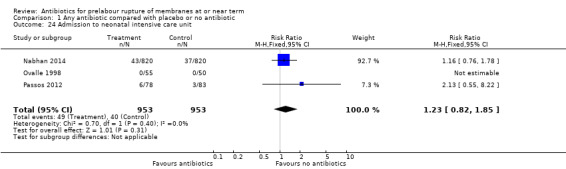
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 24 Admission to neonatal intensive care unit.
1.25. Analysis.
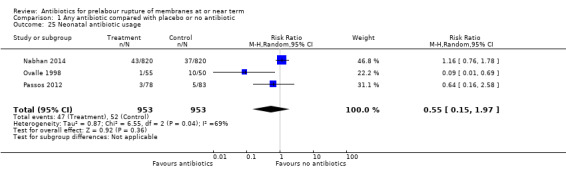
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 25 Neonatal antibiotic usage.
1.27. Analysis.
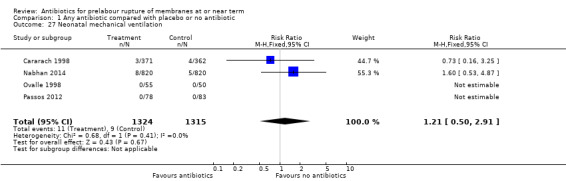
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 27 Neonatal mechanical ventilation.
1.30. Analysis.
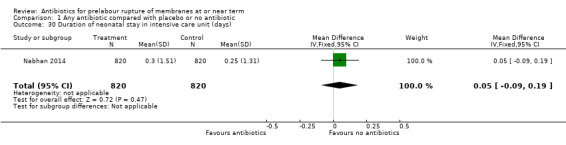
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 30 Duration of neonatal stay in intensive care unit (days).
Cost effectiveness
No data on cost effectiveness were available.
Comparison 2. Any antibiotic compared with placebo or no antibiotic subgrouped by timing of induction of labour (early induction versus late induction)
Three studies involving 2478 women were included in the subgroup analysis of timing of induction of labour (Cararach 1998; Nabhan 2014; Ovalle 1998).
Early‐onset neonatal sepsis (probable and definite)
No difference in either probable or definite early‐onset neonatal sepsis was shown in the early induction group (RR 1.47, 95% CI 0.80 to 2.70; babies = 1640; studies = one and RR 1.29, 95% CI 0.48 to 3.44; babies = 1640; studies = one, respectively) (Analysis 2.1 and Analysis 2.2). There were trends toward reduced probable and definite early‐onset neonatal sepsis in the late induction group, but neither result reached statistical significance (RR 0.14, 95% CI 0.02 to 1.13; babies = 838; studies = two, and RR 0.16, 95% CI 0.02 to 1.34; babies = 838; studies = two, respectively). A test for subgroup differences was significant (Chi² = 4.50, df = 1 (P = 0.03), I² = 77.8%), suggesting a differential effect of the intervention on probable early‐onset neonatal sepsis between the early and late induction groups.
2.1. Analysis.
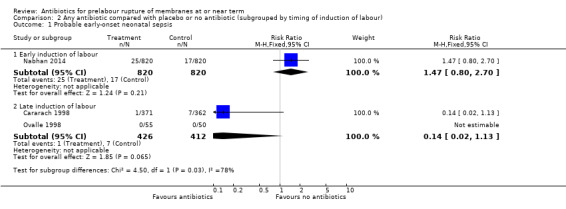
Comparison 2 Any antibiotic compared with placebo or no antibiotic (subgrouped by timing of induction of labour), Outcome 1 Probable early‐onset neonatal sepsis.
2.2. Analysis.
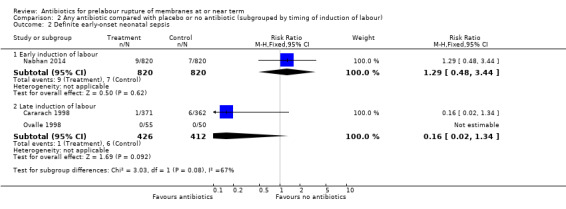
Comparison 2 Any antibiotic compared with placebo or no antibiotic (subgrouped by timing of induction of labour), Outcome 2 Definite early‐onset neonatal sepsis.
Maternal infectious morbidity (chorioamnionitis and/or endometritis)
No difference in maternal infectious morbidity (chorioamnionitis and/or endometritis) was shown in the early induction group (average RR 1.15, 95% CI 0.64 to 2.08; women = 1640; studies = one). There was a trend toward reduced maternal infectious morbidity in the late induction group (average RR 0.34, 95% CI 0.08 to 1.47; babies = 838; studies = two; Tau² = 0.70; Chi² = 2.16, df = 1 (P = 0.14); I² = 54%) (Analysis 2.3), but this result was not statistically significant.
2.3. Analysis.
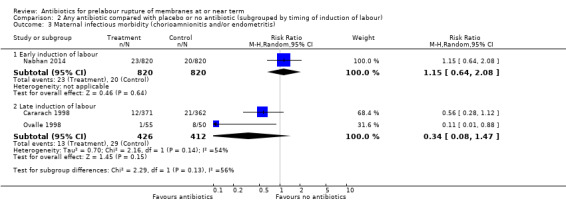
Comparison 2 Any antibiotic compared with placebo or no antibiotic (subgrouped by timing of induction of labour), Outcome 3 Maternal infectious morbidity (chorioamnionitis and/or endometritis).
Stillbirth
No difference in stillbirth was shown in one study of 1640 women in the early induction group (RR 3.00, 95% CI 0.61 to 14.82) (Analysis 2.4). No data were available for the late induction subgroup.
2.4. Analysis.
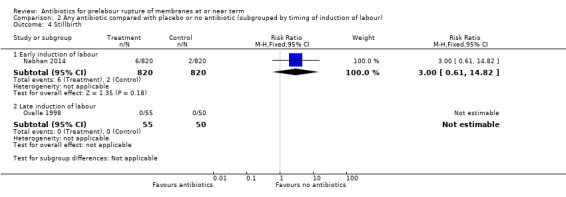
Comparison 2 Any antibiotic compared with placebo or no antibiotic (subgrouped by timing of induction of labour), Outcome 4 Stillbirth.
Perinatal mortality
No difference in perinatal mortality was shown in either the early induction or late induction groups (RR 3.00, 95% CI 0.61 to 14.82; babies = 1640; studies = one, and RR 0.98, 95% CI 0.14 to 6.89; babies = 838; studies = two, respectively) (Analysis 2.5).
2.5. Analysis.
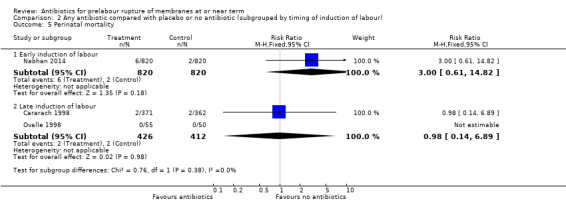
Comparison 2 Any antibiotic compared with placebo or no antibiotic (subgrouped by timing of induction of labour), Outcome 5 Perinatal mortality.
Discussion
Summary of main results
This review included four studies of 2639 women comparing antibiotics for women with term prelabour rupture of membranes with placebo or no antibiotics. The previous version of this review included two studies of 838 women. Whereas the previous version of this review showed a statistically significant reduction in endometritis, no such effect was shown in this update after an additional two studies of 1801 women. No differences were shown on the primary outcome measures of early‐onset neonatal sepsis (probable and definite), maternal infectious morbidity (chorioamnionitis and/or endometritis), stillbirth, perinatal mortality, neonatal mortality, and serious maternal outcome, though the number of cases in the control group for these outcomes was low.
Caesarean section was increased with the use of antibiotics, as was duration of maternal stay in hospital. These results appear largely driven by the Nabhan 2014 study, in which 165 women in the antibiotic group underwent caesarean section (20%), compared to 122 women in the placebo group (approximately 15%). Repeat caesarean section was significantly more common in the antibiotic group compared to the placebo group in this study. The larger incidence of caesarean section in the antibiotic group may also be partially accounted for by the increased hypertension and pre‐eclampsia in the antibiotic group in this study.
Due to heterogeneity we did not combine the data from two studies measuring postpartum antibiotic usage and postpartum pyrexia. A small study of 105 women (Ovalle 1998) reported an increase in postpartum antibiotic usage and a reduction in postpartum pyrexia, whereas a larger study of 1640 women (Nabhan 2014) reported no differences on either outcome. The increased use of postpartum antibiotic usage in Ovalle 1998 appears to be explained by the study protocol for antibiotic provision, and the reduction in postpartum pyrexia in Ovalle 1998 is likely owing to the observed reduction in maternal infectious morbidity in this study.
No difference in any measures of maternal morbidity or neonatal morbidity were shown, although one small study showed a reduction in neonatal nursery stay. No data on cost effectiveness were available.
With the additional data available in this update we were able to introduce a second comparison of antibiotics versus placebo or no antibiotics subgrouped by timing of induction, whereby we compared early induction (defined as induction less than 12 hours from rupture of membranes) with late induction (defined as induction at 12 hours or greater from rupture of membranes). Three studies involving 2478 women were included in this subgroup analysis. No difference in either probable or definite early‐onset neonatal sepsis was shown in either subgroup, although there were trends toward reduced probable and definite early‐onset neonatal sepsis in the late induction group. Similarly, no difference in maternal infectious morbidity (chorioamnionitis and/or endometritis) was found in either subgroup analysis, though there was a trend towards reduced maternal infectious morbidly in the late induction group. A test for subgroup differences confirmed a differential effect of the intervention on probable early‐onset neonatal sepsis between the early and late induction groups. Given these data, it appears that it is the difference in the timing of induction and the period of latency between membrane rupture and birth that is likely to have the greatest impact on infectious morbidity, with the least effect of antibiotic treatment seen in the Nabhan 2014 study where the period between admission and birth was shortest, and greater effect of antibiotic treatment seen in the remaining studies in these analyses where the period of latency was longer.
No differences were shown in stillbirth or perinatal mortality, and there were no cases of serious maternal outcome or neonatal mortality.
Overall completeness and applicability of evidence
The four studies included in this review showed some heterogeneity. The main source of clinical heterogeneity was in the timing of induction of labour, which we have attempted to address via subgroup analyses. Nonetheless, the conclusions from this review are limited by rare event rates across the primary outcomes. This review is underpowered to detect differences in stillbirth, perinatal mortality, and neonatal mortality and it is unlikely that future studies will be able to rectify this problem due to the sheer numbers of women and neonates that would be required to complete such clinical trials. A further limitation to the applicability of the evidence in this review is regarding the use or method of routine screening for Group B Streptococcus, which was included in only one (Passos 2012) of the included studies. The review also provides little information on possible short‐ and long‐term adverse effects from the use of antibiotics including allergic reaction and anaphylaxis for women (ACOG 2011), and the development of resistant organisms among neonates such as gram‐negative organisms (Edwards 2001; Stoll 2002). Due to unavailability of data, we are unable to assess not only the direct economic impact of routine use of antibiotics for PROM at term, but also the economic impact of treating the antibiotic‐resistant infections that may result. Due to the low number of included studies, we were also not able to investigate the likelihood of reporting biases such as publication bias.
Quality of the evidence
Overall risk of bias in this review was fair to high. Allocation to groups was not concealed in two of the four studies (Cararach 1998; Ovalle 1998), and in a third study (Passos 2012) both performance and detection bias was judged to be high risk. The largest and most recent study (Nabhan 2014) was of particularly high quality, showing low risk of bias on all domains assessed. Using the GRADE approach, quality of evidence for each of the primary outcomes yielding a point estimate (probable early‐onset neonatal sepsis; definite early‐onset neonatal sepsis; maternal infectious morbidity; stillbirth; and perinatal mortality) was judged to be low to very low. We downgraded evidence for each of these outcomes due to risk of bias and imprecision of results (seeTable 1; Table 2).
Potential biases in the review process
We are aware that the review process itself is subject to bias, and we took steps to minimise bias. At least two review authors carried out data extraction and assessed risk of bias independently; however, a different review team may not have made identical decisions.
Agreements and disagreements with other studies or reviews
In their retrospective cohort study of 3841 women at a single‐site in the United States, Tran 2007 and colleagues examined the relationship between duration of membrane rupture and maternal infection, finding that the risk chorioamnionitis and endometritis was increased at 12 hours and 16 hours respectively post membrane rupture. The study included women with singleton pregnancy and cephalic presentation, with PROM diagnosed at 37 weeks' gestation or later. Women with multiple pregnancies and obstetric complications were excluded and definitions for chorioamnionitis and endometritis were similar to those in this review. The study also found that the risk of postpartum haemorrhage was increased with durations of membrane rupture, but we were unable to investigate this in the current review due to unavailability of data. The Cochrane Review on Planned early birth versus expectant management (waiting) for prelabour rupture of membranes at term (37 weeks or more) (Dare 2006) showed that planned management (usually by induction of labour) reduced chorioamnionitis and endometritis and, similar to this review, no difference in neonatal infection was shown. It is important to note that the NICE guideline on intrapartum care states that induction of labour is appropriate approximately 24 hours after rupture of membranes (NICE guideline 55). As in this update, the guideline also states that maternal and neonatal antibiotics should not be administered in the absence of signs of maternal infection.
Authors' conclusions
Implications for practice.
This review demonstrates no convincing maternal or neonatal benefits of the routine use of antibiotics for prelabour rupture of membranes (PROM) at or near term in the absence of confirmed clinical infection. It is likely that the modest benefit for maternal endometritis seen in the previous version of this review can be largely accounted for by the late use of induction of labour in both included studies, where the duration of membrane rupture was more prolonged (related to either a policy of expectant management or a delay in induction greater than 24 hours). The low rate of maternal infection in the control population of this updated review (less than 5%) further reinforces that administration of antibiotics to women with PROM should be restricted to those who develop clinical indications for antibiotic treatment.
Implications for research.
Antibiotics may not offer any benefit under a clinical policy of immediate or early induction of labour, where the duration of membrane rupture is minimised. For women being managed expectantly or with a policy of delayed induction, there may be a role for antibiotics, but further well‐designed randomised controlled trials are needed. These trials should utilise blinding of the intervention and need to be adequately sized to address clinically important maternal and neonatal outcomes. All future trials should consider including a cost analysis to determine the economic impact of routine antibiotics for PROM at term.
What's new
| Date | Event | Description |
|---|---|---|
| 8 September 2014 | New citation required and conclusions have changed | Two new trials of 1801 women have been incorporated. The previous reduction in endometritis is no longer evident. A 'Summary of findings' table has been added to this update. |
| 31 July 2014 | New search has been performed | Search updated. Two trials identified. Methods updated. |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 23 December 2008 | New search has been performed | Search updated. No new trials identified. Two studies previously awaiting classification have been excluded (Gordon 1974; Walss Rodriguez 1988). |
| 28 August 2008 | Amended | Converted to new review format. |
| 30 September 2005 | New search has been performed | Search updated. No new trials identified. |
Acknowledgements
The review authors would like to acknowledge Dr Vicenc Cararach, Department of Obstetrics, Gynaecology and Paediatrics, Hospital Clinic, Universitat de Barcelona, Spain; Dr Alfredo Ovalle, Servicio de Obstetricia, Ginecologia y Neonatologia, Hospital San Borja Arriaran, Santagio, Chile; Dr Filipa Passos, Hospital Garcia de Orta, EPE ‐ Av. Torrado da Silva, 2801‐951 Almada, Portugal; and Professor Ashraf Nabhan, Department of Obstetrics & Gynecology, Ain Shams University, Cairo, Egypt for providing additional information and data for this review.
We acknowledge Carolina Sommariva for providing translation for an article for this review and Linda Murray for assisting with updating previous versions of this review. We would also like to thank Sonja Henderson and Leanne Jones for support and advice regarding the Cochrane Pregnancy and Childbirth Group methods and procedures, and Lynn Hampson for her assistance with searching for potentially eligible trials.
Appendices
Appendix 1. Searches carried out in the previous version
The authors conducted a systematic literature search which included electronic databases: the Cochrane Controlled Trials Register (The Cochrane Library 2001, Issue 4) and MEDLINE (1965 to 2001), using MeSH headings: pregnancy and childbirth, infant‐newborn and the search terms: term, chorioamnionitis, membrane* , rupture*, prelabour, prelabor, ROM, antibiotic*, neonat*, sepsis, early onset sepsis.
The authors also contacted recognised experts and cross referenced relevant material.
Data and analyses
Comparison 1. Any antibiotic compared with placebo or no antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Probable early‐onset neonatal sepsis | 4 | 2639 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.21, 2.33] |
| 2 Definite early‐onset neonatal sepsis | 4 | 2639 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.08, 4.26] |
| 3 Maternal infectious morbidity (chorioamnionitis and/or endometritis) | 4 | 2639 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.20, 1.15] |
| 4 Stillbirth | 3 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.61, 14.82] |
| 5 Perinatal mortality | 4 | 2639 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.60, 6.55] |
| 6 Neonatal mortality | 3 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Serious maternal outcome | 3 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Chorioamnionitis (suspected or proven) | 4 | 2639 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.34, 1.26] |
| 9 Endometritis | 4 | 2639 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.05, 2.31] |
| 10 Caesarean section | 3 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.09, 1.61] |
| 11 Operative vaginal birth | 3 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.63, 1.44] |
| 12 Internal fetal monitoring | 2 | 1745 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Epidural analgesia | 2 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.98, 1.02] |
| 14 Postpartum haemorrhage | 3 | 1906 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.14, 8.93] |
| 15 Postpartum pyrexia | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16 Postpartum septicaemia | 3 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Wound infection | 3 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.36, 1.72] |
| 18 Maternal postpartum antibiotic usage | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19 Maternal adverse effects | 4 | 2639 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 71.63] |
| 20 Neonatal meningitis | 4 | 2639 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.11] |
| 21 Neonatal pneumonia | 4 | 2639 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.96] |
| 22 Apgar score < 7 at 5 minutes | 4 | 2639 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.81, 3.36] |
| 23 Admission to neonatal special care nursery | 2 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.06, 1.74] |
| 24 Admission to neonatal intensive care unit | 3 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.82, 1.85] |
| 25 Neonatal antibiotic usage | 3 | 1906 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.15, 1.97] |
| 26 Respiratory distress syndrome | 2 | 266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Neonatal mechanical ventilation | 4 | 2639 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.50, 2.91] |
| 28 Duration of maternal stay in hospital (days) | 3 | 1906 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [0.01, 0.11] |
| 29 Duration of neonatal stay in hospital (days) | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.34, ‐0.46] |
| 30 Duration of neonatal stay in intensive care unit (days) | 1 | 1640 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.09, 0.19] |
1.6. Analysis.
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 6 Neonatal mortality.
1.7. Analysis.
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 7 Serious maternal outcome.
1.12. Analysis.
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 12 Internal fetal monitoring.
1.16. Analysis.
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 16 Postpartum septicaemia.
1.26. Analysis.
Comparison 1 Any antibiotic compared with placebo or no antibiotic, Outcome 26 Respiratory distress syndrome.
Comparison 2. Any antibiotic compared with placebo or no antibiotic (subgrouped by timing of induction of labour).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Probable early‐onset neonatal sepsis | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Early induction of labour | 1 | 1640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.80, 2.70] |
| 1.2 Late induction of labour | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.13] |
| 2 Definite early‐onset neonatal sepsis | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Early induction of labour | 1 | 1640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.48, 3.44] |
| 2.2 Late induction of labour | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.34] |
| 3 Maternal infectious morbidity (chorioamnionitis and/or endometritis) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Early induction of labour | 1 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.64, 2.08] |
| 3.2 Late induction of labour | 2 | 838 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.08, 1.47] |
| 4 Stillbirth | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Early induction of labour | 1 | 1640 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.61, 14.82] |
| 4.2 Late induction of labour | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Perinatal mortality | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Early induction of labour | 1 | 1640 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.61, 14.82] |
| 5.2 Late induction of labour | 2 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.89] |
| 6 Neonatal mortality | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Early induction of labour | 1 | 1640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Late induction of labour | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Serious maternal outcome | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Early induction of labour | 1 | 1640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Late induction of labour | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.6. Analysis.
Comparison 2 Any antibiotic compared with placebo or no antibiotic (subgrouped by timing of induction of labour), Outcome 6 Neonatal mortality.
2.7. Analysis.
Comparison 2 Any antibiotic compared with placebo or no antibiotic (subgrouped by timing of induction of labour), Outcome 7 Serious maternal outcome.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cararach 1998.
| Methods | Multicentre randomised trial. | |
| Participants | 733 women at 36 weeks' gestation or more with singleton pregnancy, MR duration less than 12 hrs and absence of uterine contractions. Setting: 11 hospitals in Spain. Exclusion criteria: fetal death or anomaly, placenta praevia, placental abruption, fetal distress, chorioamnionitis, indication for elective CS, allergy to penicillin and erythromycin. | |
| Interventions | Intervention group: antibiotics on admission following vaginal and endocervical culture. IV ampicillin 1 g every 6 hrs and IM gentamicin 80 mg every 8 hrs or IM erythromycin 500 mg every 6 hrs for women with penicillin allergy. Control group: no treatment. Admission cultures as for intervention group. |
|
| Outcomes | Maternal: mode of birth, chorioamnionitis, endometritis, postpartum antibiotics. Neonatal: Apgar score, umbilical artery and vein pH values, early neonatal infection (i.e. sepsis, meningitis, or pneumonia), respiratory complications, intracranial haemorrhage. Data were requested and received for: maternal adverse drug reaction, neonatal mechanical ventilation, perinatal death. |
|
| Notes | Management of all women enrolled: single vaginal examination until initiation of labour. Vaginal and endocervical culture on admission.
IOL with IV oxytocin after 12 hrs of MR in the absence of regular uterine contractions.
Routine antibiotic prophylaxis for women undergoing CS. Partial support received via a grant from the 'Fondo de Investigaciones Sanitarias' (FIS), Spanish Ministry of Health. Declaration of conflicts of interest not included. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation list in each participating centre". No details provided. |
| Allocation concealment (selection bias) | High risk | Open list used, no concealment of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment for neonatal outcomes only. Unblinding in cases of neonatal sepsis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Intracranial haemorrhage not reported. |
| Other bias | Low risk | None evident. |
Nabhan 2014.
| Methods | Randomised controlled trial between June 2009 and January 2011. Parallel group design. | |
| Participants | 1640 women at 36 weeks' gestation or more with prelabour rupture of membranes and singleton pregnancy. Setting: Labour and delivery ward, Department of Obstetrics, Ain Shams University, Cairo, Egypt. Exclusion criteria: rupture of membranes for more than 12 hrs, chorioamnionitis, meconium‐stained liquor on admission, fetal anomalies, maternal rheumatic valve disease. | |
| Interventions | Intervention group: single dose 1500 mg parenteral ampicillin/sulbactam. Control group: placebo (solvent without active ingredient). Both groups: no GBS cultures taken and no routine intrapartum GBS prophylaxis given according to hospital protocol. All women were managed on admission either by induction of labour or CS. | |
| Outcomes | Neonatal: primary outcome EONS. EONS defined as neonatal infection within the first 7 days of life. Definite infection defined as above plus 1 of: (1) positive blood, CSF, urine, or tracheal aspirate culture; (2) positive CSF Gram stain; (3) positive blood, CSF, or urine antigen detection test; or (4) positive chest radiograph confirming neonatal pneumonia. Probable EONS defined by clinical signs of infection plus either a complete blood count suggestive of infection or a CSF with an elevated leukocyte count, a high protein concentration, or a low glucose concentration. Additional neonatal outcomes included newborn pneumonia, fetal or early neonatal death (excluding CA), Apgar < 7 at 5 mins, admission to NICU, need for and duration of mechanical ventilation, and duration of NICU stay. Maternal outcomes were chorioamnionitis, endometritis, mode of birth, PPH, postpartum pyrexia, ADRs, duration of hospital stay. | |
| Notes | Intention‐to‐treat analyses used. Employed a policy of immediate induction, ensuring the latency between rupture of membranes and labour was less than 12 hrs. No GBS cultures taken and no routine intrapartum GBS prophylaxis as per hospital protocol. Intramaural support only, no external funding. Authors reported no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated simple randomisation procedure (1:1 allocation ratio). |
| Allocation concealment (selection bias) | Low risk | An obstetrician on the labour ward enrolled participants. For each participant, the obstetrician obtained the next randomisation number by telephone. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and (most) personnel were blinded to the group assignment (antibiotics or placebo was administered by a labour ward nurse). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Staff assessing outcomes were blinded. Only the labour ward nurse who administered antibiotics or placebo was aware of group allocation, this nurse did not assess outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 62 participants in total were lost to follow‐up due to incomplete collection of data (attrition rates acceptable: 2.6% in intervention and 5% in control group). Intention‐to‐treat analyses used. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered protocol available and outcomes reported as expected. |
| Other bias | Low risk | None evident. |
Ovalle 1998.
| Methods | Single‐centre randomised trial between August 1990 and December 1993. | |
| Participants | 105 women at 37‐42 weeks' gestation with a singleton pregnancy, duration of MR less than 12 hrs and no labour. Setting: San Borja Arriarán Hospital, Chile. Exclusion criteria: previous CS, malpresentation, fetal distress, fetal malformation, chorioamnionitis, antibiotics given within 30 days. |
|
| Interventions | Intervention: antibiotics on admission following cervicovaginal and amniotic fluid culture. IV clindamycin 600 mg every 6 hrs and IV cefuroxime 750 mg every 8 hrs for 48 hrs then oral cefuroxime 250 mg every 12 hrs and clindamycin 300 mg every 6 hrs for a further 24 hrs. Control: placebo following admission cultures. No details provided. |
|
| Outcomes | Maternal: chorioamnionitis, endometritis. Neonatal: Apgar score < 7 at 5 mins, neonatal morbidity. Data were requested and received for: neonatal sepsis, pneumonia, meningitis, neonatal mechanical ventilation, admission to SCN, maternal and neonatal length of stay, perinatal death, maternal adverse drug reaction. | |
| Notes | Management of all women enrolled: no digital vaginal examination until active labour.
Cervicovaginal culture and culture of amniotic fluid by amniocentesis on admission.
IOL with IV oxytocin within 24 hrs of MR if no labour. Antibiotic prophylaxis given to some women undergoing CS.
Neonatal antibiotics routinely given when chorioamnionitis present or positive maternal admission cultures. No funding details provided. Declaration of conflicts of interest not included. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pre‐established allocation code. No other details given. |
| Allocation concealment (selection bias) | High risk | Pharmacist allocation using a list of random numbers. No central randomisation, or sequentially numbered drug containers or opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled trial. The treating physicians were independent from those in charge of the study and were unaware of group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None evident. |
Passos 2012.
| Methods | Randomised controlled trial between October 2008 and January 2012. | |
| Participants | 161 women at 37 weeks' gestation or more with singleton pregnancy, vertex presentation, ruptured membranes for less than 12 hrs, and a negative GBS culture between 35 and 37 weeks. Setting: Department of Obstetrics, Gynecology, and Reproductive Medicine, Lisbon University Hospital. Exclusion criteria: active labour, meconium liquor, no GBS culture, indication for GBS antibiotic prophylaxis, or contraindication to expectant management (e.g. fetal distress). | |
| Interventions | Intervention group: 1 g IV ampicillin every 6 hrs and 240 mg IV gentamicin daily. Control group: no treatment. | |
| Outcomes | Maternal: primary outcome was maternal infection (chorioamnionitis or puerperal endometritis). Additional outcomes were time from PROM to admission, time from PROM to initiation of spontaneous labour, time from PROM to IOL, time from rupture of membranes to birth; mode of birth and CS for fetal distress.
Neonatal: primary outcomes was neonatal infection rate (EONS, meningitis, and pneumonia). Additional outcomes were Apgar score at 1 and 5 mins, birthweight, early‐onset neonatal infections and death. Data were requested and received for operative birth, epidural analgesia, PPH, postpartum septicaemia, wound infection, maternal adverse effects, Apgar scores, admission to SCN and NICU, EONS, neonatal use of antibiotics and mechanical ventilation. |
|
| Notes | Intention‐to‐treat analysis used, women were excluded where outcome data were not available. IOL or CS performed at the discretion of the attending physicians (no details given). Antibiotic therapy for neonates initiated only following diagnosis of EONS. No funding details provided. Authors reported no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were 'randomly assigned to the antibiotic group or to the control group, according to a computer‐generated randomisation list'. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelopes used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 2 obstetric and 1 paediatric investigators reviewed data to confirm diagnoses of maternal and neonatal infections. Only the paediatric investigator was blind to group allocation during the process. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants were excluded due to missing clinical data. 6 were excluded due to protocol violations. Intention‐to‐treat analyses used. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Trial was registered via ClinicalTrials.gov in 2012 (no prospective registration). |
| Other bias | Low risk | None evident. |
ADR: adverse drug reaction CA: congenital abnormality CS: caesarean section CSF: cerebrospinal fluid EONS: early‐onset neonatal sepsis GBS: group B streptococcus hrs: hours IM: intramuscular IOL: induction of labour IV: intravenous mins: minutes NICU: neonatal intensive care unit MR: membrane rupture PPH: postpartum haemorrhage PPROM: prelabour rupture of the membranes SCN: special care nursery
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brelje 1966 | Quasi‐random allocation used. |
| Gordon 1974 | Quasi‐random allocation used. |
| Lebherz 1963 | Antibiotic used no longer recommended for use in pregnancy. |
| Walss Rodriguez 1988 | Further information on method of randomisation and allocation to treatment was requested but is not yet available. |
Differences between protocol and review
For this update outcome measures were divided into primary and secondary outcomes. Primary outcomes were selected by the authorship team as those most representative of the clinically important measures of effectiveness and complications. Respiratory distress syndrome was added as a secondary outcome. Methods for assessing risk of bias were updated as per Chapter 8 of the Cochrane Handbook for Reviews of Intervention (Higgins 2011). These changes include evaluating the domain of blinding separately for participants and personnel (performance bias) and for outcome assessment (detection bias). The quality of the evidence was re‐assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to key outcomes.
Contributions of authors
Aleena M Wojcieszek and Owen Stock undertook data extraction and quality assessments for studies included in this review update. Aleena M Wojcieszek led revisions of the review for the current update. All authors assisted with the interpretation and final editing.
Vicki Flenady and James King worked as equal partners in the production of the original review.
Sources of support
Internal sources
Centre for Clinical Studies ‐ Mater Hospital, South Brisbane, Queensland, Australia.
J P Kelly Research Foundation, Mater Hospital, South Brisbane, Queensland, Australia.
Department of Perinatal Medicine, Royal Women's Hospital, Melbourne, Victoria, Australia.
External sources
Department of Health and Ageing, Commonwealth Government, Canberra, Australia.
Declarations of interest
None.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Cararach 1998 {published and unpublished data}
- Cararach V, Botet F, Sentis J, Almirall R, Perez‐Picanol E. Administration of antibiotics to patients with rupture of membranes at term: a prospective, randomized, multicentric study. Collaborative Group on PROM. Acta Obstetricia et Gynecologica Scandinavica 1998;77:298‐302. [PubMed] [Google Scholar]
- Cararach V, Botet F, Sentis J, Carmona F. A multicenter, prospective and randomized study in premature rupture of membranes (PROM). Maternal and perinatal complications. Proceedings of 13th World Congress of Gynaecology and Obstetrics (FIGO); 1991 Sept 15‐20; Singapore. 1991:267.
- Cararach V, Sentis J, Botet F. A multicentre prospective randomized study in premature rupture of the membranes (PROM). Respiratory and infectious complications in the newborn. Proceedings of 12th European Congress of Perinatal Medicine; 1990 September 11‐14; Lyon, France. 1990:216.
Nabhan 2014 {published data only}
- Nabhan A. Prophylactic antibiotics for spontaneous prelabor rupture of membranes at term to reduce early neonatal sepsis. http://www.anzctr.org.au/ACTRN12608000501347.aspx [accessed 2 February 2012] 2009.
- Nabhan A, Elhilaly A. Antibiotic prophylaxis in prelabor rupture of membranes at term, a randomized trial. Journal of Perinatal Medicine 2013;41(Suppl 1):Abstract no:543. [Google Scholar]
- Nabhan A, Elhilay A, Elkadi M. Antibiotic prophylaxis in prelabor spontaneous rupture of fetal membranes at or beyond 36 weeks of pregnancy. International Journal of Gynecology and Obstetrics 2014;124:59–62. [DOI: 10.1016/j.ijgo.2013.07.018] [DOI] [PubMed] [Google Scholar]
Ovalle 1998 {published and unpublished data}
- Ovalle A, Gomez R, Martinez MA, Giglio MS, Bianchi R, Diaz J, et al. Antibiotic treatment of patients with term premature rupture of membranes reduces the incidence of infection‐related complications. American Journal of Obstetrics and Gynecology 1995;172:301. [Google Scholar]
- Ovalle A, Gomez R, Martinez MA, Giglio MS, Bianchi R, Diaz J, et al. Antibiotic treatment of patients with term premature rupture of membranes: a randomized clinical trial. Prenatal and Neonatal Medicine 1998;3:599‐606. [Google Scholar]
Passos 2012 {published data only}
- Passos F. Antibiotic prophylaxis in prelabor rupture of membranes at term. http://www.clinicaltrials.gov/ct2/show/NCT01633294?term=NCT01633294&rank=1 [accessed 28 June 2014] 2012.
- Passos F, Cardoso K, Coelho AM, Graca A, Clode N, Mendes LG. Antibiotic prophylaxis in premature rupture of membranes at term: A randomized controlled trial. Obstetrics and Gynecology 2012;120(5):1045‐51. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Brelje 1966 {published data only}
- Brelje MC, Kaltreider DF, Kassir L. The use of vaginal antibiotics in premature rupture of the membranes. American Journal of Obstetrics and Gynecology 1966;94:889‐97. [DOI] [PubMed] [Google Scholar]
Gordon 1974 {published data only}
- Gordon M, Weingold AB. Treatment of patients with premature rupture of the fetal membranes: (a) prior to 32 weeks; (b) after 32 weeks. Premature rupture of the membranes ‐ a rational approach to management. In: Reid DE, Christian CD editor(s). Controversy in Obstetrics and Gynecology II. Philadelphia: WB Saunders Company, 1974:42‐4. [Google Scholar]
Lebherz 1963 {published data only}
- Lebherz TB, Hellman LP, Madding R, Anctil A, Arje SL. Double blind study of premature rupture of the membranes. American Journal of Obstetrics and Gynecology 1963;87:218‐25. [DOI] [PubMed] [Google Scholar]
Walss Rodriguez 1988 {published data only}
- Walss Rodriguez RJ, Navarro Castanon J. Prophylactic antibiotics in premature rupture of the membranes [Antibioticos profilacticos en ruptura prematura de membranas]. Ginecologia y Obstetricia de Mexico 1988;56:339‐42. [PubMed] [Google Scholar]
Additional references
ACOG 2011
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 120: Use of prophylactic antibiotics in labor and delivery. Obstetrics and Gynecology 2011;117(6):1472‐83. [DOI] [PubMed] [Google Scholar]
Cammu 1990
- Cammu H, Verlaenen H, Derde M. Premature rupture of membranes at term in multiparous women: a hazard?. Obstetrics & Gynecology 1990;76:671‐4. [PubMed] [Google Scholar]
Dare 2006
- Dare MR, Middleton P, Crowther CA, Flenady VJ, Varatharaju B. Planned early birth versus expectant management (waiting) for prelabour rupture of membranes at term (37 weeks or more). Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005302.pub2] [DOI] [PubMed] [Google Scholar]
Duff 1998
- Duff P. Premature rupture of the membranes in term patients: induction of labor versus expectant management. Clinical Obstetrics and Gynecology 1998;41(4):883‐91. [DOI] [PubMed] [Google Scholar]
Edwards 2001
- Edwards R, Clark P, Duff P. Intrapartum antibiotic prophylaxis with either penicillin or ampicillin may increase the rate of neonatal sepsis caused by ampicillin‐resistant Gram‐negative bacteria. American Journal of Obstetrics and Gynecology 2001;185:S190. [Google Scholar]
Ezra 2004
- Ezra Y, Michaelson‐Cohen R, Abramov Y, Rojansky N. Prelabor rupture of the membranes at term: when to induce labor?. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;115:23‐7. [DOI] [PubMed] [Google Scholar]
Gates 2004
- Gates S, Brocklehurst P. How should randomised trials including multiple pregnancies be analysed?. BJOG: an international journal of obstetrics and gynecology 2004;111(3):213‐29. [DOI] [PubMed] [Google Scholar]
GRADE 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.6. The Cochrane Collaboration, 2008.
Hannah 1996
- Hannah ME, Ohlsson A, Farine D, Hewson SA, Hodnett ED, Myhr TL, et al. Induction of labor compared with expectant management for prelabour rupture of the membranes at term. New England Journal of Medicine 1996;334:1005‐10. [DOI] [PubMed] [Google Scholar]
Heim 1991
- Heim K, Alge A, Marth C. Anaphylactic reaction to ampicillin and severe complications in the fetus. Lancet 1991;337:859‐60. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ingemarsson 1996
- Ingemarsson I. Controversies: premature rupture of membranes at term‐‐no advantage of delaying induction >24 hours. Journal of Perinatal Medicine 1996;24(6):573‐9. [PubMed] [Google Scholar]
Jao 2006
- Jao MS, Cheng PJ, Shaw SW, Soong YK. Anaphylaxis to cefazolin during labor secondary to prophylaxis for group B Streptococcus: a case report. Journal of Reproductive Medicine 2006;51(8):655‐8. [PubMed] [Google Scholar]
Lin 1999
- Lin FY, Weisman LE, Azimi PH, Regan JA, Philips JB, Clark P, et al. Antibiotic susceptibility of invasive group B streptococci from neonates with early‐onset disease ‐ a multi center study 1995‐97. Proceedings of Pediatric Academic Societies' Annual Meeting; 1999 May 1‐May 4, San Francisco, CA. 1999.
Marowitz 2007
- Marowitz A, Jordan R. Midwifery management of prelabor rupture of membranes at term. Journal of Midwifery and Women’s Health 2007;52(3):199–206. [DOI] [PubMed] [Google Scholar]
Marshall 2002
- Marshall JF, Vasilenko P. Classification of prematurity by gestational age: a concept now mature. Obstetrics & Gynecology 2002;99(4 Suppl):855. [Google Scholar]
Moore 2006
- Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta 2006;27(11‐12):1037‐51. [DOI] [PubMed] [Google Scholar]
Mozurkewich 2006
- Mozurkewich E. Prelabor rupture of membranes at term: induction techniques. Clinical Obstetrics and Gynecology 2006;49(3):672‐83. [DOI] [PubMed] [Google Scholar]
Neerhoff 1999
- Neerhoff GM, Cravello C, Haney EI, Silver RK. Timing of labor induction after premature rupture of membranes between 32 and 36 weeks' gestation. American Journal of Obstetrics and Gynecology 1999;180(2):349‐52. [DOI] [PubMed] [Google Scholar]
Newton 1993
- Newton ER. Chorioamnionitis and intraamniotic infection. Clinical Obstetrics and Gynecology 1993;366:795‐808. [DOI] [PubMed] [Google Scholar]
Ohlsson 2014
- Ohlsson A, Shah VS. Intrapartum antibiotics for known maternal Group B streptococcal colonization. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD007467.pub4] [DOI] [PubMed] [Google Scholar]
Reti 2007
- Reti NG, Lappas M, Riley C, Wlodek ME, Permezel M, Walker S, et al. Why do membranes rupture at term? Evidence of increased cellular apoptosis in the supracervical fetal membranes. American Journal of Obstetrics and Gynecology 2007;196(5):484.e1‐484.e10. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romero 1992
- Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, et al. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. American Journal of Obstetrics and Gynecology 1992;166(1):129‐33. [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Stoll 2002
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Changes in pathogens causing early‐onset sepsis in very‐low‐birth‐weight Infants. New England Journal of Medicine 2002;347:240–7. [DOI] [PubMed] [Google Scholar]
Tran 2007
- Tran SH, Cheng YW, Kaimal AJ, Caughey AB. Length of rupture of membranes in the setting of premature rupture of membranes at term and infectious maternal morbidity. American Journal of Obstetrics and Gynecology 2008;198(6):700.e1–700.e5. [DOI] [PubMed] [Google Scholar]
Yelland 2011
- Yelland LN, Saltera AB, Ryana P, Makridesb M. Analysis of binary outcomes from randomised trials including multiple births: when should clustering be taken into account?. Paediatric and Perinatal Epidemiology 2011;25:283–97. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Flenady 2002
- Flenady V, King JF. Antibiotics for prelabour rupture of membranes at or near term. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001807] [DOI] [PubMed] [Google Scholar]


