Abstract
Insertion sequence IS18 was detected by analysis of the spontaneous aminoglycoside resistant mutant Acinetobacter sp. 13 strain BM2716-1. Insertion of the element upstream from the silent acetyltransferase gene aac(6′)-Ij created a hybrid promoter that putatively accounts for the expression of the aminoglycoside resistance gene. The 1,074-bp IS18 element contained partially matched (20 out of 26 bases) terminal inverted repeats, one of which overlapped the 3′ end of a 935-bp open reading frame potentially encoding a protein related to the transposases of the IS30 family. IS18 was found in 6 out of 29 strains of Acinetobacter sp. 13 but not in 10 strains each of A. baumannii and A. haemolyticus.
Insertion sequences (IS) are small genetic elements, typically containing 1 to 2 kbp, able to integrate into numerous sites within genomes via transposition, a pathway independent of homologous recombination. IS elements mediate various molecular and genetic events, including gene activation, disruption, deletion, rearrangement, recombination, and transfer (for a review, see reference 6). Two IS in Acinetobacter spp. have been recently described. The first, IS1236, belongs to the IS3 family and was found because it generated, by transposition, null mutations preventing metabolism of p-hydroxybenzoate via protocatechuate in A. calcoaceticus (7). The second, IS17, is a member of the IS903 family responsible for insertion inactivation of the aminoglycoside resistance gene aac(6′)-Ig in A. haemolyticus (19).
The aac(6′)-Ij gene mediates intrinsic aminoglycoside resistance in Acinetobacter sp. 13 (12). In this report, we describe the aminoglycoside susceptible strain BM2716 of Acinetobacter sp. 13 and activation of its aac(6′)-Ij silent gene by transposition of a novel insertion sequence belonging to the IS30 family.
Identification of BM2716.
Identification of strain BM2716 at the genus level was confirmed by the transformation assay (11). Biochemical tests, including carbohydrate assimilation, indicated that this strain belonged to Acinetobacter sp. 13 (2). Furthermore, the aac(6′)-Ij gene specific for this species was detected by dot blot hybridization with an intragenic probe as described previously (12) (data not shown).
Aminoglycoside resistance of BM2716 and BM2716-1.
Despite the presence of the aminoglycoside resistance gene aac(6′)-Ij, BM2716 was susceptible to aminoglycoside antibiotics. Extracts of this strain were devoid of aminoglycoside acetyltransferase activity, as tested by the phosphocellulose paper-binding technique (9) (data not shown). Resistant clones were obtained at frequencies of 4 × 10−9 by selection on Mueller-Hinton agar containing 10 μg of tobramycin per ml and were screened by disk diffusion assay for resistance to other aminoglycosides. Among the 30 mutants tested, all but one, designated BM2716-1, were resistant to all aminoglycosides. Analysis by the paper-binding assay of crude extracts from five of these mutants did not detect any aminoglycoside acetyltransferase activity. Thus, the resistance possibly resulted from an impaired uptake of the drug. Strain BM2716-1 was less susceptible to the substrates of the AAC(6′)-I enzyme (amikacin, tobramycin, and 2′-N-ethylnetilmicin) than to the aminoglycosides that are not modified by the enzyme (gentamicin and 6′-N-ethylnetilmicin); the MICs of amikacin, gentamicin, netilmicin, and tobramycin were 2, 1, 1, and 2 μg/ml, respectively, for BM2716 and 8, 1, 8, and 16 μg/ml, respectively, for BM2716-1. As expected, BM2716-1 produced an AAC(6′)-I activity as detected by the paper-binding assay (data not shown).
Characterization of aac(6′)-Ij-like genes of BM2716 and BM2716-1.
Primers A, 5′-CTCTCGGACCCATGCAGT-3′, and B, 5′-GATGTTAAATTTAGCTT-3′, spanning the aac(6′)-Ij gene of BM2689 (12) amplified 769- and 1,846-bp fragments of BM2716 and BM2716-1 DNA, respectively. The amplification products were cloned into pUC19 linearized by SmaI (19), and the resulting plasmids, pAT677 and pAT678, conferred aminoglycoside resistance to Escherichia coli JM83 by production of an AAC(6′)-I enzyme (data not shown). The sequencing of the aac(6′)-Ij genes of BM2716 and BM2716-1 was performed on two clones obtained from independent amplifications. Double-stranded DNA sequencing by the dideoxynucleotide chain terminator technique (20) was carried out with synthetic oligonucleotides. DNA fragments were resolved by electrophoresis on 8% polyacrylamide gels containing 8 M urea. These sequences were identical and differed from that of aac(6′)-Ij of BM2689 by 11 base substitutions that resulted in four amino acid changes (Glu to Gln at position 32 [Glu32→Gln], Arg35→Gln, Val97→Ile, and Ile110→Thr). Thus, aac(6′)-Ij of BM2716 and BM2716-1 encoded an enzyme that was functional, although the gene did not confer resistance to BM2716.
Characterization of IS18.
The identity between the regions upstream from aac(6′)-I of BM2716 and BM2716-1 was interrupted 42 bp from each gene’s initiation codon due to a 1,074-bp insertion (Fig. 1). This sequence, designated IS18, displayed similarity (50 to 55% identity) to elements belonging to the IS30 family: ISAS2 of Aeromonas salmonicida (8), IS4351 of Bacteroides fragilis (18), IS30 of Escherichia hermannii (4), IS6770 of Enterococcus faecalis (21), and IS1086 of Alcaligenes eutrophus (5). IS18 was delineated by imperfect inverted repeats (IR) (20 out of 26 bp) and generated a direct 3-bp duplication of target DNA at the insertion site. A search for stop codons in the three reading frames in each DNA strand identified a large open reading frame (ORF) located between the TAG and TGA codons at coordinates 73 and 1,005, respectively. A putative ribosome-binding sequence was located 7 bp upstream from the translation start codon ATG at position 145. The deduced amino acid sequence of the coding sequence displayed 42.1, 41.1, 37.2, 36.9, and 30% identity with the putative transposases of ISAS2, IS4551, IS30, IS1086, and IS6770, respectively. Analysis of the upstream region showed a potential −35 promoter sequence at positions 80 to 85. A 12-bp IR sequence overlapping the putative −35 hexamer (Fig. 1) could function as a regulator for the transposase gene. The insertion of IS18 in BM2716-1 generated a potential promoter sequence consisting of a −35 (TTGCCG [positions 1,097 to 1,102]) motif located within the IS and a −10 (TAAAAT) motif adjacent to the site of insertion. The −35 and −10 elements of this putative hybrid promoter were separated by 17 nucleotides, the optimum spacing for promoter function (1). Transposable genetic elements as a source of transcription have been reported previously (6). With certain insertion sequences such as IS10, the activation is caused by a promoter located within the element and directed outward into the adjacent gene (3) whereas other elements such as IS1 (17), IS2 (10), and IS256 (14) create hybrid promoters upon transposition.
FIG. 1.
Sequence of IS18 and its integration site in Acinetobacter strain BM2716-1. The nucleotide sequence of IS18 is in capital letters. The start and stop codons of the coding sequence for the putative transposase are indicated by capital letters in boldface type. The putative ribosome-binding sequence (RBS) and promoter sequences (−35 and −10) are underlined. The 3-bp repeats present in identical orientation on each side of IS18 are boxed. The deduced amino acid sequence of the putative transposase is presented in single-letter code under the nucleotide sequence. The stop codon is indicated by an asterisk. The sequence of the beginning of the aac(6′)-Ij-like gene is indicated in lowercase letters, and the start codon is indicated in lowercase boldface type. Thin arrows, IR; thick arrows, 26-bp IR at the extremities of IS18.
Transposition of IS18.
Transposition of IS18 was assayed by plasmid conduction in E. coli. Plasmid pAT678 (Tra− Mob− Apr Tmr) was introduced by transformation into E. coli HB101 (recA Strr) harboring pOX38Gm (Tra+) (15). The resulting strain, HB101(pOX38Gm, pAT678), was mated with E. coli K802N (Nalr), and transconjugants were obtained on medium containing nalidixic acid (40 μg/ml) and tobramycin (10 μg/ml) at the frequency of 2 × 10−6 per donor. A transconjugant resistant to ampicillin, gentamicin, and tobramycin that may have been generated by cointegrate formation between pAT678 (Tra− Mob− Apr Tmr) carrying IS18 and pOX38Gm (Tra+) was subcultured and replica plated on nonselective medium and on media containing either ampicillin, gentamicin, or tobramycin to screen for loss of the resistance markers of pAT678. Plasmid DNA of a clone susceptible to ampicillin and tobramycin but resistant to gentamicin, likely the result of intramolecular recombination between the two IS18 copies of the cointegrate in a Rec+ host, was digested by HaeIII, ligated with T4 DNA ligase, and used as a target for inverted PCR (IPCR) (22) with primers E, 5′-CTCTATATCCACGTTGCC-3′ (positions 304 to 287), and F, 5′-TAATCCGTCAATATCTGCCAAA-3′ (positions 944 to 965), directed outward (numbering refers to the sequence in Fig. 1). The IPCR product was purified (Sephaglass BandPrep kit; Pharmacia, St. Quentin-en-Yvelines, France), cloned in pUC19, linearized by SmaI, and sequenced by the dideoxynucleotide terminator technique (20). Sequence determination of the amplification product indicated that integration of IS18 into pOX38Gm had occurred 112 bp upstream from ORF 95 (13) (Fig. 2). These results indicated that IS18 can transpose in E. coli. Insertion of IS18 into pOX38Gm generated a 3-bp target duplication, as in BM2716-1, and the duplicated nucleotides had different base contents, reflecting the new target site. Three-base-pair duplications of target DNA have also been observed with related elements ISAS2, IS4551, and IS1086, whereas IS30 generates 2-bp duplications. Interestingly, in a second IPCR experiment, we obtained an additional 435-bp fragment, which corresponded to the two ends of IS18 separated by 2 bp (GT). This observation strongly suggests that transposition of IS18 involves an (IS)2 dimer intermediate as has been shown for IS30 (16). The (IS30)2 structure results from site-specific dimerization and contains two copies of the element that are joined such that the extremities are separated by 2 bp or, occasionally, by 1 or 3 bp (16). The G+C content of IS18 was 39.7%, similar to that of the Acinetobacter genus, whereas the G+C compositions of IS30, IS1086, and IS4551 were 46, 65, and 43%, respectively. These results suggest that the elements have diverged from a common ancestor to adjust to the relative abundance of tRNA in their respective hosts.
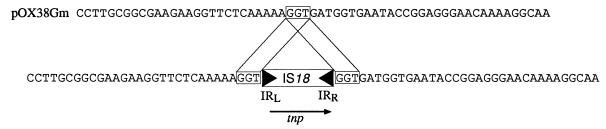
FIG. 2.
Target site before and after insertion of IS18 into pOX38Gm. The arrow indicates the ORF for the putative transposase. The target duplication is boxed.
Distribution of IS18.
The distribution of IS18 among clinical isolates of Acinetobacter was examined by PCR using primers C, 5′-ACCCAACTTTCTCAA-3′ (positions 157 to 171), and D, 5′-TGTCACACTATAAGCA-3′ (positions 825 to 809), internal to the element. A 668-bp fragment was detected in 6 out of 29 strains of Acinetobacter sp. 13 but not in 10 strains each of A. baumannii and A. haemolyticus (data not shown). These results suggest that IS18 is not widely spread among these Acinetobacter genospecies.
Conclusions.
Acinetobacter sp. 13 strain BM2716 is an aminoglycoside-susceptible strain which harbors a silent aac(6′)-Ij gene encoding a functional AAC(6′)-I enzyme. Transposition of an indigenous copy of IS18 into this strain created a putative hybrid promoter which could promote expression of the gene. IS18 is a member of the IS30 family, which is active in E. coli.
Nucleotide sequence accession number.
The nucleotide sequence of IS18 has been deposited in the GenBank data library under accession no. AF043676.
Acknowledgments
This work was supported in part by a Bristol-Myers Squibb Unrestricted Biomedical Research Grant in Infectious Diseases.
REFERENCES
- 1.Aoyama T, Ttakanami M, Ohtsuka E, Taniayama Y, Marumoto R, Sato H, Ikehara M. Essential structure of E. coli promoter: effect of spacer length between the two consensus sequences on promoter function. Nucleic Acids Res. 1983;11:5855–5864. doi: 10.1093/nar/11.17.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouvet P J M, Jeanjean S. Delineation of new proteolytic genomic species in the genus Acinetobacter. Res Microbiol. 1989;140:291–299. doi: 10.1016/0923-2508(89)90021-1. [DOI] [PubMed] [Google Scholar]
- 3.Ciampi M S, Schmid M B, Roth J R. Transposon Tn10 provides a promoter for transcription of adjacent sequences. Proc Natl Acad Sci USA. 1982;79:5016–5020. doi: 10.1073/pnas.79.16.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalrymple B, Caspers P, Arber W. Nucleotide sequence of the prokaryotic mobile genetic element IS30. EMBO J. 1984;3:2145–2149. doi: 10.1002/j.1460-2075.1984.tb02104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong Q, Sadouk A, van der Lelie D, Taghavi S, Ferhat A, Nuyten J M, Borremans B, Mergeay M, Toussaint A. Cloning and sequencing of IS1086, an Alcaligenes eutrophus insertion element related to IS30 and IS4351. J Bacteriol. 1992;174:8133–8138. doi: 10.1128/jb.174.24.8133-8138.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galas D J, Chandler M. Bacterial insertion sequences. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C: American Society for Microbiology; 1989. pp. 109–162. [Google Scholar]
- 7.Gerischer U, D’Argenio D A, Ornston L N. IS1236, a newly discovered member of the IS3 family, exhibits varied patterns of insertion into the Acinetobacter calcoaceticus chromosome. Microbiology. 1996;142:1825–1831. doi: 10.1099/13500872-142-7-1825. [DOI] [PubMed] [Google Scholar]
- 8.Gustafson C E, Chu S, Trust T J. Mutagenesis of the paracrystalline surface protein array of Aeromonas salmonicida by endogenous insertion elements. J Mol Biol. 1994;237:452–463. doi: 10.1006/jmbi.1994.1247. [DOI] [PubMed] [Google Scholar]
- 9.Haas M J, Dowding J E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- 10.Jaurin B, Normark S. Insertion of IS2 creates a novel ampC promoter in Escherichia coli. Cell. 1983;32:809–816. doi: 10.1016/0092-8674(83)90067-3. [DOI] [PubMed] [Google Scholar]
- 11.Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972;112:917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert T, Gerbaud G, Courvalin P. Characterization of the chromosomal aac(6′)-Ij gene of Acinetobacter sp. 13 and the aac(6′)-Ih plasmid gene of Acinetobacter baumannii. Antimicrob Agents Chemother. 1994;38:1883–1889. doi: 10.1128/aac.38.9.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loh S, Cram D, Skurray R. Nucleotide sequence of the leading region adjacent to the origin of transfer on plasmid F and its conservation among conjugative plasmids. Mol Gen Genet. 1989;219:177–186. doi: 10.1007/BF00261174. [DOI] [PubMed] [Google Scholar]
- 14.Maki H, Murakami K. Formation of potent hybrid promoters of the mutant llm gene by IS256 transposition in methicillin-resistant Staphylococcus aureus. J Bacteriol. 1997;179:6944–6948. doi: 10.1128/jb.179.22.6944-6948.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makris J C, Nordmann P L, Reznikoff W S. Mutational analysis of insertion sequence 50 (IS50) and transposon 5 (Tn5) ends. Proc Natl Acad Sci USA. 1988;85:2224–2228. doi: 10.1073/pnas.85.7.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olasz F, Farkas T, Kiss J, Arini A, Arber W. Terminal inverted repeats of insertion sequence IS30 serve as targets for transposition. J Bacteriol. 1997;179:7551–7558. doi: 10.1128/jb.179.23.7551-7558.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prentki P, Teter B, Chandler M, Galas D J. Functional promoters created by the insertion of transposable element IS1. J Mol Biol. 1986;191:383–393. doi: 10.1016/0022-2836(86)90134-8. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen J L, Odelson D A, Macrina F. Complete nucleotide sequence of insertion element IS4351 from Bacteroides fragilis. J Bacteriol. 1987;169:3573–3580. doi: 10.1128/jb.169.8.3573-3580.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudant E, Courvalin P, Lambert T. Loss of intrinsic aminoglycoside resistance in Acinetobacter haemolyticus as a result of three distinct types of alterations in the aac(6′)-Ig gene, including insertion of IS17. Antimicrob Agents Chemother. 1997;41:2646–2651. doi: 10.1128/aac.41.12.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorisdottir A S, Carias L L, Marshall S H, Green M, Zervos M J, Giorgio C, Mermel L A, Boyce J M, Medeiros A A, Fraimow H, Rice L B. IS6770, an enterococcal insertion-like sequence useful for determining the clonal relationship of clinical enterococcal isolates. J Infect Dis. 1994;170:1539–1548. doi: 10.1093/infdis/170.6.1539. [DOI] [PubMed] [Google Scholar]
- 22.Triglia T, Peterson M G, Kemp D J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988;16:8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]