Abstract
Background
Disrupted motivational control is a common—but poorly treated—feature of psychiatric disorders, arising via aberrant mesolimbic dopaminergic signaling. GPR88 is an orphan G protein–coupled receptor that is highly expressed in the striatum and therefore well placed to modulate disrupted signaling. While the phenotype of Gpr88 knockout mice suggests a role in motivational pathways, it is unclear whether GPR88 is involved in reward valuation and/or effort-based decision making in a sex-dependent manner and whether this involves altered dopamine function.
Methods
In male and female Gpr88 knockout mice, we used touchscreen-based progressive ratio, with and without reward devaluation, and effort-related choice tasks to assess motivation and cost/benefit decision making, respectively. To explore whether these motivational behaviors were related to alterations in the striatal dopamine system, we quantified expression of dopamine-related genes and/or proteins and used [18F]DOPA positron emission tomography and GTPγ[35S] binding to assess presynaptic and postsynaptic dopamine function, respectively.
Results
We showed that male and female Gpr88 knockout mice displayed greater motivational drive than wild-type mice, which was maintained following reward devaluation. Furthermore, we showed that cost/benefit decision making was impaired in male, but not female, Gpr88 knockout mice. Surprisingly, we found that Gpr88 deletion had no effect on striatal dopamine by any of the measures assessed.
Conclusions
Our results highlight that GPR88 regulates motivational control but that disruption of such behaviors following Gpr88 deletion occurs independently of gross perturbations to striatal dopamine at a gene, protein, or functional level. This work provides further insights into GPR88 as a drug target for motivational disorders.
Keywords: Dopamine, GPR88, Motivation, Orphan G protein–coupled receptor, Striatum, Touchscreen
Aberrant motivational control is a common feature of psychiatric disorders, with symptoms ranging from avolition and apathy to compulsive reward seeking (1, 2, 3). Current treatments for such symptoms are often ineffective, possess unwanted side effects, or, in some instances, exacerbate motivational deficits. As such, there is a significant need for novel treatments that are effective without compliance-prohibitive side effects.
The striatum is a key integrator of cognitive, motor, and limbic circuitry that collectively functions to regulate motivated behaviors (4). Midbrain dopaminergic projections and glutamatergic projections from numerous cortical and subcortical areas converge onto striatal medium spiny neurons (MSNs) to form cortico-striatal-thalamic loops, critical brain circuits for controlling movement, habit formation, and reward processing (5). Within the striatum, functional subdivisions are associated with distinct aspects of reward learning and decision making. The dorsolateral (or sensorimotor) striatum is responsible for stimulus-response associations and habitual behaviors, while the dorsomedial (or associative) striatum is important for response-outcome associations and goal-directed behavior (5). Finally, the ventral (or limbic) striatum is implicated in motivation and outcome evaluation. Concerted activity across all functional subdivisions—particularly with respect to dopamine signaling—is required for intact motivational control; therefore, striatal targets are well positioned to modulate various aspects of motivational dysfunction.
GPR88 is an orphan G protein–coupled receptor that is almost exclusively expressed in the striatum on both D1-expressing and D2-expressing MSNs (6). GPR88 expression is altered following use of drugs of abuse, highlighting a potential role in regulating motivation and reward-related pathways (7,8). Indeed, Gpr88 knockout mice display increased alcohol-seeking and risk-taking behaviors and increased appetitive motivation (9,10). However, it is unclear whether GPR88 is involved in reward valuation and/or effort-based decision making in a sex-dependent manner and if behavioral changes are due to maladaptations to the dopaminergic system. To address these questions, we probed the motivational phenotype of male and female Gpr88 knockout mice using the rodent touchscreen system, which offers a translational platform to measure cognitive behaviors with better alignment of preclinical and clinical test constructs and outcomes (11,12). We tested the effect of reward devaluation on progressive ratio (PR) breakpoint to assess the potential for altered reward valuation in Gpr88 knockout mice. We also assessed cost/benefit decision making in Gpr88 knockout mice in an effort-related choice task in which animals were given the option of a low effort/low reward or a high effort/high reward. Finally, we assessed the effect of Gpr88 deletion on the dopamine system at a gene, protein, and functional level using quantitative real-time polymerase chain reaction (qRT-PCR), Western blotting, [18F]DOPA positron emission tomography (PET) imaging, and GTPγ[35S] binding. Our work aimed to clarify the role of GPR88 in motivation and reward-related behaviors and provide further validation of its utility as a target for dysfunctional motivational control in psychiatric disorders.
Methods and Materials
Animals
Gpr88Cre/Cre mice on a C57BL/6J background were obtained from Jackson Laboratories (13). Wild-type (WT) and Gpr88Cre/Cre mice were bred in-house from heterozygote crossing and used for all behavioral procedures, qRT-PCR, and Western blotting. Gpr88 CRISPR mice (Gpr88−/−) were generated using CRISPR/Cas9 gene editing (Supplemental Methods) and then used for [18F]DOPA PET after being validated against behavioral data from Gpr88Cre/Cre mice (Figure S1). Mice had access to water and food ad libitum and were housed in a 12-hour light/dark cycle at constant temperature and humidity. All experiments were approved by The Florey Institute of Neuroscience and Mental Health Animal Ethics Committee (16-034-FINMH, 18-132-FINMH) or by the Monash University Animal Ethics Committee (no. 17661).
The animals used for each experiment were as follows:
-
•
Cohort 1: Gpr88Cre/Cre mice approximately 11 weeks of age at the start of touchscreen operant training (Gpr88Cre/Cre n = 12 males, n = 11 females; WT littermates n = 11 males, n = 10 females); PR, effort-related choice
-
•
Cohort 2: Gpr88Cre/Cre mice approximately 11 weeks of age at the start of touchscreen operant training (Gpr88Cre/Cre n = 12 male, n = 12 female; WT littermates n = 12 male, n = 12 female); PR with devaluation, qRT-PCR
-
•
Cohort 3: Gpr88Cre/Cre mice 12 to 40 weeks (Gpr88Cre/Cre n = 6 male, n = 5 female; WT littermates n = 6 male, n = 5 female); Western blotting
-
•
Cohort 4: Gpr88−/− mice 8 to 16 weeks (Gpr88−/− n = 7 male, WT littermates n = 7 male); [18F]DOPA PET
-
•
Cohort 5: Gpr88−/− mice 12 to 40 weeks (Gpr88−/− n = 6 males, n = 5 females; WT n = 6 males, n = 4 females); striatal GTPγ[35S] binding
Touchscreen Apparatus
The touchscreen automated system (Campden Instruments Ltd.) was used as previously described (Supplemental Methods) (14,15).
Behavioral Procedures
For all behavioral experiments, mice were housed under a reversed light/dark cycle condition to allow testing during the active phase. Briefly, animals were food restricted to 85% of their free-feeding body weight. For 2 days immediately prior to the beginning of operant training, mice were exposed to a small amount of the liquid reward in their home cages to prevent neophobia. Further information is provided in Supplemental Methods. Touchscreen training and task protocols were adapted from Heath et al. (14).
Touchscreen Operant Training
Operant training was conducted as previously described (Supplemental Methods) (14).
Progressive Ratio
Once mice had completed touchscreen operant training, they were tested on the PR schedule as previously described (14). The number of screen touches required for reward delivery then increased linearly by 4 (PR4: 1, 5, 9, 13, 17 touches, etc.) or by 8 (PR8: 1, 9, 17 touches, etc.) on each trial. The session ended after 60 minutes or when no screen response or magazine entry had been detected for 5 minutes. Mice were tested on the PR schedule for 4 consecutive days to ensure that all mice reached and maintained baseline performance.
PR With Devaluation
After touchscreen operant training, mice from cohort 2 underwent PR training (4 days PR4, baseline). For devaluation with chow and strawberry milk, animals were individually housed and given free access to chow or milk for 30 minutes prior to the PR test session. Chow/milk was weighed before and after the 30 minutes to determine the amount each mouse had consumed. Animals were tested on PR4 for 3 consecutive days for each devaluation procedure, with a baseline PR4 session between interventions. Mice were then given access to chow ad libitum until free-feeding weights were established, before being tested again on PR4 for 4 consecutive days.
Effort-Related Choice
Mice were tested in the effort-related choice task as previously described (14). Animals were tested on fixed ratio schedules of 16, 32, and 5 for 4 consecutive sessions as above; however, 3 preweighed pellets of standard rodent chow were now available during the session, having been randomly placed on the floor of the touchscreen testing chamber prior to each session. Therefore, mice had the option to consume freely available chow or to complete trials to receive a strawberry milk reward. Each session ended after 60 minutes or completion of 30 trials, whichever occurred first. Mice were then immediately removed, and uneaten chow was weighed to calculate the amount consumed. Because session length varied between animals, chow consumption was indexed as grams per hour.
Behavioral Measures
All touchscreen data were recorded using the ABET recording software (Lafayette Instrument Co.). For PR, the main measure of interest was the animals’ breakpoint, which is the number of touches made during the last successfully completed trial. For effort-related choice, the main measure was the number of trials completed and the amount of chow consumed during the testing session.
[18F]DOPA PET
All scanning was performed at Monash Biomedical Imaging. Mice from cohort 4 were used in the experiments (Supplemental Methods).
Striatal GTPγ[35S] Binding
Striatal membranes were prepared from cohort 5, and GTPγ[35S] binding was assessed following addition of pramipexole (Supplemental Methods).
Quantitative RT-PCR
Brain tissue of naïve and cohort 2 mice was collected, and expression of dopamine-related genes was quantified by qRT-PCR (Supplemental Methods).
Western Blotting
Striatal brain tissue from cohort 3 was collected, and expression of dopamine-related proteins was quantified by Western blotting (Supplemental Methods).
Statistical Analysis
Statistical analysis was performed using Prism version 7 or 8 (GraphPad). Repeated-measures analysis of variance and analysis of covariance were used to analyze behavioral experiments. Student t test and analysis of variance were used for qRT-PCR and Western blot analysis. When appropriate, post hoc analysis was done using the Tukey multiple comparisons test, with the significance level set at p < .05.
Results
Gpr88 Deletion Increased Motivation for a Palatable Reward
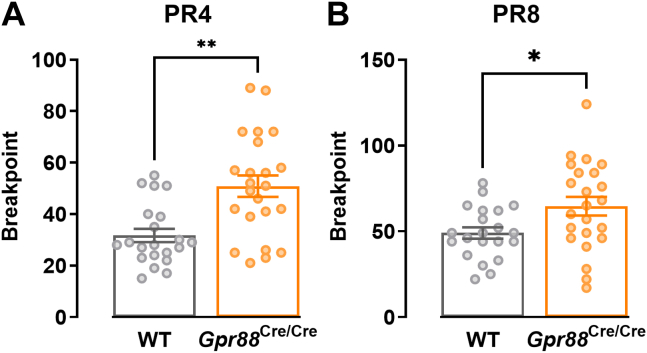
PR tasks have been used with both animals and humans to assess a subject’s ability to maintain responding for a reward as response requirements increase. The breakpoint, or the number of responses at which the subject stops responding, provides a measure of motivation encompassing the reinforcing properties of the reward and the point at which effort outweighs the benefit of obtaining that reward. We used a touchscreen-based PR task to assess motivation in Gpr88Cre/Cre mice, where the number of touches required to elicit a strawberry milk reward increased linearly by 4 (PR4) or 8 (PR8) during each trial. We found that there was no effect of sex on average breakpoint over the test sessions (Figure S2A); therefore, data from male and female mice were combined. Gpr88Cre/Cre mice had a significantly higher breakpoint than WT littermates at both reinforcement schedules (Figure 1), indicating greater motivation for a palatable reward. This finding is consistent with previous reports of increased reward-seeking behavior in Gpr88 knockout mice (9,10).
Figure 1.
Gpr88 deletion increased motivation toward a palatable reward at progressive ratio schedules of (A) 4 (determined by Mann-Whitney test, U = 107.5; p = .0012) and (B) 8 (determined by unpaired t test, t42 = 2.411; p = .0204). N = 21–23 (WT males n = 11, females n = 10; Gpr88Cre/Cre males n = 12, females n = 11). Individual data points presented with mean ± SEM. ∗p < .05, ∗∗p < .01. PR, progressive ratio; WT, wild-type.
Gpr88Cre/Cre Mice Showed Increased Motivation Despite Devaluation of the Reward
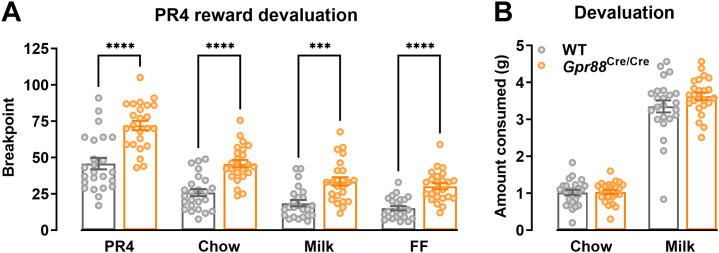
GPR88 has a reported role in feeding and metabolism (16); therefore, the increased PR breakpoint observed in Gpr88Cre/Cre mice may be explained by a metabolic, rather than a motivational, phenotype. To investigate the dependence of food intake on the PR breakpoint measure, we tested a separate cohort of Gpr88Cre/Cre mice on PR4 following reward devaluation (where animals were given free access to either chow or milk prior to touchscreen testing) and under free-feeding conditions. Again, we found no main effect of sex on breakpoint; therefore, data from male and female mice were combined (Figure S2B). In accordance with our earlier observations, Gpr88Cre/Cre mice still displayed a significantly higher breakpoint than WT mice (Figure 2A). Compared with testing under food-restricted conditions, devaluation (chow and strawberry milk) or free-feeding prior to PR4 sessions decreased breakpoint across both Gpr88Cre/Cre and WT mice, as expected (Figure 2A). Despite this, Gpr88Cre/Cre mice retained a significantly higher breakpoint than WT mice in all conditions tested (Figure 2A). Importantly, there was no significant difference in the amount of chow or milk consumed by Gpr88Cre/Cre and WT mice prior to PR4 sessions (Figure 2B). It is noteworthy that devaluation procedures shifted levels of responding in Gpr88 knockout mice at a rate similar to that of WT animals, suggesting that while reward valuation processes are disrupted, they are not abolished by Gpr88 deletion. Furthermore, we found no genotype effect on infrared beam breaks during habituation sessions (Figure S3), suggesting that the previously reported hyperactive phenotype of Gpr88 knockout mice (13) was not present in this context and therefore unlikely to contribute to the motivational phenotype. Taken together, this suggests that Gpr88 plays a role in regulating motivational processing, and loss of GPR88 increases responding for a palatable reward independently of whether energy requirements are met and does not affect mechanisms of satiety.
Figure 2.
(A)Gpr88Cre/Cre mice showed increased motivation compared with WT mice following reward devaluation with chow (t45.89 = 5.607), milk (t42.49 = 4.120), and free feeding (t40.99 = 5.794). Main effect of devaluation, F2.099,96.54 = 130.6, p < .0001; genotype, F1,46 = 42.46, p < .0001; devaluation × genotype, F3,138 = 3.39, p = .0199. (B) Consumption of chow and milk prior to testing did not significantly differ between Gpr88 knockout and WT mice. Repeated-measures two-way analysis of variance with Šídák’s multiple comparisons test; chow (t92 = 0.06545; p = .997), milk (t92 = 1.842; p = .133). N = 24 (WT males n = 12, females n = 12; Gpr88Cre/Cre males n = 12, females n = 12). Individual data points presented with mean ± SEM. ∗∗∗p < .001, ∗∗∗∗p < .0001 determined by repeated-measures two-way analysis of variance with Geisser-Greenhouse correction and Šídák’s multiple comparisons test. FF, free feeding; PR, progressive ratio; WT, wild-type.
Effort-Related Decision Making Was Impaired in Male, but Not Female, Gpr88Cre/Cre Mice
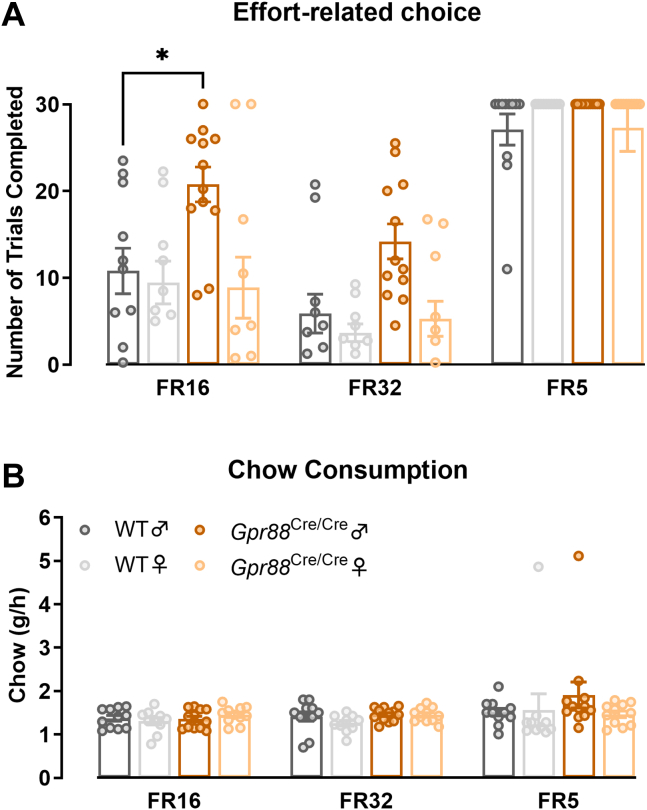
Cost/benefit analysis is a critical component that drives motivated behavior: individuals evaluate estimated costs (i.e., effort) against the estimated value of an expected reward to optimize action selection, dysfunction of which is associated with negative symptoms of psychiatric disorders such as schizophrenia (17). To further evaluate cost/benefit decision making in Gpr88Cre/Cre mice, we used a touchscreen-based, effort-related choice paradigm in which animals could either make operant touches to receive the strawberry milk reward or consume chow that was freely available. The expectation was that as the required effort to obtain the more preferred reward choice (strawberry milk) increases, animals will instead choose to consume the low-effort choice (standard chow) (14). Here, we observed sex-dependent effects (Figure 3): at fixed ratio schedules of 16 (FR16) and 32 (FR32), male Gpr88Cre/Cre mice completed a greater number of trials than male WT mice, reaching significance at FR16. Interestingly, no genotype effect was observed in female mice. When the effort required for the reward was reduced to a very low level by using an FR5 schedule, all groups completed the majority of trials without significant differences between genotypes or sexes. Because session duration varied between animals, we corrected chow consumption for time spent in the chamber. Somewhat surprisingly, despite the increased number of trials completed, male Gpr88Cre/Cre mice consumed the same amount of chow during testing as female Gpr88Cre/Cre mice and WT animals.
Figure 3.
(A) Effort-related decision making was impaired in male, but not female, Gpr88Cre/Cre mice when effort was high (repeated-measures two-way analysis of variance with Geisser-Greenhouse correction and Tukey’s multiple comparisons test, schedule × group, F6,80 = 3.064, p = .0095, p < .05) (B) despite equal consumption of chow across all fixed ratio schedules (two-way repeated-measures analysis of variance, schedule × group, F6,80 = 0.9428, p = .4694). Individual data points presented with mean ± SEM; N = 10–12. FR, fixed ratio; WT, wild-type.
Gpr88 Deletion Did Not Alter Messenger RNA or Protein Levels of Dopamine-Related Targets
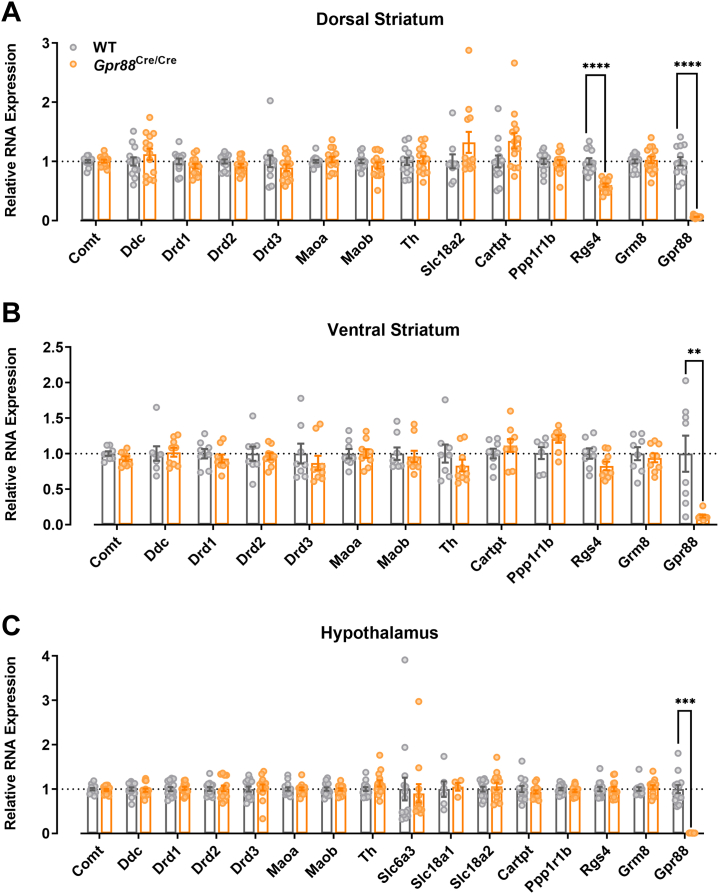
To determine whether the increased motivational phenotype in Gpr88Cre/Cre mice was associated with transcriptional changes to dopamine-related genes, we quantified expression of those genes involved in dopamine synthesis (Th, Ddc), signaling (Drd1, Drd2, Drd3, Ppp1r1b), transport (Slc6a3, Slc18a1, Slc18a2), and metabolism (Comt, Maoa, Maob) in the dorsal and ventral striatum using qRT-PCR (Table 1; Figure 4A, B).
Table 1.
Description of Genes and Proteins Quantified by qRT-PCR and/or Western Blotting
| Gene | Protein | Description |
|---|---|---|
| Comt | Catechol-O-methyltransferase | Dopamine metabolism |
| Maoa | Monoamine oxidase A | Dopamine metabolism |
| Maob | Monoamine oxidase B | Dopamine metabolism |
| Drd1 | Dopamine receptor D1 | Dopamine signaling |
| Drd2 | Dopamine receptor D2 | Dopamine signaling |
| Drd3 | Dopamine receptor D3 | Dopamine signaling |
| Ddc | L-aromatic amino acid decarboxylase | Dopamine synthesis |
| Th | Tyrosine hydroxylase | Dopamine synthesis |
| Slc6a3 | Dopamine transporter | Dopamine transport |
| Slc18a1 | Vesicular monoamine transporter 1 | Dopamine transport |
| Slc18a2 | Vesicular monoamine transporter 2 | Dopamine transport |
| Cartpt | Cocaine- and amphetamine-regulated transcript prepropeptide | Food intake, body weight, reward |
| Ppp1r1b | Dopamine- and cAMP-regulated phosphoprotein, Mr 32 kDa | Integrator of striatal neurotransmission |
| Rgs4 | Regulator of G protein signaling 4 | Inhibits signal transduction |
| Grm8 | Metabotropic glutamate receptor 8 | Negative control |
| Gpr88 | G protein–coupled receptor 88 | Positive control |
cAMP, cyclic adenosine monophosphate; qRT-PCR, quantitative real-time polymerase chain reaction.
Figure 4.
Expression of dopamine-related genes was unchanged in Gpr88Cre/Cre mice in both the (A) dorsal and (B) ventral striatum and (C) the hypothalamus. Dorsal striatum and hypothalamus N = 13–14; ventral striatum N = 8–9. Individual data points presented with mean ± SEM. ∗∗p < .01, ∗∗∗p < .001, ∗∗∗∗p < .0001 determined by multiple Mann-Whitney tests with Holm-Šídák’s correction for multiple comparisons. WT, wild-type.
In addition, we investigated genes reported to be differentially expressed in Gpr88 knockout mice (Cartpt, Rgs4) (13,16) and hypothalamic tissue, where no changes in dopamine-related genes were expected (Figure 4C). Gpr88 and Grm8 were included as positive and negative controls, respectively. Expression of Gpr88 was significantly reduced in both dorsal and ventral striatal regions and in the hypothalamus (Figure 4) (multiple Mann-Whitney test with Holm-Šídák’s correction for multiple comparisons; dorsal striatum p < .0001, ventral striatum p = .007, hypothalamus p = .0002).
No significant changes were found for any of the dopamine-related genes investigated. However, Rgs4 was significantly lower in the dorsal but not the ventral striatum, suggesting that a previous finding of striatal Rgs4 downregulation in Gpr88Cre/Cre mice was driven by changes in the dorsal region (Figure 4) (multiple Mann-Whitney test with Holm-Šídák’s correction for multiple comparisons; dorsal striatum p < .0001, ventral striatum p = .807) (13).
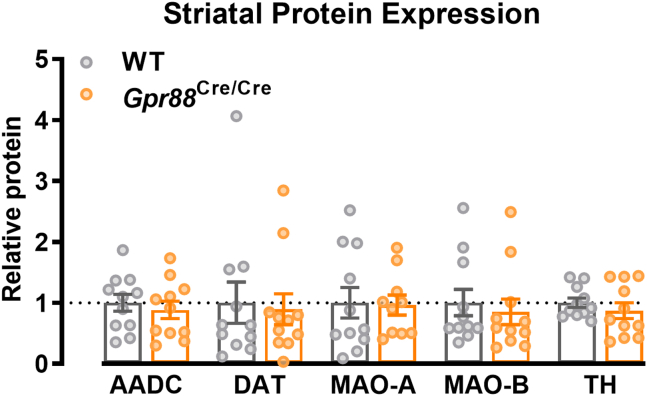
Given that messenger RNA expression is not always indicative of protein expression, we further investigated enzymes and transporters involved in mesolimbic dopamine signaling in whole striatal tissue. In particular, we quantified levels of tyrosine hydroxylase, amino acid decarboxylase, dopamine transporter, and monoamine oxidase A and B by Western blotting. Consistent with results from qRT-PCR, we found no changes in expression of dopamine-related proteins in Gpr88Cre/Cre mice, with respect to WT mice (Figure 5 and Figure S4) (two-way repeated-measures analysis of variance, genotype p = .948).
Figure 5.
Striatal expression of dopamine-related proteins was unchanged in Gpr88Cre/Cre mice (multiple Mann-Whitney tests with Holm-Šídák’s correction for multiple comparisons; AADC U = 51, p = .9256; DAT U = 58, p = .9407; MAO-A U = 50, p = .9407; MAO-B U = 49, p = .9257, TH U = 47, p = .9165; N = 11). Individual data points presented with mean ± SEM. AADC, amino acid decarboxylase; DAT, dopamine transporter; MAO-A, monoamine oxidase A; MAO-B, monoamine oxidase B; TH, tyrosine hydroxylase; WT, wild-type.
Striatal Dopamine Synthesis Capacity and Dopamine D2/D3 Receptor Function Were Unchanged in Gpr88−/− Mice
While no genotype-dependent differences were found in the expression of dopamine-related genes and proteins, an obvious limitation of these techniques is that they do not provide functional information. To address this, we investigated dopamine synthesis capacity using [18F]DOPA PET and dopamine D2/D3 receptor function using GTPγ[35S] binding.
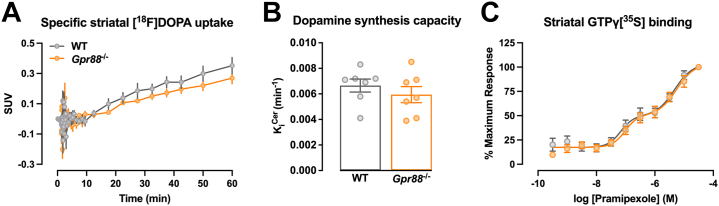
[18F]DOPA PET is commonly used in clinical studies and provides a composite measure of presynaptic dopamine function. Striatum and cerebellum uptake of [18F]DOPA was corrected for body weight and radiotracer dose, and specific striatal uptake was calculated by subtracting the cerebellum time course from the striatal time course (18). Specific striatal [18F]DOPA uptake was unchanged in Gpr88−/− mice (Figure 6A). Similarly, dopamine synthesis capacity, indexed as KiCer, was not significantly different between WT and Gpr88−/− mice (Figure 6B). The KiCer values obtained were lower than those previously reported, likely due to slight variations in experimental procedures (19).
Figure 6.
(A)Gpr88 deletion did not affect striatal dopamine synthesis capacity (unmatched t test, t12 = 0.8822, p = .395; N = 7) or (B) striatal uptake of [18F]DOPA (two-way repeated-measures analysis of variance with Geisser-Greenhouse correction, genotype, F1,12 = 0.6396, p = .439; N = 7). (C) Striatal dopamine D2/D3 receptor function was unchanged in Gpr88−/− mice (F test, p = .75; N = 10–11). Data are presented as mean ± SEM. SUV, standardized uptake value; WT, wild-type.
Having established that presynaptic dopamine content was unchanged, we studied postsynaptic dopamine D2/D3 receptor function in striatal membranes prepared from WT and Gpr88−/− mice by GTPγ[35S] binding. We found that there was no effect of sex on GTPγ[35S] binding; therefore, male and female data were combined. The dopamine D2/D3 receptor agonist pramipexole stimulated GTPγ[35S] binding in a concentration-dependent and biphasic manner, with potencies for the 2 phases of approximately 100 nM and 5 μM in membranes from both WT and Gpr88−/− mice (likely reflecting multiple Gɑi/o-coupled receptor subtypes being activated by pramipexole in the native preparation) (Figure 6C). Notably, there was no significant effect of genotype on pramipexole potencies.
Discussion
In this study, we investigated the effect of Gpr88 deletion on motivational behavior and associated changes to the striatal dopamine system. We found that male and female Gpr88Cre/Cre mice displayed increased motivation for a palatable reward, which was maintained following reward devaluation and not driven by the metabolic phenotype previously reported in Gpr88−/− mice. Interestingly, we found that Gpr88 deletion affected cost/benefit decision making in a sex-dependent manner, whereby male, but not female, Gpr88Cre/Cre mice displayed a high-effort bias. Given that the observed behavioral phenotypes are sensitive to manipulation by dopaminergic drugs, we hypothesized that changes in motivation might be driven by underlying changes to striatal dopamine, but somewhat surprisingly found no gross alterations. Taken together, this work delineates the effect of Gpr88 deletion on motivation and reward-related pathways but highlights that the disruption of these behaviors occurs independently of major perturbations to striatal dopamine at a gene, protein, or functional level.
Dopamine signaling in the striatum is strongly implicated in reward- and motivation-related pathways. In PR tasks, both D1 and D2 antagonists reduce breakpoint, while inhibiting dopamine reuptake or increasing dopamine release increases breakpoint (20, 21, 22). Despite the clear effect of Gpr88 deletion on increasing the breakpoint in the PR task, we did not identify any changes to striatal dopamine at a gene, protein, or functional level. Notwithstanding, there is some evidence to suggest that GPR88 deletion may indirectly potentiate dopamine signaling. First, Gpr88 deletion increases excitability of both D1 and D2 GABAergic (gamma-aminobutyric acidergic) MSNs, which account for approximately 95% of the striatal neuronal population (13). MSNs form reciprocal connections with midbrain dopaminergic neurons and project via the direct D1-expressing striatonigral pathway or the indirect D2-expressing striatopallidal pathway (4,23). These pathways are embedded in broader corticostriatal-thalamo-cortical loops that regulate a range of behaviors, including motivation (24,25). While activity of dopaminergic projections to the striatum may remain unchanged in Gpr88 knockout mice, supported by normal dopamine synthesis capacity (Figure 6B) and postsynaptic dopamine receptor function (Figure 6C), it is possible that increased excitability of MSNs leads to downstream potentiation of dopamine signaling.
Second, we confirmed previous reports that Gpr88 deletion downregulates striatal expression of RGS4 and found that this was specific to the dorsal striatum (Figure 4A) (13). RGS proteins are critical regulators of G protein–coupled receptor function, effectively switching off signaling following receptor activation (26). RGS4 acts at Gi-coupled receptors (27), suggesting that Gpr88 deletion may selectively increase the magnitude and duration of D2 receptor signaling, therefore leading to an imbalance in D1 versus D2 signaling pathways (28). It should be noted that RGS4 is expected to have minimal effects on D2/3 GTPγ[35S] binding due to the use of membrane preparations. Given that both D1 and D2 receptor antagonists reduce breakpoint in PR tasks, it is likely that a balance in D1/D2 receptor signaling, rather than signaling at either receptor per se, is critical for appropriate motivational control (20). This balance in D1/D2 receptor signaling is not exclusively important for regulating motivation but has been shown for a number of behaviors including working memory and locomotor activity (29,30). Indeed, Gpr88 knockout animals show increased sensitivity to the locomotor-inducing effects of amphetamine, suggesting that an imbalance of striatal dopamine D1/D2 receptor signaling may be at play (31).
Although our results show that Gpr88 deletion may indirectly potentiate dopamine signaling to regulate motivational control, a number of caveats follow. First, it is not possible to rule out phasic, state-dependent alterations in striatal dopamine release, which is important for encoding aspects of reward value (32) and was not able to be captured by the functional measures used in this study. However, given that GPR88 is not expressed in dopaminergic neurons, it is perhaps more likely that MSNs represent the main locus of dysfunction in Gpr88 knockout mice. While we did not investigate postsynaptic D1 function in Gpr88 knockout mice, our findings of decreased RGS4 expression and studies of conditional Gpr88 deletion in D1-MSNs or D2-MSNs (33) suggest that the greatest effect is in D2-MSNs. It should also be noted that GPR88 is expressed, albeit to a lesser degree, in extrastriatal regions such as the amygdala, thalamus, and hypothalamus (34); therefore, disruptions involving these regions may also contribute to the motivational phenotype of Gpr88 knockout mice. Finally, there may be an additional layer of neurodevelopmental effects in the constitutive Gpr88 knockout that could be contributing to the behavioral phenotypes observed.
While the PR task provides a basic measure of reward valuation, the effort-related choice task provides better insight into cost/benefit decision making given the competing choice between a low effort/low reward or a high effort/high reward. In this task, male, but not female, Gpr88Cre/Cre mice had a higher breakpoint than WT mice, indicating a high-effort/high-reward bias (Figure 2A). Increased synaptic dopamine and antagonism of the adenosine A2A receptor have been shown to shift preference toward the high-effort reward (22,35,36). Interestingly, these pharmacological manipulations simultaneously decrease intake of the low-effort reward, whereas male Gpr88Cre/Cre mice consumed the same amount of chow as WT animals (Figure 2B). This shows that both male and female Gpr88 knockout mice engaged with the low-effort/low-reward choice at the same level, while preference for the high effort/high reward was selectively disrupted in male Gpr88 knockout mice. The mechanisms underlying this sex-dependent impairment of cost/benefit decision making in Gpr88Cre/Cre mice is largely unclear but may be related to the metabolic phenotype of Gpr88 knockout mice, with which males display a more pronounced phenotype (16).
An important consideration in our work and related approaches is the fact that we used free-operant behavioral paradigms of reinforcement learning that involve food restriction, which affects motivation in its own right (Figure 2A). In the case of Gpr88 knockout mice, the interplay between motivation and energy requirements is of particular interest because GPR88 is expressed in the hypothalamus and has an established role in feeding, body composition, and energy expenditure (16). Lau et al. (16) reported that Gpr88 knockout mice had reduced spontaneous food intake and energy expenditure compared with WT mice without changes to body weight gain or physical activity. Surprisingly, we observed no significant differences in food intake between Gpr88Cre/Cre and WT mice (Figures 2B and 3B) but found that female, but not male, Gpr88Cre/Cre mice had a consistently lower body weight than WT mice both before and during behavioral procedures (Figure S5). Together with reports of reduced body weight in male Gpr88 knockout mice, this suggests that genotype effects on weight may be sensitive to environmental factors (37). Indeed, Gpr88 deletion reportedly increases fasting-induced food intake under a high-fat but not a normal chow diet, highlighting a complex role of GPR88 in the maintenance of energy homeostasis. We found that the increased motivation in Gpr88 knockout mice occurred independently of food intake; however, it is unclear exactly how the combination of food restriction and strawberry milk reinforcement interacts with energy homeostasis in Gpr88 knockout mice and how it may influence appetitive motivation.
Collectively, we found that GPR88 regulates motivational control of behavior but that disruption of these behaviors following Gpr88 deletion occurs independently of gross perturbations to striatal dopamine at a gene, protein, or functional level. Our study provides further insights for targeting GPR88 to address motivational and mood symptoms in neuropsychiatric disorders. While only speculative at this stage, our findings suggest that a GPR88-specific antagonist may alleviate mood symptoms without the side effects associated with overt manipulation of dopaminergic pathways.
Acknowledgments and Disclosures
This work was partially funded by Les Laboratoires Servier (to PR, RAdlFG, CMlC, ML, GDS, CJL) and supported by National Health and Medical Research Council Project (Grant No. 1104371 [to CJL and JN]) and an Australian Research Council Future Fellowship (Grant No. 140101327 [to JN]).
We thank the scientific and technical assistance of the National Imaging Facility, a National Collaborative Research Infrastructure Strategy capability; Monash Biomedical Imaging, Monash University; Ms. Yao Lu, Monash Institute of Pharmaceutical Sciences, Monash University; and Dr. Robyn M. Brown, Florey Institute of Neuroscience and Mental Health.
The mutant animals were produced via CRISPR genome editing by the Monash Genome Modification Platform, Monash University, as a node of Phenomics Australia. Phenomics Australia is supported by the Australian Government Department of Education through the National Collaborative Research Infrastructure Strategy, the Super Science Initiative, and the Collaborative Research Infrastructure Scheme.
CMlC is a full-time employee of Les Laboratoires Servier. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
PR is currently affiliated with CUREator, Brandon Capital, Melbourne, Australia.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2022.10.008.
Contributor Information
Gregory D. Stewart, Email: gregory.stewart@monash.edu.
Jess Nithianantharajah, Email: jess.n@florey.edu.au.
Supplementary Material
References
- 1.Fervaha G., Foussias G., Agid O., Remington G. Motivational deficits in early schizophrenia: Prevalent, persistent, and key determinants of functional outcome. Schizophr Res. 2015;166:9–16. doi: 10.1016/j.schres.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 2.Fervaha G., Foussias G., Takeuchi H., Agid O., Remington G. Motivational deficits in major depressive disorder: Cross-sectional and longitudinal relationships with functional impairment and subjective well-being. Compr Psychiatry. 2016;66:31–38. doi: 10.1016/j.comppsych.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Koob G.F., Everitt B.J., Robbins T.W. In: Fundamental Neuroscience. 4th ed. Larry R.S., Darwin B., Floyd E.B., Sascha du L., Anirvan G., Nicholas C.S., editors. Academic Press; New York: 2013. Reward, motivation, and addiction; pp. 871–898. [Google Scholar]
- 4.Haber S.N. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016;18:7–21. doi: 10.31887/DCNS.2016.18.1/shaber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox J., Witten I.B. Striatal circuits for reward learning and decision-making. Nat Rev Neurosci. 2019;20:482–494. doi: 10.1038/s41583-019-0189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massart R., Guilloux J.P., Mignon V., Sokoloff P., Diaz J. Striatal GPR88 expression is confined to the whole projection neuron population and is regulated by dopaminergic and glutamatergic afferents. Eur J Neurosci. 2009;30:397–414. doi: 10.1111/j.1460-9568.2009.06842.x. [DOI] [PubMed] [Google Scholar]
- 7.Le Merrer J.L., Befort K., Gardon O., Filliol D., Darcq E., Dembele D., et al. Protracted abstinence from distinct drugs of abuse shows regulation of a common gene network. Addict Biol. 2012;17:1–12. doi: 10.1111/j.1369-1600.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- 8.Walker L.C., Berizzi A.E., Chen N.A., Rueda P., Perreau V.M., Huckstep K., et al. Acetylcholine muscarinic M4 receptors as a therapeutic target for alcohol use disorder: Converging evidence from humans and rodents. Biol Psychiatry. 2020;88:898–909. doi: 10.1016/j.biopsych.2020.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben Hamida S., Mendonça-Netto S., Arefin T.M., Nasseef M.T., Boulos L.J., McNicholas M., et al. Increased alcohol seeking in mice lacking Gpr88 involves dysfunctional mesocorticolimbic networks. Biol Psychiatry. 2018;84:202–212. doi: 10.1016/j.biopsych.2018.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomson D.M., Openshaw R.L., Mitchell E.J., Kouskou M., Millan M.J., la Mannoury la Cour C.M., et al. Impaired working memory, cognitive flexibility and reward processing in mice genetically lacking Gpr88: Evidence for a key role for Gpr88 in multiple cortico-striatal-thalamic circuits. Genes Brain Behav. 2021;20 doi: 10.1111/gbb.12710. [DOI] [PubMed] [Google Scholar]
- 11.Phillips B.U., Lopez-Cruz L., Hailwood J., Heath C.J., Saksida L.M., Bussey T.J. Translational approaches to evaluating motivation in laboratory rodents: Conventional and touchscreen-based procedures. Curr Opin Behav Sci. 2018;22:21–27. [Google Scholar]
- 12.Heath C.J., O’Callaghan C., Mason S.L., Phillips B.U., Saksida L.M., Robbins T.W., et al. A touchscreen motivation assessment evaluated in Huntington’s disease patients and R6/1 model mice. Front Neurol. 2019;10:858. doi: 10.3389/fneur.2019.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quintana A., Sanz E., Wang W., Storey G.P., Güler A.D., Wanat M.J., et al. Lack of GPR88 enhances medium spiny neuron activity and alters motor- and cue-dependent behaviors. Nat Neurosci. 2012;15:1547–1555. doi: 10.1038/nn.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heath C.J., Bussey T.J., Saksida L.M. Motivational assessment of mice using the touchscreen operant testing system: Effects of dopaminergic drugs. Psychopharmacol (Berl) 2015;232:4043–4057. doi: 10.1007/s00213-015-4009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horner A.E., Heath C.J., Hvoslef-Eide M., Kent B.A., Kim C.H., Nilsson S.R.O., et al. The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc. 2013;8:1961–1984. doi: 10.1038/nprot.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau J., Farzi A., Enriquez R.F., Shi Y.C., Herzog H. GPR88 is a critical regulator of feeding and body composition in mice. Sci Rep. 2017;7:9912. doi: 10.1038/s41598-017-10058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold J.M., Strauss G.P., Waltz J.A., Robinson B.M., Brown J.K., Frank M.J. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 2013;74:130–136. doi: 10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holden J.E., Doudet D., Endres C.J., Chan G.L., Morrison K.S., Vingerhoets F.J.G., et al. Graphical analysis of 6-fluoro-L-dopa trapping: Effect of inhibition of catechol-O-methyltransferase. J Nucl Med. 1997;38:1568–1574. [PubMed] [Google Scholar]
- 19.Bonsall D.R., Kokkinou M., Veronese M., Coello C., Wells L.A., Howes O.D. Single cocaine exposure does not alter striatal pre-synaptic dopamine function in mice: An [18 F]-FDOPA PET study. J Neurochem. 2017;143:551–560. doi: 10.1111/jnc.14223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aberman J.E., Ward S.J., Salamone J.D. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav. 1998;61:341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- 21.Covelo I.R., Wirtshafter D., Stratford T.R. GABA(A) and dopamine receptors in the nucleus accumbens shell differentially influence performance of a water-reinforced progressive ratio task. Pharmacol Biochem Behav. 2012;101:57–61. doi: 10.1016/j.pbb.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sommer S., Danysz W., Russ H., Valastro B., Flik G., Hauber W. The dopamine reuptake inhibitor MRZ-9547 increases progressive ratio responding in rats. Int J Neuropsychopharmacol. 2014;17:2045–2056. doi: 10.1017/S1461145714000996. [DOI] [PubMed] [Google Scholar]
- 23.Calabresi P., Picconi B., Tozzi A., Ghiglieri V., Di Filippo M. Direct and indirect pathways of basal ganglia: A critical reappraisal. Nat Neurosci. 2014;17:1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- 24.Ikemoto S., Yang C., Tan A. Basal ganglia circuit loops, dopamine and motivation: A review and enquiry. Behav Brain Res. 2015;290:17–31. doi: 10.1016/j.bbr.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H., de Jong J.W., Tak Y., Peck J., Bateup H.S., Lammel S. Nucleus accumbens subnuclei regulate motivated behavior via direct inhibition and disinhibition of VTA dopamine subpopulations. Neuron. 2018;97:434–449.e4. doi: 10.1016/j.neuron.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Vries L., Zheng B., Fischer T., Elenko E., Farquhar M.G. The regulator of G protein signaling family. Annu Rev Pharmacol Toxicol. 2000;40:235–271. doi: 10.1146/annurev.pharmtox.40.1.235. [DOI] [PubMed] [Google Scholar]
- 27.Berman D.M., Wilkie T.M., Gilman A.G. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein α subunits. Cell. 1996;86:445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 28.Taymans J.M., Leysen J.E., Langlois X. Striatal gene expression of RGS2 and RGS4 is specifically mediated by dopamine D1 and D2 receptors: Clues for RGS2 and RGS4 functions. J Neurochem. 2003;84:1118–1127. doi: 10.1046/j.1471-4159.2003.01610.x. [DOI] [PubMed] [Google Scholar]
- 29.Cools R., D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung E.Y., Shim I. Differential DAergic control of D1 and D2 receptor agonist over locomotor activity and GABA level in the striatum. Exp Neurobiol. 2011;20:153–157. doi: 10.5607/en.2011.20.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logue S.F., Grauer S.M., Paulsen J., Graf R., Taylor N., Sung M.A., et al. The orphan, GPR88, modulates function of the striatal dopamine system: A possible therapeutic target for psychiatric disorders? Mol Cell Neurosci. 2009;42:438–447. doi: 10.1016/j.mcn.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Bromberg-Martin E.S., Matsumoto M., Hikosaka O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meirsman A.C., Ben Hamida S., Clarke E., de Kerchove d’Exaerde A., Darcq E., Kieffer B.L. GPR88 in D1R-type and D2R-type medium spiny neurons differentially regulates affective and motor behavior [published online Aug 8] eNeuro. 2019 doi: 10.1523/ENEURO.0035-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrlich A.T., Semache M., Bailly J., Wojcik S., Arefin T.M., Colley C., et al. Mapping GPR88-Venus illuminates a novel role for GPR88 in sensory processing. Brain Struct Funct. 2018;223:1275–1296. doi: 10.1007/s00429-017-1547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Randall P.A., Pardo M., Nunes E.J., López Cruz L.L., Vemuri V.K., Makriyannis A., et al. Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: Pharmacological studies and the role of individual differences. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yohn S.E., Lopez-Cruz L., Hutson P.H., Correa M., Salamone J.D. Effects of lisdexamfetamine and s-citalopram, alone and in combination, on effort-related choice behavior in the rat. Psychopharmacol (Berl) 2016;233:949–960. doi: 10.1007/s00213-015-4176-7. [DOI] [PubMed] [Google Scholar]
- 37.Rainwater A., Sanz E., Palmiter R.D., Quintana A. Striatal GPR88 modulates foraging efficiency. J Neurosci. 2017;37:7939–7947. doi: 10.1523/JNEUROSCI.2439-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.