Abstract
Background
It has been well established that socioeconomic status is associated with mental and physical health as well as brain development, with emerging data suggesting that these relationships begin in utero. However, less is known about how prenatal socioeconomic environments interact with the gestational environment to affect neonatal brain volume.
Methods
Maternal cortisol output measured at each trimester of pregnancy and neonatal brain structure were assessed in 241 mother-infant dyads. We examined associations between the trajectory of maternal cortisol output across pregnancy and volumes of cortisol receptor–rich regions of the brain, including the amygdala, hippocampus, medial prefrontal cortex, and caudate. Given the known effects of poverty on infant brain structure, socioeconomic disadvantage was included as a moderating variable.
Results
Neonatal amygdala volume was predicted by an interaction between maternal cortisol output across pregnancy and socioeconomic disadvantage (standardized β = −0.31, p < .001), controlling for postmenstrual age at scan, infant sex, and total gray matter volume. Notably, amygdala volumes were positively associated with maternal cortisol for infants with maternal disadvantage scores 1 standard deviation below the mean (i.e., less disadvantage) (simple slope = 123.36, p < .01), while the association was negative in infants with maternal disadvantage 1 standard deviation above the mean (i.e., more disadvantage) (simple slope = −82.70, p = .02). Individuals with disadvantage scores at the mean showed no association, and there were no significant interactions in the other brain regions examined.
Conclusions
These data suggest that fetal development of the amygdala is differentially affected by maternal cortisol production at varying levels of socioeconomic advantage.
Keywords: Amygdala, Cortisol, Development, Infancy, Neonatal MRI, Socioeconomic status
The developing brain is shaped by environmental factors in response to current environments and in preparation for expected future environments. In some contexts, such as poverty, adaptations to adverse early environmental factors may lead to increased susceptibility for psychopathology later in life (1,2), though alternative empirical and theoretical frameworks suggest that early adversity can promote adaptability later in life (e.g., hidden talents) (3). Each of these perspectives, however, is consistent with a robust literature linking early environmental experiences with brain development, including alterations in brain structure and associated behavior (4, 5, 6). For example, experiencing poverty in childhood has been associated with differences in brain and behavioral development. Higher family socioeconomic status (SES) has been positively associated with greater hippocampal volume in several investigations (7, 8, 9, 10). Furthermore, greater family income and parental education in a large sample of youths 3 to 20 years of age were positively associated with differences in surface area across many regions of the cortex, including regions in the frontal, temporal, and occipital lobes (11). Emphasizing the profound impact of having fundamental needs met during neurodevelopment, even small differences in family income among low-income youths had large impacts on brain structure, while correspondingly small differences in family income among high-income youths yielded only small changes in cortical surface area (11). These results emphasize the importance of including socioeconomic information when investigating environmental or physiological effects on brain development.
Despite limited research to date, associations between the prenatal maternal socioeconomic environment and infant neurodevelopment have been reported. In infants imaged shortly after birth, lower income and maternal education were associated with smaller total gray, cortical gray, and deep gray matter volumes (12,13). Similarly, family income has been shown to positively relate to rates of infant brain growth (14), and higher maternal education levels were associated with greater total gray and white matter volumes in neonates (15). The literature is not entirely consistent, however, because one small study of 37 infants has reported both larger and smaller brain volumes in occipital, temporal, and frontal cortical regions associated with low income, suggesting that there may be regional specificity in the impact of poverty on brain structure (16).
Low income has also been associated with cortisol production, one factor that may shape the developing brain in utero. While cortisol concentrations normatively increase across pregnancy (17,18), the prenatal maternal psychosocial environment may further affect cortisol concentrations during pregnancy. While specific data regarding the effects of lower SES on cortisol production in pregnancy are limited, studies have reported associations with higher levels of evening cortisol, higher levels of cortisol in hair, and greater glucocorticoid concentrations in amniotic fluid during pregnancy (19, 20, 21). Maternal material deprivation during pregnancy has further been associated with infant cortisol reactivity early in life (20). SES and cortisol have also been shown to be associated outside the context of pregnancy (22, 23, 24). There is, however, substantial diversity in the methods and results of the empirical literature assessing the influence of SES on cortisol production (25). Despite this, prior reports of an association between SES and cortisol production raise the possibility that stressors associated with low SES, or high socioeconomic disadvantage, might affect the relationship between maternal prenatal cortisol production and infant brain development, with important implications for subsequent postnatal development.
For example, the prenatal environment provides the fetus information about the maternal environment, promoting the development of biological systems that will be well adapted to the postnatal environment (26,27). Such prenatal adaptation in preparation for postnatal life makes it plausible that brain regions associated with identifying potential environmental threats, such as the amygdala, would be affected by stress-related gestational factors. Supporting this prior theoretical work, infant amygdala connectivity, microstructure, and volume have been negatively associated with maternal distress during pregnancy (28, 29, 30, 31). Notably, maternal distress was only associated with amygdala volumes in males (28). Other empirical investigations have specifically explored the effects of prenatal cortisol on offspring brain structure. In one study, maternal cortisol during pregnancy was positively associated with cortical thickness in primarily frontal brain regions during mid childhood (32). Furthermore, maternal cortisol at 15 weeks’ gestation was associated with larger right amygdala volumes in female, but not male, children (33). In these studies, increased cortisol exposure during pregnancy was associated with larger brain volumes and greater cortical thickness, consistent with the role of glucocorticoids in the maturation of the fetus late in gestation. However, this research did not specifically investigate the role of the maternal socioeconomic environment and maternal glucocorticoid production during pregnancy as interacting factors influencing offspring brain development. It is not yet established how cortisol exposure during pregnancy is associated with brain volumes in the offspring of mothers experiencing varying degrees of socioeconomic disadvantage. Furthermore, the neuroimaging data were collected in offspring later in childhood, making it difficult to eliminate the possibility that postnatal influences contributed to the associations between maternal cortisol production and the child’s subsequent brain development.
To address this gap, the present study assessed the associations between maternal prenatal cortisol, SES, and brain structure in a large sample with neuroimaging at birth. In this cohort, maternal socioeconomic disadvantage was negatively associated with total infant brain volumes at birth, emphasizing the important role of the prenatal maternal environment on infant outcomes (12). Here, we aimed to identify additional interactive associations between the prenatal maternal socioeconomic environment, maternal cortisol production, and the neonatal macrostructure of regions of the brain particularly rich in glucocorticoid receptors (34) to begin to address potential stress-related factors associated with this effect. We examined the interaction of maternal socioeconomic disadvantage and maternal prenatal cortisol as a predictor of medial prefrontal cortex, amygdala, hippocampus, and caudate volumes, controlling for characteristics known to influence neonatal brain structure. Maternal prenatal cortisol was summarized using the area under the curve with respect to ground (AUCg) (35) and investigated across the gestational period using individual maternal cortisol slopes generated by multilevel models. Building on extant literature (12,14), we hypothesized that maternal SES would moderate the association between prenatal maternal cortisol across pregnancy and subcortical brain volumes.
Methods and Materials
Participants
The study sample included 241 mother-infant dyads drawn from a larger longitudinal observational study that recruited a cohort of pregnant women planning to give birth at Barnes-Jewish Hospital in St. Louis, Missouri. All the included infants were born from 2017 to 2020 and were full-term and healthy at the time of birth (36,37) (see the Supplement for exclusion criteria). Brain imaging data were collected in the neonatal period, no later than the sixth week of life. All procedures were approved by the Human Protection Office; informed consent was obtained for each participant, and parental informed consent was obtained for each infant before participation. Table 1 includes detailed characteristics of the sample.
Table 1.
Demographic Characteristics of Participating Mothers and Infants
| Characteristic | Overall, N = 241 |
|---|---|
| Infant Sex, n (%) | |
| Female | 113 (46.9%) |
| Male | 128 (53.1%) |
| Infant Postmenstrual Age at Scan, Weeks | |
| Mean (SD) | 41.3 (1.26) |
| Median [Minimum, Maximum] | 41.0 [38.0, 45.0] |
| Infant Birth Weight, g | |
| Mean (SD) | 3250 (488) |
| Median [Minimum, Maximum] | 3180 [2270, 4610] |
| Race/Ethnicity, n (%) | |
| American Indian/Alaskan Native | 0 (0%) |
| Asian | 4 (1.7%) |
| Black | 139 (57.7%) |
| Native Hawaiian/Pacific Islander | 0 (0%) |
| Other | 5 (2.1%) |
| White | 93 (38.6%) |
| Unknown | 0 (0%) |
| First Trimester Maternal Income-to-Needs | |
| Mean (SD) | 3.08 (3.09) |
| Median [Minimum, Maximum] | 1.46 [0.430, 12.2] |
| Missing, n (%) | 2 (0.8%) |
| Socioeconomic Disadvantage Factor Score | |
| Mean (SD) | −0.124 (0.983) |
| Median [Minimum, Maximum] | 0.268 [−2.15, 1.47] |
| Psychosocial Stress Factor Score | |
| Mean (SD) | −0.142 (0.890) |
| Median [Minimum, Maximum] | −0.330 [−1.68, 3.66] |
| Health Insurance Type, n (%) | |
| Medicaid | 78 (32.4%) |
| Medicare | 6 (2.5%) |
| Individual or Group | 132 (54.8%) |
| Uninsured | 25 (10.4%) |
| Maternal Age at Delivery, Years | |
| Mean (SD) | 29.2 (5.32) |
| Median [Minimum, Maximum] | 28.8 [18.7, 41.8] |
| Average Amygdala Volume (mm3) | |
| Mean (SD) | 912 (97.8) |
| Median [Minimum, Maximum] | 912 [627, 1340] |
| Total Gray Matter Volume (mm3) | |
| Mean (SD) | 121,000 (15,000) |
| Median [Minimum, Maximum] | 120,000 [79,600, 168,000] |
Socioeconomic Disadvantage
The maternal psychosocial environment was assessed using a latent factor score from a confirmatory factor analysis that included two dimensions: maternal socioeconomic disadvantage and maternal psychosocial stress. Given our interest in the moderating effect of SES on the relationship between maternal cortisol and neonatal brain volumes, the maternal socioeconomic disadvantage score was used as a primary predictor in this study. The maternal psychosocial stress score was used in specificity analyses only (see Data Analysis Strategy below). The socioeconomic disadvantage score included information on participants’ income-to-needs ratio, neighborhood deprivation, insurance status, education status, and nutrition. Psychosocial stress scores included information about maternal depression, experiences of discrimination, life stress, and perceived stress during pregnancy. For more information on the generation of the factor scores, see reference (36) and the Supplement.
Tobacco and Cannabis Use During Pregnancy
Maternal tobacco use during pregnancy was assessed via self-report surveys completed across pregnancy visits, while maternal cannabis use was determined using a combination of drug screening at enrollment and self-reported surveys. Both were dichotomized into 0 (no tobacco/cannabis use during pregnancy) or 1 (tobacco or cannabis use during pregnancy).
Cortisol Sampling, Processing, and Data Preparation
Participants provided salivary samples using swabs and Salivette tubes (SAR-511534500; Sarstedt) every 4 hours for 24 hours in each trimester, starting at 6:00 pm. The samples were labeled with the date and time of sample collection, sealed, and stored in the participants’ home freezers prior to being delivered to study staff. The samples were then processed for analysis of cortisol concentrations by enzyme-linked immunosorbent assay (Salimetrics melatonin enzyme immunoassay kit and Salimetrics cortisol enzyme-linked immunosorbent assay kits) at Washington University School of Medicine. Raw cortisol data were hand-cleaned to ensure accuracy, and outliers were winsorized to the highest value <5 standard deviations from the mean (18.47 ng/mL) if they fell outside this cutoff. This winsorization was performed because 25 individual values fell outside the range of physiological plausibility. The AUCg was then calculated as a measure of total cortisol production over the 24-hour saliva collection period in each trimester (35). Descriptive statistics for cortisol AUCg by trimester can be found in Table 2. Additional information about cortisol collection, missingness, and analysis methods can be found in the Supplement.
Table 2.
Descriptive Statistics for Cortisol AUCg (ng/mL) in Each Trimester (T1–T3)
| Overall, N = 241 | |
|---|---|
| T1 Cortisol AUCg | |
| Mean (SD) | 3310 (1720) |
| Median [Minimum, Maximum] | 2900 [855, 12,200] |
| Missing, n (%) | 103 (42.7%) |
| T2 Cortisol AUCg | |
| Mean (SD) | 4050 (2400) |
| Median [Minimum, Maximum] | 3380 [1130, 15,400] |
| Missing, n (%) | 33 (13.7%) |
| T3 Cortisol AUCg | |
| Mean (SD) | 4950 (3200) |
| Median [Minimum, Maximum] | 4190 [463, 24,500] |
| Missing, n (%) | 24 (10.0%) |
AUCg, area under the curve with respect to ground; T, trimester.
Structural Image Acquisition and Processing
Infant imaging was performed using a Siemens 3T Prisma scanner with a 64-channel head coil, without sedation, while infants rested quietly or slept. Magnetic resonance imaging sequence parameters and our standardized preprocessing and segmentation pipeline have been described in detail previously (12). Briefly, low motion, preprocessed T2-weighted images were input into the Melbourne Children’s Regional Infant Brain atlas Surface toolkit (38,39). This automated toolkit generated spatially normalized (within group and atlas-specific) segmentations of the white and gray matter, cerebellum, brainstem, and subcortical gray matter structures and cortical surface parcellations converted to FreeSurfer-like labeling. A highly experienced team of 2 imaging scientists (DA and DM) and a pediatric neurologist (CDS) inspected and, when necessary, manually corrected all segmentations and surfaces. Amygdala, hippocampus, caudate, and medial prefrontal cortex volumetric measures were used for further analysis.
Data Analysis Strategy
To assess the impact of maternal cortisol production across pregnancy on neonatal brain volumes, we fit a multilevel regression model that included log-transformed cortisol AUCg as the dependent variable and gestational weeks at time of collection as the independent variable. Random intercepts and slopes were included because the model with random intercepts and slopes fit better than the model with only the random intercept included (χ22 = 6.74, p = .03) and indicated an effect of gestational weeks on cortisol production (Table S5). Participant-level intercept and slope coefficients were then extracted from the model results and submitted for further analysis. The final outcome models were run as linear regression models with neonatal brain regions of interest as the dependent variables, and cortisol intercept or slope across pregnancy, the socioeconomic disadvantage factor score, and their interaction as independent predictors. Covariates included infant postmenstrual age at magnetic resonance imaging scan (which combines infant gestational age at birth and chronological age at scan), sex, birth weight, and total gray matter. Additional models using gestational age in place of birth weight and excluding participants with winsorized cortisol values were also included as a robustness check for regions showing significant results. Brain regions of interest were averaged across hemispheres for each structure. All participants with two or more batches of saliva sampling were included in these models, resulting in a final analysis sample of 222 mother-infant dyads (Table S2). We also tested the specificity of the associations using the maternal psychosocial stress factor as an alternative predictor to cortisol production, assessed the possibility of sex differences because of previous research reporting female-specific effects [e.g., (33)], and evaluated hemisphere-specific effects [e.g., (33,40)] in each region of interest. Table 3 presents the demographic and behavioral variables used and their intercorrelations.
Table 3.
Mean, Standard Deviation, and Intercorrelation of Primary Study Variables and Covariates
| Variable | Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Infant Birth Weight, g | 3251.78 | 487.48 | – | – | – | – | – | – | – | – | – | – |
| 2 Infant Postmenstrual Age at Scan, wk | 41.31 | 1.26 | 0.20a (0.07 to 0.32) | – | – | – | – | – | – | – | – | – |
| 3 Maternal INR | 3.13 | 3.10 | 0.35a (0.23 to 0.46) | 0.20a (0.07 to 0.32) | – | – | – | – | – | – | – | – |
| 4 Maternal Age, y | 29.29 | 5.23 | 0.16b (0.02 to 0.28) | −0.00 (−0.14 to 0.13) | 0.46a (0.35 to 0.56) | – | – | – | – | – | – | – |
| 5 Disadvantage Factor Score | −0.14 | 0.98 | −0.38a (−0.49 to −0.26) | −0.21a (−0.33 to −0.08) | −0.91a (−0.93 to −0.88) | −0.49a (−0.58 to −0.38) | – | – | – | – | – | – |
| 6 Psychosocial Stress Factor Score | −0.17 | 0.85 | −0.14b (−0.26 to −0.01) | −0.13 (−0.26 to 0.00) | −0.36a (−0.47 to −0.24) | −0.26a (−0.38 to −0.13) | 0.41a (0.29 to 0.51) | – | – | – | – | – |
| 7 Amygdala Volume, mm3 | 910.75 | 99.79 | 0.35a (0.23 to 0.46) | 0.45a (0.34 to 0.55) | 0.33a (0.21 to 0.44) | 0.09 (−0.05 to 0.21) | −0.37a (−0.47 to −0.25) | −0.18a (−0.30 to −0.05) | – | – | – | – |
| 8 Total Gray Matter Volume, mm3 | 120,769.26 | 15,202.87 | 0.46a (0.35 to 0.56) | 0.58a (0.48 to 0.66) | 0.34a (0.21 to 0.45) | 0.10 (−0.04 to 0.22) | −0.34a (−0.45 to −0.22) | −0.21a (−0.33 to −0.08) | 0.82a (0.78 to 0.86) | – | – | – |
| 9 T1 Cortisol AUCg | 3292.04 | 1714.67 | −0.09 (−0.25 to 0.08) | −0.02 (−0.19 to 0.15) | −0.06 (−0.23 to 0.11) | −0.15 (−0.31 to 0.02) | 0.13 (−0.04 to 0.29) | 0.10 (−0.07 to 0.26) | −0.05 (−0.22 to 0.12) | −0.03 (−0.20 to 0.14) | – | – |
| 10 T2 Cortisol AUCg | 4093.92 | 2429.84 | −0.02 (−0.16 to 0.12) | 0.13 (−0.01 to 0.26) | −0.15b (−0.28 to −0.01) | −0.09 (−0.23 to 0.05) | 0.17b (0.04 to 0.31) | −0.04 (−0.18 to 0.10) | −0.03 (−0.17 to 0.11) | −0.03 (−0.17 to 0.11) | 0.20b (0.02 to 0.37) | – |
| 11 T3 Cortisol AUCg | 4914.82 | 3208.48 | −0.02 (−0.16 to 0.11) | 0.12 (−0.02 to 0.25) | −0.08 (−0.21 to 0.06) | −0.18b (−0.30 to −0.04) | 0.07 (−0.06 to 0.21) | −0.05 (−0.19 to 0.08) | −0.02 (−0.16 to 0.11) | 0.02 (−0.11 to 0.16) | 0.16 (−0.01 to 0.33) | 0.31a (0.18–0.44) |
Values in parentheses indicate the 95% CI for each correlation.
AUCg, area under the curve with respect to ground; INR, income-to-needs ratio; T, trimester.
p < .01.
p < .05.
Results
Cortisol Production, Socioeconomic Disadvantage, and Perceived Stress
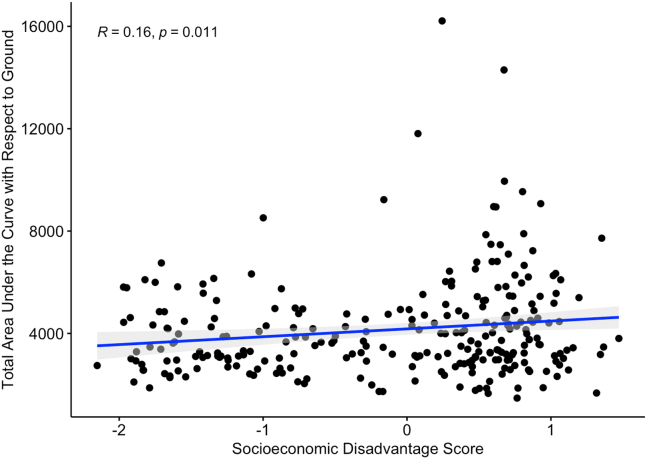
Maternal prenatal cortisol slopes were not associated with maternal socioeconomic disadvantage or psychosocial stress factor scores during pregnancy (r = 0.05, p = .40 and r = −0.04, p = .52, respectively). The maternal psychosocial stress factor during pregnancy was also not associated with total cortisol production during pregnancy (r = −0.01, p = .85). In contrast, total cortisol production during pregnancy (i.e., average AUCg across all gestational weeks) was positively associated with socioeconomic disadvantage (r = 0.16, p = .015) (Figure 1). While AUCg was used as a data reduction tool within trimesters, we chose to use the slope and intercept analytic approach, given the previously reviewed changes in cortisol secretion across pregnancy and previous literature showing timing effects.
Figure 1.
Total maternal prenatal cortisol area under the curve with respect to ground, averaged across pregnancy, as a function of socioeconomic disadvantage. Data points represent one participating mother each, and gray shading indicates the 95% confidence interval. All available data are included.
Cortisol Slope and Subcortical Brain Volumes
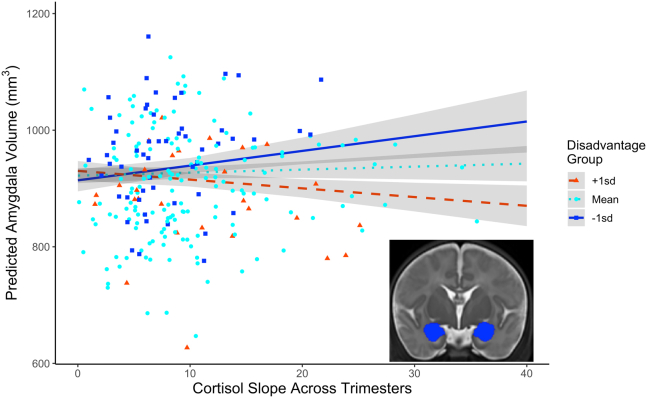
A linear regression model indicated that cortisol AUCg slope across pregnancy interacted with socioeconomic disadvantage to predict neonatal amygdala volume (false discovery rate–corrected p = .003) (Figure 2) when controlling for infant postmenstrual age at scan, sex, birth weight, maternal tobacco use, maternal cannabis use, and total gray matter volume. Complete unadjusted model results can be found in Table 4. Simple slopes decomposition indicated a significant positive relationship between cortisol AUCg slope and average neonatal amygdala volume when the socioeconomic disadvantage factor score was 1 standard deviation below the sample mean (i.e., less-disadvantaged mothers) (slope estimate = 1876.28, p < .01) and a negative relationship when the socioeconomic disadvantage score was 1 standard deviation above the sample mean (i.e., more-disadvantaged mothers) (slope estimate = −1029.68, p = .03). No association between prenatal maternal cortisol and amygdala volume was evident at mean socioeconomic disadvantage (slope estimate = 423.30, p = .27). Figure S2 shows the Johnson-Neyman plot of this interaction, including the regions of significance. This pattern of results was consistent when left and right amygdala volumes were analyzed separately, when gestational age was used as a covariate in place of child birth weight, and when participants with winsorized cortisol values were excluded (see Tables S6–S9 for model outputs). There were no significant effects in the other regions of interest (see Tables S10–S12 for model results). Models using trimester-specific cortisol AUCg instead of cortisol slope across pregnancy to predict amygdala volumes can be found in the Supplement (Tables S13–S15). Trimesters 2 and 3 showed the same pattern of results as cortisol slope.
Figure 2.
Neonatal amygdala volumes as a function of total maternal cortisol output slope across trimesters and socioeconomic disadvantage group. Lines represent model-predicted results, and points are the raw data included in the regression model. Gray shading indicates the 95% confidence interval. Outliers ± 3 SD from the mean of cortisol slope and amygdala volume have been removed for clarity; a plot including outliers can be found in Figure S3. Categorical treatment of disadvantaged group is for data visualization only. A continuous predictor was used to assess socioeconomic disadvantage in the regression model. The inset image shows a bilateral amygdala segmentation (blue) on a coronal T2 image.
Table 4.
Coefficient-Level Estimates for a Linear Regression Model Fitted to Estimate Variation in Average Amygdala Volume
| Predictor | β | 95% CI | t | p |
|---|---|---|---|---|
| Intercept | 0.01 | −0.06 to 0.08 | 3.53 | <.001 |
| Cortisol Slope | 0.04 | −0.03 to 0.12 | 0.57 | .572 |
| Disadvantage Factor | −0.14 | −0.23 to −0.05 | 1.93 | .055 |
| Infant Age at Scan | −0.03 | −0.12 to 0.06 | −0.71 | .481 |
| Infant Sex | −0.10 | −0.17 to −0.02 | −2.44 | .016 |
| Infant Birth Weight | −0.06 | −0.15 to 0.02 | −1.51 | .133 |
| Total Gray Matter Volume | 0.77 | 0.67 to 0.88 | 14.24 | <.001 |
| Maternal Tobacco Use | −0.08 | −0.16 to 0 | −2.08 | .039 |
| Maternal Cannabis Use | 0.08 | −0.01 to 0.16 | 1.81 | .071 |
| Cortisol Slope × Disadvantage Factor | −0.14 | −0.22 to −0.07 | −3.60 | <.001 |
Outcome = bilateral amygdala volume. One outlier −5 SD from the mean of cortisol area under the curve with respect to ground was removed.
Cortisol Intercept and Subcortical Brain Volumes
There were no significant effects of the cortisol AUCg intercepts extracted from the multilevel model on neonatal brain volumes (Tables S16–S19).
Specificity Analyses
To test the specificity of the interaction between prenatal maternal cortisol production and maternal disadvantage as a predictor of neonatal amygdala volume, we also tested the maternal psychosocial stress factor as an alternative predictor to cortisol production, assessed the possibility of sex differences, and evaluated hemisphere-specific effects in each region of interest. Maternal psychosocial stress did not interact with maternal socioeconomic disadvantage to predict neonatal amygdala volumes (Table S20). We also found no evidence for sex differences in the interaction between maternal cortisol production and socioeconomic disadvantage (Tables S21–S23). Finally, as observed in the models with region of interest volumes averaged across hemispheres, there were no hemisphere-specific maternal cortisol by socioeconomic interaction effects in the hippocampus, caudate, or medial prefrontal cortex (Tables S24–S29).
Discussion
In this study, we built upon previous associations of prenatal disadvantage with neonatal brain structure by further establishing the importance of prenatal maternal cortisol as a predictor of infant amygdala volumes. Our results indicate that maternal prenatal cortisol slope across trimesters interacts with socioeconomic disadvantage to predict neonatal amygdala volumes: among highly disadvantaged mothers, higher cortisol production, measured by AUCg across trimesters, was associated with smaller amygdala volumes, while among less-disadvantaged mothers, higher cortisol production was associated with larger amygdala volumes. Importantly, the sample used in this study contained a large range of income-to-needs ratios—which heavily influenced the socioeconomic disadvantage scores—further supporting the previously reported impact of family income on brain development.
Our results in the neonatal period are consistent with prior findings of higher maternal cortisol during pregnancy being related to larger amygdala volumes among children from 6 to 9 years of age and stronger amygdala functional connectivity with cortical networks in early infancy, though both effects were specific to females in the prior work (33,40). Adding to the previous research, we assessed the influence of SES on this relationship and found positive and negative relationships of prenatal maternal cortisol to amygdala volume, depending on socioeconomic disadvantage. Rising cortisol concentrations across pregnancy are a normative pattern involved in the final stages of fetal maturation (17,18). Thus, how the gestational environment affects amygdala volume in response to increasing glucocorticoid exposure may be modulated by maternal socioeconomic context. For example, improved nutrient availability and lower levels of environmental toxin exposure may promote increased amygdala volume in low socioeconomic disadvantage contexts. However, unlike prior research findings, we found that increasing maternal cortisol production across pregnancy was associated with smaller amygdala volumes in the infants of mothers with a high degree of socioeconomic disadvantage. While the reason for this is not immediately evident, the level of cortisol exposure across gestation may provide some clarity. As reviewed above, cortisol production normatively increases across pregnancy, facilitating healthy maturation of critical organ systems and the brain (17,18). In our sample, socioeconomic disadvantage is associated with greater levels of maternal cortisol production averaged across pregnancy. It may be that for highly disadvantaged mothers, the normative increase in cortisol production across pregnancy, combined with already higher levels of cortisol production, leads to greater than optimal cortisol concentrations during pregnancy, resulting in slower amygdala growth. Supporting this possibility, cortisol concentrations during trimesters 2 and 3 also interacted with socioeconomic disadvantage to predict amygdala volumes, suggesting that the level of prenatal exposure may also be an important factor in amygdala development.
There is a large body of literature that establishes a plausible mechanistic pathway by which information about the maternal prenatal environment is transmitted via glucocorticoids to the developing fetus. Despite the presence of 11β-HSD (11β-hydroxysteroid dehydrogenase type 2), a placental enzyme that provides a partial barrier between mother and fetus via conversion of cortisol to the inactive form cortisone, maternal cortisol can cross the placenta and affect glucocorticoid-rich regions like such as the amygdala (41). Previous research has also established a role for the fetal hypothalamic-pituitary-adrenal axis synthesizing glucocorticoids following stimulation of placental corticotropin-releasing hormone by maternal cortisol (18). While the potential mechanistic pathways outlining how amygdala development might be shaped by prenatal maternal cortisol production are clear, it is unclear why the effects of prenatal glucocorticoid exposure are so strong on the amygdala specifically. Consistent with theories suggesting that prenatal environments program the biological sensitivity of the offspring (42), it may be that functions served by the amygdala are especially critical to develop during the prenatal period, making the structure especially vulnerable to stress-related hormone exposure during gestation. Amygdala development being guided, in part, by prenatal stress signals related to the maternal environment may be an adaptive process that helps prepare the infant for the postnatal environment. Furthermore, animal models indicate that amygdala development outstrips hippocampal development in the perinatal period and expresses stress-responsive messenger RNA earlier than the hippocampus (43, 44, 45), providing converging evidence with our regionally specific effects. Despite this converging evidence, future research should further investigate how specific stress-related signals are to amygdala volume and assess other brain regions that may be affected.
While previous research that did not assess the effects of SES has shown increased brain volumes as a function of higher maternal cortisol during pregnancy (32,33), there are little data available on this relationship in groups experiencing socioeconomic disadvantage. In a separate but related domain, deprivation-related stressors such as institutional care have been associated with altered cortisol production and smaller amygdala volumes postnatally (46, 47, 48), though larger amygdala volumes following institutional care have also been reported (49,50). Building on this prior literature, our results suggest that the socioeconomic environment affects emotion-relevant brain structure development prenatally, emphasizing the need to develop more precision-based strategies for supporting mothers and their infants during this period.
Importantly, differences in amygdala volume and prenatal exposure to maternal cortisol have been associated with clinically relevant emotion processing later in childhood. The amygdala has long been a focus of researchers interested in the development of internalizing symptoms (51). However, the results of this research have been mixed, with both larger and smaller amygdala volumes associated with anxiety, fearfulness, and depression (51, 52, 53, 54, 55, 56, 57, 58). A number of null effects associated with subthreshold depression, diagnosed depression, and general internalizing scores have also been reported (59, 60, 61). Despite this mixed literature, the amygdala remains a structure of interest for research working to identify affective psychopathology risk during the prenatal period.
Pertinent to our results, basal maternal cortisol has been associated with infant behavioral outcomes, including negative affectivity, emotional reactivity, high distress to novelty, and subsequent childhood anxiety (62, 63, 64). Animal models support these results, showing that inhibition of 11β-HSD in pregnant rats (which increases maternal glucocorticoids crossing the placental barrier) increases anxiety-like behaviors in their offspring (65). In contrast, there is also evidence in humans that maternal cortisol production during the third trimester is linked with improved cognitive development in late childhood (32). This finding is consistent with the notion that exposure to glucocorticoids plays an important role in programming fetal brain development, particularly in regions rich in glucocorticoid receptors such as the amygdala, hippocampus, and prefrontal cortex (32,66). There are also a number of studies that have reported no associations between prenatal cortisol and offspring behavioral outcomes, suggesting alternative pathways from maternal stress to offspring behavior (67). Future research should investigate the behavioral consequences of altered amygdala volume as a function of maternal cortisol and the potential for additional physiological pathways (e.g., inflammation or maternal exposure to toxins) that may also influence subsequent infant development.
Consistent with prior research, we did not find significant effects of maternal cortisol on hippocampal volumes. Earlier work investigating postnatal exposure to maternal depression or institutional rearing had also suggested amygdala-specific structural alterations (49,50,68); however, more recent evidence is mixed and has shown postnatal associations between depriving experiences in childhood and hippocampal volume (69). Despite mixed literature on postnatal populations, research in animal models suggests that there may be reason to expect differentiation between amygdala and hippocampal volumes when exposed to prenatal maternal cortisol. The postnatal activity of the hypothalamic-pituitary-adrenal axis is programmed during gestation via changes in the density of mineralocorticoid and glucocorticoid receptors in limbic brain regions such as the hippocampus (70). It may be that these changes in receptor density make the hippocampus particularly sensitive to postnatal stress exposure, a possibility that is supported by research reporting reductions in hippocampal volume following chronic exposure to stress (71,72) and slower rates of hippocampal growth in the first 6 months of life as a function of prenatal maternal stress (73). Again, our findings largely converge with the prior literature in finding differences in amygdala, but not hippocampus, volumes as a result of prenatal cortisol exposure. Future research should seek to mechanistically establish the relative susceptibility of the amygdala and hippocampus to stress exposures in the prenatal and postnatal periods.
Despite a number of consistencies with the prior research, we did not find evidence for sex differences in the association between maternal cortisol and neonatal amygdala volume. It is possible that the significantly younger age of participants in our sample compared with that in prior research may indicate that sex differentiation occurs over postnatal development. While recent research assessing brain function and structural connectivity in neonates and children does show a sex difference in the effects of prenatal maternal cortisol (29,40,74), it is unclear whether the same applies to neonatal brain volumes. Furthermore, the previous research addressing prenatal maternal cortisol and amygdala volume did not include the role of SES as a covariate or as a focal predictor (33). It may be that the role of SES in our sample obscures the previously reported sex differences. This second possibility may be especially important. Much, if not all, of the previous research concerning the effects of prenatal cortisol has not considered the role of SES. Sex differences may exist but might only be apparent in particular regions of significance along the socioeconomic spectrum.
The strengths of this study include a sample with a wide range of socioeconomic diversity and a dataset rich in maternal characteristics, maternal cortisol production during pregnancy, and a large sample with high-quality neonatal imaging. Despite these strengths, the study is limited in ways that warrant caution in interpreting the results. Cortisol collection was not always completed during the first trimester because some women were enrolled in the study at 20 weeks’ gestation, resulting in more missing AUCg values in the first trimester than in the subsequent gestational periods. It should also be noted that participant self-report was relied upon to establish saliva sample collection times. Future research should use automated collection time devices, such as medication event monitoring system caps, to eliminate this potential source of methodological noise. Furthermore, the use of maternal cortisol slopes across pregnancy was an effective data reduction technique but lacks specificity in terms of the timing of glucocorticoid exposure. While the current literature includes findings with respect to overall maternal cortisol production and more timing-specific maternal cortisol production, more research is needed to assess the relative strengths and weaknesses of each approach. Similarly, the socioeconomic advantage and psychosocial stress scores used in these analyses effectively incorporated the rich dataset into a straightforward analysis but may obscure effects related to a single component of these scores. Future research may fruitfully distinguish the effects of different measures of socioeconomic disadvantage in ways that improve translational impact. Due to our study design, we do not have direct evidence that prenatal maternal cortisol is a mechanism linking the socioeconomic environment to neonatal brain structure. Research that more directly measures fetal glucocorticoid exposure (e.g., placental corticotropin-releasing hormone), the effects of this exposure on neonatal brain structure, and long-term behavioral outcomes would be a valuable next step toward understanding the mechanistic underpinnings of risk for psychopathology during the perinatal period. This is particularly true given the nascent state of the infant neuroimaging field because it is currently unclear what the reported neural alterations mean for long-term behavioral development or psychiatric risk. Finally, the sample included only healthy, full-term infants. This affords the opportunity to isolate the effects of prenatal maternal cortisol on neonatal brain structure to some degree but may also skew the sample to include mothers with relatively lower levels of stress during pregnancy.
In this study, we aimed to extend the previous literature establishing a role for prenatal maternal cortisol production on neonatal brain structure by investigating the moderating role of socioeconomic disadvantage. Consistent with prior research, we identified significant associations between maternal prenatal cortisol production and offspring amygdala volumes shortly after birth. Our results added to the converging literature by demonstrating that considering socioeconomic disadvantage is crucial to fully understanding the impact of maternal stress physiology on the developing fetus. Future research should investigate more granular exposures that may be associated with socioeconomic disadvantage such as exposure to environmental toxins, access to nutritious food, and maternal inflammation as potential mechanisms by which the maternal environment might directly affect the fetal brain. Furthermore, research should also probe the differential effects of maternal cortisol and maternal self-reported stress to determine whether the specificity observed in this study extends to other areas of translational interest. With this additional research, the current results will contribute to improved understanding of the prenatal environment in shaping infant neurodevelopment and emphasize the importance of considering socioeconomic disadvantage during the gestational period when developing risk screening and early intervention programs.
Acknowledgments and Disclosures
This work was supported by the National Institutes of Health (Grant No. R01 MH113883 [to JL, CDS, BBW, DMB, and CER]; Grant No. T32 MH100019 [to MPH and RT]), March of Dimes Prematurity Research Center at Washington University (to SKE, EDH, and PZ), and the Intellectual and Developmental Disabilities Research Center at Washington University (Grant No. P50 HD103525).
We thank the families, members of the March of Dimes Prematurity Research Center, the Division of Clinical Research in the Department of Obstetrics and Gynecology, the Washington University Neonatal Developmental Research Group, and Early Life Adversity and Biological Embedding study staff for their work on this study.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2023.03.002.
Supplementary Material
References
- 1.Palacios-Barrios E.E., Hanson J.L. Poverty and self-regulation: connecting psychosocial processes, neurobiology, and the risk for psychopathology. Compr Psychiatry. 2019;90:52–64. doi: 10.1016/j.comppsych.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Peverill M., Dirks M.A., Narvaja T., Herts K.L., Comer J.S., McLaughlin K.A. Socioeconomic status and child psychopathology in the United States: A meta-analysis of population-based studies. Clin Psychol Rev. 2021;83 doi: 10.1016/j.cpr.2020.101933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis B.J., Abrams L.S., Masten A.S., Sternberg R.J., Tottenham N., Frankenhuis W.E. Hidden talents in harsh environments. Dev Psychopathol. 2022;34:95–113. doi: 10.1017/S0954579420000887. [DOI] [PubMed] [Google Scholar]
- 4.Herzberg M.P., Gunnar M.R. Early life stress and brain function: Activity and connectivity associated with processing emotion and reward. Neuroimage. 2020;209 doi: 10.1016/j.neuroimage.2019.116493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miguel P.M., Pereira L.O., Silveira P.P., Meaney M.J. Early environmental influences on the development of children’s brain structure and function. Dev Med Child Neurol. 2019;61:1127–1133. doi: 10.1111/dmcn.14182. [DOI] [PubMed] [Google Scholar]
- 6.Johnson S.B., Riis J.L., Noble K.G. State of the art review: Poverty and the developing brain. Pediatrics. 2016;137 doi: 10.1542/peds.2015-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanson J.L., Chandra A., Wolfe B.L., Pollak S.D. Association between income and the hippocampus. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luby J., Belden A., Botteron K., Marrus N., Harms M.P., Babb C., et al. The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167:1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jednoróg K., Altarelli I., Monzalvo K., Fluss J., Dubois J., Billard C., et al. The influence of socioeconomic status on children’s brain structure [published correction appears in PLoS One 2012;7: PLoS One. 2012;7 doi: 10.1371/journal.pone.0042486. doi: 1371/annotation/47661de2-2c53-4396-9f88-06b5ad233566] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noble K.G., Grieve S.M., Korgaonkar M.S., Engelhardt L.E., Griffith E.Y., Williams L.M., Brickman A.M. Hippocampal volume varies with educational attainment across the life-span. Front Hum Neurosci. 2012;6:307. doi: 10.3389/fnhum.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M., et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18:773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Triplett R.L., Lean R.E., Parikh A., Miller J.P., Alexopoulos D., Kaplan S., et al. Association of prenatal exposure to early-life adversity with neonatal brain volumes at birth. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betancourt L.M., Avants B., Farah M.J., Brodsky N.L., Wu J., Ashtari M., Hurt H. Effect of socioeconomic status (SES) disparity on neural development in female African-American infants at age 1 month. Dev Sci. 2016;19:947–956. doi: 10.1111/desc.12344. [DOI] [PubMed] [Google Scholar]
- 14.Hanson J.L., Hair N., Shen D.G., Shi F., Gilmore J.H., Wolfe B.L., Pollak S.D. Family poverty affects the rate of human infant brain growth [published correction appears in PLoS One 2015;10:e0146434] PLoS One. 2013;8 doi: 10.1371/journal.pone.0080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knickmeyer R.C., Xia K., Lu Z., Ahn M., Jha S.C., Zou F., et al. Impact of demographic and obstetric factors on infant brain volumes: A population neuroscience study. Cereb Cortex. 2017;27:5616–5625. doi: 10.1093/cercor/bhw331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spann M.N., Bansal R., Hao X., Rosen T.S., Peterson B.S. Prenatal socioeconomic status and social support are associated with neonatal brain morphology, toddler language and psychiatric symptoms. Child Neuropsychol. 2020;26:170–188. doi: 10.1080/09297049.2019.1648641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin M.P., Leader L. Maternal stress and obstetric and infant outcomes: Epidemiological findings and neuroendocrine mechanisms. Aust N Z J Obstet Gynaecol. 2000;40:331–337. doi: 10.1111/j.1479-828x.2000.tb03344.x. [DOI] [PubMed] [Google Scholar]
- 18.Sandman C.A., Glynn L., Schetter C.D., Wadhwa P., Garite T., Chicz-DeMet A., Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): Priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Deuschle M., Hendlmeier F., Witt S., Rietschel M., Gilles M., Sánchez-Guijo A., et al. Cortisol, cortisone, and BDNF in amniotic fluid in the second trimester of pregnancy: Effect of early life and current maternal stress and socioeconomic status. Dev Psychopathol. 2018;30:971–980. doi: 10.1017/S0954579418000147. [DOI] [PubMed] [Google Scholar]
- 20.Thayer Z.M., Kuzawa C.W. Early origins of health disparities: Material deprivation predicts maternal evening cortisol in pregnancy and offspring cortisol reactivity in the first few weeks of life. Am J Hum Biol. 2014;26:723–730. doi: 10.1002/ajhb.22532. [DOI] [PubMed] [Google Scholar]
- 21.Bosquet Enlow M., Sideridis G., Chiu Y.M., Nentin F., Howell E.A., Le Grand B.A., Wright R.J. Associations among maternal socioeconomic status in childhood and pregnancy and hair cortisol in pregnancy. Psychoneuroendocrinology. 2019;99:216–224. doi: 10.1016/j.psyneuen.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desantis A.S., Kuzawa C.W., Adam E.K. Developmental origins of flatter cortisol rhythms: Socioeconomic status and adult cortisol activity. Am J Hum Biol. 2015;27:458–467. doi: 10.1002/ajhb.22668. [DOI] [PubMed] [Google Scholar]
- 23.Ursache A., Merz E.C., Melvin S., Meyer J., Noble K.G. Socioeconomic status, hair cortisol and internalizing symptoms in parents and children. Psychoneuroendocrinology. 2017;78:142–150. doi: 10.1016/j.psyneuen.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gustafsson P.E., Janlert U., Theorell T., Westerlund H., Hammarström A. Socioeconomic status over the life course and allostatic load in adulthood: results from the Northern Swedish Cohort. J Epidemiol Community Health. 2011;65:986–992. doi: 10.1136/jech.2010.108332. [DOI] [PubMed] [Google Scholar]
- 25.Dowd J.B., Simanek A.M., Aiello A.E. Socio-economic status, cortisol and allostatic load: A review of the literature. Int J Epidemiol. 2009;38:1297–1309. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gluckman P.D., Hanson M.A. Living with the past: Evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 27.Bateson P., Gluckman P., Hanson M. The biology of developmental plasticity and the predictive adaptive response hypothesis. J Physiol. 2014;592:2357–2368. doi: 10.1113/jphysiol.2014.271460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehtola S.J., Tuulari J.J., Scheinin N.M., Karlsson L., Parkkola R., Merisaari H., et al. Newborn amygdalar volumes are associated with maternal prenatal psychological distress in a sex-dependent way. Neuroimage Clin. 2020;28 doi: 10.1016/j.nicl.2020.102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoye D.Q., Blesa M., Sullivan G., Galdi P., Lamb G.J., Black G.S., et al. Maternal cortisol is associated with neonatal amygdala microstructure and connectivity in a sexually dimorphic manner. eLife. 2020;9 doi: 10.7554/eLife.60729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheinost D., Kwon S.H., Lacadie C., Sze G., Sinha R., Constable R.T., Ment L.R. Prenatal stress alters amygdala functional connectivity in preterm neonates. Neuroimage Clin. 2016;12:381–388. doi: 10.1016/j.nicl.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Posner J., Cha J., Roy A.K., Peterson B.S., Bansal R., Gustafsson H.C., et al. Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl Psychiatry. 2016;6:e935. doi: 10.1038/tp.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis E.P., Head K., Buss C., Sandman C.A. Prenatal maternal cortisol concentrations predict neurodevelopment in middle childhood. Psychoneuroendocrinology. 2017;75:56–63. doi: 10.1016/j.psyneuen.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buss C., Davis E.P., Shahbaba B., Pruessner J.C., Head K., Sandman C.A. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A. 2012;109:E1312–E1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joëls M., Baram T.Z. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pruessner J.C., Kirschbaum C., Meinlschmid G., Hellhammer D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 36.Luby J.L., England S.K., Barch D.M., Warner B.B., Rogers C., Smyser C.D., et al. Social disadvantage during pregnancy: Effects on gestational age and birthweight [published online Mar 13] J Perinatol. 2023 doi: 10.1038/s41372-023-01643-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stout M.J., Chubiz J., Raghuraman N., Zhao P., Tuuli M.G., Wang L.V., et al. A multidisciplinary prematurity research cohort study. medRxiv. 2021 doi: 10.1101/2021.09.28.21264264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adamson C.L., Alexander B., Ball G., Beare R., Cheong J.L.Y., Spittle A.J., et al. Parcellation of the neonatal cortex using surface-based Melbourne Children’s Regional Infant Brain atlases (M-CRIB-S) Sci Rep. 2020;10:4359. doi: 10.1038/s41598-020-61326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexander B., Murray A.L., Loh W.Y., Matthews L.G., Adamson C., Beare R., et al. A new neonatal cortical and subcortical brain atlas: The Melbourne Children’s Regional Infant Brain (M-CRIB) atlas. Neuroimage. 2017;147:841–851. doi: 10.1016/j.neuroimage.2016.09.068. [DOI] [PubMed] [Google Scholar]
- 40.Graham A.M., Rasmussen J.M., Entringer S., Ben Ward E., Rudolph M.D., Gilmore J.H., et al. Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol Psychiatry. 2019;85:172–181. doi: 10.1016/j.biopsych.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benediktsson R., Calder A.A., Edwards C.R.W., Seckl J.R. Placental 11β-hydroxysteroid dehydrogenase: A key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf) 1997;46:161–166. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- 42.Pluess M., Belsky J. Prenatal programming of postnatal plasticity? Dev Psychopathol. 2011;23:29–38. doi: 10.1017/S0954579410000623. [DOI] [PubMed] [Google Scholar]
- 43.Tottenham N., Sheridan M.A. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front Hum Neurosci. 2010;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vazquez D.M., Bailey C., Dent G.W., Okimoto D.K., Steffek A., López J.F., Levine S. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: Effect of maternal deprivation. Brain Res. 2006;1121:83–94. doi: 10.1016/j.brainres.2006.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudy J.W. Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci. 1993;107:887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- 46.Hanson J.L., Nacewicz B.M., Sutterer M.J., Cayo A.A., Schaefer S.M., Rudolph K.D., et al. Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biol Psychiatry. 2015;77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hodel A.S., Hunt R.H., Cowell R.A., Van Den Heuvel S.E., Gunnar M.R., Thomas K.M. Duration of early adversity and structural brain development in post-institutionalized adolescents. Neuroimage. 2015;105:112–119. doi: 10.1016/j.neuroimage.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.VanTieghem M., Korom M., Flannery J., Choy T., Caldera C., Humphreys K.L., et al. Longitudinal changes in amygdala, hippocampus and cortisol development following early caregiving adversity. Dev Cogn Neurosci. 2021;48 doi: 10.1016/j.dcn.2021.100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta M.A., Golembo N.I., Nosarti C., Colvert E., Mota A., Williams S.C.R., et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian adoptees study pilot. J Child Psychol Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 50.Tottenham N., Hare T.A., Quinn B.T., McCarry T.W., Nurse M., Gilhooly T., et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merz E.C., Tottenham N., Noble K.G. Socioeconomic status, amygdala volume, and internalizing symptoms in children and adolescents. J Clin Child Adolesc Psychol. 2018;47:312–323. doi: 10.1080/15374416.2017.1326122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Bellis M.D., Casey B.J., Dahl R.E., Birmaher B., Williamson D.E., Thomas K.M., et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48:51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- 53.Qin S., Young C.B., Duan X., Chen T., Supekar K., Menon V. Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol Psychiatry. 2014;75:892–900. doi: 10.1016/j.biopsych.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Plas E.A., Boes A.D., Wemmie J.A., Tranel D., Nopoulos P. Amygdala volume correlates positively with fearfulness in normal healthy girls. Soc Cogn Affect Neurosci. 2010;5:424–431. doi: 10.1093/scan/nsq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milham M.P., Nugent A.C., Drevets W.C., Dickstein D.P., Leibenluft E., Ernst M., et al. Selective reduction in amygdala volume in pediatric anxiety disorders: A voxel-based morphometry investigation. Biol Psychiatry. 2005;57:961–966. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 56.Mueller S.C., Aouidad A., Gorodetsky E., Goldman D., Pine D.S., Ernst M. Gray matter volume in adolescent anxiety: An impact of the brain-derived neurotrophic factor Val(66)met polymorphism? [published correction appears in J Am Acad Child Adolesc Psychiatry 2013;52:329] J Am Acad Child Adolesc Psychiatry. 2013;52:184–195. doi: 10.1016/j.jaac.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosso I.M., Cintron C.M., Steingard R.J., Renshaw P.F., Young A.D., Yurgelun-Todd D.A. Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry. 2005;57:21–26. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 58.Strawn J.R., Hamm L., Fitzgerald D.A., Fitzgerald K.D., Monk C.S., Phan K.L. Neurostructural abnormalities in pediatric anxiety disorders. J Anxiety Disord. 2015;32:81–88. doi: 10.1016/j.janxdis.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pannekoek J.N., Van Der Werff S.J., van den Bulk B.G., van Lang N.D.J., Rombouts S.A.R.B., Van Buchem M.A., et al. Reduced anterior cingulate gray matter volume in treatment-naïve clinically depressed adolescents. Neuroimage Clin. 2014;4:336–342. doi: 10.1016/j.nicl.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vulser H., Lemaitre H., Artiges E., Miranda R., Penttilä J., Struve M., et al. Subthreshold depression and regional brain volumes in young community adolescents. J Am Acad Child Adolesc Psychiatry. 2015;54:832–840. doi: 10.1016/j.jaac.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Koolschijn P.C.M.P., van IJzendoorn M.H., Bakermans-Kranenburg M.J., Crone E.A. Hippocampal volume and internalizing behavior problems in adolescence. Eur Neuropsychopharmacol. 2013;23:622–628. doi: 10.1016/j.euroneuro.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Rouse M.H., Goodman S.H. Perinatal depression influences on infant negative affectivity: Timing, severity, and co-morbid anxiety. Infant Behav Dev. 2014;37:739–751. doi: 10.1016/j.infbeh.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Werner E., Zhao Y., Evans L., Kinsella M., Kurzius L., Altincatal A., et al. Higher maternal prenatal cortisol and younger age predict greater infant reactivity to novelty at 4 months: an observation-based study. Dev Psychobiol. 2013;55:707–718. doi: 10.1002/dev.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis E.P., Sandman C.A. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012;37:1224–1233. doi: 10.1016/j.psyneuen.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Welberg L.A.M., Seckl J.R., Holmes M.C. Inhibition of 11beta-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 2000;12:1047–1054. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- 66.McEwen B.S., Nasca C., Gray J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van den Bergh B.R.H., van den Heuvel M.I., Lahti M., Braeken M., de Rooij S.R., Entringer S., et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci Biobehav Rev. 2020;117:26–64. doi: 10.1016/j.neubiorev.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Lupien S.J., Parent S., Evans A.C., Tremblay R.E., Zelazo P.D., Corbo V., et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci U S A. 2011;108:14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McLaughlin K.A., Weissman D., Bitrán D. Childhood adversity and neural development: A systematic review. Annu Rev Dev Psychol. 2019;1:277–312. doi: 10.1146/annurev-devpsych-121318-084950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kapoor A., Dunn E., Kostaki A., Andrews M.H., Matthews S.G. Fetal programming of hypothalamo-pituitary-adrenal function: Prenatal stress and glucocorticoids. J Physiol. 2006;572:31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vyas A., Mitra R., Shankaranarayana Rao B.S.S., Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McEwen B.S. Neurobiological and systemic effects of chronic stress. Chronic Stress (Thousand Oaks) 2017;1 doi: 10.1177/2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qiu A., Rifkin-Graboi A., Chen H., Chong Y.S., Kwek K., Gluckman P.D., et al. Maternal anxiety and infants’ hippocampal development: Timing matters. Transl Psychiatry. 2013;3 doi: 10.1038/tp.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim D.J., Davis E.P., Sandman C.A., Sporns O., O’Donnell B.F., Buss C., Hetrick W.P. Prenatal maternal cortisol has sex-specific associations with child brain network properties. Cereb Cortex. 2017;27:5230–5241. doi: 10.1093/cercor/bhw303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.