Abstract
Microplastics are created for commercial use, are shed from textiles, or result from the breakdown of larger plastic items. Recent reports have shown that microplastics accumulate in human tissues and may have adverse health consequences. Currently, there are no standardized environmental monitoring systems to track microplastic accumulation within human tissues. Using Raman spectroscopy, we investigated the temporal exposures to plastic pollution in Hawai‘i and noted a significant increase in the accumulation of microplastics in discarded placentas over the past 15 years, with changes in the size and chemical composition of the polymers. These findings provide a rare insight into the vulnerability and sensitivity of Pacific Island residents to plastic pollution and illustrate how discarded human tissues can be used as an innovative environmental plastic pollution monitoring system.
Keywords: Human placenta, Microplastic accumulation, Raman spectroscopy, Atomic force microscopy
1. Introduction
Plastic products have become a ubiquitous and essential part of modern society owing to their physiochemical properties, mass production, and convenience. Packaging containers and storage materials, single-use water bottles, sterile medical supplies, and clothing made of plastic materials have become an integral part of everyday life. Global plastic production has increased exponentially from under 2 Megatons (Mt) in the 1950s to more than 400 Mt in 2022 (Landrigan, 2023). Worldwide, only 9% of plastic waste is recycled, while 22% is polluted into the environment as macro-and microplastic waste (OECD, 2022). Macroplastic pollution is degraded by physical elements (e.g., sun, heat, wind) and chemical agents (e.g., acid, salt, chlorine) into microplastic (MP) particles (1 μm to 5 mm) which accumulate in the environment over time (Hale et al., 2020). This rapid increase in environmental pollution by commercial plastics has a combined impact on climate change, marine ecosystems, and ultimately public health.
Recently, there have been reports of MP accumulation in the human gut, lungs, and bloodstream (Ibrahim, 2021; Jenner, 2022; Leslie, 2022). However, even more alarming are the recent discoveries of MP in the human reproductive system, such as the male testis (Zhao, 2023), mammary glands (breastmilk) (Ragusa, 2022), and placental tissue (Ragusa, 2021). Nonetheless, the impact of MP accumulation in human tissues and the effect on population health is still largely unknown (Haram, 2020; Academies and of Sciences Engineering and Medicine, 2022; Sun, 2020). Currently, there are no established systems to track environmental sources, sources of plastic, circulation of plastic pollution, or MP bioaccumulation in human tissues. While it has been postulated that plastic ingestion by marine wildlife could serve as a marker of marine health and coastal environments (Savoca, 2022), there are no such bioindicators to monitor human exposure to MP (Academies and of Sciences Engineering and Medicine (U.S.), 2022).
The human placenta is a transient organ that serves as the interface between the maternal and fetal circulatory systems during pregnancy, but also as a barrier that protects the fetus from deleterious pathogens in the maternal environment (Burton and Fowden, 2015; Burton and Jauniaux, 2023; Holder, 2021). Since the placenta is expelled after birth and has been proposed to serve as a biosensor of the perinatal environment (Reiter and Lee, 2012), we hypothesized that archived placentas could be retrieved to detect, measure, and track the temporal exposure to MP pollution in pregnant women in the State of Hawai‘i. Situated geographically at the crossroads of the Continental USA and the Asia-Pacific region, Hawai‘i is ranked the most diverse state in the USA with a 76% diversity index (U.S.C. Bureau, 2021) and a population that includes Hawai‘ian/Part-Hawai‘ian, Asians, and other Pacific Islanders. One hundred percent of Hawai‘i residents live in coastal shoreline counties (N.O.A.A. Administration, 2013) and the islands harbor a geographically closed ecosystem with a linear economy while being exposed to ocean currents carrying circulating plastic debris. Microplastic intake is postulated to occur via ingestion, inhalation, and dermal contact. In Hawai‘i, seafood consumption is high compared to the national average, where almost 50% of the population eats at least 8 oz per week, which would be associated with an increase in ocean MP exposure and accumulation. Additionally, most agricultural products consumed in Hawai‘i are not grown locally but shipped in plastic containers. Another frequent exposure route is through the use of skin cosmetics containing microplastics particles (Domenech and Marcos, 2021; Enyoh, 2020; Baker et al., 2020). In summary, unique coastal communities are a rich source of data to learn about the effects of plastic use on human health.
We aimed to assess MP exposure during pregnancy, using the placenta as a bioaccumulation assay. Additionally, we determined whether the increase in plastic pollution over the past two decades parallels a rise in MP accumulation in human placentas from the largest maternity hospital in the State of Hawai‘i. The results of this study have implications for coastal communities and Native Hawai‘ian and Pacific Islander populations, which are largely under-represented in other contemporary cohort studies.
2. Material and methods
2.1. Retrieval of archived frozen placenta samples
The Hawai‘i Reproductive Biospecimen Repository (HRBR) contains over 9000 triads of maternal blood, fetal blood, and placental tissue from births at Kapi‘olani Medical Center for Women and Children (KMCWC), which is linked to clinical data from their respective electronic medical records. The HRBR was supported by National Institute on Minority Health and Disparities and was approved by the Western Institutional Review Board (WIRB Study Number #1107593). After clearance by the medical team, placentas were transported to the HRBR in phosphate buffer saline (PBS). The HRBR sample collection protocol (not plastic-free) consisted of incising 50 g of placental tissue from 2 cotyledons obtained equidistantly from the umbilical cord insertion site and the edge of the placenta. Selected cotyledons were excised, cut into smaller pieces, placed into a 50 mL polyethylene (PET), and placed into a −80 °C freezer for long-term storage without any additive or cryopreservation solutions. For the present study, a total of 20 placental samples were retrieved (n = 10/2006 and n = 10/2013) and compared to fresh placentas (n = 10) collected in 2021. The exclusion criteria included gestational diabetes, hypertension, chorioamnionitis, preeclampsia, preterm labor, any other identified maternal medical conditions, known fetal aneuploidy, fetal growth restriction, placentas sent to the Pathology Department for clinical indications, and placentas being taken home by the patient for traditional Hawai‘ian birth practice.
2.2. Prospectively collected fresh placenta samples
Since the HRBR archived samples were stored long term and defrosted in polyethylene tubes, fresh placental cotyledons (n = 10) were prospectively collected from uncomplicated scheduled Cesarean births at KMCWC in 2021 to serve as negative controls to compare with the archived specimens (WIRB Study Number #1184793). The prospectively collected samples used a protocol that avoided any contact with plastic supplies such as drapes, gloves, or containers. The fresh placentas were transferred into a surgical steel container with a surgical steel cover and taken to the research laboratory for processing. A total of two placental cotyledons (Alonso-López et al., 2021) from each placenta were excised using surgical steel scalpels and scissors with care to avoid any plastic instruments and prolonged air exposure. The cotyledons were cut into smaller fragments and were thoroughly washed in glass-filtered phosphate buffer solution (PBS, 1.6 μm Whatman GF/A, Sigma-Aldrich, St. Louis, MO, USA) in glass Petri dishes to avoid baseline plastic exposure and processed under a culture hood to reduce airborne MP contamination.
2.3. Extraction of microplastics from freshly collected and archival placental tissue
For tissue digestion, the placenta samples were processed as described by Ragusa and collaborators (Ragusa, 2021). The frozen specimens from the HRBR were thawed and weighed. Approximately 50 g of each sample, both from thawed and fresh tissue, were thoroughly rinsed in glass-filtered PBS. The cotyledons from all groups were immediately immersed into a lidded glass bottle containing glass-filtered 10% KOH solution (1:8, w/v) and incubated for 7 days at room temperature (RT) to ensure complete digestion of all organic tissue matter. The respective digestates were then filtered using a 1.6 μm pore glass fiber filter membranes (47 mm; Whatman GF/A, Sigma-Aldrich, St. Louis, MO, USA) to isolate any remaining inorganic particles (plastic debris) for the analyses. The glass filters were dried at RT in a controlled environment and stored in individual metal containers until they were analyzed. For each sample, three control (blank) filters were prepared: (Landrigan, 2023) a glass membrane filter exposed to the PBS used to rinse the placental biopsies, (OECD, 2022) a glass membrane that had been used to filter the KOH solution, and (Hale et al., 2020) a dry glass filter membrane which was left exposed to air while the other test filters were drying.
2.4. Analysis of microplastics by Raman spectroscopy
The dried glass filters were first inspected by light microscopy (20X objective Olympus Plan Phase/0.4; Olympus PlanFL N 100X/0.95) BXFM, Olympus, Japan), followed by particle characterization (XploRA Raman spectrometry; spectral range 300–2200 cm−1, 532 and 785 nm laser diodes, 600 lines per mm grating, Horiba, Japan). To standardize the area to be analyzed, a 4 cm2 square at the center of each 47 mm diameter glass filter was utilized. To determine the number of MPs per 50 g of placental tissue, assuming an even distribution of particles over the filter area, we first calculated the total filtration area of the glass filter (31 mm diameter; 754.8 mm2 area). We then used the ratio of analyzed area (400 mm2) to total filtration area as a multiplicator to extrapolate (number of MPs found per sample × multiplicator) the number of MPs per 50 g of sample. Acquired spectra were dispersed onto a 16-bit dynamic range Peltier-cooled CCD detector, and the spectrometer was calibrated to the 520.7 cm−1 line of silicone prior to spectral acquisition. Raw Raman spectra underwent polynomial baseline correction and vector normalization to reduce noise and enhance spectrum quality (Labspec 6 software, Horiba). All the Raman spectra detected were compared with those reported in the literature, including the spectral library of the KnowItAll software (Wiley Science Solutions, Hoboken, NJ, USA and the free SLOPP/SLOPPe libraries of known microplastics (Munno et al., 2020).
2.5 . Atomic force microscope (AFM) measurements and Raman spectroscopy
MP captured within the glass fiber filters was carefully isolated from the filter mesh for AFM analysis. Individual images and measurements were obtained using an Atomic Force Microscope (AFM Multiview 4000™, Nanonics, Israel) coupled with an optical microscope (Olympus, Japan) and an XploRA spectrometer (Horiba, Japan). For the Raman spectra acquisition, a 532 nm laser was focused on the center of each microplastic particle through a × 50 objective (NA = 0.7). This combination permitted lateral positioning of the AFM tip over the MPs with micrometer-scale precision, allowing the acquisition of optical imaging, topographic analysis, and diagnostic Raman spectroscopy.
All MP were imaged (256 × 256 pixels) in the tapping mode at a line scan rate of 0.2 Hz, using a scanning tip with a typical radius of curvature < 10 nm and a nominal frequency of 35 kHz. The AFM system was acoustically isolated to reduce interference by ambient noise during the measurements, and the instrument was secured on an active damping table to suppress mechanical noise. The same objective was used for collecting Raman scattered light after interaction with the sample, in backscattering geometry. The frequency calibration was set by reference to the 520 cm−1 vibrational band of a silicon wafer. Under the same conditions, the MPs were measured in the spectral range of 120–4100 cm−1. To minimize laser-induced damage to the MPs, low-power irradiation at the sample surface was used (5 mW, short exposure time, 3 s laser exposure for 15 accumulations). The diffraction grating of 600 lines per mm yielded a spectral resolution of 3 cm−1.
As described above, we used the spectral library of the KnowItAll software (Wiley Science Solutions, Hoboken, NJ, USA and the free SLOPP/SLOPPe libraries of known microplastics to characterize each particle analyzed. MP size measurements were defined by the largest dimension, i.e., length, and assessed by Image J software analysis (Munno et al., 2020).
2.6. Statistical analysis
Data analysis was performed by using the statistical software package Prism5 (Graphpad Software, Inc., San Diego, CA, USA). To determine the normal distribution of all data, a Kolmogorov-Smirnov test was performed. One-way analysis of variance (ANOVA) using the Kruskal-Wallis test with Dunns post-hoc test was performed as appropriate. The significance threshold was set at p < 0.05.
2.7. Data availability Statement
The authors declare that all the data supporting the findings of this study are available within the paper and its supplementary information files. Additional data can be requested from the corresponding author.
3. Results
3.1. Detection of microplastic accumulation and particle size over time
MP particles were found in 6 out of 10 placentas (60%) in 2006, 9 out of 10 placentas (90%) in 2013, and 10 out of 10 placentas (100%) in 2021 (Fig. 1).
Fig. 1. Temporal increase in the frequency (%) of placentas containing microplastics (MP) by year of birth in Hawai‘i:
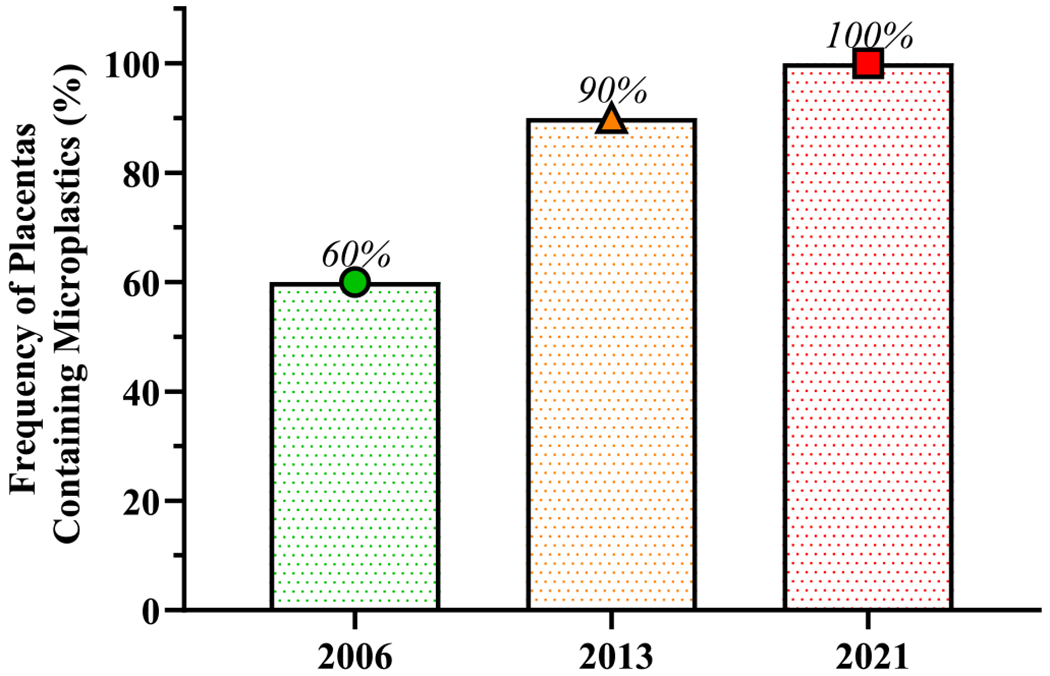
Microplastics were detected in 60% of placental samples from 2006, in 90% of 2013 samples, and in 100% of 2021 samples.
Quality controls were performed using environmental blanks (air-exposed crude filters) and procedural blanks (PBS and KOH exposed filters, respectively) as described above (Supplementary Figs. 1 and 2). We did not detect any MP contamination in the procedural blanks, whereas some of the environmental blanks contained rare pieces of MP fibers on the surface of the filter. Consequently, we disregarded surface particles in the final examination, and only analyzed particles within the glass filter itself which rendered normalization of the blanks redundant.
The average MP size from 2006 samples was 2.82 ± 0.31 μm and represented the smallest of all the years with a range of 1 to 8 μm (p < 0.05). We also noted a statistical significance when comparing 2013 samples (average of 6.24 ± 0.57 μm, range of 1 to 17 μm) with 2021 samples (average 5.14 ± 0.75 μm, range 1 to 44 μm; p < 0.0001). Filters from 2006 contained an average of 4.1 ± 1.3 MP particles per 50 g of placental tissue, filters from 2013 had 7.1 ± 0.9 MPs per sample, and filters from 2021 held 15.5 ± 3.0 MPs per sampling area. The increase in the number of MPs per 50 g of placenta tissue from 2021 reached statistical significance in comparison to 2006 samples (p < 0.001), and 2013 samples (p < 0.05) (Fig. 2). In summary, the number of MP particles per placenta and the particle size increased significantly over the years examined.
Fig. 2. Characteristics of MP accumulated in placentas from women in Hawai‘i:
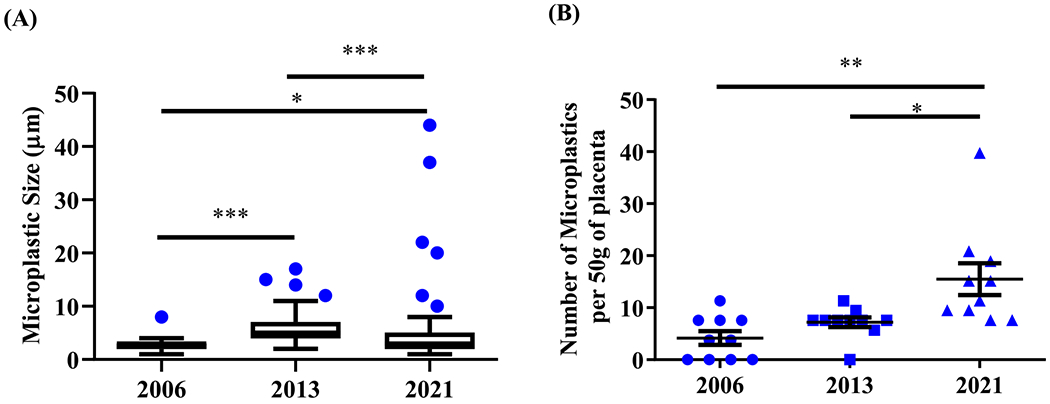
(A) Maximum length (μm) of MP particle. (B) Number of MP particles in each of the 50 g of placenta samples (calculated by using the number of MPs within the analyzed filter area multiplied by the ratio of total filtration area of the glass filter (diameter 31 mm, area 754.8 mm2) and filter area analyzed (400 mm2). (* p < 0.05, ** p < 0.001, *** p < 0.0001, One-way ANOVA).
3.2. Characterization of microplastic polymer composition by Raman spectroscopy
Using robust Raman Spectroscopy analysis techniques, MP particles were classified according to their chemical composition and subsequently identified using reference spectral libraries. Of the 142 MP particles analyzed, 22 particles were from 2006, 38 particles from 2013, and 82 particles from 2021. We were unable to assign the composition of 20 particles within the spectral libraries (KnowItAll; free SLOPP/SLOPPe). We were able to identify all polymers isolated from the 2006 HBRB samples where the most abundant were polypropylene (PP, 22.73%) and polyester (PES, 22.73%), followed by polyvinyl chloride (PVC, 18.18%), polyurethane (PU, 13.64%), polyethylene vinyl acetate (PVA, 9.09%), polyethylene terephthalate (PET, 4.54%), polyethylene (PE, 4.54%), and polyamide (PA, 4.54%). For 2013, 6 out of 38 particles (15.79%) could not be identified. Here, the most common polymers included PP (15.79%), followed by PET, PVA, and PES (10.53% each), acrylonitrile butadiene styrene (ABS, 7.89%), PE (5.26%), PU (5.26%), polycarbonate (PC, 5.26%), PA (5.26%), PVC (2.63%), polyvinyl alcohol (2.63%) and polyacrylonitrile (PAN, 2.63%). The largest number of unidentifiable polymers, 14 out of 82 (17.07%), were observed in the samples collected in 2021. As for the 2013 samples, the most common polymers were PES (13.41%), PET (12.19%), PVA (12.19%), and PP (10.97%), followed by PE (8.54%), PVC (6.1%), PU (4.88%), PC (4.88%), polystyrene (PS, 2.44%), and PA, ABS, PAN, polyethylene adipate, polymethyl methacrylate, and polyisoprene (1.22% each). All the identified MP particles were of a similar size range (Fig. 3) and colors.
Fig. 3. Identification of extracted MP by Raman spectroscopy analysis:
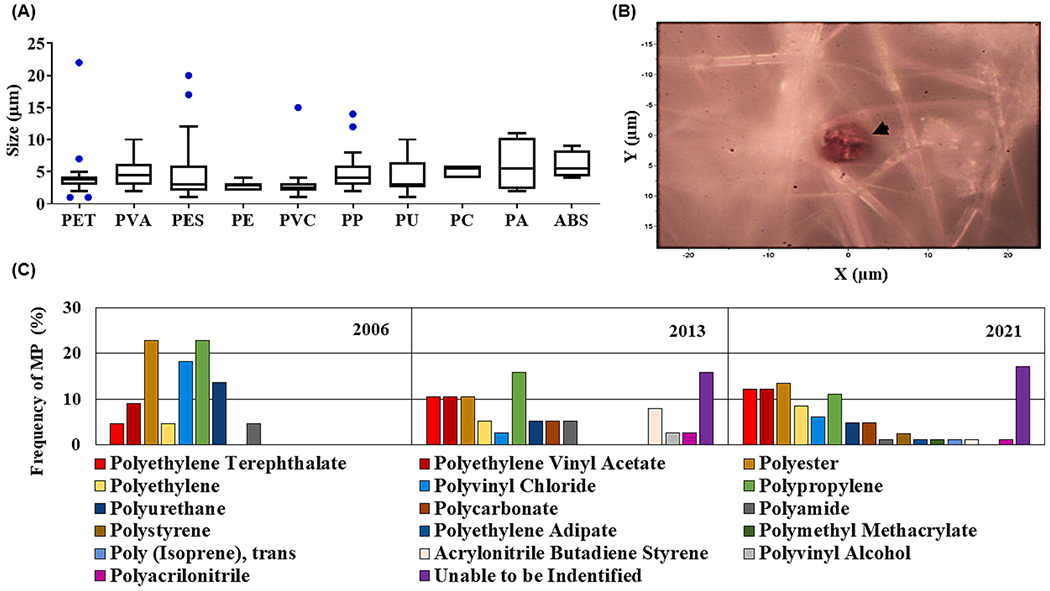
(A) Polymer name and respective size; Polyethylene terephthalate (PET), polyethylene vinyl acetate (PVA), polyester (PES), polyethylene (PE), polyvinyl chloride (PVC), polypropylene (PP), polyurethane (PU), polycarbonate (PC), polyamide (PA) and acrylonitrile butadiene styrene (ABS). (B) Arrowhead indicates a PVA red-colored MP particle trapped in glass fiber filter visualized under light microscopy (100 × objective (Olympus PlanFL N 100x/0.95). (C) Quantification of the types of MP polymers isolated by year.
Most of the MPs were transparent, followed by white, blue, red, orange, and green, while the most common shape was irregular or spherical. No plastic fibers or films were identified. In addition to the MP polymers, we were also able to identify 23 chemical additives known to be used for MPs production (Supplementary Table 1). Some of these additives were related to plasticizers, such as dioctyl phthalate (DOP) with bands at 1040, 2876, and 2932 cm−1 in several samples and bisphenol A (BPA) with bands at 1441, 1464, and 1615 cm−1. Individual spectra utilized for the characterization of the extracted MPs and additives by Raman spectroscopy and AFM analysis are listed in Supplementary Table 1 and Supplementary Fig. 3. Randomly selected MP particles were subjected to AFM imaging and exhibited variable heights (1.5 to 5.8 μm) with an uneven surface architecture. The surface topography of the MPs was highly rugous which was suggestive of visible degradation into even smaller particles (Fig. 4). Additionally, all analyzed MPs had three prominent peaks commonly masking other peaks, with variations around 440, 615, and 974 cm−1. The first two peaks are likely related to TiO2, one of the most commonly used pigments, while the latter peak has been attributed to the deformation of CH3 groups and the stretching of C–C bonds which reflect known conformational changes in plastic materials (Lee et al., 2015; Malinovskis, 2019; Nava et al., 2021).
Fig. 4. Atomic Force Microscopy (AFM) and Raman Spectra analysis of selected microplastic (MP) particles:
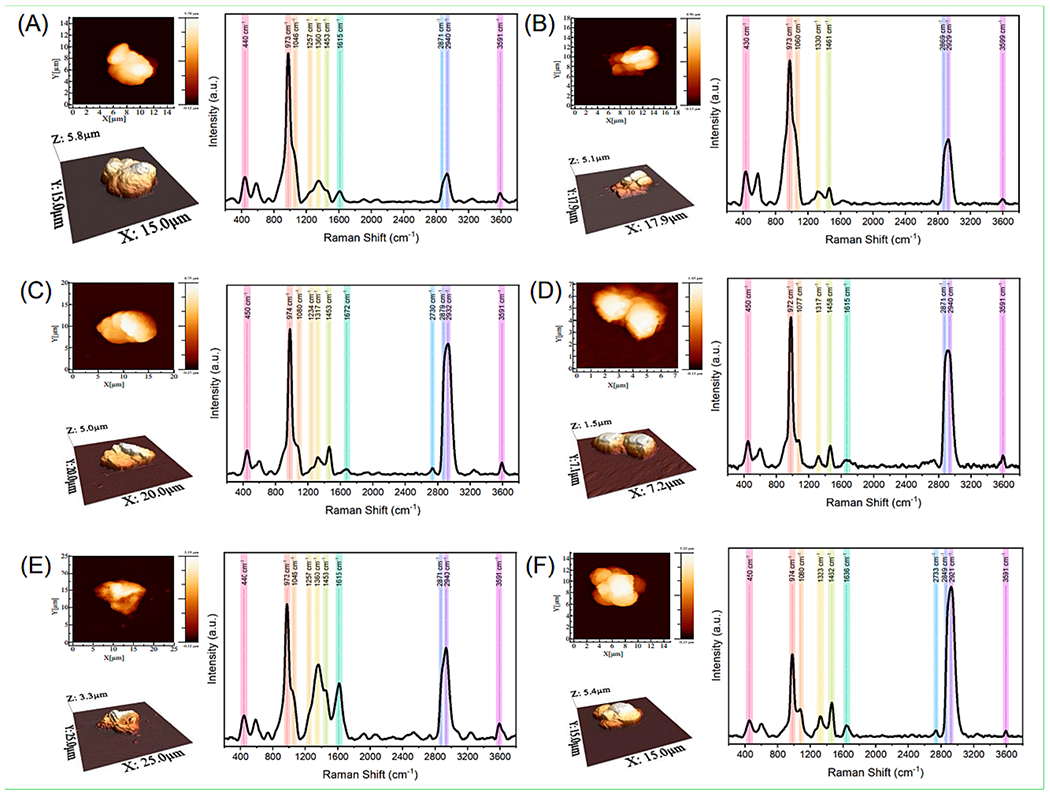
(A) Polypropylene, (B) Polyamide, (C) Polyamide, (D) Polyacrylonitrile, (E) Polypropylene, (F) Polyurethane. (Images A-B are from samples from 2006, images C-D are from 2013, and images E-F are from 2021; each letter depicts AFM 2D measurement of MP and 3D topography (photo images); Raman Spectra corresponding to assigned MP polymer (graphic images), respectively.
4. Discussion
Our results demonstrate an alarming increase, not only in the number of placentas that contain MP particles but also in the number of MP per cotyledon of placental tissue over the course of a 15-year period from a single institution in the Pacific Islands. This increase parallels the increase in global plastic production, consumption, and pollution (Geyer et al., 2017). Our findings also indicate changes in the size and chemical polymer composition of the MPs over time, with some compounds increasing over time while other materials appear to be decreasing over time. There was an increase in unidentified MPs found in placentas collected more recently, which may represent the introduction of newer chemically modified polymer compounds into the environment or a result of MP degradative by-products, which do not yet have reference standards in current databases (Liu, 2023).
These plastic materials are highly diverse in size, shape, color, density, and other physical and chemical properties, which made their identification particularly challenging (Blair et al., 2019). Among the current scientific techniques to identify chemical compounds, Raman spectroscopy is rapidly gaining prominence in the analysis of microplastics, given the higher spatial resolution (≥1 μm), broader spectral coverage, higher sensitivity to non-polar functional groups, lower water interference, and narrower spectral bands (Araujo et al., 2018). Likewise, other studies on human tissues and fluids have employed Raman spectroscopy to identify MPs (Ibrahim, 2021; Ragusa, 2021; Academies and of Sciences Engineering and Medicine (U.S.), 2022; Blair et al., 2019), in addition to laser direct infrared spectroscopy (Liu, 2022; Zhu, 2023) and double shot pyrolysis - gas chromatography/mass spectrometry (Leslie, 2022; Castelvetro, 2021).
Since each MP particle needed to be evaluated individually and manually scanned, we limited the Raman testing to MPs embedded into a standardized area of the glass filter disk, rather than the entire filter. While this could be considered a limitation of our study, other studies have employed similar approaches such as the random selection of a specific area of the filter or a percentage or number of particles on the filter (Deng, 2020; Imhof, 2016; Ossmann, 2018). MP particles in the environment are subjected to various weathering processes, and the same MP polymer at different degradation stages may result in distinctive Raman spectra, which may cause inaccurate identification when compared to the known standard spectra (Cai et al., 2018; Dong, 2020), and could also explain the lack of identification of some of the microparticles found herein. Furthermore, the presence of additives, plasticizers, novel plastic compounds, and environmental degradation byproducts can make the interpretation of Raman signatures challenging (Nava et al., 2021). We did notice the presence of well-described plasticizers associated with the polymers found in our samples, such as DOP, BPA, TiO2. So, in addition to the insult due to the presence of MPs as foreign bodies within the placenta, these chemicals are known to be harmful to human health as they may for example induce increased oxidative stress, inflammation, endocrine disruption, and metabolic disorders. (Baranowska-Wójcik et al., 2020; Bao, 2020; Eales, 2022). Low dose BPA exposure during prenatal development has been linked to a variety of effects including abnormalities in the male and female reproductive tracts, pregnancy complications, pre-malignant changes in mammary and prostate glands, and alterations in brain function (Hunt et al., 2009). Prenatal exposure to low dose phthalates has been reported to affect male and female sexual differentiation, thyroid function, metabolic dysfunction, and maternal pregnancy complications such as gestational diabetes and hypertensive disorders of pregnancy (Qian et al., 2020).
A limitation of this study is that the placentas collected in 2006 and 2013, at a time when there was little awareness of the issues surrounding MP contamination, were stored in polyethylene tubes in the HRBR and were retrieved retrospectively for our analysis. However, while there were several placenta samples from 2006 and 2013 that did not contain any MP, all ten placentas from 2021 which were prospectively collected without any contact with exogenous plastics, neither in the operating room nor at the laboratory during placental tissue processing and analysis, were found to contain contaminating MP particles.
While this study has the most significant number of samples studied to date, our sample size is too small to assign a correlation between maternal and fetal outcomes. It neither allows us to infer potential health issues arising from microplastic accumulation during pregnancy.
Ragusa and collaborators (Ragusa, 2022; Ragusa, 2021) described the presence of MPs with varying shapes, sizes, and colors in placentas and breast milk from their Italian cohort, while we discovered predominantly transparent MPs of similar size and shapes which could reflect higher rates of single-use clear plastic containers in the ecosystem of the Hawai‘ian Islands. Nevertheless, the polymers we have detected in our study are most commonly found in ocean and freshwater environments (Erni-Cassola et al., 2019; Schwarz et al., 2019). While carbon filters can remove MPs from water, this approach would only be practical for decontaminating drinking water, but not for surface water sources (Cherian et al., 2023).
Whereas Zhu and collaborators (Zhu, 2023) showed a correlation between polymer type and size of MP in a Chinese cohort, we and others found no such association in our respective cohorts (Ragusa, 2022; Ragusa, 2021; Nava et al., 2021). Overall, the most common polymers we identified were PP, PES, PVA, and PET, with variations according to the year analyzed. We did observe a temporal trend of polypropylene particles decreasing while PET appeared to increase over time (Fig. 4). Ragusa and collaborators (Ragusa, 2021) demonstrated PP as the predominant plastic polymer in the analyzed placentas of their Italian cohort, while Zhu and collaborators (Zhu, 2023) found mostly PVC, PP, and PET, and Liu and collaborators (Liu, 2022) found a prevalence of PA, PU, and PE in their placenta samples from China. These variations in plastic compounds isolated from placental tissues may reflect temporal and regional habits or differences in plastic waste management.
Our study demonstrates an increase in the number of placentas containing MP over a 15-year time frame associated with an increase in the number of MP particles per volume of placenta as well as changes in plastic polymer compositions. Together, PE, PP, PVC, PET, PU, PS, and polyester represent over 90% of all produced polymers, with an estimated annual leakage of 10Mt of plastics entering the ocean (Lau, 2020). PE and PP are the top two most produced plastic compounds; however, in Hawai‘i, only PET and PE are being collected for recycling (Beck, 2006). Excessive PE and PP exposure has been linked to immune dysfunction and abnormal neurodevelopmental outcomes in human and animal models (Alonso-López et al., 2021; Dhaka, 2022; Dievernich et al., 2022; Meng, 2014; Sax, 2010; Yuan et al., 2022).
In addition, given the low levels of DOP (a phthalate) and BPA which were found in our samples, we remain acutely aware of their endocrine disruptor properties as the MP are lodged within the human placentas with potential effects on both mother and fetus. Our findings from this study are particularly concerning as we are not able to determine the duration of exposure of these endocrine disrupting MP which are accumulating in the placentas. MPs can reach important organs, such as the brain, in a matter of minutes in a murine model (Kopatz, 2023). Of particular relevance to coastal communities such as Hawai‘i, MPs exposed to fresh and saltwater environments can be internalized by mouse macrophages in vitro by 10-fold more than MPs not exposed to water (Ramsperger, 2020).
5. Conclusion
The findings of this study, based on placental samples collected in Hawai‘i, provide insight into the vulnerability and sensitivity of Pacific Island communities to plastic pollution due to our remote location in the Pacific Ocean. Since the entire State of Hawai‘i State is designated as a coastal community (N.O.A.A. Administration, 2013), pregnant women who live there appear to be particularly vulnerable to marine plastic pollution, with yet unclear effects on maternal and fetal health. We have also demonstrated a disturbing increase in MP accumulation in human placentas from births in our referral hospital over the past 15 years. This confirms the increasing burden of human exposure to MP. These results reveal the fragile nature of our Pacific Island ecosystem and demonstrate how discarded placentas from human births can be developed as an international monitoring system to track MP exposure in diverse communities across the globe. Since fresh placentas are created and discarded with each pregnancy, placentas have the potential to serve as a non-invasive, real-time monitoring system of environmental plastic pollution and exposure during pregnancy.
Supplementary Material
Funding:
MJL received funding from the NIMHD, RMATRIX #U5MD007584, and the University of Hawai‘i Foundation / Kosasa Family Foundation.
JR received funding from NIMHD, RMATRIX #U5MD007584.
JU received funding from NIHGMS P20GM131944.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2023.108220.
Data availability
Data will be made available on request.
References
- National Academies of Sciences Engineering and Medicine (U.S.), National Academies of Sciences Engineering and Medicine (U.S.). Committee on the United States Contributions to Global Ocean Plastic Waste., National Academies of Sciences Engineering and Medicine (U.S.). Ocean Studies Board., National Academies of Sciences Engineering and Medicine (U.S.). Division on Earth and Life Studies., Reckoning with the U.S. role in global ocean plastic waste. Consensus study report (National Academies Press, Washington, DC, 2022), pp. xvi, 252 pages. [Google Scholar]
- Alonso-López O, López-Ibáñez S, Beiras R, 2021. Assessment of toxicity and biodegradability of poly(vinyl alcohol)-Based materials in marine water. Polymers (Basel) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo CF, Nolasco MM, Ribeiro AMP, Ribeiro-Claro PJA, 2018. Identification of microplastics using Raman spectroscopy: latest developments and future prospects. Water Res. 142, 426–440. [DOI] [PubMed] [Google Scholar]
- Baker KK, Watters CA, Dannemiller JE, Iwamura ST, Brooks BA, 2020. Fish consumption for the adult population of Hawai‘i, collected with a Self-Reported household survey. Hawai‘i J. Health Soc. Welf 79, 51–59. [PMC free article] [PubMed] [Google Scholar]
- Bao W, et al. , 2020. Association between bisphenol A exposure and risk of All-Cause and Cause-Specific mortality in US adults. JAMA Netw. Open 3, e2011620–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowska-Wójcik E, Szwajgier D, Oleszczuk P, Winiarska-Mieczan A, 2020. Effects of titanium dioxide nanoparticles exposure on human health-a review. Biol. Trace Elem. Res 193, 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck R, 2006. Waste Characterization Study. City and County of Honolulu (2007). [Google Scholar]
- Blair RM, Waldron S, Phoenix VR, Gauchotte-Lindsay C, 2019. Microscopy and elemental analysis characterisation of microplastics in sediment of a freshwater urban river in Scotland, UK. Environ. Sci. Pollut. Res. Int 26, 12491–12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Fowden AL, 2015. The placenta: a multifaceted, transient organ. Philos. Trans. R. Soc. Lond. B Biol. Sci 370, 20140066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, 2023. The human placenta: new perspectives on its formation and function during early pregnancy. Proc. Biol. Sci 290, 20230191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Wang J, Peng J, Wu Z, Tan X, 2018. Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments. Sci. Total Environ 628–629, 740–747. [DOI] [PubMed] [Google Scholar]
- Castelvetro V, et al. , 2021. New methodologies for the detection, identification, and quantification of microplastics and their environmental degradation by-products. Environ. Sci. Pollut. Res. Int 28, 46764–46780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian AG, Liu Z, McKie MJ, Almuhtaram H, Andrews RC, 2023. Microplastic removal from drinking water using point-of-use devices. Polymers (Basel) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, et al. , 2020. Microplastic pollution in water and sediment in a textile industrial area. Environ. Pollut 258, 113658. [DOI] [PubMed] [Google Scholar]
- Dhaka V, et al. , 2022. Occurrence, toxicity and remediation of polyethylene terephthalate plastics. A review. Environ. Chem. Lett 20, 1777–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dievernich A, Achenbach P, Davies L, Klinge U, 2022. Characterization of innate and adaptive immune cells involved in the foreign body reaction to polypropylene meshes in the human abdomen. Hernia 26, 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech J, Marcos R, 2021. Pathways of human exposure to microplastics, and estimation of the total burden. Curr. Opin. Food Sci 39, 144–151. [Google Scholar]
- Dong M, et al. , 2020. Raman spectra and surface changes of microplastics weathered under natural environments. Sci. Total Environ 739, 139990. [DOI] [PubMed] [Google Scholar]
- Eales J, et al. , 2022. Human health impacts of exposure to phthalate plasticizers: an overview of reviews. Environ. Int 158, 106903. [DOI] [PubMed] [Google Scholar]
- Enyoh CE, et al. , 2020. Microplastics exposure routes and toxicity studies to ecosystems: an overview. Environ Anal Health Toxicol 35, e2020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erni-Cassola G, Zadjelovic V, Gibson MI, Christie-Oleza JA, 2019. Distribution of plastic polymer types in the marine environment. A meta-analysis. J. Hazard Mater 369, 691–698. [DOI] [PubMed] [Google Scholar]
- Geyer R, Jambeck JR, Law KL, 2017. Production, use, and fate of all plastics ever made. Sci. Adv 3, e1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale RC, Seeley ME, La Guardia MJ, Mai L, Zeng EY, 2020. A global perspective on microplastics. J. Geophys. Res. Oceans 125. [Google Scholar]
- Haram K, et al. , 2020. Early development of the human placenta and pregnancy complications. J. Matern. Fetal Neonatal Med 33, 3538–3545. [DOI] [PubMed] [Google Scholar]
- Holder B, et al. , 2021. Nat Commun. (England, 2021) 12, 7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PA, Susiarjo M, Rubio C, Hassold TJ, 2009. the bisphenol A experience: a primer for the analysis of environmental effects on mammalian reproduction. Biol. Reprod 81, 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim YS, et al. , 2021. Detection of microplastics in human colectomy specimens. JGH Open 5, 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof HK, et al. , 2016. Pigments and plastic in limnetic ecosystems: a qualitative and quantitative study on microparticles of different size classes. Water Res. 98, 64–74. [DOI] [PubMed] [Google Scholar]
- Jenner LC, et al. , 2022. Detection of microplastics in human lung tissue using muFTIR spectroscopy. Sci. Total Environ 831, 154907. [DOI] [PubMed] [Google Scholar]
- Kopatz V, et al. , 2023. Micro- and nanoplastics breach the blood-brain barrier (BBB): biomolecular corona’s role revealed. Nanomaterials (Basel) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, et al. , 2023. The Minderoo-Monaco commission on plastics and human health. Ann. Glob. Health 89, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WWY, et al. , 2020. Evaluating scenarios toward zero plastic pollution. Science 369, 1455–1461. [DOI] [PubMed] [Google Scholar]
- Lee SY, Lee CH, Kim DY, Locquet JP, Seo JW, 2015. Preparation and photocatalytic activity of potassium- incorporated titanium oxide nanostructures produced by the wet corrosion process using various titanium alloys. Nanomaterials (Basel) 5, 1397–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie HA, et al. , 2022. Discovery and quantification of plastic particle pollution in human blood. Environ. Int 163, 107199. [DOI] [PubMed] [Google Scholar]
- Liu S, et al. , 2022. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: a pilot prospective study. Sci. Total Environ 854, 158699. [DOI] [PubMed] [Google Scholar]
- Liu S, et al. , 2022. the association between microplastics and microbiota in placentas and meconium: the first evidence in humans. Environ. Sci. Tech [DOI] [PubMed] [Google Scholar]
- Liu X, et al. , 2023. Screening for bisphenol chemicals: a strategy based on dansyl chloride derivatizatio n coupled with in-source fragmentation by high-resolution mass spectrometry. Anal. Chem 95, 6227–6234. [DOI] [PubMed] [Google Scholar]
- Malinovskis U. et al. , 2019. High-density plasmonic nanoparticle arrays deposited on nanoporous anodic alumina templates for optical sensor applications. Nanomaterials. 2019 ( 10.3390/nano9040531). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng TT, 2014. Volatile organic compounds of polyethylene vinyl acetate plastic are toxic to living organisms. J. Toxicol. Sci 39, 795–802. [DOI] [PubMed] [Google Scholar]
- Munno K, De Frond H, O’Donnell B, Rochman CM, 2020. Increasing the accessibility for characterizing microplastics: introducing new application-based and spectral libraries of plastic particles (SLoPP and SLoPP-E). Anal. Chem 92, 2443–2451. [DOI] [PubMed] [Google Scholar]
- N. O. A. A. Administration. (2013).
- Nava V, Frezzotti ML, Leoni B, 2021. Raman spectroscopy for the analysis of microplastics in aquatic systems. Appl. Spectrosc 75, 1341–1357. [DOI] [PubMed] [Google Scholar]
- OECD, 2022. Global Plastics Outlook. [Google Scholar]
- Ossmann BE, et al. , 2018. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 141, 307–316. [DOI] [PubMed] [Google Scholar]
- Qian Y, Shao H, Ying X, Huang W, Hua Y, 2020. The endocrine disruption of prenatal phthalate exposure in mother and offspring. Front. Public Health 8, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragusa A, et al. , 2021. Plasticenta: first evidence of microplastics in human placenta. Environ. Int 146, 106274. [DOI] [PubMed] [Google Scholar]
- Ragusa A, et al. , 2022. Raman microspectroscopy detection and characterisation of microplastics in human breastmilk. Polymers (Basel) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsperger A, et al. , 2020. Environmental exposure enhances the internalization of microplastic particles into cells. Sci. Adv 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter J, Lee M-J, 2012. Can loss of imprinting in the placenta serve as a biosensor of the perinatal environment? J Mol Biomark Diagn 3, e109. [Google Scholar]
- Savoca MS, et al. , 2022. Towards a North pacific ocean long-term monitoring program for plastic pollution: a review and recommendations for plastic ingestion bioindicators. Environ. Pollut 310, 119861. [DOI] [PubMed] [Google Scholar]
- Sax L, 2010. Polyethylene terephthalate may yield endocrine disruptors. Environ. Health Perspect 118, 445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz AE, Ligthart TN, Boukris E, van Harmelen T, 2019. Sources, transport, and accumulation of different types of plastic litter in aquatic environments: a review study. Mar. Pollut. Bull 143, 92–100. [DOI] [PubMed] [Google Scholar]
- Sun C, et al. , 2020. The placenta in fetal growth restriction: what is going wrong? Placenta 96, 10–18. [DOI] [PubMed] [Google Scholar]
- U. S. C. Bureau (2021).
- Yuan Z, Nag R, Cummins E, 2022. Ranking of potential hazards from microplastics polymers in the marine environment. J. Hazard. Mater 429, 128399. [DOI] [PubMed] [Google Scholar]
- Zhao Q, et al. , 2023. Detection and characterization of microplastics in the human testis and semen. Sci. Total Environ 877, 162713. [DOI] [PubMed] [Google Scholar]
- Zhu L, et al. , 2023. Identification of microplastics in human placenta using laser direct infrared spectroscopy. Sci. Total Environ 856, 159060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data supporting the findings of this study are available within the paper and its supplementary information files. Additional data can be requested from the corresponding author.
Data will be made available on request.


