Abstract
Background
Exercise has shown promise as a treatment for cocaine use disorder; however, the mechanism underlying its efficacy has remained elusive.
Methods
We used a rat model of relapse (cue-induced reinstatement) and exercise (wheel running, 2 hours/day) coupled with RNA sequencing to establish transcriptional profiles associated with the protective effects of exercise (during early withdrawal [days 1–7] or throughout withdrawal [days 1–14]) versus noneffective exercise (during late withdrawal [days 8–14]) against cocaine-seeking and sedentary conditions.
Results
As expected, cue-induced cocaine seeking was highest in the sedentary and late-withdrawal exercise groups; both groups also showed upregulation of a Grin1-associated transcript and enrichment of Drd1-Nmdar1 complex and glutamate receptor complex terms. Surprisingly, these glutamate markers were also enriched in the early- and throughout-withdrawal exercise groups, despite lower levels of cocaine seeking. However, a closer examination of the Grin1-associated transcript revealed a robust loss of transcripts spanning exons 9 and 10 in the sedentary condition relative to saline controls that was normalized by early- and throughout-withdrawal exercise, but not late-withdrawal exercise, indicating that these exercise conditions may normalize RNA mis-splicing induced by cocaine seeking. Our findings also revealed novel mechanisms by which exercise initiated during early withdrawal may modulate glutamatergic signaling in dorsomedial prefrontal cortex (e.g., via transcripts associated with non-NMDA glutamate receptors or those affecting signaling downstream of NMDA receptors), along with mechanisms outside of glutamatergic signaling such as circadian rhythm regulation and neuronal survival.
Conclusions
These findings provide a rich resource for future studies aimed at manipulating these molecular networks to better understand how exercise decreases cocaine seeking.
Keywords: Cocaine seeking, Cocaine use disorder, Exercise, Extended-access cocaine self-administration, RNA sequencing, Treatment
More than 5.1 million individuals in the United States over the age of 12 reported using cocaine in 2020 and 1.3 million met the criteria for cocaine use disorder (CUD) (1). As the supply of cocaine continues to grow, the number of users and individuals with CUD is projected to increase (2, 3, 4). New treatments are critically needed, especially because there is no U.S. Food and Drug Administration–approved treatment for CUD and current strategies result in ∼50% relapse (5). Exercise has shown promise as a nonpharmacological intervention for substance use disorders in human and animal studies. In humans, clinical trials of exercise have revealed beneficial health and anticraving effects (6, 7, 8, 9, 10, 11, 12), with recent findings showing that drug craving is immediately and persistently attenuated following an acute bout of aerobic exercise in individuals undergoing inpatient treatment for polysubstance use disorder (8). Exercise has also been shown to alleviate depression, reduce measures of anxiety, and enhance cognition (6,13, 14, 15), factors known to contribute to drug craving (16,17). Similarly, animal studies show that exercise, such as voluntary running in a wheel, markedly decreases drug use and drug seeking and, when available during withdrawal following drug self-administration, prevents the incubation of drug craving, a phenomenon that occurs in both humans and animals and is believed to reflect an enhanced vulnerability to relapse (18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30). This antirelapse effect of exercise has been observed for multiple addictive drugs, including cocaine (31, 32, 33, 34).
Despite these encouraging findings, the efficacy of exercise in humans has been variable, and while lack of power and compliance issues undoubtedly contribute to the variability (33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43), preclinical results similarly demonstrate that certain exercise regimens are not effective (18,19,28). For example, we showed that exercise (wheel running) during early withdrawal (days 1–7) or throughout withdrawal (days 1–14) prevents the development of high levels of cocaine seeking and persistently protects against cocaine seeking as assessed on withdrawal day 15. In contrast, exercise during late withdrawal (days 8–14) was not effective at reducing cocaine-seeking response on withdrawal day 15, suggesting that the timing of exercise during withdrawal is more important than the amount or recency of exercise [exercise 2 hours/day (19); exercise 1–6 hours/day (21); exercise 1–24 hours/day (22); see (44,45) for reviews]. It also suggests that effects in humans could be prolonged by initiating exercise during early withdrawal rather than after detoxification, as it typically occurs. A better understanding of the molecular mechanisms that underlie these differential effects of exercise would also help guide the development of exercise in humans with CUD because such information will help define the exercise conditions necessary for achieving an antirelapse effect.
In our previous study (18), we started to isolate the molecular changes associated with effective versus noneffective exercise conditions, focusing on neuroadaptations in the dorsomedial prefrontal cortex (dmPFC), which is known to be critically involved in cocaine seeking and its incubation over withdrawal (46, 47, 48, 49, 50, 51). We focused on markers known to be regulated by exercise and/or drug seeking, including genes involved in brain-derived neurotrophic factor (Bdnf exons I, IV, and IX and its receptor, Ntrk2), glutamate (AMPA type subunits 1, 2, and 3: Gria1, Gria2, and Gria3; NMDA type subunit 1: Grin1; metabotropic glutamate receptors mGlu1, mGlu2, and mGlu5: Grm1, Grm2, and Grm5), and dopamine signaling (D1 and D2 receptors: Drd1 and Drd2). mGlu1 and mGlu5 showed the most promise as a potential mechanism underlying the protective effect of exercise against cocaine seeking, given that dmPFC gene expression levels (Grin1 and Grm5) were positively associated with cocaine-seeking responses (or responding during extinction/reinstatement testing). Site-specific activation of mGlu5 receptors during early withdrawal also mimicked the efficacy of exercise. However, blockade of these receptors during early withdrawal did not block the efficacy of exercise, indicating that while dmPFC mGlu5 contribute to the incubation of cocaine seeking, they are not necessary for the efficacy of exercise to reduce cocaine seeking (18). Thus, the mechanism for the efficacy of exercise in reducing cocaine seeking remains elusive.
The purpose of the present study was to use RNA sequencing (RNA-seq) as an unbiased and hypothesis-generating approach to explore transcriptional changes in the dmPFC associated with cocaine seeking and the protective effects of exercise against cocaine seeking. Although several studies have characterized transcriptional changes associated with cocaine self-administration and/or cocaine seeking, most of these studies have used short-access conditions (1–2 hour/day access to the drug), which are best for studying initial vulnerability to drug use [e.g., (52, 53, 54, 55, 56), but see (57)]. Notably, to our knowledge, RNA-seq has not been used to assess changes that occur in response to cue-induced cocaine seeking following withdrawal from extended-access drug self-administration (i.e., ≥6-hour/day access to the drug), which is the gold standard for inducing addiction-like features in animals, including enhanced drug seeking/vulnerability to relapse (58). We focused on changes in transcripts rather than genes, given mounting evidence indicating that RNA mis-splicing contributes to numerous human diseases (e.g., Parkinson’s disease and dilated cardiomyopathy) (59), with preliminary support for a role in substance use disorders (e.g., alcohol use disorder) (60). Based on our previous findings in male rats showing that cocaine seeking is attenuated by exercise during early withdrawal (days 1–7) or throughout withdrawal (days 1–14) but not during late withdrawal (days 8–14) (18, 19), we predicted that the protective effects of exercise against cocaine seeking would be revealed by isolating transcriptional changes induced by early- and throughout-withdrawal exercise versus late-withdrawal exercise and sedentary conditions (relative to saline).
Methods and Materials
Subjects
Adult male Sprague Dawley rats (Charles River) (N = 21) weighing approximately 380 g at the start of the study were used as subjects. These animals were representative subsets of the sedentary (5 of 8), early (4 of 14), throughout (4 of 8), late (4 of 10), and saline (5 of 10) groups we used in our previous study on the impact of early- versus late-withdrawal–initiated exercise on cocaine seeking and associated gene expression changes (as assessed using quantitative polymerase chain reaction) (19). All procedures were approved by the University of Virginia Animal Care and Use Committee and were conducted within the guidelines set by the National Institutes of Health.
Behavioral Procedure
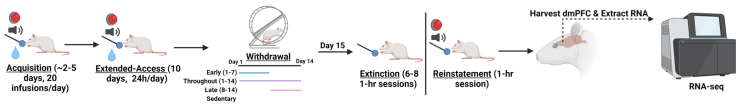
The procedures used for housing, lever pretraining, and surgical implantation of jugular catheters; cocaine self-administration during training and the extended-access period; exercise during withdrawal; and cue-induced cocaine-seeking testing are thoroughly described in our previous study (19) (Figure 1). Briefly, rats were trained to self-administer cocaine (1.5 mg/kg/infusion) under a fixed-ratio 1 schedule with a maximum of 20 infusions available per day and, once acquired (20 infusions on 2 consecutive days), were given extended (24 hours/day) access to the drug using a discrete trial procedure (2 trials/hour, 1.5 mg/kg/infusion) for 10 days. Two additional fixed-ratio 1 sessions were run to confirm patency before withdrawal. Then, rats were housed in polycarbonate cages without (sedentary; n = 5) or with 2-hour/day access to a running wheel during early withdrawal (days 1–7; n = 4), late withdrawal (days 8–14; n = 4), or throughout withdrawal (days 1–14; n = 4). Saline controls underwent the same procedures as those described for the sedentary cocaine group (n = 4) except that they previously self-administered saline instead of cocaine. Rats were returned to their operant chambers on day 14 of withdrawal and then underwent relapse testing on withdrawal day 15 using a within-session extinction/cue-induced reinstatement procedure (i.e., a minimum of six 1-hour extinction sessions wherein responding was without consequence followed by a 1-hour reinstatement session in which each response was reinforced with cocaine-associated cues, which included a stimulus light and the sound of the pump).
Figure 1.
Summary of experimental events. Male rats were trained to self-administer cocaine. Following acquisition of cocaine self-administration (2 consecutive sessions wherein all 20 infusions available were obtained), rats were given 24 hour/day, extended access to cocaine (1.5 mg/kg/infusion) under a discrete trial procedure for 10 sessions. Following the last cocaine self-administration session, rats were housed without (sedentary, n = 5) or with access to wheel running (2 hours/day) during early (days 1–7; n = 4), late (days 8–14; n = 4), or throughout (days 1–14; n = 4) a 14-day withdrawal period. Additional rats were given access to saline and housed without access to a wheel during withdrawal (n = 4). On day 15 of withdrawal, rats underwent extinction/reinstatement testing. On the morning following this 1-day test session, tissue was collected from the dmPFC and RNA-seq was performed. This graphical illustration was made using BioRender (https://biorender.com/). dmPFC, dorsomedial prefrontal cortex; RNA-seq, RNA sequencing.
Tissue Preparation and RNA-Seq
The morning following the test session, anesthetized rats were euthanized by rapid decapitation, and the dmPFC was dissected from 2-mm-thick coronal brain slices based on Paxinos and Watson (61) coordinates (bregma 3.2 mm). Brain tissue was rapidly frozen and stored at −80 °C until further processing. To generate RNA-seq libraries, total RNA was isolated from each sample using RNeasy Lipid Tissue Mini Kit (Qiagen), and 5 μg of total RNA was processed for ribosomal RNA removal using the Illumina Ribo-Zero Kit. RNA-seq libraries were prepared using the Illumina ScriptSeq v.2 RNA-seq Library Preparation Kit and purified using Beckman Coulter AMPure XP beads. The library quality and quantity were assessed by a 2100 Bioanalyzer (Agilent) prior to sequencing by the University of Virginia Core facility. Datasets were assessed for quality and consistency using FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Raw reads were then aligned to the rat rn6 genome using HiSAT2 specifying --rna-strandness FR (62). SAMtools was used to convert the resulting sequence alignment/map files to binary alignment map format, and transcripts were assembled and quantified with StringTie, using the Ensembl Rattus_norvegicus_Rnor_6.0.85 annotations (63,64). The resulting count tables were then used for identification of differentially expressed transcripts. DESeq2 (version 1.30.1) was used to normalize the raw counts and to perform differential expression analysis (65). Gene set enrichment was performed using g:Profiler (66). The RNA-seq analysis was performed using the tidyverse (version 1.3.1) and dplyr (version 1.0.8) software packages, and the term plots were made using ggplot2 (version 3.3.3). The network maps were made using Cytoscape (version 3.8.2). Volcano plots were created using the EnhancedVolcano (version 1.8.0) R package (67), and the heatmap was produced using the Pheatmap (version 1.0.12) R package (68).
Integrative Genomics Viewer
To visualize transcript changes relative to saline control animals, the merged RNA-seq datasets were loaded on the Integrative Genomics Viewer (IGV) using the rat rn6 genome (69). Differential normalized read counts for each condition were generated by subtracting the merged saline reads. IGV coverage tracks were visualized from bigwig files of paired reads, which join the 2 mates of the paired-end reads, and therefore span the entire range between the 2 mates. Exon numbering was generated by IGV using the National Center for Biotechnology Information RefSeq rat rn6 transcripts.
Data Analysis
We first confirmed that the effects of exercise in these subsets of rats were similar to those observed previously in the larger sample of sedentary, early, throughout, and late groups (19). Specifically, univariate analysis of variance was used to examine group differences in cocaine seeking during extinction (total responses), and repeated measures analysis of variance was used to examine group differences in cocaine seeking during reinstatement relative to responses during the last extinction session. Repeated measures analysis of variance was also used to verify that cocaine intake was similar between each of the cocaine groups during the extended-access phase and that levels of running were similar between each of the exercise groups during the 7-day exercise period (first 7 days for throughout exercise). Post hoc comparisons were Tukey corrected and based on either one-tailed (for predicted differences; e.g., higher cocaine seeking in sedentary vs. early and throughout exercise) or two-tailed (nonpredicted differences) distributions. The association between the efficacy of exercise to reduce cocaine-seeking responses and transcriptional changes (normalized counts) were examined using Benjamini-Hochberg–corrected Pearson correlations.
Results
Behavioral Results
Extended-Access Cocaine Self-administration and Exercise
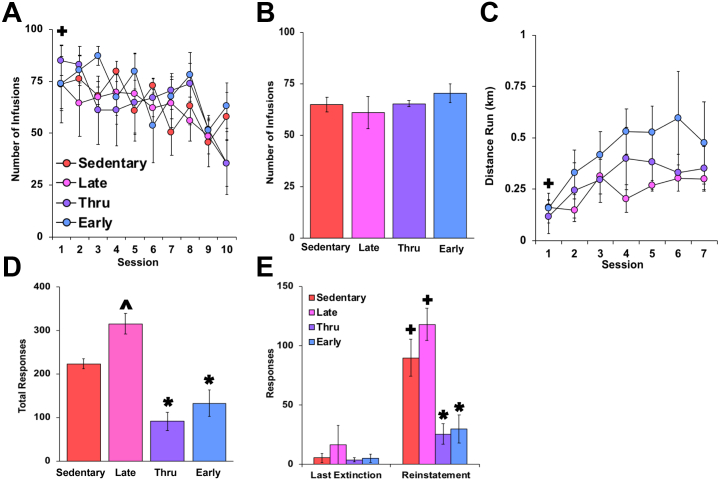
As with the larger dataset (19), results from these subsets of rats showed high levels of cocaine self-administration during the 10-day extended-access period with similar levels and patterns of intake between the groups (Figure 2A, B) (group and group by session, ps > .05). Intake was the highest in each of the groups during initial versus later sessions (session, F9,117 = 3.22, p < .01; session 1 vs. 10, p < .05). Overall levels and patterns of running during withdrawal were also similar between the 3 exercise groups (Figure 2C) (group and group by session, ps > .05). Levels of running progressively increased in each of the groups over the 7 exercise sessions (session, F6,54 = 5.18, p < .001; session 1 vs. 7, p < .01). Thus, prior to extinction/reinstatement testing, levels and patterns of cocaine intake and exercise were similar between the groups.
Figure 2.
Timing of exercise during withdrawal on cue-induced cocaine-seeking response. (A) Mean (±SEM) number of infusions for each of the 10 extended-access sessions, (B) cocaine intake averaged across the extended-access period (mg/kg), (C) distance run for the first 7 exercise sessions (km), and (D) total number of responses made on the lever formerly associated with cocaine during extinction and (E) during the last extinction session vs. the reinstatement session for male rats in the sedentary (n = 5), early (n = 4), throughout (n = 4), or late (n = 4) exercise conditions. + indicates significantly different from session 10 (B), session 7 (C), and the last extinction session (E). ∗ indicates significantly decreased vs. sedentary and late. ^ indicates significantly increased vs. sedentary. Thru, throughout.
Timing of Exercise During Withdrawal on Cue-Induced Cocaine Seeking
Also consistent with the larger dataset (19), cocaine seeking (or responding during extinction/reinstatement testing) in these subsets of rats was affected by the timing of exercise during withdrawal. For extinction, responses were significantly lower in early and throughout (Figure 2D) (group, F3,17 = 20.30, p < .001) than in both sedentary and late (ps < 0.01) and significantly higher in late than in sedentary (p < .01) conditions. Similarly, reinstatement of cocaine seeking in response to cocaine-associated cues was markedly attenuated in early and throughout versus sedentary and late (Figure 2E) (group by session, F3,13 = 10.43, p < .001) conditions. Specifically, while no group differences were observed during the last extinction session (p > .05), a significant group effect was observed within the reinstatement session (F3,13 = 11.27, p < .001), and post hoc comparisons revealed significantly lower responses in early and throughout than in sedentary and late (p < 0.05) conditions. Further analysis within each group revealed significantly higher responses during the reinstatement session than during the last extinction session for sedentary and late conditions (p < .05), but not for early and throughout conditions (p > .05). Thus, as with the larger dataset (19), results from these subsets of rats show that exercise initiated during early withdrawal, but not late withdrawal, reduces cocaine-seeking response.
Molecular Results
Transcriptional Changes Associated With Cue-Induced Cocaine Seeking
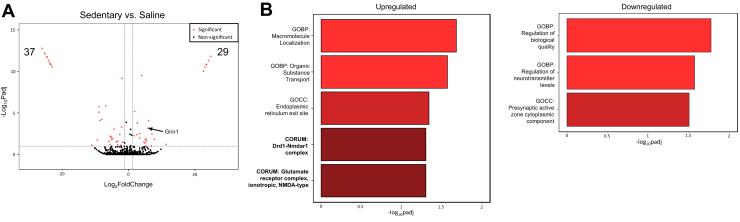
The analysis of transcriptional changes in the dmPFC of sedentary versus saline controls revealed 29 upregulated and 37 downregulated transcripts based on adjusted p = .1 and log2(fold change) > 1.3 (Figure 3A). Consistent with our previous findings (18,70), one of the upregulated transcripts was Grin1 (ENSRNOT00000044246), which encodes the NR1 subunit of the NMDA receptor. Analysis of the upregulated transcript list using Gene Ontology (GO) and CORUM, the comprehensive resource of mammalian protein complexes (which was manually created using reliable experimental evidence and utilizes the specific genes that contribute to the formation of specific quaternary protein structures) (71) revealed an enrichment of terms related to glutamate receptors, including glutamate receptor complex and Drd1-Nmdar1 complex, and macromolecule localization and organic substrate transport (Figure 3B). Interestingly, the downregulated transcript list revealed terms linked to the regulation of neurotransmission and the presynaptic active zone cytoplasmic component. Thus, cue-induced cocaine seeking appears to be associated with genes related to increased glutamate/NMDA receptor signaling and decreased regulation of presynaptic neurotransmission.
Figure 3.
Cue-induced cocaine-seeking–associated transcriptional changes in the dorsomedial prefrontal cortex. (A) Volcano plot depicting the differentially expressed transcripts in the dorsomedial prefrontal cortex of male rats in the sedentary group (n = 5) relative to the saline group (n = 5), with statistically significant differences depicted in red [padj < .1, log2(fold change) < 1.3]. (B) Bar plots depicting the enriched GO and CORUM terms using the differentially up- and downregulated transcripts in the cocaine vs. saline comparison. Bars with the same shade of red represent terms gathered from the same database. Adjusted p values were calculated using DESeq2. GO, Gene Ontology; GOBP, GO biological process; GOCC, GO cellular component; padj, adjusted p.
Transcriptional Changes Associated With the Protective Effects of Exercise Against Cocaine Seeking
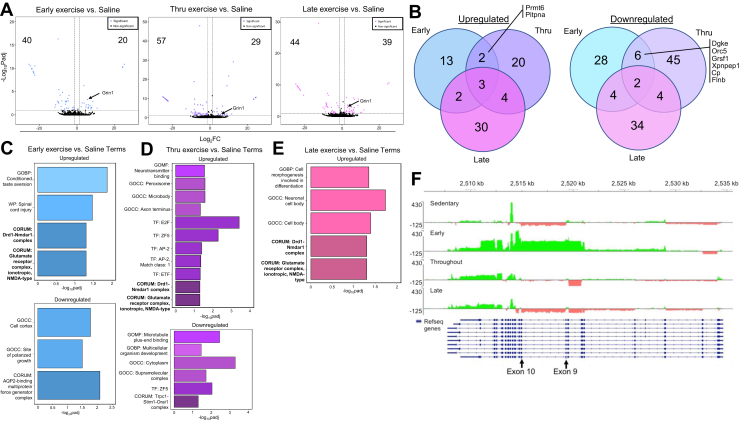
A similar strategy was used to investigate transcriptional changes associated with cue-induced cocaine seeking (vs. saline) in the dmPFC of rats that had previously exercised during early, throughout, or late withdrawal (Figure 4A). Surprisingly, despite the similarity between early and throughout exercise with regard to cocaine seeking, only 5 upregulated and 8 downregulated gene-associated transcripts overlapped between the groups, and of these, only 2 upregulated transcripts and 6 downregulated transcripts were specific to early and throughout (Figure 4B; Table 1). Notably, the Grin1 transcript was one of the three overlapping upregulated transcripts common to each of the exercise groups. We also investigated transcripts that were similarly upregulated or downregulated in early and throughout, but not late, relative to sedentary. This analysis produced results similar to those obtained from the analysis relative to saline with only 5 upregulated and 4 downregulated gene-related transcripts unique to early and throughout exercise (Table 2).
Figure 4.
Transcriptional changes associated with the protective effects of exercise against cocaine seeking. (A) Volcano plots depicting the differentially expressed transcripts in the dorsomedial prefrontal cortex of male rats in the early (n = 4), throughout (n = 4), and late (n = 4) exercise groups, each relative to the saline group (n = 5), with statistically significant transcripts depicted in blue, purple, and pink for the early, throughout, and late comparisons, respectively (padj < .1, log2FC < 1.3). Grin1 is labeled in all 3 volcano plots. (B) Venn diagrams showing the overlap of the significantly up- and downregulated transcripts in the early, thru, and late groups, each compared with the saline group. The uniquely overlapping transcripts between early and throughout exercise are shown. (C) Bar plot depicting the enriched GO, WP, and CORUM terms using the significantly up- and downregulated transcripts in the early exercise vs. saline comparison. Bars with the same shade of blue represent terms gathered from the same database. (D) Bar plot depicting the enriched GO, TF, and CORUM terms using the significantly up- and downregulated transcripts in the throughout exercise vs. saline comparison. Bars with the same shade of purple represent terms gathered from the same database. (E) Bar plot depicting the enriched GO and CORUM terms using the significantly upregulated transcripts in the throughout exercise vs. saline comparison. Bars with the same shade of pink represent terms gathered from the same database. No terms were enriched using the significantly downregulated transcripts. (F) Saline-subtracted, normalized RNA sequencing paired-read density for Grin1 in the sedentary, early, throughout, and late exercise conditions were mapped to chromosome 3 of the rat rn6 genome using the Integrated Genome Viewer. Differential transcripts above saline (green) or below saline (red) are indicated relative to known RefSeq exon transcripts (blue). Adjusted p values were calculated with DESeq2 using the Wald test for significance after fitting to a negative binomial linear model and performing the Benjamini-Hochberg procedure to limit false discoveries. GO, Gene Ontology; GOBP, GO biological process; GOCC, GO cellular component; GOMF, GO molecular function; log2FC, log2(fold change); padj, adjusted p; TF, transcription factor; thru, throughout; WP, WikiPathways.
Table 1.
Gene-Associated Transcripts That Were Similarly Up- or Downregulated in the Early and Throughout, but Not Late, Exercise Groups Relative to Saline Controls
| Gene | Transcript |
|---|---|
| Upregulated | |
| Pitpna | ENSRNOT00000090554 |
| Prmt6 | ENSRNOT00000023079 |
| Downregulated | |
| Cp | ENSRNOT00000082627 |
| Dgke | ENSRNOT00000076587 |
| Flnb | ENSRNOT00000066546 |
| Grsf1 | ENSRNOT00000086571 |
| Orc5 | ENSRNOT00000015426 |
| Xpnpep1 | ENSRNOT00000033148 |
Table 2.
Gene-Associated Transcripts That Were Similarly Up- or Downregulated in the Early and Throughout, but Not Late, Exercise Groups Relative to Sedentary Controls
| Gene | Transcript |
|---|---|
| Upregulated | |
| Ctbp2 | ENSRNOT00000023574 |
| Ten1 | ENSRNOT00000086382 |
| Elvol1 | ENSRNOT00000092972 |
| Mipep | ENSRNOT00000018845 |
| Ndufa12 | ENSRNOT00000089442 |
| Downregulated | |
| Ten1 | ENSRNOT00000083022 |
| Ggnbp2 | ENSRNOT00000085728 |
| Ybx3 | ENSRNOT00000007427 |
| AABR07030184.3 | ENSRNOT00000080772 |
To further assess similarities and differences in transcriptional profiles between the sedentary and exercise groups, we performed a GO, Kyoto Encyclopedia of Genes and Genomes (KEGG), CORUM, transcription factor database (TRANSFAC), and WikiPathways analysis on the differentially upregulated and downregulated gene-associated transcripts in each exercise group relative to saline (Figure 4C–E). To our surprise, this analysis revealed that there were 2 shared terms common for each of the exercise groups, glutamate receptor complex and Drd1-Nmdar1 complex. It is notable that these 2 terms were also found in the sedentary versus saline comparison (Figure 4C), suggesting that the efficacy of exercise is not mediated via normalization of glutamate/NMDA receptor levels in the dmPFC (but see IGV analysis below). Another notable finding from this analysis is that throughout exercise was uniquely associated with an upregulation of transcripts related to the transcription factor AP-2 (Figure 4D), which plays an important role in synaptic vesicle endocytosis and NMDA-stimulated AMPA receptor endocytosis (72,73). Finally, given the relative lack of overlapping transcripts and enrichment terms between the early and throughout exercise groups, it is likely that different mechanisms underlie the protective effects of exercise during early versus throughout withdrawal.
Given our previous work and work from others indicating a role for Grin1/NR1 in cocaine seeking and the efficacy of exercise in reducing cocaine seeking (18,70,74), Grin1 transcription was visualized using IGV. This analysis revealed that the normalized mapped read densities for the sedentary and exercise conditions varied significantly by exon (Figure 4F). For example, there was a robust loss of transcripts spanning exons 9 and 10 in the sedentary group relative to saline that was either countered or normalized with early and throughout, but not late exercise. Given that these effects mirror effects on cocaine seeking, it is possible that early and throughout exercise may normalize the RNA mis-splicing induced by cocaine seeking. Additionally, while throughout exercise normalized expression in these exon regions, early exercise led to an elevation of transcripts from these exon regions, indicating that, as suggested above, the mechanisms by which early- and throughout-withdrawal exercise exert their efficacy are different.
Transcriptional Changes Associated With the Protective Effects of Exercise Throughout Withdrawal Against Cocaine Seeking
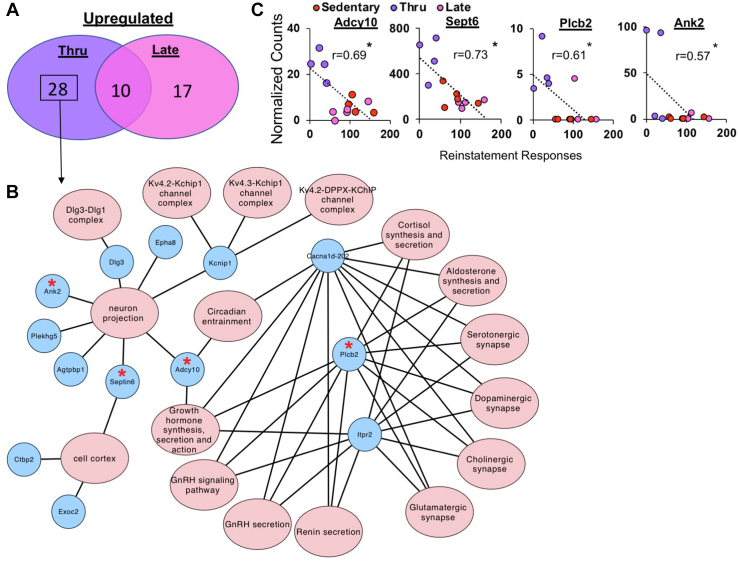
Given the relative lack of overlapping transcriptional profiles between the early and throughout exercise groups, we focused on characterizing the transcriptional profile associated with the protective effects of exercise throughout withdrawal cocaine seeking (relative to sedentary). We also compared effects with those observed in late (relative to sedentary) as a negative control for throughout exercise because the last exercise session was the day before the cue-induced cocaine-seeking test for both groups, yet only throughout exercise decreased cocaine seeking. This analysis revealed 28 upregulated gene-associated transcripts unique to throughout exercise (Figure 5A; Table 3) and further analysis of this transcript list using GO, KEGG, and CORUM revealed enrichment of terms related to neuron projection, cell cortex, growth hormone synthesis, secretion, and action; circadian entrainment; gonadotropin-releasing hormone, cortisol, renin, and aldosterone secretion; and neurotransmitter synapses, including serotonergic, dopaminergic, cholinergic, and glutamatergic synapses (Figure 5B). We also confirmed that transcriptional changes for 4 of the gene-associated transcripts unique to throughout exercise (Adcy10, Septin6, Plcb2, and Ank2) corresponded to the efficacy of exercise to reduce cocaine seeking such that higher expression levels (normalized counts) were predictive of significantly lower drug seeking (Figure 5C) (p < .05). It is notable that 3 of these transcripts are involved in neuron projection (Ank2, Septin6, and Adcy10).
Figure 5.
Transcriptional upregulation associated with the protective effects of exercise throughout withdrawal against cocaine seeking. (A) Venn diagram depicting the shared significantly upregulated transcripts between the throughout (n = 4) vs. sedentary (n = 5) and late (n = 4) vs. sedentary (n = 5) comparisons [adjusted p < .1, log2(fold change) < 1.3]. (B) Network map depicting the enriched GO, KEGG, and CORUM terms using the 28 uniquely upregulated transcripts in the throughout vs. sedentary comparison. Terms are in red ovals and genes are in blue circles. Lines connect the genes that contributed to each of the enriched terms. Red stars denote transcripts that had significant behavioral correlations. (C) The normalized counts of Adcy10, Septin6, Plcb2, and Ank2 were negatively associated with cocaine-seeking response as indicated by a significant correlation (∗). ∗p < .05. GnRH, gonadotropin-releasing hormone; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; thru, throughout.
Table 3.
Upregulated Gene-Associated Transcripts Induced by Exercise During Throughout, but Not Late, Withdrawal (Relative to Sedentary Controls)
| Upregulated Gene | Transcript |
|---|---|
| AABR07063152.1 | ENSRNOT00000089442 |
| Adcy10 | ENSRNOT00000082677 |
| Agtpbp1 | ENSRNOT00000091370 |
| Ank2 | ENSRNOT00000084756 |
| Arid5a | ENSRNOT00000089673 |
| Brpf3 | ENSRNOT00000092566 |
| Cacna1d | ENSRNOT00000047737 |
| Ctbp2 | ENSRNOT00000023574 |
| Dhrs7b | ENSRNOT00000066250 |
| Dlg3 | ENSRNOT00000088114 |
| Elovl1 | ENSRNOT00000092972 |
| Epha8 | ENSRNOT00000017559 |
| Exoc2 | ENSRNOT00000090706 |
| Gba2 | ENSRNOT00000022002 |
| Itpr2 | ENSRNOT00000040255 |
| Kcnip1 | ENSRNOT00000079031 |
| LOC103689920 | ENSRNOT00000090459 |
| Lonrf3 | ENSRNOT00000017550 |
| Mipep | ENSRNOT00000018845 |
| Nckap5l | ENSRNOT00000081434 |
| Plcb2 | ENSRNOT00000078037 |
| Plekhg5 | ENSRNOT00000082586 |
| Ppp1r18 | ENSRNOT00000082999 |
| Septin6 | ENSRNOT00000067942 |
| Ten1 | ENSRNOT00000086382 |
| Thop1 | ENSRNOT00000082486 |
| Usp19 | ENSRNOT00000074772 |
| Wbp11l1 | ENSRNOT00000082235 |
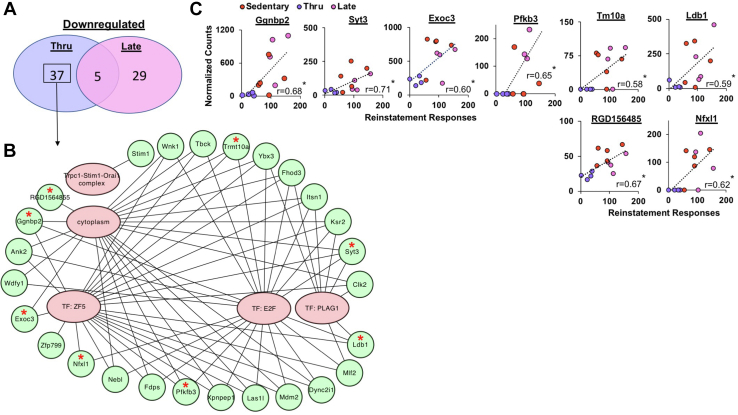
The same analysis revealed 37 downregulated gene-associated transcripts unique to throughout exercise (Figure 6A; Table 4) as well as an enrichment of terms related to cytoplasm, the Trpc1-Stim1-Orai1 complex, and several transcription factors, including ZF5, E2F, and PLAG1 (Figure 6B). Interestingly, while E2F has been implicated in neuronal migration, it also has been shown to be a positive regulator of neuronal apoptosis (75,76). The normalized transcript counts of several of the transcripts unique to throughout exercise also corresponded to the efficacy of exercise. Specifically, reinstatement responses were positively associated with normalized transcript counts for Exoc3, Ggnbp2, Syt3, Trmt10a, Ldb1, Pfkb3, RGD1564855, and Nfxl1 (ps < .05) (Figure 6C). These transcripts all intersected with at least one of the aforementioned enrichment terms. Thus, throughout exercise may serve to reduce cocaine seeking by promoting neuronal synapses and hormone secretion and preventing neuronal apoptosis.
Figure 6.
Transcriptional downregulation associated with the protective effects of exercise throughout withdrawal against cocaine seeking. (A) Venn diagram depicting the shared significantly downregulated transcripts between the throughout (n = 4) vs. sedentary (n = 5) and late (n = 4) vs. sedentary (n = 5) comparisons [adjusted p < .1, log2(fold change) < 1.3]. (B) Network map depicting the enriched GO, TF, and CORUM terms using the 37 uniquely upregulated transcripts in the throughout vs. cocaine sedentary comparison. Terms are in red ovals and genes are in green circles. Lines connect the genes that contributed to each of the enriched terms. Red stars denote genes that had significant behavioral correlations. (C) The normalized counts of Ggnbp2, Syt3, Exoc3, Pfkb3, Tm10a, Ldb1, RGD156485, and Nfxl1 were negatively associated with cocaine-seeking response as indicated by a significant correlation (∗). ∗p < .05. GO, Gene Ontology; TF, transcription factor; thru, throughout.
Table 4.
Downregulated Gene-Associated Transcripts Induced by Exercise During Throughout, but Not Late, Withdrawal (Relative to Sedentary Controls)
| Downregulated Gene | Transcript |
|---|---|
| AABR07013729.1 | ENSRNOT00000080332 |
| AABR07030184.9 | ENSRNOT00000080772 |
| AABR07046765.1 | ENSRNOT00000071767 |
| AABR07064415.1 | ENSRNOT00000082472 |
| Ank2 | ENSRNOT00000015386 |
| Clk2 | ENSRNOT00000085817 |
| Coro7 | ENSRNOT00000006067 |
| Exoc3 | ENSRNOT00000020251 |
| Fdps | ENSRNOT00000080185 |
| Fhod3 | ENSRNOT00000041961 |
| Ggnbp2 | ENSRNOT00000085728 |
| Igf1 | ENSRNOT00000081822 |
| Itsn1 | ENSRNOT00000047843 |
| Ksr2 | ENSRNOT00000071074 |
| Las1l | ENSRNOT00000016042 |
| Ldb1 | ENSRNOT00000040904 |
| LOC100911361 | ENSRNOT00000076198 |
| LOC103689920 | ENSRNOT00000084136 |
| Mdm2 | ENSRNOT00000066767 |
| Mlf2 | ENSRNOT00000088622 |
| Nebl | ENSRNOT00000068553 |
| Nfxl1 | ENSRNOT00000077080 |
| Pfkfb3 | ENSRNOT00000051067 |
| RGD1307947 | ENSRNOT00000036141 |
| RGD1564855 | ENSRNOT00000079980 |
| Rpl9 | ENSRNOT00000076914 |
| Stim1 | ENSRNOT00000088370 |
| Syt3 | ENSRNOT00000077427 |
| Tbck | ENSRNOT00000083937 |
| Ten1 | ENSRNOT00000083022 |
| Trmt10a | ENSRNOT00000014694 |
| Wdfy1 | ENSRNOT00000063834 |
| Dync2il | ENSRNOT00000006144 |
| Wnk1 | ENSRNOT00000013355 |
| Xpnpep1 | ENSRNOT00000033148 |
| Ybx3 | ENSRNOT00000007427 |
| Zfp799 | ENSRNOT00000087294 |
Discussion
The current study used RNA-seq to identify the transcriptional changes in the dmPFC associated with cue-induced cocaine seeking and its modulation by exercise. As expected (18,19), the sedentary group demonstrated high levels of cocaine-seeking response, which may be associated with increased glutamate signaling as indicated by the upregulation of a Grin1-associated transcript and an enrichment of Drd1-Nmdar1 complex and glutamate receptor complex terms. To our surprise, each of the exercise groups also showed an upregulation of the same Grin1-associated transcript and an enrichment of the same glutamate receptor–related terms (i.e., Drd1-Nmdar1 complex and glutamate receptor complex). While on the surface, these findings suggest that the protective effects of exercise against cocaine seeking are not mediated through normalization of glutamatergic signaling, particularly via gene-associated Grin1 transcripts, a closer examination revealed a robust loss of transcripts spanning exons 9 and 10 in sedentary controls (relative to saline) that was normalized with early and throughout exercise, but not late exercise, suggesting that exercise initiated during early withdrawal normalizes cocaine cue-induced RNA mis-splicing of Grin1. It is also notable that, despite similar protection against cocaine seeking by early and throughout exercise, there was minimal overlap between these groups in their transcriptional profiles. Each of these findings are discussed further below.
As expected, cue-induced cocaine seeking was high following extended-access cocaine self-administration and protracted withdrawal and appears to be associated with increased glutamate signaling in the dmPFC based on our findings showing upregulation of the Grin-1 associated transcript and an enrichment of terms related to Drd1-Nmdar1 complex and glutamate receptor complex. These findings are consistent with findings in both humans and animals showing a strong association between drug craving/seeking and dmPFC hyperactivity (46, 47, 48, 49, 50, 51) as well as work from our group (18,70) and others [e.g., (77, 78, 79, 80)], supporting the involvement of NMDA receptors in particular. Thus, targeting overactive glutamatergic signaling in the dmPFC, particularly via NMDA receptors, could serve as a potential intervention for drug craving. Drug side effects would need to be considered when selecting an NMDA receptor antagonist for treatment, but it may be possible given that high doses of methadone, a full μ opioid agonist that also acts as an NMDA receptor antagonist, are commonly prescribed for use in humans. High doses of methadone have also been shown to significantly reduce cocaine use in patients undergoing methadone maintenance treatment (81).
Surprisingly, a Grin1-associated transcript was upregulated in all the exercise groups along with the sedentary controls. While it is possible that this change was induced by extended-access cocaine self-administration independent of cue-induced cocaine seeking (77), given that NR1 has previously been associated with levels of cocaine seeking in rats (18,70) and vulnerability to substance use disorders in humans (82,83), a more likely possibility is that exercise that effectively reduces cocaine seeking/craving does so by normalizing signaling downstream of NR1/NMDA receptor signaling or, as suggested by the IGV analysis, by normalizing mis-splicing at Grin1. For example, our findings showing the loss of transcripts spanning exons 9 and 10 in the sedentary condition and normalization by early and throughout, but not late, exercise is potentially significant, considering that these exon regions are largely extracellular and include the first 5 amino acids of the ligated ion channel L-glutamate and glycine binding site. Therefore, splicing out these exons (and loss of the first 5 amino acids) could change the fidelity of NMDA receptor signaling (84). Notably, alternative splicing of 3 other Grin1 exons (5,21,22) has been shown to affect the pharmacological properties and intracellular binding partners of NMDA receptors (85). This highlights an area for future research that may provide insight on the mechanisms underlying CUD, because these splicing effects are often overlooked in RNA-seq studies and possibly missed by quantitative polymerase chain reaction, depending on the primers selected. It is also notable that the rigor of these findings is demonstrated by the fact that our RNA-seq results with Grin1 in the sedentary condition are consistent with previous gene expression findings from our group (18,70) and others (77) as well as findings in humans with substance use disorders (82).
Also, to our surprise, despite similar effects with regard to cocaine seeking, there were few transcriptomic similarities between the early and throughout exercise conditions. Of the 146 transcripts that were differentially expressed in the early and throughout exercise groups (relative to saline), only 8 were shared between the two conditions. The functional classification analysis of the differentially expressed transcripts also revealed only 2 overlapping enrichment terms between the early and throughout exercise groups (i.e., Drd1-Nmdar1 complex and glutamate receptor complex), and both terms overlapped with the sedentary and late exercise groups. Together, these findings suggest that the mechanisms underlying the protective effects of exercise against cocaine seeking are different for exercise during early versus throughout withdrawal; however, recency of exercise and methodological limitations, such as the homogeneous analyses of messenger RNA transcripts from a heterogeneous brain region with different cell- and projection-specific functions, may also have contributed to the differences observed. Thus, future research investigating the molecular changes induced by exercise during early, late, and throughout withdrawal (prior to reinstatement testing) or using techniques, such as single-cell RNA-seq, are necessary to examine these possibilities.
Our findings also reveal novel potential mechanisms by which exercise mediates its protective effects against cocaine seeking. Specifically, exercise initiated during early withdrawal was associated with the downregulation of transcripts associated with presynaptic active zone cytoplasmic component and regulation of neurotransmitter release. Additionally, the comparison that isolated transcriptional changes specific to exercise throughout withdrawal (relative to sedentary controls) revealed an upregulation of terms associated with neurotransmitter synapses, including glutamatergic synapses. One of the transcript intersections for the glutamatergic synapse enrichment term was Plcb2, which is a phosphodiesterase that catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate to the second messenger inositol 1,4,5-triphosphate (IP3). Interestingly, IP3 is downstream of mGlu5 (86), which we showed previously to be necessary for the incubation of cocaine seeking but not for the efficacy of exercise to decrease cocaine seeking (18). Thus, one possibility that we plan to address in future studies is that exercise exerts its efficacy downstream of mGlu5 via interactions with IP3.
The efficacy of exercise to reduce cocaine seeking was also associated with terms associated with circadian rhythms and neuronal survival. There is a large body of literature showing that circadian rhythms play a critical role in substance use disorder, including drug seeking/craving (87). Specifically, findings in rats show that circadian genes mutations enhance ethanol consumption and that melatonin treatment reduces motivation for cocaine and drug seeking (88,89). One interesting transcript in this regard is Adcy10, which contributed to the enrichment of the circadian entrainment term, was upregulated by throughout exercise (relative to sedentary controls), and was positively correlated with behavior. The downregulation of E2F-related transcripts, which contributed to the neuronal survival term, is also of interest, considering that it was also correlated with behavior; it has also been shown to prevent morphine- and heroin-induced apoptosis in cerebellar neurons (90, 91, 92). Our RNA-seq findings thus shed light on the potential pivotal role that circadian entrainment and neuronal survival may play in preventing cocaine-seeking response, but, as with the glutamatergic findings, we will need to provide functional confirmation in future studies.
In conclusion, these findings reveal possible glutamatergic mechanisms and other novel candidate mechanisms that may underlie cocaine seeking and the efficacy of exercise to reduce cocaine seeking. We plan to use these findings to guide our future studies, which will focus on determining the functional consequences of these molecular changes. Future studies also are needed to address mechanistic differences between early- versus throughout-withdrawal exercise because their protective effects against cocaine seeking are likely mediated via different mechanisms. Finally, these findings suggest RNA mis-splicing as a potential mechanism underlying cocaine seeking and highlight a need for further research on its role in CUD.
Acknowledgments and Disclosures
This work was supported by R01 grants from the National Institute on Drug Abuse (Grant No. DA039093 and DA024716 [to WJL]); funds from the Brain Institute at the University of Virginia (to WJL); and a Pharmacological Sciences Training Grant (Grant No. 5T32GM007055-47 [to EBT]), a Medical Scientist Training Program Grant (Grant No. T32 GM007267 [to EBT]), and a Wagner Fellowship from the University of Virginia (to EBT).
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary Material
References
- 1.Substance Abuse and Mental Health Services Administration Detailed tables: Results from the 2020 national survey on drug use. https://www.samhsa.gov/data/report/2020-nsduh-detailed-tables Available at:
- 2.Kilmer G., Midgette G. Mixed messages: Is cocaine consumption in the U.S. going up or down? Brookings: Drugs and Peace in Columbia. https://www.brookings.edu/opinions/mixed-messages-is-cocaine-consumption-in-the-u-s-going-up-or-down/ Available at:
- 3.Maxwell J.C. Is cocaine coming back? A commentary. Subst Use Misuse. 2020;55:345–348. doi: 10.1080/10826084.2019.1664592. [DOI] [PubMed] [Google Scholar]
- 4.United States Department of State International Narcotics Control Strategy Report. https://www.state.gov/international-narcotics-control-strategy-reports/ Available at:
- 5.McLellan A.T., Lewis D.C., O’Brien C.P., Kleber H.D. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 6.Ashdown-Franks G., Firth J., Carney R., Carvalho A.F., Hallgren M., Koyanagi A., et al. Exercise as medicine for mental and substance use disorders: A meta-review of the benefits for neuropsychiatric and cognitive outcomes. Sports Med. 2020;50:151–170. doi: 10.1007/s40279-019-01187-6. [DOI] [PubMed] [Google Scholar]
- 7.Cabé N., Lanièpce A., Pitel A.L. Physical activity: A promising adjunctive treatment for severe alcohol use disorder. Addict Behav. 2021;113 doi: 10.1016/j.addbeh.2020.106667. [DOI] [PubMed] [Google Scholar]
- 8.Ellingsen M.M., Clausen T., Johannesen S.L., Martinsen E.W., Hallgren M. Effects of acute exercise on drug craving in adults with poly-substance use disorder. A randomized controlled trial. Ment Health Phys Act. 2021;21 [Google Scholar]
- 9.Giménez-Meseguer J., Tortosa-Martínez J., Cortell-Tormo J.M. The benefits of physical exercise on mental disorders and quality of life in substance use disorders patients. Systematic review and meta-analysis. Int J Environ Res Public Health. 2020;17:3680. doi: 10.3390/ijerph17103680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallgren M., Vancampfort D., Hoang M.T., Andersson V., Ekblom Ö., Andreasson S., Herring M.P. Effects of acute exercise on craving, mood and anxiety in non-treatment seeking adults with alcohol use disorder: An exploratory study. Drug Alcohol Depend. 2021;220 doi: 10.1016/j.drugalcdep.2021.108506. [DOI] [PubMed] [Google Scholar]
- 11.Hallgren M., Herring M.P., Vancampfort D., Hoang M.T., Andersson V., Andreasson S., Abrantes A.M. Changes in craving following acute aerobic exercise in adults with alcohol use disorder. J Psychiatr Res. 2021;142:243–249. doi: 10.1016/j.jpsychires.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Salem B.A., Gonzales-Castaneda R., Ang A., Rawson R.A., Dickerson D., Chudzynski J., et al. Craving among individuals with stimulant use disorder in residential social model-based treatment - Can exercise help? Drug Alcohol Depend. 2022;231 doi: 10.1016/j.drugalcdep.2021.109247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ensari I., Greenlee T.A., Motl R.W., Petruzzello S.J. Meta-analysis of acute exercise effects on state anxiety: An update of randomized controlled trials over the past 25 years. Depress Anxiety. 2015;32:624–634. doi: 10.1002/da.22370. [DOI] [PubMed] [Google Scholar]
- 14.Landers D.M., Arent S.M. In: Handbook of Sport Psychology. 3rd ed. Tenenbaum G., Eklund R.C., editors. Wiley and Sons Inc; Hoboken, NJ: 2007. Physical activity and mental health. [Google Scholar]
- 15.Martinsen E.W. Physical activity in the prevention and treatment of anxiety and depression. Nord J Psychiatry. 2008;62(Suppl 47):25–29. doi: 10.1080/08039480802315640. [DOI] [PubMed] [Google Scholar]
- 16.Aharonovich E., Hasin D.S., Brooks A.C., Liu X., Bisaga A., Nunes E.V. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Poling J., Kosten T.R., Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33:191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- 18.Abel J.M., Nesil T., Bakhti-Suroosh A., Grant P.A., Lynch W.J. Mechanisms underlying the efficacy of exercise as an intervention for cocaine relapse: A focus on mGlu5 in the dorsal medial prefrontal cortex. Psychopharmacology (Berl) 2019;236:2155–2171. doi: 10.1007/s00213-019-05208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beiter R.M., Peterson A.B., Abel J.M., Lynch W.J. Exercise during early, but not late abstinence, attenuates subsequent relapse vulnerability in a rat model. Transl Psychiatry. 2016;6 doi: 10.1038/tp.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogbonmwan Y.E., Schroeder J.P., Holmes P.V., Weinshenker D. The effects of post-extinction exercise on cocaine-primed and stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2015;232:1395–1403. doi: 10.1007/s00213-014-3778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson A.B., Abel J.M., Lynch W.J. Dose-dependent effects of wheel running on cocaine-seeking and prefrontal cortex bdnf exon IV expression in rats. Psychopharmacology (Berl) 2014;231:1305–1314. doi: 10.1007/s00213-013-3321-4. [DOI] [PubMed] [Google Scholar]
- 22.Peterson A.B., Hivick D.P., Lynch W.J. Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: Impact of sex and estrous cycle in rats. Psychopharmacology (Berl) 2014;231:2661–2670. doi: 10.1007/s00213-014-3437-1. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez V., Moore C.F., Brunzell D.H., Lynch W.J. Effect of wheel-running during abstinence on subsequent nicotine-seeking in rats. Psychopharmacology (Berl) 2013;227:403–411. doi: 10.1007/s00213-012-2964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez V., Moore C.F., Brunzell D.H., Lynch W.J. Sex differences in the effect of wheel running on subsequent nicotine-seeking in a rat adolescent-onset self-administration model. Psychopharmacology (Berl) 2014;231:1753–1762. doi: 10.1007/s00213-013-3359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez V., Lycas M.D., Lynch W.J., Brunzell D.H. Wheel running exercise attenuates vulnerability to self-administer nicotine in rats. Drug Alcohol Depend. 2015;156:193–198. doi: 10.1016/j.drugalcdep.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez V., Bakhti-Suroosh A., Chen A., Brunzell D.H., Erisir A., Lynch W.J. Exercise during abstinence normalizes ultrastructural synaptic plasticity associated with nicotine-seeking following extended access self-administration. Eur J Neurosci. 2019;50:2707–2721. doi: 10.1111/ejn.14408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobieraj J.C., Kim A., Fannon M.J., Mandyam C.D. Chronic wheel running-induced reduction of extinction and reinstatement of methamphetamine seeking in methamphetamine dependent rats is associated with reduced number of periaqueductal gray dopamine neurons. Brain Struct Funct. 2016;221:261–276. doi: 10.1007/s00429-014-0905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thanos P.K., Stamos J., Robison L.S., Heyman G., Tucci A., Wang G.J., et al. Daily treadmill exercise attenuates cocaine cue-induced reinstatement and cocaine induced locomotor response but increases cocaine-primed reinstatement. Behav Brain Res. 2013;239:8–14. doi: 10.1016/j.bbr.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zlebnik N.E., Anker J.J., Gliddon L.A., Carroll M.E. Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology (Berl) 2010;209:113–125. doi: 10.1007/s00213-010-1776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zlebnik N.E., Carroll M.E. Prevention of the incubation of cocaine seeking by aerobic exercise in female rats. Psychopharmacology (Berl) 2015;232:3507–3513. doi: 10.1007/s00213-015-3999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch W.J., Abel J., Robinson A.M., Smith M.A. Exercise as a sex-specific treatment for substance use disorder. Curr Addict Rep. 2017;4:467–481. doi: 10.1007/s40429-017-0177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch W.J., Robinson A.M., Abel J., Smith M.A. Exercise as a prevention for substance use disorder: A review of sex differences and neurobiological mechanisms. Curr Addict Rep. 2017;4:455–466. doi: 10.1007/s40429-017-0178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alessi S.M., Rash C.J., Pescatello L.S. Reinforcing exercise to improve drug abuse treatment outcomes: A randomized controlled study in a substance use disorder outpatient treatment setting. Psychol Addict Behav. 2020;34:52–64. doi: 10.1037/adb0000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colledge F., Vogel M., Dürsteler-Macfarland K., Strom J., Schoen S., Pühse U., Gerber M. A pilot randomized trial of exercise as adjunct therapy in a heroin-assisted treatment setting. J Subst Abuse Treat. 2017;76:49–57. doi: 10.1016/j.jsat.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Cutter C.J., Schottenfeld R.S., Moore B.A., Ball S.A., Beitel M., Savant J.D., et al. A pilot trial of a videogame-based exercise program for methadone maintained patients. J Subst Abuse Treat. 2014;47:299–305. doi: 10.1016/j.jsat.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De La Garza R., 2nd, Yoon J.H., Thompson-Lake D.G., Haile C.N., Eisenhofer J.D., Newton T.F., Mahoney J.J., 3rd Treadmill exercise improves fitness and reduces craving and use of cocaine in individuals with concurrent cocaine and tobacco-use disorder. Psychiatry Res. 2016;245:133–140. doi: 10.1016/j.psychres.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hallgren M., Romberg K., Bakshi A.S., Andréasson S. Yoga as an adjunct treatment for alcohol dependence: A pilot study. Complement Ther Med. 2014;22:441–445. doi: 10.1016/j.ctim.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Hallgren M., Vancampfort D., Giesen E.S., Lundin A., Stubbs B. Exercise as treatment for alcohol use disorders: Systematic review and meta-analysis. Br J Sports Med. 2017;51:1058–1064. doi: 10.1136/bjsports-2016-096814. [DOI] [PubMed] [Google Scholar]
- 39.Linke S.E., Rutledge T., Myers M.G. Intermittent exercise in response to cigarette cravings in the context of an internet-based smoking cessation program. Ment Health Phys Act. 2012;5:85–92. doi: 10.1016/j.mhpa.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prapavessis H., De Jesus S., Fitzgeorge L., Faulkner G., Maddison R., Batten S. Exercise to enhance smoking cessation: The getting physical on cigarette randomized control trial. Ann Behav Med. 2016;50:358–369. doi: 10.1007/s12160-015-9761-9. [DOI] [PubMed] [Google Scholar]
- 41.Rawson R.A., Chudzynski J., Mooney L., Gonzales R., Ang A., Dickerson D., et al. Impact of an exercise intervention on methamphetamine use outcomes post-residential treatment care. Drug Alcohol Depend. 2015;156:21–28. doi: 10.1016/j.drugalcdep.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ussher M.H., Taylor A.H., Faulkner G.E. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2014;8:CD002295. doi: 10.1002/14651858.CD002295.pub5. [DOI] [PubMed] [Google Scholar]
- 43.Ussher M.H., Lewis S., Aveyard P., Manyonda I., West R., Lewis B., et al. Physical activity for smoking cessation in pregnancy: Randomised controlled trial. BMJ. 2015;350:h2145. doi: 10.1136/bmj.h2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lynch W.J., Peterson A.B., Sanchez V., Abel J., Smith M.A. Exercise as a novel treatment for drug addiction: A neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37:1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linke S.E., Ussher M. Exercise-based treatments for substance use disorders: Evidence, theory, and practicality. Am J Drug Alcohol Abuse. 2015;41:7–15. doi: 10.3109/00952990.2014.976708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin C.B., Templeton T.J., Chiu A.S., Kim J., Gable E.S., Vieira P.A., et al. Endogenous glutamate within the prelimbic and infralimbic cortices regulates the incubation of cocaine-seeking in rats. Neuropharmacology. 2018;128:293–300. doi: 10.1016/j.neuropharm.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szumlinski K.K., Shin C.B. Kinase interest you in treating incubated cocaine-craving? A hypothetical model for treatment intervention during protracted withdrawal from cocaine. Genes Brain Behav. 2018;17 doi: 10.1111/gbb.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LaLumiere R.T., Kalivas P.W. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubio F.J., Quintana-Feliciano R., Warren B.L., Li X., Witonsky K.F.R., Valle F.S.D., et al. Prelimbic cortex is a common brain area activated during cue-induced reinstatement of cocaine and heroin seeking in a polydrug self-administration rat model. Eur J Neurosci. 2019;49:165–178. doi: 10.1111/ejn.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.See R.E. Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int J Neuropsychopharmacol. 2009;12:431–436. doi: 10.1017/S1461145709000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldstein R.Z., Volkow N.D. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y., Kong F., Crofton E.J., Dragosljvich S.N., Sinha M., Li D., et al. Transcriptomics of environmental enrichment reveals a role for retinoic acid signaling in addiction. Front Mol Neurosci. 2016;9:119. doi: 10.3389/fnmol.2016.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powell G.L., Vannan A., Bastle R.M., Wilson M.A., Dell’Orco M., Perrone-Bizzozero N.I., Neisewander J.L. Environmental enrichment during forced abstinence from cocaine self-administration opposes gene network expression changes associated with the incubation effect. Sci Rep. 2020;10 doi: 10.1038/s41598-020-67966-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merritt C.R., Smith A.E., Khanipov K., Golovko G., Dineley K.T., Anastasio N.C., Cunningham K.A. Heightened cocaine-seeking in male rats associates with a distinct transcriptomic profile in the medial prefrontal cortex. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker D.M., Cates H.M., Loh Y.E., Purushothaman I., Ramakrishnan A., Cahill K.M., et al. Cocaine self-administration alters transcriptome-wide responses in the brain’s reward circuitry. Biol Psychiatry. 2018;84:867–880. doi: 10.1016/j.biopsych.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wimmer M.E., Fant B., Swinford-Jackson S.E., Testino A., Van Nest D., Abel T., Pierce R.C. H3.3 barcoding of nucleus accumbens transcriptional activity identifies novel molecular cascades associated with cocaine self-administration in mice. J Neurosci. 2019;39:5247–5254. doi: 10.1523/JNEUROSCI.0015-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duttke S.H., Montilla-Perez P., Chang M.W., Li H., Chen H., Carrette L.L., et al. Glucocorticoid receptor-regulated enhancers play a central role in the gene regulatory networks underlying drug addiction. Front Neurosci 2022. 2022;16 doi: 10.3389/fnins.2022.858427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lynch W.J. Modeling the development of drug addiction in male and female animals. Pharmacol Biochem Behav. 2018;164:50–61. doi: 10.1016/j.pbb.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scotti M.M., Swanson M.S. RNA mis-splicing in disease. Nat Rev Genet. 2016;17:19–32. doi: 10.1038/nrg.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Booven D., Li Mengying, Sunil Rao J., Blokhin I.O., Dayne Mayfield R., Barbier E., et al. Alcohol use disorder causes global changes in splicing in the human brain. Transl Psychiatry. 2021;11:1. doi: 10.1038/s41398-020-01163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paxinos G., Watson C. 6th ed. Academic Press; San Diego: 2007. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 62.Mueller R.C., Ellström P., Howe K., Uliano-Silva M., Kuo R.I., Miedzinska K., et al. A high-quality genome and comparison of short- versus long-read transcriptome of the Palaearctic duck Aythya fuligula (tufted duck) Gigascience. 2021;10 doi: 10.1093/gigascience/giab081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., et al. The Sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pertea M., Pertea G.M., Antonescu C.M., Chang T.C., Mendell J.T., Salzberg S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raudvere U., Kolberg L., Kuzmin I., Arak T., Adler P., Peterson H., Vilo J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update) Nucleic Acids Res. 2019;47:W191–W198. doi: 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blighe K., Rana S., Lewis M. EnhancedVolcano: Publication-ready volcano plots with enhanced colouring and labeling. https://bioconductor.org/packages/devel/bioc/vignettes/EnhancedVolcano/inst/doc/EnhancedVolcano.html Available at:
- 68.Kolde R. Package ‘pheatmap’. https://cran.r-project.org/web/packages/pheatmap/pheatmap.pdf Available at:
- 69.Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Towers E.B., Kilgore M., Bakhti-Suroosh A., Pidaparthi L., Williams I.L., Abel J.M., et al. Sex differences in the neuroadaptations associated with incubated cocaine-craving: A focus on the dorsomedial prefrontal cortex. Front Behav Neurosci. 2023;16 doi: 10.3389/fnbeh.2022.1027310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruepp A., Brauner B., Dunger-Kaltenbach I., Frishman G., Montrone C., Stransky M., et al. Corum: The comprehensive resource of mammalian protein complexes. Nucleic Acids Res. 2008;36:D646–D650. doi: 10.1093/nar/gkm936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim S.H., Ryan T.A. Synaptic vesicle recycling at CNS snapses without AP-2. J Neurosci. 2009;29:3865–3874. doi: 10.1523/JNEUROSCI.5639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Genoux D., Montgomery J.M. Glutamate receptor plasticity at excitatory synapses in the brain. Clin Exp Pharmacol Physiol. 2007;34:1058–1063. doi: 10.1111/j.1440-1681.2007.04722.x. [DOI] [PubMed] [Google Scholar]
- 74.Li X., Davis I.R., Lofaro O.M., Zhang J., Cimbro R., Rubio F.J. Distinct gene alterations between Fos-expressing striatal and thalamic neurons after withdrawal from methamphetamine self-administration. Brain Behav. 2019;9 doi: 10.1002/brb3.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frank C.L., Tsai L.H. Alternative functions of core cell cycle regulators in neuronal migration, neuronal maturation, and synaptic plasticity. Neuron. 2009;62:312–326. doi: 10.1016/j.neuron.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verdaguer E., Susana Gde A., Clemens A., Pallàs M., Camins A. Implication of the transcription factor E2F-1 in the modulation of neuronal apoptosis. Biomed Pharmacother. 2007;61:390–399. doi: 10.1016/j.biopha.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 77.Ploense K.L., Vieira P., Bubalo L., Olivarria G., Carr A.E., Szumlinski K.K., Kippin T.E. Contributions of prolonged contingent and non-contingent cocaine exposure to escalation of cocaine intake and glutamatergic gene expression. Psychopharmacology (Berl) 2018;235:1347–1359. doi: 10.1007/s00213-017-4798-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barry S.M., McGinty J.F. Role of Src Family Kinases in BDNF-mediated suppression of cocaine-seeking and prevention of cocaine-induced ERK, GluN2A, and GluN2B dephosphorylation in the prelimbic cortex. Neuropsychopharmacology. 2017;42:1972–1980. doi: 10.1038/npp.2017.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szumlinski K.K., Wroten M.G., Miller B.W., Sacramento A.D., Cohen M., Ben-Shahar O., Kippin T.E. Cocaine self-administration elevates GluN2B within dmPFC mediating heightened cue-elicited operant responding. J Drug Abuse. 2016;2:22. doi: 10.21767/2471-853x.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ben-Shahar O., Obara I., Ary A.W., Ma N., Mangiardi M.A., Medina R.L., Szumlinski K.K. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peles E., Kreek M.J., Kellogg S., Adelson M. High methadone dose significantly reduces cocaine use in methadone maintenance treatment (MMT) patients. J Addict Dis. 2006;25:43–50. doi: 10.1300/J069v25n01_07. [DOI] [PubMed] [Google Scholar]
- 82.Daneshparvar H., Sadat-Shirazi M.S., Fekri M., Khalifeh S., Ziaie A., Esfahanizadeh N., et al. NMDA receptor subunits change in the prefrontal cortex of pure-opioid and multi-drug abusers: A post-mortem study. Eur Arch Psychiatry Clin Neurosci. 2019;269:309–315. doi: 10.1007/s00406-018-0900-8. [DOI] [PubMed] [Google Scholar]
- 83.Enoch M.A., Rosser A.A., Zhou Z., Mash D.C., Yuan Q., Goldman D. Expression of glutamatergic genes in healthy humans across 16 brain regions; altered expression in the hippocampus after chronic exposure to alcohol or cocaine. Genes Brain Behav. 2014;13:758–768. doi: 10.1111/gbb.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamakura T., Shimoji K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog Neurobiol. 1999;59:279–298. doi: 10.1016/s0301-0082(99)00007-6. [DOI] [PubMed] [Google Scholar]
- 85.Hansen K.B., Yi F., Perszyk R.E., Furukawa H., Wollmuth L.P., Gibb A.J., Traynelis S.F. Structure, function, and allosteric modulation of NMDA receptors. J Gen Physiol. 2018;150:1081–1105. doi: 10.1085/jgp.201812032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piers T.M., Kim D.H., Kim B.C., Regan P., Whitcomb D.J., Cho K. Translational concepts of mGluR5 in synaptic diseases of the brain. Front Pharmacol. 2012;3:199. doi: 10.3389/fphar.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tamura E.K., Oliveira-Silva K.S., Ferreira-Moraes F.A., Marinho E.A.V., Guerrero-Vargas N.N. Circadian rhythms and substance use disorders: A bidirectional relationship. Pharmacol Biochem Behav. 2021;201 doi: 10.1016/j.pbb.2021.173105. [DOI] [PubMed] [Google Scholar]
- 88.Perreau-Lenz S., Vengeliene V., Noori H.R., Merlo-Pich E.V., Corsi M.A., Corti C., Spanagel R. Inhibition of the casein-kinase-1-ε/δ/ prevents relapse-like alcohol drinking. Neuropsychopharmacology. 2012;37:2121–2131. doi: 10.1038/npp.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takahashi T.T., Vengeliene V., Spanagel R. Melatonin reduces motivation for cocaine self-administration and prevents relapse-like behavior in rats. Psychopharmacology (Berl) 2017;234:1741–1748. doi: 10.1007/s00213-017-4576-y. [DOI] [PubMed] [Google Scholar]
- 90.Liu L.W., Lu J., Wang X.H., Fu S.K., Li Q., Lin F.Q. Neuronal apoptosis in morphine addiction and its molecular mechanism. Int J Clin Exp Med. 2013;6:540–545. [PMC free article] [PubMed] [Google Scholar]
- 91.Tan M., Li Z., Ma S., Luo J., Xu S., Lu A., et al. Heroin activates Bim via c-Jun N-terminal kinase/c-Jun pathway to mediate neuronal apoptosis. Neuroscience. 2013;233:1–8. doi: 10.1016/j.neuroscience.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 92.Pu H., Wang X., Zhang J., Ma C., Su Y., Li X., et al. Cerebellar neuronal apoptosis in heroin-addicted rats and its molecular mechanism. Int J Clin Exp Pathol. 2015;8:8260–8267. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.