Abstract
BACKGROUND:
Moyamoya is managed by surgical revascularization, but no standardized method has yet been universally adopted.
OBJECTIVE:
To describe a new indirect bypass technique for pediatric moyamoya, wide arterial sparing encephalo-duro-synangiosis (WASEDS), which provides a much wider area of revascularization with minimal compromise to the middle meningeal arterial tree compared with traditional procedures. Initially used as a salvage technique after failed encephalo-duro-arterio-synangiosis, its success later motivated its use as a first-line procedure.
METHODS:
Clinical and radiographic records of patients who underwent WASEDS for moyamoya from 2009 to 2020 were reviewed. Brain perfusion relative cerebral blood volume on the side of the WASEDS procedure was calculated. Two-tailed paired t tests were performed to identify the statistically significant differences (P ≤ .05).
RESULTS:
WASEDS was successfully performed on 8 patients for a total of 14 cerebral hemispheres. Age ranged from 2 to 25 years. There were no mortalities. The average clinical and radiographic follow-up was 49.79 months (range 2-126 months), demonstrating improvement in neurological condition and no postoperative stroke and significant diminution or cessation of transient ischemic attacks in all patients. Relative cerebral blood volume increased 9.24% after the WASEDS procedure (P = .012). There were no neurological complications. There were 2 pseudomeningoceles related to the extensive dural openings.
CONCLUSION:
WASEDS is a safe and effective indirect revascularization technique for both primary and salvage techniques. It provides an extensive area of cortical revascularization with no compromise of the middle meningeal vasculature and subjective reports of early improvement in cognition and behavior. The main disadvantage is elevated risk of pseudomeningocele secondary to the large craniotomy.
KEY WORDS: Moyamoya, Direct bypass, Indirect bypass, Encephalo-duro-synangiosis
ABBREVIATIONS:
- CA
contrast agent
- CBV
cerebral blood volume
- EDAS
encephalo-duro-arterio-synangiosis
- EGDS
encephalogaleodurosynangiosis
- EMAS
encephalomyoarterial-synangiosis
- FMT
first-moment mean transit time
- MMA
middle meningeal artery
- MRP
MR perfusion
- OA
occipital artery
- rCBV
relative cerebral blood volume
- STA
superficial temporal artery
- TIA
transient ischemic attack
- WASEDS
wide arterial sparing encephalo-duro-synangiosis.
Moyamoya is managed by surgical augmentation of cerebral blood flow because there are no medical therapies that prevent stenosis progression.1 Surgically enhancing cerebral blood flow improves cerebral perfusion and reduces ischemic events.2,3 Surgical options include direct and indirect bypass or combinations. No standardized method has been universally adopted because of the multitude of operative techniques available, surgeon preference, and the lack of randomized trials.3-7
We describe a new indirect wide-arterial-sparing-encephalo-duro-synangiosis (WASEDS) technique, in which multiple dural incisions are made over a large region of the brain without compromising large/medium-sized middle meningeal vessels. The technique arose from the need for salvage surgery after failed encephalo-duro-arterio-synangiosis (EDAS) in 3 cases. Favorable early results, and our recognition of several advantages over other techniques, motivated us to use WASEDS as first-line surgical therapy in 5 additional cases.
WASEDS follows established brain revascularization principles. Specifically, it provides ischemic cortex with vascularized tissue, which in this case are the dura and middle meningeal artery (MMA) branches.5 In brain ischemia, the MMAs form spontaneous transdural brain collaterals and terminal MMA-ACA collaterals.5,8 In addition, the angiography literature reveals that after EDAS, MMA collaterals are often more robust than collaterals arising from the transposed superficial temporal artery (STA).9 We observed that in EDAS, the dura is opened parallel to the STA course, which inevitably sacrifices more than 1 major MMA branch that runs in an oblique direction to the STA. Such injury may presumably lead to disruption of already formed distal collaterals (eg, MMA-ACA). Notably, WASEDS preserves the MMAs but also 1 STA division in case future revascularization surgery is needed.
METHODS
The diagnosis of moyamoya disease was confirmed preoperatively by MRI/magnetic resonance angiography, MR perfusion (MRP), and diagnostic angiography. After showing success in 3 salvage cases that failed EDAS, WASEDS was offered to consecutive children with new diagnosis of moyamoya that presented to the senior surgeon. Patients with moyamoya that presented to other neurosurgeons at out institution were not included. The diagnosis was based on angiography and symptoms of transient ischemic attack (TIA), stroke, or others related to ischemia. Informed consent emphasized WASEDS as a new procedure with advantages observed in a small number of patients, but with potential disadvantages of large craniotomy including bleeding and pseudomeningocele, and absence of long-term results. After Institutional Review Board approval, we retrospectively reviewed 8 patients treated with WASEDS since 2009.
Surgical Technique
Patients underwent general anesthesia and were placed supine on the operating table with optimization of volume status and avoidance of hyperventilation.
In salvage procedures for failed EDAS, a new incision was made to expose the posterior hemisphere, starting in a T fashion from the middle of the prior frontotemporal incision. In first-time procedures, a bicoronal incision was used for both unilateral and bilateral cases. The STA was mapped and marked using a hand-held doppler. One STA branch was preserved in case future revascularization procedures were needed. In most cases, the incision was made 0.5 to 1 cm anterior to the posterior STA division, thus sacrificing the anterior division. If the anterior division was dominant, it was preserved, and the posterior division divided. The subgaleal dissection exposed the skull from anterior frontal to posterior parietal (except in salvage cases, in which only the posterior hemisphere was exposed). A large fronto-temporo-parietal craniotomy was elevated (temporo-parietal in salvage procedures), with a 2 to 3 cm strip remaining intact over the superior sagittal sinus in bilateral cases. The root of the MMA was protected while raising the bone flap to prevent dural devascularization.
Coagulation of the small MMA branches was minimized, and bleeding areas were covered with thrombin-soaked gelfoam. Under the surgical microscope (Video), full-thickness dural slits along all major MMA branches were created in a tree-like fashion, including the main trunk and as many medium-sized and small-sized branches as possible, covering all the exposed dura (Figure 1). The arachnoid was opened in multiple locations along the dural slits, and a large gelfoam was used to cover the dura before the bone flaps were reattached before closure. Aspirin was continued throughout the perioperative period.
FIGURE 1.
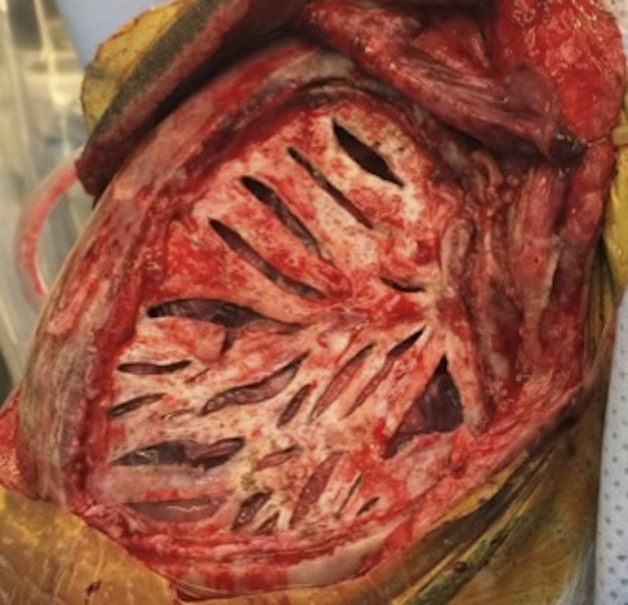
Surgical images of the final product of wide arterial sparing encephalo-duro-synangiosis. Dural slits along most of the large-sized and medium-sized middle meningeal artery branches were created, followed by arachnoid opening (see Video).
Imaging Technique
The scanning protocol involved fluid-attenuated inversion recovery, T2-weighted imaging, diffusion-weighted imaging, precontrast and postcontrast T1-weighted imaging, and MRP. Dynamic susceptibility contrast MRI for MRP with repetition time 1500 ms, flip angle 60°, field of view 24 × 24 cm, acquisition matrix 128 × 128, slice thickness 7 mm with 2 mm gap, 15 to 19 slices covering half of the cerebellum to the top of the cerebrum, and 50 phases were used. Studies were performed on 1.5T or 3T GE scanners (GE Healthcare) with echo time = 45 or 27 ms, respectively, to maximize T2* sensitivity at a given field strength, as published.10
Imaging Analysis
Qualitative and quantitative perfusion analysis was performed for each hemisphere treated with WASEDS. Relative cerebral blood volume (rCBV) maps were calculated from dynamic time series as reported.11 The time curve for each pixel was normalized by the intensity of its value before contrast agent (CA) injection. The normalized curve was then nonlinearly transformed to the changes in transverse relaxation rate because of CA passage. The results were then converted to a CA concentration curve using the proportionality relationship between them.11 After the conversion, rCBV was calculated using the area under the curve. To account for variations between acquisitions, the rCBV maps were normalized by their average values in untreated regions (cerebellum).
We extracted brain from the source images using semiautomatic brain contouring and split it into cerebral hemispheres using Jim software (Jim 8.0, Xinapse Systems). We thresholded rCBV maps in Matlab (The MathWorks) to eliminate large blood vessels and adjacent areas with vessel “blooming” artifact. Additional manual delineation was performed in the posterior fossa to isolate the cerebellar hemispheres from adjacent brainstem. Areas of prior infarct/encephalomalacia were manually excluded, isolating viable brain tissue for analysis. Brain perfusion was calculated as the average rCBV of the treated cerebral hemisphere/average rCBV of the cerebellum. Two-tailed paired t tests were performed (significance: P ≤ .05).
RESULTS
WASEDS was performed on 14 cerebral hemispheres in 8 patients, including 2 with down syndrome and 1 with sickle cell anemia. Age at surgery ranged from 2 to 25 years (9.25 ± 8.51 years; mean ± SD) with a follow-up range of 12 to 126 months (57.40 ± 37.92 months; mean ± SD).
Clinical Presentations
The first 3 patients underwent WASEDS as a salvage procedure after EDAS failure. Patient 1 presented with recurrent strokes, patient 2 with headache, vertigo and dystonia, and patient 3 with recurrent TIAs. Salvage WASEDS surgery was performed 3 years after EDAS in patients 1 and 2, and 9 years after EDAS in patient 3. In the remaining 5 patients, the procedure was performed as the initial intervention. Four of these presented with either ischemic stroke or TIAs, and one presented with intractable headaches and delayed language development (Table 1).
TABLE 1.
Clinical Presentations and Outcomes of 8 Patients Undergoing WASEDS Procedure
| Patient | Age, y, sex | Additional diagnosis | Presenting symptoms | Surgical history | WASEDS | Follow-up (mo) | Outcome | Surgical complications |
|---|---|---|---|---|---|---|---|---|
| 1 | 6, F | – | Left hemiparesis; right frontal, parietal, temporal, and occipital ischemic infarcts | Bilateral EDAS, 3 y prior | Bilateral, 1 wk apart | 126 | No new CVA/TIA; unchanged baseline hemiparesis; subjective improvement in cognition, behavior and school performance at 6 wk | None |
| 2 | 18, F | – | Headache, vertigo, intermittent dystonia | Left EDAS, 3 y prior | Left | 92 | No new CVA/TIA; complete resolution of symptoms | None |
| 3 | 25, F | – | Left hemiparesis, monthly TIAs | Bilateral EDAS, 9 y prior | Right | 44 | No new CVA; significant reduction of TIAs: preoperative: several prolonged episodes of motor TIAs per week. Postoperative: 1 mild episode per year, maybe suggestive of migraine | None |
| 4 | 3, F | Sickle cell | Left hemiparesis; right frontal and lacunar ischemic infarcts | None | Bilateral, 3 mo apart | Left 87, right 72 | No new CVA/TIA; subjective improvement in school performance at 3 mo | Inflicted wound dehiscence resulting in superficial infection requiring wound revisions |
| 5 | 4, F | Down syndrome | Left hemiparesis; multifocal ischemic infarcts in bilateral hemispheres | None | Bilateral, same day | 15 | No new CVA/TIA; improvement in left hemiparesis; subjective improvement in vocabulary and school performance at 4 mo | None |
| 6 | 2, F | Down syndrome | Left hemiparesis; right frontal ischemic infarct | None | Bilateral, same day | 66 | No new CVA; single TIA concurrent with dehydration/gastrointestinal illness; improvement of left hemiparesis; subjective improvement in language and vocabulary at 1 mo | Pseudomeningocele requiring temporary lumboperitoneal shunt |
| 7 | 13, M | Autism | TIAs (expressive aphasia), headaches | None | Bilateral, same day | 43 | No new CVA; headaches and speech improved; 1 delayed TIA referable to right hemisphere resolved with increased aspirin therapy (Figure 2); subjective improvement in writing, speech, and behavior at 6 wk | Limited superficial wound swelling suggesting infection resolved with oral antibiotics |
| 8 | 3, M | – | Delayed language development, frequent anger spells, and intractable headaches | None | Bilateral, 4 mo apart | Left 17, right 13 | No new CVA/TIA; improved language skills, progressive improvement then complete resolution of headaches, and behavioral problems | Pseudomeningocele requiring temporary lumboperitoneal shunt |
CVA, cerebrovascular accident, EDAS, encephalo-duro-arterio-synangiosis; TIA, transient ischemic attack; WASEDS, wide arterial sparing encephalo-duro-synangiosis.
Clinical Outcomes
WASEDS was technically successful with neurological improvement in all 8 patients. One patient had significant diminution of the frequency of motor TIAs from several prolonged episodes/week preoperatively, to 1 mild episode/year postoperatively, with suspicion that the postoperative episodes represent complicated migraine. Another patient had 1 delayed TIA postoperatively and none after increasing the aspirin dose (Figure 2). There were no mortalities. There were 2 superficial wound problems and 2 pseudomeningoceles requiring temporary lumboperitoneal shunt placement with an on-off valve. These function as temporary lumbar drains that provide prolonged (weeks) drainage. The shunt valves were turned off 2 and 4 months postoperatively, respectively, with no pseudomeningocele recurrence (Table 1; Figure 2). No formal neuropsychological testing was obtained. However, unsolicited reports from the family and/or school teachers revealed that 5 of the younger children made cognitive and behavioral improvements within weeks to months of surgery (Table 1).
FIGURE 2.
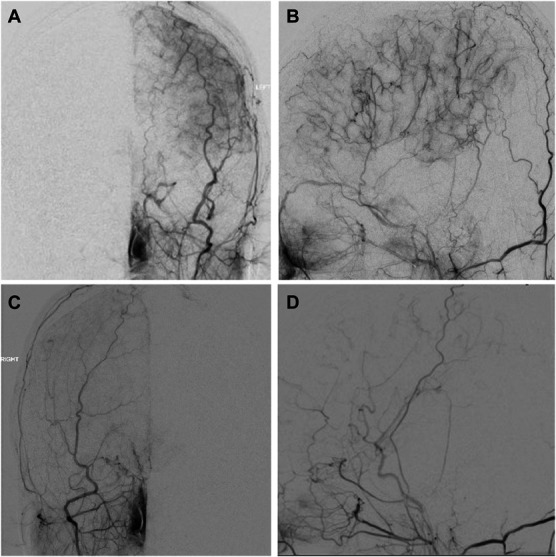
Post-wide arterial sparing encephalo-duro-synangiosis angiography performed on patient 7. A, AP and B, lateral right ECA injections and C, AP and D, lateral left ECA injections. Diagnostic images demonstrate extensive collateral formation, left greater than right. The cause of the asymmetry is unclear and may not only depend on technical issues but also on preoperative ischemia severity and other biological factors. Regardless, both hemispheres showed improved perfusion, and the patient had 1 postoperative transient ischemic attack, otherwise made significant clinical improvement. AP, anterior-posterior; ECA, external carotid artery.
Imaging Outcomes
The analysis includes 13 of the 14 WASEDS procedures. One treated hemisphere was excluded because of lack of MRP after WASEDS. Imaging was obtained at a range of 96 to 560 days postoperatively (mean 229 days). The mean pretreatment and post-treatment rCBV cerebral hemisphere/rCBV cerebellar hemispheres ratio was 0.912 and 1.00 mL of blood/100 g of brain tissue, respectively, or a 9.24% increase (paired t test, P = .012; Figure 3), along with significant increase in perfusion on qualitative assessment and marked enlargement of the MMA on MR imaging in select cases (Figure 4).
FIGURE 3.
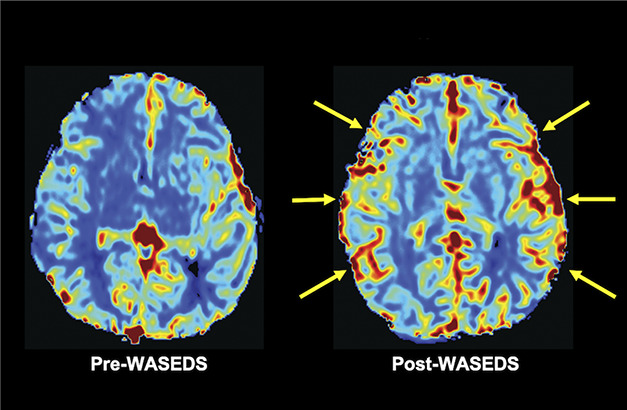
MR perfusion images of patient 1. Pre-WASEDs and post-WASEDS CBV perfusion maps with increased bilateral CBV post-WASEDs (yellow arrows). CBV, cerebral blood volume; WASEDS, Wide arterial sparing encephalo-duro-synangiosis.
FIGURE 4.
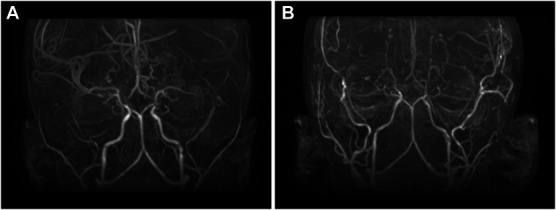
Three-year-old boy (case 8) with several months of intractable headaches, intermittent emesis, and severe anger issues, all of which improved markedly within weeks of bilateral wide arterial sparing encephalo-duro-synangiosis, and progressively resolved over 2 years. Note significant enlargement of the middle meningeal arteries (A, preoperative magnetic resonance angiography and B, postoperative magnetic resonance angiography).
Representative Case Illustration
Patient 1 is a 6-year-old woman who presented with right hemiparesis and MRI findings of multifocal left hemispheric infarcts. Magnetic resonance angiography and angiography showed bilateral severe stenosis of the distal ICA with near absence of the left middle and anterior cerebral arteries and enlarged collaterals. One month after presentation, she underwent bilateral EDAS, 5 weeks apart. She continued to have significant ischemic strokes requiring prolonged periods of rehabilitation, despite aspirin use and adequate hydration, including 1 presentation with a dense left hemiparesis and subacute infarction on MRI. MRP demonstrated elevated bilateral first-moment mean transit time (FMT), most significantly involving the right parietal, occipital, and posterior/inferior temporal lobes (Figure 5). Bilateral EDAS arteries were patent. Three years after EDAS, she underwent bilateral WASEDS, 1 week apart, without complication. Six weeks postoperatively, unsolicited reports from the family and teachers described significant improvement in cognitive function, behavior, and school performance. Four months postoperatively, imaging revealed significant improvement in left hemisphere perfusion, and overall interval improvement of FMT in the right parietal, occipital, and temporal lobes, with persistent prolongation of FMT in the frontal lobes (Figure 5). In the 11 years of follow-up after WASEDS, the patient had no further TIAs or strokes.
FIGURE 5.
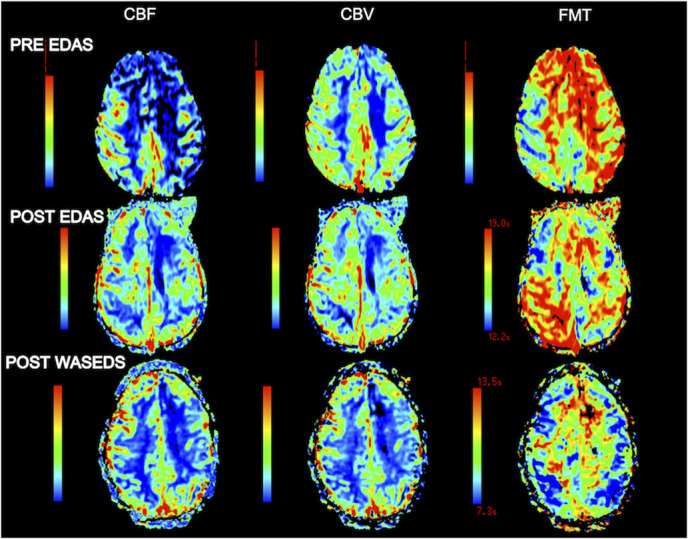
MR perfusion images of patient 1. Pre-EDAS images demonstrate prolonged FMT in both hemispheres. After EDAS, there is improvement in the left hemisphere but persistent FMT prolongation on the right, predominantly in the parietal and occipital regions. Imaging 4 months after WASEDS shows significant improvement in left hemisphere perfusion and bilateral interval improvement in FMT. CBV, cerebral blood volume; EDAS, encephalo-duro-arterio-synangiosis; FMT, first-moment transit time; WASEDS, wide arterial sparing encephalo-duro-synangiosis.
DISCUSSION
Surgery is the mainstay of treatment for symptomatic moyamoya disease.12 With both direct and indirect procedures, revascularization results in cessation of strokes or TIA events in the majority.3,4,13 Reported rates of stroke after EDAS are less than 5% in the general population and 10% in children younger than 2 years.3,14,15 To date, there is no evidence of superiority of 1 procedure over another, leaving the choice of procedure to surgeon preference (Table 23,16-29 and 3 30-38). Montaser et al21 recently reported the use of pericranial grafts to revascularize anterior cerebral artery territories lacking an overlying artery, termed pial pericranial dural revascularization. They note that although EDAS has been successful for most, looking beyond this technique may be beneficial for larger surface area and multiple territory revascularization.21
TABLE 2.
Literature Review of Direct and Indirect Revascularization Options and Their Outcomes for Moyamoya Disease
| Paper | Country | Intervention | No. of patients (no. of hemispheres) | Neurological outcome | Mean follow-up | Complications |
|---|---|---|---|---|---|---|
| Matsushima et al16 | Japan | STA-MCA + EMS (7) or EDAS alone (13) | 16 (20) | Symptoms disappeared in 3 (23%) sides with EDAS and 7 (100%) sides with direct bypass | 1 y | Increased TIAs in 2 EDAS patients and 1 direct bypass patients; 1 patient developed seizures |
| Mizoi et al22 | Japan | STA-MCA bypass ± EMS or EDAS; 1 patient with OA | 23 (41) | No ischemic or hemorrhagic events in 21 (91%) patients | 3.4 y | 2 (10%) pediatric patients with postoperative TIAs, 1 patient with skin flap necrosis |
| Ishikawa et al23 | Japan | STA-MCA bypass + EDAMS (48), EDAMS alone (16) | 34 (64) | Ischemic events persisted in 9 (56%) sides of the indirect group and in 5 (10%) sides in the combined group | 6.6 y | Perioperative ischemic events in 11 (17%) surgeries |
| Sakamoto et al24 | Japan | STA-MCA double anastomoses with EMS | 10 (19) | No patients with any significant postoperative ischemic episodes | 4 y | 4 patients had minor TIA within 1 month of surgery associated with hyperventilation |
| Matsushima et al25 | Japan | EDAS (18), EDAS + EMS (35) STA-MCA bypass with EMS (19) | 50 (72) | Symptoms resolved in 56% EDAS, 63% EDAS + EMS, and 74% direct bypass | 1 y | 1 patient had stroke after EDAS, 2 patients had EDH after MCI, 2 patients had stroke after direct bypass |
| Suzuki et al26 | Japan | STA-MCA bypass + EDAS, EMS, and burr holes (20), EDAS + EMS and burr holes (10), STA-MCA bypass on 1 side and EDAS on the other (6) | 36 (NRa) | TIA frequency decreased and completely disappeared within 1 y in 25 of 31 patients (81%). Symptoms were less frequent in the remaining 6 patients | NRa | 1 patient had complete stroke after indirect bypas, initial patients with skin necrosis |
| Golby et al27 | USA | STA-MCA bypass ± EDAS (19), MMA-MCA bypass (1), omental transposition (1) | 12 (21) | Neurological conditions stable to improved in all patients | 35 mo | None |
| Kim et al28 | Korea | Bifrontal EGPS with EDAS or EMS | 67 (NRa) | Complete disappearance of TIA in 63% | 21.4 mo | 3 patients had infarction because of damage of the draining vein into the superior sagittal sinus, 1 patient with postoperative arterial ischemic infarct, 1 patient with osteomyelitis of bone flap |
| Scott et al3 | USA | Pial synangiosis | 143 (271) | Of 126 patients with >1 y follow-up 7 patients had late-onset neurological problems, 6 patients required salvage surgeries | 5.1 y | 11 patients (7.7%) had stroke and 3 patients had severe TIAs within 30 days of surgery, 4 patients SDH, 1 patient died from IPH |
| Kim et al, 200729 | Korea | EDAS (16), EDAMS (8), STA-MCA bypass with EDAMS (12) | 24 (36) | Excellent to good results in 67%, 80% with EDAMS, and 100% with STA-MCA-EDAMS | NRa | None |
| Park et al17 | Korea | EDAS with bifrontal EGPS | 17 (NRa) | Excellent to good results in 15 patients (88.2%) | 11.5 mo | 1 patient with subgaleal/subdural hematoma resulting in poor revascularization |
| Tripathi et al18 | India | EDAS | 8 (15) | No episode of stroke or TIA for any patients | 2 y | 1 patient developed seizures temporarily postoperatively |
| Kim et al19 | Korea | EDAS + EGPS | 410 (NRa) | 81% of patients had a favorable clinical outcome | 61 mo | 2 patients died (0.5%), 15% had postoperative strokes, 6% had wound complications, 4% had EDH, 2% had SDH, 0.5% had IPH |
| Ng et al20 | UK | STA-MCA (49), EDAS (82) | 73 (134) | 5 patients (3.8%) had clinical or radiological acute ischemic symptoms. 79.2% had resolved or improved TIA symptoms | 2.8 y | 3 patients had intracranial hemorrhage |
| Montaser et al21 | USA | PiPeD revascularization | 21 (25) | No postoperative strokes. Radiographic revascularization in 20/22 hemispheres (90.9%) | 1 patient (4.7%) had superficial infection |
EDAS, encephalo-duro-arterio-synangiosis; EGPS, encephalogaleoperiosteal-synangiosis; EMS, encephalo-myo-synangiosis; IPH, intraparenchymal hematoma; MCA, middle cerebral artery; MMA, middle meningeal artery; OA, occipital artery; PiPeD, Pial pericranial dural; SDH, subdural hematoma; STA, superficial temporal artery; TIA, transient ischemic attack.
NR means information not reported.
TABLE 3.
Literature Review of Salvage Revascularization Surgery Options and Their Outcomes for Moyamoya Disease
| Paper | Country | Intervention | No. of patients (no. of hemispheres) | Neurological outcome | Mean follow-up | Complications |
|---|---|---|---|---|---|---|
| Cahan30 | USA | Direct STA-MCA bypass | 1 (1) | No new ischemic symptoms | 1 y | None |
| Miyamoto et al31 | Japan | Direct (STA-MCA or OA-MCA) bypass with EMS (16), omental transposition (3) | 11 (19) | Complete resolution of TIAs and improved motor and intellectual function in 10 of 11 patients (91%) | NRa | None |
| Matsushima et al32 | Japan | EMS (2), EMAS (1) | 3 (3) | Complete resolution of TIAs in 2 of 3 patients (67%), 1 markedly decreased TIAs which gradually disappeared | NR | None |
| Touho et al33 | Japan | Direct STA-MCA bypass with or without EMS | 31 (28) | Clinical improvement in 27 of 28 patients with complete resolution of TIAs (96%), 1 patient with fixed deficit unchanged | 42.4 mo | None |
| Touho et al34 | Japan | Omental transplantation | 5 | Complete resolution of TIAs in 4 of 5 patients (80%), continued but markedly decreased TIAs in 1 patient | 15.2 mo | None |
| Hayashi et al35 | Japan | Direct OA-PCA bypass with EGDS and burr holes | 3 (3) | Complete resolution of TIAs in 3 of 3 patients (67%) | 9 mo | 1 patient with “transient visual impairment” in the early postoperative period for 1 mo because of cerebral edema |
| Pandey and Steinberg36 | USA | Direct (STA-MCA, OA-PCA, or OA-MCA) bypass (14) or EMS (4) or repeat EDAS (2) or omental transposition (3) | 16 (23) | 12 patients (80%) had no TIA or strokes, 2 patients with occasional TIAs (before surgery), 1 patient had frequent TIAs/failed anastomsis and had rpt EMS with burr holes | 34 mo | Death due to reperfusion hemorrhage from high-flow vein graft |
| Navarro et al37 | USA | Laparoscopic pedicle omental transposition | 3 (4) | Complete resolution of ischemic symptoms in 3 of 3 patients (100%) | 1 y | None |
| Hori et al38 | Germany | Radial artery graft ECA-MCA bypass | 4 (4) | Complete resolution of TIAs and/or stroke in 4 of 4 patients, 1 patient had graft failure but transdural collaterals had formed | 8.5 mo | None |
ECA, external carotid artery; EGDS, encephalogaleodurosynangiosis; EMAS, encephalomyoarterial-synangiosis; EMS, encephalo-myo-synangiosis; MCA, middle cerebral artery; OA, occipital artery; PCA, posterior cerebral artery; STA, superficial temporal artery; TIA, transient ischemic attack.
NR means information not reported.
In our experience, WASEDS is a safe and effective indirect cerebral revascularization procedure with several advantages: (1) It is an effective salvage technique after failure of other surgical treatment; (2) on the surface, the larger craniotomy and extent of dural slits may provide wider cortical coverage than traditional procedures, and imaging shows extensive collateralization across the hemisphere with enlargement of associated MMA vasculature (Figure 4); and (3) unlike EDAS or external carotid to internal carotid bypass, it preserves large-sized and medium-sized MMA branches, thus preserving their terminal collateralization with intracranial vasculature.
Compared with EDAS, WASEDS seems to have a higher risk of postoperative pseudomeningocele (2 of 8 patients). Pseudomeningocele development is likely related to the extensive dural slits in the setting of wide craniotomy. To avoid prolonged lumbar drainage or permanent shunting, these were treated with temporary lumboperitoneal shunts with an on-off valve. The valve can be turned off percutaneously and eventually removed once the tissues heal. If superior revascularization is confirmed in larger studies, this would weigh favorably against the pseudomeningocele risk.
WASEDS as a Salvage Procedure
The original motivation for developing WASEDS was EDAS failure. Only a handful of reports describe management options after surgical failure (Table 3). Reasons for failure include donor vessel occlusion, inadequate collateral formation, or development of new ischemic zones outside the surgical territory.37 In our series, all 3 patients had persistent symptoms or strokes without STA occlusion.
Repeat revascularization attempts are challenging for various reasons including the technical particularities of reoperations (scar tissue, arachnoiditis, complex incisions, etc.) and the availability of donor and/or recipient tissue or vessels.36,37 Salvage procedures include direct (eg, external carotid to internal carotid bypass) and indirect (eg, burr holes in previously unexposed areas or synangiosis with extracranial vascularized tissues) revascularization techniques. We found that WASEDS is a safe and effective salvage option. It offers excellent clinical results and relative technical simplicity compared with other techniques because no dissection is needed to transpose vessels, muscle, or other tissues. Its limitations include increased risk of bleeding and cerebrospinal fluid leak because of size of craniotomy.
WASEDS as First-Line Surgery
WASEDS provides vascular supply to ischemic tissue through the dura and MMA branches. It improves cerebral perfusion on imaging in line with other revascularization procedures. Interestingly, the spontaneous collateralization of MMA branches is presumably more robust than those from the transposed STA in EDAS.9 In addition, EDAS requires sacrifice of at least 1 or 2 MMA branches during dural opening, which may disrupt some of the collaterals, whereas WASEDS follows the course of the MMA branch without compromise.
With equipoise in preventing ischemic symptoms and stroke, advances in revascularization techniques should consider studying other factors relevant to the ischemic brain, namely higher cortical function. In the adult literature, clinicians are becoming acutely aware of the relationship between cerebral ischemia and cognitive function, with stroke becoming the second most cited cause of acquired cognitive impairment.39,40 In the younger moyamoya population, “silent” lacunar infarcts have been correlated with deteriorating global cognitive function and dementia, and cognitive decline has been reported in up to 50% of untreated children.1,7,41,42 Furthermore, a cohort of 27 children with moyamoya disease revealed that although ischemic symptoms peak in the first 4 years of life and decline thereafter, intellectual deterioration and neurological deficits continue to increase.41 This may suggest that with traditional procedures, the revascularized region reduces TIA risk and focal stroke damage, but is not generous enough to halt cognitive decline.
In this series, WASEDS use as first-line therapy compares favorably with the literature in preventing stroke and TIA. However, we also observed that, unlike EDAS, young children may experience discernible improvement in cognition and behavior as early as 3 weeks postoperatively. Obviously, this is anecdotal and could be entirely coincidental. However, these reports encouraged us to use WASEDS more frequently and to incorporate formal neuropsychological testing in our routine evaluation and follow-up. If we were to postulate that the success of cranial revascularization relies in part on the size of the cortical surface exposed to new vascular supply, WASEDS covers a much larger cortical area than what is traditionally covered by the STA vessel in EDAS and the temporalis muscle in encephalo‐myo‐synangiosis.3 Multiple burr holes with dural opening presumably provide a wide area of revascularization. However, the spaces not covered by the burr holes may not show evidence of neovascularization. In addition, opening the dura at a burr hole site may inadvertently injure an MMA branch, thus possibly jeopardizing existing distal collaterals.
Study Limitations
The small sample size precludes any generalization of results, and a larger cohort is required before more definitive conclusions can be drawn. MRP studies are still evolving technically and show enough variability that clinical parameters remain the cornerstone of measuring outcome. Furthermore, the study does not address nuance of timing and indications for surgery.
CONCLUSION
WASEDS is a safe and effective indirect revascularization technique for moyamoya disease. We propose that this surgical approach provides more extensive areas of cortical revascularization than traditional indirect procedures, without interruption of spontaneous, naturally established collaterals. WASEDS is particularly useful in the setting of failed initial indirect revascularization. Surgeons are cautioned about the potentially increased risk of bleeding and cerebrospinal fluid leak from the large craniotomy without dural closure.
Footnotes
Beverly Aagaard-Kienitz and Bermans Iskandar contributed equally to this work.
Contributor Information
Demi Dawkins, Email: demi.dawkins@gmail.com.
Beverly Aagaard-Kienitz, Email: BKienitz@uwhealth.org.
Kelly Capel, Email: KCapel@uwhealth.org.
Laura Eisenmenger, Email: LEisenmenger@uwhealth.org.
Alexey Samsonov, Email: samsonov@wisc.edu.
Yiping Li, Email: liyip218@gmail.com.
Carolina Sandoval-Garcia, Email: csandova@umn.edu.
Funding
This study did not receive any funding or financial support. Dr Eisenmenger's effort on this work was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), Grant UL1TR002373 and KL2TR002374, as well as the Wisconsin Alzheimer's Disease Research Center Grant P30-AG062715.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1.Jodi LS. Understanding and treating moyamoya disease in children. Neurosurg Focus. 2009;26(4):1-11. [DOI] [PubMed] [Google Scholar]
- 2.Fung LWE, Thompson D, Ganesan V. Revascularisation surgery for paediatric moyamoya: a review of the literature. Childs Nerv Syst. 2005;21(5):358-364. [DOI] [PubMed] [Google Scholar]
- 3.Scott RM, Smith JL, Robertson RL, Madsen JR, Soriano SG, Rockoff MA. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg. 2004;100(2 suppl):142-149. [DOI] [PubMed] [Google Scholar]
- 4.Kim T, Oh C, Bang J, Kim JE, Cho W-S. Moyamoya disease: treatment and outcomes. J Stroke. 2016;18(1):21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsushima Y, Inaba Y. The specificity of the collaterals to the brain through the study and surgical treatment of moyamoya disease. Stroke. 1986;17(1):117-122. [DOI] [PubMed] [Google Scholar]
- 6.Hwang JK, Park EK, Kim J, Kang H, Kim DS, Shim KW. The feasibility of performing multiple burr hole surgery in pediatric moyamoya patients as a response to failed mEDAS. Childs Nerv Syst. 2021;37(7):2233-2238. [DOI] [PubMed] [Google Scholar]
- 7.Ravindran K, Wellons JC, Dewan MC. Surgical outcomes for pediatric moyamoya: a systematic review and meta-analysis. J Neurosurg Pediatr. 2019;24(6):663-672. [DOI] [PubMed] [Google Scholar]
- 8.Robertson RL, Burrows P, Barnes P, Robson C, Poussaint T, Scott RM. Angiographic changes after pial synangiosis in childhood moyamoya disease. AJNR Am J Neuroradiol. 1997;18(5):837-845. [PMC free article] [PubMed] [Google Scholar]
- 9.King J, Armstrong D, Vachhrajani S, Dirks P. Relative contributions of the middle meningeal artery and superficial temporal artery in revascularizaiton surgery for moyamoya syndrome in children: the results of superselective angiography. Neurosurg Pediatr. 2010;5(2):184-189. [DOI] [PubMed] [Google Scholar]
- 10.Welker K, Boxerman J, Kalnin A, Kaufmann T, Shiroishi M, Wintermark M. ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. Am J Neuroradiol 2015;36(6):E41-E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostergaard L, Weisskoff R, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: mathematical approach and statistical analysis. Magn Reson Med. 1996;36(5):715-725. [DOI] [PubMed] [Google Scholar]
- 12.Smith ER, Scott RM. Surgical management of moyamoya syndrome. Skull Base. 2005;15(1):15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman R, Lee M, Achrol A, Bell-Stephens T, Kelly M, Do H. Clinical outcome after 450 revascularization procedures for moyamoya disease. J Neurosurg. 2009;111(5):927-935. [DOI] [PubMed] [Google Scholar]
- 14.Jackson E, Lin N, Manjila S, Scott RM, Smith ER. Pial synangiosis in patients with moyamoya younger than 2 years of age. J Neurosurg Pediatr. 2014;13(4):420-425. [DOI] [PubMed] [Google Scholar]
- 15.Adelson P, Scott RM. Pial synangiosis for moyamoya syndrome in children. Pediatr Neurosurg. 1995;23(1):26-33. [DOI] [PubMed] [Google Scholar]
- 16.Matsushima T, Inoue T, Suzuki S, Fujii K, Fukui M, Hasuo K. Surgical treatment of moyamoya disease in pediatric patients—comparison between the results of indirect and direct revascularization procedures. Neurosurgery. 1992;31(3):401-405. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Yang SY, Chung YN, et al. Modified encephaloduroarteriosynangiosis with bifrontal encephalogaleoperiosteal synangiosis for the treatment of pediatric moyamoya disease: technical note. J Neurosurg. 2007;106(3 suppl):237-242. [DOI] [PubMed] [Google Scholar]
- 18.Tripathi P, Tripath V, Naik R, Patel J. Moya moya cases treated with encephaloduroarteriosynangiosis. Indian Pediatr. 2007;44(2):123-127. [PubMed] [Google Scholar]
- 19.Kim SK, Cho BK, Phi JH, et al. Pediatric moyamoya disease: an analysis of 410 consecutive cases. Ann Neurol. 2010;68(1):92-101. [DOI] [PubMed] [Google Scholar]
- 20.Ng J, Thompson D, Lumley JPS, Saunders DE, Ganesan V. Surgical revascularisation for childhood moyamoya. Childs Nerv Syst. 2012;28(7):1041-1048. [DOI] [PubMed] [Google Scholar]
- 21.Montaser A, Driscoll J, Smith H, et al. Long-term clinical and radiographic outcomes after pial pericranial dural revascularization: a hybrid surgical technique for treatment of anterior cerebral territory ischemia in pediatric moyamoya disease. J Neurosurg Pediatr. 2021;28(3):351-359. [DOI] [PubMed] [Google Scholar]
- 22.Mizoi K, Kayama T, Yoshimoto T, Nagamine Y. Indirect revascularization for moyamoya disease: is there a beneficial effect for adult patients? Surg Neurol. 1996;45(6):541-548. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa T, Houkin K, Kamiyama H, Abe H. Effects of surgical revascularization on outcome of patients with pediatric moyamoya disease. Stroke. 1997;28(6):1170-1173. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto H, Kitano S, Yasui T, et al. Direct extracranial–intracranial bypass for children with Moyamoya disease. Clin Neurol Neurosurg. 1997;99(suppl 2):128-133. [PubMed] [Google Scholar]
- 25.Matsushima T, Inoue T, Ikezaki K, et al. Multiple combined indirect procedure for the surgical treatment of children with moyamoya disease. A comparison with single indirect anastomosis with direct anastomosis. Neurosurg Focus. 2008;5(5):E6. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki Y, Negoro M, Shibuya M, Yoshida J, Negoro T, Watanabe K. Surgical treatment for pediatric moyamoya disease: use of the superficial temporal artery for both areas supplied by the anterior and middle cerebral arteries. Neurosurgery. 1997;40(2):324-330. [DOI] [PubMed] [Google Scholar]
- 27.Golby A, Marks M, Thompson R, Steinberg GK. Direct and combined revascularization in pediatric moyamoya disease. Neurosurgery. 1999;45(1):50-58. [DOI] [PubMed] [Google Scholar]
- 28.Kim CY, Wang KC, Kim SK, Chung YN, Kim HS, Cho BK. Encephaloduroarteriosynangiosis with bifrontal encephalogaleo(periosteal)-synangiosis in the pediatric moyamoya disease: the surgical technique and its outcomes. Childs Nerv Syst. 2003;19(5-6):316-324. [DOI] [PubMed] [Google Scholar]
- 29.Kim DS, Kang SG, Yoo DS, Huh PW, Cho KS, Park CK. Surgical results in pediatric moyamoya disease: angiographic revascularization and the clinical results. Clin Neurol Neurosurg. 2007;109(2):125-131. [DOI] [PubMed] [Google Scholar]
- 30.Cahan LD. Failure of encephalo-duro-arterio-synangiosis procedure in Moyamoya disease. Pediatr Neurosci. 1985;12(1):58-62. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto S, Kikuchi H, Karasawa J, Nagata I, Yamazoe N, Akiyama Y. Pitfalls in the surgical treatment of moyamoya disease. J Neurosurg. 2009;68(4):537-543. [DOI] [PubMed] [Google Scholar]
- 32.Matsushima T, Fujiwara S, Nagata S, Fujii K, Fukui M, Hasuo K. Reoperation for moyamoya disease refractory to encephalo-duro-arterio-synangiosis. Acta Neurochir. 1990;107(3-4):129-132. [DOI] [PubMed] [Google Scholar]
- 33.Touho H, Karasawa J, Ohnishi H, Yamada K, Shibamoto K. Surgical reconstruction of failed indirect anastomosis in childhood moyamoya disease. Neurosurgery. 1993;32(6):935-940. [DOI] [PubMed] [Google Scholar]
- 34.Touho H, Karasawa J, Tenjin H, Ueda S. Omental transplantation using a superficial temporal artery previously used for encephaloduroarteriosynangiosis. Surg Neurol. 1996;45(6):550-558. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi T, Shirane R, Tominaga T. Additional surgery for postoperative ischemic symptoms in patients with moyamoya disease: the effectiveness of occipital artery-posterior cerebral artery bypass with an indirect procedure: technical case report. Neurosurgery. 2009;64(1):E195-E196. [DOI] [PubMed] [Google Scholar]
- 36.Pandey P, Steinberg GK. Outcome of repeat revascularization surgery for moyamoya disease after an unsuccessful indirect revascularization: clinical article. J Neurosurg. 2011;115(2):328-336. [DOI] [PubMed] [Google Scholar]
- 37.Navarro R, Chao K, Gooderham P, Bruzoni M, Dutta S, Steinberg GK. Less invasive pedicled omental-cranial transposition in pediatric patients with moyamoya disease and failed prior revascularization. Neurosurgery 2014;10 Suppl 1:1-14. [DOI] [PubMed] [Google Scholar]
- 38.Hori S, Acker G, Vajkoczy P. Radial artery grafts as rescue strategy for patients with moyamoya disease for whom conventional revascularization failed. World Neurosurg. 2016;85:77-84. [DOI] [PubMed] [Google Scholar]
- 39.Barba R, Martínez-Espinosa S, Rodríguez-García E, Pondal M, Vivancos J, Del Ser T. Poststroke dementia: clinical features and risk factors. Stroke. 2000;31(7):1494-1501. [DOI] [PubMed] [Google Scholar]
- 40.Tang EYH, Amiesimaka O, Harrison SL, et al. Longitudinal effect of stroke on cognition: a systematic review. J Am Heart Assoc. 2018;7(2):e006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurokawa T, Tomita S, Ueda K, Narazaki O, Hanai T, Hasuo K. Prognosis of occlusive disease of the circle of Willis (moyamoya disease) in children. Pediatr Neurol. 1985;1(5):274-277. [DOI] [PubMed] [Google Scholar]
- 42.Vermeer SE, Prins ND, Den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215-1222. [DOI] [PubMed] [Google Scholar]


