Abstract
Background
Adversity has been linked to accelerated maturation. Molar eruption is a simple and scalable way to identify early maturation, but its developmental correlates remain unexplored. Thus, we examined whether accelerated maturation as indexed by molar eruption is associated with children’s mental health or cognitive skills.
Methods
Molar eruption was evaluated from T2-weighted magnetic resonance imaging in 117 children (63 female; ages 4–7 years). Parents reported on child mental health with the Child Behavior Checklist. Children completed standardized assessments of fluid reasoning, working memory, processing speed, crystallized knowledge, and math performance. Relationships between molar eruption and developmental outcomes were examined using linear models, with age, gender, and stress risk as covariates.
Results
Earlier molar eruption was positively associated with children’s externalizing symptoms (false discovery rate-corrected p [pFDR] = .027) but not internalizing symptoms, and the relationship with externalizing symptoms did not hold when controlling for stress risk. Earlier molar eruption was negatively associated with fluid reasoning (pFDR < .001), working memory (pFDR = .033), and crystallized knowledge (pFDR = .001). The association between molar eruption and both reasoning and crystallized knowledge held when controlling for stress risk. Molar eruption also partially mediated associations between stress risk and both reasoning (proportion mediated = 0.273, p = .004) and crystallized knowledge (proportion mediated = 0.126, p = .016).
Conclusions
Accelerated maturation, as reflected in early molar eruption, may have consequences for cognitive development, perhaps because it constrains brain plasticity. Knowing the pace of a child’s maturation may provide insight into the impact of a child’s stress history on their cognitive development.
Keywords: Accelerated maturation, Adversity, Cognition, Fluid reasoning, Mental health, Molar eruption
Children who mature too quickly are more likely to experience mental health challenges, including externalizing problems such as aggression and delinquency (1, 2, 3) and internalizing problems including depression and anxiety disorders (4, 5, 6, 7, 8). Early adversity, including low socioeconomic status and exposure to adverse childhood experiences (ACEs), is a major risk factor for accelerated maturation of the body (9, 10, 11, 12, 13, 14, 15) and brain (16, 17, 18, 19, 20). According to evolutionary theories (21,22), accelerated development may represent an adaptive trade-off in harsh environments because it can lead to earlier attainment of adult-like abilities and increased reproductive fitness, but at the cost of later health. Multiple theories have been put forth to explain the relationship between accelerated development and psychopathology (23). First, early-maturing children may show a mismatch between their physical and social cognitive development, leading peers and adults to place unwarranted expectations of socioemotional maturity onto early physically maturing children (1,5,24). Second, early cortical thinning in brain regions that support functions such as emotion regulation (18) and attention (3) may disrupt the development of these skills. It is also possible that mental health symptoms such as increased attention to threat (25) can contribute to faster maturation through physiological processes such as oxidative stress and inflammation (26,27). Finally, accelerated maturation and psychopathology could both be downstream effects of early adversity with no causal links between them.
Accelerated maturation has been measured in a number of ways, including pubertal timing and epigenetic age. Existing methods of detecting accelerated maturation can be costly and difficult to obtain at scale (e.g., DNA methylation) or cannot be detected until later in adolescence (e.g., pubertal timing). Accordingly, associations between early maturation and mental health or other important developmental outcomes in early childhood have been less well established (7). Identifying developmental risk earlier in childhood can provide opportunities for intervention before the full symptom consequences are realized. In response to these challenges, we have put forth a new scalable biomarker of accelerated maturation: molar eruption (28).
The first permanent molars erupt around age 6 years, before puberty begins, and are easier and less expensive to assess than epigenetic or brain age. Molar eruption is typically characterized through radiographs and dental examinations obtained through routine dental care and can also be captured in existing magnetic resonance imaging (MRI) datasets. Using MRI, we found that children from lower-income backgrounds and children with more ACEs showed earlier permanent molar eruption than their more advantaged peers (28). We also observed differences in molar eruption timing by race/ethnicity (28,29), which is consistent with pervasive racial disparities in income and other contextual stressors that have roots in structural racism. We replicated associations between molar eruption and income in a large epidemiological dataset with dental examination data (30) and also showed that second molar eruption was correlated with menarche status in girls (28). A recent unpublished finding supported the stress sensitivity of dental milestones: children with higher salivary cortisol were found to have lost more of their primary teeth (31). Thus, the timing of dental development may serve as an early indicator of a broad pattern of stress-accelerated maturation. However, it remains unknown whether molar eruption is associated with important developmental outcomes.
In addition to mental health consequences, early maturation may have consequences for cognitive development. Complex cognitive skills including reasoning and working memory develop slowly, perhaps because the brain networks that support them require extended environmental input to reach optimal configurations (32,33). A faster pace of brain development may shorten windows of peak neuroplasticity, reducing the brain’s sensitivity to future experiences (34). Reduced plasticity as a consequence of accelerated maturation may have negative implications for cognition and learning. Consistent with this hypothesis, slower structural brain development is associated with higher general intelligence (35). In addition, one recent study found that children with a faster pace of epigenetic aging demonstrated lower scores on tests of verbal intelligence and perceptual reasoning (36). If accelerated molar eruption is indicative of a broader pattern of accelerated brain development following stress exposure, we may expect earlier molar eruption to be associated with lower cognitive skills.
In this study, we examined how accelerated maturation, as indexed by molar eruption, is associated with mental health and cognition in childhood. To test associations between maturation and mental health, we examined parent report of internalizing and externalizing symptoms. To test associations between maturation and cognition, we explored 5 cognitive tests: tests of reasoning, working memory, processing speed, crystallized knowledge, and math performance. We examined associations between each developmental domain and molar eruption status characterized from T2-weighted MRI. We tested associations between maturation and developmental outcomes with and without controlling for measures of stress risk. We also assessed whether accelerated maturation mediates the relationship between stress risk and developmental outcomes. To summarize, we examined whether molar eruption is a marker of developmental risk above and beyond stress exposure because children differ in how their brains and bodies respond to stress (37).
Methods and Materials
Participants
This study was approved by the institutional review board at the University of Pennsylvania. All parents provided written informed consent. Children and parents were recruited from the greater Philadelphia area as part of 2 larger neuroimaging studies. Participants were recruited through advertisements on public transportation, partnerships with local schools, outreach programs, community family events, and advertisements on social media. Participants were screened and were excluded from participating in the studies if they had a diagnosed psychiatric, neurological, or learning disorder; were born more than 6 weeks premature; were adopted; or had any contraindications for MRI scanning. These exclusions were put into place because associations between the environment, molar eruption, and developmental outcomes may be different in children with diagnoses than they are in typically developing children.
Molar Eruption
A detailed description of the molar eruption scale and rating procedure, as well as example scans, can be found in McDermott et al. (28). Molar eruption was rated from each subject’s raw T2-weighted MRI scan by a dental student (KH) at the University of Pennsylvania School of Dental Medicine, in consultation with a faculty member (MM) with expertise in oral and maxillofacial radiology. They developed a novel scale for classifying molar eruption status in MRI that was based on previous experience in dental imaging and similar to other fine-grained dental scales (38), which rely on dental radiographs. The eruption status of each first permanent molar was rated on a scale from 1 (unerupted) to 4 (fully erupted) (see Supplemental Methods for further information).
The eruption status of all 4 first molars was averaged to create a single continuous molar eruption variable that was used in analyses. We analyzed molar eruption data from 117 participants (63 female, 54 male) between the ages of 4.05 and 7.32 years (28). The final sample size was obtained after excluding 3 subjects who had extremely delayed molar eruption (Cook’s Distance > 4/N for the molar eruption by age regression) and restricting analyses to include only subjects within the range for which there was variability in molar eruption, as we preregistered (https://osf.io/2edyx). For visualization purposes and inclusion in a zero-order correlation table (Table S2), we created an adjusted molar eruption variable by regressing age and gender on molar eruption status and extracting the residuals. Positive molar eruption residuals indicate accelerated molar eruption relative to chronological age.
Psychiatric Symptoms
In one of the two neuroimaging studies included in the current sample, parents (n = 80) completed the Child Behavior Checklist (CBCL) to identify behavioral and emotional problems (39,40). Parents of children under age 6 years completed the preschool version of the CBCL, and parents of children ages 6 and above completed the school-age version of the CBCL. We initially preregistered analyzing the 3 “AAA” subscales that are shared between the 2 versions of the CBCL: aggressive behavior, anxious/depressed, and attention problems (https://osf.io/2edyx). However, based on the distribution of the data (Figure S1), we decided to analyze the more commonly used internalizing and externalizing subscales, which we initially planned to use in exploratory analyses. T scores for these composite scales were used in all analyses.
Cognitive Assessment
To assess cognition, we administered 4 subtests from the Wechsler Preschool & Primary Scale of Intelligence (WPPSI-IV) (41): Matrix Reasoning (n = 116), Picture Memory (n = 106), Bug Search (n = 76), and Information (n = 115). Missing subtest scores are due to differences in study protocols, administration errors, or lack of child understanding or compliance. Children ages 5 years and older also completed the Numeration subtest of the KeyMath-3 Diagnostic Assessment (n = 80) (42). Matrix Reasoning is a measure of fluid reasoning that requires children to select missing pieces to complete a visual pattern. Picture Memory is a measure of working memory in which children must memorize and then identify pictures. Bug Search measures processing speed by requiring children to scan and mark simple visual information. Information consists of general, crystallized knowledge questions. Finally, Numeration measures the child’s understanding of number concepts. Scaled scores were used in all analyses.
Covariates
Parents reported their child’s date of birth, gender, race and ethnicity, family income, and exposure to ACEs. Age and gender were included as covariates in all analyses of molar eruption because molar eruption shows strong relationships with both variables (age: β = 0.842, 95% CI [0.743, 0.942], p < .001; gender: β = 0.349, 95% CI [0.159, 0.539], p < .001, controlling for age) (28). Poverty, ACEs, and racism-based stress are all environmental risk factors that can contribute to chronic stress and increase biological aging (28,43,44). To streamline analyses, we used exploratory factor analysis to generate a single score representing stress risk, which we used as a covariate and as the exposure variable in mediation analyses.
Parents were asked to indicate which of the following race categories applied to their child: American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, White, or Other. Parents were separately asked to indicate whether their child was Hispanic or Latino (Table S1). For our factor analysis, to capture risk for racism-based stress, we recoded race and ethnicity into 2 categories: a racial/ethnic majority group (non-Hispanic White; n = 36, 31%) and a racial/ethnic minority group (n = 81, 69%). Parents reported their total annual family income in 1 of 11 income brackets (less than $5000, $5000–$11,999, $12,000–$15,999, $16,000–$24,999, $25,000–$34,999, $35,000–$49,999, $50,000–$74,999, $75,000–$99,999, $100,000–$149,999, $150,000–$199,999, and $200,000 or more). Annual family income was estimated as the median value of each income bracket. Family income in this sample ranged from $2500 to $200,000 (mean = $80,051, SD = $66,735, n = 108) (Table S1). For context, the median income in Philadelphia County during the years of this study was $49,127 (45). Parents completed the child version of the ACEs questionnaire (46), which asks parents about their child’s lifetime experiences with 10 types of experiences with household dysfunction, parent separation, abuse, and neglect. An ACEs score is calculated by summing the binary responses to each of the 10 items. ACEs scores in this sample ranged from 0 to 7 (mean = 1.0, SD = 1.3, n = 111) (Table S1). For the factor analysis, ACEs were grouped into 3 categories: 0 ACEs (n = 52, 47%), 1 ACE (n = 36, 32%), and 2 or more ACEs (n = 23, 21%). It is possible that some parents failed to report ACE exposure because they did not witness events, did not remember events, or did not want to disclose sensitive data to researchers. We attempted to minimize the last possibility by allowing parents to complete questionnaires privately on tablet computers and ensuring that they understood that data are deidentified. Despite these limitations, parent report is the most standard way of reporting ACEs (47) and is less intrusive and time-consuming than other possible methods, including requesting sensitive health and Child Protective Service records. Furthermore, the rates of exposure to ACEs that we observe in our data are consistent with the national prevalence rates of ACEs among children in this age range. Nationally, 65% of children under the age of 5 have experienced no ACEs, and 52% of children between the ages of 6 and 11 years have experienced no ACEs (48).
To generate a single variable capturing children’s stress risk, we conducted a one-factor exploratory factor analysis of income, ACEs, and race (as a proxy for racism-based stress), using the psych package in R (49). All 3 variables showed acceptable loadings onto the single factor (income: λ = 0.92, ACEs: λ = −0.37, race: λ = 0.71). Individual factor scores extracted from the exploratory factor analysis were reverse coded so that a higher score represents greater purported stress risk (i.e., lower income, more ACEs, and non-White race). Greater stress risk was significantly associated with earlier eruption of the first permanent molars (β = 0.163, 95% CI [0.072, 0.254], p = .001).
Statistical Analyses
We examined associations between molar eruption and developmental outcomes using linear regression in R. All analyses included age and gender as covariates because molar eruption shows strong relationships with both variables (28,29). Missing data were handled by complete case analysis within each model. First, we tested the association between CBCL internalizing and externalizing symptoms and molar eruption. We re-ran both regressions with the factor score of stress risk as a covariate. Next, we tested associations between each cognitive test and molar eruption and again re-ran each regression with stress risk as a covariate. We report standardized effect sizes for independent variables, obtained by running the linear models after centering and scaling all variables. To correct for multiple comparisons, we used the Benjamini-Hochberg false discovery rate (FDR) (50). FDR correction was applied separately across the 2 CBCL composite scales and across all 5 cognitive tests. FDR correction was also applied separately for models with and without controlling for stress risk.
Next, we conducted mediation analyses to investigate whether molar eruption mediates the associations between the early environment and developmental outcomes. Because our data are cross-sectional and observational, we cannot draw causal conclusions from a mediation analysis; instead, we ran these analyses to understand whether accelerated maturation explains variance in the relationship between environmental measures and developmental outcomes. We used the R mediation package for causal mediation analysis (51), with stress risk as the independent variable and each cognitive test as the dependent variable. First, we tested associations between stress risk and cognition, and then we conducted mediation analyses for cognitive tests that were significantly related to stress risk. The “mediate” function estimates the average causal mediation effect (ACME) and the average direct effect, which together sum to the total effect of the independent variable on the dependent variable. The proportion mediated, which we report, represents the size of the ACME relative to the total effect.
Results
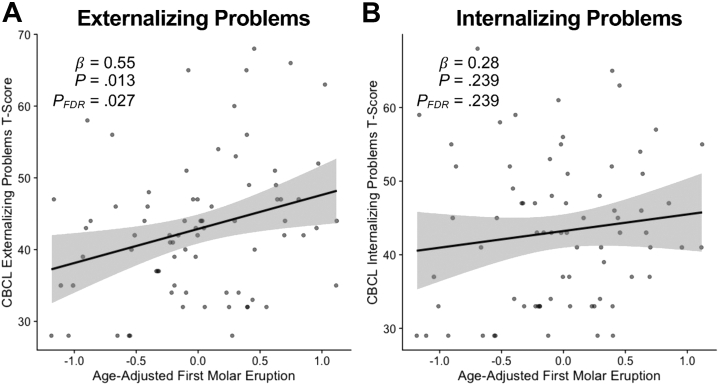
Children with accelerated molar maturation showed more externalizing symptoms (Table S3; Figure 1) (β = 0.545, 95% CI [0.117, 0.973], p = .013, FDR-corrected p = .027). Internalizing symptoms were not related to molar eruption (Table S3; Figure 1) (β = 0.283, 95% CI [−0.192, 0.758], p = .239, FDR-corrected p = .239). The association between molar eruption and externalizing symptoms was no longer significant after controlling for stress risk (FDR-corrected p = .069).
Figure 1.
Scatterplots show relationships between adjusted molar eruption and parent-reported (A) externalizing problems and (B) internalizing problems. Positive molar eruption residuals indicate accelerated molar eruption relative to chronological age and gender. CBCL, Child Behavior Checklist; FDR, false discovery rate.
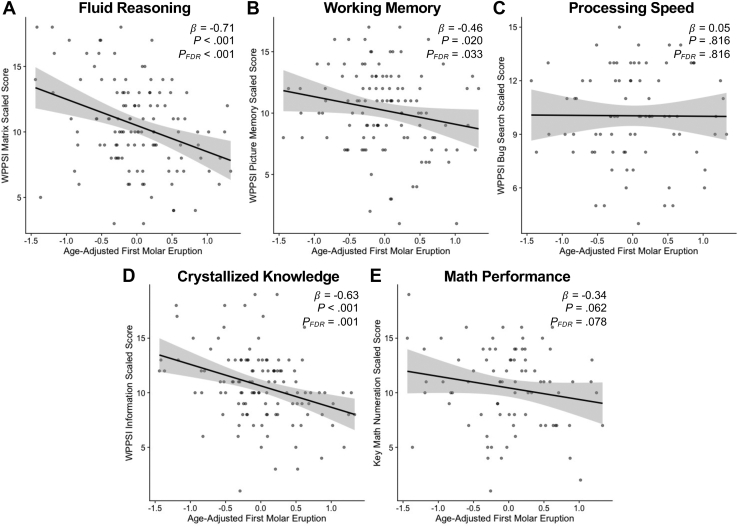
Accelerated molar eruption was also associated with lower performance on tests of fluid reasoning (Table S4; Figure 2A) (WPPSI-IV Matrix Reasoning, β = −0.707, 95% CI [−1.052, −0.362], p < .001, FDR-corrected p < .001), working memory (Figure 2B) (WPPSI-IV Picture Memory, β = −0.459, 95% CI [−0.843, −0.075], p = .020, FDR-corrected p = .033), and crystallized knowledge (Figure 2D) (WPPSI-IV Information, β = −0.634, 95% CI [−0.984, −0.284], p < .001, FDR-corrected p = .001). Molar eruption was marginally associated with math performance (Figure 2E) (KeyMath-3 Numeration, β = −0.339, 95% CI [−0.696, 0.018], p = .062, FDR-corrected p = .078) and was not associated with processing speed (Figure 2C) (WPPSI-IV Bug Search, β = 0.049, 95% CI [−0.369, 0.468], p = .816). Associations between molar eruption status and fluid reasoning held when controlling for stress risk (Table S4) (β = −0.561, 95% CI [−0.916, −0.206], p = .002, FDR-corrected p = .011). Molar eruption was also associated with crystallized knowledge after controlling for stress risk (β = −0.402, 95% CI [−0.738, −0.066], p = .019, FDR-corrected p = .049). Associations between molar eruption and working memory, math performance, and processing speed were not significant after adding stress risk to the models (Table S4).
Figure 2.
Scatterplots show relationships between adjusted molar eruption and scaled scores on cognitive tests of (A) fluid reasoning, (B) working memory, (C) processing speed, (D) crystallized knowledge, and (E) math performance. Positive molar eruption residuals indicate accelerated molar eruption relative to chronological age and gender. FDR, false discovery rate; WPPSI, Wechsler Preschool & Primary Scale of Intelligence.
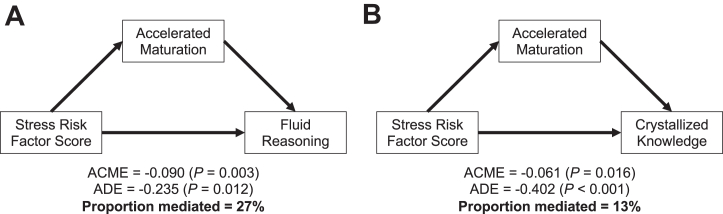
Finally, we explored relationships among stress, cognition, and accelerated maturation by investigating whether molar eruption mediated associations between stress risk and cognition. Mediation was tested for developmental outcomes that were significantly related to the stress risk factor score. Stress risk was not associated with internalizing or externalizing behavior or with processing speed (ps > .05). Higher stress risk was significantly related to performance on fluid reasoning (β = −0.322, 95% CI [−0.498, −0.147], p < .001), working memory (β = −0.292, 95% CI [−0.478, −0.106], p = .002), crystallized knowledge (β = −0.473, 95% CI [−0.637, −0.309], p < .001), and math performance (β = −0.444, 95% CI [−0.646, −0.242], p < .001), and thus, we tested whether molar eruption mediated these associations. Molar eruption partially mediated the relationships between stress risk and both fluid reasoning (proportion mediated = 0.273, p = .004) (Figure 3) and crystallized knowledge (proportion mediated = 0.126, p = .016) (Figure 3) but not the relationship between stress risk and working memory (p = .172) or math performance (p = .491).
Figure 3.
Causal mediation analyses. Accelerated maturation, as indexed by molar eruption, partially mediates the associations between stress risk and both fluid reasoning and crystallized knowledge. ACME, average causal mediation effect; ADE, average direct effect.
Discussion
Molar eruption timing, characterized by MRI, showed significant associations with mental health and cognition in early childhood. More specifically, children with earlier molar eruption demonstrated higher externalizing problems and lower performance on assessments of fluid reasoning, crystallized knowledge, and working memory. Fluid reasoning and crystallized knowledge remained significantly related to molar eruption after controlling for a composite factor of stress risk variables, suggesting that understanding a child’s maturational state may provide unique predictive power for the development of reasoning skills and acquisition of crystallized knowledge. Furthermore, molar eruption significantly mediated the associations between stress risk and both fluid reasoning and crystallized knowledge. Although we cannot draw causal conclusions using cross-sectional observational data, these findings are broadly consistent with the hypothesis that early adversity can accelerate maturational processes in the brain, leading to reduced plasticity and negatively affecting cognition and learning.
The strongest relationship that we observed was the relationship between early maturation and fluid reasoning. There are multiple reasons why fluid reasoning may show the strongest relationship with accelerated development after controlling for stress exposure variables. Fluid reasoning may depend most strongly on generating novel ideas and thinking flexibly and therefore may be most sensitive to overly stabilized neural networks. Fluid reasoning may also be less dependent than crystallized skills on income-related experiences other than stress, such as language and explicit instruction. It is also possible that associations between accelerated development and domains of cognition will shift across development. Fluid reasoning predicts change over time in crystallized abilities and academic knowledge (52), and the strength of association between fluid reasoning and academic skills increases with age (53). Given this complex developmental cascade of cognitive skills, we may expect accelerated maturation to be predictive of different cognitive domains at different ages.
We did not find a significant association between molar eruption and either processing speed or math performance. Processing speed is supported by white matter maturation (54) because myelination increases action potential conduction speed. In contrast, processing speed performance also relies on attention and inhibitory control (55), which can be impaired by early life stress (56). If accelerated structural brain development increases conduction speed but limits attentional control, there may be no observed association between accelerated maturation and processing speed. We also did not observe strong associations between molar eruption and math performance. Notably, the math assessment was only scaled for children ages 5 years and older, and a test of processing speed was only administered to a subgroup of participants; thus, both subtests had a reduced sample size. Math performance in this age range may also be more strongly driven by explicit instruction in the home that may vary by family income. Future longitudinal research is needed to explore whether associations between accelerated maturation and cognition vary across development.
Finally, we observed a significant association between molar eruption and children’s externalizing problems, although this association did not hold when controlling for a composite factor for stress risk. This finding is in contrast to studies that have shown a correspondence between accelerated maturation and both internalizing and externalizing problems in adolescence (1,57, 58, 59). There are several possible explanations for our limited mental health findings. First, the CBCL was only administered in 1 of the 2 studies included in this sample, so analyses of mental health included a smaller sample than analyses of cognition. Second, we relied on parent report of child symptoms. Childhood externalizing behaviors are more salient to observers, and thus parents can be more reliable at reporting externalizing than internalizing problems (60). Furthermore, the majority of psychiatric illnesses peak in onset during adolescence (61), and thus the mental health consequences of accelerated maturation may not emerge until later in development. Our results suggest that cognitive vulnerability associated with accelerated maturation may precede mental health vulnerability, although future longitudinal research is needed to further explore this developmental cascade.
This study has a number of limitations. First, our data are cross-sectional, and therefore, we cannot make conclusions about whether early maturation constrains cognitive development or mental health over time. Future longitudinal research is needed to illuminate co-occurring trajectories of physical maturation, cognitive development, and mental health. It is possible that poor mental health or cognitive difficulties lead to accelerated maturation (27) or that there are reciprocal interactions between the pace of maturation and health across the lifespan. Second, this study used a limited selection of cognitive measures. It is possible that accelerated maturation plays an adaptive or protective role for other skills that we did not measure, such as fear conditioning (62). Third, we cannot directly compare how molar eruption is related to mental health versus cognition because the 2 sets of developmental outcomes were measured with different methods (parent-report vs. test-based assessment). Fourth, it is important to note that observed associations between molar eruption and cognition are neither diagnostic nor deterministic. Early maturation does not guarantee cognitive or mental health challenges, and future work is needed before conclusions can be drawn about the utility of dental development as a screening tool for cognitive risk. Molar eruption is also not the mechanism linking stress exposure and cognition, but rather a putative biomarker of the impact of a child’s experiences on their development. Fifth, there may be pathways other than psychosocial stress that link income, ACEs, and race with the timing of molar eruption. Finally, future research is needed to establish the mechanisms of accelerated dental development and the extent to which molar eruption timing is predictive of accelerated maturation across other domains, such as epigenetic aging or brain age.
Despite these limitations, this work provides insight into the potential developmental consequences of accelerated maturation. Children vary greatly in how they perceive, internalize, and respond to stressors in their lives (37). Thus, knowing the pace or status of a child’s maturation may reflect the developmental impact of their stress history beyond what can be measured by “objective” questionnaires alone. There are several important future directions for this research. First, it is unknown to what extent a child’s maturational trajectory is established early in life or even prenatally (63) or can be recalibrated across development. Understanding when a child’s maturational trajectory is established and perhaps whether there are variable sensitive periods across biological domains may inform decisions about intervention timing. Although a reduced plasticity account may suggest that children who develop faster would be less responsive to interventions, some interventions have instead found that the most academically at-risk children benefit the most (64,65). Thus, it is important to understand whether children who are growing up more quickly are more or less likely to benefit from educational or psychosocial interventions. Overall, our results suggest that dental milestones may make an important contribution toward a holistic understanding of risk and resilience during child development.
Acknowledgments and Disclosures
This work was supported by the Jacobs Foundation Early Career Award (to APM), National Institute on Drug Abuse (Award No. 1R34DA050297-01 [to APM]), and National Science Foundation Graduate Research Fellowships (to CLM, UAT, and ALB).
We thank the families who participated in this research. We also thank Jasmine Forde, Katrina Simon, Sophie Sharp, Yoojin Hahn, Jamie Bogert, Alexis Broussard, Ava Cruz, Samantha Ferleger, Destiny Frazier, Jessica George, Abigail Katz, Sun Min Kim, Hunter Liu, Dominique Martinez, Ortal Nakash, Emily Orengo, Christina Recto, Leah Sorcher, and Alexis Uria for their help with data acquisition. Finally, we thank Elizabeth Brannon, Ph.D., Stephanie Bugden, Ph.D., and Nuwar Ahmed for sharing magnetic resonance imaging and behavioral data.
A previous version of this article was published as a preprint on PsyArXiv: https://psyarxiv.com/t2qg5/.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2023.02.006.
Supplementary Material
References
- 1.Negriff S., Susman E.J. Pubertal timing, depression, and externalizing problems: A framework, review, and examination of gender differences. J Res Adolesc. 2011;21:717–746. [Google Scholar]
- 2.Kaltiala-Heino R., Marttunen M., Rantanen P., Rimpelä M. Early puberty is associated with mental health problems in middle adolescence. Soc Sci Med. 2003;57:1055–1064. doi: 10.1016/s0277-9536(02)00480-x. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin K.A., Sheridan M.A., Winter W., Fox N.A., Zeanah C.H., Nelson C.A. Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biol Psychiatry. 2014;76:629–638. doi: 10.1016/j.biopsych.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamlat E.J., McCormick K.C., Young J.F., Hankin B.L. Early pubertal timing predicts onset and recurrence of depressive episodes in boys and girls. J Child Psychol Psychiatry. 2020;61:1266–1274. doi: 10.1111/jcpp.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendle J., Leve L.D., Van Ryzin M.V., Natsuaki M.N. Linking childhood maltreatment with girls’ internalizing symptoms: Early puberty as a tipping point. J Res Adolesc. 2014;24:689–702. doi: 10.1111/jora.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridout K.K., Ridout S.J., Price L.H., Sen S., Tyrka A.R. Depression and telomere length: A meta-analysis. J Affect Disord. 2016;191:237–247. doi: 10.1016/j.jad.2015.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tollenaar M.S., Beijers R., Garg E., Nguyen T.T.T., Lin D.T.S., MacIsaac J.L., et al. Internalizing symptoms associate with the pace of epigenetic aging in childhood. Biol Psychol. 2021;159 doi: 10.1016/j.biopsycho.2021.108021. [DOI] [PubMed] [Google Scholar]
- 8.Suarez A., Lahti J., Czamara D., Lahti-Pulkkinen M., Knight A.K., Girchenko P., et al. The epigenetic clock at birth: Associations with maternal antenatal depression and child psychiatric problems. J Am Acad Child Adolesc Psychiatry. 2018;57:321–328.e2. doi: 10.1016/j.jaac.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boynton-Jarrett R., Harville E.W. A prospective study of childhood social hardships and age at menarche. Ann Epidemiol. 2012;22:731–737. doi: 10.1016/j.annepidem.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colich N.L., Platt J.M., Keyes K.M., Sumner J.A., Allen N.B., McLaughlin K.A. Earlier age at menarche as a transdiagnostic mechanism linking childhood trauma with multiple forms of psychopathology in adolescent girls. Psychol Med. 2020;50:1090–1098. doi: 10.1017/S0033291719000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendle J., Ryan R.M., McKone K.M. Early childhood maltreatment and pubertal development: Replication in a population-based sample. J Res Adolesc. 2016;26:595–602. doi: 10.1111/jora.12201. [DOI] [PubMed] [Google Scholar]
- 12.Negriff S., Saxbe D.E., Trickett P.K. Childhood maltreatment, pubertal development, HPA axis functioning, and psychosocial outcomes: An integrative biopsychosocial model. Dev Psychobiol. 2015;57:984–993. doi: 10.1002/dev.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jovanovic T., Vance L.A., Cross D., Knight A.K., Kilaru V., Michopoulos V., et al. Exposure to violence accelerates epigenetic aging in children. Sci Rep. 2017;7:8962. doi: 10.1038/s41598-017-09235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridout K.K., Levandowski M., Ridout S.J., Gantz L., Goonan K., Palermo D., et al. Early life adversity and telomere length: A meta-analysis. Mol Psychiatry. 2018;23:858–871. doi: 10.1038/mp.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumner J.A., Colich N.L., Uddin M., Armstrong D., McLaughlin K.A. Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biol Psychiatry. 2019;85:268–278. doi: 10.1016/j.biopsych.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busso D.S., McLaughlin K.A., Brueck S., Peverill M., Gold A.L., Sheridan M.A. Child abuse, neural structure, and adolescent psychopathology: A longitudinal study. J Am Acad Child Adolesc Psychiatry. 2017;56:321–328.e1. doi: 10.1016/j.jaac.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., et al. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gold A.L., Sheridan M.A., Peverill M., Busso D.S., Lambert H.K., Alves S., et al. Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. J Child Psychol Psychiatry. 2016;57:1154–1164. doi: 10.1111/jcpp.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gur R.E., Moore T.M., Rosen A.F.G., Barzilay R., Roalf D.R., Calkins M.E., et al. Burden of environmental adversity associated with psychopathology, maturation, and brain behavior parameters in youths. JAMA Psychiatry. 2019;76:966–975. doi: 10.1001/jamapsychiatry.2019.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackey A.P., Finn A.S., Leonard J.A., Jacoby-Senghor D.S., West M.R., Gabrieli C.F.O., Gabrieli J.D.E. Neuroanatomical correlates of the income-achievement gap. Psychol Sci. 2015;26:925–933. doi: 10.1177/0956797615572233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belsky J. Early-life adversity accelerates child and adolescent development. Curr Dir Psychol Sci. 2019;28:241–246. [Google Scholar]
- 22.Belsky J., Steinberg L., Draper P. Childhood experience, interpersonal development, and reproductive strategy: And evolutionary theory of socialization. Child Dev. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 23.Bateson M., Nettle D. Why are there associations between telomere length and behaviour? Philos Trans R Soc Lond B Biol Sci. 2018;373 doi: 10.1098/rstb.2016.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendle J. Why puberty matters for psychopathology. Child Dev Perspect. 2014;8:218–222. [Google Scholar]
- 25.O’Donovan A., Slavich G.M., Epel E.S., Neylan T.C. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci Biobehav Rev. 2013;37:96–108. doi: 10.1016/j.neubiorev.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolkowitz O.M., Mellon S.H., Epel E.S., Lin J., Dhabhar F.S., Su Y., et al. Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress – Preliminary findings. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wade M., Fox N.A., Zeanah C.H., Nelson C.A., Drury S.S. Telomere length and psychopathology: Specificity and direction of effects within the Bucharest early intervention project. J Am Acad Child Adolesc Psychiatry. 2020;59:140–148.e3. doi: 10.1016/j.jaac.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDermott C.L., Hilton K., Park A.T., Tooley U.A., Boroshok A.L., Mupparapu M., et al. Early life stress is associated with earlier emergence of permanent molars. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2105304118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pahel B.T., Vann W.F., Divaris K., Rozier R.G. A contemporary examination of first and second permanent molar emergence. J Dent Res. 2017;96:1115–1121. doi: 10.1177/0022034517716395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (US) National health and nutrition examination survey. 2020. https://www.cdc.gov/nchs/nhanes.htm Available at:
- 31.Roubinov D., Meaney M.J., Boyce W.T. Change of pace: How developmental tempo varies to accommodate failed provision of early needs. Neurosci Biobehav Rev. 2021;131:120–134. doi: 10.1016/j.neubiorev.2021.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gopnik A. Childhood as a solution to explore–exploit tensions. Philos Trans R Soc Lond B Biol Sci. 2020;375 doi: 10.1098/rstb.2019.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gopnik A., Frankenhuis W.E., Tomasello M. Introduction to special issue: ‘life history and learning: how childhood, caregiving and old age shape cognition and culture in humans and other animals. ’ Philos Trans R Soc Lond B Biol Sci. 2020;375 doi: 10.1098/rstb.2019.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tooley U.A., Bassett D.S., Mackey A.P. Environmental influences on the pace of brain development. Nat Rev Neurosci. 2021;22:372–384. doi: 10.1038/s41583-021-00457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw P., Greenstein D., Lerch J., Clasen L., Lenroot R., Gogtay N., et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 36.Raffington L., Tanksley P.T., Sabhlok A., Vinnik L., Mallard T., King L.S., et al. Socially stratified epigenetic profiles are associated with cognitive functioning in children and adolescents. Psychol Sci. 2023;34:170–185. doi: 10.1177/09567976221122760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith K.E., Pollak S.D. Social relationships and children’s perceptions of adversity. Child Dev Perspect. 2021;15:228–234. doi: 10.1111/cdep.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demirjian A., Goldstein H., Tanner J.M. A new system of dental age assessment. Hum Biol. 1973;45:211–227. [PubMed] [Google Scholar]
- 39.Achenbach T.M., Rescorla L.A. ASEBA; Burlington, VT: 2001. Manual for the ASEBA School-Age Forms and Profiles. [Google Scholar]
- 40.Achenbach T.M., Rescorla L.A. ASEBA; Burlington, VT: 2000. Manual for the ASEBA Preschool Forms and Profiles. [Google Scholar]
- 41.Wechsler D. 4th ed. Psychological Corporation; San Antonio, TX: 2012. Wechsler Preschool and Primary Scale of Intelligence. [Google Scholar]
- 42.Connolly A.J. 3rd ed. Pearson, Inc; Minneapolis, MN: 2007. KeyMath Diagnostic Assessment. [Google Scholar]
- 43.Geronimus A.T., Hicken M., Keene D., Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danese A., McEwen B.S. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 45.United States Census Bureau QuickFacts: Philadelphia County, Pennsylvania. https://www.census.gov/quickfacts/fact/table/philadelphiacountypennsylvania/AFN120212 Available at:
- 46.Murphy A., Steele H., Steele M., Allman B., Kastner T., Dube S.R. In: Integrated Early Childhood Behavioral Health in Primary Care: A Guide to Implementation and Evaluation. Briggs R.D., editor. Springer International Publishing; Cham, Germany: 2016. The clinical adverse childhood experiences (ACEs) questionnaire: Implications for trauma-informed behavioral healthcare; pp. 7–16. [Google Scholar]
- 47.Bethell C.D., Carle A., Hudziak J., Gombojav N., Powers K., Wade R., Braveman P. Methods to assess adverse childhood experiences of children and families: Toward approaches to promote child well-being in policy and practice. Acad Pediatr. 2017;17:S51–69. doi: 10.1016/j.acap.2017.04.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bethell C.D., Davis M.B., Gombojav N., Stumbo S., Powers K. Issue brief: Adverse childhood experiences among US children. Child and Adolescent Health Measurement Initiative. Johns Hopkins Bloomberg School of Public Health. http://cahmi.org/projects/adverse-childhood-experiences-aces Available at:
- 49.Revelle W. psych: Procedures for Psychological: Psychometric, and Personality Research, version 2.2.9. 2022. https://CRAN.R-project.org/package=psych Available at:
- 50.Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B (Methodol) 1995;57:289–300. [Google Scholar]
- 51.Tingley D., Yamamoto T., Hirose K., Keele L., Imai K. mediation: R package for causal mediation analysis. J Stat Softw. 2014;59:1–38. [Google Scholar]
- 52.Ferrer E., McArdle J.J. An experimental analysis of dynamic hypotheses about cognitive abilities and achievement from childhood to early adulthood. Dev Psychol. 2004;40:935–952. doi: 10.1037/0012-1649.40.6.935. [DOI] [PubMed] [Google Scholar]
- 53.Peng P., Wang T., Wang C., Lin X. A meta-analysis on the relation between fluid intelligence and reading/mathematics: Effects of tasks, age, and social economics status. Psychol Bull. 2019;145:189–236. doi: 10.1037/bul0000182. [DOI] [PubMed] [Google Scholar]
- 54.Ferrer E., Whitaker K.J., Steele J.S., Green C.T., Wendelken C., Bunge S.A. White matter maturation supports the development of reasoning ability through its influence on processing speed. Dev Sci. 2013;16:941–951. doi: 10.1111/desc.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goth-Owens T.L., Martinez-Torteya C., Martel M.M., Nigg J.T. Processing speed weakness in children and adolescents with non-hyperactive but inattentive ADHD (ADD) Child Neuropsychol. 2010;16:577–591. doi: 10.1080/09297049.2010.485126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawson G.M., Hook C.J., Farah M.J. A meta-analysis of the relationship between socioeconomic status and executive function performance among children. Dev Sci. 2018;21 doi: 10.1111/desc.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malouff J.M., Schutte N.S. A meta-analysis of the relationship between anxiety and telomere length. Anxiety Stress Coping. 2017;30:264–272. doi: 10.1080/10615806.2016.1261286. [DOI] [PubMed] [Google Scholar]
- 58.Ullsperger J.M., Nikolas M.A. A meta-analytic review of the association between pubertal timing and psychopathology in adolescence: Are there sex differences in risk? Psychol Bull. 2017;143:903–938. doi: 10.1037/bul0000106. [DOI] [PubMed] [Google Scholar]
- 59.Suarez A., Lahti J., Czamara D., Lahti-Pulkkinen M., Girchenko P., Andersson S., et al. The epigenetic clock and pubertal, neuroendocrine, psychiatric, and cognitive outcomes in adolescents. Clin Epigenetics. 2018;10:96. doi: 10.1186/s13148-018-0528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duhig A.M., Renk K., Epstein M.K., Phares V. Interparental agreement on internalizing, externalizing, and total behavior problems: A meta-analysis. Clin Psychol Sci Pract. 2000;7:435–453. [Google Scholar]
- 61.Solmi M., Radua J., Olivola M., Croce E., Soardo L., Salazar de Pablo G., et al. Age at onset of mental disorders worldwide: Large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2022;27:281–295. doi: 10.1038/s41380-021-01161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bath K.G., Manzano-Nieves G., Goodwill H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm Behav. 2016;82:64–71. doi: 10.1016/j.yhbeh.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ciceri G., Cho H., Kshirsagar M., Baggiolini A., Aromolaran K.A., Walsh R.M., et al. An epigenetic barrier sets the timing of human neuronal maturation. Dev Biology. 2022 doi: 10.1101/2022.06.02.490114. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diamond A., Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333:959–964. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mackey A.P., Park A.T., Robinson S.T., Gabrieli J.D.E. A pilot study of classroom-based cognitive skill instruction: Effects on cognition and academic performance. Mind Brain Educ. 2017;11:85–95. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.