ABSTRACT
Background:
Polytetrafluoroethylene (PTFE) patch is commonly used during surgical closure for atrial septal defect (ASD) and ventricular septal defect (VSD). However, this patch has several limitations such as its inability to grow or remodel, especially in children and young adults. To tackle these limitations, we have tried to use fibronectin and human adipose-derived mesenchymal stem cells (hAMSCs) in the PTFE patch.
Objective:
To understand the impact of fibronectin to enhance hAMSCs cell-to-cell adherence and cell-to-patch surface attachment into PTFE patches used in the surgical closure of ASD or VSD.
Materials and Methods:
The hAMSCs were plated and fixated with 15 mL methanol and cluster of differentiation (CD) 90+, CD105+, and CD45 − antibodies were labeled with fluorescein isothiocyanate, rinsed with phosphate-buffered saline, and analyzed under a fluorescence microscope. Fibronectin solution (0.1%) was used to soak patch scaffolds for approximately 2-h duration and then dried for 20 min in the treatment group. The samples were examined with a scanning electron microscope (SEM).
Results:
SEM examination showed incomplete attachment of the cells even after 10 days in the control group at 1.14 ± 1.13. In contrast, the treatment group showed more cells attached to the patch surface at 31.25 ± 13.28 (P ≤ 0.0001). The observation at 5 days was 17.67 ± 20.21, at 7 days was 12.11 ± 10.94, and at 10 days was 18.83 ± 23.25. There was no significant statistical difference in mean cell per view among each treatment group (P = 0.802).
Conclusion:
Our work demonstrates that fibronectin has a positive impact on hAMSC attachment seeded onto the PTFE patch. These properties, in combination with their developmental plasticity, have generated tremendous interest in regenerative medicine.
Keywords: Atrial septal defect, fibronectin, human adipose-derived mesenchymal stem cells, polytetrafluoroethylene patch, surgical closure, ventricular septal defect
INTRODUCTION
Acyanotic congenital heart diseases (CHDs) such as atrial septal defect (ASD) and ventricular septal defect (VSD) are the most common CHDs (50% of all CHDs).[1] Usually, a patch of prosthetic material or pericardium is sewn over the ASD or VSD to close it completely. Unfortunately, materials used for patch repair or reconstruction have limitations such as their inability to grow, repair, and remodel. Aneurysm formation, thrombosis, and the inability of patches to grow or remodel are important sources of morbidity and mortality after the repair or reconstruction of cardiovascular structures, especially in children and young adults.[2] Tissue engineering has the potential to become an important in vitro innovative approach for the intended surgical replacement of congenital defects. By transplanting multipotential human adipose-derived mesenchymal stem cells (hAMSCs) onto the widely used polytetrafluoroethylene (PTFE) patch, we tried a new solution for surgical prosthetic material. Fibronectin as an extracellular matrix (ECM) protein was added to enhance cell-to-cell adherence and cell-to-patch surface attachment. The objective of the current study is to study the impact of fibronectin to enhance hAMSCs cell-to-cell adherence and cell-to-patch surface attachment into PTFE patch to close ASD or VSD.
MATERIALS AND METHODS
Ethical approval
This study was approved by the Health Research Ethics Committee, Dr. Soetomo General Hospital, Surabaya (Institution Review Board number: IORG0007195) on March 6, 2018 (Ref number: 60/Panke. KKE/III/2018). This study was carried out in strict accordance with the principles expressed in the Guidance Document on Good In Vitro Method Practices.
Scaffold materials
Cardiovascular synthetic patches made of PTFE (Gore-Tex®) were used. Reconstruction of congenital cardiac defects often requires synthetic materials such as PTFE when native tissue is not adequate. PTFE itself is completely harmless because it is not digested, metabolized, or absorbed by the host and PTFE has a proven track record about its safety profile. It is a durable, easy-to-handle, and sterile synthetic patch designed for optimal tissue ingrowth while minimizing aneurysmal dilation in a wide variety of cardiovascular applications including cardiac, great vessel, and peripheral vascular reconstructions and surgeries. The patch was sliced into 0.8 cm × 0.8 cm pieces.
Mesenchymal stem cell culture and harvesting
Mesenchymal stem cells (MSCs) were obtained by thawing of cryopreserved hAMSCs. They were thawed at 37°C and transferred in 5 mL of standard cell media and gently homogenized. 500 μL warm phosphate-buffered saline (PBS) was added into the medium well for 2 min. Resuspension with PBS plus 500 μL trypsin/ethylenediaminetetraacetic acid solution (0,5%) and incubated for 5–10 min 37°C and 5% of CO2 until they reached 80% of confluence. The cell suspension was transferred to a sterile centrifuge tube and centrifuged at 300 g for 5 min. After centrifugation, 200 μL warm PBS solution was added to each centrifuge tube. After another centrifugation, the supernatant was aspirated, and the cells were resuspended in approximately 500 μL of stromal medium. 1 mL aliquot cell dilution in trypsin blue was submitted to counting cells using a hematocytometer. The cells were then placed onto suitable culture plates. For each sample, the culture was done at passage number 5. Using a light microscope, the cell culture showed spindle-like shaped morphology consistent with MSCs.
Identification of human adipose-derived mesenchymal stem cell phenotype
All samples were analyzed for the expression of surface markers, using monoclonal antibodies against cluster of differentiation (CD) antigens, conjugated with fluorochromes, and analyzed after thawing by indirect immunofluorescence. Cultured cells were fixated with 15 mL methanol and CD90+, CD105+, and CD45− antibodies were labeled with fluorescein-isothiocyanate, rinsed with PBS, and analyzed under a fluorescence microscope for 15 min.
Fibronectin addition onto the polytetrafluoroethylene patch surface
Fibronectin solution 0.1% was used to soak patch scaffolds for approximately 2-h duration. After that, the solution was dried for 20 min for the treatment group. As for the control group, the fibronectin solution was not added to the culture. To improve the adhesion capabilities in the PTFE patch, hAMSCs were seeded on fibronectin-coated ventricular ECM scaffolds. Co-transplantation of hAMSCs and fibronectin seems to be highly efficient regarding the improvement of acute retention.
Human adipose-derived mesenchymal stem cell direct seeding into polytetrafluoroethylene patch
Approximately 2 μL (±10, 000 cells) were seeded onto the PTFE patch surface in M-96 medium wells. The cells were cultured in Dulbecco’s Modified Eagle Medium (Gibco Life Technologies) supplemented with 10% fetal calf serum (Sigma-Aldrich, Germany) and 1% penicillin–streptomycin–glutamine solution (Gibco Life Technologies). The medium was changed every 2 days. The cell-seeded scaffolds were placed in 96 medium wells under dynamic cell culture conditions and divided into 5-, 7-, or 10-day observation groups. After the required observing days, the samples were harvested and prepared for scanning electron microscope (SEM) examination. The analysis of patches includes a qualitative and quantitative examination by counting the mean number of cells per field of view.
Structure examination with scanning electron microscope
The samples were fixed with 2% glutaraldehyde, followed by gradual dehydration using alcohol. Afterward, the specimens were critical point dried in CO2 using a Hitachi HCP-2 critical point dryer (Hitachi, Tokyo, Japan) and were subsequently gold sputtered using a vacuum evaporator. The samples were examined with a Jeol SEM (Hitachi, Tokyo, Japan), and the images were captured by a Nikon camera.
Statistical analysis
Data results were expressed as mean ± standard deviation. Data and graphs were produced with MS Excel 2010 (Microsoft Corp., USA). Comparisons between the fibronectin-added hAMSCs seeded patch and control group were performed with Mann–Whitney U-test and one-way analysis of variance test using SPSS Statistics 25.0 (IBM Corp., USA). Statistical significance was set at P < 0.05.
RESULTS
The International Society for Cellular Therapy clearly stated criteria for minimum expression of hAMSC immunophenotyping, which included CD73+, CD90+, CD44+, and CD105+ and CD45-negative expression based on phase 2 immunocytochemistry (ICC). In our samples, data show a positive expression for CD105+ and a negative expression for CD45-based on ICC [Figure 1].
Figure 1.

Cell culture characteristics based on immunocytochemistry CD90+and CD105+ (positive expression showed green luminescence) whereas CD45− (negative expression does not show)
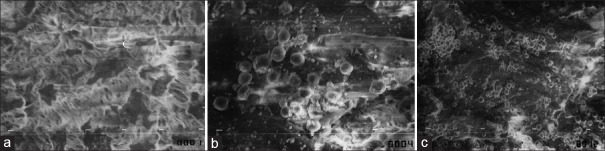
Data from SEM examination after transplantation of PTFE with MSCs showed more hAMSCs attached to the patch surface at 31.25 ± 13.28 (P = 0.000) whereas incomplete attachment of the hAMSCs even after 10 days on the control group at 1.14 ± 1.13 [Figure 2]. Observation at 5 days was 17.67 ± 20.21, at 7 days was 12.11 ± 10.94, and at 10 days was 18.83 ± 23.25. There is a significant statistical difference in the mean cell per view between the treatment group and the control group (P = 0.002) [Figure 3].
Figure 2.
Scanning electron micrographs: Control group without fibronectin addition (a), Treatment group after 5 days (b) (×1000), Treatment group after 5 days (c) (×350)
Figure 3.

Human adipose-derived mesenchymal stem cell attachment onto polytetrafluoroethylene patch surface: Control versus treatment group (mean number of cell per field of view)
DISCUSSION
Stem cell therapy in tissue engineering has been a promising strategy to reconstruct, repair, or replace tissues in congenital defects. The important components are the cells used, scaffold material, and supportive signaling molecules. The hAMSCs have been recommended by the International Fat Applied Technology Society for having good differentiation capacity, easy accessibility in terms of isolation procedure, and proliferation ability, and it does not change throughout a patient’s life.[3] Therefore, hAMSCs were used in this study for their superiority over bone marrow MSCs.
Many factors affect the efficacy of solubilized proteins such as fibronectin on surfaces including the distance between the protein and substratum,[4,5] the immediate environment surrounding the functional domain,[6] and the configuration of the protein and its secondary structure.[7] Those factors may affect the accessibility of the active part of the protein to the cell, the activity of the peptide, and the topography of the surface.
PTFE patch has low seeding efficiency and difficulty to withhold cells,[8] and this might be due to the hydrophobic and lightweight character of the patch itself, not allowing it to sink into the culture media. Adsorption or immobilization of specific ECM proteins can, therefore, be used to enhance cell attachment. In order to mimic the in vivo environment, purified components of the ECM are frequently used in the laboratory to coat cell culture plastic or glass to enhance cell adhesion.[9] Cell culture studies using human coronary artery smooth muscle cells onto polyurethane scaffold demonstrated that fibronectin-conjugated scaffolds had improved cell attachment and infiltration depth compared with scaffolds without fibronectin conjugation and with those scaffolds on which fibronectin was merely adsorbed at 4-day cell culture.[10] This study shows that undifferentiated hAMSCs seeded onto those patch scaffolds can attach more with fibronectin addition. On the other hand, the control group showed the original structure of the patch rather than a homogenous and confluent surface.
The smaller size of scaffolds and many greater numbers of cells used to seed may improve the attachment and retainability of cells onto the patch surface. However, the molecular interactions between hAMSCs and fibronectin protein remain unknown.
The longer duration of cell culture did not show any statistically significant difference. This may imply that after fibronectin addition, 5 days of cell culture has shown good cell distribution and cell attachment onto the patch surface as expected. The findings of this early study may add an important point in creating a biomimetic patch in vitro. However, the potency of hAMSCs seeded on patches to induce differentiation into grown cardiac endothelial cells still needs further research. Its physical and chemical durability still needs further evaluation in terms of ECM formation and endothelial coverage before expected clinical application in the future.
CONCLUSIONS
Fibronectin has a positive impact on hAMSC attachment seeded onto the PTFE patch. These properties, in combination with their developmental plasticity, have generated tremendous interest because of the potential use of hAMSCs in regenerative medicine to replace damaged tissues. The longer duration of cell culture did not provide a significant difference in cell-to-patch surface attachment.
Author contributions
A. K. R. conceived and planned the experiments, carried out the experiments, planned and carried out the simulations, and contributed to sample preparation. I. G. R. S. contributed to the project administration and funding. A. A. and M. A. contributed to the interpretation of the results. R. A. N. initially wrote the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to express special gratitude to all staff from the Institute of Tropical Diseases Universitas Airlangga who allowed us to do this research. We would like to Budi Santoso as Dean of the Faculty of Medicine Universitas Airlangga and Djoni Wahyuhadi as Director of Dr. Soetomo General Hospital for their support in this research.
A preprint has previously been published in Preprints.org according to the following link: https://www.preprints.org/manuscript/202107.0544/v1.[11]
REFERENCES
- 1.Hoffman JI, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J. 2004;147:425–39. doi: 10.1016/j.ahj.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Yang C, Sodian R, Fu P, Lüders C, Lemke T, Du J, et al. In vitro fabrication of a tissue engineered human cardiovascular patch for future use in cardiovascular surgery. Ann Thorac Surg. 2006;81:57–63. doi: 10.1016/j.athoracsur.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 4.Houseman BT, Mrksich M. The microenvironment of immobilized Arg-Gly-Asp peptides is an important determinant of cell adhesion. Biomaterials. 2001;22:943–55. doi: 10.1016/s0142-9612(00)00259-3. [DOI] [PubMed] [Google Scholar]
- 5.Tong YW, Shoichet MS. Enhancing the neuronal interaction on fluoropolymer surfaces with mixed peptides or spacer group linkers. Biomaterials. 2001;22:1029–34. doi: 10.1016/s0142-9612(00)00338-0. [DOI] [PubMed] [Google Scholar]
- 6.Dori Y, Bianco-Peled H, Satija SK, Fields GB, McCarthy JB, Tirrell M. Ligand accessibility as means to control cell response to bioactive bilayer membranes. J Biomed Mater Res. 2000;50:75–81. doi: 10.1002/(sici)1097-4636(200004)50:1<75::aid-jbm11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 7.Ochsenhirt SE, Kokkoli E, McCarthy JB, Tirrell M. Effect of RGD secondary structure and the synergy site PHSRN on cell adhesion, spreading and specific integrin engagement. Biomaterials. 2006;27:3863–74. doi: 10.1016/j.biomaterials.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Rosenman JE, Kempczinski RF, Pearce WH, Silberstein EB. Kinetics of endothelial cell seeding. J Vasc Surg. 1985;2:778–84. [PubMed] [Google Scholar]
- 9.Kleinman HK, Luckenbill-Edds L, Cannon FW, Sephel GC. Use of extracellular matrix components for cell culture. Anal Biochem. 1987;166:1–13. doi: 10.1016/0003-2697(87)90538-0. [DOI] [PubMed] [Google Scholar]
- 10.Dubey G, Mequanint K. Conjugation of fibronectin onto three-dimensional porous scaffolds for vascular tissue engineering applications. Acta Biomater. 2011;7:1114–25. doi: 10.1016/j.actbio.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Suryawan IG, Ratri AK, Andrianto A, Ardiana M, Nugraha RA. Positive impact of fibronectin in stimulation human adipose-derived mesenchymal stem cells attachment seeded into polytetrafluoroethylene patch for future surgical closure of atrial and ventricular septal defect. Preprints. 2021:2021070544. doi: 10.4103/apc.apc_9_23. [doi: 10.20944/preprints202107.0544.v1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.