Abstract
Background
Matrix metalloproteinases (MMPs) are a diverse set of enzymes associated with inflammation. MMP-9 is of particular interest because it has been associated with autoimmune and cardiopulmonary disorders, tobacco smoking, and obesity, prevalent in psychiatric populations.
Methods
Sensitive enzyme immunoassays measured MMP-9 in blood samples from 1121 individuals (mean age = 35.6 [SD = 13.0] years; 47.7% male; 440 with schizophrenia, 399 with bipolar disorder, and 282 without a psychiatric disorder). We estimated the odds of diagnosis associated with MMP-9, demographic variables, tobacco smoking, and obesity, and also the partial explained variance using regression methods. We also determined the association between psychiatric medications and MMP-9 levels.
Results
Individuals with elevated MMP-9 levels had higher odds of schizophrenia or bipolar disorder compared with the nonpsychiatric group adjusted for demographic variables. Partial correlation analyses indicated the demographic-adjusted variance associated with MMP-9, smoking, obesity, and their interaction explained 59.6% for schizophrenia and 39.9% for bipolar disorder. Levels of MMP-9 were substantially lower in individuals receiving valproate, particularly relatively high doses.
Conclusions
Individuals with higher levels of MMP-9 have significantly higher odds of schizophrenia or bipolar disorder. Individuals receiving valproate had substantially lower levels of MMP-9, possibly related to its ability to inhibit histone deacetylation. A substantial portion of the variance in clinical disorders associated with MMP-9 can be attributed to smoking or obesity. Interventions to reduce smoking and obesity might reduce the morbidity and mortality associated with elevated MMP-9 levels and improve the health outcomes of individuals with these disorders.
Keywords: Bipolar disorder, Endopeptidase, Immune, Schizophrenia, Tobacco smoking, Valproate
Matrix metalloproteinases (MMPs) constitute a diverse set of calcium-dependent endopeptidases with a wide range of biological functions. While there are many human metalloproteinases, MMP-9, also known as gelatinase B, has been of particular interest in neuroscience because it interacts with several arms of the immune system, alters blood-brain barrier functioning, regulates glutamate receptors, induces glial cell activation, and modulates physiological and morphological synaptic plasticity (1, 2, 3, 4). Increased levels of MMP-9 have also been associated with increased rates of morbidity and mortality related to cardiopulmonary, vascular, and neoplastic disorders (5,6).
Individual levels of MMP-9 are determined by both genetic and environmental factors (6). While there are a number of genetic polymorphisms associated with baseline MMP-9 levels, environmental factors can alter MMP-9 levels through gene methylation and other epigenetic mechanisms (7,8). Environmental and anthropometric factors associated with elevated MMP-9 levels include tobacco smoking (9) and obesity (10), factors that are common in individuals with mental illness and are potentially modifiable (11). Previous studies, including ones employing broadly inclusive proteomic approaches (12), have reported increased levels of MMP-9 in some individuals with psychiatric disorders, but these studies often employed limited sample sizes and generally did not define the role of potentially modifiable covariates or medications. We measured circulating MMP-9 levels in a cohort of individuals with schizophrenia or bipolar disorder as well as individuals without a psychiatric disorder. We employed statistical models to define the attenuation of the association of MMP-9 levels with diagnostic group after accounting for tobacco smoking and obesity and psychiatric medications.
Methods and Materials
Study Participants
Participants were individuals diagnosed with schizophrenia or bipolar disorder or persons without a psychiatric disorder who were enrolled during the period January 1, 2008, to September 1, 2021, in the Stanley Research Program at Sheppard Pratt in Baltimore, Maryland, for a study of the association between infection, immune activation, and psychiatric disorders. All participants provided written informed consent after the study procedures were explained. The study was approved by the Sheppard Pratt Institutional Review Board and the Institutional Review Board of Johns Hopkins University School of Medicine. All participating individuals signed informed consent.
The inclusion criterion for individuals with schizophrenia was a diagnosis of schizophrenia, schizophreniform disorder, or schizoaffective disorder. The inclusion criterion for individuals with bipolar disorder was a diagnosis of bipolar disorder including bipolar I disorder, bipolar II disorder, or bipolar disorder not otherwise specified. The psychiatric participants were recruited from inpatient and day hospital programs of Sheppard Pratt and from affiliated psychiatric rehabilitation programs. The diagnosis of each psychiatric participant was established by the research team including a board-certified psychiatrist and based on the Structured Clinical Interview for DSM-IV Axis I Disorders (13) and available medical records. The inclusion criterion for the individuals without a psychiatric disorder was the absence of a current or past psychiatric disorder as determined by screening with the Structured Clinical Interview for DSM-IV Axis I Disorders, Non-Patient Edition (14). Persons in this group were recruited from posted announcements at local health facilities and universities in the same geographic area where the psychiatric participants were recruited. Methods for the recruitment and evaluation of the control population have been previously published (15).
Participants in all groups met the following additional criteria: age 18 to 65 years (except the nonpsychiatric comparison individuals, who were age 20–60), proficient in English, absence of any history of intravenous substance abuse, absence of intellectual disability by history, absence of HIV infection, absence of serious medical disorder that would affect cognitive functioning, and absence of a primary diagnosis of alcohol or substance use disorder per DSM-IV criteria.
Immunoassay Measures
Each participant had a blood sample drawn from which MMP-9 was measured by means of sensitive solid-phase immunoassays. Details relating to blood collection and storage are provided in the Supplement. Immune reagents for the measurement of MMP-9 levels were obtained from Fisher R&D Systems (Bio-Techne Corporation). Results were standardized across assays using control samples with known concentrations of MMP-9 and were further normalized to account for the testing of serum or plasma. The sensitivity of the immunoassay is 0.156 ng/mL and the dynamic range was 0.3 to 20 ng/mL. The validation of this assay has been previously described (16).
Demographic and Clinical Measures
Demographic and background information was obtained by interview during the study visit at which time the blood sample was drawn. Age, sex, and race were determined by self-report. Body mass index (BMI) was calculated based on height and measured weight, and participants were asked about their current tobacco smoking. Cognitive functioning was measured by the Repeatable Battery for the Assessment of Neuropsychological Status (17). In the case of individuals with a psychiatric disorder, psychiatric medications at the time of assessment were determined from the medical record. An individual was considered to be taking valproate if they were prescribed valproic acid, sodium valproate, or semisodium valproate because these forms are largely interchangeable under physiological conditions (18). Psychiatric symptoms were measured by the Positive and Negative Syndrome Scale (19).
Statistical Analyses
Demographic and clinical characteristics among groups were compared with chi-square analyses for dichotomous variables and one-way analysis of variance for linear variables.
In exploratory locally weighted scatterplot smoothing plot analyses, we determined that log-transformed MMP-9 was appropriate as an independent variable for the analyses, consistent with its linear relationship with the logit of the dependent variables, and log-transformed values were used for all analyses. Separate logistic regression analyses were performed for the psychiatric diagnostic groups, comparing each of them with the comparison group of individuals without a psychiatric disorder as the dependent variable. For each psychiatric group, we constructed 5 sequential logistic regression models employing log (MMP-9) level as the independent variable. Model 1 included age in years as a continuous variable, as well as male vs female and White vs other race as dichotomous covariates. Model 2 employed model 1 covariates as well as obesity defined as BMI ≥30 as an additional dichotomous covariate. Model 3 added tobacco smoking at the time of evaluation as a dichotomous covariate to model 1. Model 4 added both obesity and tobacco smoking to model 1. Model 5 added an interaction term for obesity and tobacco smoking to model 4. We performed the same analyses separately for each sex as shown in Figures S1 and S2.
We further used each psychiatric group versus the nonpsychiatric comparison group in separate models as dependent variables in linear regression analyses in models with the same covariates. For models 2 to 5, we examined the extent to which the partial r2 for MMP-9 was reduced compared with model 1, interpreting it as the fraction of variance of the age-, sex-, and race-adjusted psychiatric condition explained by MMP-9 that was attributable to the additional covariate to estimate the contributions of obesity and tobacco smoking to the MMP-9 levels measured in each diagnostic group. This was accomplished by linear regression models constructed to define the change in the partial r2 of MMP-9 associated with the covariates. The partial correlations for MMP-9 in models 2 to 5 were presented as a percentage of the partial correlation in model 1. Given that dichotomous variables violate the normality assumption for residuals for linear regression, we used robust standard errors for all linear regression analyses and also for logistic regression analyses for consistency.
We also employed linear regression models to explore the relationship between psychiatric medications and MMP-9 levels in the pooled sample of the individuals with either psychiatric disorder. Exploratory analyses were performed using medication categories exploring the relationship between log MMP-9 levels and the receipt of any antipsychotic, an atypical antipsychotic, an antidepressant, an anticholinergic, or a mood-stabilizing medication employing age, sex, race, diagnostic group, tobacco smoking, and obesity as covariates. For medications that showed an association at the p < .05 level, we further examined the association of log-transformed MMP-9 levels with the dosage of the medications using linear regression with the same covariates. The specific medications in each group are shown in Table S1. We then employed similar linear regression models to examine individual medications in categories that showed an association with log MMP-9 levels (p < .05) using the same covariates. We further examined the quantitative relationship between relevant dosage intervals of medications with significant associations in these analyses and log MMP-9 employing multinomial logistic regression models and the same covariates. We also examined the association between MMP-9 levels and other clinical variables such as symptom severity and cognitive functioning.
All analyses were performed with Stata versions 16.1 and 17.0 (Stata Corp LP). The results of the statistical analyses were not corrected for multiple comparisons.
Results
The sample consisted of 1121 persons: 440 with a diagnosis of schizophrenia, 399 with a diagnosis of bipolar disorder, and 282 in the nonpsychiatric comparison group. Characteristics of the study participants are displayed in Table 1. The groups differed significantly on demographic, clinical, and medication variables, as has been shown in previous studies (20).
Table 1.
Characteristics of Study Participants
| Schizophrenia Group, n= 440 | Bipolar Disorder Group, n = 399 | Nonpsychiatric Group, n = 282 | |
|---|---|---|---|
| Demographic Variables | |||
| Age, yearsa | 39.3 ± 13.3 | 34.1 ± 13.0 | 31.8 ± 11.0 |
| Sex, malea | 290 (66%) | 131 (33%) | 114 (40%) |
| Race, Whiteb,c | 202 (46%) | 283 (71%) | 128 (45%) |
| Education, yearsd | 12.2 ± 2.1 | 13.8 ± 2.4 | 15.7 ± 2.6 |
| Maternal education, yearse | 12.8 ± 2.6 | 13.7 ± 3.0 | 14.1 ± 2.7 |
| Place of birth outside United States or Canadaf,g | 21 (5%) | 19 (5%) | 30 (11%) |
| Clinical Variables | |||
| Current tobacco smokera | 264 (60%) | 140 (35%) | 38 (14%) |
| Body mass index >30a | 213 (48%) | 144 (36%) | 75 (27%) |
| Psychiatric Medications | |||
| Antipsychotica | 422 (96%) | 322 (81%) | – |
| Antidepressant | 169 (38%) | 162 (41%) | – |
| Mood stabilizera | 186 (42%) | 323 (81%) | – |
| Anticholinergica | 173 (39%) | 57 (14%) | – |
Values are mean ± SD or n (%).
Significant difference among or between groups, p < .001.
Significant difference among groups, p < .01.
Almost all of the non-White individuals were Black.
n = 439 in the schizophrenia group.
n = 422 in the schizophrenia group, 396 in the bipolar disorder group, and 280 in the nonpsychiatric group.
n = 437 in the schizophrenia group.
Significant difference among groups, p < .05.
The odds ratios associated with 1-log-unit-higher MMP-9 levels for each diagnostic group compared with the control individuals without a psychiatric diagnosis and their 95% confidence intervals are depicted in Figure 1. This figure also displays the odds ratios associated with sequential models associated with the demographic covariates of age, sex, and race as well as differing levels of obesity and tobacco smoking and the interaction between these two factors. The odds ratio of MMP-9 levels decreased from 8.27 (SE = 2.72) in the model that contained age, sex, and race as covariates to 4.71 (SE = 1.61) in the model that contained these covariates as well as obesity, tobacco smoking, and their interaction. Compared with schizophrenia versus the comparison group, there were lower odds ratios associated with MMP-9 levels for bipolar disorder versus the comparison group, with some reduction in odds in models containing the additional covariates (Figure 1).
Figure 1.
Odds ratios associated with MMP-9 levels in individuals with schizophrenia or bipolar disorder compared with individuals without a psychiatric disorder, adjusted for different covariates. Model 1 included age in years as a continuous variable, as well as male vs female and White vs other race as dichotomous covariates. Model 2 employed model 1 covariates as well as obesity defined as body mass index ≥30 as an additional dichotomous covariate. Model 3 added tobacco smoking at the time of evaluation as a dichotomous covariate to model 1. Model 4 added both obesity and tobacco smoking to model 1. Model 5 added an interaction term for obesity and tobacco smoking to model 4. MMP-9, matrix metalloproteinase 9.
We further examined the fractional effects of smoking, obesity, and their interaction on reducing the partial correlation of MMP-9 levels for each psychiatric group (Figure 2). In the case of schizophrenia, 59.6% of the partial r2 attributable to MMP-9 with age, sex, and race adjustment alone was lost when smoking, obesity, and their interaction were added to the model, while the remaining 40.4% of the original partial r2 remained attributable to MMP-9 and thus could not be explained by these factors. In the case of bipolar disorder, 39.9% of the age-, sex-, and race-adjusted explanatory fraction of MMP-9 was lost when explained by smoking, obesity, and their interaction, while 60.1% of the elevation could not be explained by these factors. Levels of MMP-9 were not significantly correlated with symptom severity as measured by the Positive and Negative Syndrome Scale score (for the psychiatric groups) or cognitive functioning as measured by the Repeatable Battery for the Assessment of Neuropsychological Status (for the entire sample) when adjusted for age, sex, race, and diagnosis (all p > .05), nor were there significant associations within either sex group.
Figure 2.
Residual percentage of MMP-9 levels in individuals with schizophrenia or bipolar disorder remaining after accounting for covariates. Model 1 included age in years as a continuous variable, as well as male vs female and White vs other race as dichotomous covariates. Model 2 employed model 1 covariates as well as obesity defined as body mass index ≥30 as an additional dichotomous covariate. Model 3 added tobacco smoking at the time of evaluation as a dichotomous covariate to model 1. Model 4 added both obesity and tobacco smoking to model 1. Model 5 added an interaction term for obesity and tobacco smoking to model 4. MMP-9, matrix metalloproteinase 9.
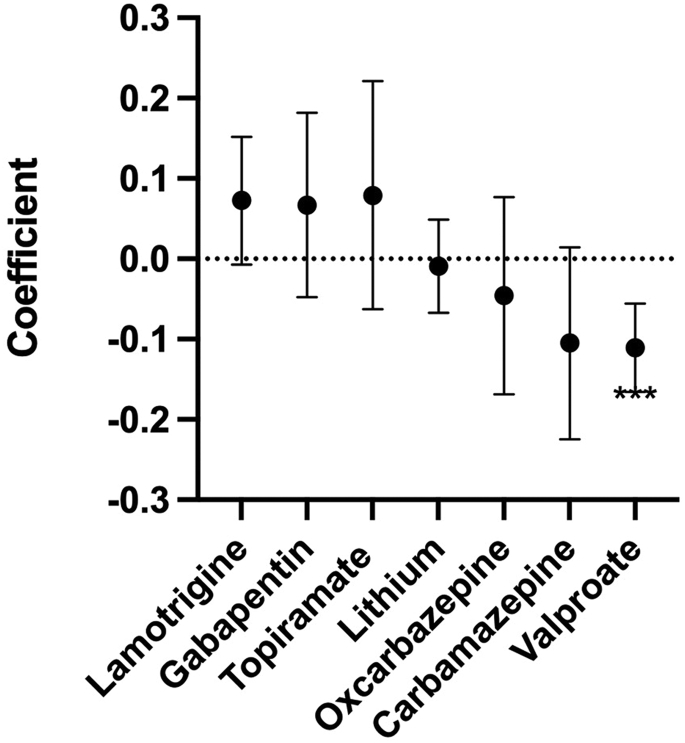
We employed linear regression models to examine the relationship between medications and log MMP-9 levels. As depicted in Figure 3, the levels of MMP-9 were reduced in individuals receiving mood-stabilizing medications but not in individuals receiving antipsychotic, antidepressant, or anticholinergic medications. Further exploration of individual mood-stabilizing medications (Figure 4) indicated that there was a significant association between reduced levels of MMP-9 in individuals who were receiving valproate but not in individuals who were receiving lithium or other mood-stabilizing medications. Furthermore, there was a significant association between the dose of valproate and lower levels of MMP-9, with lower levels becoming evident at doses ≥500 mg/day (Figure 5). The largest odds were associated with doses of ≥1500 mg/day (odds ratio, 0.12; 95% CI, 0.06–0.25; p < 10−7; adjusted for age, sex, race, tobacco smoking, obesity, and clinical diagnosis).
Figure 3.
Regression coefficients of MMP-9 levels associated with medication categories adjusted for age, sex, race, diagnosis, smoking, and obesity. ∗p = .044. MMP-9, matrix metalloproteinase 9.
Figure 4.
Regression coefficients of MMP-9 levels associated with the administration of individual mood-stabilizing medications adjusted for age, sex, race, diagnosis, smoking, and obesity. The one individual receiving primidone was not included in the analysis. ∗∗∗p < .001. MMP-9, matrix metalloproteinase 9.
Figure 5.
Regression coefficient of MMP-9 levels associated with daily dose ranges of valproate adjusted for age, sex, race, diagnosis, smoking and obesity. ∗p < .02. ∗∗p < .005. ∗∗∗p < 2 × 10−5. MMP-9, matrix metalloproteinase 9.
Discussion
Our study documents that individuals with higher levels of MMP-9 are more likely to have a diagnosis of schizophrenia or bipolar disorder as compared with individuals without a psychiatric disorder. A substantial portion of the explanation of the psychiatric disorder by the MMP-9 level could be attributed to tobacco smoking or obesity, particularly in individuals with schizophrenia in whom these factors are highly prevalent. In individuals with this disorder, approximately 60% of the increase in MMP-9 levels could be attributed to smoking and obesity along with basic demographic factors. Individuals with bipolar disorder also displayed effects of smoking and obesity on MMP-9 levels, albeit at levels of attribution. Mechanisms underlying the residual effects of diagnosis are not known with certainty but may be related to unmeasured genetic or environmental factors. The latter might include increased levels of exposure to infectious agents, environmental toxins, immune stimulators, or allergens (21,22). In the case or infectious agents, it is of note that increased levels of MMP-9 have been reported in some groups of individuals infected with coronaviruses or Epstein-Barr virus, infectious agents that have been found to have increased prevalence in some individuals with schizophrenia or bipolar disorder (4,23, 24, 25, 26).
The results of this study are consistent with those of several other studies in showing elevated levels of MMP-9 in individuals with schizophrenia (27, 28, 29, 30, 31). Several additional studies have not shown this association, but they have had small samples sizes and/or did not adjust for smoking status or obesity (32,33). Further studies should include smoking and obesity and other environmental factors in order to maximize reproducibility across studies. MMP-9 levels have also been associated with decreased cognitive functioning in individuals with schizophrenia (34,35).
There are fewer studies looking at MMP-9 blood levels in persons with bipolar disorder than in schizophrenia, and these studies have inconsistent findings. In bipolar disorder, no differences in MMP-9 serum levels were found in a study of mood-stabilized persons with bipolar disorder compared with control participants (36) or in another small bipolar disorder sample (37), but elevated MMP-9 levels were reported in persons with acute bipolar depression (38) and in those with bipolar disorder, all of whom had bipolar II disorder (39). It is of note that in our study the odds ratios of MMP-9 levels associated with bipolar disorder were substantially lower than those in individuals with schizophrenia.
Specific MMP modulators directed at lowering MMP-9 levels have been proposed as novel therapeutic interventions for psychiatric disorders based on the finding of increased levels as well as conceptual models, suggesting that MMP-9 contributes to schizophrenia pathogenesis (40). However, the efficacy of therapeutic agents tested to date has been limited (41). Our findings show that tobacco smoking and obesity contribute to increased levels of MMP-9 in individuals with schizophrenia or other psychiatric disorders. Thus, it is likely that programs directed at decreasing the rates of tobacco smoking or obesity would decrease the levels of MMP-9 and also benefit psychiatric disorders both independently and through reductions in MMP-9. While smoking cessation is more challenging to accomplish in persons with serious mental illness than in the general population, numerous trials have shown success in enabling persons in this population to obtain abstinence from tobacco. Effective interventions involve a combination of smoking cessation medication and behavioral counseling that is tailored to address the cognitive deficits and reduced motivation found in some persons with serious mental illness (42). Smoking cessation outcomes also may benefit from an extended period of treatment (42). Similar findings have emerged from weight loss trials in this population. Effective weight loss interventions have involved an adaptation of programs available for the general population and that include regular contact with and booster support from the interventionist, specific tools to promote weight loss, and materials that are tailored for persons with serious mental illness (43).
It is also of note that the receipt of valproate was associated with significantly lower levels of MMP-9 in a dose-dependent manner (Figures 4 and 5). Valproate has a number of pharmacological activities that inform its use in the treatment of psychiatric disorders (18). Recently, valproate has been found to have histone deacetylation activity, which can alter immune functioning through the regulation of MHC (major histocompatibility complex) class I molecules (44,45). This activity of valproate appears to differ from that of lithium, a mood stabilizer which was not associated with lower levels of MMP-9 in our study (46). There are no previous studies documenting an association between MMP-9 levels and the use of valproate. However, our results are consistent with a recent study showing that valproate downregulates MMP-9 messenger RNA and the subsequent shedding of MHC class I–related molecules in cell culture, suggesting that valproate may lower MMP-9 levels through its effect on MHC class I and other immune mediators (47). The mechanism by which valproate is associated with lower MMP-9 levels should be further explored with the goal of optimizing the use of psychiatric medications that can alter the levels of MMP-9 and other potentially toxic proteinases.
Strengths of our study include the relatively large sample size and the measurement and adjustment of important covariates such as smoking status, obesity, and medications. In addition, ours is among the first to examine MMP-9 levels across diagnostic groups using standardized measures.
Limitations of our study include the cross-sectional nature of the analyses. Further studies would benefit from examining MMP-9 longitudinally to determine the association between MMP-9 levels and psychiatric symptom severity and acute illness status, particularly in individuals with changes in tobacco smoking, BMI, or psychiatric medications. Also, in the current study, we cannot rule out the effect of residual confounders, variables not included or accounted for in the analyses. We recognize that partial correlation analyses for dichotomous outcomes violate the assumption of normality; however, we believe that our analysis can give an approximate magnitude of the partial explanatory contributions of MMP-9 and other variables. Our study would have benefited from measures of genetic polymorphisms. Further studies would also benefit from multiple markers in larger sample sizes and additional variables, including physical comorbidities and alcohol and other substances. It would also be important to study rates of cardiovascular disease and mortality as they relate to MMP-9 levels, especially in aging populations.
Another limitation of our study is that we measured MMP-9 in the blood as opposed to in the brain or cerebrospinal fluid. However, there are a number of studies suggesting that blood levels of MMP-9 are informative of processes that affect the central nervous system. For example, several studies have documented that increased levels of MMP-9 are associated with alterations in the blood-brain barrier (48,49). This barrier is altered in many individuals with schizophrenia and other psychiatric disorders and can be associated with an altered immune response within the CNS due to the altered permeability to immune active blood cells and soluble mediators (50,51). Animal model studies indicate that this alteration may function through hedgehog signaling, a pathway that can also alter immune functioning within the CNS through astrocyte activation and other mechanisms (52,53). Furthermore, the levels of MMP-9 in the blood and cerebrospinal fluid were found to be correlated with each other and predictive of outcome in individuals with subarachnoid hemorrhage (54). Elevated blood levels of MMP-9 have also been associated with poorer outcome following ischemic stroke (55) and traumatic brain injury (56). Additional studies should be performed for the direct measurement of the CNS effects of increased levels of MMP-9 in individuals with psychiatric disorders.
Acknowledgments and Disclosures
This work was supported by the Stanley Medical Research Institute (Grant No. 07-1690 [to FD]).
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2023.02.007.
Supplementary Material
References
- 1.D'Amico F., Candido S., Libra M. Interaction between matrix metalloproteinase-9 (MMP-9) and neutrophil gelatinase-associated lipocalin (NGAL): A recent evolutionary event in primates. Dev Comp Immunol. 2021;116 doi: 10.1016/j.dci.2020.103933. [DOI] [PubMed] [Google Scholar]
- 2.Bratcher P.E., Weathington N.M., Nick H.J., Jackson P.L., Snelgrove R.J., Gaggar A. MMP-9 cleaves SP-D and abrogates its innate immune functions in vitro. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin W., Li J., Zhu R., Gao S., Fan J., Xia M., et al. Melatonin protects blood-brain barrier integrity and permeability by inhibiting matrix metalloproteinase-9 via the NOTCH3/NF-κB pathway. Aging (Albany NY) 2019;11:11391–11415. doi: 10.18632/aging.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avila-Mesquita C.D., Couto A.E.S., Campos L.C.B., Vasconcelos T.F., Michelon-Barbosa J., Corsi C.A.C., et al. MMP-2 and MMP-9 levels in plasma are altered and associated with mortality in COVID-19 patients. Biomed Pharmacother. 2021;142 doi: 10.1016/j.biopha.2021.112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lahdentausta L., Leskelä J., Winkelmann A., Tervahartiala T., Sorsa T., Pesonen E., et al. Serum MMP-9 diagnostics, prognostics, and activation in acute coronary syndrome and its recurrence. J Cardiovasc Transl Res. 2018;11:210–220. doi: 10.1007/s12265-018-9789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang T., Fu W., Song S., Han Y., Yao L., Lu Y., et al. Matrix metalloproteinase-9 gene polymorphisms and their interaction with environment on subarachnoid hemorrhage risk. Exp Biol Med (Maywood) 2018;243:749–753. doi: 10.1177/1535370218775042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labrie M., St-Pierre Y. Epigenetic regulation of mmp-9 gene expression. Cell Mol Life Sci. 2013;70:3109–3124. doi: 10.1007/s00018-012-1214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhari O.K., Rani A., Kampani G., Kaur C., Sengupta A. Matrix metalloproteinase-9 gene polymorphism and its methylation in stroke patients. Malays J Med Sci. 2021;28:32–41. doi: 10.21315/mjms2021.28.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bchir S., Nasr H.B., Bouchet S., Benzarti M., Garrouch A., Tabka Z., et al. Concomitant elevations of MMP-9, NGAL, proMMP-9/NGAL and neutrophil elastase in serum of smokers with chronic obstructive pulmonary disease. J Cell Mol Med. 2017;21:1280–1291. doi: 10.1111/jcmm.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritter A.M., de Faria A.P., Barbaro N., Sabbatini A.R., Corrêa N.B., Brunelli V., et al. Crosstalk between obesity and MMP-9 in cardiac remodelling-a cross-sectional study in apparent treatment-resistant hypertension. Blood Press. 2017;26:122–129. doi: 10.1080/08037051.2016.1249336. [DOI] [PubMed] [Google Scholar]
- 11.Dickerson F.B., Brown C.H., Daumit G.L., Fang L., Goldberg R.W., Wohlheiter K., et al. Health status of individuals with serious mental illness. Schizophr Bull. 2006;32:584–589. doi: 10.1093/schbul/sbj048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickerson F., Schroeder J., Stallings C., Origoni A., Bahn S., Yolken R. Multianalyte markers of schizophrenia and bipolar disorder: A preliminary study. Schizophr Res. 2015;168:450–455. doi: 10.1016/j.schres.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 13.First M., Gibbon M., Spitzer R.L., Williams J.B.W. Biometrics Research; New York: 1996. User's Guide for the SCID-I, Structured Clinical Interview for DSM IV Axis I Disorders. [Google Scholar]
- 14.First M., Gibbon M., Spitzer R.L., Williams J.B.W. Biometrics Research; New York: 1998. Structured Clinical Interview for DSM-IV Axis I Disorders, Non-Patient Edition. [Google Scholar]
- 15.Dickerson F., Stallings C., Sullens A., Origoni A., Leister F., Krivogorsky B., et al. Association between cognitive functioning, exposure to Herpes Simplex Virus type 1, and the COMT Val158Met genetic polymorphism in adults without a psychiatric disorder. Brain Behav Immun. 2008;22:1103–1107. doi: 10.1016/j.bbi.2008.04.156. [DOI] [PubMed] [Google Scholar]
- 16.Lourbakos A., Yau N., de Bruijn P., Hiller M., Kozaczynska K., Jean-Baptiste R., et al. Evaluation of serum MMP-9 as predictive biomarker for antisense therapy in Duchenne. Sci Rep. 2017;7 doi: 10.1038/s41598-017-17982-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Randolph C., Tierney M.C., Mohr E., Chase T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 18.Haddad P.M., Das A., Ashfaq M., Wieck A. A review of valproate in psychiatric practice. Expert Opin Drug Metab Toxicol. 2009;5:539–551. doi: 10.1517/17425250902911455. [DOI] [PubMed] [Google Scholar]
- 19.Kay S.R., Fiszbein A., Opler L.A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 20.Dickerson F., Adamos M.B., Katsafanas E., Khushalani S., Origoni A., Savage C.L.G., et al. The association among smoking, HSV-1 exposure, and cognitive functioning in schizophrenia, bipolar disorder, and non-psychiatric controls. Schizophr Res. 2016;176:566–571. doi: 10.1016/j.schres.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Roomi M.W., Kalinovsky T., Monterrey J., Rath M., Niedzwiecki A. In vitro modulation of MMP-2 and MMP-9 in adult human sarcoma cell lines by cytokines, inducers and inhibitors. Int J Oncol. 2013;43:1787–1798. doi: 10.3892/ijo.2013.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naik S.P., P A.M., B S.J., Madhunapantula S.V., Jahromi S.R., Yadav M.K. Evaluation of inflammatory markers interleukin-6 (IL-6) and matrix metalloproteinase-9 (MMP-9) in asthma. J Asthma. 2017;54:584–593. doi: 10.1080/02770903.2016.1244828. [DOI] [PubMed] [Google Scholar]
- 23.Stępień E., Dworzański J., Dworzańska A., Drop B., Polz-Dacewicz M. Serum level of MMP-3 and MMP-9 in patients with diabetes mellitus type 2 infected with Epstein-Barr virus. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232113599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickerson F.B., Severance E.G., Yolken R.H. Non-SARS coronaviruses in individuals with psychiatric disorders. Curr Top Behav Neurosci. 2023;61:265–278. doi: 10.1007/7854_2022_386. [DOI] [PubMed] [Google Scholar]
- 25.Baranova A., Cao H., Zhang F. Severe COVID-19 increases the risk of schizophrenia. Psychiatry Res. 2022;317 doi: 10.1016/j.psychres.2022.114809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickerson F., Katsafanas E., Origoni A., Squire A., Khushalani S., Newman T., et al. Exposure to Epstein Barr virus and cognitive functioning in individuals with schizophrenia. Schizophr Res. 2021;228:193–197. doi: 10.1016/j.schres.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamori H., Hashimoto R., Ishima T., Kishi F., Yasuda Y., Ohi K., et al. Plasma levels of mature brain-derived neurotrophic factor (BDNF) and matrix metalloproteinase-9 (MMP-9) in treatment-resistant schizophrenia treated with clozapine. Neurosci Lett. 2013;556:37–41. doi: 10.1016/j.neulet.2013.09.059. [DOI] [PubMed] [Google Scholar]
- 28.Domenici E., Willé D.R., Tozzi F., Prokopenko I., Miller S., McKeown A., et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali F.T., Abd El-Azeem E.M., Hamed M.A., Ali M.A.M., Abd Al-Kader N.M., Hassan E.A. Redox dysregulation, immuno-inflammatory alterations and genetic variants of BDNF and MMP-9 in schizophrenia: Pathophysiological and phenotypic implications. Schizophr Res. 2017;188:98–109. doi: 10.1016/j.schres.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Devanarayanan S., Nandeesha H., Kattimani S., Sarkar S. Relationship between matrix metalloproteinase-9 and oxidative stress in drug-free male schizophrenia: A case control study. Clin Chem Lab Med. 2016;54:447–452. doi: 10.1515/cclm-2015-0212. [DOI] [PubMed] [Google Scholar]
- 31.Seitz-Holland J., Seethaler M., Makris N., Rushmore J., Cho K.K., Rizzoni E., et al. The association of matrix metalloproteinase 9 (MMP9) with hippocampal volume in schizophrenia: A preliminary MRI study. Neuropsychopharmacology. 2022;47:524–530. doi: 10.1038/s41386-021-00997-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niitsu T., Ishima T., Yoshida T., Hashimoto T., Matsuzawa D., Shirayama Y., et al. A positive correlation between serum levels of mature brain-derived neurotrophic factor and negative symptoms in schizophrenia. Psychiatry Res. 2014;215:268–273. doi: 10.1016/j.psychres.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Rahimi S., Sayad A., Moslemi E., Ghafouri-Fard S., Taheri M. Blood assessment of the expression levels of matrix metalloproteinase 9 (MMP9) and its natural inhibitor, TIMP1 genes in Iranian schizophrenic patients. Metab Brain Dis. 2017;32:1537–1542. doi: 10.1007/s11011-017-0043-z. [DOI] [PubMed] [Google Scholar]
- 34.Keshri N., Nandeesha H., Rajappa M., Menon V. Matrix metalloproteinase-9 increases the risk of cognitive impairment in schizophrenia. Nord J Psychiatry. 2021;75:130–134. doi: 10.1080/08039488.2020.1808901. [DOI] [PubMed] [Google Scholar]
- 35.Kudo N., Yamamori H., Ishima T., Nemoto K., Yasuda Y., Fujimoto M., et al. Plasma levels of matrix metalloproteinase-9 (MMP-9) are associated with cognitive performance in patients with schizophrenia. Neuropsychopharmacol Rep. 2020;40:150–156. doi: 10.1002/npr2.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Södersten K., Pålsson E., Ishima T., Funa K., Landén M., Hashimoto K., et al. Abnormality in serum levels of mature brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in mood-stabilized patients with bipolar disorder: A study of two independent cohorts. J Affect Disord. 2014;160:1–9. doi: 10.1016/j.jad.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Chiarani F., Fries G.R., Stertz L., Ceresér K.M., Wyse A.T., Kapczinski F.P., et al. Expression of matrix metalloproteinases in patients with bipolar disorder. Braz J Psychiatry. 2013;35:375–379. doi: 10.1590/1516-4446-2012-1004. [DOI] [PubMed] [Google Scholar]
- 38.Rybakowski J.K., Remlinger-Molenda A., Czech-Kucharska A., Wojcicka M., Michalak M., Losy J. Increased serum matrix metalloproteinase-9 (MMP-9) levels in young patients during bipolar depression. J Affect Disord. 2013;146:286–289. doi: 10.1016/j.jad.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 39.Lee S.Y., Wang T.Y., Lu R.B., Wang L.J., Chang C.H., Chiang Y.C., et al. Plasma BDNF and cytokines correlated with protein biomarkers for bipolar II disorder. J Pers Med. 2021;11:1282. doi: 10.3390/jpm11121282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bitanihirwe B.K.Y., Woo T.W. A conceptualized model linking matrix metalloproteinase-9 to schizophrenia pathogenesis. Schizophr Res. 2020;218:28–35. doi: 10.1016/j.schres.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 41.Chaves Filho A.J.M., Mottin M., Lós D.B., Andrade C.H., Macedo D.S. The tetrapartite synapse in neuropsychiatric disorders: Matrix metalloproteinases (MMPs) as promising targets for treatment and rational drug design. Biochimie. 2022;201:79–99. doi: 10.1016/j.biochi.2022.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Evins A.E., Cather C., Pratt S.A., Pachas G.N., Hoeppner S.S., Goff D.C., et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: A randomized clinical trial. JAMA. 2014;311:145–154. doi: 10.1001/jama.2013.285113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daumit G.L., Dickerson F.B., Wang N.Y., Dalcin A., Jerome G.J., Anderson C.A., et al. A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med. 2013;368:1594–1602. doi: 10.1056/NEJMoa1214530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim T., Song S., Park Y., Kang S., Seo H. HDAC inhibition by valproic acid induces neuroprotection and improvement of PD-like behaviors in LRRK2 R1441G transgenic mice. Exp Neurobiol. 2019;28:504–515. doi: 10.5607/en.2019.28.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun T., Li Y., Yang W., Wu H., Li X., Huang Y., et al. Histone deacetylase inhibition up-regulates MHC class I to facilitate cytotoxic T lymphocyte-mediated tumor cell killing in glioma cells. J Cancer. 2019;10:5638–5645. doi: 10.7150/jca.34471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leu S.J., Yang Y.Y., Liu H.C., Cheng C.Y., Wu Y.C., Huang M.C., et al. Valproic acid and lithium meditate anti-inflammatory effects by differentially modulating dendritic cell differentiation and function. J Cell Physiol. 2017;232:1176–1186. doi: 10.1002/jcp.25604. [DOI] [PubMed] [Google Scholar]
- 47.Yamanegi K., Yamane J., Kobayashi K., Ohyama H., Nakasho K., Yamada N., et al. Downregulation of matrix metalloproteinase-9 mRNA by valproic acid plays a role in inhibiting the shedding of MHC class I-related molecules A and B on the surface of human osteosarcoma cells. Oncol Rep. 2012;28:1585–1590. doi: 10.3892/or.2012.1981. [DOI] [PubMed] [Google Scholar]
- 48.Barr T.L., Latour L.L., Lee K.Y., Schaewe T.J., Luby M., Chang G.S., et al. Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke. 2010;41:e123–e128. doi: 10.1161/STROKEAHA.109.570515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vafadari B., Salamian A., Kaczmarek L. MMP-9 in translation: From molecule to brain physiology, pathology, and therapy. J Neurochem. 2016;139:91–114. doi: 10.1111/jnc.13415. [DOI] [PubMed] [Google Scholar]
- 50.Severance E.G., Gressitt K.L., Alaedini A., Rohleder C., Enning F., Bumb J.M., et al. IgG dynamics of dietary antigens point to cerebrospinal fluid barrier or flow dysfunction in first-episode schizophrenia. Brain Behav Immun. 2015;44:148–158. doi: 10.1016/j.bbi.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollak T.A., Drndarski S., Stone J.M., David A.S., McGuire P., Abbott N.J. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5:79–92. doi: 10.1016/S2215-0366(17)30293-6. [DOI] [PubMed] [Google Scholar]
- 52.Wu M.Y., Gao F., Yang X.M., Qin X., Chen G.Z., Li D., et al. Matrix metalloproteinase-9 regulates the blood brain barrier via the hedgehog pathway in a rat model of traumatic brain injury. Brain Res. 2020;1727 doi: 10.1016/j.brainres.2019.146553. [DOI] [PubMed] [Google Scholar]
- 53.Ballester A., Guijarro A., Bravo B., Hernández J., Murillas R., Gallego M.I., et al. Hedgehog signalling modulates immune response and protects against experimental autoimmune encephalomyelitis. Int J Mol Sci. 2022;23:3171. doi: 10.3390/ijms23063171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chou S.H., Feske S.K., Simmons S.L., Konigsberg R.G., Orzell S.C., Marckmann A., et al. Elevated peripheral neutrophils and matrix metalloproteinase 9 as biomarkers of functional outcome following subarachnoid hemorrhage. Transl Stroke Res. 2011;2:600–607. doi: 10.1007/s12975-011-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhong C., Yang J., Xu T., Xu T., Peng Y., Wang A., et al. Serum matrix metalloproteinase-9 levels and prognosis of acute ischemic stroke. Neurology. 2017;89:805–812. doi: 10.1212/WNL.0000000000004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lima R., Simon D., Silva W., Nabinger D.D., Regner A. Prognostic utility of early plasma matrix metalloproteinases -2 and -9 concentrations after severe traumatic brain injury. Rev Bras Ter Intensiva. 2020;32:418–425. doi: 10.5935/0103-507X.20200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.