Abstract
Background:
Familial hypercholesterolemia (FH) genetic variants confer risk for coronary artery disease (CAD) independent of low-density lipoprotein cholesterol (LDL-C) when considering a single measurement. In real clinical settings, longitudinal LDL-C data are often available through the electronic health record (EHR). It is unknown whether genetic testing for FH variants provide additional risk-stratifying information once longitudinal LDL-C is considered.
Methods:
We used the extensive EHR data available through the Million Veteran Program to conduct a nested case-control study. The primary outcome was CAD, derived from EHR codes for acute myocardial infarction and coronary revascularization. Incidence density sampling was used to match case/control exposure windows, defined by the date of the first LDL-C measurement to the date of the first CAD code of the index case. Adjustments for the first, maximum, or mean LDL-C were analyzed. FH variants in LDLR, APOB, and PCSK9 were assessed by custom genotype array.
Results:
In a cohort of 23,091 predominantly prevalent cases at enrollment and 230,910 matched controls, FH variant carriers had an increased risk for CAD (odds ratio [OR], 1.53; 95% confidence interval [CI], 1.24–1.89). Adjusting for mean LDL-C led to the greatest attenuation of the risk estimate, but significant risk remained (OR, 1.33; 95% CI, 1.08–1.64). The degree of attenuation was not affected by the number and the spread of LDL-C measures available.
Conclusions:
The risk associated with carrying an FH variant cannot be fully captured by the LDL-C data available in the EHR, even when considering multiple LDL-C measurements spanning more than a decade.
Keywords: familial hypercholesterolemia, coronary artery disease, electronic health record, biobank, longitudinal study
Familial hypercholesterolemia (FH) is a monogenic disorder that causes elevated low-density lipoprotein cholesterol (LDL-C) from birth, leading to increased risk for cardiovascular disease. Early identification and treatment of individuals with FH may significantly improve outcomes.1,2 However, FH is underdiagnosed and undertreated.3 Current practice relies on family history, physical exam, and cholesterol screening to identify FH, but many FH variant carriers do not meet criteria for the clinical diagnosis of FH.4
Prior studies suggest that carrying an FH variant confers independent risk for coronary artery disease (CAD) after adjustment for a single baseline LDL-C measurement.5,6 These observations have supported efforts to increase clinical genetic testing for FH.7 However, clinicians often have access to multiple historical LDL-C measurements documented in the medical record. It is unknown whether FH variants continue to confer independent risk after accounting for longitudinal LDL-C exposure.
Estimating the risk among FH variant carriers while accounting for multiple LDL-C measurements over many years is challenging given the relatively small size of most observational cohort studies. However, the maturation of biobanks within large-scale integrated healthcare systems with extensive electronic health records (EHR) provides unprecedented opportunities. We analyzed linked genetic and EHR-derived data for >400,000 participants in the Million Veteran Program (MVP)8 to test the hypothesis that clinically measured longitudinal LDL-C exposure can account for the CAD risk associated with carrying an FH variant.
Methods
The VA Institutional Review Board approved the MVP study protocol in accordance with the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all participants. The individual-level data of veteran participants is only available upon approval from the United States Department of VA Institutional Review Board.
Full methods are now available in the Data Supplement.
Results
FH variant carriers in the MVP population
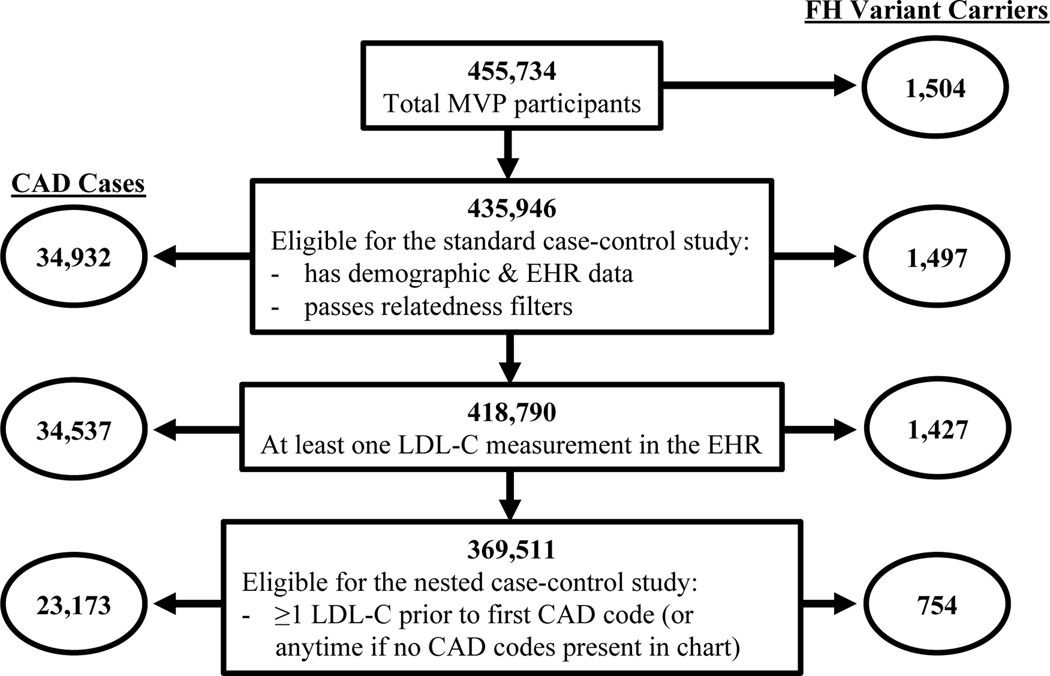
We identified 55 FH variants (51 LDLR, 2 APOB, 2 PCSK9) among 455,734 MVP participants (Table I in the Data Supplement). FH variants were defined by 1) ClinVar annotations of LDLR, APOB and PCSK9; 2) predicted loss-of-function variants in LDLR; and 3) predicted pathogenic missense variants in LDLR. Additionally, we assessed two missense variants in APOB that were previously found to be associated with severe hypercholesterolemia in MVP 9 but were labeled as “uncertain” or “conflicting evidence” in ClinVar. We found that one of these variants was strongly associated with CAD (Table II in the Data Supplement), and thus we chose to keep it in our analysis as an FH variant. All identified FH variants were directly genotyped. In total, we found 1,504 carriers of these variants, for an approximate prevalence of 1 in 303 (Table 1). After excluding individuals with missing demographic data and filtering for relatedness, we were left with 435,946 unrelated individuals, including 1,497 FH carriers (Figure 1).
Table 1.
Prevalence of FH variant carriers in the Million Veteran Program
| Ancestry group | ||||||
|---|---|---|---|---|---|---|
| All | African | Asian | European | Hispanic | Unclassified | |
|
| ||||||
| n | 455,734 | 87,163 | 4,553 | 318,694 | 34,151 | 11,173 |
| FH variant carriers | 1,504 | 258 | 11 | 1,095* | 111 | 29 |
| LDLR LoF | 165 | 20 | 3 | 130 | 10 | 2 |
| LDLR missense | 944 | 222 | 6 | 606 | 91 | 19 |
| APOB | 383 | 16 | 2 | 349 | 8 | 8 |
| PCSK9 | 13 | 0 | 0 | 11 | 2 | 0 |
| Prevalence | 1:303 | 1:338 | 1:414 | 1:291 | 1:308 | 1:385 |
| (95% CI) | (1:288–319) | (1:301–385) | (1:260–1,010) | (1:275–309) | (1:259–378) | (1:283–605) |
One individual was found to be a carrier of both an LDLR missense variant and an APOB variant.
LoF = loss of function. CI = confidence interval.
Figure 1.
Summary of the study cohort at each stage of analysis. FH = familial hypercholesterolemia. CAD = coronary artery disease. LDL-C = low-density lipoprotein cholesterol.
LDL-C metrics and association with FH carrier status
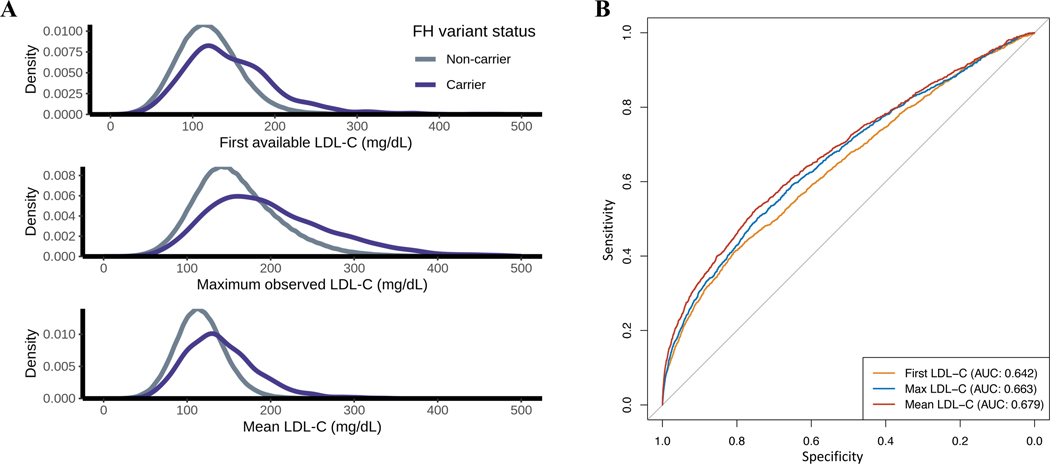
The majority of participants (418,790 or 96.1%) had at least one LDL-C measurement in the EHR, and the median number of LDL-C measurements per individual was 12 (interquartile range [IQR] 6–21). In total, ~6.3 million LDL-C measurements were used in this study. MVP participants carrying FH variants showed a wide range of LDL-C values (Figure 2A). The prevalence of FH variant carriers among subjects with severe hypercholesterolemia (LDL-C > 190 mg/dL) varied dramatically depending on which LDL-C metric was used to define severe hypercholesterolemia (Table 2). In general, however, LDL-C metrics offered only modest discriminatory power for predicting FH carrier status, with mean LDL-C performing better than the other metrics (Figure 2B).
Figure 2.
FH Variant Carrier Status and LDL-C metrics. (A) Density distributions of the first, maximum, and mean low-density lipoprotein cholesterol (LDL-C) measurements observed in the electronic health record (EHR) for individuals with and without familial hypercholesterolemia (FH) genetic variants. (B) Receiver operating characteristic curve for predicting FH variant carrier status using each LDL-C metric with adjustment for age at measurement. For Mean LDL-C, the age at each measurement was used to calculate a mean age across all measurements. To convert LDL-C values from mg/dL to mmol/L, divide by 38.67.
Table 2.
Prevalence of FH variant carriers by LDL-C level, defined by the first available, the maximum observed, or the mean of all measures.
| LDL-C (mg/dL) | n | FH variant carriers (%) |
|---|---|---|
|
| ||
| First | ||
| ≤130 | 264,734 | 640 (0.2) |
| 131–190 | 135,800 | 535 (0.4) |
| >190 | 18,256 | 252 (1.4) |
| >250 | 1,816 | 70 (3.9) |
| Maximum | ||
| ≤130 | 124,964 | 236 (0.2) |
| 131–190 | 191,581 | 500 (0.3) |
| >190 | 102,245 | 691 (0.7) |
| >250 | 24,089 | 321 (1.3) |
| Mean | ||
| ≤130 | 293,585 | 642 (0.2) |
| 131–190 | 119,689 | 607 (0.5) |
| >190 | 5,516 | 178 (3.2) |
| >250 | 244 | 28 (11.5) |
To convert LDL-C values from mg/dL to mmol/L, divide by 38.67.
LDL-C = low-density lipoprotein cholesterol
FH = familial hypercholesterolemia
FH genetic variants, LDL-C exposure, and risk for CAD
We first conducted a standard case-control study of CAD in order to provide comparison to prior sequencing-based population studies of FH variant carriers.4,6 We identified 34,932 CAD cases. A majority of cases (29,300; 84%) were prevalent at the time of enrollment with a mean time from first CAD code to enrollment of 7.6 ± 4.9 years. For incident cases, the mean time from enrollment to the date of the first CAD code was 2.0 ± 1.5 years. We compared cases to 291,408 controls defined as having no codes suggestive of CAD documented across the full span of EHR data. All traditional risk factors were more prevalent among cases compared to controls (Table III in the Data Supplement). The odds ratio (OR) for CAD among FH carriers was 1.7 (95% confidence interval [CI], 1.4–2.0). The OR for premature CAD (male <55 and female <65) was 3.0 (95% CI, 1.7–5.0), consistent with other population studies4,6 (Figure I in the Data Supplement). When adjusting for LDL-C using the first available measurement, the risk attenuated but remained significant for all CAD (OR, 1.4; 95% CI, 1.2–1.6) and for premature CAD (OR, 2.1; 95% CI, 1.2–3.7).
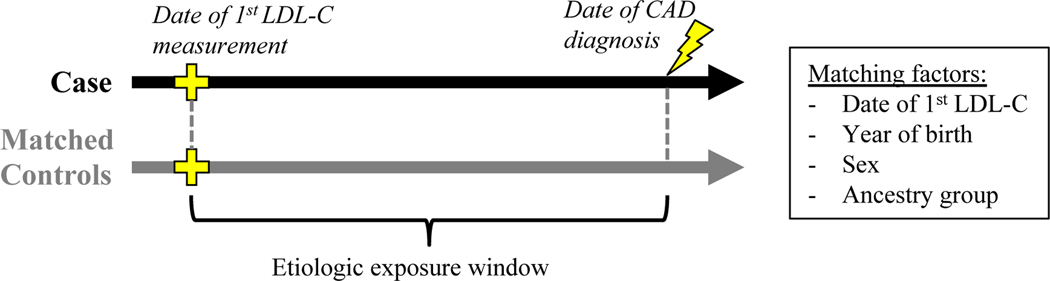
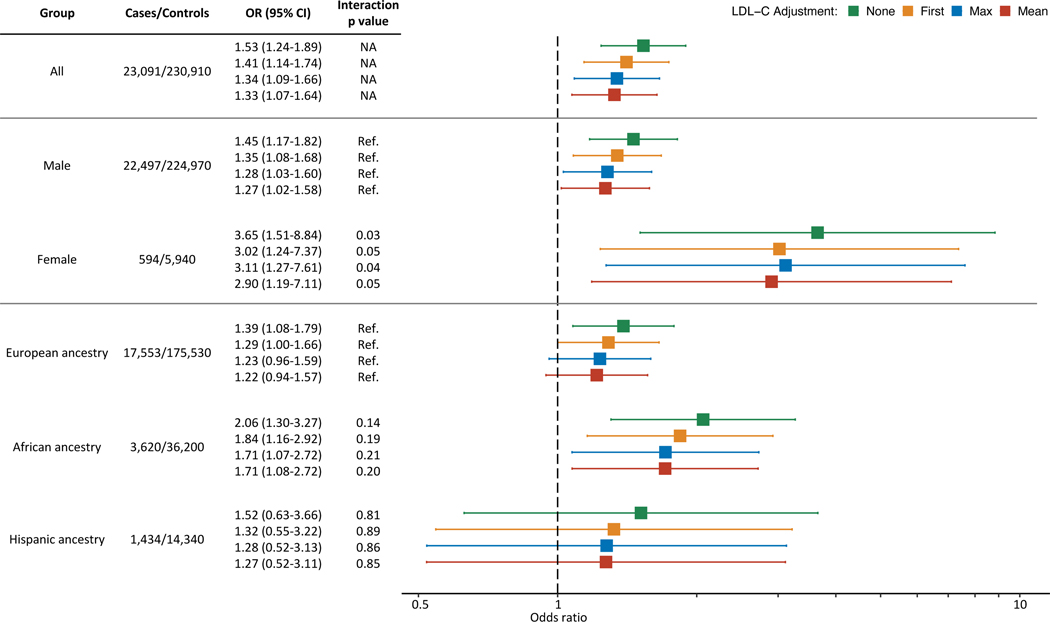
We next conducted a nested case-control study10 designed to measure the risk of CAD while adjusting for longitudinal LDL-C exposure. Cases were restricted to those with ≥1 LDL-C measurement prior to the first diagnosis of CAD (n = 23,173). The median number of prior measurements was 6 (IQR 2–12), and the median span of prior measurements was 49 months (IQR 12–100). Both FH variant carriers and non-carriers had similarly extensive prior LDL-C data (Figure II in the Data Supplement). For each case, we matched 10 random controls, matching on date of first LDL-C, sex, year of birth, and ancestry. We used the principle of incidence density sampling in order to allow measurement of LDL-C exposure over matched etiologic exposure windows for all subjects in a given set (Figure 3). Three LDL-C metrics over the exposure window were considered: first (earliest available measurement), max (highest observed measurement during the exposure window), and mean (average of all LDL-C observed during the exposure window). In total, 23,091 cases (99.6%) were successfully matched to 10 controls (Table 3). The OR for CAD among FH variant carriers was 1.53 (95% CI, 1.24–1.89). When adding an adjustment for the first, the maximum observed, or the mean LDL-C prior to the index date, the OR progressively attenuated, but the risk among FH variant carriers remained significant (Figure 4, Table IV in the Data Supplement). We observed the same pattern of incomplete attenuation when analyzing the subset of matched sets restricted to incident cases occurring after enrollment (Figure III in the Data Supplement). In an additional sensitivity analysis, we assessed the impact of using alternative approaches to statin correction (see Methods in the Data Supplement). Our results were robust across each approach, which included no statin correction, a more aggressive statin correction, a less aggressive statin correction, and a variable statin correction based on LDL-C level (Table V in the Data Supplement).
Figure 3.
Illustration of case-control sets with matched etiologic exposure windows. Incidence density sampling was used to generate matched sets for the nested case-control study. For each case, the index date was set to the date of the first CAD code. Any subject with no CAD codes before or within 1 month after the index date was eligible to serve as a control, and 10 random controls were selected, matching on the date of the first LDL-C measurement, the year of birth, sex, and ancestry. FH = familial hypercholesterolemia. CAD = coronary artery disease. LDL-C = low-density lipoprotein cholesterol.
Table 3.
Characteristic of the nested case-control cohort
| Characteristic | CAD cases | Matched Controls |
|---|---|---|
|
| ||
| Demographics | ||
| n | 23,091 | 230,910 |
| Male | 22,497 (97.4) | 224,970 (97.4) |
| Age at enrollment (years) | 66.3 ± 9.1 | 66.3 ± 9.0 |
| Ancestry group | ||
| African | 3,620 (15.7) | 36,200 (15.7) |
| Asian | 144 (0.6) | 1,440 (0.6) |
| European | 17,553 (76.0) | 175,530 (76.0) |
| Hispanic | 1,434 (6.2) | 14,340 (6.2) |
| Unclassified | 340 (1.5) | 3,400 (1.5) |
| Lipid Data | ||
| Age at first LDL-C (years) | 57.3 ± 9.0 | 57.2 ± 9.0 |
| First LDL-C to index date (years) | 5.7 ± 4.6 | 5.7 ± 4.5 |
| LDL-C (mg/dL) | ||
| First | 131.6 ± 42.5 | 125.2 ± 38.5 |
| Maximum prior to index date | 164.0 ± 54.8 | 151.0 ± 48.5 |
| Mean prior to index date | 130.7 ± 36.5 | 124.1 ± 32.9 |
| Medical history | ||
| Hypertension | ||
| prior to first LDL-C | 11,447 (49.6) | 93,759 (40.6) |
| prior to index date | 17,938 (77.7) | 148,201 (64.2) |
| Diabetes | ||
| prior to first LDL-C | 5,634 (24.4) | 33,379 (14.5) |
| prior to index date | 9,438 (40.9) | 61,175 (26.5) |
| Tobacco | ||
| prior to first LDL-C | 4,115 (17.8) | 32,561 (14.1) |
| prior to index date | 8,320 (36.0) | 62,993 (27.3) |
| Statin use | ||
| prior to first LDL-C | 3,882 (16.8) | 29,076 (12.6) |
| prior to index date | 14,644 (63.4) | 113,023 (48.9) |
| FH variant carrier | 103 (0.4) | 651 (0.3) |
| Case type | ||
| Prevalent cases | 17,642 (76.4) | NA |
| Index date to enrollment (years) | 5.7 (4.1) | NA |
| Incident cases | 5,449 (23.6) | NA |
| Enrollment to index date (years) | 2.0 (1.5) | NA |
Values are n (%) or mean ± SD. To convert LDL-C values from mg/dL to mmol/L, divide by 38.67.
CAD = coronary artery disease
LDL-C = low-density lipoprotein cholesterol
FH = familial hypercholesterolemia
NA = not applicable
Figure 4.
Association Between FH Variants and CAD with Adjustments for Historical LDL-C Exposure. Risk of coronary artery disease (CAD) associated with familial hypercholesterolemia (FH) genetic variants in the full cohort (top segment) and with stratification by sex (middle segment) and ancestry (bottom segment). Interaction P values are listed where appropriate, and “Ref.” denotes the reference group. Odds ratios (OR) were estimated using logistic regression, adjusting for the indicated low-density lipoprotein cholesterol (LDL-C) metric in addition to the nested case-control matching factors, tobacco use, hypertension, diabetes, statin prescription, and number of LDL-C measurements during the exposure window.
We next tested for modifiers of the CAD risk associated with carrying an FH variant. We found a significant interaction between sex and carrier status (P = 0.03). The interaction remained significant with adjustments for LDL-C (Figure 4). Stratified analyses showed an OR for CAD of 3.65 (CI, 1.51–8.84) among female FH variant carriers and 1.46 (CI, 1.17–1.82) among male carriers (Figure 4). Importantly, female subjects were younger than male subjects on average. We also found that female FH carriers tend to have higher LDL-C than male FH carriers, while female and male non-carriers have relatively similar LDL-C. Statin use and CAD risk factors are less prevalent among female subjects compared to males (Table VI in the Data Supplement). We did not find a statistically significant interaction between ancestry and FH carrier status. Though, we saw a trend towards significance for African ancestry, and stratified analysis showed a higher risk estimate within the African-ancestry group (Figure 4). Notably, MVP subjects with African ancestry tended to be younger than those with European ancestry (Table VII in the Data Supplement).
Lastly, we sought to determine if the incomplete attenuation pattern we observed in this study was primarily driven by subjects with the limited historical LDL-C data. We therefore generated matched sets of subjects with extensive LDL-C data. In a matched cohort requiring ≥5 LDL-C measures spanning ≥5 years prior to the index date (9,786 cases, 97,860 controls) and in a matched cohort requiring ≥10 LDL-C measures spanning ≥10 years (3,615 cases, 36,150 controls), we did not observe any notable differences in the degree of attenuation of the risk for CAD (Tables VIII and IX in the Data Supplement).
Discussion
In this study, we aimed to determine if the longitudinal LDL-C exposure observed in medical records can account for the increased CAD risk among carriers of FH genetic variants. We adopted a nested case-control design and carefully matched the etiologic exposure window of case-control sets using the principal of incidence density sampling. We showed that adjusting for longitudinal LDL-C exposure using multiple measurements does not fully attenuate the CAD risk associated with carrying an FH variant, even when extensive LDL-C records are available.
We found evidence of a modification of effect of FH variant carrier status by sex. Among female subjects, the CAD risk was higher with and without LDL-C adjustment. This difference may be due to less survival bias among the female participants, who were younger than the male participants and had fewer risk factors. Other sex differences may also contribute. For example, across childhood and adolescence, untreated girls with FH demonstrate consistently higher LDL-C levels than untreated boys,11 and adult women with FH may be undertreated compared to men.12 We observed patterns in MVP consistent with these prior findings, but additional studies are needed to better understand sex differences while accounting for several potential confounders.
A strength of MVP is the genetic diversity, which is more reflective of the U.S. population than European biobanks. To our knowledge, this study is the largest to date to estimate the CAD risk associated with FH variant carrier status among persons with significant African ancestry. We found that carrying an FH variant conferred greater CAD risk among this group compared to subjects of European ancestry. This difference may reflect selection biases that occur with stratification. However, racial disparities in the treatment of FH may contribute. For example, in an analysis of self-reported race/ethnicity in the CASCADE-FH registry, U.S. Blacks were more likely to be undertreated compared to white patients.12 In our cohort, statin use among FH carriers of African and European ancestry was similar (Supplemental material Table S7), but additional work is needed to assess timing and adequacy of treatment.
In sum, our observations support the notion that genetic testing adds important predictive value to standard clinical assessment, even when longitudinal LDL-C measures are considered. This finding is consistent with a recently proposed framework that recommends both LDL-C measurement and genetic assessment to identify the highest risk patients.13 Our study suggests that among adults, typical LDL-C monitoring does not optimally stratify subjects by their lifelong exposure to LDL-C. The cholesterol exposure pattern of FH carriers versus non-carriers is most distinct during childhood.14 We hypothesize that much of the excess risk associated with FH variants accumulates during childhood and early adulthood, a time when a majority are not treated. Thus, adult FH carriers and non-carriers who demonstrate similar patterns of LDL-C may have already separated their risk trajectories in the decades prior to LDL-C monitoring.
Pediatric guidelines recommend screening LDL-C in children to identify FH early in life.15 It is possible that if childhood LDL-C data were available, adjustment for LDL-C exposure over a greater fraction of one’s lifetime may supplant the predictive power of FH variant carrier status. However, evaluation of lifelong LDL-C measurements is not currently feasible in most clinical settings, whereas genetic testing is rapidly becoming widely available.
The cost effectiveness of genetic testing for FH remains a debate. Cascade screening is one cost-effective strategy,16 but it is underutilized in the United States.17 Universal screening may ultimately prove cost-effective when considering the possibility of simultaneously testing for actionable genetic variants across multiple syndromes. For example, ~1% of UK Biobank subjects harbor pathogenic variants for FH, hereditary breast or ovarian cancer syndrome, or Lynch syndrome.18 As genetic testing becomes more informative for a wider spectrum of diseases, and as the cost continues to decline, we expect genetic risk assessment to become an integral part of primary prevention. The existence of effective, safe, and inexpensive primary prevention strategies such as lifestyle counseling and statins affords CAD a major advantage in this respect. Efforts are underway within MVP to implement return of actionable results to research participants, and the presence of an FH variant is one such actionable result being explored.
Study Limitations
We note several limitations of our study. First, a majority of the CAD cases are prevalent, occurring up to 20 years prior to enrollment. While we implemented a prospective analysis, our risk estimates still suffer from survivor bias because only prevalent cases that survived to enroll in MVP could be observed. Moreover, MVP participants tend to be older at enrollment and have more CAD risk factors when compared to other biobanks, further enhancing survivor bias. Thus, our study likely underestimates the risk of FH variants. However, underestimating the risk of FH is not expected to alter our main conclusion regarding patterns of risk attenuation.
A second limitation of our study is the use of a genotyping array rather than gene sequencing to identify FH variants. Although the MVP array is designed to detect rare protein-altering variants and known disease-causing variants, we expect to miss some variants that would be identified through sequencing. In particular, we were not able to evaluate for copy number variants, which likely account for 5–10% of FH variants at the LDLR locus.19,20 Based on prior U.S. data4 as well as a recent global meta-analysis,21 we may reasonably estimate the expected prevalence of FH variant carriers in our cohort to be no more than 1 in ~250–300. We observed a prevalence of 1 in 303 in this study. Thus, we expect the number of missed carriers to be quite small and to have minimal impact on our analysis. Corroborating this supposition, we found that our risk estimates for CAD are consistent with other population studies that identified FH carriers through sequencing (Figure I in the Data Supplement).
A third limitation of our analysis is that it does not capture care provided outside of the VA. Lab measurements, prescriptions, and diagnoses that only occurred in non-VA settings may be missed. However, we do not expect such missing data to be substantial or to alter our basic conclusions.
A fourth limitation of our study is that we used extensive prescription data to account for statin use, but we did not account for non-statin LDL-lowering medications. The best approach for adjusting longitudinal LDL-C data for different classes and combinations of medications is unknown and will require future research efforts. Importantly, PCSK9 inhibitors were not available or prescribed in the VA healthcare system for nearly all of the study period.
Lastly, the MVP cohort is predominantly male. Our risk estimates are less precise in women due to a small sample size. Larger studies of FH among women are needed to confirm our findings and to better understand potential sex differences.
In conclusion, FH genetic variants confer significant risk for CAD that is independent of LDL-C exposure as defined by longitudinal measurements in the EHR. We believe that the residual risk associated of FH variants reflects the limitations of clinical phenotyping for capturing genetic risk. Whereas FH variants impact LDL-C exposure continuously throughout life, clinical measurements of LDL-C can only sample a fraction of this exposure.
Supplementary Material
Acknowledgments:
We greatly appreciate the participation of the Million Veterans Program participants and the support of the Million Veterans Program staff.
Sources of Funding:
This work was supported by funding from the Department of Veterans Affairs Office of Research and Development, Million Veteran Program Grants 2I01BX003362 and 1I01BX004821. This publication does not represent the views of the Department of Veteran Affairs or the United States Government.
Non-standard Abbreviations and Acronyms
- CAD
Coronary artery disease
- EHR
Electronic health record
- FH
Familial hypercholesterolemia
- MVP
Million Veteran Program
Footnotes
Disclosures: None
References:
- 1.Versmissen J, Oosterveer DM, Yazdanpanah M, Defesche JC, Basart DCG, Liem AH, Heeringa J, Witteman JC, Lansberg PJ, Kastelein JJP, et al. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ 2008;337:a2423. doi: 10.1136/bmj.a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luirink IK, Wiegman A, Kusters DM, Hof MH, Groothoff JW, de Groot E, Kastelein JJP, Hutten BA. 20-Year Follow-up of Statins in Children with Familial Hypercholesterolemia. N Engl J Med 2019;381:1547–1556. doi: 10.1056/NEJMoa1816454. [DOI] [PubMed] [Google Scholar]
- 3.Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013;34:3478–3490a. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abul-Husn NS, Manickam K, Jones LK, Wright EA, Hartzel DN, Gonzaga-Jauregui C, O’Dushlaine C, Leader JB, Lester Kirchner H, Lindbuchler DM, et al. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science 2016;354. doi: 10.1126/science.aaf7000. [DOI] [PubMed] [Google Scholar]
- 5.Khera AV, Won H-H, Peloso GM, Lawson KS, Bartz TM, Deng X, van Leeuwen EM, Natarajan P, Emdin CA, Bick AG, et al. Diagnostic Yield and Clinical Utility of Sequencing Familial Hypercholesterolemia Genes in Patients With Severe Hypercholesterolemia. J Am Coll Cardiol 2016;67:2578–2589. doi: 10.1016/j.jacc.2016.03.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trinder M, Francis GA, Brunham LR. Association of Monogenic vs Polygenic Hypercholesterolemia With Risk of Atherosclerotic Cardiovascular Disease. JAMA Cardiol 2020. doi: 10.1001/jamacardio.2019.5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturm AC, Knowles JW, Gidding SS, Ahmad ZS, Ahmed CD, Ballantyne CM, Baum SJ, Bourbon M, Carrié A, Cuchel M, et al. Clinical Genetic Testing for Familial Hypercholesterolemia: JACC Scientific Expert Panel. J Am Coll Cardiol 2018;72:662–680. doi: 10.1016/j.jacc.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 8.Gaziano JM, Concato J, Brophy M, Fiore L, Pyarajan S, Breeling J, Whitbourne S, Deen J, Shannon C, Humphries D, et al. Million Veteran Program: A mega-biobank to study genetic influences on health and disease. J Clin Epidemiol 2016;70:214–223. doi: 10.1016/j.jclinepi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Sun YV, Damrauer SM, Hui Q, Assimes TL, Ho Y-L, Natarajan P, Klarin D, Huang J, Lynch J, DuVall SL, et al. Effects of Genetic Variants Associated with Familial Hypercholesterolemia on Low-Density Lipoprotein-Cholesterol Levels and Cardiovascular Outcomes in the Million Veteran Program. Circ Genomic Precis Med 2018;11. doi: 10.1161/CIRCGEN.118.002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Essebag V, Genest J, Suissa S, Pilote L. The nested case-control study in cardiology. Am Heart J 2003;146:581–590. doi: 10.1016/S0002-8703(03)00512-X. [DOI] [PubMed] [Google Scholar]
- 11.Holven KB, Narverud I, van Lennep JR, Versmissen J, Øyri LKL, Galema-Boers A, Langslet G, Ulven SM, Veierød MB, Retterstøl K, et al. Sex differences in cholesterol levels from birth to 19 years of age may lead to increased cholesterol burden in females with FH. J Clin Lipidol 2018;12:748–755.e2. doi: 10.1016/j.jacl.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Amrock SM, Duell PB, Knickelbine T, Martin SS, O’Brien EC, Watson KE, Mitri J, Kindt I, Shrader P, Baum SJ, et al. Health disparities among adult patients with a phenotypic diagnosis of familial hypercholesterolemia in the CASCADE-FH™ patient registry. Atherosclerosis 2017;267:19–26. doi: 10.1016/j.atherosclerosis.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Khera AV, Hegele RA. What Is Familial Hypercholesterolemia, and Why Does It Matter? Circulation 2020;141:1760–1763. doi: 10.1161/CIRCULATIONAHA.120.046961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wald DS, Bestwick JP, Wald NJ. Child-parent screening for familial hypercholesterolaemia: screening strategy based on a meta-analysis. BMJ 2007;335:599. doi: 10.1136/bmj.39300.616076.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011;128 Suppl 5:S213–256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerr M, Pears R, Miedzybrodzka Z, Haralambos K, Cather M, Watson M, Humphries SE. Cost effectiveness of cascade testing for familial hypercholesterolaemia, based on data from familial hypercholesterolaemia services in the UK. Eur Heart J 2017;38:1832–1839. doi: 10.1093/eurheartj/ehx111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad ZS, Andersen RL, Andersen LH, O’Brien EC, Kindt I, Shrader P, Vasandani C, Newman CB, deGoma EM, Baum SJ, et al. US physician practices for diagnosing familial hypercholesterolemia: data from the CASCADE-FH registry. J Clin Lipidol 2016;10:1223–1229. doi: 10.1016/j.jacl.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel AP, Wang M, Fahed AC, Mason-Suares H, Brockman D, Pelletier R, Amr S, Machini K, Hawley M, Witkowski L, et al. Association of Rare Pathogenic DNA Variants for Familial Hypercholesterolemia, Hereditary Breast and Ovarian Cancer Syndrome, and Lynch Syndrome With Disease Risk in Adults According to Family History. JAMA Netw Open 2020;3:e203959. doi: 10.1001/jamanetworkopen.2020.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Ban MR, Hegele RA. Multiplex ligation-dependent probe amplification of LDLR enhances molecular diagnosis of familial hypercholesterolemia. J Lipid Res 2005;46:366–372. doi: 10.1194/jlr.D400030-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Iacocca MA, Wang J, Dron JS, Robinson JF, McIntyre AD, Cao H, Hegele RA. Use of next-generation sequencing to detect LDLR gene copy number variation in familial hypercholesterolemia. J Lipid Res 2017;58:2202–2209. doi: 10.1194/jlr.D079301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beheshti SO, Madsen CM, Varbo A, Nordestgaard BG. Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J Am Coll Cardiol 2020;75:2553–2566. doi: 10.1016/j.jacc.2020.03.057. [DOI] [PubMed] [Google Scholar]
Supplementary References
- 22.Justice AC, Lasky E, McGinnis KA, Skanderson M, Conigliaro J, Fultz SL, Crothers K, Rabeneck L, Rodriguez-Barradas M, Weissman SB, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: A comparison of disease measurement strategies. Med Care 2006;44:S52–60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- 23.Goulet JL, Erdos J, Kancir S, Levin FL, Wright SM, Daniels SM, Nilan L, Justice AC. Measuring performance directly using the veterans health administration electronic medical record: a comparison with external peer review. Med Care 2007;45:73–79. doi: 10.1097/01.mlr.0000244510.09001.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, Brown ST, Freiberg MS, Gibert CL, Goetz MB, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res Off J Soc Res Nicotine Tob 2011;13:1233–1239. doi: 10.1093/ntr/ntr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goulet JL, Brandt C, Crystal S, Fiellin DA, Gibert C, Gordon AJ, Kerns RD, Maisto S, Justice AC. Agreement between electronic medical record-based and self-administered pain numeric rating scale: clinical and research implications. Med Care 2013;51:245–250. doi: 10.1097/MLR.0b013e318277f1ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGinnis KA, Tate JP, Williams EC, Skanderson M, Bryant KJ, Gordon AJ, Kraemer KL, Maisto SA, Crystal S, Fiellin DA, et al. Comparison of AUDIT-C collected via electronic medical record and self-administered research survey in HIV infected and uninfected patients. Drug Alcohol Depend 2016;168:196–202. doi: 10.1016/j.drugalcdep.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calhoun PS, Wilson SM, Hertzberg JS, Kirby AC, McDonald SD, Dennis PA, Bastian LA, Dedert EA, VA Mid-Atlantic MIRECC Workgroup, Beckham JC. Validation of Veterans Affairs Electronic Medical Record Smoking Data Among Iraq- and Afghanistan-Era Veterans. J Gen Intern Med 2017;32:1228–1234. doi: 10.1007/s11606-017-4144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klarin D, Lynch J, Aragam K, Chaffin M, Assimes TL, Huang J, Lee KM, Shao Q, Huffman JE, Natarajan P, et al. Genome-wide association study of peripheral artery disease in the Million Veteran Program. Nat Med 2019;25:1274–1279. doi: 10.1038/s41591-019-0492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golden SE, Hooker ER, Shull S, Howard M, Crothers K, Thompson RF, Slatore CG. Validity of Veterans Health Administration structured data to determine accurate smoking status. Health Informatics J 2020;26:1507–1515. doi: 10.1177/1460458219882259. [DOI] [PubMed] [Google Scholar]
- 30.McGinnis KA, Justice AC, Bailin S, Wellons M, Freiberg M, Koethe JR. High concordance between chart review adjudication and electronic medical record data to identify prevalent and incident diabetes mellitus among persons with and without HIV. Pharmacoepidemiol Drug Saf 2020;29:1432–1439. doi: 10.1002/pds.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Barradas MC, McGinnis KA, Akgün K, Tate JP, Brown ST, Butt AA, Fine M, Goetz MB, Graber CJ, Huang L, et al. Validation for using electronic health records to identify community acquired pneumonia hospitalization among people with and without HIV. Pneumonia Nathan Qld 2020;12:6. doi: 10.1186/s41479-020-00068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu F, Kent WJ, Clawson H, Kuhn RM, Diekhans M, Haussler D. The UCSC Known Genes. Bioinformatics 2006;22:1036–1046. doi: 10.1093/bioinformatics/btl048. [DOI] [PubMed] [Google Scholar]
- 33.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, et al. A global reference for human genetic variation. Nature 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Do R, Stitziel NO, Won H-H, Jørgensen AB, Duga S, Angelica Merlini P, Kiezun A, Farrall M, Goel A, Zuk O, et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature 2015;518:102–106. doi: 10.1038/nature13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, Maglott DR. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res 2014;42:D980–985. doi: 10.1093/nar/gkt1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Wu C, Li C, Boerwinkle E. dbNSFP v3.0: A One-Stop Database of Functional Predictions and Annotations for Human Nonsynonymous and Splice-Site SNVs. Hum Mutat 2016;37:235–241. doi: 10.1002/humu.22932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med Off J Am Coll Med Genet 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen W-M. Robust relationship inference in genome-wide association studies. Bioinforma Oxf Engl 2010;26:2867–2873. doi: 10.1093/bioinformatics/btq559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang H, Hui Q, Lynch J, Honerlaw J, Assimes TL, Huang J, Vujkovic M, Damrauer SM, Pyarajan S, Gaziano JM, et al. Harmonizing Genetic Ancestry and Self-identified Race/Ethnicity in Genome-wide Association Studies. Am J Hum Genet 2019;105:763–772. doi: 10.1016/j.ajhg.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet Lond Engl 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 43.Pearce N Analysis of matched case-control studies. BMJ 2016;352:i969. doi: 10.1136/bmj.i969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.