Abstract
Background:
We investigated whether lower dietary acid load in women living with HIV (WLWH) receiving antiretroviral therapy (ART) is associated with lower kidney function decline.
Setting:
1,608 WLWH receiving ART in WIHS cohort with available diet data and a baseline estimated glomerular filtration rate (eGFR)≥15 ml/min/1.73 m2.
Methods:
A brief dietary instrument conducted from 2013–2016 under the Food Insecurity Sub-Study was used for assessing fruits and vegetables (FV) and protein intake. A mixed effects model with random-intercept and slope was used to estimate subjects’ annual decline rate in eGFR and the association between FV intake, adjusting for socio-demographics, serum albumin, comorbidities, time on ART, ART drugs, HIV markers, and baseline eGFR. We evaluated whether markers of inflammation mediated the effect of FV intake on decline in eGFR, using causal mediation analysis.
Results:
We found a dose-response relationship for the association of FV intake and eGFR decline, with lesser annual decline in eGFR in the middle and highest tertiles of FV intake. An increase of 5 servings of FV intake per day was associated with a lower annual eGFR decline (−1.18 [−1.43, −0.94]). On average, 39% of the association between higher FV intake and slower eGFR decline was explained by decreased levels of inflammation.
Conclusion:
Plant-rich diets was associated with slower decline in kidney function. Inflammation is a potential path through which diet may affect kidney function. The findings support an emerging body of literature on the potential benefits of plant-rich diets for prevention of chronic kidney disease.
Keywords: dietary intake, fruits and vegetables, kidney function, inflammation, HIV
Introduction
Antiretroviral therapy (ART) has resulted in a marked decrease in AIDS-related conditions and improved survival among people with HIV (PWH).1 However, with increased longevity, non-AIDS related comorbidities such as chronic kidney disease (CKD) have become increasingly important causes of morbidity and mortality in this population. PWH receiving ART are at disproportionately increased risk of CKD due to development of comorbidities associated with aging such as diabetes and hypertension as well as potential for nephrotoxicity from prolonged ART exposure.
The kidneys play a central role in the regulation of body fluids, electrolytes, and acid-base balance. Diets in industrialized societies are shifting from relatively alkali-predominant toward more acid-predominant diets because they are deficient in fruits and vegetables, and high in sulfur-rich proteins and phosphorus.2 This dietary shift may play a role in the regulation of chronic inflammation. The consumption of more fruits and vegetables typically lowers the dietary acid content while meat, eggs, cheese, grain products, sugar, and rice are relatively strong net acidifying foods.2–6 Nutrition has been known to strongly influence acid-base balance, and imbalances in endogenous acid-base equilibrium due to diet may lead to inflammation.7,8 Consumption of a diet abundant in acid precursors over time results in multiple derangements including lower buffering capacity, and as CKD progresses, a decreased ability of the kidneys to excrete the excess acid.9 Such diets are believed to have a proinflammatory effect and can induce metabolic acidosis in individuals with reduced glomerular filtration rate (GFR), including otherwise healthy older persons.10,11 On the other hand, diets high in base precursors such as fiber may have an anti-inflammatory effect and have been shown to ameliorate metabolic acidosis.12
Dietary acid load is a measure of balance between acid-inducing foods and base-inducing foods.2,13 Changing from standard industrialized society diets to a low-phosphorous vegan diet has shown to improve metabolic acidosis in patients with advanced CKD.14 Moreover, added fruits and vegetables has reduced urine net acid excretion, consistent with reduced net endogenous acid production10, in individuals with CKD stage 2 (estimated GFR, 60–89 ml/min per 1.73 m2) but without metabolic acidosis.15 Further, our research along with other epidemiological and small clinical studies have previously shown a direct association between high dietary acid load due to higher consumption of animal protein and lower potassium enriched fruits and vegetables and CKD progression.16–18 Together, these studies suggest that added fruits and vegetables can reduce the acid load, improve metabolic acidosis in CKD and slow progression to end-stage kidney disease. To the best of our knowledge, it is unknown if lowering the acid load in the diet among women living with HIV (WLWH) on antiretroviral therapy (ART) slows kidney function decline and if reduced inflammation may be on the causal path of this association.
In this study, we examined the association of fruits and vegetables intake (marker of low dietary acid load) and protein intake (marker of high dietary acid load) with decline in kidney function, among WLWH receiving ART. We hypothesized that a low dietary acid load in PLW receiving ART for a long duration slows decline in kidney function and that reduced inflammation mediates this association.
Materials and Methods
Participants Characteristics and Procedures
Data were collected from WLWH participating in the Women’s Interagency HIV Study (WIHS).19,20 WIHS is a multi-site prospective study investigating HIV disease progression, comorbidities, and the behavioral impact of HIV among women in the US, now part of the MACS-WIHS Combined Cohort Study (MWCCS, https://statepi.jhsph.edu/mwccs/). Established in 1994, data were collected from participants in six US sites (Bronx, Brooklyn, Washington DC, Chicago, Los Angeles, and San Francisco) every 6 months via interviews, physical assessments, and laboratory tests. To further enhance the representativeness of the cohort, new participants were enrolled between 2013–2015 at sites in the US South; including Atlanta, Birmingham, Jackson, Miami, and Chapel Hill.20 The median age of WIHS WLWH is 50 years. Around 75% of WIHS WLWH identify as African American, 14% as White, and 14% as Hispanic. All participants provided written informed consent, with study activities being approved by each site’s Institutional Review Board.
This secondary data analysis used retrospective data collected under the food insecurity sub-study (grant # R01MH095683) from 2013 to 2016 biannually from nine sites across the United States participating in the WIHS. To fit within the data collection procedures of WIHS and limit respondent burden, we used a brief dietary instrument to assess dietary behavior rather than a method intended to estimate quantitatively the amounts of nutrients.
For this study, we restricted analyses to WLWH on ART at the time of the first available dietary instrument and who continued with ART till the end of the follow up period (n=1,624). We further excluded women with an eGFR at baseline of less than 15 ml/min/1.73 m2 (due to the lack of information on participant’s dialysis history) or who did not have available dietary data and data on markers of inflammation for analysis resulting in a sample size of 1,608 (Supplemental Figure 1).
Primary exposure variables
Dietary intake was measured using an adapted version of the Year 2000 National Health Interview Survey multifactor brief dietary instrument.21 For many studies, including studies in the WIHS, the brief dietary instrument has been found to successfully provide information about differences and changes in dietary behavior. The diet quality instrument was conducted among all WIHS women from 2013 to 2016 annually under the Food Insecurity Sub-Study. This dietary instrument assessed the dietary intake frequencies as times per day, week, month, or year of 18 food line items, representing 50 foods or food groups. All intake frequencies were converted to daily intake frequencies. Missing values (<1%) were imputed under the assumption of missing at random to ensure complete cases. The dietary instrument was used for assessing fruits and vegetable intake and protein intake (Supplementary Table 1).
Outcomes
The primary outcome was eGFR slope in each rolling window, expressed in mL/min/1.73 m2 per year, that is the annual change in eGFR in each of the three time periods ranging from each participant’s first visit to end of 12 months, 13 months to 24 months, and finally 25 months to end of 36 months. We calculated eGFR using the 2009 CKD Epidemiology Collaboration creatinine equation.22 Serum creatinine was measured semi-annually at the clinical labs of each WIHS site using the modified Jaffe method, traceable to isotope dilution mass spectrometry.
Covariates
We selected clinical and sociodemographic covariates based on previous literature and theory.16,23,24 The socio-demographic covariates were age at enrollment, race/ethnicity, self-reported educational attainment categorized as < high school, ≥high school, race/ethnicity, and annual household income (≤$36,000 [ref] vs. >$36,000). Clinical covariates were body weight (kg), body height (m), serum albumin, hypertension, diabetes status, and smoking. Hypertension status was a composite variable defined as systolic blood pressure>=140 or diastolic blood pressure>=90, self-report, or use of anti-hypertensive medicines at visit. Diabetes status was defined as if ever self-reported anti-diabetic medication or fasting glucose>=126 mg/dL or HbA1C>=6.5% or self-report. HIV-related clinical covariates included current (i.e., at index visit) CD4+ count (cells per μL), current CD8+ count (cells per μL), current HIV-1 RNA (copies/mL), and years on ART. We also evaluated current ART drug regimen that included tenofovir dixoproxil fumarate, or other drugs influencing creatinine such as dolutegravir, rilpivirine and cobicistat. Across all covariates, <1% of the data were missing.
Inflammatory Markers
We used serum markers of monocyte activation (soluble (s) CD14, sCD163) and systemic inflammation (IL-6, TNF-R1). To increase the power for testing associations, we created a summary index for individual inflammatory markers by rescaling each individual biomarker to have a mean of zero and a standard deviation (SD) of 1 so that normalized z-scores were obtained for each biomarker.25 The z-scores for serum measures of inflammation were averaged to create the inflammation status variable.
Statistical Analysis
The outcome measure for this study was the annual rate of eGFR decline. Characteristics at study participants’ first visit were examined according to tertiles of fruits and vegetables intake using χ2 tests for categorical variables and ANOVA for numeric variables. Our first analysis examined the association between fruits and vegetables intake and inflammation in our study population using a mixed-effects model adjusted for all covariates. We used a mixed effects model with random intercepts and slopes to estimate the subjects’ annual decline in eGFR. We used a mixed effects model with age as the time scale to examine the independent predictive value of fruits and vegetables intake and (ii) protein intake (categorically as tertile intake with the lowest tertile as the reference category) for decline of eGFR, adjusting for potential covariates and baseline eGFR. We further examined the intake of fruits and vegetables as a continuous variable (considering an increase of 5 servings for all participants) with decline of eGFR. We used age as the time scale since that allows the model to compute risk estimates for individuals with the same age irrespective of the years of follow up.26,27 Since age is a risk factor for CKD as well as its progression, we would expect the risk to change more as a function of age than as a function of “time on study”. Covariates for adjustment were chosen if they were associated with progression of CKD in univariate analyses (P<0.10).
Mediation Analysis:
We tested the mediation effect of inflammation by examining the indirect effects in mediation analysis by using a method described by Bauer et al.28 The outcome model has random intercepts and random slopes for the exposure, the mediator, and their interaction. In this analysis, we calculated the indirect effect and test the importance of the indirect path– that lower intake of fruits and vegetables affects decline in GFR through the increased levels of inflammation. The mediator was lagged relative to the exposure, and hence data on inflammation were used from 2014 and 2015 only.
Results
Descriptive Statistics
There was a wide range in serum creatinine among women with HIV ranging from 0.48 to 8.69 mg/dl with a mean ± SD of 1.20 ± 0.85 mg/dl. Participant demographics are summarized in Table 1 and Supplementary Table 2. Our population was comprised mainly of women with the mean age of 47.6 years and blacks (68.2%). One-third (33.2%) of the women did not complete high school while 86.3% had an annual household income of less than $36,000. Hypertension (49.4%) was common in this cohort.
Table 1:
Baseline Characteristics of women living with HIV by tertiles of fruits and vegetables intake (N=1,608)
| Total | Lowest Tertile (0–1.2 servings/day, n=413)) | Middle Tertile (1.2–2.3 servings/day, n=626) | Highest Tertile (≥2.3 servings/day, n=569) | Linear Trend Test | |
|---|---|---|---|---|---|
| Age (in yrs), mean±SE | 47.6±0.2 | 47.7±0.4 | 47.6±0.3 | 46.7±0.6 | 0.18 |
| Race | 0.08 | ||||
| White, non-Hispanic | 193 (12) | 32 (7.7) | 94 (15) | 67 (11.8) | |
| Black, non-Hispanic | 1096 (68.2) | 306 (74.1) | 405 (64.7) | 385 (67.7) | |
| Hispanic | 241 (15) | 54 (13.1) | 103 (16.5) | 84 (14.8) | |
| Other | 78 (4.8) | 21 (5.1) | 24 (3.8) | 33 (5.7) | |
| Education Level (<high school), % | 33.2 | 35.6 | 33.4 | 24.2 | 0.004 |
| Annual Household Income (<36,000), % | 86.3 | 88.5 | 86.3 | 79.4 | 0.02 |
| Body Mass Index (kg/m2), mean ±SE | 31.0±0.2 | 31.1±0.4 | 31.1±0.3 | 30.8±0.7 | 0.76 |
| Diabetes Status (yes), % | 19.1 | 34.2 | 53.9 | 11.9 | 0.18 |
| Hypertension Status (yes), % | 49.4 | 39.3 | 50.6 | 10.1 | 0.03 |
| Smoking Status (yes), % | 38.6 | 38.7 | 53.2 | 8.1 | 0.02 |
| Serum Albumin (gm/dL), mean±SE | 4.1±0.02 | 4.1±0.03 | 4.1±0.02 | 4.2±0.03 | 0.05 |
| eGFR (ml/min/1.73 m2), mean±SE | 94.1±0.6 | 93.1±1.0 | 94.2±0.8 | 98.2±1.8 | 0.041 |
| Antiretroviral Drugs, % | 0.04 | ||||
| Tenofovir Dixoproxil Fumarate | 43.7 | 52.3 | 37.3 | 10.4 | |
| TDF/Emtricitabine | 28.5 | 55.8 | 35.7 | 8.4 | |
| Dolutegravir | 25.8 | 57.4 | 34.8 | 7.8 | |
| Rilpivirine | 0.3 | 56.1 | 35.6 | 8.3 | |
| Cobicistat | 1.7 | 58.3 | 25.0 | 16.7 | |
| CD4 cell count, cells/μL, mean±SE | 592.6±7.5 | 580.8±13.0 | 583.2±22.4 | 600.6±10.1 | 0.02 |
| CD8 cell count, cells/μL, mean±SE | 783.9±9.7 | 791.0±16.9 | 784.6±13.2 | 756.4±25.4 | 0.03 |
| Viral Load, copies/ml, median (Q1–Q3) | 38.0 [20.0–173.5] | 42.6 [22.5–140.0] | 38.1 [21.2–172.0] | 35.6 [20.0–140.0] | 0.035 |
P value for trend for continuous dependent variables were calculated with the use of linear regression.
Figure 1 shows that the intake of fruits and vegetables was relatively low, and the median servings per day was 1.28 (25th–75th percentile: 0.8–2.0). The median intake of protein was 3.1 servings/day (25th–75th percentile: 2.1 to 4.4 servings/day) (Figure 2).
Figure 1:
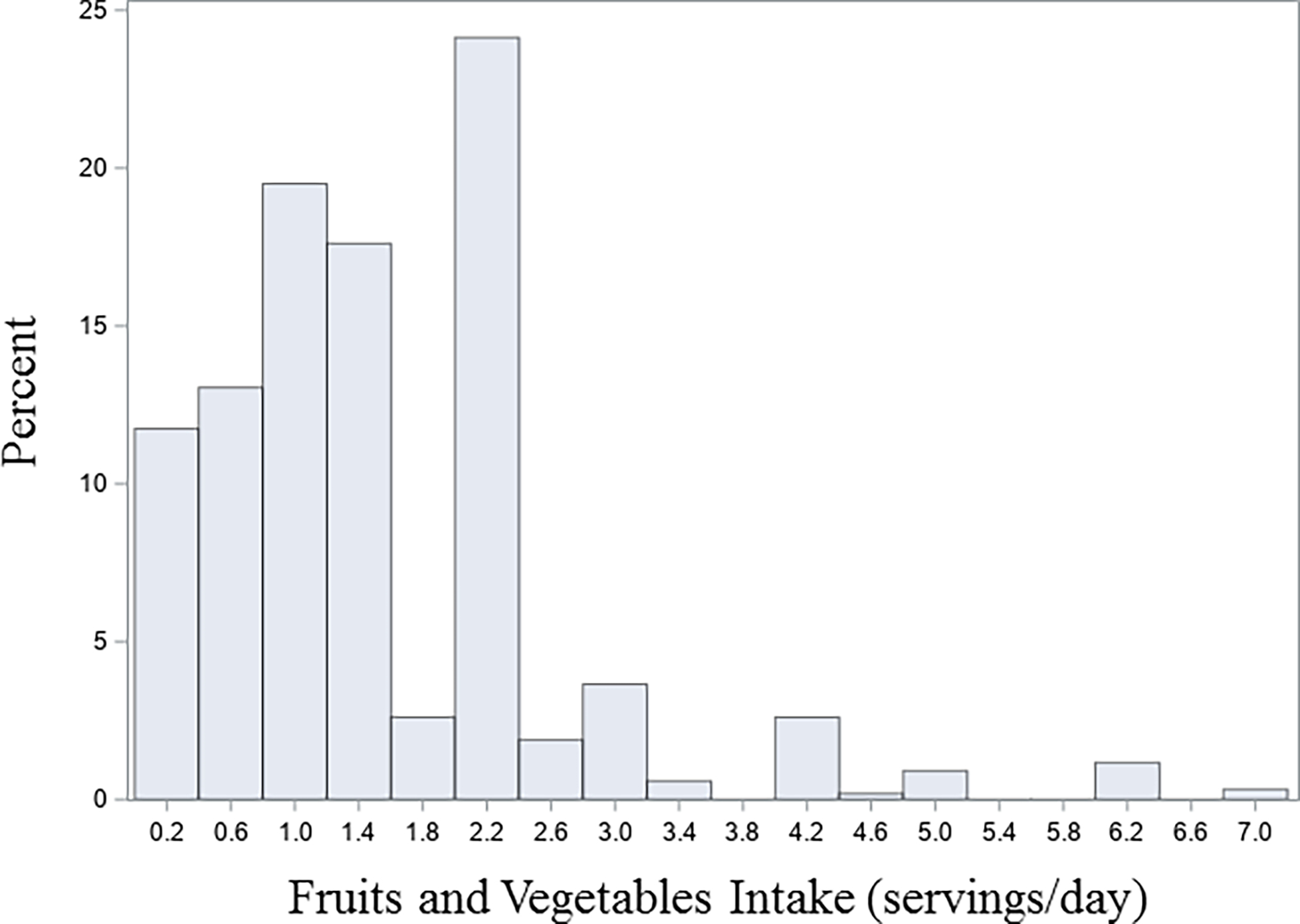
Frequency distribution of intake of fruits and vegetables per day in 1,608 women living with HIV
Figure 2:

Frequency distribution of intake of protein per day in 1,608 women living with HIV HIV-positive women
Association of fruits and vegetables intake and protein intake with decline in kidney function using mixed-effects model
Unadjusted analysis demonstrated that moderate and high FV intake was associated with a slower annual eGFR decline (over a median 2 year of follow-up, Table 2). Adjustment for age and race attenuated the decline in eGFR to −1.09 (−2.03, −0.13) in the highest tertile of intake and to −0.32 (−0.84, 0.19) in the middle tertile. On adjustment for all covariates, and baseline eGFR, the association between the middle and higher tertile of fruits and vegetables intake and decline in eGFR was lessened. A dose-response relationship was noted in the fully adjusted model (β [95% CI]: −0.60 (−1.14, −0.06) in the middle tertile and −1.27 (−2.04, −0.45) in the highest tertile; ptrend=0.001).
Table 2:
Association between the intake of fruits and vegetables (in tertiles and for an increase of 5 servings per day) and eGFR decline in women living with HIV
| Fruits and Vegetables Intake, β (95% CI) | ||||
|---|---|---|---|---|
| Lowest Tertile (0–1.2 servings/day) | Middle Tertile (1.2–2.3 servings/day) | Highest Tertile (≥2.3 servings/day) | Continuous (increase of 5 servings/day) | |
| Unadjusted | β=0 (Reference) | −0.35 (−0.86, −0.16) | −1.06 (−2.01, −0.16) | −1.42 (−1.80, −1.06) |
| Model 1 | β=0 (Reference) | −0.32 (−0.84, 0.19) | −1.09 (−2.03, −0.13) | −1.36 (−1.68, −1.04) |
| Model 2 | β=0 (Reference) | −0.27 (−0.80, 0.26) | −1.02 (−1.94, 0.10) | −1.30 (−1.63, −0.97) |
| Model 3 | β=0 (Reference) | −0.30 (−0.82, 0.25) | −1.00 (−1.95, 0.12) | −1.35 (−1.67, −0.98) |
| Model 4 | β=0 (Reference) | −0.65 (−1.04, 0.02) | −1.37 (−2.23, −0.42) | −1.24 (−1.55, −0.94) |
| Model 5 | β=0 (Reference) | −0.61 (−1.13, −0.08) | −1.32 (−2.17, −0.48) | −1.20 (−1.46, −0.91) |
| Model 6 | β=0 (Reference) | −0.60 (−1.14, −0.06) | −1.27 (−2.04, −0.45) | −1.18 (−1.43, −0.94) |
Model 1: Unadjusted+ age+ race; Model 2: Model 1+ education+ household income; Model 3: Model 2+ serum albumin+ hypertension+ diabetes+ smoking; Model 4: Model 3+ time on antiretroviral drugs+ ART drugs; Model 5: Model 4+CD4 count+CD8 count+ log viral load; Model 6: Model 5+ baseline kidney function
On testing the association between the increase in servings of fruits and vegetables per day (continuously per increase in 5 servings/day) in all participants and annual decline in eGFR, we noted the estimated decrease in the decline in eGFR as −1.18 (−1.43, −0.94).
On examining the association of protein intake and decline in eGFR, the highest tertile of protein intake was associated with a lower decline in eGFR (−0.85 [−1.49, −0.14] when adjusted for the potential confounders, Table 3).
Table 3:
Association between the intake of protein (in tertiles) and eGFR decline in women living with HIV
| Protein Intake, β (95% CI) | |||
|---|---|---|---|
| Lowest Tertile (0–2.3 servings/day) | Middle Tertile (2.3–3.7 servings/day) | Highest Tertile (≥3.7 servings/day) | |
| Unadjusted | β=0 (Reference) | 0.08 (−0.54, 0.71) | −0.41 (−1.06, 0.23) |
| Model 1 | β=0 (Reference) | 0.07 (−0.56, 0.69) | −0.45 (−1.10, 0.18) |
| Model 2 | β=0 (Reference) | 0.10 (−0.53, 0.74) | −0.43 (−1.09, 0.23) |
| Model 3 | β=0 (Reference) | 0.13 (−0.49, 0.75) | −0.47 (−1.13, 0.19) |
| Model 4 | β=0 (Reference) | −0.07 (−0.69, 0.57) | −0.70 (−1.36, −0.03) |
| Model 5 | β=0 (Reference) | −0.08 (−0.70, 0.54) | −0.82 (−1.50, −0.15) |
| Model 6 | β=0 (Reference) | −0.10 (−0.71, 0.53) | −0.85 (−1.49, −0.14) |
Model 1: Unadjusted+ age+ race; Model 2: Model 1+ education+ household income; Model 3: Model 2+ serum albumin+ hypertension+ diabetes+ smoking; Model 4: Model 3+ time on antiretroviral drugs+ ART drugs; Model 5: Model 4+CD4 count+CD8 count+ log viral load; Model 6: Model 5+ baseline kidney function
Association between FV intake and inflammation and kidney function
Unadjusted analysis showed that higher intake of FV was associated with lower levels of inflammation (as measured by the summary index for inflammatory markers, β [95% CI]: −0.17 [−0.29, −0.03]). After adjustment for potential confounders, higher intake of FV remained associated with lower levels of inflammation (−0.22 [−0.35, −0.06]). Furthermore, increased levels of inflammation markers were associated with a greater decline in eGFR (3.55 (2.85–4.35]). The association remained upon adjustment for cofounders (2.05 [1.60–2.58]).
Association of higher intake of fruits and vegetables and slower decline in kidney function mediated by decreased levels of inflammation
We determined the estimated average indirect effect of higher intake of fruits and vegetables on slower decline in kidney function to be −0.45 (95% CI= −0.72, −0.12) and the estimated average total effect of higher intake of fruits and vegetable on slower decline in kidney function to be −1.17 (95% CI= −1.48, −0.82), Figure 3. The effect estimates indicate that higher intake of fruits and vegetables reduce inflammation and, on average, about 39% of the total effect of higher intake of fruits and vegetables (decreased diet acid load) on slower decline in eGFR was indirect and mediated by the lower state of inflammation. Mediation for each marker of inflammation considered separately was consistent with the mediation by the summary index of inflammation, with the percent mediated being 26.1, 18.2, 8.6, and 16.5 for CD14, sCD163, IL-6, and TNF-R1, respectively (Supplementary Table 3).
Figure 3:

Decreased levels of inflammation markers at months after first visit mediate the effect of higher intake of fruits and vegetables at time of first visit on improvement in kidney functional markers three years later in 1,608 women living with HIV
Note. Associations are presented as path coefficients (adjusted), see results section.
Discussion
The present study examined the association between intake of FV and risk of eGFR decline among women receiving ART among a large geographically diverse cohort of women with HIV in the United States. We found that repeated higher intake of FVs was associated with a slower rate of decline in eGFR, suggesting lower dietary acid load diet due to a daily higher intake of FVs may be associated with slowing decline in kidney function.
Diet composition is known to influence acid–base balance by providing acid or base precursors. Depending on the quantity of the acid load and kidney function, diets high in acid load can induce an acid-retaining state, which may be associated with the development of metabolic alterations such as hypertension, progression of CKD, and other complications.16,29,30 To our knowledge, no prior study has investigated the association between a lower diet dependent acid load and kidney function in people living with HIV. Findings from the current study corroborate findings from prior studies that have shown progressive GFR decline by acid-inducing diets in subjects with relatively mild CKD (eGFR=60–89 ml/min per 1.73 m2. The predominant anions in FVs are citrate and malate, and when metabolized, they release bicarbonate and thus contribute alkali to the body exhibiting beneficial metabolic effects. Furthermore, FVs that are high in fiber promote the growth of saccharolytic bacteria31, which produce short chain fatty acids (alkali) and other anti-inflammatory compounds, and lower the generation of uremic toxins.32 Uremic toxins, such as P-cresol or indoxyl sulfate, are involved in the onset and progression of CKD through promotion of fibrosis in the kidney.33 Antioxidants in FVs may neutralize reactive oxygen species, which play a role in CKD progression, and reduce DNA damage34, and glucosinolates in cruciferous vegetables induce detoxifying enzymes.35 High FV intake may also indirectly reduce risk of CKD progression by displacement of unhealthy foods high in saturated fat, trans fat, glycemic load and sodium.
Lower financial means and living in certain communities may affect the ability of individuals to obtain a diet rich in fruits and vegetables. Studies of the food and low socio-economic status environment suggest that low-income individuals often live in neighborhoods where there are few full-service grocery stores and may not have easy access to transportation to allow for shopping at such stores in outlying areas. Limited access to nutritious food and relatively easier access to less nutritious food may be linked to poor diets and, ultimately, to diet-related diseases. Given this, effects on eGFR reduction due to FV would have greatly diminished when adjusted for income and education. However, the association remained significant.
We found that higher protein intake was associated with a slower decline in kidney function. Though prior studies suggest that high protein intake may promote kidney damage by chronically increasing glomerular pressure and hyperfiltration36–38, growing evidence show that the more important determinant of the effect of dietary protein on CKD progression is the quality of the ingested protein (i.e., whether it induces acid-production [like most animal protein] or base production [like most fruit and vegetable protein]) when ingested rather than the quantity of protein ingested.3,39–42 This study did not evaluate the association between the quality of the ingested protein and decline in kidney function which may explain this unexpected finding.
The mediating role of circulating biomarkers of serum macrophage inflammation and systemic inflammation in the association between the lower intake of fruits and vegetables and decline in kidney function suggests that inflammation might be one of the paths through which diet can affect kidney function. Serum levels of CD14 and CD163 in HIV-infected participants helps identify individuals with ongoing inflammation despite successful ART.43 Increased generation of IL-6 and TNF-R1 is noted in metabolic alterations such as CKD and is largely caused by chronic inflammation and oxidative stress. The kidneys play a central role in maintaining homeostasis in the body and can be the target of inflammatory disorders caused by the immune system’s response to the presence of HIV.44 Healthier diets (e.g., rich in fruits and vegetables such as the Mediterranean diet) typically have been associated with lower inflammation levels, whereas Western-style diets (e.g., high in fat and simple carbohydrates) have been associated with higher levels of inflammatory markers.45–48 In our study, we found a higher intake of FV to be associated with lower levels of the markers of systemic inflammation in women living with HIV.
Several limitations of our study should be considered. First, dietary assessment was limited to 18 self-reported food line items, and only intake frequency was queried; referent serving sizes were not provided; details of the foods comprising each line item are unknown; and nutrient content is not available. We used a dietary screener that was validated and developed by the National Cancer Institute21 for use in similar communities. Despite the lack of information about total dietary and energy intakes, there is precedent for using key indicator foods to examine diet quality.49,50 Second, our findings cannot be generalized to men or younger women. Lastly, we did not have the necessary dietary components to estimate diet-dependent acid load and have used intake of fruits and vegetables and protein as a marker for dietary acid load.
These limitations are counterbalanced by several strengths of this study. This is the first longitudinal study examining decline in kidney function in association with lowering of diet-dependent acid load intake (higher intake of fruits and vegetables and lower intake of protein) in HIV-positive women. Prior studies by Banerjee et al.52,53 have documented the impact of food insecurity and unhealthy diets on clinical conditions of CKD and its progression using the National Health and Nutrition Examination Survey linked to US Renal Data System Registry.
The findings from this study show an association between higher intake of fruits and vegetables and slower decline in kidney function, supporting an emerging body of literature on the potential benefits of plant-rich diets for primary CKD prevention. This result suggests inflammation is a potential path through which diet may affect kidney function. This is the first study in a population living with HIV that has examined the association of diet dependent acid load characterized by higher intake of fruits and vegetables with kidney function, a population in whom comorbid kidney diseases become increasingly common with age. Our results suggest that the alkali-based diet in people with HIV receiving antiretroviral therapy slows decline of kidney function despite potential nephrotoxic effects of ART. Findings from our study, if confirmed in clinical trials, may have application for both population-wide and high-risk approaches to CKD prevention and management in various settings.
Supplementary Material
Research in Context.
Evidence before this study
Although small clinical studies and cohort studies in chronic kidney disease patients have shown that diets rich in fruits and vegetables can slow the progression of kidney damage, a search of PubMed conducted before commencement of this study using the terms “kidney disease progression” AND “HIV” AND “fruits and vegetables” for studies published in English between Jan 1, 2005, and July 31, 2019, yielded no results suggesting that no prior study in HIV population had examined if lowering the acid load in the diet among subjects on antiretroviral therapy slows kidney function decline. Over the past four decades, advances in HIV treatment have contributed to a longer life expectancy for people living with HIV. However, with prolonged survival, recipients of ART face adverse consequences beyond HIV itself, including diseases associated with aging such as diabetes, hypertension, and chronic kidney disease. Apart from beneficial impact of early ART initiation on HIV associated nephropathy, studies exploring other treatment strategies for slowing kidney disease progression have not been conducted rigorously.
Added value of this study
We analyzed data from the Women’s Interagency HIV study (WIHS, now part of the MWCCS) in women living with HIV receiving ART. We used a mixed effects model with age as the time scale to examine the independent predictive value of fruits and vegetables (FV) intake for decline of eGFR. The primary finding shows a dose-response relationship for the association of FV intake and eGFR decline suggesting plant-based diets may be effective in slowing decline in kidney function. Causal mediation analysis showed that 39% of the association between higher FV intake and slower eGFR decline was explained by decreased levels of inflammation. This is the first study in a population living with HIV that has examined the association of diet dependent acid load with kidney function, a population in whom comorbid kidney diseases become increasingly common with age.
Implications of all the available evidence
The potentially deleterious effect of a diet low in plant-based foods on kidney health mediated through increased levels of inflammatory markers, supported by our findings and those of other investigators in chronic kidney disease, suggests the potential utility of the modulation of inflammatory properties of diet, in strategies to prevent kidney disease. If confirmed in clinical trial, this knowledge may have application for both population-wide and high-risk approach to CKD prevention and control in various settings.
ACKNOWLEDGEMENTS
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS), now the MACS/WIHS Combined Cohort Study (MWCCS). The authors gratefully acknowledge the contributions of the study participants and dedication of the staff at the MWCCS sites. This study was funded by Women’s Interagency HIV Study (WIHS) sub-study grants from the National Institute of Mental Health, R01MH104114 and R01MH095683. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos, David Hanna, and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Topper), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora and Michelle Floris-Moore), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR003098 (JHU ICTR), UL1-TR001881 (UCLA CTSI), P30-AI-050409 (Atlanta CFAR), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), P30-AI-027767 (UAB CFAR), and P30-MH-116867 (Miami CHARM).
Footnotes
Disclosures: TB, EAF, JMT, LAS, AA, TW, DM, MC, AAA, IO, LM, GS, MAF, MF, PCT, and SDW, no conflicts of interest.
Data Sharing
Data described in the manuscript, code book, and analytic code will be made available upon request pending [e.g., application and approval, payment, other].
REFERENCES
- 1.Phair J and Palella F. Renal disease in HIV infected Individuals. Curr Opin HIV AIDS. 2011. Jul; 6(4): 285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remer T: Influence of nutrition on acid-base balance—metabolic aspects. Eur J Nutr 40: 214–220, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Remer T, Manz F: Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 95: 791–797, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Adeva MM, Souto G: Diet-induced metabolic acidosis. Clin Nutr 30: 416–421, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Gannon RH, Millward DJ, Brown JE, et al. Estimates of daily net endogenous acid production in the elderly UK population: analysis of the National Diet and Nutrition Survey (NDNS) of British adults aged 65 years and over. Br J Nutr. 2008; 100(3):615–623. [DOI] [PubMed] [Google Scholar]
- 6.Murakami K, Sasaki S, Takahashi Y, Uenishi K. Association between dietary acid-base load and cardiometabolic risk factors in young Japanese women. Br J Nutr. 2008; 100(3):642–651. [DOI] [PubMed] [Google Scholar]
- 7.Galland L. Diet and inflammation. Nutr Clin Pract 2010; 25: 634–40. [DOI] [PubMed] [Google Scholar]
- 8.Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation—emphasis on the metabolic syndrome. J Am Coll Cardiol 2006; 48: 677–85. [DOI] [PubMed] [Google Scholar]
- 9.Wesson DE, Simoni J. Increased tissue acid mediates a progressive decline in the glomerular filtration rate of animals with reduced nephron mass. Kidney Int 2009; 75: 929–935. [DOI] [PubMed] [Google Scholar]
- 10.Frassetto LA, Todd KM, Morris RC Jr, Sebastian A: Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr 68: 576–583, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol 2011; 6: 160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snelson M, Clarke RE, Coughlan MT. Stirring the Pot: Can Dietary Modification Alleviate the Burden of CKD? Nutrients. 2017;9(3):265. Published 2017 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemann J Jr, Adams ND, Wilz DR, Brenes LG. Acid and mineral balances and bone in familial proximal renal tubular acidosis. Kidney Int 2000; 58: 1267–1277. [DOI] [PubMed] [Google Scholar]
- 14.Barsotti G, Morelli E, Cupisti A, Meola M, Dani L, Giovannetti S: A low-nitrogen low-phosphorus Vegan diet for patients with chronic renal failure. Nephron 74: 390–394, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Goraya N, Simoni J, Jo C-H, Wesson DE: Dietary acid reduction with fruits and vegetables or sodium bicarbonate reduces kidney injury in individuals with moderately reduced GFR due to hypertensive nephropathy. Kidney Int 81: 86–93, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Banerjee T, Crews D, Wesson D, Saran R, Tilea A, Williams D, Rios Burrows N, Powe N High dietary acid load predicts End Stage Renal Disease among Chronic Kidney Disease adults. Journal of American Society of Nephrology 2015; 26 (7): 1693–1700; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scialla JJ, Appel LJ, Astor BC, et al. Estimated net endogenous acid production and serum bicarbonate in African Americans with chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(7): 1526–1532; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goraya N, Simoni J, Jo C-H, et al. Comparison of treating the metabolic acidosis of CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol 2013; 8(3): 371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12(9):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adimora AA, Ramirez C, Benning L, Greenblatt RM, Kempf M-C, Tien PC, et al. Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol 2018; 47(2):393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Cancer Institute (NCI). The Multifactor Screener in the 2000 National Health Interview Survey Cancer Control Supplement (NHIS 2000). Epidemiology and Genomics Research Program. National Cancer Institute, Division of Cancer Control and Population Sciences. Updated November 20, 2019 [Internet]. Available from: https://epi.grants.cancer.gov/nhis/multifactor/. [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee T, Carrero JJ, McCulloch C, Burrows NR, Siegel KR, Morgenstern H, Saran R, Powe NR; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. Dietary Factors and Prevention: Risk of End-Stage Kidney Disease by Fruit and Vegetable Consumption. Am J Nephrol. 2021;52(5):356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whittle HJ, Sheira LA, Frongillo EA, Palar K, Cohen J, Merenstein D, Wilson TE, Adedimeji A, Cohen MH, Adimora AA, Ofotokun I. Longitudinal associations between food insecurity and substance use in a cohort of women with or at risk for HIV in the United States. Addiction. 2019. Jan;114(1):127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prizment AE, Anderson KE, Visvanathan K, Folsom AR. Association of Inflammatory Markers with Colorectal Cancer Incidence in the Atherosclerosis Risk in Communities Study. Cancer Epidemiol Biomarkers Prev 2011; 20(2): 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of time-scale. Am J Epidemiol 1997; 145:72–80. [DOI] [PubMed] [Google Scholar]
- 27.Collett D Modelling Survival Data in Medical Research, Chapman & Hall, London, 1994. 347 pp. [Google Scholar]
- 28.Bauer DJ, Preacher KJ, Gil KM (2006). Conceptualizing and Testing Random Indirect Effects and Moderated Mediation in Multilevel Models: New Procedures and Recommendations. Psychological Methods Vol. 11, No. 2, 142–163. [DOI] [PubMed] [Google Scholar]
- 29.Parohan M, Sadeghi A, Nasiri M, Maleki V, Khodadost M, Pirouzi A, Sadeghi O. Dietary acid load and risk of hypertension: A systematic review and dose-response meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2019;29(7):665–675. [DOI] [PubMed] [Google Scholar]
- 30.Lee KW, Shin D. Positive association between dietary acid load and future insulin resistance risk: findings from the Korean Genome and Epidemiology Study. Nutr J 2020; 19, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson JW, Baird P, Davis RH Jr. et al. Health benefits of dietary fibre. Nutr Rev 2009;67:188–205 [DOI] [PubMed] [Google Scholar]
- 32.Carrero JJ, González-Ortiz A, Avesani CM, Bakker SJL, Bellizzi V, Chauveau P, Clase CM, Cupisti A, Espinosa-Cuevas A, Molina P, Moreau K, Piccoli GB, Post A, Sezer S, Fouque D. Plant-based diets to manage the risks and complications of chronic kidney disease. Nature Rev Nephrol (In Press). [DOI] [PubMed] [Google Scholar]
- 33.Niwa T Role of indoxyl sulfate in the progression of chronic kidney disease and cardiovascular disease: experimental and clinical effects of oral sorbent AST-120. Ther Apher Dial 15, 120–124, doi: 10.1111/j.1744-9987.2010.00882.x(2011). [DOI] [PubMed] [Google Scholar]
- 34.Lampe JW. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. Am J Clin Nutr 1999;70(Suppl 3):475–90S. [DOI] [PubMed] [Google Scholar]
- 35.Broekmans WM, Klopping-Ketelaars IA, Schuurman CR. et al. Fruits and vegetables increase plasma carotenoids and vitamins and decrease homocysteine in humans. J Nutr 2000;130:1578–83 [DOI] [PubMed] [Google Scholar]
- 36.Metges CC, Barth CA: Metabolic consequences of a high dietary-protein intake in adulthood: assessment of the available evidence. J Nutr. 2000, 130 (4): 886–889. [DOI] [PubMed] [Google Scholar]
- 37.Brenner BM, Meyer TW, Hostetter TH: Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982, 307 (11): 652–659. 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Parra E, Gracia_iguacel C, Egido J, Ortiz A: Phosphorus and nutrition in chronic kidney disease. Int J Nephrol. 2012, Volume 2012: Article ID 597605–5 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Remer T, Manz F: Estimation of the renal acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. 1994, 59: 1356–1361. [DOI] [PubMed] [Google Scholar]
- 40.Goraya N, Simoni J, Jo C-H, Wesson DE: Comparison of treating the metabolic acidosis of CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. 2013, 8 (3): 371–381. 10.2215/CJN.02430312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goraya N, Simoni J, Jo C, Wesson DE: Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012, 81: 86–93. 10.1038/ki.2011.313. [DOI] [PubMed] [Google Scholar]
- 42.Scialla JJ, Appel LJ, Wolf M, Yang W, Zhang X, Sozio SM, Miller ER, Bazzano LA, Cuevas M, Glenn MJ, Lustigova E, Kallem RR, Porter AC, Townsend RR, Weir MR, Anderson CA, Chronic Renal Insufficiency Cohort-CRIC Study Group: Plant protein intake is associated with fibroblast growth factor 23 and serum bicarbonate levels in patients with chronic kidney disease: the Chronic Renal Insufficiency Cohort study. J Renal Nutr. 2012, 22 (4): 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunt Peter. Soluble CD163 and Clinical Outcomes in Treated HIV Infection: Insights into Mechanisms. The Journal of Infectious Diseases 2016. DOI: 10.1093/infdis/jiw264 [DOI] [PubMed] [Google Scholar]
- 44.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, Levin A. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013. Jul 13; 382(9887):158–69. [DOI] [PubMed] [Google Scholar]
- 45.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr 2007;137:992–8. [DOI] [PubMed] [Google Scholar]
- 46.Esposito K, Giugliano D. Diet and inflammation: a link to metabolic and cardiovascular diseases. Eur Heart J 2006;27:15–20. [DOI] [PubMed] [Google Scholar]
- 47.Johansson-Persson A, Ulmius M, Cloetens L, Karhu T, Herzig KH, Önning G. A high intake of dietary fiber influences C—reactive protein and fibrinogen, but not glucose and lipid metabolism, in mildly hypercholesterolemic subjects. Eur J Nutr 2014;53:39–48. [DOI] [PubMed] [Google Scholar]
- 48.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2004;80:1029–35. [DOI] [PubMed] [Google Scholar]
- 49.Wolfe WS, Frongillo EA, Cassano PA. Evaluating brief measures of fruit and vegetable consumption frequency and variety: cognition, interpretation, and other measurement issues. J Am Diet Assoc. 2001;101(3):311–18. [DOI] [PubMed] [Google Scholar]
- 50.Heimendinger J, Van Duyn MA, Chapelsky D, Foerster S, Stables G. The national 5 A Day for Better Health Program: a large-scale nutrition intervention. J Public Health Manag Pract. 1996;2(2):27–35. [PubMed] [Google Scholar]
- 51.Hever J Plant-based diets: a physician’s guide. Perm J. 2016;20(3):15–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banerjee T, Crews DC, Wesson DE, Dharmarajan S, Saran R, Ríos Burrows N, Saydah S, Powe NR; CDC CKD Surveillance Team. Food Insecurity, CKD, and Subsequent ESRD in US Adults. Am J Kidney Dis. 2017; 70(1):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banerjee T, Crews D, Wesson D, Saran R, Tilea A, Williams D, Rios Burrows N, Powe N. Dietary acid load and chronic kidney disease among adults in the United States. BMC Nephrology 2014, 15, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending [e.g., application and approval, payment, other].


