Summary
One of the most captivating questions in neuroscience revolves around the brain’s ability to efficiently and durably capture and store information. It must process continuous input from sensory organs while also encoding memories that can persist throughout a lifetime. What are the cellular-, subcellular-, and network-level mechanisms that underlie this remarkable capacity for long-term information storage? Furthermore, what contributions do distinct types of GABAergic interneurons make to this process? As the hippocampus plays a pivotal role in memory, our review focuses on three aspects: (1) delineation of hippocampal interneuron types and their connectivity, (2) interneuron plasticity, and (3) activity patterns of interneurons during memory-related rhythms, including the role of long-range interneurons and disinhibition. We explore how these three elements, together showcasing the remarkable diversity of inhibitory circuits, shape the processing of memories in the hippocampus.
Keywords: interneurons, hippocampus, memory, plasticity, oscillations, disinhibition, long-range projections, connectivity
In this review, Tzilivaki et al. summarize current information on hippocampal GABAergic interneurons with an emphasis on their connectivity patterns, mechanisms of plasticity induction, and regulation of oscillatory rhythms during memory processing.
Introduction
Over the past decades, a multitude of exciting research has advanced our understanding of how the mammalian brain learns and stores new information. For a long time, research has focused on the role of pyramidal neurons during memory processes.1 However, recent discoveries about the role of inhibitory neurons in learning and memory formation have led to a growing appreciation of inhibitory gamma-aminobutyric acid-expressing (GABAergic) interneurons.2,3,4,5,6
In this review, we first highlight recent advances regarding the diversity of hippocampal interneurons, including an overview of their positions in the microcircuitry. Next, we summarize knowledge of synaptic and intrinsic plasticity phenomena in interneurons. For a few types, we discuss their contribution to the induction and regulation of oscillatory rhythms, which likely reflect important roles during memory encoding and consolidation. We end by posing a number of critical questions that should be answered in order to reach a deeper understanding of how interneuron diversity impacts hippocampal memory formation.
Interneuron types and microcircuits
“Classic” interneuron classifications
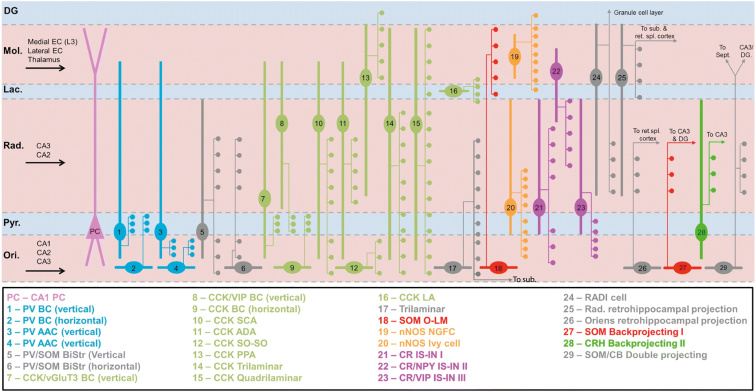
A classic distinction in cortical circuits is between the majority (80%–90%) of excitatory glutamatergic pyramidal cells (PCs) and a minority of inhibitory GABAergic interneurons. The latter group forms a strikingly heterogeneous population of interneuronal types that were defined over the course of more than a century of research,7,8,9,10,11,12 based on morphology (e.g., basket cells [BCs]), electrophysiological properties (e.g., fast-spiking [FS]), connectivity/targeting (e.g., axo-axonic [AAC]), or the expression of particular molecular markers (e.g., somatostatin [SOM]). Decades of work in the rodent hippocampal area CA1, including recordings in anesthetized and awake behaving animals, resulted in 29 different types (Figure 1), many of which have different roles in vivo.13,14,15,16,17,18,19,20,21,22,23,24 About a decade ago, cortical interneurons were found to be divided into three largely non-overlapping classes with different developmental origins25,26: parvalbumin- (PV) and SOM-expressing interneurons, derived from the medial ganglionic eminence (MGE), and serotonin receptor 3a (5HT3aR)-expressing interneurons, derived from the caudal ganglionic eminence (CGE).
Figure 1.
Hippocampal interneuron diversity
Overview of interneuron types in CA1, based on a combination of morphologies and a small number of molecular markers. For each type, axon terminations are indicated by colored dots, and dendritic distribution by thicker lines. External inputs are indicated by black arrows in the appropriate layers. Note that types 17 and 24–29 include external projections, indicated by arrows; other projections have also been reported but are not included here. To indicate the somatic and dendritic location of pyramidal cells relative to the interneuronal axon terminals, one schematic pyramidal cell (PC, pink) is included. Figure modified with permission from Booker and Vida.7
For MGE-derived interneurons, the dichotomy between SOM and PV cells has proven valuable, with many studies showing functional differences between “dendrite-targeting,” slow-firing SOM interneurons on the one hand, and “soma-targeting,” FS BC interneurons on the other.27,28,29,30,31 However, within these two classes, there is considerable diversity in terms of morphology and firing properties.10,16,24 The fact that the hippocampus has a single PC layer and inputs segregated into three further layers leads to some unique morphological cell types compared with the neocortex. For instance, the oriens-lacunosum-moleculare (O-LM) cell with dendrites in stratum oriens (SO) extends an axon into stratum lacunosum-moleculare (SLM), where it branches extensively and targets the apical dendrites of PCs.32,33,34 The bistratified cell (BiC) is defined by axons limited to stratum radiatum (SR) and SO.34,35 These two interneuron types have been reported to co-express SOM and PV22,36 (Figure 1). The other two stereotypic PV interneurons in the hippocampus are the AAC cells (also known as chandelier cells), targeting PC axon initial segments,37,38 and the FS BCs, targeting PC somata and proximal dendrites.10 Both display FS responses to depolarizing input and fire at high rates in vivo.22,39 In contrast, SOM cells typically have lower firing rates despite their low firing threshold.40
For CGE-derived interneurons, three main subclasses have been defined based on the expressions of Lamp5, Sncg, or vasoactive intestinal peptide-expressing (VIP).41 Lamp5 interneurons include ivy and neurogliaform cells (NGFCs). The ivy cell is the most common interneuron type in CA142; it has a distinct morphology with a relatively extensive axonal cloud extending over several hippocampal layers and co-expresses neuronal nitric oxide synthase (nNOS), suggesting retrograde signaling via nitric oxide (NO). NGFCs morphologically resemble compact ivy cells, with a distinctive “bushy” dendritic tree and a dense axonal cloud.43 Cells whose dendrites fall within the axonal tree of these two major cell types are inhibited via “volume transmission” of GABA, which induces a combination of slow GABA-A receptor-mediated synaptic currents and spillover-mediated activation of extra-synaptic GABA-B receptors.44,45 Paradoxically, although ivy cells express Lamp5 (a marker for a major subclass of CGE interneurons, as mentioned above), they appear to stem from the MGE, along with a subpopulation of nNOS-expressing NGFCs.46 Sncg cells were shown to include many cholecystokinin (CCK)-positive cell types.47 However, CCK cells are a remarkably diverse group in the hippocampus48,49 (Figure 1) that extends beyond Sncg cells.13 The majority of cells in the VIP subclass are interneuron-specific (IS), specifically targeting other interneurons rather than PCs. Three types of IS interneurons have been described, mostly in the hippocampus (Figure 1), and they are likely to play important disinhibitory roles.50
Overall, the above studies highlight the extensive diversity of the most important interneuron types in the hippocampus. Although recent single-cell transcriptomic datasets have led to a more extensive taxonomy of hippocampal interneurons,13,41,47,51 with up to ∼100 types having been described, the functional significance of these transcriptomic types remains mostly uninvestigated. Indeed, the question of what constitutes the most useful way to classify a cell type is non-trivial and depends on the question one wishes to address.52,53,54,55,56 Although this new method of classification presents an exciting and important avenue for future research, there is still much work to be done to determine how these transcriptomic cell types relate to the more traditional classifications such as morphological or molecular markers. Therefore, in this review, we focus on published work at the level of interneuron types described above.
Long-range projecting interneurons
Although GABAergic cells are mainly considered to be locally targeting interneurons, there is also a diverse group of long-range projecting (LRP) interneurons.57,58 In the hippocampus, three main projection patterns have been described, which are likely correlated to different functional roles.
The first group, retrohippocampal-projecting interneurons in CA1, is characterized by long-range projections limited to the entorhinal cortex (EC) and nuclei of the subicular complex (areas often described as “retrohippocampal”). This group includes “trilaminar” cells (Figure 1), which are SOM- and PV-negative, muscarinic type 2 acetylcholine receptor (M2R)-expressing interneurons that send projections to the subiculum.57,59,60 VIP+ LRP interneurons co-expressing M2Rs and calbindin61,62 not only project to the subiculum, where they target both inhibitory interneurons and PCs but also make local synaptic contacts with O-LM cells, BiCs, and BCs. Hence, they may also mediate local disinhibition (see below).
Back-projecting interneurons form the second group of LRP interneurons in CA1, with projections “back” to large parts of the upstream areas of CA3 and dentate gyrus (DG).63 This group is heterogeneous in terms of their precise targets, expression profiles, and firing patterns during memory-related rhythms (see section activity patterns of hippocampal interneurons in theta rhythm and SWRs). Two types with axons extending into the molecular layer of DG appear to be SOM+ (Figure 1).64,65 SOM+ interneurons co-expressing nNOS may also include back-projecting cells.63 Note that SOM-negative interneurons back-projecting to CA3 have also been reported,66 expressing corticotropin-releasing hormone (CRH) and CCK (Figure 1).
Extrahippocampally projecting interneurons form the third main group of LRP interneurons. This group includes a population of SOM cells that project to one or more extrahippocampal areas, including the ipsilateral medial septum (MS), the medial EC (MEC), the striatum, and contralateral DG.67 This population includes some “double projection” SOM- and calbindin-expressing cells (Figure 1) that target retrohippocampal areas and the MS57; in some cases, these cells additionally express neuropeptide Y (NPY).57,68 A partial overlap with SOM cells was described for so-called theta-off-ripple-on (TORO) cells,20 with multiple extrahippocampal targets including subiculum, lateral EC (LEC) and MEC, retrosplenial cortex, MS, and others. These cells were classified not only based on their functional properties (as implied by their name, see section activity patterns of hippocampal interneurons in theta rhythm and SWRs) but also on the robust expression of M2R. SOM-negative “radiatum-retrohippocampal neurons” (Figure 1) project to retrohippocampal areas, the retrosplenial cortex, and indusium griseum.57 More recently, another type of SOM-negative interneuron expressing nNOS and projecting onto many different extrahippocampal areas has also been described.69
Connectivity and microcircuits
The role of each interneuron type is not only determined by its expression profiles, morphologies, and intrinsic electrical properties but also by its connectivity. In the last decade, there have been huge advances in techniques enabling connectomic studies, both structurally and functionally. On the structural side, a series of studies revealed the connectivity between CA1 pyramidal neurons and eleven prominent interneuron types70: a CA1 PC receives roughly 1,120 inhibitory synapses, including about 420 from ivy cells; 180 from PV BCs; 140 from NGFCs; BiCs and CCK BCs each contribute about 100 synapses; O-LM cells about 80; and AAC cells, 30. More recently, EM reconstructions have allowed unprecedented insights into the connectivity patterns of interneurons in a number of brain regions and mammalian species.71,72 Unfortunately, to the best of our knowledge, such data are not yet available for the entire hippocampus.73,74 Although considerable progress has been made in terms of linking EM reconstruction to the function of the reconstructed neurons,75 in general, this “connectomics” approach is still restricted by difficulties in labeling particular cell types in electron microscopy. Furthermore, the volume that can be reconstructed is still limited. These limitations mean that one can only determine the connectivity of cells with nearby somata, with limited access to transcriptomic data for the connected cells.
Recent advances in patch-clamp methodology now allow the simultaneous recording from up to 12 neurons and have yielded large datasets that reveal details of local microcircuits.76,77 Although most of these studies have focused on excitatory connectivity in the hippocampal formation, a series of studies have elucidated connectivity for several subclasses of interneurons in the subicular complex78,79,80 and the MEC.81 For example, sharp-wave ripple (SWR) events propagating from area CA1 into the subiculum are associated with selective activation of burst-firing but not regular-firing PCs. In line with this observation, regular-firing, but not burst-firing, PCs are more strongly interconnected with FS PV interneurons.78 Such a specific inhibitory innervation of principal cell types has also been shown in EC82 and CA183 and is hypothesized to separate different information streams.84 Consistent with this idea, burst-firing and regular-firing cells in the subiculum have different long-range projection targets,85,86 and the described connectivity scheme may help to selectively mediate the information conveyed during SWRs to specific downstream brain areas. Within area CA1, there are also two main populations of PCs that populate different sublayers of stratum pyramidale (SP).87,88 One type (the “deep” PC) seems selectively inhibited during SWRs.89,90 The inhibition of deep PCs appears to be mediated mainly by PV BCs, whereas inhibition of superficial PCs is mediated more by CCK BCs.83,90 Thus, both the subiculum and CA1 demonstrate a division of labor in terms of interneuron innervation between PC subpopulations. However, the exact contribution of different interneuron types to the delineation of distinct output streams remains unclear.
In addition to controlling information streams mediated by excitatory pathways, interneurons can also inhibit other interneurons, leading to disinhibition. Such disinhibition has been demonstrated to contribute to learning in neocortical areas.50,91 In contrast, the role of disinhibition in the hippocampus during high-level computations, such as memory formation, is only beginning to be unraveled. Hippocampal disinhibitory signals can be mediated by different circuit elements (Table 1), either by IS interneurons (IS inhibition, [1]), non-IS interneurons that contact inhibitory neurons of the same type (non-IS homotypic inhibition, [2]), non-IS interneurons contacting other interneurons (non-IS heterotypic inhibition, [3]), or inhibitory inputs from other brain regions, such as the MS or EC, onto different types of hippocampal interneurons (extrahippocampal long-range GABAergic input,58 [4]). In the following paragraphs, we briefly summarize the main characteristics of hippocampal disinhibitory interneurons (Table 1).
Table 1.
Hippocampal disinhibitory connectivity motifs
| Mode of disinhibition | Presynaptic interneuron | Molecular marker | CA1 target interneuron | Method | Publication(s) |
|---|---|---|---|---|---|
| Interneuron-specific (IS) inhibition | IS-1 | CR | CB IN (SCA, PPA); CCK/VIP BC; IS-1 | immunocytochemistry; electron microscopy | Acsády et al.92 and Gulyás et al.93 |
| IS-3 | paired recording, in vitro | Luo et al.94 | |||
| IS-2 | VIP | SCA, PPA, IS-2, CCK/VIP BC | immunocytochemistry; electron microscopy |
Gulyás et al.93 | |
| IS-3 | paired recording, in vitro | Luo et al.94 | |||
| IS-3 | VIP; CR | O-LM, BiC, CCK/VIP BC, O-O | paired recording, in vitro | Tyan et al.95 | |
| IS-3 | paired recording, in vitro | Luo et al.94 | |||
| VIP-LRPa | VIP; M2R; CB | O-LM, BiC, CCK BC | paired recording, in vitro | Francavilla et al.61 | |
| Non-IS homotypic inhibition | CCK-BC | CCK; CB1Rb | CCK BC | paired recording, in vitro | Daw et al.96 |
| PV BC | PV | PV BC | paired recording, in vitro | Daw et al.96 and Cobb et al.97 | |
| SCA | CCK; CB | CCK SCA | paired recording, in vitro | Ali98 | |
| NGFC | NPY; nNOS | NGFC | paired recording, in vitro | Price et al.99 | |
| Non-IS heterotypic inhibition | O-LM | SOM; mGluR1α; (PV) | CCK BC, SCA, PPA, NGFC | paired recording, in vitro | Elfant et al.100 |
| CCK-BC | CCK; CB1Rb | PV BC | paired recording, in vitro | Karson et al.101 | |
| PV BC | PV | CCK BC | optogenetics and Ca2+ imaging, in vitro and in vivo | Dudok et al.13 | |
| TORO | M2R | PV- and CCK-expressing IN | immunocytochemistry | Szabo et al.20 | |
| AAC | paired recording, in vitro | Szabo et al.20 | |||
| Extrahippocampal GABAergic, long-range input | septo-hippocampal | not specified | PV- and CB-expressing IN | immunocytochemistry, anterograde tracing; electron microscopy | Freund and Antal102 |
| PV | not specifiedc | antero- and retrograde, and AAV tracing; immunocytochemistry; electron microscopy |
Unal et al.103 | ||
| PV; SATB1; HCN4 | BiC | juxtacellular labeling, electrophysiology immunocytochemistry | Unal et al.104 | ||
| GAD67 (not further specified) | PV-, CB-, CR-, SOM-expressing INd | immunocytochemistry; optogenetics, in vivo and in vitro | Sans-Dublanc et al.105 | ||
| mGluR8a; in terminals | trilaminar IN (M2R+) | anterograde labeling | Katona et al.59 | ||
| not specifiede | TORO | optogenetics and Ca2+ imaging, in vivo and in vitro | Szabo et al.20 | ||
| PFC-hippocampal | PV; Som; VIP; CR; NPYf | VIP-expressing IN | optogenetics and Ca2+ imaging, in vivo and in vitro | Malik et al.106 |
Abbreviations: IN, interneuron; SCA, Schaffer-collateral associated; PPA, perforant path associated; O-O, Oriens-oriens; SATB1, special AT-rich sequence binding protein 1; HCN4, hyperpolarization-activated cyclic nucleotide-gated channel isoform 4; GAD67, glutamate decarboxylase isoform 67.
VIP-LRP INs target both PCs and INs in the subiculum61 and thus, strictly speaking, these cells can only be considered interneuron-specific within CA1.
Based on the available literature, it is unclear whether these CCK-expressing BCs belong to the VIP or VGlut3-expressing group.
Postsynaptic targets in the subiculum included PV-, CB-, CR-, NECAB1-, CCK- and Reelin-expressing IN, among others.
The majority of postsynaptic somata was found in the pyramidal cell layer. Fractions of postsynaptic somata expressing PV: 44%, CB: 22%, CR: 18%, SOM: 9%, CCK: 4%, VIP: 3%.
Medial septum GABAergic neurons were labeled using Dlx5/6-Cre transgenic mice and Cre-dependent viral constructs.
Prefrontal cortex GABAergic neurons were labeled using Dlxi12b-Cre transgenic mice and Cre-dependent viral constructs.
(1) Freund and colleagues first characterized IS interneurons and showed that these cells express calretinin (CR) (IS-1), VIP (IS-2), or both (IS-3). Postsynaptic local targets of IS interneurons include not only other types of IS cells and pyramidal neuron dendrite-targeting interneurons (like BiCs, O-LM cells, and others) but also CCK/VIP-expressing BCs. In addition to these “classical” types, another IS interneuron was recently characterized, namely VIP-expressing interneurons with long-range projections to the subiculum61 (VIP-LRP), indicating a possible role of VIP-LRP INs in coordinating hippocampal and subicular activity during mnemonic processing. In addition to IS cells, disinhibition mediated by non-IS interneurons is also well documented in CA1. (2) For homotypic connections, paired intracellular recordings from hippocampal slices have shown synaptic transmission between PV interneurons, CCK interneurons, and NGFCs. (3) For heterotypic connections, bidirectional synaptic connections between PV and CCK BCs have been identified anatomically, and paired recordings in vitro showed inhibition of PV BCs by CCK BCs.101 More recently, Dudok et al.13 used a combination of immunocytochemistry, optogenetics, and in vivo Ca2+ imaging to uncover PV to CCK BC connections. Consistent with this, reciprocal signaling between both types of BCs, CCK BCs, and PV cells can express complementary activity levels depending on the brain state. These findings are of particular significance, given the importance of PV interneuron-mediated inhibition in hippocampus-dependent memory formation associated with theta and ripple oscillations107,108,109,110 (see section activity patterns of hippocampal interneurons in theta rhythm and SWRs). (4) Currently, most of our knowledge about the effects of disinhibition on hippocampus-dependent function is based on GABAergic extrahippocampal “long-range” input controlling CA1 interneurons. Significant inhibitory projections to CA1 emanate from the MS, primarily from PV cells that target interneurons, including PV types as well as trilaminar and TORO cells.59,102,107,108,109,110 As shown in functional Ca2+ imaging in behaving mice, septo-hippocampal (S-H) GABAergic projections surrounding and inhibiting TORO cells are active during theta-associated locomotion,20 and S-H inhibitory projections contribute to the encoding of sensory saliency.111 Moreover, a role of disinhibition via GABAergic S-H projections has been directly demonstrated for contextual fear memory, where these inputs show activity during memory retrieval, thereby suppressing target CA1 interneurons. The acute chemogenetic suppression of S-H GABAergic inputs resulted in increased activity of PV interneurons in CA1 and suppressed fear memory retrieval. Thus, memory recall seems to depend on GABAergic input from the MS acting on PV interneurons, disinhibiting PCs, and increasing their activity.107,108,109,110 Consistent with this significant role of S-H disinhibition in hippocampal memory formation, S-H signaling has been shown to decay during normal aging and in a mouse model of Alzheimer’s disease.112 This finding indicates that impaired disinhibition may participate in the pathology of some forms of dementia.
Above, we outlined progress in understanding interneuron diversity in the hippocampus. We discussed the main methods by which interneurons are currently classified and how new methods might extend future classifications. Additionally, we presented the current knowledge on the connectivity of interneurons and their role in the topology of local and larger brain circuits. This topology determines the flow of information within and between brain regions. However, to ultimately understand memory formation, knowledge of topology alone is not sufficient. Another important feature of neuronal networks is their capacity for plasticity. In the next section, we therefore discuss the current knowledge of plasticity mechanisms exerted by different interneuron subclasses in the hippocampus.
Plasticity of synaptic transmission and intrinsic excitability in hippocampal interneurons
Although plasticity induction in PCs is a well-studied phenomenon that is assumed to underlie memory-related processes in the hippocampus and beyond,113 the plasticity of hippocampal interneurons has only recently started to be elucidated. Thus far, the best-studied form of synaptic plasticity in hippocampal interneurons is the plasticity of glutamatergic synapses formed by PCs onto interneurons (PC to IN synaptic plasticity). PC to IN synaptic plasticity (such as long-term potentiation [LTP] and long-term depression [LTD]) typically refers to changes in the amount and/or properties of interneuron postsynaptic receptors, as discussed in recent reviews.114,115,116,117 Importantly, plasticity has also been documented at GABAergic synapses onto PCs.114,115,116,117,118 This IN to PC synaptic plasticity, also known as presynaptic inhibitory plasticity, involves changes in the postsynaptic GABA receptors119 or in the amount of GABA release114,115,116,117,118 and can result in either LTP or LTD. Moreover, in addition to synaptic plasticity, changes in the properties of voltage-dependent conductances that mediate the integration of incoming signals (e.g., potassium channels) can evoke alterations in the excitability of a neuron. This plasticity of intrinsic excitability (IE) can be input- or cell-specific and was recently described in just a few interneuron types.119,120,121,122 This section aims to summarize the current knowledge on different forms of plasticity “to and from” interneurons, with an emphasis on hippocampal areas. We start by discussing studies related to synaptic plasticity (Figure 2), followed by recent advances in intrinsic plasticity mechanisms (Figure 3).
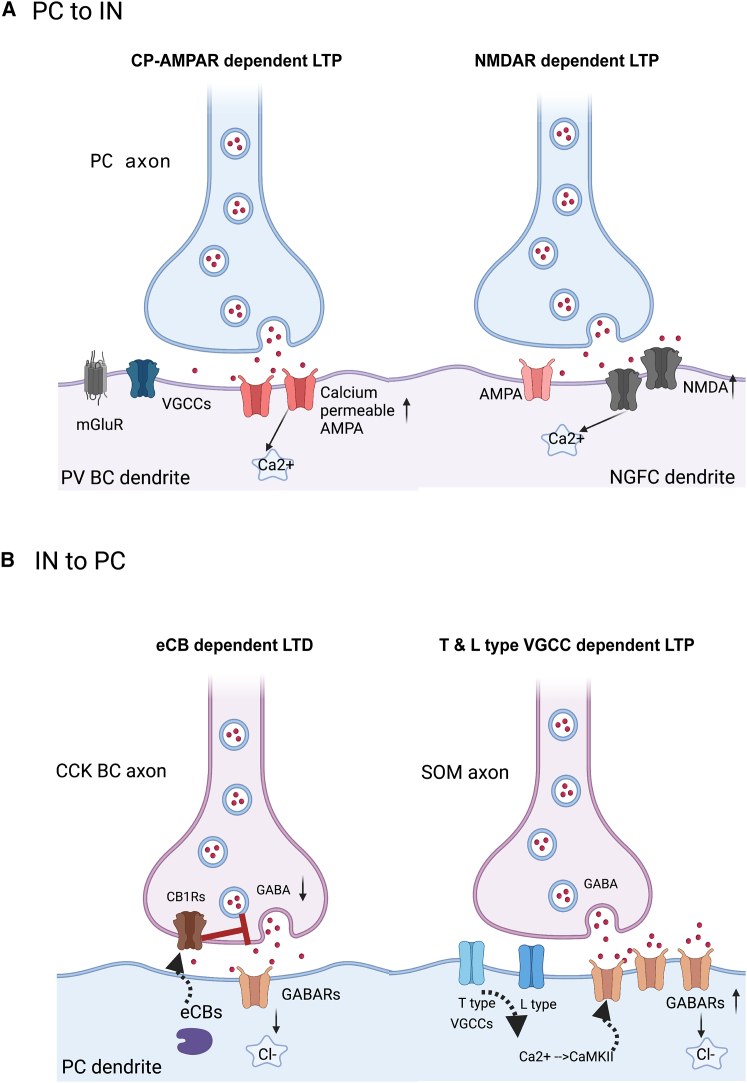
Figure 2.
Best-characterized forms of synaptic plasticity in hippocampal interneurons
(A) PC to IN synaptic LTP formation has been shown to be either CP-AMPAR dependent, e.g., in PV BCs (left) or NMDAR dependent, e.g., in NGFCs (right). Red dots represent glutamate release.
(B) IN to PC plasticity examples. Induction of presynaptic LTD in CCK BC to PC synapses (left): postsynaptic (PC) eCB signaling mediates the activation of presynaptic CB1Rs that in turn regulate the reduction of GABA release from the CCK-BC axonal terminals. T and L type VGCC-dependent LTP in SOM to PC synapses (right): coincident activation of both presynaptic SOM cell and postsynaptic PC cell leads to activation of T and L type VGCCs in PC dendrites that induces the activation of CaMKII. CaMKII activation leads in inhibitory LTP in SOM to PC synapses. Red dots represent GABA release. Figure created with BioRender and designed based on data from published work.123,124,125,126,127
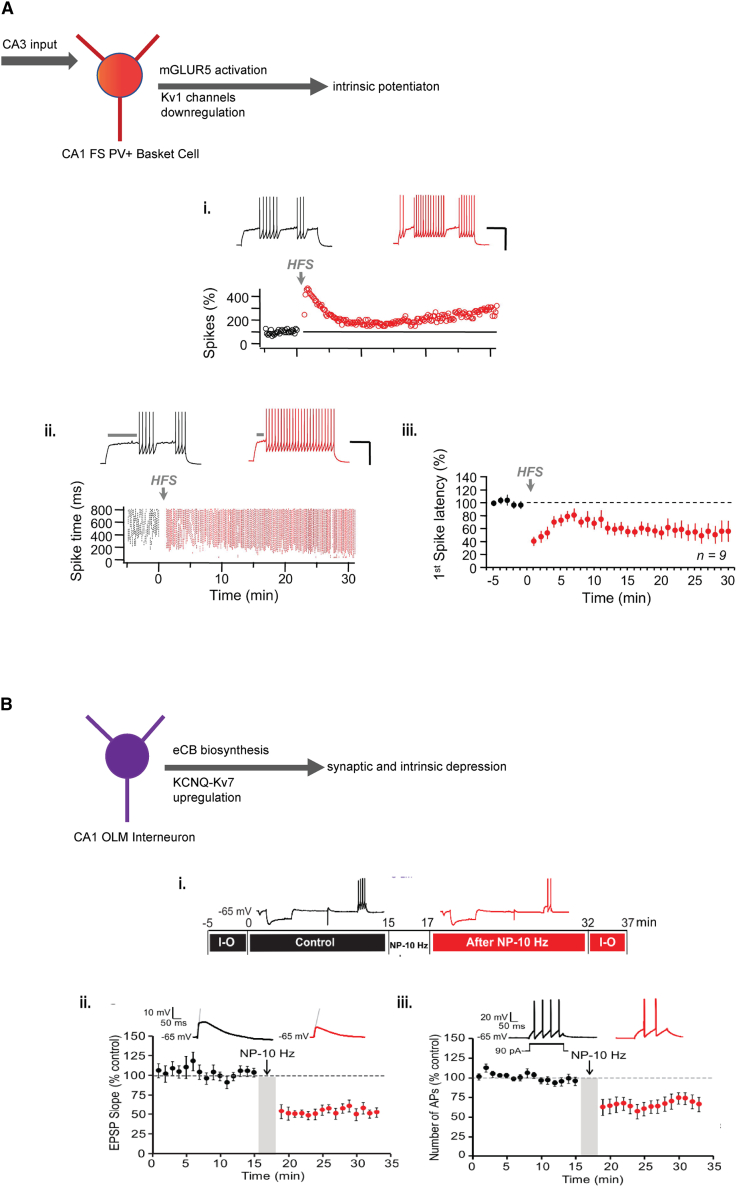
Figure 3.
Intrinsic excitability examples in hippocampal interneurons
(A) Induction mechanisms of synaptically generated LTP-IE in PV BCs in the CA1 after activation of CA3 Schaffer-collateral inputs (high-frequency stimulation). Top: graphical illustration of the experimental protocol and main findings. Induction of intrinsic excitability in CA1 FS BCs resulted in: (i) higher spiking activity and reduced first spike latency (iii). (ii) Shows the spike timing over time in a representative example (n = 9 FS BCs, scale bars: 200 ms and 40 mV).
(B) Induction of presynaptic LTD in CA1 O-LM interneurons triggers a Kv7 potassium channel dependent decrease in their intrinsic excitability. Top: graphical illustration of the experimental design and findings. (i) The induction of presynaptic LTD was induced via a protocol of negative pairing between a single postsynaptic action potential, followed by a presynaptic (pyramidal) stimulation with a delay of 5, 10, or 20 Hz. Negative pairing at 10 Hz induces presynaptic LTD (measured as changes in the EPSP slope) and (ii) and decreases in the intrinsic excitability (measured as changes in the number of spikes) (iii) in CA1 O-LM interneurons. Pooled data. (A) Campanac et al.119 and (B) Incontro et al.121 modified with permission.
PC to IN synaptic plasticity
Studies on PC to IN synaptic plasticity date back to the 1980s, when LTP induction in interneurons was established via tetanic stimulation in the CA1 area in vivo.128 This interest was recently renewed for both hippocampal and cortical interneurons in rodents.117,119,120,121,122,124,129,130 Several of these studies highlight the diversity of plasticity induction forms resulting from the abundance of active membrane mechanisms and their heterogeneous distribution across different interneuron types or even subtypes.54,115 For example, in hippocampal interneurons in the SLM and specifically in NGFCs, LTP was found to be NMDAR dependent124 (Figure 2A, right), enabling the induction of synaptic plasticity similar to that reported for PCs. This form of synaptic NMDAR-dependent plasticity is also known as Hebbian plasticity for both PCs and interneurons. However, apart from NGFCs, the specific interneuron types that exhibit this form of Hebbian LTP remain unknown.115,124 In addition to NMDAR-dependent plasticity, in several interneuron types, including the PV BCs and the O-LM cells in CA1, LTP induction is primarily mediated by calcium-permeable AMPARs (CP-AMPARs, often referred to as anti-Hebbian synaptic plasticity; Figure 2A, left).122,125,131,132 Another NMDA-independent induction of LTP, depending on metabotropic glutamate type 1 receptors (mGluR1s), has been documented for CA1 SO interneurons.126,133,134 However, the exact subtypes of interneurons were not described. Although initially characterized as Hebbian, a later study126 reported that the interaction between mGluR1s and muscarinic receptors (mAChRs) results in CP-AMPAR-dependent rather than NMDAR-dependent postsynaptic LTP. Induction of LTD, on the other hand, is significantly less studied in PC to IN synapses in the hippocampus; it has been suggested to be mediated by mGluR1 or NMDA receptors.11,116 Taken together, both LTP induction (NMDAR or CP-AMPAR dependent) (Figure 2A) and LTD induction have been reported in some broadly defined hippocampal interneuron types, mainly the PV class. However, further work is needed to map the different forms of synaptic plasticity induction across all hippocampal interneuron subtypes (e.g., in distinct PV subtypes, SOM, and VIP classes) in order to delineate the mechanisms that mediate each form of plasticity.
IN to PC synaptic plasticity
Both LTP and LTD have been described in IN to PC connections across multiple brain regions, including hippocampal areas.118,135 It has been shown that depolarization of pyramidal neurons can regulate an increase or a decrease in GABA release, which enhances (LTP) or reduces (LTD) the resulting inhibitory postsynaptic potentials (iPSPs) onto PC dendrites. This retrograde signaling from PCs onto presynaptic interneurons is mediated by molecules such as endocannabinoids (eCBs), NO, and brain-derived neurotrophic factor (BDNF)118,127,136 that can influence the GABA release machinery in interneurons by activating receptors on the interneuronal axon terminals (e.g., cannabinoid receptors [CBRs]). In contrast to PC to IN plasticity forms, where most is known about LTP induction, for IN to PC synaptic plasticity, the most characterized form is the induction of LTD in GABAergic axonal terminals. The leading retrograde signal messengers for this IN to PC LTD (often called presynaptic iLTD) consist of eCBs, which decrease GABA release via the activation of CBRs127 (Figure 2B, left). For CCK-positive BCs, in particular, CBR-inverse agonists can increase GABA release.137,138 Although CBRs are abundant in regular spiking CCK-positive BCs, this is not the case for FS BCs,136,139 suggesting that eCB-dependent IN to PC LTD is interneuron type dependent. Other studies suggest that IN to PC iLTD can also be achieved by alternative mechanisms. Specifically, in CA2 PV-positive interneurons, GABA release is reduced following stimulation of the CA3 Schaffer collaterals through the activation of delta opioid receptors in their dendrites.140 In this study, the induction of PC to IN plasticity was associated with the ability of CA2 PV-positive interneurons to control the CA3 to CA2 transmission.
In addition, a recent in vitro study showed the induction of either LTP or LTD upon coincident activation of both inhibitory presynaptic and excitatory postsynaptic neurons in CA1. In particular, CA1 PV interneuron to PC synapses in SP undergo LTD upon activation of postsynaptic T type voltage-gated calcium channels (VGCCs) and calcineurin, which in turn modulate the amount of GABA receptors in the excitatory postsynaptic cell. In contrast, under the same stimulation protocol, CA1 SOM interneuron to PC synapses in SO and SLM layers undergo LTP (Figure 2B, right). Although in this case, T type VGCCs were activated, similar to PV-PC synapses, the joint activation of L type VGGCs (located in the PC dendrites) resulted in the activation of calcium–calmodulin (CaM)-dependent protein kinase II (CaMKII) and, in turn, in SOM to PC LTP.123
Altogether, the abovementioned data demonstrate the plethora of mechanisms that lead to synaptic plasticity induction to and from distinct interneurons. However, our knowledge remains restricted to the PV and SOM subclasses, as well as CCK and NGFC types. Future studies should delineate the mechanisms and forms of synaptic plasticity in other interneuron types, including ivy cells and VIP cells, among others, as well as the possibility of plasticity induction in interneuron-interneuron synapses.
Intrinsic excitability
Multiple ion channels and transporters, located at input (dendrites) or output sites (axons) of neurons, engage in plasticity of IE. Below, we summarize findings related to IE of hippocampal interneurons and discuss the potential advantages of this plasticity form in memory processing.
In an earlier study on DG BCs, it was shown that after tetanic stimulation of glutamatergic inputs, interneurons exhibited a long-term change in the rate of electrogenic Na+/K+-ATPase pump function. As a result, interneurons responded to stimuli with spikes instead of the previously generated subthreshold excitatory postsynaptic potentials (EPSPs). This Na+/K+ ATPase-dependent intrinsic plasticity in DG BCs also required the activation of the CP-AMPARs and the rise of intracellular Ca2+ stores but not NMDA receptors.141
Later, FS BCs in the hippocampus (and neocortical L2/3) were also found to exhibit plasticity of their IE. In this case, activation of ErbB4, a Neuregulin 1 (NRG1) receptor that is present in FS PV BCs in the SO of CA1 and CA3,142 resulted in enhanced voltage-dependent excitability mediated by the downregulation of Kv1.1 potassium channels. Specifically, ERbB4 influences the tyrosine phosphorylation of the Kv1.1 channel protein, a biochemical reaction that, in turn, tunes the near-threshold responsiveness (the slope of spike initiation) and spike threshold of FS BCs.143 NRG1 receptors have been shown to modulate gamma oscillations in the hippocampus.144 However, NRG1-mediated intrinsic plasticity of FS BCs during gamma oscillatory events has not yet been documented. Another mechanism of long-term (>30 min) potentiation of IE (LTP-IE) in CA1 FS PV BCs was demonstrated following a brief high-frequency stimulation of CA3 Schaffer collaterals, which activated metabotropic glutamate receptor subtype 5 (mGluR5) receptors,119 leading to the downregulation of Kv1 potassium channels. This LTP-IE facilitated feedforward inhibition in CA1 circuits (Figure 3A). The authors of this study also proposed a link between IE and regulation of oscillatory activity of BCs; they found that LTP-IE enhanced the firing of these interneurons within the gamma band frequency. A similar mGluR5-dependent IE in DG PV neurons was observed after the induction of bursts of synaptic stimulation of the mossy fiber pathway at gamma frequency.145 These two studies demonstrate a clear link between inhibitory IE and memory-related oscillations. IE has also been found in hippocampal SOM cells. When rodents were trained in a hippocampus-dependent trace eyeblink conditioning task, SOM INs expressed LTP-IE, mediated via a reduction in small conductance calcium-activated potassium (SK) channels.146 This reduction led to an attenuation of afterhyperpolarization (AHP), which was also found in pyramidal neurons in the same study. Notably, induction of IE plasticity was recently found in both rodent and human NGFCs in the SLM of the hippocampus.147 For the mouse NGFCs, Kv4 potassium channels were shown to effectively influence action potential timing and threshold. Expressed in the somatodendritic domain of these neurons, Kv4 channels were proposed to evoke somatic depolarization-driven excitability (STP-SE) and enhanced dendritic integration. A recent study on CA1 O-LM interneurons121 uncovered a surprising link between the induction of both presynaptic and IE plasticity. Direct interaction between eCBs and Kv7 potassium channels on the dendrites of these interneurons resulted in LTD of IE (LTD-IE) after synaptic LTD of GABA release in the presynaptic interneuron axonal boutons (Figure 3B). Overall, this study revealed that eCB biosynthesis can evoke both synaptic depression as well as depression of the IE by the interaction between CB1 receptors and Kv7 potassium channels, respectively (Figure 3B). Work from the same group also showed that in vitro low-frequency stimulation at 5 Hz (theta frequency-related stimulation) induces LTP-IE in conjunction with synaptic LTP in CA1 O-LM cells.122 Although synaptic LTP was mediated by CP-AMPARs, LTP-IE was evoked after mGluR1-dependent downregulation of hyperpolarization-activated cyclic nucleotide-gated (HCN) and Kv7 potassium channels.
In conclusion, the data above indicate that, alongside synaptic plasticity, the excitability of hippocampal interneurons can be regulated through modifications of their intrinsic properties, often in conjunction with synaptic-level modifications (e.g., mGluR5 upregulation or eCBs-mediated reduction of GABA release). This flexibility of interneurons to change their excitability can have a significant impact on their function at the hippocampal circuit level. For example, LTP-IE of CA1 PV BCs can result in increased feedforward inhibition and consequently a decrease in PC firing.119 Therefore, this plasticity may represent a dynamic mechanism to control the excitatory drive within the CA1. According to the authors, LTP-IE in PV cells could mediate their response during gamma oscillations. What could that mean for PCs or other interneuron classes that receive inhibition from PV cells? One possibility might be that their activity during gamma oscillations could serve as a network reconfiguration mechanism by modulating the excitation to inhibition balance. As the strength of the PV cell drive increases, a possible decrease in the strength of PCs or other interneurons drive could result in an overall change in the functional connectivity motifs. In general, dissection of how LTP-IE in PV cells influences gamma activity would prove valuable. Although data suggest that the plasticity of synaptic transmission in PV cells can affect the induction of gamma activity,148 it is not known whether the LTP-IE in these neurons could also affect gamma activity induction. Finally, PV cells are highly active during other oscillatory states in the hippocampus, namely theta and SWRs, and thus, IE plasticity may also contribute to these events.
Data from O-LM interneurons indicate that IE plasticity can be expressed in these cells as either depression121 (Figure 3B) or potentiation.122 CA1 O-LM cells control the excitation of distal dendritic compartments of PCs; thus, they have been proposed to modulate the integration of different excitatory inputs to CA1 PCs. The best-studied pathways include intrahippocampal inputs from the CA3 and sensory inputs from the EC through the temporo-ammonic pathway.149 An obvious question is whether O-LM IE plasticity could influence how PCs integrate these inputs. LTP-IE in O-LM cells will increase the strength of inhibitory synapses on distal dendrites of PCs. As a result, sensory inputs on these dendrites might be attenuated. As plasticity of IE changes in PV cells, it will be important to understand the influence of O-LM IE plasticity on memory-related oscillations. In particular, what might be the advantage of such cooperation between synaptic and intrinsic plasticity? How might this plasticity modulate both intra- and extrahippocampal inputs during memory encoding?121,150,151 It would be interesting to test the effect of O-LM cells' IEon the modulation of intrahippocampal and sensory inputs during spatial memory encoding.
In the above section, we discussed the diverse mechanisms of plasticity in hippocampal interneurons. Although a complete understanding of how interneurons exert control over functional circuits is still beyond our current grasp, combining knowledge of interneuron types, their connectivity schemes, and the plasticity mechanisms they exhibit, we can start to build a picture of how interneurons may modulate certain points of these circuits. In the next section, we summarize the current state of knowledge of the functions of interneurons during synchronous activities related to hippocampal-dependent memory processing.
Activity patterns of hippocampal interneurons in theta rhythm and SWRs
In the hippocampus, the predominant electrographic signatures vary depending on the behavioral context of the animal: theta oscillations (∼4–10 Hz) are present during locomotive behaviors and rapid eye movement sleep.152,153 Conversely, during inactive wakefulness and slow-wave sleep, ripples and SWRs (∼120–250 Hz) predominate.154,155,156 Theta oscillatory activity has been linked to learning and memory encoding in the hippocampus, whereas SWR-related activity was associated with the ability of the hippocampus to consolidate and recall memories that are stored during theta oscillations.157 Below, we summarize the firing patterns of the best-studied local and LRP CA1 interneurons and their involvement in these key hippocampal rhythms.
Local interneuron firing patterns
In the rodent brain, about 10% of all neurons fire during an SWR epoch,158,159 making this network pattern the most synchronous type of neuronal network oscillation in the mammalian brain.7 However, the activity levels of excitatory and inhibitory cells during SWRs show significant differences: up to 30% of CA1 PCs are recruited into the active network, and they exhibit the strongest relative gain in activation compared with non-SWR periods (∼9-fold increase159). At the same time, up to 70% of inhibitory interneurons were recorded in or near the PC layer of CA1 discharge, showing a 4-fold increase in firing rate compared with levels during non-SWR epochs.160 In absolute terms, despite the much higher increase in PCs, the mean firing rate during SWRs is still much lower in PCs (∼10 Hz) than in inhibitory interneurons (up to 100 Hz).160 The landmark studies of Somogyi, Klausberger, and collaborators, in anesthetized and later in freely moving rats,15,16,21,22,161 together with more recent work,13,14,23,24 have provided a detailed account of the activity patterns of different interneuron types during theta and ripple oscillations (Figure 4).
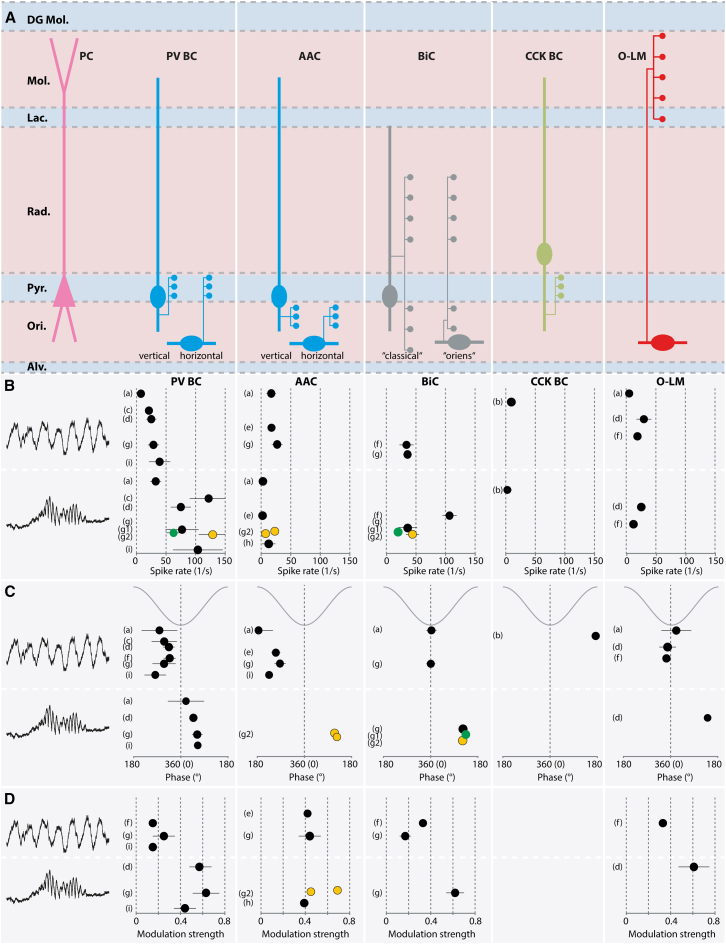
Figure 4.
Activity of CA1 local interneurons during theta and ripple oscillations
(A) Most relevant interneuron types in CA1 separated by morphology and molecular markers. For reference, the somatodendritic layout of a schematic pyramidal cell (PC, left) is included. Note that for three interneuron subtypes, two separate morphological subclasses have been described.
(B–D) Properties of the interneuron classes displayed in (A), during theta oscillations (upper row) and ripples (lower row). Data were obtained from different studies (a–i), with the following experimental conditions: (a) anesthetized rat22; (b) anesthetized rat162; (c) freely moving rat15; (d) awake head-fixed mouse23; (e) anesthetized and freely moving rat21; (f) freely moving rat16; (g) awake head-fixed mouse24 (for PV BCs: black, population average, [g1, green] vertical and [g2, yellow] horizontal orientation; for AACs: two individual horizontal cells reported [g2, yellow]; for BiCs: black, population average, [g1, green], “classical” and [g2, yellow] “oriens” orientation); (h) awake head-fixed mouse14; (i) awake head-fixed mouse.20 (B) Spike rates. Note that the reported spike rates might differ, even for a given interneuron subtype, depending on the experimental conditions. In some studies, individual neurons were reported and therefore no error margins are displayed. (C) Preferred spike phases. Phase values are given with a full cycle of oscillations as a reference. (D) Modulation strength, reflecting how precisely the spikes of a cell are bound to a particular phase of an oscillation with values ranging from 0 (no preferred spike phase) to 1 (all spikes occur with the same phase angle). The error bars shown represent the margins of error reported in the indicated studies.
Of the total population of interneurons in the CA1 region, about 30% express the Ca2+-binding protein PV,11 and these cells have been particularly associated with rhythm generation due to their tight inhibitory control of principal neurons. Compared with other interneuron types, PV cells discharge in a strongly theta-modulated manner16,22,24 (Figure 4D). The largest group of these, at ∼14% of all CA1 interneurons, are the FS BCs. During ripples, FS BCs discharge at ∼75 to ∼130 Hz with intermediate modulation strength (a measure of phase-coupling of spikes to an oscillation); their involvement is also particularly high (95% ± 6% of them spike during recorded ripples24). A second PV-expressing group, the AACs, accounts for ∼4% of CA1 interneurons.11 Recent Ca2+ imaging experiments on genetically labeled AACs confirmed the strong and reliable involvement of these cells in locomotion-associated theta oscillations, demonstrating greater activation than other interneurons recorded simultaneously in the CA1 network. However, during SWRs, AACs fell into two groups: about half showed a moderate but statistically significant increase in Ca2+ transients during ripples, whereas the other half displayed no change compared with pre-ripple periods.14,163 These findings are consistent with previous juxtacellular recordings in which a subset of AACs, whose somata are located outside the PC layer and whose dendrites extend “horizontally” in the SO (“external AACs,” E-AACs164) increase their discharge rates moderately (Figure 4B) and exhibit strong modulation strength during ripples24 (Figure 4D). BiCs comprise the third group of PV-expressing interneurons, which account for about 6% of inhibitory neurons in CA1.11 During ripples, BiCs fire at high rates with high modulation strength16,24 (Figures 4B and 4D). The involvement of BiCs is high, spiking in 68% ± 13% of recorded ripples.24 Finally, a fourth group of PV-expressing interneurons are O-LM cells, which constitute about 4.5% of all inhibitory interneurons in CA1.11 In mice, these neurons increase their discharge rate almost 3-fold during ripples compared with non-ripple periods and show robust phase locking23 (Figures 4B and 4D). It should be noted, however, that in behaving rats, O-LM cell involvement during ripples has been shown to be less reliable.16
CCK BCs account for approximately 9% of CA1 interneurons.11 Unlike their PV-expressing counterparts, CCK BCs exhibit a slow-firing pattern, and their action is subject to multiple neuromodulatory systems, including cannabinoids, acetylcholine, and serotonin.139 During theta rhythm, CCK BCs fire much slower than PV BCs (Figure 4B). During ripples, they participate only in an “episode-dependent” manner, with no consistent change in discharge rate compared with non-ripple periods.162 A more recent study13 largely confirmed these findings and showed that PV- and CCK-expressing BCs are inversely coupled in their activity rates; the highest activity rates for CCK BCs were observed during brain states corresponding to non-theta, non-ripple, “irregular” activity.
In summary, accumulating evidence has established the contribution of local circuit interneurons in rhythm generation in the hippocampal—mainly CA1—region (Figure 4). Furthermore, the targeted manipulation of specific interneuron subclasses has demonstrated the importance of these cells in regulating basic properties of hippocampal rhythms, such as oscillation frequency and power. Of note, a specific role for PV cells in generating oscillations in the hippocampus has been shown: the targeted deletion of the GluN1 subunit of NMDA receptors in PV-expressing interneuron types reduced the power of theta oscillations, both in movement and during REM sleep, and impaired memory performance in working and spatial memory tasks.107 In an in vitro model of SWRs in CA3, activation of PV-expressing interneurons with either optogenetic stimulation165 or current injection during whole-cell recordings166 can trigger SWRs, demonstrating the importance of PV interneurons in their initiation. In contrast, dampening of the excitatory drive to PV-expressing interneurons by selective ablation of the AMPA receptor subunit GluA1 increases ripple power,167 demonstrating a complex, balanced contribution of both PCs and PV interneurons to ripple initiation.
LRP interneuron firing patterns
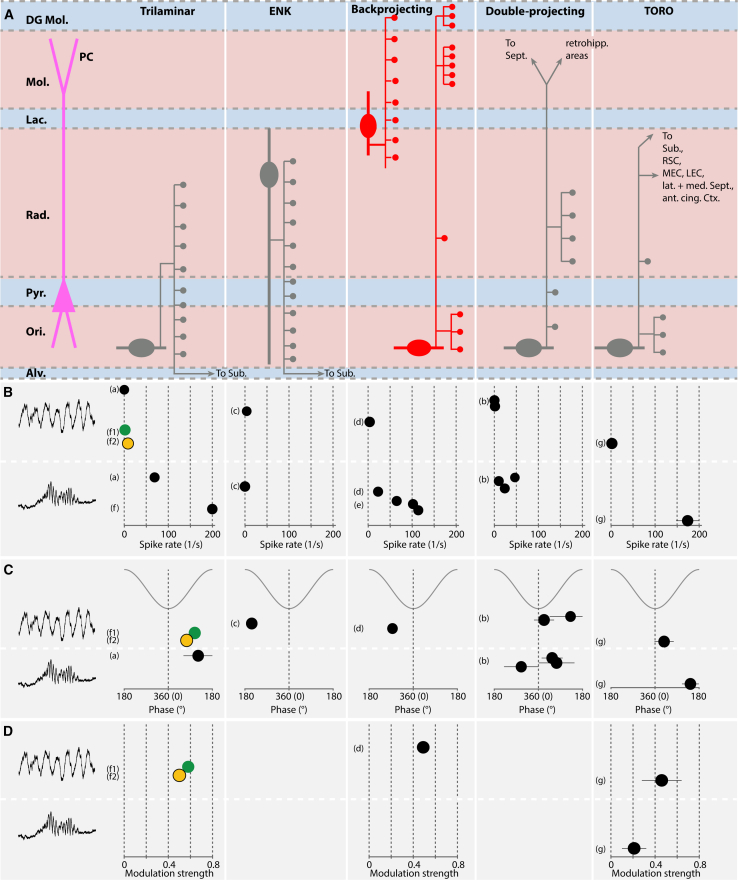
Since the formation of memory involves the transfer of information between the hippocampus and other cortical areas, the recently described involvement of LRP interneurons in hippocampal rhythms is of particular interest. Here, we summarize these findings (Figure 5) for the three main groups of LRP interneurons described above:
-
1.
Retrohippocampal interneurons. the activity of trilaminar LRP interneurons during hippocampal rhythms has been described for identified cells in anesthetized60 and freely behaving rats.59 During theta oscillations, they show low spike rates (Figure 5). During slow-wave sleep, they exhibit an irregular, relatively slow-frequency spiking (∼22 Hz59) that is interrupted by barrages of high-frequency action potential bursts60 at frequencies associated with ripples. However, in the absence of a local field potential (LFP) recording, it was only suspected but not proven by Katona and coworkers59 that the spiking activity of >200 Hz during slow-wave sleep co-occurs with SWRs. Therefore, the question of how these cells fire during ripples in drug-free conditions remains open. For VIP-LRP interneurons, calcium imaging in head-fixed awake mice showed the highest activity during behavioral immobility, compared with theta and SWR episodes,61 ruling out a major contribution for local disinhibition by VIP interneurons during these oscillations. So far, only one identified enkephalin-positive subicular-projecting interneuron has been recorded; in the anesthetized rat, this cell spiked at ∼4 Hz during theta oscillation and strongly decreased its firing to virtual silence during ripples168 (Figure 5).
-
2.
Back-projecting interneurons. the SOM+ LRP interneurons in CA1 SO that project to the DG discharge at ∼4 Hz during theta rhythm; during SWRs, their discharge rate increases to ∼23 Hz64 (Figure 5B). Two recently recorded DG-projecting SOM+ LRP interneurons with somata located in CA1 SR massively increased their spiking during SWRs65 (Figure 5B).
-
3.
Extrahippocampally projecting interneurons. (1) optogenetic activation of extrahippocampally projecting SOM+ cells at theta frequency induced oscillatory activity in the MEC, indicating their contribution to the synchronization of the hippocampus and MEC.67 (2) Double projection SOM cells, targeting both retrohippocampal areas and the MS, were recorded in anesthetized rats.57 These cells discharged at low frequency during theta rhythm and strongly increased their discharge rates during ripples (Figure 5). (3) TORO cells remain largely silent during theta rhythm and also dramatically increase their discharge rates during ripples20 (Figure 5). They are activated by CA3 Schaffer collateral inputs and suppressed by GABAergic and cholinergic inputs from MS.20
Figure 5.
Activity of CA1 long-range projecting interneurons during theta and ripple oscillations
(A) Most relevant long-range projecting interneuron types in CA1. For reference, the somatodendritic layout of a schematic pyramidal cell (PC, left) is included. ENK indicates an enkephalin expressing cell. Extrahippocampal projection target areas are indicated for some of the cell types: Sub, subiculum; RSC, retrosplenial cortex; MEC/LEC, medial/lateral entorhinal cortex; lat/med Sept., lateral/medial septum; ant. cing. Ctx, anterior cingular cortex.
(B–D) Properties of the interneuron classes displayed in (A), during theta oscillations (upper row) and ripples (lower row). The data are compiled from various studies. (a–g), with the following experimental conditions: (a) anesthetized rat60; (b) anesthetized rat (for theta and ripple, two and three individual cells, respectively, reported)57; (c) anesthetized rat, one cell reported168; (d) freely moving rat,64 one cell reported; (e) awake/head-fixed mouse,65 (for ripples, three individual cells, termed “extrinsic interneurons” located in CA1 and CA3 radiatum, and hilus, reported); (f) freely moving rat59 (for theta, during REM sleep [f1, green] and during movement [f2, yellow]; for ripples: value reflects activity recorded during slow-wave sleep; however, LFP was not independently monitored; it remains uncertain whether the ∼200 Hz activity actually reflects ripple-related activation of this cell type); (g) awake head-fixed mouse.20 (B) Spike rates. (C) Preferred spike phases. Phase values are given with a full cycle of oscillations as a reference. (D) Modulation strength as in Figure 4D. The error bars shown represent the margins of error reported in the indicated studies.
In this section, we have discussed the current state of knowledge about the contributions of different hippocampal interneurons to oscillatory activity, particularly theta and SWRs. Overall, although great progress has been made in all areas of the interneuron field covered by this review, we still lack a full understanding of how these individual elements come together to ultimately serve memory processing. A major challenge for the future is to provide a holistic model based on the domains we have discussed above. In the final section, we raise three open questions that we consider important for enabling us to reach this goal.
Perspectives: Inhibitory diversity, oscillations, plasticity, and hippocampal memory processing
What is the contribution of different hippocampal interneuron types to mnemonic functions?
Genetic manipulations in PV-Cre mouse lines have shown that inhibition of hippocampal PV interneurons disrupts memory function and memory-related oscillations.107,108 For the other broad subclasses of interneurons (SOM, Lamp5, Sncg, and VIP), less is known. VIP cells in CA1 have also been implicated in hippocampus-dependent goal learning.169 Interestingly, at least a subset of VIP cells in the neighboring CA2 region appears to be implicated in the encoding of social memory in a process involving regulation of feedforward inhibition via delta opioid receptors and the release of enkephalin.170 This study not only highlights the role of CA2 in social memory but also points to the role of further transmitters like enkephalin. Many interneurons co-release other transmitters alongside GABA that typically work on slower timescales (e.g., CCK, NO, and SOM), and in general, not much is known about their function (but see Racine et al.171).
Sncg (CCK) cells have also been implicated in similar types of plasticity of feedforward inhibition in CA1, reducing their GABA release after sustained Schaffer collateral activation,172 but the direct significance of this for memory has not been shown to the best of our knowledge. For hippocampal SOM cells, a link with memory has also been found.173,174,175 Finally, the Lamp5 subclass is the least studied and currently lacks a functional role in memory.
Recent mouse transcriptomic studies indicate a huge diversity of interneurons beyond the level of the cell types discussed thus far. Focusing on the PV subclass as an example, CA1 appears to contain at least seven47 and as many as 14 PV-expressing transcriptomic subtypes.41 Discovering the extent to which these subtypes are also differentially related to mnemonic functions remains a major challenge. Although influences from neighboring cells and other external factors may cause cells with the same expression pattern to have different phenotypes, a recent study showed that in visual cortex, the morphological and electrical properties of these cells are linked to transcriptomic subtype identity.176 Importantly, the link between transcriptomic and morphoelectric subtypes was not 1:1. Most of the visual cortex PV transcriptomic subtypes are also present in the hippocampus (10/14), albeit at different proportions, and it seems plausible that in CA1 there is also no 1:1 link between these transcriptomic subtypes and subtypes based on morphoelectric properties. One study focusing on morphologically defined CA1 PV interneurons found almost no correlation with transcriptomic subtypes, suggesting a continuum rather than distinct, discrete types.177 It remains to be seen if this result will also hold for larger sample sizes and broader sequencing. Perhaps transcriptomic subtypes that do not correlate to morphoelectric properties are still functionally important, e.g., by virtue of particular receptors and/or neuromodulatory elements, including distinct hormones and neuropeptides.47
The preceding summary of PV cell subtypes illustrates a broader issue: which aspects of a particular cell determine its function, and to what extent are interneuron “types” consistent across a wide range of functional domains? It will be exciting to see future studies attempting to correlate more detailed transcriptomic descriptions of hippocampal interneurons with memory processes, for example, looking at different memory contents (likely linked to different pathways), temporal stages (encoding, consolidation, and recall), timescales (working vs. long-term memory), and states (development/aging, diseases, and behavioral variables such as attention, arousal, reward, etc.).
It is perhaps not surprising that we still lack community consensus on how to define neuron types, despite recent progress.53,54 The most useful level of analysis is likely to vary depending on the brain state and the particular aspect of mnemonic function one is trying to explain. Currently, there is little data at the transcriptomic level with respect to the mechanisms underlying memory-associated oscillations or interneuron-associated plasticity, but the authors believe this will form an important angle of future research in order to eventually tackle the challenge of linking interneuron types to mnemonic processes.
Local and long-range connectivity motifs: How do they serve memory oscillations?
In the section activity patterns of hippocampal interneurons in theta rhythm and SWRs, we summarized studies comparing the contribution of subtypes of interneurons during theta and SWR oscillations. Still, most of this work has been correlative, and only a limited scope of associated brain states and mnemonic functions has been studied.
For the diverse subclasses (e.g., PV), it is highly possible that different subtypes (namely, somatic-, axonal-, and dendritic-targeting PV cells) will exhibit different activity phenotypes during theta and SWRs. A number of recent studies support such a possibility by showing that PV BCs and PV AACs cells, for example, exhibit distinct activation patterns during locomotion and running (related to theta activity), as well as during immobility and rest periods (related to SWR activity), in vivo.13,14 Work from the same group has also revealed that both types of hippocampal BCs (PV- and CCK-positive populations) are active during different memory-related stages.13 This new finding is crucial because it shows that even interneurons that target the same postsynaptic compartment and mediate perisomatic inhibition but have different intrinsic properties (e.g., fast vs. regular spiking for PV and CCK cells) can contribute to memory-related oscillations in different ways. Moreover, we consider it important to highlight that apart from their differences in connectivity or intrinsic properties, interneurons—even the ones belonging to the same subclass—are different in terms of their subcellular features and, as a result, can exhibit different computational capabilities and synaptic integration profiles178,179,180 that could uniquely impact their contribution in the generation and propagation of oscillatory activity.181 Therefore, a detailed description of interneuronal activity during hippocampal rhythms is needed to finally understand how and when the different classes and subtypes of these cells contribute to memory formation at the network level.
It is also important to mention that to fully understand the mechanisms underlying memory-associated oscillations, we will require more studies investigating the connections between different cell types, considering not only the variability of interneurons but also of principal cells (i.e., deep vs. superficial PCs83,84). This applies both at the microcircuit level, as our review of disinhibition has demonstrated, and at the inter-regional level, as highlighted by our review of LRP interneurons.
Below, as an example of such a mechanism, we present a view on how disinhibition could support SWRs. We also speculate on how long-range connectivity motifs could potentially aid the transfer of memories within and outside of the hippocampus.
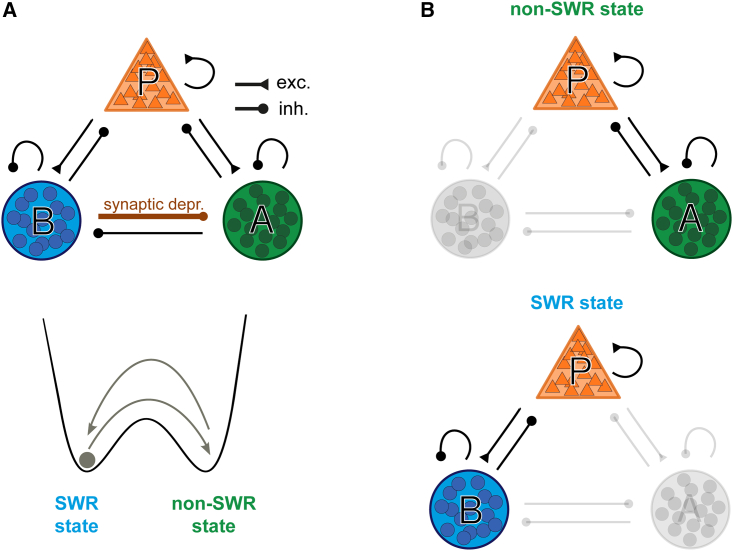
A possible role of disinhibition in hippocampal SWRs
Given the abundance of disinhibitory circuitries in the hippocampus, is there a role for disinhibition in ripples? Recently, a theoretical model was presented182 in which disinhibition is a vital component to explain the emergence of SWR in the hippocampus (Figure 6). This model is based on a simplified spiking network consisting of excitatory neurons (P) and two types of interneurons, PV BCs (B) and a proposed class of interneuron (A) whose activity exhibits an “anti-SWR” spiking pattern. Thus, in contrast to what is known for PV BCs, A neurons are active before and after SWRs but silent during them. Both classes of interneurons are reciprocally connected. B-to-A synapses are equipped with a mechanism for short-term depression (Figure 6A). Oscillations with SWR-like properties can arise spontaneously in the network or can be induced by cell stimulation (by activation of P or B cells or inactivation of A cells). The fundamental trigger for the development of SWRs is the disinhibition of P cells due to the suppression of A cells by active B cells. As a result, the network state fluctuates and is determined either by the higher activity of P and A cells (non-SWR state) or by the higher activity of P and B cells (SWR state) (Figure 6B). Although the model is based on biological data, such as CA3 network connectivity and synaptic properties, key assumptions such as the existence of A cells playing a causal role in SWR initiation and the mechanism of B-to-A synaptic depression have yet to be directly demonstrated. In this context, it will be interesting to further characterize the already identified “anti”-modulated SWR interneurons in CA1.14,160,168 Overall, disinhibitory circuit motifs are abundant in this region, and the data already available suggest that disinhibition is likely to play an important role in the context of hippocampus-dependent memory formation and its associated rhythms.
Figure 6.
A possible role of disinhibition in the generation of SWRs
(A) Top: the three-population network model consists of excitatory cells (P) and two groups of interneurons (PV BCs and anti-SWR cells, B and A, respectively). Arrows ending with a triangle show excitatory connections (exc.), and arrows ending with a circle show inhibitory connections (inh.). The connection of PV BCs to anti-SWR cells involves a mechanism of short-term synaptic depression (dark red). Bottom: schematic representation of the network behavior by a particle (gray dot) moving in a potential landscape. The network dynamics switch between non-SWR and SWR states (arrows). Text color represents the dominant interneuron type that is active in both states, i.e., dominance of B cells in the SWR state and dominance of A cells in the non-SWR state. External factors (i.e., current injection or dynamic synaptic depression) can be used to trigger transitions between the two states.
(B) Schematic of the dominant subnetworks in non-SWR and SWR states. Top: non-SWR state: the interaction between P and A cells determines the network activity, while B cells are almost silent. Despite the low firing rate of P cells, their inputs to the A cells are required to keep the A cells active. Bottom: SWR state: active P and B cells dominate the network activity, whereas A cells are almost silent. Figure modified with permission from Evangelista et al. 182
Extrahippocampal memory communication through inhibitory long-range projections
Recent studies have shown that certain GABAergic interneurons in the hippocampus connect with other cortical areas.20,58,67 What is the contribution of these inhibitory long-distance projections in oscillogenesis and the transition of memory-related information from the hippocampus to other brain areas? To answer this question, we first need to classify and characterize their properties. In other words, a detailed description of the anatomical, morphological, and intrinsic features of these “special” interneurons is needed to selectively target them and modulate their activity during memory processing and its associated rhythms in vivo. Another question that arises from the existence of these neurons is what differentiates these LRP interneurons from their local-targeting counterparts. Is there a set of exclusive developmental, morphological, or molecular features that could differentiate local and long-distance projecting interneurons? If yes, then do LRP interneurons consist of a distinct functional class among hippocampal inhibitory elements? The data available so far indicate that they express similar molecular markers as local interneurons (e.g., VIP,61,62 SOM,67 or calbindin/M2Rs20), making their classification based on single molecular profiling quite challenging. Differentiation appears to be possible by combining multiple markers, but this remains to be confirmed. These interneurons seem to play important roles during hippocampal memory oscillations. For example, TORO cells were found to transmit information to other areas, specifically during SWRs in CA1, suggesting a potential mechanism contributing to the transmission of memory-related information outside the hippocampus.20 What is the local contribution to ripple oscillogenesis of these far-projecting inhibitory interneurons? What is the role of ripple-associated inhibition by TORO cells in distant brain regions? Hopefully, future research will shed light on these exciting questions.
Another intriguing possibility is that the hippocampus not only “sends” memory-related information but also “receives” such information through targeted inhibitory transmission. In support of such a possibility, a recent study demonstrated that LRP interneurons from the prefrontal cortex (PFC) target CA1 VIP+ cells during memory encoding.106 Such communication could be a key mechanism that facilitates flexible changes in hippocampal circuitries through cross-area modulation of local disinhibition. Overall, although little is currently known about the functional role of LRP GABAergic interneurons, they are likely to provide a powerful toolset for transferring and receiving memory-related information in the hippocampus and beyond.
Is there a link between plasticity induction in hippocampal interneurons and their role in controlling circuit dynamics and memory-related rhythms?
Synaptic and intrinsic plasticity may provide an ideal mechanism to enable interneuronal regulation of memory under distinct oscillatory events. Given that synaptic and intrinsic plasticity is a widely accepted mechanism for the reconfiguration of network excitability, it is highly possible that the “plasticity state” of interneurons will define whether they will be active (through potentiation) or silent (through depression). In other words, when and at which level they will participate in distinct rhythmic activity patterns during memory formation. As discussed in the section plasticity of synaptic transmission and intrinsic excitability in hippocampal interneurons, multiple distinct mechanisms of synaptic (PC to IN or IN to PC) and intrinsic plasticity have been found in a few hippocampal interneuron subtypes.
Most available data account for the PV class. Interestingly, a direct link between PV interneuron plasticity and memory formation has been reported. Network-level plasticity of PV+ interneurons has, thus far, been associated with different memory functions through changes in the molecular composition of FS BC subpopulations in the hippocampus, namely varying levels of PV and GAD67 concentration.183,184 Specifically, a network with low-PV concentration in FS BCs was shown to enhance spatial learning (associated with theta activity), whereas learning was reduced by the high-PV-network configuration.183 Moreover, such long- but not short-term (learning-induced) plasticity of local PV FS BCs was specifically required for long-term, but not short/intermediate-term, memory consolidation (associated with SWR activity184). Furthermore, the IE of PV FS BCs, driven by CA3 Schaffer collateral inputs, showed an increase in their activity during gamma oscillatory activity.119 Finally, plasticity in CA1 PV interneurons was recently found to depend on the γ isoform of CaMKII, which is also critical for memory-related changes in firing rate and theta oscillations.185 The above findings pave the way for linking plasticity of PV interneurons with memory states. However, these findings apply to the entire PV interneuron class, including BiCs, FS BCs, AACs, and O-LM cells. Thus, it would be very interesting to test whether the above data applies to all or specific PV subtypes. Given their molecular heterogeneity, it is possible that plasticity induction in PV subtypes, as well as in other diverse subclasses, e.g., VIP cells, may be regulated by distinct molecules. Transcriptomic data may, in the future, provide a powerful tool for predicting plasticity induction mechanisms based on gene regulation. Finding a link between the induction of interneuron plasticity and oscillatory activity will bring us a considerable step closer to understanding how cell assemblies, or engrams, and ultimately memories are formed.
Conclusion and outlook
Despite their relatively low numbers compared with excitatory cells, interneurons are undoubtedly a major determinant of normal brain function. In this review, we have attempted to describe the diversity of hippocampal interneurons under the memory prism. We discussed known differences in molecular, anatomical, electrophysiological, and connectivity profiles of the numerous local and distant projecting hippocampal interneuron subtypes and pointed out current knowledge gaps. Furthermore, we summarized the various forms and underlying mechanisms of both synaptic plasticity and plasticity of IE that have been reported in some interneuron subtypes in the hippocampus. Finally, we provided an overview of the local and distant projecting interneurons in the two most predominant memory-related rhythms—theta rhythm and SWRs.
Although much progress has been made in the last decades, we currently still lack a complete understanding of both the single-neuron characteristics as well as the functional roles of the numerous hippocampal interneurons at the circuit and behavioral levels. With increasingly sophisticated methods allowing for more comprehensive cell characterization, the first step toward this goal will be to provide a full overview of the interneuron palette. Further technological advances enabling targeted manipulations of these subtypes in behaving animals will pave the way for the next steps. The time is ripe for such an exciting endeavor.
Acknowledgments
Research in the D.S. lab is supported by the Einstein Foundation Berlin, the European Research Council (ERC) under the Europeans Union’s Horizon 2020 research and innovation program (BrainPlay grant, agreement no. 810580), the German Research Foundation (Deutsche Forschungsgemeinschaft [DFG], SFB-958 – project 184695641, project 431572356, FOR 3004 – project 415914819, SFB 1315 – project 327654276 and under Germany’s Excellence Strategy – Exc-2049-390688087 NeuroCure), and the Federal Ministry of Education and Research (BMBF, SmartAge – project 01GQ1420B). A.T. is supported by the DFG with the SFB1315-2 TP A01 Brenda Milner Award and the Einstein Center for Neurosciences Berlin PhD Fellowship. P.P. is supported by the NIH (1R01MH124867-02), the European Commission (H2020-FETOPEN-2018-2019-2020-01, FET-Open Challenging Current Thinking, NEUREKA GA-863245), and the Einstein Foundation Berlin, Germany, visiting fellowship EVF-2019-508. We thank Antje Fortströer for helpful proofreading and Michael Hadler for comments on an earlier version of the manuscript.
Author contributions
Conceptualization, A.T., J.J.T., N.M., and D.S.; original writing, A.T., J.J.T., N.M., and D.S.; figure preparation, A.T., J.J.T., and N.M.; diting all authors.
Declaration of interests
The authors declare no competing interests.
References
- 1.Holtmaat A., Caroni P. Functional and structural underpinnings of neuronal assembly formation in learning. Nat. Neurosci. 2016;19:1553–1562. doi: 10.1038/nn.4418. [DOI] [PubMed] [Google Scholar]
- 2.Cummings K.A., Lacagnina A.F., Clem R.L. GABAergic microcircuitry of fear memory encoding. Neurobiol. Learn. Mem. 2021;184:107504. doi: 10.1016/j.nlm.2021.107504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giorgi C., Marinelli S. Roles and transcriptional responses of inhibitory neurons in learning and memory. Front. Mol. Neurosci. 2021;14:689952. doi: 10.3389/fnmol.2021.689952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topolnik L., Tamboli S. The role of inhibitory circuits in hippocampal memory processing. Nat. Rev. Neurosci. 2022;23:476–492. doi: 10.1038/s41583-022-00599-0. [DOI] [PubMed] [Google Scholar]
- 5.Tzilivaki A., Kastellakis G., Schmitz D., Poirazi P. GABAergic interneurons with nonlinear dendrites: from neuronal computations to memory engrams. Neuroscience. 2022;489:34–43. doi: 10.1016/j.neuroscience.2021.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Lucas E.K., Clem R.L. GABAergic interneurons: the orchestra or the conductor in fear learning and memory? Brain Res. Bull. 2018;141:13–19. doi: 10.1016/j.brainresbull.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booker S.A., Vida I. Morphological diversity and connectivity of hippocampal interneurons. Cell Tissue Res. 2018;373:619–641. doi: 10.1007/s00441-018-2882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFelipe J., López-Cruz P.L., Benavides-Piccione R., Bielza C., Larrañaga P., Anderson S., Burkhalter A., Cauli B., Fairén A., Feldmeyer D., et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat. Rev. Neurosci. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fishell G., Kepecs A. Interneuron types as attractors and controllers. Annu. Rev. Neurosci. 2020;43:1–30. doi: 10.1146/annurev-neuro-070918-050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klausberger T., Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelkey K.A., Chittajallu R., Craig M.T., Tricoire L., Wester J.C., McBain C.J. Hippocampal gabaergic inhibitory interneurons. Physiol. Rev. 2017;97:1619–1747. doi: 10.1152/physrev.00007.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tremblay R., Lee S., Rudy B. Gabaergic interneurons in the neocortex: from cellular properties to circuits. Neuron. 2016;91:260–292. doi: 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudok B., Klein P.M., Hwaun E., Lee B.R., Yao Z., Fong O., Bowler J.C., Terada S., Sparks F.T., Szabo G.G., et al. Alternating sources of perisomatic inhibition during behavior. Neuron. 2021;109:997–1012.e9. doi: 10.1016/j.neuron.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudok B., Szoboszlay M., Paul A., Klein P.M., Liao Z., Hwaun E., Szabo G.G., Geiller T., Vancura B., Wang B.S., et al. Recruitment and inhibitory action of hippocampal axo-axonic cells during behavior. Neuron. 2021;109:3838–3850.e8. doi: 10.1016/j.neuron.2021.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lapray D., Lasztoczi B., Lagler M., Viney T.J., Katona L., Valenti O., Hartwich K., Borhegyi Z., Somogyi P., Klausberger T. Behavior-dependent specialization of identified hippocampal interneurons. Nat. Neurosci. 2012;15:1265–1271. doi: 10.1038/nn.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katona L., Lapray D., Viney T.J., Oulhaj A., Borhegyi Z., Micklem B., Klausberger T., Somogyi P. Sleep and movement differentiates actions of two types of somatostatin-expressing GABAergic interneuron in rat hippocampus. Neuron. 2014;82:872–886. doi: 10.1016/j.neuron.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Aguilera A., Wheeler D.W., Jurado-Parras T., Valero M., Nokia M.S., Cid E., Fernandez-Lamo I., Sutton N., García-Rincón D., de la Prida L.M., et al. An update to Hippocampome.org by integrating single-cell phenotypes with circuit function in vivo. PLoS Biol. 2021;19:e3001213. doi: 10.1371/journal.pbio.3001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somogyi P., Katona L., Klausberger T., Lasztóczi B., Viney T.J. Temporal redistribution of inhibition over neuronal subcellular domains underlies state-dependent rhythmic change of excitability in the hippocampus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20120518. doi: 10.1098/rstb.2012.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tukker J.J., Fuentealba P., Hartwich K., Somogyi P., Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J. Neurosci. 2007;27:8184–8189. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szabo G.G., Farrell J.S., Dudok B., Hou W.H., Ortiz A.L., Varga C., Moolchand P., Gulsever C.I., Gschwind T., Dimidschstein J., et al. Ripple-selective GABAergic projection cells in the hippocampus. Neuron. 2022;110:1959–1977.e9. doi: 10.1016/j.neuron.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viney T.J., Lasztoczi B., Katona L., Crump M.G., Tukker J.J., Klausberger T., Somogyi P. Network state-dependent inhibition of identified hippocampal CA3 axo-axonic cells in vivo. Nat. Neurosci. 2013;16:1802–1811. doi: 10.1038/nn.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klausberger T., Magill P.J., Márton L.F., Roberts J.D., Cobden P.M., Buzsáki G., Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 23.Varga C., Golshani P., Soltesz I. Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proc. Natl. Acad. Sci. USA. 2012;109:E2726–E2734. doi: 10.1073/pnas.1210929109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varga C., Oijala M., Lish J., Szabo G.G., Bezaire M., Marchionni I., Golshani P., Soltesz I. Functional fission of parvalbumin interneuron classes during fast network events. eLife. 2014;3 doi: 10.7554/eLife.04006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S., Hjerling-Leffler J., Zagha E., Fishell G., Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J. Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudy B., Fishell G., Lee S., Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Filippo R., Rost B.R., Stumpf A., Cooper C., Tukker J.J., Harms C., Beed P., Schmitz D. Somatostatin interneurons activated by 5-HT2A receptor suppress slow oscillations in medial entorhinal cortex. eLife. 2021;10 doi: 10.7554/eLife.66960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jouhanneau J.S., Kremkow J., Poulet J.F.A. Single synaptic inputs drive high-precision action potentials in parvalbumin expressing GABA-ergic cortical neurons in vivo. Nat. Commun. 2018;9:1540. doi: 10.1038/s41467-018-03995-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan A.G., Poort J., Chadwick A., Blot A., Sahani M., Mrsic-Flogel T.D., Hofer S.B. Distinct learning-induced changes in stimulus selectivity and interactions of GABAergic interneuron classes in visual cortex. Nat. Neurosci. 2018;21:851–859. doi: 10.1038/s41593-018-0143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miao C., Cao Q., Moser M.B., Moser E.I. Parvalbumin and somatostatin interneurons control different space-coding networks in the medial entorhinal cortex. Cell. 2017;171:507–521.e17. doi: 10.1016/j.cell.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Royer S., Zemelman B.V., Losonczy A., Kim J., Chance F., Magee J.C., Buzsáki G. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci. 2012;15:769–775. doi: 10.1038/nn.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulyás A.I., Miles R., Hájos N., Freund T.F. Precision and variability in postsynaptic target selection of inhibitory cells in the hippocampal CA3 region. Eur. J. Neurosci. 1993;5:1729–1751. doi: 10.1111/j.1460-9568.1993.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 33.McBain C.J., DiChiara T.J., Kauer J.A. Activation of metabotropic glutamate receptors differentially affects two classes of hippocampal interneurons and potentiates excitatory synaptic transmission. J. Neurosci. 1994;14:4433–4445. doi: 10.1523/JNEUROSCI.14-07-04433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sik A., Penttonen M., Ylinen A., Buzsáki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J. Neurosci. 1995;15:6651–6665. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buhl E.H., Halasy K., Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- 36.Katona I., Acsády L., Freund T.F. Postsynaptic targets of somatostatin-immunoreactive interneurons in the rat hippocampus. Neuroscience. 1999;88:37–55. doi: 10.1016/s0306-4522(98)00302-9. [DOI] [PubMed] [Google Scholar]
- 37.Somogyi P. A specific “axo-axonal” interneuron in the visual cortex of the rat. Brain Res. 1977;136:345–350. doi: 10.1016/0006-8993(77)90808-3. [DOI] [PubMed] [Google Scholar]
- 38.Somogyi P., Freund T.F., Cowey A. The axo-axonic interneuron in the cerebral cortex of the rat, cat and monkey. Neuroscience. 1982;7:2577–2607. doi: 10.1016/0306-4522(82)90086-0. [DOI] [PubMed] [Google Scholar]
- 39.Buhl E.H., Han Z.S., Lörinczi Z., Stezhka V.V., Karnup S.V., Somogyi P. Physiological properties of anatomically identified axo-axonic cells in the rat hippocampus. J. Neurophysiol. 1994;71:1289–1307. doi: 10.1152/jn.1994.71.4.1289. [DOI] [PubMed] [Google Scholar]
- 40.Kawaguchi Y., Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J. Neurosci. 1996;16:2701–2715. doi: 10.1523/JNEUROSCI.16-08-02701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao Z., van Velthoven C.T.J., Nguyen T.N., Goldy J., Sedeno-Cortes A.E., Baftizadeh F., Bertagnolli D., Casper T., Chiang M., Crichton K., et al. A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell. 2021;184:3222–3241.e26. doi: 10.1016/j.cell.2021.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuentealba P., Begum R., Capogna M., Jinno S., Márton L.F., Csicsvari J., Thomson A., Somogyi P., Klausberger T. Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron. 2008;57:917–929. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cajal S.R. Vol. 2. A. Maloine; 1911. Histologie Du Système Nerveux de l’Homme et Des Vertébrés. [Google Scholar]
- 44.Armstrong C., Krook-Magnuson E., Soltesz I. Neurogliaform and Ivy cells: a major family of nNOS expressing GABAergic neurons. Front. Neural Circuits. 2012;6:23. doi: 10.3389/fncir.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szabadics J., Tamás G., Soltesz I. Different transmitter transients underlie presynaptic cell type specificity of GABAA, slow and GABAA, fast. Proc. Natl. Acad. Sci. USA. 2007;104:14831–14836. doi: 10.1073/pnas.0707204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tricoire L., Pelkey K.A., Daw M.I., Sousa V.H., Miyoshi G., Jeffries B., Cauli B., Fishell G., McBain C.J. Common origins of hippocampal Ivy and nitric oxide synthase expressing neurogliaform cells. J. Neurosci. 2010;30:2165–2176. doi: 10.1523/JNEUROSCI.5123-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris K.D., Hochgerner H., Skene N.G., Magno L., Katona L., Bengtsson Gonzales C., Somogyi P., Kessaris N., Linnarsson S., Hjerling-Leffler J. Classes and continua of hippocampal CA1 inhibitory neurons revealed by single-cell transcriptomics. PLoS Biol. 2018;16:e2006387. doi: 10.1371/journal.pbio.2006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cope D.W., Maccaferri G., Márton L.F., Roberts J.D.B., Cobden P.M., Somogyi P. Cholecystokinin-immunopositive basket and Schaffer collateral-associated interneurones target different domains of pyramidal cells in the CA1 area of the rat hippocampus. Neuroscience. 2002;109:63–80. doi: 10.1016/s0306-4522(01)00440-7. [DOI] [PubMed] [Google Scholar]
- 49.Lasztóczi B., Tukker J.J., Somogyi P., Klausberger T. Terminal field and firing selectivity of cholecystokinin-expressing interneurons in the hippocampal CA3 area. J. Neurosci. 2011;31:18073–18093. doi: 10.1523/JNEUROSCI.3573-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guet-McCreight A., Skinner F.K., Topolnik L. Common principles in functional organization of VIP/calretinin cell-driven disinhibitory circuits across cortical areas. Front. Neural Circuits. 2020;14:32. doi: 10.3389/fncir.2020.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeisel A., Muñoz-Manchado A.B., Codeluppi S., Lönnerberg P., La Manno G., Juréus A., Marques S., Munguba H., He L., Betsholtz C., et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 52.Zeng H. What is a cell type and how to define it? Cell. 2022;185:2739–2755. doi: 10.1016/j.cell.2022.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuste R., Hawrylycz M., Aalling N., Aguilar-Valles A., Arendt D., Armañanzas R., Ascoli G.A., Bielza C., Bokharaie V., Bergmann T.B., et al. A community-based transcriptomics classification and nomenclature of neocortical cell types. Nat. Neurosci. 2020;23:1456–1468. doi: 10.1038/s41593-020-0685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petilla Interneuron Nomenclature Group. Ascoli G.A., Alonso-Nanclares L., Anderson S.A., Barrionuevo G., Benavides-Piccione R., Burkhalter A., Buzsáki G., Cauli B., Defelipe J., et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Battaglia D., Karagiannis A., Gallopin T., Gutch H.W., Cauli B. Beyond the frontiers of neuronal types. Front. Neural Circuits. 2013;7:13. doi: 10.3389/fncir.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bota M., Swanson L.W. The neuron classification problem. Brain Res. Rev. 2007;56:79–88. doi: 10.1016/j.brainresrev.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jinno S., Klausberger T., Marton L.F., Dalezios Y., Roberts J.D., Fuentealba P., Bushong E.A., Henze D., Buzsáki G., Somogyi P. Neuronal diversity in GABAergic long-range projections from the hippocampus. J. Neurosci. 2007;27:8790–8804. doi: 10.1523/JNEUROSCI.1847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melzer S., Monyer H. Diversity and function of corticopetal and corticofugal GABAergic projection neurons. Nat. Rev. Neurosci. 2020;21:499–515. doi: 10.1038/s41583-020-0344-9. [DOI] [PubMed] [Google Scholar]
- 59.Katona L., Hartwich K., Tomioka R., Somogyi J., Roberts J.D.B., Wagner K., Joshi A., Klausberger T., Rockland K.S., Somogyi P. Synaptic organisation and behaviour-dependent activity of mGluR8a-innervated GABAergic trilaminar cells projecting from the hippocampus to the subiculum. Brain Struct. Funct. 2020;225:705–734. doi: 10.1007/s00429-020-02029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferraguti F., Klausberger T., Cobden P., Baude A., Roberts J.D., Szucs P., Kinoshita A., Shigemoto R., Somogyi P., Dalezios Y. Metabotropic glutamate receptor 8-expressing nerve terminals target subsets of GABAergic neurons in the hippocampus. J. Neurosci. 2005;25:10520–10536. doi: 10.1523/JNEUROSCI.2547-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Francavilla R., Villette V., Luo X., Chamberland S., Muñoz-Pino E., Camiré O., Wagner K., Kis V., Somogyi P., Topolnik L. Connectivity and network state-dependent recruitment of long-range VIP-GABAergic neurons in the mouse hippocampus. Nat. Commun. 2018;9:5043. doi: 10.1038/s41467-018-07162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo X., Muñoz-Pino E., Francavilla R., Vallée M., Droit A., Topolnik L. Transcriptomic profile of the subiculum-projecting VIP GABAergic neurons in the mouse CA1 hippocampus. Brain Struct. Funct. 2019;224:2269–2280. doi: 10.1007/s00429-019-01883-z. [DOI] [PubMed] [Google Scholar]
- 63.Sik A., Ylinen A., Penttonen M., Buzsáki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994;265:1722–1724. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- 64.Katona L., Micklem B., Borhegyi Z., Swiejkowski D.A., Valenti O., Viney T.J., Kotzadimitriou D., Klausberger T., Somogyi P. Behavior-dependent activity patterns of GABAergic long-range projecting neurons in the rat hippocampus. Hippocampus. 2017;27:359–377. doi: 10.1002/hipo.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szabo G.G., Du X., Oijala M., Varga C., Parent J.M., Soltesz I. Extended interneuronal network of the dentate gyrus. Cell Rep. 2017;20:1262–1268. doi: 10.1016/j.celrep.2017.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hooper A., Maguire J. Characterization of a novel subtype of hippocampal interneurons that express corticotropin-releasing hormone. Hippocampus. 2016;26:41–53. doi: 10.1002/hipo.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melzer S., Michael M., Caputi A., Eliava M., Fuchs E.C., Whittington M.A., Monyer H. Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science. 2012;335:1506–1510. doi: 10.1126/science.1217139. [DOI] [PubMed] [Google Scholar]
- 68.Jinno S., Kosaka T. Colocalization of parvalbumin and somatostatin-like immunoreactivity in the mouse hippocampus: quantitative analysis with optical dissector. J. Comp. Neurol. 2000;428:377–388. doi: 10.1002/1096-9861(20001218)428:3<377::AID-CNE1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 69.Christenson Wick Z., Tetzlaff M.R., Krook-Magnuson E. Novel long-range inhibitory nNOS-expressing hippocampal cells. eLife. 2019;8 doi: 10.7554/eLife.46816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bezaire M.J., Soltesz I. Quantitative assessment of CA1 local circuits: knowledge base for interneuron-pyramidal cell connectivity. Hippocampus. 2013;23:751–785. doi: 10.1002/hipo.22141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gour A., Boergens K.M., Heike N., Hua Y., Laserstein P., Song K., Helmstaedter M. Postnatal connectomic development of inhibition in mouse barrel cortex. Science. 2021;371 doi: 10.1126/science.abb4534. [DOI] [PubMed] [Google Scholar]
- 72.Schneider-Mizell C.M., Bodor A.L., Collman F., Brittain D., Bleckert A., Dorkenwald S., Turner N.L., Macrina T., Lee K., Lu R., et al. Structure and function of axo-axonic inhibition. eLife. 2021;10 doi: 10.7554/eLife.73783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bloss E.B., Cembrowski M.S., Karsh B., Colonell J., Fetter R.D., Spruston N. Single excitatory axons form clustered synapses onto CA1 pyramidal cell dendrites. Nat. Neurosci. 2018;21:353–363. doi: 10.1038/s41593-018-0084-6. [DOI] [PubMed] [Google Scholar]
- 74.Mishchenko Y., Hu T., Spacek J., Mendenhall J., Harris K.M., Chklovskii D.B. Ultrastructural analysis of hippocampal neuropil from the connectomics perspective. Neuron. 2010;67:1009–1020. doi: 10.1016/j.neuron.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turner N.L., Macrina T., Bae J.A., Yang R., Wilson A.M., Schneider-Mizell C., Lee K., Lu R., Wu J., Bodor A.L., et al. Reconstruction of neocortex: organelles, compartments, cells, circuits, and activity. Cell. 2022;185:1082–1100.e24. doi: 10.1016/j.cell.2022.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guzman S.J., Schlögl A., Frotscher M., Jonas P. Synaptic mechanisms of pattern completion in the hippocampal CA3 network. Science. 2016;353:1117–1123. doi: 10.1126/science.aaf1836. [DOI] [PubMed] [Google Scholar]
- 77.Perin R., Berger T.K., Markram H. A synaptic organizing principle for cortical neuronal groups. Proc. Natl. Acad. Sci. USA. 2011;108:5419–5424. doi: 10.1073/pnas.1016051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Böhm C., Peng Y., Maier N., Winterer J., Poulet J.F., Geiger J.R., Schmitz D. Functional diversity of subicular principal cells during hippocampal ripples. J. Neurosci. 2015;35:13608–13618. doi: 10.1523/JNEUROSCI.5034-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peng Y., Barreda Tomás F.J., Klisch C., Vida I., Geiger J.R.P. Layer-specific organization of local excitatory and inhibitory synaptic connectivity in the rat presubiculum. Cereb. Cortex. 2017;27:2435–2452. doi: 10.1093/cercor/bhx049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sammons R.P., Tzilivaki A., Schmitz D. Local microcircuitry of pas shows distinct and common features of excitatory and inhibitory connectivity. Cereb. Cortex. July 2021;32:76–92. doi: 10.1093/cercor/bhab195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuchs E.C., Neitz A., Pinna R., Melzer S., Caputi A., Monyer H. Local and distant input controlling excitation in layer II of the medial entorhinal cortex. Neuron. 2016;89:194–208. doi: 10.1016/j.neuron.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Varga C., Lee S.Y., Soltesz I. Target-selective GABAergic control of entorhinal cortex output. Nat. Neurosci. 2010;13:822–824. doi: 10.1038/nn.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee S.H., Marchionni I., Bezaire M., Varga C., Danielson N., Lovett-Barron M., Losonczy A., Soltesz I. Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron. 2014;82:1129–1144. doi: 10.1016/j.neuron.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soltesz I., Losonczy A. CA1 pyramidal cell diversity enabling parallel information processing in the hippocampus. Nat. Neurosci. 2018;21:484–493. doi: 10.1038/s41593-018-0118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cembrowski M.S., Phillips M.G., DiLisio S.F., Shields B.C., Winnubst J., Chandrashekar J., Bas E., Spruston N. Dissociable structural and functional hippocampal outputs via distinct subiculum cell classes. Cell. 2018;173:1280–1292.e18. doi: 10.1016/j.cell.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 86.Kim Y., Spruston N. Target-specific output patterns are predicted by the distribution of regular-spiking and bursting pyramidal neurons in the subiculum. Hippocampus. 2012;22:693–706. doi: 10.1002/hipo.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Valero M., de la Prida L.M. The hippocampus in depth: a sublayer-specific perspective of entorhinal-hippocampal function. Curr. Opin. Neurobiol. 2018;52:107–114. doi: 10.1016/j.conb.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 88.Geiller T., Royer S., Choi J.S. Segregated cell populations enable distinct parallel encoding within the radial axis of the CA1 pyramidal layer. Exp. Neurobiol. 2017;26:1–10. doi: 10.5607/en.2017.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stark E., Roux L., Eichler R., Senzai Y., Royer S., Buzsáki G. Pyramidal cell-interneuron interactions underlie hippocampal ripple oscillations. Neuron. 2014;83:467–480. doi: 10.1016/j.neuron.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valero M., Cid E., Averkin R.G., Aguilar J., Sanchez-Aguilera A., Viney T.J., Gomez-Dominguez D., Bellistri E., de la Prida L.M. Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat. Neurosci. 2015;18:1281–1290. doi: 10.1038/nn.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abs E., Poorthuis R.B., Apelblat D., Muhammad K., Pardi M.B., Enke L., Kushinsky D., Pu D.L., Eizinger M.F., Conzelmann K.K., et al. Learning-related plasticity in dendrite-targeting layer 1 interneurons. Neuron. 2018;100:684–699.e6. doi: 10.1016/j.neuron.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Acsády L., Arabadzisz D., Freund T.F. Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide-immunoreactive interneurons in rat hippocampus. Neuroscience. 1996;73:299–315. doi: 10.1016/0306-4522(95)00610-9. [DOI] [PubMed] [Google Scholar]
- 93.Gulyás A.I., Hájos N., Freund T.F. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J. Neurosci. 1996;16:3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luo X., Guet-McCreight A., Villette V., Francavilla R., Marino B., Chamberland S., Skinner F.K., Topolnik L. Synaptic mechanisms underlying the network state-dependent recruitment of VIP-expressing interneurons in the CA1 hippocampus. Cereb. Cortex. 2020;30:3667–3685. doi: 10.1093/cercor/bhz334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tyan L., Chamberland S., Magnin E., Camiré O., Francavilla R., David L.S., Deisseroth K., Topolnik L. Dendritic inhibition provided by interneuron-specific cells controls the firing rate and timing of the hippocampal feedback inhibitory circuitry. J. Neurosci. 2014;34:4534–4547. doi: 10.1523/JNEUROSCI.3813-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Daw M.I., Tricoire L., Erdelyi F., Szabo G., McBain C.J. Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. J. Neurosci. 2009;29:11112–11122. doi: 10.1523/JNEUROSCI.5760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cobb S.R., Halasy K., Vida I., Nyiri G., Tamás G., Buhl E.H., Somogyi P. Synaptic effects of identified interneurons innervating both interneurons and pyramidal cells in the rat hippocampus. Neuroscience. 1997;79:629–648. doi: 10.1016/s0306-4522(97)00055-9. [DOI] [PubMed] [Google Scholar]
- 98.Ali A.B. Presynaptic inhibition of GABAA receptor-mediated unitary IPSPs by cannabinoid receptors at synapses between CCK-positive interneurons in rat hippocampus. J. Neurophysiol. 2007;98:861–869. doi: 10.1152/jn.00156.2007. [DOI] [PubMed] [Google Scholar]
- 99.Price C.J., Cauli B., Kovacs E.R., Kulik A., Lambolez B., Shigemoto R., Capogna M. Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J. Neurosci. 2005;25:6775–6786. doi: 10.1523/JNEUROSCI.1135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elfant D., Pál B.Z., Emptage N., Capogna M. Specific inhibitory synapses shift the balance from feedforward to feedback inhibition of hippocampal CA1 pyramidal cells. Eur. J. Neurosci. 2008;27:104–113. doi: 10.1111/j.1460-9568.2007.06001.x. [DOI] [PubMed] [Google Scholar]
- 101.Karson M.A., Tang A.H., Milner T.A., Alger B.E. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J. Neurosci. 2009;29:4140–4154. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freund T.F., Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- 103.Unal G., Joshi A., Viney T.J., Kis V., Somogyi P. Synaptic targets of medial septal projections in the hippocampus and extrahippocampal cortices of the mouse. J. Neurosci. 2015;35:15812–15826. doi: 10.1523/JNEUROSCI.2639-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Unal G., Crump M.G., Viney T.J., Éltes T., Katona L., Klausberger T., Somogyi P. Spatio-temporal specialization of GABAergic septo-hippocampal neurons for rhythmic network activity. Brain Struct. Funct. 2018;223:2409–2432. doi: 10.1007/s00429-018-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sans-Dublanc A., Razzauti A., Desikan S., Pascual M., Monyer H., Sindreu C. Septal GABAergic inputs to CA1 govern contextual memory retrieval. Sci. Adv. 2020;6:eaba5003. doi: 10.1126/sciadv.aba5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Malik R., Li Y., Schamiloglu S., Sohal V.S. Top-down control of hippocampal signal-to-noise by prefrontal long-range inhibition. Cell. 2022;185:1602–1617.e17. doi: 10.1016/j.cell.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Korotkova T., Fuchs E.C., Ponomarenko A., von Engelhardt J., Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 108.Murray A.J., Sauer J.F., Riedel G., McClure C., Ansel L., Cheyne L., Bartos M., Wisden W., Wulff P. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat. Neurosci. 2011;14:297–299. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ognjanovski N., Schaeffer S., Wu J., Mofakham S., Maruyama D., Zochowski M., Aton S.J. Parvalbumin-expressing interneurons coordinate hippocampal network dynamics required for memory consolidation. Nat. Commun. 2017;8:15039. doi: 10.1038/ncomms15039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xia F., Richards B.A., Tran M.M., Josselyn S.A., Takehara-Nishiuchi K., Frankland P.W. Parvalbumin-positive interneurons mediate neocortical-hippocampal interactions that are necessary for memory consolidation. eLife. 2017;6 doi: 10.7554/eLife.27868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaifosh P., Lovett-Barron M., Turi G.F., Reardon T.R., Losonczy A. Septo-hippocampal GABAergic signaling across multiple modalities in awake mice. Nat. Neurosci. 2013;16:1182–1184. doi: 10.1038/nn.3482. [DOI] [PubMed] [Google Scholar]
- 112.Rubio S.E., Vega-Flores G., Martínez A., Bosch C., Pérez-Mediavilla A., del Río J., Gruart A., Delgado-García J.M., Soriano E., Pascual M. Accelerated aging of the GABAergic septohippocampal pathway and decreased hippocampal rhythms in a mouse model of Alzheimer’s disease. FASEB J. 2012;26:4458–4467. doi: 10.1096/fj.12-208413. [DOI] [PubMed] [Google Scholar]
- 113.Govindarajan A., Kelleher R.J., Tonegawa S. A clustered plasticity model of long-term memory engrams. Nat. Rev. Neurosci. 2006;7:575–583. doi: 10.1038/nrn1937. [DOI] [PubMed] [Google Scholar]
- 114.Lamsa K.P., Heeroma J.H., Somogyi P., Rusakov D.A., Kullmann D.M. Anti-Hebbian long-term potentiation in the hippocampal feedback inhibitory circuit. Science. 2007;315:1262–1266. doi: 10.1126/science.1137450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kullmann D.M., Lamsa K.P. Long-term synaptic plasticity in hippocampal interneurons. Nat. Rev. Neurosci. 2007;8:687–699. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- 116.Kullmann D.M., Lamsa K.P. LTP and LTD in cortical GABAergic interneurons: emerging rules and roles. Neuropharmacology. 2011;60:712–719. doi: 10.1016/j.neuropharm.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 117.Lamsa K., Lau P. Long-term plasticity of hippocampal interneurons during in vivo memory processes. Curr. Opin. Neurobiol. 2019;54:20–27. doi: 10.1016/j.conb.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 118.Castillo P.E., Younts T.J., Chávez A.E., Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Campanac E., Gasselin C., Baude A., Rama S., Ankri N., Debanne D. Enhanced intrinsic excitability in basket cells maintains excitatory-inhibitory balance in hippocampal circuits. Neuron. 2013;77:712–722. doi: 10.1016/j.neuron.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 120.Debanne D., Inglebert Y., Russier M. Plasticity of intrinsic neuronal excitability. Curr. Opin. Neurobiol. 2019;54:73–82. doi: 10.1016/j.conb.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 121.Incontro S., Sammari M., Azzaz F., Inglebert Y., Ankri N., Russier M., Fantini J., Debanne D. Endocannabinoids tune intrinsic excitability in O-LM interneurons by direct modulation of postsynaptic Kv7 channels. J. Neurosci. 2021;41:9521–9538. doi: 10.1523/JNEUROSCI.1279-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sammari M., Inglebert Y., Ankri N., Russier M., Incontro S., Debanne D. Theta patterns of stimulation induce synaptic and intrinsic potentiation in O-LM interneurons. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2205264119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Udakis M., Pedrosa V., Chamberlain S.E.L., Clopath C., Mellor J.R. Interneuron-specific plasticity at parvalbumin and somatostatin inhibitory synapses onto CA1 pyramidal neurons shapes hippocampal output. Nat. Commun. 2020;11:4395. doi: 10.1038/s41467-020-18074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mercier M.S., Magloire V., Cornford J.H., Kullmann D.M. Long-term potentiation in neurogliaform interneurons modulates excitation-inhibition balance in the temporoammonic pathway. J. Physiol. 2022;600:4001–4017. doi: 10.1113/JP282753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Griguoli M., Cellot G., Cherubini E. In hippocampal oriens interneurons anti-Hebbian long-term potentiation requires cholinergic signaling via α7 nicotinic acetylcholine receptors. J. Neurosci. 2013;33:1044–1049. doi: 10.1523/JNEUROSCI.1070-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Le Duigou C., Savary E., Kullmann D.M., Miles R. Induction of anti-Hebbian LTP in CA1 stratum oriens interneurons: interactions between group I metabotropic glutamate receptors and M1 muscarinic receptors. J. Neurosci. 2015;35:13542–13554. doi: 10.1523/JNEUROSCI.0956-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chevaleyre V., Castillo P.E. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 128.Buzsáki G., Eidelberg E. Direct afferent excitation and long-term potentiation of hippocampal interneurons. J. Neurophysiol. 1982;48:597–607. doi: 10.1152/jn.1982.48.3.597. [DOI] [PubMed] [Google Scholar]
- 129.Hainmueller T., Krieglstein K., Kulik A., Bartos M. Joint CP-AMPA and group I mGlu receptor activation is required for synaptic plasticity in dentate gyrus fast-spiking interneurons. Proc. Natl. Acad. Sci. USA. 2014;111:13211–13216. doi: 10.1073/pnas.1409394111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Holly E.N., Davatolhagh M.F., Choi K., Alabi O.O., Vargas Cifuentes L., Fuccillo M.V. Striatal low-threshold spiking interneurons regulate goal-directed learning. Neuron. 2019;103:92–101.e6. doi: 10.1016/j.neuron.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hu H., Gan J., Jonas P. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science. 2014;345:1255263. doi: 10.1126/science.1255263. [DOI] [PubMed] [Google Scholar]
- 132.Kullmann D.M., Moreau A.W., Bakiri Y., Nicholson E. Plasticity of inhibition. Neuron. 2012;75:951–962. doi: 10.1016/j.neuron.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 133.Perez Y., Morin F., Lacaille J.C. A hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc. Natl. Acad. Sci. USA. 2001;98:9401–9406. doi: 10.1073/pnas.161493498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Topolnik L., Congar P., Lacaille J.C. Differential regulation of metabotropic glutamate receptor- and AMPA receptor-mediated dendritic Ca2+ signals by presynaptic and postsynaptic activity in hippocampal interneurons. J. Neurosci. 2005;25:990–1001. doi: 10.1523/JNEUROSCI.4388-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tsetsenis T., Younts T.J., Chiu C.Q., Kaeser P.S., Castillo P.E., Südhof T.C. Rab3B protein is required for long-term depression of hippocampal inhibitory synapses and for normal reversal learning. Proc. Natl. Acad. Sci. USA. 2011;108:14300–14305. doi: 10.1073/pnas.1112237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Capogna M., Castillo P.E., Maffei A. The ins and outs of inhibitory synaptic plasticity: neuron types, molecular mechanisms and functional roles. Eur. J. Neurosci. 2021;54:6882–6901. doi: 10.1111/ejn.14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee S.H., Ledri M., Tóth B., Marchionni I., Henstridge C.M., Dudok B., Kenesei K., Barna L., Szabó S.I., Renkecz T., et al. Multiple forms of endocannabinoid and endovanilloid signaling regulate the tonic control of GABA release. J. Neurosci. 2015;35:10039–10057. doi: 10.1523/JNEUROSCI.4112-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Losonczy A., Biró A.A., Nusser Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc. Natl. Acad. Sci. USA. 2004;101:1362–1367. doi: 10.1073/pnas.0304752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Freund T.F. Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 140.Nasrallah K., Therreau L., Robert V., Huang A.J.Y., McHugh T.J., Piskorowski R.A., Chevaleyre V. Routing hippocampal information flow through parvalbumin interneuron plasticity in area CA2. Cell Rep. 2019;27:86–98.e3. doi: 10.1016/j.celrep.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 141.Ross S.T., Soltesz I. Long-term plasticity in interneurons of the dentate gyrus. Proc. Natl. Acad. Sci. USA. 2001;98:8874–8879. doi: 10.1073/pnas.141042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Neddens J., Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2010;20:724–744. doi: 10.1002/hipo.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li K.X., Lu Y.M., Xu Z.H., Zhang J., Zhu J.M., Zhang J.M., Cao S.X., Chen X.J., Chen Z., Luo J.H., et al. Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat. Neurosci. 2011;15:267–273. doi: 10.1038/nn.3006. [DOI] [PubMed] [Google Scholar]
- 144.Fisahn A., Neddens J., Yan L., Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb. Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dasgupta D., Sikdar S.K. Calcium permeable AMPA receptor-dependent long lasting plasticity of intrinsic excitability in fast spiking interneurons of the dentate gyrus decreases inhibition in the granule cell layer. Hippocampus. 2015;25:269–285. doi: 10.1002/hipo.22371. [DOI] [PubMed] [Google Scholar]
- 146.McKay B.M., Oh M.M., Disterhoft J.F. Learning increases intrinsic excitability of hippocampal interneurons. J. Neurosci. 2013;33:5499–5506. doi: 10.1523/JNEUROSCI.4068-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chittajallu R., Auville K., Mahadevan V., Lai M., Hunt S., Calvigioni D., Pelkey K.A., Zaghloul K.A., McBain C.J. Activity-dependent tuning of intrinsic excitability in mouse and human neurogliaform cells. eLife. 2020;9 doi: 10.7554/eLife.57571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zarnadze S., Bäuerle P., Santos-Torres J., Böhm C., Schmitz D., Geiger J.R., Dugladze T., Gloveli T. Cell-specific synaptic plasticity induced by network oscillations. eLife. 2016;5 doi: 10.7554/eLife.14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leão R.N., Mikulovic S., Leão K.E., Munguba H., Gezelius H., Enjin A., Patra K., Eriksson A., Loew L.M., Tort A.B., et al. OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat. Neurosci. 2012;15:1524–1530. doi: 10.1038/nn.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.O’Keefe J., Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 151.O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp. Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- 152.Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- 153.Vanderwolf C.H. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr. Clin. Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 154.Buzsáki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- 155.Buzsáki G., Horváth Z., Urioste R., Hetke J., Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 156.Suzuki S.S., Smith G.K. Spontaneous EEG spikes in the normal hippocampus. I. Behavioral correlates, laminar profiles and bilateral synchrony. Electroencephalogr. Clin. Neurophysiol. 1987;67:348–359. doi: 10.1016/0013-4694(87)90123-4. [DOI] [PubMed] [Google Scholar]
- 157.Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 158.Buzsáki G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25:1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Csicsvari J., Hirase H., Mamiya A., Buzsáki G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron. 2000;28:585–594. doi: 10.1016/s0896-6273(00)00135-5. [DOI] [PubMed] [Google Scholar]
- 160.Csicsvari J., Hirase H., Czurkó A., Mamiya A., Buzsáki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving Rat. J. Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Klausberger T., Márton L.F., Baude A., Roberts J.D.B., Magill P.J., Somogyi P. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat. Neurosci. 2004;7:41–47. doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- 162.Klausberger T., Marton L.F., O’Neill J., Huck J.H., Dalezios Y., Fuentealba P., Suen W.Y., Papp E., Kaneko T., Watanabe M., et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J. Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Geiller T., Vancura B., Terada S., Troullinou E., Chavlis S., Tsagkatakis G., Tsakalides P., Ócsai K., Poirazi P., Rózsa B.J., et al. Large-scale 3D two-photon imaging of molecularly identified CA1 interneuron dynamics in behaving mice. Neuron. 2020;108:968–983.e9. doi: 10.1016/j.neuron.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ganter P., Szücs P., Paulsen O., Somogyi P. Properties of horizontal axo-axonic cells in stratum oriens of the hippocampal CA1 area of rats in vitro. Hippocampus. 2004;14:232–243. doi: 10.1002/hipo.10170. [DOI] [PubMed] [Google Scholar]
- 165.Schlingloff D., Káli S., Freund T.F., Hájos N., Gulyás A.I. Mechanisms of sharp wave initiation and ripple generation. J. Neurosci. 2014;34:11385–11398. doi: 10.1523/JNEUROSCI.0867-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ellender T.J., Nissen W., Colgin L.L., Mann E.O., Paulsen O. Priming of hippocampal population bursts by individual perisomatic-targeting interneurons. J. Neurosci. 2010;30:5979–5991. doi: 10.1523/JNEUROSCI.3962-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rácz A., Ponomarenko A.A., Fuchs E.C., Monyer H. Augmented hippocampal ripple oscillations in mice with reduced fast excitation onto parvalbumin-positive cells. J. Neurosci. 2009;29:2563–2568. doi: 10.1523/JNEUROSCI.5036-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fuentealba P., Tomioka R., Dalezios Y., Márton L.F., Studer M., Rockland K., Klausberger T., Somogyi P. Rhythmically active enkephalin-expressing GABAergic cells in the CA1 area of the hippocampus project to the subiculum and preferentially innervate interneurons. J. Neurosci. 2008;28:10017–10022. doi: 10.1523/JNEUROSCI.2052-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Turi G.F., Li W.K., Chavlis S., Pandi I., O’Hare J., Priestley J.B., Grosmark A.D., Liao Z., Ladow M., Zhang J.F., et al. Vasoactive intestinal polypeptide-expressing interneurons in the hippocampus support goal-oriented spatial learning. Neuron. 2019;101:1150–1165.e8. doi: 10.1016/j.neuron.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Leroy F., de Solis C.A., Boyle L.M., Bock T., Lofaro O.M., Buss E.W., Asok A., Kandel E.R., Siegelbaum S.A. Enkephalin release from VIP interneurons in the hippocampal CA2/3a region mediates heterosynaptic plasticity and social memory. Mol. Psychiatry. 2022;27:2879–2900. doi: 10.1038/s41380-021-01124-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Racine A.S., Michon F.X., Laplante I., Lacaille J.C. Somatostatin contributes to long-term potentiation at excitatory synapses onto hippocampal somatostatinergic interneurons. Mol. Brain. 2021;14:130. doi: 10.1186/s13041-021-00830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Basu J., Srinivas K.V., Cheung S.K., Taniguchi H., Huang Z.J., Siegelbaum S.A. A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron. 2013;79:1208–1221. doi: 10.1016/j.neuron.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.He L., Caudill M.S., Jing J., Wang W., Sun Y., Tang J., Jiang X., Zoghbi H.Y. A weakened recurrent circuit in the hippocampus of Rett syndrome mice disrupts long-term memory representations. Neuron. 2022;110:1689–1699.e6. doi: 10.1016/j.neuron.2022.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lovett-Barron M., Kaifosh P., Kheirbek M.A., Danielson N., Zaremba J.D., Reardon T.R., Turi G.F., Hen R., Zemelman B.V., Losonczy A. Dendritic inhibition in the hippocampus supports fear learning. Science. 2014;343:857–863. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sharma V., Sood R., Khlaifia A., Eslamizade M.J., Hung T.Y., Lou D., Asgarihafshejani A., Lalzar M., Kiniry S.J., Stokes M.P., et al. eIF2α controls memory consolidation via excitatory and somatostatin neurons. Nature. 2020;586:412–416. doi: 10.1038/s41586-020-2805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gouwens N.W., Sorensen S.A., Baftizadeh F., Budzillo A., Lee B.R., Jarsky T., Alfiler L., Baker K., Barkan E., Berry K., et al. Integrated morphoelectric and transcriptomic classification of cortical gabaergic cells. Cell. 2020;183:935–953.e19. doi: 10.1016/j.cell.2020.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Que L., Lukacsovich D., Luo W., Földy C. Transcriptional and morphological profiling of parvalbumin interneuron subpopulations in the mouse hippocampus. Nat. Commun. 2021;12:108. doi: 10.1038/s41467-020-20328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tzilivaki A., Kastellakis G., Poirazi P. Challenging the point neuron dogma: FS basket cells as 2-stage nonlinear integrators. Nat. Commun. 2019;10:3664. doi: 10.1038/s41467-019-11537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cornford J.H., Mercier M.S., Leite M., Magloire V., Häusser M., Kullmann D.M. Dendritic NMDA receptors in parvalbumin neurons enable strong and stable neuronal assemblies. eLife. 2019;8 doi: 10.7554/eLife.49872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Katona G., Kaszás A., Turi G.F., Hájos N., Tamás G., Vizi E.S., Rózsa B. Roller Coaster Scanning reveals spontaneous triggering of dendritic spikes in CA1 interneurons. Proc. Natl. Acad. Sci. USA. 2011;108:2148–2153. doi: 10.1073/pnas.1009270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chiovini B., Turi G.F., Katona G., Kaszás A., Pálfi D., Maák P., Szalay G., Szabó M.F., Szabó G., Szadai Z., et al. Dendritic spikes induce ripples in parvalbumin interneurons during hippocampal sharp waves. Neuron. 2014;82:908–924. doi: 10.1016/j.neuron.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 182.Evangelista R., Cano G., Cooper C., Schmitz D., Maier N., Kempter R. Generation of sharp wave-ripple events by disinhibition. J. Neurosci. 2020;40:7811–7836. doi: 10.1523/JNEUROSCI.2174-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Donato F., Rompani S.B., Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–276. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- 184.Karunakaran S., Chowdhury A., Donato F., Quairiaux C., Michel C.M., Caroni P. PV plasticity sustained through D1/5 dopamine signaling required for long-term memory consolidation. Nat. Neurosci. 2016;19:454–464. doi: 10.1038/nn.4231. [DOI] [PubMed] [Google Scholar]
- 185.He X., Li J., Zhou G., Yang J., McKenzie S., Li Y., Li W., Yu J., Wang Y., Qu J., et al. Gating of hippocampal rhythms and memory by synaptic plasticity in inhibitory interneurons. Neuron. 2021;109:1013–1028.e9. doi: 10.1016/j.neuron.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]