Abstract
Background
There is paucity of data on incidence and pattern of drug resistance in spinal TB. This prospective observational study was conducted to document the incidence and drug-resistance pattern among primary and presumptive resistant cases.
Methods
59 consecutive cases diagnosed clinico-radiologically (imaging) were grouped into Group A (n = 51, primary cases) and Group B (n = 8, presumptive resistant cases) based on pre-defined criteria (INDEX-TB guidelines). Tissue samples obtained percutaneously (37.29%, 22/59) and on surgery (62.71%, 37/59) were subjected to genotypic DST (CBNAAT, LPA) and phenotypic DST (BACTEC MGIT 960 culture and sensitivity using fixed critical concentration of drugs).
Results
Etiological diagnosis was ascertained in all. 13/51 (25.49%) in Group A, while 3/8 (37.5%) in Group B and 16/59 (27.12%) overall demonstrated drug resistance. 12/16 (75%) had no prior history of ATT intake. 4 demonstrated INH (Isoniazid) mono-resistance. 12 polydrug resistance demonstrated: 5MDR, 3pre-XDR, while RIF + FQ (fluoroquinolones), FQ + Lz (linezolid), only SLID (second-line injectable drugs), and only FQ resistance observed in 1 case each. Isolated RIF (Rifampicin) resistance and XDR pattern were not observed. Overall frequency of RIF resistance was 16.4% (9/55) and INH was 25% (12/48) with low-(n–2) and high-level INH resistance (n–10). Among second-line drugs, FQ resistance was more than SLID resistance and within FQ, levofloxacin resistance was more frequent than moxifloxacin. MGIT demonstrated positive growth in 16/59 samples, out of which 1 sample was positive for nontuberculous mycobacteria (M. chelonae) but on genotypic testing demonstrated MTB resistant to RIF and FQ.
Conclusion
This is the first report on incidence and drug-resistant pattern in culture-positive/negative cases. High (25.49%) primary drug resistance is worrisome. This being the first study in spinal TB cases which document prevalent drug-resistant pattern as evaluated for consecutive culture-positive/negative cases. The tissue obtained must be submitted for AFB culture and molecular tests to ascertain drug resistance in culture-positive/negative cases. However, in the presence of insufficient tissue sample histology and CBNAAT can ascertain etiological diagnosis in 100% cases. INH resistance is more than RIF with isolated RIF resistance unreported.
Keywords: Spinal TB, Primary drug-resistant TB, Genotypic DST, Phenotypic DST, MDR-TB
Introduction
The early diagnosis and treatment has improved outcome for spinal tuberculosis (STB). Mean delay of 6–8 months is reported for diagnosis of spinal tuberculosis [1–3]. The etiological diagnosis in spinal TB is ascertained by histology, demonstration of acid-fast bacilli on smear/culture. The culture has low yield since organism is fastidious, slow-growing, and disease is paucibacillary [4]. No single modality is able to conclusively diagnose all cases of TB spine [5, 6]. The molecular methods can reduce the delay and provide fairly accurate diagnosis [7, 8].
Drug-resistant TB, an emerging health problem, has poorer outcomes due to difficult demonstration of drug resistance and complex treatment. Insensitive diagnostic methods for detection of drug-resistant TB has hampered global control of TB [9]. Drug resistance is detected through culture-based (phenotypic) or nucleic acid-based (genotypic) drug susceptibility testing (DST) with 'gold standard' being indirect 1% proportion method (phenotypic DST) [10]. The phenotypic DST takes long time to identify drug resistance in STB [11], while genotypic tests provide diagnosis and DST within few days [12].
Clinical data on drug-resistant STB are limited with knowledge regarding diagnosis and management been extrapolated from pulmonary TB. Scarce data are available on use of molecular tests for diagnosis of drug-resistant STB (primary and acquired resistance). Hence, this study was conducted to elucidate the incidence and patterns of drug resistance amongst primary and presumptive drug-resistant spinal tuberculosis patients.
Patients and Methods
This prospective observational study was conducted (November 2018–March 2020) at tertiary care center. India lies in endemic zone for tuberculosis. Following institutional board review, ethical committee approval and informed consent from patients, 59 consecutive cases of STB diagnosed on clinico-imaging basis were enrolled [5]. Using the STARD’s methodology, required sample size was 100 but due to resource constraints and time availability, 59 consecutive cases were enrolled for study. No patient was immunocompromised. The patients without classical clinico-radiological/imaging findings were excluded. 59 cases were divided into 2 groups (a) Primary STB (n = 51) that included patients who were not on anti-tubercular therapy (ATT) or ATT intake was < 5 months (b) Presumptive drug-resistant STB (n = 8) included bacteriologically confirmed/clinically diagnosed STB patients, who after 5 months of ATT intake had persisting/worsening local/systemic symptoms and signs, deterioration of the lesion (s), appearance of new lesion (s), non-healing ulcer/sinus, new abscesses/lymphadenopathy, and wound dehiscence post-operatively [13, 14].
Detailed clinical history including ATT intake, physical examination, and neurological grade was documented [15]. Blood reports, X-rays, and MRI findings were recorded. Samples were procured by fluoroscope-guided transpedicular biopsy, cold abscess aspiration, or on surgical debridement. The indications for surgery were neural complications developing/getting worse/remaining stationary during the course of non-operative treatment (3–4 weeks), paraplegia of rapid onset, neural arch disease, severe paraplegia (flaccid paraplegia, paraplegia in flexion and complete sensory and motor loss for > 6 months), pan-vertebral disease, severe kyphotic deformity at presentation or in children at high risk of progression of kyphosis [13].
The tissue samples (pus, granulation tissue or bone) were sent for AFB (acid-fast bacilli) smear, genotypic DST [CBNAAT (Cartridge-Based Nucleic Acid Amplification Test), LPA (line probe assay)1st and 2nd lines], phenotypic DST [BACTEC™ MGIT (Mycobacteria Growth Indicator Tube) 960 culture and DST using fixed critical concentration of drugs] at National Reference Laboratory for TB in Department of Microbiology, National Institute of TB and Respiratory Diseases, New Delhi and histopathological examination at our institute. Standard operating procedures were followed for sample collection, transport, and processing. The LPA is done as part of this study to define drug resistance pattern and not as routine test to ascertain diagnosis of TB. The patients were managed with supportive braces, good nutritious diet, and ATT (daily dosage regimen for Drug sensitive and programmatic management of drug-resistant TB (PMDT) guidelines, 2017 for Drug-resistant STB) [16, 17].
Genotypic DST is based on detection of specific nucleotide sequences and/or mutations in the genes for various antibiotics within M. tuberculosis (MTB) genome known to be associated with resistance. CBNAAT, based on real-time PCR (polymerase chain reaction), can detect Mycobacterium tuberculosis complex (MTBC) and resistance to Rifampicin (RIF) [18]. The LPA based on reverse hybridization of DNA can identify members of MTBC, while simultaneously identifying drug-resistant strains by detecting the most common single nucleotide polymorphism (SNP) [19]. First-line LPA (GenoType MTBDRplus) can identify the MTBC and its resistance to both Isoniazid (INH) and RIF [19]. Second-line LPA (GenoType MTBDRsl) can identify the MTBC and its resistance to Fluoroquinolones (FQ) and Aminoglycosides (AG)/Second-Line Injectable Drugs (SLID) [19]. Phenotypic DST assess inhibition of MTB growth in the presence of antibiotics and defines resistance based on response of organism when exposed to that drug. Oxygen consumption by bacilli during the process of growth as measured by fluorescence is detected with BACTEC™ MGIT 960 instrument which is based on liquid culture using Middlebrook 7H9 broth [20]. Comparison of fluorescence is done between drug-containing tubes and control tube to determine susceptibility results. For Ethionamide (ETH), Linezolid (Lz), and Para-amino Salicylic Acid (PAS) susceptibility testing was exclusive to phenotypic DST due to non-availability of nucleic acid-based DST (Table 1).
Table 1.
Critical concentration, solvent, and diluents of drugs for DST by MGIT
| Drugs | Critical concentrations (μg/ml) | Solvent | Diluent |
|---|---|---|---|
| Isoniazid (INH) | 0.1 | DW (Distilled water) | DW |
| Rifampicin (RIF) | 1.0 | DMSO (Dimethyl sulfoxide) | 95% Ethanol |
| Kanamycin (Km) | 2.5 | DW | DW |
| Amikacin (Ak) | 1.0 | DW | DW |
| Capreomycin (Cap) | 2.5 | DW | DW |
| Ethionamide (ETH) | 5.0 | DMSO | DW |
| Levofloxacin (Lev) | 1.0 | 0.1N NaOH (Sodium hydroxide) | DW |
| Moxifloxacin (Mox) | 1.0 | 0.1N NaOH | DW |
| Linezolid (Lz) | 1.0 | DMSO | DW |
| Para-amino salicylic acid (PAS) | 4.0 | Ethanol | DW |
The data were entered into excel spreadsheet (Microsoft Inc, Washington, US) and analyzed using SPSS software v23.0 (IBM, NewYork, US). Quantitative data were presented as Mean ± SD, while qualitative data were represented as percentage. Fisher’s exact test and Chi-square test were used for determination of statistical significance.
Results
The mean age was 25.98 (10–75 years) with 43 (72.88%) females and 16 (27.12%) males. Tuberculosis diagnosis was ascertained by molecular methods in 91.53% (54/59), AFB smear 20.34% (12/59), liquid culture 27.12% (16/59), histology 96.61% (57/59), and in 100% (59/59) cases when all tests were used in tandem (Table 2). The diagnosis of TB was also ascertained in 59/59 (100%) by CBNAAT and histology in tandem.
Table 2.
Diagnostic accuracy of various tests
| Tests | Diagnostic accuracy |
|---|---|
| AFB smear | 20.34% (12/59) |
| Liquid culture | 27.12% (16/59) |
| CBNAAT | 91.53% (54/59) |
| First-line LPA | 79.66% (47/59) |
| Second-line LPA | 61.02% (36/59) |
| LPA (overall) | 79.66% (47/59) |
| Tissue diagnosis | 96.61% (57/59) |
| Molecular tests, tissue examination, and AFB smear in tandem | 100% (59/59) |
| CBNAAT and histology in tandem | 100% (59/59) |
DST results
Primary cases (n = 51): Drug resistance was demonstrated in 13/51 (25.49%), of which 9/13 cases never took ATT in past, while 4 cases were treated for pulmonary TB in the past 6–35 years (mean 13.75) ago and not for present disease. 3/13 cases demonstrated INH mono-resistant, while 10/13 polydrug resistant. MDR (RIF + INH resistance) was found in 3 patients, RIF + INH + FQ (Pre-XDR) in 3. One patient each showed only SLID, only FQ, RIF + FQ and FQ + Lz (Table 3). Overall RIF resistance (RR) was 14.6% (7/48), while INH was 21.4% (9/42) (Table 4).
Table 3.
Drug-resistance pattern
| Primary cases (n = 51) | Presumptive drug-resistance cases (n = 8) | Overall (n = 59) | |
|---|---|---|---|
| Resistance cases | 13/51 (25.49%) | 3/8 (37.5%) | 16/59 (27.12%) |
| Mono-drug resistance | 3 | 1 | 4 |
| Only INH resistance = 3 | Only INH resistance = 1 | Only INH resistance = 4 | |
| Only RIF resistance = 0 | Only RIF resistance = 0 | Only RIF resistance = 0 | |
| Poly-drug resistance | 10 | 2 | 12 |
| INH + RIF (MDR-TB) = 3 | INH + RIF (MDR-TB) = 2 | INH + RIF (MDR-TB) = 5 | |
| RIF + FQ = 1 | INH + RIF + FQ (Pre-XDR TB) = 0 | RIF + FQ = 1 | |
| FQ + Lz = 1 | INH + RIF + SLID (Pre-XDR TB) = 0 | FQ + Lz = 1 | |
| SLID (Km, Cap, Ak) = 1 | INH + RIF + FQ + SLID (XDR TB) = 0 | SLID (Km, Cap, Ak) = 1 | |
| FQ (Mox, Lev) = 1 | FQ (Mox, Lev) = 1 | ||
| INH + RIF + FQ (Pre-XDR TB) = 3 | INH + RIF + FQ (Pre-XDR TB) = 3 | ||
| INH + RIF + SLID (Pre-XDR TB) = 0 | INH + RIF + SLID (Pre-XDR TB) = 0 | ||
| INH + RIF + FQ + SLID (XDR TB) = 0 | INH + RIF + FQ + SLID (XDR TB) = 0 |
Table 4.
Individual drug-resistance frequency
| Drug | Primary cases | Presumptive drug-resistant cases | Overall |
|---|---|---|---|
| RIF (Rifampicin) | 7/48 (14.6%) | 2/7 (28.6%) | 9/55 (16.4%) |
| INH (Isoniazid) | 9/42 (21.4%) | 3/6 (50.0%) | 12/48 (25.0%) |
| Km (Kanamycin) | 1/34 (2.94%) | 0/6 (0.0%) | 1/40 (2.5%) |
| Cap (Capreomycin) | 1/34 (2.94%) | 0/6 (0.0%) | 1/40 (2.5%) |
| Ak (Amikacin) | 1/34 (2.94%) | 0/6 (0.0%) | 1/40 (2.5%) |
| Mox (Moxifloxacin) | 5/34 (14.7%) | 0/6 (0.0%) | 5/40 (12.5%) |
| Lev (Levofloxacin) | 6/34 (15.0%) | 0/6 (0.0%) | 6/40 (15.0%) |
| Lz (Linezolid) | 1/15 (6.7%) | Could not be evaluated | 1/15 (6.7%) |
| PAS (Para-amino salicylic acid) | 0/15 (0.0%) | Could not be evaluated | 0/15 (0.0%) |
| ETH (Ethionamide) | 0/15 (0.0%) | Could not be evaluated | 0/15 (0.0%) |
Presumptive drug-resistant cases (n = 8): Drug resistance could be demonstrated in 3/8 (37.5%) cases. None had history of ATT intake in past; however for present disease, they were on ATT. One case demonstrated INH mono-resistance, while two were MDR (RIF + INH (Table 3). RR was 28.6% (2/7) and INH was 50% (3/6) (Table 4).
Overall (n = 59): Overall, 16/59 (27.12%) demonstrated drug resistance. 4/16 demonstrated INH mono-resistance (Fig. 1). 5 were MDR (Fig. 2) while 3 were Pre-XDR. RIF + FQ and FQ + Lz resistance was present in 1 case each. Only SLID and only FQ resistance (Fig. 3) was observed in 1 case each (Table3). Isolated Rifampicin resistance was not observed. Overall RR was 16.4% (9/55), while INH resistance was 25% (12/48) (Table 4). Out of proven drug resistance, 9 in Group A and 3 in Group B overall 12/16 had not taken ATT in the past.
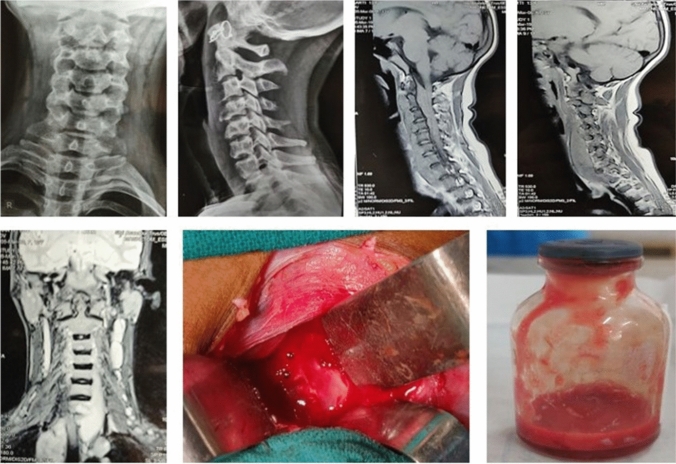
Fig. 1.
13 year old female diagnosed as Potts spine C4-C5 with quadriparesis underwent Decompression alone on March 14, 2019. On DST, only low-level INH resistance, AFB smear negative, Liquid culture positive, Histology consistent with TB
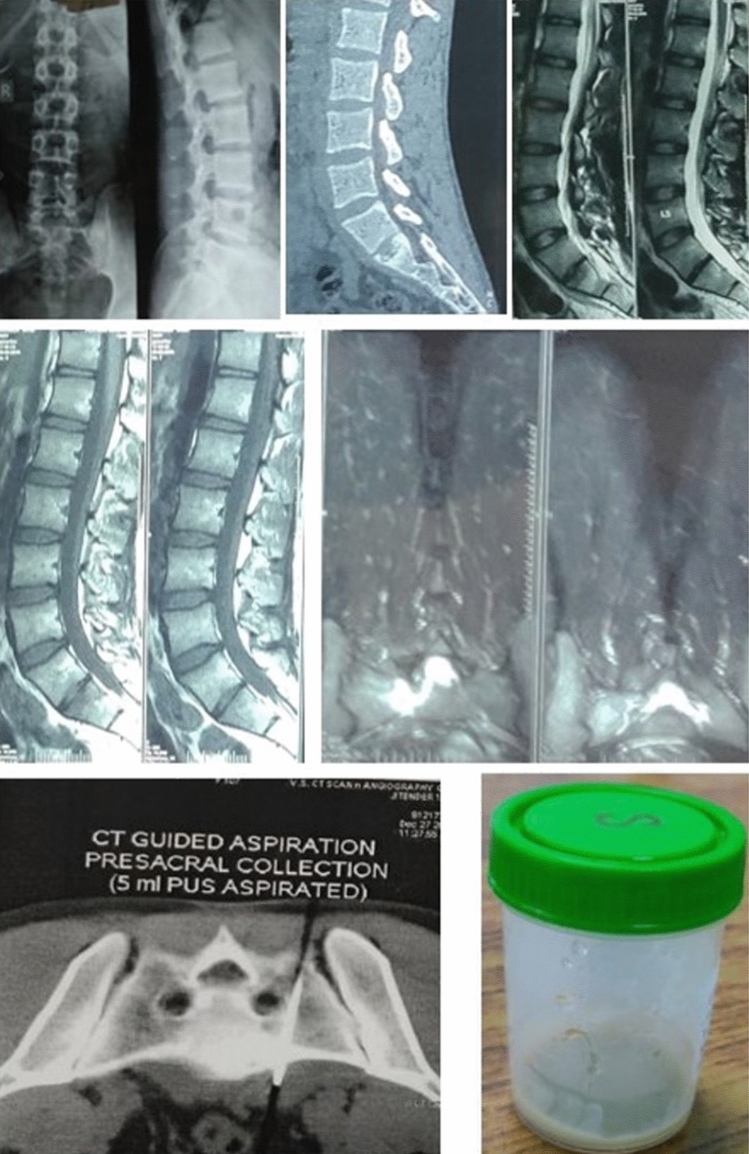
Fig. 2.
18 year old male diagnosed as Pott’s spine S1-S2 with intact neurology underwent CT guided aspiration of pre-sacral collection on December 27, 2019. On DST- RIF & INH (high level) resistance, AFB smear negative, Liquid culture no growth, Cytology consistent with TB
Fig. 3.
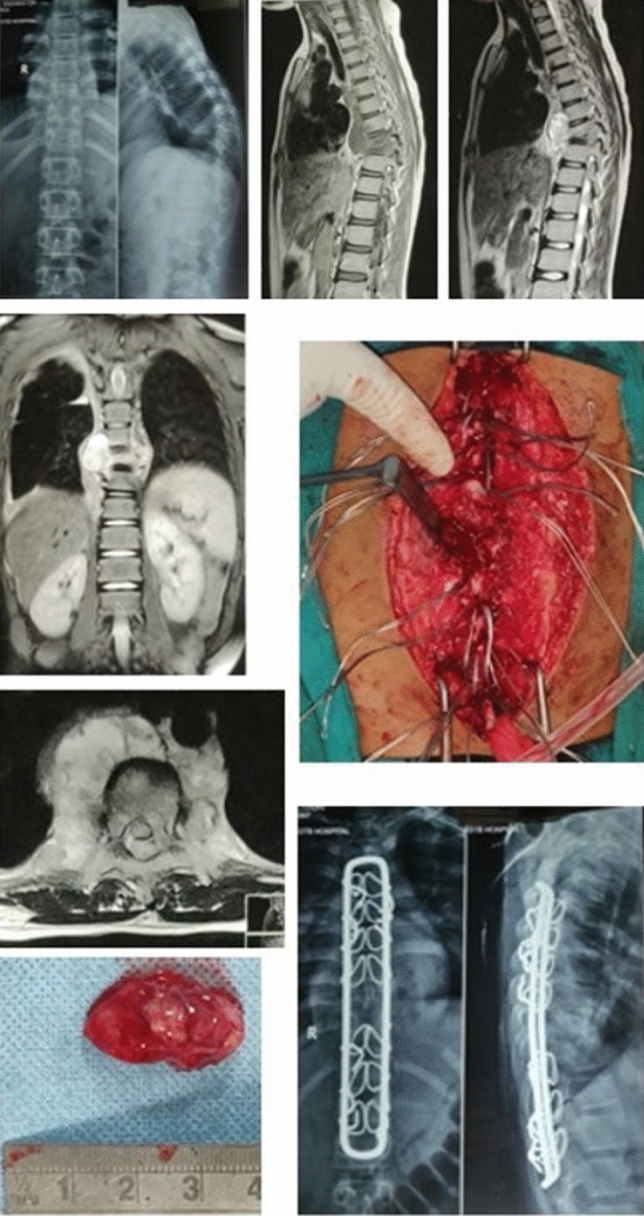
12 year old female diagnosed as Pott’s spine D8-D9 with grade IV paraplegia underwent Decompression with instrumentation on August 05, 2019. On DST- Only FQ resistance, AFB smear negative, Liquid culture no growth, Histology consistent with TB
Discussion
The growth of tubercular bacilli on culture has been gold standard for diagnosis of TB [21]. Osseous lesions are paucibacillary (< 104 colony-forming units/mL), therefore difficult to diagnose on AFB staining/culture [4]. Hence, reliance is placed on clinical examination, imaging diagnosis, AFB smear, tubercular culture, molecular methods, histology/cytology and therapeutic response [22]. No single diagnostic method can conclusively ascertain diagnosis in all STB cases [5, 6]. Molecular tests do not require mycobacterial growth. They are based on detection of specific nucleotide sequences and/or mutations in various genes associated with resistance within genome of MTBC. Hence, early detection is possible, from low volume of clinical specimen [12]. However, they cannot differentiate between living/dead organisms, thus cannot predict disease activity.
Drug-resistant STB is emerging health problem in developing/developed countries. 17% of all new TB cases worldwide harbor some drug resistance [23]. Global control is hampered by slow diagnostic methods, especially for detection of drug-resistant forms [9]. It is imperative to promptly recognize and initiate timely treatment of drug-resistant disease [24].
The main factors contributing to development of drug resistance are inadequate and incomplete treatment leading to off and on phenomenon and non-adherence to treatment [25, 26]. Unlike pulmonary tuberculosis, STB is a deep-seated lesion and has inherent difficulty in tissue/pus sample procurement. STB is paucibacillary with fastidious, slow-growing organism; hence, probability of mycobacterial growth and culture sensitivity is low even with adequate tissue samples [11].
With increased understanding of genetic basis of drug resistance, rapid molecular or genotypic DST kits have been developed. These tests do not require growth of mycobacterium, thus the time for diagnosis and DST has reduced substantially in comparison to phenotypic (culture-based) DST methods [12]. Rapid molecular assays for detection of TB and drug resistance in clinical specimens are based on detection of specific nucleotide sequences and/or mutations in the MTB genome [10].
CBNAAT detected MTB in 91.53% (54/59) patients and demonstrated 14.8% (8/54) RIF resistance. Vadwai.et.al. obtained RIF resistance rate of 98% (39/40) on 40 culture-positive extrapulmonary MDR-TB samples [27]. Gu.et.al compared CBNAAT with MGIT DST in detecting RIF resistance in 24 pus samples from culture-positive osteoarticular tuberculosis patients with sensitivity and specificity of 100% [28]. Sharma.et.al, reported retrospective analysis of STB cases, with 100% (7/7) sensitivity of CBNAAT to detect RR, among 43 CBNAAT and culture-positive cases [29]. These studies report sensitivity of CBNAAT to demonstrate RR among culture-positive MDR-TB cases, and hence cannot be compared with our data as we report occurrence of RR in consecutive series of cases.
First-Line LPA detected MTB in 79.66% (47/59) and demonstrated resistance in 12/47 (25.5%) cases. Only RR was detected in 1/47 (2.1%) with additional resistance to FQ by second-line LPA. Isolated INH resistance was detected in 4/47 (8.5%). and 7/47 (14.9%) demonstrated resistance to both RIF and INH. 2/11 with INH resistance detected low-level INH resistance while 9 had high level. On Genotypic DST, two main molecular mechanisms of INH resistance are detected: (a)Loss of INH activation by katG-high-level INH resistance and (b) Increase in inhA expression or modification of inhA target-low-level resistance to INH and cross-resistance to ethionamide [30]. On Phenotypic DST, high-level INH resistance is classified as resistance when minimum inhibitory concentration (MIC) > 2 μg/mL and low-level resistance when MIC is 0.2–1 μg/mL [31]. Higher dose of Isoniazid (10–15 mg/kg/day) instead of usual dose (4–6 mg/kg/day) can be given in low-level INH-resistant cases. Gu.et.al. compared GenoType MTBDR plus assay with MGIT DST in detecting RIF and INH resistance among 24 pus from culture-positive osteoarticular TB cases with sensitivity 83.33% and 85.71% for RIF and INH resistance, respectively [28]. Ramachandran et al. in a review article on rapid molecular diagnostics for MDR-TB in India reported 97.7% sensitivity and of 91.8% specificity by LPA in AFB smear-positive cases, while 94.0% sensitivity and 98.0% specificity by CBNAAT in detecting MTB in AFB smear-negative samples [32, 33]. Hence, CBNAAT provides significant diagnostic edge in smear-negative cases.
Second-Line LPA detected MTB in 36/59 (61.02%) samples and demonstrated resistance in 6/36 (16.7%). Only SLID resistance in 1/36 (2.8%), while only FQ resistance in 5/36 (13.9%). Brossier et.al. reported sensitivities and specificities of GenoType MTBDRsl test in 49 cases (41MDR-TB and 8XDR-TB) as FQ 87%, 96%; amikacin (Ak)100%, 100%; kanamycin (Km)77%, 100%; and capreomycin (Cap) 80%, 98%, respectively [34]. Kiet et.al. analyzed 41 FQ-resistant isolates and 21 MDR-TB + FQ-sensitive isolates and reported sensitivities (FQ 75.6%, Km 100%) and specificities (FQ100%, Km100%) of MTBDRsl test. [35]
Genotypic DST detects resistances having known gene loci; therefore, resistances originating from mutations of other genes/gene regions and unknown resistance mechanisms are not detected. These kits screen nucleic acid sequence only, while amino acid sequences are not screened. Hence, mutations in probe region (not causing an amino acid exchange (silent mutations) will go undetected. Therefore, solitary genotypic drug-resistance detection would underestimate drug-resistance rates. Molecular tests cannot differentiate between members of MTBC, and between DNA from viable/nonviable bacteria, therefore cannot be used for monitoring the progression/success of treatment with ATT.
Liquid culture (MGIT) was positive for MTB in 27% (16/59) cases only, hence its utility for drug-resistance demonstration is limited. Mycobacterial growth was positive 15/16 cases. One culture showed Mycobacterium Chelonae (nontuberculous mycobacteria, NTM), which on genotypic testing demonstrated MTB (RIF, FQ resistant). This suggests mixed nature of infection (both NTM and MTB infection). Phenotypic DST of these 15 samples showed RR (1/15), INH resistance (3/15), Moxifloxacin resistance (1/15), Levofloxacin resistance (2/15), Lz resistance (1/15). Jain.et.al. reported 15 suspected drug-resistant (therapeutically refractory) cases where mycobacterial culture was positive 3 (20%) and drug resistance was documented in two [14]. LiL.et.al. reported 249 histologically proven STB with 127 (51%) positive culture. 39/127 (30.7%) culture-positive patients documented drug resistance. 4/39 were excluded from study due to loss to follow up while 12/35 had MDR-TB, 16/35 mono-drug resistance and 7 had resistance to additional ATT. The resistance rate demonstrated was INH (54.3%), RIF (48.6%), and streptomycin (34.3%) [36]. Mohan K et.al. analyzed 686 culture-positive patients of STB and found 111 patients (16.2%) having drug-resistant strains to at least one drug. 87/111 patients had MDR, 3 as XDR strains, while the remaining 21 patients demonstrated mono-drug-resistant strains. Prevalence of INH resistance was 15.0% (93/686), RIF 13.5% (93/686), and streptomycin was11.2% (77/686) [37]. PawarUM et.al. evaluated 238 histologically proven STB patients and found 28/238 (11.7%) having MDR strains [3]. XuLan et.al. evaluated 152 histologically proven STB patients and reported 76 culture-positive samples. 23/76 (30.3%) patient reported drug-resistant TB [1]. All these studies have shown higher percentage of proven and individual drug resistance only in culture-positive samples, while we are reporting drug resistance in all samples (culture ±) as percentage of drug resistance in primary and suspected drug-resistance patients. Phenotypic DST is able to detect known and unknown resistances irrespective of mutations/resistance mechanisms; however, it is time taking. These tests require high biosafety laboratory infrastructures and has high turnaround time of 2–6 weeks for detection of MTB with added 3 weeks for DST, limiting its role in early diagnosis/interventions in drug-resistant TB [19].
We detected unusual, alarming incidence of primary drug resistance in Group A in STB (25.49%, 13/51). 9/13 never took ATT in past, while 4 took ATT in the past but not for present illness. Among Group B (presumptive drug resistance), 3/8 cases who demonstrated resistance had no history of ATT intake. Bhosale et al. reported 28.6% primary drug resistance in STB in only culture-positive cases [38], which is the only study available where primary drug-resistance STB in only culture positive consecutive cases has been reported.
Both primary (Group A) and presumptive drug resistance (Group B) demonstrated maximum drug resistance against INH, followed by RIF. Although sample size in presumptive drug resistance (Group B) is small to draw conclusion, higher RIF resistance was demonstrated in comparison to primary cases. INH resistance demonstrated was 21.4% (9/42) in primary cases, while 50% (3/6) in presumptive drug-resistance cases. RIF resistance was 14.6% (7/48) in primary cases, while 28.6% (2/7) in presumptive drug-resistance cases. For statistical significance testing, p-value was calculated using Fisher's exact test and Chi-square test which revealed no significant difference (p-value > 0.05) in DST patterns in two groups.
In this study, maximum drug resistance was against INH 25% (12/48) followed by RIF 16.4% (9/55). Isolated resistance to RIF was not observed. In one case, RIF resistance was present with additional FQ resistance instead of INH resistance. RIF resistance is considered as a surrogate marker for MDR-TB, but certain strains may have only mono-resistance to RIF or resistance to some additional drugs. Above assumption may lead to full-fledged MDR-TB treatment and over-estimation of MDR-TB cases [39]. Among FQ (Fig. 3), Lev (levofloxacin) resistance was demonstrated in 15% (6/40), while Moxifloxacin resistance was observed in 12.5% (5/40) cases.
The occurrence of spontaneous (rare) mutations during replication of TB bacillus is a natural phenomenon, but the preferential selection and propagation of such resistant mutants is man-made and largely preventable [23]. Detection of all forms of drug resistance, not only MDR/XDR-TB, should be an integral part of diagnosis and management of all patients of STB. Therefore, the assumption that resistance is only due to non-compliance/inadequate chemotherapy is not true since all mechanisms of antibiotic resistance in TB bacilli are still unknown and some patients may have some inherent or de novo resistance. Therefore, all new cases should be considered as potentially resistant case and DST should be part of initial workup and individualized chemotherapy regimen should be initiated for every patient to yield optimum outcome.
Since, outcome of STB is improved by early diagnosis/intervention, genotypic DST should be used preferably as first line test to get an insight about the resistance pattern for initiation of early and appropriate ATT regimen. Genotypic DST must be reinforced by phenotypic DST since phenotypic tests detect resistance independent of the underlying mechanism and not all mutations conferring resistance to anti-tubercular drugs are known. Therefore, wherever possible, DST should be performed to guide anti-tubercular therapy to achieve higher rates of healing.
To conclude, we report alarmingly high percentage of primary drug resistance (25.49%) among patients of STB. Even in presumptive drug-resistance cases, all three proven cases never took ATT in past raising either a suspicion of primary drug resistance or faulty ATT intake in the present disease. This is the first study where incidence and resistance pattern has been evaluated for both culture-positive as well as culture-negative cases to document prevalent drug resistance in all cases. All the available studies have performed DST only on culture-positive cases. Molecular tests should be a part of routine testing to formulate individualized ATT regimen instead of blanket therapy. We reiterate that no single diagnostic test can conclusively diagnosis all cases of spinal TB and tissue should be submitted for AFB smear, culture, histology, and molecular methods in tandem to ascertain diagnosis of TB and culture sensitivity. However by subjecting tissue to only CBNAAT and histology, the diagnosis of TB and sensitivity for RIF can be ascertained.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Informed consent
For this type of study informed consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Manish Yadav, Email: manishpurnia@gmail.com.
Anil K. Jain, Email: profakjain@gmail.com
Ritu Singhal, Email: drritugo@gmail.com.
Manish Chadha, Email: mchadha@hotmail.com.
Vinod Kumar Arora, Email: drvinodkumarora@gmail.com.
Aayush Bhargava, Email: aayushbhargava@gmail.com.
References
- 1.Xu L, Jian-Zhong X, Xue-Mei L, Bao-Feng G. Drug susceptibility testing guided treatment for drug resistant spinal tuberculosis: a retrospective analysis of 19 patients? Int Surg. 2013;98(2):175–80. doi: 10.9738/INTSURG-D-12-00004.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z, Luo F, Zhou Q, Dai F, Sun D, Xu J. The outcomes of chemotherapy only treatment on mild spinal tuberculosis. J OrthopSurg Res. 2016;11:49. doi: 10.1186/s13018-016-0385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawar UM, Kundnani V, Agashe V, Nene A, Nene A. Multidrug-resistant tuberculosis of the spine–is it the beginning of the end. A study of twenty-five culture proven multidrug-resistant tuberculosis spine patients. Spine. 2009;34(22):806–810. doi: 10.1097/BRS.0b013e3181af7797. [DOI] [PubMed] [Google Scholar]
- 4.Lee JY. Diagnosis and treatment of extrapulmonary tuberculosis. TubercRespir Dis. 2015;78(2):47–55. doi: 10.4046/trd.2015.78.2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain AK, Jena SK, Singh MP, Dhammi IK, Ramachadran VG, Dev G. Evaluation of clinico-radiological, bacteriological, serological, molecular and histological diagnosis of osteoarticular tuberculosis. Ind J Ortho. 2008;42(2):173–177. doi: 10.4103/0019-5413.40253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abhimanyu S, Jain AK, Myneedu VP, Arora VK, Chadha M, Sarin R. The Role of cartridge-based nucleic acid amplification test (CBNAAT), Line Probe Assay (LPA), Liquid Culture, Acid-Fast Bacilli (AFB) smear and histopathology in the diagnosis of osteoarticular tuberculosis. Indian J Orthop. 2021;55(Suppl 1):157–166. doi: 10.1007/s43465-020-00326-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kent PT, Kubica GP. Public health mycobacteriology. A guide for the level III laboratory. Atlanta, GA: Centers for Disease Control, U.S. Department of Health and Human Services; 1985. https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB86216546.xhtml.
- 8.Lee CN, Heifets LB. Determination of minimal inhibitory concentrations of antituberculosis drugs by radiometric and conventional methods. American Review of Respiratory Disease. 1987;136(2):349–352. doi: 10.1164/ajrccm/136.2.349. [DOI] [PubMed] [Google Scholar]
- 9.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. New England Journal of Medicine. 2010;363(11):1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oommen S, Banaji N. Laboratory diagnosis of tuberculosis: advances in technology and drug susceptibility testing. Indian Journal of Medical Microbiology. 2017;35(3):323. doi: 10.4103/ijmm.IJMM_16_204. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Zhang Z, Luo F, Xu J, Cheng P, Wu Z, et al. Management of drug-resistant spinal tuberculosis with a combination of surgery and individualized chemotherapy: A retrospective analysis of thirty-five patients. International Orthopaedics. 2012;36:277–283. doi: 10.1007/s00264-011-1398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organisation . Rapid implementation of the Xpert MTB/RIF diagnosis test. Technical and operational “How to” practical considerations. World Health Organization 2014; WHO/HTM/TB/20112. Geneva: World Health Organization; 2014. [Google Scholar]
- 13.Central TB division: Index TB guidelines-Guidelines for extrapulmonary tuberculosis for India. 2016. https://tbcindia.gov.in/showfilephp?lid=3245.
- 14.Jain AK, Dhammi IK, Modi P, Kumar J, Sreenivasan R, Saini NS. Tuberculosis spine: therapeutically refractory disease. Indian Journal of Orthopaedics. 2012;46:171–178. doi: 10.4103/0019-5413.93685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain AK, Sinha S. Evaluation of paraplegia grading systems in tuberculosis of the spine. Spinal Cord. 2005;43(6):375–380. doi: 10.1038/sj.sc.3101718. [DOI] [PubMed] [Google Scholar]
- 16.Central TB division: Technical and Operational Guidelines for TB Control in India-Chapter 4-Treatment of TB Part 1.2016. https://www.tbcindia.gov.in/showfile.php?lid=3219.
- 17.Central TB division: Guidelines on PMDT in India.2017. https://www.tbcindia.gov.in/index1.php?lang=1&level=2&sublinkid=4780&lid=3306.
- 18.World Health Organisation . Xpert MTB/RIF assay for diagnosis of pulmonary and extrapulmonary TB in adults and children Policy update WHO/HTM/TB/ 201316. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 19.World Health Organization . Molecular line probe assays for rapid screening of patients at risk of multidrug resistant tuberculosis (MDR-TB) Geneva: World Health Organization; 2008. [Google Scholar]
- 20.Grady J, Maeurer M, Mwaba P, Kapata N, Bates M, Hoelscher M, et al. New and improved diagnostics for detection of drug-resistant pulmonary tuberculosis. Current Opinion in Pulmonary Medicine. 2011;17(3):134–141. doi: 10.1097/MCP.0b013e3283452346. [DOI] [PubMed] [Google Scholar]
- 21.Garg RK, Somvanshi DS. Spinal tuberculosis: a review. Journal of Spinal Cord Medicine. 2011;34(5):440–454. doi: 10.1179/2045772311Y.0000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain AK, Rajasekaran S, Jaggi KR, Myneedu VP. Tuberculosis of the Spine. Journal of Bone and Joint Surgery. 2020;102(7):617–628. doi: 10.2106/JBJS.19.00001. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Anti-tuberculosis drug resistance in the world report no4WHO/HTM/TB/2008394. Geneva: World Health Organization.
- 24.Moon MS. Tuberculosis of spine: current views in diagnosis and management. Asian Spine J. 2014;8(1):97–111. doi: 10.4184/asj.2014.8.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bastian I, Rigouts L, Van Deun A, Portaels F. Directly observed treatment, short-course strategy and multidrug- resistant tuberculosis: are any modifications required? Bulletin of the World Health Organization. 2000;78:238–251. [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma SK, Mohan A. Multidrug-resistant tuberculosis. Indian Journal of Medical Research. 2004;120:354–376. [PubMed] [Google Scholar]
- 27.Vadwai V, Boehme C, Nabeta P, Shetty A, Alland D, Rodrigues C. Xpert MTB/RIF: a new pillar in diagnosis of extrapulmonary tuberculosis? J ClinMicrobiol. 2011;49(7):2540–2545. doi: 10.1128/JCM.02319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu Y, Wang G, Dong W, Li Y, Ma Y, Shang Y, Qin S, Huang H. Xpert MT/RIF and GenoTypeMTBDRplus assays for the rapid diagnosis of bone and joint tuberculosis. International Journal of Infectious Diseases. 2015;36:27–30. doi: 10.1016/j.ijid.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Sharma A, Chhabra HS, Mahajan R, Chabra T, Batra S. Magnetic Resonance imaging and GeneXpert®: a rapid and accurate diagnostic tool for the management of tuberculosis of the Spine. Asian Spine J. 2016;10(5):850–856. doi: 10.4184/asj.2016.10.5.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain A, Singh PK, Chooramani G, Dixit P, Malhotra HS. Drug resistance and associated genetic mutations among patients with suspected MDR-TB in Uttar Pradesh India. Int J Tuberc Lung Dis. 2016;20(7):870–875. doi: 10.5588/ijtld.15.0874. [DOI] [PubMed] [Google Scholar]
- 31.Isaac A, Kunimoto D. Treatment Outcomes in Low-Level Isoniazid Resistant Tuberculosis. Open Forum Infectious Diseases. 2016;3(1):559. doi: 10.1093/ofid/ofw172.422. [DOI] [Google Scholar]
- 32.Lawn SD, Zumla AI. Diagnosis of extrapulmonary tuberculosis using the Xpert® MTB/RIF assay. Expert Rev Anti Infect Ther. 2012;10(6):631–635. doi: 10.1586/eri.12.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomasicchio M, Theron G, Pietersen E, Streicher E, Stanley-Josephs D, Van Helden P, Warren R, Dheda K. The diagnostic accuracy of the MTBDRplus and MTBDRsl assays for drug-resistant TB detection when performed on sputum and culture isolates. Sci Rep. 2015;6:17850. doi: 10.1038/srep17850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brossier F, Veziris N, Aubry A, Jarlier V, Sougakoff W. Detection by GenoTypeMTBDRsl test of complex mechanisms of resistance to second-line drugs and ethambutol in multidrug-resistant Mycobacterium tuberculosis complex isolates. Journal of Clinical Microbiology. 2010;48:1683–1689. doi: 10.1128/JCM.01947-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiet VS, Lan NT, An DD, Dung NH, Hoa DV, van Vinh CN. Evaluation of the MTBDRsl test for detection of second-line-drug resistance in Mycobacterium tuberculosis. J Clin Microbiol. 2010;48:2934–2939. doi: 10.1128/JCM.00201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi J, Tang X, Xu Y, Zhou T, Pan X, Lin H, et al. Single-stage internal fixation for thoracolumbar spinal tuberculosis using 4 different surgical approaches. J Spinal Disord Techn. 2014;27:E247–E257. doi: 10.1097/BSD.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 37.Mohan K, Rawall S, Pawar UM, et al. Drug resistance patterns in 111 cases of drug-resistant tuberculosis spine. Eur Spine J. 2013;22:647–652. doi: 10.1007/s00586-012-2154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhosale S, Prabhakar A, Srivastava S, Raj A, Purohit S, Marathe N. Pattern of drug resistance in primary spinal tuberculosis: a single-center study from India. Global Spine J. 2021;11(7):1070–1075. doi: 10.1177/2192568220941445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma SK, Kohli M, Yadav RN, Chaubey J, Bhasin D, Sreenivas V, et al. Evaluating the diagnostic accuracy of Xpert MTB/RIF assay in pulmonary tuberculosis. PLoS ONE. 2015;10(10):e0141011. doi: 10.1371/journal.pone.0141011. [DOI] [PMC free article] [PubMed] [Google Scholar]