Abstract
Primary cutaneous gamma-delta T cell lymphoma (PCGDTL) is a rare and diagnostically challenging primary skin lymphoma. We present a case of a 78-year-old otherwise healthy man who developed non-healing nodules on his right posterior calf. Initial biopsy showed a dense, atypical lymphoid infiltrate with gamma-delta and cytotoxic T-cell immunophenotypes. The diagnosis of PCGDTL was rendered, however concurrent flow cytometry revealed expression of aberrant B-cell markers, including CD19 and cytoplasmic CD79a. Subsequent immunohistochemical studies corroborated this result. We report the extremely rare phenomenon of aberrant B-cell marker expression in PCGDTL, the first formally reported case to our knowledge.
Keywords: T- cell lymphoma, cutaneous, gamma, delta
Introduction
Primary cutaneous gamma-delta T cell lymphoma (PCGDTL) is a rare subtype of non-Hodgkin lymphoma that is diagnostically challenging.1,2 PCGDTL is derived from a clonal proliferation of γδ T-cells with a cytotoxic phenotype.2 Cases of PCGDTL comprise less than 1% of all primary cutaneous lymphomas, creating a paucity of large-scale studies.1 The median age of onset is between 50 to 60 years old with no gender predilection.1,2 PCGDTL commonly presents as multifocal deep plaques, nodules, patches, and/or superficial plaques, frequently with ulceration.1 B symptoms are reported in 40-60% of patients and imaging is required to rule out extracutaneous involvement.1 Histologically, specimens of PCGDTL can show epidermotropic, dermal, or subcutaneous infiltrates of atypical, small and medium-sized lymphocytes.3 Additional findings include angioinvasion, necrosis, and ulceration.3 The infiltrate usually stains with CD3, TCR-gamma/delta, CD56, and cytotoxic proteins such as TIA-1.3 The tumor cells often stain negative with CD5, CD4, and CD8, but expression patterns can be variable.3 Overall five-year survival is estimated at less than 20%, with better prognosis in the cases showing predominantly epidermal involvement.2,4-6 Since PCDTCL carries a poor prognosis and is diagnostically challenging, deepening our understanding of its histologic spectrum is helpful. T-cell lymphomas bearing aberrant B cell marker expression is an exceptionally rare event.7-10 Aberrant B-cell marker expression was rarely described in some T-cell lymphomas, including hepatosplenic T-cell lymphoma, primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder, systemic and extra-nodal NK/T-cell lymphomas, adult T-cell leukemia/lymphoma, angioimmunoblastic T-cell lymphoma, mycosis fungoides, cutaneous peripheral T-cell lymphoma not otherwise specified, and cutaneous and systemic anaplastic large-cell lymphoma.11,12 In this report, we describe an extremely rare case of PCGDTL with aberrant B-cell markers, the first of its kind to be formally reported to our knowledge.
Case Presentation
A 78-year-old otherwise healthy man developed a non-healing nodule on the right posterior calf. Initial outside workup and biopsies were non-diagnostic. Intensive wound care including an Unna boot was attempted and failed. The patient reported developing similar ulcerating nodules on the right calf several months prior to presentation that resolved with intensive wound care. Over an interval of three months, the nodule grew significantly in size and a second nodule developed more inferiorly on the right posterior calf. He otherwise felt well and denied any B symptoms.
On physical exam, two ulcerated purple-pink plaques were identified, with a much larger, superior plaque. CT imaging demonstrated two masses extending into the soft tissue, the larger of which measured 4.5 * 4.5 * 2.0 cm (Figure 1). Both nodules showed fluorodeoxyglucose (FDG) avidity on FDG PET/CT imaging and mild avidity of a right inguinal lymph node (1.9 * 1.0 cm, SUV 3.0).
Figure 1.
A: Large non-healing plaques on the right posterior lower calf are seen, the larger of which is uncovered and superior. B: A closer view of the larger, growing plaque on the right posterior calf.
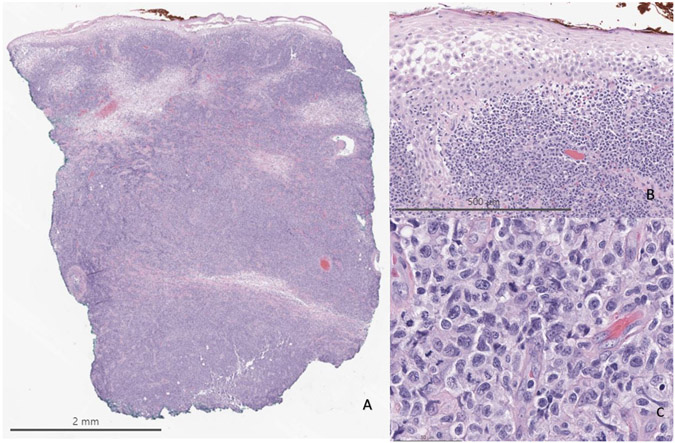
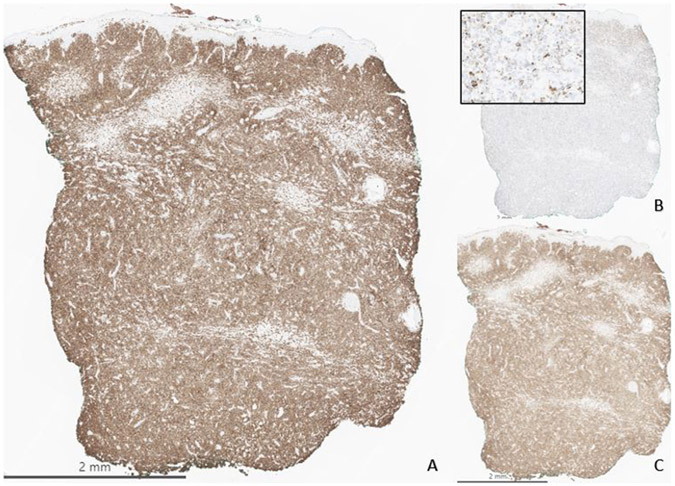
A punch biopsy of the large plaque on the right posterior calf was performed for further classification. A dense, high-grade, malignant lymphoid infiltrate extended from superficial to the deep dermis (Figure 2). Epidermotropism was absent. The infiltrate was composed of medium to large cells with irregular nuclear contours, numerous mitoses, and prominent nucleoli. Initial immunohistochemical workup showed that it stained positive with CD3, cytotoxic markers (granzyme B and TIA1), TCR delta, and variably with CD7 (Figure 3). It was negative with CD4, CD5, CD8, TCR alpha, TCR beta, CD20, and EBER in-situ hybridization (Figure 4). PAX5, CD56, and CD30 highlighted scattered cells. Additional immunostains for CD34, TdT, CD117, PU.1, CD68, CD13, and CD33 were all negative in the neoplastic cells. Concurrent flow cytometry performed with fresh skin tissue from the same site showed an abnormal gamma-delta T cell population representing over 75% of white blood cells (Figure 5). Importantly, this abnormal T cell population showed co-expression of B-cell markers including cytoplasmic CD79a and CD19. No abnormal mature B-cell population was detected in the concurrent flow sample. Based on the flow cytometry results, further immunohistochemistry workup was performed and confirmed the co-expression of CD19 and CD79a in the abnormal T-cell infiltrate (Figure 6). A needle core biopsy of the right groin lymph node revealed lymphoid tissue with no evidence of involvement by the patient’s previously diagnosed T cell lymphoma, but a minute abnormal clonal B cell population with CLL/SLL-like immunophenotype was detected (0.037% of WBC) by flow cytometry only, likely representing peripheral blood contamination with low level monoclonal B-cell lymphocytosis. The bone marrow was also negative for involvement by the T-cell lymphoma, and the same CLL/SLL-like B-cell was detected at low level by flow cytometry (0.0071%). Upon further work up, HTLV-I/II antibody tests were negative.
Figure 2.
A: A dense infiltrate of atypical lymphoid cells is seen with extension into the deep dermis (Hematoxylin–eosin, 10x original magnification). B: No epidermotropism of the lymphoid infiltrate is appreciated (100x original magnification). C: The infiltrate is composed of atypical medium to large cells with numerous mitoses and prominent nucleoli (400x original magnification).
Figure 3.
The infiltrate is positive for CD3 (A, 10x original magnification), cytotoxic markers including TIA and granzyme (B, granzyme, 10x original magnification), and for T-cell receptor delta (C, 10x original magnification).
Figure 4.
The infiltrate stains negative with CD4 (A, 10x original magnification), CD8 (B, 10x original magnification), CD20 (C, 10x original magnification), and T-cell receptor alpha (D, 10x original magnification).
Figure 5.
Flow cytometry of tissue shows that the CD3 positive T cells have aberrant expression of CD19 and cCD79a, and the gamma-delta receptor bearing T cells also have CD19 aberrant expression. CD7 has bimodal expression within the CD3 T-cell population.
Figure 6.
Follow up immunohistochemistry workup given the flow cytometry results shows that the infiltrate stains with CD79a (A) and CD19 (B), bearing aberrant B cell markers.
In addition, T-cell receptor clonality studies were performed and demonstrated restricted clonal rearrangement of the T cell receptor gamma (TRG) gene without rearrangement of the beta (TRB) locus. B-cell receptor IGH gene rearrangement studies targeting the FR1, FR2 and FR3 regions showed low level amplification, an expected result due to the low number of B cells in this T-cell lymphoma, with no evidence of clonality. MSK-IMPACT HEME (Integrated Mutation Profiling of Actionable Cancer Targets) was conducted to identify specific mutations in 468 genes. Mutations were called based on a paired analysis using a matched, patient’s normal sample. Only somatic variants were reported. Forty-one mutations and many chromosomal level losses and/or gains were detected (see Supplement 1). One chromosomal level alteration in the clinically validated arm showed a gain of chromosome 7q. Deletions in CDKN2A and CDKN2B were noted, along with BRAF and JAK1 mutations, in keeping with a T-cell lymphoma.14 Mutations in JAK3 and MAPK1 were also detected, albeit not the exact actionable mutations previously described within PCGDTL.12
The patient was treated with radiation therapy to the right calf with electron radiation therapy, 3,600 cGy in 12 fractions, with a complete response. The patient was most recently seen nine months following his diagnosis and had no evidence of disease. Long term follow up will be essential in this patient given the poor outcomes.
Discussion
PCGDTL is a rare and diagnostically challenging primary skin lymphoma with a poor prognosis.1,2 To the best of our knowledge, we present the first formally diagnosed case of PCGDTL with aberrant expression of B-cell markers including CD19 and cCD79a. Aberrant B-cell marker expression has been rarely described in some T-cell lymphomas, including hepatosplenic T-cell lymphoma, primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder, systemic and extra-nodal NK/T-cell lymphomas, adult T-cell leukemia/lymphoma, angioimmunoblastic T-cell lymphoma, mycosis fungoides, cutaneous peripheral T-cell lymphoma not otherwise specified, and cutaneous and systemic anaplastic large-cell lymphoma.7-10,12 In addition, rare instances of γδ T-cell lymphomas with aberrant B-cell marker expression have been reported, however to the best of our knowledge not with a proper diagnosis of PCGDTL.10-12,15 Our case lacked CD20 expression.
Although early and accurate diagnosis of PCGDTL aids in avoiding significant repercussions, reaching the diagnosis comes with many pitfalls. Both malignant and benign T-cell lymphocytic proliferations can show the γδ T-cell receptor (TCR) phenotype, and while it typically correlates with cytotoxic immunophenotype, it does not necessarily exhibit malignant behavior.11 The γδ TCR phenotype was reported after burns, and in inflammatory conditions like psoriasis, allergic contact dermatitis, pityriasis lichenoides, and lupus.11,16-17 In inflammatory conditions, the γδ T cells rarely account for more than 10% of the T-cell infiltrate.17 PCGDTL commonly has a double negative CD4 and CD8 immunophenotype, but this can vary.11 CD56 tends to be predominately expressed in dermal or subcutaneous variants of PCGDTL.11 TCR-silent phenotypes have also been reported.11 Other markers including CD4, CD5, CD7, and CD30 can show variable expression and, not-uncommonly, immunophenotypic shifts.11 Finally, although driver mutations have been described, translocations are extraordinarily rare.12,18 Understanding the entire immunophenotypic spectrum of the disease can be very helpful, including the aberrant B-cell markers described in this unusual case presentation.
In conclusion, we present a very unusual case of PCGDTL with aberrant B cell marker expression. Due to its rarity, limited availability of large-scale studies, and diverse immunophenotypic presentations, the diagnosis of PCGDTL is challenging to many dermatopathologists. Appreciation for the full range of presentations, including aberrant B cell markers, can assist dermatopathologists in rendering this difficult diagnosis.
Supplementary Material
Funding statements/Acknowledgements
Supported by: MSK NIH Funded Grant# P30 CA08748
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
This case was presented at the 59th Annual Meeting of the American Society of Dermatopathology.
Citations
- 1.Muhsen IN, El Fakih R, Hamadani M, et al. Clinical, Diagnostic and Prognostic Characteristics of Primary Cutaneous Gamma Delta T-cell Lymphomas. Clin Hematol Int. 2022;4(1-2):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberti-Violetti S, Maronese CA, et al. Primary Cutaneous Gamma-Delta T Cell Lymphomas: A Case Series and Overview of the Literature. Dermatopathology (Basel). 2021;8(4):515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subtil A. Diagnosis of Cutaneous Lymphoid Infiltrates. Sham, Switzerland: Springer Nature Switzerland AG; 2019. [Google Scholar]
- 4.Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toro JR, Liewehr DJ, Pabby N, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood J Am Soc Hematol. 2003;101:3407–3412. [DOI] [PubMed] [Google Scholar]
- 6.Merrill ED, Agbay R, Miranda RN, et al. Primary cutaneous T-cell lymphomas showing gamma-delta (γδ) phenotype and predominantly epidermotropic pattern are clinicopathologically distinct from classic primary cutaneous γδ T-cell lymphomas. Am J Surg Pathol. 2017;41:204–215. [DOI] [PubMed] [Google Scholar]
- 7.Jain H, Shetty D, Jain H, et al. A rare case of hepatosplenic γδ T-cell lymphoma expressing CD19 with ring chromosome 7 and trisomy 8. Cancer Genet. 2018;228-229:17–20 [DOI] [PubMed] [Google Scholar]
- 8.Rizzo K, Stetler-Stevenson M, et al. Novel CD19 expression in a peripheral T cell lymphoma: A flow cytometry case report with morphologic correlation. Cytometry B Clin Cytom. 2009;76(2):142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao X, Teruya-Feldstein J, Raffeld M, et al. Peripheral T-cell lymphoma with aberrant expression of CD79a and CD20: a diagnostic pitfall. Mod Pathol. 2001;14(2):105–10. [DOI] [PubMed] [Google Scholar]
- 10.Matnani RG, Stewart RL, Pulliam J, et al. Peripheral T-Cell Lymphoma with Aberrant Expression of CD19, CD20, and CD79a: Case Report and Literature Review. Case Rep Hematol. 2013;2013:183134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres-Cabala CA, Huen A, Iyer SP, et al. Gamma/Delta Phenotype in Primary Cutaneous T-cell Lymphomas and Lymphoid Proliferations: Challenges for Diagnosis and Classification. Surg Pathol Clin. 2021;14(2):177–194. [DOI] [PubMed] [Google Scholar]
- 12.Mark E, Sutton M, Gru A. Primary Cutaneous Anaplastic Large-Cell Lymphoma With Aberrant CD20 Expression: Case Report and Review of the Literature. Am J Dermatopathol. 2022;44(12):971–978. [DOI] [PubMed] [Google Scholar]
- 13.Daniels J, Doukas PG, Escala MEM, at al. Cellular origins and genetic landscape of cutaneous gamma delta T cell lymphomas. Nat Commun. 2020;11(1):1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syrykh C, Gorez P, Péricart S et al. Molecular diagnosis of T-cell lymphoma: a correlative study of PCR-based T-cell clonality assessment and targeted NGS. Blood Adv. 2021;5(22):4590–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ju JY, Sorensen EP, Walsh JS, et al. CD5 + Gamma Delta T-Cell Lymphoproliferative Disorder/Lymphoma Without Cytotoxic Granules in the Skin. Am J Dermatopathol. 2022;44(9):680–682. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Escala ME, Sidiropoulos M, Deonizio J, et al. γδ T-cell-rich variants of pityriasis lichenoides and lymphomatoid papulosis: benign cutaneous disorders to be distinguished from aggressive cutaneous γδ T-cell lymphomas. Br J Dermatol. 2015;172(2):372–9. [DOI] [PubMed] [Google Scholar]
- 17.Hocker TL, Wada DA, El-Azhary R, et al. Expression of T-cell receptor-γδ in normal human skin, inflammatory dermatoses and mycosis fungoides. J Cutan Pathol. 2012;39(4):419–24. [DOI] [PubMed] [Google Scholar]
- 18.Fadl A, Bennani NN, Comfere N, et al. Primary cutaneous gamma/delta T-cell lymphoma with simultaneous JAK2 and TP63 rearrangements: a new double-hit? Histopathology. 2023. Jun 12; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.