Summary
Background
Deep brain stimulation (DBS) is an approved treatment option for Parkinson’s Disease (PD), essential tremor (ET), dystonia, obsessive-compulsive disorder and epilepsy in the United States. There are disparities in access to DBS, and clear understanding of the contextual factors driving them is important. Previous studies aimed at understanding these factors have been limited by single indications or small cohort sizes. The aim of this study is to provide an updated and comprehensive analysis of DBS utilization for multiple indications to better understand the factors driving disparities in access.
Methods
The United States based National Inpatient Sample (NIS) database was utilized to analyze the surgical volume and trends of procedures based on indication, using relevant ICD codes. Predictors of DBS use were analyzed using a logistic regression model. DBS-implanted patients in each indication were compared based on the patient-, hospital-, and outcome-related variables.
Findings
Our analysis of 104,356 DBS discharges from 1993 to 2017 revealed that the most frequent indications for DBS were PD (67%), ET (24%), and dystonia (4%). Although the number of DBS procedures has consistently increased over the years, radiofrequency ablation utilization has significantly decreased to only a few patients per year since 2003. Negative predictors for DBS utilization in PD and ET cohorts included age increase and female sex, while African American status was a negative predictor across all cohorts. Significant differences in patient-, hospital-, and outcome-related variables between DBS indications were also determined.
Interpretation
Demographic and socioeconomic-based disparities in DBS use are evident. Although racial disparities are present across all indications, other disparities such as age, sex, wealth, and insurance status are only relevant in certain indications.
Funding
This work was supported by Alan & Susan Hudson Cornerstone Chair in Neurosurgery at University Health Network.
Keywords: Deep brain stimulation, Disparities, National Inpatient Sample, Surgical volume, Trends
Research in context.
Evidence before this study
We conducted a search across PubMed and Google Scholar to identify studies addressing disparities in the utilization of deep brain stimulation (DBS). The search employed the terms: ("deep brain stimulation" OR "DBS") AND ("accessibility" OR "disparity"), focusing on articles published before March 2023 and without language limitations. Additionally, we expanded our search by referencing the bibliographies of relevant articles and exploring citations of those articles. In total, we identified 12 relevant articles. Notably, the majority of these studies are confined to a single DBS indication; Parkinson’s disease. However, two studies deviate from this trend: one lacks a specific indication, while the other, a small-scale single-center study, assesses referral rates without inter-indication analysis. Specific studies demonstrate limitations, encompassing factors like small cohort sizes, geographical coverage restrictions, a constrained range of insurance providers, or reliance on the initial stages of DBS usage (prior to 2010). Despite these inherent constraints, these studies collectively highlight the underutilization of DBS to varying extents. Nonetheless, a comprehensive and current analysis of DBS accessibility and its application across diverse indications is currently lacking.
Added value of this study
Over the years, there has been a consistent increase in the number of DBS procedures in the US, with annual admissions exceeding 5500. Among the main indications for DBS, Parkinson's Disease (PD) accounted for the majority, comprising 67% of cases, followed by essential tremor at 24% and dystonia at 4%. In this study, we conducted an analysis of disease-specific data, which has not been done previously, to gain a better understanding of the disparities specific to each indication. Our findings reveal that racial disparities exist across all primary DBS indications, while other disparities, such as age, sex, wealth, and insurance status, are specific to certain indications.
Implications of all the available evidence
Unveiling disparities in healthcare requires diligent investigation to grasp their full context and extent, as they may not be readily apparent. Achieving equitable healthcare for all individuals necessitates a comprehensive understanding of the circumstances under which health disparities arise. By combining the findings from existing literature with the results of this study, we have gained valuable insights into the disparities specific to certain indications and influenced by non-medical factors. This knowledge will inform future research to explore additional suspected factors and facilitate targeted interventions once identified.
Introduction
It is well-known that disparities in healthcare access for many patients including those with neurological disorders exist and can be based on various factors, such as race, ethnicity, income, education, gender, sexual orientation, disability and geographic location.1 These disparities may not be immediately apparent and often require further investigation to fully understand their context and scope. A thorough understanding of the contexts in which health disparities occur is crucial for addressing them and ensuring equitable healthcare for all individuals. Moreover, this knowledge can be used to increase awareness of these disparities and inform strategies for their elimination.2
Deep brain stimulation (DBS), a neurosurgical procedure that involves the placement of electrodes within the brain to deliver electrical stimulation for the treatment of several neurological disorders, is no exception to these disparities.3 The first modern use of DBS in the US occurred in the early 1970s for the treatment of facial pain.4 However, DBS did not gain widespread adoption until it received approval from the Food and Drug Administration (FDA) in 1997 for essential tremor (ET) and Parkinson’s Disease (PD) tremor.5 While DBS surgery can be associated with severe adverse effects including intracranial hemorrhage and infection, the incidence of these occurrences is low and the procedure is considered to have a good safety profile.6, 7, 8, 9, 10 Nevertheless, despite a growing body of evidence that DBS is a relatively safe and effective treatment, underutilization of DBS persists.6, 7, 8, 9, 10, 11 Our understanding of disparities in this field has been largely derived from previous reports that analyzed cohorts that are (1) small and often consisting of fewer than 1000 DBS cases12, 13, 14, 15, 16, 17, 18; (2) comprising a single DBS indication such as Parkinson’s Disease (PD)3,11,16, 17, 18, 19, 20, 21; (3) located in restricted geographic areas such as Denmark,12 Ontario,13 Calgary,14 Miami,16 or Hawaii18; (4) limited to only one type of insurance provider such as Medicare19; and (5) from early experiences just after the adoption of the DBS treatment (cases prior to 2010).3,14,19,20 An updated and comprehensive analysis of DBS availability and application for multiple indications is lacking, and this study aims to fill this gap.
In this study, we hypothesize that the disparities in access to DBS treatment are not uniform across different indications for DBS, as the clinical presentation and outcomes of these distinct DBS cohorts differ. To test this hypothesis, we examined the volume of DBS procedures using discharge records from the National Inpatient Sample (NIS) database spanning the period from 1993 to 2017 and performed regression analysis to identify potential disparities in DBS utilization for the most common indications as the primary objective of the study. The study’s secondary objectives encompassed: (1) identifying trends and indications of DBS and radiofrequency [RF] ablation, (2) comparing patient, hospital and outcome characteristics between DBS patients with different indications, and (3) examining the characteristics of DBS procedures such as the number of single admission procedures or revision surgeries.
Methods
Data source
In this institutional review board exempt study, we obtained the de-identified retrospective data from the 1993 to 2017 NIS database (https://www.hcup-us.ahrq.gov/nisoverview.jsp, Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, USA). The NIS is the largest publicly available database of all-payer inpatient healthcare data in the US, and is used to generate estimates of inpatient utilization, access, charges, quality, and outcomes at the national and regional levels. The NIS database comprises the records of a total of 184,939,218 inpatient hospital discharges from 1993 to 2017, representing a stratified 20% sample of all non-federal hospitals in the US. The dataset encompasses all non-federal short-term general and other specialty hospitals, excluding Veterans Affairs and Indian Health Service hospitals, as well as short-term rehabilitation hospitals (post-1998), long-term acute care hospitals (post-2012), and other Federal hospitals. Missing values within the data are notably sparse, with overall rates remaining below 1 percent for all documented data attributes, except for race, total charges, and median income. The underlying rationale for this variation is rooted in the incomplete reporting of race information across certain states. Specifically, some states, such as Minnesota, North Dakota, and West Virginia, either entirely omitted race data, while others like California, Louisiana, and Utah partially reported race data due to the sensitivity of certain medical conditions (e.g., HIV and AIDS) or suppression of Hispanic ethnicity information. Notably, diverse hospitals in California were not mandated to report total charges, and reporting was voluntary for Kansas hospitals, while Maryland hospitals didn't provide such data. The absence of the median household income quartile was observed when ZIP Code data was either missing or couldn't be matched with the data source providing median household income. The discharge records in the database have been weighted by HCUP in order to extrapolate annual patient discharge information, and they have been coded by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) diagnostic and procedure codes for the period from 1993 to third quarter of 2015 (2015q1–3), and using ICD-10 codes for the final quarter of 2015 (2015q4) through 2017. The unweighted HCUP data were weighted by using weights given in NIS database for each individual observation prior to conducting any analyses in the study. The manuscript was prepared in accordance with the STROBE checklist.22
Volume analysis and characteristics of DBS surgeries
Surgical volume analysis was performed by selecting cases using the relevant ICD codes from all the procedure fields in the dataset (see Supplementary Table S1 for a list of all the ICD codes used in this study). Patients with a principal diagnosis of epilepsy and phacomatoses were excluded from the analysis of DBS surgical volume, since the “implantation of intracranial neurostimulator lead” code includes the insertion of stereoelectroencephalography (sEEG) electrodes. The included patients were classified according to their principal diagnosis codes (Supplementary Table S1). Indications falling outside FDA approval or Humanitarian Device Exemption (HDE) were categorized as off-label. In addition, patients with principal diagnosis codes unrelated to a known DBS or RF ablation procedure were excluded. Patients with principal diagnosis codes related to revision surgery were also excluded and were reported separately. For the included DBS patients and the excluded epilepsy patients, we collected data on the number of cases in which a code for neurostimulator lead insertion and an implantable pulse generator (IPG) were documented during the same hospital admission.
Identifying predictors of DBS use and comparing characteristics between different DBS cohorts
We retrieved discharge records by querying relevant ICD-10 disease codes in all diagnosis fields for the top three DBS indications, which were determined through analysis of surgical volume (Supplementary Table S1). Patients in each sub-dataset were dichotomized for DBS status by filtering neurostimulator lead insertion codes in all procedure fields. The following variables were analyzed for the identification of DBS-use disparities: patient-related (age, sex, race, patient location [NCHS urban-rural code], primary payer, median household income for patient’s zip code) and hospital-related (census division, bed size, location/teaching status, control/ownership). Subsequently, DBS-implanted patients in each of the three disease groups were compared based on the patient- and hospital-related variables, mentioned above, as well as on all patient refined diagnosis-related group (APR-DRG) risk of mortality and severity of illness subclasses and outcome-related variables (length of stay, inpatient mortality, total charges and patient disposition).
Statistical analysis
We presented categorical variables as proportions and continuous variables as mean and standard deviation (SD). The trend of the total number of procedures was plotted using Mann–Kendall test. Welch’s one-way analysis of variance (ANOVA) was used to compare continuous variables between the three DBS cohorts, and post-hoc pairwise comparisons were performed by using the Student’s t-test for equal variance and Games–Howell test for non-equal variance. Proportions were compared using the chi-square test. Following a significant chi-square test, post-hoc tests were conducted to test significant differences among all pairs of population, by performing all chi-square tests for all pairs of populations and then adjusting the resulting P-values for inflation due to multiple comparisons using the Bonferroni correction. Predictors of DBS use were analyzed by multivariable binary logistic regression through the purposeful selection of variables method. The binary dependent variable was the DBS status, while the independent predictor variables encompassed age, sex, race, income quartile of the patient's zip code, payer status, patient location, hospital type, size, control, and division. Univariable regression analyses were conducted for each variable, and variables with results yielding a P value less than 0.25 were subsequently considered for inclusion in the multivariable analysis. No additional adjustments or removal of variables were made during the multivariable analysis, as the purpose of constructing these models is not prediction but rather to demonstrate the effects of variables on the outcome, irrespective of their predictive ability. The results were reported as odds ratios (OR) and 95% confidence intervals (CI).
The Multivariate Imputation by Chained Equations (MICE) R package's md.pattern() function was employed to detect instances of missing data. We calculated the proportions of missing values in the PD, ET, and dystonia cohorts for various variables: race (3.3/4.5/4.9%), income by zip code (1.5/1.4/2.1%), total charges (0.9/1.7/1.2%), and hospital type (0.2/0.2/0.7%), respectively. Remaining variables exhibited negligible missing values, typically below 0.1%. Nonetheless, imputations were performed for all variables, irrespective of their missing value proportion. Employing the same MICE R package, multiple imputations were conducted using the mice() function. We employed five multiple imputations in a single iteration. All variables utilized in our logistic regression model were employed as predictors by the imputation process to fill in the missing values. For continuous, binary, and ordinal variables, Bayesian linear regression, logistic regression, and polytomous logistic regression imputation methods were respectively utilized. We conducted logistic regression diagnostics for each model to assess linearity between continuous predictor variables and the logit of the outcome. We also examined the presence of influential observations using the ‘augment()’ function from the broom package and checked for multicollinearity among predictors using the ‘vif()’ function from the car package. The scatter plot indicated a linear association between the age variable and the DBS utilization outcome in logit scale. Furthermore, no collinearity among predictors was detected. In terms of influential observations, data points with absolute standardized residuals exceeding 3 were deemed significant (nPD = 192; nET = 55; nDystonia = 19) and were consequently excluded from the model dataset. The values of the Area Under the Receiver Operating Characteristic Curve (AUC-ROC)—PD: 0.9, ET: 0.82, dystonia: 0.85—signify good discrimination power in the models. A P value < 0.05 was considered statistically significant. The HCUP dataset was extracted using SPSS Statistics v24.0 (IBM, NY, USA) and all data were analyzed using R Studio (2022.02.3+492, “Prairie Trillium” https://www.rstudio.com).
Role of the funding source
The funders had no role in study design, data collection, data analysis, or the initial writing of the report.
Results
Analysis of DBS procedure volume and characteristics
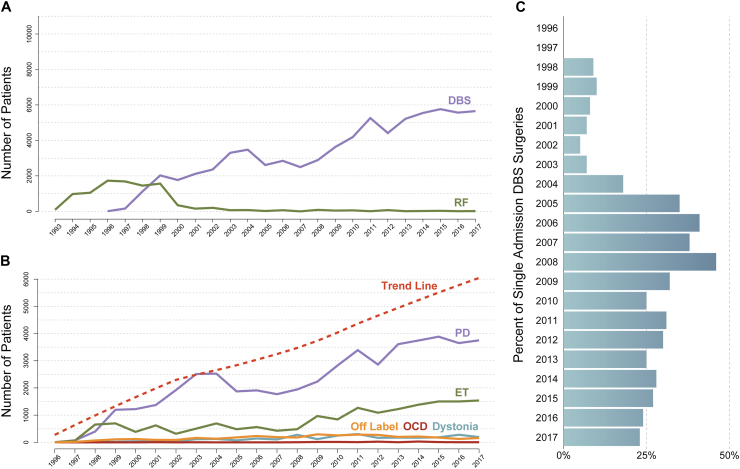
Between 1996 and 2017, a total of 104,356 discharges with codes for “neurostimulator lead implantation” were identified, including 72,427 for DBS indications, 24,865 for epilepsy and phacomatoses, 2925 for revision procedures, and 4139 for unrelated primary diagnosis codes. No DBS discharges were identified from 1993 to 1995. The annual volume of DBS and RF admissions were presented in Fig. 1A. The most common indications for DBS procedure were PD (n = 48,674/72,437, 67.2%), ET (n = 17,248/72,437, 23.8%) and dystonia (n = 2803/72,437, 3.8%) (Fig. 1B). In the DBS patient cohort (excluding those with epilepsy), 24.7% (19,673/79,491) of admissions with neurostimulator lead implantation code also had a concurrent code for IPG implantation, while this percentage was 8.2% (1941/23,633) in the excluded epilepsy cohort (Fig. 1C). Moreover, 6.3% (825/12,915) of admissions had the ICD-10 code for robotic surgery. Among the revision procedures, 156/2925 (5.3%) were due to infections, 35/2925 (1.2%) involved disruption of the surgical wound, 2528/2925 (86.4%) were mechanical complications, and 206/2925 (7.1%) were other complications. Detailed tables of discharge volumes for DBS and RF procedures by indication were provided in Supplementary Table S2.
Fig. 1.
A) Number of patients per year who underwent deep brain stimulation (DBS) or radiofrequency (RF) ablation surgeries. The number of DBS procedures has consistently increased over the years. However, the utilization of radiofrequency ablation has significantly decreased, with only a few patients per year undergoing this procedure since 2003. B) The number of patients per year who underwent DBS, as enumerated by Food and Drug Administration (FDA)-approved indications (Parkinson’s disease [PD] and essential tremor [ET]), Humanitarian Device Exemption (HDE) indications (dystonia and obsessive-compulsive disorder [OCD]), and off-label use. The trend line depicting a progressive increase over time showcases the total number of patients undergoing DBS per year. The volume of PD cases experienced an exponential increase until 2003, followed by a decline until 2008. However, a reversal in this trend occurred in 2009, leading to a continuous rise until 2017. As for ET DBS, there was an initial upward trend until 1998, followed by a plateau that persisted for over a decade. It wasn't until 2009 that the number of ET-DBS procedures started to increase again, albeit not as dramatically as observed in PD cases. For dystonia, OCD, and off-label indications, the maximum number of DBS cases per year reached 306, 30, and 296, respectively. C) Single admissions for concurrent neurostimulator lead and implantable pulse generator (IPG) placements expressed as a percentage of all DBS neurostimulator lead placement procedure admissions over years. DBS surgery involves two stages: stage 1 includes the implantation of the leads, and stage 2 entails the implantation of the IPG. While some surgeons opt for combining both stages in a single admission, others prefer to stage the procedures across multiple admissions. Notably, 24.7% of admissions completed the entire DBS procedure (stage 1 + stage 2) within a single admission.
Predictors of DBS use
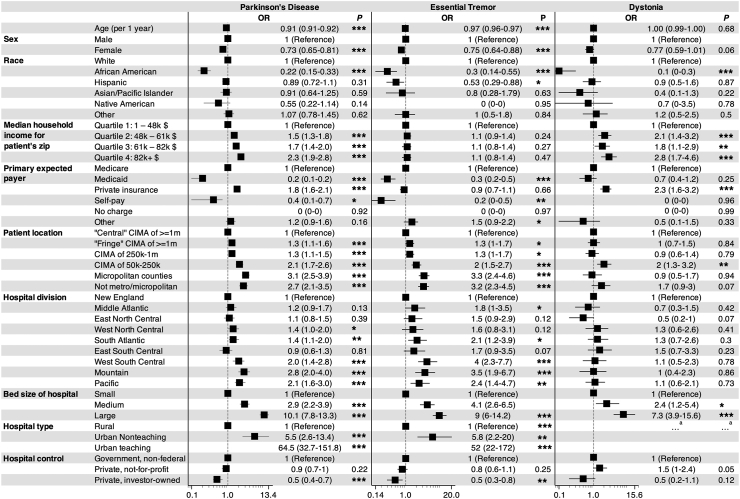
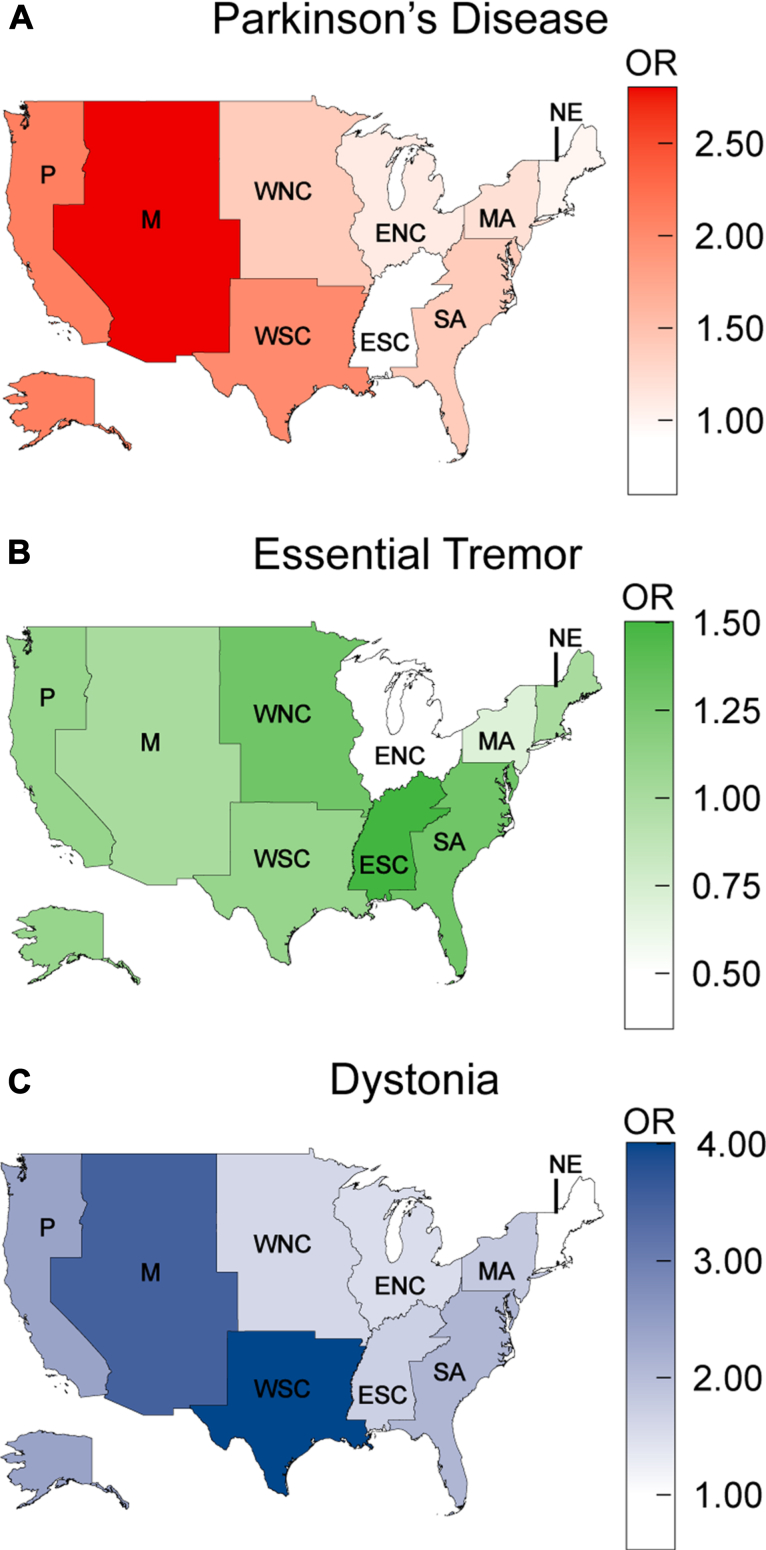
In this study, increasing age and female sex were found to be negative predictors of DBS use in PD and ET cohorts but not in dystonia cohort (Fig. 2). For each year of increased age, the odds of undergoing a DBS procedure for a patient with PD and ET decreased by 0.91 (95% CI = 0.91, 0.92; P < 0.001) and 0.97 (95% CI = 0.96, 0.97; P < 0.001), respectively. Likewise, females with PD (OR: 0.73; 95% CI = 0.65, 0.81; P < 0.001) and ET (OR: 0.75; 95% CI = 0.64, 0.88; P < 0.001) exhibited significantly lower odds ratios for DBS implantation compared to males. African American status (PD–OR: 0.22; 95% CI = 0.15, 0.33; P < 0.001; ET–OR: 0.3; 95% CI = 0.14, 0.55; P < 0.001; dystonia–OR: 0.1; 95% CI = 0, 0.3; P < 0.001) was a strong negative predictor for all three DBS cohorts, while Hispanic status (OR: 0.53; 95% CI = 0.29, 0.88; P < 0.05) was a negative predictor for only the ET cohort. Increasing median household income in the patient’s zip code was a positive predictor for PD and dystonia cohorts, but was not found to be predictive of DBS use in the ET cohort. Relative to Medicare, private insurance positively predicted DBS use in the PD (OR: 1.8; 95% CI = 1.6, 2.1; P < 0.001) and dystonia (OR: 2.3; 95% CI = 1.6, 3.2; P < 0.001) cohorts, but was not significant in the ET cohort (OR: 0.9; 95% CI = 0.7, 1.1; P = 0.66). Medicaid (PD–OR: 0.2; 95% CI = 0.1, 0.2; P < 0.001; ET–OR: 0.3; 95% CI = 0.2, 0.5; P < 0.001) and self-pay (PD–OR: 0.4; 95% CI = 0.1, 0.7; P < 0.05; ET–OR: 0.2; 95% CI = 0, 0.5; P < 0.01) predicted nonuse in PD and ET cohorts. Patients living in smaller or more remote locations were more likely to receive DBS in the PD and ET cohorts. Among hospital-related variables, larger bed size and urban status positively, and investor-owned private status negatively predicted the use of DBS. In comparison to the New England census region, the South Atlantic, West South Central, Mountain and Pacific regions were positive predictors of DBS use in the PD and ET cohorts, while the Middle Atlantic region was a positive predictor only for the ET group and the West North Central region was a positive predictor only for the PD cohort (Fig. 3).
Fig. 2.
Predictors of deep brain stimulation (DBS) utilization. Reference odds ratios (ORs) are universally accepted as 1. OR values below 1 serve as negative predictors, whereas values above 1 serve as positive predictors of DBS utilization. The 95% confidence intervals were given in parenthesis. aHospital type was removed to allow for logistic regression model convergence. ∗∗∗ represents a P-value <0.001, ∗∗ represents a P-value <0.01, and ∗ represents a P-value <0.05. CIMA: counties in metro areas.
Fig. 3.
Map of United States partitioned by census regions, with odds ratios (OR) denoting the geographic differences in deep brain stimulation procedure utilization for Parkinson’s disease (A), essential tremor (B), and dystonia (C). New England (NE) serves as the reference census region, denoted by an OR of 1. P: Pacific; M: Mountain; WNC/ENC: West/East North Central; WSC/ESC: West/East South Central; SA/MA: South/Middle Atlantic.
Comparison of DBS patients with different indications
The means or proportions of patient-, hospital-, and outcome-related variables for the PD, ET, and dystonia groups and the PD-DBS, ET-DBS, and dystonia-DBS subgroups were presented in Table 1. Out of all admissions, the procedure of neurostimulator lead implantation was performed in 1.1% (8655/752,245) of PD patient admissions, 2.1% (3690/172,485) of ET patient admissions, and 1.7% (1270/73,080) of dystonia patient admissions. There were significant differences between the three distinct DBS cohorts for all patient-related variables and APR-DRG scores (P < 0.001). The census division of the hospital and the patient disposition were the only hospital- and outcome-related variables that significantly differed between the groups. The DBS-implanted patients with dystonia (53 ± 18 years) were significantly younger than those with PD (65 ± 8 years), and the patients with PD were younger than the patients with ET (67 ± 10 years). The female/male ratio is significantly lower in PD-DBS group (30.5%) compared to the ratio in all DBS implanted patients for the three indications. The ET-DBS cohort had a higher proportion of white individuals (P < 0.001) and a lower proportion of Hispanic individuals (P < 0.001) compared to the proportions in the entire DBS patient population. In addition, the proportion of individuals in the lowest income quartile (quartile 1) was higher (P < 0.01) and the proportion of individuals in the highest income quartile (quartile 4) was lower (P < 0.001) in the ET-DBS cohort. In contrast, quartile 4 individuals were higher in PD-DBS cohort (P < 0.01). Dystonia-DBS group had a higher proportion of individuals with private insurance and Medicaid coverage compared to those with Medicare coverage (P < 0.001), whereas the opposite was true for ET-DBS patients (P < 0.001). The PD-DBS cohort had a lower proportion of individuals with moderate APR-DRG scores (P < 0.001) and a higher proportion of individuals with minor scores (P < 0.001), while the ET-DBS had the opposite distribution (P < 0.001).
Table 1.
Comparison of patient, hospital and outcome characteristics across different DBS-cohorts.
| PD | ET | Dystonia | PD-DBS | ET-DBS | Dystonia-DBS | P | |
|---|---|---|---|---|---|---|---|
| Age (year) | 76.57 ± 9.74 n = 752,225 |
72.87 ± 12.79 n = 172,480 |
55.36 ± 22.59 n = 73,005 |
65.11 ± 8.93 n = 8655 |
67.57 ± 10.61 n = 3690 |
53.68 ± 18.95 n = 1270 |
<0.001; Post-hoc PD-ET∗∗∗ PD-Dystonia∗∗∗ ET-Dystonia∗∗∗ |
| Female n (%) | 314,700 (41.8) n = 752,040 |
93,560 (54.3) n = 172,430 |
43,405 (59.4) n = 73,060 |
2640 (30.5)∗∗∗n = 8650 | 1645 (44.6)∗∗∗n = 3685 | 700 (55.1)∗∗∗n = 1270 | <0.001 |
| Race, n (%) | n = 726,835 | n = 164,760 | n = 69,520 | n = 8260 | n = 3420 | n = 1175 | <0.001 |
| White | 582,185 (80.1) | 148,520 (90.1) | 49,270 (70.9) | 7020 (85.0)∗∗∗ | 3220 (94.2)∗∗∗ | 1010 (86.0) | |
| African American | 50,735 (7.0) | 6590 (4.0) | 10,855 (15.6) | 145 (1.8) | 45 (1.3) | 20 (1.7) | |
| Hispanic | 55,790 (7.7) | 5440 (3.3) | 5845 (8.4) | 565 (6.8)∗∗ | 75 (2.2)∗∗∗ | 80 (6.8) | |
| Asian or Pacific Islander | 17,900 (2.5) | 1365 (0.8) | 1330 (1.9) | 215 (2.6)∗ | 25 (0.7) | 15 (1.3) | |
| Native American | 2515 (0.3) | 405 (0.2) | 420 (0.6) | 35 (0.4) | 0 | 5 (0.4) | |
| Other | 17,710 (2.4) | 2440 (1.5) | 1800 (2.6) | 280 (3.4) | 55 (1.6) | 45 (3.8) | |
| Median household income for patient's zip code, n (%) | n = 741,145 | n = 170,130 | n = 71,510 | n = 8480 | n = 3625 | n = 1240 | <0.001 |
| Quartile 1: 1–47,999$ | 193,165 (26.1) | 37,225 (21.9) | 22,030 (30.8) | 1370 (16.2) | 780 (21.5)∗∗ | 180 (14.5) | |
| Quartile 2: 48,000–60,999$ | 192,115 (25.9) | 46,945 (27.6) | 18,275 (25.6) | 2165 (25.5) | 1045 (28.8) | 365 (29.4) | |
| Quartile 3: 61,000–81,999$ | 183,315 (24.7) | 45,900 (27.0) | 16,840 (23.5) | 2295 (27.1) | 1010 (27.9) | 305 (24.6) | |
| Quartile 4: 82,000+ $ | 172,550 (23.3) | 40,060 (23.5) | 14,365 (20.1) | 2650 (31.2)∗∗ | 790 (21.8)∗∗∗ | 390 (31.5) | |
| Primary expected payer, n (%) | n = 751,545 | n = 172,235 | n = 72,985 | n = 8650 | n = 3675 | n = 1270 | <0.001 |
| Medicare | 656,080 (87.3) | 135,945 (78.9) | 40,920 (56.1) | 5565 (64.3) | 2610 (71.0)∗∗∗ | 610 (48.0)∗∗∗ | |
| Medicaid | 23,965 (3.2) | 7715 (4.5) | 14,465 (19.8) | 195 (2.3)∗∗∗ | 115 (3.1) | 125 (9.8)∗∗∗ | |
| Private insurance | 55,770 (7.4) | 23,990 (13.9) | 14,015 (19.2) | 2555 (29.5) | 785 (21.4)∗∗∗ | 510 (40.2)∗∗∗ | |
| Self-pay | 3655 (0.5) | 1635 (0.9) | 1500 (2.1) | 50 (0.6) | 15 (0.4) | 0 | |
| No charge | 295 (0) | 140 (0.1) | 145 (0.2) | 0 | 0 | 0 | |
| Other | 11,780 (1.6) | 2810 (1.6) | 1940 (2.7) | 285 (3.3) | 150 (4.1) | 25 (2.0) | |
| Patient location: NCHS urban-rural code, n (%) | n = 750,680 | n = 172,065 | n = 72,535 | n = 8605 | n = 3685 | n = 1260 | <0.001 |
| "Central" counties of metro areas of ≥1 million population | 209,930 (28.0) | 41,235 (24.0) | 22,215 (30.6) | 2310 (26.8)∗ | 730 (19.8)∗∗ | 320 (25.4) | |
| "Fringe" counties of metro areas of ≥1 million population | 193,460 (25.8) | 43,850 (25.5) | 16,815 (23.2) | 2260 (26.3) | 820 (22.3) | 325 (25.8) | |
| Counties in metro areas of 250,000–999,999 population | 146,825 (19.6) | 37,075 (21.5) | 15,755 (21.7) | 1785 (20.7) | 855 (23.2) | 245 (19.4) | |
| Counties in metro areas of 50,000–249,999 population | 72,545 (9.7) | 19,805 (11.5) | 6900 (9.5) | 910 (10.6) | 470 (12.8) | 185 (14.7) | |
| Micropolitan counties | 72,305 (9.6) | 17,400 (10.1) | 6260 (8.6) | 790 (9.2) | 485 (13.2)∗ | 80 (6.3) | |
| Not metropolitan or micropolitan counties | 55,615 (7.4) | 12,700 (7.4) | 4590 (6.3) | 550 (6.4) | 325 (8.8) | 105 (8.3) | |
| Census division of hospital, n (%) | n = 752,245 | n = 172,485 | n = 73,080 | n = 8655 | n = 3690 | n = 1270 | <0.001 |
| New England | 37,255 (5.0) | 8760 (5.1) | 4265 (5.8) | 280 (3.2) | 90 (2.4) | 70 (5.5) | |
| Middle Atlantic | 116,560 (15.5) | 19,495 (11.3) | 9370 (12.8) | 1245 (14.4)∗∗ | 345 (9.3)∗ | 125 (9.8) | |
| East North Central | 124,705 (16.6) | 33,180 (19.2) | 12,180 (16.7) | 1160 (13.4) | 515 (14.0) | 125 (9.8) | |
| West North Central | 52,795 (7.0) | 17,660 (10.2) | 6465 (8.8) | 775 (9.0) | 450 (12.2) | 180 (14.2) | |
| South Atlantic | 150,460 (20.0) | 33,080 (19.2) | 15,100 (20.7) | 1500 (17.3) | 670 (18.2) | 280 (22.0) | |
| East South Central | 50,460 (6.7) | 9840 (5.7) | 4240 (5.8) | 375 (4.3) | 210 (5.7) | 85 (6.7) | |
| West South Central | 81,680 (10.9) | 13,780 (8.0) | 7210 (9.9) | 915 (10.6) | 425 (11.5) | 100 (7.9) | |
| Mountain | 39,095 (5.2) | 12,440 (7.2) | 4765 (6.5) | 855 (9.9) | 460 (12.5) | 95 (7.5) | |
| Pacific | 99,235 (13.2) | 24,250 (14.1) | 9485 (13.0) | 1550 (17.9) | 525 (14.2) | 210 (16.5) | |
| Bed size of hospital, n (%) | n = 752,245 | n = 172,485 | n = 73,080 | n = 8655 | n = 3690 | n = 1270 | 0.3 |
| Small | 158,775 (21.1) | 34,025 (19.7) | 13,345 (18.3) | 325 (3.8) | 140 (3.8) | 55 (4.3) | |
| Medium | 225,320 (30.0) | 47,450 (27.5) | 19,970 (27.3) | 1285 (14.8) | 675 (18.3) | 200 (15.7) | |
| Large | 368,150 (48.9) | 91,010 (52.8) | 39,765 (54.4) | 7045 (81.4) | 2875 (77.9) | 1015 (79.9) | |
| Location/teaching status of hospital, n (%) | n = 752,245 | n = 172,485 | n = 73,080 | n = 8655 | n = 3690 | n = 1270 | 0.09 |
| Rural | 84,025 (11.2) | 17,475 (10.1) | 5815 (8.0) | 45 (0.5) | 25 (0.7) | 0 | |
| Urban nonteaching | 209,235 (27.8) | 42,385 (24.6) | 14,870 (20.3) | 355 (4.1) | 170 (4.6) | 15 (1.2) | |
| Urban teaching | 458,985 (61.0) | 112,625 (65.3) | 52,395 (71.7) | 8255 (95.4) | 3495 (94.7) | 1255 (98.8) | |
| Control/ownership of hospital, n (%) | n = 752,245 | n = 172,485 | n = 73,080 | n = 8655 | n = 3690 | n = 1270 | 0.28 |
| Government, non-federal | 75,255 (10.0) | 16,895 (9.8) | 9085 (12.4) | 1275 (14.7) | 570 (15.4) | 140 (11.0) | |
| Private, not-for-profit | 557,420 (74.1) | 137,685 (79.8) | 54,970 (75.2) | 6925 (80.0) | 2965 (80.4) | 1080 (85.0) | |
| Private, investor-owned | 119,570 (15.9) | 17,905 (10.4) | 9025 (12.3) | 455 (5.3) | 155 (4.2) | 50 (3.9) | |
| All patient refined DRG: risk of mortality subclass, n (%) | n = 752,135 | n = 172,470 | n = 72,995 | n = 8655 | n = 3690 | n = 1270 | <0.001 |
| Minor likelihood of dying | 81,150 (10.8) | 46,000 (26.7) | 29,350 (40.2) | 7575 (87.5)∗∗ | 2965 (80.4)∗∗∗ | 1120 (88.2) | |
| Moderate likelihood of dying | 293,125 (39.0) | 61,105 (35.4) | 21,565 (29.5) | 895 (10.3)∗ | 605 (16.4)∗∗∗ | 120 (9.4) | |
| Major likelihood of dying | 278,675 (37.0) | 50,200 (29.1) | 15,750 (21.6) | 115 (1.3) | 80 (2.2) | 25 (2.0) | |
| Extreme likelihood of dying | 99,185 (13.2) | 15,165 (8.8) | 6330 (8.7) | 70 (0.8) | 40 (1.1) | 5 (0.4) | |
| All patient refined DRG: severity of illness subclass, n (%) | n = 752,135 | n = 172,470 | n = 72,995 | n = 8655 | n = 3690 | n = 1270 | <0.001 |
| Minor loss of function (includes cases with no comorbidity or complications) | 49,790 (6.6) | 25,310 (14.7) | 7610 (10.4) | 6335 (73.2)∗∗∗ | 2330 (63.1)∗∗∗ | 805 (63.4) | |
| Moderate loss of function | 285,175 (37.9) | 68,805 (39.9) | 30,910 (42.3) | 2075 (24.0)∗∗∗ | 1200 (32.5)∗∗∗ | 380 (29.9) | |
| Major loss of function | 317,470 (42.2) | 62,900 (36.5) | 26,175 (35.8) | 200 (2.3) | 120 (3.3) | 65 (5.1) | |
| Extreme loss of function | 99,700 (13.3) | 15,455 (9.0) | 8300 (11.4) | 45 (0.5) | 40 (1.1) | 20 (1.6) | |
| Outcomes | |||||||
| Length of stay (days) | 5.80 ± 7.03 n = 752,120 |
5.15 ± 6.01 n = 172,470 |
8.42 ± 13.64 n = 73,075 |
1.69 ± 1.97 n = 8655 |
1.59 ± 1.81 n = 3690 |
2.54 ± 9.68 n = 1270 |
0.17 |
| Total charges (USD, 2015–2017) | 52,729 ± 69,215 n = 745,650 |
51,923 ± 64,255 n = 169,605 |
65,948 ± 139,356 n = 72,155 |
101,494 ± 72,268 n = 8500 |
94,212 ± 60,176 n = 3665 |
111,139 ± 154,242 n = 1265 |
0.02; Post-hoc NS |
| Inpatient mortality (%) | 26,420 (3.5) n = 750,985 |
2945 (1.7) n = 172,335 |
930 (1.3) n = 72,990 |
0 n = 8650 |
5 (0.1) n = 3685 |
0 n = 1270 |
0.26 |
| Disposition of patient, n (%) | n = 750,985 | n = 172,335 | n = 72,990 | n = 8650 | n = 3685 | n = 1270 | <0.01 |
| Routine | 201,010 (26.7) | 78,950 (45.8) | 38,080 (52.2) | 7330 (84.7)∗ | 3275 (88.9) | 1125 (88.6) | |
| Transfer to short-term hospital | 16,410 (2.2) | 3010 (1.7) | 1945 (2.7) | 0 | 0 | 5 (0.4)∗ | |
| Transfer other: includes skilled nursing facility (SNF), intermediate care facility (ICF), another type of facility | 352,440 (46.9) | 51,570 (29.9) | 20,820 (28.5) | 485 (5.6) | 195 (5.3) | 60 (4.7) | |
| Home health care (HHC) | 151,410 (20.1) | 34,905 (20.3) | 10,525 (14.4) | 835 (9.7)∗∗ | 210 (5.7)∗ | 80 (6.3) | |
| Against medical advice (AMA) | 3720 (0.5) | 935 (0.5) | 680 (0.9) | 0 | 0 | 0 | |
| Died | 26,420 (3.5) | 2945 (1.7) | 930 (1.3) | 0 | 5 (0.1) | 0 | |
| Discharge alive, destination unknown | 205 (0) | 20 (0) | 10 (0) | 0 | 0 | 0 |
The columns labeled PD, ET, and dystonia represent the data of admissions for all patients diagnosed with Parkinson's Disease (PD), Essential Tremor (ET), and dystonia, respectively. In contrast, the columns labeled PD-DBS, ET-DBS, and dystonia-DBS specifically indicate admissions within the specified disease cohorts that underwent deep brain stimulation (DBS) implantation procedures during the corresponding admission period.
The values in each cell represent the exact count and proportion of the specified characteristic within the given cohort, unless noted otherwise. “The total number of discharges” is presented as an exact count (n), while the “age”, “length of stay” and “total charges” are presented as mean ± standard deviation.
Statistical analysis was only conducted among DBS cohorts to compare the proportion of the given characteristic in a specified cohort (i.e., the PD-DBS cohort) with the proportion of that characteristic in the entire patient population (i.e., all DBS-implanted patients).
The statistical significance of the results is indicated as follows: ∗∗∗ represents a P-value <0.001, ∗∗ represents a P-value <0.01, and ∗ represents a P-value <0.05.
Discussion
Our investigation utilized an administrative database study to explore the impact of patient demographics and diagnosis on the use of DBS. In contrast to prior studies that focused on a single indication for DBS,3,11,16, 17, 18, 19, 20, 21 we broadened the scope of our investigation to encompass multiple indications. This approach may aid in the detection of non-disease-specific factors that contribute to disparities. Our study initially showed that, on average, there were 5547 admissions annually for DBS lead insertion procedures in the US over the past 5 years of the study period. Among all DBS admissions during the study period, 67% were for PD, 24% were for ET, and 4% were for dystonia. The most notable finding of this study was that African American status was the only factor that negatively predicted the use of DBS across all three top DBS indications. We also identified some non-uniform disparities across different DBS indications. While DBS utilization for PD and ET was lower among women, older patients, and those from central and larger counties, these demographic factors did not significantly impact DBS utilization for dystonia. Interestingly, disparities for PD and dystonia were observed among individuals with private insurance and those from wealthier neighborhoods, but not for ET. The PD-, ET- and dystonia-DBS cohorts differed significantly from one another in terms of age, gender, income, insurance status, geographical location, disease severity, and disposition.
Trends in DBS use
The volume of DBS procedures has shown a consistent upward trend over the years, with only temporary minor dips in certain years. In contrast to the sharp rise in DBS procedures since its adoption, RF volume began to consistently drop in 1999 and eventually became almost non-existent within a few years. It is noteworthy that the outpatient procedures of sub/thalamotomies and pallidotomies, which can be performed using radiosurgery, are not included in this database due to their outpatient nature. Until 1998, the most common indication for DBS was ET. However, in 1999 there was a shift towards PD as the most frequent indication, which coincided with the prospective demonstration of the effect of STN DBS in PD in North America in the same year.23 The volume of PD cases saw an exponential increase until 2003, after which there was a decline until 2008. However, this trend was reversed in 2009, coinciding with the demonstration of the superiority of DBS over best medical therapy alone.24 After an initial rising trend in ET DBS up to 1998, there was a plateau that lasted for more than a decade. In 2009, the number of ET-DBS procedures began to rise again, but the increase was not as dramatic as that observed in PD. Dystonia and off-label indications made up only a small percentage of all DBS procedures. Despite having an FDA HDE approval, DBS procedures for OCD treatment remained limited, with only a modest number of cases performed each year.
Disparities in DBS use
African Americans were found to have received DBS at rates 4.5, 3.3, and 10 times lower than white patients for the treatment of PD, ET and dystonia, respectively. Despite accounting for 7% of PD cases, 4% of ET cases, and 15.6% of dystonia cases, only 1.8%, 1.3% and 1.7% of DBS procedures were performed on African Americans for these diagnoses. In contrast, white patients, who accounted for 80.1% of PD, 90.1% of ET, and 70.9% of dystonia cases, received a larger share of DBS procedures (85%, 94.2%, and 86% of cases performed for PD, ET, and dystonia, respectively). The multifactorial reasons underlying this racial disparity may arise at two distinct stages for DBS surgery: Firstly, during the initial screening and referral process by neurologists or primary physicians; and secondly, during the assessment of patients for surgery by the surgical team. Further, these factors can be broadly classified as medical and non-medical. Medical comorbidities are crucial determinants of the selection process of DBS candidates, both at the level of initial screening and surgical assessment, as they can impact surgical complication rates.25 The number of comorbidities tends to rise as age increases, potentially explaining the age discrepancy in DBS utilization for PD and ET.26 The effect of comorbidities on race disparity was hypothesized, as there is evidence that African Americans are more likely to experience comorbidities such as obesity and diabetes compared to white patients.11,27 However, previous studies aimed at investigating this hypothesis found that the number of comorbidities does not significantly contribute to racial disparities in DBS utilization for PD patients.3,11 Our findings revealed that the ET-DBS cohort had a significantly higher proportion of patients with moderate number of comorbidities compared to the PD-DBS cohort (32.5% vs %24%), indicating more liberal selection criteria for DBS surgery among ET patients than PD patients. However, despite the liberal selection criteria for comorbidities, we still observed consistent disparities in DBS utilization rates across both indications. These results suggest that medical comorbidities may not be the sole explanation for race disparities. Furthermore, racial disparity between African Americans and whites exists for other surgeries that are performed even in the presence of a high number of comorbidities.28 Another medical hypothesis suggested by previous studies is that African Americans with PD have more severe disease on initial presentation, which could be a contraindication for DBS surgery and contribute to disparity.19 However, our study revealed a similar discrepancy in ET cases, and the extent of disease severity during the initial presentation cannot explain the racial disparity in DBS utilization in ET.
Non-medical factors may include implicit or explicit biases, patient preferences or mistrust, and discrepancies in the tendency to refer among referring physicians. The documented presence of unconscious/implicit or conscious/explicit biases among certain physicians is a known factor and may contribute to the racial disparity against African Americans in DBS utilization.29 These biases stem from negative stereotypes, and research shows that the reported levels of negative stereotypes toward minority groups are unevenly distributed. For instance, while white persons hold more negative views towards black persons, Hispanics, and Asians compared to themselves, black persons are viewed more negatively than all other groups, and Hispanics are viewed twice as negatively as Asians.30 Our findings are consistent with this pattern, revealing that racial discrimination is solely directed towards African Americans across all three indications, whereas Hispanics are only significantly affected in the case of ET, and other minority groups are not significantly impacted. Patient preference may play a significant role in driving various disparities, including those related to race or sex. Our study revealed that male patients are 1.3 times more likely than female patients to undergo DBS surgery for both PD and ET. Previous studies have suggested that women may be less inclined to pursue DBS surgery due to various reasons, including concerns about potential complications.16,31 This condition may be a key factor driving sex disparities and may also contribute to racial disparities in the utilization of DBS. African American patients may be less likely to pursue DBS surgery due to factors such as medical mistrust, inadequate communication between patients and physicians about the surgical process and complications, lack of targeted marketing of DBS devices to African Americans, fear of potential complications, and the financial and time burden of the procedure and follow-up care. Additionally, it is possible that a greater proportion of African American patients prefer to receive care from neurologists, who may have a lower tendency to suggest DBS surgery as a treatment option. Unfortunately, the HCUP dataset is limited in its ability to investigate all these non-medical factors. Consequently, future studies should focus on examining the rates of DBS surgery offered to minority groups and identifying the factors that may lead to a decline in the offer of DBS surgery in order to gain a better understanding of these disparities.
Wealth disparity is evident in the utilization of DBS for PD and dystonia, whereby having private insurance and residing in wealthier neighborhoods are positive predictors of undergoing the procedure. Interestingly, this trend does not hold true for ET, as individuals with private insurance or living in neighborhoods with a higher median household income are not more likely to receive DBS for this indication. One possible explanation for this phenomenon is that ET patients may perceive DBS as a more favorable long-term investment compared to PD patients. The tremor-suppressive effects of DBS have been demonstrated to persist for more than a decade in ET patients.32 Similarly, long-term motor improvements have been observed in PD patients who have undergone DBS.33 However, it is important to note that PD is a progressive disease with clinical manifestations extending beyond tremors including cognitive decline, depression, pain, speech and gait problems that DBS has little to no effect on, ultimately resulting in the debilitation of most PD patients. Furthermore, the burden of managing follow-up visits for programming is typically more arduous for patients with PD and their caregivers compared to those with ET. PD patients usually require 4–5 programming visits within the first 6 months after surgery, while only 1–2 appointments are often sufficient for ET patients to achieve optimal programming.34,35 The comparatively higher frequency of battery replacement surgeries required for patients with PD and dystonia with bilateral implants, compared to those with ET who have unilateral implants, represents an additional burden for PD and dystonia patients to overcome.36 All these challenges may lead some patients with PD to decide against undergoing the procedure, whereas patients with ET may still choose to proceed with the surgery despite financial constraints. The economic considerations of DBS surgeries also play a significant role in determining the location where the surgery is performed, as hospital status and location have been found to be significant predictors of DBS surgeries. Not-for-profit or government-owned large urban teaching hospitals tend to be the most frequent sites for DBS procedures, likely due to the comparatively lower reimbursement rates for such procedures. It is therefore not surprising that privately owned or for-profit hospitals tend to focus on other services, given the typical duration of the DBS procedure and the reimbursement rates for other neurosurgical operations. Certain census divisions, including Mountain, Pacific, and West South Central, serve as positive predictors of DBS use in contrast to the reference division, New England. Such geographical disparities may arise from an inequity between the high population density and the limited number of hospital beds within specific divisions, potentially leading to extended waitlists for DBS surgery.
Limitations
This study has some limitations, as the dataset only represents a 20% stratified sample of non-federal hospitals in the US rather than the complete number of cases discharged, which makes it unreliable to analyze rare indications with a volume of fewer than 10 cases per year. Although federal hospitals constitute only around 3% of the total number of hospitals in the US, their omission from the dataset could lead to the exclusion of relevant cohorts. Performing staged DBS procedures within the same year, which involves implanting one electrode during each hospital admission, may inflate the rate of DBS procedures. The use of a single ICD code for all types of intracranial electrode implantation can lead to the misidentification of cases that are not DBS but rather sEEG, subdural grids, or other procedures, such as Gasserian ganglion stimulation for trigeminal neuralgia. Due to the inability to distinguish between DBS and other types of electrode implantation, we excluded epilepsy cases, even though epilepsy is a known DBS indication. Although it is possible that procedures and diagnoses were misclassified during hospital discharge or the data collection process, any potential errors are expected to have a minimal impact on the overall significance of the analysis, given the large volume of patients studied. The dataset is limited in its ability to reflect some outpatient conditions that may shed light on the trends and origins of disparities, such as referral rates, characteristics of the referred patients or reasons for patients rejecting surgical options. In addition, the study did not cover high-intensity focused ultrasound treatments because they are not yet assigned a specific ICD procedure code, and the majority of the cases are treated on an outpatient basis.
Conclusion
The number of DBS procedures in the US has demonstrated a consistent increase over the years, surpassing 5500 admissions annually. Among the main DBS indications, PD accounted for the majority (67%), followed by ET at 24% and dystonia at 4%. Analyzing disease-specific data reveals the presence of racial disparities across all primary DBS indications, while other disparities, such as age, sex, wealth, and insurance status, only exist for certain indications.
Contributors
Conception of the work: CS, KY; Acquisition of the data: CS, KY, CIM, VM, OF, JG; Data interpretation: CS, AV, CC, GD, JG; Data Verification: AL, DHAP, CTC, STL, JG; Statistical analysis: CS, JG; Writing the first draft: CS, CRC, AY, MP, MML, NS, MC, BS; Study supervision: MH, SKK, RPM, AF, AML; Critical Revision and final approval: All authors. All authors attest they meet the ICMJE criteria for authorship and have approved the final article.
Data sharing statement
The study employed publicly accessible HCUP data, available upon request. https://hcup-us.ahrq.gov.
Declaration of interests
CS has been receiving fellowship grants from Michael and Amira Dan Foundation and Turkish Neurosurgical Society. STL is a recipient of the Parkinson Canada Clinical Movement Disorder Fellowship. AML is scientific director for Functional Neuromodulation and a consultant to Medtronic, Abbott, Boston Scientific, Insightec and Focused Ultrasound Foundation. AF reports the following: consultancies from Abbvie, Medtronic, Boston Scientific, Sunovion, Merz, UCB and Ipsen; membership in advisory boards of Abbvie, Boston Scientific, Ceregate, Inbrain and Inbrain Pharma; receiving honoraria from Abbvie, Medtronic, Boston Scientific, Sunovion, Merz, UCB and Ipsen; receiving grants from Dystonia Medical Research Foundation, MSA coalition, University of Toronto, Weston foundation, Abbvie, Medtronic and Boston Scientific. SKK receives honoraria, consulting, and/or speaker fees from Abbott, Boston Scientific, inBrain, Medtronic, Novo Nordisk, Parkinson Canada, and Movement Disorders Society; and research support from Parkinson Canada, CIHR, MJFF, FUS Foundation, MitoO2, Toronto Western Hospital Foundation, Weston Foundation, and RR Tasker Chair in Stereotactic and Functional Neurosurgery. KY received a consulting fee from Insightec. DHAP received payment or honoraria from Boston Scientific Corporation. All other authors report no disclosures relevant to the manuscript.
Acknowledgements
We acknowledge Jetan H. Badhiwala and Erdogan Ozalp for their help in data processing.
Funding: This work was supported by Alan & Susan Hudson Cornerstone Chair in Neurosurgery at University Health Network.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100599.
Appendix A. Supplementary data
References
- 1.Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94:666–668. [PMC free article] [PubMed] [Google Scholar]
- 2.Brown A.F., Ma G.X., Miranda J., et al. Structural interventions to reduce and eliminate health disparities. Am J Public Health. 2019;109:S72–S78. doi: 10.2105/AJPH.2018.304844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan A.K., McGovern R.A., Brown L.T., et al. Disparities in access to deep brain stimulation surgery for Parkinson disease: interaction between African American race and Medicaid use. JAMA Neurol. 2014;71:291. doi: 10.1001/jamaneurol.2013.5798. [DOI] [PubMed] [Google Scholar]
- 4.Hosobuchi Y., Adams J.E., Rutkin B. Chronic thalamic stimulation for the control of facial anesthesia Dolorosa. Arch Neurol. 1973;29:158–161. doi: 10.1001/archneur.1973.00490270040005. [DOI] [PubMed] [Google Scholar]
- 5.Schwalb J.M., Hamani C. The history and future of deep brain stimulation. Neurotherapeutics. 2008;5:3–13. doi: 10.1016/j.nurt.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Limousin P., Foltynie T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat Rev Neurol. 2019;15:234–242. doi: 10.1038/s41582-019-0145-9. [DOI] [PubMed] [Google Scholar]
- 7.Krauss J.K., Lipsman N., Aziz T., et al. Technology of deep brain stimulation: current status and future directions. Nat Rev Neurol. 2021;17:75–87. doi: 10.1038/s41582-020-00426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lozano A.M., Lipsman N., Bergman H., et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15:148–160. doi: 10.1038/s41582-018-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cagnan H., Denison T., McIntyre C., Brown P. Emerging technologies for improved deep brain stimulation. Nat Biotechnol. 2019;37:1024–1033. doi: 10.1038/s41587-019-0244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh E.J., Golubovsky J.L., Rammo R., et al. Estimating the risk of deep brain stimulation in the modern era: 2008 to 2020. Oper Surg. 2021;21:277–290. doi: 10.1093/ons/opab261. [DOI] [PubMed] [Google Scholar]
- 11.Cramer S.W., Do T.H., Palzer E.F., et al. Persistent racial disparities in deep brain stimulation for Parkinson’s disease. Ann Neurol. 2022;92:246–254. doi: 10.1002/ana.26378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henriksen T., Dalhoff K.P., Hansen H.E., Brenneche A.W., Lønberg U.S., Danielsen E.H. Access and use of device-aided therapies for Parkinson’s disease in Denmark. Mov Disord Clin Pract. 2020;7:656–663. doi: 10.1002/mdc3.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crispo J.A.G., Lam M., Le B., et al. Disparities in deep brain stimulation use for Parkinson’s disease in Ontario, Canada. Can J Neurol Sci. 2020;47:642–655. doi: 10.1017/cjn.2020.79. [DOI] [PubMed] [Google Scholar]
- 14.Setiawan M., Kraft S., Doig K., et al. Referrals for movement disorder surgery: under-representation of females and reasons for refusal. Can J Neurol Sci. 2006;33:53–57. doi: 10.1017/s0317167100004698. [DOI] [PubMed] [Google Scholar]
- 15.Honey C.M., Malhotra A.K., Tamber M.S., Prud’homme M., Mendez I., Honey C.R. Canadian assessment of deep brain stimulation access: the Canada study. Can J Neurol Sci. 2018;45:553–558. doi: 10.1017/cjn.2018.268. [DOI] [PubMed] [Google Scholar]
- 16.Shpiner D.S., Di Luca D.G., Cajigas I., et al. Gender disparities in deep brain stimulation for Parkinson’s disease. Neuromodulation. 2019;22:484–488. doi: 10.1111/ner.12973. [DOI] [PubMed] [Google Scholar]
- 17.Jost S.T., Strobel L., Rizos A., et al. Gender gap in deep brain stimulation for Parkinson’s disease. NPJ Parkinsons Dis. 2022;8:47. doi: 10.1038/s41531-022-00305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe G., Morden F.T.C., Gao F., Morita M., Bruno M.K. Utilization and gender disparities of deep brain stimulation surgery amongst Asian Americans, Native Hawaiians, and Other Pacific islanders with Parkinson’s disease in Hawai`i. Clin Neurol Neurosurg. 2022;222 doi: 10.1016/j.clineuro.2022.107466. [DOI] [PubMed] [Google Scholar]
- 19.Willis A.W., Schootman M., Kung N., Wang X.-Y., Perlmutter J.S., Racette B.A. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology. 2014;82:163–171. doi: 10.1212/WNL.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hariz G.-M., Nakajima T., Limousin P., et al. Gender distribution of patients with Parkinson’s disease treated with subthalamic deep brain stimulation; a review of the 2000–2009 literature. Parkinsonism Relat Disord. 2011;17:146–149. doi: 10.1016/j.parkreldis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Fana M., Everett G., Fagan T., Mazzella M., Zahedi S., Clements J.M. Procedural outcomes of deep brain stimulation (DBS) surgery in rural and urban patient population settings. J Clin Neurosci. 2020;72:310–315. doi: 10.1016/j.jocn.2019.08.117. [DOI] [PubMed] [Google Scholar]
- 22.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 23.Kumar R., Lozano A.M., Kim Y.J., et al. Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Neurology. 1998;51:850–855. doi: 10.1212/wnl.51.3.850. [DOI] [PubMed] [Google Scholar]
- 24.Deuschl G., Schade-Brittinger C., Krack P., et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez R.L., Fernandez H.H., Haq I., Okun M.S. Pearls in patient selection for deep brain stimulation. Neurol. 2007;13:253–260. doi: 10.1097/NRL.0b013e318095a4d5. [DOI] [PubMed] [Google Scholar]
- 26.Divo M.J., Martinez C.H., Mannino D.M. Ageing and the epidemiology of multimorbidity. Eur Respir J. 2014;44:1055–1068. doi: 10.1183/09031936.00059814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen S.A., Nash C.C., Byrne E.N., Mitchell L.E., Greaney M.L. Black/White disparities in obesity widen with increasing rurality: evidence from a national survey. Health Equity. 2022;6:178–188. doi: 10.1089/heq.2021.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Best M.J., McFarland E.G., Thakkar S.C., Srikumaran U. Racial disparities in the use of surgical procedures in the US. JAMA Surg. 2021;156:274. doi: 10.1001/jamasurg.2020.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman E.N., Kaatz A., Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28:1504–1510. doi: 10.1007/s11606-013-2441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Priest N., Slopen N., Woolford S., et al. Stereotyping across intersections of race and age: racial stereotyping among white adults working with children. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamberg K., Hariz G.-M. The decision-making process leading to deep brain stimulation in men and women with Parkinson’s disease – an interview study. BMC Neurol. 2014;14:89. doi: 10.1186/1471-2377-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cury R.G., Fraix V., Castrioto A., et al. Thalamic deep brain stimulation for tremor in Parkinson disease, essential tremor, and dystonia. Neurology. 2017;89:1416–1423. doi: 10.1212/WNL.0000000000004295. [DOI] [PubMed] [Google Scholar]
- 33.Moro E., Lozano A.M., Pollak P., et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease: study on subthalamic and pallidal stimulation. Mov Disord. 2010;25:578–586. doi: 10.1002/mds.22735. [DOI] [PubMed] [Google Scholar]
- 34.Bronstein J.M., Tagliati M., Alterman R.L., et al. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol. 2011;68:165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Picillo M., Lozano A.M., Kou N., Munhoz R.P., Fasano A. Programming deep brain stimulation for tremor and dystonia: the Toronto Western Hospital algorithms. Brain Stimul. 2016;9:438–452. doi: 10.1016/j.brs.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Sarica C., Iorio-Morin C., Aguirre-Padilla D.H., et al. Implantable pulse generators for deep brain stimulation: challenges, complications, and strategies for practicality and longevity. Front Hum Neurosci. 2021;15 doi: 10.3389/fnhum.2021.708481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.