Abstract
Background
Hippocampal abnormalities are among the most consistent findings in schizophrenia. Numerous studies have reported deficits in hippocampal volume, function, and connectivity in the chronic stage of illness. While hippocampal volume and function deficits are also present in the early stage of illness, there is mixed evidence of both higher and lower functional connectivity. Here, we use graph theory to test the hypothesis that hippocampal network connectivity is broadly lowered in early psychosis and progressively worsens over 2 years.
Methods
We examined longitudinal resting-state functional connectivity in 140 participants (68 individuals in the early stage of psychosis, 72 demographically similar healthy control individuals). We used an anatomically driven approach to quantify hippocampal network connectivity at 2 levels: 1) a core hippocampal-medial temporal lobe cortex (MTLC) network; and 2) an extended hippocampal-cortical network. Group and time effects were tested in a linear mixed effects model.
Results
Early psychosis patients showed elevated functional connectivity in the core hippocampal-MTLC network, but contrary to our hypothesis, did not show alterations within the broader hippocampal-cortical network. Hippocampal-MTLC network hyperconnectivity normalized longitudinally and predicted improvement in positive symptoms but was not associated with increasing illness duration.
Conclusions
These results show abnormally elevated functional connectivity in a core hippocampal-MTLC network in early psychosis, suggesting that selectively increased hippocampal signaling within a localized cortical circuit may be a marker of the early stage of psychosis. Hippocampal-MTLC hyperconnectivity could have prognostic and therapeutic implications.
Keywords: Functional connectivity, Graph theory, Hyperconnectivity, Medial temporal lobe, Resting-state fMRI, Schizophrenia
Hippocampal deficits are among the most robust and well-replicated brain abnormalities in schizophrenia (1, 2, 3). Postmortem and neuroimaging studies have demonstrated involvement of the hippocampus at all stages of illness (4, 5, 6, 7, 8, 9) and implicated a fundamental relationship between smaller hippocampal volumes and elevated hippocampal metabolism in the progression of psychosis (10, 11, 12, 13). Early hippocampal dysfunction begins in the CA1 region (13, 14, 15) and may remain relatively stable from psychosis onset through the early course of illness (6,7,16,17), followed by a spread to other subfields (13,15) and cortical regions by way of long-range efferent projections (18) during a period of progressive deterioration (15,19, 20, 21). These findings identify the hippocampus as a critical early biomarker for detection and therapeutic intervention (12,22,23). However, the emergence and progression of hippocampal functional connectivity remains to be fully characterized.
Disrupted functional integration across brain regions has been proposed as a mechanism underlying psychosis (24,25). Widespread dysconnectivity is considered a hallmark feature of chronic schizophrenia (26), and many studies indicate that this view extends to hippocampal connections (27, 28, 29, 30, 31, 32). In contrast, in early psychosis, functional connectivity studies have produced mixed results, including findings of higher (33,34), lower (34,35), and normal (35) hippocampal-cortical connectivity. Mixed connectivity findings may stem from interneuron hypofunction in the hippocampus that results in increased hippocampal metabolism, glutamatergic tone, and disinhibition (36). In the early stages of psychotic illness, this hippocampal disinhibition may contribute to both elevated and lowered connectivity (34), consistent with the disruptive and compensatory connectivity patterns identified in temporal lobe epilepsy (37). Investigation of hippocampal network connectivity as a whole, as opposed to individual connections, may clarify the patterns associated with specific illness stages. The introduction of quantitative network analysis has facilitated examination of aberrant connectivity across entire networks (38). These studies have suggested that with advancing stages of psychotic illness, aberrant connectivity patterns may change from mainly hyperconnectivity to hypoconnectivity (33), although it remains unclear whether this shift in connectivity patterns is also present in the hippocampal network.
One reason for this gap in knowledge may be the distinct architecture of hippocampal connections. The hippocampus binds sensory information from distributed parts of the cortex to form holistic representations of experiences. Polymodal sensory information converges on the hippocampus via the medial temporal lobe cortices (MTLCs)—perirhinal, parahippocampal, and entorhinal (39,40)—culminating in highly integrated information processing at the level of the hippocampus. Processed information is then relayed to a distributed cortical network via return circuits through the MTLC (40), suggesting that the hippocampus occupies an important position in whole-brain networks. However, data-driven whole-brain network analyses often do not identify the hippocampus as a central hub because its unique connectional architecture results neither in a disproportionately high number of structural connections nor in a central location in the whole-brain connectome (41, 42, 43). For example, data-driven approaches have generally partitioned the default mode network, which includes a number of regions that support hippocampal memory, into the following 3 distinct subnetworks (44): a hippocampal-MTLC subnetwork; a midline cortical subnetwork; and a subnetwork comprising the dorsal medial prefrontal, lateral temporal, and ventrolateral prefrontal cortex (45). Although early research included the hippocampal-MTLC subnetwork as a key component of the default mode network (46,47), recent findings have largely focused on cortical subnetworks. However, when communication capacity is considered, the centrality of the hippocampal-MTLC network embedded in the whole-brain connectome becomes clear (47, 48, 49).
To address this gap, we used an anatomically guided approach to examine hippocampal network connectivity in both a core hippocampal-MTLC network and an extended hippocampal-cortical network in a cohort of early psychosis patients. To characterize network interactions, we used the graph theory metric of modularity, an index of the balance between functional integration and segregation of networks. Higher modularity scores indicate greater cohesion of connections into a distinct network, while lower modularity scores indicate lower cohesion. Using a similar approach, we previously demonstrated a decreased modularity and broad hypoconnectivity in hippocampal networks in patients with chronic schizophrenia (28). Because hippocampal deficits are already prominent at the earliest stages of illness, we hypothesized that early psychosis would also be associated with decreased modularity and widespread network hypoconnectivity. To determine whether hippocampal network connectivity changes during the early stage of illness, we used a prospective 2-year longitudinal study design to examine hippocampal network function across the initial years of illness. Patients completed a neuroimaging assessment within the first 2 years of illness and follow-up assessments every 8 months for 2 years. We hypothesized that early psychosis patients would show a greater hippocampal network hypoconnectivity over 2 years, which is in line with findings of broad hypoconnectivity in chronic illness.
Methods and Materials
Participants
Participants (N= 140) were 68 individuals in the early stage of a psychotic disorder and 72 healthy control participants enrolled between May 2013 and February 2018 into a prospective 2-year longitudinal neuroimaging study. Most participants (57 early psychosis, 66 healthy control subjects) were included at 2 or more visits. Details regarding participant attrition are included in the Supplement. Groups were recruited with the goal of having similar age, sex, race, and parental education across groups (Table 1). Early psychosis patients included in the analysis had similar demographic and clinical characteristics as those who were excluded.
Table 1.
Participant Characteristics
| Demographics | Early Psychosis Group, n = 68 | Healthy Control Group, n = 72 | Early Psychosis vs. Healthy Control |
|
|---|---|---|---|---|
| Statistic | p | |||
| Age at Enrollment, Years | 21 ± 3.3 | 22 ± 2.8 | F1,139 = 0.76 | .38 |
| Sex, % Male | 79% | 74% | χ21 = 0.65 | .42 |
| Race, Black/Other/White | 15/1/52 | 12/4/56 | χ22 = 2.17 | .34 |
| Ethnicity, % Non-Hispanic | 99% | 93% | χ22 = 2.55 | .11 |
| Handedness, % Right | 94% | 92% | χ21 = 0.32 | .57 |
| Premorbid IQ, WTAR | 103 ± 15.0 | 113 ± 9.7 | F1,130 = 19.67a | <.001b |
| Participant’s Education, Years | 13 ± 2.2 | 15 ± 1.9 | F1,139 = 13.66 | <.001b |
| Parental Education, Years | 15 ± 2.8 | 15 ± 2.3 | F1,139 = 0.54 | .46 |
| Clinical Characteristics | Study Entry, n = 52c |
2-Year Follow-up, n = 56 |
Study Entry vs. 2 Years |
|
| Diagnosis, SZF/SZ/BPd | 35/14/3 | 15/41/0 | − | − |
| Duration of Illness, Months | 7.2 ± 5.9 (1–24) | 31.8 ± 5.7 (25–48) | − | − |
| CPZ, mg | 337 ± 155.9 (75–750) | 299 ± 197.2 (50–879) | F1,79 = 0.93 | .34 |
| Antipsychotic Treatment, % | 85% | 64% | χ21 = 5.80 | .02b |
| PANSS Total | 66 ± 20.7 (34–114) | 52 ± 15.1 (33–104) | F1,107 = 15.32 | <.001b |
| PANSS-positive | 16 ± 7.0 (7–36) | 13 ± 4.7 (7–27) | F1,107 = 7.68 | .007b |
| PANSS-negative | 17 ± 7.1 (7–33) | 12 ± 6.2 (7–32) | F1,107 = 14.55 | <.001b |
| PANSS-general | 32 ± 9.6 (18–59) | 27 ± 7.0 (17–46) | F1,107 = 11.85 | .001b |
| HAM-D | 11 ± 8.0 (1–29) | 7 ± 6.3 (0–28) | F1,106 = 9.42e | .003b |
| YMRS | 2 ± 4.1 (0–19) | 2 ± 3.0 (0–15) | F1,106 = 0.31e | .58 |
Values are presented as mean ± SD, mean ± SD (range), percentage, or n. Demographic data are presented for included study participants.
BP, bipolar disorder with psychotic features; CPZ, chlorpromazine equivalent; HAM-D, Hamilton Depression Rating Scale−17 item; PANSS, Positive and Negative Syndrome Scale; SZF, schizophreniform; SZ, schizophrenia; WTAR, Wechsler Test of Adult Reading; YMRS, Young Mania Rating Scale.
WTAR scores are reported for native English speakers (early psychosis patients, n = 66; healthy control subjects, n = 65).
Significant p values (p ≤ .05).
All data meeting quality thresholds were included. Exclusions are detailed by visit and group in the Supplement.
All participants met criteria for a schizophrenia-spectrum disorder diagnosis at 2-year follow-up.
One patient missing HAM-D and YMRS at study entry.
Patients in the early stage of a psychotic disorder (<2 years following psychosis onset) were recruited from the Vanderbilt Psychiatric Hospital inpatient units and outpatient clinics. To specifically target early neuropathology (50), a majority of early psychosis patients were recruited during the initial months following onset (mean = 7 ± 6 months, range = 1–24 months). Diagnoses were determined using the Structured Clinical Interview for DSM-IV-TR (51,52) augmented by extensive review of all available medical records and finalized by a senior psychiatrist (SH) during diagnostic consensus meetings. Patients were required to meet criterion A for schizophrenia at study entry. Only patients with either schizophreniform disorder or schizophrenia/schizoaffective disorder (hereafter referred to as schizophrenia) at 2-year follow-up were included in this analysis. Healthy control participants were recruited from the community via advertisements. Exclusion criteria for all participants were younger than 16 or older than 65 years of age, premorbid IQ less than 75, a history of significant head injury, presence of a major medical or neurologic illness, any contraindications for magnetic resonance imaging (MRI), substance abuse within the past month, and uncorrected vision deficits. In addition, healthy control participants were excluded for any history of Axis I disorders or prior psychotropic medication use and for having a first-degree relative with a psychotic illness.
Data from participants in this cohort have been included in previous reports (6,7,19,53, 54, 55, 56, 57, 58, 59), but the functional connectivity analyses presented here have not. All participants provided written informed consent and received monetary compensation for their time. The study was approved by the Vanderbilt University Institutional Review Board.
Clinical Measures
Diagnostic assessments were collected during in-person interviews at study entry and 2-year visits. The onset of psychosis symptoms was determined using the Symptom Onset Scale (60). The duration of psychosis was calculated as the time between symptom onset and study enrollment. Chlorpromazine (CPZ)-equivalent doses were calculated for patients on antipsychotic medication (61,62). Premorbid IQ was estimated using the Wechsler Test of Adult Reading (63,64,65). Clinical symptom severity was assessed at each study visit using the Positive and Negative Syndrome Scale (PANSS) (66,67), the Hamilton Depression Rating Scale (HAM-D) (68), and the Young Mania Rating Scale (69). Clinical and cognitive characteristics of the participants are reported in Table 1 and Table S3.
Imaging Data
Acquisition and Preprocessing
High resolution T1-weighted structural and 7-minute echo-planar resting-state functional MRI (fMRI) data were collected at each study visit. Imaging data were acquired on 2 identical 3T Philips Intera Achieva MRI scanners using a 32-channel head coil at the Vanderbilt University Institute for Imaging Science. Images were processed on the Vanderbilt University Institute of Imaging Science Center for Computational Imaging XNAT platform (70) in MATLAB (version 2018a; The MathWorks, Inc.) using SPM12 (http://www.fil.ion.ucl.ac.uk/spm). Resting-state data were motion-corrected, coregistered to the participant’s structural image, and normalized to a Montreal Neurological Institute T1 template image (see the Supplement for full preprocessing details).
Data Quality
Data quality and exclusions are detailed in the Supplement. Overall motion in the sample was low (median framewise displacement [FD] = 0.042 mm) and was higher in healthy control participants than in patients (p = .002). To ensure that differences did not influence graph theory comparisons, FD was included as a covariate of no interest for all between-group comparisons.
Modularity Analysis
Regions of Interest
Hippocampal network connectivity was calculated for the following 2 predefined networks: 1) a core hippocampal-MTLC network consisting of the hippocampus, rhinal cortex, and parahippocampal cortex (71); and 2) an extended hippocampal-cortical network consisting of functionally connected prefrontal, posterior parietal, temporal, thalamic, and amygdala regions that interact with the core hippocampal network to support hippocampally guided behavior (72, 73, 74). The hippocampal-MTLC network had 12 regions of interest (ROIs) divided into 2 a priori modules/communities (left and right, separately). The hippocampal-cortical network had 66 ROIs divided into 4 modules/communities (left–right and anterior–posterior, separately). Community assignments are detailed by anatomical brain region in Table S1 and visualized in Figure 1. To provide a comparison to the hippocampal network, connectivity was also calculated for the visual network (75) (Table S2). The visual network was chosen in light of considerable visual processing (76) and visual ROI connectivity (77,78) deficits in schizophrenia.
Figure 1.
(A) Hippocampal networks are visualized on medial and lateral brain surfaces. (B) Area under the curve (AUC) of modularity values across sparsity thresholds (80%–99%) are plotted as least-squares mean by group and time. Early psychosis patients had higher modularity than healthy control participants in the hippocampal–medial temporal lobe cortex (MTLC) network driven by elevated modularity at study entry (t0). (C) Modularity values increased at higher sparsity thresholds across participants and for both hippocampal networks. Elevations in early psychosis patients were greater at higher thresholds. (D) Modularity values in the hippocampal-MTLC network significantly decreased over time in early psychosis patients, with the largest decrease occurring between study entry (t0) and 8-month follow-up (t1). Modularity remained stable from the 8-month through 2-year follow-up (t1–t3). Error bars are 95% confidence intervals. Asterisks indicate significant effects.
Network Construction
Networks were calculated for a 66 × 66 region connectivity matrix. For each participant, temporal connectivity was obtained by extracting the average time series for each ROI and calculating the ROI-to-ROI Pearson’s correlation coefficients. To remove potential sources of noise, the fMRI signal was bandpass filtered (0.01–0.10 Hz), and 12 motion parameters, 6 principal components of white matter and cerebrospinal fluid, and mean gray matter time series signal were removed (79). Correlation coefficients were Fischer r-to-z transformed and corrected for the number of time points. Both positive and negative connections were considered connectivity in brain networks. The absolute value of connections was calculated to create a non-negative graph matrix. Connectivity matrices were thresholded for noise (r ≥ 0.2) and binarized to create unweighted networks. The number of retained edges was similar between groups (p = .99). Functional connectivity matrices are displayed in Figure S2.
Network Architecture
The architecture of hippocampal networks was investigated as described subsequently. First, communities were assigned a priori as shown in Table S1, and modularity (Q), or cohesion, of each network was calculated according to Newman’s metric (80). Second, to examine communication within hippocampal networks, we calculated characteristic path length, global efficiency, and degree density. Short characteristic path length, high global efficiency, and low degree density indicate ability to communicate quickly across the network as a whole (81,82). Finally, we calculated the nodal descriptive statistics of degree (number of connected edges) and local efficiency to identify ROIs with altered nodal properties associated with module integrity. To evaluate the stability of networks over connection strengths, graph matrices were thresholded at 80%–99% sparsity, corresponding to values ranging from mild to strong connectivity (|r| ∼ 0.34–0.57). For all network measures, area under the curve served as the dependent variable in the models described subsequently. Visual network modularity was calculated using methods described previously (see the Supplement for full details).
Statistical Analysis
Statistical analyses were conducted in Statistical Analysis System (version 3.8; SAS Studio). Hippocampal network modularity served as the dependent variable in the primary analysis. Effects of group, time, and the interaction of group by time were modeled using linear mixed effects analyses, with group included as a fixed factor, time included as a repeated factor (t0 = study entry, t1 = t0 + 8 months, t2 = t0 + 16 months, t3 = t0 + 24 months), and participant included as a random factor. FD, race, and scanner were included as covariates of no interest. For linear mixed effects models, hypothesis tests were performed using Satterthwaite’s adjustment for degrees of freedom, and Cohen’s d effect sizes were calculated according to the procedure used by Vandekar et al. (83). Secondary analyses of network communication (characteristic path length, global efficiency, and degree density) and nodal characteristics (degree and local efficiency) were conducted using the primary model described above, with each serving as the dependent variable. Three exploratory linear mixed effects analyses were conducted to examine associations between clinical features and hippocampal network modularity, with modularity entered as the dependent variable; clinical feature (duration of illness, PANSS subscale scores, or HAM-D score) and time included as the independent variables; and FD, race, and scanner included as covariates of no interest. Duration of illness models did not include a time covariate. Spearman correlations were used to test for associations between modularity, demographic variables, and clinical features within visits. Associations were considered significant at p ≤ .05.
Results
Demographic and Clinical Features
Demographic and clinical characteristics of study participants are presented in Table 1. For simplicity, comparisons of characteristics at study entry versus 2-year follow-up are presented; comparisons across all 4 study visits produced similar results and can be found in Table S3. The early psychosis sample was young (mean age = 21 years, range = 16−31), primarily male (79%), White (76%), and educated (mean years of education = 13, range = 9−22). Psychosis and depression symptom severity decreased significantly over time (ps ≤ .007). Fewer patients were taking antipsychotic medication at follow-up visits versus at study entry (p = .02), although among those taking medication, the CPZ-equivalent dosage was similar across visits (p = .34).
Hippocampal Networks
Modularity
Modularity values were higher in early psychosis patients than in healthy control participants, with significant differences detected in the hippocampal-MTLC network (F1,136 = 3.95, β = −0.005, standard error [StdErr] = 0.003, d = 0.29, p = .05; Figure 1). Higher modularity in patients was largely driven by elevated group values at study entry (t0, control vs. patient: t = −1.77, d = 0.25, p = .08); later time points were similar across groups (t1–t3, control vs. patient: ps ≥ .39). Hippocampal-MTLC network modularity decreased over time in all participants (F3,240 = 2.79, β = 0.014, StdErr = 0.005, d = 0.39, p = .04; no group × time interaction), with the largest decrease occurring between t0 and t1 (t0 vs. t1: t = 3.02, p = .003; t1 vs. t2: t = −1.53, p = .13; t2 vs. t3: t = 0.59, p = .56). In contrast, modularity of the hippocampal-cortical network was similar between groups and did not differ over time (ps ≥ .12). In comparison, visual network modularity was stably elevated in early psychosis patients (F1,136 = 10.27, β = −0.009, StdErr = 0.003, d = 0.60, p = .002; no time or time × group interaction, ps ≥ .59). Comparing elevations at study entry, between-group effects were similar for the hippocampal-MTLC (d = 0.25) and visual networks (d = 0.30) (Supplemental Results).
Network Characteristics
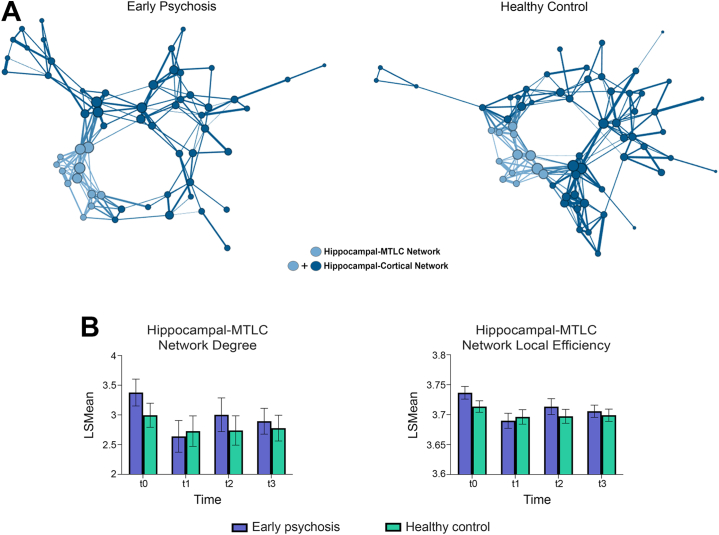
To understand the dynamics that contribute to elevated hippocampal-MTLC network modularity in early psychosis, we next examined measures of network communication. Early psychosis patients had a greater number of connections per node (degree: F1,136 = 7.38, β = −0.17, StdErr = 0.08, d = 0.42, p = .007) and greater local efficiency of connections (F1,136 = 12.23, β = −0.01, StdErr = 0.004, d = 0.56, p < .001) than healthy control participants (Figure 2). The overall density of connections also tended to be higher in patients than in healthy control participants (degree density: F1,136 = 3.66, β = −0.016, StdErr = 0.01, d = 0.27, trend p = .06; Figure S5). Similar to modularity, local efficiency was highest at study entry and lower at all subsequent visits (F3,242 = 9.28, β = 0.03, StdErr = 0.005, d = 0.84, p < .001). Hippocampal-MTLC modularity was strongly correlated with the number and local efficiency of connections (ps ≤ .001). Full statistical results are detailed in the Supplement. Together, these findings indicate that the hippocampal-MTLC network is more densely integrated in early psychosis patients than in healthy individuals.
Figure 2.
(A) Network graphs are displayed for the early psychosis and healthy control groups at study entry (t0). Graphs were drawn in Gephi (https://gephi.org/) using the Yifan Hu Multilevel layout algorithm on the weighted network at 90% sparsity. Node sizes and connection weights are scaled by number and weight of connections, respectively. Node color denotes the assigned network. To reduce the number of connections visualized, displayed connections are present in ≥20% of individuals. (B) On average, mean degree and local efficiency of the hippocampal–medial temporal lobe cortex (MTLC) network was higher in early psychosis patients than in healthy control participants. Error bars are 95% confidence intervals. LSMean, least-squares mean.
Clinical Correlates of Hippocampal Network Modularity
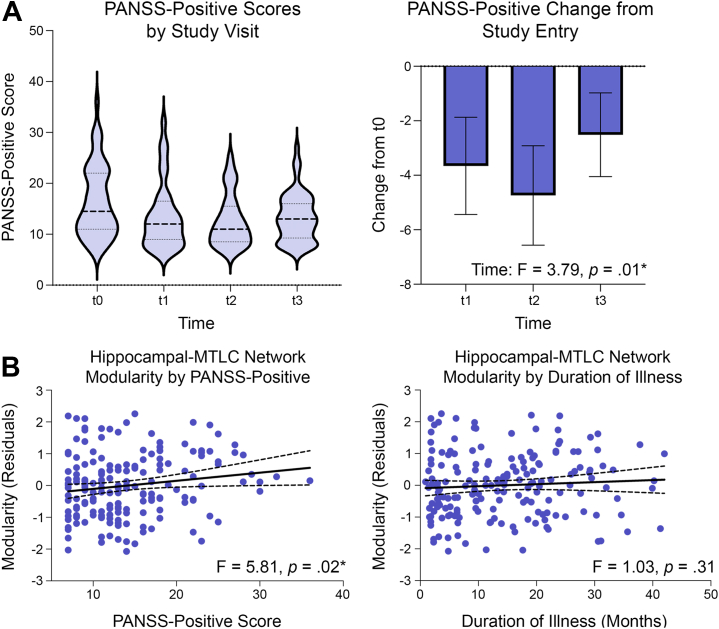
To understand why hippocampal-MTLC network modularity was elevated in patients at study entry but normalized over follow-up visits, we performed exploratory analyses to test the following 2 competing hypotheses: that network modularity 1) varied with symptom severity (i.e., clinical state) or 2) decreased with greater duration of illness (i.e., clinical stage). Analyses across PANSS subscales (positive, negative, general) revealed that positive symptom severity was selectively associated with modularity values (PANSS-positive, F1,105 = 5.16, p = .03; PANSS-negative and general subscale ps ≥ .25) (Figure 3). Follow-up analysis of the PANSS positive subscale showed that higher PANSS-positive symptoms significantly predicted higher modularity (F1,104 = 5.81, p = .02) (Figure 3). There was no linear interaction between positive symptoms and time. To determine whether higher PANSS-positive symptoms at study entry drove associations, PANSS-positive scores at study entry were included as a covariate in the linear mixed model. Controlling for the association between modularity and positive symptom severity at study entry, higher positive symptoms significantly predicted higher modularity across visits in patients (PANSS-positive, F1,90 = 4.31, p = .04). This suggests that higher modularity at any visit was associated with higher positive symptom severity regardless of severity at study entry. The association was similar when including duration of illness or HAM-D score as a covariate, and neither covariate independently predicted modularity in patients (duration of illness: F1,106 = 1.03, p = .31; HAM-D: F1,101 = 1.45, p = .23). Examining correlations at individual time points, we found that positive symptom severity was positively correlated with hippocampal-MTLC modularity at study entry and 16-month follow-up (t0: r = 0.31, p = .02; t1: r = −0.02, p = .92; t2: r = 0.36, p = .04; t3: r = 0.10, p = .48, Table S11). Duration of illness was not correlated with hippocampal-MTLC modularity (ps ≥ .20). Taken together, these findings suggest that variation in hippocampal-MTLC connectivity over visits is explained by changes in positive symptom severity, not increasing duration of illness. Hippocampal-MTLC network modularity was also positively correlated with CPZ dose at study entry (t0: r = 0.30, p = .05), but was not correlated with age, subject education, premorbid IQ, duration of illness, or mood symptoms (ps ≥ .27). Including CPZ dose as a covariate in the model did not alter the association between positive symptoms and modularity.
Figure 3.
(A) Positive symptoms were highest at study entry (t0) and were significantly lower at all subsequent visits (t1–t3). (B) Associations between hippocampal–medial temporal lobe cortex (MTLC) modularity, clinical state, and illness duration are visualized across visits. More severe positive symptoms in individual participants were associated with corresponding elevations in modularity of the hippocampal-MTLC network. In contrast, increasing duration of illness over study visits was not associated with changes in hippocampal-MTLC modularity. Dotted lines are 95% confidence intervals. Asterisks indicate significant effects. PANSS, Positive and Negative Syndrome Scale.
Discussion
Our goal was to characterize hippocampal network connectivity over 2 years in the early stage of psychosis. We found that resting-state hippocampal functional connectivity in a core MTL network was strengthened in acute early psychosis patients, with stronger connectivity positively correlated with severity of positive symptoms. In contrast, we did not observe alterations within a broader hippocampal-cortical network. Hippocampal-MTLC network hyperconnectivity normalized longitudinally and was associated with improvement in positive symptoms. Collectively, these results highlight a potential neuroimaging marker for identifying abnormal connectivity in early psychosis patients that relates to clinical symptom severity and clinical improvement, and they provide insights into hippocampal network dynamics during the early stages of psychosis.
Schizophrenia is associated with progressive, but likely nonlinear, declines in hippocampal structure across different illness stages (6,15,17,19,20). Hippocampal function may also vary by illness stage (84,85). A primary goal of this study was to determine whether hippocampal network connectivity was disrupted in the early stage of a psychotic disorder. Contrary to our hypothesis of predominantly lowered connectivity, we identified a pattern of elevated hippocampal-MTLC network cohesiveness in early psychosis patients, with the magnitude of elevations being similar to that of the visual network. Visual processing deficits (76) and connectivity abnormalities in early visual processing regions (77,86,87) are prominent in schizophrenia. Hippocampal network elevations are consistent with findings of interneuron hypofunction in patients with schizophrenia (5,18,88) that could lead to hippocampal hyperactivity (7), elevated glutamatergic tone (13), and sustained hippocampal signaling (54,55) in early illness. Given that hyperconnectivity has been associated with psychosis symptoms and cognitive impairments (89), abnormally elevated integration of hippocampal-MTLC functional connections is likely indicative of a pathological state arising from disinhibition in the hippocampus.
Contrary to our hypothesis of widespread deficits, we did not detect connectivity differences across the broader hippocampal-cortical network. One possible explanation for this is that widespread deficits develop in later illness stages as a result of a prolonged period of abnormal hippocampal signaling that entrains abnormal communication within cortical regions (90), culminating in widespread asynchronous communication (24). Alternatively, it is possible that medication effects normalize hippocampal-cortical interactions during early illness (34,35,91,92). Because the vast majority of patients in this study were taking antipsychotic medication during one or more study visits (94%), we were unable to test whether hippocampal-cortical modularity values differed in participants who were not exposed to antipsychotics, although modularity values were not associated with antipsychotic dose. Importantly, connectivity within the MTL may not be normalized even with antipsychotic treatment (91), suggesting that hippocampal-MTLC connectivity may represent a core illness-related feature of psychosis.
The second key goal of the study was to determine whether hippocampal network connectivity changes over 2 years of follow-up. Longitudinal analyses revealed the following 3 key findings: 1) hippocampal-MTLC hyperconnectivity normalized in our longitudinal sample, with connectivity in early psychosis patients resembling that of healthy control participants at 2-year follow-up; 2) connectivity was not associated with duration of illness at study entry, and normalization was not associated with progression of illness; and 3) connectivity normalization was correlated with improvement in psychosis symptoms over longitudinal follow-up. Together, these findings suggest that hippocampal-MTLC network hyperconnectivity may be a marker of current clinical state. Alterations in hippocampal function and connectivity have been proposed as a foundational mechanism in the formation of psychotic symptoms (93, 94, 95). Abnormally elevated resting activity in the hippocampus and MTLC, particularly parahippocampal cortex, has been associated with the presence or severity of positive symptoms in schizophrenia in a number of studies (96, 97, 98, 99, 100). Additional findings have linked alterations in hippocampal structure and function to the pathophysiology of positive symptoms in traumatic brain injury (101,102), stroke (103), Alzheimer disease (104), and temporal lobe epilepsy (105, 106, 107), indicating a central, transdiagnostic role for the hippocampus in the formation of positive symptoms. Longer duration of active psychosis has been associated with lower rates of remission and delayed treatment response (108), indicating that acute psychosis may reflect an ongoing pathophysiological process (109,110). Our findings suggest that hippocampal-MTLC network connectivity may be a neurobiological process that is amenable to early interventions (90).
We need to consider several limitations of our study. First, given the drawbacks of data-driven approaches to delineating the hippocampal network, we used an anatomically guided approach instead. Cortical nodes were comprised of regions that showed functional connectivity with the parahippocampal and perirhinal cortex in an independent sample (72). This approach excluded some direct hippocampal connections (e.g., ventral striatum) (111) that are altered in early psychosis. Second, hippocampal-MTLC network connectivity decreased across visits in all participants, which may reflect hippocampal habituation (112) to repeated scanning and/or lowered state anxiety (113) across visits. Future longitudinal studies may consider using naturalistic viewing during resting-state acquisitions to limit arousal confounds (114) and the collection of state-based anxiety measures during scanning. Third, graph theory metrics show increased reliability with longer resting-state fMRI acquisitions (i.e., 20–100 minutes) (115). Future studies may consider using longer acquisition times, although shorter acquisitions (e.g., 7 minutes, as acquired here) can be helpful in minimizing in-scanner head motion, a major confound for resting-state fMRI analyses (116). Finally, because the majority of patients were taking antipsychotic medications during the study, alterations in connectivity related to disease state and medication use are intrinsically linked. Because antipsychotic use affects hippocampal connectivity (34,35), additional longitudinal studies of hippocampal network function in medication-naïve patients are warranted.
In conclusion, this study established that functional connectivity was elevated within a core hippocampal-MTLC network in early psychosis and associated with the severity of positive symptoms. Hippocampal hyperconnectivity normalized longitudinally and predicted clinical improvement. These results suggest that hippocampal-MTLC hyperconnectivity may be a useful biomarker development target (23). Quantitative metrics of hippocampal network function may be particularly useful in the classification of psychosis patients by clinical stage (117) and prediction of treatment response (35). Longer-term prospective studies are necessary to determine whether additional hippocampal connectivity changes occur during progression to chronic stages of psychosis.
Acknowledgments and Disclosures
This work was supported by the Charlotte and Donald Test Fund, The National Institute of Mental Health (Grant Nos. R01-MH70560 [to SH] and R01-MH123563 [to SNV]), the Vanderbilt Psychiatric Genotype/Phenotype Project, and the Vanderbilt Institute for Clinical and Translational Research (Grant No. 1-UL-1-TR000445 from the National Center for Research Resources/National Institutes of Health).
This work was conducted in part using the resources of the Center for Computational Imaging at Vanderbilt University Institute of Imaging Science and the Advanced Computing Center for Research Education at Vanderbilt University, Nashville, TN.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2022.10.002.
Supplementary Material
References
- 1.Haijma S.V., Van Haren N., Cahn W., Koolschijn P.C.M.P., Hulshoff Pol H.E., Kahn R.S. Brain volumes in schizophrenia: A meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Erp T.G.M., Hibar D.P., Rasmussen J.M., Glahn D.C., Pearlson G.D., Andreassen O.A., et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21:547–553. doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heckers S., Konradi C. Hippocampal pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:529–553. doi: 10.1007/7854_2010_43. [DOI] [PubMed] [Google Scholar]
- 4.Kraguljac N.V., White D.M., Reid M.A., Lahti A.C. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70:1294–1302. doi: 10.1001/jamapsychiatry.2013.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roeske M.J., Konradi C., Heckers S., Lewis A.S. Hippocampal volume and hippocampal neuron density, number and size in schizophrenia: A systematic review and meta-analysis of postmortem studies. Mol Psychiatry. 2021;26:3524–3535. doi: 10.1038/s41380-020-0853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McHugo M., Armstrong K., Roeske M.J., Woodward N.D., JU Blackford, Heckers S. Hippocampal volume in early psychosis: A 2-year longitudinal study. Transl Psychiatry. 2020;10:306. doi: 10.1038/s41398-020-00985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McHugo M., Avery S., Armstrong K., Rogers B.P., Vandekar S.N., Woodward N.D., et al. Anterior hippocampal dysfunction in early psychosis: A 2-year follow-up study. Psychol Med. 2021:1–10. doi: 10.1017/S0033291721001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvo A., Roddy D.W., Coughlan H., Kelleher I., Healy C., Harley M., et al. Reduced hippocampal volume in adolescents with psychotic experiences: A longitudinal population-based study. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho N.F., Holt D.J., Cheung M., Iglesias J.E., Goh A., Wang M., et al. Progressive decline in hippocampal CA1 volume in individuals at ultra-high-risk for psychosis who do not remit: Findings from the longitudinal youth at risk study. Neuropsychopharmacology. 2017;42:1361–1370. doi: 10.1038/npp.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bossong M.G., Antoniades M., Azis M., Samson C., Quinn B., Bonoldi I., et al. Association of hippocampal glutamate levels with adverse outcomes in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2019;76:199–207. doi: 10.1001/jamapsychiatry.2018.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provenzano F.A., Guo J., Wall M.M., Feng X., Sigmon H.C., Brucato G., et al. Hippocampal pathology in clinical high-risk patients and the onset of schizophrenia. Biol Psychiatry. 2020;87:234–242. doi: 10.1016/j.biopsych.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Lieberman J.A., Girgis R.R., Brucato G., Moore H., Provenzano F., Kegeles L., et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: A selective review and hypothesis for early detection and intervention. Mol Psychiatry. 2018;23:1764–1772. doi: 10.1038/mp.2017.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schobel S.A., Chaudhury N.H., Khan U.A., Paniagua B., Styner M.A., Asllani I., et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schobel S.A., Lewandowski N.M., Corcoran C.M., Moore H., Brown T., Malaspina D., Small S.A. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho N.F., Iglesias J.E., Sum M.Y., Kuswanto C.N., Sitoh Y.Y., De Souza J., et al. Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol Psychiatry. 2017;22:142–152. doi: 10.1038/mp.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makowski C., Bodnar M., Shenker J.J., Malla A.K., Joober R., Chakravarty M.M., Lepage M. Linking persistent negative symptoms to amygdala–hippocampus structure in first-episode psychosis. Transl Psychiatry. 2017;7:e1195. doi: 10.1038/tp.2017.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieberman J., Chakos M., Wu H., Alvir J., Hoffman E., Robinson D., Bilder R. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- 18.Heckers S., Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. 2015;167:4–11. doi: 10.1016/j.schres.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McHugo M., Talati P., Woodward N.D., Armstrong K., JU Blackford, Heckers S. Regionally specific volume deficits along the hippocampal long axis in early and chronic psychosis. NeuroImage Clin. 2018;20:1106–1114. doi: 10.1016/j.nicl.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velakoulis D., Wood S.J., Wong M.T.H., McGorry P.D., Yung A., Phillips L., et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: A magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 21.Pujol N., Penadés R., Junqué C., Dinov I., Fu C.H.Y., Catalán R., et al. Hippocampal abnormalities and age in chronic schizophrenia: Morphometric study across the adult lifespan. Br J Psychiatry. 2014;205:369–375. doi: 10.1192/bjp.bp.113.140384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieberman J.A., Small S.A., Girgis R.R. Early detection and preventive intervention in schizophrenia: From fantasy to reality. Am J Psychiatry. 2019;176:794–810. doi: 10.1176/appi.ajp.2019.19080865. [DOI] [PubMed] [Google Scholar]
- 23.Kraguljac N.V., McDonald W.M., Widge A.S., Rodriguez C.I., Tohen M., Nemeroff C.B. Neuroimaging biomarkers in schizophrenia. Am J Psychiatry. 2021;178:509–521. doi: 10.1176/appi.ajp.2020.20030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friston K.J. The disconnection hypothesis. Schizophr Res. 1998;30:115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- 25.Northoff G., Duncan N.W. How do abnormalities in the brain’s spontaneous activity translate into symptoms in schizophrenia? From an overview of resting state activity findings to a proposed spatiotemporal psychopathology. Prog Neurobiol. 2016;145-146:26–45. doi: 10.1016/j.pneurobio.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Stephan K.E., Friston K.J., Frith C.D. Dysconnection in Schizophrenia: From abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edmiston E.K., Song Y., Chang M., Yin Z., Zhou Q., Zhou Y., et al. Hippocampal resting state functional connectivity in patients with schizophrenia and unaffected family members. Front Psychiatry. 2020;11:278. doi: 10.3389/fpsyt.2020.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avery S.N., Rogers B.P., Heckers S. Hippocampal network modularity is associated with relational memory dysfunction in schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:423–432. doi: 10.1016/j.bpsc.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y., Shu N., Liu Y., Song M., Hao Y., Liu H., et al. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res. 2008;100:120–132. doi: 10.1016/j.schres.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 30.Kühn S., Gallinat J. Resting-state brain activity in schizophrenia and major depression: A quantitative meta-analysis. Schizophr Bull. 2013;39:358–365. doi: 10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters H., Shao J., Scherr M., Schwerthöffer D., Zimmer C., Förstl H., et al. More consistently altered connectivity patterns for cerebellum and medial temporal lobes than for amygdala and striatum in schizophrenia. Front Hum Neurosci. 2016;10:1–11. doi: 10.3389/fnhum.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samudra N., Ivleva E.I., Hubbard N.A., Rypma B., Sweeney J.A., Clementz B.A., et al. Alterations in hippocampal connectivity across the psychosis dimension. Psychiatry Res. 2015;233:148–157. doi: 10.1016/j.pscychresns.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anticevic A., Hu X., Xiao Y., Hu J., Li F., Bi F., et al. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci. 2015;35:267–286. doi: 10.1523/JNEUROSCI.2310-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson E.A., Kraguljac N.V., Maximo J.O., Briend F., Armstrong W., Ver Hoef L.W., et al. Hippocampal dysconnectivity and altered glutamatergic modulation of the default mode network: A combined resting-state connectivity and magnetic resonance spectroscopy study in schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7:108–118. doi: 10.1016/j.bpsc.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blessing E.M., Murty V.P., Zeng B., Wang J., Davachi L., Goff D.C. Anterior hippocampal-cortical functional connectivity distinguishes antipsychotic naïve first-episode psychosis patients from controls and may predict response to second-generation antipsychotic treatment. Schizophr Bull. 2020;46:680–689. doi: 10.1093/schbul/sbz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egerton A., Grace A.A., Stone J., Bossong M.G., Sand M., McGuire P. Glutamate in schizophrenia: Neurodevelopmental perspectives and drug development. Schizophr Res. 2020;223:59–70. doi: 10.1016/j.schres.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Chiang S., Haneef Z. Graph theory findings in the pathophysiology of temporal lobe epilepsy. Clin Neurophysiol. 2014;125:1295–1305. doi: 10.1016/j.clinph.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bullmore E., Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki W.A., Amaral D.G. Cortical inputs to the CA1 field of the monkey hippocampus originate from the perirhinal and parahippocampal cortex but not from area TE. Neurosci Lett. 1990;115:43–48. doi: 10.1016/0304-3940(90)90515-b. [DOI] [PubMed] [Google Scholar]
- 40.Witter M.P., Wouterlood F.G., Naber P.A., Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]
- 41.Buckner R.L., Sepulcre J., Talukdar T., Krienen F.M., Liu H., Hedden T., et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honey C.J., Sporns O., Cammoun L., Gigandet X., Thiran J.P., Meuli R., Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagmann P., Cammoun L., Gigandet X., Meuli R., Honey C.J., Wedeen V.J., Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward A.M., Schultz A.P., Huijbers W., Van Dijk K.R.A., Hedden T., Sperling R.A. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp. 2014;35:1061–1073. doi: 10.1002/hbm.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnett A.J., Reilly W., Dimsdale-Zucker H.R., Mizrak E., Reagh Z., Ranganath C. Intrinsic connectivity reveals functionally distinct cortico-hippocampal networks in the human brain. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 47.Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mišić B., Goñi J., Betzel R.F., Sporns O., McIntosh A.R. A network convergence zone in the hippocampus. PLoS Comput Biol. 2014;10 doi: 10.1371/journal.pcbi.1003982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kernbach J.M., Yeo B.T.T., Smallwood J., Margulies D.S., Thiebaut de Schotten M., Walter H., et al. Subspecialization within default mode nodes characterized in 10,000 UK Biobank participants. Proc Natl Acad Sci U S A. 2018;115:12295–12300. doi: 10.1073/pnas.1804876115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newton R., Rouleau A., Nylander A.G., Loze J.Y., Resemann H.K., Steeves S., Crespo-Facorro B. Diverse definitions of the early course of schizophrenia-a targeted literature review. NPJ Schizophr. 2018;4:21. doi: 10.1038/s41537-018-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.First M., Spitzer R.R.L., Gibbon M., Williams J. Biometrics Research, New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for the DSM-IV-TR Axis I Disorders, research version, patient ed (SCID-I/P) [Google Scholar]
- 52.American Psychiatric Association . 5th ed. CRS Publishers and Distributors; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 53.McHugo M., Talati P., Armstrong K., Vandekar S.N., JU Blackford, Woodward N.D., Heckers S. Hyperactivity and reduced activation of anterior hippocampus in early psychosis. Am J Psychiatry. 2019;176:1030–1038. doi: 10.1176/appi.ajp.2019.19020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avery S.N., McHugo M., Armstrong K., Blackford J.U., Woodward N.D., Heckers S. Disrupted habituation in the early stage of psychosis. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:1004–1012. doi: 10.1016/j.bpsc.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avery S.N., McHugo M., Armstrong K., Blackford J.U., Woodward N.D., Heckers S. Stable habituation deficits in the early stage of psychosis: A 2-year follow-up study. Transl Psychiatry. 2021;11:20. doi: 10.1038/s41398-020-01167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Avery S.N., Armstrong K., McHugo M., Vandekar S., Blackford J.U., Woodward N.D., Heckers S. Relational memory in the early stage of psychosis: A 2-year follow-up study. Schizophr Bull. 2021;47:75–86. doi: 10.1093/schbul/sbaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Armstrong K., Avery S., Blackford J.U., Woodward N., Heckers S. Impaired associative inference in the early stage of psychosis. Schizophr Res. 2018;202:86–90. doi: 10.1016/j.schres.2018.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Avery S.N., Armstrong K., Blackford J.U., Woodward N.D., Cohen N., Heckers S. Impaired relational memory in the early stage of psychosis. Schizophr Res. 2019;212:113–120. doi: 10.1016/j.schres.2019.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suh D.Y., Vandekar S.N., Heckers S., Avery S.N. Visual exploration differences during relational memory encoding in early psychosis. Psychiatry Res. 2020;287 doi: 10.1016/j.psychres.2020.112910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perkins D.O.O., Leserman J., Jarskog L.F.F., Graham K., Kazmer J., Lieberman J.A.A. Characterizing and dating the onset of symptoms in psychotic illness: The symptom onset in Schizophrenia (SOS) inventory. Schizophr Res. 2000;44:1–10. doi: 10.1016/s0920-9964(99)00161-9. [DOI] [PubMed] [Google Scholar]
- 61.Gardner D.M., Murphy A.L., O’Donnell H., Centorrino F., Baldessarini R.J. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- 62.Leucht S., Samara M., Heres S., Patel M.X., Woods S.W., Davis J.M. Dose equivalents for second-generation antipsychotics: The minimum effective dose method. Schizophr Bull. 2014;40:314–326. doi: 10.1093/schbul/sbu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dykiert D., Deary I.J. Retrospective validation of WTAR and NART scores as estimators of prior cognitive ability using the Lothian Birth Cohort 1936. Psychol Assess. 2013;25:1361–1366. doi: 10.1037/a0033623. [DOI] [PubMed] [Google Scholar]
- 64.Dalby J.T., Williams R. Preserved reading and spelling ability in psychotic disorders. Psychol Med. 1986;16:171–175. doi: 10.1017/s0033291700002609. [DOI] [PubMed] [Google Scholar]
- 65.Holdnack H.A. Wechsler test of adult reading: WTAR. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 66.Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 67.Kay S.R., Opler L.A., Lindenmayer J.P. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Res. 1988;23:99–110. doi: 10.1016/0165-1781(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 68.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 70.Harrigan R.L., Yvernault B.C., Boyd B.D., Damon S.M., Gibney K.D., Conrad B.N., et al. Vanderbilt University Institute of Imaging Science Center for Computational Imaging XNAT: A multimodal data archive and processing environment. Neuroimage. 2016;124:1097–1101. doi: 10.1016/j.neuroimage.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Squire L.R., Stark C.E.L., Clark R.E. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 72.Libby L.A., Ekstrom A.D., Ragland J.D., Ranganath C. Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. J Neurosci. 2012;32:6550–6560. doi: 10.1523/JNEUROSCI.3711-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ranganath C., Ritchey M. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- 74.Ritchey M., Libby L.A., Ranganath C. Cortico-hippocampal systems involved in memory and cognition: The PMAT framework. Prog Brain Res. 2015;219:45–64. doi: 10.1016/bs.pbr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 75.Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Green M.F., Lee J., Wynn J.K., Mathis K.I. Visual masking in schizophrenia: Overview and theoretical implications. Schizophr Bull. 2011;37:700–708. doi: 10.1093/schbul/sbr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van de Ven V., Rotarska Jagiela A., Oertel-Knöchel V., Linden D.E.J. Reduced intrinsic visual cortical connectivity is associated with impaired perceptual closure in schizophrenia. NeuroImage Clin. 2017;15:45–52. doi: 10.1016/j.nicl.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sharma A., Kumar A., Singh S., Bhatia T., Beniwal R.P., Khushu S., et al. Altered resting state functional connectivity in early course schizophrenia. Psychiatry Res Neuroimaging. 2018;271:17–23. doi: 10.1016/j.pscychresns.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burgess G.C., Kandala S., Nolan D., Laumann T.O., Power J.D., Adeyemo B., et al. Evaluation of denoising strategies to address motion-correlated artifacts in resting-state functional magnetic resonance imaging data from the human connectome project. Brain Connect. 2016;6:669–680. doi: 10.1089/brain.2016.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Newman M.E.J. Modularity and community structure in networks. Proc Natl Acad Sci U S A. 2006;103:8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Achard S., Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bullmore E., Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- 83.Vandekar S., Tao R., Blume J. A robust effect size index. Psychometrika. 2020;85:232–246. doi: 10.1007/s11336-020-09698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uhlhaas P.J. Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr Opin Neurobiol. 2013;23:283–290. doi: 10.1016/j.conb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 85.Li T., Wang Q., Zhang J., Rolls E.T., Yang W., Palaniyappan L., et al. Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr Bull. 2017;43:436–448. doi: 10.1093/schbul/sbw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oh K.H., Oh I.S., Tsogt U., Shen J., Kim W.S., Liu C., et al. Diagnosis of schizophrenia with functional connectome data: A graph-based convolutional neural network approach. BMC Neurosci. 2022;23:5. doi: 10.1186/s12868-021-00682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rolls E.T., Cheng W., Feng J. Brain dynamics: The temporal variability of connectivity, and differences in schizophrenia and ADHD. Transl Psychiatry. 2021;11:70. doi: 10.1038/s41398-021-01197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Konradi C., Yang C.K., Zimmerman E.I., Lohmann K.M., Gresch P., Pantazopoulos H., et al. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131:165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Driesen N.R., McCarthy G., Bhagwagar Z., Bloch M., Calhoun V., D’Souza D.C., et al. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 2013;18:1199–1204. doi: 10.1038/mp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Small S.A., Schobel S.A., Buxton R.B., Witter M.P., Barnes C.A. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chopra S., Francey S.M., O’Donoghue B., Sabaroedin K., Arnatkeviciute A., Cropley V., et al. Functional connectivity in antipsychotic-treated and antipsychotic-naive patients with first-episode psychosis and low risk of self-harm or aggression: A secondary analysis of a randomized clinical trial. JAMA Psychiatry. 2021;78:994–1004. doi: 10.1001/jamapsychiatry.2021.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.González-Vivas C., Soldevila-Matías P., Sparano O., García-Martí G., Martí-Bonmatí L., Crespo-Facorro B., et al. Longitudinal studies of functional magnetic resonance imaging in first-episode psychosis: A systematic review. Eur Psychiatry. 2019;59:60–69. doi: 10.1016/j.eurpsy.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 93.Lodge D.J., Grace A.A. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32:507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lodge D.J., Grace A.A. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zierhut K.C., Graßmann R., Kaufmann J., Steiner J., Bogerts B., Schiltz K. Hippocampal CA1 deformity is related to symptom severity and antipsychotic dosage in schizophrenia. Brain. 2013;136:804–814. doi: 10.1093/brain/aws335. [DOI] [PubMed] [Google Scholar]
- 96.Liddle P.F., Friston K.J., Frith C.D., Hirsch S.R., Jones T., Frackowiak R.S.J. Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry. 1992;160:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- 97.Heckers S. Neural models of schizophrenia. Dial Clin Neurosci. 2000;2:267–279. doi: 10.31887/DCNS.2000.2.3/sheckers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Delavari F., Sandini C., Zöller D., Mancini V., Bortolin K., Schneider M., et al. Dysmaturation observed as altered hippocampal functional connectivity at rest is associated with the emergence of positive psychotic symptoms in patients with 22q11 deletion syndrome. Biol Psychiatry. 2021;90:58–68. doi: 10.1016/j.biopsych.2020.12.033. [DOI] [PubMed] [Google Scholar]
- 99.Horga G., Parellada E., Lomeña F., Fernández-Egea E., Mané A., Font M., et al. Differential brain glucose metabolic patterns in antipsychotic-naive first-episode schizophrenia with and without auditory verbal hallucinations. J Psychiatry Neurosci. 2011;36:312–321. doi: 10.1503/jpn.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Uscătescu L.C., Kronbichler L., Stelzig-Schöler R., Pearce B.G., Said-Yürekli S., Reich L.A., et al. Effective connectivity of the hippocampus can differentiate patients with schizophrenia from healthy controls: A spectral DCM approach. Brain Topogr. 2021;34:762–778. doi: 10.1007/s10548-021-00868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bray M.J.C., Sharma B., Cottrelle’s J., Peters M.E., Bayley M., Green R.E.A. Hippocampal atrophy is associated with psychotic symptom severity following traumatic brain injury. Brain Commun. 2021;3:fcab026. doi: 10.1093/braincomms/fcab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fujii D.E., Armstrong N.P., Ahmed I. In: The Spectrum of Psychotic Disorders: Neurobiology, Etiology and Pathogenesis. Fujii D., Ahmed I., editors. Cambridge University Press; Cambridge: 2007. Psychotic disorder due to traumatic brain injury; pp. 249–261. [Google Scholar]
- 103.Stangeland H., Orgeta V., Bell V. Poststroke psychosis: A systematic review. J Neurol Neurosurg Psychiatry. 2018;89:879–885. doi: 10.1136/jnnp-2017-317327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee K., Lee Y.M., Park J.M., Lee B.D., Moon E., Jeong H.J., et al. Right hippocampus atrophy is independently associated with Alzheimer’s disease with psychosis. Psychogeriatrics. 2019;19:105–110. doi: 10.1111/psyg.12369. [DOI] [PubMed] [Google Scholar]
- 105.Irwin L.G., Fortune D.G. Risk factors for psychosis secondary to temporal lobe epilepsy: A systematic review. J Neuropsychiatry Clin Neurosci. 2014;26:5–23. doi: 10.1176/appi.neuropsych.12120403. [DOI] [PubMed] [Google Scholar]
- 106.Elliott B., Joyce E., Shorvon S. Delusions, illusions and hallucinations in epilepsy: 2. Complex phenomena and psychosis. Epilepsy Res. 2009;85:172–186. doi: 10.1016/j.eplepsyres.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 107.Elliott B., Joyce E., Shorvon S. Delusions, illusions and hallucinations in epilepsy: 1. Elementary phenomena. Epilepsy Res. 2009;85:162–171. doi: 10.1016/j.eplepsyres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 108.Perkins D.O., Gu H., Boteva K., Lieberman J.A. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: A critical review and meta-analysis. Am J Psychiatry. 2005;162:1785–1804. doi: 10.1176/appi.ajp.162.10.1785. [DOI] [PubMed] [Google Scholar]
- 109.Loebel A.D., Lieberman J.A., Alvir J.M.J., Mayerhoff D.I., Geisler S.H., Szymanski S.R. Duration of psychosis and outcome in first-episode schizophrenia. Am J Psychiatry. 1992;149:1183–1188. doi: 10.1176/ajp.149.9.1183. [DOI] [PubMed] [Google Scholar]
- 110.Lieberman J.A., Perkins D., Belger A., Chakos M., Jarskog F., Boteva K., Gilmore J. The early stages of schizophrenia: Speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50:884–897. doi: 10.1016/s0006-3223(01)01303-8. [DOI] [PubMed] [Google Scholar]
- 111.Groenewegen H.J., Vermeulen-Van der Zee E.V., te Kortschot A., Witter M.P. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- 112.Murty V.P., Ballard I.C., MacDuffie K.E., Krebs R.M., Adcock R.A. Hippocampal networks habituate as novelty accumulates. Learn Mem. 2013;20:229–235. doi: 10.1101/lm.029728.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Satpute A.B., Mumford J.A., Naliboff B.D., Poldrack R.A. Human anterior and posterior hippocampus respond distinctly to state and trait anxiety. Emotion. 2012;12:58–68. doi: 10.1037/a0026517. [DOI] [PubMed] [Google Scholar]
- 114.Finn E.S., Bandettini P.A. Movie-watching outperforms rest for functional connectivity-based prediction of behavior. Neuroimage. 2021;235 doi: 10.1016/j.neuroimage.2021.117963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gordon E.M., Laumann T.O., Gilmore A.W., Newbold D.J., Greene D.J., Berg J.J., et al. Precision functional mapping of individual human brains. Neuron. 2017;95:791–807.e7. doi: 10.1016/j.neuron.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parkes L., Fulcher B., Yücel M., Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage. 2018;171:415–436. doi: 10.1016/j.neuroimage.2017.12.073. [DOI] [PubMed] [Google Scholar]
- 117.Schmidt A., Diwadkar V.A., Smieskova R., Harrisberger F., Lang U.E., McGuire P., et al. Approaching a network connectivity-driven classification of the psychosis continuum: A selective review and suggestions for future research. Front Hum Neurosci. 2014;8:1047. doi: 10.3389/fnhum.2014.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.