Abstract
Background:
Chest physiotherapy is an established cornerstone of care for people with cystic fibrosis (pwCF), but is often burdensome. Guidelines recommend at least one chest physiotherapy session daily, using various airway clearance techniques (ACTs). Exercise (with huffs and coughs) may offer an alternative ACT, however the willingness of pwCF to be randomised into a trial needs testing. The ‘ExACT-CF: Exercise as an Airway Clearance Technique in people with Cystic Fibrosis’ trial will test the feasibility of recruiting pwCF to be randomised to continue usual care (chest physiotherapy) or replace it with exercise ACT (ExACT) for 28-days. Secondary aims include determining the short-term clinical impact (and safety) of stopping routine chest physiotherapy and replacing it with ExACT, and effects on physical activity, sleep, mood, quality of life and treatment burden, alongside preliminary health economic measures and acceptability.
Methods:
Multi-centre, two-arm, randomised (1:1 allocation using minimisation), pilot trial at two sites. Fifty pwCF (≥10 years, FEV 1 >40% predicted, stable on Elexacaftor/Tezacaftor/Ivacaftor (ETI)) will be randomised to an individually-customised ExACT programme (≥once daily aerobic exercise of ≥20-minutes duration at an intensity that elicits deep breathing, with huffs and coughs), or usual care. After baseline assessments, secondary outcomes will be assessed after 28-days, with additional home lung function and exacerbation questionnaires at 7, 14 and 21-days, physical activity and sleep monitoring throughout, and embedded qualitative and health-economic components. Feasibility measures include recruitment, retention, measurement completion, adverse events, interviews exploring the acceptability of trial procedures, and a trial satisfaction questionnaire.
Discussion:
Co-designed with the UK CF community, the ExACT-CF pilot trial is the first multi-centre RCT to test the feasibility of recruiting pwCF stable on ETI into a trial investigating ExACT. This pilot trial will inform the feasibility, design, management, likely external validity for progression to a main phase randomised controlled trial.
Registration:
Clinicaltrials.gov ( NCT05482048).
Keywords: airway clearance therapy, cystic fibrosis, exercise, feasibility, physical activity, physiotherapy, randomised controlled trial
Plain English summary
Cystic fibrosis (CF) is the UK’s most common inherited genetic condition and affects > 10,500 people. CF causes problems with the movement of salt and water in the body, resulting in sticky mucus building up, mostly in the lungs and gut. Thick mucus in the airways leads to repeated infections which can damage the lungs. Chest physiotherapy is routinely prescribed to keep pwCF healthy, by loosening and clearing sticky, thick mucus from the airways. However, many find it time-consuming and burdensome. People with CF (pwCF) have asked if doing exercise could have the same effect for clearing mucus.
Surveys show that many pwCF have occasionally replaced chest physiotherapy with exercise for airway clearance. We also showed that many pwCF, their families, physiotherapists and doctors in the UK consider that hard exercise with huffs and coughs may be able to clear mucus from the airways. We now need to know if they would be willing to take part in research that asks some to stop chest physiotherapy and do intense exercise with huffs and coughs instead.
We will study 50 pwCF (> 10 years old) for 28 days. We will ask half to continue their usual care, and half to stop chest physiotherapy and do exercise that gets them breathing deeply (with huffs and coughs) instead. We will see if people are willing to start and continue with such a study and what they think of the processes. We will also see how stopping chest physiotherapy and replacing it with exercise affects measurements of their lung function. Within the study we will talk with pwCF and members of their CF team to understand their experiences. This information will tell us whether a larger study can answer whether certain forms of exercise can safely be used as an alternative to chest physiotherapy.
Introduction
Background and rationale
Cystic fibrosis (CF) is a multisystem genetic disease characterised by thickening of mucus within lumina including airways, biliary tree, pancreatic ducts and the digestive tract. Removal of thickened airway secretions using a combination of airway clearance techniques (ACTs), for example chest physiotherapy, along with mucoactive agents is key to preventing recurrent infections and airway inflammation, which can cause lung damage leading to respiratory failure and death. Chest physiotherapy has been a cornerstone of CF clinical care for > 60 years 1 Chest physiotherapy may be considered to be traditional airway clearance therapy (tACT), and is considered to act by loosening, mobilising, and expectorating thickened, infected airway secretions. Chest physiotherapy for ACT is a standard of care in the UK and internationally 2, 3 , and persons with CF (pwCF) typically start tACT following diagnosis and continue once or twice daily lifelong 4, 5 .
There are many accepted chest physiotherapy tACTs to manage the respiratory health of pwCF, including active cycle of breathing techniques (ACBT), autogenic drainage (AD), positive expiratory pressure (PEP) and oscillating PEP devices, postural drainage, and percussion techniques, with the choice depending on preference, age, airway pathophysiology and adverse events. tACTs have evolved over time 6 , driven largely by increased life expectancy and a need for independence. However, pwCF consider tACTs burdensome 7 ; those who are younger and with less advanced lung disease perceive little benefit 8 and self-reported adherence is as low as 30% 9 . With new highly-effective small molecules therapies (e.g. Elexacaftor/Ivacaftor/Tezacaftor [ETI]; Kaftrio ®) 10, 11 changing the landscape of CF management, pwCF are actively seeking to reduce their treatment burden 7 . Cochrane Reviews have reported short-term effects (specifically, increased mucus transport) of tACT, but no evidence supporting long-term benefit 12 . Moreover, non-superiority between tACTs using various techniques and devices 13– 16 and tACT without any adjuncts (conventional chest physiotherapy by percussion and drainage) 12 mean there remains no globally agreed definitive treatment strategy for tACT prescription in CF.
Exercise is an alternative ACT thought to produce shearing forces within the lung parenchyma, which enhances mucociliary clearance and the removal of viscous secretions 17 , in a multi-mechanistic way, including mechanical vibration, tachypnoea, coughing and changing the viscosity of sputum 17– 19 . Recent evidence suggests that some pwCF are already using exercise as a substitute for tACTs 20 , despite there being no agreed recommendation for this. A recent qualitative study around patient and caregiver opinions on ACT commented that a personalised ACT approach that includes exercise as ACT is likely to be effective 21 . A preference for Exercise as an ACT (ExACT) may already exist in pwCF; 96% report exercising, with 48% omitting tACT if they have exercised 22 and 16% report exercise as their primary ACT (UK CF Registry data). Persons with CF using ExACT as a substitute for tACTs report better lung function, lower perceived severity of respiratory disease and sputum load 20 , however systematic trial design limitations, with small sample size, short intervention duration and all except one study being a randomised cross-over design, characterise the existing evidence base 23 . More recently, a Cochrane Review on this topic concluded that there is currently insufficient evidence regarding whether or not exercise can replace other methods of airway clearance 17 , although promisingly no studies reported any negative effects of ExACT. To date, no studies have evaluated whether replacing tACTs with ExACT impacts upon quality of life or antibiotic treatment need.
Whether exercise could be undertaken as an alternative form of ACT, without impacting health, is a top 10 James Lind Alliance research priority for pwCF 24 . It has been recommended that this priority question be resolved by randomised, controlled trials 17, 23, 25, 26 . To operationalise and develop this priority question for a trial setting, we established benchmark equivalence for ExACT as a comparator to tACTs using Delphi methodology and a UK-based expert panel, comprising pwCF, caregivers, specialist physiotherapists and physicians 27 . It was agreed that ExACT should be aerobic activity (typically weight-bearing modalities that induce body vibration), of at least 20 minutes duration, and intense enough to elicit deep breathing, with assessment breaths, huffs and coughs incorporated to remove loose secretions, in line with a recent systematic review 23 . Importantly, it was agreed that at present ExACT should only be considered as a substitute for tACT during times of stable CF 27 .
The ‘Exercise as an Airway Clearance Technique in people with Cystic Fibrosis (ExACT-CF)’ study is a randomised pilot trial of ExACT (at least once daily) for 28 days as an alternative to chest physiotherapy (tACT) in pwCF. Although there are precedents within the literature for stopping routine chest physiotherapy in favour of alternative ACTs 28, 29 for periods of 3 to 12 months, the intervention period is 28 days, in line with recent CF trials with washout periods of 28 days, in which treatments with significant benefits (e.g. Ivacaftor modulator therapy) have been stopped 30 .
Objectives
This multi-centre randomised pilot trial RCT is designed to answer the primary research question: is it feasible to randomise pwCF into a trial where ExACT replaces routine chest physiotherapy (tACT) for airway clearance? The primary objective is to test study recruitment and retention rates, and adherence rates to replacing traditional chest physiotherapy with an ExACT intervention in pwCF, plus an understanding of why potential participants declined to take part. The primary hypothesis is that it will be feasible to randomise pwCF to usual care or ExACT across 2 sites, that this will be acceptable to pwCF and their clinical care teams, and that a sufficient recruitment rate will be achieved to render an upscaled ExACT-CF trial feasible. We further hypothesise that lung clearance index (LCI 2.5), alongside other clinical and patient-reported outcome measures, will be feasible outcomes measured to be included in a larger, definitive trial.
The secondary objectives are to (1) compare the ExACT intervention to usual care (chest physiotherapy) to obtain preliminary data regarding the short-term clinical impact and safety of stopping chest physiotherapy, also known as traditional airway clearance therapy (tACT), for 28 days, by measuring LCI 2.5, spirometry (FEV 1, FVC – %predicted), exacerbation frequency, and rates of adverse and/or serious adverse events; (2) explore participants' preference for exercise types, intensities and durations undertaken as ExACT in place of chest physiotherapy as airway clearance; (3) assess the feasibility, acceptability and ability to collect specific health-related quality of life (HRQoL) and resource use data for a definitive trial, and conduct exploratory health economics analysis to inform a full trial; and (4) audit and analyse the returned data for completeness and revise questionnaires, where appropriate, to improve data capture in a full trial.
This paper describes the design of the study, based on the protocol version 1.2 as of 22 nd October 2022.
Methods
Reporting of this randomised clinical trial protocol has followed the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines 31, 32 and Template for Intervention Description and Replication (TIDieR) checklist and guide 33 .
Trial design
ExACT-CF is designed as a multi-centre, two-arm, open-label, randomised (1:1 allocation using minimisation), pilot trial comparing exercise (with huffs and coughs) as an airway clearance technique (ExACT) versus usual care (chest physiotherapy, tACT), with an embedded qualitative component. Following informed consent/assent, enrolled participants will be required to complete baseline assessments. Participants will then be randomised to receive either the ExACT intervention or usual care (tACT).
Participants, interventions, and outcomes
Study setting
The randomised pilot trial will be conducted at the adult and paediatric CF units at the University Hospital Southampton (UHS) and Southampton Children’s Hospital (SCH), respectively (England, UK), and the paediatric and adult CF units at the Royal Hospital for Children and Young People (RHCYP) and the Western General Hospital (WGH), respectively (Scotland, UK). Each of these centres are sub-regional referral centres providing multidisciplinary care for people with CF. A number of components will also be conducted remotely to limit participant travel and time burden.
Eligibility criteria
To be eligible for enrolment, participants must have a confirmed diagnosis of CF based on sweat chloride ≥ 60 mmol/L and CFTR genotype which includes at least one phe508del allele; be clinically stable; have a baseline lung function of ≥ 40% predicted; be ≥ 10 years of age; be established (≥ 3 months) on Elexacaftor/Tezacaftor/Ivacaftor (ETI, Kaftrio ®); and be able to co-operate with the study requirements. Participants will be excluded from the study if they are medically unstable (as advised by the recruiting physician), e.g. frequent exacerbations (> 3/year in preceding 2 years), variable lung function (FEV 1 < 40% predicted and/or excursions of ≥ 20% from baseline); have any health contraindications to exercise (e.g. arthritis, cardiac disease); have insufficient cooperation and understanding in regard to the trial; not be willing to give consent to participate; have been previously randomised into this trial or are concurrently participating in an interventional trial. To note, participants will not be excluded due to any pathogen as long as they are deemed clinically stable.
Informed consent/assent
Participants will be recruited from the CF units at each study site (adult and paediatric CF centres in Southampton and Edinburgh). Members of the study team will screen for participant eligibility using the inclusion/exclusion criteria. After confirming eligibility, potential participants will be approached by an appropriately trained member of the team to determine their interest in entering the study. This individual will give a comprehensive verbal explanation of the trial (explaining the intervention and highlighting any possible benefits or risks relating to participation). Potential participants will be given adequate time throughout the discussion to ask any questions, and these will be suitably addressed. They will also be given a written information sheet about the trial and be given sufficient time (minimum 24 hours) to read and consider the study information prior to deciding whether to take part. If additional time is needed to consider participation, a follow-up at a more convenient time can be requested.
If the participant is willing to take part, then they will be asked to sign the consent form. No clinical trial procedures will be conducted prior to taking consent. Fully informed written consent (adults) and assent (minors) will be obtained by a member of the research team in accordance with Good Clinical Practice (GCP). For children and adolescents, assent will be taken and consent also obtained from their parent/guardian. An optional item will be included in the consent form asking participants, who would consider being interviewed, for their consent to share their contact details with the qualitative researcher. If participants give their consent, their contact details will be sent securely to the qualitative embedded study (ExACT-Q) researcher. For participants who decline to participate, they will be provided with a short information sheet on the qualitative sub-study, and consent will be sought to invite them to participate in a short 10-minute decliner interview via videocall (audio and audio+video options). This trial does not involve collecting biological specimens for storage.
Interventions
Explanation for the choice of comparators
The ExACT intervention group will be compared to the chest physiotherapy (tACT) group receiving usual care, which is currently recommended as at least once daily tACT. Its selection as a comparator is therefore justified.
Intervention description
Participants allocated to the intervention will receive the ExACT programme, facilitated by their CF physiotherapist and members of the research team. Participants will be advised to replace all chest physiotherapy (tACT) sessions with intense exercise combined with huffs and coughs - also referred to as forced expiratory techniques (FET). This intervention has been co-developed with the CF community using both involvement mechanisms and a UK-based Delphi consensus survey with people with CF, caregivers, specialist physiotherapists and physicians 27 . Participants will be instructed to perform ExACT at least once per day at a time that suits them.
In summary, ExACT is exercise that can replace chest physiotherapy for airway clearance in people with CF. An ExACT session should be made up of a recommended activity, should last at least 20 minutes, and should result in deep breathing that stops the participant talking in sentences. Assessment breaths should be performed before the exercise session (to see where any secretions are) and again at the end of the session to ensure that secretions have been cleared. Huffs and coughs should be performed on a number of occasions alongside the exercise, to ensure any loosened secretions are effectively cleared. ExACT guidance and a compendium of exercises ( Figure 1) has been produced to facilitate written and verbal instruction on the type, duration, frequency and intensity of exercise that is considered adequate replacement for routine chest physiotherapy, informed by the published e-Delphi consensus undertaken by our study group.
Figure 1. The ExACT intervention – guidance on how to undertake an ExACT session and a compendium of suggested ExACT activities.
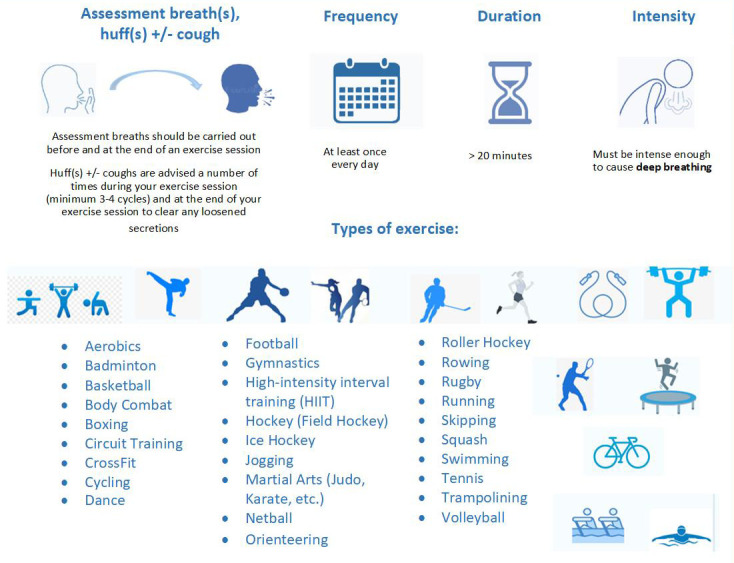
N.B. The exercise airway clearance therapy intervention to be used in this study was co-developed in collaboration with the UK-based cystic fibrosis community 27 , and further refined with the support from a global group of physiotherapists.
The compendium has further undergone peer review by the UK CF Trust patient involvement group and a worldwide CF physiotherapy expert group. Rather than be an exhaustive list of exercise that may be undertaken as ExACT, the compendium is intended to be a guide for study participants and their specialist teams. ExACT needs to consider 5 key factors: 1) exercise type, 2) exercise duration, 3), exercise frequency, 4) exercise intensity, and 5) building in assessment breaths, huffs +/- coughs. Importantly, huffs +/- coughs must be combined with exercise to be classified as exercise for airway clearance.
Exercise type: There is consensus that the best exercises for ExACT are those with weight-bearing/vibration (e.g. jogging, interval training, playing football, etc.) or those where breathing control is central to the exercise e.g. swimming ( Figure 1). Exercise frequency: ExACT should be undertaken at least once per day – in line with current physiotherapy for airway clearance recommendations. Exercise duration: ExACT should be of at least 20 minutes duration. Exercise intensity: ExACT should be performed at moderate-to-vigorous intensity. There is no need to use heart rate monitors or any other equipment to measure this intensity – put simply it is exercise at a level that causes one to breathe deeply, and makes it impossible to talk in sentences.
Assessment breaths, huffs and coughs during exercise: Assessment breaths should be performed to assess whether any secretions are present and where any secretions are before an exercise session is started and again at the end of the session to ensure that secretions have been cleared. Huffs and coughs should be performed on a number of occasions during and at the end of an exercise session to ensure any loosened secretions are effectively cleared.
Adult participants and parents of children/adolescents will receive guidance regarding how to complete the home-based sessions. Each day, the participant will choose ExACT activities to complete instead of their chest physiotherapy. Participants, or their parent/guardian for children and adolescents, will document the number of ExACT sessions via a daily diary (within the participant facing module of the trial database) along with their perceived effort as well as logging sessions using their Garmin Vivosmart 4 ® wearable device.
The clinical team will support exercise advice in line with standard care if participants request this. The trial has no other changes to CF care. All medications, nutritional support and other treatments continue. If participants deviate from the protocol and complete any traditional chest physiotherapy sessions, these will also be reported on the bespoke trial platform. The participant log will be reviewed by a research nurse or member of the research team throughout the study. Although all routine chest physiotherapy is being replaced by ExACT, chest physiotherapy is permissible in the event of a chest exacerbation and a protocol deviation will be recorded.
All individuals who will be responsible for explaining the intervention will complete training led by the study researchers (ET, ZS and DU). Each instructor will: review the intervention and usual care standard operating procedures, receive guidance about setting up the activity monitoring and lung function assessment devices, completing assessments/worksheets and the trial database, be given standardised instructions regarding each component of the intervention and advice to navigate individual modifications or adaptations.
For quality assurance purposes and program fidelity, 1) video/audio records will be taken of a subset of visits with physiotherapists/research nurses and members of the research team at each study site, with independent assessors rating role play sessions where mock ExACT guidance is delivered, to assess competence and adherence; 2) video/audio recordings will be taken of a random selection (10–20%) of sessions where ExACT training is delivered to participants. An independent reviewer will rate (i) adherence to ExACT guidelines and (ii) participant understanding; 3) a log will be completed by all trainers for each participant in ExACT, including a checklist of all aspects to be delivered, which trainers will tick if covered, and space to record deviations from the ExACT protocol and to assess patients’ confidence/understanding; 4) physiotherapists/research nurses will self-report their confidence in delivering ExACT; and finally 5) qualitative interviews with physiotherapists/research nurses to report when, why and frequency of when they adapted ExACT and understand how they view/experience participants' confidence to comply with the EXACT protocol. The rationale for evaluating the fidelity across the above groups is that, if implemented within clinical care, ExACT would be used independently by adults with CF and with support in younger patients. However, over time and after initial guidance and training, ExACT would likely be used with reduced physiotherapy input, which also presents a more cost-effective option.
Criteria for discontinuing of modifying allocated interventions
Although all routine chest physiotherapy is being stopped and replaced with ExACT in the intervention study arm, chest physiotherapy is permissible in the event of a chest exacerbation and a protocol deviation will be recorded. Any departure from the approved protocol will be considered to be a protocol deviation. Any protocol deviation, non-compliance or breach shall be reported to the Chief Investigator and the Sponsor immediately, and data made available to the Data Monitoring and Ethics Committee (DMEC).
Strategies to improve adherence to interventions
Adherence to the intervention will be monitored via use of the Garmin wearable device and data entered into the bespoke participant facing module of the trial database through the 28 day study period. The study is assessing the feasibility of participants adhering to an intervention that relies on self-efficacy. There will be no prompts or coaching in respect of the intervention during the 28 day study. There will be prompts (via daily email) for participants to enter data into a daily diary within the participant-facing database; as well as verbal reminders at the day 7,14 and 21 virtual visits.
Relevant concomitant care permitted or prohibited during the trial
Usual concomitant care will continue for both ExACT intervention and usual care (tACT) groups. Concurrent participation in interventional trials is not permitted for the duration of the ExACT-CF trial.
Outcomes
Key outcome measures of this pilot study will inform whether or not to proceed with a full trial, and if so, any revisions to be made to the study protocol beforehand. These outcomes include retention rate, adherence (with ExACT intervention, and also with uploading of data) and participants’ experiences of participation and perspectives (including those of decliners) on joining this and future trials of exercise for airway clearance in CF.
A candidate primary outcome of a full RCT is measurement of lung health (by LCI 2.5) to establish the non-inferiority of ExACT, compared with usual care (tACT) in people with CF. This will be an exploratory outcome for this trial. There has been recent growing interest in outcome measures for both observational and interventional studies in CF including LCI 34 .
Baseline data collection will include demographics (date of birth, sex, initials) and relevant medical history. Anthropometric characteristics (height, weight, body mass index (BMI), BMI z-score), basic medical information (current medications), lung function (spirometry and LCI), physical activity, sleep and quality of life will be measured at baseline and 28 day follow-up, with additional home-based spirometry, physical activity, sleep, and pulmonary exacerbation assessment each week.
Primary outcome
The primary outcome is the feasibility of randomising (1:1) 50 people with CF to 28 days of ExACT or usual care (tACT). Feasibility will be assessed in terms of the following outcomes: the proportion of people with CF that accept invitation to be randomised and attend their baseline visit; the proportion of people with CF that attend their baseline visit who also attend the day 28 visit; the proportion of people with CF who complete the study and submit the necessary data; the proportion of people with CF that would chose the intervention over usual care (chest physiotherapy) in the future; and thematic analysis of semi-structured interviews of participants and healthcare professional involved with the study, with respect to their perceptions and experiences of the ExACT intervention and study procedures (consent, randomisation, participant information sheets, etc.)
Secondary outcome measures
Information on the short-term clinical impact and safety of stopping routine chest physiotherapy for 28 days will be obtained by analysing predefined plausible secondary outcomes. Preliminary safety data will include the between group differences in change in LCI 2.5 from baseline to day 28 follow-up, between group differences in the change in FEV 1 (%predicted) and FVC (%predicted) from baseline to day 7, 14, 21 and day 28 follow-up, between group differences in rates of pulmonary exacerbation from baseline to day 28, and between group differences in adverse event and serious adverse event rates from baseline to 28 day follow-up. Additional information regarding the short-term clinical impact of stopping routine chest physiotherapy and replacing with ExACT will include: between group differences in the duration, type and intensity of physical activity and exercise (steps per day, minutes of moderate-to-vigorous activity, number of exercise sessions, and sleep duration, by daily upload and device-based activity monitoring (Garmin Vivosmart4 ®) undertaken by people with CF and any change from baseline to 28 day follow-up, and between group differences in change in mood, quality of life, treatment burden.
Pulmonary function
Lung clearance index (LCI 2.5) will be measured at baseline and 28 days follow-up. LCI will be measured by a nitrogen multiple breath washout (N 2-MBW) technique (Exhalyser-D device, EcoMedics AG, Duernten, Switzerland) using the updated software version [Spiro Ware 3.3.1]. To ensure standardisation across sites operator training, quality control and over-reading will be provided by a central over-reading centre (Royal Brompton Hospital, London, UK) 35 . LCI is derived from a MBW test; a passive breathing test measuring ventilation inhomogeneity within the lungs. LCI is considered more sensitive than spirometry (FEV 1) in early lung disease and is reported as LCI 2.5 (the rate at which an inert gas, nitrogen, reduces to 1/40th of the starting concentration when breathing 100% oxygen). LCI 2.5 is sensitive to change 36 and correlates with ventilatory efficiency during exercise, as well as exercise capacity 37 and may offer a better outcome measure for ACT trials that FEV 1, which reportedly lacks sensitivity to measure AC T efficacy 38 .
Spirometry will be performed using a Spirobank Smart device (Intermedical UK Limited, Kent, UK) that enables home-based assessment. Baseline and day 28 will be performed face-to-face in clinic under supervision, with day 7, 14 and 21 measurements performed at home using the same device. All spirometry will be performed in accordance with American Thoracic Society/European Respiratory Society standards and utilising appropriate reference values, with FEV 1 and FVC (%predicted and Z-scores) recorded at baseline (day 0), day 7, 14, 21 and day 28. Standard operating procedure guidance for participants and researchers has been developed to standardise test conduct across sites. For home-based assessments, participants will send a PDF copy of their results (generated within the application) to a central email address for analysis and addition to the study database in pseudoanonymised format. At the day 28 visit, a member of the research team will download an anonymised data file containing all spirometry assessments undertaken by the participant during the 28 day period. This will enable any additional spirometry manoeuvres (completed outside of research visits) undertaken to also be captured.
Physical activity
Device-based physical activity and exercise monitoring: Physical activity and exercise will be monitored using a wristwatch monitor (Garmin Vivosmart 4 ®, Garmin, Olathe, KS, USA) before participants attend their baseline visit (7 day assessment to be used for randomisation) and throughout the study. Monitors will be worn during waking hours and encouraged at night. Total numbers of steps and details of any ‘activities’ (e.g. duration, distance walked/run/cycled or swum, and heart rate) will allow the frequency, duration and intensity (sedentary/light/moderate/vigorous) of any periods of physical activity and exercise to be assessed. The Garmin Vivosmart 4 ® device provides a measure of the intensity of physical activity by tracking heart rate. Moderate and vigorous physical activity reflects a fraction (typically 50–85%) of the individual predicted maximal heart rate (maximal heart rate = 220-age).
Participants in both study arms will specifically be asked to log all structured activities (e.g. planned walks, running, swimming, cardio etc.) using their wrist-worn wearable device and to also provide a self-reported, session specific score using the Borg scale of reported perceived exertion (0-10 Likert scale). This will be particularly important for those in the ExACT arm, who will be asked to log all ExACT sessions to compare this data to Delphi consensus recommendations 27 regarding the duration and intensity of ExACT sessions for people with CF e.g. testing the fidelity of the intervention. Participants will be prompted to sync their watch with the Garmin platform, which will automatically download their data. Participants will receive daily prompts to complete their diaries via the database.
Questionnaire based physical activity: The Habitual Activity Estimation Scale (HAES) 39 , a questionnaire used to assess physical activity in paediatric and adult CF populations., will be completed at baseline and 28 day follow-up.
Sleep: An estimation of total sleep time will also be obtained from the wrist worn wearable device. The sleep and physical activity / exercise data will be accessed via Garmin Connect and exported to the study database.
Quality of life (QoL): QoL will be assessed using the CF Questionnaire-Revised (CFQ-R) 40 , a disease-specific health-related QoL (HRQoL) measure for children, adolescents and adults with CF. It is a profile measure of HRQoL with several different domains, and was designed to measure impact on overall health, daily life, perceived wellbeing and symptoms. The CFQ-R has undergone extensive reliability and validity testing and will be used to calculate a score on a 0-100 scale, with higher scores indicating better HRQoL. The CFQ-R comprises 4 versions, and the most appropriate of the following two versions will be chosen based on the participant’s age, namely the CFQ-14+ version for participants aged ≥ 14 years and the parent-completed version for children aged 10–13 years.
Treatment burden: Treatment burden will be measured using the subscale of the CFQ-R.
Mood: Mood will be measured using the Hospital Anxiety and Depression Scales (HADS), which was designed to measure anxiety and depression in a general medical population of patients. The questionnaire comprises seven questions for anxiety and seven questions for depression, and takes 2–5 minutes to complete. Normative data exist for the HADS questionnaire exist for those aged ≥ 13 years 41 . The HADS will be administered only to the subset of participants aged ≥ 13 years in the ExACT-CF study. Although the anxiety and depression questions are interspersed within the questionnaire, these must be scored separately. Cut-off scores are available for quantification.
Pulmonary exacerbations: Information on pulmonary exacerbations in the preceding 7 days will be ascertained using, with permission, a modified version of the ‘Exacerbations, infections of upper respiratory tract, antibiotic use and adverse effects of exercise’ questionnaire developed by Hebestreit et al. and used previously within the ACTIVATE-CF trial 42 , an international, multi-centre, randomised controlled trial of a partially supervised conditioning programme in CF.
Monitoring of ACTs: Participants will have daily automated contact via a daily automated e-mail to log each airway clearance (ExACT and/or tACT) session as well as additional exercise for exercise sessions, linked directly to a participant-facing arm of an online study database. Alongside this, participants will be able to subjectively rate how hard they felt they were exercising (Borg Rating of Perceived Exertion 43 ) to help us gauge the intensity of exercise. The physical activity monitor detailed above will also offer insight into intensity by providing heart rate data. These data, alongside the information gathered from physical activity and exercise monitoring, will allow estimation of adherence to ExACT. Participants will also be asked to measure the duration of traditional airway clearance sessions using their wrist worn wearable device.
Economic evaluation: Self-reported health service use and social/economic outcomes will be collected by a health economist using bespoke question sets (health utilisation questionnaires) that will inform future economic analyses. If data collection is confirmed as feasible, then data collected within this study will be used to conduct an exploratory ‘short-term’ within trial health economic analysis to provide an estimate of cost-effectiveness and support to either continue usual care (chest physiotherapy, tACT) for airway clearance or to stop this and replace it with exercise as an airway clearance technique (ExACT).
In this randomised pilot trial, the health economics objectives are to: (i) design and test an optimal mechanism for the capture of resource use and cost data in community NHS settings and NHS secondary care, (ii) estimate expected effect size and variance of relevant outcomes including health-related utility and quality-adjusted life years (QALYs), and (iii) identify and measure the potential cost implications of exercise as an airway clearance technique in people with CF.
All health-care resource use data will be collected for the trial period of 28 days from randomisation using participant-completed health utilisation questionnaires administered at baseline and Day 28, along with data obtained directly from hospital records. This will include use of primary and secondary care services along with prescription medications. Health-related utility will be measured using the EQ-5D instrument 44 and healthcare-related resource use and costs using participant questionnaires at baseline and 28 day follow-up. These costs will be ratified by the study team through scrutiny of the patient pathway in both arms of the trials using available medical records to populate CRFs. We will assign unit costs using standard national costing sources where available, or through consultation with relevant service business managers. Costs will be summarised from the NHS and personal social services perspective.
Embedded qualitative evaluation: Semi-structured video-call interviews (maximum 1 hour) will be used to explore the experiences of a subset ( n = 10–15) of people with CF (and their parent/guardian if < 18 years) along with physiotherapists, clinicians and other health professionals (e.g. research nurses) involved in the trial. Individuals who decline to participate in this randomised pilot trial will also be approached to be interviewed to gain insight into the reasons for this. Interviews with participants of this trial will focus on their experiences of the trial processes (e.g. randomisation); trial materials (e.g. participant information sheets, questionnaires, etc); and the acceptability and fidelity of ExACT in order to identify barriers/facilitators to inform a definitive trial, while those who declined to participate will be asked for their reasons for non-participation.
In addition to evaluating the experience of individuals involved in this trial, this qualitative sub-study will also help refine what exercise is considered equivalent to chest physiotherapy, building on earlier UK-based e-Delphi consensus work 27 . To ensure representative views, a purposive maximum variation sample (sampling for age, gender, ethnicity, recruitment site, study arm, completers/drop out and decliners, roles) will be sought. Interviews will be digitally recorded via Zoom (audio and audio+video options), transcribed verbatim and stored securely, and thematically analysed. All interviews will take place 1–3 months following participation (or invitation to participate) in the trial.
Participant timeline
Figure 2 displays the participant timeline.
Figure 2. SPIRIT overview or enrolment, interventions, and assessments.
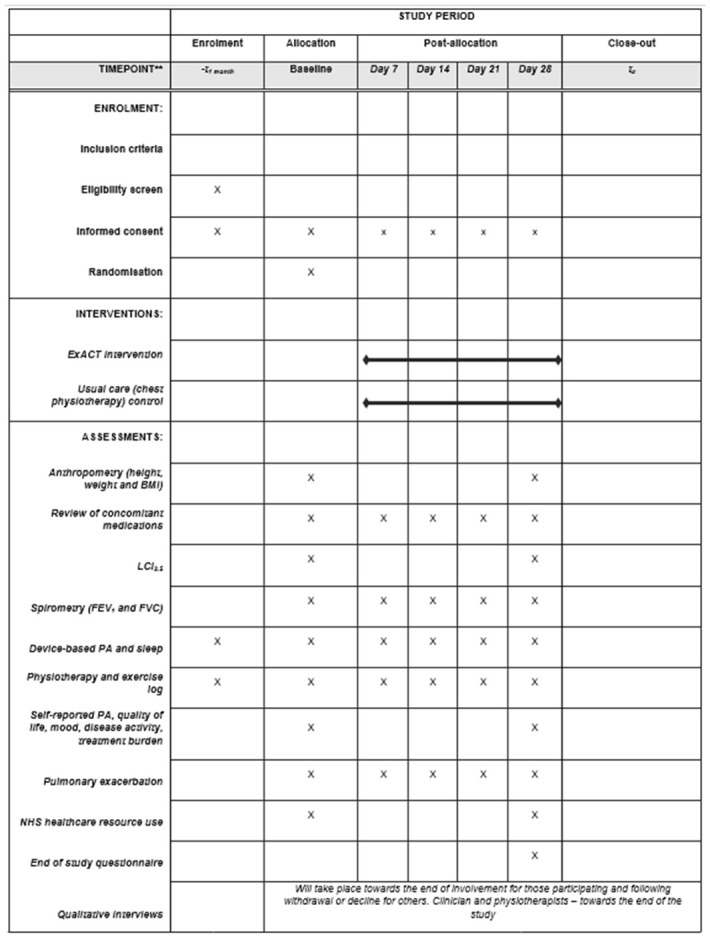
N.B. BMI, body mass index; ExACT, exercise as an airway clearance technique; FEV 1, forced expiratory volume in 1 second; FVC, forced vital capacity; LCI, lung clearance index; PA, physical activity.
Sample size
Although formal sample size calculations are not required for this feasibility study, we provide justification for the sample size chosen in regard to safety. We will aim to recruit 50 people with CF into the study. In this pilot trial, the key feasibility metrics will help to inform whether we will be able to recruit an adequate sample size in the main phase ExACT-CF RCT.
Recruitment
Participants will be recruited from the CF clinics at each study site (UHS, SCH, RHCYP, WGH). Patients who meet the entry criteria may be recruited by the investigator or a trained member of the local site team who has delegated responsibility. Following eligibility screening, the lead CF consultant with the respective site will confirm patient eligibility and provide medical clearance for intense exercise and study involvement. Eligible and interested participants (and parents/guardians for paediatric participants) will be given a participant information sheet and consent form. To support recruitment, a number of visits and outcome measures will be home-based, the intervention will be carried out independently at home at times that suit the participant, and funding will be provided for travel and parking for all trial visits.
Assignment of interventions: allocation
Sequence generation
Participants will be randomly assigned to either the ExACT group or usual care (chest physiotherapy) control group using central web-based randomisation (Edinburgh Clinical Trials Unit (ECTU) bespoke database) and will randomise 1:1 using minimisation to ensure the groups are balanced for age (< 20 years and ≥ 20 years), lung function (< 70% predicted and ≥ 70% predicted) and baseline physical activity, as measured as measured in the 7 days preceding baseline by Garmin Vivosmart 4 ® device (< 3-hours moderate and/or vigorous physical activity per week versus ≥ 3 hours). Allocation will be concealed until participants are enrolled and assigned. The web-based database will allocate participants a unique trial identification number and their identification details then entered onto the trial identification log, to be stored in the Investigator Site File. The only situation where a participant who fails screening could be re-considered for study participation would be in the event of an injury precluding participation which subsequently resolves. Data would be explicitly collected for any subject who is a re-screen.
Allocation concealment mechanism
Participants will be centrally randomised using a web-based database. Allocation concealment will be ensured until participants are enrolled and assigned to their trial arm.
Implementation
Participants will be enrolled in the study by the research staff responsible for recruitment in clinics. Following enrolment, the recruiting researcher will create a record for each enrolled participant in the bespoke trial database. Following completion of the baseline assessment, participants will be randomised to receive either usual care or the ExACT intervention.
Who will be blinded after assignment to interventions?
Neither researchers who access physical activity downloads nor participants can be blinded in regards to the exercise intervention.
Data collection, management, and analysis
Plans for assessment and collection of outcomes
The baseline/randomisation visit must occur ≤ 4 weeks after the patient has successfully passed screening. Upon consent, participants will be provided with a wearable activity monitor (Garmin Vivosmart 4 ®). A reminder will be issued at Day -7 to request that the monitor is worn in order to capture physical activity data for the 7 days preceding their baseline visit. This will enable measurement of levels of moderate and/or vigorous physical activity to be quantified for inclusion within the minimisation algorithm at randomisation. A device that has not been worn by the participant will be considered as < 3 hours of moderate and/or vigorous physical activity for minimisation purposes. All study assessments will be conducted by trained research nurses and members of the research team at each respective site. All LCI assessors will be required to meet predetermined inter- and intra-observer reliability standards for each outcome. For the measurement of physical activity and sleep using the wrist-worn wearable device, data will be downloaded and processed into physical activity metrics offline by one of the study researchers (ET) and subsequently imported into the bespoke trial database. Similarly, for spirometry measures, data will be manually entered into the trial database. All questionnaires will be completed on paper or online within the bespoke trial database, with participants given the option to either complete those at 28 day follow-up prior to attending or at this study visit. A daily diary of airway clearance and exercise undertaken, linked to the study database, will be expected to be completed daily throughout the 28 day period. Automated text reminders will be sent the following morning in the event of non-completion.
Plans to promote participant retention and complete follow up
Participants will be kept up to date with progress via the trial Twitter page and website.
Data management
All data will be recorded using standardised data entry forms created in a bespoke trial database (ECTU), with paper case report forms (CRFs) also available. Electronic participant data will be stored on the bespoke password-protected trial database and, where hard copies, in locked cabinets in secure facilities at UHS and UoE. Only study staff directly involved in the research will have access to data files.
Confidentiality
To ensure confidentiality, all participants will be allocated an individual identification number. Access to participant information will only be provided to immediate trial staff, unless requested by legislative or regulatory agencies. Identifiable information will need to be collected to follow-up participants and their families. Participants and parents/guardians (for minors) will be informed at enrolment that this identifiable information is being collected and that is will not be disclosed to anyone outside the immediate study team. Specifically, identifiable information collected include name, address, and date of birth. All identifiable information will be removed from the database at the end of the study. All publicly accessible data will be presented in anonymised form only. Field notes will only reference the participants’ study identification number. Data will be stored for 10 years after completion of the study.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in the current trial and for future use
This trial does not involve collecting biological specimens for storage.
Statistical methods
Statistical methods for primary and secondary outcomes
Feasibility analysis
Feasibility measures will be presented descriptively by arm. We will present quantitative data with summary statistics and 95% confidence intervals as appropriate. We will report the number of patients eligible, approached, consented and randomised in total and on a per-site level. A trial CONSORT diagram will be prepared, illustrating the participants’ pathway through the study. We will report the number of participants who remain in the study and complete data collection/upload data at the various data collection time points. Adherence to the ExACT intervention will be self-reported by subjects with data on the type, and duration collected. These will be cross-validated using start and stop facility on the Garmin Vivosmart 4 ® device to enable duration and intensity (peak heart rate and time spent undertaking moderate-to-vigorous intensity physical activity) of each ExACT session to be measured.
This study is not powered for formal statistical comparisons, but we will report estimates of clinical efficacy and safety outcomes in both the intervention and the control arm. In brief, between group comparisons and within group comparisons (baseline to 28 days) will be made for the continuous variables measured in the study e.g. FEV 1, LCI 2.5, CFQ-R etc. A test of normality will be used to determine whether parametric or non-parametric analyses are appropriate for these comparisons.
Interim analyses
Interim analyses are not planned.
Methods for additional analyses
Health economic and qualitative analyses are also planned as described below.
Determination of feasibility of performing a definitive main trial
The progression criteria have been informed by locally available data from centres on the number of eligible participants presenting annually, in line with existing guidelines around the design of studies of this nature 45 . This was used to inform the minimum recruitment thresholds required to be achieved during the main trial. Given the nature of this external pilot trial, the progression criteria will not be considered on a simple stop/go basis, but rather a red/amber/green traffic light system has been adopted, that enables exploration of whether the trial can proceed with modifications. More specifically, stop/red (e.g., when there are intractable issues that cannot be remedied), amend/amber (where there are remediable issues, thereafter proceeding with caution – e.g. measurement of LCI 2.5 may be adapted with training and so should not be stop/go for future trial), or continue/green (where there are no concerning issues that threaten the success of the trial). These pre-specified criteria are set in order to judge the viability of completing the main trial within the planned timetable and budget and usually address the key areas of uncertainty or risk that could influence the success of the trial 45 .
Previous researchers in CF have undertaken feasibility work in this area 28 , and they defined feasibility a priori as the recruitment of at least 30% eligible participants, with randomisation of at least 80% participants following a wash-in period, along with 80% of randomised participants completing all study interventions. In this trial, the proportion of eligible participants (number eligible/number of people with CF in clinic) - pre-screening that participant meets inclusion/exclusion criteria (genotype, existing treatment, clinical stability, competing studies), will first be established. The primary feasibility metrics (recruitment, retention/follow up, adherence rate) will then be reported as point estimates alongside 95% CIs, and assessed against the pre-specified traffic light progression criteria: 1) proportion recruited/randomised (number randomised/number screened and approached): 60% green, 30–59% amber, < 30% red; 2) retention/follow-up (number providing primary clinical outcome data/number randomised): 85% green, 71–84% amber, < 70% red; and 3) adherence rate (number undertaking satisfactory airway clearance*/number randomised): 80% green, 60–79% amber, < 60% red.
*Undertaking airway clearance on ≥5 days out of 7 as ExACT or tACT would be satisfactory
In summary, ‘red’ would be an indication for consideration of study non-progression to main trial, whilst ‘amber’ would indicate a requirement for study re-design. Additional factors determining trial progression would include: rates of recruitment/recruitment trajectory, fidelity to trial protocol, ability of investigators to implement trial protocol (in particular measurement of LCI 2.5), and safety of the ExACT intervention, as assessed by change in LCI 2.5 between groups as well as Serious Adverse Event (SAE) reporting. Exacerbation frequency and FEV 1 whilst also being measured are unlikely to inform safety in a trial of such short duration.
Health economics analysis
The exploratory economic evaluation will adopt an NHS viewpoint and the focus of most data collection will be on direct health care costs for this feasibility study. Intervention-related costs (e.g. associated with initial ExACT exercise session training), health care resource use including secondary care (e.g. hospitalisations: outpatient, day case, inpatient stay, use of emergency department (ED) services) and primary and community care (e.g. GP appointments and home visits for primary care, use of other community care services) to 28 days will be compared between the ExACT intervention and usual care (tACT). The relative importance of other types of health care costs (e.g. medicines) will be captured in other planned study assessments –Table 1 ‘Review of concomitant medications’. Direct non-health care costs, participants’ out of pocket expenses, carer time costs/informal care and productivity losses are excluded from any formal health economic analysis, but the importance of these types of economic costs to CF study participants and their families will be explored as part of the planned qualitative study/semi-structured interview discussions. Use of health care resources will be valued and the associated costs estimated by assigning unit costs from standard published UK sources where available (including Personal Social Service Research Unit (PSSRU) unit costs and NHS reference costs). Costs related to intervention delivery will be estimated from trial records. The effects of the intervention (and usual care) will be estimated in terms of QALYs at 28 days using the EQ-5D-5L health-related quality of life data collected at baseline and 28 days and the area under the curve method. Published UK tariffs will be used to convert these data to quality of life weights.
Descriptive analyses will provide estimates of total and average resource use, costs, outcomes (and associated 95% confidence intervals as appropriate), comparisons of cost profiles by arm (and levels of missing data for NHS resource use and EQ-5D-5L health utilities data). A Health Economics Analysis Plan (HEAP) will be developed describing all the health economics analyses to be carried out.
Qualitative analysis
The interviews will be recorded within Zoom videoconferencing software on a secure University of Portsmouth or University of Edinburgh account. The recording will be uploaded to a password-protected computer and then deleted from the recorder. The recording will be sent to a professional transcription company that will sign a confidentiality agreement. Interviews will be analysed thematically following the framework approach. NVivo qualitative data management software will facilitate management of the dataset. Repeated readings of transcripts and listening to recordings will assist familiarisation with the data and identification of the initial framework. Using the initial framework, existing and new codes will be added to develop the main themes which will then be reviewed, defined and named. Thematic analysis will facilitate the identification of major issues and barriers for patients and practitioners, and enable relationships between the themes to be developed.
Oversight and monitoring
Composition of the coordinating centre and trial steering committee
The University Hospital Southampton NHS Foundation Trust in Southampton (England, UK) will provide sponsorship for this study and the joint Chief Investigators (ZS and DU) will be responsible for the day-to-day running of the trial. A Research Fellow based at the University of Edinburgh (UoE) will review the eligibility, enrolment, and consent for each participant; independently verify data entered into the bespoke trial database; monitor the assessment time points for scheduling; and review the data for completeness. A Research Fellow from UoE and the chief investigators will independently review the study data, ensure assessment equipment is maintained, provide support to the research nurses and physiotherapists at each site, and oversee the organisation at each study site and their respective activities.
A trial steering committee, comprising the chief investigators, their mentor (SC), and independent physiotherapists, a statistician, specialist CF consultant physicians and Patient and Public Involvement (PPI) representatives (people with CF and a parent), will provide overall supervision for the ExACT-CF pilot trial on behalf of the Trial Sponsor and the funder. They will also ensure that the trial is conducted according to the guidelines for Good Clinical Practice (GCP), Research Governance Framework for Health and Social Care and all relevant regulations and local policies. A trial management group, comprising the research team and research nurses from all sites, will meet monthly via a videoconference, to ensure protocols are properly established and implemented and that project milestones are completed on time.
Composition of the data monitoring committee, its role, and reporting structure
An independent Data Monitoring and Ethics Committee (DMEC), comprising experienced specialist CF physicians, a statistician and person with CF, has been formed and will be responsible for protect the safety interests of patients, ensuring the ethical conduct, validity and scientific merit of the trial, evaluating ongoing safety data and assess the risk of the patients, and making recommendations for the conduct of the trial in the light of the study data accumulated throughout the entire duration of the project.
Adverse event reporting and harms
Surveillance for adverse events will occur for the duration that participants are involved in the ExACT program (28 days). This will be the responsibility of the chief investigators, who will ensure that all adverse events, including serious adverse events (SAEs) events, are documented and accurately reported. All the study staff will be trained in the requirements and procedures of reporting evens.
An AE is defined as any untoward medical occurrence during the study. The event does not necessarily have a causal relationship with the treatment. This includes any newly-occurring event or worsening of a pre-existing condition (e.g., increase in its severity or frequency) that presents after a participant has provided fully informed consent form. A SAE is an AE which meets any of the following criteria:
Fatal (death, regardless of cause that occurs during participation in the study)
Life threatening, such that the study participant was at risk of death when the event occurred
In-patient hospitalisation during the course of the study
Permanent or significant disability/incapacity
Important medical event that, based on appropriate medical judgement, may seriously jeopardise the participant or may require medical or surgical intervention to prevent one of the outcomes listed above (e.g. severe bronchospasm requiring intensive treatment in the emergency department)
All AEs and SAEs will be carefully described with a description of the event, dates of onset and resolution, action taken, outcome, and whether any concomitant medications were administered. Furthermore, all AEs and SAEs will be graded in regard to:
Severity (1 – mild, 2 – moderate, 3 – severe, 4 – life threatening, 5 – death)
Causality (related, possibly related, unlikely related, not related to the intervention)
Episodes of hospitalisation and other infection episodes will be documented and measurements (LCI 2.5, lung function [FEV 1], exacerbation questionnaires) and monitoring processes within this pilot trial will enable observation of early signals and ensure participant safety oversight. We plan to gain insight into clinical impact on lung health by measuring LCI 2.5 as a key secondary outcome - which appears a possible candidate outcome for a trial comparing ACTs, given the initial consequence of impaired airway clearance may be expected to be retention of secretions, which in turn would affect regional ventilation to those affected areas. LCI 2.5 is, we believe, the best available physiological test for the early detection of such changes. Weekly pulmonary exacerbation questionnaires and home spirometry measurements will also enable regular monitoring of participants in the ExACT-CF pilot trial.
Participants and/or their caregivers will be asked to notify the study coordinators of any serious adverse events (SAEs) that may occur during the study period. Any SAEs will be reported to the chief investigator within 24-hours of being made aware of the event. In line with procedures, the chief investigator will complete the SAE form with as much information regarding the event that is available to them at the time and a copy placed within the participant’s e-CRF. Telephone reports will be followed by a full report including copies of relevant medical records and other documents. Any SAEs identified will be reported to the sponsor.
All data will be available to the independent DMEC, who will advise the trial steering group in the event of concerns. Furthermore, it has been clarified that participants in the ExACT intervention arm can resume chest physiotherapy (tACT) if exercise is not possible e.g. chest exacerbation, abdominal pain (distal intestinal obstruction syndrome - DIOS), musculoskeletal injury. Finally, participants will remain under regular review by their CF teams throughout the study, and are free to withdraw at any time.
Frequency and plans for auditing trial conduct
The study sponsor (University Hospital Southampton NHS Foundation Trust) will be responsible for the overall coordination of the trial and will work closely with the clinical chief/associate investigators to monitor the project staff and trial conduct at each site. All study related documents will be made available on request for monitoring and audit by UHS, the relevant REC or other licensing bodies to ensure adherence to ICH GCP, UK Policy Framework for Health and Social Care Research, applicable contracts/agreements and national regulations.
Plans for communicating important protocol amendments to relevant parties (e.g., trial participants, ethical committees)
Any protocol amendments will be submitted to the lead ethics committee (Sheffield REC) for review. Any amended versions of the study protocol will be distributed to the local sites by the Chief Investigators and any necessary updates made on clinicaltrials.gov website.
Dissemination plans
The results of the ExACT-CF randomised pilot trial will be disseminated through open access peer-reviewed journal publications, conference presentations, CF networks (e.g. Association of Chartered Physiotherapists in CF, UK CF Medical Association) and specialist societies such (e.g. European CF Society, European Respiratory Society). Additionally, with support from our involvement team, findings from this randomised pilot trial will be disseminated to public audiences with input from, and acknowledgement of, the PPI advisory group. Public audiences will include patient and caregiver groups and meetings through e.g. the CF Trust and CF Warriors global exercise charity (#1178063). We will also keep the public, patients and their caregivers informed about study progress via social media platforms (e.g. Twitter), and will hold education webinars virtually as an open forum to share our findings and for Q&A.
Research ethics approval
Ethics approval has been obtained for this study from the Yorkshire and Humber -Sheffield NHS Research Ethics Committee (REC #22/YH/0223) and the study has been registered on ClinicalTrials.gov website ( NCT05482048). The trial will be conducted in compliance with the principles of the Declaration of Helsinki (1996), the principles of GCP and in accordance with all applicable regulatory requirements including but not limited to the UK Policy Framework for Health and Social Care Research.
Declaration of interests
There are no conflicts of interests for to declare for this trial.
Access to data
The Chief Investigators will act as custodians for the final trial dataset which will be pseudo-anonymised and stored on a password protected database.
Discussion
Airway clearance is a long‐established treatment for pwCF, dating back almost to when CF was identified 6 , yet tACT (chest physiotherapy) is consistently reported to be the most burdensome treatment for pwCF, with self‐reported adherence lower than other treatments such as medication 9 . CFTR modulators are changing the landscape of CF care and ways to reduce the burden of treatment for pwCF is a priority 6 . Aerobic capacity is thought to be one of the best predictors of survival for people with CF 46– 48 and exercise training is already part of the physiotherapy management of pwCF 2 , however it is currently recommended in addition to airway clearance, rather than this purpose alone 5 . However, pwCF, their caregivers and clinicians, are asking if exercise can be used as an airway clearance technique - ExACT 24 , many would take part in a trial evaluating ExACT 27 , and are supportive of adopting ExACT into clinical practice if evidence were supportive 27 .
There is currently insufficient evidence regarding whether or not exercise can replace other methods of airway clearance 17 , with a mandate for well-conducted, multi-centre trial. Prior to a larger, fully powered study, a pilot trial is needed to determine the feasibility of randomising pwCF into a trial replacing traditional ACTs with ExACT in CF and to establish any safety impacts of stopping ACT over the short-term (28 days). It is anticipated that the ExACT-CF pilot work will refine the ExACT intervention, as well as optimising trial design and outcome selection for a definitive study.
By implementing a pragmatic patient-focused intervention that is supported by the UK CF community 27 , in line with recent recommendations 17 , the ExACT-CF pilot trial has been co-designed with the CF community to aid recruitment, improve adherence and remove socioeconomic barriers conferred by activities that require access to expensive equipment. Recently recommended 17, 49 alternative outcome measures, such as LCI 2.5, that may be more sensitive to changes in lung disease or in response to intervention 50 in the clinical trial environment, and patient-centres outcomes, such as preference and quality life, have also been included.
As outlined above, tACT is considered burdensome 7 and time consuming 51 for pwCF. Many people with CF are already using 20 , or are keen to use 27 , ExACT within their clinical management. If ExACT can replace tACT, significant time savings may be achievable, as well as additional health benefits. The qualitative substudy of this present randomised pilot trial offers a possibility of exploring the behaviours of pwCF to inform the design, management, and delivery of a future larger trial. This randomised pilot trial is needed to provide feasibility and early safety data and inform the design and management of a larger study to investigate the efficacy and cost-effectiveness of ExACT for pwCF who are stable on CFTR modulator therapy, contributing to future challenges of clinical care and its delivery for pwCF 52 .
Trial status
The recruitment of participants is planned to commence at all study sites in December 2022 and is planned to continue until June 2023 (protocol version 1.2, 24 th October 2022).
Acknowledgements
We would like to thank Mrs Lisa Morrison, Dr Robert Gray, Professor Gary Connett, Dr Julian Legg , Dr Mark Allenby, Dr Thomas Daniels, Dr Mary Carroll, and Dr Carlos Enchevarria for their help in the development of this study application and subsequent protocol. We wish to also thank all participants and their families, the clinical CF Teams at each of the recruiting sites, the NIHR Research Design Service, Shobonna Akhter and Debbie Miller from the CF Trust Clinical Trial Accelerator Programme, research nurses, and all who have volunteered their time to join the Trial Steering Committee and independent Data Monitoring and Ethics Committee. Finally, we also give thanks to the network of people with cystic fibrosis and physiotherapists from across the globe who gave their time to inform the design of this trial through involvement activities.
Funding Statement
This project is funded by the National Institute for Health and Care Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number NIHR203185). The views expressed are those of the author(s) and not necessarily those of the NIHR and Department of Health and Social Care. The funding body is not involved in the design of the study; the collection, analysis, and interpretation of the data; or the writing of the manuscript. This study is a UK Clinical Trials Accelerator Platform Supported Study and is also supported by the National Institute for Health and Care Research ARC Wessex.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
Data availability
Underlying data
No data are associated with this article.
Extended data
Figshare: Participant Information Sheets for the ExACT-CF pilot trial, https://doi.org/10.6084/m9.figshare.21640631.v1 53
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
References
- 1. Doyle B: Physical therapy in the treatment of cystic fibrosis. Phys Ther Rev (1948). Oxford University Press,1959;39(1):24–7. 10.1093/ptj/39.1.24 [DOI] [PubMed] [Google Scholar]
- 2. Standards of care and good clinical practice for the physiotherapy management of cystic fibrosis: CF Trust Physiotherapy Guidelines. 4th ed,2020. Reference Source [Google Scholar]
- 3. Kerstan M, Maguire I,, International Physiotherapy Group for Cystic Fibrosis : Physiotherapy for people with cystic fibrosis: From infant to adult.2019. Reference Source [Google Scholar]
- 4. Daniels T, Morrison L, Lewis S: Association of chartered physiotherapists in cystic fibrosis standard of care and good clinical practice for the physiotherapy management of cystic fibrosis. Draft. 2016;2016. [Google Scholar]
- 5. Flume PA, Robinson KA, O'Sullivan BP, et al. : Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care. 2009;54(4):522–37. [PubMed] [Google Scholar]
- 6. Rowbotham NJ, Daniels TE: Airway clearance and exercise for people with cystic fibrosis: balancing longevity with life. Pediatr Pulmonol. 2022;57 Suppl 1:S50–S59. 10.1002/ppul.25734 [DOI] [PubMed] [Google Scholar]
- 7. Davies G, Rowbotham NJ, Smith S, et al. : Characterising burden of treatment in cystic fibrosis to identify priority areas for clinical trials. J Cyst Fibros. 2020;19(3):499–502. 10.1016/j.jcf.2019.10.025 [DOI] [PubMed] [Google Scholar]
- 8. Williams B, Mukhopadhyay S, Dowell J, et al. : Problems and solutions: accounts by parents and children of adhering to chest physiotherapy for cystic fibrosis. Disabil Rehabil. 2007;29(14):1097–105. 10.1080/09638280600948060 [DOI] [PubMed] [Google Scholar]
- 9. Myers LB, Horn SA: Adherence to chest physiotherapy in adults with cystic fibrosis. J Health Psychol. 2006;11(6):915–26. 10.1177/1359105306069093 [DOI] [PubMed] [Google Scholar]
- 10. Middleton PG, Mall MA, Dřevínek P, et al. : Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809–19. 10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heijerman HG, McKone EF, Downey DG, et al. : Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940–48. 10.1016/S0140-6736(19)32597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Main E, Prasad A, Schans C: Conventional chest physiotherapy compared to other airway clearance techniques for cystic fibrosis. Cochrane Database Syst Rev. 2005;2005(1):CD002011. 10.1002/14651858.CD002011.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burnham P, Stanford G, Stewart R: Autogenic drainage for airway clearance in cystic fibrosis. Cochrane Database Syst Rev. 2021;12(12):CD009595. 10.1002/14651858.CD009595.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morrison L, Milroy S: Oscillating devices for airway clearance in people with cystic fibrosis. Cochrane Database Syst Rev. 2020;4(4):CD006842. 10.1002/14651858.CD006842.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mckoy NA, Wilson LM, Saldanha IJ, et al. : Active cycle of breathing technique for cystic fibrosis. Cochrane Database Syst Rev. 2016;7(7):CD007862. 10.1002/14651858.CD007862.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McIlwaine M, Button B, Nevitt SJ: Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database Syst Rev. 2019;2019(11):CD003147. 10.1002/14651858.CD003147.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heinz KD, Walsh A, Southern KW, et al. : Exercise versus airway clearance techniques for people with cystic fibrosis. Cochrane Database Syst Rev. 2022;6(6):CD013285. 10.1002/14651858.CD013285.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hebestreit A, Kersting U, Basler B, et al. : Exercise inhibits epithelial sodium channels in patients with cystic fibrosis. Am J Respir Crit Care Med. 2001;164(3):443–46. 10.1164/ajrccm.164.3.2007168 [DOI] [PubMed] [Google Scholar]
- 19. Kriemler S, Radtke T, Christen G, et al. : Short-term effect of different physical exercises and physiotherapy combinations on sputum expectoration, oxygen saturation, and lung function in young patients with cystic fibrosis. Lung. 2016;194(4):659–64. 10.1007/s00408-016-9888-x [DOI] [PubMed] [Google Scholar]
- 20. Ward N, Stiller K, Holland AE, et al. : Exercise is commonly used as a substitute for traditional airway clearance techniques by adults with cystic fibrosis in Australia: a survey. J Physiother. 2019;65(1):43–50. 10.1016/j.jphys.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 21. Almulhem M, Harnett N, Graham S, et al. : Exploring the impact of elexacaftor-tezacaftor-ivacaftor treatment on opinions regarding airway clearance techniques and nebulisers: TEMPO a qualitative study in children with cystic fibrosis, their families and healthcare professionals. BMJ Open Respir Res. 2022;9(1):e001420. 10.1136/bmjresp-2022-001420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rowbotham N, Smith S, Davies G, et al. : Can exercise replace airway clearance techniques in cystic fibrosis? A survey of patients and healthcare professionals. J Cyst Fibros. 2020;19(4):e19–e24. 10.1016/j.jcf.2019.10.026 [DOI] [PubMed] [Google Scholar]
- 23. Ward N, Morrow S, Stiller K, et al. : Exercise as a substitute for traditional airway clearance in cystic fibrosis: a systematic review. Thorax. 2021;76(8):763–71. 10.1136/thoraxjnl-2020-215836 [DOI] [PubMed] [Google Scholar]
- 24. Rowbotham NJ, Smith S, Leighton PA, et al. : The top 10 research priorities in cystic fibrosis developed by a partnership between people with CF and healthcare providers. Thorax. 2018;73(4):388–90. 10.1136/thoraxjnl-2017-210473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dwyer T: Huff and puff of exercise for airway clearance in cystic fibrosis: how clear is the evidence? Thorax. BMJ Publishing Group Ltd,2021; thoraxjnl-2020-216622 10.1136/thoraxjnl-2020-216622 [DOI] [PubMed] [Google Scholar]
- 26. Radtke T: Role of physical activity and airway clearance therapy in cystic fibrosis: moving forward in a rapidly changing landscape. Thorax. 2022; thorax-2022-219429. 10.1136/thorax-2022-219429 [DOI] [PubMed] [Google Scholar]
- 27. Saynor Z, Cunningham S, Morrison L, et al. : Exercise as airway clearance therapy (ExACT) in cystic fibrosis: a UK-based e-Delphi survey of patients, caregivers and health professionals. Thorax. 2022;1–4. 10.1136/thorax-2022-219213 [DOI] [PubMed] [Google Scholar]
- 28. Ward N, Stiller K, Rowe H, et al. : Airway clearance by exercising in mild cystic fibrosis (ACE-CF): a feasibility study. Respir Med. 2018;142:23–28. 10.1016/j.rmed.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 29. McIlwaine MP, Alarie N, Davidson GF, et al. : Long-term multicentre randomised controlled study of high frequency chest wall oscillation versus positive expiratory pressure mask in cystic fibrosis. Thorax. 2013;68(8):746–51. 10.1136/thoraxjnl-2012-202915 [DOI] [PubMed] [Google Scholar]
- 30. Donaldson SH, Pilewski JM, Griese M, et al. : Tezacaftor/ivacaftor in subjects with cystic fibrosis and F508del/F508del-CFTR or F508del/G551D-CFTR. Am J Respir Crit Care Med. 2018;197(2):214–24. 10.1164/rccm.201704-0717OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan AW, Tetzlaff JM, Altman DG, et al. : SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–07. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. : SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoffmann TC, Glasziou PP, Boutron I, et al. : Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 34. Stanford GE, Jones M, Charman SC, et al. : Clinimetric analysis of outcome measures for airway clearance in people with cystic fibrosis: a systematic review. Ther Adv Respir Dis. 2022;16: 17534666221122572. 10.1177/17534666221122572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saunders C, Jensen R, Robinson PD, et al. : Integrating the multiple breath washout test into international multicentre trials. J Cyst Fibros. 2020;19(4):602–07. 10.1016/j.jcf.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 36. Ratjen F, Davis SD, Stanojevic S, et al. : Inhaled hypertonic saline in preschool children with cystic fibrosis (SHIP): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2019;7(9):802–09. 10.1016/S2213-2600(19)30187-0 [DOI] [PubMed] [Google Scholar]
- 37. Avramidou V, Hatziagorou E, Kampouras A, et al. : Lung clearance index (LCI) as a predictor of exercise limitation among CF patients. Pediatr Pulmonol. 2018;53(1):81–87. 10.1002/ppul.23833 [DOI] [PubMed] [Google Scholar]
- 38. Chapman N, Watson K, Hatton T, et al. : Methods Used to Evaluate the Immediate Effects of Airway Clearance Techniques in Adults with Cystic Fibrosis: A Systematic Review and Meta-Analysis. J Clin Med. 2021;10(22):5280. 10.3390/jcm10225280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wells GD, Wilkes DL, Schneiderman-Walker J, et al. : Reliability and validity of the habitual activity estimation scale (HAES) in patients with cystic fibrosis. Pediatr Pulmonol. 2008;43(4):345–53. 10.1002/ppul.20737 [DOI] [PubMed] [Google Scholar]
- 40. Quittner AL, Buu A, Messer MA, et al. : Development and validation of The Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest. 2005;128(4):2347–54. 10.1378/chest.128.4.2347 [DOI] [PubMed] [Google Scholar]
- 41. Jörngården A, Wettergen L, von Essen L: Measuring health-related quality of life in adolescents and young adults: Swedish normative data for the SF-36 and the HADS, and the influence of age, gender, and method of administration. Health Qual Life Outcomes. 2006;4:91. 10.1186/1477-7525-4-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hebestreit H, Kriemler S, Schindler C, et al. : Effects of a partially supervised conditioning program in cystic fibrosis: an international multicenter, randomized controlled trial (ACTIVATE-CF). Am J Respir Crit Care Med. 2022;205(3):330–39. 10.1164/rccm.202106-1419OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Borg G: Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–8. [PubMed] [Google Scholar]
- 44. Herdman M, Gudex C, Lloyd A, et al. : Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Avery KN, Williamson PR, Gamble C, et al. : Informing efficient randomised controlled trials: exploration of challenges in developing progression criteria for internal pilot studies. BMJ Open. 2017;7(2):e013537. 10.1136/bmjopen-2016-013537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hebestreit H, Hulzebos EH, Schneiderman JE, et al. : Cardiopulmonary exercise testing provides additional prognostic information in cystic fibrosis. Am J Respir Crit Care Med. 2019;199(8):987–95. 10.1164/rccm.201806-1110OC [DOI] [PubMed] [Google Scholar]
- 47. Nixon PA, Orenstein DM, Kelsey SF, et al. : The prognostic value of exercise testing in patients with cystic fibrosis. N Engl J Med. 1992;327(25):1785–88. 10.1056/NEJM199212173272504 [DOI] [PubMed] [Google Scholar]
- 48. Pianosi P, Leblanc J, Almudevar A: Peak oxygen uptake and mortality in children with cystic fibrosis. Thorax. 2005;60(1):50–54. 10.1136/thx.2003.008102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hatziagorou E, Kampouras A, Avramidou V, et al. : Toward the establishment of new clinical endpoints for cystic fibrosis: the role of lung clearance index and cardiopulmonary exercise testing. Front Pediatr. 2021;9:635719. 10.3389/fped.2021.635719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perrem L, Rayment JH, Ratjen F: The lung clearance index as a monitoring tool in cystic fibrosis: ready for the clinic? Curr Opin Pulm Med. 2018;24(6):579–85. 10.1097/MCP.0000000000000515 [DOI] [PubMed] [Google Scholar]
- 51. Sawicki GS, Sellers DE, Robinson WM: High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros. 2009;8(2):91–96. 10.1016/j.jcf.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bell SC, Mall MA, Gutierrez H, et al. : The future of cystic fibrosis care: a global perspective. Lancet Respir Med. 2020;8(1):65–124. 10.1016/S2213-2600(19)30337-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saynor Z, Urquhart D: Participant Information Sheets for the ExACT-CF pilot trial. figshare. Online resource.2022. 10.6084/m9.figshare.21640631.v1 [DOI] [Google Scholar]


