Abstract
Additive manufacturing of polymers is gaining momentum in health care industries by providing rapid 3D printing of customizable designs. Yet, little is explored about the cytotoxicity of leachable toxins that the 3D printing process introduced into the final product. We studied three printable materials, which have various mechanical properties and are widely used in stereolithography 3D printing. We evaluated the cytotoxicity of these materials through exposing two fibroblast cell lines (human and mouse derived) to the 3D-printed parts, using overlay indirect contact assays. All the 3D-printed parts were measured toxic to the cells in a leachable manner, with flexible materials more toxic than rigid materials. Furthermore, we attempted to reduce the toxicity of the 3D-printed material by employing three treatment methods (further curing, passivation coating, and Soxhlet solvent extraction).
The Soxhlet solvent extraction method was the most effective in removing the leachable toxins, resulting in the eradication of the material's toxicity. Passivation coating and further curing showed moderate and little detoxification, respectively. Additionally, mechanical testing of the materials treated with extraction methods revealed no significant impacts on its mechanical performances. As leachable toxins are broadly present in 3D-printed polymers, our cytotoxicity evaluation and reduction methods could aid in extending the selections of biocompatible materials and pave the way for the translational use of 3D printing.
Keywords: additive manufacturing, biocompatibility, cytotoxicity, delamination, passivation, Soxhlet extraction, stereolithography, further curing
Introduction
3D printing has broad applications in additive manufacture1–4 as it enabled the rapid prototyping of complex 3D parts through computer-aided precision and cost-effective manufacturing.5,6 Lately, 3D printing has emerged in the biological and biomedical field7–9 and has shown a great potential for fabricating 3D polymers for bioscaffolds,10,11 biodevices,12,13 and medical implants.14–17 The orthopedic transplant, explicitly customized to a patient's 3D anatomy of the pelvic bone, is a well-commercialized example. It can be 3D printed in the clinic in just hours,18 in contrast to weeks with traditional manufacturing techniques (e.g., injection molding).19
Despite 3D printing's continued growth, concerns have been raised about its cytotoxicity that risks its biological and biomedical applications.20–22 Although various photocurable polymers have been explored in the 3D printing of bioapplications, only a few are certified biocompatible (i.e., based on ISO 10993-5:2009 or USP class testing standards).23 Moreover, these polymers are certified safe only with traditional manufacturing methods, such as subtractive manufacturing or formative manufacturing,24–26 which employ a well-optimized curing process to ensure complete homogeneous polymerization.
Unlike the traditional manufacturing methods, 3D printing is branded with rapid prototyping, which inevitably leads to rushed curing and subsequently incomplete polymerization.23,27–31 Rush curing requires the use of excess additives (e.g., metal photoinitiators), which are often cytotoxic and sometimes undisclosed.6,32,33 Due to incomplete polymerization, these excess additives and uncrosslinked cytotoxic monomers or oligomers remain embedded in the final products.23,34,35
Leaching of these cytotoxins can render the 3D print cytotoxic,20,32,36 causing cell apoptosis/necrosis.37 In 3D-printed implants, these leachable toxins further cause glossitis, stomatitis, and pulpitis.38 Such cytotoxicity concerns may not be significant with traditional manufacturing methods due to its vastly different curing conditions. Hence, the cytotoxicity of the polymers that are made through rushed curing methods should be re-evaluated with care in the new context of 3D printing.
Recent studies have started to address these cytotoxicity issues of the 3D-printed parts and explore detoxification methods. These methods often focus on furthering the polymerization through postprinting treatments but have their own limitations.39,40 For instance, heating the 3D-printed part to 233°C was shown to reduce the cytotoxicity of the polymer to cancer cell cultures. Still, it adversely affected the polymers' appearance by impairing the materials' transparency.20,41 Postprint UV curing helped to further the polymerization but was limited to UV-opaque polymers.35
Alternatively, an emergent extraction method, using supercritical carbon dioxide, showed more effective removal of cytotoxic impurities from 3D-printed implants.42 This pointed out an interesting new direction using the extraction approach, although their cytotoxicity and mechanical evaluations were incomplete.42 Furthermore, the use of supercritical carbon dioxide treatments requires specialized equipment and a sophisticated setting. Thus, simple and accessible methods to evaluate and reduce the 3D-printed polymers' cytotoxicity are desired by bioapplications.
In this study, we characterized three accessible post-treatment methods and compared their effectiveness in reducing the leachable cytotoxicity of 3D-printed polymers. Various acrylic-based resins widely used in stereolithography (SLA) 3D printing were selected. The in vitro cytotoxicity was characterized with two fibroblast cell lines, following the ISO-10993-5 indirect-contact cytotoxicity assay.43
Furthermore, we evaluated the effects of three novel detoxification techniques on the mechanical properties of the 3D-printed polymers. The tested regions and SLA are among the most popular polymer materials and printing techniques due to their balanced advantages in mechanical properties, cost-effectiveness, and printing resolutions.8,44,45 Our study provided a systematic strategy to effectively establish and reduce these 3D-printed polymers' cytotoxicity, paving the way for the translational use of 3D bioprinting and broadening the applications for biofabrication.
Materials and Methods
Materials
Stratasys Objet TangoPlus (FLX930) and Objet VeroClear (RGD810) 3D-printed materials were tested. The Dow Corning SYLGARD 184 Silicone Encapsulant Kit (part number 184 SIL ELAST KIT 0.5KG) was used to prepare a Polydimethylsiloxane (PDMS) passivation material. Ethyl Alcohol, 200 Proof (cat. no. 111000200; Pharmco-aaper, CT) was used during sterilization of the polymers before cytotoxicity testing as well as an extraction solvent. NCTC clone 929 [L cell, L-929, a derivative of Strain L] (ATCC CCL-1, VA) and HEL 299 (ATCC CCL-137) mammalian cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM; cat. no. SH3002201; HyClone, MA).
Methylcellulose overlay medium was prepared by dissolving 8.4 g methylcellulose powder 4000 cP (cat. no. 9004-67-5; Sigma-Aldrich, MO) into 500 mL DMEM supplemented with 10% fetal bovine serum (cat. no. SH3008803; HyClone) at 4°C until homogeneous. A 0.1% Crystal Violet stain solution was prepared by dissolving 1.2 g Crystal Violet powder (cat. no. 3039294; EMD Millipore, MA) into 286 mL ethanol. Once dissolved, 37 mL of formaldehyde (cat. no. 7230673; EMD Millipore) and 676 mL sterile water were added to the solution and stir mixed until homogeneous.
SLA 3D-printing
Polymer materials were printed in two shapes: ring and dog bone. The ring shape was used for the cytotoxicity test (10 × 3 × 5 mm diameter × thickness × height), and the dog-bone shape was used for the mechanical test (40 × 5 × 2 mm length × neck × thickness). First, STL files of both shapes were designed in AutoCAD software (AutoDesk) and SLA printed using Objet 260 Connex3 3D printer (Stratasys). Three Stratasys prepolymer materials were used, VeroClear (RGD), TangoPlus (FLX), and a 50:50 (vol/vol) mixture (HYB) of the two (Fig. 1). After printing, the materials were cleaned in deionized (DI) water (3 × 2 h) to remove the support material residue.
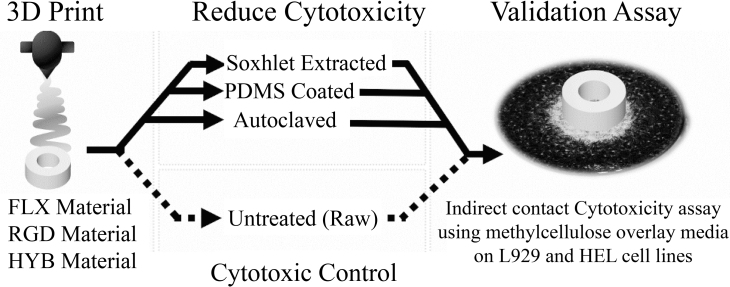
FIG. 1.
Experimental flowchart. Left: Two materials (TangoPlus [FLX] and VeroClear [RGD]) and a 50:50 (vol/vol) mixture (HYB) of the two were 3D printed in the form of millimeter-sized plastic rings. Center: The rings were treated using three different methods to reduce cytotoxicity. Untreated (Raw) materials were used as control. Right: An indirect-contact cytotoxicity assay was used to evaluate the treatment method on two different fibroblast cell lines (HEL and L929). FLX, Flexible; HYB, Hybrid; PDMS, polydimethylsiloxane; RGD, Rigid.
Detoxification treatments
Printed ring structures were treated with one of the following methods to reduce cytotoxicity: steam autoclaving, PDMS passivation, and Soxhlet solvent extraction. Untreated ring structures served as a control group (Fig. 1).
Steam autoclaving
The 3D-printed rings were autoclaved at 121°C for 20 min, followed by a 30-min exhaust cycle to remove the trapped stream.
PDMS passivation
PDMS was prepared as previously described.46,47 Briefly, a prepolymer solution of 10:1 ratio of PDMS: curing agent was prepared and degassed thoroughly. The 3D-printed rings were immersed into the PDMS until all areas of the rings were covered by PDMS. Then, PDMS was cured for 2 h at 80°C, followed by 12-h curing at room temperature. After curing, the thickness of the coating was measured using a Vernier caliper (Neiko, China). The rings were sterilized under UV light for 30 min in a biosafety hood for further use.
Soxhlet solvent extraction
The treatment was carried as previously described.48 Briefly, 3D-printed rings were treated in a 500-mL Soxhlet extraction apparatus using ethanol (200 Proof) for 24 h at 30 min per cycle (approx.) speed. After the Soxhlet extraction, the rings were air dried in the biosafety cabinet.
Cell culture and cytotoxicity assay
Two cell lines, L929 (mouse fibroblast) and HEL (human fibroblast), were used for the cytotoxicity assay. L929 cells were maintained as described earlier.46 The cells were passaged at least three times to ensure cell-line stability before they were used for the assay. HEL cells were cultured following the ATCC's product sheet for HEL 299 (ATCC® CCL-137™), similar to the culture method as the L929 cells.
For the cytotoxicity assay, each cell line was cultured in six-well plates. When the cells were 100% confluent, the culture medium was replaced with 1.5 mL methylcellulose to condition the culture. All 3D-printed polymer rings—treated and untreated—were immersed in 70% (v/v) ethanol for 2 h and 30 min treated with UV in a biosafety cabinet. Each ring was gently placed at the center of a methylcellulose-conditioned culture well. The subsequent cultures were live recorded for 72 h, every 20 min, using an environment-controlled live-cell imaging system (Juli™Stage; NanoEnTek, Inc., Korea). The cell death area around the polymer was analyzed from the time-lapse images at different time points, using the software ImageJ (V1.53a; NIH).
Furthermore, after 72 h of incubation, the rings and the methylcellulose overlay were carefully removed. For cytotoxicity analysis, cells were incubated at room temperature with 1 mL 0.1% Crystal Violet stain solution for 45 min. The Crystal Violet was then removed, and the wells were washed thrice with sterile DI water and air dried.
Mechanical testing
Mechanical properties of Soxhlet extraction samples were tested. Only the Soxhlet group was chosen as it showed promising results in the cytotoxicity study. Half of the dog bone-shaped samples were treated with Soxhlet, and the other half served as untreated (raw) controls from all three material types. After extraction, the specimens were air dried in the biosafety cabinet and tested in tension along the machine's direction at room temperature and humidity.
On Soxhlet-treated and untreated dog bone-shaped 3D-printed specimens, Quasi-static tensile tests were performed in displacement-controlled mode at 2 mm/min (corresponding to 5%/min) on Intron 5542 Dynamic Mechanical Analysis Machine equipped with 5 kN load cell and grips (Anton-Paar, VA), and the strain was measured using a video-extensometer Shimadzu DVE 201. For calibration, the elastic modulus of poly-lactic acid (PLA) was determined from these settings, which was ∼2.9 GPa, which agreed with previously reported data.49
The resulting stress–strain curves were used to derive the mechanical property data. The elastic modulus was determined from the slope of the stress–strain curve in the linear strain region 0.025–0.050, 0.05–0.6, and 0.1–0.8 for rigid (RGD), hybrid (HYB), and flexible (FLX) specimens, respectively. The yield strength was determined as the stress of the intersection point between the stress–strain curve and the proportional line offset by 0.2% strain. The toughness was calculated by using the area underneath the stress–strain curve. Note: the toughness value for untreated RGD was underestimated as the maximum loading has been achieved before the specimen could fail. The resilience was calculated by using the area underneath the proportional fragment of the stress–strain curve. From each type, four specimens were tested as replicates.
SEM imaging was performed as previously mentioned46 for the untreated and Soxhlet extraction-treated samples of RGD and FLX materials.
Data and statistical analyses
Progression of the cell death was quantified using time-lapse imaging of the cell monolayer and Crystal Violet imaging data. Data were reported in two formats: Bar graphs—data reported as the mean ± standard error; Box plot analysis—data reported as mean, median, and interquartile range. The tensile test (stress–strain) data were further analyzed using MS Excel (Microsoft, CA) to obtain elastic modulus, yield strength, resilience, and toughness data of the material.
All statistical analyses were performed using SAS software (SAS Institute, Inc., NC). The data groups were statistically compared using Student's t-test (two-tailed), assuming unequal variances to determine significance probabilities. Probabilities of significance were determined for: *p < 0.05, **p < 0.01, and ***p < 0.001. The effect of 3D printed material types, cell lines, and postprint treatments and their interactions were examined using a two-way ANOVA test. Interaction plots revealed interactions between different factors.
Results and Discussion
3D-printed polymer and material properties
This study employs SLA printing, which uses inkjet heads to deposit tiny droplets of prepolymer resin and polymerize them with selective UV light exposure.50 SLA printers offer many biocompatible and dental materials. Herein, we choose two broadly used acrylic polymers for SLA printing (i.e., VeroClear, TangoPlus) as they are cost-effective, readily available, and have versatile mechanical properties.51,52 Besides, the leaching kinetics of their toxins shows moderate-level cytotoxicity, which is representative of similar acrylic polymers that are widely used in SLA printing.23,53 Although their liquid form is considered to be toxic, their polymerized solid form is claimed to be safe34,51,52 and envisioned in many biomedical applications.34
We 3D printed millimeter-sized rings of three test materials; (1) primarily made up of VeroClear,52 (2) primarily made up of TangoPlus,51 and (3) an intermediate of both (50:50 vol/vol mixture of VeroClear and TangoPlus).51,52 VeroClear resulted in a RGD material, whereas TangoPlus resulted in rubber-like FLX materials. The HYB of these two resulted in a semi-FLX material. These outcomes were consistent with the material properties reported by the company.35,51,52
Furthermore, through microscopic inspection of these printed materials (Supplementary Fig. S1), a string pattern of polymer deposition and granule structure from the laser polymerization were revealed. RGD displayed the highest level of granule structures, whereas the FLX displayed the lowest. The granule structure levels are proportional to the crystalline levels, and polymerization extends28 (highest in RGD and lowest in FLX), which also defines the stiffness of the materials. This variation in the polymerization leaves a varied proportion of uncured monomers and unknown additives in the final product,54,55 which would result in different cytotoxicity of the polymer.
Cytotoxicity of untreated materials
Cytotoxicity is often the foremost biocompatibility test for clinical use, as required by the Federal Food and Drug Administration (FDA) and ISO.56,57 In this study, we selected an overlay indirect-contact assay to test the cytotoxicity of our 3D-printed materials, as described in ISO-10993-5.43 We chose this method because it is most suitable for assessing the cytotoxicity from leachable toxins, which spread to the surrounding cells through diffusion.
Two fibroblast cell lines, L929 and HEL, were selected to test the materials' cytotoxicity and compared side by side. L929 and HEL cell lines (mouse fibroblast and a human fibroblast, respectively) are broadly used for similar overlay indirect-contact cytotoxicity assay.43,58–61 Test materials were kept on methylcellulose-covered confluent fibroblast cells (Fig. 2A). The methylcellulose overlay acted as a cushion between the test material and the cell monolayer below to avoid the physical distress acting on the cells while allowing the diffusion of leachable toxins (Fig. 2B). Additionally, studies showed that many biocompatible-labeled polymers failed the cytotoxicity test when tested for >24 h.62 Thus, all our cytotoxicity tests were performed for a prolonged time of 72 h to ensure the full coverage of polymers' cytotoxicity level.
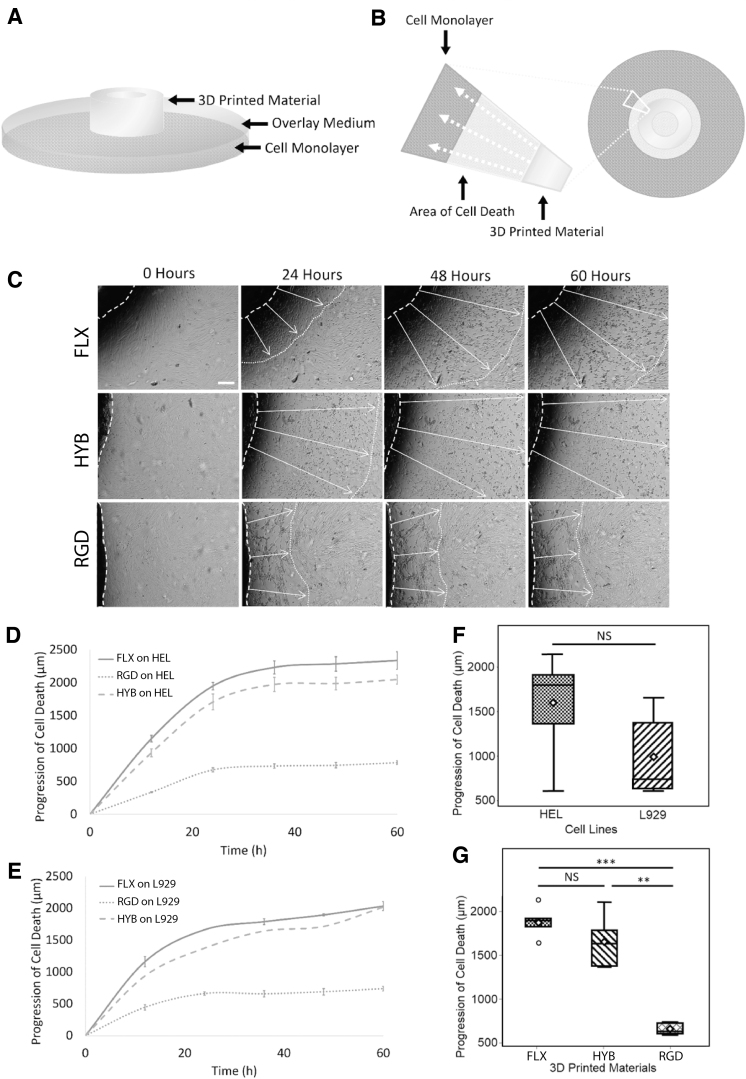
FIG. 2.
Cytotoxicity test of untreated (raw) materials. (A) Assay setup. A methylcellulose overlay media acted as a cushion between the test material and the cell monolayer. (B) Schematics of an area of cell death. Cytotoxins created an area of cell death around the test material as they diffused through the overlay medium in the direction of white arrows. (C) Representative time-lapse images. The progression of cell death spread from the material outward, shown in time-lapse images as white arrows. Scale bar: 250 μm. (D) HEL cell death as a function of time. On the human fibroblast cell line (HEL), the death progression curves showed that the FLX and HYB materials were more cytotoxic than the RGD material. (E) L929 cell death as a function of time. A mouse fibroblasts cell-line (L929) was also used in a second assay, which showed similar trends seen in the HEL cells. Both cell lines' death was slowed down after hour 36. (F) Overall cytotoxicity for both the cell lines showed at hour 36; the human fibroblasts were more sensitive to the materials' cytotoxicity, although insignificant. (G) Collective cytotoxicity of all three materials at hour 36 showed that RGD was significantly less toxic than FLX materials (FLX and MIX). Three samples were recorded in eight different locations each for D–G results.
The overlay indirect-contact assay showed substantial cytotoxicity from all three materials and both cell lines (Fig. 2). The cytotoxicity level was quantified by measuring the area size of the cell death zone around the polymer (Fig. 2C) at different time points. In the 72-h long time-lapse imaging, necrosis-like cell death was easily identified in darker color phase and fasciculate morphology with amorphous granular debris compared with the healthy cells with bright-phased color and smooth cell membrane (Fig. 2C, Supplementary Fig. S2, and Supplementary Video S1).
The cell death zone formed a coaxial circle around the polymer ring. This death zone continuously progressed outward, indicating cytotoxic leachates diffused into the overlay media (Fig. 2B). The perpendicular distance of the cell death zone's frontier to the edge of the 3D-printed ring was measured as the progression of cell death, representing the leachate cytotoxicity level.
The progression of cell death (all materials and cell lines) as a function of time showed a consistent logarithmic increase (Fig. 2D, E), which is similar to the previously published studies.63,64 This result indicates the presence of leachable toxins in the 3D-printed polymers. Additionally, similar trends in the progression profiles across all cell line and material type conditions, which slowed down at around hour 36 (Fig. 2D, E), suggest similar diffusion kinetics of the toxins and maximum observed toxicity of the material in the given testing time.
Two cell lines performed similarly in the cytotoxicity test (Fig. 2D–G), with the human cell line being more sensitive than the mouse one. Overall, the HEL cell line exhibited a higher sensitivity toward cytotoxicity than the L929 cell line by showing consistently 50% larger areas of cell death (Fig. 2C). More cell lines will be tested in the follow-up studies to determine whether this sensitivity difference can be attributed to the species or not.
The cytotoxicity showed polymer material dependency, as further compared through t-tests. Among different polymers, FLX and HYB had similar cytotoxicity, which was significantly (∼75%) higher than RGD (Fig. 2G). This result indicates that significantly more leachable toxins were in the FLX and HYB than RGD, and the diffusivity of the leachates was not diluted or blocked by the presence of RGD in the HYB material. We attribute this variation in leachability to the crosslinking density difference between FLX and RGD.
In polymer chemistry, it is common to lower the mechanical strength of polymers by reducing the crosslinking density.54,55 Although not disclosed, we believe FLX and RGD were also engineered using this strategy: FLX is a rubber-like material with a lower mechanical strength than RGD, likely by bearing a lower crosslinking density. This lower crosslinking density leads to more abundant uncrosslinked monomers/oligomers and subsequently higher cytotoxicity in FLX.
The progression of cell death as a function of time further confirmed this phenomenon (Fig. 2D, E), where a similar diffusion profile of toxic molecules showed more significant damage to cells in FLX and HYB compared with RGD. We also noticed that 50:50 HYB behaved much more like FLX than RGD in cytotoxicity. This behavior could be attributed to the structural similarity between two materials that provided a leaching path, while the half-diluted leachable toxins in HYB were still abundant. These results demonstrated that the RGD material has lower cytotoxicity than the FLX material (FLX and HYB).
Polymer treatments and reduced cytotoxicity
We then explored three strategies to reduce cytotoxicity and thus remedy the use of these biomaterials by either removing the leachable toxins or blocking the leaching.
(1) Autoclaving, a standard sterilization method, has been previously reported to further crosslink various polymers by providing high heat and humidity.55,65,66 We utilized this method to further cure the 3D-printed polymer rings, aiming to reduce the uncrosslinked monomers and oligomers. This method has a similar premise to the prolonged UV treatment, which was reported to reduce the degree of uncured monomers and, in extension, its cytotoxicity.62 Visual inspection of autoclaved polymer printouts showed no melting or deformation of the rings after the treatment (data not shown), confirming that autoclaving conditions were safe to the printing materials.
(2) PDMS coating: PDMS is a biocompatible polymer often used to protect surgical tools through protective passivation.67 We coated the 3D-printed polymer rings with a thin PDMS layer (0.1–0.2 mm thickness) to block the pores on the polymer surface and reduce the leaching of toxins into the cell medium.
(3) Soxhlet extraction is an extraction method that reflux solvent in cycles. Soxhlet extraction can dissolve and extract a large amount of compound with limited solubility by avoiding its saturation in a solvent. Through Soxhlet extraction, 3D-printed polymer rings were washed by ethanol for up to 24 h (about 48 reflux cycles). There were no visible defects or swelling in the rings post-treatment.
Post-treatment cytotoxicity was measured using the time-lapse images as described in the previous sections. From the time-lapse images, the best time point for efficient cell survival analysis was determined to be 36 h as the cell death in untreated samples peaked and started to plateau after 36 h (Fig. 2D, E). In untreated (raw) samples, the dead cell zone was coaxially progressing out from the polymer (Fig. 3A: Top Left), as seen by the dark and shrunken apoptotic cells near the polymer's edge (Fig. 3A: Bottom left), whereas the detoxification treatment reduced the cell death zone to virtually zero, which was seen by the bright-colored healthy cell zone near the edge of the polymer (Fig. 3A: Top and Bottom right).
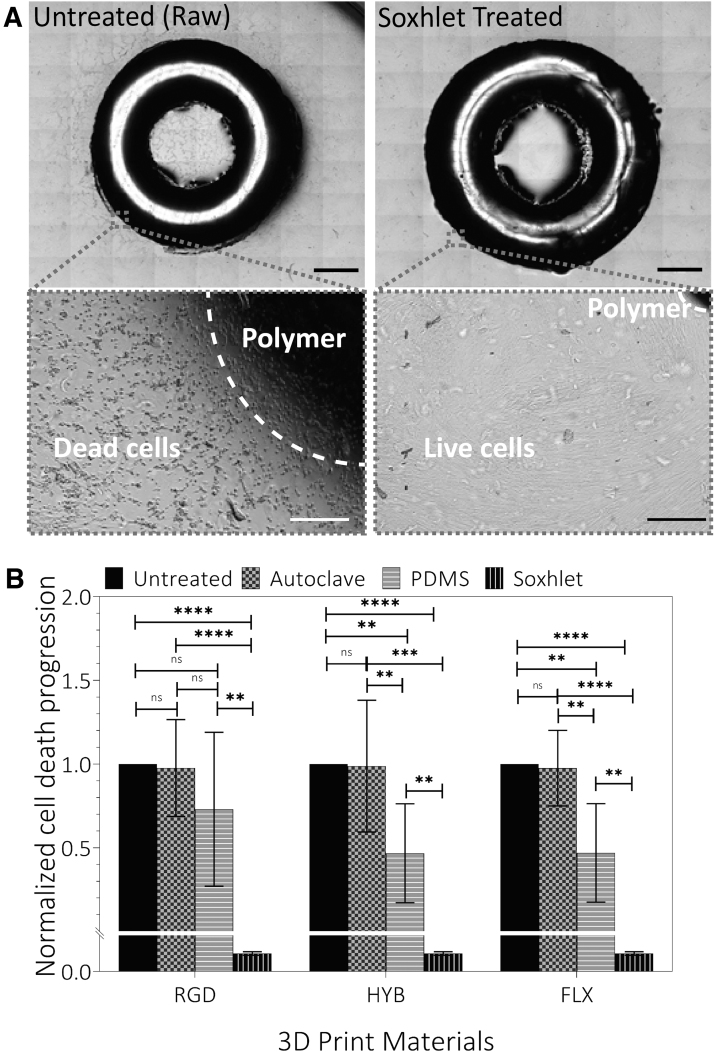
FIG. 3.
Cytotoxicity test of treated materials. (A) Representative stitched snapshots of the indirect contact cytotoxicity assay. Treated samples (Soxhlet extraction) showed drastically less cell death than the untreated (raw) sample. Darker zones indicate the areas of cell death caused by cytotoxicity of the material, while lighter zones show live cells. Both snapshots show RGD material on L929 cells after 36 h. The blowup images (bottom) show typical apoptotic (shrink) and healthy cell morphologies that led to dark and bright tones, respectively, in the stitched images. Scale bars: Top: 2 mm; Bottom: 0.1 mm. (B) Progression of cell death after 36 h, measured as the distance from each treated and untreated ring, showing little detoxification with autoclaved samples, partial detoxification with PDMS-coated samples, and complete detoxification with Soxhlet extracted samples. The areas of cell death were normalized to the control. Note: Y-axis was segmented to show the Soxhlet extraction value as it was close to zero. Statistical analysis: Student t-test. **p < 0.01; ***p < 0.001; ****p < 0.0001. Sample size: Three samples were recorded in eight different locations each. NS, not significant; PDMS, polydimethylsiloxane.
In effect, all three treatment methods have demonstrated various degrees of detoxification effect (Fig. 3B). The Soxhlet extraction demonstrated the most effective detoxification effect among all treatment methods by reducing the cytotoxicity almost entirely (reduced by ∼100% for all the materials) (Fig. 3B). Time-lapse imaging showed no visible cell death around the Soxhlet-extracted materials so that cells continued to grow unaffected by the ring's presence (Fig. 3A, B).
Leaching of cytotoxins (uncured monomer/oligomer and additives) contributes majorly to these materials' cytotoxicity. They are often low in solubility and thus easy to saturate the solvent in the static extraction method. On the other hand, circulation in the Soxhlet extraction recondenses the solvent, avoids saturation, and can keep extracting the leachable cytotoxins until almost completely gone. Thus, all treated materials unanimously showed close to zero cytotoxicity during biological testing.
Besides, complete removal of toxic chemicals implies long-term safety of the material as well. Despite the excellent performance, Soxhlet extraction has a few technical constraints such as time-consuming drying and extraction process, sample-size limitations, and low scalability.68 However, by establishing a commendable performance, we envisioned a broader use of this method for 3D print materials' detoxification, which would enable future technic adoption of this method to eliminate these limitations.
The second effective treatment method was PDMS passivation, which showed moderately significant detoxification for FLX/semi-FLX materials (FLX/HYB: ∼53% reduction for both materials) and insignificant detoxification for RGD material (RGD: 26% reduction) (Fig. 3B). The detoxification effect of PDMS passivation was better for FLX and HYB, likely because PDMS blocked both the leaching of additives and the uncured monomers/oligomers. However, the blocking effect was limited by the polymer's property.
In contrast to FLX and HYB, PDMS treatment showed no significant reduction of cytotoxicity in the RGD material, which can be attributed to the mismatch of material properties (e.g., surface and mechanical) between PDMS and the material. PDMS coating was meant to block the small pores on the 3D-printed material's surface by establishing compliant passivation. RGD's elastic modulus (refer to Treatment Influence on the Mechanical Properties section) is 1000-fold higher than the PDMS (0.5–3.7).69
The mismatch of elastic modulus and thermal coefficient70 between PDMS and RGD could undermine this material adhesion71 and introduce delamination during the heated curing process. In contrast, FLX's mechanical property, similar to PDMS, could aid in the proper conformation of the PDMS–3D material interface. Besides, we speculate that the lower surface energy72,73 of the hydrophobic PDMS layer74 could prevent its strong adhesion on the hydrophilic surface of the RGD.75 This hypothesis was consistent with the delamination and gaps of PDMS coating on RGD as frequently observed in our experiments (data not shown). When placed in the media culture, these phenomena could create coating defects and gaps, allowing the leaching of toxins.
Autoclaving was the least effective by showing marginal detoxification (∼1–2% reduction for all materials), which was statistically nonsignificant compared with the untreated materials. The incompetence of autoclaving was not a complete surprise, as the uncured monomers and oligomers may not be the only toxicity sources. The additives, which are abundant in epoxy curing, cannot be reduced using this method. Furthermore, the employed high heat and pressure might have also introduced an aging effect to the materials and broken surface polymers into leachable oligomers.
The above measurements were based on time-lapse imaging as a novel approach to study cytotoxicity, requiring no additional steps (e.g., staining). We also compared it with the conventional method, such as the live/dead assay using Crystal Violet staining (Supplementary Fig. S3). In general, the Crystal Violet method showed slightly higher cell death. For example, with the Soxhlet-treated samples, Crystal Violet staining showed a small area of cell death near the ring.
We believe this seemingly higher death was due to the process of manually removing the ring, which induced undesired cell detachment. For instance, all cell death with Soxhlet-extracted rings was directly located underneath the rings (Fig. 3B). Nevertheless, the trend of cytotoxicity reduction through all three methods was similar in both evaluation studies, which further validated the reduction of cytotoxicity with our methods.
Synergic effects of treatment methods with polymer types and cell lines
In the previous sections, we demonstrated that the cytotoxicity was cell line independent but material and treatment dependent. To identify any synergic effects of these conditions (i.e., treatment methods, cell lines, material types), we conducted interaction analysis (Fig. 4) based on the progression of cell death and time-lapse imaging.
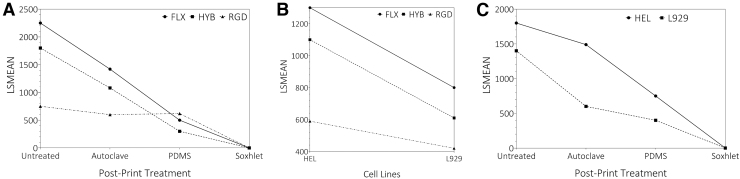
FIG. 4.
Interaction plots of cytotoxicity (in terms of the progression of cell death zone) across material types, treatment methods, and cell lines. (A) Treatments versus materials. FLX and HYB materials seem to have a similar trend for each treatment, whereas the RGD material shows less response to the PDMS coating. The PDMS-coated treatment was more effective (although partially) with FLX and HYB to reduce cytotoxicity than RGD. Finally, the Soxhlet extraction treatment significantly reduced cytotoxicity in all three material types, proving to be a highly effective treatment method. (B) Cell lines versus Materials. Similar trends were observed between all the materials between both the cell lines. RGD was significantly less toxic compared with the HYB and FLX. (C) Treatments vs. cell lines. Similar trends between HEL and L929 cell lines were seen across material treatment methods. Between two cell lines, HEL was slightly more sensitive to the cytotoxin, although insignificant. LSMEAN, Least-Square Means.
Provided the same cell line and treatment methods, FLX and HYB showed overall similar cytotoxicity, which was usually higher than the RGD material. Among the polymer materials, a significant difference was detected between the RGD and FLX material (FLX and HYB) (p = 0.1199 for FLX/HYB, <0.0001 for FLX/RGD, and 0.0002 for HYB/RGD), indicating that the FLX and HYB materials were more similar than RGD. This overall trend is likely attributed to the high crosslinking rate required to increase the polymer rigidness. Therefore, the RGD has a much lower content of uncured monomers/oligomers as the leachable toxins than rubber-like materials (FLX and partially HYB) (Fig. 4B).
Only one interaction was identified, which appeared in polymer versus treatment methods (Fig. 4A). The significantly higher Least-Square Means at PDMS-RGD suggests that PDMS coating had a synergic effect on the RGD material, remarkably different from other materials. Combined with the area of cell death (Fig. 3B), PDMS coating worked very poorly on RGD material. As mentioned earlier, we believe this is due to the mechanical mismatch: RGD is stiff and nonadhesive, while PDMS is soft. This mismatch may lead to ineffective coating and thus the failure in blocking the monomer leaching. This theory is also consistent with the delamination of PDMS coating we frequently observed only with RGD (data not shown).
No interaction was observed with either cell line versus material type (Fig. 4B) or cell line vs. treatment method (Fig. 4C), indicating no synergic effect between these combinations. Provided other conditions were the same, the human cell line (HEL) was more sensitive to the material's cytotoxicity than the mouse cell line (L929) with a p-value (0.051) slightly nonsignificant (Fig. 4B). Thus, either cell line could be a suitable model for cytotoxicity study.
The interaction plots regarding the treatment methods (Fig. 4A, C) demonstrated the same trend as shown in Figure 3B. The autoclaved and PDMS-coated treatments partially reduced the cytotoxicity, whereas the Soxhlet extraction method almost eradicated the cytotoxicity. These results are also consistent with the t-tests against the toxicity level of untreated materials, showing an insignificant reduction effect of autoclaving (p > 0.05), a moderately significant reduction effect of PDMS coating (p = 0.0142), and a significant reduction effect of Soxhlet extraction (p = 0.003). These results support that the Soxhlet extraction treatment is the strongest in detoxification in all conditions.
Treatment influence on the mechanical properties
Because 3D-printed biomedical devices are often used to provide structural and mechanical support, it is crucial to evaluate their mechanical properties.76 Thus, we performed comprehensive tensile tests, aiming to evaluate mechanical impacts by the Soxhlet extraction that removed these monomers/oligomers.
The stress–strain curves of RGD and HYB showed a typical polymer behavior with elastic deformation followed by plastic deformation (Supplementary Fig. S4), whereas FLX showed an interesting tendon-like deformation containing a toe region followed by the linear region. The presence of the toe regions suggests the reorganization process straighten the crimp pattern of FLX polymers.77 However, the stress–strain curve's shape and deformation type remained similar within each material type after the extraction (Supplementary Fig. S4).
Based on the stress–strain curves, four mechanical properties (elastic modulus, yield strength, resilience, and toughness) were derived for all three printing material types (RGD, HYB, FLX) (Fig. 5). The determined values mostly matched their brand names for untreated samples, with RGD being the strongest, HYB being median, and FLX being the weakest (Fig. 5). The only exception was the resilience value, which was slightly higher for HYB compared with RGD.
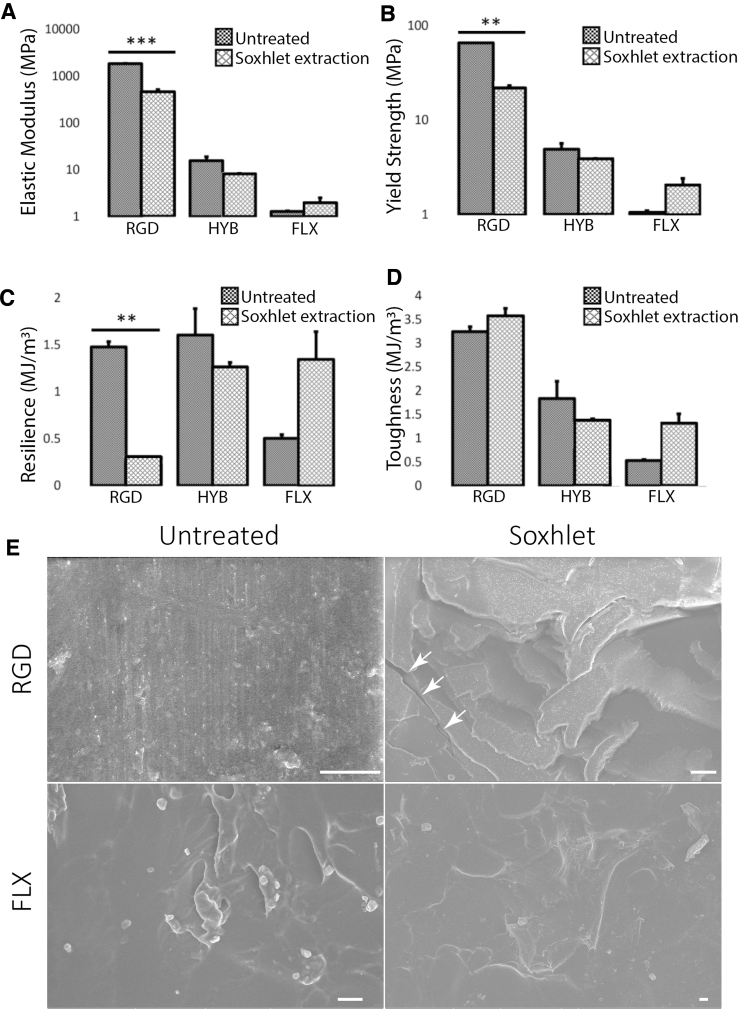
FIG. 5.
The effect of Soxhlet extraction detoxification treatment on the mechanical properties of 3D printed materials, including (A) elastic modulus, (B) yield strength, (C) resilience, and (D) toughness. Three samples were tested for results A–D. The effect is material type dependent. After the Soxhlet extraction, RGD showed a reduction in elastic modulus, yield strength, resilience, and a slight increase in toughness. HYB showed a small reduction in all parameters. FLX showed an increase in all parameters. However, only the changes with RGD are statistically significant (marked with asterisks). The changes after treatments (unmarked) are nonsignificant. (E) SEM images of RGD and FLX polymer surface before (Untreated) and after Soxhlet treatment (Soxhlet). The white arrow in the RGD Soxhlet denotes microfractures. FLX Soxhlet did not show any microdefects after Soxhlet treatment. Scale bars: 10 μm.
On the other hand, after extraction, all four mechanical property values changed. The changing pattern was observed to be material type-dependent (Fig. 5). RGD was the most influenced. For RGD, all values have significantly decreased after the Soxhlet extraction by 1.5–5-folds, except for toughness, which increased after Soxhlet treatment. The roughness value did not change because the machine had reached the maximum loading limit before the fracture point of the untreated RGD material. The SEM inspection (Fig. 5E) showed substantially more defects and microfractures with RGD than FLX. We also observed that a good fraction of RGD samples got delaminated into layers after the Soxhlet extraction.
These results suggested that RGD has undergone significant structural changes and became much more brittle upon the Soxhlet extraction (Supplementary Fig. S3). Very consistently, the interaction plots (Supplementary Fig. S5) also showed a synergy between the treatments and materials, where the Soxhlet extraction treatment particularly weakened RGD.
All FLX's mechanical values showed an interestingly opposite effect by increasing after the Soxhlet extraction, although barely nonsignificantly (p ∼ 0.6). This mechanical enhancement was likely attributed to the potential reorganization of the soft fiber component in FLX, as implied by the more toeing deformation of its stress–strain curve (Supplementary Fig. S4). This phenomenon is likely because the Soxhlet extraction removed the impurity and cleared the space for the fiber components in FLX to better reorganize in the toeing deformation and therefore improve its elasticity.
As a HYB of RGD and FLX, all HYB values also showed a combined effect with only marginal decreases. The variation was also nonsignificant, suggesting little influence the Soxhlet extraction had on this material's mechanical properties.
In short, only RGD raised concern after the extraction with significantly reduced mechanical properties and became exceedingly more brittle. Yet, the values suggest that the extracted RGD was still stronger than FLX and HYB and was still usable for biological applications. In contrast, there was no significant change with HYB, whereas the FLX material became even stronger and more elastic after the extraction. Therefore, we concluded that the Soxhlet extraction method should not jeopardize most material's mechanical performance, at least with the HYB and FLX materials.
Overall, we have presented a successful assay to evaluate and an effective treatment method to reduce 3D-printed materials' cytotoxicity. This study is an important first step to lead to biocompatible bioprinting. Recent regulatory addendums (ANSI/AAMI/ISO 10993-5:2009) asserted that qualitative cytotoxicity testing is suitable for material screening purposes.78 On top of that, the international standard (ISO 10993) and FDA79,80 recommends more than one evaluation test (e.g., sensitization, irritation, acute systemic toxicity, chronic toxicity, genotoxicity, hemocompatibility, risks based on degradation, and carcinogenicity) to test the material's long-term biocompatibility based on the device's nature of body contact and usage duration. Further study is under investigation for the thorough characterization of a broad range of Soxhlet extraction's biocompatibility.
Conclusion
In summary, we studied two commonly used 3D printing inks (VeroClear and TangoPlus) and showed strong cytotoxicity of printed materials using the indirect-contact assay. Among the three printed materials, the FLX/semi-FLX material (FLX and HYB) was more cytotoxic than the RGD one. Two employed cell lines (HEL and L929) showed consistent cytotoxicity profiles. We then explored three cytotoxicity reduction techniques—further curing (Autoclave), passivation (PDMS), and washing (Soxhlet extraction). We identified that the Soxhlet extraction method had a most substantial remedy effect by eradicating the cytotoxicity of all materials. Although the Soxhlet extraction method was found to weaken RGD's certain mechanical performances, the effect was not significant enough to prevent its use in biological applications.
Taken together, our study facilitated the understanding of the benefits and limitations of 3D printed materials, which is crucial to the successful translation of additive manufacturing into biomaterial fabrication. The postprinting detoxification approaches we established will further open an avenue to safely employ current 3D printing designs for many biological applications.
Supplementary Material
Acknowledgments
The authors thank the Institute of Antiviral Research and Dr. Justin Julander for providing the cell lines. They also thank Ty Nicholas and Zachary Ellsworth for the initial discussions.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Clyde was supported by the College of Engineering Undergraduate Research Program (EURP). Huang, Rengarajan, and Clyde were also partially supported by NIH NIGMS (grant number: R15GM132877).
Supplementary Material
References
- 1. Correa D, Papadopoulou A, Guberan C, et al. 3D-printed wood: Programming hygroscopic material transformations. 3D Print Addit Manuf 2015;2:106–116. [Google Scholar]
- 2. Schelly C, Anzalone G, Wijnen B, et al. Open-source 3-D printing technologies for education: Bringing additive manufacturing to the classroom. J Vis Lang Comput 2015;28:226–237. [Google Scholar]
- 3. Mcloughlin L, Fryazinov O, Moseley M, et al. Virtual sculpting and 3D printing for young people with disabilities. IEEE Comput Graphics Appl 2016;36:22–28. [DOI] [PubMed] [Google Scholar]
- 4. Zhang H, Zhang W, Xiao L, et al. Use of surface-enhanced Raman scattering (SERS) probes to detect fatty acid receptor activity in a microfluidic device. Sensors 2019;19:1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bak D. Rapid prototyping or rapid production? 3D printing processes move industry towards the latter. Assem Autom 2003;23:340–345. [Google Scholar]
- 6. Gross BC, Erkal JL, Lockwood SY, et al. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem 2014;86(7):3240–3253. [DOI] [PubMed] [Google Scholar]
- 7. Chua CK, Yeong WY, An J. 3D printing for biomedical engineering. Mater 2017;10(3):243. 10.3390/ma10030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jammalamadaka U, Tappa K. Recent advances in biomaterials for 3D printing and tissue engineering. J Funct Biomater 2018;9:22. 10.3390/jfb9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang H, Guzman AR, Wippold JA, et al. An ultra high-efficiency droplet microfluidics platform using automatically synchronized droplet pairing and merging. Lab Chip 2020;20:3948–3959. [DOI] [PubMed] [Google Scholar]
- 10. Placone JK, Engler AJ. Recent advances in extrusion-based 3D printing for biomedical applications. Adv Healthc Mater 2018;7:1701161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen C, Rengarajan V, Kjar A, et al. A matrigel-free method to generate matured human cerebral organoids using 3D-Printed microwell arrays. Bioact Mater 2021;6:1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng 2015;9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang H, Chen Z, Dai J, et al. A low-cost mobile platform for whole blood glucose monitoring using colorimetric method. Microchem J 2021;162:105814. [Google Scholar]
- 14. Sutradhar A, Park J, Carrau D, et al. Designing patient-specific 3D printed craniofacial implants using a novel topology optimization method. Med Biol Eng Comput 2016;54:1123–1135. [DOI] [PubMed] [Google Scholar]
- 15. Mommaerts MY, Büttner M, Vercruysse Jr H, et al. Orbital wall reconstruction with two-piece puzzle 3D printed implants. Craniomaxillofac Ttrauma Reconstr 2016;9:055–061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mobbs RJ, Coughlan M, Thompson R, et al. The utility of 3D printing for surgical planning and patient-specific implant design for complex spinal pathologies: Case report. J Neurosurg Spine 2017;26:513–518. [DOI] [PubMed] [Google Scholar]
- 17. Guo R, Merkel A, Sterling J, et al. Substrate modulus of 3D-printed scaffolds regulates the regenerative response in subcutaneous implants through the macrophage phenotype and Wnt signaling. Biomaterials 2015;73:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hughes AJ, DeBuitleir C, Soden P, et al. 3D printing aids acetabular reconstruction in complex revision hip arthroplasty. Adv Orthop 2017;2017. Article ID 8925050, 7 pages, 2017. 10.1155/2017/8925050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu L, Jiang G. 3D printing techniques in environmental science and engineering will bring new innovation. Environ Sci Technol 2017;51:3597–3599. [DOI] [PubMed] [Google Scholar]
- 20. Inoue Y, Ikuta K. Detoxification of the photocurable polymer by heat treatment for microstereolithography. Procedia CIRP 2013;5:115–118. [Google Scholar]
- 21. Grover WH, Oskui SM. Treatment for reducing the toxicity of 3D-printed parts. U.S. Patent No. 10,201,964. (last accessed February 12, 2019).
- 22. D'Urso PS, Effeney DJ, Earwaker WJ, et al. Custom cranioplasty using stereolithography and acrylic. Br J Plast Surg 2000;53:200–204. [DOI] [PubMed] [Google Scholar]
- 23. Kreß S, Schaller-Ammann R, Feiel J, et al. 3D printing of cell culture devices: Assessment and prevention of the cytotoxicity of photopolymers for stereolithography. Materials 2020;13:3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pereira T, Kennedy JV, Potgieter J. A comparison of traditional manufacturing vs additive manufacturing, the best method for the job. Procedia Manuf 2019;30:11–18. [Google Scholar]
- 25. Campbell T, Williams C, Ivanova O, et al. Could 3D Printing Change the World. Technologies, Potential, and Implications of Additive Manufacturing, Washington, DC: Atlantic Council, 2011; p. 3. [Google Scholar]
- 26. Bhushan B, Caspers M. An overview of additive manufacturing (3D printing) for microfabrication. Microsyst Technol 2017;23:1117–1124. [Google Scholar]
- 27. Hanna SD. Detoxification of solid freeform fabrication materials. U.S. Patent No. 6,996,245. (last accessed February 7, 2006).
- 28. Bagheri A, Jin J. Photopolymerization in 3D printing. ACS Appl Polym Mater 2019;1:593–611. [Google Scholar]
- 29. Kjar A, Huang Y. Application of micro-scale 3D printing in pharmaceutics. Pharmaceutics 2019;11:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mathew E, Pitzanti G, Larrañeta E, et al. 3D Printing of Pharmaceuticals and Drug Delivery Devices. Pharmaceutics. 2020;12(3):266. 10.3390/pharmaceutics12030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Revilla-León M, Meyers MJ, Zandinejad A, et al. A review on chemical composition, mechanical properties, and manufacturing work flow of additively manufactured current polymers for interim dental restorations. J Esthet Restor Dent 2019;31:51–57. [DOI] [PubMed] [Google Scholar]
- 32. Ligon SC, Liska R, Stampfl J, et al. Polymers for 3D printing and customized additive manufacturing. Chem Rev 2017;117:10212–10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carve M, Wlodkowic D. 3D-printed chips: Compatibility of additive manufacturing photopolymeric substrata with biological applications. Micromachines 2018;9:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Priyadarshini BM, Dikshit V, Zhang Y. 3D-printed bioreactors for in vitro modeling and analysis. Int J Bioprint 2020;6:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oskui SM, Diamante G, Liao C, et al. Assessing and reducing the toxicity of 3D-printed parts. Environ Sci Technol Lett 2016;3:1–6. [Google Scholar]
- 36. Leonhardt S, Klare M, Scheer M, et al. Biocompatibility of photopolymers for additive manufacturing. Curr Dir Biomed Eng 2016;2:113–116. [Google Scholar]
- 37. Cimpan MR, Matre R, Cressey LI, et al. The effect of heat-and auto-polymerized denture base polymers on clonogenicity, apoptosis, and necrosis in fibroblasts: Denture base polymers induce apoptosis and necrosis. Acta Odontol Scand 2000;58:217–228. [DOI] [PubMed] [Google Scholar]
- 38. Kim S-H, Watts DC. Degree of conversion of bis-acrylic based provisional crown and fixed partial denture materials. J Korean Acad Prosthodont 2008;46:639–643. [Google Scholar]
- 39. Papanu JS, Hess DW, Soane (Soong) DS, et al. Swelling of poly(methyl methacrylate) thin films in low molecular weight alcohols. J Appl Polym Sci 1990;39:803–823. [Google Scholar]
- 40. Xu Y, Xepapadeas AB, Koos B, et al. Effect of post-rinsing time on the mechanical strength and cytotoxicity of a 3D printed orthodontic splint material. Dent Mater 2021;37:e314–e327. [DOI] [PubMed] [Google Scholar]
- 41. Alifui-Segbaya F, Varma S, Lieschke GJ, et al. Biocompatibility of photopolymers in 3D printing. 3D Print Addit Manufact 2017;4:185–191. [Google Scholar]
- 42. Popov VK, Evseev AV, Ivanov AL, et al. Laser stereolithography and supercritical fluid processing for custom-designed implant fabrication. J Mater Sci 2004;15:123–128. [DOI] [PubMed] [Google Scholar]
- 43. Wallin RF, Arscott E. A practical guide to ISO 10993-5: Cytotoxicity. Med Device Diagn Ind 1998;20:96–98. [Google Scholar]
- 44. Stansbury JW, Idacavage MJ. 3D printing with polymers: Challenges among expanding options and opportunities. Dental Mater 2016;32:54–64. [DOI] [PubMed] [Google Scholar]
- 45. Weber M, Petzoldt F, Hartwig T, et al. IFAM advancing MIM technology. Met Powder Rep (UK) 1997;52:22–26. [Google Scholar]
- 46. Rengarajan V, Geng J, Huang Y. Fabrication of tapered 3D microstructure arrays using dual-exposure lithography (DEL). Micromachines 2020;11:903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rengarajan V. Fabrication of 3D hydrogel-based microscale tissue analog chip with integrated optofluidics. 2016. Dissertations. 1550. https://digitalcommons.njit.edu/dissertations/1550.
- 48. Lai Y, Ruscio D, Vanderbilt D. Method of purification of polymeric medical device materials using continuous soxhlet extraction. U.S. Patent No. 6,790,318. (last accessed September 14, 2004).
- 49. Du Y, Wu T, Yan N, et al. Fabrication and characterization of fully biodegradable natural fiber-reinforced poly (lactic acid) composites. Compos Part B Eng 2014;56:717–723. [Google Scholar]
- 50. Fischer F. FDM and Polyjet 3D printing. Popul Plast Packaging 2015;60. [Google Scholar]
- 51. Safety Data Sheet TANGOPLUS FLX930. http://www.advancedtek.com/wp-content/uploads/2016/12/SDS-Objet-TangoPlus-FLX930-US.pdf. (last accessed December 30, 2021).
- 52. SAFETY DATA SHEET VeroClear™, RGD810. 2020. https://www.stratasys.com/-/media/4514C58E09744D1A9F8425496B720C89.pdf. (last accessed December 30, 2021).
- 53. Manapat JZ, Chen Q, Ye P, et al. 3D printing of polymer nanocomposites via stereolithography. Macromol Mater Eng 2017;302:1600553. [Google Scholar]
- 54. Safranski DL, Gall K. Effect of chemical structure and crosslinking density on the thermo-mechanical properties and toughness of (meth) acrylate shape memory polymer networks. Polymer 2008;49:4446–4455. [Google Scholar]
- 55. Shokuhfar A, Arab B. The effect of cross linking density on the mechanical properties and structure of the epoxy polymers: Molecular dynamics simulation. J Mol Model 2013;19:3719–3731. [DOI] [PubMed] [Google Scholar]
- 56. Wallin RF. A practical guide to ISO 10993: Part 1&# 151; introduction to the standards. 1998.
- 57. Health C for D and R. Use of International Standard ISO 10993-1, ‘Biological evaluation of medical devices—Part 1: Evaluation and testing within a risk management process’. U.S. Food and Drug Administration (2020). Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-international-standard-iso-10993-1-biological-evaluation-medical-devices-part-1-evaluation-and Accessed August 12, 2021.
- 58. Wadajkar AS, Ahn C, Nguyen KT, et al. In vitro cytotoxicity evaluation of four vital pulp therapy materials on l929 fibroblasts. Int Sch Res Notices 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Poskus LT, Lima RSMS, Lima IR, et al. Cytotoxicity of current adhesive systems: In vitro testing on cell culture of L929 and balb/c 3T3 fibroblasts. Rev Odonto Ciênc 2009;24:129–134. [Google Scholar]
- 60. Liu X, Rodeheaver DP, White JC, et al. A comparison of in vitro cytotoxicity assays in medical device regulatory studies. Regul Toxicol Pharmacol 2018;97:24–32. [DOI] [PubMed] [Google Scholar]
- 61. Inagaki Y, Matsumoto Y, Tang W, et al. Dividing phase-dependent cytotoxicity profiling of human embryonic lung fibroblast identifies candidate anticancer reagents. Drug Discov Ther 2016;10:195–200. [DOI] [PubMed] [Google Scholar]
- 62. Zhu F, Friedrich T, Nugegoda D, et al. Assessment of the biocompatibility of three-dimensional-printed polymers using multispecies toxicity tests. Biomicrofluidics 2015;9:061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pusnik M, Imeri M, Deppierraz G, et al. The agar diffusion scratch assay-A novel method to assess the bioactive and cytotoxic potential of new materials and compounds. Sci Rep 2016;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kramer N, Walzl A, Unger C, et al. In vitro cell migration and invasion assays. Mutat Res 2013;752:10–24. [DOI] [PubMed] [Google Scholar]
- 65. Gautriaud E, Stafford KT, Adamchuk J, et al. Effect of sterilization on the mechanical properties of silicone rubbers. BioProcess Int 2010;8:S42–S49. [Google Scholar]
- 66. Halpern JM, Gormley CA, Keech MA, et al. Thermomechanical properties, antibiotic release, and bioactivity of a sterilized cyclodextrin drug delivery system. J Mater Chem B 2014;2:2764–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Walther C, Raddatz G. Process for coating surgical needles. U.S. Patent No. 5,985,355. (last accessed November 16, 1999).
- 68. De Castro ML, Priego-Capote F. Soxhlet extraction: Past and present panacea. J Chromatogr A 2010;1217:2383–2389. [DOI] [PubMed] [Google Scholar]
- 69. Wang Z, Volinsky AA, Gallant ND. Crosslinking effect on polydimethylsiloxane elastic modulus measured by custom-built compression instrument. J Appl Polym Sci 2014;131. [Google Scholar]
- 70. You JH, Bolt H. Analysis of singular interface stresses in dissimilar material joints for plasma facing components. J Nuclear Mater 2001;299:1–8. [Google Scholar]
- 71. Yang H, He T, Yan X. Adhesion strategies for heterogeneous soft materials—A review. Eng Res Express 2021. 10.1088/2631-8695/ac342e. [DOI]
- 72. Jofre-Reche JA, Pulpytel J, Arefi-Khonsari F, et al. Increased adhesion of polydimethylsiloxane (PDMS) to acrylic adhesive tape for medical use by surface treatment with an atmospheric pressure rotating plasma jet. J Phys D Appl Phys 2016;49:334001. [Google Scholar]
- 73. Niu X, Peng S, Liu L, et al. Characterizing and patterning of PDMS-based conducting composites. Adv Mater 2007;19:2682–2686. [Google Scholar]
- 74. Seghir R, Arscott S. Extended PDMS stiffness range for flexible systems. Sens Actuat A Phys 2015;230:33–39. [Google Scholar]
- 75. Lu Z, Jiang X, Zuo X, et al. Improvement of cytocompatibility of 3D-printing resins for endothelial cell adhesion. RSC Adv 2016;6:102381–102388. [Google Scholar]
- 76. Cazón A, Morer P, Matey L. PolyJet technology for product prototyping: Tensile strength and surface roughness properties. Proc Inst Mech Eng B 2014;228:1664–1675. [Google Scholar]
- 77. Iannace S, Sabatini G, Ambrosio L, et al. Mechanical behaviour of composite artificial tendons and ligaments. Biomaterials 1995;16:675–680. [DOI] [PubMed] [Google Scholar]
- 78. Register F. Federal Register, Vol. 81, No.143. Federal Register Available at: https://thefederalregister.org/81-FR/Issue-143. (last accessed December 30, 2021).
- 79. ISO. Biological evaluation of medical devices-Part 1: Evaluation and testing within a risk management process. ISO 10993-1: 2009. 2009.
- 80. Stang K, Krajewski S, Neumann B, et al. Hemocompatibility testing according to ISO 10993-4: Discrimination between pyrogen-and device-induced hemostatic activation. Mater Sci Eng C 2014;42:422–428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.